Abstract
Background:
Severe injury can provoke systemic processes that lead to organ dysfunction, and hemolysis of both native and transfused red blood cells (RBC’s) may contribute. Hemolysis can release erythrocyte proteins, such as hemoglobin and arginase-1, the latter with the potential to disrupt arginine metabolism and limit physiologic nitric oxide (NO) production. We aimed to quantify hemolysis and arginine metabolism in trauma patients and measure association with injury severity, transfusions, and outcomes.
Methods:
Blood was collected from injured patients at a Level I Trauma Center enrolled in the COMBAT trial. Proteomics and metabolomics were performed on plasma fractions through liquid chromatography coupled with mass spectrometry. Abundances of erythrocyte proteins comprising a hemolytic profile as well as haptoglobin, L-arginine, ornithine, and L-citrulline (NO surrogate marker) were analyzed at different timepoints and correlated with transfusions and adverse outcomes.
Results:
More critically injured patients, non-survivors, and those with longer ventilator requirement had higher levels of hemolysis markers with reduced L-arginine and L-citrulline. In logistic regression, elevated hemolysis markers, reduced L-arginine, and reduced L-citrulline were significantly associated with these adverse outcomes. An increased number of blood transfusions was significantly associated with elevated hemolysis markers and reduced L-arginine and L-citrulline independently of new injury severity score and arterial base excess.
Conclusions:
Severe injury induces intravascular hemolysis, which may mediate post-injury organ dysfunction. In addition to native RBC’s, transfused RBC’s can lyse and may exacerbate trauma-induced hemolysis. Arginase-1 released from RBC’s may contribute to the depletion of L-arginine and the subsequent reduction in the NO necessary to maintain organ perfusion.
Keywords: Trauma, Hemolysis, Transfusion, L-arginine, Nitric Oxide
Introduction:
Trauma continues to be a major source of morbidity and mortality worldwide. Many severely injured patients survive the initial resuscitation period only to experience a dysregulated, systemic inflammatory response and protracted physiological derangement that lead to organ dysfunction and adverse clinical outcomes. Despite being an ongoing focus in trauma research, the mechanisms driving this pathology remain elusive. Multiple investigations have reported trauma-associated intravascular hemolysis, and this process may play a role in prolonged thromboinflammatory dysregulation [1–4].
In the mid-20th century, Hardaway et al. first described trauma-associated hemolysis while experimenting with a trauma/hemorrhagic shock model in dogs [1]. Later in the 1980’s, Stahl detected decreased haptoglobin levels in a small group of patients sustaining major abdominal trauma, and then near the turn of the century, Gando and Tedo provided evidence of hemolysis in 53 severely injured patients [2–3]. The mechanisms behind this trauma-induced hemolysis are unknown, but complement deposition and microcirculatory fracturing due to endothelial damage have been suggested to contribute [5–7]. Not only can native red blood cells (RBC’s) lyse, but evidence suggests that transfused RBC’s lyse in the setting of trauma as well [3,6,8]. One cannot ignore the impact of the RBC storage lesion, but these transfused cells may be more susceptible to lysis once infused into an already pro-hemolytic trauma milieu [6,9]. Undeniably this has significant implications for resuscitation of the injured patient given the frequency of transfusion in this setting.
A body of work has demonstrated the deleterious effects of hemolysis, and trauma is a disease process that is certainly not immune to these effects [10–13]. Release of free hemoglobin carries substantial pathologic capability, and this is perhaps best exemplified in the unsuccessful trials of hemoglobin-based blood substitutes [10]. Free hemoglobin (Hgb) reacts with and depletes nitric oxide (NO) [11]. NO is vital to vascular homeostasis, protecting the endothelium and helping to maintain tissue perfusion, an important property in trauma with hemorrhagic shock [11]. Free heme and Hgb are also pro-inflammatory and can undergo reactions generating reactive oxygen species (ROS) [12–13]. Hemoglobin, however, is not the only intracellular RBC protein that can be released into circulation from hemolysis; RBC’s also contain high concentrations of the enzyme arginase-1 [14–15]. Arginase-1 converts the amino acid L-arginine into ornithine and urea. It competes with nitric oxide synthase (NOS), which also uses L-arginine as a substrate in the production of NO and L-citrulline. It is therefore possible that hemolysis increases the concentration of circulating arginase-1, which has the potential to deplete the available extracellular L-arginine for NO synthesis. Indeed, there is extensive pre-clinical literature describing reduced L-arginine after traumatic injury and numerous benefits, including improved survival, with repletion of L-arginine to enhance NO synthesis in this setting [16–19]. Furthermore, these observations parallel findings supporting that trauma patients have suppressed NO production [20]. In a murine model, increased plasma arginase and decreased arginine after trauma were accompanied by significantly reduced citrulline, a surrogate for NO production [17]. Given the normal levels of liver enzymes measured in this study, the liver was eliminated as a potential source of the elevated plasma arginase; the authors speculated the vascular endothelium and neutrophils may be sources but failed to consider the RBC and hemolysis [17].
Trauma-induced hemolysis is an under-investigated area of trauma research and has significant implications for the management of injured patients. Release of arginase-1, depletion of L-arginine, and reduced production of NO has been largely ignored as a potential mechanism of hemolysis-driven organ dysfunction that warrants investigation. While modern mass spectrometry methods have been used to measure elevated products of hemolysis in the post-shock mesenteric lymph of a small number of injured patients, to date, there are no reported hemolysis-focused proteomics analyses of trauma patients [21]. Therefore, the present study employed discovery proteomics to quantify hemolysis in severely injured patients. We further used metabolomics to probe the pathways of L-arginine metabolism. We hypothesized that more critically injured patients would have increased hemolysis early in their course, this hemolysis would be associated with increased blood transfusions and adverse outcomes, and there would be evidence that the release of arginase-1 dysregulates L-arginine metabolism, suppressing production of NO.
Methods:
This study was a post hoc analysis of data collected as part of the Control Of Major Bleeding After Trauma (COMBAT) trial, a single center, randomized controlled trial of pre-hospital plasma for traumatic hemorrhagic shock (NCT01838863). Institutional Review Board (IRB) approval of our research protocol was obtained under exemption from informed consent requirements for emergency research (21 CFR 50.24). The STrengthening the Reporting of OBservational Studies in Epidemiology (STROBE) checklist was used to ensure abidance to the Enhancing the QUAlity and Transparency Of Health Research (EQUATOR) guidelines for reporting observational studies (see Checklist, Supplemental Digital Content 1, STROBE Checklist). Adherence to the reporting standards for clinical trials and a CONSORT flow diagram have been previously published for the COMBAT trial [22].
Patient and Sample Collection
The inclusion criteria for the COMBAT trial have been previously published [22]. Briefly, adult trauma patients with hypotension in the field due to hemorrhage were included, and blood samples were collected in the field and immediately upon emergency department (ED) arrival, followed by samples at six, 12, and 24 hours. Under IRB approval, blood samples from an appropriately matched cohort of 100 prospectively collected healthy volunteers (median age 30 years, 52% male) were used as a control group.
Sample Measurement (Proteomics and Metabolomics)
Whole blood samples were collected on ice, centrifuged to yield platelet-free plasma, and flash frozen in liquid nitrogen as previously described [22]. Banked plasma samples were digested with trypsin in the S-Trap 96-well plate (Protifi, Huntington, NY) after reduction and alkylation. Each sample was loaded onto individual Evotips for desalting and washing. The Evosep One system (Evosep, Odense, Denmark) was used to separate peptides on a Pepsep column. The system was coupled to the timsTOF Pro mass spectrometer (Bruker Daltonics, Bremen, Germany) via the nano-electrospray ion source (Captive Spray, Bruker Daltonics). Raw data files were converted to peak lists and queried for peptide identification against the human proteome (Uniprot database) using Spectronaut (Biognosys, Schlieren, Switzerland). Peptide identifications were summed (area under the curve) to generate protein level abundance quantities. Metabolites were extracted from plasma samples for mass spectrometric analysis using previously described methods [23]. Briefly, metabolites were extracted using a methanol, acetonitrile, and water (5:3:1) solution. Extracts were vortexed vigorously and centrifuged to pellet insoluble fractions. Supernatants were transferred to autosampler vials and loaded into a Vanquish UHLPC coupled to a Q-Exactive mass spectrometer (Thermo Fisher Scientific, Bremen, Germany) with a Kinetex C18 column (Phenomenex). Raw data was processed using Maven (Princeton University, Princeton, NJ, USA) equipped with the KEGG database.
