Abstract
Objective
Inflammatory bowel disease (IBD) is not only a chronic inflammatory disorder of the gastrointestinal tract but also accompanied by systemic inflammation. The onset of hypertension is closely related to systemic inflammation. However, the relationship between IBD and hypertension has not been investigated. We aimed to investigate the potential association between IBD and the incidence of hypertension.
Method
We retrieved IBD onset and the incidence of hypertension from a public database UK Biobank. The association between the onset of IBD and subsequent incidence of hypertension was analyzed using a multivariate Cox regression analysis, and propensity score matching was performed for sensitivity analysis.
Result
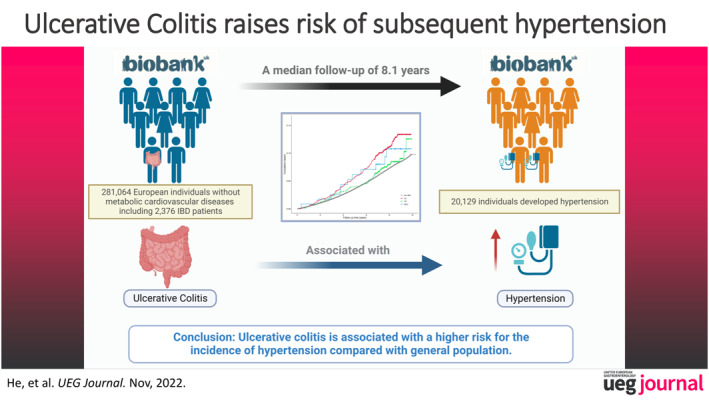
Of a total of 281,064 participants included in the study, 2376 (0.8%) were diagnosed with IBD at baseline, and 20,129 (7.2%) in the whole cohort developed hypertension with a median follow‐up duration of 8.1 years (interquartile range [IQR] 7.3–8.8 years). Patients with IBD had a higher cumulative risk of hypertension compared with general population (10.9% in ulcerative colitis [UC], 7.7% in Crohn's disease [CD], and 9.3% in IBD unclassified [IBD‐U] vs. 7.1% in non‐IBD, p < 0.001). Multivariate Cox regression analysis identified that UC, rather than CD or IBD‐U, was independently associated with subsequent occurrence of hypertension (HR 1.30, 95% CI: 1.11–1.52, p = 0.001). In propensity matching analysis, UC also showed its robustness as a risk factor for the prediction of hypertension (HR 1.56, 95% CI: 1.21–2.03, p = 0.001).
Conclusion
In IBD patients, UC rather than CD is associated with a higher risk for the incidence of hypertension compared with general population. Close monitoring of hypertension might be required in clinical practice.
Keywords: epidemiology, hypertension, inflammatory bowel disease, predictor, UK Biobank, ulcerative colitis
Key summary.
The established knowledge on this subject
Inflammatory bowel disease (IBD) is accompanied by various extraintestinal manifestations induced by systemic inflammatory stimulation.
Systemic inflammation is closely related to the onset of hypertension.
What are the significant and/or new findings of this study?
Data from a large cohort UK Biobank revealed that IBD patients had a higher risk of hypertension than normal population.
Ulcerative colitis was an independent risk predictor of subsequent hypertension.
Close monitoring and early management of hypertension may be required in clinical practice for patients with IBD.
INTRODUCTION
Inflammatory bowel disease (IBD) is a chronic intestinal disease that mainly includes Crohn's disease (CD), ulcerative colitis (UC), and IBD unclassified (IBD‐U). Although the pathogenesis of IBD remains unclear, it involves a complex interplay between genetic, environmental, epithelial, microbial, and immune factors, resulting in not only a local inflammation limited to intestine but also a systemic inflammation. 1 IBD patients are often accompanied by various extraintestinal manifestations such as erythema nodosum, arthritis, and thromboembolism, which may be due to systemic inflammatory stimulation.
Studies have pointed out that systemic inflammation is involved in atherosclerosis, and that systemic inflammation increases the risk of acute arterial events, which can be alleviated by anti‐inflammatory treatment. 2 , 3 , 4 , 5 , 6 Hypertension is an early and common manifestation of atherosclerosis and various cardiovascular events. Although the etiology of hypertension is not completely clear, many studies and theories have pointed out that it occurs much earlier than acute arterial events and often causes arterial stiffness under the continuous stimulation of systemic inflammation. 7 , 8 , 9 , 10
IBD has been considered a potential risk factor for acute arterial events in addition to traditional cardiovascular risk factors. 11 , 12 However, these studies have mainly focused on hard endpoints such as cardiovascular and cerebrovascular events, and few studies focused on the relationship between IBD and hypertension. Therefore, we analyzed the data from the UK Biobank, a large prospective, population‐based and multiple‐centered study focusing on genetic and nongenetic risk factors for diseases in general population. We aimed to explore the association between IBD and subsequent incidence of hypertension and to provide a reference for the necessity of routine screening and early intervention in clinical practice.
MATERIALS AND METHODS
Study population
The study was conducted under the guideline of STROBE Checklist (Supporting Information S2). In this prospective, observational cohort study, we used the data from the UK Biobank. This large cohort has collected health‐related information from more than 500,000 participants ranging from 37 to 69 years old during the time of recruitment between 2006 and 2010. Information regarding the study design, data collection, and protocol has been previously published online. 13 Briefly, each approved participant received a 90‐min session which includes questionnaires, physical examinations, blood sample collections, and interviews at the recruitment. The cohort then matched the data of all participants with the NHS Central Register database and obtained their subsequent medical records, including diagnosis and death registration.
All 502,505 participants with eligible data between 2006 and 2017 were initially included in this study. The exclusion criteria were listed as follows: (a) Diagnosis of hypertension at recruitment; (b) History of cardiovascular diseases, diabetes, or stroke at recruitment; (c) New diagnosis of IBD during follow up; (d) Lack of baseline or follow‐up data. The screening progress of participants is shown in Figure 1. Finally, a towtal of 281,064 eligible participants were included.
FIGURE 1.
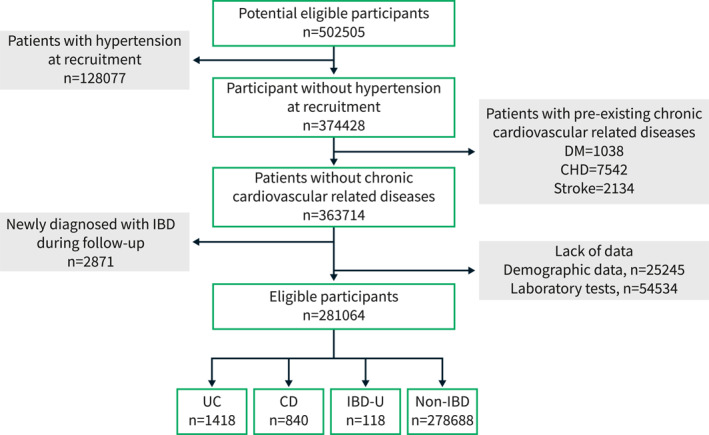
Screening process and criteria of eligible participants in the study.
