Abstract
The type 2a sarco/endoplasmic reticulum Ca2+-ATPase (SERCA2a) plays a central role in the intracellular Ca2+ homeostasis of cardiac myocytes, pumping Ca2+ from the cytoplasm into the sarcoplasmic reticulum (SR) lumen to maintain relaxation (diastole) and prepare for contraction (systole). Diminished SERCA2a function has been reported in several pathological conditions, including heart failure. Therefore, development of new drugs that improve SERCA2a Ca2+ transport is of great clinical significance. In this study, we characterized the effect of a recently identified N-aryl-N-alkyl-thiophene-2-carboxamide (or compound 1) on SERCA2a Ca2+-ATPase and Ca2+ transport activities in cardiac SR vesicles, and on Ca2+ regulation in a HEK293 cell expression system and in mouse ventricular myocytes. We found that compound 1 enhances SERCA2a Ca2+-ATPase and Ca2+ transport in SR vesicles. Fluorescence lifetime measurements of fluorescence resonance energy transfer between SERCA2a and phospholamban indicated that compound 1 interacts with the SERCA-phospholamban complex. Measurement of endoplasmic reticulum Ca2+ dynamics in HEK293 cells expressing human SERCA2a showed that compound 1 increases endoplasmic reticulum Ca2+ load by enhancing SERCA2a-mediated Ca2+ transport. Analysis of cytosolic Ca2+ dynamics in mouse ventricular myocytes revealed that compound 1 increases the action potential-induced Ca2+ transients and SR Ca2+ load, with negligible effects on L-type Ca2+ channels and Na+/Ca2+ exchanger. However, during adrenergic receptor activation, compound 1 did not further increase Ca2+ transients and SR Ca2+ load, but it decreased the propensity toward Ca2+ waves. Suggestive of concurrent desirable effects of compound 1 on RyR2, [3H]-ryanodine binding to cardiac SR vesicles shows a small decrease in nM Ca2+ and a small increase in μM Ca2+. Accordingly, compound 1 slightly decreased Ca2+ sparks in permeabilized myocytes. Thus, this novel compound shows promising characteristics to improve intracellular Ca2+ dynamics in cardiomyocytes that exhibit reduced SERCA2a Ca2+ uptake, as found in failing hearts.
Significance
SERCA2a plays a central role in the intracellular Ca2+ homeostasis of cardiac myocytes, pumping Ca2+ from the cytosol into the sarcoplasmic reticulum (SR) lumen to maintain SR Ca2+ load. In this study, we characterized the effect N-aryl-N-alkyl-thiophene-2-carboxamide or compound 1 on SERCA2a function. Compound 1 enhances SERCA2a-mediated Ca2+ transport in SR vesicles, increases endoplasmic reticulum Ca2+ load and endoplasmic reticulum Ca2+ uptake in HEK293 cells expressing human SERCA2a, and enhances SR Ca2+ load and Ca2+ transients in cardiomyocytes. Thus, this novel compound can be used to improve intracellular Ca2+ dynamics and contraction in cardiomyocytes that exhibit reduced SERCA2a function, as found in failing hearts.
Introduction
The sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) plays a central role in Ca2+ signaling by removing cytosolic Ca2+ and maintaining the sarco/endoplasmic reticulum (SR/ER) Ca2+ load. This pump uses the energy of ATP hydrolysis to translocate two Ca2+ ions from the cytosol into the SR/ER lumen, creating a steep Ca2+ gradient between the ER/SR lumen and the cytosol. This gradient is critical for Ca2+ signaling, as activation of SR/ER Ca2+ release channels produces a robust cytosolic Ca2+ transient that is essential for regulation of many cellular processes, including growth, migration, secretion, gene regulation, metabolism, neurotransmission, and contraction (1). Due to its critical role in Ca2+ homeostasis, SERCA expression and function are tightly regulated by posttranslational modifications, hormones, regulatory peptides, and microRNAs (2).
There are 10 isoforms of SERCA expressed in different tissues of mammalian species (3). The type 2a isoform or SERCA2a plays a central role in the cardiac contractile cycle. The rate of SERCA2a Ca2+ transport determines the heart’s relaxation rate during diastole when the heart refills with blood. SERCA2a activity also plays a major role in setting SR Ca2+ load, which determines the magnitude of SR Ca2+ release and the strength of cardiac contraction during systole (4). In ventricular myocardium, SERCA2a activity is predominantly regulated by the small transmembrane protein phospholamban (PLB). Under resting conditions, PLB binds to SERCA2a and inhibits SR Ca2+ transport by decreasing the pump’s apparent affinity for cytosolic Ca2+ (5). During adrenergic stress, phosphorylation of PLB by cAMP-dependent protein kinase (PKA) relieves SERCA2a inhibition by PLB, enhancing SR Ca2+ uptake (6). SERCA2a regulation by PKA is the major mechanism behind accelerated relaxation and increased contraction strength during adrenergic receptor activation. Diminished SERCA function and altered regulation have been reported in several pathological conditions, such as cardiac and skeletal myopathies (7,8,9). Consequently, the Ca2+ pump has become a promising therapeutic target for treating muscle diseases associated with disrupted Ca2+ homeostasis. Improving SERCA function has been also shown to be a viable therapeutic strategy in animal models of type 2 diabetes and Parkinson disease (10,11).
Existing strategies to improve SERCA function during pathological conditions include in vivo gene delivery (12,13,14,15) and small-molecule allosteric SERCA activators (9,10,11,16,17,18). Although SERCA2a gene therapy showed promising results in initial clinical studies (19,20), it failed to reach primary and secondary endpoints in a subsequent clinical trial on heart failure (CUPID 2) (21). Although the exact reasons are unclear, the probable culprits were protocol changes versus CUPID 1. The pharmacological approach has been shown to be effective in activating SERCA function. However, its clinical application is still limited by the scarcity of available lead compounds. Therefore, there is a high demand for developing new drugs that can effectively restore normal Ca2+ homeostasis in the diseased heart by enhancing SERCA2a function. Using both fluorescence resonance energy transfer (FRET) and biochemical measurements of Ca2+-ATPase activity, we have recently identified several new SERCA modulators, including compound 1 (22). In the present study, we took advantage of a sequence of complementary approaches that connect the effects of compound 1 on SERCA2a function and on ER/SR Ca2+ regulation to arrhythmia mitigation in the cardiomyocyte. Our results indicate that new compound 1 may improve intracellular Ca2+ dynamics in cardiomyocytes by increasing SERCA2a-mediated Ca2+ transport and reducing SR Ca2+ leak.
Materials and methods
Vectors and stable cell lines
pCMV R-CEPIA1er was a gift from Dr. Masamitsu Iino (Addgene, USA). The vector encoding the human type 2 ryanodine receptor (RyR2) cDNA fused to GFP at the N-terminus domain was kindly provided by Dr. Christopher George (University of Cardiff, UK). Human wild-type SERCA2a (SERCA2aWT) cDNA was cloned into the mCerluean-M1 (mCer) modified plasmid (Addgene, USA) using KpnI and NotI restriction enzymes, yielding SERCA2a fused to mCer at the N-terminus. The sequence for human PLB was synthesized by GenScript and inserted at the 3′ end of mMaroon1 to create a mMaroon1-PLB fusion construct. SERCA2a cDNA was also cloned into the inducible expression vector pcDNA5/FRT/TO for the generation of SERCA2a stable cell line. The sequences were all verified by single pass primer extension analysis (ACGT Inc., USA).
Chemical synthesis
Compound 1 has been identified in a screen of DIVERSet-CL (ChemBridge, San Diego, CA), and initially tested as repurchased from the ChemBridge Hit2Lead online chemical store (compound ID, DS81260867) and validated via mass spectrometry (MS), 1H and 13C NMR. To obtain sufficient quantities for this project, we have re-synthesized compound 1 (Fig. 1 a). Briefly, compound 1 was successfully synthesized from 2-methoxybenzylamine, 1-bromopropane, and 4,5-dimethylthiophene-2-carboxylic acid in a two-step synthetic procedure. First, the propyl group was transferred from 1-bromopropane to 2-methoxybenzylamine by N-alkylation in DMSO. The obtained N-(2-methoxybenzyl)propan-1-amine was then coupled to 4,5-dimethylthiophene-2-carboxylic acid employing HBTU/HOBt under microwave irradiation to obtain N-(2-methoxybenzyl)-4,5-dimethyl-N-propylthiophene-2-carboxamide (i.e., compound 1) in 65% yield.
