Abstract
Crystalline light chain cast nephropathy is a rare distinct morphologic variant of light chain cast nephropathy which is the most common renal lesion associated with multiple myeloma. It is often related to high myeloma tumor burden, severe acute kidney injury, and an unfavorable prognosis. A 79-year-old Japanese man was referred to our medical center with anemia, proteinuria, and acute exacerbation of the serum creatinine accompanying anuria. A renal biopsy showed crystalline cast filling the tubular lumens, injured tubular cells, and inflammatory cells infiltration of interstitium. Serum and urine immunofixation detected a monoclonal protein (IgA-λ and Bence-Jones Protein–λ, respectively), and bone marrow examination observed 64% of plasma cells. IgA-λ type multiple myeloma-associated crystalline light chain cast nephropathy and accompanying acute kidney injury were confirmed. Hydration and emergency hemodialysis were immediately introduced, and the treatment with bortezomib and dexamethasone was initiated. The patient showed successful recovery in renal manifestations. We suggest that early use with bortezomib-based therapy should be considered for patients with acute kidney injury caused by multiple myeloma-associated crystalline light chain cast nephropathy.
Keywords: Acute kidney injury, Bortezomib, Crystalline cast, Light chain cast nephropathy, Multiple myeloma
Introduction
Crystalline light chain cast nephropathy (Crystalline LCCN) is a rare morphologic variant of light chain cast nephropathy (LCCN) which is the most common renal lesion associated with multiple myeloma (MM) [1, 2]. It occurs by free light chains forming casts in urine tubules due to its structural characteristics and causes acute kidney injury (AKI) by obstructing tubular lumens and inducing the inflammatory reaction around them which leads to tubular atrophy and interstitial inflammation [1–5]. Clinically, crystalline LCCN is often associated with advanced MM with severe AKI and higher early mortality [3]. Here, we report a rare case of a 79-year-old Japanese man with IgA-λ type MM-associated crystalline LCCN and succeeding AKI, who finally showed renal recovery and dialysis independence with the early use of bortezomib and dexamethasone. In addition, we reviewed the literature of such cases. Given the renal outcomes in those cases, early intervention with bortezomib-based therapy should be considered for successful renal recovery from crystalline LCCN-related AKI.
Case report
A 79-year-old Japanese man was referred to our medical center with anemia, proteinuria, and acute exacerbation of the serum creatinine. He had a baseline creatinine level of 0.75 mg/dL. He complained of vomiting, fatigue, and anuria lasting 4 days. There was no complaint of chilling, headache, shortness of breath, or abdominal pain. The patient was taking eplerenone for his hypertension; he had no history of any drug abuse or known allergies. He had smoked for 40 years and has been daily consuming alcohol. Physical examination revealed that his respiratory rate, heart rate, blood pressure, O2 saturation at room air, and body temperature were 18 breaths per minute, 65 beats per minute, 130/76 mm Hg, 97%, and 36.5 °C, respectively. There were no notable examination findings, including tenderness of abdomen or bones, hepatosplenomegaly, lymphadenopathy, pretibial edema, or paresthesia. Laboratory findings (Table 1) showed anemia (hemoglobin 8.8 × 104 g/dL) and a normal white blood cell level (4880 × 106 /L). The total protein was 8.4 g/dL, while the albumin level was 3.3 g/dL. A severe renal function loss (blood urea nitrogen: 45.0 mg/dL; serum creatinine: 7.92 mg/dL; estimated glomerular filtration rate: 6 ml/min/1.73 m2) was noted. Serum corrected calcium level was high (10.3 mg/dL). Additionally, the immunoglobulin levels show significant elevation of IgA (3218 mg/dL) with suppressions of IgG and IgM (283 mg/dL and 16 mg/dL, respectively). Serum-free light chain λ was markedly elevated (9480 mg/L), while κ was low (13.0 mg/L). Serum β2 microglobulin was high (21.0 mg/L). Urinalysis showed severe proteinuria (urine protein creatinine ratio17.04 g/g Cr) with a few granular casts. Hyaline casts were undetected. Serum and urine immunofixation detected a monoclonal protein (IgA-λ and Bence-Jones Protein–λ, respectively). Bone marrow examination observed 64% of plasma cells without amyloid deposition; chromosomal abnormalities in plasma cells were unclear. Viral screenings for hepatitis B showed he had an inactive HBV. Radiography showed punched-out lesions on the skull and the right humerus. Plain computed tomography of the abdomen showed no abnormality of kidneys. Renal biopsy showed 24 glomeruli with mild mesangial proliferation. There was rod-shaped and rhomboid crystalline cast filling the tubular lumens, injured tubular cells, and inflammatory cells infiltration of interstitium (Fig. 1a, b). The glomeruli did not show significant pathological changes. Congo red staining was not suggestive of amyloidosis. Immunofluorescence microscopy showed λ chain staining of the crystals within tubules and of tubule basement membranes on formalin-fixed tissue (Fig. 2). Electron microscopy detected rod-shaped and rhomboid intraluminal crystalline inclusions (Fig. 3), while there were no electron-dense deposits nor granular deposits on basement membranes.