Assessment of IBD
Participants were divided into four groups according to the baseline status of IBD (UC, CD, IBD‐U, and non‐IBD) based on self‐report at baseline interview or data extraction from medical records of the International Classification of Disease (ICD) and the time when they first presented. The ICD‐10 codes for UC and CD were K51 and K50, respectively. Those who did not clearly report their IBD subphenotype or had diagnostic records of both UC and CD at recruitment were classified as IBD‐U.
Follow‐up and the outcome event
The follow‐up started from the enrollment of the study till the occurrence of the outcome event or the end of the available date (31 March 2017). The primary outcome was the occurrence of hypertension determined by the primary care, hospital admission or death register during follow‐up. The ICD codes for hypertension included I10, I11, I12, I13, and I15 in the 10th edition.
Covariates
A variety of covariates recorded at baseline were also collected. Demographic characteristics included age, gender, race (white or other), education phase (ranked as 5 grades as followed: 0 for national vocational qualification or equivalent, 1 for the certificate of secondary education or equivalent, 2 for O levels or equivalent, 3 for A levels or equivalent, and 4 for college/university degree), smoking status (never, former or current), drinking status (never, former or current), and an indicator of poverty, the Townsend deprivation index (TDI). Anthropometric characteristics included average handgrip strength (defined as the average grip strength of two hands), body mass index (BMI), and waist to hip rate. Blood samples and laboratory tests were obtained in accordance of standardized protocols at recruitment, including triglycerides (TG), total cholesterol (TC), high‐density lipoprotein cholesterol (HDL‐C), low‐density lipoprotein cholesterol (LDL‐C), glucose, creatine, albumin, lymphocyte and C‐reactive protein (CRP). 14 Physical activity score was evaluated by a modified version of the International Physical Activity Questionnaire. Overall health rating was self‐reported and divided into four levels: poor, fair, good, and excellent. Anxiety status was determined by whether there was a medical consultation due to tension or anxiety at recruitment. Family history of heart diseases and medication histories were self‐reported. The medication histories of concern included anti‐hypertension drugs, lipid‐lowering drugs, aspirin, steroids, and immunosuppressants including methotrexate, thiopurine, tacrolimus, cyclosporin, and adalimumab.
Statistical analysis
Baseline characteristics of participants with different IBD states were summarized, in which continuous variables were used as means with standard deviations (SD) or medians with interquartile range (IQR), and categorical variables were expressed as frequencies with proportions. Multiple comparisons were adjusted with the Bonferroni correction. A Kaplan Meier survival analysis with log‐rank tests between groups was performed to compare the cumulative risk for hypertension. A multivariate Cox regression model was established to determine the associated factors for hypertension. Subgroup analysis and interactive tests were subsequently performed to explore the interactive relationship between IBD and other categorical variables.
Sensitivity analyses were performed to verify the robustness of the result. Firstly, to explore the association between the prevalence of IBD and hypertension at baseline, we performed a univariate logistics analysis between the subpopulations with and without hypertension at baseline. Secondly, we further adjusted variables including age >60 years (in replace of the continuous age variable), baseline CRP level, and a specified immunosuppressants use history in the multivariate model. In addition, a propensity score matching (PSM) (nearest neighbor ratio of 1:1 without replacement and a caliper width of 0.02) was performed. A two‐tailed p‐value <0.05 was considered significant. Analyses were performed by SPSS Statistics 26 (SPSS) and R v4.1.0.
RESULTS
IBD patients have a higher incidence of hypertension than normal population
A total of 281,064 eligible participants were included in the study and their characteristics are summarized in Table 1. Among them, 120,765 (43.0%) were female and 267,934 (95.3%) were Caucasian. The mean age of the participants was 55 ± 8 years old, and 36.2% of them were more than 60 years old. Most of the participants (93.0%) were current drinkers and nearly a half (57.1%) were non‐smokers.
TABLE 1.
Characteristics of IBD patients and non‐IBD participants in UK Biobank
| Characteristics | Total N 0 = 281,064 | IBD | Non‐IBD N 4 = 278,688 | p value | ||
|---|---|---|---|---|---|---|
| UC N 1 = 1418 | CD N 2 = 840 | IBD‐U N 3 = 118 | ||||
| Age, years | 55.2 (8.1) | 56.0 (8.0) | 54.7 (8.1) | 55.1 (8.1) | 55.2 (8.1) | 0.001 |
| ≥60 | 101,655 (36.2%) | 587 (41.4%) | 289 (34.4%) | 79 (66.9%) | 100,740 (36.1%) | <0.001 |
| Female | 120,765 (43.0%) | 629 (44.4%) | 350 (41.7%) | 43 (36.4%) | 119,743 (43.0%) | 0.290 |
| White | 267,934 (95.3%) | 1368 (96.5%) | 819 (97.5%) | 113 (95.8%) | 265,634 (95.3%) | 0.004 |
| Education phase a | 0.006 | |||||
| 0 | 38,793 (13.8%) | 220 (15.5%) | 141 (16.8%) | 29 (24.6%) | 38,403 (13.8%) | |
| 1 | 100,594 (35.8%) | 461 (32.5%) | 250 (29.8%) | 46 (39.0%) | 99,837 (35.8%) | |
| 2 | 33,440 (11.9%) | 156 (11.0%) | 99 (11.8%) | 12 (10.2%) | 33,173 (11.9%) | |
| 3 | 60,981 (21.7%) | 333 (23.5%) | 194 (23.1%) | 15 (12.7%) | 60,439 (21.7%) | |
| 4 | 47,256 (16.8%) | 248 (17.5%) | 156 (18.6%) | 16 (13.6%) | 46,836 (16.8%) | |
| TDI | −1.5 (3.0) | −1.6 (2.9) | −1.3 (3.0) | −1.1 (3.0) | −1.5 (3.0) | 0.051 |
| Drinking status | 0.001 | |||||
| Non‐drinker | 10,913 (3.9%) | 61 (4.3%) | 46 (5.5%) | 5 (4.2%) | 10,801 (3.9%) | |
