Abstract
Background
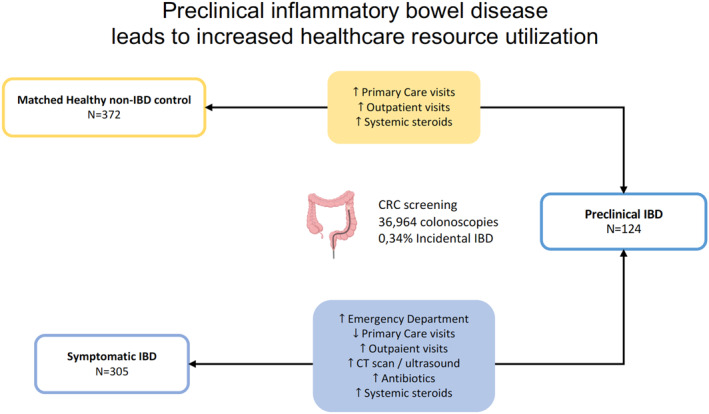
Previous data support that the inflammatory process underlying ulcerative colitis (UC) and Crohn's disease (CD) can start years before the diagnosis. The aim of this study was to determine if patients with an incidental diagnosis of UC or CD demonstrate an increase in healthcare utilization in the years preceding the symptomatic onset of the disease.
Methods
We performed a multicenter, retrospective, hospital‐based, case‐control study. Patients with an incidental diagnosis of UC or CD during the colorectal cancer screening program at 9 hospitals were included. Cases were matched 1:3 and compared separately with two control populations: one including healthy non‐IBD subjects adjusted by gender, age, and date, excluding those with visits to Gastroenterology; and a second control cohort of UC/CD patients with symptomatic onset.
Results
A total of 124 patients with preclinical inflammatory bowel disease (IBD) were included (87 UC, 30 CD, 7 IBD unclassified; median age 56 years). Patients with preclinical IBD showed an increase in the number of visits to Primary Care up to 3 and 5 years before diagnosis (aIRR 1.59, 95% CI [1.37–1.86], p = 0.001; aIRR 1.43, 95% CI [1.24–1.67], p = 0.01) and more frequent use of steroids (aOR 2.84, 95% CI [1.21–6.69], p = 0.03; aOR 2.25, 95% CI [1.06–4.79], p = 0.04) compared to matched non‐IBD healthy controls, respectively. In contrast, patients with a symptomatic onset visited Primary Care less frequently, but they had an increase in the number of visits to Emergency Department, specialist care, sick‐leaves, CT/ultrasound examinations, and use of antibiotics or systemic steroids.
Conclusions
There is an increased need for medical assistance and use of systemic steroids during the presymptomatic phase of IBD. These results will help in establishing new tools for early identification of IBD in the future.
Keywords: Crohn's disease, diagnostic tests, early, primary care, ulcerative colitis
Key summary.
Established knowledge
The inflammatory process associated with inflammatory bowel disease (IBD) precedes the diagnosis, during the so‐called preclinical period.
A range of protein signatures, fecal markers, mucosal barrier function abnormalities, and antimicrobial antibodies have been described during this period.
Significant and/or new findings of this study
The subclinical inflammatory process preceding the onset of IBD is associated to an increase in the number of visits to Primary Care and to specialized care.
Individuals with preclinical IBD have more frequent use of systemic steroids before diagnosis.
INTRODUCTION
Ulcerative colitis (UC) and Crohn's disease (CD) are two chronic and immune‐mediated conditions that lead to an uncontrolled inflammatory process in the gut, and they are included under the term inflammatory bowel disease (IBD). 1 The pathophysiology of both disorders is still not fully understood, but it is considered that these lesions are secondary to a dysregulation of the immune system arising in predisposed individuals, where some environmental factors including smoking habits, breastfeeding, or dietary patterns, among others, play a crucial role. 2 Their timing and exact magnitude of their effect are still unknown, despite the clear influence of some of them on the natural history of the disease.
Recent data supports that the inflammatory process associated with IBD precedes the diagnosis, during the so‐called preclinical period. 3 Here, the effect of triggering factors would lead to microscopic infiltration of the mucosa, followed by macroscopic damage, gastrointestinal symptoms, and the diagnosis at the end of this process. 4 A wide range of alterations on protein signatures, 5 fecal markers, 6 mucosal barrier function, 7 and antimicrobial antibodies 8 , 9 have been already described during this period. Recently, it has also been observed that these patients have an increase on the healthcare assistance and societal costs that precedes the diagnosis. 10 , 11
The potential impact on the prognosis by reducing the diagnostic delay has increased the interest on the preclinical period, a phase where significant advances have been already made in other immune‐mediated diseases. 12 , 13 , 14 Colorectal cancer screening programs have posed the opportunity to perform a high number of endoscopic examinations on otherwise asymptomatic subjects, with recent reports describing case series where subclinical endoscopic inflammatory findings can be identified and associated with the later onset of symptoms after several months to years. 15 , 16 Thus, research on this subgroup of patients has the potential to unveil significant findings that can lead to actions towards the prevention of overt IBD.
Hence, the primary aim of our study was to determine if the presence of subclinical endoscopic lesions are associated to an increase on healthcare utilization in the years preceding the symptomatic onset of the disease.
