Abstract
We often encounter patients with congestive heart failure refractory to conventional diuretics therapy, and Kampo Goreisan is receiving great concern to mediate body water balance particularly for such a cohort. However, its detailed biological mechanism remains uncertain. We had two hospitalized patients with congestive heart failure receiving tolvaptan. Following the administration of Goreisan, both urine cyclic adenosine monophosphate concentration and urine aquaporin-2 concentration decreased, accompanied by incremental diluted urine volume. Although further studies are warranted to establish therapeutic strategy, Goreisan might be a promising therapeutic tool for those with congestive heart failure refractory to conventional diuretics including tolvaptan, via pleiotropic effects including suppression of aquaporin-incorporated water reabsorption system.
Keywords: Hemodynamics, Diuretics, Kampo
Introduction
Goreisan, which is called Wulingsan in China, is introduced in the “Treatise on Febrile Diseases” published in the early third century and is one of the most traditional herbal medicine formulas, consisting of predominantly five herbs: Alisma orientale, Polyporus umbellatus, Wolfiporia cocos, Cinnamomum cassia, and Atractylodes lancea. Goreisan is widely prescribed to treat impaired body water balance. Of note, Goreisan is reimbursed in Japan for systemic edema or nephrotic syndrome accompanying thirst and oliguria.
Tolvaptan has recently been introduced to treat systemic/pulmonary congestion refractory to conventional diuretics [1]. Methodology to assess systemic/pulmonary congestion utilizing multi-modalities has also been accumulating thus far. Nevertheless, the number of patients with refractory congestive heart failure is increasing.
Herbal medicines, including Goreisan, are receiving great concern to manage such refractory congestion [2]. In the animal experimental studies, Goreisan seems to mediate the expression of aquaporin families, one of the major mediators of systemic water balance [3]. However, the detailed pathophysiological implication of Goreisan in combination with other diuretics including tolvaptan in patients with congestive heart failure remains unknown.
Case report
Case 1
The patient was 85-year-old man with chronic heart failure with preserved ejection fraction accompanying hypertension and persistent atrial fibrillation. He was admitted to our hospital complaining of dyspnea on exertion and bilateral leg edema for 3 months against the administration of sacubitril/valsartan 150 mg/day, bisoprolol 2.5 mg/day, furosemide 60 mg/day, and spironolactone 25 mg/day.
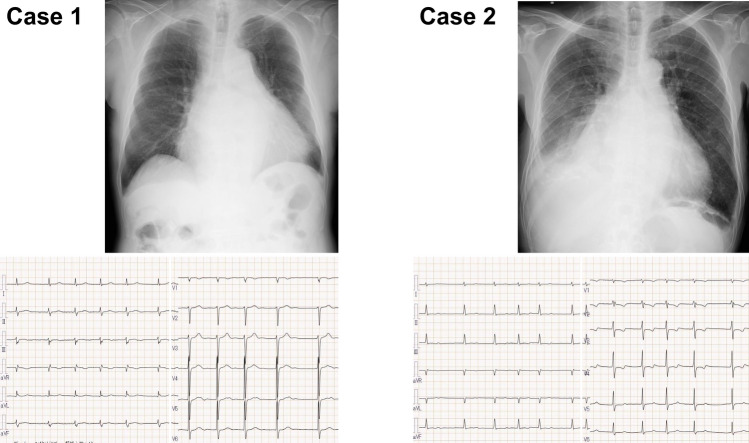
On admission (Table 1), blood pressure was 82/56 mmHg and pulse rate was 74 bpm. Body height was 164.5 cm and body weight was 74.5 kg. New York Heart Association functional class was III degree. He had cardiomegaly and mild bilateral congestion in chest X-ray and atrial fibrillation and 71 bpm of heart rate without obvious ST-segment abnormality in electrocardiogram (Fig. 1). Plasma B-type natriuretic peptide was 198.8 pg/mL and estimated glomerular filtration ratio was 44.0 mL/min/1.73m2.
