Abstract
The sarco(endo)plasmic reticulum calcium ATPase (SERCA) is an ion transporter that creates and maintains intracellular calcium stores. SERCA is inhibited or stimulated by several membrane micropeptides including another-regulin, dwarf open reading frame, endoregulin, phospholamban (PLB), and sarcolipin. We previously showed that these micropeptides assemble into homo-oligomeric complexes with varying affinity. Here, we tested whether different micropeptides can interact with each other, hypothesizing that coassembly into hetero-oligomers may affect micropeptide bioavailability to regulate SERCA. We quantified the relative binding affinity of each combination of candidates using automated fluorescence resonance energy transfer microscopy. All pairs were capable of interacting with good affinity, similar to the affinity of micropeptide self-binding (homo-oligomerization). Testing each pair at a 1:5 ratio and a reciprocal 5:1 ratio, we noted that the affinity of hetero-oligomerization of some micropeptides depended on whether they were the minority or majority species. In particular, sarcolipin was able to join oligomers when it was the minority species but did not readily accommodate other micropeptides in the reciprocal experiment when it was expressed in fivefold excess. The opposite was observed for endoregulin. PLB was a universal partner for all other micropeptides tested, forming avid hetero-oligomers whether it was the minority or majority species. Increasing expression of SERCA decreased PLB-dwarf open reading frame hetero-oligomerization, suggesting that SERCA-micropeptide interactions compete with micropeptide-micropeptide interactions. Thus, micropeptides populate a regulatory network of diverse protein assemblies. The data suggest that the complexity of this interactome increases exponentially with the number of micropeptides that are coexpressed in a particular tissue.
Significance
There is growing recognition of the physiological importance of micropeptides translated from ostensibly “noncoding RNAs” transcribed from small open reading frames. Of particular interest are the “regulin” family of micropeptides, which have been shown to modulate the function of the SERCA calcium transporter. This ion pump plays a key role in creating and maintaining intracellular calcium stores needed for cell signaling. Tissue-specific expression of regulin micropeptides provides finely tuned regulation of SERCA to meet the varying needs of different cells under different physiological conditions. Here, we find that the regulins can coassemble into hetero-oligomeric complexes, and we compare the relative affinity of different combinations of micropeptides. We propose that hetero-oligomerization affects the bioavailability and physiological potency of these regulatory species.
Introduction
Recently, there has been increasing recognition of the biological importance of an overlooked class of small proteins composed of fewer than 100 amino acids. Thousands of small open reading frames previously assumed to be noncoding have been shown to express physiologically functional protein species (1,2,3). These proteins, termed microproteins or micropeptides, have been implicated in a wide range of physiological and pathological ([4,5,6) processes. This includes, but is not limited to, dilated cardiomyopathy in the case of cardiac-expressed microprotein deletion (7) and metastasis of hepatoma cells in the downregulation of mitochondrial micropeptides (8). One class of micropeptides that has come under intensive scrutiny is a family of membrane micropeptides that modulate the function of the sarco(endo)plasmic reticulum calcium ATPase (SERCA) (1). This calcium pump transports Ca2+ into the endoplasmic reticulum, creating a Ca2+ reservoir that is the foundation of intracellular signaling in many diverse cell types. Tissue-specific expression of membrane micropeptides provides a mechanism to appropriately tune SERCA function for each physiological context.
We have previously shown that SERCA-modulating membrane micropeptides bind to the pump as monomers, but they also assemble into homo-oligomers that do not interact with SERCA (9). The oligomers may serve as an inactive pool of micropeptides that is held in reserve. Since some micropeptides are coexpressed together in the same tissues (10), we reasoned that these structurally similar proteins might interact with each other to form hetero-oligomers, thereby modulating the bioavailability of both micropeptides. We used a medium-throughput fluorescence resonance energy transfer (FRET) microscopy assay to determine the hetero-oligomerization affinity of pairs of micropeptides including another-regulin (ALN), dwarf open reading frame (DWORF), endoregulin (ELN), phospholamban (PLB), and sarcolipin (SLN). PLB is expressed abundantly in the ventricles of the heart and in some skeletal muscle fiber types. SLN is primarily expressed in skeletal muscle and the atria of the heart. ALN is found in almost all tissues. ELN is mainly found in endo/epithelial cells. DWORF is found in heart and soleus muscle tissue. DWORF has been shown to stimulate SERCA function (11), and the other micropeptides are inhibitory (10). While some of these micropeptides are not natively coexpressed together in the same tissue, we considered that expression patterns may change in disease (12,13) or with therapeutic gene delivery (14). Therefore, we performed pairwise tests of each combination for a thorough comparison of hetero-oligomerization affinities.
Materials and methods
Cell culture and transfection
pEGFP-C1 was used as an expression vector and human sequences of all micropeptides (PLB, SLN, DWORF, ALN, and ELN) were labeled using either mCerulean or YFP on the N-terminal end (15) with a 5 amino acid linker of sequence SGLRS. We previously showed that SERCA singly or doubly labeled with fluorescent protein still retains normal ATPase activity (16,17) and Ca2+ transport function (16,18). In addition, SERCA fused to two fluorescent proteins can be regulated by PLB fused to a third fluorescent protein (16). The data suggest that the fluorescent protein tags are benign for the function of the transporter and the micropeptide. AAV-293 cells (Agilent, Santa Clara, CA, USA) were cultured in DMEM cell culture medium with 10% fetal bovine serum (Thermo Fisher Scientific, Waltham, MA, USA). The cells were then transfected using Lipofectamine 3000 transfection kit (Invitrogen Life Technologies, Carlsbad, CA, USA) per the manufacturer’s instructions and plated on 24-well cell culture plates. After 24 h of protein expression, the cells were trypsinized (Thermo Fisher Scientific) and replated onto poly-D-lysine-coated 24-well glass bottom chamber plates before imaging the following day. Prior to imaging, cells were incubated at 37°C in DMEM with 10% fetal bovine serum. The expression of micropeptides in vivo varies widely across different tissues (10,19). Our previous studies of DWORF and PLB suggest that the expression levels achieved in AAV-293 cells are somewhat lower than native myocardium (20), thus we do not consider the present experimental system to be overexpression.