| Former drinker | 8681 (3.1%) | 60 (4.2%) | 37 (4.4%) | 11 (9.3%) | 8573 (3.1%) | |
| Current drinker | 261,470 (93.0%) | 1297 (91.5%) | 757 (90.1%) | 102 (86.4%) | 259,314 (93.0%) | |
| Smoking status | <0.001 | |||||
| Non‐smoker | 160,440 (57.1%) | 729 (51.4%) | 390 (46.4%) | 63 (53.4%) | 159,258 (57.1%) | |
| Former smoker | 90,758 (32.3%) | 607 (42.8%) | 318 (37.9%) | 43 (36.4%) | 89,790 (32.2%) | |
| Current smoker | 29,866 (10.6%) | 82 (5.8%) | 132 (15.7%) | 12 (10.2%) | 29,640 (10.5%) | |
| Anthropometrics | ||||||
| BMI, kg/m2 | 26.6 (4.3) | 26.4 (4.2) | 26.0 (4.1) | 26.5 (4.1) | 26.6 (4.3) | <0.001 |
| Waist‐hip ratio | 0.9 (0.1) | 0.9 (0.1) | 0.9 (0.1) | 0.9 (0.1) | 0.9 (0.1) | 0.470 |
| Hand grip strength, kg | 30.9 (11.0) | 30.3 (10. 7) | 29. 8 (11.2) | 29.7 (11.9) | 31.0 (11.0) | 0.001 |
| Physical activity score | 44.9 (41.2) | 43.5 (40.3) | 41.4 (37.4) | 40.0 (36.8) | 45.0 (41.2) | 0.015 |
| Laboratory tests | ||||||
| Lymphocyte, 109/L | 1.9 (1.5–2.3) | 1.8 (1.4–2.2) | 1.6 (1.2–2.1) | 1.8 (1.5–2.1) | 1.9 (1.5–2.3) | <0.001 |
| Albumin, g/L | 45.2 (43.5–46.9) | 44.8 (43.0–46.6) | 44.0 (41.9–46.1) | 45.1 (43.3–46.8) | 45.2 (43.6–46.9) | <0.001 |
| TC, mmol/L | 5.8 (5.1–6.5) | 5.6 (5.0–6.3) | 5.28 (4.6–6.0) | 5.8 (5.3–6.6) | 5.8 (5.1–6.5) | <0.001 |
| Creatine, umol/L | 69.5 (60.9–79.6) | 69.6 (60.0–80.2) | 69.6 (61.0–81.0) | 67.7 (60.8–77.6) | 69.5 (60.9–79.6) | 0.285 |
| Glucose, mmol/L | 4.9 (4.6–5.2) | 4.9 (4.6–5.3) | 4.9 (4.6–5.3) | 4.8 (4.5–5.2) | 4.9 (4.6–5.2) | 0.239 |
| HDL‐C, mmol/L | 1.4 (1.2–1.7) | 1.5 (1.2–1.8) | 1.4 (1.2–1.7) | 1.5 (1.2–1.8) | 1.4 (1.2–1.7) | <0.001 |
| LDL‐C, mmol/L | 3.6 (3.1–4.2) | 3.5 (3.0–4.1) | 3.2 (2.7–3.8) | 3.7 (3.2–4.3) | 3.6 (3.1–4.2) | <0.001 |
| TG, mmol/L | 1.4 (1.0–2.0) | 1.4 (1.0–2.0) | 1.7 (1.1–2.4) | 1.5 (1.0–2.2) | 1.4 (1.0–2.0) | <0.001 |
| CRP, mg/L | 1.2 (0.6–2.5) | 1.7 (0.8–3.3) | 2.0 (0.9–3.9) | 1.5 (0.6–7.2) | 1.2 (0.6–2.4) | <0.001 |
| Anxiety status | 91,431 (32.5%) | 464 (32.7%) | 262 (31.2%) | 50 (42.4%) | 90,655 (32.5%) | 0.116 |
| Over‐all health rating | 2.0 (0.7) | 2.3 (0.7) | 2.5 (0.8) | 2.5 (0.8) | 2.0 (0.7) | <0.001 |
| Family history of HD | 105,390 (37.5%) | 548 (38.6%) | 314 (37.4%) | 44 (37.3%) | 104,484 (37.5%) | 0.847 |
| Medication history | ||||||
| Anti‐hypertension | 2695 (1.0%) | 21 (1.5%) | 4 (0.5%) | 0 (0.0%) | 2663 (1.0%) | 0.097 |
| Lipid‐lowering | 18,866 (6.7%) | 91 (6.4%) | 29 (3.5%) | 6 (5.1%) | 18,717 (6.7%) | 0.785 |
| Aspirin | 18,235 (6.5%) | 79 (5.6%) | 39 (4.6%) | 3 (2.5%) | 18,114 (6.5%) | 0.021 |
| Steroids | 3092 (1.1%) | 80 (5.6%) | 67 (8%) | 5 (4.2%) | 2940 (1.1%) | <0.001 |
| Immunosuppresants | 1836 (0.7%) | 152 (10.7%) | 171 (20.4%) | 8 (6.8%) | 1505 (0.5%) | <0.001 |
| Methotrexate | 1209 (0.4%) | 11 (0.8%) | 16 (1.9%) | 4 (3.4%) | 1178 (0.4%) | |
| Thiopurine | 461 (0.2%) | 134 (9.4%) | 149 (17.7%) | 4 (3.4%) | 174 (0.1%) | |
| Tacrolimus | 21 (0%) | 2 (0.1%) | 0 (0%) | 0 (0%) | 19 (0%) | |
| Cyclosporine | 43 (0%) | 3 (0.2%) | 0 (0%) | 0 (0%) | 40 (0%) | |
| Adalimumab | 41 (0%) | 0 (0%) | 4 (0.5%) | 0 (0%) | 37 (0%) | |
| Using ≥2 drugs | 61 (0%) | 2 (0.1%) | 2 (0.2%) | 0 (0%) | 57 (0%) | |
| Follow up and outcome | ||||||
| Follow‐up time, years | 8.1 (7.3–8.8) | 8.1 (7.3–8.8) | 8.0 (7.2–8.8) | 8.2 (7.1–8.9) | 8.1 (7.3–8.8) | <0.001 |
| Hypertension | 20,129 (7.2%) | 155 (10.9%) | 65 (7.7%) | 11 (9.3%) | 19,898 (7.1%) | <0.001 |
Note: Data was expressed as mean (standard deviation) or median (interquartile range) or numbers (percentage %). The p values less than 0.1 were highlighted with bold font.
Abbreviations: BMI, body mass index; CD, Crohn's disease; CRP, C‐reactive protein; HD, heart diseases; HDL‐C, high‐density lipoprotein; IBD, inflammatory bowel disease; IBD‐U, inflammatory bowel disease unclassified; LDL‐C, low‐density lipoprotein; TC, total cholesterol; TDI, Townsend deprivation index; TG, triglycerides; UC, ulcerative colitis.
Education phase was ranked as 5 grades as followed: 0 for National Vocational Qualification or equivalent, 1 for Certificate of Secondary Education or equivalent, 2 for O levels or equivalent, 3 for A levels or equivalent, and 4 for College/University degree.
In the whole cohort, 2376 (0.8%) participants were diagnosed with IBD at baseline, including 1418 UC, 840 CD, and 118 IBD‐U patients, respectively. The percentage of the elderly (>60 years) in UC and IBD‐U patients was higher than that of in non‐IBD participants (41.4% and 66.9% vs. 36.1%, p < 0.001), respectively. There were fewer current drinkers in patients with IBD than those in non‐IBD participants (86.4%–91.5% vs. 93.0%, p = 0.001), but patients with CD were more likely to be a current smoker compared to non‐IBD participants (15.7% vs. 10.5%, p < 0.001). Physically, IBD patients had a slightly weaker handgrip strength (29.7–30.3 vs. 31.0, p = 0.001) and a lower physical activity score (44.8–45.1 vs. 54.2, p = 0.015) than non‐IBDs. Other parameters including lymphocyte, albumin, TC, HDL‐C, LDL‐C, TG and CRP showed significant but narrow differences among the 4 groups (Table 1), respectively. As for medication history, the percentages of participants with a medication history were relatively low, such as anti‐hypertension drugs (1.0%), steroids (1.1%), and immunosuppressants (0.7%), except for lipid‐lowering drugs (6.7%) and aspirin (6.5%). Interestingly, IBD patients had a higher rate of usage with steroids (4.2%–8.0% vs. 1.1%, p < 0.001) and immunosuppressants (6.8%–20.4% vs. 0.5%, p < 0.001) than non‐IBD participants, but less with aspirin (2.5%–5.6% vs. 6.5%, p = 0.021). Thiopurine was mostly used in the population with UC (134 (9.4%)) and CD (149 (17.7%)), while other immunosuppresants, including methotrexate, tacrolimus, cyclosporine, adalimumab, and combination of ≥2 drugs, were mostly used in the non‐IBD population.
