Abstract
Zinc deficiency is one cause of anemia. However, it has been reported that some patients who were treated with zinc supplementation to resolve this anemia subsequently experienced copper deficiency, which lead to continued anemia, as well as leukocytopenia and other symptoms. However, only two patients with copper deficiency induced by zinc supplementation undergoing peritoneal dialysis have been reported. Here, we report the case of a 59 year-old man with copper deficiency after zinc supplementation undergoing peritoneal dialysis (PD). He took meals only once a day and drank about 750 mL/day of wine every day. He had been receiving zinc supplementation for 4 months. He was diagnosed with severe leukocytopenia and worsening anemia at a planned outpatient visit; in addition, his copper levels had markedly decreased. Thus, zinc supplementation was discontinued, and the patient was instructed to take cocoa for copper supplementation. Because of severe leukocytopenia, he was admitted to our hospital, and granulocyte colony-stimulating factor was administered. Red blood cell transfusions were performed for anemia. After discontinuing zinc supplementation, his white blood cell count and hemoglobin levels improved.
To avoid Cu deficiency, patients’ dietary history should be checked in detail and Cu should be monitored carefully when Zn is supplemented in patients undergoing PD.
Keywords: Copper deficiency, Peritoneal dialysis, Leukocytopenia, Zinc supplementation
Introduction
Zinc (Zn) deficiency is a known cause of anemia. In patients with chronic kidney disease (CKD), Zn deficiency induces erythropoietin-resistant anemia. A previous study reported that oral Zn supplementation reduced the erythropoietin responsiveness index in patients on hemodialysis (HD) [1]. The amount of Zn is rich in oysters, meats (especially liver), and eggs [2]. However, patients with CKD are required to consume mildly low-protein diets, even if they start dialysis or receive kidney transplantation. Therefore, patients with CKD could not consume these Zn-rich foods, and medication to supply Zn is required for these patients. Although Zn supplementation is an important treatment for anemia caused by Zn deficiency, it has several adverse effects, including copper (Cu) deficiency.
Cu deficiency causes anemia, leukocytopenia, myelopathy, and other symptoms [3]. In patients on HD, Cu and ceruloplasmin levels are low compared with those in healthy controls [4]. Also in these patients, Zn and Cu levels are negatively associated, and the association is stronger in patients on HD than in control patients [5].
However, reports on Cu deficiency in patients undergoing peritoneal dialysis (PD) are rare. One study [6] reported that a child on continuous ambulatory peritoneal dialysis (CAPD) had Cu deficiency accompanied with severe neutropenia. Another [7] reported that an adult on CAPD had Cu deficiency accompanied with a history of an increasingly unsteady gait and an inability to stand. Here, we report a case of Cu deficiency accompanied by leukocytopenia induced by Zn supplementation during PD.
Case report
A 59 year-old man undergoing PD was admitted to our hospital because of leukopenia. He had started PD approximately 7 months prior and did not have any history of gastrointestinal surgery. Although renal biopsy had not been performed, the etiology of his renal failure was suspected to be chronic glomerulonephritis due to proteinuria since there was no history of diabetes. He was under automated peritoneal dialysis (APD) with a total of 8 L of Dianeal-N PD4® 1.5% and with four exchanges during the night. He consumed meals only once a day and drank about 750 mL of wine every day.
The patient received 150 μg of epoetin beta pegor subcutaneously administered once per month for treatment of renal anemia. He did not have liver cirrhosis or splenomegaly. Poor response of hemoglobin (Hb) level (7.9 g/dL) to erythropoiesis-stimulating agent (ESA) led us to further investigate the underlying cause of anemia, which turned out to be a low Zn level (37 μg/dL; normal range 80–130 μg/dL). Causes of ESA-resistant anemia such as iron deficiency (ferritin 608 ng/dL and C-reactive protein (CRP) 0.01 mg/dL), blood loss (negative fecal occult blood), inflammation associated with infections and autoimmune reactions (CRP 0.01 mg/dL), severe hyperparathyroidism (intact parathyroid hormone (iPTH) 183 pg/mL), malignant tumors (no malignant tumors detected in abdominal CT scan), hemolysis (lactate dehydrogenase 204 U/L and total bilirubin 0.6 mg/dL), abnormal hemoglobinopathy (Hb level was normal at the first visit of our hospital, suggesting that he did not have genetic abnormal hemoglobinopathy), hypersplenism (splenomegaly was not detected in abdominal CT scan), or administration of ACE inhibitors (ACE inhibitor was not administered) were ruled out (right column in Table 1). Anti-erythropoietin antibodies or carnitine were not measured. We cannot rule out anti-erythropoiesis-stimulating agent antibodies-induced anemia or carnitine deficiency anemia.
