Abstract
Background
Psychological stress and increased permeability are implicated as contributing factors in the initiation and worsening of gastrointestinal diseases. A link between stress and intestinal permeability has been shown in animal models as well as in human small intestine, but stress effects on the human colorectal mucosal barrier has not been reported.
Objective
To investigate the potential effects of acute psychological stress on colorectal mucosal barrier function and to explore stress‐induced molecular events in the rectal mucosa under healthy conditions.
Methods
Endoscopic biopsies were taken from the rectosigmoid region of healthy volunteers, who had been subjected to dichotomous listening stress and after a control session, respectively. Paracellular and transcellular permeability were assessed in modified Ussing chambers. RNA expression (microarray technology confirmed by quantitative real‐time polymerase chain reaction) and biological pathway analysis were used to investigate the local mucosal response to acute stress.
Results
Dichotomous listening stress induced a subjective and objective stress response, and significantly increased paracellular but not transcellular permeability. We also identified a stress‐induced reduction in RNA expression of genes related to immune cell activation and maturation (CR2, CD20, TCLA1, BANK1, CD22, FDCSP), signaling molecules of homing of immune cells to the gut (chemokines: CCL21, CXCL13, and CCL19, and receptors: CCR7, CXCR5), and innate immunity (DUOX2). Eight of the 10 top down‐regulated genes are directly involved in B cell activation, signaling and migration. The systemic stress response correlated positively with paracellular permeability and negatively with DUOX2 expression.
Conclusion
Dichotomous listening stress increases paracellular permeability and modulates immune cell activity in the rectal mucosa. Further studies are warranted to identify the primary mechanisms of stress‐mediated reduction of mucosal defensive activity and barrier dysfunction, and their potential implications for gastrointestinal disorders.
Keywords: intestinal permeability, microarray, mucosal immunity, psychological stress
Key summary.
Current knowledge
Stress and barrier dysfunction are contributing factors in gastrointestinal diseases.
There is a link between psychological stress and increased intestinal permeability, but this has not been studied in the human colorectal mucosa.
What are the new findings?
Dichotomous listening stress increases paracellular permeability in the colorectal mucosa of healthy volunteers.
Modifications of immune activity, mainly implicating B‐cells, is involved in the initial mucosal response to mild psychological stress.
INTRODUCTION
Psychological stress is recognized as a contributing factor in several gastrointestinal (GI) conditions, including irritable bowel syndrome (IBS) and inflammatory bowel disease (IBD). IBS patients have more stressful life events 1 , 2 and have altered visceral sensitivity in response to acute stress. 3 Additionally, a recent large Genome‐Wide Association Study confirmed that neuronal pathways, potentially involving enteric nerves, play a dominant role in the genetics of IBS. 4 In ulcerative colitis, persistent life stress increases the number of exacerbations, and stress resilience is protective in IBD. 5 , 6
Intestinal barrier dysfunction is also an established factor in the pathophysiology of both IBD 7 , 8 , 9 and IBS. 10 , 11 In animal models, it has been established that stress and barrier dysfunction are linked: both acute and chronic psychological stress increase GI permeability in rats and predispose to inflammation by inducing IBS‐ and UC‐like colonic mucosal phenotypes in a mast cell‐dependent manner. 12 , 13 In humans, acute stress increases small intestinal secretion 14 and permeability via mast cells, 15 but stress effects on human colorectal mucosal function have not been reported. With the differences in microanatomy, mucus composition, microbiome and microbial load, enteric nerve innervation, etc., the potential for dissimilarities between the physiological and pathophysiological effects of stress in small bowel and colorectal mucosa is obvious. It is also unknown how mucosal responses to acute and chronic stressors are linked in humans, but it was shown that healthy women with high background stress had distorted small bowel epithelial responses to acute stress, a potential initial step in the development of prolonged GI dysfunction and disease. 16 Psychological stress also perturbs intestinal immunity, 17 affecting circulating lymphocytes 18 and activating mucosal mast cells. 15 However, early stress‐induced immune responses in human colorectal mucosa have not been fully elucidated.
The aim of this study was to investigate the effects of acute psychological stress on human colorectal mucosal barrier function and gain insights into stress‐induced modulation of mucosal immunity. Healthy volunteers were submitted to acute dichotomous listening stress 19 and a control session, and colorectal mucosal biopsies were obtained to assess the impact of stress on barrier function 20 and gene expression.
MATERIALS AND METHODS
Participants and clinical assessment
Healthy volunteers were recruited by public advertisement. Exclusion criteria were chronic GI disorders or symptoms, other organic diseases, first‐degree relatives with IBD, medication known to affect GI permeability, and pregnancy. Use of NSAIDs was not allowed and alcohol was discouraged, 2 weeks and 3 days, respectively, before the procedures.
The Regional Committee of Human Ethics in Linköping approved the study (Dnr M183‐08) and all subjects gave their written informed consent.
Study design
See overview of study in Figure 1. Before each session, all participants completed validated questionnaires related to life event stress, 21 current perceived stress the week preceding the experiments 22 and the individual's tendency for stress/anxiety. 23 During each of the control and the stress sessions, objective and subjective stress variables were monitored to assess the response to acute stress. After each control and stress session, rectal biopsies were obtained to evaluate intestinal mucosal response to acute stress by assessing epithelial barrier function (transepithelial electrical resistance [TER], paracellular and transcellular permeability) and by quantifying changes in gene expression profile in colonic biopsies. All samples were codified and blindly analyzed.
FIGURE 1.