There were 20,129 (7.2%) participants, including 231 IBD patients and 19,898 non‐IBD participants, who developed hypertension with a median follow‐up of 8.1 years (IQR 7.3–8.8 years). The incidence of hypertension in IBD patients was significantly higher than those without IBD (10.9% in UC, 7.7% in CD, and 9.3% in IBD‐U vs. 7.1% in non‐IBD, p < 0.001, Table 1). Survival analysis with intergroup log‐rank tests showed that patients with UC had a higher cumulative rate of hypertension compared to non‐IBD participants (p < 0.001, Figure 2), but not in those with CD (p = 1.000) or IBD‐U (p = 1.000).
FIGURE 2.
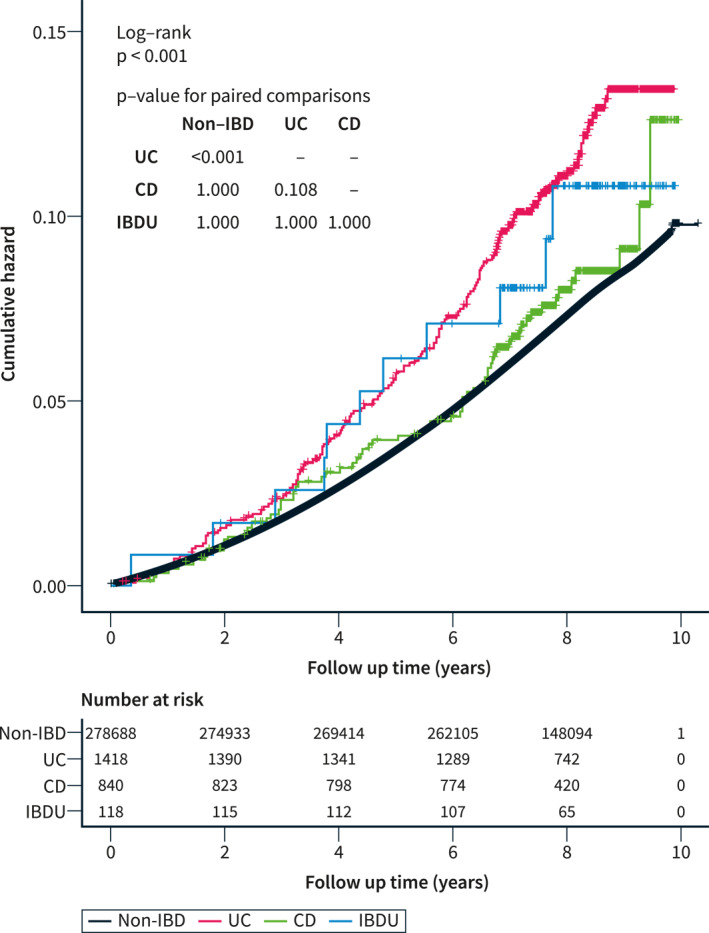
The Kaplan–Meier curves of cumulative risk for the incidence of hypertension between UC, CD, IBD‐U patients, and non‐IBD population. Log‐rank tests between groups were adjusted with Bonferroni correction. CD, Crohn's disease; IBD‐U, inflammatory bowel disease unclassified; UC, ulcerative colitis.
UC is a risk predictor of subsequent hypertension
We then established three multivariate models to explore whether the diagnosis of UC, CD, or IBD‐U was independently associated with subsequent hypertension (Table 2). Age, sex, and race were adjusted in Model 1. Education, smoking status, drinking status, TDI, handgrip strength, BMI, waist to hip rate, physical activity score, and laboratory tests were further adjusted in Model 2 based on Model 1. Then, we further adjusted Model 2 by overall health rating, anxiety status, family history of heart diseases, and medication history, and eventually obtained Model 3. We found that UC, rather than CD or IBD‐U, showed a significant risk for subsequent hypertension in all three Models (HR 1.30, p = 0.001 in Model 1; HR 1.49, p < 0.001 in Model 2, and HR 1.30, p = 0.001 in Model 3, Table 2). Additionally, the multivariate analysis also identified both the usage of steroids (HR 1.33, 95% CI: 1.20–1.47, p < 0.001, Supplementary Table 1) and immunosuppressive agents (HR 1.32, 95% CI: 1.16–1.50, p < 0.001, Supplementary Table 1) as risk factors for hypertension.
TABLE 2.
Multivariate analysis for incidence of hypertension in patents with UC, CD and IBD‐U
| Diseases | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| HR with 95% CI | p value | HR with 95% CI | p value | HR with 95% CI | p value | |
| UC | 1.30 (1.11–1.53) | 0.001 | 1.49 (1.27–1.75) | <0.001 | 1.30 (1.11–1.52) | 0.001 |
| CD | 0.89 (0.70–1.14) | 0.346 | 1.08 (0.88–1.38) | 0.542 | 0.88 (0.69–1.13) | 0.314 |
| IBD‐U | 1.09 (0.60–1.97) | 0.778 | 1.25 (0.69–2.26) | 0.460 | 1.12 (0.62–2.02) | 0.708 |
Note: Model 1: adjusted for age, sex, race. Model 2: further adjusted for education, smoking status, drinking status, Townsend deprivation index at recruitment, hand grip strength, BMI, waist to hip rate, physical activity score, lab tests (TG, TC, HDL, LDL, glucose, Creatine, Albumin, Lymphocyte). Model 3: further adjusted for over‐all health rating, anxiety status, family history of heart diseases and medication history (including anti‐hypertension medicine, lip‐lowing medicine, aspirin, steroids and immunosuppresants). The p values less than 0.05 were highlighted with bold font.
Abbreviations: BMI, body mass index; CD, Crohn's disease; CI, confidence interval; HR, hazard ratio; IBD‐U, inflammatory bowel disease unclassified; TC, total cholesterol; TG, triglycerides; UC, ulcerative colitis.
Subgroup analysis and interaction tests were also performed between UC status and categorical characteristics adjusted in Model 3 (Figure 3). The hazard ratios of UC for hypertension differed between the sub‐populations with (HR 1.01, 95% CI: 0.75–1.36, p = 0.933) and without anxiety (HR 1.46, 95% CI: 1.21–1.76, p < 0.001), and a significant interaction effect was found between UC and anxiety (p = 0.040). For the use of anti‐hypertension drugs, UC was the risk predictor for hypertension only in the subpopulation without the drug history (HR 1.36, 95% CI: 1.15–1.59, p < 0.001) and the interaction between UC and the use of anti‐hypertension drugs was relatively borderline (p = 0.078). For immunosuppressive agents, UC also showed its the risk only in the subpopulation without the drug history (HR 1.35, 95% CI: 1.14–1.59, p < 0.001). For the use of steroids, UC was a risk factor for hypertension in both those with (HR 1.77, 95% CI: 1.07–2.93, p = 0.027) and without (HR 1.27, 95% CI: 1.07–1.50, p = 0.006) steroid use. No significant interaction effect with UC was observed in the use of immunosuppressive agents or steroids.