Table 1.
Laboratory data 5 months before admission and at the time of Cu deficiency diagnosis
| 5 months before admission | At the time of Cu deficiency diagnosis | |
|---|---|---|
| Complete blood count | ||
| White blood cells (/μL) | 3300 | 1600 |
| Red blood cells (/μL) | 199 × 104 | 202 × 104 |
| Hemoglobin (g/dL) | 6.9 | 6.7 |
| Hematocrit (%) | 19.9 | 19.8 |
| Mean corpuscular volume (fl) | 99.9 | 97.8 |
| Mean cell hemoglobin (pg) | 34.5 | 33.1 |
| Mean cell hemoglobin concentration (g/dL) | 34.5 | 33.8 |
| Platelets (/μL) | 21.4 × 104 | 17.8 × 104 |
| Blood chemistry | ||
| Total bilirubin (mg/dL) | 0.7 | 0.6 |
| Aspartate transaminase (U/L) | 27 | 31 |
| Alanine transaminase (U/L) | 12 | 13 |
| Lactate dehydrogenase (U/L) | 225 | 204 |
| Total protein (g/dL) | 5.7 | 5.4 |
| Albumin (g/dL) | 3.0 | 3.1 |
| C-reactive protein (mg/dL) | 0.05 | 0.01 |
| Ferritin (ng/dL) | 1380 | 608 |
| Fe (μg/dL) | 145 | 174 |
| Unsaturated iron binding capacity (μg/dL) | 79 | 16 |
| Total iron binding capacity (μg/dL) | 224 | 190 |
| Zn (μg/dL) | 37 | 105 |
| Cu (μg/dL) | 75 | 12 |
| Vitamin B12 (pg/mL) | 330 | 156 |
| Folic acid (ng/mL) | 4.4 | 20.0 |
| iPTH (pg/mL) | 139 | 183 |
The laboratory data obtained 5 months prior to admission are shown in Table 1. One hundred and fifty mg of polaprezinc containing 34 mg of Zn was prescribed for 4 months.
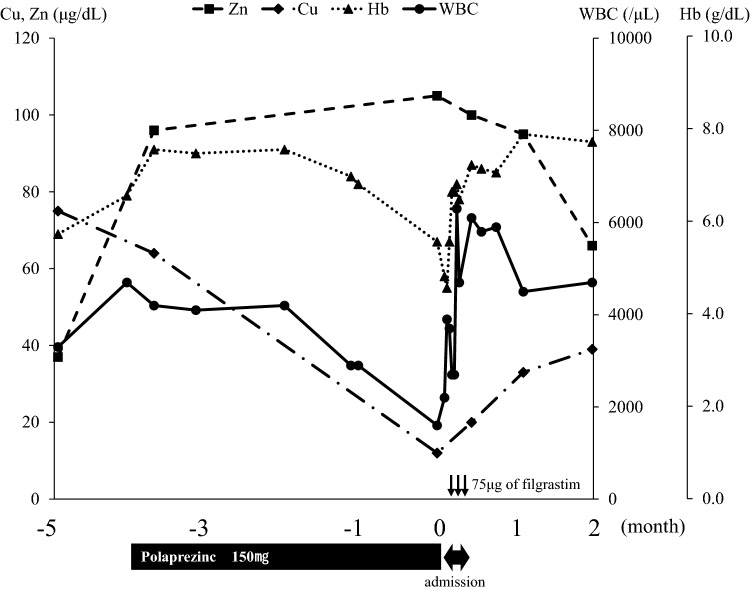
The patient’s Zn and Hb levels were elevated after the start of polaprezinc treatment. During the supplementation, folic acid deficiency was diagnosed, and the patient was supplemented with folic acid. However, 4 months after starting polaprezinc (4 days before admission), white blood cell count (WBC) and Hb levels decreased to 1600/μL and 6.7 g/dL, respectively. The following laboratory data were confirmed: folic acid was high (> 20 ng/mL; normal range 3.9–12.9 ng/mL) because he had been taking prescribed folic acid for folic acid deficiency anemia; Zn level recovered and was increased (105 μg/dL; normal range 80–130 μg/dL); vitamin B12 level was slightly decreased (156 pg/mL; normal range 233–914 pg/mL); Cu was markedly decreased (Cu 12 μg/dL; normal range 70–132 μg/dL); and cerulopasmin level on admission was also markedly decreased (2.7 mg/dL; normal range 21–37 mg/dL). Therefore, Cu deficiency was considered to be the cause of leukocytopenia and severe anemia. The laboratory data at the time of Cu deficiency diagnosis are shown in Table 1. Although his Cu level was low, the patient did not have any neurological abnormalities. Three days later (the day before admission), he visited our hospital and laboratory data showed that his WBC was 2200/μL, neutrophil count was 500/μL, and Hb was 5.8 g/dL. Polaprezinc was then discontinued and the patient was instructed to take cocoa for Cu supplementation. To increase the WBC, 75 μg of filgrastim was administered subcutaneously. The next day, the patient was admitted to our hospital. Filgrastim administration was repeated on