MATERIALS AND METHODS
We performed a multicentric, retrospective, hospital‐based, case‐control study at 9 hospitals in the Basque Country region (Spain). The Basque Health Service (Osakidetza) provides healthcare to all citizens and residents (2,177,270 individuals) within the Basque Country (Spain), including a personal identification number. This number was used to retrieve clinical information from each participant in the study.
All asymptomatic subjects with a diagnosis of UC or CD during the colorectal cancer screening program between October 2010 and January 2021 were included. The Basque colorectal cancer screening program invites all persons between 50 and 69 years to perform a fecal occult blood test, and this is followed by a complete colonoscopy in those with a positive result. The so‐called preclinical cohort included all patients in whom diagnosis of IBD was established by a combination clinical data, requiring the presence of endoscopic findings suggestive of IBD with further confirmation by histology, following the recommendations from the European Crohn's and Colitis Organization 17 , 18 and using the same criteria as in our previous reports. 4 , 16 Moreover, all subjects with abnormal findings suggestive of IBD in this context are referred to the Gastroenterology clinic, so alternative diagnosis were ruled out. We excluded patients with acute inflammatory infiltrate with no signs of chronicity, presence any gastrointestinal pathogen, previous diagnosis of microscopic colitis or gastrointestinal symptoms suggestive of IBD before the index endoscopic examination. Subjects with missing data were not considered in the analyses. In the current study, we have included all patients described in our initial report 16 and this cohort has been prospectively enriched with all new cases fulfilling the inclusion criteria since then. The follow‐up period has been updated and described until the last visit available in all subjects.
Cases were matched 1:3 with two control populations. First, healthy non‐IBD controls from the same region and hospital, adjusted by gender, age and date, excluding individuals with any visit to Gastroenterology Department. A second control population included all consecutive patients with a new diagnosis of IBD between January 2018 and January 2020 that were evaluated at Hospital Universitario de Galdakao after the symptomatic onset of the disease—described elsewhere 11 —in order to provide a more detailed overview of the years preceding the diagnosis, starting from the presence of subclinical inflammation until the onset of symptoms.
The primary endpoints included the number of outpatient visits to specialist care, Primary Care, or to the Emergency Department, and also hospital admissions (defined as a stay 24 h at the hospital facilities), radiological examinations (ultrasound [US], computed tomography [CT] scan, magnetic resonance imaging [MRI]), all‐cause sick‐leaves, and the prescription of antibiotics (Anatomical Therapeutic Chemical [ATC] code J01) or systemic steroids (ATC code H02 A) up to 5 years before the diagnosis. This information was obtained from the administrative data that is automatically registered and linked to the electronic health records of each patient (Osakidetza Business Intelligence), and this was retrieved for each claim during the study period. All drugs must be prescribed through an electronic application (Presbide), and this grants obtaining information on the type of drug used, the start date of the prescription and the adherence. Hence, all data regarding the main outcome was obtained from the administrative database of the electronic health records from each participant. The same tools automatically provide a range of scores including the Charlson comorbidity index, 19 and this information was compiled from all three cohorts.
The Ethics Committee from the Basque Country approved the final version of the protocol (PI2021195, 10/Nov/2021). Due to the study design informed consent was not mandatory, and it was requested from patients on regular follow‐up.
Statistical analysis
Descriptive statistics were used, followed by chi‐square tests and a multivariable hurdle or negative binomial regression model, adjusted by smoking habits, and Charlson comorbidity index, 19 in order to perform a per year analysis. The multivariate models comparing preclinical IBD and symptomatic patients was adjusted for gender, age at diagnosis, smoking habits, and Charlson index. All the analysis included also an evaluation of the information on each outcome combined up to 3 and 5 years, excluding the 12 months prior to diagnosis in order to avoid the influence of the diagnostic workup. All p‐values <0.05 were considered as statistically significant.
RESULTS
A total of 124 patients with preclinical IBD were identified (Figure 1). This cohort included 87 UC (70%), 30 CD (24%), and 7 IBD unclassified (6%) patients, with a median age of 56 years (interquartile range [IQR], 53–62), 44% of them were female, and 35% former smokers. This cohort was compared to 372 matched healthy non‐IBD controls and 305 IBD patients with a symptomatic debut of the disease. Baseline characteristics including comorbidities are summarized in Table 1. Notably, a higher proportion of patients with preclinical IBD were former smokers, had prior cerebrovascular disease or history of any type of malignancy. According to the Charlson comorbidity index, more patients in this subgroup had a score 2 compared to non‐IBD controls. Subjects with symptomatic onset of IBD showed certain baseline differences compared to the preclinical cohort.
FIGURE 1.
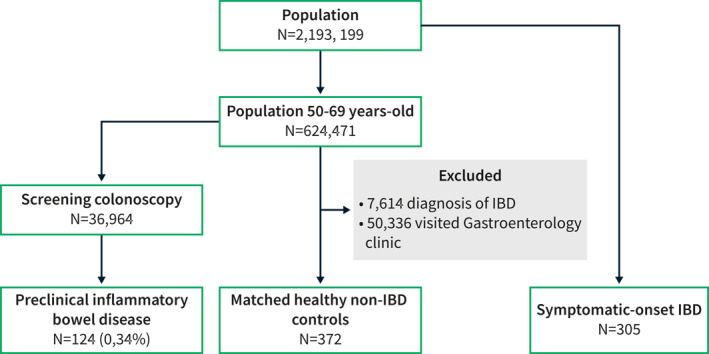
Flowchart of patients included in the study
TABLE 1.
