Abstract
Immunoglobulin G (IgG) nephropathy refers to a rare group of diseases characterized by deposits of IgG in the mesangial region. However, IgG nephropathy is controversial as a single disease entity, and its pathogenesis remains to be elucidated. In the present report, we discuss a case of IgG nephropathy in which we observed activation of the classical complement pathway.
A 47-year-old woman was admitted to our hospital with nephrotic syndrome. Light-microscopic examination revealed neither proliferative nor sclerotic lesions in the glomeruli. However, unusual and large deposits were observed in the paramesangial area. An immunofluorescence study revealed predominant IgG and C1q and slight C3 deposits in the paramesangial area, suggesting immune-complex-type glomerular disease. An electron microscopic study also revealed different sizes of non-organized electron-dense deposits with a similar pattern of distribution, which were accompanied by foot process effacement. Clinically, there was no evidence of systemic diseases, such as infectious or autoimmune diseases (including systemic lupus erythematosus). Based on these findings, she was diagnosed with IgG nephropathy and treated with prednisolone. Steroid therapy was effective, and complete remission was maintained.
Additional immunological examination revealed that IgG deposits were polyclonal and consisted mainly of the IgG1 and IgG3 subclasses. Furthermore, staining was positive for C4d and C5b-9. The present findings indicate that the pathogenesis of IgG nephropathy in our patient may have involved activation of the classical complement pathway.
Keywords: Antigen–antibody complex, Complement activation, Complement C1q, Immunoglobulin G, Nephrotic syndrome
Introduction
Primary glomerulonephritis (GN) with predominant glomerular immunoglobulin G (IgG) deposits (IgG nephropathy) was first described as a distinct disease entity by Sato et al. in 1993 [1]. The authors reported cases of four adults and two children with proteinuria and hematuria. A common pathological feature of IgG nephropathy is the predominance of mesangial IgG deposits; among the immunoglobulins, IgG was the only one to form deposits in the initial case. Subsequent reports described cases with glomerular IgG deposits and co-deposits of other immunoglobulins and complements [2–5].
Glomerular IgG deposits can be observed in patients with various renal diseases, including primary or secondary GN (e.g., lupus nephritis, post-infectious GN, and cryoglobulinemic GN). In most previously reported cases of IgG nephropathy, complements were co-deposited with polyclonal IgG. Therefore, the pathogenesis of IgG nephropathy is thought to involve an immune complex-mediated form of GN [2].
Among the differential diagnoses of IgG nephropathy, physicians must exclude infectious and autoimmune diseases. Conversely, IgG-dominant immune complex-mediated GN without extrarenal signs has been described as renal-limited “lupus-like” nephritis [6]. This differs from IgG nephropathy, because it has multiple pathological findings that strongly suggest lupus nephritis, including combined mesangial, subendothelial, and subepithelial immune deposits; the presence of endothelial tubuloreticular inclusions; and “full-house” immunofluorescence (IF) staining findings.
In addition, several cases of IgG nephropathy cannot be distinguished from those of C1q nephropathy, because co-dominant deposits of C1q among complements are often observed [7]. Therefore, IgG nephropathy may be confused with C1q nephropathy during diagnosis.
C1q nephropathy was first proposed by Janette and Hipp in 1985. They reported cases showing dominant or co-dominant deposition of C1q in the mesangial region, without serological detection of systemic lupus erythematosus (SLE) [8]. Although the classical pathway of complement activation and inflow of large amounts of proteins to the glomeruli or other regions have been suggested as mechanisms of C1q nephropathy, many aspects of the process remain unknown [9]. In addition, the symptoms, histological findings, and treatment courses of C1q nephropathy are heterogeneous [10–13]; this indicates that instead of being a single disease entity, it may represent multiple diseases.
In this report, we discuss a case of IgG nephropathy in which additional immunological examination revealed activation of the classical complement pathway.