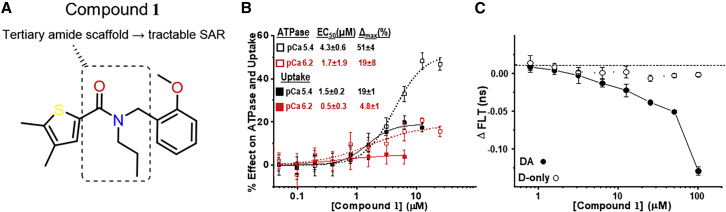
Figure 1.
Compound 1 chemical structure and concentration response curves of Ca2+-transport and Ca2+-ATPase activity and FRET of SERCA-PLB interaction. (A) Chemical structure of compound 1. (B) Effect of compound 1 on Ca2+-transport and Ca2+-ATPase activity of SR vesicles isolated from porcine heart tissue. Measurements were carried out in the presence of compound 1 at the indicated concentrations. Data are represented as means ± SEM (n = 3) and were fitted using the Hill function, yielding compound potency (EC50) and maximum effect (Δmax) as indicated for ATPase (open symbols) and uptake (solid symbols), at Vmax [Ca2+] (black) and intermediate [Ca2+] (red). (C) Fluorescence lifetime (FLT) response of the SERCA-PLB FRET biosensor to a range of [compound 1]. Measurements were conducted in 384-well microplates read in an FLT plate reader. Samples were obtained from a stable HEK cell line expressing the FRET biosensor (solid symbols) or a donor-alone control (open symbols). Null controls containing the corresponding volume of DMSO were read at the same time. Data are represented as means ± SEM (n = 3). To see this figure in color, go online.
SERCA2a Ca-ATPase and Ca2+-uptake measurements using porcine cardiac SR vesicles
Cardiac SR vesicles were isolated from porcine ventricular tissue, as described (23). To determine compound effect on the Ca2+-dependent ATP hydrolysis activity of SERCA2a, we used an NADH-coupled enzyme-linked assay in 384-well plate format, as previously described (16). Briefly, each well contained a solution (termed “assay mix”) consisting of 50 mM MOPS (pH 7.0), 100 mM KCl, 5 mM MgCl2, 1 mM EGTA, 0.2 mM NADH, 1 mM phosphoenol pyruvate, 10 IU/mL of pyruvate kinase, 10 IU/mL of lactate dehydrogenase, 1 μM of the calcium ionophore A23187, and added CaCl2 to obtain pCa 6.2 (intermediate Ca) and pCa 5.4 (Vmax Ca). Next, 10 μg/mL of cardiac SR, CaCl2, the tested small-molecule, and the assay mix were incubated for 20 min at 21°C. The assay was started upon the addition of ATP, to a final concentration of 5 mM (80 μL total assay volume). The time-dependence of 340-nm absorbance was measured in a SpectraMax Plus384 microplate spectrophotometer (Molecular Devices, Sunnyvale, CA).
To determine compound effect on the Ca2+-uptake activity of SERCA2a, we used an oxalate-supported assay, as previously described (24). A solution consisting of 50 mM MOPS (pH 7.0), 100 mM KCl, 30 mg/mL sucrose, 1 mM EGTA, 10 mM potassium oxalate, 2μM Fluo-4, 30 μg/mL cardiac SR vesicles, and CaCl2 calculated to reach the desired free [Ca2+] (intermediate or Vmax Ca, as above) was dispensed into 384-well plates containing the tested small molecule and incubated at 21°C for 20 min while protected from light. To start the reaction, MgATP was added to a final concentration of 5 mM, and the decrease in 485-nm excited fluorescence was monitored at 520 nm for 15 min using an FLIPR Tetra (Molecular Devices, San Jose, CA).
[3H]ryanodine binding to SR vesicles
Cardiac SR vesicles were isolated from porcine cardiac ventricle using previously described protocols (25). Using 96-well microplates, SR samples (3 mg/mL) were preincubated 30 min with DMSO or 10 μM compound 1 (dissolved in DMSO, final DMSO 0.02% in the well) in media containing 20 mM PIPES (pH 7.0), 150 mM KCl, 5 mM GSH, 2 mM DTT, 1 μg/mL aprotinin/leupeptin, 0.1 mg/mL BSA, 5 mM ATP, 1 mM EGTA plus CaCl2 (calculated using MaxChelator to yield 0.1 or 30 μM free Ca2+). Binding of [3H]ryanodine was determined after a 3-h incubation at 37°C. Nonspecific and maximal [3H]ryanodine binding to SR were separately assessed by addition of 40 μM nonradioactively labeled ryanodine or 5 mM adenylyl-imidodiphosphate, respectively. These controls were each present in four wells/plate. Samples were filtered through grade GF/B Glass Microfiber filters (Brandel, Gaithersburg, MD) using a 96-channel Brandel harvester, and filters placed in 4 mL of Ecolite scintillation cocktail (MP Biomedicals, Solon, OH). [3H] retained on the filter was determined using a PerkinElmer Tri-Carb 4810 scintillation counter.
Isolation of ventricular myocytes
Male and female C57Bl6/J mice, (13 animals, Jackson Laboratories) were housed according to approved IACUC guidelines. Mice aged between 2 and 5 months were anesthetized using isoflurane (1%). After thoracotomy, hearts were quickly excised, immersed in Ca2+ free buffer, mounted on a Langendorff apparatus, and retrogradely perfused with a solution (37°C) containing Liberase H (Roche), according to a procedure described previously (26). The left ventricle was excised from the digested heart, placed in stop buffer containing BSA 1 mg/mL, cut into several pieces (average size 1 mm), and gently triturated into single cells. Myocytes were pelleted by gravity (∼0.1 mL) and resuspended in low-Ca2+ Tyrode buffer (in mM: NaCl 140; KCl 4; CaCl2 0.1; MgCl2 1; glucose 10; Hepes 10; pH 7.4). [Ca2+] was gradually adjusted to 1 mM. Isolated cardiomyocytes were stored at room temperature (20°C). All chemicals and reagents were purchased from Sigma-Aldrich (St Louis, MO, USA).
Culturing and transfection of HEK293 cells
HEK293 cells at 60%–80% confluency were transiently co-transfected with plasmids containing the cDNA of RyR2, SERCA2a, and R-CEPIA1er. Experiments were conducted 48 h after transfection to obtain the optimal level of recombinant proteins expression. Flp-In T-Rex-293 stably expressing SERCA2a were co-transfected with plasmids containing the cDNA of RyR2 and R-CEPIA1er, using the same conditions for the HEK293 cells. The surface membrane was permeabilized with saponin (0.005%). Experiments were conducted after washing out saponin with an experimental solution (in mM): K-aspartate 100; KCl 15; KH2PO4 5; MgATP 5; EGTA 0.35; CaCl2 0.22; MgCl2 0.75; HEPES 10; dextran (MW: 40,000) 2% and pH 7.2. Free [Ca2+] and [Mg2+] of this solution were 200 nM and 1 mM, respectively.
Confocal microscopy
Changes in the cytosolic [Ca2+] ([Ca2+]i) and the luminal ER [Ca2+] ([Ca2+]ER) were measured with laser scanning confocal microscopy (Radiance 2000 MP, Bio-Rad, UK) equipped with a ×40 oil-immersion objective lens (N.A. = 1.3).