days 4 and 6 after admission. On days 1 and 2, 2 units/day of red blood cell concentrate were transfused for anemia. After the transfusion, the patient’s Hb level was elevated to 8.0 g/dL and remained fairly consistent. On day 7, the patient was discharged from our hospital and underwent outpatient visits. Temporal changes in WBC, Hb, Cu, and Zn are shown in Fig. 1. As shown in Fig. 1, after discharge from our hospital, Cu levels gradually increased, and Zn levels gradually decreased. Subsequently, his WBC and Hb levels returned to normal levels.
Fig. 1.
The change of WBC, Hb, Zn and Cu. The x-axis indicates the elapsed months after the day of admission
Discussion
This was a case of leukocytopenia that was primarily caused by Cu deficiency after Zn supplementation over a relatively short period. The main reason for Cu deficiency in this case can be considered an adverse effect of Zn supplementation.
Under physiological conditions, Cu is absorbed in the proximal small intestine and stomach. Two transport proteins, the high-affinity Cu transporter 1 (CTR1) and ATP7A, play an important role in Cu absorption. Dietary Cu is absorbed through CTR1 in enterocytes. Subsequently, Cu in enterocytes is transported to the circulation by ATP7A [3]. Recently, it has been suggested that divalent metal transporter 1 and low-affinity Cu transporter 2 may also play a role in Cu absorption [8].
When Zn is supplemented, intracellular Zn is increased in enterocytes and activates metal-responsive transcription factor I (MTF-I). Activated MTF-I induces metallothionein (MT) gene expression, and MT binds to Cu. Although the excess Cu in a cell is excreted throughout ATP7A, Cu bound to MT does not undergo ATP7A-dependent Cu excretion. Thus, the Cu level in the enterocytes is increased and the enterocytes are sloughed off into the intestinal lumen, leading to a lower rate of uptake of Cu [8].
The reason for the relatively short period between Zn supplementation and the emergence of Cu deficiency in our case may be due to the relatively lower Cu level (75 μg/dL) at the start of Zn supplementation. The patient did not receive enteral nutrition or total parenteral nutrition. Although the inhibition of absorption from the intestinal tract was not investigated in detail, precipitated calcium carbonate was prescribed for hyperphosphatemia, suggesting that the inhibition of absorption from the intestinal tract was less likely. The patient was on peritoneal dialysis, drank a relatively high amount of alcohol, and took meals only once a day under Zn supplementation. Thus, the intake of Cu was probably low. These multiple risk factors might have led to the Cu deficiency. However, we could not completely rule out the underlying diseases causing anemia such as anti-erythropoiesis-stimulating agent antibodies-induced anemia or carnitine deficiency anemia because bone marrow aspiration was not performed and anti-erythropoiesis-stimulating agent antibodies and carnitine were not measured.