Automated FRET microscopy
Imaging was performed using a wide-field fluorescence microscope as described previously (21). Cells were washed with phosphate-buffered saline and immediately subjected to automated fluorescence imaging at room temperature in ambient air using a Nikon Eclipse Ti2 inverted microscope. The image acquisition of a field of view for each sample was performed with a 40× objective, numerical aperture 0.95, and 50 ms exposure time for Cer, YFP, and FRET (Cer excitation, YFP emission) channels. 64 images were taken of each well of the 24-well plate, with 800 μm between each image in an 8 × 8 grid. Acquisition of a complete grid in one well required about 3 min, so a full 24-well plate required about 72 min. We acquired eight independent measurements of each pairing of micropeptides, transfected on different days. The fluorescence intensity of cells was quantified from images using automated analysis with a custom script in FIJI software. Cells were selected for analysis based on area (55–2,200 μm2) and circularity (0.40–1, with 1 being a perfect circle). Selected cells were scored for fluorescence intensity in Cer, YFP, and FRET channels. FRET efficiency was calculated using the acceptor sensitization method according to , where G = FFRET − a × FYFP – d × FCer, where FFRET, FYFP, and FCer are the matching fluorescence intensity from FRET, YFP, and Cer images, respectively, and G represents FRET intensity corrected for the bleed through of the channels. The parameters a and d are bleed-through constants calculated as a = FFRET/FCer for a control sample transfected with only YFP-SERCA and d = FFRET/FYFP for a control sample transfected with only Cer-SERCA. Values used here were G = 4.74, a = 0.075, and b = 0.88. As described previously (9,22), the FRET efficiency measured for each cell was plotted as a function of that cell’s YFP fluorescence intensity, which was taken as an index of the expression level (membrane concentration) of the YFP-labeled micropeptide ([micropeptide]) in arbitrary units (AU). The relationship between measured FRET and expression was fit by a hyperbolic fit of the form
where FRETmax is the maximum FRET at the highest concentration of micropeptide and KD is the apparent dissociation constant. High FRETmax values reflect a more compact hetero-oligomer structure that brings the donor and acceptor fluorescent tags into close proximity. Differences between different pairs of micropeptides are likely due to differences in the lengths of the cytoplasmic domain of different micropeptides. In our previous study (9), we proposed that this parameter is also affected by the prevailing relative position of the cytoplasmic domain, that is, whether it tends to interact with the surface of the membrane. In mixed oligomers composed of different micropeptide species with varying cytoplasmic domain lengths, there are too many uncertainties to interpret this value quantitatively for insight into the hetero-oligomer quaternary structure.
Competition for micropeptide binding by SERCA
To test whether PLB-DWORF interactions were increased or decreased by coexpression of SERCA, AAV-293 cells were plated onto 60 mm plates and transfected with 0.5 μg plasmid DNA encoding mCer-PLB and 1.5 μg of DNA for YFP-DWORF and varying concentrations of plasmid DNA encoding unlabeled SERCA (0, 0.5, 1, 3, and 5 μg). The cells were imaged, on the same wide-field fluorescence microscope as previously described, 2 days posttransfection on a poly-D-lysine-coated 2-well glass-bottom chamber. Cells were scored using FIJI, and each transfection was analyzed with curve fitting in OriginPro 9.1.
Progressive acceptor photobleaching
As previously described (9), 10 images of Cer- and YFP-expressing cells were acquired at 10 s intervals to establish a baseline, then cells were subjected to progressive acceptor photobleaching, during which images of Cer and YFP were obtained after 10 s intervals of exposure to intense illumination through a 510/25 nm bandpass filter for selective photobleaching of YFP. The fluorescence of the donor was plotted against the fluorescence of the acceptor at the same time point during progressive bleaching. A linear relationship was taken to indicate a dimer formation, and a supralinear relationship was taken to indicate higher-order oligomers containing multiple YFP-labeled proteins. Curvature of the progressive acceptor photobleaching plot arises from the nature of energy transfer to multiple acceptors around the oligomer (23). In previous studies, we have exploited a Matlab model of this FRET theory (9,24) to analyze these data more quantitatively. However, that model assumes a symmetric ring-shaped distribution of fluorophores, which is not a valid assumption in a mixed hetero-oligomer. Moreover, while the degree of curvature of this plot depends on the number of subunits in the oligomer, in practice, it is difficult to discriminate between n = 3 and higher-order (n > 3) oligomers (9). With these methodological limitations, we limit the present analysis to qualitative scoring of the donor versus acceptor plots as linear or curved for simple comparison of the stoichiometry (dimer versus higher-order oligomer) of micropeptide complexes in the membranes of living cells.
Time-correlated single-photon counting (TCSPC)
TCSPC data acquisition was performed as previously described (25). TCSPC histograms were obtained using AAV-293 cells expressing mCyRFP1-PLB alone or mCyRFP1-PLB coexpressed with mMaroon-DWORF with increasing expressions of unlabeled human SERCA2a. To excite mCyRFP1, the excitation laser beam was filtered through 482/18 nm bandpass filter and 1.0 neutral density filter and focused on the sample with a 1.2 numerical aperture water-immersion objective. Emitted fluorescence was passed through a 525/50 nm bandpass filter and detected with a PMA hybrid detector and HydraHarp 400 TCSPC module (PicoQuant, Berlin, Germany), with a target fluorescence intensity of approximately 100,000 photons per second and a total acquisition time of 60 s per histogram. Fluorescence decay histograms were analyzed using global analysis in SymPhoTime 64 software with fixed lifetime corresponding to non-FRET donor (τ1 = 3.46 ns) plus a second freely variable global lifetime τ2 corresponding to the donors that participate in FRET, with variable amplitudes for both lifetimes.