Measurement of [Ca2+]i
To record [Ca2+]i, we used the high-affinity Ca2+ indicator Fluo-4 (Molecular Probes/Invitrogen, Carlsbad, CA, USA). To load the cytosol with Fluo-4, ventricular myocytes were incubated at room temperature with 10 μM Fluo-4 AM for 15 min in Tyrode solution (in mM: NaCl 140; KCl 4; CaCl2 2; MgCl2 1; glucose 10; Hepes 10; pH 7.4), followed by a 20-min wash. Fluo-4 was excited with the 488-nm line of an argon laser, and the emission signal was collected at wavelengths above 515 nm. Changes in [Ca2+]i were expressed as changes in F/F0, where F0 is the Fluo-4 signal at the resting condition. Ca2+ sparks were measured with Fluo-4 in permeabilized ventricular myocytes as described previously (27,28). Permeabilized myocytes were studied in an internal solution composed of (in mM): K-aspartate 100; KCl 15; KH2PO4 5; MgATP 5; EGTA 0.35; CaCl2 0.1; MgCl2 0.75; phosphocreatine 10; HEPES 10; Fluo-4 pentapotassium salt 0.04 mM; dextran (MW: 40,000) 8%, and pH 7.2 (KOH). Free [Ca2+] and [Mg2+] in this solution were 100 nM and 1 mM, respectively. Sparks were detected and analyzed using the SparkMaster algorithm (29).
Measurement of [Ca2+]ER
[Ca2+]ER was recorded as changes in fluorescence intensity of the genetically encoded ER-targeted Ca2+ sensor R-CEPIA1er in HEK293 cells (30,31). R-CEPIA1er was excited with a 514-nm line of the argon laser, and signal was collected at >560 nm. Line scan images were collected at a speed of 20 ms/line. The R-CEPIA1er signal (F) was converted to [Ca2+]ER by the following formula: [Ca2+]SE = Kd × [(F – Fmin)/(Fmax – F)]. Fmax was recorded in 5 mM Ca2+ and 5 μM ionomycin, and Fmin was recorded after ER Ca2+ depletion with 5 mM caffeine. The Kd (Ca2+ dissociation constant) was 564 μM (32). SERCA-mediated Ca2+ uptake was calculated as the first derivative of [Ca2+]ER refilling (d[Ca2+]ER/dt) after RyR2 inhibition with ruthenium red (15 μM) and tetracaine (1 mM). ER Ca2+ uptake rate was plotted as a function of [Ca2+]ER to analyze maximum ER Ca2+ uptake rate and maximum ER Ca2+ load. All 2D images and line scan measurements for [Ca2+]SR were analyzed with ImageJ software (NIH, USA).
Microplate FLT-FRET measurements in live HEK293-6E cells
Using a previously reported FRET biosensor that concatenates (fuses) RFP (acceptor), SERCA, GFP (donor), and PLB in a single polypeptide chain (24), we have determined the profile of fluorescence lifetime FRET (FLT-FRET) response to a range of compound 1 concentrations (concentration response curves, CRCs). This SERCA2a-PLB biosensor was generated starting from a previously developed RFP-SERCA2a construct (33), which was fused to a flexible linker (47 residues) followed by GFP-PLB. A donor-only construct was created using the same approach. Each biosensor DNA was subcloned into the puromycin-resistant expression vector pTT22, and stable clones expressing the SERCA2a-PLB fusion biosensor at high levels were generated as described (24). These clones were used to acquire the FRET CRC measurements in live HEK293-6E cells. Stable cell lines over-expressing either donor-acceptor or donor-only biosensor were cultured in F-17 media (Thermo Fisher) with 200 nM/mL GlutaMAX (Invitrogen), 25 μg/mL Geneticin (Thermo Fisher), and 2.0 μg/mL puromycin (Invitrogen). On the day of testing, cells were harvested and centrifuged at 300 g for 5 min. Cell pellets were resuspended and washed in PBS (Thermo Fisher) three times before being passed through a 70-μm cell strainer. Cell concentration and viability were evaluated by a Countess automated cell counter (Invitrogen) using the Trypan Blue method. Samples for CRCs were prepared in 384-well format using a Mosquito HV liquid dispenser (SPT Labtech, Melbourne, UK). Next, 1 μL of DMSO or compound dissolved in DMSO was dispensed into 384-well black polystyrene plates (Greiner Bio-One) yielding a final assay concentration range of 0–100 μM. Finally, 50 μL of cells at a density of 2.0 × 106 cells/mL were dispensed into each well using a Multidrop Combi (Thermo Fisher Scientific). Plates were sealed, incubated at room temperature, and FLT measurements were taken 120 min using a high-throughput FLT plate reader (Fluorescence Innovations, Minnesota, USA) provided by Photonic Pharma, as previously described (24).
Statistics
Data are presented as mean ± standard error of the mean (SEM) of n measurements. When only two groups were compared, statistical significance was determined by Student's t-test. Significance between multiple groups was determined by one-way ANOVA followed by a Newman-Keuls post hoc test. p < 0.05 was considered statistically significant. Statistical analysis and graphical representation of averaged data were carried out on OriginPro7.5 software (OriginLab, USA).
Results
Effects of compound 1 on SERCA ATPase activity and Ca2+ transport in SR vesicles
Using an enzyme-linked NADH-coupled Ca2+-ATPase assay of rabbit fast skeletal muscle SR, we have carried out a high-throughput screen of 40,000 drug-like small-molecules in the DIVERSet-CL library (ChemBridge, San Diego, CA) (22). In that screen, compound 1 (10 μM) was identified as an enhancer of the Ca2+-ATPase activity as measured at pCa 5.4 ([Ca2+] that produces the maximum ATP hydrolysis rate; Vmax) in SR from rabbit fast skeletal muscle. Here, we used the same enzyme-linked assay to measure the response of Ca2+-ATPase activity of porcine cardiac SR over a range of compound 1 concentrations, thus constructing CRCs (Fig. 1 b). To gain further insight into this compound’s mode of action, we compared CRCs acquired at the Vmax Ca2+ (pCa 5.4) and at intermediate Ca2+ (pCa 6.2). We observed robust (>50%) ATPase activation at Vmax Ca2+, with a low-μM EC50 (5.4 ± 0.3 μM). However, when ATPase was measured at pCa 6.2, maximum activation by compound 1 reached only ∼20%. This behavior was not surprising for a compound that was discovered using a Vmax screen. A similar pattern was found in the CRCs of Ca2+-uptake, where we observed ∼20% activation at Vmax Ca2+ and a much smaller effect at intermediate Ca2+. At the Vmax Ca2+, the uptake CRC had a lower EC50 than the ATPase CRC, which placed the uptake curve above the ATPase curve in the range of 0.3–3 μM compound 1. This suggests an enhanced coupling ratio (transported Ca2+ per hydrolyzed ATP) at Vmax Ca2+. This was not observed for the intermediate-Ca2+ profiles, where the enhancement of Ca2+ uptake was too small to bring the curve above the ATPase CRC. This indicates a decreased coupling ratio at the intermediate Ca2+. For all CRCs, we observed biphasic profiles, with the rollover occurring between 1 and 10 μM of [compound 1]. This behavior (hormesis) is frequently encountered in biology and especially in pharmacology (34).
To determine whether compound 1 directly interacts with the SERCA-PLB complex, we measured its effect on the fluorescence lifetime of the concatenated SERCA-PLB FRET biosensor. We observed a decrease in FLT (increase in FRET), with EC50 ≈ 10 μM (Fig. 1c). The effect of compound 1 on the FLT of the donor-only control was much smaller than on the donor-acceptor construct, thus suggesting that compound 1 directly binds to the targeted complex where it causes a structural change that shortens the distance between the FRET probes.