The characteristics of the cases of Cu deficiency in patients on PD during Zn supplementation, including our case, are summarized in Table 2 [6, 7]. The main symptoms of Cu deficiency improved relatively quickly after the initiation of Cu supplementation. Several risk factors for Cu deficiency have been reported, such as Zn supplementation [9, 10], surgery to remove foregut [11–13], chronic diarrhea [14, 15], chronic peritoneal dialysis [6, 7], HD [4], and total parenteral nutrition [16]. Cu is present in many foods, especially in oysters, cocoa, and liver [17]. Patients with CKD, including those on chronic dialysis, generally consume a low-protein diet. Thus, dietary intake of Cu may be low. In patients on CAPD, Cu is excreted in the dialysate [18]. Furthermore, in non-anuric patients on CAPD, Cu excretion in urine is higher than that in healthy subjects [18]. Additionally, a previous case report suggested that excessive alcohol consumption could be a risk factor for Cu deficiency [19]. In the report, a relatively high amount of alcohol consumption (about 750 mL/day of wine) might have affected the incidence of Cu deficiency.
Table 2.
Characteristics of the cases of Cu deficiency in patients on PD during Zinc supplementation
| Becton DL et al.[6] | Saly DL et al.[7] | The present case | |
|---|---|---|---|
| Sex | Male | Female | Male |
| Age (years) | 16 | 61 | 59 |
| PD vintage | 8 years | Not available | 7 months |
| Zinc supplementation | Yes | Yes | Yes |
| Complete blood count | |||
| White blood cells (/μL) | 2200 | Unremarkable change | 1600 |
| Hemoglobin (g/dL) | 9.6 (With RBC transfusion) | 8.3 | 6.7 |
| Mean corpuscular volume (fl) | 84 | 84 | 97.8 |
| Platelets (/μL) | 17.7 × 104 | Unremarkable change | 17.8 × 104 |
| Blood chemistry | |||
| Albumin (g/dL) | 2.9 | Not available | 3.1 |
| Ferritin (ng/dL) | Mildly elevated | Iron study were normal | 608 |
| Zn (μg/dL) | Normal | 134 | 105 |
| Cu (μg/dL) | 12 | < 10 | 12 |
| Ceruloplasmin (mg/dL) | 2.0 | < 4 | 2.7 |
| Vitamin B12 (pg/mL) | Not available | 1207 | 156 |
| Folic acid (ng/mL) | Normal | 20.3 | 20.0 |
| Treatment | |||
| Cu supplementation | Orally | Intravenously and orally | Orally |
| Duration for the recovery of the main symptoms | 2 weeks | 5 days | 7 days |
As for the Cu deficiency in patients on HD, in a randomized controlled trial (RCT), serum Cu levels reportedly decreased significantly in the acetate hydrate group, but not in the polaprezinc group [20]. Moreover, Cu deficiency-induced pancytopenia caused by polaprezinc was reported in a patient on HD [21]. However, we could not determine whether patients on PD are more prone to Cu deficiency than patients on HD based on the previous case report and the RCT, which included only patients on HD. To compare the occurrence of Cu deficiency by Zn supplementation between PD and HD patient populations, a cohort study is necessary.
The mechanism of leukocytopenia due to Cu deficiency is not clearly understood. Previous studies have reported that progenitor cells were preserved before Cu supplementation in patients with Cu deficiency who had severe neutropenia. This indicates that Cu enzymes play an important role in the maturation of hematopoietic cells, and a lack of Cu may result in ineffective granulopoiesis [22, 23].
Furthermore, an experimental study reported that a decrease in cellular chelatable Cu content inhibited the differentiation and self-renewal of CD34-positive cells [24].
The mechanisms of anemia due to Cu deficiency are as follows: Cu deficiency decreases RBC survival time [9, 25] and interferes with heme synthesis by suppressing iron transport and utilization because Cu is an important component of the ferroxidase enzymes, hephaestin, and ceruloplasmin [3, 9, 26]; hephaestin facilitates oxidation of ferrous iron (Fe2+) to ferric iron (Fe3+); ceruloplasmin plays an important role in iron transport; and Cu deficiency leads to impaired ferroxidase enzymes, which cause impaired hemoglobin synthesis [3].
Although Cu deficiency sometimes causes anemia and leukopenia, platelet counts are typically normal or only mildly low [3], which is consistent with our present case. Bone marrow morphology in patients with Cu deficiency is reportedly similar to that in patients with myelodysplastic syndromes [15, 27].
Here, we describe the case of a patient on PD with Cu deficiency caused by Zn supplementation. To avoid Cu deficiency, patients’ dietary histories should be checked in detail, and Cu should be monitored carefully when Zn is supplemented in patients undergoing PD.
Acknowledgements
None.
