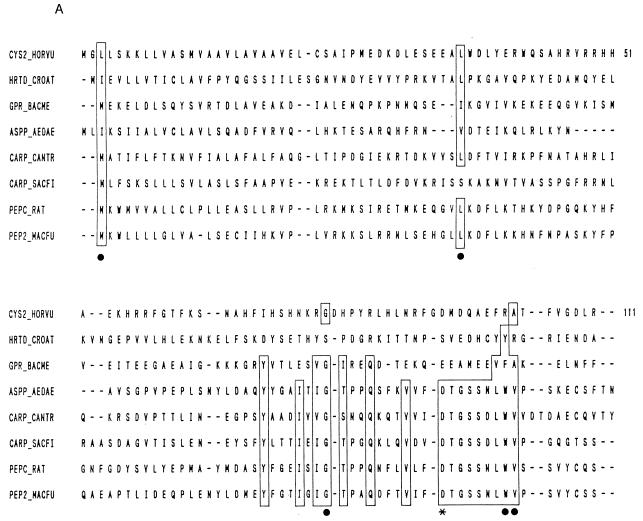
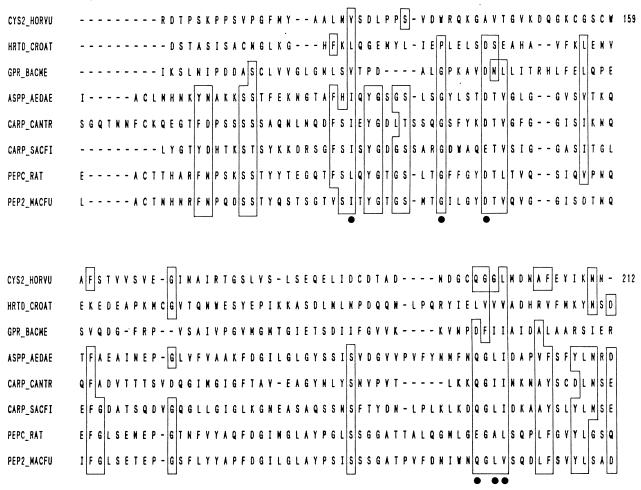
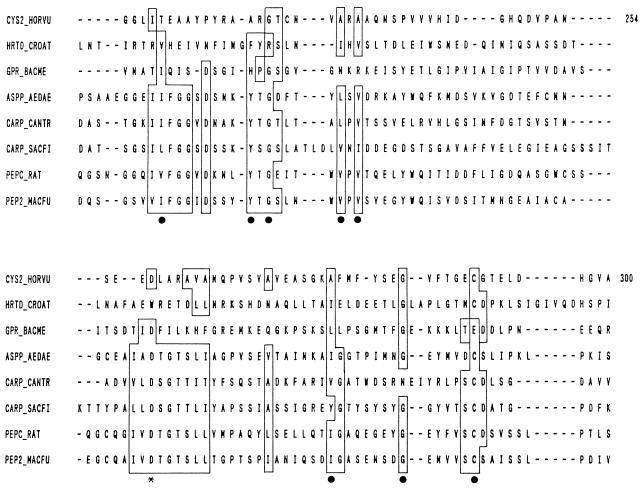
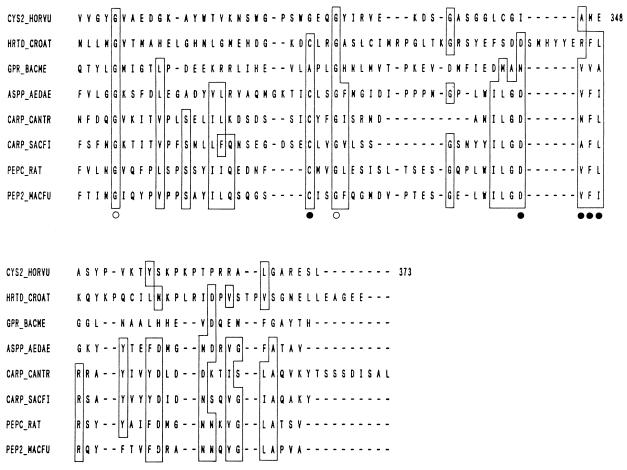
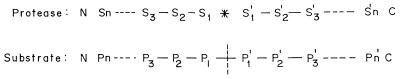Abstract
Proteases represent the class of enzymes which occupy a pivotal position with respect to their physiological roles as well as their commercial applications. They perform both degradative and synthetic functions. Since they are physiologically necessary for living organisms, proteases occur ubiquitously in a wide diversity of sources such as plants, animals, and microorganisms. Microbes are an attractive source of proteases owing to the limited space required for their cultivation and their ready susceptibility to genetic manipulation. Proteases are divided into exo- and endopeptidases based on their action at or away from the termini, respectively. They are also classified as serine proteases, aspartic proteases, cysteine proteases, and metalloproteases depending on the nature of the functional group at the active site. Proteases play a critical role in many physiological and pathophysiological processes. Based on their classification, four different types of catalytic mechanisms are operative. Proteases find extensive applications in the food and dairy industries. Alkaline proteases hold a great potential for application in the detergent and leather industries due to the increasing trend to develop environmentally friendly technologies. There is a renaissance of interest in using proteolytic enzymes as targets for developing therapeutic agents. Protease genes from several bacteria, fungi, and viruses have been cloned and sequenced with the prime aims of (i) overproduction of the enzyme by gene amplification, (ii) delineation of the role of the enzyme in pathogenecity, and (iii) alteration in enzyme properties to suit its commercial application. Protein engineering techniques have been exploited to obtain proteases which show unique specificity and/or enhanced stability at high temperature or pH or in the presence of detergents and to understand the structure-function relationships of the enzyme. Protein sequences of acidic, alkaline, and neutral proteases from diverse origins have been analyzed with the aim of studying their evolutionary relationships. Despite the extensive research on several aspects of proteases, there is a paucity of knowledge about the roles that govern the diverse specificity of these enzymes. Deciphering these secrets would enable us to exploit proteases for their applications in biotechnology.
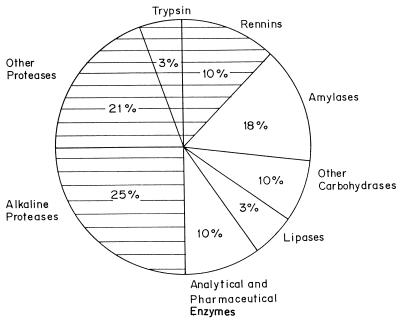
Proteases are the single class of enzymes which occupy a pivotal position with respect to their applications in both physiological and commercial fields. Proteolytic enzymes catalyze the cleavage of peptide bonds in other proteins. Proteases are degradative enzymes which catalyze the total hydrolysis of proteins. Advances in analytical techniques have demonstrated that proteases conduct highly specific and selective modifications of proteins such as activation of zymogenic forms of enzymes by limited proteolysis, blood clotting and lysis of fibrin clots, and processing and transport of secretory proteins across the membranes. The current estimated value of the worldwide sales of industrial enzymes is $1 billion (72). Of the industrial enzymes, 75% are hydrolytic. Proteases represent one of the three largest groups of industrial enzymes and account for about 60% of the total worldwide sale of enzymes (Fig. 1). Proteases execute a large variety of functions, extending from the cellular level to the organ and organism level, to produce cascade systems such as hemostasis and inflammation. They are responsible for the complex processes involved in the normal physiology of the cell as well as in abnormal pathophysiological conditions. Their involvement in the life cycle of disease-causing organisms has led them to become a potential target for developing therapeutic agents against fatal diseases such as cancer and AIDS. Proteases have a long history of application in the food and detergent industries. Their application in the leather industry for dehairing and bating of hides to substitute currently used toxic chemicals is a relatively new development and has conferred added biotechnological importance (235). The vast diversity of proteases, in contrast to the specificity of their action, has attracted worldwide attention in attempts to exploit their physiological and biotechnological applications (64, 225). The major producers of proteases worldwide are listed in Table 1.
FIG. 1.
Distribution of enzyme sales. The contribution of different enzymes to the total sale of enzymes is indicated. The shaded portion indicates the total sale of proteases.
TABLE 1.
Major protease producers
| Company | Country | Market share (%) |
|---|---|---|
| Novo Industries | Denmark | 40 |
| Gist-Brocades | Netherlands | 20 |
| Genencor International | United States | 10 |
| Miles Laboratories | United States | 10 |
| Others | 20 |
SCOPE OF THE REVIEW
Since proteases are enzymes of metabolic as well as commercial importance, there is a vast literature on their biochemical and biotechnological aspects (64, 128, 192, 235, 309). However, the earlier reviews did not deal with the molecular biology of proteases, which offers new possibilities and potentials for their biotechnological applications. This review aims at analyzing the updated information on biochemical and genetic aspects of proteases, with special reference to some of the advances made in these areas. We also attempt to address some of the deficiencies in the earlier reviews and to identify problems, along with possible solutions, for the successful applications of proteases for the benefit of mankind. The genetic engineering approaches are also discussed, from the perspective of making better use of proteases. The reference to plant and animal proteases has been made to complete the overview. However, the major emphasis of the review is on the microbial proteases.
SOURCES OF PROTEASES
Since proteases are physiologically necessary for living organisms, they are ubiquitous, being found in a wide diversity of sources such as plants, animals, and microorganisms.
Plant Proteases
The use of plants as a source of proteases is governed by several factors such as the availability of land for cultivation and the suitability of climatic conditions for growth. Moreover, production of proteases from plants is a time-consuming process. Papain, bromelain, keratinases, and ficin represent some of the well-known proteases of plant origin.
Papain.
Papain is a traditional plant protease and has a long history of use (250). It is extracted from the latex of Carica papaya fruits, which are grown in subtropical areas of west and central Africa and India. The crude preparation of the enzyme has a broader specificity due to the presence of several proteinase and peptidase isozymes. The performance of the enzyme depends on the plant source, the climatic conditions for growth, and the methods used for its extraction and purification. The enzyme is active between pH 5 and 9 and is stable up to 80 or 90°C in the presence of substrates. It is extensively used in industry for the preparation of highly soluble and flavored protein hydrolysates.
Bromelain.
Bromelain is prepared from the stem and juice of pineapples. The major supplier of the enzyme is Great Food Biochem., Bangkok, Thailand. The enzyme is characterized as a cysteine protease and is active from pH 5 to 9. Its inactivation temperature is 70°C, which is lower than that of papain.
Keratinases.
Some of the botanical groups of plants produce proteases which degrade hair. Digestion of hair and wool is important for the production of essential amino acids such as lysine and for the prevention of clogging of wastewater systems.
Animal Proteases
The most familiar proteases of animal origin are pancreatic trypsin, chymotrypsin, pepsin, and rennins (23, 97). These are prepared in pure form in bulk quantities. However, their production depends on the availability of livestock for slaughter, which in turn is governed by political and agricultural policies.
Trypsin.
Trypsin (Mr 23,300) is the main intestinal digestive enzyme responsible for the hydrolysis of food proteins. It is a serine protease and hydrolyzes peptide bonds in which the carboxyl groups are contributed by the lysine and arginine residues (Table 2). Based on the ability of protease inhibitors to inhibit the enzyme from the insect gut, this enzyme has received attention as a target for biocontrol of insect pests. Trypsin has limited applications in the food industry, since the protein hydrolysates generated by its action have a highly bitter taste. Trypsin is used in the preparation of bacterial media and in some specialized medical applications.
TABLE 2.
Specificity of proteases
| Enzyme | Peptide bond cleaveda |
|---|---|
| Trypsin | -Lys (or Arg) ↓----- |
| Chymotrypsin, subtilisin | -Trp (or Tyr, Phe, Leu)↓------ |
| Staphylococcus V8 protease | -Asp (or Glu)↓------ |
| Papain | -Phe (or Val, Leu)-Xaa↓----- |
| Thermolysin | ----↓Leu (or Phe) ------ |
| Pepsin | -Phe (or Tyr, Leu)↓- Trp (or Phe, Tyr) |
The arrow indicates the site of action of the protease. Xaa, any amino acid residue.
Chymotrypsin.
Chymotrypsin (Mr 23,800) is found in animal pancreatic extract. Pure chymotrypsin is an expensive enzyme and is used only for diagnostic and analytical applications. It is specific for the hydrolysis of peptide bonds in which the carboxyl groups are provided by one of the three aromatic amino acids, i.e., phenylalanine, tyrosine, or tryptophan. It is used extensively in the deallergenizing of milk protein hydrolysates. It is stored in the pancreas in the form of a precursor, chymotrypsinogen, and is activated by trypsin in a multistep process.
Pepsin.
Pepsin (Mr 34,500) is an acidic protease that is found in the stomachs of almost all vertebrates. The active enzyme is released from its zymogen, i.e., pepsinogen, by autocatalysis in the presence of hydrochloric acid. Pepsin is an aspartyl protease and resembles human immunodeficiency virus type 1 (HIV-1) protease, responsible for the maturation of HIV-1. It exhibits optimal activity between pH 1 and 2, while the optimal pH of the stomach is 2 to 4. Pepsin is inactivated above pH 6.0. The enzyme catalyzes the hydrolysis of peptide bonds between two hydrophobic amino acids.
Rennin.
Rennet is a pepsin-like protease (rennin, chymosin; EC 3.4.23.4) that is produced as an inactive precursor, prorennin, in the stomachs of all nursing mammals. It is converted to active rennin (Mr 30,700) by the action of pepsin or by its autocatalysis. It is used extensively in the dairy industry to produce a stable curd with good flavor. The specialized nature of the enzyme is due to its specificity in cleaving a single peptide bond in κ-casein to generate insoluble para-κ-casein and C-terminal glycopeptide.
Microbial Proteases
The inability of the plant and animal proteases to meet current world demands has led to an increased interest in microbial proteases. Microorganisms represent an excellent source of enzymes owing to their broad biochemical diversity and their susceptibility to genetic manipulation. Microbial proteases account for approximately 40% of the total worldwide enzyme sales (72). Proteases from microbial sources are preferred to the enzymes from plant and animal sources since they possess almost all the characteristics desired for their biotechnological applications.
Bacteria.