Results
Relative binding affinities of micropeptides
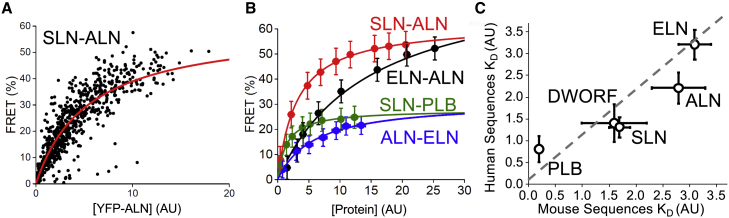
We have previously described the use of FRET microscopy to quantify the relative affinity of protein-protein interactions (9). Here, this approach was adapted to a 24-well microtiter plate format for improved throughput to evaluate multiple combinations of fluorescently labeled membrane proteins. Briefly, AAV-293 cells were transfected with plasmid DNA encoding Cer- and YFP-labeled proteins (human protein sequences) at 1:5 ratios and subjected to automated FRET microscopy at 48 h posttransfection. Previous studies have shown that fluorescent protein tags are benign for SERCA and micropeptide function (16,17,18) (see materials and methods). The populations of transfected cells expressed micropeptides over a wide range of protein concentrations as quantified from cellular fluorescence intensity. FRET was low for cells expressing protein at a low concentration, increasing to a maximum for cells expressing protein at a high concentration (Fig. 1 A and B). This relationship between observed FRET and protein concentration was well described by a hyperbolic function of the form FRET = FRETmax[protein]/(KD+[protein]), where FRETmax is the maximal FRET, revealing the intrinsic FRET efficiency of the bound complex, and KD is the apparent dissociation constant (the protein concentration that yields half-maximal FRET efficiency). We have previously interpreted FRETmax values to gain insight into the overall architecture of a protein complex (9,22). Here, we do not make structural conclusions from the FRETmax parameter since mixed hetero-oligomers are not expected to conform to assumptions for theory of FRET within a symmetric, ring-shaped oligomer (23). We quantified the relative affinity of 25 pairings in total, including reciprocal experiments where each micropeptide was tested as a minority (donor-labeled) or majority (acceptor-labeled) partner. All combinations interacted to some degree as demonstrated by concentration-dependent FRET. All binding curves, composed of data obtained from individual cells, are provided in Fig. S1. Donor versus acceptor plots for these binding curves are provided in Fig. S2, ensuring the ratio of donor- to acceptor-tagged constructs is similar for each combination. Pooled data compared in Fig. 1 B include isotherms exemplifying high intrinsic FRET efficiency (FRETmax) and low affinity (high KD) (ELN-ALN); high FRETmax and high affinity (low KD) (SLN-ALN); low FRETmax and high affinity (SLN-PLB); and low FRETmax and low affinity (ALN-ELN). Each micropeptide also assembled into homo-oligomers as previously described (9). In our previous study of micropeptide homo-oligomerization (9), we used mouse sequences to engineer fluorescent protein fusion constructs. Here, we used the human sequences and observed very similar apparent affinities for homo-oligomerization. Fig. 1 C compares the previously published KDs obtained from mouse sequences (x axis values) (9) with the KDs observed for human sequences (y axis values) in the present study. The data indicate good reproducibility of the measurements over time and suggest that the mouse sequences provide satisfactory models for human micropeptide regulatory interactions.
Figure 1.
FRET-based binding assays. (A) FRET increased with increasing protein expression. Example binding curve for SLN ALN with hyperbolic function of best fit shown in red. (B) Examples of different combinations of micropeptides that showed high or low FRETmax and dissociation constants (KDs). Data are mean FRET pooled from cells expressing a similar level of protein. X/Y standard deviation denoted using brackets. (C) The apparent homo-oligomerization affinities of micropeptide for human protein sequences (measured here) were similar to values previously obtained with mouse sequences. The gray dotted line represents 1:1 correspondence.
In addition to FRET arising from micropeptide hetero-oligomerization, we anticipate nonspecific FRET will occur between noninteracting micropeptides that are concentrated in close proximity in the membrane (26,27,28). We have previously quantified nonspecific FRET using competition to eliminate specific donor-acceptor interactions, observing nonspecific FRET efficiencies of 3.5% (25) and 4% (29). To quantify nonspecific FRET in the current experimental context, we quantified FRET from YFP-SLN to mCherry-PLB, disrupting this complex with competition by ELN. ELN was labeled with mCer, which cannot act as a FRET acceptor for YFP but can be used to quantify the expression of competitor micropeptide. We observed that increasing expression of mCer-ELN decreased SLN-PLB FRETmax toward a minimum of 9% (Fig. S3), which we take to represent FRET between noninteracting SLN and PLB. We consider this value of nonspecific FRET between YFP and mCherry (R0 = 58.58 Å) to be an overestimate of nonspecific FRET for the present conditions. The Cer/YFP pair have a shorter Förster radius (R0 = 51.11 Å) and so should exhibit decreased proximity FRET.
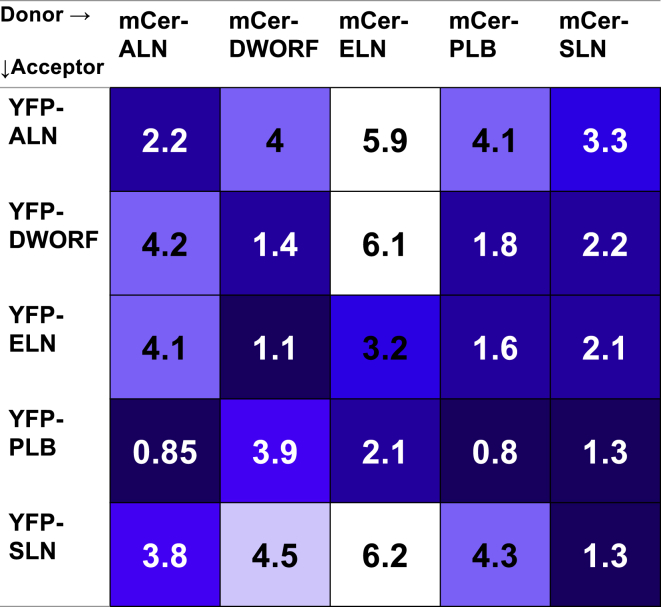
Mean KD values obtained for all 25 micropeptide combinations are summarized in Fig. 2. In general, homo-oligomerization of a given micropeptide was slightly more avid compared with that peptide’s hetero-oligomer interactions. However, DWORF, ELN, and PLB engaged in some hetero-oligomer interactions that showed binding affinities as good as or better than that of the homo-oligomer. PLB, in particular, was a universal partner for all other micropeptides, forming avid hetero-oligomers whether it was in the minority or majority group. Comparison of reciprocal pairings revealed that some micropeptides interacted best when they were the minority species in the 1:5 expression ratio. SLN showed consistently higher relative binding affinities when it was the minority species, with all average KD values under 3.3 AU. When expressed in excess, SLN showed lower relative affinities with average hetero-oligomerization KD values over 3.8 AU. Conversely, ELN showed high affinity (KD values below 4.1 AU) in 5:1 ratios but low affinities (KD values above 6 AU) in 1:5 pairings.