Main characteristics of patients and both control populations
| Cases | Controls | ||
|---|---|---|---|
| Preclinical IBD | Healthy non‐IBD controls a | Symptomatic IBD | |
| N = 124 | N = 372 | N = 305 | |
| Sex, male | 70 (56) | 210 (56) | 142 (46) |
| N (%) | |||
| Age, years | 57 (5.6) | 57 (5.6) | 49 (15.7) |
| Mean (SD) | |||
| Ulcerative colitis, N (%) | 87 (70) | ‐ | 160 (52) |
| Crohn's disease, N (%) | 30 24 | ‐ | 145 (48) |
| IBD‐U, N (%) | 7 6 | ‐ | 0 |
| Smoking habits, N (%) | |||
| Former smoker | 43 (35) | 89 24 | 66 22 |
| No smoker | 32 26 | 126 (34) | 168 (55) |
| Active smoker | 11 9 | 48 13 | 52 17 |
| Extraintestinal manifestations before index date, N (%) | 3 (2%) | 11 (3%) | 18 (6%) |
| Charlson comorbidity index at diagnosis | 0.71 (1.11) | 0.53 (1.02) | 0.37 (0.82) |
| Mean (SD) | |||
| Charlson comorbidity index at diagnosis | |||
| 0 | 79 (64) | 253 (68) | 229 (75) |
| 1 | 16 13 | 74 20 | 55 18 |
| 2 | 29 23 | 45 12 | 21 7 |
Abbreviations: IBD, inflammatory bowel disease; IBD‐U, inflammatory bowel disease unclassified; SD, standard deviation.
Matched by sex, age and date of diagnosis.
Preclinical IBD versus healthy non‐IBD controls
The main results comparing the preclinical IBD cohort with the matched healthy non‐IBD controls are summarized in Table 2 and Figure 2. Patients demonstrated an increase in the number of visits to Primary Care during the combined periods including 3 and 5 years before the diagnosis (adjusted incidence rate ratio [aIRR] 1.59, 95% confidence interval [CI] [1.37–1.86], p = 0.001; aIRR 1.43, 95% CI [1.24–1.67], p = 0.01; respectively). This difference was also observed in the comparison of visits to specialized care (adjusted odds ratio [aOR] 62.74, 95% CI [26.10–151.41], p < 0.0001; aOR 66.90, 95% CI [28.16–158.97], p < 0.001; 3 and 5 years before the diagnosis, respectively). In contrast, patients underwent US and/or CT scan less frequently in the preceding 5‐year period (aIRR 0.53, 95% CI [0.30–0.93], p = 0.03), but no differences were observed when considering the 3‐year interval. We found no differences in the remaining resources including the number of hospital admissions, Emergency Department visits, number of MRI examinations, sick‐leaves, and the prescriptions of antibiotics (Table 2).
TABLE 2.
Comparison of the main outcomes between preclinical inflammatory bowel disease (IBD) and the matched healthy non‐IBD cohort
| Years prior to diagnosis (grouped) | aOR (95% CI) | aIRR (95% CI) | |
|---|---|---|---|
| Hospital admission | −3 | 1.32 (0.77–2.26) | 0.96 (0.46–2.03) |
| −5 | 1.58 (0.97–2.58) | 0.75 (0.29–1.94) | |
| Emergency department | −3 | 0.85 (0.55–1.33) | 0.87 (0.41–1.85) |
| −5 | 0.73 (0.47–1.12) | 0.91 (0.50–1.66) | |
| Primary care | −3 | − | 1.59 (1.37–1.86) |
| −5 | − | 1.43 (1.24–1.67) | |
| Outpatient visits | −3 | 62.74 (26.10–151.41) | − |
| −5 | 66.90 (28.16–158.97) | − | |
| Sick‐leaves | −3 | 1.00 (0.64–1.58) | 1.24 (0.69–2.22) |
| −5 | 0.90 (0.60–1.37) | 1.11 (0.69–1.80) | |
| CT scan/ultrasound | −3 | 1.07 (0.67–1.70) | 0.51 (0.26–1.02) |
| −5 | 1.24 (0.80–1.91) | 0.53 (0.30–0.93) | |
| MRI | −3 | 0.73 (0.39–1.36) | 1.27 (0.36–4.52) |
| −5 | 0.62 (0.34–1.13) | 1.10 (0.40–3.02) | |
| Antibiotics | −3 | 0.96 (0.59–1.56) | 1.03 (0.62–1.73) |
| −5 | 0.92 (0.57–1.50) | 1.14 (0.70–1.85) | |
| Systemic steroids | −3 | 2.84 (1.21–6.69) | 0.49 (0.17–1.46) |
| −5 | 2.25 (1.06–4.79) | 0.51 (0.18–1.45) |
Note: Multivariate models adjusted for gender, age at diagnosis, smoking habits, and Charlson index. Bold values were suggested by reviewers to highlight statistically significant results.