Table 1.
Laboratory data
| Hemogram and infection |
|---|
| White blood cell 4880/μL (3040–8540) |
| Red blood cell 276 × 104/μL (378–499 × 104) |
| Hemoglobin 8.8 g/dL (10.8–14.9) |
| Hematocrit 27.2% (35.6–45.4) |
| Platelet 12.3 × 104/μL (15.0–36.1 × 104) |
| Hepatitis B virus surface antigen (–) |
| Hepatitis B virus surface antibody ( +) |
| Hepatitis B virus core antibody ( +) |
| Hepatitis B virus—DNA (–) |
| Hepatitis C virus antibody (–) |
| Immunological examinations |
| C-reactive protein 1.97 mg/dL (< 0.3) |
| IgG 283 mg/dL (870–1700) |
| IgA 3218 mg/dL (90–400) |
| IgM 16 mg/dL (35–220) |
| Serum-free light chain κ 13.0 mg/L (3–20) |
| Serum-free light chain λ 9480 mg/L (5–25) |
| Serum-free light chain ratio < 0.01 |
| Complement 3 69 mg/dL (65–135) |
| Complement 4 29 mg/dL (13–35) |
| CH50 41.6 U/mL (28–53) |
| Antinuclear antibody (–) |
| Myeloperoxidase anti-neutrophil cytoplasmic antibody (–) |
| Proteinase 3 anti-neutrophil cytoplasmic antibody (–) |
| C1q immune complex (–) |
| Cryoglobulin (–) |
| Blood chemistry |
| Total protein 8.4 g/dL (6.6–8.0) |
| Albumin 3.3 g/dL (4.1–5.0) |
| BUN 45.0 mg/dL (8–20) |
| sCr 7.92 mg/dL (0.5–0.8) |
| eGFR 6 mg/min/1.73m2 |
| Uric acid 10.2 mg/dL (2.9–5.2) |
| Na 133 mEq/L (138–146) |
| K 4.4 mEq/L (3.6–4.9) |
| Cl 98 mEq/L (99–109) |
| Ca 9.6 mg/dL (8.7–10.0) |
| P 4.5 mg/dL (2.5–4.6) |
| AST 22 IU/L (13–33) |
| ALT 14 IU/L (6–27) |
| LDH 168 IU/L (119–229) |
| ALP 64 IU/L (115–379) |
| Total bilirubin 0.4 mg/dL (0.3–1.2) |
| β2 microglobulin 21.0 mg/L (1–2) |
| Urinalysis |
| Urine protein creatinine ratio 17.04 |
| RBC 1–4/HPF |
| WBC 10–19/HPF |
| Granular casts 1–9/LPF |
| Hyaline casts < 1/LPF |
| Serum immunofixation |
| IgA-λ |
| Urine immunofixation |
| Bence-Jones Protein –λ |
| Bone marrow examination |
| Plasma cells 64% |
| Amyloid deposition (–) |
IgG immunoglobulin G, IgA immunoglobulin A, IgM immunoglobulin M, BUN blood urea nitrogen, sCr serum creatinine, eGFR estimated Glomerular Filtration Rate, Na sodium, K potassium, Cl chloride, Ca calcium, P phosphate, AST aspartate transaminase, ALT alanine transaminase, LDH lactate hydrogenase, ALP alkaline phosphatase
Fig. 1.