Abbreviations
- Zn
Zinc
- CKD
Chronic kidney disease
- Cu
Copper
- HD
Hemodialysis
- PD
Peritoneal dialysis
- CAPD
Continuous ambulatory peritoneal dialysis
- APD
Automated peritoneal dialysis
- Hb
Hemoglobin
- ESA
Erythropoiesis-stimulating agent
- CRP
C-reactive protein
- iPTH
Intact parathyroid hormone
- WBC
White blood cell count
- CTR1
Copper transporter 1
- MTF-I
Metal-responsive transcription factor I
- MT
Metallothionein
- RCT
Randomized controlled trial
Author contributions
All authors discussed this case and read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Because of case report, approval from a local ethics committee is not necessary.
Consent for publication
Written informed consent was obtained from the patient.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kobayashi H, Abe M, Okada K, Tei R, Maruyama N, Kikuchi F, et al. Oral zinc supplementation reduces the erythropoietin responsiveness index in patients on hemodialysis. Nutrients. 2015;7:3783–95. doi: 10.3390/nu7053783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy EW, Willis BW, Watt BK. Provisional tables on the zinc content of foods. J Am Diet Assoc. 1975;66:345–55. doi: 10.1016/S0002-8223(21)14515-8. [DOI] [PubMed] [Google Scholar]
- 3.Myint ZW, Oo TH, Thein KZ, Tun AM, Saeed H. Copper deficiency anemia: review article. Ann Hematol Annals Hematol. 2018;97:1527–34. doi: 10.1007/s00277-018-3407-5. [DOI] [PubMed] [Google Scholar]
- 4.Eleftheriadis T, Pissas G, Antoniadi G, Filippidis G, Golfinopoulos S, Spanoulis A, et al. Serum copper and ferroportin in monocytes of hemodialysis patients are both decreased but unassociated. Int Urol Nephrol. 2014;46:1825–31. doi: 10.1007/s11255-014-0725-y. [DOI] [PubMed] [Google Scholar]
- 5.Nishime K, Kondo M, Saito K, Miyawaki H, Nakagawa T. Zinc burden evokes copper deficiency in the hypoalbuminemic hemodialysis patients. Nutrients. 2020;12:1–10. doi: 10.3390/nu12020577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becton DL, Schultz WH, Kinney TR. Severe neutropenia caused by copper deficiency in a child receiving continuous ambulatory peritoneal dialysis. J Pediatr. 1986;108:735–7. doi: 10.1016/S0022-3476(86)81056-3. [DOI] [PubMed] [Google Scholar]
- 7.Saly DL, Brewster UC, Sze GK, Louis ED, Shirali AC. An element of unsteadiness. N Engl J Med. 2017;377:1379–85. doi: 10.1056/NEJMcps1701934. [DOI] [PubMed] [Google Scholar]
- 8.van den Berghe PVE, Klomp LWJ. New developments in the regulation of intestinal copper absorption. Nutr Rev. 2009;67:658–72. doi: 10.1111/j.1753-4887.2009.00250.x. [DOI] [PubMed] [Google Scholar]
- 9.Willis MS, Monaghan SA, Miller ML, McKenna RW, Perkins WD, Levinson BS, et al. Zinc-induced copper deficiency: a report of three cases initially recognized on bone marrow examination. Am J Clin Pathol. 2005;123:125–31. doi: 10.1309/V6GVYW2QTYD5C5PJ. [DOI] [PubMed] [Google Scholar]
- 10.Rowin J, Lewis SL. Copper deficiency myeloneuropathy and pancytopenia secondary to overuse of zinc supplementation. J Neurol Neurosurg Psychiatr. 2005;76:750–1. doi: 10.1136/jnnp.2004.046987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griffith DP, Liff DA, Ziegler TR, Esper GJ, Winton EF. Acquired copper deficiency: a potentially serious and preventable complication following gastric bypass surgery. Obes (Silver Spring) 2009;17:827–31. doi: 10.1038/oby.2008.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar P, Hamza N, Madhok B, De Alwis N, Sharma M, Miras AD, et al. Copper deficiency after gastric bypass for morbid obesity: a systematic review. Obes Surg. 2016;26:1335–42. doi: 10.1007/s11695-016-2162-8. [DOI] [PubMed] [Google Scholar]
