Abstract
Introduction
Evidence supporting transmural remission (TR) as a long‐term treatment target in Crohn's disease (CD) is still unavailable. Less stringent but more reachable targets such as isolated endoscopic (IER) or radiologic remission (IRR) may also be acceptable options in the long‐term.
Methods
Multicenter retrospective study including 404 CD patients evaluated by magnetic resonance enterography and colonoscopy. Five‐year rates of hospitalization, surgery, use of steroids, and treatment escalation were compared between patients with TR, IER, IRR, and no remission (NR).
Results
20.8% of CD patients presented TR, 23.3% IER, 13.6% IRR and 42.3% NR. TR was associated with lower risk of hospitalization (odds‐ratio [OR] 0.244 [0.111–0.538], p < 0.001), surgery (OR 0.132 [0.030–0.585], p = 0.008), steroid use (OR 0.283 [0.159–0.505], p < 0.001), and treatment escalation (OR 0.088 [0.044–0.176], p < 0.001) compared to no NR. IRR resulted in lower risk of hospitalization (OR 0.333 [0.143–0.777], p = 0.011) and treatment escalation (OR 0.260 [0.125–0.540], p < 0.001), while IER reduced the risk of steroid use (OR 0.442 [0.262–0.745], p = 0.002) and treatment escalation (OR 0.490 [0.259–0.925], p = 0.028) compared to NR.
Conclusions
TR improved clinical outcomes over 5 years of follow‐up in CD patients. Distinct but significant benefits were seen with IER and IRR. This suggests that both endoscopic and radiologic remission should be part of the treatment targets of CD.
Keywords: Crohn's disease, endoscopy, inflammatory bowel disease, MRI enterography, transmural remission
Key summary.
What is already known?
Transmural remission (TR) is associated with improved clinical outcomes at 1‐year of follow‐up compared to isolated endoscopic remission (IER) and no remission (NR).
Few patients can reach TR with available therapies.
What is new here?
The clinical benefits of TR remain over 5‐year of follow‐up.
Significant clinical benefits can be obtained from IER remission in terms of steroid‐free remission and treatment escalation, and from isolated radiologic remission in terms of hospitalization and treatment escalation.
This suggests that both endoscopic and radiologic evaluation should be performed when assessing disease activity and treatment response.
INTRODUCTION
Crohn's disease (CD) is a chronic and potentially debilitating multisystemic disorder. Over time, a substantial percentage of patients will progress from inflammatory to stricturing and penetrating phenotypes. 1 , 2 Disease progression is associated with reduced treatment response and increased rates of steroid dependence, hospitalization, and surgery. 1 , 2 , 3 Current evidence‐based recommendations support a treat‐to‐target strategy aiming for both clinical and endoscopic remission. 3 This is based on substantial evidence associating endoscopic remission with long‐term symptomatic remission, and reduced rates of hospitalization and surgery. 4 , 5 However, disease progression may still occur despite endoscopic remission. 6 , 7 Unlike other conditions affecting the bowel, inflammation in CD is not limited to the mucosa. In fact, transmural inflammation plays a key role in the development of complications such as strictures, fistulas, and abscesses. 8 , 9 Several studies support the importance of controlling transmural inflammation, showing improved clinical outcomes in patients reaching both endoscopic and radiologic remission. 10 , 11 , 12 However, most studies followed patients for less than 1 year. This may be insufficient, as disease complications may take longer to develop. It is unknown if the benefits of transmural remission (TR) will persist over longer periods of time. On the other hand, only a low percentage of patients will reach TR with the current available therapies. This raises the question if “less stringent” targets such as isolated endoscopic remission (IER) and radiologic remission could be acceptable alternatives in the long‐term.
In the present study, we aim at answering these questions by comparing the long‐term outcomes of transmural, endoscopic, radiologic, and NR in a multicenter cohort of patients with CD.
MATERIAL AND METHODS
Study design
This was a retrospective multicenter study conducted in nine gastroenterology departments in Portugal. Patients were included if they met the following criteria: a confirmed diagnosis of ileal or ileocolonic CD according to the European Crohn and colitis Organization recommendations, 13 a magnetic resonance enterography (MRE) and ileocolonoscopy performed within a 6‐month interval, and at least 5 years of follow‐up from the latest of the two examinations. Patients were excluded if they had isolated colonic disease, inaccessible small bowel by colonoscopy, inadequate MRE examination according to the radiologist, or a CD‐related bowel surgery occurring between examinations. The most proximal extent of small bowel inflammation was defined by MRE. Patients unable to reach those segments by colonoscopy (or by a complementary method such as capsule endoscopy or balloon enteroscopy) and without other signs of disease activity, were excluded.
Clinical information was retrieved from local databases and included patients' demographics, disease characteristics, treatments, disease course, and endoscopic and radiologic data. The study was approved by each local Ethics committee.