Table 1.
Clinical data on admission
| Case 1 | Case 2 | |
|---|---|---|
| Demographics | ||
| Age, years | 85 | 81 |
| Body mass index, kg/m2 | 27.5 | 21.9 |
| Blood pressure, mmHg | 82/56 | 84/62 |
| Pulse rate, beats/min | 74 | 79 |
| Laboratory data | ||
| Hemoglobin, g/dL | 11.4 | 10.3 |
| Serum albumin, g/dL | 4.2 | 3.0 |
| Serum sodium, mEq/L | 138 | 131 |
| Serum osmolality, mOsm/kg・H2O | 296 | 285 |
| Blood urea nitrogen, mg/dL | 20.4 | 40.0 |
| Serum creatinine, mg/dL | 1.21 | 2.76 |
| Estimated glomerular filtration ratio, mL/min/1.73m2 | 44.0 | 18.1 |
| Serum C-reactive protein, mg/dL | 0.02 | 0.67 |
| Plasma arginine vasopressin, pg/mL | 6.5 | 5.5 |
| Plasma B-type natriuretic peptide, pg/mL | 198.8 | 86.0 |
| Serum N-terminal pro-brain natriuretic peptide, pg/mL | 1208 | 803 |
| Echocardiography | ||
| Left ventricular end-diastolic diameter, mm | 59 | 40 |
| Left ventricular end-systolic diameter, mm | 41 | 15 |
| Left ventricular ejection fraction, % | 57 | 68 |
| Left atrial dimension, mm | 63 | 55 |
| Deceleration time, msec | 215 | 118 |
| Early diastolic left ventricular filling velocity, cm/sec | 151 | 79 |
| Tricuspid regurgitant pressure gradient, mmHg | 20 | 20 |
| Inferior vena cava, inspiratory, mm | 19 | 24 |
| Inferior vena cava, expiratory, mm | 28 | 29 |
Fig. 1.
Chest X-ray and electrocardiogram findings in Case 1 and 2
We performed an invasive hemodynamic assessment, displaying no significant coronary artery stenosis, 18 mmHg of mean right atrial pressure, 20 mmHg of left ventricular end-diastolic pressure, and 4.12 mL/min/m2 of cardiac index.
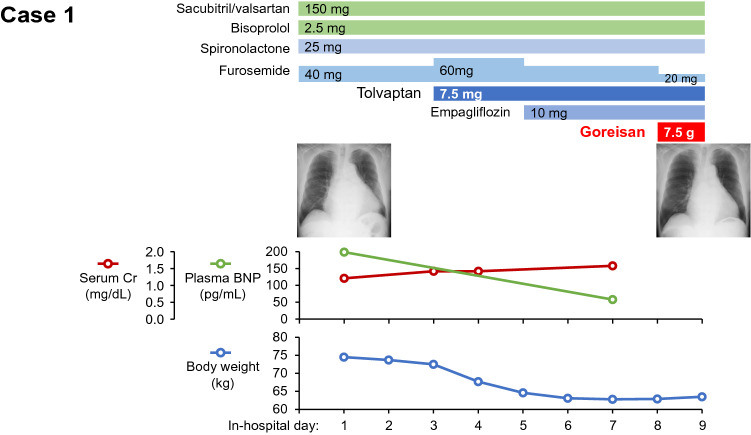
We initiated tolvaptan 7.5 mg and empagliflozin 10 mg (Fig. 2). Urine output increased and his body weight decreased gradually down to 62.8 kg, accompanied by amelioration of heart failure symptoms. Increases in blood urea nitrogen (from 20.4 mg/dL to 47.0 mg/dL) and serum creatinine (from 1.21 mg/dL to 1.58 mg/dL), indicating intra-vascular hypovolemia, let us decrease the dose of furosemide down to 20 mg. Instead, we initiated Goreisan (Tsumura &Co. Tokyo, Japan) 7.5 g/day on day 8.