- 13.Tan JC, Burns DL, Jones HR. Severe ataxia, myelopathy, and peripheral neuropathy due to acquired copper deficiency in a patient with history of gastrectomy. JPEN J Parenter Enteral Nutr. 2006;30:446–50. doi: 10.1177/0148607106030005446. [DOI] [PubMed] [Google Scholar]
- 14.Goodman BP, Mistry DH, Pasha SF, Bosch PE. Copper deficiency myeloneuropathy due to occult celiac disease. Neurologist. 2009;15:355–6. doi: 10.1097/NRL.0b013e31819428a8. [DOI] [PubMed] [Google Scholar]
- 15.Halfdanarson TR, Kumar N, Hogan WJ, Murray JA. Copper deficiency in celiac disease. J Clin Gastroenterol. 2009;43:162–4. doi: 10.1097/MCG.0b013e3181354294. [DOI] [PubMed] [Google Scholar]
- 16.Fuhrman MP, Herrmann V, Masidonski P, Eby C. Pancytopenia after removal of copper from total parenteral nutrition. J Parenter Enter Nutr. 2000;24:361–6. doi: 10.1177/0148607100024006361. [DOI] [PubMed] [Google Scholar]
- 17.Lindow CW. The copper content of plant and animal foods. J Biol Chem. 1929;82:465–71. doi: 10.1016/S0021-9258(20)78293-1. [DOI] [Google Scholar]
- 18.Xiang S, Yao Y, Wan Y, Liang W, Meng R, Jin Q, et al. Comparative study on trace element excretions between nonanuric and anuric patients undergoing continuous ambulatory peritoneal dialysis. Nutrients. 2016;8(12):126. doi: 10.3390/nu8120826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shibazaki S, Uchiyama S, Tsuda K, Taniuchi N. Copper deficiency caused by excessive alcohol consumption. BMJ Case Rep. 2017;2017:12–5. doi: 10.1136/bcr-2017-220921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okamoto T, Hatakeyama S, Konishi S, Okita K, Tanaka Y, Imanishi K, et al. Comparison of zinc acetate hydrate and polaprezinc for zinc deficiency in patients on maintenance hemodialysis: a single-center, open-label, prospective randomized study. Ther Apher Dial. 2020;24:568–77. doi: 10.1111/1744-9987.13461. [DOI] [PubMed] [Google Scholar]
- 21.Kadoya H, Uchida A, Kashihara N. A case of copper deficiency-induced pancytopenia with maintenance hemodialysis outpatient treated with polaprezinc. Ther Apher Dial. 2016;20:422–3. doi: 10.1111/1744-9987.12398. [DOI] [PubMed] [Google Scholar]
- 22.Hirase N, Abe Y, Sadamura S, Yufu Y, Muta K, Umemura T, et al. Anemia and neutropenia in a case of copper deficiency: Role of copper in normal hematopoiesis. Acta Haematol Karger Publishers. 1992;87:195–7. doi: 10.1159/000204758. [DOI] [PubMed] [Google Scholar]
- 23.Zidar BL, Shadduck RK, Zeigler Z, Winkelstein A. Observations on the anemia and neutropenia of human copper deficiency. Am J Hematol Am J Hematol. 1977;3:177–85. doi: 10.1002/ajh.2830030209. [DOI] [PubMed] [Google Scholar]
- 24.Peled T, Glukhman E, Hasson N, Adi S, Assor H, Yudin D, et al. Chelatable cellular copper modulates differentiation and self-renewal of cord blood-derived hematopoietic progenitor cells. Exp Hematol. 2005;33:1092–100. doi: 10.1016/j.exphem.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 25.Dunlap WM, James GW, Hume DM. Anemia and neutropenia caused by copper deficiency. Ann Intern Med. 1974;80:470–6. doi: 10.7326/0003-4819-80-4-470. [DOI] [PubMed] [Google Scholar]
- 26.Nagano T, Toyoda T, Tanabe H, Nagato T, Tsuchida T, Kitamura A, et al. Clinical features of hematological disorders caused by copper deficiency during long-term enteral nutrition. Intern Med. 2005;44:554–9. doi: 10.2169/internalmedicine.44.554. [DOI] [PubMed] [Google Scholar]
- 27.Gregg XT, Reddy V, Prchal JT. Copper deficiency masquerading as myelodysplastic syndrome. Blood. 2002;100:1493–5. doi: 10.1182/blood-2002-01-0256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.