Most commercial proteases, mainly neutral and alkaline, are produced by organisms belonging to the genus Bacillus. Bacterial neutral proteases are active in a narrow pH range (pH 5 to 8) and have relatively low thermotolerance. Due to their intermediate rate of reaction, neutral proteases generate less bitterness in hydrolyzed food proteins than do the animal proteinases and hence are valuable for use in the food industry. Neutrase, a neutral protease, is insensitive to the natural plant proteinase inhibitors and is therefore useful in the brewing industry. The bacterial neutral proteases are characterized by their high affinity for hydrophobic amino acid pairs. Their low thermotolerance is advantageous for controlling their reactivity during the production of food hydrolysates with a low degree of hydrolysis. Some of the neutral proteases belong to the metalloprotease type and require divalent metal ions for their activity, while others are serine proteinases, which are not affected by chelating agents.
Bacterial alkaline proteases are characterized by their high activity at alkaline pH, e.g., pH 10, and their broad substrate specificity. Their optimal temperature is around 60°C. These properties of bacterial alkaline proteases make them suitable for use in the detergent industry.
Fungi.
Fungi elaborate a wider variety of enzymes than do bacteria. For example, Aspergillus oryzae produces acid, neutral, and alkaline proteases. The fungal proteases are active over a wide pH range (pH 4 to 11) and exhibit broad substrate specificity. However, they have a lower reaction rate and worse heat tolerance than do the bacterial enzymes. Fungal enzymes can be conveniently produced in a solid-state fermentation process. Fungal acid proteases have an optimal pH between 4 and 4.5 and are stable between pH 2.5 and 6.0. They are particularly useful in the cheesemaking industry due to their narrow pH and temperature specificities. Fungal neutral proteases are metalloproteases that are active at pH 7.0 and are inhibited by chelating agents. In view of the accompanying peptidase activity and their specific function in hydrolyzing hydrophobic amino acid bonds, fungal neutral proteases supplement the action of plant, animal, and bacterial proteases in reducing the bitterness of food protein hydrolysates. Fungal alkaline proteases are also used in food protein modification.
Viruses.
Viral proteases have gained importance due to their functional involvement in the processing of proteins of viruses that cause certain fatal diseases such as AIDS and cancer. Serine, aspartic, and cysteine peptidases are found in various viruses (236). All of the virus-encoded peptidases are endopeptidases; there are no metallopeptidases. Retroviral aspartyl proteases that are required for viral assembly and replication are homodimers and are expressed as a part of the polyprotein precursor. The mature protease is released by autolysis of the precursor. An extensive literature is available on the expression, purification, and enzymatic analysis of retroviral aspartic protease and its mutants (147). Extensive research has focused on the three-dimensional structure of viral proteases and their interaction with synthetic inhibitors with a view to designing potent inhibitors that can combat the relentlessly spreading and devastating epidemic of AIDS.
Thus, although proteases are widespread in nature, microbes serve as a preferred source of these enzymes because of their rapid growth, the limited space required for their cultivation, and the ease with which they can be genetically manipulated to generate new enzymes with altered properties that are desirable for their various applications.
CLASSIFICATION OF PROTEASES
According to the Nomenclature Committee of the International Union of Biochemistry and Molecular Biology, proteases are classified in subgroup 4 of group 3 (hydrolases) (114a). However, proteases do not comply easily with the general system of enzyme nomenclature due to their huge diversity of action and structure. Currently, proteases are classified on the basis of three major criteria: (i) type of reaction catalyzed, (ii) chemical nature of the catalytic site, and (iii) evolutionary relationship with reference to structure (12).
Proteases are grossly subdivided into two major groups, i.e., exopeptidases and endopeptidases, depending on their site of action. Exopeptidases cleave the peptide bond proximal to the amino or carboxy termini of the substrate, whereas endopeptidases cleave peptide bonds distant from the termini of the substrate. Based on the functional group present at the active site, proteases are further classified into four prominent groups, i.e., serine proteases, aspartic proteases, cysteine proteases, and metalloproteases (85). There are a few miscellaneous proteases which do not precisely fit into the standard classification, e.g., ATP-dependent proteases which require ATP for activity (183). Based on their amino acid sequences, proteases are classified into different families (5) and further subdivided into “clans” to accommodate sets of peptidases that have diverged from a common ancestor (236). Each family of peptidases has been assigned a code letter denoting the type of catalysis, i.e., S, C, A, M, or U for serine, cysteine, aspartic, metallo-, or unknown type, respectively.
Exopeptidases
The exopeptidases act only near the ends of polypeptide chains. Based on their site of action at the N or C terminus, they are classified as amino- and carboxypeptidases, respectively.
Aminopeptidases.
Aminopeptidases act at a free N terminus of the polypeptide chain and liberate a single amino acid residue, a dipeptide, or a tripeptide (Table 3). They are known to remove the N-terminal Met that may be found in heterologously expressed proteins but not in many naturally occurring mature proteins. Aminopeptidases occur in a wide variety of microbial species including bacteria and fungi (310). In general, aminopeptidases are intracellular enzymes, but there has been a single report on an extracellular aminopeptidase produced by A. oryzae (150). The substrate specificities of the enzymes from bacteria and fungi are distinctly different in that the organisms can be differentiated on the basis of the profiles of the products of hydrolysis (31). Aminopeptidase I from Escherichia coli is a large protease (400,000 Da). It has a broad pH optimum of 7.5 to 10.5 and requires Mg2+ or Mn2+ for optimal activity (48). The Bacillus licheniformis aminopeptidase has a molecular weight of 34,000. It contains 1 g-atom of Zn2+ per mol, and its activity is enhanced by Co2+ ions. On the other hand, aminopeptidase II from B. stearothermophilus is a dimer with a molecular weight of 80,000 to 100,000 (272) and is activated by Zn2+, Mn2+, or Co2+ ions.
TABLE 3.
Classification of proteases
| Protease | Mode of actiona | EC no. |
|---|---|---|
| Exopeptidases | ||
| Aminopeptidases | •↓-○-○-○-○--- | 3.4.11 |
| Dipeptidyl peptidase | •-•↓-○-○-○--- | 3.4.14 |
| Tripeptidyl peptidase | •-•-•↓-○-○--- | 3.4.14 |
| Carboxypeptidase | ---○-○-○-○-○↓-• | 3.4.16–3.4.18 |
| Serine type protease | 3.4.16 | |
| Metalloprotease | 3.4.17 | |
| Cysteine type protease | 3.4.18 | |
| Peptidyl dipeptidase | ---○-○-○-○↓-•-• | 3.4.15 |
| Dipeptidases | •↓-• | 3.4.13 |
| Omega peptidases | ∗-•↓-○-○--- | 3.4.19 |
| ---○-○-○↓-•-∗ | 3.4.19 | |
| Endopeptidases | ----○-○-○↓-○-○-○--- | 3.4.21–3.4.34 |
| Serine protease | 3.4.21 | |
| Cysteine protease | 3.4.22 | |
| Aspartic protease | 3.4.23 | |
| Metalloprotease | 3.4.24 | |
| Endopeptidases of unknown catalytic mechanism | 3.4.99 |
Open circles represent the amino acid residues in the polypeptide chain. Solid circles indicate the terminal amino acids, and stars signify the blocked termini. Arrows show the sites of action of the enzyme.
Carboxypeptidases.
The carboxypeptidases act at C terminals of the polypeptide chain and liberate a single amino acid or a dipeptide. Carboxypeptidases can be divided into three major groups, serine carboxypeptidases, metallocarboxypeptidases, and cysteine carboxypeptidases, based on the nature of the amino acid residues at the active site of the enzymes. The serine carboxypeptidases isolated from Penicillium spp., Saccharomyces spp., and Aspergillus spp. are similar in their substrate specificities but differ slightly in other properties such as pH optimum, stability, molecular weight, and effect of inhibitors. Metallocarboxypeptidases from Saccharomyces spp. (61) and Pseudomonas spp. (174) require Zn2+ or Co2+ for their activity. The enzymes can also hydrolyze the peptides in which the peptidyl group is replaced by a pteroyl moiety or by acyl groups.
Endopeptidases
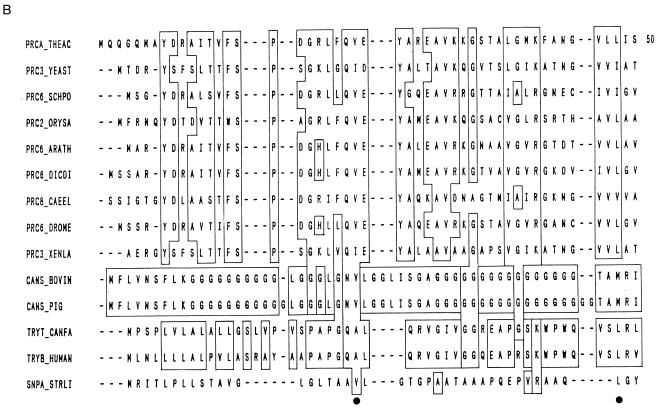
Endopeptidases are characterized by their preferential action at the peptide bonds in the inner regions of the polypeptide chain away from the N and C termini. The presence of the free amino or carboxyl group has a negative influence on enzyme activity. The endopeptidases are divided into four subgroups based on their catalytic mechanism, (i) serine proteases, (ii) aspartic proteases, (iii) cysteine proteases, and (iv) metalloproteases. To facilitate quick and unambiguous reference to a particular family of peptidases, Rawlings and Barrett have assigned a code letter denoting the catalytic type, i.e., S, C, A, M, or U (see above) followed by an artibrarily assigned number (236).
Serine proteases.
Serine proteases are characterized by the presence of a serine group in their active site. They are numerous and widespread among viruses, bacteria, and eukaryotes, suggesting that they are vital to the organisms. Serine proteases are found in the exopeptidase, endopeptidase, oligopeptidase, and omega peptidase groups. Based on their structural similarities, serine proteases have been grouped into 20 families, which have been further subdivided into about six clans with common ancestors (12). The primary structures of the members of four clans, chymotrypsin (SA), subtilisin (SB), carboxypeptidase C (SC), and Escherichia d-Ala–d-Ala peptidase A (SE) are totally unrelated, suggesting that there are at least four separate evolutionary origins for serine proteases. Clans SA, SB, and SC have a common reaction mechanism consisting of a common catalytic triad of the three amino acids, serine (nucleophile), aspartate (electrophile), and histidine (base). Although the geometric orientations of these residues are similar, the protein folds are quite different, forming a typical example of a convergent evolution. The catalytic mechanisms of clans SE and SF (repressor LexA) are distinctly different from those of clans SA, SB, and SE, since they lack the classical Ser-His-Asp triad. Another interesting feature of the serine proteases is the conservation of glycine residues in the vicinity of the catalytic serine residue to form the motif Gly-Xaa-Ser-Yaa-Gly (25).
Serine proteases are recognized by their irreversible inhibition by 3,4-dichloroisocoumarin (3,4-DCI), l-3-carboxytrans 2,3-epoxypropyl-leucylamido (4-guanidine) butane (E.64), diisopropylfluorophosphate (DFP), phenylmethylsulfonyl fluoride (PMSF) and tosyl-l-lysine chloromethyl ketone (TLCK). Some of the serine proteases are inhibited by thiol reagents such as p-chloromercuribenzoate (PCMB) due to the presence of a cysteine residue near the active site. Serine proteases are generally active at neutral and alkaline pH, with an optimum between pH 7 and 11. They have broad substrate specificities including esterolytic and amidase activity. Their molecular masses range between 18 and 35 kDa, for the serine protease from Blakeslea trispora, which has a molecular mass of 126 kDa (76). The isoelectric points of serine proteases are generally between pH 4 and 6. Serine alkaline proteases that are active at highly alkaline pH represent the largest subgroup of serine proteases.
(i) Serine alkaline proteases.
Serine alkaline proteases are produced by several bacteria, molds, yeasts, and fungi. They are inhibited by DFP or a potato protease inhibitor but not by tosyl-l-phenylalanine chloromethyl ketone (TPCK) or TLCK. Their substrate specificity is similar to but less stringent than that of chymotrypsin. They hydrolyze a peptide bond which has tyrosine, phenylalanine, or leucine at the carboxyl side of the splitting bond. The optimal pH of alkaline proteases is around pH 10, and their isoelectric point is around pH 9. Their molecular masses are in the range of 15 to 30 kDa. Although alkaline serine proteases are produced by several bacteria such as Arthrobacter, Streptomyces, and Flavobacterium spp. (21), subtilisins produced by Bacillus spp. are the best known. Alkaline proteases are also produced by S. cerevisiae (189) and filamentous fungi such as Conidiobolus spp. (219) and Aspergillus and Neurospora spp. (165).
(ii) Subtilisins.
Subtilisins of Bacillus origin represent the second largest family of serine proteases. Two different types of alkaline proteases, subtilisin Carlsberg and subtilisin Novo or bacterial protease Nagase (BPN′), have been identified. Subtilisin Carlsberg produced by Bacillus licheniformis was discovered in 1947 by Linderstrom, Lang, and Ottesen at the Carlsberg laboratory. Subtilisin Novo or BPN′ is produced by Bacillus amyloliquefaciens. Subtilisin Carlsberg is widely used in detergents. Its annual production amounts to about 500 tons of pure enzyme protein. Subtilisin BPN′ is less commercially important. Both subtilisins have a molecular mass of 27.5 kDa but differ from each other by 58 amino acids. They have similar properties such as an optimal temperature of 60°C and an optimal pH of 10. Both enzymes exhibit a broad substrate specificity and have an active-site triad made up of Ser221, His64 and Asp32. The Carlsberg enzyme has a broader substrate specificity and does not depend on Ca2+ for its stability. The active-site conformation of subtilisins is similar to that of trypsin and chymotrypsin despite the dissimilarity in their overall molecular arrangements. The serine alkaline protease from the fungus Conidiobolus coronatus was shown to possess a distinctly different structure from subtilisin Carlsberg in spite of their functional similarities (218).
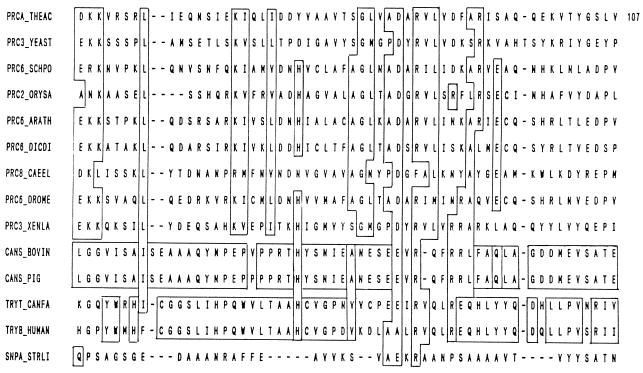
Aspartic proteases.