Magnetic resonance enterography
MREs were performed and locally read by experienced radiologists amongst participating centers using a similar protocol. Following a fasting period of 4–6 h before the examination, patients drank 1.5–2 L of a solution of water and mannitol (2.5%) over a period of 40–45 min to allow optimal luminal distension. Intravenous hyoscine 10 mg was then given to reduce peristalsis. Sequences obtained included conventional axial and coronal T1‐ and T2‐weighted images, balanced fast field echo with short repetition time (0.3 mm/s), T2 fast spin echo with and without fat suppression and, following intravenous gadolinium contrast injection, fat‐suppressed 3D T1‐weighted breath‐hold gradient eco images with acquisitions at 30, 60, and 90 s in coronal orientation and at 110 s in axial orientation. Examinations were classified as active or inactive based on the presence of abnormal bowel wall thickening (defined as a bowel thickness >3 mm) plus either increased T2 mural intensity or increased contrast enhancement on T1 gadolinium sequences. Other signs of disease activity (creeping fat, peri‐enteric vascularization, strictures, abscess, and fistulae) were recorded but not used for this definition.
Colonoscopy
Colonoscopies were performed in participating centers by experienced endoscopists or by supervised residents. In non‐operated patients, examinations were classified as active or inactive based on the presence of ulcers (>5 mm) in any gastrointestinal (GI) segment, following the recommendations of the STRIDE‐II consensus. 3 In patients with previous bowel surgery, active disease was defined by a modified Rutgeerts score ≥i2b. The Rutgeerts score is an endoscopic scoring system developed to predict progression of disease in operated CD patients by grading the severity of endoscopic lesions in the neo‐terminal ileum and ileocolonic anastomosis. 14 The modified Rutgeerts score subdivides i2 lesions into i2a (lesions confined to the ileocolonic anastomosis, including anastomotic stenosis) and i2b (lesions in the neoterminal ileum with normal intervening mucosa). 15 Patients with anastomotic stricture, forbidding assess to the neoterminal ileum, were excluded.
Definitions of remission
Transmural remission was defined as an inactive colonoscopy and MRE; radiologic remission as an inactive MRE with active colonoscopy; endoscopic remission as an inactive colonoscopy with active MRE; and NR as an active endoscopy and MRE.
Study outcomes
We evaluated the cumulative rates and time until hospitalization, surgery, steroid use, and treatment escalation over the following 5 years of follow‐up. The baseline was defined as the date of the latest examination (MRE or colonoscopy). Hospitalization was defined as any admission related to CD activity, excluding infections and other unrelated adverse events. Surgery included any bowel resection related to CD (excluding perianal‐related surgeries). Steroid use was defined as the use of an equivalent dose of prednisolone ≥20 mg/day or oral budesonide ≥6 mg/day for more than 1 week. Treatment escalation included the need to start an immunomodulator (IM) (azathioprine, mercaptopurine, or methotrexate) or any CD‐approved biologic (infliximab, adalimumab, vedolizumab or ustekinumab). Considering that dose/interval escalation could be triggered by reasons other than clinical activity (e.g., control of subclinical inflammation and/or proactive therapeutic drug monitoring) we did not include these in the definition of treatment escalation. In addition, switching between immunomodulators or between biologics because of drug intolerance was not considered as treatment escalation for the outcome analysis.
Statistical analysis
Continuous variables were expressed as median (range) and compared using the Mann‐Whitney U test. Categorical variables were described using frequencies and percentages and compared in pairs using the chi‐square test. The presence or absence of overall inflammation by MRE and colonoscopy was correlated using Spearman's rho. Logistic regression and Cox proportional‐hazards regression were used respectively to investigate factors associated with the occurrence, and the time until the occurrence, of the negative outcome over the study period. Variables with a p value below 0.1 in univariate analysis were used in the multivariate analysis. Tested variables included gender, age at evaluation, disease duration, location and phenotype, presence of upper GI disease, perianal disease, previous surgery, time between examinations, IM and biologic use at baseline, and type of remission. Results were expressed as odds‐ratio (OR) or hazards‐ratio (HR) with 95% confidence interval. Results of the Cox proportional‐hazards regression were plotted using Kaplan‐Meier survival curves.
The significance level was chosen at 0.05. Statistical analysis was performed using IBM Statistical Package for the Social Sciences v26.0.
RESULTS
General characteristics and demographics
We included 404 patients in the analysis, 213 (52.7%) females. At baseline, the median age was 34.0 years (24.0–45.0) and the median disease duration was 4.0 years (1.0–11.0). Two hundred and forty‐six patients (60.9%) had isolated ileal disease, 158 patients (39.1%) had non‐stricturing/non‐penetrating phenotype, and 121 patients (30.0%) had at least one previous bowel surgery. One hundred ninety‐seven patients (48.8%) were under immunomodulators (azathioprine: 187, methotrexate: 9, mercaptopurine: 1) and 86 patients (21.3%) were under biologics (infliximab: 49, adalimumab: 37). A list of patients' demographics and disease characteristics is presented in Table 1.