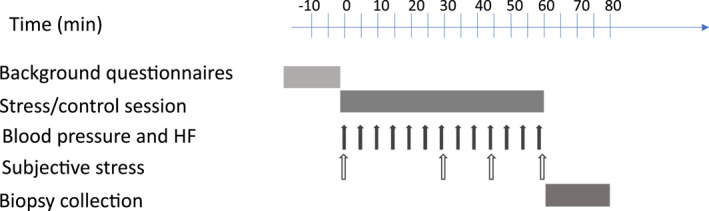
Overview of experimental design. Background questionnaires: Current background stress, Life event stress and Trait anxiety (see Table S2 in Supporting information S1) were filled in by all volunteers before each session. Stress and control sessions were conducted using a designed software. Subjective stress was measured using a visual analog scale (VAS; see Table 1). Biopsies were collected by endoscopy immediately following the sessions (see Supporting Information S1)
Dichotomous listening stress
Each participant performed a stress and a control session (serving as its own control) with an interval of at least 2 weeks between sessions. Procedures were done between 7:30–09:00 AM in all participants and lasted 1 h. Acute stress was induced by dichotomous listening 19 : subjects were exposed to two different audiobooks, one in each ear for 60 min. Which story they should focus on alternated during the session. Volunteers were required to, in alternating periods, either answer listening comprehension questions or mark every tenth word using a custom‐made software that gives immediate feedback. The test leader supervised them and gave continuous feedback on their performance. Prior to the stress session, subjects were informed that the task was challenging, that their results would be compared with other participants, and that if they scored more than 75% correct answers, they would get a reward. During the control session subjects listened to calm music from a selection as they pleased.
Assessment of the stress response
Psychological response: Perceived stress was assessed by each participant every 15 min on a visual analog scale (VAS) from 0, no stress, to 100, maximum stress.
Biological response: Heart rate (HF) and blood pressure (BP) were measured with OMRON M10‐IT (Omron) at baseline and every 5 min during the stress procedure.
In previous studies using laboratory psychological stress 17 , 19 there have been non‐responders in whom the stressor did not elicit a stress response. We defined responders as those who had both subjective and objective responses to the stress session.
Biological samples
Immediately following the stress/control session, rectal biopsies were collected via flexible sigmoidoscopy. From every session 6–8 biopsies were directly put in ice‐cold oxygenated Krebs buffer (115 mM NaCl, 1.25 mM CaCl2, 1.2 mM MgCl2, 2 mM KH2PO4, and 25 mM NaHCO3, pH 7.35) for barrier measurements, 2–3 biopsies were placed in RNAlater (Invitrogen, Fisher Scientific, Gothenburg, Sweden) and maintained at −80°C for subsequent mRNA analysis, and 2–3 were fixed in 4% paraformaldehyde in Phosphate‐buffered saline for histological evaluation.
Ussing chamber experiments (see also Supplementary S1)
Rectal biopsies were mounted in modified Ussing chambers as previously described. 20 Tissue viability was monitored by potential difference (PD) during experiments and forskolin response at the end of experiments. The transcellular marker horseradish peroxidase (HRP) (type VI; Sigma Chemical Co) and the paracellular probe 51Chromium(Cr)‐EDTA (Perkin Elmer) were added to the mucosal sides and serosal samples were collected during 120 min. 51Cr‐EDTA permeability was measured by gamma‐counting (1282 Compugamma) and HRP passage was analyzed with QuantaBlu™ Flourigenic peroxidase substrate kit (Pierce). 8
RNA isolation, microarray and biological pathway analysis
In randomly selected biopsies, microarray technology (Applied Biosystems), was applied to identify consistent differential gene expression due to experimental stress. 24 Total mRNA was isolated with the RNAeasy Mini Kit (Qiagen). Quality and quantity of mRNA were assessed with the Agilent 2100 Bioanalyzer. Sampling for high quality mRNA expression was achieved in 10 included individuals and paired analysis was feasible in samples from seven individuals. To identify the gene expression profile, we applied hierarchical clustering on the complete set of differentially expressed genes using average linkage and correlation as measures of similarity. Genes with similar expression profiles were grouped together in a hierarchical way and differentially expressed genes (p < 0.05) were submitted to pathway and network analysis using the Ingenuity Pathway Analysis (IPA) methodology (IPA 7.0, Ingenuity Systems). 24 Genes with a mean fold‐change of ≤0.7 and ≥1.4, obtained comparing stress versus control were analyzed by IPA software. The list of differentially expressed genes in stress versus control, linked to their approved nomenclature (http://www.genenames.org), and fold‐change was uploaded into the IPA application. For network analysis, IPA provided a score according to the fit of supplied genes and the list of biological functions involved.
Quantitative real‐time PCR
To further validate microarray results, cDNA synthesis and amplification was performed using 1 μg of total RNA with the High‐Capacity Reverse Transcription Reagents Kit (Applied Biosystems). Q‐RT‐PCR was performed on an ABI PRISM® 7500 FAST Sequence Detection System (Applied Biosystems) using validated TaqMan Gene Expression Assays, and the human Peptidylprolyl isomerase A (PPIA) (cyclophilin A) gene as endogenous control (Applied Biosystems). Sampling for validation Q‐RT‐PCR was achieved in 10 included subjects, and full paired analysis was feasible in seven individuals. The selection of genes to quantify (based on microarray, plus tryptase and four barrier‐related proteins) is shown in supplementary material (Tables S1a and S1b). Each sample, including distilled water as negative control, was run in triplicate and data were analyzed by the 2‐ΔΔCt method, as previously described. 25 The expression of each gene was normalized to the endogenous control and the fold change after stress compared to control was calculated individually.
Statistical analysis
Data are expressed as mean (±SD) or median (Q1–Q3). Normality of data distribution was tested by the Kolmogorov‐Smirnov test. Normally distributed parametric data were compared by paired Student's t‐test; otherwise, the Wilcoxon signed ranks test was used. p < 0.05 was considered statistically significant. Statistical analysis was performed using GraphPad Prism 8 software (GraphPad Software Inc.). Correlations between variables was assessed by the Spearman test. The Benjamini and Hochberg method was used to adjust p‐values in the gene expression analysis and adjusted to obtain strong control over the “false discovery rate” (FDR).
RESULTS
Study population
Twenty‐one (8 male, 13 female) healthy volunteers were recruited, and 16 were included in the full analysis (Figure 2). All 16 participants were medication free, including NSAIDs and antibiotics. Also, all included volunteers scored inside the normal range for the scales measuring background stress levels (Table S2), and there were no sex differences.
FIGURE 2.