FIGURE 3.
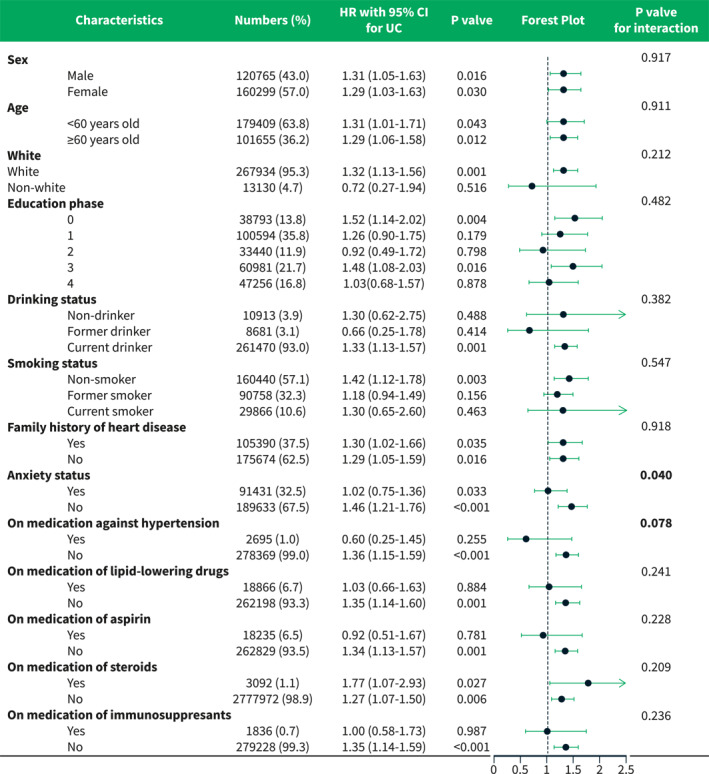
Forest plot of subgroup analysis and interaction tests between categorical variables and UC for the risk of developing hypertension in patients with UC. UC, ulcerative colitis.
Sensitivity analysis
Firstly, we explored the association between the prevalence of IBD and the previously diagnosed hypertension events at baseline. When stratifying the whole population with concurrent hypertension at baseline during the screening process (128,077 vs. 374,428 people, Figure 1), we found that the subpopulation with hypertension at baseline had a relatively higher prevalence of IBD at baseline (1141.3 vs. 1035.6 per 100,000 people) and a higher incidence during follow‐up (53.9 vs. 40.0 per 100,000 person years) than those without. The prevalence and the incidence in the two subpopulations were close to the values reported in previous studies, with the prevalence of 725–1421 per 100,000 people and the incidence of 28.6–69.5 per 100,000 person years. 15 , 16 Furthermore, the cross‐sectional univariate logistics analysis showed that prevalence of hypertension was significantly associated with prevalence of IBD at baseline (OR 1.10, 95% CI 1.04–1.17, p = 0.001), suggesting that the prevalence of IBD might also be associated with previously diagnosed hypertension events.
Considering that the onset ages of IBD and hypertension were different, and that most of the population recruited in the study were middle‐aged, we divided the population into two groups by the age of 60 years old and adjusted it in the multivariate model (in replace of the continuous variable of age) to observe the overall trend. Similar to the continuous one, the age >60 years was also a risk factor for subsequent hypertension (HR 2.17, 95% CI: 2.10–2.24, p < 0.001, Supplementary Table 2). We also adjusted the baseline CRP level in the model, an index reflecting the levels of systemic inflammation or the disease activity, and identified it as a risk factor for subsequent hypertension (HR 1.01, 95% CI: 1.01–1.02, p < 0.001, Supplementary Table 2). It was notable that UC, rather than CD or IBD‐U, still remained as an independent risk factor for subsequent hypertension after the additional adjustment of these variables (all p = 0.001, Supplementary Table 2).
Thirdly, to clarify the effect of different immunosuppressants on the onset of hypertension, we further adjusted the specified immunosuppressants in the multivariate analysis (Supplementary Table 3). The results showed that methotrexate (HR 1.35, p < 0.001 in all three subpopulation) and the combination of immunosuppressants (HR 2.09, p = 0.008 in all three subpopulations) were significantly associated with the onset of hypertension. The use of thiopurine was also a risk factor of subsequent hypertension development in the subpopulations of CD (HR 1.34, 95% CI 1.02–1.77, p = 0.033) and IBD‐U (HR 1.30, 95% CI 1.00–1.69, p = 0.052), but not UC. The use of other immunosuppressants did not show significant effect. Our main result that UC increased the risk of subsequent hypertension showed a good robustness (HR 1.31, 95% CI 1.11–1.53, p = 0.001), regardless of the adjustment of the use of different immunosuppressive agents.
In addition, we performed a 1:1 PSM for sensitivity analysis to eliminate other potential confounding factors. Finally, 1329 UC patients, 758 CD patients, and 117 IBD‐U patients were paired with the same number of non‐IBD participants, respectively. Baseline characteristics in the three paired groups had no significant difference (Supplementary Table 4–6). Subsequent survival analysis (p < 0.001, Figure 4a) and univariate COX regression analysis (HR 1.56, 95% CI: 1.21–2.03, p = 0.001, Supplementary Table 7) showed that only UC was a predictive risk factor for the onset of hypertension.
FIGURE 4.
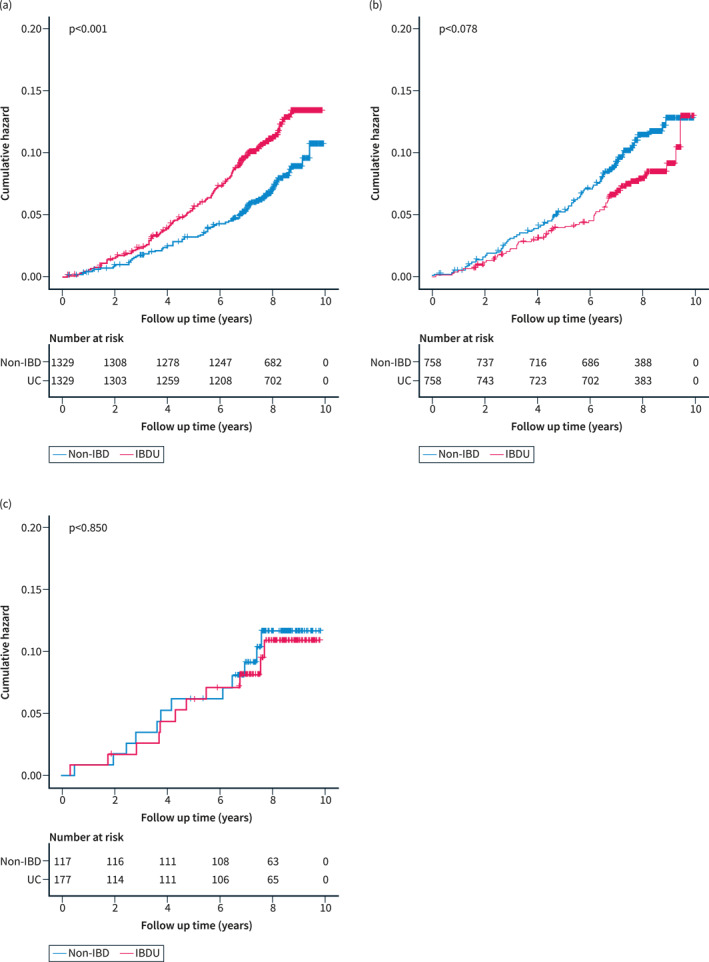
Survival curves of cumulative risk with log‐rank tests of (a) UC, (b) CD, and (c) IBD‐U patients matched with the same number of non‐IBD participants after propensity score matching, respectively. CD, Crohn's disease; IBD‐U, inflammatory bowel disease unclassified; UC, ulcerative colitis.