Fig. 2.
Clinical course of case 1. Cr creatinine, BNP B-type natriuretic peptide. Goreisan was administered on day 8 in addition to furosemide and tolvaptan
One day following the initiation of Goreisan, urine volume increased mildly, accompanied by a decrease in urine cyclic adenosine monophosphate (cAMP) from 2.47 nmol/mg Cr to 2.13 nmol/mg Cr and urine aquaporin-2 from 1.16 ng/mg Cr to 0.52 ng/mg Cr as well as a decrease in urine osmolality from 395 mOsm/kg H2O to 216 mOsm/kg H2O (Table 2). He was discharged on foot on day 9 without any heart failure symptoms. His heart failure was well managed with maintained urine volume following the index discharge. Goreisan was terminated and the dose of furosemide was further decreased at 2 weeks following the index discharge.
Table 2.
Urine data before and 1 day after Goreisan administration
| Case 1 | Case 2 | |||
|---|---|---|---|---|
| Pre-Goreisan | Post-Goreisan | Pre-Goreisan | Post-Goreisan | |
| Urine cAMP, nmol/mg Cr | 2.47 | 2.13 | 3.87 | 2.53 |
| Urine aquaporin-2, ng/mg Cr | 1.16 | 0.52 | 0.13 | 0.060 |
| Urine osmolality, mOsm/kg H2O | 395 | 216 | 303 | 287 |
| Urine specific gravity | 1.014 | 1.007 | 1.020 | 1.015 |
cAMP cyclic adenosine monophosphate
Case 2
The patient was 81-year-old man with persistent atrial fibrillation and chronic heart failure with preserved ejection fraction predominantly accompanying right heart failure and severe tricuspid regurgitation. Medication lists were candesartan 8 mg/day, eplerenone 50 mg/day, azosemide 60 mg/day, and tolvaptan 7.5 mg/day. He was admitted to our hospital complaining of severe fatigue, dyspnea at rest, and appetite loss for 1 week. He could not take any medications for 2 days before admission.
On admission, his body height was 165.0 cm and body weight was 59.6 kg (Table 1). Systolic blood pressure was 84/62 mmHg and pulse rate was 79 bpm. Oxygen saturation at room condition was 97%. Chest X-ray displayed cardiomegaly, predominantly due to the enlargement of right second arch, and right pleural effusion, as well as bilateral mild congestion (Fig. 1). Atrial fibrillation and 80 bpm of heart rate was observed in electrocardiogram. Left ventricular end-diastolic diameter was 40 mm and left ventricular ejection fraction was 68%. Tricuspid regurgitation was severe with enlarged right-side heart and dilated inferior vena cava (expiratory/inspiratory 29/24 mm). Plasma B-type natriuretic peptide was 86.0 pg/mL and serum creatinine level was 2.76 mg/dL (Table 1).
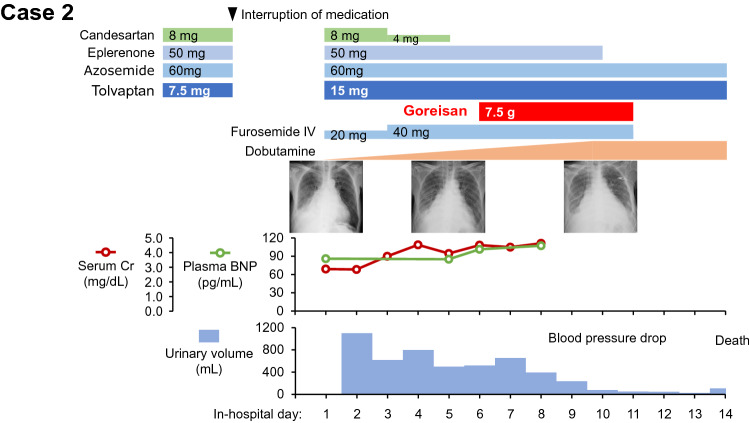
Given extreme bilateral leg pitting edema and coldness of limbs, intravenous furosemide administration and continuous intravenous dobutamine were initiated (Fig. 3). Tolvaptan was up-titrated. Urine volume remained unchanged, and we decided to initiate Goreisan 7.5 g/day on day 6.