Abbreviations: aIRR, adjusted incidence rate ratio; CI, confidence interval; CT, computed tomography; IaOR, adjusted odds ratio; MRI, magnetic resonance imaging.
FIGURE 2.
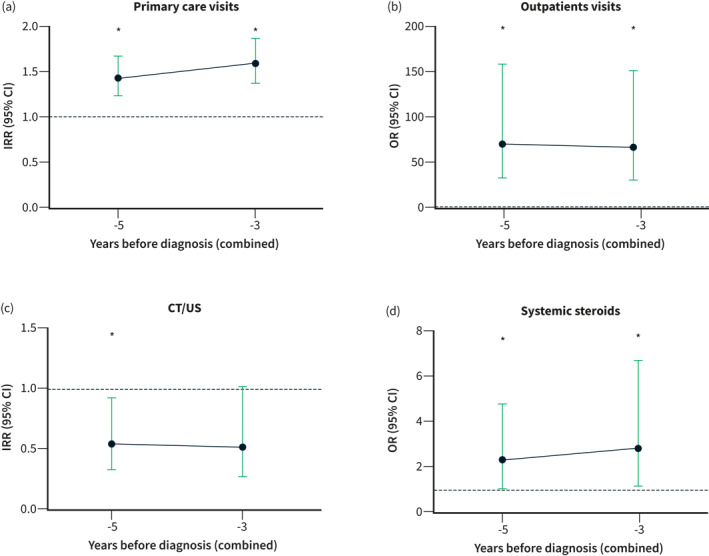
Patients with an incidental diagnosis of inflammatory bowel disease (IBD) demonstrate a higher probability of attending to Primary Care consultations (a), specialist care visits (b), and receiving systemic steroids (d), while radiological examinations (CT/US) were less frequently used 5 years before the diagnosis as compared to healthy non‐IBD controls (c). IRR: incidence rate ratio, OR: odds ratio.
A total of 97 patients (78%) with preclinical IBD received medical therapy at some point after diagnosis. Aminosalicylates was the most frequently prescribed treatment (77% oral and 42% topical), and therapy was usually initiated while patients were asymptomatic (73%). A total of 72 patients (58%) developed symptomatic disease after a median follow‐up of 87.5 months (IQR, 58–111). These symptoms started a median of 9 months (IQR, 1–30) after the index colonoscopy.
Use of steroids
We also observed that systemic steroids were more frequently prescribed to patients during the preclinical period compared to healthy non‐IBD controls (aOR 2.84, 95% CI [1.21–6.69], p = 0.03; aOR 2.25, 95% CI [1.06–4.79], p = 0.04; 3 and 5 years before the diagnosis, respectively; Figure 2d). We performed a more detailed analysis in order to identify additional factors associated with its use. A total of 13 patients (10.5%) received systemic steroids before the incidental diagnosis of IBD (Supplementary Table S1 and S2). The most frequently prescribed drugs were prednisone (69%) and dexamethasone (23%). They were usually prescribed by Primary Care physicians (62%), at the Emergency Department, or by rheumatologists (15% each).
We also explored the potential relationship between receiving steroids and the subsequent risk of developing symptoms during follow‐up. Patients with UC receiving steroids during the presymptomatic period showed a trend, although not statistically significant, towards reduced probability of developing symptomatic disease (25% vs. 62%, p = 0.06; aOR 0.21, 95% CI [0.038–1.087]; log‐rank = 0.9), and patients with preclinical CD did not show a difference on this risk (100% vs. 65%, p = 0.67; aOR 1.07, 95% CI [0.94–1.21]; log‐rank = 0.51).
Preclinical IBD versus symptomatic IBD controls
We also compared the resources utilization in the incidental IBD cohort with a cohort in whom the final diagnosis was obtained after the development of gastrointestinal symptoms during the same period (n = 305, Table 1).
The multivariable analysis adjusted by gender, age at diagnosis, smoking habits, and Charlson index revealed significant differences in the primary endpoints between both groups. The main results are shown on Table 3 and Figure 3. Patients with symptomatic IBD had an increased number of visits to specialist care, Emergency Department, CT/US examinations, and prescriptions of both antibiotics and systemic steroids during the whole study period (3 and 5 years grouped analysis). Meanwhile, sick‐leaves were only increased in the combined analysis including the 5‐year period before diagnosis. In contrast, patients with a symptomatic debut of IBD had a reduced number of visits to Primary Care during the presymptomatic period. No statistically significant differences in the number of hospital admissions or MRI procedures were found.
TABLE 3.