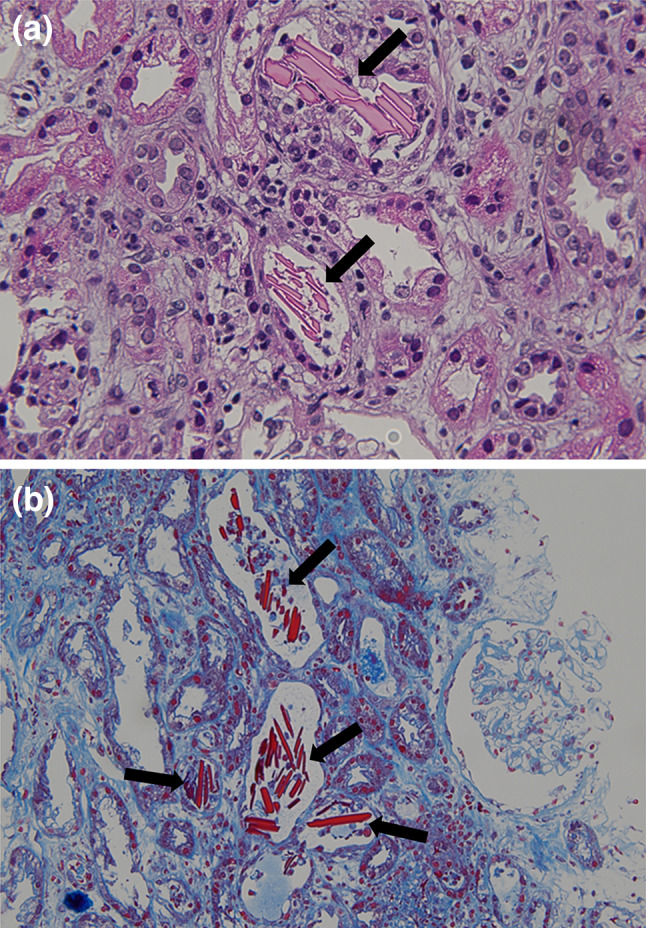
Light microscopy of the kidney showed crystalline cast filling the tubular lumens (arrow), injured tubular cells, and inflammatory cells infiltration of interstitium. (a Hematoxylin–eosin, × 400. b Masson trichrome, × 200.)
Fig. 2.
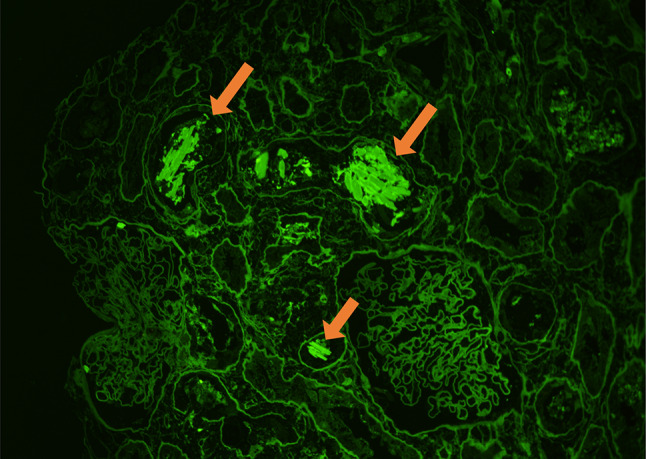
Immunofluorescence staining of the kidney showed λ chain staining of the crystals within tubules (arrow). (Anti-lambda immunofluorescence, × 200)
Fig. 3.