Effect of compound 1 on ER Ca2+ load and uptake in HEK293 cells expressing human SERCA2a
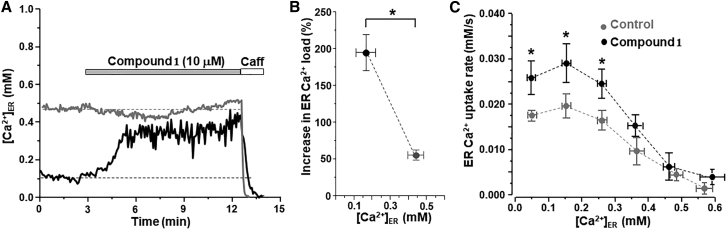
A recently developed assay (31) was used to determine the effect of compound 1 on ER Ca2+ dynamics in a human SERCA2a stable cell line. Changes in [Ca2+]ER were recorded with the ER-targeted Ca2+ sensor R-CEPIA1er (32). To manipulate [Ca2+]ER, cells were transfected with the Ca2+ release channel ryanodine receptor (RyR2). Experiments were conducted in permeabilized cells to control the cytosolic environment and to exclude sarcolemmal Ca2+ transporters from ER Ca2+ regulation. The R-CEPIA1er fluorescence signal was calibrated with ionomycin to convert the fluorescent signal to [Ca2+]ER. Due to differences in RyR2 expression level and therefore in ER Ca2+ leak, resting [Ca2+]ER (or ER Ca2+ load) varied significantly among the studied cells, allowing us to observe the effects of compound 1 on cells with a range of ER Ca2+ loads. Application of compound 1 (10 μM) increased [Ca2+]ER particularly in cells with depleted ER Ca2+ load (black trace, Fig. 2 a). Compound 1 increased [Ca2+]ER by 194% ± 25% (n = 18) in cells with depleted ER Ca2+ load (0.17 ± 0.04 mM; Fig. 2 b). In cells with high ER Ca2+ load, compound 1 was less effective to increase [Ca2+]ER (gray trace, Fig. 2 a). At high ER Ca2+ load (0.45 ± 0.04 mM; Fig. 2 b), compound 1 increased [Ca2+]ER by 55% ± 7% (n = 17).
Figure 2.
Effect of compound 1 on ER Ca2+ load and ER Ca2+ uptake in HEK293 cells expressing human SERCA2a. (A) Effect of compound 1 (10 μM) on [Ca2+]ER in two HEK293 cells with different ER Ca2+ load. Compound 1 increased [Ca2+]ER in cells with depleted ER Ca2+ load (black trace). However, compound 1 did not change [Ca2+]ER in cells with high ER Ca2+ load (gray trace). Caffeine (Caff) application was used to completely deplete [Ca2+]ER. (B) A relative increase in ER Ca2+ load induced by compound 1 in cells with initially depleted ER Ca2+ load (n = 18 cells) and cells with high ER Ca2+ load (n = 17 cells). ∗p < 0.05 versus high load. (C) Effect of compound 1 (10 μM) on SERCA2a-mediated Ca2+ uptake. ER Ca2+ uptake was analyzed as the first derivative (d[Ca2+]ER/dt) and plotted as a function of ER Ca2+ load (control 16 cells and DS 17 cells). ∗p < 0.05 versus control.
Next, we studied the effect of compound 1 on SERCA2a function throughout a wide range of [Ca2+]ER: from completely depleted to maximal ER Ca2+ loads. SERCA2a-mediated Ca2+ transport was analyzed from the rate of [Ca2+]ER reuptake after full Ca2+ depletion induced by RyR2 activation with caffeine followed by RyR2 blockage with ruthenium red and tetracaine, as described before (31). To quantify the rate of SERCA Ca2+ transport, the first derivative of ER Ca2+ uptake (d[Ca2+]ER/dt) was plotted against the corresponding [Ca2+]ER. Compound 1 (10 μM) significantly increased ER Ca2+ uptake rate, particularly at low [Ca2+]ER (between 0.05 and 0.26 mM; Fig. 2 c). These results illustrate that compound 1 can increase ER Ca2+ load by enhancing SERCA2a-mediated Ca2+ uptake.
Effect of compound 1 on Ca2+ signaling in ventricular myocytes
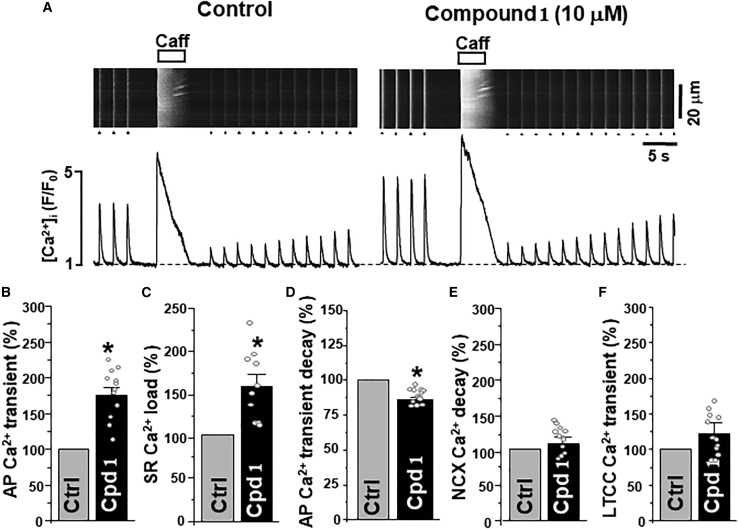
To assess the effect of compound 1 on cardiac Ca2+ regulation, action potential (AP)- and caffeine-induced Ca2+ transients were measured in mouse ventricular myocytes (Fig. 3 a). To evoke AP-induced Ca2+ transients, myocytes were electrically stimulated at 0.5 Hz. The amplitude of Ca2+ transient during caffeine (5 mM) application was used as an index of SR Ca2+ load. Compound 1 (10 μM) increased AP-induced Ca2+ transients to 175% ± 12% (n = 12 cells; Fig. 3b) and SR Ca2+ load to 153% ± 13% (n = 10 cells; Fig. 3 c). Moreover, compound 1 increased the rate of AP-induced Ca2+ transient decay by 14% ± 2% (n = 12 cells; Fig. 3 d). The decay rate was measured as the time constant of exponential decay, which changed from 308 ± 17 to 267 ± 19 ms (n = 12). These effects developed within 5 min and were reversible after washout. However, compound 1 did not change the rate of [Ca2+]i decay during the caffeine application (Fig. 3 e). As this decay is mainly mediated by NCX-dependent Ca2+ extrusion, these results suggest that 1 does not affect NCX function. The first AP-induced Ca2+ transient after complete depletion of [Ca2+]SR with caffeine is mainly mediated by Ca2+ current via LTCC (35). Analysis of LTCC-mediated Ca2+ transients suggests that compound 1 does not affect LTCC activity (Fig. 3 f). Therefore, the increase of AP-induced Ca2+ transients and SR Ca2+ load during the compound 1 application is likely to be mediated by enhanced SERCA2a-mediated Ca2+ uptake.
Figure 3.
Effect of compound 1 on Ca2+ signaling in ventricular myocytes. (A) Confocal line scan images of [Ca2+]i with corresponding F/F0 profiles of AP-induced (marked by triangles) and caffeine-induced Ca2+ transients (or SR Ca2+ load) in control conditions and during compound 1 (10 μM) application. Effect of compound 1 (Cpd 1; n = 12 myocytes) on AP-induced Ca2+ transient amplitude (B), SR Ca2+ load (C), AP-induced Ca2+ transient decay (D), NCX-mediated [Ca2+]i decay rate (E), and LTCC-mediated Ca2+ transient amplitude (F). ∗p < 0.05 versus control.