TABLE 1.
Patients' characteristics at baseline
| Total | TR | IER | IRR | NR | |
|---|---|---|---|---|---|
| n = 404 | n = 84 (20.8) | n = 94 (23.3) | n = 55 (13.6) | n = 171 (42.3) | |
| Age (years) | 34.0 (24.0–45.0) | 35.0 (25.5–45.0) | 33.5 (24.0–43.0) | 33.0 (23.0–53.0) | 33.0 (23.0–44.0) |
| Disease duration (years) | 4.0 (1.0–11.0) | 6 (2.0–14.0) | 4.0 (1.0–9.0) | 3.0 (1.0–10.0) | 4.0 (1.0–10.0) |
| Time between exams (months) | 2.0 (0.0–4.0) | 2 (1.0–4.0) | 3 (0–4.0) | 1 (1.0–3.0) | 1 (0–3.0) |
| Female gender (%) | 213 (52.7) | 52 (61.9) a | 52 (55.3) | 30 (54.5) | 79 (46.2) a |
| Disease location | |||||
| Ileal (L1) | 246 (60.9) | 50 (59.5) | 53 (56.4) | 32 (58.2) | 111 (64.9) |
| Ileo‐colonic (L3) | 158 (39.1) | 34 (40.5) | 41 (43.6) | 23 (41.8) | 60 (35.1) |
| Disease behavior | |||||
| Inflammatory (B1) | 158 (39.1) | 41 (48.8) b | 39 (41.5) | 25 (45.5) c | 53 (31.0) b , c |
| Stricturing (B2) | 134 (33.2) | 27 (32.1) b | 31 (33.0) | 16 (29.1) c | 60 (35.1) b , c |
| Penetrating (B3) | 112 (27.7) | 16 (19.0) b | 24 (25.5) | 14 (25.5) c | 58 (33.9) b , c |
| Perianal disease (%) | 81 (20.0) | 19 (22.6) | 17 (18.1) | 9 (16.4) | 36 (21.1) |
| Upper GI disease (%) | 74 (18.3) | 15 (17.9) | 19 (20.2) | 9 (16.4) | 31 (18.1) |
| Previous surgery (%) | 121 (30.0) | 34 (40.5) d , e | 25 (26.6) d , f | 24 (43.6) e , f | 38 (22.2) e |
| IM at baseline (%) | 197 (48.8) | 45 (53.6) | 47 (50.0) | 29 (52.7) | 76 (44.4) |
| Biologic at baseline (%) | 86 (21.3) | 26 (31.0) b | 19 (20.2) | 14 (25.5) | 27 (15.8) b |
| Biologic exposure (%) | |||||
| None | 302 (74.8) | 56 (66.7) b | 68 (72.3) | 40 (72.7) | 138 (80.7) b |
| 1 biologic | 80 (19.8) | 24 (28.6) b | 19 (20.2) | 12 (21.8) | 25 (14.6) b |
| 2 biologics | 22 (5.4) | 4 (4.8) b | 7 (7.4) | 3 (5.5) | 8 (4.7) b |
Note: Continuous variables are expressed as median (interquartile range). Comparisons with a p value < 0.1 are presented.
Abbreviations: GI, gastrointestinal; IER, isolated endoscopic remission; IM, immunomodulator; IRR, isolated radiologic remission; NR, no remission; TR, transmural remission. Significant comparisons are highlighted in bold.
p = 0.02.
p = 0.008.
p = 0.072.
p = 0.057.
p = 0.003.
p = 0.046.
Radiologic and endoscopic inflammation
The median time between MRE and colonoscopy was 2.0 (0–4.0) months. At baseline, MRE activity was present in 265 patients (65.6%) and endoscopic activity in 226 patients (55.9%). There was a weak but significant correlation between both diagnostic methods (rho = 0.239, p < 0.001).
Transmural, radiologic, endoscopic, and no remission
Eighty‐four patients (20.8%) were classified as having TR, 94 patients (23.3%) IER remission, 55 patients (13.6%) isolated radiologic remission, and 171 patients (42.3%) NR. Most baseline characteristics were similar between the different types of remission. However, significant differences were found amongst groups in terms of phenotype and previous bowel surgery (Table 1). Interestingly, while the proportion of patients using immunomodulators at baseline did not differ between groups, there was a higher use of biologics in patients with TR compared to NR (31.0% vs. 15.8%, p = 0.008), with a numerical difference for isolated radiologic remission (31.0% vs. 25.5%, p = 0.112), and IER remission (31.0% vs. 20.2%, p = 0.121).
Long‐term outcomes according to the type of remission at baseline
The 5‐year outcomes between the different types of remission are presented in Figure 1. Kaplan‐Meier curves for each outcome are shown in Figure 2.