Case report
A 47-year-old Japanese woman noticed systemic edema and a weight gain of 3 kg a week before admission to our hospital. Three days prior to admission, she consulted a local physician; her laboratory test results revealed proteinuria in the qualitative analysis (3+) and a serum albumin level of 2.0 mg/dL. Accordingly, she was referred to our hospital on suspicion of nephrotic syndrome. She had a history of hysteromyoma and liver hemangioma. However, she had no medication, no allergies, and no relevant family history. Physical examination findings at admission were as follows: height, 155 cm; body weight, 47.5 kg (increased by 5 kg from before admission); consciousness, clear; blood pressure, 82/40 mmHg; heart rate, 75 bpm; and body temperature, 36.7 °C. Edema was present in the bilateral lower thighs. No other notable physical abnormalities were detected. Arthritic pain and exanthema were not observed. Chest and abdominal computed tomography revealed a small amount of bilateral pleural effusion, a large amount of ascites, and edema in the gastrointestinal tract. The kidney sizes were normal. Table 1 presents the laboratory findings on admission.
Table 1.
Laboratory values
| Parameter | Value | Parameter | Value |
|---|---|---|---|
| Hematology | Immunology/Infection | ||
| WBC count, /μL | 7600 | IgG, mg/dL | 390 |
| RBC count, 104/μL | 561 | IgA, mg/dL | 306 |
| Hemoglobin, g/dL | 17.9 | IgM, mg/dL | 369 |
| Hematocrit, % | 52.4 | C3, mg/dL | 140 |
| Platelet count, 104/μL | 25.3 | C4, mg/dL | 43 |
| IC-C1q, mg/dL | < 1.5 | ||
| Blood chemistry | MPO-ANCA | negative | |
| AST, U/L | 29 | PR3-ANCA | negative |
| ALT, U/L | 20 | anti-GBM Ab | negative |
| LDH, U/L | 227 | Cryoglobulin | negative |
| Total protein, g/dL | 4.3 | ANA | negative |