Figure 2.
Comparison of micropeptide hetero-oligomerization affinities. Values are apparent KDs for each combination of micropeptides labeled with donors (columns) and acceptors (rows). Colors depict relative binding affinity from dark blue (high affinity) to white (low affinity).
SERCA binding competes with micropeptide-micropeptide interactions
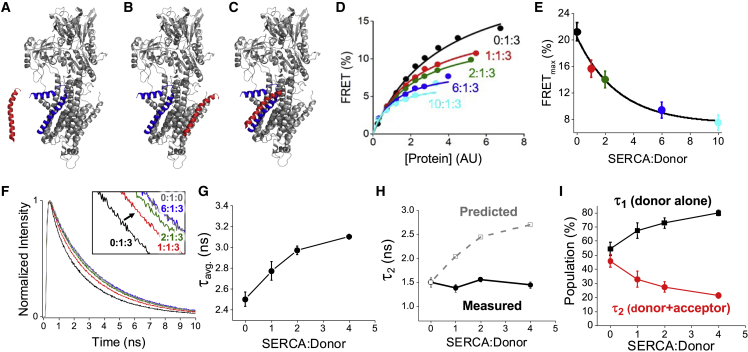
The micropeptides investigated here were previously shown to bind to SERCA as monomers (9), and oligomerization and SERCA binding have been regarded as competing protein-protein interactions (11,14). Fig. 3 A shows a conceptual cartoon in which SERCA can bind to PLB (blue) or DWORF (red) but not to both at the same time. However, we have not tested directly whether different micropeptides could simultaneously occupy distinct binding sites on SERCA (30) (Fig. 3 B) or whether multiple micropeptides could bind together at a single site (Fig. 3 C), as has been proposed for PLB and SLN (31). To determine how the oligomerization equilibrium and SERCA-binding equilibrium interact, we quantified FRET between micropeptides as a function of increasing expression of unlabeled SERCA (Fig. 3 A). We focused on PLB (an inhibitor of SERCA (32,33)) and DWORF (an activator of SERCA (11,34–35,36)), which are expressed together in cardiac muscle (11). If SERCA interacts with individual monomeric micropeptides (Fig. 3 A), one would predict that excess SERCA would compete with hetero-oligomer interactions and decrease DWORF-PLB FRET. Conversely, if multiple micropeptides can interact with SERCA simultaneously (Fig. 3 B and C), one would predict that increasing expression of SERCA would increasingly bring unbound micropeptides into close proximity, increasing DWORF-PLB FRET. Fig. 3, D and E, shows that increasing expression of unlabeled SERCA resulted in a progressive decrease in DWORF-PLB FRETmax in a pattern that is consistent with previous competition experiments (25,29). The data suggest that SERCA binds either PLB or DWORF but cannot bind both at the same time. We also considered the possibility that SERCA binds to a PLB-DWORF hetero-oligomer (Fig. 3 C) but alters the structure of that hetero-oligomer in a way that increases the separation distance between the donor and acceptor. This would also reduce the value of the FRETmax parameter as in Fig. 3 E. To evaluate this possibility, we engineered a new FRET pair, mCyRFP1-labeled PLB (donor) and mMaroon-DWORF (acceptor), and performed fluorescence lifetime measurements using TCSPC. This approach allows us to quantify the populations of distinct FRET species including donors that are not bound to acceptors and do not engage in FRET (37). The mCyRFP1 donor excited-state lifetime is 3.46 ns, and we observed that the average fluorescence lifetime of mCyRFP1-PLB was reduced to 2.5 ns when coexpressed with mMaroon-DWORF, consistent with quenching of the donor by FRET. Increasing coexpression of unlabeled SERCA resulted in an increase in the average fluorescence lifetime (τavg) of the mCyRFP1 donor (Fig. 3 F and G), indicating a decrease in PLB-DWORF FRET. Multiexponential decay analysis revealed two donor subpopulations including a non-FRET donor population with a 3.4 ns lifetime (τ1) and a FRET species with a fluorescence lifetime of approximately 1.5 ns (τ2). If the change in average lifetime observed in Fig. 3, F and G, was due to a conformational change of the hetero-oligomer that occurred with SERCA binding, we predicted that the value of τ2 would increase with increasing expression of unlabeled SERCA, as shown in Fig. 3 H (gray open squares). Instead, τ2 stayed the same (Fig. 3 H, black circles), but the population of this FRET species decreased (Fig. 3 I, red circles), while the population of the non-FRET donor species increased (Fig. 3 I, black squares), indicating disruption of the DWORF-PLB complexes with increasing competition by SERCA. We conclude that multiple micropeptides do not bind to distinct sites simultaneously (Fig. 3 B), nor does SERCA bind the PLB-DWORF hetero-oligomer (Fig. 3 C). Rather, the data are consistent with the model that SERCA binds individual monomeric micropeptides (Fig. 3 A). Thus, SERCA binding competes for PLB-DWORF hetero-oligomerization, and inversely, we hypothesize that hetero-oligomerization reduces the bioavailability of the active, monomeric species of micropeptide that binds and regulates SERCA.
Figure 3.
Competition of micropeptide hetero-oligomerization and SERCA-binding interactions. (A) SERCA binds to DWORF (red) or PLB (blue) but not both. (B) SERCA binds DWORF and PLB at distinct binding sites. (C) SERCA binds a PLB-DWORF hetero-oligomer. (D) FRET from Cer-DWORF to YFP-PLB with increasing coexpression of SERCA. Labels indicate the ratio of competitor:donor:acceptor. Data represent the mean. The average Y SE for each point is 0.04%. (E) FRETmax decreased with increasing expression of SERCA, consistent with depolymerization of the hetero-oligomer complex. Data are mean ± SD. (F) Normalized decay curves show a right shift of the curves (longer average lifetime) with additional added competitor. Inset: enlarged view, labels indicate competitor:donor:acceptor ratio. (G) Fluorescence lifetime measurements suggest that FRET from PLB to DWORF is decreased with increasing SERCA expression. (H) Multiexponential decay analysis revealed the fluorescence lifetime (τ2) of the donor population that participates in FRET. A structure change of the PLB-DWORF complex that decreases FRET (as in F) is predicted to alter τ2 as shown by gray open squares. Instead, the measured value of τ2 did not increase with increasing SERCA expression (black circles). (I) Competition with SERCA increased τ1 (non-FRET species) at the expense of τ2 (FRET species). The data suggest binding of PLB and DWORF to SERCA decreased PLB-DWORF hetero-oligomerization.