Grouped comparison of the 3 and 5 years prior to diagnosis in symptomatic patients with inflammatory bowel disease (IBD) compared to the preclinical IBD cohort
| Years prior to diagnosis (grouped) | aOR (95% CI) | aIRR (95% CI) | |
|---|---|---|---|
| Hospital admission | −3 | 1.50 (0.73–3.11) | 1.01 (0.32–3.21) |
| −5 | 1.60 (0.88–2.90) | 2.52 (0.66–9.64) | |
| Emergency department | −3 | 3.27 (1.87–5.70) | 0.97 (0.36–2.60) |
| −5 | 5.11 (2.99–8.74) | 1.08 (0.55–2.15) | |
| Primary care | −3 | − | 0.53 (0.37–0.76) |
| −5 | − | 0.54 (0.36–0.78) | |
| Outpatient visits | −3 | − | 28.98 (19.84–42.37) |
| −5 | − | 38.42 (26.75–55.26) | |
| Sick‐leaves | −3 | 0.95 (0.54–1.67) | 1.44 (0.75–2.76) |
| −5 | 0.81 (0.43–1.36) | 1.71 (1.02–2.85) | |
| CT scan/ultrasound | −3 | 1.23 (0.66–2.30) | 4.28 (1.18–15.58) |
| −5 | 0.79 (0.46–1.38) | 2.93 (1.32–6.51) | |
| MRI | −3 | 2.00 (0.90–4.48) | 1.48 (0.58–3.78) |
| −5 | 2.10 (0.97–4.53) | 1.17 (0.49–2.84) | |
| Antibiotics | −3 | 9.07 (4.82–17.04) | 3.89 (1.39–10.89) |
| −5 | 11.46 (6.00–21.88) | 1.92 (0.79–4.64) | |
| Systemic steroids | −3 | 4.40 (1.67–11.52) | 7.82 (0.35–175.56) |
| −5 | 4.08 (1.74–9.55) | 16.65 (0.26–171.86) |
Note: Multivariate models adjusted for gender, age at diagnosis, smoking habits, and Charlson index. Bold values were suggested by reviewers to highlight statistically significant results.
Abbreviations: aIRR, adjusted incidence rate ratio; aOR, adjusted odds ratio; CI, confidence interval; CT, computed tomography; MRI, magnetic resonance imaging.
FIGURE 3.
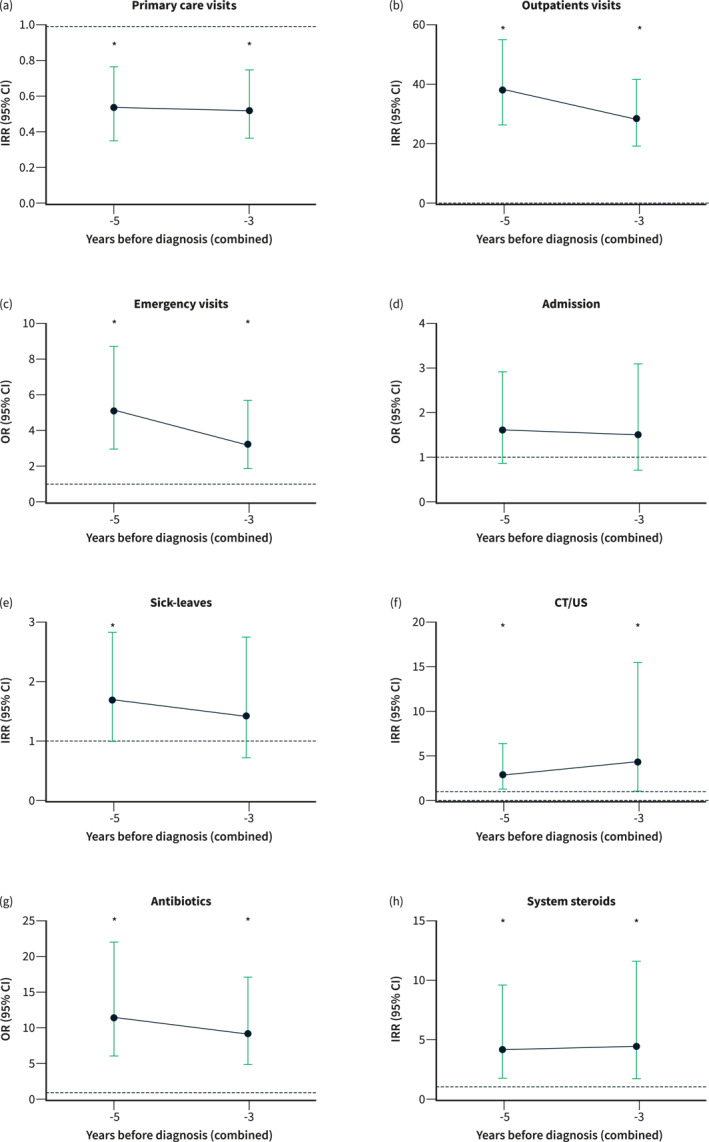
Patients with a symptomatic debut of inflammatory bowel disease (IBD) had less frequent visits to Primary Care (a), while they showed increased use of outpatient visits to specialist care (b), Emergency Department visits (c), CT/US procedures (f), and prescriptions of antibiotics (g) or systemic steroids (h) during the whole study period. Sick‐leaves were only increased in the combined 5‐years grouped analysis (e). No differences in hospital admissions were found (d). IRR: incidence rate ratio, OR: odds ratio.
Remarkably, both symptomatic UC and CD cohorts had a consistent increase in the number of outpatient visits to specialist care, Emergency Department, along with an increase in the prescriptions of systemic steroids and antibiotics during the whole observation period. We also observed that the estimates were significantly increased only in symptomatic UC patients at some point for MRI, CT/US, and sick‐leaves during the study period.