| Albumin, g/dL | 0.9 | Immunoelectrophoresis | WNL |
| T-Cho, mg/dL | 654 | HBsAg | negative |
| TG, mg/dL | 479 | HCVAb | negative |
| BUN, mg/dL | 17.6 | ASO, IU/mL | 15 |
| Creatinine, mg/dL | 0.58 | ASK, IU/mL | 40 |
| HbA1c, % | 5.6 | ||
| CRP, mg/dL | 0.05 | Urinalysis | |
| Urine protein | 4+ | ||
| Urine occult blood | 2+ | ||
| Spot urine PCR, g/g | 24.59 | ||
| Selectivity index | 0.075 |
AST aspartate transaminase, Alb albumin, ALT alanine aminotransferase, ANA antinuclear antibodies, ANCA anti-neutrophil cytoplasm antibodies, anti-GBMAb anti-glomerular basement membrane antibody, Anti-ds-DNA Ab anti-double stranded DNA antibodies, ASK anti-streptokinase antibody, ASO anti-streptolysin O antibody, BUN blood urea nitrogen, CRP C-reactive protein, HbA1c hemoglobin A1c, HBsAg hepatitis B surface antigen, HCVAb hepatitis C virus antibody, IC-C1q C1q
binding IgG immune complex, IgA immunoglobulin A, IgG immunoglobulin G, IgM immunoglobulin M, LDH lactate dehydrogenase, LDL-Chol low-density lipoprotein cholesterol, PCR protein–creatinine ratio, Plt platelets, RBC red blood cells, RF rheumatoid factor, T-Chol total cholesterol, TG triglycerides, WBC white blood cells
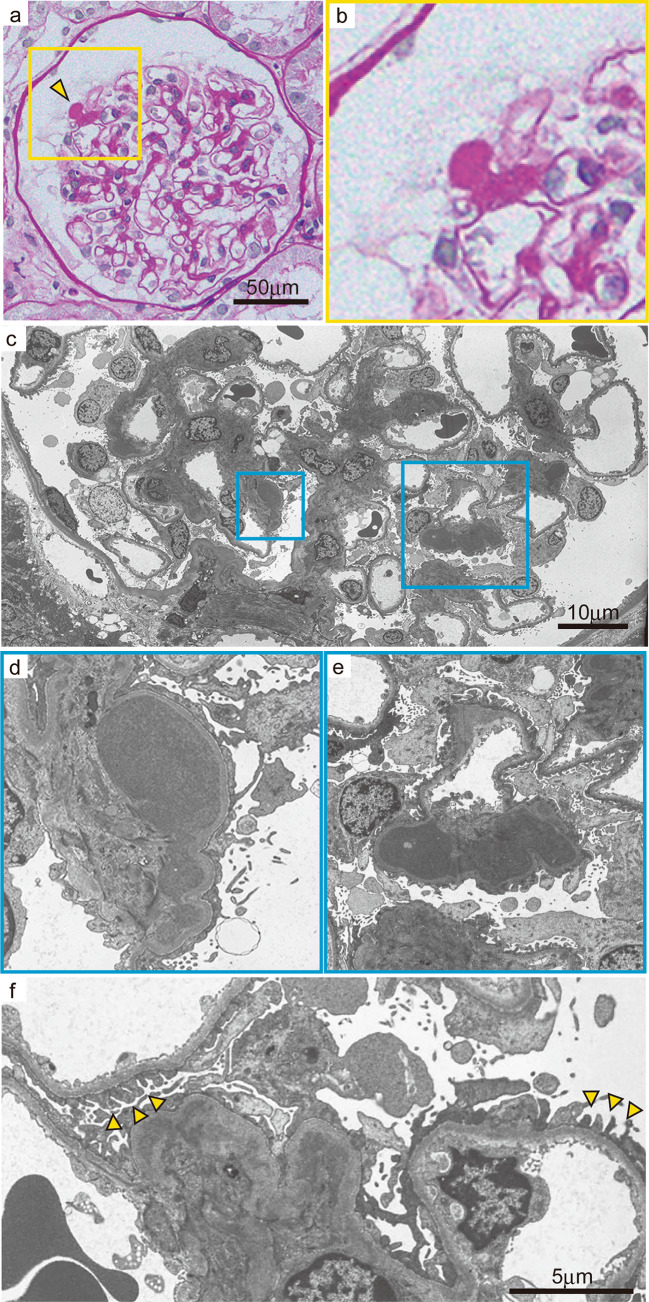
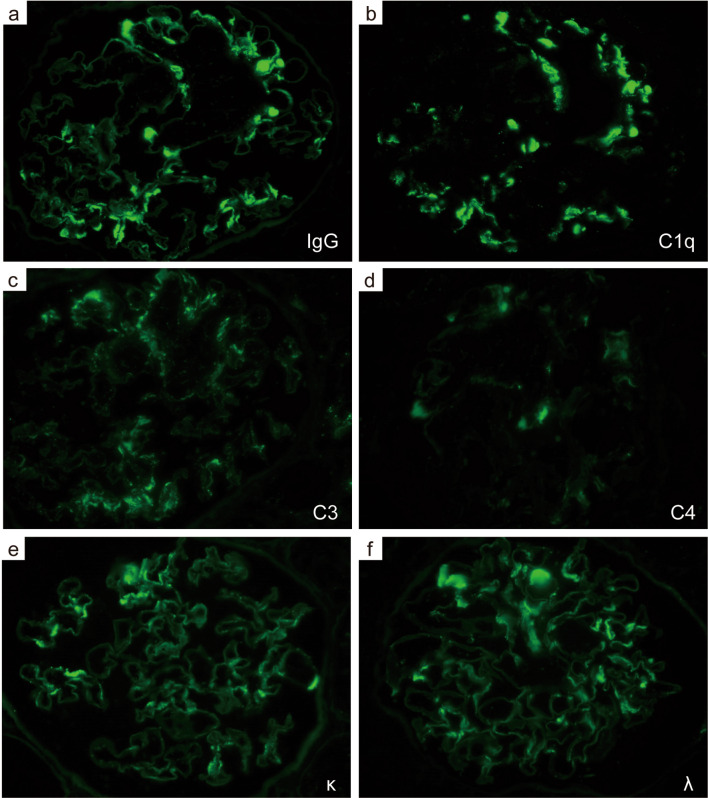
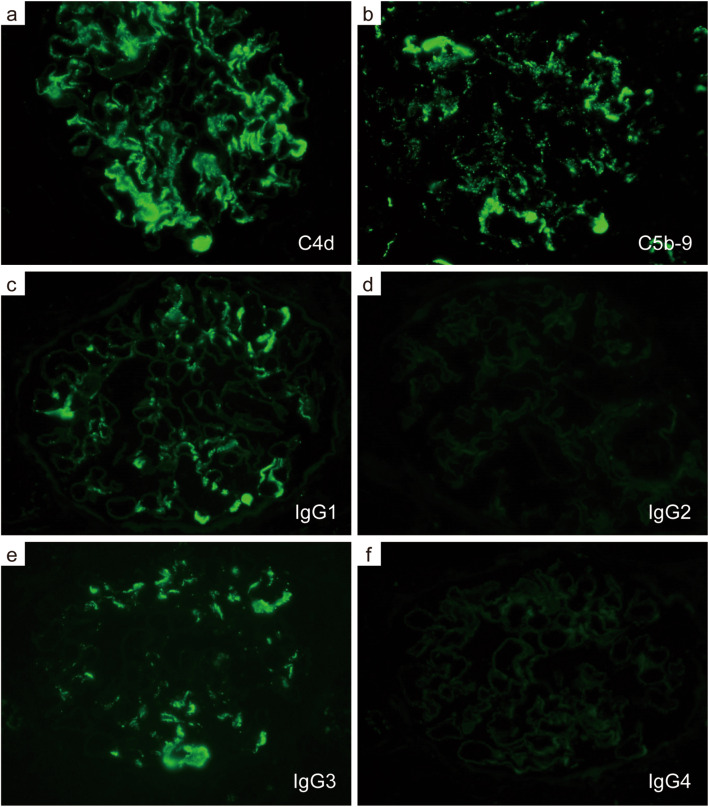
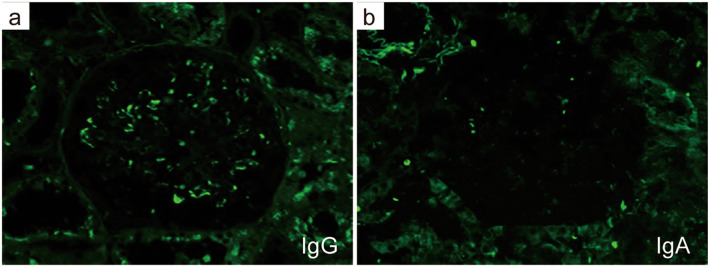
The patient was diagnosed with nephrotic syndrome, and a renal biopsy was performed. Light microscopy revealed adhesion lesions in only one of the 19–22 glomeruli analyzed. There were neither proliferative nor sclerotic lesions. No interstitial fibrosis, tubular atrophy or arteriolar abnormalities were observed. However, unique and large deposits were found mainly in the paramesangial lesions (Fig. 1a, b). Electron microscopy revealed non-organized