Hetero-oligomer complex stoichiometry
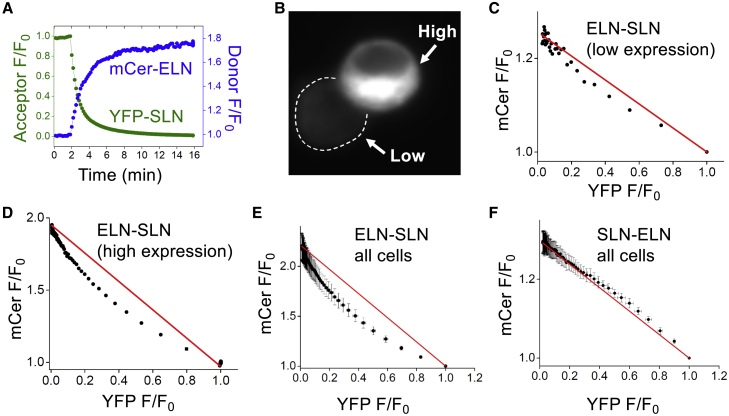
To investigate the stoichiometry of hetero-oligomer complexes, we subjected select combinations of micropeptides to progressive acceptor photobleaching. In this experiment, incremental photobleaching of the acceptor gradually abolishes FRET, resulting in a progressive increase in the brightness of the donor fluorescence (Fig. 4 A). Plotting the fluorescence of the donor as a function of the acceptor reveals whether FRET arises from a complex containing a single acceptor or multiple acceptors, yielding linear or curved donor/acceptor (D/A) relationships, respectively (22). This provides information about oligomer size since dimers (containing one acceptor) give rise to a linear D/A relationship and higher-order oligomers (n > 2) yield a curved D/A plot. In our previous analysis of micropeptide homo-oligomerization, we observed that most micropeptides showed linear D/A plots when expressed at a low concentration, and D/A relationship nonlinearity increased with increasing protein expression (9). In the present study, we observed a similar pattern for hetero-oligomerization when comparing cells expressing Cer-ELN and YFP-SLN at low and high concentrations (Fig. 4 B). Quantification of the progressive acceptor photobleaching data revealed a more linear D/A relationship for a low-expressing cell (Fig. 4 C) but marked curvature for the high-expressing cell in the same microscopic field (Fig. 4 D). The data suggest a pathway of assembly of high-order ELN-SLN oligomers in which an ELN-SLN dimer forms first at low micropeptide concentration followed by oligomerization into larger complexes at high protein expression. Fig. 4 E provides the summary of all measurements of ELN-SLN combination. Interestingly, the reciprocal combination of SLN with an excess of ELN yielded a linear D/A relationship (Fig. 4 F) for both low- and high-expressing cells (Fig. S4). The data suggest that the SLN-ELN combination yields a hetero-dimer containing a single acceptor even at the highest protein concentrations achieved here.
Figure 4.
Evaluation of hetero-oligomer stoichiometry with progressive acceptor photobleaching. (A) YFP photobleaching abolishes FRET, dequenching mCer donor fluorescence. (B) Fluorescence microscopy of cells expressing Cer-ELN and YFP-SLN at 10-fold different concentrations. The dotted line delineates the margin of the low-expressing cell. (C) Progressive acceptor photobleaching revealed a linear donor/acceptor relationship for the low-expressing cell in (B) consistent with a hetero-dimer. (D) The high-expressing cell in (B) showed a curved donor/acceptor relationship consistent with a higher-order hetero-oligomer that contains more than one FRET acceptor. (E) As in (C) and (D). Data are mean ± SE for n = 10 cells. (F) The reciprocal experiment, with progressive acceptor photobleaching of cells expressing Cer-SLN and YFP-ELN, showed a linear donor/acceptor relationship consistent with a hetero-dimer. Data are mean ± SE for n = 10 cells.
Discussion
Relative binding affinities of micropeptides
The principal finding of this study is that all the micropeptides tested here can interact with each other to some degree. Most combinations bind with an affinity of hetero-oligomerization that is comparable to the homo-oligomerization affinity of the respective peptides (9) and similar to the affinity of SERCA-micropeptide interactions. The data suggest that micropeptides populate a network of different regulatory protein complexes in the membrane (Fig. 5) and that the complexity of this network increases exponentially with the number of micropeptides that are coexpressed in a particular tissue (10). It was noteworthy that reciprocal combinations did not always show the same binding affinity, that is, some micropeptides had a preference for forming hetero-oligomers as the majority species, while others bound better as a minority constituent. The reason for this asymmetry is unclear, but we speculate that different micropeptides form homo-oligomeric starting structures that may be more or less accommodating to the addition of heteromeric species as a minority protomer in the oliogomeric complex. For example, SLN showed higher apparent hetero-oligomerization affinity when combined with other micropeptides at a 1:5 SLN:micropeptide ratio compared with a 5:1 ratio. This suggests that SLN can be incorporated as a minor component in a preformed oligomer but cannot easily accommodate other micropeptides into its oligomer ring. Conversely, ELN showed high affinity in 5:1 ELN:micropeptide ratios but low affinities in 1:5 ratio expression. We speculated that this micropeptide forms loosely specific oligomers that can permit addition of other micropeptides, but as a minority constituent, it binds weakly to other species of oligomers that may have more strict structural requirements. As the universal partner, PLB interacted avidly with other species regardless of whether it was combined with other micropeptides as a minority donor-labeled protein or in excess as an acceptor-labeled protein. These oligomeric structures may be worthwhile subjects for future physical or computational studies. A recent molecular dynamics study showed that PLB pentamers have stable ring-shaped architecture, while SLN homo-oligomers collapsed into an assymetric cluster of a dimer and a trimer (38). These distinct arrangements of protomers may be the basis for differences in ability of “host” oligomers to accommodate additional heteromeric participants, accounting for the different affinities (Fig. 2) and stoichiometry (Fig. 4) of 1:5 and 5:1 combinations of partner micropeptides. Computational studies may also be the best approach to understanding the disparate stoichiometry of reciprocal pairs, as in Fig. 4. Again, SLN provides a peculiar example of oligomer assembly: with SLN as a homo-oligomer, we have previously observed dimers (39) and higher-order species (9). Here, we observed SLN as a minority protomer forming only hetero-dimers with ALN (Fig. 4 F), but as the majority species interacting with ALN, it exhibited D/A plot curvature consistent with higher-order hetero-oligomers (Fig. 4 E). Future molecular dynamics simulations of hetero-oligomer complexes will benefit from the determination of the structures of the newly discovered micropeptides. Other orthogonal approaches that could provide additional insight into micropeptide hetero-oligomerization include surface plasmon resonance, size-exclusion chromatography, SDS-polyacrylamide gel electrophoresis, analytical ultracentrifugation, and isothermal calorimetry. These methods may yield a more precise estimate of the stoichiometry of the higher-order oligomer species detected in the present study by progressive acceptor photobleaching. It is a limitation of our approach that we can only distinguish between dimers (n = 2) and higher-order oligomers (n > 2). While these alternative approaches require solubilization/purification of the membrane proteins, previous studies have shown that some micropeptide interactions are preserved in detergent micelles (40,41,42,43,44).