Effect of compound 1 on Ca2+ regulation under adrenergic activation and increased SR Ca2+ leak
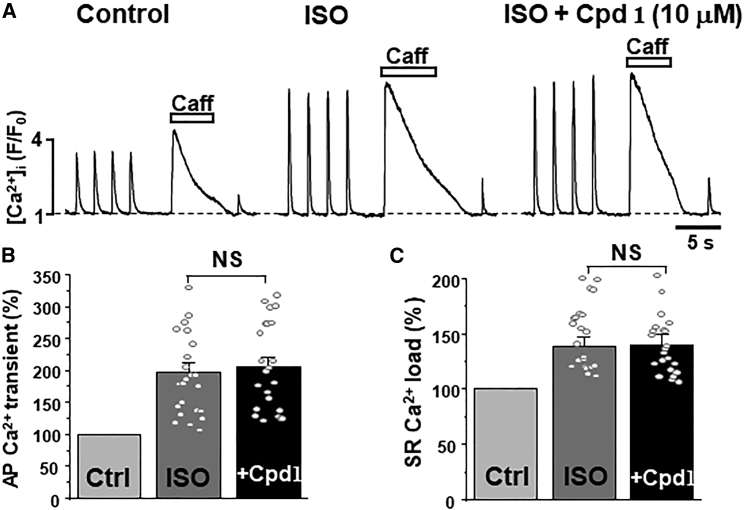
Beta adrenergic receptor activation with isoproterenol (ISO; 0.1 μM) increased the AP-induced Ca2+ transient amplitude to 197% ± 16% of control (n = 22 cells; Fig. 4 a and b). This effect is largely mediated by an increase in SR Ca2+ uptake and sarcolemmal Ca2+ current due to PLB and LTCC phosphorylation by PKA (36). As expected, ISO increased both SR Ca2+ load to 139% ± 8% of control (n = 22 cells; Fig. 4 c) and LTCC-mediated Ca2+ transients to 220% ± 45% of control (n = 22 cells; Fig. 4 A). We then tested whether compound 1 could further increase SR Ca2+ load during adrenergic receptor stimulation. This analysis revealed that compound 1 (10 μM) had a minimal additional effect on the AP-induced Ca2+ transient amplitude (Fig. 4 b) and SR Ca2+ load (Fig. 4 c) already increased by ISO.
Figure 4.
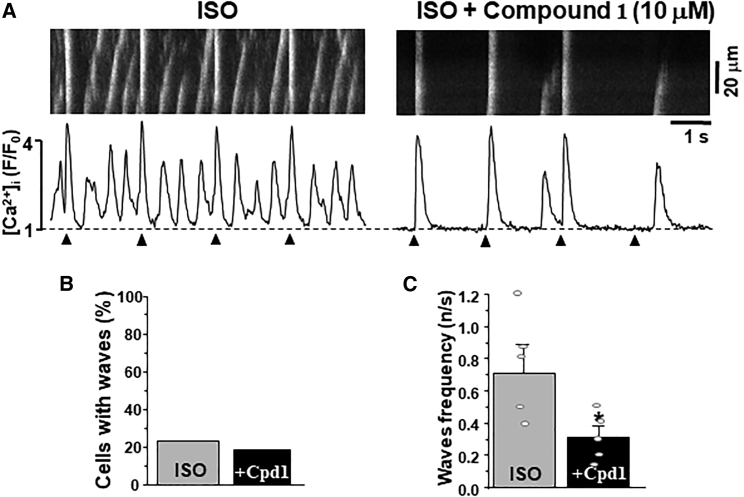
Effect of compound 1 on Ca2+ signaling during adrenergic receptor activation in ventricular myocytes. (A) F/F0 profiles of changes in [Ca2+]i during AP- and caffeine-induced Ca2+ transients in control conditions, during adrenergic receptor activation with ISO (0.1 μM) and during the consecutive application of compound 1 (10 μM). Effect of ISO and compound 1 in the presence of ISO on AP-induced Ca2+ transient amplitude (B) and SR Ca2+ load (C). A total of 22 myocytes were studied in these experiments. Among the studied cells, five myocytes exhibited Ca2+ waves during ISO application (Fig. 5).
In ventricular myocytes, SR Ca2+ overload during adrenergic receptor activation is frequently associated with spontaneous Ca2+ waves (Fig. 5 a) (37,38,39). We found that 5 myocytes out of 22 studied (or ∼22%) exhibited Ca2+ waves during ISO application. Vehicle time-control ISO experiments showed that these Ca2+ waves appeared 5 min after ISO application and remained consistent during adrenergic stimulation. Application of compound 1 did not increase the fraction of myocytes with Ca2+ waves during adrenergic stimulation (Fig. 5 b). Importantly, in cells that produced Ca2+ waves during ISO application, compound 1 significantly reduced Ca2+ wave frequency (Fig. 5 c). This effect was associated with a decrease of the Ca2+ wave propagation velocity from 253.6 ± 38.7 μm/s (ISO alone) to 155.3 ± 27.3 μm/s (ISO and compound 1; n = 5 cells).
Figure 5.
Effect of compound 1 on Ca2+ waves during adrenergic receptor activation in ventricular myocytes. (A) Confocal line scan images of [Ca2+]i with corresponding F/F0 profiles of AP-induced (marked by triangles) and spontaneous Ca2+ waves during ISO (0.1 μM) and during the consecutive application of compound 1 (10 μM). (B) Effect of compound 1 (Cpd 1) on the Ca2+ wave occurrence during adrenergic receptor activation with ISO. (C) Effect of compound 1 (Cpd 1) on the Ca2+ wave frequency during adrenergic receptor activation. ∗p < 0.05 versus control.
It has been shown that several heart pathologies, including heart failure and myocardial infarction, are associated with increased RyR2-mediated Ca2+ leak (40,41,42,43). Thus, we analyzed an effect of Compound 1 on Ca2+ regulation under the conditions of increased SR Ca2+ leak. In these experiments, RyR2-mediated Ca2+ leak was selectively increased by treating cells with a low dose of the RyR agonist caffeine. Application of 250 μM caffeine to myocytes paced at 0.5 Hz produced an immediate increase in Ca2+ transient amplitude, with afterward a decline of SR Ca2+ release to a new steady-state level. Under these conditions, compound 1 (10 μM) was still effective to increase Ca2+ transient amplitude by 38.2% ± 5.7% and SR Ca2+ load by 8.6 ± 2.3 (n = 8 cells; Fig. 6).
Figure 6.
Effect of compound 1 on Ca2+ signaling under conditions of increased SR Ca2+ leak. (A) F/F0 profiles of changes in [Ca2+]i during AP- and caffeine-induced Ca2+ transients in control conditions, during RyR2 activation with caffeine (250 μM) and during the consecutive application of compound 1 (10 μM). Effect of caffeine and compound 1 in the presence of caffeine on AP-induced Ca2+ transient amplitude (B) and SR Ca2+ load (C).
Effect of compound 1 on RyR2 function
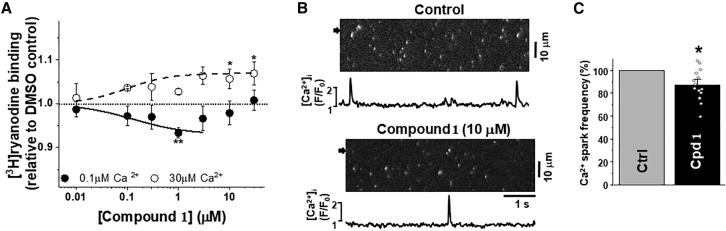
The observation that compound 1 inhibited myocyte Ca2+ waves led to the hypothesis that effects on RyR2-mediated Ca2+ leak might contribute to its mechanism of action. To test this, we first used an established index of RyR channel open state, i.e., [3H]ryanodine binding to SR vesicles. We performed this assay using isolated cardiac SR (as described in materials and methods), and we observed that compound 1 is slightly inhibitory in 0.1 μM Ca2+ and slightly activatory in 30 μM Ca2+ (Fig. 7 a). These results suggest that RyR2 leak might also be inhibited in cells.
Figure 7.
Effect of compound 1 on measures of RyR2 function. (A) Concentration response curves of [3H]ryanodine binding to RyR2. Using SR isolated from porcine heart in the presence of compound 1 concentrations ranging 0–100 μM, we measured [3H]ryanodine binding to RyR2 in 0.1 μM Ca2+ (solid symbol) or 30 μM Ca2+ (open symbol). Data are shown normalized relative to the values sans-drug DMSO control (dotted line), means ± SD, n = 4–6. ∗p < 0.05, ∗∗p < .01, ∗∗∗p < .001 for samples versus DMSO control using Student’s unpaired t-test. (B) Ca2+ sparks in permeabilized ventricular myocytes. Confocal line scan images of [Ca2+]i with corresponding F/F0 profiles of Ca2+ sparks in control conditions and during compound 1 (10 μM) application. F/F0 profiles were obtained by averaging the fluorescence over the 1-μm-wide regions indicated by the black arrows. (C) Effect of compound 1 on Ca2+ spark frequency. 14 myocytes were studied in these experiments. ∗p < 0.05 versus control.