DISCUSSION
To the best of our knowledge, this is the first study to link IBD with hypertension. To ensure that the study is representative enough of the general population, we focused on this large cohort based on the general population instead of hospitalized patients. Besides, we excluded cases with cardiovascular or cerebrovascular history (hypertension, heart disease, stroke, and diabetes) to get a cohort with a relatively healthy baseline status. Moreover, unlike cross‐sectional studies, we longitudinally collected IBD status at baseline and evaluated the endpoint event after follow‐up, which could avoid reverse causalities in the study (considering hypertension as a risk factor for IBD). In addition, recognized cardiovascular risk factors such as age, sex, and waist hips are also risk factors for hypertension with a narrow but stable confidence interval in the model, suggesting that the population could well represent the general population. 17 , 18
Based on this large general population, we found that IBD patients had a significantly higher prevalence of hypertension than those without. Although there is no similar study with hypertension as the endpoint, several studies have found a positive correlation between IBD and cardiovascular and cerebrovascular diseases, which is partly consistent with our study. 19 , 20 , 21 However, these studies chose major cardiovascular and cerebrovascular events as the endpoint, which might underestimate the endothelial dysfunction caused by the disease. On the contrary, hypertension occurs earlier and more frequently than those hard endpoints and may be more sensitive to spot early vascular endothelial injuries which have long‐term systemic harmful effects.
As one of the main phenotypes of IBD, CD was once considered to have a wider range of lesions and more frequent extraintestinal manifestations compared with UC. 22 , 23 As a result of systemic inflammation and endothelial dysfunction, the incidence of hypertension should have reflected a similar trend. However, we found a significant association only between UC and subsequent hypertension, suggesting that UC, rather than CD or IBD‐U, was an independent risk factor for hypertension. The result was still robust when verified by PSM analysis. Previous population‐based cohort studies have identified UC but not CD as a significant risk factor of cardiovascular events including acute arterial events, ischemic heart disease, heart failure and venous thromboembolism. 24 , 25 , 26 , 27 Moreover, UC rather than CD was identified as a risk factor of diabetes mellitus, a risk equivalent of coronary heart disease leading to endothelial dysfunction. 28 , 29 Our result was consistent with these studies. Further studies are warrant to explore the intriguing pathogenesis of the association between IBD and cardiovascular events, which may involve systemic inflammation, vascular endothelial dysfunction, distribution of intestinal microbial population, thrombosis susceptibility and lipid abnormality. 30
If systemic inflammation does increase the risk of endothelial damage and hypertension, it is reasonable to believe that medication therapies might reduce the risk. However, we identified the use of steroids and immunosuppressive agents as risk factors for hypertension in our study. Subgroup analysis with interaction tests suggested no significant interaction effect with UC in either of them, indicating that steroids and immunosuppressants independently raised the risk of hypertension. The use of glucocorticoids has been widely reported to induce hypertension, which is consistent with our result. 31 , 32 The effect of immunosuppressants on blood pressure varied with different types of drugs. Previous studies have reported that treatment of cyclosporine and tacrolimus might cause hypertension, that methotrexate and anti‐tumor necrosis factor α agents could lower blood pressure, and that the effect of thiopurine on blood pressure has not been well investigated. 33 , 34 , 35 , 36 , 37 Our results based on types of immunosuppressants seemed to differ from the studies above. Inevitable confounding bias might be blamed, considering the relatively small size of population with a history of immunosuppressive drugs. Our main finding that UC posed an independent risk for hypertension was still robust regardless of the adjustment of different immunosuppressants use.
For other therapies not included in our study, there is still not enough evidence to clarify their impact on the onset of hypertension. Some related studies indicated that anti‐inflammatory medications like 5‐aminosalicylic acid could reduce the risk of acute artery events. 25 , 38 Another longitudinal study showed that bowel resection reduced arterial stiffness in patients with UC. 39 Further studies, with more detailed prescription data such as dosage and time, are needed to explore the impact of these therapeutic factors on the onset of hypertension.
Additionally, we spotted that the anxiety status was a risk factor for hypertension, which is consistent with the current view. 40 Subgroup analysis with the interaction test further suggested that UC might not only cause subsequent hypertension directly but also lead to hypertension indirectly by affecting the mental state of patients. Anxiety and depression are common in patients with IBD, and they are considered to be associated with not only the disease activity but the discontinuation of treatment. 41 , 42 In terms of pathophysiological mechanism, previous studies have linked IBD to anxiety and depression through many aspects, including pro‐inflammatory factors affecting brain structure, intestinal microbiome changing brain function through the gut‐brain axis, and the shared genetic correlations of IBD and mental illness. 43 Since UC and anxiety may interact in the onset of hypertension, antidepressants and psychological interventions seem to be capable to prevent hypertension complications in patients with IBD. However, it is worth noting that current findings of the impact of antidepressants on the course of IBD are mixed, and the effects of psychological intervention on IBD improvement are still unclear. 44 , 45 Therefore, further research is needed to clarify the question.
Inevitably, there are certain limitations in our study. First, as an observational study, we could only find the association between IBD and hypertension but not a proven causal relationship. Second, the number of CD and IBD‐U patients enrolled in the study is inadequate, and the evaluation of disease activity at baseline and follow‐up (e.g., longitudinal blood samples, endoscopy and radiographic data) is unavailable. Third, we could only obtain the diagnostic information of IBD and hypertension through the ICD code without other inspection materials at diagnosis, and there was a potential bias of misdiagnosis. Similarly, the endpoint event was determined only by the diagnosis at its first occurrence, and records of multiple diagnoses during follow‐up were not accessible. Other longitudinal variables including follow‐up blood pressure measurement and subsequent use of anti‐hypertension drugs were also unavailable. Lastly, we had no access to the prescription data of other biologic agents in the UK Biobank.
In summary, our study demonstrates IBD patients, especially UC patients, have a higher risk of subsequent hypertension than the general population. It is recommended to strengthen the management of patients with IBD to minimize disease activity and prevent cardiovascular events in clinical practice.
AUTHOR CONTRIBUTIONS
Ren Mao and Xiaodong Zhuang conceived the idea and obtained research data. Jinyu Tan and Fan Hu finished the literature retrieval. Jinshen He and Shaozhao Zhang performed the statistical analysis and drafted the manuscript. Xiaomin Wu and Yu Wang modified figures and tables. Ren Mao, Xiaodong Zhuang, Yun Qiu, Fen Liu, Zishan Liu, Longyuan Zhou, Shixian Hu, Minhu Chen and Xinxue Liao supervised and revised the manuscript critically. All of the authors read and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
ETHICS APPROVAL
The cohort study has been approved by the NHS National Research Ethics Service and all participants enrolled have provided written informed consent. This research was conducted using the UK Biobank Resource under Application Number 56,925, which is available for details at https://www.ukbiobank.ac.uk/enable‐your‐research/approved‐research/.