Outcome Measures
Patients were followed for 28 days. Outcome measures included mortality as well as 28-day ventilator-free days (VFD). Massive transfusion (MT) was defined as receiving >10 RBC units within six hours post-injury or death within six hours post-injury after receiving at least one RBC unit.
Variables of Interest
There have been several studies to characterize the human erythrocyte proteome [14–15]. Based on these prior analyses, we mined our proteomics data to establish a set of well covered proteins representing a proteomic signature of hemolysis in our plasma samples. This set of analytes contained a mixture of cytoplasmic and membrane-associated RBC proteins [14–15]. The hemolytic protein profile (with Uniprot gene names) was comprised of hemoglobin beta subunit (HBB), bisphosphoglycerate mutase (BPGM), peroxiredoxin-2 (PRDX2), LDHA (lactate dehydrogenase A chain), LDHB (lactate dehydrogenase B chain), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), catalase (CAT), carbonic anhydrase 1 (CA1), erythrocyte band 7 integral membrane protein (STOM), band 3 anion transport protein (SLC4A1), and arginase-1 (ARG1). We also measured haptoglobin (HP), which is cleared from circulation after binding free hemoglobin, as an additional index of hemolysis. The Uniprot gene names are used in subsequent references to these proteins. From our metabolomics data, we also analyzed L-arginine, L-citrulline, and ornithine to investigate L-arginine metabolism. As L-citrulline is produced stoichiometrically with NO in the NOS-catalyzed conversion of L-arginine to NO, it was used as a surrogate for NO production in this study as has been done in multiple prior studies [17,24]. To characterize patients by injury severity and degree of hemorrhagic shock, we included the new injury severity score (NISS) and the clinical laboratory value of the arterial base excess (BE) measured on ED arrival. For some of our analyses, patients were stratified into “shock/injury phenotype” groups defined by combinations of NISS and BE using the cutoffs NISS≥25 versus <25 and BE<−10 vs ≥10 mEq/L; these cutoffs were based on previous studies, including the original description of the COMBAT randomized controlled trial [22].
Statistical Analyses
All statistical analyses were conducted using Prism vs 9.3.1 (GraphPad Software, LLC). Spearman correlation, Mann-Whitney tests, Kruskal-Wallis tests, and Dunn’s tests were used as appropriate. Simple logistic regression with receiver operating characteristics (ROC) curve analysis was employed to measure association of analytes with outcomes and massive transfusion groups. Multiple logistic regression was used to adjust for NISS and BE. To categorize patients into low and high VFD groups, the median VFD among all patients with plasma samples at the ED timepoint was used as a cutoff.
Results:
There were 118 (94%) patients of the 125 patients included in the COMBAT trial with proteomics and metabolomics data for analysis. Of those 118, there were 107 (91%) with plasma samples at the ED timepoint, which comprised the patient cohort for the majority of our analyses. Based on eligibility criteria for the study, this was a severely injured patient cohort with median NISS 27 and BE −9.0 mEq/L. For temporal trends and logistic analyses at timepoints beyond the initial ED timepoint, the 13 (11%) non-survivors were excluded to avoid survival bias. Not every patient had plasma samples at every analyzed timepoint and therefore the composition of groups of patients with samples at the different timepoints (“timepoint groups”) differed slightly. However, missingness was minimal enough that these groups of patients were similar, thus we did not anticipate selection bias when comparing different timepoint groups for temporal trend analyses (Table 1). The only difference observed was the group of patients with samples at 24 hours having slightly fewer ventilator-free days (VFD) than the other timepoint groups. Despite this small difference when using VFD as a continuous variable, when considering the categorical groups of fewer and greater VFD defined by the above-described cutoff, the proportions of patients in the fewer versus greater VFD groups were no different across timepoint groups (Table 1). Additional patient characteristics and outcomes for all patient groups can be viewed in Table 1.
Table 1.
Baseline characteristics and outcomes
| ED (all patients)† | ED | 6hr | 12hr | 24hr | |
|---|---|---|---|---|---|
| Total n | 107 | 94 | 83 | 73 | 68 |
| COMBAT Study Group | |||||
| Experimental | 56 (52%) | 50 (53%) | 41 (49%) | 37 (51%) | 38 (56%) |
| Control | 51 (48%) | 44 (47%) | 42 (51%) | 36 (49%) | 30 (44%) |
| Age (median/IQR) | 33 (26–49) | 34.5 (25–49.3) | 34 (25–49) | 34 (25–49) | 34.5 (25–49.8) |
| Male Sex | 86 (80%) | 75 (80%) | 70 (84%) | 57 (78%) | 57 (84%) |
| Mechanism | |||||
| Blunt | 55 (51%) | 48 (51%) | 43 (52%) | 42 (58%) | 37 (54%) |
| Penetrating | 52 (49%) | 46 (49%) | 40 (48%) | 31 (42%) | 31 (46%) |
| NISS (median/IQR) | 27 (11–41) | 22 (10–34) | 27 (11–34) | 27 (12.5–4) | 27 (12.3–41) |
| BE (median/IQR) | −9 (−13 to −5.75) | −9 (−12 to −5) | −9.5 (−12.8 to −6) | −10 (−13 to −6.85) | −10.2 (−13.25 to −6.95) |
| Missing BE | 14 (13%) | 11 (12%) | 7 (8%) | 5 (7%) | 3 (4%) |
| Injury/Shock Groups | |||||
| Group 1 (NISS<25, BE≥−10) | 24 (26%) | 24 (29%) | 22 (29%) | 19 (28%) | 17 (26%) |
| Group 2 (NISS<25, BE<−10) | 14 (15%) | 14 (17%) | 13 (17%) | 10 (15%) | 13 (20%) |
| Group 3 (NISS>25, BE≥−10) | 29 (31%) | 26 (31%) | 21 (28%) | 18 (26%) | 16 (25%) |
| Group 4 (NISS>25, BE<−10) | 26 (28%) | 19 (23%) | 20 (26%) | 21 (31%) | 19 (29%) |
| MT | 25 (23%) | 12 (13%) | 14 (17%) | 14 (19%) | 13 (19%) |
| VFD (median/IQR) | 26 (14–28) | 27 (22–28) | 26 (21–28) | 25 (18–28) | 25 (14.8–27)* |
| VFD <26 | 50 (47%) | 37 (39%) | 36 (43%) | 37 (51%) | 37 (54%) |
| Mortality | 13 (12%) | N/A | N/A | N/A | N/A |
Abbreviations/Definitions: ED (emergency department), Experimental (randomized to pre-hospital plasma in COMBAT trial), Control (randomized to normal saline in COMBAT trial), IQR (interquartile range), NISS (new injury severity score), BE (base excess measured on ED arrival), MT (massive transfusion), VFD (ventilator-free days)
Only the 24hr group had statistically fewer ventilator-free days (VFD) compared to the other timepoint groups (P<0.05); otherwise there were no differences between timepoint groups for any of the variables.