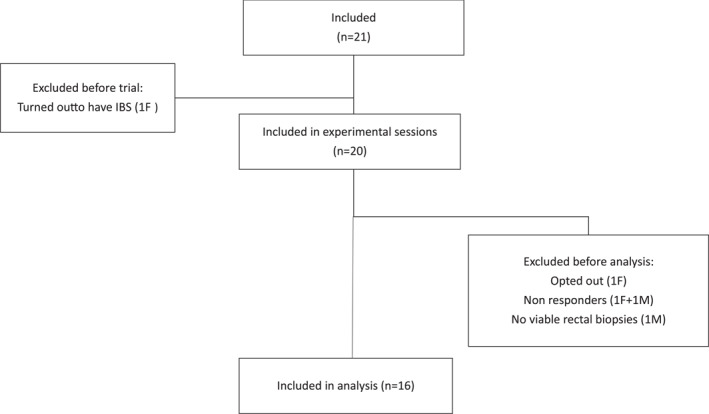
Flow chart of participants in the study. Sixteen of the 21 recruited healthy volunteers were included in the permeability studies M = male; F = female. IBS, irritable bowel syndrome
Dichotomous listening induced a systemic stress response
Dichotomous listening increased both objective and subjective stress as compared to control sessions. There were significant differences between control and stress in systolic blood pressure, diastolic blood pressure, and heart rate and subjective stress (VAS) (Table 1). Correlations between trait anxiety and current perceived stress (r = 0.63, p = 0.03; n = 16), and HF and SysBP (r = 0.49, p = 0.05; n = 16), supported the validity of the stress model.
TABLE 1.
Subjective stress and cardiovascular variables during control sessions and stress sessions, respectively
| Control (median; range) | Stress (median; range) | p‐value | |
|---|---|---|---|
| Subjective stress (VAS 0–100) | 8.1 (0.5–50) | 54.9 (19.5–74.3) | <0.0001 |
| Heart frequency (bpm) | 72.3 (51.9–85.1) | 81.6 (57.5–102) | <0.0001 |
| Systolic blood pressure (mmHg) | 109.8 (97–129) | 122.6 (103–159) | <0.0001 |
| Diastolic blood pressure (mmHg) | 72.4 (65.2–93.8) | 79.4 (61.2–83.3) | <0.0001 |
Note: Subjective stress was measured using a visual analog scale where 0 = no stress and 100 = maximum stress (arbitrary units).
Abbreviations: mmHg, millimeter mercury; ppm, beats per minute.
Dichotomous listening increased paracellular permeability
In paired comparisons between stress and control sessions in the same individuals, paracellular permeability as measured by 51Cr‐EDTA was significantly increased by stress, p < 0.05 (Figure 3a); 51Cr‐EDTA permeation was increased in 10/11 participants after stress (Figure 3b), but there was no significant difference between stress and control sessions in TER (Figure 3c,d).
FIGURE 3.
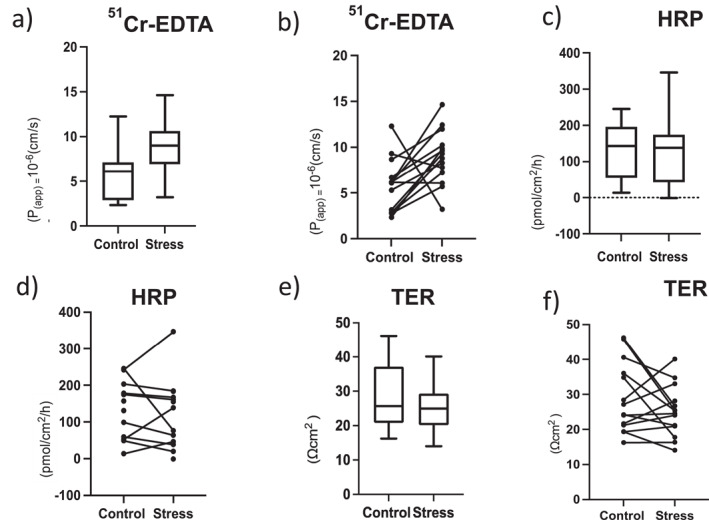
Assessment of barrier function in rectal mucosa in control and stress sessions. Results are presented as box plot and paired line chart (control and stress in same individual), respectively, for all variables. (a and b) Paracellular permeability studied using 51Cr‐EDTA (n = 16 subjects, 14 paired analyses, p = 0.0245); (c and d) Transcellular antigen passage studied using horseradish peroxidase (HRP) (n = 14 subjects, 8 paired analyses); (e and f) Transepithelial resistance (TER) (n = 12 subjects, 10 paired analyses). *p < 0.05
Transepithelial passage of HRP (Figure 3e,f) was not significantly changed by stress neither in absolute (n = 14) nor in paired (n = 10) analysis. Despite this, the delta value between stress and control sessions in HRP flux correlated to Δ51Cr‐EDTA‐permeability (r = 0.63, p = 0.044; n = 11). There were no differences between men and women in the permeability response to stress.
Dichotomous listening modulated mucosal gene expression profile
Microarray analysis in biopsies from stress and control sessions identified 185 genes that responded to experimental stress. Among these, 171 were eligible for gene interaction and biological function analysis, and 83 were differentially expressed, that is, p‐value ≤ 0.05 after FDR adjustment.
The stress‐induced expression profile was mainly characterized by a reduction of immune‐related genes (Table 2). The only gene found to be up‐regulated was phosphoenolpyruvate carboxykinase 1 (PCK1), involved in the gluconeogenesis process (Table 2). The nuclear factor kappa B subunit 2 (NFkB2) was identified as the only significantly affected (down‐regulated) transcription factor (p < 0.001). The list of differentially expressed genes was overlaid onto the molecular network contained in the IPA, which identified seven networks, based on focus genes interrelationships (Table S3). The most significant network (score 32) was associated with functions related to canonical pathways of the network “Inflammatory Response, Cell Signaling, Cellular Function and Maintenance” (Figure 4). Additionally, the biofunction analysis of the immune response, based on stress‐induced changes in gene expression, revealed a gene profile associated with migration and chemotaxis of immune cells, mainly B and T lymphocytes, dendritic cells and phagocytes (Table S4).
TABLE 2.