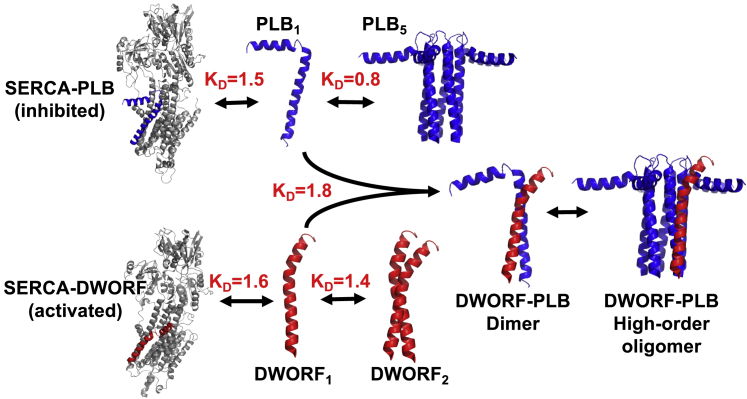
Figure 5.
A conceptual diagram depicting regulatory interactions of SERCA (gray) with PLB (blue) and DWORF (red). Homo-oligomeric and hetero-oligomeric binding states for PLB and DWORF with KD values displayed for each interaction. Structures: SERCA, PDB: 5A3Q; PLB1, PDB: 2KB7; DWORF1, PDB: 7MPA; PLB5, PDB: 2KYV.
A regulatory network of competing membrane protein-protein interactions
In addition, the data suggest that SERCA binding occurs at the expense of other micropeptide interactions. The implication is that each membrane protein-protein interaction competes with and equilibrates with all the other interactions of the SERCA regulatory network (Fig. 5). Progressive acceptor photobleaching experiments (Fig. 4) suggested an assembly pathway of micropeptide monomers forming dimers first (at low concentration), proceeding to higher-order hetero-oligomers when the concentration is increased.
Since SERCA is regulated by monomeric micropeptides (9), presumably hetero-oligomerization partially depletes the active, monomeric species. The prototypical example of oligomerization in competition with SERCA binding is provided by PLB. Previous studies have shown that mutations that decrease PLB homo-oligomerization increase PLB binding to SERCA and enhance PLB inhibitory potency (22,45,46,47), while stabilization of the pentamer by oxidative cross-linking (29) or phosphorylation (48) decreases binding to SERCA. However, it is important to note that the degree of oligomerization is not the only determinant of micropeptide potency, and avid micropeptide oligomerization is not incompatible with strong binding to SERCA. Indeed, comparing across different micropeptides, we previously observed a positive correlation between oligomerization affinity and SERCA binding (9)—that is, micropeptides that show avid oligomerization also bind particularly well to SERCA. This pattern suggests that the specific structural elements that support the SERCA-micropeptide interaction are also critical determinants of functional potency.
In hetero-oligomerization, we expect the differential oligomerization affinities of different micropeptides to confer additional regulatory network complexity. For example, a particular micropeptide’s regulatory potency may depend on relatively loose homo-oligomerization when expressed alone, but then its bioavailability may become strongly limited by avid hetero-oligomerization upon expression of another species. Conversely, one may speculate that high-affinity oligomers of one micropeptide could be destabilized by the intrusion of a different, poorly oligomeric species, potentiating the avidly oligomeric micropeptide for increased modulation of SERCA function.
Summary
Overall, we suggest that the SERCA transporter regulatory interactome may be more elaborate than previously appreciated. Tissue-specific expression of a micropeptides will regulate SERCA directly but also indirectly affect the availability of competing micropeptides through hetero-oligomerization. This may be a general feature of transport ATPases that are functionally modulated by interactions with oligomer-forming microproteins. For example, another likely candidate to exploit this regulatory mechanism is the sodium pump (Na/K-ATPase). Like SERCA, Na/K-ATPase is modulated by tissue-specific expression of regulatory micropeptides (37,49), some of which have been shown to form oligomers (50). This multifaceted regulatory scheme provides many points of control for fine regulation of pump function to meet the diverse needs of different tissues and appropriately respond to varying physiological stresses.
Author contributions
Performed research, T.A.P., G.T.H., M.P.P., E.E.C., and S.R.C.; designed research, M.P.P., E.E.C., and S.L.R.; analyzed data, T.A.P., G.T.H., M.P.P., E.E.C., and S.R.C.; wrote the paper, T.A.P. and S.L.R.
Acknowledgments
This investigation was supported by the National Institutes of Health (NIH) R01HL092321 and R01HL143816 from the National Heart, Lung, and Blood Institute (NHLBI) to S.L.R.
Declaration of interests
The authors declare no competing interests.
Editor: Eleonora Grandi.
Footnotes
Supporting material can be found online at https://doi.org/10.1016/j.bpj.2022.12.014.