Fig. 3.
Clinical course of case 2. IV intravenous injection, Cr creatinine, BNP B-type natriuretic peptide. Goreisan was administered on day 6 in addition to furosemide, azosemide, and tolvaptan
One day following the initiation of Goreisan, urine volume increased slightly from 520 to 655 mL. Urine cAMP decreased from 3.87 nmol/mg Cr to 2.53 nmol/mg Cr and urine aquaporin-2 also decreased from 0.13 ng/mg Cr to 0.060 ng/mg Cr (Table 2). Urine was diluted with decreased urine osmolality from 303 mOsm/kg H2O to 287 mOsm/kg H2O.
Heart failure symptoms improved transiently, but systemic hemodynamics gradually deteriorated against up-titration of dobutamine. He and his relatives wished for palliative care and was expired peacefully on day 14.
Discussion
According to a few experimental studies, Goreisan seems to mediate aquaporin-incorporated signal cascades to adjust body fluid balance. In rats with 0.9% saline loading, mRNA of vasopressin type-2 receptor remained unchanged following the administration of Goreisan [4]. In the murine inner medullary collecting duct cell line, Goreisan suppressed the signal cascade of cAMP [5]. In rats with 5/6 nephrectomy, Goreisan suppressed the expression of aquaporin-2 [3].
We observed for the first time the detailed trends in the above-described aquaresis-related biomarkers following Goreisan administration in the clinical setting. As observed in the above experimental studies, Goreisan seems to suppress cAMP-aquaporin-2 system and inhibit the reabsorption of free water in patients with congestive heart failure. Goreisan seems to be effective even during tolvaptan therapy, which also suppresses cAMP-aquaporin-2 system. Urine volume consistently further increased following the administration of Goreisan upon tolvaptan therapy.
We focused on the impact of Goreisan on the aquaporin-2-incorporated aquaresis system in the collecting ducts [6]. However, Goreisan might have other pleiotropic effects: suppression of aquaporin-3 and aquaporin-4 [3, 4]; anti-inflammatory effect to improve endothelial function and ameliorate peripheral permeability [7, 8]; suppression of renin–angiotensin–aldosterone system [9, 10]. Further studies are warranted to investigate them.
Goreisan might have another clinical implication in reducing the dose of conventional loop diuretics, which has a negative prognostic impact in the heart failure cohort via intra-vascular hypotension, reduction in peripheral circulation, and inappropriate activation of renin–angiotensin–aldosterone system [11, 12]. In case 1, we could reduce the dose of loop diuretics following the administration of Goreisan. We observed just a very short period, and clinical implication, including the impact upon urine biomarkers, of Goreisan in patients with congestive heart failure requires further studies. Also, Impact of Goreisan on those without apparent congestion remains unknown.
Conclusion
We reported for the first time the clinical courses of Goreisan therapy upon tolvaptan and loop diuretics therapy in patients with congestive heart failure. In the same manner with tolvaptan, urine cAMP and urine aquaporin-2 further decreased accompanied by an incremental volume of diluted urine, following Goreisan therapy. Although further studies are warranted to establish a therapeutic strategy, Goreisan might be a promising therapeutic tool in patients with congestive heart failure refractory to tolvaptan and loop diuretics therapy.
Declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Ethical approval
All procedures performed in this case report were in accordance with the 1964 Helsinki Declaration and its later amendments.