Aspartic acid proteases, commonly known as acidic proteases, are the endopeptidases that depend on aspartic acid residues for their catalytic activity. Acidic proteases have been grouped into three families, namely, pepsin (A1), retropepsin (A2), and enzymes from pararetroviruses (A3) (13), and have been placed in clan AA. The members of families A1 and A2 are known to be related to each other, while those of family A3 show some relatedness to A1 and A2. Most aspartic proteases show maximal activity at low pH (pH 3 to 4) and have isoelectric points in the range of pH 3 to 4.5. Their molecular masses are in the range of 30 to 45 kDa. The members of the pepsin family have a bilobal structure with the active-site cleft located between the lobes (259). The active-site aspartic acid residue is situated within the motif Asp-Xaa-Gly, in which Xaa can be Ser or Thr. The aspartic proteases are inhibited by pepstatin (63). They are also sensitive to diazoketone compounds such as diazoacetyl-dl-norleucine methyl ester (DAN) and 1,2-epoxy-3-(p-nitrophenoxy)propane (EPNP) in the presence of copper ions. Microbial acid proteases exhibit specificity against aromatic or bulky amino acid residues on both sides of the peptide bond, which is similar to pepsin, but their action is less stringent than that of pepsin. Microbial aspartic proteases can be broadly divided into two groups, (i) pepsin-like enzymes produced by Aspergillus, Penicillium, Rhizopus, and Neurospora and (ii) rennin-like enzymes produced by Endothia and Mucor spp.
Cysteine/thiol proteases.
Cysteine proteases occur in both prokaryotes and eukaryotes. About 20 families of cysteine proteases have been recognized. The activity of all cysteine proteases depends on a catalytic dyad consisting of cysteine and histidine. The order of Cys and His (Cys-His or His-Cys) residues differs among the families (12). Generally, cysteine proteases are active only in the presence of reducing agents such as HCN or cysteine. Based on their side chain specificity, they are broadly divided into four groups: (i) papain-like, (ii) trypsin-like with preference for cleavage at the arginine residue, (iii) specific to glutamic acid, and (iv) others. Papain is the best-known cysteine protease. Cysteine proteases have neutral pH optima, although a few of them, e.g., lysosomal proteases, are maximally active at acidic pH. They are susceptible to sulfhydryl agents such as PCMB but are unaffected by DFP and metal-chelating agents. Clostripain, produced by the anaerobic bacterium Clostridium histolyticum, exhibits a stringent specificity for arginyl residues at the carboxyl side of the splitting bond and differs from papain in its obligate requirement for calcium. Streptopain, the cysteine protease produced by Streptococcus spp., shows a broader specificity, including oxidized insulin B chain and other synthetic substrates. Clostripain has an isoelectric point of pH 4.9 and a molecular mass of 50 kDa, whereas the isoelectric point and molecular mass of streptopain are pH 8.4 and 32 kDa, respectively.
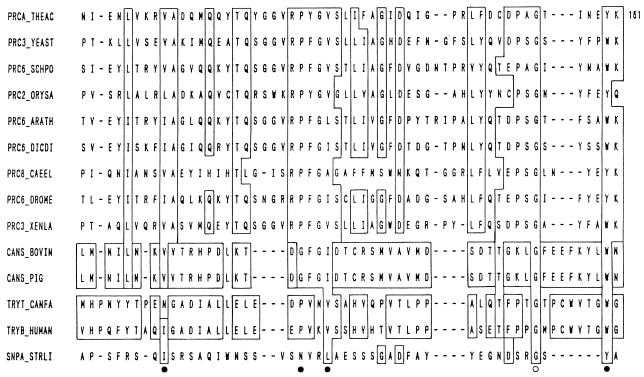
Metalloproteases.
Metalloproteases are the most diverse of the catalytic types of proteases (13). They are characterized by the requirement for a divalent metal ion for their activity. They include enzymes from a variety of origins such as collagenases from higher organisms, hemorrhagic toxins from snake venoms, and thermolysin from bacteria (92, 210, 253, 311, 314). About 30 families of metalloproteases have been recognized, of which 17 contain only endopeptidases, 12 contain only exopeptidases, and 1 (M3) contains both endo- and exopeptidases. Families of metalloproteases have been grouped into different clans based on the nature of the amino acid that completes the metal-binding site; e.g., clan MA has the sequence HEXXH-E and clan MB corresponds to the motif HEXXH-H. In one of the groups, the metal atom binds at a motif other than the usual motif.
Based on the specificity of their action, metalloproteases can be divided into four groups, (i) neutral, (ii) alkaline, (iii) Myxobacter I, and (iv) Myxobacter II. The neutral proteases show specificity for hydrophobic amino acids, while the alkaline proteases possess a very broad specificity. Myxobacter protease I is specific for small amino acid residues on either side of the cleavage bond, whereas protease II is specific for lysine residue on the amino side of the peptide bond. All of them are inhibited by chelating agents such as EDTA but not by sulfhydryl agents or DFP.
Thermolysin, a neutral protease, is the most thoroughly characterized member of clan MA. Histidine residues from the HEXXH motif serve as Zn ligands, and Glu has a catalytic function (311). Thermolysin produced by B. stearothermophilus is a single peptide without disulfide bridges and has a molecular mass of 34 kDa. It contains an essential Zn atom embedded in a cleft formed between two folded lobes of the protein and four Ca atoms which impart thermostability to the protein. Thermolysin is a very stable protease, with a half-life of 1 h at 80°C.
Collagenase, another important metalloprotease, was first discovered in the broth of the anaerobic bacterium Clostridium hystolyticum as a component of toxic products. Later, it was found to be produced by the aerobic bacterium Achromobacter iophagus and other microorganisms including fungi. The action of collagenase is very specific; i.e., it acts only on collagen and gelatin and not on any of the other usual protein substrates. Elastase produced by Pseudomonas aeruginosa is another important member of the neutral metalloprotease family.
The alkaline metalloproteases produced by Pseudomonas aeruginosa and Serratia spp. are active in the pH range from 7 to 9 and have molecular masses in the region of 48 to 60 kDa. Myxobacter protease I has a pH optimum of 9.0 and a molecular mass of 14 kDa and can lyse cell walls of Arthrobacter crystellopoites, whereas protease II cannot lyse the bacterial cells. Matrix metalloproteases play a prominent role in the degradation of the extracellular matrix during tissue morphogenesis, differentiation, and wound healing and may be useful in the treatment of diseases such as cancer and arthritis (26).
In summary, proteases are broadly classified as endo- or exoenzymes on the basis of their site of action on protein substrates. They are further categorized as serine proteases, aspartic proteases, cysteine proteases, or metalloproteases depending on their catalytic mechanism. They are also classified into different families and clans depending on their amino acid sequences and evolutionary relationships. Based on the pH of their optimal activity, they are also referred to as acidic, neutral, or alkaline proteases.
MECHANISM OF ACTION OF PROTEASES
The mechanism of action of proteases has been a subject of great interest to researchers. Purification of proteases to homogeneity is a prerequisite for studying their mechanism of action. Vast numbers of purification procedures for proteases, involving affinity chromatography, ion-exchange chromatography, and gel filtration techniques, have been well documented. Preparative polyacrylamide gel electrophoresis has been used for the purification of proteases from Conidiobolus coronatus (220). Purification of staphylocoagulase to homogeneity was carried out from culture filtrates of Staphylococcus aureus by affinity chromatography with a bovine prothrombin-Sepharose 4B column (109) and gel filtration (335). A number of peptide hydrolases have been isolated and purified from E. coli by DEAE-cellulose chromatography (217).
The catalytic site of proteases is flanked on one or both sides by specificity subsites, each able to accommodate the side chain of a single amino acid residue from the substrate. These sites are numbered from the catalytic site S1 through Sn toward the N terminus of the structure and Sl′ through Sn′ toward the C terminus. The residues which they accommodate from the substrate are numbered Pl through Pn and P1′ through Pn′, respectively (Fig. 2).
FIG. 2.
Active sites of proteases. The catalytic site of proteases is indicated by ∗ and the scissile bond is indicated by ; S1 through Sn and S1′ through Sn′ are the specificity subsites on the enzyme, while P1 through Pn and P1′ through Pn′ are the residues on the substrate accommodated by the subsites on the enzyme.
Serine Proteases
Serine proteases usually follow a two-step reaction for hydrolysis in which a covalently linked enzyme-peptide intermediate is formed with the loss of the amino acid or peptide fragment (60). This acylation step is followed by a deacylation process which occurs by a nucleophilic attack on the intermediate by water, resulting in hydrolysis of the peptide. Serine endopeptidases can be classified into three groups based mainly on their primary substrate preference: (i) trypsin-like, which cleave after positively charged residues; (ii) chymotrypsin-like, which cleave after large hydrophobic residues; and (iii) elastase-like, which cleave after small hydrophobic residues. The Pl residue exclusively dictates the site of peptide bond cleavage. The primary specificity is affected only by the Pl residues; the residues at other positions affect the rate of cleavage. The subsite interactions are localized to specific amino acids around the Pl residue to a unique set of sequences on the enzyme. Some of the serine peptidases from Achromobacter spp. are lysine-specific enzymes (179), whereas those from Clostridium spp. are arginine specific (clostripain) (71) and those from Flavobacterium spp. are post proline-specific (329). Endopeptidases that are specific to glutamic acid and aspartic acid residues have also been found in B. licheniformis and S. aureus (52).
The recent studies based on the three-dimensional structures of proteases and comparisons of amino acid sequences near the primary substrate-binding site in trypsin-like proteases of viral and bacterial origin suggest a putative general substrate binding scheme for proteases with specificity towards glutamic acid involving a histidine residue and a hydroxyl function. However, a few other serine proteases such as peptidase A from E. coli and the repressor LexA show distinctly different mechanism of action without the classic Ser-His-Asp triad (12). Some of the glycine residues are conserved in the vicinity of the catalytic serine residue, but their exact positions are variable (25).
The chymotrypsin-like enzymes are confined almost entirely to animals, the exceptions being trypsin-like enzymes from actinomycetes and Saccharopolyspora spp. and from the fungus Fusarium oxysporum.
A few of the serine proteases belonging to the subtilisin family show a catalytic triad composed of the same residues as in the chymotrypsin family; however, the residues occur in a different order (Asp-His-Ser). Some members of the subtilisin family from the yeasts Tritirachium and Metarhizium spp. require thiol for their activity. The thiol dependance is attributable to Cys173 near the active-site histidine (122).
The carboxypeptidases are unusual among the serine-dependent enzymes in that they are maximally active at acidic pH. These enzymes are known to possess a Glu residue preceding the catalytic Ser, which is believed to be responsible for their acidic pH optimum. Although the majority of the serine proteases contain the catalytic triad Ser-His-Asp, a few use the Ser-base catalytic dyad. The Glu-specific proteases display a pronounced preference for Glu-Xaa bonds over Asp-Xaa bonds (8).
Aspartic Proteases
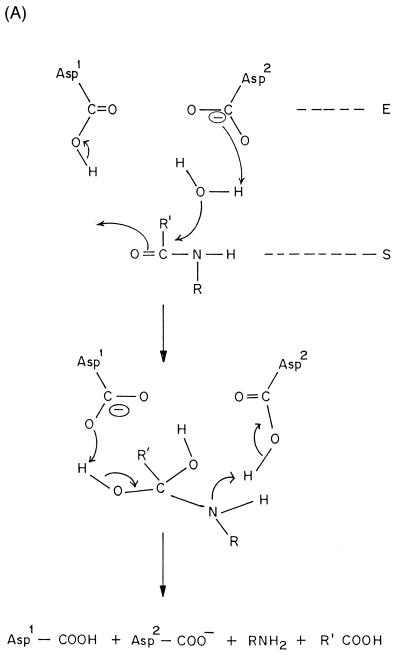
Aspartic endopeptidases depend on the aspartic acid residues for their catalytic activity. A general base catalytic mechanism has been proposed for the hydrolysis of proteins by aspartic proteases such as penicillopepsin (121) and endothiapepsin (215). Crystallographic studies have shown that the enzymes of the pepsin family are bilobed molecules with the active-site cleft located between the lobes and each lobe contributing one of the pair of aspartic acid residues that is essential for the catalytic activity (20, 259). The lobes are homologous to one another, having arisen by gene duplication. The retropepsin molecule has only one lobe, which carries only one aspartic residue, and the activity requires the formation of a noncovalent homodimer (184). In most of the enzymes from the pepsin family, the catalytic Asp residues are contained in an Asp-Thr-Gly-Xaa motif in both the N- and C-terminal lobes of the enzyme, where Xaa is Ser or Thr, whose side chains can hydrogen bond to Asp. However, Xaa is Ala in most of the retropepsins. A marked conservation of cysteine residue is also evident in aspartic proteases. The pepsins and the majority of other members of the family show specificity for the cleavage of bonds in peptides of at least six residues with hydrophobic amino acids in both the Pl and Pl′ positions (132).
The specificity of the catalysis has been explained on the basis of available crystal structures (166). The structural and kinetic studies also have suggested that the mechanism involves general acid-base catalysis with lytic water molecule that directly participates in the reaction (Fig. 3A). This is supported by the crystal structures of various aspartic protease-inhibitor complexes and by the thiol inhibitors mimicking a tetrahedral intermediate formed after the attack by the lytic water molecule (120).
FIG. 3.
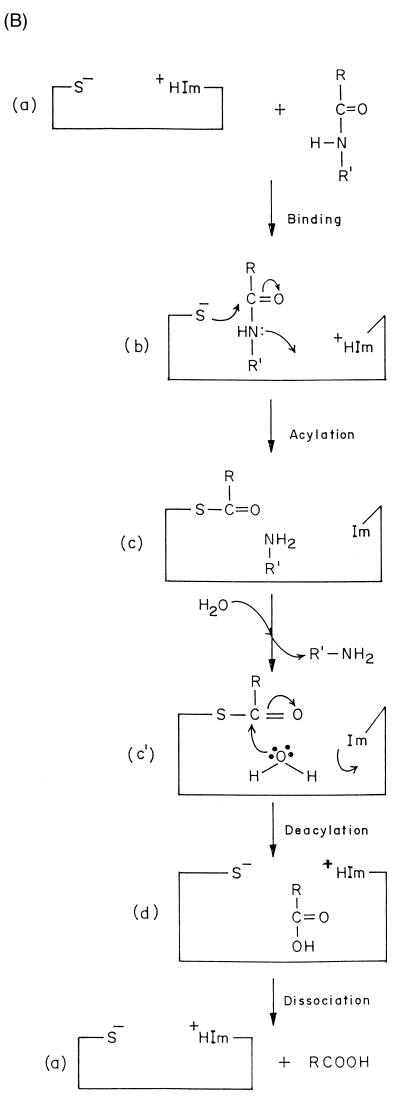
Mechanism of action of proteases. (A) Aspartic proteases. (B) Cysteine proteases. Im and +HIm refer to the imidazole and protonated imidazole, respectively.