Includes all patients with plasma samples at the ED timepoint; the other timepoint groups exclude non-survivors
More critically injured patients had higher levels of hemolysis markers, including ARG1, and reduced L-arginine on ED arrival (Fig. 1). In addition to ARG1, Fig. 1 displays HBB and SLC4A1 as erythrocyte-specific cytoplasmic and membrane proteins, respectively, but all analyzed RBC proteins followed this same pattern by shock/injury phenotype (see Figure, Supplemental Digital Content 2, Extended hemolysis markers by shock/injury phenotype). Ornithine levels were also elevated on ED arrival in severely injured patients in hemorrhagic shock. Temporal trend analysis revealed that hemolysis persisted longer in more critically injured patients, and this trend paralleled reductions in L-arginine and L-citrulline (Fig. 2).
Figure 1.
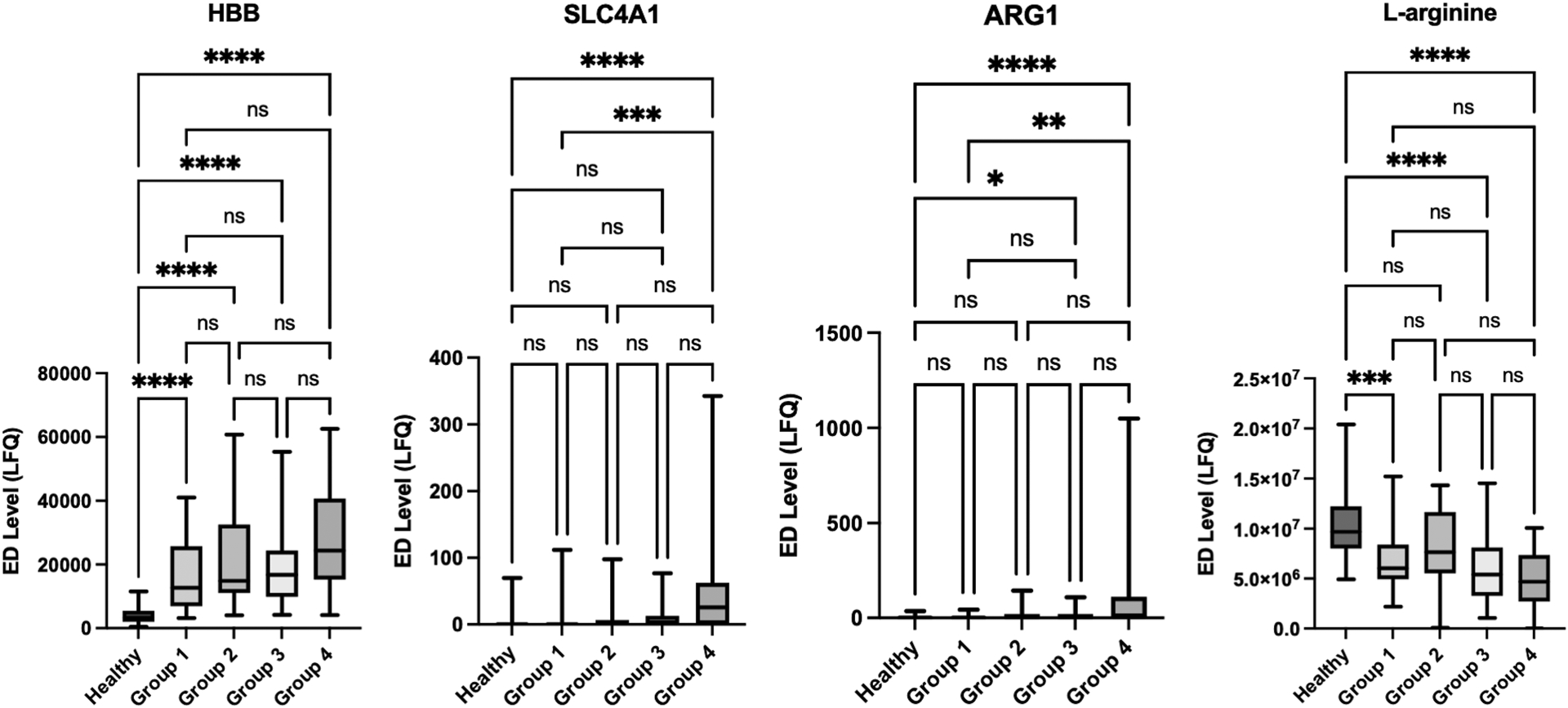
More critically injured patients had increased hemolysis on emergency department (ED) arrival with elevated plasma arginase-1 (ARG1) and decreased L-arginine. Hemoglobin beta (HBB) and band 3 anion transport protein (SLC4A1) are erythrocyte-specific cytoplasmic and membrane proteins, respectively. ED Level refers to the label-free quantification (LFQ) of analyte abundance. Healthy denotes the group of prospectively collected healthy volunteer samples. Shock/injury phenotypes are defined in Table 1 (Groups 1–4). Plots contain boxes displaying the median and interquartile range (IQR) with whiskers denoting the range.
****P<0.0001, ***P<0.001, **P<0.01, *P<0.05, ns (non-significant)
Figure 2.
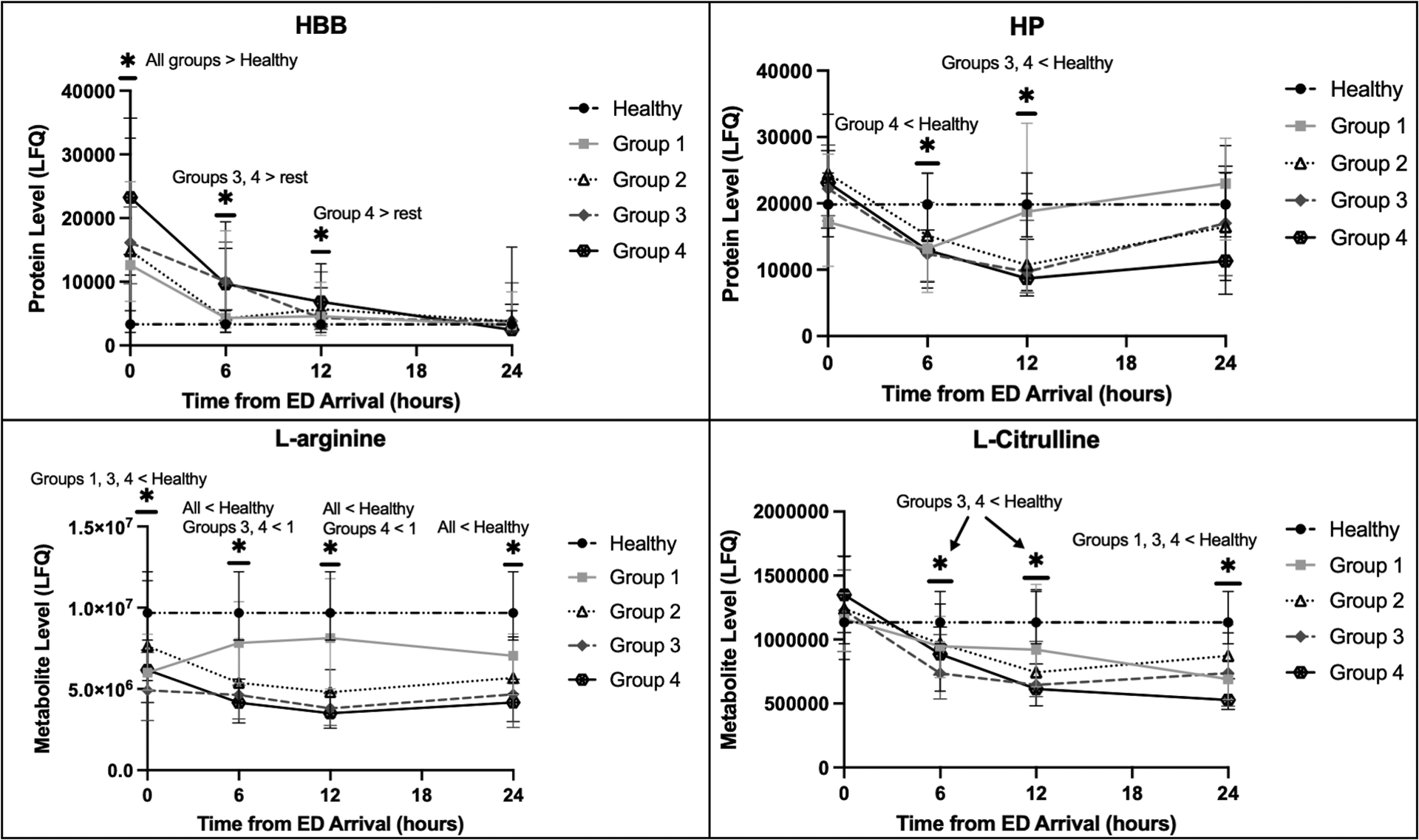
Temporal trends of the hemolysis indicators hemoglobin beta (HBB) and haptoglobin (HP) as well as L-arginine and L-citrulline by shock/injury phenotypes, demonstrating hemolysis persisted longer in more critically injured patients, which paralleled persistent reductions in L-arginine and L-citrulline. HBB was elevated early, which was followed by significant decreases in HP, consistent with HP binding free hemoglobin and being cleared from circulation. The Y-axis indicates the label-free quantification (LFQ) of analyte abundances. The healthy volunteer reference level is shown. Shock/injury phenotypes are defined in Table 1 (Groups 1–4).