Supporting information
Supporting Information S1
Supporting Information S2
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (NSFC grant No. 81970483, 82170537 and 82222010), and the Young S&T Talent Training Program of Guangdong Provincial Association for S&T (GDSTA) (No. SKXRC202201) to Ren Mao.
He J, Zhang S, Qiu Y, Liu F, Liu Z, Tan J, et al. Ulcerative colitis increases risk of hypertension in a UK biobank cohort study. United European Gastroenterol J. 2023;11(1):19–30. 10.1002/ueg2.12351
Jinshen He and Shaozhao Zhang contributed equally.
Contributor Information
Xiaodong Zhuang, Email: maor5@mail.sysu.edu.cn.
Ren Mao, Email: maor5@mail.sysu.edu.cn.
DATA AVAILABILITY STATEMENT
Data from UK Biobank is available to researchers through an open application. A detailed procedure is described at http://www.ukbiobank.ac.uk/using‐the‐resource/.
REFERENCES
- 1. Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14(5):329–42. 10.1038/nri3661 [DOI] [PubMed] [Google Scholar]
- 2. Mehta NN, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. 2010;31(8):1000–6. 10.1093/eurheartj/ehp567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Avina‐Zubieta JA, Thomas J, Sadatsafavi M, Lehman AJ, Lacaille D. Risk of incident cardiovascular events in patients with rheumatoid arthritis: a meta‐analysis of observational studies. Ann Rheum Dis. 2012;71(9):1524–9. 10.1136/annrheumdis-2011-200726 [DOI] [PubMed] [Google Scholar]
- 4. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–31. 10.1056/nejmoa1707914 [DOI] [PubMed] [Google Scholar]
- 5. Tardif J‐C, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, et al. Efficacy and safety of low‐dose colchicine after myocardial infarction. N Engl J Med. 2019;381(26):2497–505. 10.1056/nejmoa1912388 [DOI] [PubMed] [Google Scholar]
- 6. Engelen SE, Robinson AJB, Zurke YX, Monaco C. Therapeutic strategies targeting inflammation and immunity in atherosclerosis: how to proceed? Nat Rev Cardiol. 2022;19(8):522–42. 10.1038/s41569-021-00668-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cicalese SM, da Silva JF, Priviero F, Webb RC, Eguchi S, Tostes RC. Vascular stress signaling in hypertension. Circ Res. 2021;128(7):969–92. 10.1161/circresaha.121.318053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harrison DG, Coffman TM, Wilcox CS. Pathophysiology of hypertension: the mosaic theory and beyond. Circ Res. 2021;128(7):847–63. 10.1161/circresaha.121.318082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zanoli L, Mikhailidis DP, Bruno RM, Abreu MT, Danese S, Eliakim R, et al. Aortic stiffening is an extraintestinal manifestation of inflammatory bowel disease: review of the literature and expert panel statement. Angiology. 2020;71(8):689–97. 10.1177/0003319720918509 [DOI] [PubMed] [Google Scholar]
- 10. Safar ME, Asmar R, Benetos A, Blacher J, Boutouyrie P, Lacolley P, et al. Interaction between hypertension and arterial stiffness. Hypertension. 2018;72(4):796–805. 10.1161/hypertensionaha.118.11212 [DOI] [PubMed] [Google Scholar]
- 11. Singh S, Singh H, Loftus EV, Pardi DS. Risk of cerebrovascular accidents and ischemic heart disease in patients with inflammatory bowel disease: a systematic review and meta‐analysis. Clin Gastroenterol Hepatol. 2014;12(3):382–93.e1. 10.1016/j.cgh.2013.08.023 [DOI] [PubMed] [Google Scholar]
- 12. Ghoneim S, Shah A, Dhorepatil A, Butt MU, Waghray N. The risk of cerebrovascular accidents in inflammatory bowel disease in the United States: a population‐based national study. Clin Exp Gastroenterol. 2020;13:123–9. 10.2147/ceg.s250182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779. 10.1371/journal.pmed.1001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Elliott P, Peakman TC. The UK Biobank sample handling and storage protocol for the collection, processing and archiving of human blood and urine. Int J Epidemiol. 2008;37(2):234–44. 10.1093/ije/dym276 [DOI] [PubMed] [Google Scholar]
- 15. Freeman K, Ryan R, Parsons N, Taylor‐Phillips S, Willis BH, Clarke A. The incidence and prevalence of inflammatory bowel disease in UK primary care: a retrospective cohort study of the IQVIA Medical Research Database. BMC Gastroenterol. 2021;21(1):139. 10.1186/s12876-021-01716-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pasvol TJ, Horsfall L, Bloom S, Segal AW, Sabin C, Field N, et al. Incidence and prevalence of inflammatory bowel disease in UK primary care: a population‐based cohort study. BMJ Open. 2020;10(7):e036584. 10.1136/bmjopen-2019-036584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2019;140(11):e596–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhou B, Perel P, Mensah GA, Ezzati M. Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat Rev Cardiol. 2021;18(11):785–802. 10.1038/s41569-021-00559-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sun H‐H, Tian F. Inflammatory bowel disease and cardiovascular disease incidence and mortality: a meta‐analysis. Eur J Prev Cardiol. 2018;25(15):1623–31. 10.1177/2047487318792952 [DOI] [PubMed] [Google Scholar]
- 20. Panhwar MS, Mansoor E, Al‐Kindi SG, Sinh P, Katz J, Oliveira GH, et al. Risk of myocardial infarction in inflammatory bowel disease: a population‐based national study. Inflamm Bowel Dis. 2019;25(6):1080–7. 10.1093/ibd/izy354 [DOI] [PubMed] [Google Scholar]
- 21. Lee MT, Mahtta D, Chen L, Hussain A, Al Rifai M, Sinh P, et al. Premature atherosclerotic cardiovascular disease risk among patients with inflammatory bowel disease. Am J Med. 2021;134(8):1047–51. 10.1016/j.amjmed.2021.02.029 [DOI] [PubMed] [Google Scholar]
- 22. Herzog D, Fournier N, Buehr P, Rueger V, Koller R, Heyland K, et al. Age at disease onset of inflammatory bowel disease is associated with later extraintestinal manifestations and complications. Eur J Gastroenterol Hepatol. 2018;30(6):598–607. 10.1097/meg.0000000000001072 [DOI] [PubMed] [Google Scholar]
- 23. Rogler G, Singh A, Kavanaugh A, Rubin DT. Extraintestinal manifestations of inflammatory bowel disease: current concepts, treatment, and implications for disease management. Gastroenterology. 2021;161(4):1118–32. 10.1053/j.gastro.2021.07.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Alayo Q, Loftus EV, Jr , Yarur A, Alvarado D, Ciorba MA, de las Fuentes L, et al. Inflammatory bowel disease is associated with an increased risk of incident acute arterial events: analysis of the United Kingdom Biobank. Clin Gastroenterol Hepatol. 2022. 10.1016/j.cgh.2022.08.035 [DOI] [PubMed] [Google Scholar]