Metalloproteases
The mechanism of action of metalloproteases is slightly different from that of the above-described proteases. These enzymes depend on the presence of bound divalent cations and can be inactivated by dialysis or by the addition of chelating agents. For thermolysin, based on the X-ray studies of the complex with a hydroxamic acid inhibitor, it has been proposed that Glu143 assists the nucleophilic attack of a water molecule on the carbonyl carbon of the scissile peptide bond, which is polarized by the Zn2+ ion (98). Most of the metalloproteases are enzymes containing the His-Glu-Xaa-Xaa-His (HEXXH) motif, which has been shown by X-ray crystallography to form a part of the site for binding of the metal, usually zinc.
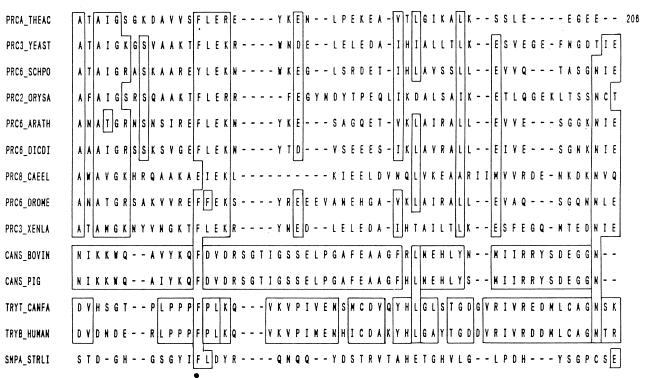
Cysteine Proteases
Cysteine proteases catalyze the hydrolysis of carboxylic acid derivatives through a double-displacement pathway involving general acid-base formation and hydrolysis of an acyl-thiol intermediate. The mechanism of action of cysteine proteases is thus very similar to that of serine proteases.
A striking similarity is also observed in the reaction mechanism for several peptidases of different evolutionary origins. The plant peptidase papain can be considered the archetype of cysteine peptidases and constitutes a good model for this family of enzymes. They catalyze the hydrolysis of peptide, amide ester, thiol ester, and thiono ester bonds (226). The initial step in the catalytic process (Fig. 3B) involves the noncovalent binding of the free enzyme (structure a) and the substrate to form the complex (structure b). This is followed by the acylation of the enzyme (structure c), with the formation and release of the first product, the amine R′-NH2. In the next deacylation step, the acyl-enzyme reacts with a water molecule to release the second product, with the regeneration of free enzyme.
The enzyme papain consists of a single protein chain folded to form two domains containing a cleft for the substrate to bind. The crystal structure of papain confirmed the Cys25-His159 pairing (11). The presence of a conserved aspargine residue (Asn175) in the proximity of catalytic histidine (His159) creating a Cys-His-Asn triad in cysteine peptidases is considered analogous to the Ser-His-Asp arrangement found in serine proteases.
Studies of the mechanism of action of proteases have revealed that they exhibit different types of mechanism based on their active-site configuration. The serine proteases contain a Ser-His-Asp catalytic triad, and the hydrolysis of the peptide bond involves an acylation step followed by a deacylation step. Aspartic proteases are characterized by an Asp-Thr-Gly motif in their active site and by an acid-base catalysis as their mechanisms of action. The activity of metalloproteases depends on the binding of a divalent metal ion to a His-Glu-Xaa-Xaa-His motif. Cysteine proteases adopt a hydrolysis mechanism involving a general acid-base formation followed by hydrolysis of an acyl-thiol intermediate.
PHYSIOLOGICAL FUNCTIONS OF PROTEASES
Proteases execute a large variety of complex physiological functions. Their importance in conducting the essential metabolic and regulatory functions is evident from their occurrence in all forms of living organisms. Proteases play a critical role in many physiological and pathological processes such as protein catabolism, blood coagulation, cell growth and migration, tissue arrangement, morphogenesis in development, inflammation, tumor growth and metastasis, activation of zymogens, release of hormones and pharmacologically active peptides from precursor proteins, and transport of secretory proteins across membranes. In general, extracellular proteases catalyze the hydrolysis of large proteins to smaller molecules for subsequent absorption by the cell whereas intracellular proteases play a critical role in the regulation of metabolism. In contrast to the multitude of the roles contemplated for proteases, our knowledge about the mechanisms by which they perform these functions is very limited. Extensive research is being carried out to unravel the metabolic pathways in which proteases play an integral role; this research will continue to contribute significantly to our present state of information. Some of the major activities in which the proteases participate are described below.
Protein Turnover
All living cells maintain a particular rate of protein turnover by continuous, albeit balanced, degradation and synthesis of proteins. Catabolism of proteins provides a ready pool of amino acids as precursors of the synthesis of proteins. Intracellular proteases are known to participate in executing the proper protein turnover for the cell. In E. coli, ATP-dependent protease La, the lon gene product, is responsible for hydrolysis of abnormal proteins (38). The turnover of intracellular proteins in eukaryotes is also affected by a pathway involving ATP-dependent proteases (91). Evidence for the participation of proteolytic activity in controlling the protein turnover was demonstrated by the lack of proper turnover in protease-deficient mutants.
Sporulation and Conidial Discharge
The formation of spores in bacteria (142), ascospores in yeasts (58), fruiting bodies in slime molds (205) and conidial discharge in fungi (221) all involve intensive protein turnover. The requirement of a protease for sporulation has been demonstrated by the use of protease inhibitors (41). Ascospore formation in yeast diploids was shown to be related to the increase in protease A activity (58). Extensive protein degradation accompanied the formation of a fruiting body and its differentiation to a stalk in slime molds. The alkaline serine protease of Conidiobolus coronatus was shown to be involved in forcible conidial discharge by isolation of a mutant with less conidial formation (221). Formation of the less active protease by autoproteolysis represents a novel means of physiological regulation of protease activity in C. coronatus (219).
Germination
The dormant spores lack the amino acids required for germination. Degradation of proteins in dormant spores by serine endoproteinases makes amino acids and nitrogen available for the biosynthesis of new proteins and nucleotides. These proteases are specific only for storage proteins and do not affect other spore proteins. Their activity is rapidly lost on germination of the spores (227). Microconidal germination and hyphal fusion also involve the participation of a specific alkaline serine protease (159). Extracellular acid proteases are believed to be involved in the breakage of cell wall polypeptide linkages during germination of Dictyostelium discoideum spores (118) and Polysphondylium pallidum microcysts (206).
Enzyme Modification
Activation of the zymogenic precursor forms of enzymes and proteins by specific proteases represents an important step in the physiological regulation of many rate-controlling processes such as generation of protein hormones, assembly of fibrils and viruses, blood coagulation, and fertilization of ova by sperm. Activation of zymogenic forms of chitin synthase by limited proteolysis has been observed in Candida albicans, Mucor rouxii, and Aspergillus nidulans. Kex-2 protease (kexin; EC 3.4.21.61), originally discovered in yeast, has emerged as a prototype of a family of eukaryotic precursor processing enzymes. It catalyzes the hydrolysis of prohormones and of integral membrane proteins of the secretory pathway by specific cleavage at the carboxyl side of pairs of basic residues such as Lys-Arg or Arg-Arg (12). Furin (EC 3.4.21.5) is a mammalian homolog of the Kex-2 protease that was discovered serendipitously and has been shown to catalyze the hydrolysis of a wide variety of precursor proteins at Arg-X-Lys or Arg-Arg sites within the constitutive secretory pathway (266). Pepsin, trypsin, and chymotrypsin occur as their inactive zymogenic forms, which are activated by the action of proteases.
Proteolytic inactivation of enzymes, leading to irreversible loss of in vivo catalytic activity, is also a physiologically significant event. Several enzymes are known to be inactivated in response to physiological or developmental changes or after a metabolic shift. Proteinases A and B from yeast inactivate several enzymes in a two-step process involving covalent modification of proteins as a marking mechanism for proteolysis.
Proteolytic modification of enzymes is known to result in a protein with altered physiological function; e.g., leucyl-l-RNA synthetase from E. coli is converted into an enzyme which catalyzes leucine-dependent pyrophosphate exchange by removal of a small peptide from the native enzyme.
Nutrition
Proteases assist the hydrolysis of large polypeptides into smaller peptides and amino acids, thus facilitating their absorption by the cell. The extracellular enzymes play a major role in nutrition due to their depolymerizing activity. The microbial enzymes and the mammalian extracellular enzymes such as those secreted by pancreas are primarily involved in keeping the cells alive by providing them with the necessary amino acid pool as nutrition.
Regulation of Gene Expression
Modulation of gene expression mediated by protease has been demonstrated (241). Proteolysis of a repressor by an ATP-requiring protease resulted in a derepression of the gene. A change in the transcriptional specificity of the B subunit of Bacillus thuringiensis RNA polymerase was correlated with its proteolytic modification (154). Modification of ribosomal proteins by proteases has been suggested to be responsible for the regulation of translation (128).
Besides the general functions described so far, the proteases also mediate the degradation of a variety of regulatory proteins that control the heat shock response, the SOS response to DNA damage, the life cycle of bacteriophage (75), and programmed bacterial cell death (303). Recently, a new physiological function has been attributed to the ATP-dependent proteases conserved between bacteria and eukaryotes. It is believed that they act as chaperones and mediate not only proteolysis but also the insertion of proteins into membranes and the disassembly or oligomerization of protein complexes (275). In addition to the multitude of activities that are already assigned to proteases, many more new functions are likely to emerge in the near future.
APPLICATIONS OF PROTEASES
Proteases have a large variety of applications, mainly in the detergent and food industries. In view of the recent trend of developing environmentally friendly technologies, proteases are envisaged to have extensive applications in leather treatment and in several bioremediation processes. The worldwide requirement for enzymes for individual applications varies considerably. Proteases are used extensively in the pharmaceutical industry for preparation of medicines such as ointments for debridement of wounds, etc. Proteases that are used in the food and detergent industries are prepared in bulk quantities and used as crude preparations, whereas those that are used in medicine are produced in small amounts but require extensive purification before they can be used.
Detergents
Proteases are one of the standard ingredients of all kinds of detergents ranging from those used for household laundering to reagents used for cleaning contact lenses or dentures. The use of proteases in laundry detergents accounts for approximately 25% of the total worldwide sales of enzymes. The preparation of the first enzymatic detergent, “Burnus,” dates back to 1913; it consisted of sodium carbonate and a crude pancreatic extract. The first detergent containing the bacterial enzyme was introduced in 1956 under the trade name BIO-40. In 1960, Novo Industry A/S introduced alcalase, produced by Bacillus licheniformis; its commercial name was BIOTEX. This was followed by Maxatase, a detergent made by Gist-Brocades. The biggest market for detergents is in the laundry industry, amounting to a worldwide production of 13 billion tons per year. The ideal detergent protease should possess broad substrate specificity to facilitate the removal of a large variety of stains due to food, blood, and other body secretions. Activity and stability at high pH and temperature and compatibility with other chelating and oxidizing agents added to the detergents are among the major prerequisites for the use of proteases in detergents. The key parameter for the best performance of a protease in a detergent is its pI. It is known that a protease is most suitable for this application if its pI coincides with the pH of the detergent solution. Esperase and Savinase T (Novo Industry), produced by alkalophilic Bacillus spp., are two commercial preparations with very high isoelectric points (pI 11); hence, they can withstand higher pH ranges. Due to the present energy crisis and the awareness for energy conservation, it is desirable to use proteases that are active at lower temperatures. A combination of lipase, amylase, and cellulase is expected to enhance the performance of protease in laundry detergents.
All detergent proteases currently used in the market are serine proteases produced by Bacillus strains. Fungal alkaline proteases are advantageous due to the ease of downstream processing to prepare a microbe-free enzyme. An alkaline protease from Conidiobolus coronatus was found to be compatible with commercial detergents used in India (219) and retained 43% of its activity at 50°C for 50 min in the presence of Ca2+ (25 mM) and glycine (1 M) (16).
Leather Industry
Leather processing involves several steps such as soaking, dehairing, bating, and tanning. The major building blocks of skin and hair are proteinaceous. The conventional methods of leather processing involve hazardous chemicals such as sodium sulfide, which create problems of pollution and effluent disposal. The use of enzymes as alternatives to chemicals has proved successful in improving leather quality and in reducing environmental pollution. Proteases are used for selective hydrolysis of noncollagenous constituents of the skin and for removal of nonfibrillar proteins such as albumins and globulins. The purpose of soaking is to swell the hide. Traditionally, this step was performed with alkali. Currently, microbial alkaline proteases are used to ensure faster absorption of water and to reduce the time required for soaking. The use of nonionic and, to some extent, anionic surfactants is compatible with the use of enzymes. The conventional method of dehairing and dewooling consists of development of an extremely alkaline condition followed by treatment with sulfide to solubilize the proteins of the hair root. At present, alkaline proteases with hydrated lime and sodium chloride are used for dehairing, resulting in a significant reduction in the amount of wastewater generated. Earlier methods of bating were based on the use of animal feces as the source of proteases; these methods were unpleasant and unreliable and were replaced by methods involving pancreatic trypsin. Currently, trypsin is used in combination with other Bacillus and Aspergillus proteases for bating. The selection of the enzyme depends on its specificity for matrix proteins such as elastin and keratin, and the amount of enzyme needed depends on the type of leather (soft or hard) to be produced. Increased usage of enzymes for dehairing and bating not only prevents pollution problems but also is effective in saving energy. Novo Nordisk manufactures three different proteases, Aquaderm, NUE, and Pyrase, for use in soaking, dehairing, and bating, respectively.
Food Industry
The use of proteases in the food industry dates back to antiquity. They have been routinely used for various purposes such as cheesemaking, baking, preparation of soya hydrolysates, and meat tenderization.
Dairy industry.