*P<0.05 for the designated comparisons at each timepoint
Compared to patients who received fewer RBC transfusions, MT patients (>10 RBC units in six hours) had evidence of increased hemolysis from 6–12 hours as indicated by elevated HBB and decreased HP levels (Fig. 3A). In logistic regression, levels of nearly all analyzed hemolysis proteins at six hours were significantly positively associated with MT with several having individual areas under the ROC curve (AUC) greater than 0.70. All but one of the significantly associated hemolysis proteins at six hours remained significantly associated after adjustment for NISS and BE in multiple logistic regression (Fig. 3B). Likewise, MT patients had reduced L-arginine and L-citrulline (Fig. 3A). L-arginine levels at six hours were significantly negatively associated with receiving >10 RBC units in six hours with an ROC AUC approaching 0.9; this association was also independent of NISS and BE in multiple logistic regression (Fig. 3B). The associations at six hours were also observed at 12 hours; notably, elevated ARG1 and decreased L-arginine and L-citrulline at 12 hours were independently associated with having received >10 RBC units in the first six hours (see Table, Supplemental Digital Content 3, Association between MT and hemolysis at 12 hours).
Figure 3.
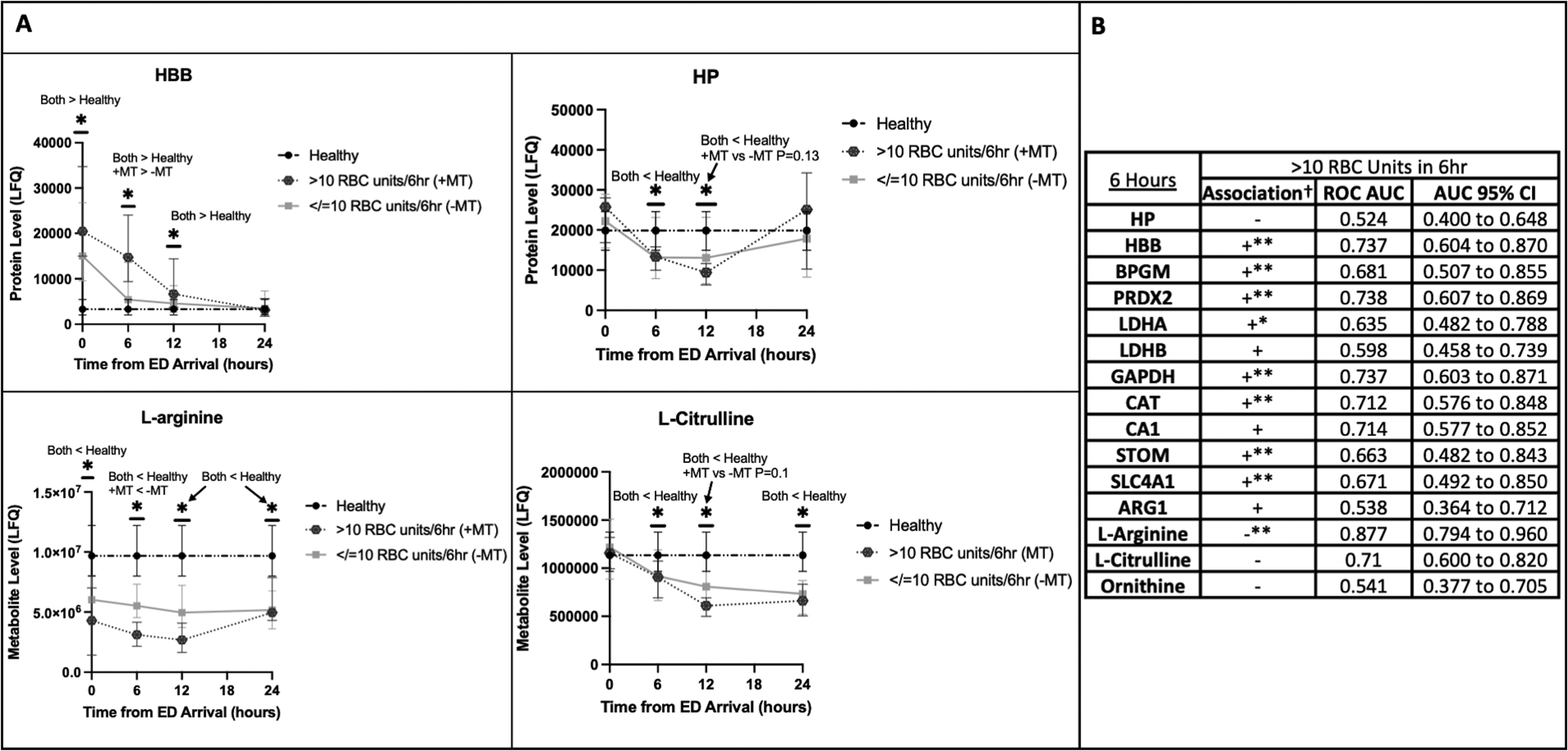
Increased number of red blood cell (RBC) transfusions in the first six hours was associated with increased hemolysis and decreased L-arginine and L-citrulline.
A: Temporal trends of the hemolysis indicators hemoglobin beta (HBB) and haptoglobin (HP) as well as L-arginine and L-citrulline for patients who received >10 RBC units in six hours (+MT [massive transfusion]) and those who received 10 or fewer (-MT). The healthy control reference level is shown. The Y-axis indicates the label-free quantification (LFQ) of analyte abundances.
*P<0.05 for the designated comparisons at each timepoint.
B: Logistic regression with receiver operating characteristic (ROC) curve areas under the curves (AUC) and corresponding 95% confidence intervals (CI) for association of individual analyte levels at six hours with having received >10 RBC units in six hours.
†Association in simple logistic regression is designated with “+” if the odds ratio (OR) is >1.0 and “−” if the OR is <1.0. Lack of clinical significance for the label-free quantification of analytes renders actual OR values meaningless.
*The 95% CI for the OR in simple logistic regression falls entirely above or below 1.0, and the association is considered statistically significant.
**The OR for individual analytes remains statistically significant after adjustment for new injury severity score (NISS) and base excess (BE) in multiple logistic regression.