FIGURE 1.
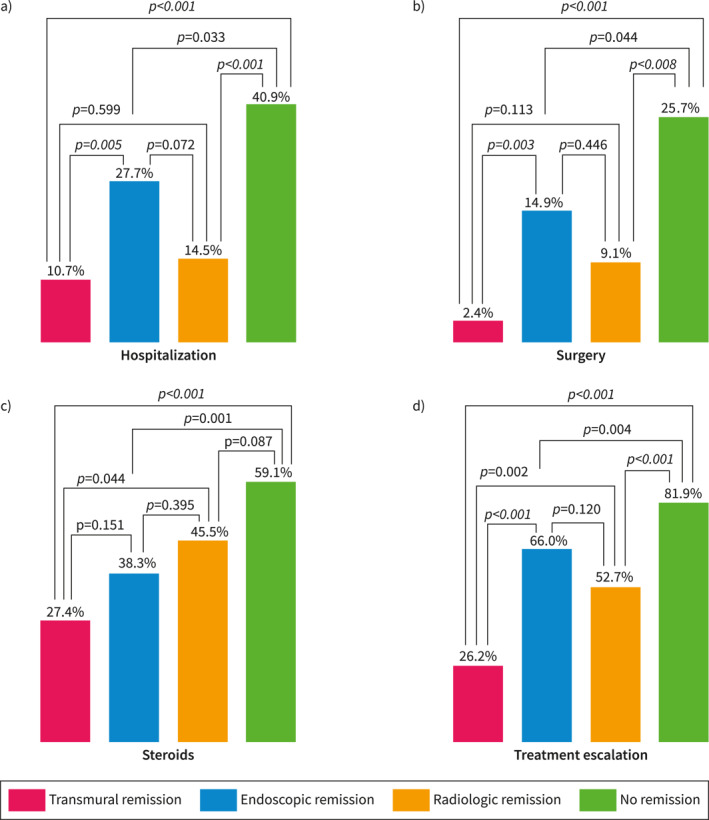
Five‐year clinical outcomes according to the type of remission at baseline.
FIGURE 2.
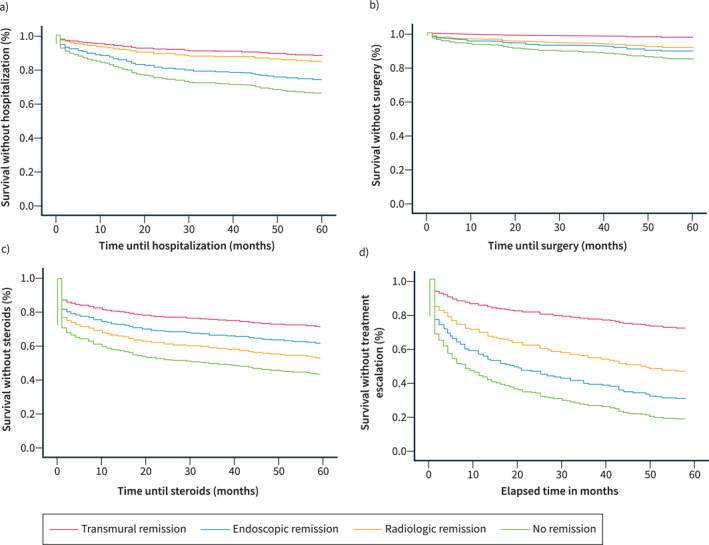
Long‐term clinical benefit according to the type of remission at baseline in respect to (a) hospitalization, (b) surgery, (c) steroids, and (d) treatment escalation.
Multivariate logistic regression analysis and cox‐proportional regression analysis for each outcome are available as Supporting Information (supplementary Tables 1‐8).
Hospitalization
Transmural remission was associated with lower rates of hospitalization compared to NR (10.7% vs. 40.9%, p < 0.001) and IER remission (10.7% vs. 27.7%, p = 0.005). The rates of hospitalization were similar between TR and isolated radiologic remission (10.7% vs. 14.5%, p = 0.599). In multivariate logistic regression analysis, TR (OR 0.244 [0.111–0.538], p < 0.001), and isolated radiologic remission (OR 0.333 [0.143–0.777], p = 0.011) were associated with lower risk of hospitalization compared to NR. Cox‐proportional regression analysis showed longer time until hospitalization in patients with TR (HR 0.303 [0.149–0.617], p = 0.001), and isolated radiologic remission (HR 0.402 [0.190–0.850], p = 0.017) compared to NR.
Surgery
Transmural remission demonstrated lower rates of surgery compared to NR (2.4% vs. 25.7%, p < 0.001), and IER remission (2.4% vs. 14.9%, p = 0.003), but not to isolated radiologic remission (2.4% vs. 9.1%, p = 0.113). In logistic regression, only TR was associated with a lower risk of surgery compared to NR (OR 0.132 [0.030–0.585], p = 0.008). In Cox‐proportional regression, only TR showed longer time until surgery compared to NR (HR 0.160 [0.038–0.673], p = 0.012).