The major application of proteases in the dairy industry is in the manufacture of cheese. The milk-coagulating enzymes fall into three main categories, (i) animal rennets, (ii) microbial milk coagulants, and (iii) genetically engineered chymosin. Both animal and microbial milk-coagulating proteases belong to a class of acid aspartate proteases and have molecular weights between 30,000 to 40,000. Rennet extracted from the fourth stomach of unweaned calves contains the highest ratio of chymosin (EC 3.4.23.4) to pepsin activity. A world shortage of calf rennet due to the increased demand for cheese production has intensified the search for alternative microbial milk coagulants. The microbial enzymes exhibited two major drawbacks, i.e., (i) the presence of high levels of nonspecific and heat-stable proteases, which led to the development of bitterness in cheese after storage; and (ii) a poor yield. Extensive research in this area has resulted in the production of enzymes that are completely inactivated at normal pasteurization temperatures and contain very low levels of nonspecific proteases. In cheesemaking, the primary function of proteases is to hydrolyze the specific peptide bond (the Phe105-Met106 bond) to generate para-κ-casein and macropeptides. Chymosin is preferred due to its high specificity for casein, which is responsible for its excellent performance in cheesemaking. The proteases produced by GRAS (genetically regarded as safe)-cleared microbes such as Mucor michei, Bacillus subtilis, and Endothia parasitica are gradually replacing chymosin in cheesemaking. In 1988, chymosin produced through recombinant DNA technology was first introduced to cheesemakers for evaluation. Genencor International increased the production of chymosin in Aspergillus niger var. awamori to commercial levels. At present, their three recombinant chymosin products are available and are awaiting legislative approval for their use in cheesemaking (72).
Whey is a by-product of cheese manufacture. It contains lactose, proteins, minerals, and lactic acid. The insoluble heat-denatured whey protein is solubilized by treatment with immobilized trypsin.
Baking industry.
Wheat flour is a major component of baking processes. It contains an insoluble protein called gluten, which determines the properties of the bakery doughs. Endo- and exoproteinases from Aspergillus oryzae have been used to modify wheat gluten by limited proteolysis. Enzymatic treatment of the dough facilitates its handling and machining and permits the production of a wider range of products. The addition of proteases reduces the mixing time and results in increased loaf volumes. Bacterial proteases are used to improve the extensibility and strength of the dough.
Manufacture of soy products.
Soybeans serve as a rich source of food, due to their high content of good-quality protein. Proteases have been used from ancient times to prepare soy sauce and other soy products. The alkaline and neutral proteases of fungal origin play an important role in the processing of soy sauce. Proteolytic modification of soy proteins helps to improve their functional properties. Treatment of soy proteins with alcalase at pH 8 results in soluble hydrolysates with high solubility, good protein yield, and low bitterness. The hydrolysate is used in protein-fortified soft drinks and in the formulation of dietetic feeds.
Debittering of protein hydrolysates.
Protein hydrolysates have several applications, e.g., as constituents of dietetic and health products, in infant formulae and clinical nutrition supplements, and as flavoring agents. The bitter taste of protein hydrolysates is a major barrier to their use in food and health care products. The intensity of the bitterness is proportional to the number of hydrophobic amino acids in the hydrolysate. The presence of a proline residue in the center of the peptide also contributes to the bitterness. The peptidases that can cleave hydrophobic amino acids and proline are valuable in debittering protein hydrolysates. Aminopeptidases from lactic acid bacteria are available under the trade name Debitrase. Carboxypeptidase A has a high specificity for hydrophobic amino acids and hence has a great potential for debittering. A careful combination of an endoprotease for the primary hydrolysis and an aminopeptidase for the secondary hydrolysis is required for the production of a functional hydrolysate with reduced bitterness.
Synthesis of aspartame.
The use of aspartame as a noncalorific artificial sweetener has been approved by the Food and Drug Administration. Aspartame is a dipeptide composed of l-aspartic acid and the methyl ester of l-phenylalanine. The l configuration of the two amino acids is responsible for the sweet taste of aspartame. Maintenance of the stereospecificity is crucial, but it adds to the cost of production by chemical methods. Enzymatic synthesis of aspartame is therefore preferred. Although proteases are generally regarded as hydrolytic enzymes, they catalyze the reverse reaction under certain kinetically controlled conditions. An immobilized preparation of thermolysin from Bacillus thermoprotyolyticus is used for the enzymatic synthesis of aspartame. Toya Soda (Japan) and DSM (The Netherlands) are the major industrial producers of aspartame.
Pharmaceutical Industry
The wide diversity and specificity of proteases are used to great advantage in developing effective therapeutic agents. Oral administration of proteases from Aspergillus oryzae (Luizym and Nortase) has been used as a digestive aid to correct certain lytic enzyme deficiency syndromes. Clostridial collagenase or subtilisin is used in combination with broad-spectrum antibiotics in the treatment of burns and wounds. An asparginase isolated from E. coli is used to eliminate aspargine from the bloodstream in the various forms of lymphocytic leukemia. Alkaline protease from Conidiobolus coronatus was found to be able to replace trypsin in animal cell cultures (36).
Other Applications
Besides their industrial and medicinal applications, proteases play an important role in basic research. Their selective peptide bond cleavage is used in the elucidation of structure-function relationship, in the synthesis of peptides, and in the sequencing of proteins.
In essence, the wide specificity of the hydrolytic action of proteases finds an extensive application in the food, detergent, leather, and pharmaceutical industries, as well as in the structural elucidation of proteins, whereas their synthetic capacities are used for the synthesis of proteins.
GENETIC ENGINEERING OF MICROBIAL PROTEASES
Gene cloning is a rapidly progressing technology that has been instrumental in improving our understanding of the structure-function relationship of genetic systems. It provides an excellent method for the manipulation and control of genes. More than 50% of the industrially important enzymes are now produced from genetically engineered microorganisms (96). Several reports have been published in the past decade (Table 4) on the isolation and manipulation of microbial protease genes with the aim of (i) enzyme overproduction by the gene dosage effect, (ii) studying the primary structure of the protein and its role in the pathogenicity of the secreting microorganism, and (iii) protein engineering to locate the active-site residues and/or to alter the enzyme properties to suit its commercial applications. Protease genes from bacteria, fungi, and viruses have been cloned and sequenced (Table 4).
TABLE 4.
Cloning, sequencing, and/or expression of protease genes or cDNAs from microbial sources
| Source of protease gene | Reference(s) | ||
|---|---|---|---|
| Bacteria | |||
| Bacilli | |||
| B. subtilis 168 | |||
| apr | 270 | ||
| npr | 90, 295, 297, 323 | ||
| epr | 27, 263 | ||
| bpr | 265 | ||
| mpr | 264 | ||
| Isp-1 | 138 | ||
| B. subtilis (Natto) 16 | 319 | ||
| B. subtilis N 515-N (nprX) | 157 | ||
| Alkalophilic Bacillus strain | 129 | ||
| B. alkalophilus PB92 | 300 | ||
| Bacillus sp. strain Y | 293 | ||
| Alkalophilic Bacillus sp. NKS-21 | 318 | ||
| Alkalophilic Bacillus sp. LG-12 | 251 | ||
| Bacillus sp. EA (Npr) | 249 | ||
| Lactococci | |||
| Streptococcus cremoris Wg2 | 139–141 | ||
| Lactococcus lactis subsp. cremoris H2 | 317 | ||
| Streptococcus lactis NCDO 763 | 137 | ||
| L. lactis subsp. cremoris SK11 | 50 | ||
| L. lactis subsp. lactis VC 317 | 153 | ||
| L. lactis subsp. cremoris Wg2 | 58 | ||
| Lactobacillus delbruckii subsp. bulgaricus | 69 | ||
| Streptomyces | |||
| S. griseus | 89 | ||
| S. griseus ATCC 10137 | 107 | ||
| S. cacaoi YM15 | 32 | ||
| S. fradiae ATCC 14544 | 135 | ||
| S. lividans 66 | 17, 18, 163 | ||
| Serratia | |||
| Serratia sp. strain E-15 | 198 | ||
| S. marcescens SM6 | 24 | ||
| S. marcescens | 187 | ||
| S. marcescens ATCC 27117 | 134 | ||
| Pseudomonas | |||
| P. aeruginosa IFO 3455 | 7, 254 | ||
| P. aeruginosa PAO1 | 83 | ||
| P. aeruginosa | 82 | ||
| P. nalgiovense | 68 | ||
| Aeromonas | |||
| A. hydrophila SO 212 | 238, 239 | ||
| A. hydrophila D13 | 238 | ||
| Vibrio | |||
| V. anguillarum NB10 | 185 | ||
| V. parahaemolyticus | 155 | ||
| V. vulnificus | 34 | ||
| V. proteolyticus | 43 | ||
| V. angionolyticus | 45 | ||
| V. cholerae | 86 | ||
| E. coli | |||
| Membrane proteases | |||
| lspA, lep | 42, 333 | ||
| sppA | 108, 276 | ||
| ompT | 80 | ||
| ATP-dependent proteases | |||
| La/Lon | 3, 35, 334 | ||
| Clp/Ti | 181 | ||
| Miscellaneous | |||
| Lysobacter enzymogenes 495 | 260 | ||
| L. enzymogenes | 57 | ||
| Achromobacter lyticus M497-1 | 208 | ||
| A. lyticus | 169 | ||
| Erwinia sp. | 2, 307 | ||
| Rhodocyclus gilatinosa APR 3-2 | 116 | ||
| Bacteroids nodosus | 194 | ||
| Xanthomonas campestris pv. campestris | 168 | ||
| Treponema denticola ATCC 33520 | 176 | ||
| Staphylococcus aureus V8 | 29 | ||
| Thermus aquaticus YT-1 | 148 | ||
| Thermomonospora fusca YX | 152 | ||
| Alteromonas sp. strain O-7 | 298, 299 | ||
| IgA family of proteases | |||
| N. gonorrhoeae | 62, 224, 232 | ||
| N. meningitidis | 169 | ||
| H. influenzae | 228 | ||
| Streptococcus sanguis ATCC 10556 | 70 | ||
| S. pneumoniae | 229, 308 | ||
| Fungi | |||
| Filamentous fungi | |||
| Acidic proteases | |||
| Mucor pusillus rennin (MPR) | 94, 296 | ||
| Mucor miehei aspartyl protease (MMAP) | 51, 79 | ||
| R. niveus aspartic protease (RNAP) | 100, 101 | ||
| A. awamori aspergillopepsin A | 15 | ||
| A. oryzae aspergillopepsin A | 74 | ||
| A. fumigatus aspergillopepsin F | 156, 237 | ||
| A. oryzae M-9 | 284 | ||
| A. satoi ATCC 14332 | 257 | ||
| A. niger var. macrosporus | |||
| Proctase A | 114, 125, 283 | ||
| Proctase B | 113, 175 | ||
| Alkaline proteases (Alp) | |||
| Aspergillus | |||
| A. oryzae ATCC 20386 | 195, 207, 286, 288 | ||
| A. oryzae Thailand industrial strain | 33 | ||
| A. soya | 211 | ||
| A. fumigatus | 123, 237 | ||
| A. flavus | 233 | ||
| A. nidulans | 131 | ||
| Acremonium | |||
| A. chrysogenum ATCC 11550 | 115 | ||
| Fusarium | 136, 193 | ||
| Serine proteases | |||
| Tritirachium album Limber | |||
| Proteinase K | 81 | ||
| Proteinase T | 247 | ||
| Metalloproteases | |||
| A. fumigatus MEP | 124, 262 | ||
| A. flavus MEP-20 | 234 | ||
| A. fumigatus MEP-20 | 234 | ||
| Yeasts | |||
| Acidic proteases | |||
| S. fibuligera (PEP1) | 95, 320 | ||
| S. cerevisiae (PEP4) | 4, 315 | ||
| S. cerevisiae (BAR1) | 177 | ||
| S. cerevisiae (YAP3) | 53 | ||
| C. albicans (SAP) | 106, 170, 196 | ||
| C. tropicalis (ACP) | 294 | ||
| Y. lipolytica 148 (AXP) | 331 | ||
| Wild-type yeast | 332 | ||
| Alkaline proteases | |||
| Y. lipolytica (AEP) XRP2 | 44, 201 | ||
| Serine proteases | |||
| Kluyveromyces lactis KEX-1 | 285 | ||
| S. cerevisiae KEX-2 | 188, 212 | ||
| Other proteases | |||
| Yeast carboxypeptidase (CPY) | |||
| S. cerevisiae PRC1 | 202 | ||
| Vacoular protease B | |||
| S. cerevisiae PRB1 | 190 | ||
| Yeast proteasome PRG1 | 65 | ||
| Viruses | |||
| Animal viruses | |||
| Herpesviruses | |||
| HSV-1 | 47 | ||
| HSV-2 | 271 | ||
| MCMV | 172 | ||
| HHV-6 | 292 | ||
| Adenoviruses | |||
| Ad4 | 102 | ||
| Ad12 | 103 | ||
| Ad3 | 104 | ||
| Ad40 | 306 | ||
| Ad41 | 306 | ||
| Retroviruses | |||
| RSV | 252 | ||
| ASLV | 144 | ||
| ARV-2 | 248 | ||
| M-MuLv | 256 | ||
| SRV-I | 231 | ||
| HTLV-2 | 255 | ||
| BLV | 245 | ||
| M-PMV | 105, 268 | ||
| SIVmac | 40 | ||
| ARV | 144 | ||
| HTLV-I | 246 | ||
| HIV-1 | 46, 78, 171, 222 | ||
| Picornaviruses | |||
| Human rhinovirus type 14 | 162 | ||
| Foot-and-mouth disease virus | 6 | ||
| Encephalomyocarditis virus | 6 | ||
| Poliovirus | 6 | ||
| Plant viruses | |||
| Bean yellow mosaic virus | 22 | ||
| Zucchini yellow mosaic virus (Singapore isolate) | 316 |
Bacteria
The objective of cloning bacterial protease genes has been mainly the overproduction of enzymes for various commercial applications in the food, detergent and pharmaceutical industries. The virulence of several bacteria is related to the secretion of several extracellular proteases. Gene cloning in these microbes was studied to understand the basis of their pathogenicity and to develop therapeutics against them. Proteases play an important role in cell physiology, and protease gene cloning, especially in E. coli, has been attempted to study the regulatory aspects of proteases.
Bacilli.
(i) B. subtilis as a host for cloning of protease genes from Bacillus spp.
The ability of B. subtilis to secrete various proteins into the culture medium and its lack of pathogenicity make it a potential host for the production of foreign polypeptides by recombinant DNA technology. Several Bacillus spp. secrete two major types of protease, a subtilisin or alkaline protease and a metalloprotease or neutral protease, which are of industrial importance. Studies of these extracellular proteases are significant not only from the point of view of overproduction but also for understanding their mechanism of secretion. Table 5 describes the cloning of genes for several neutral (npr) and alkaline (apr) proteases from various bacilli into B. subtilis.
TABLE 5.