All plasma hemolysis proteins analyzed were elevated on ED arrival in patients who ultimately died compared to those who survived (Fig. 4A; also see Figure, Supplemental Digital Content 4, Extended hemolysis markers by mortality). In logistic regression, nearly all hemolysis proteins were significantly positively associated with mortality, and most had ROC AUC >0.80 and remained significantly associated after adjustment for NISS and BE (Fig. 4B). Additionally, alongside elevated ARG1 on ED arrival, ornithine was elevated while L-citrulline was reduced in non-survivors versus survivors (Fig. 4A). While decreased L-arginine on ED arrival was non-significantly associated with mortality, elevated ornithine and reduced L-citrulline were both significantly associated with mortality in logistic regression with ornithine having an ROC AUC approaching 0.80 (Fig. 4B).
Figure 4.
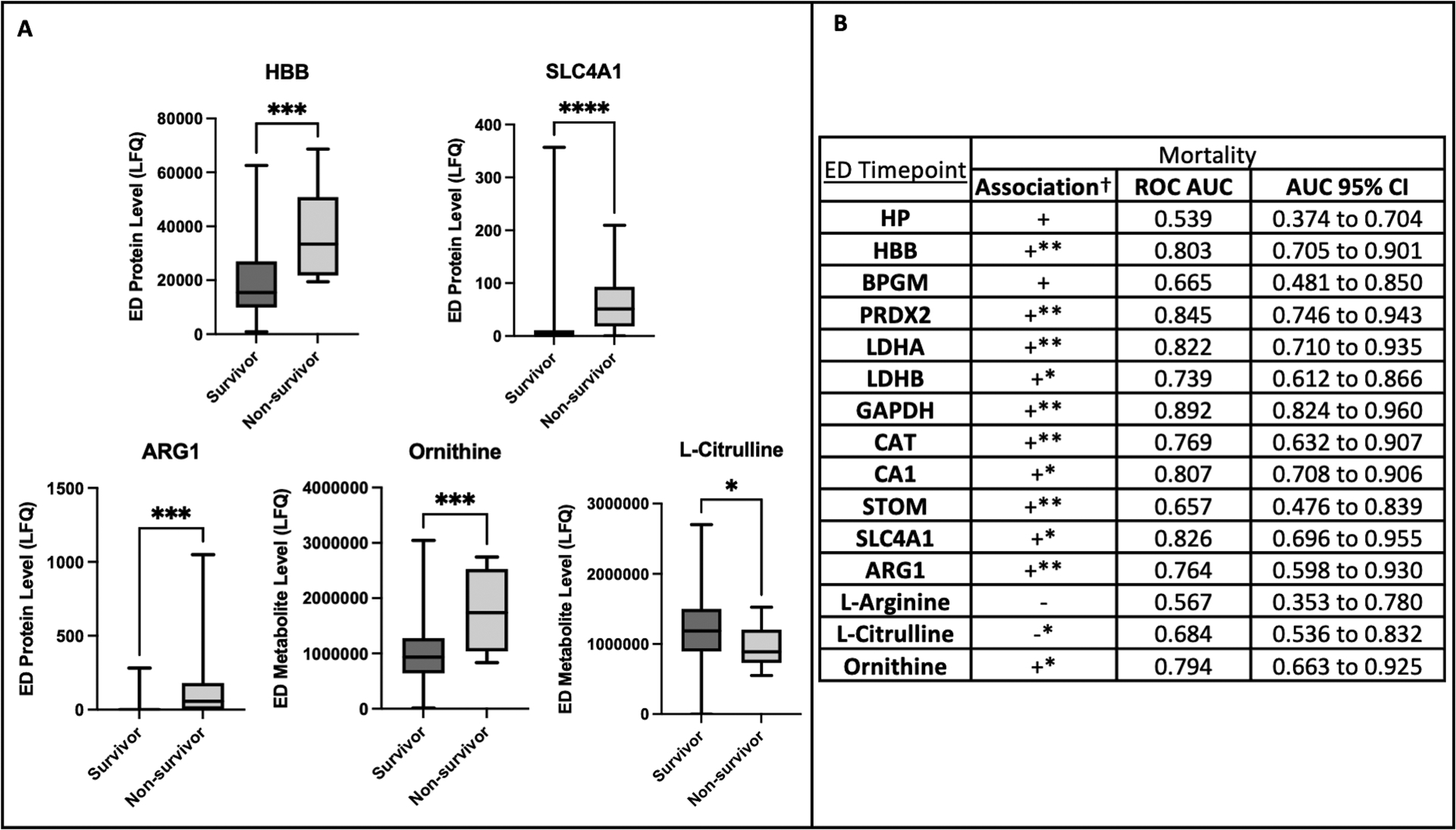
Non-survivors had increased hemolysis on emergency department (ED) arrival with elevated arginase-1 (ARG1), elevated ornithine, and decreased L-citrulline. A: Comparison of ED levels by mortality. Hemoglobin beta (HBB) and band 3 anion transport protein (SLC4A1) are erythrocyte-specific cytoplasmic and membrane proteins, respectively. Protein/metabolite level refers to the label-free quantification (LFQ) of analyte abundance. Plots contain boxes displaying the median and interquartile range (IQR) with whiskers denoting the range.
****P<0.0001, ***P<0.001, *P<0.05.
B: Logistic regression with receiver operating characteristic (ROC) curve areas under the curves (AUC) and corresponding 95% confidence intervals (CI) for association of individual analyte levels on ED arrival with mortality.
†Association in simple logistic regression is designated with “+” if the odds ratio (OR) is >1.0 and “−” if the OR is <1.0. Lack of clinical significance for the label-free quantification of analytes renders actual OR values meaningless.
*The 95% CI for the OR in simple logistic regression falls entirely above or below 1.0, and the association is considered statistically significant.
**The OR for individual analytes remains statistically significant after adjustment for new injury severity score (NISS) and base excess (BE) in multiple logistic regression.
Compared to patients with more VFD, patients with fewer VFD had more hemolysis over the first 24 hours as evidenced by initially higher HBB levels with subsequently persistently lower HP from six hours onward (Fig. 5A). This is also reflected in nearly all hemolysis proteins at the ED timepoint and several at six and 12 hours being significantly positively associated with having <26 VFD in logistic regression, several remaining significantly associated after adjustment for NISS and BE in multiple logistic regression. Notably, by six hours, HP was significantly negatively associated with having <26 VFD, and this negative association remained significant at 12 hours even after adjustment for NISS and BE (Fig. 5B; also see Tables, Supplemental Digital Content 5, Association of hemolysis at six and 12 hours with having <26 VFD). Decreased L-arginine and L-citrulline over this time period were also associated with having <26 VFD (Fig. 5; also see Tables, Supplemental Digital Content 5, Association of hemolysis at six and 12 hours with having <26 VFD). Elevated ARG1 and ornithine on ED arrival were both independently associated with having <26 VFD (Fig. 5B). While L-arginine levels at ED, six hours, and 12 hours were significantly negatively associated with fewer VFD, by six hours, this association remained significant after adjustment for NISS and BE (Fig. 5; also see Tables, Supplemental Digital Content 5, Association of hemolysis at six and 12 hours with having <26 VFD).
Figure 5.