electron-dense deposits (EDDs) of different sizes with podocyte foot process (FP) effacement in the paramesangial area; podocyte FP was maintained in some areas without deposits (Fig. 1c–f). IF analyses revealed IgG (++), C1q (++), C3 (+), and C4 (+/−) in the paramesangial area (Fig. 2a–d). Light chain (κ and λ) staining revealed no significant differences in intensity (Fig. 2e, f). An additional IF study of C4d, C5b-9 (Fig. 3a, b) and IgG subclasses (Fig. 3c–f) revealed positive C4d and C5b-9 staining in the paramesangial area. Patterns of IgG1 and IgG3 positivity were similar to those observed for IgG, while staining for IgG2 and IgG4 was negative. Furthermore, to rule out masked IgA deposits, we performed an IF study for IgA and IgG on pronase-digested paraffin tissue (Fig. 4a, b). Staining was negative for IgA, but positive for IgG in a similar distribution on frozen section. These findings suggested that our patient had an immune-complex-type glomerular disease in which there were no proliferative lesions.
Fig. 1.
Light and electron microscopy findings for large, unique deposits found mainly in the paramesangial area. a PAS stain, scale bar: 50 µm. b Enlarged view of Fig. 1a. Arrowhead shows a unique large deposit in the paramesangial area. c Electron microscopy image shows non-organized electron-dense deposits (EDDs) of different sizes in the mesangium and paramesangium, scale bar: 10 µm. d, e Enlarged view of Fig. 1c shows diffuse foot process effacement of podocytes with paramesangial deposits. f Arrowheads indicate that FP was maintained in some areas without EDDs, scale bar: 5 µm
Fig. 2.
Immunofluorescence findings. Immunofluorescence staining for immunoglobulin and complements (a IgG, b C1q, c C3, d C4, e κ, f λ, ×400). IgG immunoglobulin G
Fig. 3.