Supporting material
References
- 1.Makarewich C.A. The hidden world of membrane microproteins. Exp. Cell Res. 2020;388:111853. doi: 10.1016/j.yexcr.2020.111853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vitorino R., Guedes S., et al. Akimitsu N. The role of micropeptides in biology. Cell. Mol. Life Sci. 2021;78:3285–3298. doi: 10.1007/s00018-020-03740-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen J., Brunner A.D., et al. Weissman J.S. Pervasive functional translation of noncanonical human open reading frames. Science. 2020;367:1140–1146. doi: 10.1126/science.aay0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polenkowski M., Burbano de Lara S., et al. Tran D.D.H. Identification of novel micropeptides derived from hepatocellular carcinoma-specific long noncoding RNA. Int. J. Mol. Sci. 2021;23:58. doi: 10.3390/ijms23010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ye M., Zhang J., et al. Dong K. Emerging role of long noncoding RNA-encoded micropeptides in cancer. Cancer Cell Int. 2020;20:506. doi: 10.1186/s12935-020-01589-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hasenfuss G., Pieske B. Calcium cycling in congestive heart failure. J. Mol. Cell. Cardiol. 2002;34:951–969. doi: 10.1006/jmcc.2002.2037. [DOI] [PubMed] [Google Scholar]
- 7.Makarewich C.A., Munir A.Z., et al. Olson E.N. The cardiac-enriched microprotein mitolamban regulates mitochondrial respiratory complex assembly and function in mice. Proc. Natl. Acad. Sci. USA. 2022;119 doi: 10.1073/pnas.2120476119. e2120476119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiao M.H., Lin Y.F., et al. Fang J.H. Downregulation of a mitochondrial micropeptide, MPM, promotes hepatoma metastasis by enhancing mitochondrial complex I activity. Mol. Ther. 2022;30:714–725. doi: 10.1016/j.ymthe.2021.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh D.R., Dalton M.P., et al. Robia S.L. Newly discovered micropeptide regulators of SERCA form oligomers but bind to the pump as monomers. J. Mol. Biol. 2019;431:4429–4443. doi: 10.1016/j.jmb.2019.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson D.M., Makarewich C.A., et al. Olson E.N. Widespread control of calcium signaling by a family of SERCA-inhibiting micropeptides. Sci. Signal. 2016;9:ra119. doi: 10.1126/scisignal.aaj1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Makarewich C.A., Munir A.Z., et al. Olson E.N. The DWORF micropeptide enhances contractility and prevents heart failure in a mouse model of dilated cardiomyopathy. Elife. 2018;7:e38319. doi: 10.7554/eLife.38319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Babu G.J., Bhupathy P., et al. Periasamy M. Differential expression of sarcolipin protein during muscle development and cardiac pathophysiology. J. Mol. Cell. Cardiol. 2007;43:215–222. doi: 10.1016/j.yjmcc.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shanmugam M., Molina C.E., et al. Babu G.J. Decreased sarcolipin protein expression and enhanced sarco(endo)plasmic reticulum Ca2+ uptake in human atrial fibrillation. Biochem. Biophys. Res. Commun. 2011;410:97–101. doi: 10.1016/j.bbrc.2011.05.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Makarewich C.A., Bezprozvannaya S., et al. Olson E.N. Gene therapy with the DWORF micropeptide attenuates cardiomyopathy in mice. Circ. Res. 2020;127:1340–1342. doi: 10.1161/CIRCRESAHA.120.317156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koushik S.V., Chen H., et al. Vogel S.S. Cerulean, venus, and VenusY67C FRET reference standards. Biophys. J. 2006;91:L99–L101. doi: 10.1529/biophysj.106.096206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hou Z., Hu Z., et al. Robia S.L. 2-Color calcium pump reveals closure of the cytoplasmic headpiece with calcium binding. PLoS One. 2012;7:e40369. doi: 10.1371/journal.pone.0040369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gruber S.J., Cornea R.L., et al. Thomas D.D. Discovery of enzyme modulators via high-throughput time-resolved FRET in living cells. J. Biomol. Screen. 2014;19:215–222. doi: 10.1177/1087057113510740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raguimova O.N., Smolin N., et al. Robia S.L. Redistribution of SERCA calcium pump conformers during intracellular calcium signaling. J. Biol. Chem. 2018;293:10843–10856. doi: 10.1074/jbc.RA118.002472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson D.M., Anderson K.M., et al. Olson E.N. A micropeptide encoded by a putative long noncoding RNA regulates muscle performance. Cell. 2015;160:595–606. doi: 10.1016/j.cell.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cleary S.R., Fang X., et al. Robia S.L. Inhibitory and stimulatory micropeptides preferentially bind to different conformations of the cardiac calcium pump. J. Biol. Chem. 2022;298:102060. doi: 10.1016/j.jbc.2022.102060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hou Z., Robia S.L. Relative affinity of calcium pump isoforms for phospholamban quantified by fluorescence resonance energy transfer. J. Mol. Biol. 2010;402:210–216. doi: 10.1016/j.jmb.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelly E.M., Hou Z., et al. Robia S.L. Phospholamban oligomerization, quaternary structure, and sarco(endo)plasmic reticulum calcium ATPase binding measured by fluorescence resonance energy transfer in living cells. J. Biol. Chem. 2008;283:12202–12211. doi: 10.1074/jbc.M707590200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li M., Reddy L.G., et al. Thomas D.D. A fluorescence energy transfer method for analyzing protein oligomeric structure: application to phospholamban. Biophys. J. 1999;76:2587–2599. doi: 10.1016/S0006-3495(99)77411-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hou Z., Kelly E.M., Robia S.L. Phosphomimetic mutations increase phospholamban oligomerization and alter the structure of its regulatory complex. J. Biol. Chem. 2008;283:28996–29003. doi: 10.1074/jbc.M804782200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blackwell D.J., Zak T.J., Robia S.L. Cardiac calcium ATPase dimerization measured by cross-linking and fluorescence energy transfer. Biophys. J. 2016;111:1192–1202. doi: 10.1016/j.bpj.