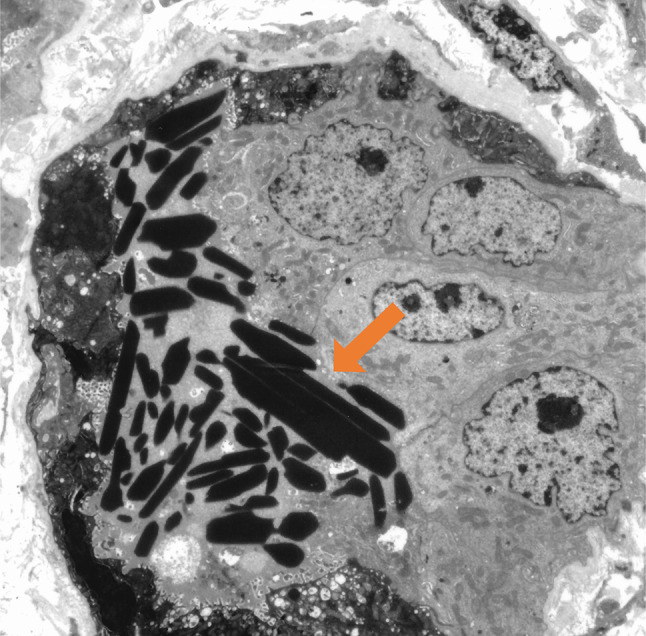
Electron microscopy showed intraluminal crystalline inclusions (arrow). (× 2000)
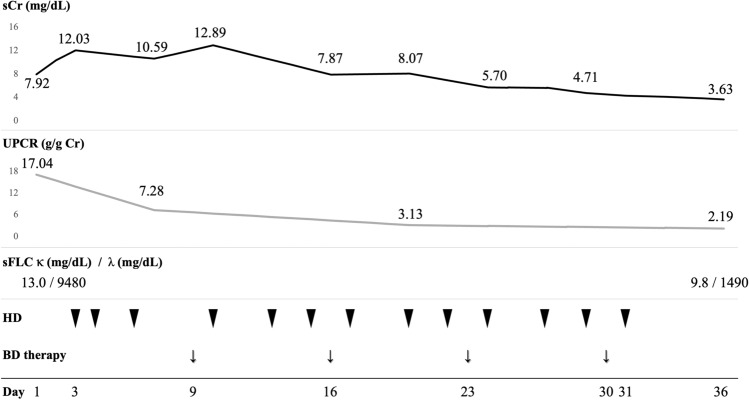
The diagnosis of IgA-λ type MM-associated crystalline LCCN and accompanying AKI was made. Figure 4 shows the clinical course of this patient. Given his rapid exacerbation of renal function, hydration, and emergency hemodialysis had been initiated on Day 3. Hemodialysis was provided with the non-high-cutoff (non-HCO) dialyzer for three hours and continued twice weekly after the acute phase. With the use of hemodialysis, urine protein creatinine ratio improved from 17.04 to 7.28 g/g Cr in a short time before the initiation of anti-myeloma therapy. Weekly administration of anti-myeloma therapy, 1.3 mg/m2 of bortezomib, and 20 mg of dexamethasone was started on Day 9. During the anti-myeloma treatment, the patient did not show significant side effects, including bortezomib-induced neuropathy or lung injury, and the treatment was continued without dose reduction. His serum creatinine and proteinuria continuously recovered; urine output was retrieved on Day 18; dialysis was stopped on Day 31. The duration of dialysis was 29 days. The serum-free light chain ratio moderately improved, and he achieved a partial response according to the International Myeloma Working Group criteria after four weeks of the above therapy [6]. The patient keeps on the therapy and remains independent from dialysis; serum creatinine which was 3.63 mg/dL on Day 36 settled at around 1.30 mg/dL 4 months after the beginning of bortezomib-based therapy.
Fig. 4.