Immunofluorescence analysis of complement activation and IgG subclasses. Immunofluorescence staining for complements involved in the classical pathway (a C4d, b C5b-9, ×400) Immunofluorescence staining for immunoglobulin subclasses (c IgG1, d IgG2, e IgG3, f IgG4, ×400)
Fig. 4.
Immunofluorescence study on pronase-digested paraffin tissue.IgA staining was negative except for a non-specific binding on red blood cells, while IgG was positive in a similar distribution on frozen section (a IgG, b IgA×400)
Serologically, tests for antinuclear antibodies and anti-DNA antibodies were negative. Moreover, immunological tests revealed normal serum complement levels. There was no clinical evidence of autoimmune diseases and no symptoms indicating infection prior to admission, such as fever, cough, and other clinical symptoms. In addition, laboratory findings revealed no elevation of infection markers (including C-reactive protein and white blood cells), and both the ASO and ASK titers were within their normal ranges. Histologically, IF study in our case revealed only a weak positivity for C3; C3 co-dominant glomerular staining is observed typically in infectious-related glomerulonephritis. A “hump-shaped” appearance was also not detected in electron microscopy findings. Therefore, infection-related glomerulonephritis was ruled out. Based on these results, the patient was diagnosed with IgG nephropathy. Due to the severe nephrotic syndrome, reduced effective circulating volume resulted in low blood pressure and hypouresis. A steroid pulse therapy (methylprednisolone: 500 mg/day for 3 days) was administered, followed by the oral administration of prednisolone at 40 mg/day. Her response to steroids was favorable, and proteinuria disappeared on day 15 of treatment. Oral prednisolone was gradually tapered. Complete remission was maintained, and the patient was discharged 47 days after admission. The course after discharge was uneventful, and the dose of steroids was tapered gradually until treatment was discontinued.
Discussion
IgG nephropathy is a rare disease. Although some individual cases and case series have been reported (Table 2), no long-term follow-up studies have been performed. Many case reports are from Japan [1–3, 14], most of which describe patients with favorable courses of the disease. On the other hand, a French study by Fakhouri et al. [4] reported that half of the patients developed chronic kidney disease and recurrence in the kidney graft following kidney transplantation, with further research indicating that there may be differences in the outcomes among human races [15].
Table 2.
Clinical and pathological characteristics of IgG nephropathy and its relatives in published cases
| Authors [ref no.] | Year | n | Country | Age | Clinical findings | Pathological findings (IF or IHCa) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IgG | IgA | IgM | C1q | C3 | C4 | ||||||
| Original articles | Frequency (%) of positive cases in the mesangium | ||||||||||
| Sato et al. [1] | 1993 | 6 | Japan | 6–52 | Renal dysfunction (n=1) | 100 | 0 | 0 | 66.7 | 50 | 0 |
| Yoshikawa et al. [2] | 1994 | 10 | Japan | 1–5 | NS (n=4) | 100 | 0 | 20 | 80 | 30 | 10 |
| Fakhouri et al. [4] | 2002 | 14 | France | 13–47 | NS (n=1), renal dysfunction (n=4; four patients progressed to ESKD within 3–15 years) | 100 | 0 | 31.3 | 100 | 69.2 | 66.7 |
| Jalalah [15] | 2009 | 2 | Saudi Arabia | 4, 14 | NS (n=1), hematuria (n=1) | 100 | 0 | 100 | 0 | 50 | 0 |
| Lim et al. [7] | 2009 | 14 | Korea | 8–52 | Renal dysfunction (n=2), hypocomplementemia (n=6) | 100 | 35.7 | 50 | 78.6 | 78.6 | 28.6 |
| Case reports | Intensity | ||||||||||
| Kano et al. [3] | 1996 | 1 | Japan | 8 | NS, CR after steroid therapy | 1+a | 0a | ND | 2+a | ND | ND |
| (three kidney biopsies per case) | 2+a | 0a | 0a | 2+a | ND | ND | |||||
| 2+ | 0 | 1+ | 1+ | ND | ND | ||||||
| Pickering et al. [22] | 1996 | 1 | New Zealand | 23 | Microscopic hematuria | + | 0 | 0 | + | + | ND |
| Sepandj et al. [23] | 1998 | 1 | Canada | 48 | Steroid-resistant NS | + | ND | ND | + | ND | ND |
| Onitsuka et al. [14] | 2000 | 1 | Japan | 48 | Graft biopsy (original disease: unknown) | + | 0 | + | + | + | ND |
| Assadi [24] | 2008 | 1 | USA | 17 | Proteinuria with the Down’s syndrome | + | 0 | 0 | ND | 0 | ND |
| Kharroubi et al. [25] | 2018 | 1 | Tunisia | 50 | Frequent relapse of NS | + | 0 | ND | weak | 0 | ND |
| Present case | 2021 | 1 | Japan | 47 | NS, CR after steroid therapy | 2+ | 0 | 0 | 2+ | 1+ | ± |