Steroids
Lower steroid requirements were seen in patients with TR compared to NR (27.4% vs. 59.1%, p < 0.001), and isolated radiologic remission (27.4% vs. 45.5%, p = 0.044). The difference was not significant against IER remission (27.4% vs. 38.3%, p = 0.151). In logistic regression analysis, lower risk of steroid use was seen in patients with TR (OR 0.283 [0.159–0.505], p < 0.001), and IER remission (OR 0.442 [0.262–0.745], p = 0.002) compared to NR. Similarly, Cox‐proportional regression analysis showed longer time until needing steroids only with TR (HR 0.415 [0.263–0.655], p < 0.001), and IER remission (HR 0.590 [0.403–0.865], p = 0.007) compared to NR.
Treatment escalation
Patients with TR required less treatment escalation compared to NR (26.2% vs. 81.9%, p < 0.001), IER remission (26.2% vs. 66.0%, p < 0.001), and isolated radiologic remission (26.2% vs. 52.7%, p = 0.002). Logistic regression showed lower risk of treatment escalation in patients with TR (OR 0.088 [0.044–0.176], p < 0.001), IER remission (OR 0.490 [0.259–0.925], p = 0.028), and isolated radiologic remission (OR 0.260 [0.125–0.540], p < 0.001) compared to NR. Finally, Cox‐proportional regression showed longer time until treatment escalation in patients with TR (HR 0.204 [0.128–0.327], p < 0.001), IER remission (HR 0.709 [0.521–0.965], p = 0.029), and isolated radiologic remission (HR 0.469 [0.309–0.710], p < 0.001) compared to NR.
A separate analysis for immunomodulators and biologics is presented as Supporting Information (supplementary Tables 17‐20, supplementary Figure 1‐2).
Endoscopic and radiologic remission
Obtaining either endoscopic or radiologic remission resulted in lower rates of hospitalization (18.5% vs. 40.9%, p < 0.001), surgery (9.0% vs. 25.7%, p < 0.001), steroid use (36.1% vs. 59.1%, p < 0.001), and treatment escalation (48.5% vs. 81.9%, p < 0.001) compared to NR.
In multivariate logistic regression analysis, radiologic inflammation was associated with a higher risk of hospitalization (OR 2.881 [1.568–5.294], p = 0.001), and surgery (OR 3.000 [1.240–7.257], p = 0.015).
On the contrary, endoscopic inflammation was not a significant predictor for hospitalization (OR 1.455 [0.877–2.415], p = 0.146) or surgery (OR 1.624 [0.829–3.183], p = 0.158). Endoscopic and radiologic inflammation were both independent predictors for the need of steroids (OR 2.236 [1.464–3.417], p < 0.001 and OR 1.587 [1.013–2.486], p = 0.044), and treatment escalation (OR 2.354 [1.443–3.876], p = 0.001 and OR 4.650 [2.773–7.797], p < 0.001).
Similar results were seen for predicting the time until an adverse outcome (Supporting Information, supplementary Tables 9‐16).
DISCUSSION
CD is a progressive inflammatory condition that follows a chronic relapsing course, eventually resulting in complications requiring surgery such as strictures, fistulas, and abscesses. 1 Treatment aiming only at clinical remission has not been shown to decrease the rate of significant outcomes such as hospitalization and surgery. 3 On the other hand, there is sufficient clinical evidence associating endoscopic remission with higher rates of steroid‐free clinical remission, and lower risk of relapse, hospitalization, and surgery. 16 Nevertheless, surgery may still be required despite endoscopic remission. 1 , 6 A couple of years ago, we published the first study showing that TR, a combined endoscopic and radiologic target, resulted in better clinical outcomes than endoscopic remission alone. 10 Since then, other publications have confirmed these results. 11 , 12 However, previous studies were limited by small sample size and insufficient follow‐up. In a population‐based study, the cumulative risk of a first intestinal resection doubled (24% vs. 49%) over the first 10 years of diagnosis. 17 In another study, the risk of surgery increased from 16.7% within the first year of diagnosis to 31.7% 5 years after diagnosis. 18 Therefore, longer follow‐up may be required to adequately assess the benefits of a particular form of remission. In the current study, we present for the first time the 5‐year follow‐up data of a large multicenter cohort of CD patients with different types of remission. Our results confirm that even over a longer follow‐up, TR results in lower rates of hospitalization, surgery, use of steroids, and treatment escalation compared to other forms of remission. Similarly, the time until any of the former events was longer in patients with TR.