Therefore, we determined whether compound 1 affects a cellular measure of RyR2-mediated Ca2+ leak, by studying its effect on Ca2+ sparks in permeabilized ventricular myocytes. After sarcolemma permeabilization, Ca2+ sparks were observed at a stable frequency of 11.5 ± 3.1 sparks×s−1×(100 μm)−1. Fig. 7 b shows Fluo-4 line scan images and corresponding F/F0 profiles of Ca2+ sparks recorded in control conditions and in the presence of 1 (10 μM). Compound 1 produced a small decrease in Ca2+ spark frequency to 90% ± 5% (n = 14 cells; Fig. 7 c). This inhibitory effect on Ca2+ sparks developed within 3 min, remained stable during compound 1 application, and was reversible after washout.
Discussion
SERCA is an essential component of Ca2+ signaling in virtually all eukaryotic cells. Consequently, defects in SERCA function can cause significant health problems. For example, mutations in the skeletal muscle SERCA1a isoform cause Brody myopathy, a disease characterized by impaired muscle relaxation (44). Alterations in the cardiac SERCA2a regulation contribute to heart pathologies, such as hypertrophy and heart failure (45,46,47,48). Mutations of the nonmuscle SERCA2b lead to Darier’s disease, an autosomal dominant inherited disorder of skin cells (49). It is not surprising that the Ca2+ pump has attracted attention as an important target for therapeutic approaches for numerous diseases (8,50).
The small molecule evaluated here, compound 1 (Fig. 1 a), has been identified as an enhancer of both the Ca2+-ATPase and Ca2+-transport activity of SERCA at physiologically relevant Ca2+ concentrations (Fig. 1 b). For these reasons, compound 1 has the potential to correct myocyte dysfunction, when that is attributable to diminished SR Ca2+ uptake capacity, as found in various forms of heart failure (48,51,52,53). Examination of compound 1 also reveals favorable physicochemical properties and Ca2+-transport activities. In general, very polar compounds (calculated partition coefficient, clogP <1 and total polar surface area, tPSA >130 Å2) are likely to have poor permeability that restricts their oral bioavailability and tissue distribution, whereas highly lipophilic molecules (clogP >5) are typically poorly soluble, have reduced receptor selectivity, and are rapidly metabolized. Compound 1 has desirable physicochemical properties (MW = 317.4, clogP = 4.5, tPSA = 30 Å2) and does not possess any structural alerts for toxicity or promiscuity (e.g., reactive quinones or metal chelating motifs). For these reasons, compound 1 is very attractive for further development. Exploration of structure-activity relationships (SARs) of compound 1 by modification of the thiophene and methoxybenzyl moieties could further improve the potency and physicochemical properties. This will be the focus of a future study.
To evaluate effects of compound 1 on SERCA2a function in the cellular environment, we used a newly developed approach that directly measures Ca2+ dynamics within the ER lumen (31). Pump function was studied in a human SERCA2a HEK stable cell line transfected with the human Ca2+ release channel, RyR2. Transient RyR2 DNA transfection led to a variability in the RyR2 expression level among cells, which we exploited in this study. Since resting ER Ca2+ load is set by a balance between ER Ca2+ uptake and ER Ca2+ leak, this caused significant variability in ER Ca2+ load between cells. Compound 1 increased ER Ca2+ load only in cells with initially depleted Ca2+ load (Fig. 2 a and b). In cells with ER Ca2+ load approaching the maximal level, compound 1 was less effective to increase [Ca2+]ER. The maximum ER Ca2+ load is determined by the pump’s catalytic efficiency (i.e., ATPase-Ca2+ transport coupling) and the pump’s thermodynamic limit set by the ATP/ADP ratio. Our results suggest that compound 1 increases ER Ca2+ uptake primarily by enhancing the SERCA2a Ca2+ transport kinetics, but not its catalytic efficiency. As a result, a more active pump in the presence of compound 1 can more efficiently compensate ER Ca2+ leak. The direct measurements of SERCA2a-mediated Ca2+ transport further confirmed that compound 1 stimulates ER Ca2+ uptake, particularly at low [Ca2+]ER (Fig. 2 c).
Analysis of intracellular Ca2+ dynamics in ventricular myocytes revealed that compound 1 increases the Ca2+ transients induced by AP. This effect was associated with the increased SR Ca2+ load and the rate of AP-induced Ca2+ transient decay, indicating that compound 1 can stimulate SERCA Ca2+ transport in cardiomyocytes (Fig 3). Further analysis also revealed that small molecule 1 has an insignificant effect on Ca2+ extrusion by NCX and Ca2+ entry via LTCC. Since myocardial contraction is directly proportional to cytosolic [Ca2+], this effect of compound 1 on SERCA2a function should cause a positive inotropic effect, which should increase cardiac output without changes in the heart rate. This effect on cardiac Ca2+ regulation would be particularly beneficial during pathological heart conditions associated with reduced SERCA2a Ca2+ uptake, as found in heart failure with reduced ejection fraction. Importantly, compound 1 did not further increase AP-induced Ca2+ transients and SR Ca2+ load during adrenergic receptor activation (Fig. 4). Results in Fig. 2 c suggest that this lack of additional stimulatory effect on Ca2+ release is due to the SR Ca2+ load approaching its maximal level (i.e., thermodynamic limit) during adrenergic receptor stimulation and PKA activation. This was similar to the Ca2+ load dependence of compound 1 action observed in the HEK cells experiments (Fig. 2). In cardiomyocytes, SR Ca2+ overload is frequently associated with spontaneous SR Ca2+ release in a form of propagating Ca2+ waves (37,38,39). In myocytes with diastolic Ca2+ waves, compound 1 reduced Ca2+ wave frequency. It appears that in these myocytes, SR Ca2+ load could not reach maximal level, due to elevated diastolic SR Ca2+ release (during spontaneous Ca2+ waves) that offsets SERCA Ca2+ transport (37). This suggests that in cells with arrhythmogenic behavior, compound 1 was effective to stimulate SERCA Ca2+ uptake, which prevented wave Ca2+ formation by breaking the positive feed-forward of increased spontaneous Ca2+-induced Ca2+ release.
In cardiomyocytes, an increase in SR Ca2+ load is typically accompanied by increased Ca2+ spark activity (54). Here, we found that compound 1 has the opposite effect, slightly inhibiting Ca2+ sparks (Fig. 7 b), and this is consistent with the small inhibition of [3H]-ryanodine binding to isolated cardiac SR in diastolic (nM) Ca2+ (Fig. 7 a). Moreover, compound 1 causes a small activation of [3H]-ryanodine binding in systolic (μM) Ca2+ (Fig 7 a), which should contribute to the higher amplitude of AP-induced Ca2+-transients. This represents an ideal profile for a therapeutic agent targeting RyR2 (i.e., inhibition of Ca2+ leak and unchanged or higher AP-triggered Ca2+ release). By serendipity, the compound 1 mechanism of action seems to involve synergistic effects on both SERCA2a and RyR2.
In conclusion, the novel compound 1 shows properties consistent with a potential to improve intracellular Ca2+ dynamics in cardiomyocytes with reduced SERCA2a Ca2+ uptake, as found in failing hearts. Future studies will focus on evaluating the ability of compound 1 to improve Ca2+ cycling in cardiomyocytes from animal models of heart failure or derived from iPSCs procured from patients with relevant cardiomyopathies. SAR studies will follow to improve potency and efficacy. The biochemical assays of SERCA and RyR2 function featured here (Figs. 1 and 7, respectively) have the throughput necessary to drive the SAR work (where we expect that hundreds of compounds will be tested). The downstream cellular assays (Figs. 2, 3, 4, 5, and 6) will be essential to establish compound safety and efficacy in the targeted cardiomyopathies.