Top regulated genes by acute experimental stress in the colorectal mucosa of healthy volunteers
| Gene symbol | Gene/protein name | Fold change | FDR | Main function of gene product |
|---|---|---|---|---|
| PCK1 | Phosphoenolpyruvate carboxykinase | 1.83 | 0.050 | Acts as the rate‐limiting enzyme in gluconeogenesis and controls levels of metabolic intermediates in the citric acid cycle. Regulates formation and maintenance of memory CD8(+) T‐cells. |
| CR2 | Complement C3d receptor 2 | −2.18 | 0.021 | Receptor for complement C3, on human B‐cells a and T‐cells. Participates in B‐cell activation. |
| CXC13 | C‐X‐C motif chemokine 13 | −1.97 | 0.050 | Chemotactic for B‐lymphocytes but not for T‐lymphocytes, monocytes and neutrophils. Binds to chemokine receptor CXCR5. |
| CCL21 | C‐C motif chemokine 21 | −1.82 | 0.029 | Chemotactic for activated T‐cells, B‐cells, and dendritic cells. Binds to atypical chemokine receptor CCR7. |
| MS4A1 | Membrane spanning 4‐domains A1 | −1.78 | 0.049 | B‐lymphocyte‐specific membrane protein that plays a role in the development, differentiation, and activation of B‐lymphocytes. |
| BANK1 | B cell scaffold protein with ankyrin repeat | −1.70 | 0.029 | Involved in B‐cell receptor (BCR)‐induced Ca(2+) mobilization from intracellular stores. |
| CCL19 | C‐C motif chemokine ligand 19 | −1.62 | 0.022 | Potent chemotactic activity for T‐cells and B‐cells. Binds to chemokine receptor CCR7 and ACKR4. |
| DOUX2 | Dual oxidase 2 | −1.59 | 0.050 | Generates hydrogen peroxide; playing role in Lactoperox‐idase‐mediated antimicrobial defense at mucosal surface. |
| TCLA1 | T cell leukemia/lymphoma 1 family AKT coactivator A | −1.51 | 0.050 | Phosphorylation and activation of AKT1, AKT2 and AKT3. Stabilizes mitochondrial membrane potential and promotes cell survival. |
| CD22 | CD22 molecule | −1.51 | 0.044 | Mediates B‐cell–B‐cell interactions. Involved in localization of B‐cells in lymphoid tissues and regulation of B‐cell antigen receptor signaling. |
Note: Gene symbol and name for the up‐ and down‐regulated genes and their fold‐change expression respect to basal state are indicated. Data are based on analysis of mRNA expression in seven individuals in control and stress sessions, respectively. p values were adjusted to obtain strong control over the “false discovery rate” (FDR) using the Benjamini and Hochberg method. Main protein functions from The Human Protein Atlas (https://www.proteinatlas.org). PCK1 was the only up‐regulated gene, all others were significantly down‐regulated.
FIGURE 4.
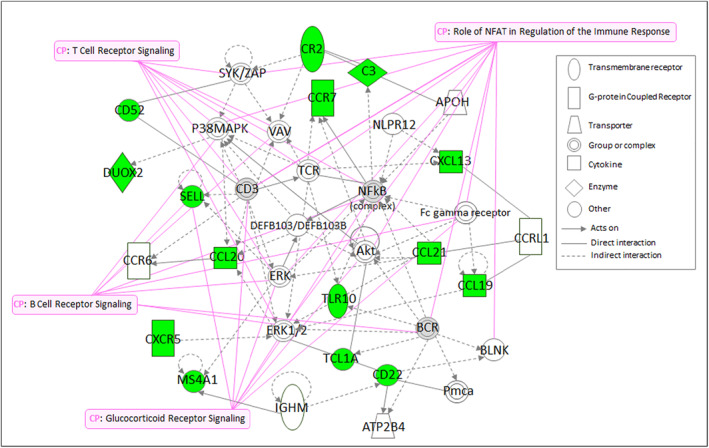
Functional analysis of mucosal transcriptional signature in the colonic mucosa of healthy volunteers induced by dichotomous listening stress. Relationships between differentially expressed genes in the first highest scored network as analyzed by the Ingenuity Pathway Analysis (IPA) application and associated canonical pathways (CP) based on gene expression during control and stress sessions, respectively, in seven individuals. Node (gene) and edge (gene relationship) symbols are described. The color indicates downregulation (green). Genes in uncolored nodes were not identified as differentially expressed and were integrated into the computationally generated networks based on the evidence stored in the IPA knowledge memory indicating relevance for this network
Validation by q‐RT‐PCR of the top differentially expressed genes (Table 2) confirmed that acute stress increased the expression of the PCK1 gene. Reduced expression was confirmed of genes relating to immune cell (mainly B‐cell) activation and maturation (CR2, MS4A1(CD20), TCLA1, BANK1, CD22, FDCSP), and signaling molecules of homing of immune cells to the gut (chemokines: CCL21, CXCL13, CCL19, and receptors: CCR7, CXCR5) and innate immunity (DUOX2), as illustrated in Figure 5. However, after stress, the gene expression of tryptase in the rectal mucosa was not significantly modified (Control: 1 [0.4–1.7]; Stress: 1 [0.4–2.2], fold change; p = 0.41) (Figure 5). Also, no significant changes were found in mRNA expression of the apical junctional proteins, JAM‐A, claudin 2, ZO1 and ZO3 (Table S5).
FIGURE 5.