Unfortunately, in line with our previous results, TR was achieved in only a small subset of patients. Taking this limitation into account, we evaluated the potential benefits of less stringent targets such as IER and radiologic remission. Our results suggest that both forms of remission are associated with different, although meaningful clinical benefits. Compared to patients without remission, isolated radiologic remission resulted in lower rates of hospitalization and treatment escalation while IER remission associated with lower rates of steroid use and treatment escalation. Nevertheless, neither form of remission decreased the rates of surgery compared to NR. We hypothesize that endoscopic and radiologic inflammation may influence clinical outcomes in different ways. In fact, radiologic activity may be more important than endoscopic activity in predicting both hospitalization and surgery. This is in line with the published evidence linking radiologic inflammation with the development of parietal and extramural complications which in turn may result in hospitalization and/or surgery (e.g., strictures, abscess). 8 , 9 On the other hand, endoscopic activity may be more important in respect to the need for steroid therapy. As clinical symptoms in CD such as diarrhea result from disruption of the intestinal epithelium barrier secondary to mucosal inflammation. Endoscopic remission, by restoring the epithelial function, may lead to less symptomatic flares requiring steroids. 19
Our findings challenge the perception that a single method such as colonoscopy or MRE can be used to adequately monitor treatment response and prognosis in CD. It remains to be proven if endoscopic or radiologic improvement might also be acceptable as long‐term treatment targets. In a recent metanalysis, partial and complete endoscopic remission resulted in similar rates of hospitalization and surgery. 16 In a retrospective study, radiologic response was associated with a decreased risk of surgical or endoscopic interventions. 20 In another study radiologic response at week 12 predicted steroid‐free clinical remission up to week 52. 21
Unfortunately, using endoscopy and MRE to systematically assess for TR as part of a treat‐to‐target strategy may not be feasible in clinical practice. In fact, both methods are expensive, require experienced personnel, have potential complications, low patient acceptability, and may not be widely available. One potential solution is the replacement of both diagnostic modalities with accurate but less invasive and less expensive techniques such as bowel ultrasound and fecal calprotectin. 22 , 23 , 24 , 25 A strategy using both non‐invasive modalities may offer an acceptable, cost‐effective, and patient‐friendly approach to disease monitoring in CD.
Although our study presents many strengths including sample size and follow‐up, we acknowledge several limitations. First, we chose a retrospective and multicenter design in order to recruit a large sample of patients with the desired follow‐up. Aside from potential bias associated with retrieving data from clinical registries, we were unable to use endoscopic and radiologic scores to define transmural, radiologic, and endoscopic remission. Nevertheless, in clinical practice, these scores are seldom used. In fact, expert consensus recommend the use of simpler definitions such as the absence of ulceration to definite endoscopic remission. 3 By simplifying the definition of endoscopic and radiologic activity, we avoided potential bias associated with the interpretation of data retrieved from endoscopic and radiologic reports. For the same reasons, we opted to include patients with up to 6 months between examinations. While this could hypothetically influence our results it was not significant in the multivariate analysis. Also, considering that patients were recruited from different centers we cannot exclude an underlying effect of individual treatment decisions.
As we excluded patients with isolated colonic disease, we cannot extrapolate our findings to this group of patients. This was necessary as the MRI protocols of participating centers did not include colonography. Up until recently the evidence for colonography in IBD was scarce and it was not recommended by international guidelines. Of note, MRI colonography requires filling the colon with large volumes of water through rectal enemas which may not only increase patient discomfort but also results in increased cost and duration of the examination. Another potential limitation was the exclusion of dose and interval escalation from the definition of treatment intensification. While we realize that some patients may benefit from this treatment strategy, this decision was taken to avoid overestimating escalation rates in all groups, as most of participating centers practiced some form of proactive treatment escalation. Finally, examinations were performed according to usual clinical practice. Therefore, it is expected that the reasons for performing colonoscopy and MRE were probably different across treatment groups (e.g., assessment of deep remission in patients with TR vs. evaluation of a disease flare in patients with NR). Again, this is understandable considering the retrospective design of our study, and hardly poses a limitation, as even in clinical practice it is expected that most patients without signs of endoscopic and radiologic activity will be in clinical remission, while patients with endoscopic or radiologic activity will most likely be symptomatic and at risk for requiring steroids, immunosuppressants, hospitalization and surgery.
In conclusion, our study suggests that both endoscopic and radiologic assessment are important to define the prognosis of CD. We provide further evidence that TR associates with improved long‐term outcomes even after 5‐year of follow‐up. In patients unable to obtain TR, obtaining at least endoscopic remission can decrease the need for steroids, and treatment escalation, while obtaining radiologic remission may decrease the need for hospitalization and treatment escalation.
AUTHOR CONTRIBUTIONS
Samuel Raimundo Fernandes acts as the guarantor of the article. The authors from each center were responsible for collecting the data. Samuel Raimundo Fernandes and Luís Correia were responsible for designing the study, analyzing the data, and writing the manuscript. Rui Tato Marinho, Helena Cortez‐Pinto, and Fernando Magro reviewed the final manuscript. All authors approved the final version of the article including the authorship list.