Cloning of protease genes in B. subtilis
| Source of proteases | Type of protease | Expression (fold) | Characterization of gene | Reference(s) |
|---|---|---|---|---|
| B. amyloliquefaciens F | Neutral | 50 | —a | 99, 178 |
| B. stearothermophilus F TELNE | Neutral | 5 | nprS sequenced; homologous to nprM | 203 |
| B. amyloliquefaciens IFO 14141 | Neutral | 15 | Partially sequenced | 133, 330 |
| B. cereus | Neutral (metalloprotease) | √b | — | 1 |
| B. stearothermophilus CU21 | Thermostable, neutral | √ | nprT sequenced | 67, 279 |
| B. stearothermophilus 313-1 | Thermostable, neutral | 29 | — | 324 |
| B. stearothermophilus HY-69 | Thermostable, neutral | √ | — | 325 |
| B. stearothermophilus MK232 and YG185-hyperproducing mutant of MK232 | Highly thermostable, neutral | nprM sequenced; deduced amino acid sequence homologous to thermolysin (B. thermoproteolyticus) except for two substitutions, Asp37 to Asn37 and Glu119 to Gln119 | 145, 146 | |
| B. stearothermophilus | Thermostable, metalloprotease | √ | 258 | |
| B. brevis | Metalloprotease | — | √ | 9 |
| B. licheniformis | Alkaline and neutral | √ | — | 209 |
| B. amyloliquefaciens | Alkaline and neutral | — | apr, npr sequenced | 304 |
| B. licheniformis | Alkaline | √ | — | 88 |
| B. pumilus IFO 12092 | Alkaline | √ | — | 289 |
| B. amyloliquefaciens | Subtilisin | √ | √ | 313 |
| B. natto | — | 350 | — | 197 |
| B. licheniformis ATCC 14580 | C-terminal glutamic acid specific (BLase) | √ | — | 127 |
—, no data is available.
√, expression of the gene was observed.
(ii) B. subtilis.
B. subtilis 168 secretes at least six extracellular proteases into the culture medium at the end of the exponential phase. The structural genes encoding the alkaline protease (apr) or subtilisin (270), neutral protease A and B (nprA and nprB) (90, 297, 323), minor extracellular protease (epr) (27, 263), bacillopeptidase F (bpr) (265), and metalloprotease (mpr) (264) have been cloned and characterized. These proteases are synthesized in the form of a “prepro” enzyme. To increase the expression of subtilisin and neutral proteases, Henner et al. replaced the natural promoters of apr and npr genes with the amylase promoter from B. amyloliquefaciens and the neutral protease promoter from B. subtilis, respectively (90). To understand the regulation of npr A gene expression, Toma et al. cloned the genes from B. subtilis 168 (normal producer) and Basc 1A341 (overproducer) (295). The two genes were found to be highly homologous except for a stretch of 66 bp close to the promoter region, which is absent in the Basc 1A341 gene. The epr gene shows partial homology to the apr gene and to the major intracellular serine protease (Isp-1) gene of B. subtilis (138). The epr gene was mapped at a locus different from the apr and npr loci on the B. subtilis chromosome and was shown not to be required for growth or sporulation, similar to apr or npr genes. Deletion of 240 amino acids (aa) from the C-terminal region of the epr gene product did not abolish the enzyme activity (27, 263). The deduced amino acid sequence of the mature bpr gene product is similar to those of other serine proteases of B. subtilis, i.e., subtilisin, Isp-1, and Epr. B. subtilis strains containing mutations in five extracellular protease genes (apr, npr, epr, mpr, and bpr) have been constructed (264) with the aim of expressing heterologous gene products in B. subtilis. The total amino acid sequence of B. subtilis Isp-1 deduced from the nucleotide sequence showed considerable homology (45%) to subtilisin. Highly conserved sequences are present around the essential amino acids, Ser, His, and Asp, indicating that the genes for both the intra- and extracellular serine proteases have a common ancestor.
In 1995, Yamagata et al. cloned and sequenced a 90-kDa serine protease gene (hspK) from B. subtilis (Natto) 16 (319). The large size of the enzyme may represent an ancient form of bacterial serine protease.
Analysis of DNA sequences of subtilisin BPN′ from B. amyloliquefaciens (304, 313) and subtilisin Carlsberg from B. licheniformis (119) revealed that the two sequences are highly conserved in the coding region for the mature protein and must therefore have a common ancestral precursor. Yoshimoto et al. characterized the gene encoding subtilisin amylosacchariticus from B. subtilis subsp. amylosacchariticus (327, 328). The sequence was highly homologous to that of subtilisin E from B. subtilis 168 (269). The gene was expressed in B. subtilis ISW 1214 by using the vector pHY300PLK, with 20-fold-higher activity than that of the host and 4-fold-higher activity than that of B. subtilis subsp. amylosacchariticus.
(iii) Alkalophilic Bacillus spp.
Bacillusproteases with an extremely alkaline pH optimum are generally used in detergent powders and are preferred over the subtilisins (optimal pH, 8.5 to 10.0). The information on these enzymes is helpful in designing new subtilisins. Kaneko et al. cloned and sequenced the ale gene, encoding alkaline elastase YaB, a new subtilisin from an alkalophilic Bacillus strain (129). The deduced amino acid sequence showed 55% homology to subtilisin BPN′. Almost all the positively charged residues have been predicted to be present on the surface of the alkaline elastase YaB molecule, facilitating its binding to elastin. The deduced amino acid sequence of the highly alkaline serine protease from another alkalophilic strain, B. alcalophilus PB92, showed considerable homology to YaB (300). The cloned gene was further used to increase the production level of the protease by gene amplification through chromosomal integration. Increased enzyme production and gene stabilization was observed when nontandem duplication occurred.
A gene encoding ISP-1 was characterized from alkalophilic Bacillus sp. strain NKS-21 (318). The nucleotide sequence was 50% homologous to genes encoding ISP-1 from B. subtilis, B. polymyxa, and the alkalophilic Bacillus sp. strain 221.
(iv) Other bacilli.
A gene encoding the highly thermostable neutral proteinase (Npr) from Bacillus sp. strain EA1 was shown to be closely related to an npr gene from B. caldolyticus YP-T, except for a single-amino-acid change in the gene product (249). The enzyme from Bacillus sp. strain EA1 was more thermostable than the enzyme from B. caldolyticus YP-T; this can be attributed to the single-amino-acid change.
Lactococci.
Lactococci (Lactococcus lactis subsp. lactis and cremoris, previously Streptococcus lactis and Streptococcus cremoris, respectively), the dairy starter cultures, have a complex proteolytic system which enables them to grow in milk by degrading casein into small peptides and free amino acids. This leads to the development of the texture and flavor of various dairy products. The importance of the cell envelope-located proteolytic system for dairy product quality has resulted in an increased fundamental research of the involved enzymes and their genes. On the basis of differences in caseinolytic specificity, the lactococcal proteases have been classified into two main groups: the PI-type protease, which degrades predominantly β-casein, and the PIII-type protease which degrades αS1-, β-, and κ-casein (305). Most of the genetic studies have focused on the PI-type protease genes. Lactococcal protease genes are located mostly on plasmids, which differ considerably in size and genetic organization in different strains (49). Curing experiments have suggested that plasmid pWV05 of S. cremoris Wg2 specifies proteolytic activity. The entire plasmid was subcloned in E. coli (140). A 4.3-MDa HindIII fragment of the plasmid, specifying the proteolytic activity, was cloned in B. subtilis and in a protease-deficient S. lactis strain. In S. lactis, the recombinant plasmid enabled the cells to grow normally in milk with rapid acid production. The HindIII fragment specifying the proteolytic activity of S. cremoris Wg2 was fully sequenced (141). The nucleotide sequence revealed two open reading frames (ORFs), ORF-1, a small ORF containing 295 codons, and ORF-2, a large ORF containing 1,772 codons. The protein specified by ORF-2 contained regions of extensive homology to subtilisins. The amino acids Asp32, His64, and Ser221, involved in the formation of the active site, were well conserved. Deletion analysis of the proteinase gene of S. cremoris Wg2 showed that deletion of the C-terminal 343 aa did not influence the enzyme specificity of β-casein degradation (139). L. lactis subsp. cremoris H2 carries plasmid pDI21, containing the gene for the protease-positive phenotype (Prt+). The 6.5-kbp HindIII DNA fragment of pDI21 encoding the protease was cloned in E. coli as well as in L. lactis subsp. lactis 4125 (317). Protease that specifically degrades β-casein was expressed in both the transformed organisms. S. lactis NCDO 763 harbors plasmid pLP763, containing the gene for Prt+, which enables it to grow to a higher density in milk. The deduced amino acid sequence (1,902 aa) of the Prt+ phenotype was homologous to that of the serine protease from S. cremoris Wg2, suggesting that the genes encoding both products must have been derived from a common ancestral gene (137).
The PIII-type protease is found only in L. lactis subsp. cremoris AM1 and SK11. These strains are related, and they both contain the proteases encoded by the 78-kbp plasmid psk111. The L. lactis subsp. cremoris SK11 prtP gene was cloned and expressed in E. coli as well as in other subspecies of L. lactis (50). The location and orientation of the prtP gene on psk111 was determined by deletion analysis. A region at the C terminus of the prtP product, which is involved in cell envelope attachment, was identified. A deletion derivative of prtP specifying a C-terminally truncated protease was able to express and fully secrete the protease in the medium and showed the capacity to degrade αS1-, β-, and κ-casein. The N-terminal catalytic domain of the matrix enzyme shows significant sequence homology to the serine proteases of the subtilisin family (subtilases). Comparison with the known sequences of prt genes from L. lactis SK11, Wg2, and NCDO 763 indicated that the VC317 protease (153) is a natural hybrid of the SK11 and Wg2 proteases.
Stabilization of lactococcal protease genes (prtP, encoding the cell envelope-associated serine protease, and prtM, which activates the prtP gene product) is essential for the dairy industry. The plasmid-located prtP and prtM genes of L. lactis subsp. cremoris Wg2 were integrated (Campbell-like integration) into the L. lactis subsp. lactis MG1363 chromosome by using the insertion vector pKL9610 (158). Two transformants, MG610 and MG611, carrying different numbers (two and eight, respectively) of stable tandemly integrated plasmid copies, were obtained. Strain MG611 produced 11 times as much protease activity as did strain MG610 and about 1.5 times as much as did strain MG1363 (carrying five copies of the autonomously replicating plasmid).
A plasmid-free strain, L. lactis subsp. cremoris BC101, produces cell envelope-associated protease that is very similar or identical to the envelope protease encoded by the plasmid-linked prtP gene in other strains such as Wg2 and SK11. The prtP and prtM genes in this plasmid-free strain were identified on chromosomal DNA by pulsed-field gel electrophoresis (204). The chromosomal protease gene was shown to be organized in a fashion similar to that of the plasmid-linked protease gene. Recently, Gilbert et al. cloned and sequenced the prtB chromosomal gene from Lactobacillus delbrueckii subsp. bulgaricus, encoding a protease of 1,946 residues with a predicted molecular mass of 212 kDa (69). The deduced amino acid sequence showed significant homology to the N-terminal and catalytic domains of lactococcal PrtP cell surface proteases.
Streptomyces.
Streptomyces griseus , an organism used for the commercial production of pronase, secretes two extracellular serine proteases: proteases A and B. The enzymes are 61% homologous on the basis of amino acid identity. The genes encoding protease A (sprA) and protease B (sprB) were isolated from the S. griseus genomic library, and their proteolytic activity was demonstrated in S. lividans (89). The DNA sequences suggest that each protease is initially secreted as a precursor, which is then processed to remove an N-terminal propeptide from the mature protease. The strong homology between the coding regions of the two protease genes suggests that sprA and sprB must have originated by gene duplication. Protease B is one of the major extracellular proteases secreted by S. griseus ATCC 10137, and its gene was expressed in S. lividans by Hwang et al. (107). Their nucleotide sequencing of the gene further revealed that the deduced amino acid sequence was identical to that reported earlier by Henderson et al. (89). However, the nucleotide sequence of the 3′-flanking region was G rich and may be responsible for the reduced level of protease in S. griseus ATCC 10137 compared to the level in protease B-overproducing strains of S. griseus.
The npr gene for neutral metalloprotease from S. cacaoi YM15 was expressed in S. lividans (32). The deduced ORF encoded a 550-aa (60-kDa) protein, whereas the Npr secreted into the medium is 35 kDa, suggesting that it has undergone substantial processing since separating from the precursor.
S. fradiae ATCC 14544 secretes a novel, acidic-amino-acid-specific serine protease (SFase) into the culture medium. The deduced amino acid (135) sequence revealed a mature protein of 187 aa and shows 82% homology to the acidic-amino-acid-specific protease from S. griseus (277). Genes coding for a novel protease (163), a chymotrypsin-like serine protease (SAM-P20) (17), and SlpD and SlpE (homologs of the Tap [major tripeptidyl aminopeptidase] mycelium-associated proteases) (18) were cloned from S. lividans 66.
Serratia.
The gram-negative bacteria belonging to the family Enterobacteriaceae are known to secrete large amounts of extracellular proteases into the surrounding medium. Serratia sp. strain E-15 produces a potent extracellular metalloprotease, which is widely used as an anti-inflammatory agent. The gene encoding the protease from Serratia sp. strain E-15 was expressed both in E. coli and in S. marcescens (198). Nucleotide sequence analysis revealed three zinc ligands (essential for proteolytic activity) and an active site, as predicted by comparing the deduced amino acid sequence with that of B. thermoproteolyticus thermolysin and B. subtilis neutral protease.
In another study, the extracellular serine protease (SSP) of S. marcescens was excreted through the outer membrane of E. coli. The nucleotide sequence of the cloned SSP gene, together with the determination of the N and C termini of the excreted enzymes, suggested that this protease is produced as a 112-kDa preproenzyme composed of an N-terminal signal sequence, the mature protease, and a large C-terminal domain (187).
Pseudomonas.
Pseudomonas aeruginosa is an opportunistic pathogen and can cause fatal infections in compromised hosts. This virulence is related to the secretion of several extracellular proteins (167). P. aeruginosa secretes two proteases, an alkaline protease and an elastase. The alkaline protease genes (apr) from P. aeruginosa IFO 3455 and PAO1 were cloned in E. coli (7, 83, 254). The DNA fragment (8.8 kbp) coding for the alkaline protease from strain PAO1 was expressed in E. coli under the control of a tac promoter. Active enzyme was found to be synthesized and secreted into the medium in the absence of cell lysis.