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King C., Raicu V., Hristova K. Understanding the FRET signatures of interacting membrane proteins. J. Biol. Chem. 2017;292:5291–5310. doi: 10.1074/jbc.M116.764282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.King C., Sarabipour S., et al. Hristova K. The FRET signatures of noninteracting proteins in membranes: simulations and experiments. Biophys. J. 2014;106:1309–1317. doi: 10.1016/j.bpj.2014.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.King C., Stoneman M., et al. Hristova K. Fully quantified spectral imaging reveals in vivo membrane protein interactions. Integr. Biol. 2016;8:216–229. doi: 10.1039/c5ib00202h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ha K.N., Masterson L.R., et al. Robia S.L. Lethal Arg9Cys phospholamban mutation hinders Ca2+-ATPase regulation and phosphorylation by protein kinase A. Proc. Natl. Acad. Sci. USA. 2011;108:2735–2740. doi: 10.1073/pnas.1013987108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alford R.F., Smolin N., et al. Robia S.L. Protein docking and steered molecular dynamics suggest alternative phospholamban-binding sites on the SERCA calcium transporter. J. Biol. Chem. 2020;295:11262–11274. doi: 10.1074/jbc.RA120.012948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacLennan D.H., Asahi M., Tupling A.R. The regulation of SERCA-type pumps by phospholamban and sarcolipin. Ann. N. Y. Acad. Sci. 2003;986:472–480. doi: 10.1111/j.1749-6632.2003.tb07231.x. [DOI] [PubMed] [Google Scholar]
- 32.Bidwell P., Blackwell D.J., et al. Robia S.L. Phospholamban binds with differential affinity to calcium pump conformers. J. Biol. Chem. 2011;286:35044–35050. doi: 10.1074/jbc.M111.266759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hicks M.J., Shigekawa M., et al. Katz A.M. Mechanism by which cyclic adenosine 3’:5’-monophosphate-dependent protein kinase stimulates calcium transport in cardiac sarcoplasmic reticulum. Circ Res. 1979;44:384–391. doi: 10.1161/01.res.44.3.384. [DOI] [PubMed] [Google Scholar]
- 34.Nelson B.R., Makarewich C.A., et al. Olson E.N. A peptide encoded by a transcript annotated as long noncoding RNA enhances SERCA activity in muscle. Science. 2016;351:271–275. doi: 10.1126/science.aad4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fisher M.E., Bovo E., Aguayo-Ortiz R., Cho E.E., Pribadi M.P., et al. Dalton M.P. Dwarf open reading frame (DWORF) is a direct activator of the sarcoplasmic reticulum calcium pump SERCA. Elife. 2021 doi: 10.7554/eLife.65545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li A., Yuen S.L., Stroik D.R., Kleinboehl E., Cornea R.L., Thomas D.D. The transmembrane peptide DWORF activates SERCA2a via dual mechanisms. J Biol Chem. 2021;296:100412. doi: 10.1016/j.jbc.2021.100412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seflova J., Habibi N.R., et al. Robia S.L. Fluorescence lifetime imaging microscopy reveals sodium pump dimers in live cells. J. Biol. Chem. 2022;298:101865. doi: 10.1016/j.jbc.2022.101865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu A.Y., Aguayo-Ortiz R., et al. Michel Espinoza-Fonseca L. Homologous cardiac calcium pump regulators phospholamban and sarcolipin adopt distinct oligomeric states in the membrane. Comput. Struct. Biotechnol. J. 2022;20:380–384. doi: 10.1016/j.csbj.2021.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Autry J.M., Rubin J.E., et al. Thomas D.D. Oligomeric interactions of sarcolipin and the Ca-ATPase. J. Biol. Chem. 2011;286:31697–31706. doi: 10.1074/jbc.M111.246843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akin B.L., Hurley T.D., et al. Jones L.R. The structural basis for phospholamban inhibition of the calcium pump in sarcoplasmic reticulum. J. Biol. Chem. 2013;288:30181–30191. doi: 10.1074/jbc.M113.501585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones L.R., Simmerman H.K., et al. Wegener A.D. Purification and characterization of phospholamban from canine cardiac sarcoplasmic reticulum. J. Biol. Chem. 1985;260:7721–7730. [PubMed] [Google Scholar]
- 42.Simmerman H.K., Lovelace D.E., Jones L.R. Secondary structure of detergent-solubilized phospholamban, a phosphorylatable, oligomeric protein of cardiac sarcoplasmic reticulum. Biochim. Biophys. Acta. 1989;997:322–329. doi: 10.1016/0167-4838(89)90203-3. [DOI] [PubMed] [Google Scholar]
- 43.Reddy L.G., Jones L.R., et al. Stokes D.L. Functional reconstitution of recombinant phospholamban with rabbit skeletal Ca(2+)-ATPase. J. Biol. Chem. 1995;270:9390–9397. doi: 10.1074/jbc.270.16.9390. [DOI] [PubMed] [Google Scholar]
- 44.Zamoon J., Nitu F., et al. Veglia G. Mapping the interaction surface of a membrane protein: unveiling the conformational switch of phospholamban in calcium pump regulation. Proc. Natl. Acad. Sci. USA. 2005;102:4747–4752. doi: 10.1073/pnas.0406039102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Autry J.M., Jones L.R. Functional Co-expression of the canine cardiac Ca2+ pump and phospholamban in Spodoptera frugiperda (Sf21) cells reveals new insights on ATPase regulation. J. Biol. Chem. 1997;272:15872–15880. doi: 10.1074/jbc.272.25.15872. [DOI] [PubMed] [Google Scholar]
- 46.Kimura Y., Kurzydlowski K., et al. MacLennan D.H. Phospholamban inhibitory function is activated by depolymerization. J. Biol. Chem. 1997;272:15061–15064. doi: 10.1074/jbc.272.24.15061. [DOI] [PubMed] [Google Scholar]
- 47.Zvaritch E., Backx P.H., et al. MacLennan D.H. The transgenic expression of highly inhibitory monomeric forms of phospholamban in mouse heart impairs cardiac contractility. J. Biol. Chem. 2000;275:14985–14991. doi: 10.1074/jbc.275.20.14985. [DOI] [PubMed] [Google Scholar]
- 48.Cornea R.L., Jones L.R., et al. Thomas D.D. Mutation and phosphorylation change the oligomeric structure of phospholamban in lipid bilayers. Biochemistry. 1997;36:2960–2967. doi: 10.1021/bi961955q. [DOI] [PubMed] [Google Scholar]
- 49.Yap J.Q., Seflova J., et al. Robia S.L. FXYD proteins and sodium pump regulatory mechanisms. J. Gen. Physiol. 2021;153:e202012633. doi: 10.1085/jgp.202012633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Song Q., Pallikkuth S., et al. Robia S.L. Phosphomimetic mutations enhance oligomerization of phospholemman and modulate its interaction with the Na/K-ATPase. J. Biol. Chem. 2011;286:9120–9126. doi: 10.1074/jbc.M110.198036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.