DISCUSSION
Patients with IBD have an increased need of medical assistance in the years prior to the diagnosis, 10 , 11 and our results show that this is observed during the presymptomatic phase of the disease. Our findings also confirm that subclinical endoscopic lesions are present years before the symptomatic onset and the diagnosis of the disease, and they are associated to an increased number of consultations on Primary Care or specialist care and more frequent use of systemic steroids. In addition, we also observed that as the disease progresses, the medical assistance shifts towards an increased need of specialist care and Emergency Department visits, linked to more radiological examinations, sick‐leaves and prescriptions of certain drugs.
The incidental diagnosis of IBD in subjects undergoing colorectal cancer screening examinations has been previously described. 4 , 16 Together with studies on first‐degree relatives and biobank analysis, this is a population where the initial mechanisms leading to IBD can be identified. 3 While prospective studies on first‐degree relatives are of great importance, studies on this subgroup usually lack the correlation with endoscopic or histologic findings. This is a fundamental aspect of our cohort, where the mucosal inflammatory process was confirmed in all patients. Furthermore, the follow‐up over a median of 7 years provides useful information about the long‐term risk of progression to symptomatic disease in approximately two thirds of patients.
Previous reports have described that patients with IBD show increased costs up to 10 years before diagnosis. 10 Data from this Danish population‐based study demonstrated that the average costs increase progressively while work productivity decreases until the diagnosis with a peak in the year before diagnosis. Similar findings were also observed in another hospital‐based study, where items linked to the increased medical assistance during the prediagnostic period were identified. 11 The present analysis has provided further details, as asymptomatic subjects in whom mucosal damage has been detected also demonstrate an increased need of medical assistance and drug prescriptions. Thus, our results support the presence of undiagnosed bowel lesions at the initial stages of IBD, and this has a significant impact as they lead to healthcare resources utilization. This is expected to be followed by the expansion of the inflammatory process and conclude with the onset of gastrointestinal symptoms. Therefore, our findings provide a time frame and some clues about where we should focus future strategies that will prompt early identification of these patients.
Our results are supported by the recent observation that certain immunological and metabolic pathways are already altered years before the diagnosis of the disease. 5 , 8 , 20 We can hypothesize that the preliminary disturbances would lead to subtle or unspecific symptoms, or even extraintestinal manifestations, for an indeterminate period of time. The latter is supported by the prescription of steroids by rheumatologists, probably reflecting the onset of articular comorbidities before the initiation of the gastrointestinal disease. Furthermore, the use of steroids during this period may interfere during the progression towards the full spectrum of IBD. In our study, these drugs were prescribed during the so‐called disease initiation and expansion stages, 3 so they could have the potential of modifying certain immunological disturbances at this point. This hypothesis is supported by the evidence from other immune‐mediated diseases like rheumatoid arthritis, where the use of steroids in patients with undifferentiated arthritis or very early arthritis can reduce the risk of developing the full spectrum of the disease. 21 , 22 This has been also explored in secondary‐prevention strategies were methotrexate 23 or abatacept 24 have been prescribed. Our findings provide preliminary data of a possible benefit of this strategy in IBD, but this question will need to be explored in dedicated trials.
Research on biomarkers associated with IBD phenotype or disease activity have been increasing in recent years, but its role in the very early stages and the possible prediction of the future course of the disease is still controversial. 25 Our findings suggest that Primary Care providers should be one of the main targets for the risk stratification of patients at higher risk of developing IBD. In this setting, fecal calprotectin or other non‐invasive biomarkers will have the opportunity to individualize the risk of disease‐modification strategies. Further, these subjects would benefit from those initiatives that ensure appropriate referral processes and short waiting times in order to initiate adequate and tailored management, but this aspect seems to be still insufficient in some regions. 26 , 27
This study has some limitations that should be considered. First, this is a retrospective registry‐based study using administrative data from a single region, that may limit our ability of describing more in detail certain outcomes like steroid use and generalizing our results. Also different approaches during the diagnostic process at each center could have included some bias, but all centers are included under the Basque Health Service and follow the same Spanish and European guidelines. Similarly, the absence of information on biomarkers, disease extension, behaviour, or the indication for antibiotic therapy might be a shortcoming. Second, there are no clear criteria for stablishing a definitive diagnosis of IBD in special situations like in our cohort, and our study focused on patients with a disease onset over 50 years old. Third, although the cohorts might have certain differences regarding the disease characteristics, this study focused on the diagnostic process from 9 hospitals in our region, so this is expected to have a small impact on our findings. In addition, the evaluation of costs may have provided further information. Besides, our approach allowed us to perform a description of multiple resources with a wide overview of the possible needs of this specific subgroup of patients in a real‐world setting.
In conclusion, patients with subclinical IBD have an increase use of healthcare resources, that is most remarkable on Primary Care and specialized consultations. This is associated with a more frequent use of systemic steroids, that may halt the expansion of the inflammatory process. This phase is followed by a progressive increased need of medical assistance in the 5 years prior to diagnosis, thus providing some clues that can aid in the early identification of these patients and introducing an opportunity to apply disease‐modification interventions that will have the potential of improve the prognosis of IBD.
AUTHOR CONTRIBUTIONS
Study design: Iago Rodríguez‐Lago and Manuel Barreiro‐de Acosta. Collecting and/or interpreting data: All authors. Drafting the manuscript: Iago Rodríguez‐Lago and Manuel Barreiro‐de Acosta. Revision for relevant intellectual content: José Luis Cabriada and Manuel Barreiro‐de Acosta. All authors have approved the final version of this manuscript.