CONFLICT OF INTEREST
The authors have no conflicts of interest or funding related to the present study.
Supporting information
Supporting Information S1
ACKNOWLEDGMENTS
We acknowledge Dr. Maria Teresa Brito, Dr. Sónia Bernardo, Dr. Ana Rita Gonçalves, Dr. Ana Valente, Dr. Paula Moura Santos, Dr. Rita Vale Rodrigues, Dr. João Cortez‐Pinto, Dr. João Silva, Dr. José Venâncio, Dr. Mariana Roque, Dr. Paula Campos, and Dr. João Leitão for their contribution to the paper.
Fernandes SR, Serrazina J, Botto IA, Leal T, Guimarães A, Garcia JL, et al. Transmural remission improves clinical outcomes up to 5 years in Crohn's disease. United European Gastroenterol J. 2023;11(1):51–9. 10.1002/ueg2.12356
[Correction added on 12 January 2023, after first online publication: Surname of the author ‘Helena Cortez‐Pinto’ has been hyphenated; ORCID has been added for the aforementioned author.]
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Cosnes J, Cattan S, Blain A, Beaugerie L, Carbonnel F, Parc R, et al. Long‐term evolution of disease behavior of Crohn's disease. Inflamm Bowel Dis. 2002;8(4):244–50. 10.1097/00054725-200207000-00002 [DOI] [PubMed] [Google Scholar]
- 2. Magro F, Rodrigues‐Pinto E, Coelho R, Andrade P, Santos‐Antunes J, Lopes S, et al. Is it possible to change phenotype progression in Crohn's disease in the era of immunomodulators? Predictive factors of phenotype progression. Am J Gastroenterol. 2014;109(7):1026–36. 10.1038/ajg.2014.97 [DOI] [PubMed] [Google Scholar]
- 3. Turner D, Ricciuto A, Lewis A, D'Amico F, Dhaliwal J, Griffiths AM, et al. STRIDE‐II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the international organization for the study of IBD (IOIBD): determining therapeutic goals for treat‐to‐target strategies in IBD. Gastroenterology. 2021;160(5):1570–83. 10.1053/j.gastro.2020.12.031 [DOI] [PubMed] [Google Scholar]
- 4. Frøslie KF, Jahnsen J, Moum BA, Vatn MH, IBSEN Group . Mucosal healing in inflammatory bowel disease: results from a Norwegian population‐based cohort. Gastroenterology. 2007;133(2):412c22. 10.1053/j.gastro.2007.05.051 [DOI] [PubMed] [Google Scholar]
- 5. Shah SC, Colombel JF, Sands BE, Narula N. Systematic review with meta‐analysis: mucosal healing is associated with improved long‐term outcomes in Crohn's disease. Aliment Pharmacol Ther. 2016;43(3):317c33. 10.1111/apt.13475 [DOI] [PubMed] [Google Scholar]
- 6. Laharie D, D'Haens G, Nachury M, Lambrecht G, Bossuyt P, Bouhnik Y, et al. Steroid‐free deep remission at one year does not prevent Crohn's disease progression: long‐term data from the TAILORIX trial. Clin Gastroenterol Hepatol. 2022;20(9):2074–82. 10.1016/j.cgh.2021.11.030 [DOI] [PubMed] [Google Scholar]
- 7. Moum B, Hovde Ø, Høivik ML. What have we learnt about the role of the environment and natural course of IBD in the new millennium? 20‐year follow‐up of the IBSEN cohort. Dig Dis. 2014;32(suppl 1):2–9. 10.1159/000367818 [DOI] [PubMed] [Google Scholar]
- 8. Crespi M, Dulbecco P, De Ceglie A, Conio M. Strictures in Crohn's disease: from pathophysiology to treatment. Dig Dis Sci. 2020;65(7):1904–16. 10.1007/s10620-020-06227-0 [DOI] [PubMed] [Google Scholar]
- 9. Scharl M, Rogler G. Pathophysiology of fistula formation in Crohn's disease. World J Gastrointest Pathophysiol. 2014;5(3):205–12. 10.4291/wjgp.v5.i3.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fernandes SR, Rodrigues RV, Bernardo S, Cortez‐Pinto J, Rosa I, da Silva JP, et al. Transmural healing is associated with improved long‐term outcomes of patients with Crohn's disease. Inflamm Bowel Dis. 2017;23(8):1403–9. 10.1097/mib.0000000000001143 [DOI] [PubMed] [Google Scholar]
- 11. Castiglione F, Imperatore N, Testa A, De Palma GD, Nardone OM, Pellegrini L, et al. One‐year clinical outcomes with biologics in Crohn's disease: transmural healing compared with mucosal or no healing. Aliment Pharmacol Ther. 2019;49(8):1026–39. 10.1111/apt.15190 [DOI] [PubMed] [Google Scholar]