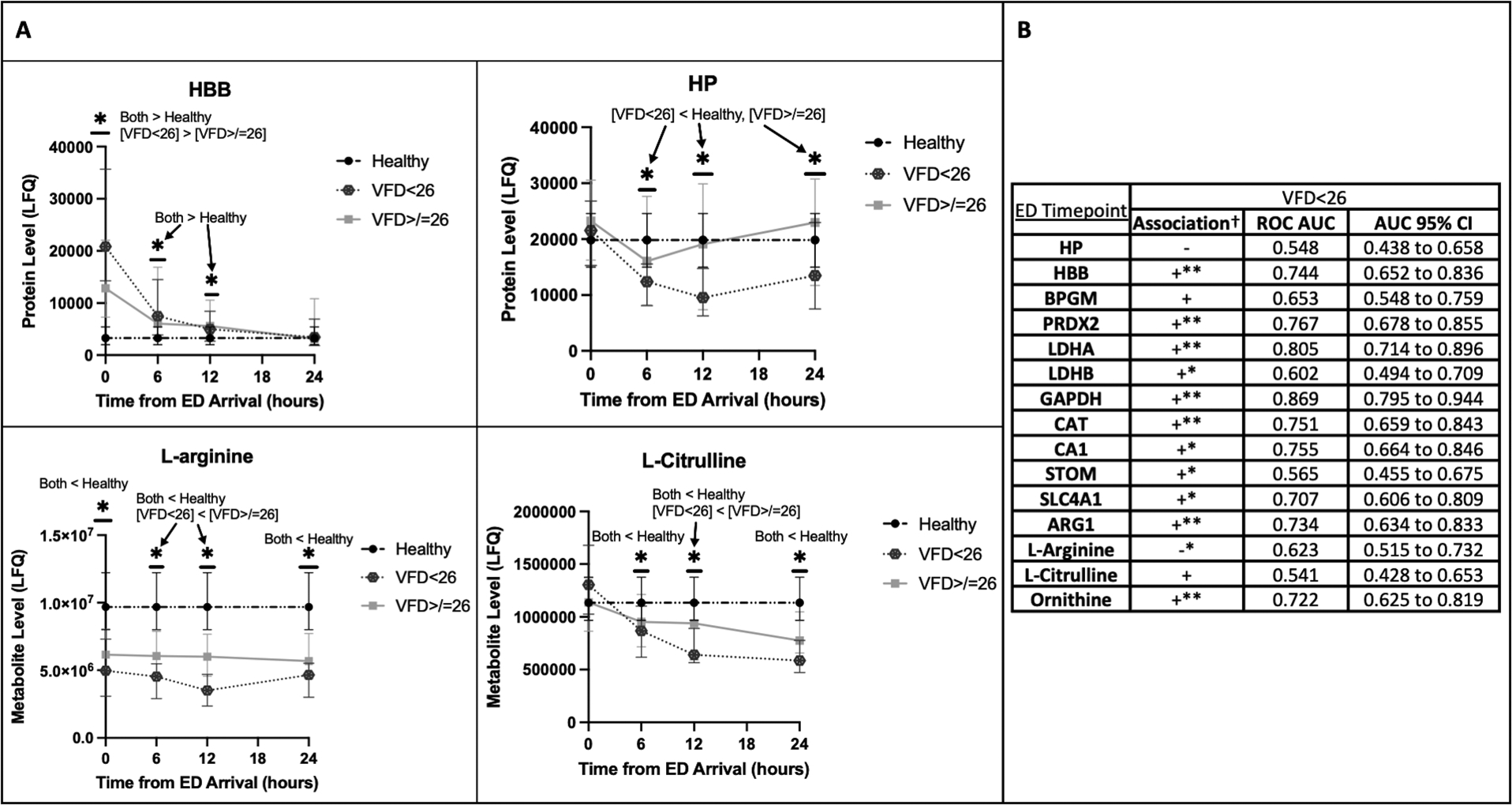
Increased hemolysis and decreased L-arginine and L-citrulline were associated with longer ventilator requirement.
A: Temporal trends of the hemolysis indicators hemoglobin beta (HBB) and haptoglobin (HP) as well as L-arginine and L-citrulline for patients with <26 ventilator-free days (VFD) and those with ≥26 VFD. The healthy control reference level is shown. The Y-axis indicates the label-free quantification (LFQ) of analyte abundances.
*P<0.05 for the designated comparisons at each timepoint.
B: Logistic regression with receiver operating characteristic (ROC) curve areas under the curves (AUC) and corresponding 95% confidence intervals (CI) for association of individual analyte levels on ED arrival with having <26 VFD.
†Association in simple logistic regression is designated with “+” if the odds ratio (OR) is >1.0 and “−” if the OR is <1.0. Lack of clinical significance for the label-free quantification of analytes renders actual OR values meaningless.
*The 95% CI for the OR in simple logistic regression falls entirely above or below 1.0, and the association is considered statistically significant.
**The OR for individual analytes remains statistically significant after adjustment for new injury severity score (NISS) and base excess (BE) in multiple logistic regression.
Discussion:
We employed untargeted, modern mass spectrometry-based proteomics to measure intravascular hemolysis in trauma patients, which is more severe in more critically injured patients and associated with adverse outcomes. The presented data further suggest transfused RBC’s can lyse and may exacerbate this trauma-induced hemolysis. With the additional use of discovery metabolomics, we have also illuminated released arginase-1 with depletion of L-arginine and consequent reduced production of NO as a potential mechanism of hemolysis-driven organ dysfunction in this setting.
The question remains of what in the post-injury, thromboinflammatory milieu is causing this hemolysis. There are several plausible mechanisms supported by prior work. Complement C4d, a stable split-product of C4 activation, has been shown to be deposited early on the RBC’s of trauma patients with prolonged deposition in the more severely injured [5]. This C4d deposition has been demonstrated to limit RBC deformability in a simulated microcirculation, and this functional effect has been attributed to increased RBC calcium influx and phosphorylation of band 3 [5]. While not investigated in these prior experiments, the observed RBC cytoskeletal dysfunction induced by C4d deposition may promote microcirculatory hemolysis, and band 3 phosphorylation has previously been linked to hemolysis [25]. Future studies using peptide-level proteomics to analyze band 3 phosphorylation will help clarify this mechanism. To further support the involvement of complement, a recent study demonstrated complement C4 activation in injured patients was associated with adverse outcomes [26]. Complement activation, therefore, may be the link between severe injury and hemolysis. It is also possible that trauma-associated endothelial damage contributes to RBC shear stress and lysis within the capillaries, but this has yet to be confirmed experimentally [7]. Beyond native RBC lysis, prior work and the presented data here suggest transfused RBC’s can lyse when subjected to this trauma-induced, hemolytic milieu [3,6,8]. Stored RBC’s may even be more susceptible to complement deposition [6,9]. Additionally, while recent studies from the REDS (Recipient Epidemiology and Donor Evaluation Study) group have shown a role for donor biology and exposures in mediating hemolysis of transfused RBC’s, our study here highlights the importance of determining how recipient biology may also impact hemolytic propensity of the allogeneic, transfused erythrocyte [27–28]. Indeed, personalized omics signatures of donors and recipients will be the subject of future studies from our group, but additional investigation is undoubtedly needed on endogenous versus transfused RBC lysis in trauma, which may unveil critical implications for resuscitation of the severely injured patient.
While demonstrating trauma-induced hemolysis is associated with adverse outcomes, these data also shed light on a largely ignored process that may be contributing to the downstream pathology of hemolysis in these injured patients. Arginase-1 is an enzyme normally located in the cytoplasm of RBC’s, shown here to be elevated in the plasma of trauma patients as part of a hemolytic protein profile. We observed early ARG1 elevations alongside decreased L-arginine, increased ornithine, and decreased L-citrulline. We presume the increased plasma arginase was consuming circulating L-arginine in the formation of ornithine and urea, contributing, at least in part, to an L-arginine-deficient state. As L-arginine is the substrate for NOS, its depletion may have limited the production of NO necessary to maintain organ perfusion in the setting of trauma and hemorrhagic shock, perhaps at least partially explaining the association with adverse outcomes. Our observation of reduced L-citrulline supports that this process is occurring in these patients. This is consistent with the findings by Pribis et al. who reported plasma arginase activity was increased, and arginine and citrulline were decreased in a murine model of trauma as well as Jacob et al. who detected reduced plasma and urinary nitrate/nitrite levels in injured patients [17,20]. The former study was unable to identify the source of the increased plasma arginase activity, concluding it was not arginase release from the liver, but we contend the source may, in fact, be the lysed RBC. Additionally, transfusion of blood cold stored for 42 days has been shown to hemolyze in recipients, which was associated with increased arginase-1 activity at the expense of NO synthesis [29].