CONFLICTS OF INTEREST
Iago Rodríguez‐Lago has received financial support for travelling and educational activities or has served as an advisory board member for MSD, Pfizer, Abbvie, Takeda, Janssen, Tillotts Pharma, Kern, Celltrion, Roche, Ferring, Dr. Falk Pharma, Galapagos, Otsuka Pharmaceutical and Adacyte. Financial support for research from Tillotts Pharma. Rebeca Higuera received financial support for travelling and educational activities or has served as an advisory board member for MSD, Pfizer, Abbvie, Takeda, Janssen, Tillotts Pharma, Ferring, Chiesi, Dr. Falk Pharma and Shire. Manuel Barreiro‐de Acosta has received financial support for travelling and educational activities or has served as an advisory board member for Pfizer, MSD, Takeda, AbbVie, Kern, Janssen, Fresenius Kabi, Biogen, Ferring, Faes Farma, Shire Pharmaceuticals, Dr. Falk Pharma, Chiesi, Gebro Pharma, Otsuka Pharmaceuticals, and Tillotts Pharma. The remaining authors declare no conflicts of interest.
Supporting information
Supporting Information S1
ACKNOWLEDGEMENTS
Iago Rodríguez‐Lago is supported by a research grant from Gobierno Vasco‐Eusko Jaurlaritza [Grant No 2020222004].
Rodríguez‐Lago I, Aguirre U, Ramírez de la Piscina P, Muñagorri A, Zapata E, Higuera R, et al. Subclinical bowel inflammation increases healthcare resources utilization and steroid use before diagnosis of inflammatory bowel disease. United European Gastroenterol J. 2023;11(1):9–18. 10.1002/ueg2.12352
José Luis Cabriada and Manuel Barreiro‐de Acosta authors share last authorship.
DATA AVAILABILITY STATEMENT
Original data from this study will be provided upon reasonable request.
REFERENCES
- 1. Chang JT. Pathophysiology of inflammatory bowel diseases. N Engl J Med. 2020;383(27):2652–64. 10.1056/nejmra2002697 [DOI] [PubMed] [Google Scholar]
- 2. Maaser C, Langholz E, Gordon H, Burisch J, Ellul P, Ramirez VH, et al. European crohn's and colitis organisation topical review on environmental factors in IBD. J Crohns Colitis. 2017;11(8):905–20. [DOI] [PubMed] [Google Scholar]
- 3. Torres J, Halfvarson J, Rodriguez‐Lago I, Hedin CRH, Jess T, Dubinsky M, et al. Results of the seventh scientific workshop of ECCO: precision medicine in IBD‐prediction and prevention of inflammatory bowel disease. J Crohns Colitis. 2021;15(9):1443–54. 10.1093/ecco-jcc/jjab048 [DOI] [PubMed] [Google Scholar]
- 4. Rodriguez‐Lago I, Ramirez C, Merino O, Azagra I, Maiz A, Zapata E, et al. Early microscopic findings in preclinical inflammatory bowel disease. Dig Liver Dis. 2020;52(12):1467–72. 10.1016/j.dld.2020.05.052 [DOI] [PubMed] [Google Scholar]
- 5. Torres J, Petralia F, Sato T, Wang P, Telesco SE, Choung RS, et al. Serum biomarkers identify patients who will develop inflammatory bowel diseases up to 5 Years before diagnosis. Gastroenterology. 2020;159(1):96–104. 10.1053/j.gastro.2020.03.007 [DOI] [PubMed] [Google Scholar]
- 6. Galipeau HJ, Caminero A, Turpin W, Bermudez‐Brito M, Santiago A, Libertucci J, et al. Novel fecal biomarkers that precede clinical diagnosis of ulcerative colitis. Gastroenterology. 2021;160(5):1532–45. 10.1053/j.gastro.2020.12.004 [DOI] [PubMed] [Google Scholar]
- 7. Turpin W, Lee SH, Raygoza Garay JA, Madsen KL, Meddings JB, Bedrani L, et al. Increased intestinal permeability is associated with later development of crohn's disease. Gastroenterology. 2020;159(6):2092–100:e5. [DOI] [PubMed] [Google Scholar]
- 8. Choung RS, Princen F, Stockfisch TP, Torres J, Maue AC, Porter CK, et al. Serologic microbial associated markers can predict Crohn's disease behaviour years before disease diagnosis. Aliment Pharmacol Ther. 2016;43(12):1300–10. 10.1111/apt.13641 [DOI] [PubMed] [Google Scholar]
- 9. Israeli E, Grotto I, Gilburd B, Balicer RD, Goldin E, Wiik A, et al. Anti‐Saccharomyces cerevisiae and antineutrophil cytoplasmic antibodies as predictors of inflammatory bowel disease. Gut. 2005;54(9):1232–6. 10.1136/gut.2004.060228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vadstrup K, Alulis S, Borsi A, Gustafsson N, Nielsen A, Wennerstrom ECM, et al. Cost burden of crohn's disease and ulcerative colitis in the 10‐year period before diagnosis‐A Danish register‐based study from 2003‐2015. Inflamm Bowel Dis. 2020;26(9):1377–82. 10.1093/ibd/izz265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rodriguez‐Lago I, Agirre U, Intxaurza N, Cantero D, Cabriada JL, Barreiro‐de Acosta M. Increased use of healthcare resources during the preclinical period of inflammatory bowel disease. Dig Liver Dis. 2021;53(7):927–30. 10.1016/j.dld.2021.04.002 [DOI] [PubMed] [Google Scholar]