The LasA protease (elastin degrading) of P. aeruginosa is also an important contributor to the pathogenesis of this bacterium. The enzyme shows a high level of staphylolytic activity. The lasA gene from strain FRD1 was overexpressed in E. coli (82). It encodes a precursor, prepro-LasA, of about 45 kDa. N-terminal sequence analysis allowed the identification of a 31-aa signal peptide. pro-LasA (42 kDa) does not undergo autoproteolytic processing and possesses little anti-staphylococcal activity. The digestion of pro-LasA either by trypsin or by culture filtrate of the P. aeruginosa lasA deletion mutant yielded the active (20-kDa) staphylolytic protease.
Aeromonas.
Aeromonas hydrophila and the related aeromonads are opportunistic pathogens of humans and fish. The pathogenicity of the microbe may involve several extracellular enzymes, and it has been suggested that the proteases excreted by Aeromonas spp. play an important role in invasiveness and in establishment of the infection. Two distinct types of extracellular proteases, a temperature-stable metalloprotease and a temperature-labile serine protease, are found in various strains of A. hydrophila and other aeromonads (160). Structural genes encoding extracellular proteases from two different A. hydrophila strains, SO2/2 and D13, were cloned in E. coli C600-1 by using pBR322 (238). A temperature-stable protease is secreted into the periplasm of E. coli and exhibits properties identical to those of the protease purified from A. hydrophila SO2/2 culture supernatant. A gene for the temperature-labile serine protease was also expressed from A. hydrophila SO2/2 into E. coli C600-1 and S. lividans 1326 (239).
Vibrio.
To facilitate genetic analyses of the role of proteases in the pathogenesis of various Vibrio species, the genes encoding the Zn2+-metalloprotease from V. anguillarum NB 10 (185), V. parahaemolyticus (155), and V. vulnificus (34) were cloned and sequenced. The conserved Zn2+-binding domains were identified by measuring homology to other metalloproteases. The nucleotide sequence of the nprV gene encoding the extracellular neutral protease, vibriolysin (NprV), of V. proteolyticus revealed an ORF encoding 609 aa including a putative signal peptide sequence followed by a long prosequence of 172 aa (43). Comparative analysis of the mature NprV with the sequences of the neutral proteases from bacilli revealed extensive regions of conserved amino acid homology with respect to the active site and zinc- and calcium-binding residues. NprV was overproduced in B. subtilis by placing the DNA encoding the pro-NprV and the mature NprV downstream of the Bacillus promoter and signal sequences.
In one of the studies, the nucleotide sequence analysis of the structural gene, hap, for the extracellular haemagglutinin protease of V. cholerae revealed that the enzyme is produced as a large precursor, with the amino-terminal signal sequence following a propeptide (86). The deduced amino acid sequence of the mature enzyme showed 61.5% identity to the P. aeruginosa elastase.
E. coli.
(i) Membrane proteases.
In a bacterium, a protein that is to be exported across the cytoplasmic membrane is synthesized as a large precursor with a signal peptide at its amino terminus (19). The processing of this precursor involves two sequential events: (i) removal of the signal peptide from the precursor through an endo-type cleavage and (ii) digestion of the cleaved signal peptide. The membrane proteases involved are (i) signal peptidases (lipoprotein signal peptidase [Lsp] and leader peptidase [Lep]) and (ii) signal peptide peptidase (protease IV). The genes lspA (333), lep (42), and sppA (108, 276) for protease IV of E. coli have been characterized and mapped on E. coli chromosomal DNA. Protease IV was shown to be a tetramer of the sppA gene product.
(ii) ATP-dependent proteases.
ATP-dependent proteolysis plays a major role in the turnover of both abnormal proteins and a variety of regulatory proteins in both prokaryotic and eukaryotic cells. Three families of ATP-dependent proteases are found in E. coli: La (or Lon), Clp (or Ti), and FtsH (or HflB) proteases. Lon and Clp are soluble proteins, whereas FtsH is a membrane-anchored protein.
In vitro studies on ATP-dependent proteolysis have shown that the major ATP-dependent activity in the extracts of E. coli cells is the Lon protease (73). The lon gene of E. coli K-12 has been cloned (334), sequenced (3, 35), and shown to be dispensable by insertional mutagenesis of the gene (180). Extracts from Lon-deficient E. coli cells still catalyze ATP-dependent proteolysis mediated by a soluble two-component protease, Clp. Two dissimilar components of Clp are (i) the ClpA regulatory polypeptide, with two ATP-binding sites and an intrinsic ATPase activity, and (ii) the ClpP subunit, with a proteolytic active site. Clp is a serine protease, and its nucleotide sequence (181) showed little homology to the known classes of serine proteases representing a unique family of serine proteases (182).
The cleavage of proteins such as casein and albumin by Clp proteases requires both ClpP and the regulatory subunit ClpA and ATP. However, it has been observed that ClpP can independently catalyze endoproteolytic cleavage of short peptides at a lower rate than in the presence of ClpA and ATP. The gene encoding ClpP is, at 10 min on the E. coli map, nearer to the gene encoding the ATP-dependent Lon protease of E. coli and farther from the gene encoding ClpA. Primer extension experiments indicate that the transcription initiates immediately upstream of the coding region for ClpP, with a major transcription start at 120 bases in front of the start of translation. ClpP insertion mutants have been isolated, and strains devoid of ClpP are viable in the presence as well as the absence of Lon protease. Genetic evidence is available demonstrating that ClpA and ClpP act together in vivo (181). Processing of ClpP appears to involve an intermolecular autocatalytic cleavage reaction which is shown to be independent of ClpA (182). A speculative model for the chaperone-like function of ATP-dependent proteases has been postulated by Suzuki et al. (275). The dual function of the ATP-dependent protease is determined by the affinity of the protein for the subunit or domain. Based on this, the ATP-dependent protease may regulate the subunit stoichiometry of protein complexes.
Miscellaneous.
Among the bacterial representatives of the trypsin family, α-lytic protease, an extracellular enzyme of the gram-negative soil bacterium Lysobacter enzymogenes 495, is of particular interest. Nucleotide sequence analysis and S1 mapping of the structural gene for the α-lytic protease from L. enzymogenes 495 indicated that the enzyme is synthesized as a prepro-protein (41 kDa) that is subsequently processed to its mature extracellular form (20 kDa) (260). The gene was further expressed in E. coli by fusing the promoter and signal sequence of the E. coli phoA gene to the proenzyme portion of the α-lytic protease gene (261). Following induction, an active enzyme was produced both intra- and extracellularly. Fusion of the mature protein domain alone resulted in the production of an inactive enzyme, indicating that the large N-terminal pro-protein region is necessary for activity. Epstein and Wensink also cloned and sequenced the gene for α-lytic protease, a 19.8-kDa serine protease secreted by L. enzymogenes (57). The nucleotide sequence contains an ORF which codes for the 198-residue mature enzyme and a potential prepro-peptide, also of 198 residues.
Achromobacter protease I (API) is a mammalian-type, lysine-specific serine protease that specifically hydrolyzes the lysyl peptide bond. The nucleotide sequence analysis of API from Achromobacter lyticus M497-1 revealed that the gene codes for a single polypeptide chain of 653 aa (208). The 263-aa mature protein, which was identified by protein sequencing, was found to be flanked N-terminally by 205 aa including a signal peptide and C-terminally by 180 aa. E. coli carrying a recombinant plasmid containing the API gene overproduced and secreted the protein (API′) into the periplasm. The N-terminal amino acid sequence of API′ was the same as that of mature API, whereas the enzyme retained the C-terminal extended polypeptide chain. The structural gene for β-lytic protease was cloned from A. lyticus, and the nucleotide sequence analysis of the gene revealed a mature enzyme of 179 aa, with additional 195 aa at the N-terminal end of the enzyme, which includes the signal peptide (161).
Characterization of a serine protease gene that cleaves specifically on the carbonyl side of acidic residues from Staphylococcus aureus V8 revealed a 68-residue N-terminal extension which includes a 19- to 29-residue signal peptide, the mature protein, and the C-terminal region with several repeated acidic amino acid-rich tripeptides (29). The C terminus may function as a competitive inhibitor of the prepro-protein form of the enzyme, perhaps to prevent activity prior to secretion.
Aqualysin I, an alkaline serine protease, is secreted into the culture medium by an extreme thermophile, Thermus aquaticus YT-1. Aqualysin I shows high DNA sequence homology to the subtilisin-type serine proteases, especially in the regions containing the active-site residues (Asp32, His64, and Ser221) of subtilisin BPN′ (148). The nucleotide sequence also revealed that the enzyme is produced as a large precursor, containing the N-terminal portion, the protease, and the C-terminal portion.
The gene (tfgA) for the major extracellular protease of Thermomonospora fusca YX was isolated, sequenced, and expressed in Streptomyces lividans (152). The ORF encoded 375 residues including a 31-residue potential signal sequence, an N-terminal 150-residue prosequence, and the 194-residue mature protease belonging to chymotrypsin family.
Alteromonas sp. strain O-7, a marine bacterium, excretes alkaline serine proteases or subtilases (AprI and AprII) into the growth medium. The results of the deduced amino acid sequence analysis of genes for both AprI and AprII indicated that both the enzymes are produced as large precursors consisting of four domains: the signal sequence, the N-terminal pro-region, the mature AprI or AprII, and the C-terminal extension (298, 299). The amino acid sequence of mature AprI shows high sequence homology to that of class I subtilase, while the sequence of AprII shows high sequence homology to that of class II subtilase. Repeated sequences were observed in the C-terminal pro-region, showing high homology to sequences from the C-terminal pro-region of other known gram-negative bacteria (V. angiolyticum, Xanthomonas campestris, and V. proteolyticus).
IgA family of proteases.
Immunoglobulin A1 (IgA1) proteases form a very heterogenous group of extracellular endopeptidases produced by a number of bacterial pathogens that colonize human mucosal surfaces. The enzymes specifically cleave human IgA1, which participates in the immune system surveillance in the human mucosa. A number of reports (62, 224, 232) on the cloning of the iga gene, encoding the IgA1 protease from Neisseria gonorrhoeae, are available. Nucleotide sequence analysis revealed that the enzyme is produced as a large precursor with three functional domains, i.e., the N-terminal leader peptide, the protease, and the carboxy-terminal “helper” domain. An overall structural similarity to the iga gene from N. meningitidis was also demonstrated (169).
Comparison of the deduced amino acid sequence of the iga gene of Haemophilus influenzae serotype b with that of a similar protease from N. gonorrhoeae revealed several domains with a high degree of homology (228). An enzyme secretion mechanism analogous to that for N. gonorrhoeae IgA1 protease was proposed for H. influenzae IgA1 protease. Limited diversity has been found among the IgA1 protease genes of H. influenzae, serotype b strains (230), information that is useful from the point of view of vaccine preparation.
Cloning of streptococcal IgA1 genes from Streptococcus sanguis ATCC 10556 (70) and S. pneumoniae (229, 308) has been reported. Hybridization experiments with an S. sanguis IgA1 protease gene probe showed no detectable homology to chromosomal DNA of gram-negative bacteria secreting IgA1 proteases. The gene encoding IgA1 protease from S. pneumoniae was identified by using the S. sanguis protease probe. However, the iga gene was found to be highly heterogenous among streptococcal species.
From the foregoing, it can be seen that subtilisins (270) and neutral proteases (279, 323) of various Bacillus species, the α-lytic protease from L. enzymogenes (57, 260), and proteases A and B from S. griseus (89) have long polypeptide extensions at their N termini. The IgA protease of N. gonorrhoeae (224) and the protease of S. marcescens (322) have C-terminal extensions. Achromobacter protease I (208), aqualysin I from T. aquaticus (148), and AprI and AprII from Alteromonas sp. strain O-7 (298, 299) bear long peptide chains at both the N and C termini. The function of the pre-peptide portion (signal peptide) in these precursors is possibly to assist in the transport of the secretory protein across the cytoplasmic membrane. The exact role of the pro-peptide region is not known; possibly the long peptide serves to inhibit the mature protease to which it is connected (29, 57). It is also possible that the pro-peptide helps the protease to fold into its active form (111, 261).
Fungi
As in bacteria, cloning of the protease genes of fungi has been attempted from both the commercial and pathogenicity points of view.
Filamentous fungi.
(i) Acidic proteases.
(a) Mucor. Two closely related species of zygomycete fungus, Mucor pusillus and Mucor miehei, secrete aspartate proteases, also known as mucor rennins, into the medium. The enzymes possess high milk-clotting activity and low proteolytic activity, enabling them to be used as substitutes for calf chymosin in the cheese industry.
Sequencing of the cloned gene encoding M. pusillus rennin (MPR) revealed an ORF without introns, encoding possible pre-pro-sequences (66 aa) upstream of the mature MPR sequence (296). The deduced amino acid sequence showed a high degree of homology to that of M. miehei rennin (MMR). The gene encoding M. miehei aspartyl protease (MMAP) has also been cloned and sequenced (79). The deduced primary translation product showed an N-terminal extension which appears to comprise a signal peptide of 22 aa and a propeptide of 47 aa. Fungal aspartyl proteases are structurally related to each other and to the gastric aspartyl proteases chymosin and pepsin; therefore, they may be activated in a manner similar to their gastric counterparts. When the gene encoding the prepro-form of MPR was cloned in S. cerevisiae under the control of the yeast GAL7 promoter, an inactive zymogen of the enzyme with the 44-aa pro-sequence was identified in the medium during the initial stage of cultivation (94). In vitro conversion of the zymogen to mature MRP was shown to proceed autocatalytically under the acidic conditions.
(b) Rhizopus. Rhizopus niveus, belonging to the zygomycete class, also secretes aspartyl protease abundantly. The gene encoding R. niveus aspartic protease (RNAP) was cloned and sequenced (100). Comparison of the deduced amino acid sequence with the amino acid sequence of rhizopuspepsin of R. chinensis (282) revealed that the RNAP gene has an intron within its coding region. A prepro-sequence of 66 aa upstream of the mature enzyme was also revealed. High-level secretion of RNAP-I was achieved by subcloning the RNAP-I gene into Saccharomyces cerevisiae (101). Yeast cells carrying the intact RNAP-I gene under the control of the glyceraldehyde-3-phosphate dehydrogenase gene promoter of S. cerevisiae were unable to synthesize RNAP-I. On removal of the intron of the RNAP-I gene, the cell secreted the enzyme with high efficiency.