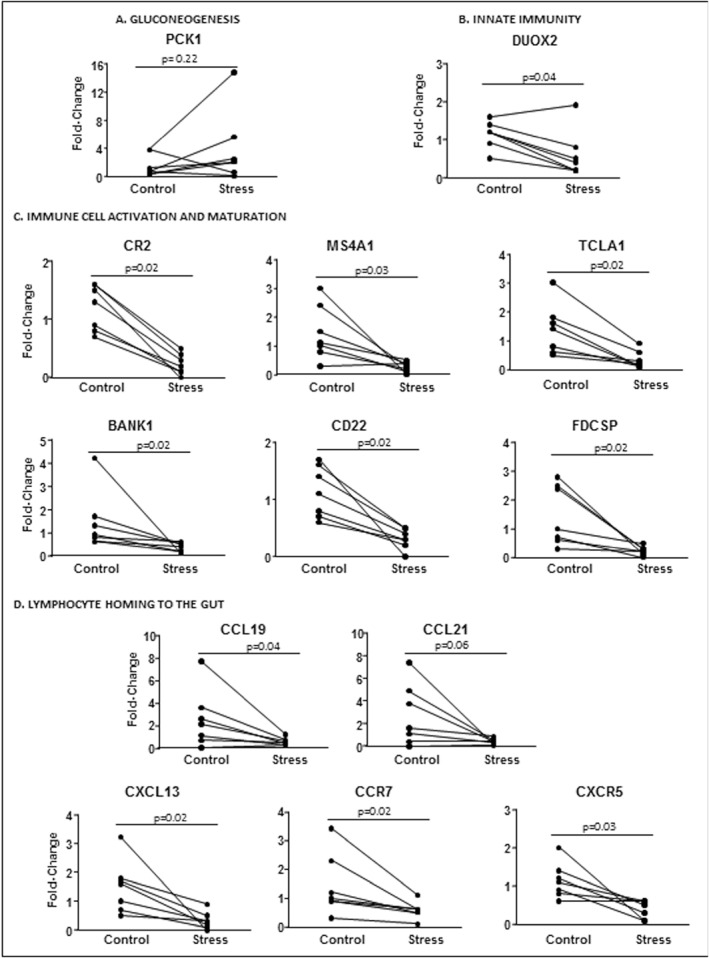
Validation of microarray data by quantitative real‐time polymerase chain reaction (Q‐RT–PCR) of the top regulated genes by experimental stress, grouped by biological function. Quantitative gene expression in the colonic mucosa of seven individuals with paired analysis, before and after stress, of (a) Gluconeogenesis. (b) Innate immunity. (c) Immune cell activation and maturation. (d) Lymphocyte homing to the gut. BANK1, B‐cell scaffold protein with ankyrin repeats 1; CCL19, C‐C motif chemokine ligand 19; CCL21, C‐C motif chemokine ligand 21; CCR7, C‐C motif chemokine receptor 7; CD22, CD22 molecule; CR2, Complement component 3d receptor 2; CXCL13, C‐X‐C motif chemokine ligand 13; CXCR5, C‐X‐C motif chemokine receptor 5; DUOX2, Dual oxidase 2; FDCSP, Follicular dendritic cell secreted protein; MS4A1, Membrane spanning 4‐domains A1; PCK1, Phosphenolpuruvate carboxy kinase 1; PPIA, Peptidylprolyl isomerase A (Cyclophyllin A); TCLA1, T‐cell leukemia/lymphoma 1A. The PPIA was used as a house‐keeping gene. p‐values are indicated for each analysis
Association between systemic and mucosal response to acute stress
Changes in mucosal function and/or immunity following stress were in some cases statistically correlated to systemic stress response. The increase in 51Cr‐EDTA permeability correlated to increased heart rate during stress (r = 0.52, p = 0.036; n = 13). Moreover, subjective stress during dichotomous listening (VAS) correlated with decrease in FDCSP gene expression (r = 0.93, p = 0.017; n = 6), and perceived stress during the 2 weeks preceding the experiment correlated with acute stress‐induced reduction in DUOX2 (r = 0.85, p = 0.023; n = 7).
DISCUSSION
In this study we show, for the first time, that mild acute laboratory stress increases paracellular permeability in rectal mucosa and impacts mucosal immunity. We studied healthy volunteers reporting normal background stress levels, using a dichotomous listening stress protocol. Dichotomous listening is known to induce mild psychological stress, with previously documented effects on several GI functions in humans, for example, absorption and secretion, 14 sensitivity and motility, 26 and mucosal blood flow, 17 but the effects of dichotomous listening on intestinal permeability have not previously been reported. During the stress session, significant but moderate increases in subjective (VAS) and objective (heart rate, BP) systemic stress parameters were found, which validated that the present experimental protocol induced psychological stress and suggests activation of the sympathetic‐adrenomedullary system. Additionally, mucosal expression of PCK‐1, a key enzyme in gluconeogenesis, was increased after stress, suggesting higher local energy requirements. Thus, the used methodology was relevant for studies of the effects of low‐grade acute stress on mucosal function.
Following dichotomous listening, all individuals had permeability values within the range found in healthy volunteers in line with our previous publications. 8 , 11 , 20 In other words, at a group level, there were limited differences between control and stress sessions regarding paracellular permeability and no changes in transcellular permeability. On the other hand, we did find clearly measurable changes in paracellular permeability using paired comparisons (10/11 subjects had increased permeability after stress). On the other hand, no significant changes were seen in TER during stress. This could relate to there being different regulation of permeability pathways, that is, the leak and the pore pathways, 27 with molecules being able to pass through the paracelllular pathway without the complete tight junction (TJ) network being open at the same time and thereby not affecting TER. The findings could also be a result of differences in mucosal TER between stress and control situation being relatively small in most subjects, and conventional TER measurements not being sensitive enough to fully detect differences between tissues. 28 Notably, systemic stress affected physiological variables related to barrier function: increased heart rate was correlated to the increase in paracellular permeability and perceived stress the weeks preceding the experiment correlated to reduction in DUOX2, an enzyme that plays a fundamental role in mucosal host defense against pathogens by the production of reactive oxygen species. Interestingly, mutations in DUOX2 have been identified in patients at very early onset of IBD. 29 Based on our findings, our model may be a useful approach to further identify mechanisms underlying stress‐mediated reduction in relevant innate defensive responses predisposing to GI disease. With changes in paracellular permeability, we had also expected changes in mRNA expression of proteins of the apical junctional complex. The lack of significant changes in JAM‐A, CLDN2, ZO1 and ZO3 does, however, not rule out changes in phosphorylation, in subunits, in localization etc. More studies will be needed to look further into the TJ‐related alterations by stress. Potentially, more sustained and/or more pronounced stress would be needed to show changes in mRNA expression of the TJ proteins. Also, the stress responsiveness of the TJ in the small intestinal and colorectal mucosa would need comparative studies to elucidate differences related to anatomical regions.