It would seem that arginine administration has therapeutic utility in trauma, and, indeed, the benefits of L-arginine administration on NO production and outcome have been demonstrated in multiple animal models of trauma and hemorrhagic shock [16–19]. In one study, D-arginine in the resuscitation fluid did not improve organ blood flow following trauma-hemorrhage while L-arginine did [19]. As D-arginine is not a substrate for NOS and L-arginine is, the beneficial effects of L-arginine are presumably due to its conversion to NO by NOS [30]. Despite the multiple studies indicating a benefit to L-arginine supplementation in critically injured patients, the literature is still undecided [31]. Part of this controversy is due to compelling evidence against L-arginine supplementation in septic patients [32–33]. However, while overlapping in many ways related to the systemic inflammatory response, sepsis and trauma are still very different disease processes. Trauma may be dominated by arginase-1 activity while sepsis induces NOS activity, the latter being able to generate pathologic amounts of NO and contribute to a host of deleterious effects [32–33]. Trauma-induced hemolysis, therefore, may be the tipping factor. Nevertheless, this study calls for revisiting the arginine-NO axis and reinvigorates the hypothesis that L-arginine and NO donors may have therapeutic potential in a subset of trauma patients.
This study has several limitations. Broadly, we present correlative human data analyzed retrospectively and therefore are unable to control for all variables contributing to illness severity and medical decision-making. Furthermore, disruption of arginine-NO metabolism is a multifactorial phenomenon, and the influence of different NOS isoforms adds a layer of complexity that we did not pursue in this investigation [31]. While the presented analysis is novel and provides compelling evidence, well-designed in vitro and in vivo experiments are necessary to fully elucidate the mechanisms of trauma-induced hemolysis and its downstream impacts on organ dysfunction. Importantly, while there is emerging evidence that RBC’s play a more vital role in clotting than previously appreciated, the relationship between hemolysis and coagulopathy was beyond the scope of this study [34]. Future analyses examining the omics signature of hemolysis-associated coagulopathy in conjunction with the use of microfluidics technology may prove enlightening.
In summary, with the use of discovery proteomics, we have documented hemolysis in trauma patients, as early as on ED arrival, worse in more critically injured patients, and independently associated with RBC transfusions. This trauma-induced hemolysis is associated with adverse clinical outcomes, and, in addition to the deleterious effects of circulating free hemoglobin, arginase-1 released from RBC’s may contribute to the depletion of L-arginine and the subsequent reduction in the NO production necessary to maintain organ perfusion. This study lays the groundwork for future experiments to identify the mediators of trauma-induced hemolysis and explore the differential contribution of native and transfused RBC lysis. It further highlights the need to more intensely evaluate the impact of increased plasma arginase-1 on NO and outcomes and offers exciting potential to therapeutically intervene on this pathway.
Supplementary Material
STrengthening the Reporting of OBservational Studies in Epidemiology (STROBE) Checklist.
Association between massive transfusion criteria (>10 RBC units in six hours) and hemolysis at 12 hours in logistic regression. Receiver operating characteristic (ROC) curve areas under the curves (AUC) and corresponding 95% confidence intervals (CI) for the individual analytes are also shown.
Emergency department (ED) levels of additional hemolysis proteins by shock/injury (S/I) phenotype (Groups 1–4, defined in Table 1) with comparisons to healthy volunteers. Protein identifications for Uniprot gene names can be found in the methods section. ED Level refers to the label-free quantification (LFQ) of analyte abundance. Plots contain boxes displaying the median and interquartile range (IQR) with whiskers denoting the range.
****P<0.0001, ***P<0.001, **P<0.01, *P<0.05, ns (non-significant)
Emergency department (ED) levels of additional hemolysis proteins by mortality. Protein/metabolite level refers to the label-free quantification (LFQ) of analyte abundance. Plots contain boxes displaying the median and interquartile range (IQR) with whiskers denoting the range. ****P<0.0001, ***P<0.001, **P<0.01, *P<0.05
Association between hemolysis at six and 12 hours with having <26 ventilator-free days (VFD) in logistic regression. Receiver operating characteristic (ROC) curve areas under the curves (AUC) and corresponding 95% confidence intervals (CI) for the individual analytes are also shown.
Funding Disclosure and Acknowledgements
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health (T32 GM008315 and P50 GM049222) and the Department of Defense (USAMRAA, W81XWH-12-2-0028). The Control of Major Bleeding after Trauma (COMBAT W81XWH-12-2-2008) was funded by the Department of Defense. Other important funding sources include a series of grants through the Trans-agency Research Consortium for Trauma Induced Coagulopathy (TACTIC UM1-HL120877) from the National Heart, Lung and Blood Institute, NIH. The current major funding source is an RM-1 grant (1RM1GM131968-01) that runs through May of 2024. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other sponsors of the project.
Footnotes
Conflict of Interest Statement: The authors report no conflicts of interest.
Supplemental Digital Content (SDC):
SDC 1 (checklist): STROBE Checklist
SDC 2 (figure): Box plots of extended hemolysis markers by shock/injury phenotype
SDC 3 (table): Table of associations in logistic regression between MT and hemolysis at 12 hours
SDC 4 (figure): Box plots of extended hemolysis markers by mortality
SDC 5 (tables): Tables of associations in logistic regression between hemolysis at six and 12 hours with having <26VFD
References
- 1.Hardaway RM, Johnson DG, Elovitz MJ, Houchin DN, Jenkins EB, Burns JW, Jackson DR. Influence of trauma and hemolysis on hemorrhagic shock in dogs. J Trauma. 4:624–641, 1964. [DOI] [PubMed] [Google Scholar]
- 2.Stahl WM. Acute phase protein response to tissue injury. Crit Care Med. 15(6):545–550, 1987. [DOI] [PubMed] [Google Scholar]
- 3.Gando S, Tedo I. The effects of massive transfusion and haptoglobin therapy on hemolysis in trauma patients. Surg Today. 24(9):785–790, 1994. [DOI] [PubMed] [Google Scholar]
- 4.Moore HB, Moore EE, Gonzalez E, Hansen KC, Dzieciatkowska M, Chapman MP, Sauaia A, West B, Banerjee A, Silliman CC. Hemolysis exacerbates hyperfibrinolysis, whereas platelolysis shuts down fibrinolysis: evolving concepts of the spectrum of fibrinolysis in response to severe injury. Shock. 43(1):39–46, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muroya T, Kannan L, Ghiran IC, Shevkoplyas SS, Paz Z, Tsokos M, Dalle Lucca JJ, Shapiro NI, Tsokos GC. C4d deposits on the surface of RBCs in trauma patients and interferes with their function. Crit Care Med. 42(5):e364–e372, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu X, Patel RP, Weinberg JA, Marques MB, Ramos TN, Barnum SR. Membrane attack complex generation increases as a function of time in stored blood. Transfus Med. 24(2):114–116, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buzio M, Pigella S, Memore L, Olivero G. Emolisi e politrauma. Descrizione di un caso clinico [Hemolysis and multiple trauma. A clinical case report]. Minerva Chir. 52(4):485–487, 1997. [PubMed] [Google Scholar]