Clinical course of the patient. sCr serum creatinine, UPCR urine protein creatinine ratio, sFLC serum-free light chain, BD bortezomib plus dexamethasone, HD hemodialysis
Discussion
Here, we have reported the first detailed description of an early treatment course of MM-associated crystalline LCCN and accompanying AKI. Crystalline LCCN is a rare morphologic variant of LCCN, the most common MM-associated renal lesion, and is caused by light chain crystallization which is mainly associated with λ restriction in monoclonal gammopathy. Crystalline nephropathy can be classified intracellularly (as light chain proximal tubulopathy and as crystal-storing histiocytosis), or extracellularly (as crystalline LCCN and as crystalglobulin-induced nephropathy) [1, 2, 5, 7, 8]. Extracellular crystallization normally induces AKI and is more fatal [7]. Lin ZS et al. reported that crystalline LCCN is associated with high myeloma tumor burden, AKI with a median eGFR of 5.59 (range, 2.27–26.04) mL/min/1.73 m2, and has higher early mortality (death ≤ 6 months from diagnosis) than ordinary LCCN patients (50.0 vs 11.1%) [3]. Crystalline LCCN differs from non-crystalline LCCN in a way of cast formation; crystalline cast is considered to form based on structural characteristics of the pathogenic free light chains through hydrophobic and hydrogen bonds, in addition to the tubular luminal environment such as injured tubular cells, ionic and in tubule and tubular fluid flow rates; non-crystalline cast normally derives from the interaction between light chains and Tamm-Horsfall glycoprotein [1, 3, 4]. Light chain casts in crystalline LCCN fill the tubular lumens, obstruct them, and induce the inflammatory reaction around them, leading to tubular atrophy and interstitial inflammation [1, 5]. Based on the above-mentioned pathogeneses, AKI in crystalline LCCN is thought to be attributed to “post-glomerular” failure by crystalline cast obstruction of tubules and to renal failure caused by tubular atrophy and interstitial inflammation.
Our observation is that crystalline LCCN-related AKI successfully resolved shortly after hemodialysis and anti-myeloma therapy in our case. To the best of our knowledge, there have only been six reported cases, including ours, of having achieved dialysis independence with the use of anti-myeloma therapy in AKI patients with MM-associated crystalline LCCN [3, 4, 7, 9]. These reports are summarized in (Table 2), five of which were treated with bortezomib-based regimens. This could mean that a bortezomib-based regimen played a significant role in renal recovery and dialysis independence. Bortezomib-based therapy is thought to be currently the best therapy against plasma cell clones to achieve hematologic response, such as a reduction of the involved free light chain which can further improve the kidney response [5]. In addition, bortezomib is a reversible inhibitor of the 26 S proteasome and prevents the proteasomal cleavage of nuclear factor-kappa B (NF-κB) proteins which activate inflammatory pathway leading to free light chains’ toxicity of renal tubules [10, 11]. Clinically, bortezomib is known to be a well-tolerated and effective agent in MM patients with severe renal dysfunction and its potency does not appear to be affected by dialysis [12, 13]. In our case, we selected bortezomib as an anti-myeloma therapy based on the above pharmacological and clinical aspects; we avoided immunomodulatory imide drugs, cyclophosphamide, and a monoclonal antibody against CD38 in consideration of renal manifestations and a risk of adverse effects, including immunosuppression. We assessed our bortezomib-based therapy helped resolve renal AKI in its early stage by reducing the free light chain burden and providing an anti-inflammatory effect. While dose reduction or cessation may had been required when adverse effects, including severe peripheral neuropathy or lung injury, are recognized [12–15], the patient did not show any notable adverse events.
Table 2.