In the present study, using a broad gene expression analysis following mild psychological stress, alterations of rectal mucosal B‐cell immunity were demonstrated. During disease, the intestinal mucosa of both IBS and IBD show higher number of B cells and plasma cells and increased production of antibodies. 30 , 31 The importance of neuro‐immune interactions in the control of humoral immunity was recently highlighted, 32 and several studies have shown a link between stress and B cell responses. Previous studies of circulating lymphocytes in various types of acute stress models in humans, 33 , 34 including dichotomous listening, 17 have shown increased numbers and/or activity of natural killer (NK) cells and CD8+ T cells, together with a decrease in CD19+ B cells. At a mucosal level, stress in rodents induce decreased colonic secretion of IgA 35 and studies on college students showed decreased secretory IgA concentrations in saliva at examinations. 36 Together, these previous studies suggest that stress diminishes B‐cell activity. Our present data, from analyses of biopsies suggest rapid downregulation of rectal mucosal NFkB2 and B cell activity in response to mild acute stress, since 7 of 10 top down‐regulated genes are directly involved in B cell activation, signaling and migration (Table 2). Despite changes in mucosal immunity and barrier function, mRNA expression of mast cell tryptase was unchanged by stress in the present study. Several previous studies, in animal models 12 , 13 as well as in humans, 15 , 37 highlighted the importance of neuroimmune interaction involving corticotrpin‐releasing hormone (CRH), mast cells and eosinophils in the stress response of the mucosal barrier. Animal models of acute stress (e.g., water avoidance restraint stress, crowding stress, etc.) are different and stronger stressors than the mild experimental stress used in the present study. In addition, there are species differences in the neuro‐immune signaling in the colorectal mucosa related to CRH and mast cells. 8 In humans, small intestinal permeability was increased following psychological stress by public speech at examination via mechanisms involving CRH and mast cells, 15 whereas a milder stressor (anticipation of electroshock) had no effects on permeability. 15 We previously showed that CRH increases HRP‐uptake in colonic mucosa via mast cells, 8 , 37 while in the present study we found no significant changes in HRP permeability. No detailed analysis of CRH‐mast cell interaction was performed in our study, but the unaltered mRNA expression of tryptase following dichotomous listening, may imply mast cells not being a major player. Together, these findings may suggest differences in nerve‐mast cell interactions between the small intestine 15 and the colorectum in humans, as previously implied. 38 Another potential explanation is that, in the colorectal region, mild stress using dichotomous listening acts through different mechanisms than stronger stressors and with less pronounced effects on barrier function.
Even though our results are obtained from healthy individuals and most likely are part of a homeostatic response, the stress‐related decrease in expression of follicular dendritic cell secreted protein, that binds to activated B cells, regulating antibody production, 39 the receptors CCR7 and CXCR5, and respective ligands CCL19, CCL21 and CXCL13, may predispose the intestinal mucosa to deficient defensive activity, as those molecules play a key role in the homing of lymphocytes and dendritic cells. These immune alterations might, together with increased permeability, facilitate antigen penetration following acute stress and, thereby, favor mucosal inflammation in vulnerable individuals exposed to persistent stress.
Dichotomous listening is experimental and may result in other responses than real life stressors do, which is a limitation of studies using experimental stress protocols. It is, for example, well established that physical and psychological experimental stressors may affect the intestinal tract in different ways. 15 , 26 Vanuytsel et al. 15 also showed that the mucosal stress response was related to the systemic cortisol response, with increased small intestinal permeability only in individuals with elevated salivary cortisol. The authors did not report effects on the sympathetic‐adrenomedullary system which was activated in the present study as demonstrated by increased blood pressure and heart rate and recently shown to play an important role in modulating intestinal barrier function in mice. 40 Future studies to investigate mechanisms should, therefore, include assessment of the sympathetic‐adrenomedullary system as well as of the HPA‐axis during dichotomous listening.
The consequences of the humoral immune inhibition were not elucidated in our study, and we do not know if dichotomous listening reduced antibody production or the release of other defensive mediators in the intestinal mucosa. Further research is needed to better phenotype the mucosal immune response triggered by stress and to identify potential therapeutic targets at early stages of colorectal dysfunction. However, the fact that mild acute psychological stress leads to rapid changes in paracellular permeability and humoral immunity in healthy rectal mucosa lends further support to the importance of stress as a contributing factor in GI diseases in predisposed individuals.
In conclusion, we report, for the first time, that paracellular permeability was altered in rectal mucosa of healthy volunteers immediately following dichotomous listening stress. Additionally, indications of stress‐induced reduction in mucosal B cell‐activity were demonstrated. Further studies of the importance of humoral immunity in the early stress response of colorectal mucosa are therefore warranted to delineate mechanisms of stress‐mediated mucosal barrier dysfunction. Also, our model may be used in studies of patients with IBS and IBD to further define the role of stress in the initiation and/or perpetuation of these diseases.
CONFLICT OF INTEREST
The authors disclose no conflicts of interest to study participants and no financial arrangements with any company whose product figures in the submitted manuscript. Maria Vicario is an employee of Nestlé Research, Switzerland.
Supporting information
Supporting Information S1
ACKNOWLEDGMENTS
The authors acknowledge PM Olsson and Johan Hedström for designing the software used to control the dichotomous listening and control sessions. This work was supported by grants from the Swedish Research Council (VR‐Medicine and Health, 2014‐02537, 2017‐02475) (Johan D. Söderholm, Åsa V. Keita). Supported in part by Fondo de Investigación Sanitaria and CIBERehd, Instituto de Salud Carlos III, Subdirección General de Investigación Sanitaria, Ministerio de Economía y Competitividad: PI19/01643 (Maria Vicario); PI17/0190 (Javier Santos); Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas: CB06/04/0021 (Javier Santos & Maria Vicario). Futurum, Region Jönköping County 12406 and 11460, (Linda Gerdin, Mats Persborn) Medical Research Council of Southeast Sweden, 37841 (Mats Persborn, Linda Gerdin).