Informed consent
Informed consent was obtained from the patients for the publication of this case report.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Matsuzaki M, Hori M, Izumi T, Fukunami M. Efficacy and safety of tolvaptan in heart failure patients with volume overload despite the standard treatment with conventional diuretics: a phase III, randomized, double-blind, placebo-controlled study (QUEST study) Cardiovasc Drugs Ther. 2011;25(Suppl 1):S33–45. doi: 10.1007/s10557-011-6304-x. [DOI] [PubMed] [Google Scholar]
- 2.Li Z, Ren L, Gu R, Zhou C, Tong X, Hu J. The efficacy and safety of Wulingsan modified formulas for chronic heart failure patients: a systematic review and meta-analysis. J Thorac Dis. 2022;14:1232–1242. doi: 10.21037/jtd-22-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jo M, Fujimoto T, Kaneko M, Oka H, Shibahara N. Effect of goreisan on urinary concentrating ability and expression of aquaporin-2 in 5/6 nephrectomized rats. J Trad Med. 2013;30:145–157. [Google Scholar]
- 4.Kurita K, Nakamura K, Tabuchi M, et al. Effects of Gorei-san, a traditional Japanese kampo medicine, on aquaporin 1, 2, 3, 4, and V2R mRNA expression in rat kidney and forebrain. J Med Sci. 2011;11:30–38. doi: 10.3923/jms.2011.30.38. [DOI] [Google Scholar]
- 5.Lee YJ, Lee SM, Cui X, Yoon JJ, Oh HC, Kim YC, et al. Quantitative evaluation of Oryeongsan and its action on water regulation in renal inner medullary collecting duct cells. J Ethnopharmacol. 2016;185:310–318. doi: 10.1016/j.jep.2016.03.030. [DOI] [PubMed] [Google Scholar]
- 6.Imamura T, Kinugawa K, Fujino T, Inaba T, Maki H, Hatano M, et al. Increased urine aquaporin-2 relative to plasma arginine vasopressin is a novel marker of response to tolvaptan in patients with decompensated heart failure. Circ J. 2014;78:2240–2249. doi: 10.1253/circj.CJ-14-0244. [DOI] [PubMed] [Google Scholar]
- 7.Oh YC, Jeong YH, Ha JH, Cho WK, Ma JY. Oryeongsan inhibits LPS-induced production of inflammatory mediators via blockade of the NF-kappa B, MAPK pathways and leads to HO-1 induction in macrophage cells. BMC Complement Altern Med. 2014;14:242. doi: 10.1186/1472-6882-14-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shang C, Lin H, Fang X, Wang Y, Jiang Z, Qu Y, et al. Beneficial effects of cinnamon and its extracts in the management of cardiovascular diseases and diabetes. Food Funct. 2021;12:12194–12220. doi: 10.1039/D1FO01935J. [DOI] [PubMed] [Google Scholar]
- 9.Ahn YM, Cho KW, Kang DG, Lee HS. Oryeong san (Wuling san), a traditional Chinese herbal medicine, induces natriuresis and diuresis along with an inhibition of the renin-angiotensin-aldosterone system in rats. J Ethnopharmacol. 2012;141:780–785. doi: 10.1016/j.jep.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 10.Kim SK, Lee S, Lee MK, Lee S. A systems pharmacology approach to investigate the mechanism of Oryeong-san formula for the treatment of hypertension. J Ethnopharmacol. 2019;244:112129. doi: 10.1016/j.jep.2019.112129. [DOI] [PubMed] [Google Scholar]
- 11.Neuberg GW, Miller AB, O'Connor CM, Belkin RN, Carson PE, Cropp AB, et al. Diuretic resistance predicts mortality in patients with advanced heart failure. Am Heart J. 2002;144:31–38. doi: 10.1067/mhj.2002.123144. [DOI] [PubMed] [Google Scholar]
- 12.Eshaghian S, Horwich TB, Fonarow GC. Relation of loop diuretic dose to mortality in advanced heart failure. Am J Cardiol. 2006;97:1759–1764. doi: 10.1016/j.amjcard.2005.12.072. [DOI] [PubMed] [Google Scholar]