- 25. Rungoe C, Basit S, Ranthe MF, Wohlfahrt J, Langholz E, Jess T. Risk of ischaemic heart disease in patients with inflammatory bowel disease: a nationwide Danish cohort study. Gut. 2013;62(5):689–94. 10.1136/gutjnl-2012-303285 [DOI] [PubMed] [Google Scholar]
- 26. Aniwan S, Pardi DS, Tremaine WJ, Loftus EV, Jr . Increased risk of acute myocardial infarction and heart failure in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2018;16(10):1607–15. 10.1016/j.cgh.2018.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Harvey PR, Coupland B, Mytton J, De Silva S, Trudgill NJ. Venous thromboembolism following discharge from hospital in patients admitted for inflammatory bowel disease. J Crohns Colitis. 2022. [DOI] [PubMed] [Google Scholar]
- 28. Dregan A, Charlton J, Chowienczyk P, Gulliford MC. Chronic inflammatory disorders and risk of type 2 diabetes mellitus, coronary heart disease, and stroke: a population‐based cohort study. Circulation. 2014;130(10):837–44. 10.1161/circulationaha.114.009990 [DOI] [PubMed] [Google Scholar]
- 29. Expert Panel on Detection E . Treatment of High Blood Cholesterol in A . Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486–97. 10.1001/jama.285.19.2486 [DOI] [PubMed] [Google Scholar]
- 30. Cainzos‐Achirica M, Glassner K, Zawahir HS, Dey AK, Agrawal T, Quigley EM, et al. Inflammatory bowel disease and atherosclerotic cardiovascular disease: JACC review topic of the week. J Am Coll Cardiol. 2020;76(24):2895–905. [DOI] [PubMed] [Google Scholar]
- 31. Mebrahtu TF, Morgan AW, West RM, Stewart PM, Pujades‐Rodriguez M. Oral glucocorticoids and incidence of hypertension in people with chronic inflammatory diseases: a population‐based cohort study. CMAJ. 2020;192(12):E295–301. 10.1503/cmaj.191012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Costello RE, Yimer BB, Roads P, Jani M, Dixon WG. Glucocorticoid use is associated with an increased risk of hypertension. Rheumatology. 2021;60(1):132–9. 10.1093/rheumatology/keaa209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chiasson VL, Talreja D, Young KJ, Chatterjee P, Banes‐Berceli AK, Mitchell BM. FK506 binding protein 12 deficiency in endothelial and hematopoietic cells decreases regulatory T cells and causes hypertension. Hypertension. 2011;57(6):1167–75. 10.1161/hypertensionaha.110.162917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Filho AG, Kinote A, Pereira DJ, Renno A, dos Santos RC, Ferreira‐Melo SE, et al. Infliximab prevents increased systolic blood pressure and upregulates the AKT/eNOS pathway in the aorta of spontaneously hypertensive rats. Eur J Pharmacol. 2013;700(1–3):201–9. 10.1016/j.ejphar.2012.11.059 [DOI] [PubMed] [Google Scholar]
- 35. Kvien TK, Zeidler HK, Hannonen P, Wollheim FA, Førre Ø, Hafström I, et al. Long term efficacy and safety of cyclosporin versus parenteral gold in early rheumatoid arthritis: a three year study of radiographic progression, renal function, and arterial hypertension. Ann Rheum Dis. 2002;61(6):511–16. 10.1136/ard.61.6.511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mangoni AA, Baghdadi LR, Shanahan EM, Wiese MD, Tommasi S, Elliot D, et al. Methotrexate, blood pressure and markers of arterial function in patients with rheumatoid arthritis: a repeated cross‐sectional study. Ther Adv Musculoskelet Dis. 2017;9(9):213–29. 10.1177/1759720x17719850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yoshida S, Takeuchi T, Kotani T, Yamamoto N, Hata K, Nagai K, et al. Infliximab, a TNF‐alpha inhibitor, reduces 24‐h ambulatory blood pressure in rheumatoid arthritis patients. J Hum Hypertens. 2014;28(3):165–9. 10.1038/jhh.2013.80 [DOI] [PubMed] [Google Scholar]
- 38. Kirchgesner J, Nyboe Andersen N, Carrat F, Jess T, Beaugerie L. Risk of acute arterial events associated with treatment of inflammatory bowel diseases: nationwide French cohort study. Gut. 2020;69(5):852–8. 10.1136/gutjnl-2019-318932 [DOI] [PubMed] [Google Scholar]
- 39. Zanoli L, Tuttolomondo A, Geraci G, Castellino P. Bowel resection reduces aortic pulse wave velocity in patients with ulcerative colitis. A longitudinal study. Eur J Intern Med. 2020;82:126–7. 10.1016/j.ejim.2020.08.001 [DOI] [PubMed] [Google Scholar]
- 40. Lim LF, Solmi M, Cortese S. Association between anxiety and hypertension in adults: a systematic review and meta‐analysis. Neurosci Biobehav Rev. 2021;131:96–119. 10.1016/j.neubiorev.2021.08.031 [DOI] [PubMed] [Google Scholar]
- 41. Dolovich C, Bernstein CN, Singh H, Nugent Z, Tennakoon A, Shafer LA, et al. Anxiety and depression leads to anti‐tumor necrosis factor discontinuation in inflammatory bowel disease. Clin Gastroenterol Hepatol. 2021;19(6):1200–8.e1201. 10.1016/j.cgh.2020.07.013 [DOI] [PubMed] [Google Scholar]
- 42. Barberio B, Zamani M, Black CJ, Savarino EV, Ford AC. Prevalence of symptoms of anxiety and depression in patients with inflammatory bowel disease: a systematic review and meta‐analysis. Lancet Gastroenterol Hepatol. 2021;6(5):359–70. 10.1016/s2468-1253(21)00014-5 [DOI] [PubMed] [Google Scholar]
- 43. Bisgaard TH, Allin KH, Keefer L, Ananthakrishnan AN, Jess T. Depression and anxiety in inflammatory bowel disease: epidemiology, mechanisms and treatment. Nat Rev Gastroenterol Hepatol. 2022;19(11):717–26. 10.1038/s41575-022-00634-6 [DOI] [PubMed] [Google Scholar]
- 44. Gracie DJ, Irvine AJ, Sood R, Mikocka‐Walus A, Hamlin PJ, Ford AC. Effect of psychological therapy on disease activity, psychological comorbidity, and quality of life in inflammatory bowel disease: a systematic review and meta‐analysis. Lancet Gastroenterol Hepatol. 2017;2(3):189–99. 10.1016/s2468-1253(16)30206-0 [DOI] [PubMed] [Google Scholar]
- 45. Mikocka‐Walus A, Prady SL, Pollok J, Esterman AJ, Gordon AL, Knowles S, et al. Adjuvant therapy with antidepressants for the management of inflammatory bowel disease. Cochrane Database Syst Rev. 2019;4:CD012680. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Supporting Information S2
Data Availability Statement
Data from UK Biobank is available to researchers through an open application. A detailed procedure is described at http://www.ukbiobank.ac.uk/using‐the‐resource/.