Author contributions
C.C.A., R.L.C., D.D.T., and A.V.Z. conceived and supervised the study. R.N., E.B., S.L.Y., L.M.T., K.B., R.L.C, D.D.T., and A.V.Z designed and performed experiments. R.N., E.B., S.L.Y., L.M.T., and K.B. performed analysis. R.N., E.B., C.C.A., R.L.C., D.D.T., and A.V.Z. wrote the manuscript. All the authors read and approved the manuscript version to be published.
Acknowledgments
This work was supported by the National Institutes of Health Grants R01HL151990 (to A.V.Z.), R01HL139065 (to D.D.T. and R.L.C.), R01HL138539 (to R.L.C.), and R37AG026160 (to D.D.T.). The authors would like to thank Dr. Christopher George (University of Cardiff, UK) for providing the vector encoding the human RyR2. The authors also would like to thank Dr. Masamitsu Iino for donating the R-CEPIA1er vector.
Declaration of interests
R.L.C. and D.D.T. hold equity in, and serve as executive officers for, Photonic Pharma LLC, which had no role in this study. These relationships have been reviewed and managed by the University of Minnesota.
Editor: Eleonora Grandi
Footnotes
Roman Nikolaienko and Elisa Bovo contributed equally to this work.
References
- 1.Berridge M.J., Lipp P., Bootman M.D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 2.Stammers A.N., Susser S.E., et al. Duhamel T.A. The regulation of sarco(endo)plasmic reticulum calcium-ATPases (SERCA)1. Can. J. Physiol. Pharmacol. 2015;93:843–854. doi: 10.1139/cjpp-2014-0463. [DOI] [PubMed] [Google Scholar]
- 3.Periasamy M., Kalyanasundaram A. SERCA pump isoforms: their role in calcium transport and disease. Muscle Nerve. 2007;35:430–442. doi: 10.1002/mus.20745. [DOI] [PubMed] [Google Scholar]
- 4.Bers D.M. 2d edition. Springer Netherlands; 2001. Excitation-Contraction Coupling and Cardiac Contractile Force. [Google Scholar]
- 5.Cantilina T., Sagara Y., et al. Jones L.R. Comparative studies of cardiac and skeletal sarcoplasmic reticulum ATPases. Effect of a phospholamban antibody on enzyme activation by Ca2+ J. Biol. Chem. 1993;268:17018–17025. [PubMed] [Google Scholar]
- 6.Periasamy M., Bhupathy P., Babu G.J. Regulation of sarcoplasmic reticulum Ca2+ ATPase pump expression and its relevance to cardiac muscle physiology and pathology. Cardiovasc. Res. 2008;77:265–273. doi: 10.1093/cvr/cvm056. [DOI] [PubMed] [Google Scholar]
- 7.Goonasekera S.A., Lam C.K., et al. Sargent M.A. Mitigation of muscular dystrophy in mice by SERCA overexpression in skeletal muscle. J. Clin. Invest. 2011;121:1044–1052. doi: 10.1172/JCI43844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayward C., Banner N.R., et al. Harding S.E. The current and future landscape of SERCA gene therapy for heart failure: a clinical perspective. Hum. Gene Ther. 2015;26:293–304. doi: 10.1089/hum.2015.018. [DOI] [PubMed] [Google Scholar]
- 9.Qaisar R., Bhaskaran S., et al. Van Remmen H. Restoration of SERCA ATPase prevents oxidative stress-related muscle atrophy and weakness. Redox Biol. 2019;20:68–74. doi: 10.1016/j.redox.2018.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang S., Dahl R., et al. Lebeche D. Small molecular allosteric activator of the sarco/endoplasmic reticulum Ca 2+ -ATPase (SERCA) attenuates diabetes and metabolic disorders. J. Biol. Chem. 2016;291:5185–5198. doi: 10.1074/jbc.M115.705012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dahl R. A new target for Parkinson’s disease: small molecule SERCA activator CDN1163 ameliorates dyskinesia in 6-OHDA-lesioned rats. Bioorg. Med. Chem. 2017;25:53–57. doi: 10.1016/j.bmc.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Hajjar R.J., Schmidt U., et al. Rosenzweig A. Modulation of ventricular function through gene transfer in vivo. Proc. Natl. Acad. Sci. USA. 1998;95:5251–5256. doi: 10.1073/pnas.95.9.5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Del Monte F., Harding S.E., et al. Hajjar R.J. Restoration of contractile function in isolated cardiomyocytes from failing human hearts by gene transfer of SERCA2a. Circulation. 1999;100:2308–2311. doi: 10.1161/01.cir.100.23.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lyon A.R., Bannister M.L., et al. Harding S.E. SERCA2a gene transfer decreases sarcoplasmic reticulum calcium leak and reduces ventricular arrhythmias in a model of chronic heart failure. Circ. Arrhythm. Electrophysiol. 2011;4:362–372. doi: 10.1161/CIRCEP.110.961615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Penny W.F., Hammond H.K. Randomized clinical trials of gene transfer for heart failure with reduced ejection fraction. Hum. Gene Ther. 2017;28:378–384. doi: 10.1089/hum.2016.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cornea R.L., Gruber S.J., et al. Thomas D.D. High-throughput FRET assay yields allosteric SERCA activators. J. Biomol. Screen. 2013;18:97–107. doi: 10.1177/1087057112456878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrandi M., Barassi P., et al. Ferrari P. Istaroxime stimulates SERCA2a and accelerates calcium cycling in heart failure by relieving phospholamban inhibition. Br. J. Pharmacol. 2013;169:1849–1861. doi: 10.1111/bph.12278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gruber S.J., Cornea R.L., et al. Thomas D.D. Discovery of enzyme modulators via high-throughput time-resolved FRET in living cells. J. Biomol. Screen. 2014;19:215–222. doi: 10.1177/1087057113510740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jessup M., Greenberg B., et al. Patients A.H. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease ( CUPID ) A phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca 2 ؉ -ATPase in patients with advanced heart failure. Circulation. 2011;124:304–313. doi: 10.1161/CIRCULATIONAHA.111.022889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zsebo K., Yaroshinsky A., et al. Hajjar R.J. Long-term effects of AAV1/SERCA2a gene transfer in patients with severe heart failure: analysis of recurrent cardiovascular events and mortality. Circ. Res. 2014;114:101–108. doi: 10.1161/CIRCRESAHA.113.302421. [DOI] [PubMed] [Google Scholar]
- 21.Greenberg B., Butler J., et al. Zsebo K.M. Calcium upregulation by percutaneous administration of gene therapy in patients with cardiac disease (CUPID 2): a randomised, multinational, double-blind, placebo-controlled, phase 2b trial. Lancet. 2016;387:1178–1186. doi: 10.1016/S0140-6736(16)00082-9. [DOI] [PubMed] [Google Scholar]
- 22.Bidwell P.A., Yuen S.L., et al. Thomas D.D. A large-scale high-throughput screen for modulators of SERCA activity. Biomolecules. 2022;12:1789. doi: 10.3390/biom12121789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fruen B.R., Bardy J.M., et al. Louis C.F. Differential Ca2+ sensitivity of skeletal and cardiac muscle ryanodine receptors in the presence of calmodulin. Am. J. Physiol. Cell Physiol. 2000;279:724–733. doi: 10.1152/ajpcell.2000.279.3.C724. [DOI] [PubMed] [Google Scholar]