- 8.Nishiyama T, Hanaoka K. Free hemoglobin concentrations in patients receiving massive blood transfusion during emergency surgery for trauma. Can J Anaesth. 47(9):881–885, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Baek JH, D’Agnillo F, Vallelian F, Pereira CP, Williams MC, Jia Y, Schaer DJ, Buehler PW. Hemoglobin-driven pathophysiology is an in vivo consequence of the red blood cell storage lesion that can be attenuated in guinea pigs by haptoglobin therapy. J Clin Invest. 122(4):1444–1458, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Natanson C, Kern SJ, Lurie P, Banks SM, Wolfe SM. Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death: a meta-analysis [published correction appears in JAMA. 2008 Sep 17;300(11): 1300]. JAMA. 299(19):2304–2312, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gladwin MT, Kanias T, Kim-Shapiro DB. Hemolysis and cell-free hemoglobin drive an intrinsic mechanism for human disease. J Clin Invest. 122(4):1205–1208, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porto BN, Alves LS, Fernández PL, Dutra TP, Figueiredo RT, Graça-Souza AV, Bozza MT. Heme induces neutrophil migration and reactive oxygen species generation through signaling pathways characteristic of chemotactic receptors. J Biol Chem. 282(33):24430–24436, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Belcher JD, Chen C, Nguyen J, Milbauer L, Abdulla F, Alayash AI, Smith A, Nath KA, Hebbel RP, Vercellotti GM. Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood. 123(3):377–390, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kakhniashvili DG, Bulla LA Jr, Goodman SR. The human erythrocyte proteome: analysis by ion trap mass spectrometry. Mol Cell Proteomics. 3(5):501–509, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Bryk AH, Wiśniewski JR. Quantitative Analysis of Human Red Blood Cell Proteome. J Proteome Res. 16(8):2752–2761, 2017. [DOI] [PubMed] [Google Scholar]
- 16.Daughters K, Waxman K, Nguyen H. Increasing nitric oxide production improves survival in experimental hemorrhagic shock. Resuscitation. 31(2):141–144, 1996. [DOI] [PubMed] [Google Scholar]
- 17.Pribis JP, Zhu X, Vodovotz Y, Ochoa JB. Systemic arginine depletion after a murine model of surgery or trauma. JPEN J Parenter Enteral Nutr. 36(1):53–59, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anaya-Prado R, Toledo-Pereyra LH, Walsh J, Guo RF, Reuben J, Ward PA. Exogenous nitric oxide donor and related compounds protect against lung inflammatory response after hemorrhagic shock and resuscitation. J Trauma. 57(5):980–988, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Angele MK, Smail N, Wang P, Cioffi WG, Bland KI, Chaudry IH. L-arginine restores the depressed cardiac output and regional perfusion after trauma-hemorrhage. Surgery. 124(2):394–402, 1998. [PubMed] [Google Scholar]
- 20.Jacob TD, Ochoa JB, Udekwu AO, Wilkinson J, Murray T, Billiar TR, Simmons RL, Marion DW, Peitzman AB. Nitric oxide production is inhibited in trauma patients. J Trauma. 35(4):590–597, 1993. [DOI] [PubMed] [Google Scholar]
- 21.Dzieciatkowska M, Wohlauer MV, Moore EE, Damle S, Peltz E, Campsen J, Kelher M, Silliman C, Banerjee A, Hansen KC. Proteomic analysis of human mesenteric lymph. Shock. 35(4):331–338, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore HB, Moore EE, Chapman MP, McVaney K, Bryskiewicz G, Blechar R, Chin T, Burlew CC, Pieracci F, West FB, et al. Plasma-first resuscitation to treat haemorrhagic shock during emergency ground transportation in an urban area: a randomised trial. Lancet. 392(10144):283–291, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nemkov T, Reisz JA, Gehrke S, Hansen KC, D’Alessandro A. High-Throughput Metabolomics: Isocratic and Gradient Mass Spectrometry-Based Methods. Methods Mol Biol. 1978:13–26, 2019. [DOI] [PubMed] [Google Scholar]
- 24.Sud A, Khullar M, Wanchu A, Bambery P. Increased nitric oxide production in patients with systemic sclerosis. Nitric Oxide. 4(6):615–619, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Pantaleo A, Ferru E, Pau MC, Khadjavi A, Mandili G, Mattè A, Spano A, De Franceschi L, Pippia P, Turrini F. Band 3 Erythrocyte Membrane Protein Acts as Redox Stress Sensor Leading to Its Phosphorylation by p (72) Syk. Oxid Med Cell Longev. 2016:6051093, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaid TR Jr, Hansen KC, Sauaia A, Moore EE, DeBot M, Cralley AL, Erickson C, Silliman CC, Banerjee A, Ghasabyan A, et al. Post-injury Complement C4 Activation is Associated with Adverse Outcomes and is Potentially Influenced by Plasma Resuscitation [published online ahead of print, 2022 May 25]. J Trauma Acute Care Surg. 10.1097/TA.0000000000003713, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanias T, Lanteri MC, Page GP, Guo Y, Endres SM, Stone M, Keating S, Mast AE, Cable RG, Triulzi DJ, et al. Ethnicity, sex, and age are determinants of red blood cell storage and stress hemolysis: results of the REDS-III RBC-Omics study. Blood Adv. 1(15):1132–1141, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nemkov T, Stefanoni D, Bordbar A, Issaian A, Palsson BO, Dumont LJ, Hay A, Song A, Xia Y, Redzic JS, et al. Blood donor exposome and impact of common drugs on red blood cell metabolism. JCI Insight. 6(3):e146175, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D’Alessandro A, Reisz JA, Zhang Y, Gehrke S, Alexander K, Kanias T, Triulzi DJ, Donadee C, Barge S, Badlam J, et al. Effects of aged stored autologous red blood cells on human plasma metabolome. Blood Adv. 3(6):884–896, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Albina JE, Reichner JS. Nitric oxide in inflammation and immunity. New Horiz. 3(1):46–64, 1995. [PubMed] [Google Scholar]
- 31.Darwiche SS, Pfeifer R, Menzel C, Ruan X, Hoffman M, Cai C, Chanthaphavong RS, Loughran P, Pitt BR, Hoffman R, et al. Inducible nitric oxide synthase contributes to immune dysfunction following trauma. Shock. 38(5):499–507, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalil AC, Danner RL. L-Arginine supplementation in sepsis: beneficial or harmful?. Curr Opin Crit Care. 12(4):303–308, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Heyland DK, Samis A. Does immunonutrition in patients with sepsis do more harm than good?. Intensive Care Med. 29(5):669–671, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Weisel JW, Litvinov RI. Red blood cells: the forgotten player in hemostasis and thrombosis. J Thromb Haemost. 17(2):271–282, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
STrengthening the Reporting of OBservational Studies in Epidemiology (STROBE) Checklist.
Association between massive transfusion criteria (>10 RBC units in six hours) and hemolysis at 12 hours in logistic regression. Receiver operating characteristic (ROC) curve areas under the curves (AUC) and corresponding 95% confidence intervals (CI) for the individual analytes are also shown.
Emergency department (ED) levels of additional hemolysis proteins by shock/injury (S/I) phenotype (Groups 1–4, defined in Table 1) with comparisons to healthy volunteers. Protein identifications for Uniprot gene names can be found in the methods section. ED Level refers to the label-free quantification (LFQ) of analyte abundance. Plots contain boxes displaying the median and interquartile range (IQR) with whiskers denoting the range.
****P<0.0001, ***P<0.001, **P<0.01, *P<0.05, ns (non-significant)
Emergency department (ED) levels of additional hemolysis proteins by mortality. Protein/metabolite level refers to the label-free quantification (LFQ) of analyte abundance. Plots contain boxes displaying the median and interquartile range (IQR) with whiskers denoting the range. ****P<0.0001, ***P<0.001, **P<0.01, *P<0.05
Association between hemolysis at six and 12 hours with having <26 ventilator-free days (VFD) in logistic regression. Receiver operating characteristic (ROC) curve areas under the curves (AUC) and corresponding 95% confidence intervals (CI) for the individual analytes are also shown.