- 12. Martins P, Fonseca JE. How to investigate: pre‐clinical rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2019;33(4):101438. 10.1016/j.berh.2019.101438 [DOI] [PubMed] [Google Scholar]
- 13. Carter LM, McGonagle D, Vital EM, Wittmann M. Applying early intervention strategies to autoimmune skin diseases. Is the window of opportunity preclinical? A dermato‐rheumatology perspective. J Invest Dermatol. 2022;142(3 Pt B):944–50. 10.1016/j.jid.2021.11.018 [DOI] [PubMed] [Google Scholar]
- 14. Haville S, Deane KD. Pre‐RA: can early diagnosis lead to prevention? Best Pract Res Clin Rheumatol. 2022;36(1):101737. 10.1016/j.berh.2021.101737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rodriguez‐Lago I, Zabana Y, Barreiro‐de Acosta M. Diagnosis and natural history of preclinical and early inflammatory bowel disease. Ann Gastroenterol. 2020;33(5):443–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rodriguez‐Lago I, Merino O, Azagra I, Maiz A, Zapata E, Higuera R, et al. Characteristics and progression of preclinical inflammatory bowel disease. Clin Gastroenterol Hepatol. 2018;16(9):1459–66. 10.1016/j.cgh.2017.11.006 [DOI] [PubMed] [Google Scholar]
- 17. Annese V, Daperno M, Rutter MD, Amiot A, Bossuyt P, East J, et al. European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohn's colitis. 2013;7(12):982–1018. 10.1016/j.crohns.2013.09.016 [DOI] [PubMed] [Google Scholar]
- 18. Magro F, Langner C, Driessen A, Ensari A, Geboes K, Mantzaris GJ, et al. European consensus on the histopathology of inflammatory bowel disease. J Crohns Colitis. 2013;7(10):827–51. 10.1016/j.crohns.2013.06.001 [DOI] [PubMed] [Google Scholar]
- 19. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 20. Park S, Lee HJ, Han KD, Soh H, Moon JM, Hong SW, et al. Proteinuria is associated with the development of crohn's disease: a nationwide population‐based study. J Clin Med. 2021;10(4):799. 10.3390/jcm10040799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Verstappen SM, McCoy MJ, Roberts C, Dale NE, Hassell AB, Symmons DP, et al. Beneficial effects of a 3‐week course of intramuscular glucocorticoid injections in patients with very early inflammatory polyarthritis: results of the STIVEA trial. Ann Rheum Dis. 2010;69(3):503–9. 10.1136/ard.2009.119149 [DOI] [PubMed] [Google Scholar]
- 22. Machold KP, Landewe R, Smolen JS, Stamm TA, van der Heijde DM, Verpoort KN, et al. The Stop Arthritis Very Early (SAVE) trial, an international multicentre, randomised, double‐blind, placebo‐controlled trial on glucocorticoids in very early arthritis. Ann Rheum Dis. 2010;69(3):495–502. 10.1136/ard.2009.122473 [DOI] [PubMed] [Google Scholar]
- 23. van Dongen H, van Aken J, Lard LR, Visser K, Ronday HK, Hulsmans HM, et al. Efficacy of methotrexate treatment in patients with probable rheumatoid arthritis: a double‐blind, randomized, placebo‐controlled trial. Arthritis Rheum. 2007;56(5):1424–32. 10.1002/art.22525 [DOI] [PubMed] [Google Scholar]
- 24. Emery P, Durez P, Dougados M, Legerton CW, Becker JC, Vratsanos G, et al. Impact of T‐cell costimulation modulation in patients with undifferentiated inflammatory arthritis or very early rheumatoid arthritis: a clinical and imaging study of abatacept (the ADJUST trial). Ann Rheum Dis. 2010;69(3):510–6. 10.1136/ard.2009.119016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zilbauer M, Heuschkel R. Disease prognostic biomarkers in inflammatory bowel diseases‐A reality check. J Crohns Colitis. 2022;16(1):162–5. 10.1093/ecco-jcc/jjab118 [DOI] [PubMed] [Google Scholar]
- 26. Burns EE, Mathias HM, Heisler C, Cui Y, Kits O, Veldhuyzen van Zanten S, et al. Access to inflammatory bowel disease speciality care: the primary healthcare physician perspective. Fam Pract. 2021;38(4):416–24. 10.1093/fampra/cmab006 [DOI] [PubMed] [Google Scholar]
- 27. Barreiro‐de Acosta M, Gutierrez A, Zabana Y, Beltran B, Calvet X, Chaparro M, et al. Inflammatory bowel disease integral care units: evaluation of a nationwide quality certification programme. The GETECCU experience. United Eur Gastroenterol J. 2021;9(7):766–72. 10.1002/ueg2.12105 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1
Data Availability Statement
Original data from this study will be provided upon reasonable request.