ref no reference number, IF immunofluorescence, IHC immunohistochemistry, NS nephrotic syndrome, ESKD end-stage kidney disease, CR complete remission, ND not determined
Asterisks indicate IHC staining intensity
Classically, IgG nephropathy refers to a group of diseases characterized by deposits of IgG alone in the mesangial region [1]. However, this definition has expanded to include co-deposition of complement protein (mainly C1q) in cases in which IgG deposits are predominant. In most cases, pathological findings include mesangial matrix expansion, with or without proliferation. IF studies often show IgG and C1q positivity. The ratio of double-positive staining for IgG and C1q ranges from 0% [15] to 100% [3, 4]. In addition, positive staining for other complements is observed in many cases. Therefore, some researchers have suggested that IgG nephropathy is a subtype of immune-complex-type GN [2, 15]. The diagnosis of IgG nephropathy is mainly one of exclusion, i.e., other secondary diseases such as infectious and autoimmune diseases must be excluded. Our patient showed no clinical evidence of SLE, neither at the time of diagnosis nor beyond 7 years of follow-up.
In our case, IgG nephropathy and C1q nephropathy should be considered as differential diagnoses because IF studies revealed positive findings for both IgG and C1q. The definition of IgG nephropathy is focused on the presence of IgG deposits only, and it is often problematic to differentiate the disease from C1q nephropathy characterized by dominant or co-dominant deposition of C1q in the mesangial region and absence of SLE on serology [8]. Iskandar et al. [16] and Markowitz et al. [12] reported C1q nephropathy cases showing a clinical course similar to that of minimal change disease (MCD)/focal segmental glomerulosclerosis (FSGS). In such cases, C1q deposition is thought to be a non-specific immunoreaction accompanying the inflow of large amounts of proteins to the mesangial region [12].
Because many cases with co-dominant staining for both IgG and C1q have been reported, the disease entity was expanded, causing confusion during diagnosis. Lim et al. reported that among patients diagnosed with C1q nephropathy, many cases involved a similar extent of IgG and C1q deposition [7]. Therefore, many cases of overlap between IgG nephropathy and C1q nephropathy have been identified, with researchers proposing new terminology (i.e., IgG/C1q nephropathy) to avoid confusion. On the other hand, Fogo et al. [17] stated that C1q nephropathy should be diagnosed based on dominant staining for C1q. In addition, an absence of reticular aggregates and a lack of IgG-dominant deposits are also important in the diagnosis of C1q nephropathy. Based on this perspective, our case should be diagnosed as IgG nephropathy.
Clinically, our patient exhibited acute-onset nephrotic syndrome with massive proteinuria and a good response to steroid therapy, resembling MCD. However, electron microscopy revealed unusual and large paramesangial deposits with FP effacement. Furthermore, podocyte FP was rather maintained in some areas without deposits. This indicates that podocyte damage may not be diffuse and may be more severe around the deposits. Previously, complement activation has been reported to be involved in not only proliferative GN, but also in other proteinuric glomerular diseases such as FSGS and diabetic nephropathy [18]. Therefore, additional immunostaining was conducted to assess the pathological conditions of polyclonal IgG and complement deposits. C4d is considered to be a useful tool for diagnosing immune-complex-mediated GN [19]. We hypothesized that verifying complement components, such as C4d or C5b-9, may clarify the involvement of complement activation in IgG nephropathy. The results were positive for C1q, C4d, C3, and C5b-9, which are structural components of the classical pathway for complement activation. This indicates that the classical pathway may have been activated. Moreover, IgG subclass staining was positive for IgG1 and IgG3 in our case. Complement “C1q” binds strongly to IgM, IgG1, and IgG3; weakly to IgG2; and does not bind to IgG4, IgA, IgD, or IgE [9] [20, 21]. Fakhouri et al. [4] also reported positive staining for IgG1 and IgG3 in patients with IgG nephropathy. Based on these, we suggested that activation of the classical complement pathway occurred via an antigen-antibody complex reaction with IgG.