- 12. Lafeuille P, Hordonneau C, Vignette J, Blayac L, Dapoigny M, Reymond M, et al. Transmural healing and MRI healing are associated with lower risk of bowel damage progression than endoscopic mucosal healing in Crohn's disease. Aliment Pharmacol Ther. 2021;53(5):577–86. [DOI] [PubMed] [Google Scholar]
- 13. Maaser C, Sturm A, Vavricka SR, Kucharzik T, Fiorino G, Annese V, et al. ECCO‐ESGAR guideline for diagnostic assessment in IBD part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019;13(2)144–64. [DOI] [PubMed] [Google Scholar]
- 14. Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn's disease. Gastroenterology. 1990;99(4):956–63. 10.1016/0016-5085(90)90613-6 [DOI] [PubMed] [Google Scholar]
- 15. Gecse K, Lowenberg M, Bossuyt P, Rutgeerts PJ, Vermeire S, Stitt L, et al. Sa1198 agreement among experts in the endoscopic evaluation of postoperative recurrence in Crohn's disease using the Rutgeerts score. Gastroenterology. 2014;146(5):S‐227–. 10.1016/s0016-5085(14)60802-7 [DOI] [Google Scholar]
- 16. Reinink AR, Lee TC, Higgins PD. Endoscopic mucosal healing predicts favorable clinical outcomes in inflammatory bowel disease: a meta‐analysis. Inflamm Bowel Dis. 2016;22(8):1859–69. 10.1097/mib.0000000000000816 [DOI] [PubMed] [Google Scholar]
- 17. Dhillon SL, Loftus EV Jr, Tremaine WJ, Jewell DA, Harmsen WS, Zinsmeister AR, et al. The natural history of surgery for Crohns disease in a population‐based cohort from Olmsted County, Minnesota (Abstract 825). Am J Gastroenterol. 2005;100(suppl l):S305. 10.14309/00000434-200509001-00825 [DOI] [Google Scholar]
- 18. Toh JWT, Wang N, Young CJ, Rickard MJ, Keshava A, Stewart P, et al. Major abdominal and perianal surgery in Crohn's disease: long‐term follow‐up of Australian patients with Crohn's disease. Dis Colon Rectum. 2018;61(1):67–76. 10.1097/dcr.0000000000000975 [DOI] [PubMed] [Google Scholar]
- 19. Anbazhagan AN, Priyamvada S, Alrefai WA, Dudeja PK. Pathophysiology of IBD associated diarrhea. Tissue Barriers. 2018;6(2):e1463897. 10.1080/21688370.2018.1463897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hallé E, Azahaf M, Duveau N, Lambin T, Nachury M, Branche J, et al. Radiological response is associated with better outcomes and should be considered a therapeutic target in Crohn's disease. Dig Dis Sci. 2020;65(9):2664–74. 10.1007/s10620-019-05979-8 [DOI] [PubMed] [Google Scholar]
- 21. Messadeg L, Hordonneau C, Bouguen G, Goutorbe F, Reimund JM, Goutte M, et al. Early transmural response assessed using magnetic resonance imaging could predict sustained clinical remission and prevent bowel damage in patients with Crohn's disease treated with anti‐tumour necrosis factor therapy. J Crohns Colitis. 2020;14(11):1524–34. 10.1093/ecco-jcc/jjaa098 [DOI] [PubMed] [Google Scholar]
- 22. Buisson A, Gonzalez F, Poullenot F, Nancey S, Sollellis E, Fumery M, et al. Comparative acceptability and perceived clinical utility of monitoring tools: a nationwide survey of patients with inflammatory bowel disease. Inflamm Bowel Dis. 2017;23(8):1425–33. 10.1097/mib.0000000000001140 [DOI] [PubMed] [Google Scholar]
- 23. Kopylov U, Yung DE, Engel T, Vijayan S, Har‐Noy O, Katz L, et al. Diagnostic yield of capsule endoscopy versus magnetic resonance enterography and small bowel contrast ultrasound in the evaluation of small bowel Crohn's disease: systematic review and meta‐analysis. Dig Liver Dis. 2017;49(8):854–63. 10.1016/j.dld.2017.04.013 [DOI] [PubMed] [Google Scholar]
- 24. Rokkas T, Portincasa P, Koutroubakis IE. Fecal calprotectin in assessing inflammatory bowel disease endoscopic activity: a diagnostic accuracy meta‐analysis. J Gastrointestin Liver Dis. 2018;27(3):299–306. 10.15403/jgld.2014.1121.273.pti [DOI] [PubMed] [Google Scholar]
- 25. Fernandes SR, Serrazina J, Rodrigues IC, Bernardo S, Rita Goncalves A, Valente A, et al. Proactive therapeutic drug monitoring is more effective than conventional management in inducing fecal calprotectin remission in inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2021;33(12):1539–46. 10.1097/meg.0000000000002111 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