- 24.Schaaf T.M., Kleinboehl E., et al. Thomas D.D. Live-cell cardiac-specific high-throughput screening platform for drug-like molecules that enhance Ca2+ transport. Cells. 2020;9:1170. doi: 10.3390/cells9051170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rebbeck R.T., Nitu F.R., et al. Cornea R.L. S100A1 does not compete with calmodulin for ryanodine receptor binding but structurally alters the ryanodine receptor/calmodulin complex. J. Biol. Chem. 2016;291:15896–15907. doi: 10.1074/jbc.M115.713107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuo I.Y., Kwaczala A.T., et al. Ehrlich B.E. Decreased polycystin 2 expression alters calcium-contraction coupling and changes β-adrenergic signaling pathways. Proc. Natl. Acad. Sci. USA. 2014;111:16604–16609. doi: 10.1073/pnas.1415933111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zima A.V., Kockskämper J., et al. Blatter L.A. Pyruvate modulates cardiac sarcoplasmic reticulum Ca2+ release in rats via mitochondria-dependent and -independent mechanisms. J. Physiol. 2003;550:765–783. doi: 10.1113/jphysiol.2003.040345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zima A.V., Picht E., et al. Blatter L.A. Partial inhibition of sarcoplasmic reticulum ca release evokes long-lasting ca release events in ventricular myocytes: role of luminal ca in termination of ca release. Biophys. J. 2008;94:1867–1879. doi: 10.1529/biophysj.107.114694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Picht E., Zima A.V., et al. Bers D.M. SparkMaster: automated calcium spark analysis with ImageJ. Am. J. Physiol. Cell Physiol. 2007;293:C1073–C1081. doi: 10.1152/ajpcell.00586.2006. [DOI] [PubMed] [Google Scholar]
- 30.Bovo E., Martin J.L., et al. Zima A.V. R-CEPIA1er as a new tool to directly measure sarcoplasmic reticulum [Ca] in ventricular myocytes. Am. J. Physiol. Heart Circ. Physiol. 2016;311:H268. doi: 10.1152/ajpheart.00175.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bovo E., Nikolaienko R., et al. Zima A.V. Novel approach for quantification of endoplasmic reticulum Ca 2+ transport. Am. J. Physiol. Heart Circ. Physiol. 2019;316:H1323–H1331. doi: 10.1152/ajpheart.00031.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki J., Kanemaru K., et al. Iino M. Imaging intraorganellar Ca2+ at subcellular resolution using CEPIA. Nat. Commun. 2014;5:4153. doi: 10.1038/ncomms5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schaaf T.M., Peterson K.C., et al. Thomas D.D. High-throughput spectral and lifetime-based FRET screening in living cells to identify small-molecule effectors of SERCA. SLAS Discov. 2017;22:262–273. doi: 10.1177/1087057116680151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calabrese E.J. The emergence of the dose-response concept in biology and medicine. Int. J. Mol. Sci. 2016;17:2034. doi: 10.3390/ijms17122034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bovo E., Dvornikov A.V., et al. Zima A.V. Mechanisms of Ca2+ handling in zebrafish ventricular myocytes. Pflugers Arch. 2013;465:1775–1784. doi: 10.1007/s00424-013-1312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bers D.M. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 37.Bovo E., Lipsius S.L., Zima A.V. Reactive oxygen species contribute to the development of arrhythmogenic Ca2+ waves during β-adrenergic receptor stimulation in rabbit cardiomyocytes. J. Physiol. 2012;590:3291–3304. doi: 10.1113/jphysiol.2012.230748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Curran J., Brown K.H., et al. Shannon T.R. Spontaneous Ca waves in ventricular myocytes from failing hearts depend on Ca2+-calmodulin-dependent protein kinase II. J. Mol. Cell. Cardiol. 2010;49:25–32. doi: 10.1016/j.yjmcc.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Venetucci L.A., Trafford A.W., Eisner D.A. Increasing ryanodine receptor open probability alone does not produce arrhythmogenic calcium waves: threshold sarcoplasmic reticulum calcium content is required. Circ. Res. 2007;100:105–111. doi: 10.1161/01.RES.0000252828.17939.00. [DOI] [PubMed] [Google Scholar]
- 40.Mochizuki M., Yano M., et al. Matsuzaki M. Scavenging free radicals by low-dose carvedilol prevents redox-dependent Ca2+ leak via stabilization of ryanodine receptor in heart failure. J. Am. Coll. Cardiol. 2007;49:1722–1732. doi: 10.1016/j.jacc.2007.01.064. [DOI] [PubMed] [Google Scholar]
- 41.Zima A.V., Bovo E., et al. Blatter L.A. Ca2+ spark-dependent and -independent sarcoplasmic reticulum Ca2+ leak in normal and failing rabbit ventricular myocytes. J. Physiol. 2010;588:4743–4757. doi: 10.1113/jphysiol.2010.197913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Belevych A.E., Terentyev D., et al. Györke S. The relationship between arrhythmogenesis and impaired contractility in heart failure: role of altered ryanodine receptor function. Cardiovasc. Res. 2011;90:493–502. doi: 10.1093/cvr/cvr025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Respress J.L., van Oort R.J., et al. Wehrens X.H.T. Role of RyR2 phosphorylation at S2814 during heart failure progression. Circ. Res. 2012;110:1474–1483. doi: 10.1161/CIRCRESAHA.112.268094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Odermatt A., Taschner P.E., et al. MacLennan D.H. Mutations in the gene-encoding SERCA1, the fast-twitch skeletal muscle sarcoplasmic reticulum Ca2+ ATPase, are associated with Brody disease. Nat. Genet. 1996;14:191–194. doi: 10.1038/ng1096-191. [DOI] [PubMed] [Google Scholar]
- 45.Hasenfuss G., Reinecke H., et al. Drexler H. Relation between myocardial function and expression of sarcoplasmic reticulum Ca2+-ATPase in failing and nonfailing human myocardium. Circ. Res. 1994;75:434–442. doi: 10.1161/01.res.75.3.434. [DOI] [PubMed] [Google Scholar]
- 46.Kiss E., Ball N.A., et al. Walsh R.A. Differential changes in cardiac phospholamban and sarcoplasmic reticular Ca 2+ -ATPase protein levels. Circ. Res. 1995;77:759–764. doi: 10.1161/01.res.77.4.759. [DOI] [PubMed] [Google Scholar]
- 47.O’Rourke B., Kass D.A., et al. Marbán E. Mechanisms of altered excitation-contraction coupling in canine tachycardia-induced heart failure, I: experimental studies. Circ. Res. 1999;84:562–570. doi: 10.1161/01.res.84.5.562. [DOI] [PubMed] [Google Scholar]
- 48.Schmidt U., Hajjar R.J., et al. Gwathmey J.K. Contribution of abnormal sarcoplasmic reticulum ATPase activity to systolic and diastolic dysfunction in human heart failure. J. Mol. Cell. Cardiol. 1998;30:1929–1937. doi: 10.1006/jmcc.1998.0748. [DOI] [PubMed] [Google Scholar]
- 49.Savignac M., Edir A., et al. Hovnanian A. Darier disease: a disease model of impaired calcium homeostasis in the skin. Biochim. Biophys. Acta. 2011;1813:1111–1117. doi: 10.1016/j.bbamcr.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 50.Kranias E.G., Hajjar R.J. Modulation of cardiac contractility by the phopholamban/SERCA2a regulatome. Circ. Res. 2012;110:1646–1660. doi: 10.1161/CIRCRESAHA.111.259754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Currie S., Smith G.L. Enhanced phosphorylation of phospholamban and downregulation of sarco/endoplasmic reticulum Ca2+ ATPase type 2 (SERCA 2) in cardiac sarcoplasmic reticulum from rabbits with heart failure. Cardiovasc. Res. 1999;41:135–146. doi: 10.1016/s0008-6363(98)00241-7. [DOI] [PubMed] [Google Scholar]
- 52.Pieske B., Kretschmann B., et al. Hasenfuss G. Alterations in intracellular calcium handling associated with the inverse force-frequency relation in human dilated cardiomyopathy. Circulation. 1995;92:1169–1178. doi: 10.1161/01.cir.92.5.1169. [DOI] [PubMed] [Google Scholar]
- 53.Jiang M.T., Lokuta A.J., et al. Valdivia H.H. Abnormal Ca2+ release, but normal ryanodine receptors, in canine and human heart failure. Circ. Res. 2002;91:1015–1022. doi: 10.1161/01.res.0000043663.08689.05. [DOI] [PubMed] [Google Scholar]
- 54.Lukyanenko V., Viatchenko-Karpinski S., et al. Györke S. Dynamic regulation of sarcoplasmic reticulum Ca(2+) content and release by luminal Ca(2+)-sensitive leak in rat ventricular myocytes. Biophys. J. 2001;81:785–798. doi: 10.1016/S0006-3495(01)75741-4. [DOI] [PMC free article] [PubMed] [Google Scholar]