Case summary of MM-associated crystalline LCCN in which dialysis independence was achieved
| Case | Age, Gender |
sCr (mg/dl) |
UPCR | Light chain isotype |
Anti-myeloma therapy |
The time from symptom onset to anti-myeloma therapy initiation |
The duration of dialysis |
Hematological outcome |
|---|---|---|---|---|---|---|---|---|
|
Haider M [9] |
57, Male |
7.39 | 1.2 | λ | BD | 2 weeks | 4 weeks |
being evaluated for ASCT |
|
Lin ZS [3] |
59.5 [41, 73], N/A |
N/A | N/A | N/A | VAD | N/A | N/A |
CR at 3 months; clinical relapse and death |
|
Lin ZS [3] |
59.5 [41, 73], N/A |
N/A | N/A | N/A |
bortezomib- based |
N/A | N/A | PR at 13 months |
|
Matsumura M [4] |
73, Female |
5.59 | 9.1 | λ |
bortezomib, dexamethasone, lenalidomide, and pomalidomide |
N/A | 2 weeks | Died at 6 months |
| Chou A [7] | 74, Female | 8.62 | 0.55 g (proteinuria per day) | κ | CyBorD | 3 weeks | 2 months with 5 sessions of plasmapheresis | PR |
| Our case |
79, Male |
7.92 | 17.04 | λ | BD | 5 days | 3 weeks | PR at 1 month |
N/A not available, sCr serum creatinine, UPCR urine protein creatinine ratio, VAD vinorelbine, pirarubicin, and dexamethasone, CyBorD cyclophosphamide, bortezomib, and dexamethasone, BD bortezomib and dexamethasone, ASCT autologous stem cell transplant, CR complete remission ,PR, partial remission
In the literature in Table 2, serum creatinine ranges from 5.59 to 8.62 mg/dl, and the time from symptom onset to anti-myeloma therapy initiation is identified in three cases ranging from 5 days to no more than 3 weeks. On the other hand, Toly-Ndour C et al. and Chung-Kuan Wu et al. reported the cases in which crystalline LCCN patients who had AKI with more than 12 mg/dl of initial serum creatinine did not regain renal function and did not leave dialysis despite the use of bortezomib-based treatment [16, 17]. It implies that a high value of initial serum creatinine and a substantial therapeutic delay can be deadly to renal outcomes regardless of anti-myeloma intervention.
Clinically, it should be noted that, in LCCN with or without crystallization, the presence of numerous casts and diffuse tubular atrophy are considered independent predictors of renal outcomes in patients with a hematological response (partial response or above) [18]. Considering the necessity of eliminating those precipitating factors, the use of anti-myeloma therapy, including bortezomib, should be considered as soon as the underlying AKI etiology is detected in patients with MM-associated crystalline LCCN.
In addition, hydration and emergency hemodialysis might have provided a largely supportive role in our case. We did not use high-cutoff (HCO) dialysis, although some studies suggest that the combination of bortezomib-based chemotherapy with free light chain removal using HCO dialysis may increase renal recovery rate or dialysis independence rate in patients with MM-associated LCCN [19–21]. In our case, even before the initiation of anti-myeloma therapy, proteinuria significantly improved. Given that early time course and the final achievement of renal recovery, including dialysis independence, “post-glomerular” AKI might have been resolved by crystalline casts being spontaneously washed out during a series of non-HCO hemodialysis. This was supposedly because we made an early diagnosis and the initial amount of crystalline cast filling the tubules was rather small.
This case report has several limitations. First, the follow-up duration of our case is only 4 months; the long-term renal outcomes after renal recovery from crystalline LCCN-related AKI are unclear, and an overall prognosis is uncertain because a progression or a relapse of MM can occur with or without renal manifestations. Long-term follow-up studies are expected in the future. Second, the efficacy of daratumumab, a monoclonal antibody against CD38, for crystalline LCCN remains unknown. Daratumumab has shown high efficacy for patients with MM and AL amyloidosis [22, 23] and would be an additional option to reduce plasma cell clones and provide better outcomes in patients with crystalline LCCN. On the other hand, the serious adverse events with the use of daratumumab are cytopenia and infections, such as pneumonia [22, 23]. In our case, daratumumab was on hold to introduce anti-myeloma treatment more securely to the elderly frail patient.
Conclusion
We report an adult case of IgA-λ type MM-associated crystalline LCCN and accompanying AKI. Emergency hemodialysis was immediately introduced, and the treatment with bortezomib and dexamethasone was initiated. The patient showed successful recovery in renal manifestations and became independent from dialysis. Crystalline LCCN is often associated with advanced MM with severe AKI and higher early mortality. In addition, it is known that the presence of numerous casts and diffuse tubular atrophy is independent predictor of renal outcomes. We suggest that early anti-myeloma intervention should be considered for AKI patients with MM-associated crystalline LCCN. Bortezomib is a well-tolerated, efficient agent in MM patients with severe renal dysfunction, and bortezomib-based therapy should be a good option as an anti-myeloma treatment in such cases.
Declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee at which the studies were conducted and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Doshi M, Lahoti A, Danesh FR, Batuman V, Sanders PW. Paraprotein–related kidney disease: kidney injury from paraproteins—what determines the site of injury? Clin J Am Soc Nephrol. 2016;11(12):2288–2294. doi: 10.2215/CJN.02560316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leung N, Bridoux F, Batuman V, Chaidos A, Cockwell P, D’Agati VD, Dispenzieri A, Fervenza FC, Fermand JP, Gibbs S, Gillmore JD. The evaluation of monoclonal gammopathy of renal significance: a consensus report of the international kidney and monoclonal gammopathy research group. Nature Rev Nephrol. 2019;15(1):45–59. doi: 10.1038/s41581-018-0077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin ZS, Zhang X, Yu XJ, Wang S, Wang SX, Dong YJ, Zhou FD, Zhao MH. Crystalline appearance in light chain cast nephropathy is associated with higher early mortality in patients with newly diagnosed multiple myeloma. Int Immunopharmacol. 2021;98:107875. doi: 10.1016/j.intimp.2021.107875. [DOI] [PubMed] [Google Scholar]
- 4.Matsumura H, Furukawa Y, Nakagaki T, Furutani C, Osanai S, Noguchi K, Odaka M, Yohda M, Ohtani H, Michishita Y, Kawabata Y. Multiple myeloma-associated ig light chain crystalline cast nephropathy. Kidney Int Rep. 2020;5(9):1595–1602. doi: 10.1016/j.ekir.2020.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leung N, Bridoux F, Nasr SH. Monoclonal gammopathy of renal significance. N Engl J Med. 2021;384(20):1931–1941. doi: 10.1056/NEJMra1810907. [DOI] [PubMed] [Google Scholar]
- 6.Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, Munshi N, Lonial S, Bladé J, Mateos MV, Dimopoulos M. International Myeloma working group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17(8):e328–e346. doi: 10.1016/S1470-2045(16)30206-6. [DOI] [PubMed] [Google Scholar]
- 7.Chou A, Long C, Vonthethoff L, Ho SJ, Pettit F, Badve SV. Crystalglobulinemia in multiple myeloma: a rare case report of survival and renal recovery. Can J Kidney Health Dis. 2020;7:2054358120922629. doi: 10.1177/2054358120922629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stokes MB, Valeri AM, Herlitz L, Khan AM, Siegel DS, Markowitz GS, D’Agati VD. Light chain proximal tubulopathy: clinical and pathologic characteristics in the modern treatment era. J Am Soc Nephrol. 2016;27(5):1555–1565. doi: 10.1681/ASN.2015020185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haider M, Salvatore SP, Kaplan J, Seshan SV. Acute kidney injury due to tubular intraluminal monoclonal light chain crystals mimicking acute pyelonephritis. Ren Fail. 2014;36(2):300–305. doi: 10.3109/0886022X.2013.844643. [DOI] [PubMed] [Google Scholar]
- 10.Fabbrini P, Finkel K, Gallieni M, Capasso G, Cavo M, Santoro A, Pasquali S. Light chains removal by extracorporeal techniques in acute kidney injury due to multiple myeloma: a position statement of the onconephrology work group of the Italian society of nephrology. J Nephrol. 2016;29(6):735–746. doi: 10.1007/s40620-016-0347-9. [DOI] [PubMed] [Google Scholar]
- 11.Wong AH, Shin EM, Tergaonkar V, Chng WJ. Targeting NF-κB signaling for multiple myeloma. Cancers. 2020;12(8):2203. doi: 10.3390/cancers12082203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chanan-Khan AA, Kaufman JL, Mehta J, Richardson PG, Miller KC, Lonial S, Munshi NC, Schlossman R, Tariman J, Singhal S. Activity and safety of bortezomib in multiple myeloma patients with advanced renal failure: a multicenter retrospective study. Blood. 2007;109(6):2604–2606. doi: 10.1182/blood-2006-09-046409. [DOI] [PubMed] [Google Scholar]