Gerdin L, González‐Castro AM, Ericson A‐C, Persborn M, Santos J, Walter SA, et al. Acute psychological stress increases paracellular permeability and modulates immune activity in rectal mucosa of healthy volunteers. United European Gastroenterol J. 2023;11(1):31–41. 10.1002/ueg2.12329
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Bradford K, Shih W, Videlock EJ, Presson AP, Naliboff BD, Mayer EA, et al. Association between early adverse life events and irritable bowel syndrome. Clin Gastroenterol Hepatol. 2012;10(4):385–90e1‐3. 10.1016/j.cgh.2011.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Parker CH, Naliboff BD, Shih W, Presson AP, Videlock EJ, Mayer EA, et al. Negative events during adulthood are associated with symptom severity and altered stress response in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2019;17(11):2245–52. 10.1016/j.cgh.2018.12.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Murray CD, Flynn J, Ratcliffe L, Jacyna MR, Kamm MA, Emmanuel AV. Effect of acute physical and psychological stress on gut autonomic innervation in irritable bowel syndrome. Gastroenterology. 2004;127(6):1695–703. 10.1053/j.gastro.2004.08.057 [DOI] [PubMed] [Google Scholar]
- 4. Eijsbouts C, Zheng T, Kennedy NA, et al. Genome‐wide analysis of 53,400 people with irritable bowel syndrome highlights shared genetic pathways with mood and anxiety disorders. Nat Genet. 2021;53:1543–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Levenstein S, Prantera C, Varvo V, Scribano ML, Andreoli A, Luzi C, et al. Stress and exacerbation in ulcerative colitis: a prospective study of patients enrolled in remission. Am J Gastroenterol. 2000;95(5):1213–20. 10.1111/j.1572-0241.2000.02012.x [DOI] [PubMed] [Google Scholar]
- 6. Melinder C, Hiyoshi A, Fall K, Halfvarson J, Montgomery S. Stress resilience and the risk of inflammatory bowel disease: a cohort study of men living in Sweden. BMJ Open. 2017;7(1):e014315. 10.1136/bmjopen-2016-014315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gitter AH, Wullstein F, Fromm M, Schulzke JD. Epithelial barrier defects in ulcerative colitis: characterization and quantification by electrophysiological imaging. Gastroenterology. 2001;121(6):1320–8. 10.1053/gast.2001.29694 [DOI] [PubMed] [Google Scholar]
- 8. Wallon C, Persborn M, Jonsson M, Wang A, Phan V, Lampinen M, et al. Eosinophils express muscarinic receptors and corticotropin‐releasing factor to disrupt the mucosal barrier in ulcerative colitis. Gastroenterology. 2011;140(5):1597–607. 10.1053/j.gastro.2011.01.042 [DOI] [PubMed] [Google Scholar]
- 9. Zeissig S, Burgel N, Gunzel D, Richter J, Mankertz J, Wahnschaffe U, et al. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn's disease. Gut. 2007;56(1):61–72. 10.1136/gut.2006.094375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Piche T, Barbara G, Aubert P, Bruley des Varannes S, Dainese R, Nano JL, et al. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut. 2009;58(2):196–201. 10.1136/gut.2007.140806 [DOI] [PubMed] [Google Scholar]
- 11. Bednarska O, Walter SA, Casado‐Bedmar M, Strom M, Salvo‐Romero E, Vicario M, et al. Vasoactive intestinal polypeptide and mast cells regulate increased passage of colonic bacteria in patients with irritable bowel syndrome. Gastroenterology. 2017;153(4):948–960e3. 10.1053/j.gastro.2017.06.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Soderholm JD, Yang PC, Ceponis P, Vohra A, Riddell R, Sherman PM, et al. Chronic stress induces mast cell‐dependent bacterial adherence and initiates mucosal inflammation in rat intestine. Gastroenterology. 2002;123(4):1099–108. 10.1053/gast.2002.36019 [DOI] [PubMed] [Google Scholar]
- 13. Vicario M, Guilarte M, Alonso C, Yang P, Martinez C, Ramos L, et al. Chronological assessment of mast cell‐mediated gut dysfunction and mucosal inflammation in a rat model of chronic psychosocial stress. Brain Behav Immun. 2010;24(7):1166–75. 10.1016/j.bbi.2010.06.002 [DOI] [PubMed] [Google Scholar]
- 14. Barclay GR, Turnberg LA. Effect of psychological stress on salt and water transport in the human jejunum. Gastroenterology. 1987;93(1):91–7. 10.1016/0016-5085(87)90319-2 [DOI] [PubMed] [Google Scholar]
- 15. Vanuytsel T, van Wanrooy S, Vanheel H, Vanormelingen C, Verschueren S, Houben E, et al. Psychological stress and corticotropin‐releasing hormone increase intestinal permeability in humans by a mast cell‐dependent mechanism. Gut. 2014;63(8):1293–9. 10.1136/gutjnl-2013-305690 [DOI] [PubMed] [Google Scholar]
- 16. Alonso C, Guilarte M, Vicario M, Ramos L, Ramadan Z, Antolin M, et al. Maladaptive intestinal epithelial responses to life stress may predispose healthy women to gut mucosal inflammation. Gastroenterology. 2008;135(1):163–172e1. 10.1053/j.gastro.2008.03.036 [DOI] [PubMed] [Google Scholar]
- 17. Mawdsley JE, Macey MG, Feakins RM, Langmead L, Rampton DS. The effect of acute psychologic stress on systemic and rectal mucosal measures of inflammation in ulcerative colitis. Gastroenterology. 2006;131(2):410–9. 10.1053/j.gastro.2006.05.017 [DOI] [PubMed] [Google Scholar]
- 18. Marsland AL, Herbert TB, Muldoon MF, Bachen EA, Patterson S, Cohen S, et al. Lymphocyte subset redistribution during acute laboratory stress in young adults: mediating effects of hemoconcentration. Health Psychol. 1997;16(4):341–8. 10.1037/0278-6133.16.4.341 [DOI] [PubMed] [Google Scholar]