In summary, we discussed a case of IgG nephropathy characterized by unusual non-organized EDDs with podocyte injury. Activation of the classical complement pathway appeared to be involved in the pathogenesis of the disease in our patient.
Acknowledgements
We thank Ms. Arimi Ishikawa and Ms. Asae Kurosawa for their technical assistance.
Author contribution
MA, HA, TK, AM, and YS treated the patient and evaluated the clinical images. AM and AS were responsible for the pathological diagnosis. MA, AM, and TK contributed to clinical data acquisition. MA, AM, and AS interpreted the data and prepared the manuscript. All authors read and approved the final version of this manuscript.
Funding
No funding to declare.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Ethical approval
No experimental study was conducted. All procedures performed in this case were in accordance with the ethical standards of the institutional committee and with the 1975 Helsinki declaration and its later amendments or comparable ethical standards.
Consent for publication
Written informed consent was obtained from the patient for the publication of this case report and any accompanying images. This work does not contain any studies with animals performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sato M, Kojima H, Nabeshima K, Nakajima Y, Koshikawa S. Primary glomerulonephritis with predominant mesangial immunoglobulin G deposits–a distinct entity? Nephron. 1993;64:122–8. doi: 10.1159/000187291. [DOI] [PubMed] [Google Scholar]
- 2.Yoshikawa N, Iijima K, Shimomura M, Nakamura H, Ito H. IgG-associated primary glomerulonephritis in children. Clin Nephrol. 1994;42:281–7. [PubMed] [Google Scholar]
- 3.Kano K, Ueda Y, Iidaka K, Ichimura T. Glomerulonephritis with predominant paramesangial IgG deposition. Pathol Int. 1996;46:306–9. doi: 10.1111/j.1440-1827.1996.tb03616.x. [DOI] [PubMed] [Google Scholar]
- 4.Fakhouri F, Darré S, Droz D, Lemaire M, Nabarra B, Machet MC, Chauveau D, Lesavre P, Grünfeld JP, Noël LH, Knebelmann B. Mesangial IgG glomerulonephritis: a distinct type of primary glomerulonephritis. J Am Soc Nephrol. 2002;13:379–87. doi: 10.1681/ASN.V132379. [DOI] [PubMed] [Google Scholar]
- 5.Jourde-Chiche N, Moal V, Daniel L, Purgus R, Legis T, Jr, Vacher-Coponat H, Moussi-Frances J, Berland Y. Early IgG glomerulonephritis recurrence in a kidney transplant recipient. Clin Nephrol. 2008;70:340–3. doi: 10.5414/CNP70340. [DOI] [PubMed] [Google Scholar]
- 6.Huerta A, Bomback AS, Liakopoulos V, Palanisamy A, Stokes MB, D'Agati VD, Radhakrishnan J, Markowitz GS, Appel GB. Renal-limited 'lupus-like' nephritis. Nephrol Dial Transplant. 2012;27:2337–42. doi: 10.1093/ndt/gfr663. [DOI] [PubMed] [Google Scholar]
- 7.Lim BJ, Hong SW, Jeong HJ. IgG nephropathy - confusion and overlap with C1q nephropathy. Clin Nephrol. 2009;72:360–5. doi: 10.5414/CNP72360. [DOI] [PubMed] [Google Scholar]
- 8.Jennette JC, Hipp CG. C1q nephropathy: a distinct pathologic entity usually causing nephrotic syndrome. Am J Kidney Dis. 1985;6:103–10. doi: 10.1016/S0272-6386(85)80150-5. [DOI] [PubMed] [Google Scholar]
- 9.Mii A, Shimizu A, Masuda Y, Fujita E, Aki K, Ishizaki M, Sato S, Griesemer A, Fukuda Y. Current status and issues of C1q nephropathy. Clin Exp Nephrol. 2009;13:263–74. doi: 10.1007/s10157-009-0159-5. [DOI] [PubMed] [Google Scholar]