- 13.Piro E, Molica S. A systematic review on the use of bortezomib in multiple myeloma patients with renal impairment: what is the published evidence? Acta Haematol. 2011;126(3):163–168. doi: 10.1159/000328417. [DOI] [PubMed] [Google Scholar]
- 14.Miyakoshi S, Kami M, Yuji K, Matsumura T, Takatoku M, Sasaki M, Narimatsu H, Fujii T, Kawabata M, Taniguchi S, Ozawa K. Severe pulmonary complications in Japanese patients after bortezomib treatment for refractory multiple myeloma. Blood. 2006;107(9):3492–3494. doi: 10.1182/blood-2005-11-4541. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Chen S, Hu Y, Cai J. Bortezomib-induced severe pulmonary complications in multiple myeloma: a case report and literature review. Oncol Lett. 2016;11(3):2255–2260. doi: 10.3892/ol.2016.4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toly-Ndour C, Peltier J, Piedagnel R, Coppo P, Sachon E, Ronco P, Rondeau E, Callard P, Aucouturier P. Acute renal failure with lambda light chain-derived crystals in a patient with IgD myeloma. Nephrol Dial Transplant. 2011;26(9):3057–3059. doi: 10.1093/ndt/gfr377. [DOI] [PubMed] [Google Scholar]
- 17.Wu CK, Yang AH, Lai HC, Lin BS. Combined proximal tubulopathy, crystal-storing histiocytosis, and cast nephropathy in a patient with light chain multiple myeloma. BMC Nephrol. 2017;18(1):1–6. doi: 10.1186/s12882-017-0584-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ecotière L, Thierry A, Debiais-Delpech C, Chevret S, Javaugue V, Desport E, Belmouaz S, Quellard N, Kaaki S, Goujon JM, Fermand JP. Prognostic value of kidney biopsy in myeloma cast nephropathy: a retrospective study of 70 patients. Nephrol Dial Transplant. 2016;31(1):64–72. doi: 10.1093/ndt/gfv283. [DOI] [PubMed] [Google Scholar]
- 19.Bridoux F, Carron PL, Pegourie B, Alamartine E, Augeul-Meunier K, Karras A, Joly B, Peraldi MN, Arnulf B, Vigneau C, Lamy T. Effect of high-cutoff hemodialysis vs conventional hemodialysis on hemodialysis independence among patients with myeloma cast nephropathy: a randomized clinical trial. JAMA. 2017;318(21):2099–2110. doi: 10.1001/jama.2017.17924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hutchison CA, Cook M, Heyne N, Weisel K, Billingham L, Bradwell A, Cockwell P. European trial of free light chain removal by extended haemodialysis in cast nephropathy (EuLITE): a randomised control trial. Trials. 2008;9(1):1–7. doi: 10.1186/1745-6215-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sathick IJ, Drosou ME, Leung N. Myeloma light chain cast nephropathy, a review. J Nephrol. 2019;32(2):189–198. doi: 10.1007/s40620-018-0492-4. [DOI] [PubMed] [Google Scholar]
- 22.Mateos MV, Dimopoulos MA, Cavo M, Suzuki K, Jakubowiak A, Knop S, Doyen C, Lucio P, Nagy Z, Kaplan P, Pour L. Daratumumab plus bortezomib, melphalan, and prednisone for untreated myeloma. N Engl J Med. 2018;378(6):518–528. doi: 10.1056/NEJMoa1714678. [DOI] [PubMed] [Google Scholar]
- 23.Kastritis E, Palladini G, Minnema MC, Wechalekar AD, Jaccard A, Lee HC, Sanchorawala V, Gibbs S, Mollee P, Venner CP, Lu J. Daratumumab-based treatment for immunoglobulin light-chain amyloidosis. N Engl J Med. 2021;385(1):46–58. doi: 10.1056/NEJMoa2028631. [DOI] [PubMed] [Google Scholar]