- 19. McRae S, Younger K, Thompson DG, Wingate DL. Sustained mental stress alters human jejunal motor activity. Gut. 1982;23(5):404–9. 10.1136/gut.23.5.404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wallon C, Braaf Y, Wolving M, Olaison G, Soderholm JD. Endoscopic biopsies in Ussing chambers evaluated for studies of macromolecular permeability in the human colon. Scand J Gastroenterol. 2005;40(5):586–95. 10.1080/00365520510012235 [DOI] [PubMed] [Google Scholar]
- 21. Holmes THR, Rahe RH. The social readjustment rating scale. J Psychosom Res. 1967;11(2):213–18. 10.1016/0022-3999(67)90010-4 [DOI] [PubMed] [Google Scholar]
- 22. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–96. 10.2307/2136404 [DOI] [PubMed] [Google Scholar]
- 23. Gaudry E, Vagg P, Spielberger CD. Validation of the state‐trait distinction in anxiety research. Multivariate Behav Res. 1975;10(3):331–41. 10.1207/s15327906mbr1003_6 [DOI] [PubMed] [Google Scholar]
- 24. Martinez C, Vicario M, Ramos L, Lobo B, Mosquera JL, Alonso C, et al. The jejunum of diarrhea‐predominant irritable bowel syndrome shows molecular alterations in the tight junction signaling pathway that are associated with mucosal pathobiology and clinical manifestations. Am J Gastroenterol. 2012;107(5):736–46. 10.1038/ajg.2011.472 [DOI] [PubMed] [Google Scholar]
- 25. Schmittgen TD, Livak KJ. Analyzing real‐time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–8. 10.1038/nprot.2008.73 [DOI] [PubMed] [Google Scholar]
- 26. Rao SS, Hatfield RA, Suls JM, Chamberlain MJ. Psychological and physical stress induce differential effects on human colonic motility. Am J Gastroenterol. 1998;93(6):985–90. 10.1111/j.1572-0241.1998.00293.x [DOI] [PubMed] [Google Scholar]
- 27. Shen L, Weber CR, Raleigh DR, Yu D, Turner JR. Tight junction pore and leak pathways: a dynamic duo. Annu Rev Physiol. 2011;73(1):283–309. 10.1146/annurev-physiol-012110-142150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Keita AV, Gullberg E, Ericson AC, Salim SY, Wallon C, Kald A, et al. Characterization of antigen and bacterial transport in the follicle‐associated epithelium of human ileum. Lab Invest. 2006;86(5):504–16. 10.1038/labinvest.3700397 [DOI] [PubMed] [Google Scholar]
- 29. Hayes P, Dhillon S, O'Neill K, Thoeni C, Hui KY, Elkadri A, et al. Defects in NADPH oxidase genes NOX1 and DUOX2 in very early onset inflammatory bowel disease. Cell Mol Gastroenterol Hepatol. 2015;1:489–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brandtzaeg P, Carlsen HS, Halstensen TS. The B‐cell system in inflammatory bowel disease. Adv Exp Med Biol. 2006;579:149–67. [DOI] [PubMed] [Google Scholar]
- 31. Vicario M, Gonzalez‐Castro AM, Martinez C, Lobo B, Pigrau M, Guilarte M, et al. Increased humoral immunity in the jejunum of diarrhoea‐predominant irritable bowel syndrome associated with clinical manifestations. Gut. 2015;64(9):1379–88. 10.1136/gutjnl-2013-306236 [DOI] [PubMed] [Google Scholar]
- 32. Zhang X, Lei B, Yuan Y, Zhang L, Hu L, Jin S, et al. Brain control of humoral immune responses amenable to behavioural modulation. Nature. 2020;581(7807):204–8. 10.1038/s41586-020-2235-7 [DOI] [PubMed] [Google Scholar]
- 33. McGregor BA, Murphy KM, Albano DL, Ceballos RM. Stress, cortisol, and B lymphocytes: a novel approach to understanding academic stress and immune function. Stress. 2016;19(2):185–91. 10.3109/10253890.2015.1127913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huang CJ, Webb HE, Garten RS, Kamimori GH, Acevedo EO. Psychological stress during exercise: lymphocyte subset redistribution in firefighters. Physiol Behav. 2010;101(3):320–6. 10.1016/j.physbeh.2010.05.018 [DOI] [PubMed] [Google Scholar]
- 35. Zoppi S, Madrigal JL, Perez‐Nievas BG, Marin‐Jimenez I, Caso JR, Alou L, et al. Endogenous cannabinoid system regulates intestinal barrier function in vivo through cannabinoid type 1 receptor activation. Am J Physiol Gastrointest Liver Physiol. 2012;302(5):G565–71. 10.1152/ajpgi.00158.2011 [DOI] [PubMed] [Google Scholar]
- 36. Jemmott JB, 3rd , Magloire K. Academic stress, social support, and secretory immunoglobulin A. J Pers Soc Psychol. 1988;55(5):803–10. 10.1037/0022-3514.55.5.803 [DOI] [PubMed] [Google Scholar]
- 37. Wallon C, Yang PC, Keita AV, Ericson AC, McKay DM, Sherman PM, et al. Corticotropin‐releasing hormone (CRH) regulates macromolecular permeability via mast cells in normal human colonic biopsies in vitro. Gut. 2008;57(1):50–8. 10.1136/gut.2006.117549 [DOI] [PubMed] [Google Scholar]
- 38. Keita AV, Carlsson AH, Cigehn M, Ericson AC, McKay DM, Soderholm JD. Vasoactive intestinal polypeptide regulates barrier function via mast cells in human intestinal follicle‐associated epithelium and during stress in rats. Neurogastroenterol Motil. 2013;25(6):e406–17. 10.1111/nmo.12127 [DOI] [PubMed] [Google Scholar]
- 39. Al‐Alwan M, Du Q, Hou S, Nashed B, Fan Y, Yang X, et al. Follicular dendritic cell secreted protein (FDC‐SP) regulates germinal center and antibody responses. J Immunol. 2007;178(12):7859–67. 10.4049/jimmunol.178.12.7859 [DOI] [PubMed] [Google Scholar]
- 40. Mallesh S, Ten Hove AS, Schneider R, Schneiker B, Efferz P, Kalff JC, et al. Sympathetic innervation modulates mucosal immune homeostasis and epithelial host defense. Cells. 2022;11(16):2606. 10.3390/cells11162606 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