- 10.Lau KK, Gaber LW, Delos Santos NM, Wyatt RJ. C1q nephropathy: features at presentation and outcome. Pediatr Nephrol. 2005;20:744–9. doi: 10.1007/s00467-004-1810-8. [DOI] [PubMed] [Google Scholar]
- 11.Fukuma Y, Hisano S, Segawa Y, Niimi K, Tsuru N, Kaku Y, Hatae K, Kiyoshi Y, Mitsudome A, Iwasaki H. Clinicopathologic correlation of C1q nephropathy in children. Am J Kidney Dis. 2006;47:412–8. doi: 10.1053/j.ajkd.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 12.Markowitz GS, Schwimmer JA, Stokes MB, Nasr S, Seigle RL, Valeri AM, D'Agati VD. C1q nephropathy: a variant of focal segmental glomerulosclerosis. Kidney Int. 2003;64:1232–40. doi: 10.1046/j.1523-1755.2003.00218.x. [DOI] [PubMed] [Google Scholar]
- 13.Hisano S, Fukuma Y, Segawa Y, Niimi K, Kaku Y, Hatae K, Saitoh T, Takeshita M, Iwasaki H. Clinicopathologic correlation and outcome of C1q nephropathy. Clin J Am Soc Nephrol. 2008;3:1637–43. doi: 10.2215/CJN.00830208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Onitsuka S, Tanabe K, Toma H, Yamaguchi Y. Mesangial proliferative glomerulonephritis with predominant mesangial IgG deposition in renal allograft. Nephron. 2000;86:404–6. doi: 10.1159/000045824. [DOI] [PubMed] [Google Scholar]
- 15.Jalalah SM. IgG glomerulonephritis: a morphologic study of a rare entity. Saudi J Kidney Dis Transpl. 2009;20:798–801. [PubMed] [Google Scholar]
- 16.Iskandar SS, Browning MC, Lorentz WB. C1q nephropathy: a pediatric clinicopathologic study. Am J Kidney Dis. 1991;18:459–65. doi: 10.1016/S0272-6386(12)80114-4. [DOI] [PubMed] [Google Scholar]
- 17.Fogo AB, Lusco MA, Najafian B, Alpers CE. AJKD atlas of renal pathology: C1q nephropathy. Am J Kidney Dis. 2015;66:e13–4. doi: 10.1053/j.ajkd.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 18.van de Lest NA, Zandbergen M, Wolterbeek R, Kreutz R, Trouw LA, Dorresteijn EM, Bruijn JA, Bajema IM, Scharpfenecker M, Chua JS. Glomerular C4d deposition can precede the development of focal segmental glomerulosclerosis. Kidney Int. 2019;96:738–49. doi: 10.1016/j.kint.2019.04.028. [DOI] [PubMed] [Google Scholar]
- 19.Drachenberg CB, Papadimitriou JC, Chandra P, Haririan A, Mendley S, Weir MR, Rubin MF. Epidemiology and pathophysiology of glomerular C4d staining in native kidney biopsies. Kidney Int Rep. 2019;4:1555–67. doi: 10.1016/j.ekir.2019.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooper NR. The classical complement pathway: activation and regulation of the first complement component. Adv Immunol. 1985;37:151–216. doi: 10.1016/S0065-2776(08)60340-5. [DOI] [PubMed] [Google Scholar]
- 21.Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol. 2014;5:520. doi: 10.3389/fimmu.2014.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pickering WP, Bailey RR, Gardner J. IgG nephropathy: an uncommon form of primary glomerulonephritis? N Z Med J. 1996;109:365. [PubMed] [Google Scholar]
- 23.Sepandj F, McFarlane C, Trillo A. Nephrotic syndrome secondary to primary immunoglobulin-G mesangioproliferative glomerulonephritis. Nephrol Dial Transplant. 1998;13:1889–90. doi: 10.1093/oxfordjournals.ndt.a027901. [DOI] [PubMed] [Google Scholar]
- 24.Assadi FK. IgG-associated mesangial glomerulonephritis in a patient with Down syndrome. Med Sci Monit. 2004;10:CS54–6. [PubMed] [Google Scholar]
- 25.Kharroubi M, Ben Fatma L, Rais L, Jebali H, Mami I, Zouaghi MK. Primary glomerulonephritis with predominant mesangial Immunoglobulin G deposits. Tunis Med. 2018;96:442–4. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.