(c) Aspergillus. The pepA gene encoding the aspartic protease, aspergillopepsin A, from Aspergillus awamori (15), the pepA gene from A. oryzae (74), and the cDNA coding for an elastinolytic aspartic protease, aspergillopepsin F, from A. fumigatus (156, 237) were cloned and sequenced. The nucleotide sequence data revealed that the ORFs encoding aspartic proteases in these aspergilli are composed of four exons. Prepro-peptides of 69, 78, and 70 aa were found to precede 395-, 326-, and 323-aa mature proteins of A. awamori, A. oryzae, and A. fumigatus, respectively. The amino acid sequence of aspergillopepsin F shows 70, 66, and 67% homology to the sequences of those from A. oryzae, A. awamori, and A. saitoi, respectively. The primary structure of aspergillopepsin I from A. satoi ATCC 14332 (now designated A. phoenicis) was deduced from the nucleotide sequence of the gene (257). The cDNA of the gene was also cloned and expressed in yeast cells.
Two types of acid endopeptidases, acid proteases A and B (commercially named proctase A and B), are known to be secreted into the medium by A. niger var. macrosporus. Protease B is a typical aspartic protease, inhibited by pepstatin, whereas protease A is not inhibited by pepstatin. Sequencing of the protease A gene revealed an 846-bp structural gene without any introns encoding the precursor form of the enzyme (114, 125, 283). The precursor, of 282 residues, includes an N-terminal prepro-sequence of 59 residues, the L chain of 39 residues, an intervening sequence of 11 residues, and the H chain of 173 residues linked in that order. The deduced amino acid sequence (394 residues) of the prepro-form of protease B showed 98% homology to the sequences of aspergillopepsin I from A. awamori and A. saitoi and 68% homology to that of aspergillopepsin I from A. oryzae (113, 175). The cDNA was expressed in E. coli, and the purified pro-protease B showed protease activity under acidic conditions (pH 2 to 4).
(ii) Alkaline proteases.
(a) Aspergillus.Alkaline protease (Alp) produced by A. oryzae, a filamentous ascomycete used in the manufacture of soy sauce, is considered to play an important role in producing the flavor of soy sauce by hydrolyzing the raw materials. Tatsumi et al. constructed the cDNA library of A. oryzae ATCC 20386 in pUC119 and isolated a cDNA (1,100 bp) encoding the mature region of Alp (286). The nucleotide sequence of the cDNA lacked most of the DNA sequences corresponding to the prepro-region. The entire cDNA coding for prepro-Alp was cloned and expressed in S. cerevisiae (288). The character of the Alp secreted from S. cerevisiae was shown to be identical to that of the native Alp. The predicted mature Alp consists of 282 aa and shows homology to other serine proteases of subtilisin families from bacteria as well as from fungi. Alp has a 121-aa prepro-region wherein the N-terminal 21 residues show the characteristics of a signal peptide. Alp expressed in S. cerevisiae was secreted with the N terminus processed correctly, analogous to the expression in S. cerevisiae of aspartic protease from M. pusillus (321). The prepro-Alp cDNA of A. oryzae was further cloned into an osmophilic yeast, Zygosaccharomyces rouxii (207). The recombinant Z. rouxii secreted a large amount of Alp (about 300 mg/liter) into the culture medium. The Alp gene is 1,374 nucleotides long and contains three introns, one in the pro-region and two in the mature protein region (195).
A gene encoding Alp from the A. oryzae Thailand industrial strain was isolated from the genomic library by using oligodeoxyribonucleotide probes based on the A. oryzae ATCC 20386 cDNA sequence (33). By comparison with the published cDNA sequence (286), Alp from A. oryzae Thailand was found to be encoded by four exons. Transformation of the alpA gene in the high-level-Alp-producing A. oryzae strain U212, obtained by UV mutagenesis, resulted in the production of up to five times as much Alp as in the parental strain. A. fumigatus and A. flavus, the agents of invasive aspergillosis, secrete highly homologous serine proteases. The genomic as well as cDNA clones encoding elastinolytic Alp from both A. fumigatus (123, 237) and A. flavus (233) were sequenced. The A. nidulans prtA gene coding for Alp was isolated by using the gene encoding A. oryzae Alp (131). The nucleotide sequence of prtA was determined, and the deduced amino acid sequence showed a high degree of similarity to Alp from A. fumigatus, A. flavus, and A. oryzae. prtA transcription was shown to be dependent on the medium composition.
(b) Acremonium. Acremonium chrysogenum ATCC 11550 (Cephalosporium acremonium) produces a considerable amount of extracellular Alp. The cDNA and genomic DNA encoding Alp were isolated from the A. chrysogenum cDNA and genomic DNA libraries, respectively (115). The nucleotide sequence of the gene was determined. The deduced amino acid sequence showed 57% homology to that of A. oryzae Alp. Cloning of the entire cDNA encoding A. chrysogenum Alp into S. cerevisiae directed the secretion of enzymatically active Alp into the culture medium.
(c) Fusarium. The transfer of the Fusarium alkaline protease gene (136) into A. chrysogenum resulted in transformants producing large amount of Alp (193). Southern hybridization analysis, as well as PCR of genomic DNAs from these transformants, showed chromosomal integration of the full-length alp gene. The enzyme secreted by A. chrysogenum had properties identical to that of the native Fusarium Alp, indicating that the Alp promoter, signal sequence, and introns functioned correctly in A. chrysogenum.
(iii) Serine proteases.
(a) Tritirachium.Proteinase K is a serine endoproteinase excreted by the fungus Tritirachium album Limber. The enzyme is able to hydrolyze native proteins rapidly and is active in the presence of detergents (urea, sodium dodecyl sulfate, etc.), making the proteinase K one of the most useful tools in molecular biology. The enzyme exhibits strong similarity to the bacterial subtilisins. The genomic DNA as well as the cDNA encoding proteinase K from T. album Limber have been cloned in E. coli, and the entire nucleotide sequence of the coding region, including the 5′- and 3′-flanking regions, has been determined (81). The nucleotide sequence analysis revealed that the primary secreted product is a zymogen containing a 15-aa signal sequence and a 90-aa pro-peptide. The pro-peptide is presumably removed in the later steps of the secretion process or upon secretion into the medium. The proteinase K gene was shown to be composed of two exons and one 63-bp intron located in the proregion. The pro-proteinase K gene was expressed in E. coli under the control of the tac promoter.
The coding sequence of proteinase T from T. album Limber (247) was shown to be interrupted by two introns. The deduced amino acid sequence showed 53% identity to that of proteinase K. The presence of four cysteines in the mature proteinase, probably in the form of two disulfide bonds, explains the thermal stability of proteinase T. The proteinase T cDNA was expressed in E. coli, and the authenticity of the product was confirmed by Western blotting and N-terminal analysis of the recombinant product.
(iv) Metalloproteases.
(a) Aspergillus.Jaton-Ogay et al. (124) and Sirakova et al. (262) cloned and sequenced the gene as well as the cDNA encoding the 42-kDa elastinolytic metalloproteinase (MEP) of A. fumigatus. Comparison of the nucleotide sequences revealed that the genomic and the cDNA sequences are analogous except for four introns interrupting the ORF. The enzyme was shown to be produced in a prepro-form, with a 384-aa mature protease region. In another study, no intron was found in the ORF of A. flavus mep20 (encoding a 23-kDa MEP) whereas a 59-bp intron was present in the gene from A. fumigatus (a homolog of mep20) (234). The MEP20 proteins of A. flavus and A. fumigatus have 68% identity.
Yeasts.
(i) Acidic proteases.
The yeast Saccharomycopsis fibuligera produces an extracellular acid protease (PEP1). DNA coding for the secretable acid protease gene of S. fibuligera was isolated (95, 320). The enzyme produced by Saccharomyces cerevisiae cells that are transformed with a plasmid carrying the cloned gene showed enzymatic properties similar to those of the S. fibuligera protease.
Two different groups of workers (4, 315) from the United States worked simultaneously on the PEP4 gene of S. cerevisiae, which encodes an aspartyl protease implicated in the posttranslational regulation of the yeast vacuolar hydrolases. The PEP4 gene was isolated from a genomic library by complementation of the PEP4-3 mutation. The nucleotide sequence was deduced, and the predicted amino acid sequence showed substantial homology to that of the aspartyl protease family.
The deduced primary translation product (587 aa) of bar1, the structural gene for the barrier activity of S. cerevisiae, has a putative signal peptide and nine potential asparagine-linked glycosylation sites (177). Marked sequence similarity to pepsin-like proteases was observed.
A gene for yeast aspartyl protease 3 (YAP3) allowing KEX-2-independent MFα pro-pheromone processing was isolated from S. cerevisiae (53). The nucleotide sequence of the YAP3-encoding gene was determined, and the deduced amino acid sequence was shown to exhibit extensive homology to a number of aspartyl proteases, including the PEP4 and BAR1 proteins of S. cerevisiae. A potential transmembrane domain similar to that found in the KEX-2 gene product was also located.
Candida albicans and Candida tropicalis are the medically more important opportunistic pathogens causing infections in immunocompromised patients. Their secretory proteolytic activity is considered to be a major virulence factor. The deduced amino acid sequence of the acid protease (ACP) from C. tropicalis shows similarity to the amino acid sequence of the pepsin family (294). The aspartyl proteinase gene (106, 170) and cDNA (196) from various C. albicans strains were cloned and sequenced. The genes for secreted aspartic proteases (the SAP1, SAP2, SAP3, and SAP4 genes) in C. albicans constitute a multigene family. Three putative new members, SAP5, SAP6, and SAP7, were also isolated and sequenced. Evidence was also obtained for the existence of SAP multigene families in other Candida species such as C. tropicalis, C. parapsilosis, and C. guilliermondii (191).
The amino acid sequence of an acid extracellular protease (AXP) from Yarrowia lipolytica 148 deduced from the nucleotide sequence revealed a putative 17-aa pre-peptide, a 27-aa pro-peptide, and a 353-aa mature protein (37 kDa) (331). AXP showed homology to proteases of several fungal genera. The transcription of both AXP and the alkaline extracellular protease (AEP) genes in Y. lipolytica was shown to be regulated by the pH of the culture (331).
A gene encoding an extracellular protease was cloned from a wild-type yeast into brewer’s yeast, S. cerevisiae (332). Such genetically engineered strains carrying the gene for an extracellular protease were shown to exhibit chill-proofing activity in beer. Proteins remaining in beer after its brewing from malt tend to form hazes during chilling due to their poor solubility at lower temperatures. Acid proteases assist in reducing the haze formation by degrading the proteins in beer without affecting foam stability or organoleptic properties such as taste.
(ii) Alkaline protease.
The XRP2 gene for AEP from Y. lipolytica encodes a putative 22-aa pre-peptide followed by a 135-aa pro-peptide containing a possible N-linked glycosylation site and the two Lys-Arg peptidase-processing sites (44, 201). The mature protease (297 aa) contains two potential glycosylation sites.
(iii) Serine proteases.
(a) Kluyveromyces. The KEX-1 gene product is required for the production of a killer toxin by Kluyveromyces lactis. The deduced amino acid sequence (700 aa) encoded by KEX-1 showed an internal domain with a striking homology to the sequences of the subtilisin-type proteinases (285).
(b) S. cerevisiae. The KEX-2 gene, encoding a subtilisin-like endoprotease responsible for posttranslational processing of certain gene products, contains a 2,442-bp ORF encoding a polypeptide of 814 aa (188, 212). The deduced amino acid sequence revealed a region near the N terminus that has extensive homology to the subtilisin family of serine proteases. A putative membrane-spanning domain near the C terminus was also detected. The wild type and the C-terminal deletion derivatives showed similar substrate specificities, with the highest activity being against Arg-Arg dipeptides.
(iv) Other proteases.
Yeast carboxypeptidase (CPY) is a glycosylated yeast vacuolar protease that is used commercially in peptide synthesis. CPY is encoded by the PRC1 gene. To increase the production of CPY in S. cerevisiae, PRC1 was placed under the control of the strongly regulated yeast GAL1 promoter on the multicopy plasmids and introduced into νpl1 mutant strains (202). About a 200-fold increase in the level of secreted CPY (40 mg/liter) was obtained compared to the level in a νpl1 mutant carrying a single copy of the wild-type PRC-1 gene. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis revealed two forms of secreted active CPY, probably due to the different levels of glycosylation. The structural gene PRB1, encoding the vacuolar protease B of S. cerevisiae, was cloned by complementation of the prb1-1122 mutation (190).
PRG1, a yeast gene encoding the 32-kDa proteasome, which shows 55.6% sequence homology to 80% of the RING10 gene product (human proteasome), was identified (65). Genomic disruption of PRG1 revealed that it is essential for yeast growth. The results strongly indicate that the antigen-processing system present in vertebrates has evolved from a basic cellular process present in all organisms.
Viruses
Gene cloning of viral proteases has been undertaken for the isolation and overexpression of the gene and for subsequent screening of inhibitory compounds that may be used in the development of chemotherapeutic agents. Viral protease is responsible for processing of polyprotein precursors into the structural proteins of the mature virion. Among viruses, reports on cloning of protease genes are limited mainly to animal viruses (Table 5).
Animal viruses.
(i) Herpesviruses.
Each member of the herpesvirus family encodes a unique serine protease in association with a capsid assembly protein, with the associated ORFs being designated UL80 and UL26 in human cytomegalovirus (HCMV) and in herpes simplex virus type-1 (HSV-1), respectively. The UL26 gene encodes a protease responsible for the C-terminal cleavage of the nucleocapsid-associated proteins (ICP35c and ICP35d) to their posttranslationally modified counterparts (ICP35e and ICP35f). The protease expressed in E. coli exhibited autoprocessing and specifically cleaved the ICP35 protein assembly (47). Similarly, genes encoding proteases from HSV-2, murine cytomegalovirus (MCMV), and human herpesvirus 6 (HHV-6) have been studied (172, 271, 292). Such studies assist in the investigation of the role of proteolytic processing in the virus.
(ii) Adenoviruses.
Adenoviruses code for a serine-centered, neutral protease specific for selected Gly-Ala bonds in several virus-encoded precursor proteins that are required for virion maturation and infectivity. To determine the functional domains of this key enzyme, protease genes from various types of adenoviruses have also been cloned and sequenced (102–104, 306).
(iii) Retroviruses.
The genomic organization of retroviruses is 5′-LTR-gag-pro-pol-env-LTR-3′ (where LTR is a long terminal repeat). The pro/prt gene product is an aspartyl protease, which is responsible for processing the gag and pol polyprotein precursors into the structural proteins of the mature virion. Comparison of the genomic organization of certain retroviruses revealed that prt lies in the carboxyl terminus of gag in Rous sarcoma virus (RSV) (252) and avian sarcoma leukosis virus (ASLV) (144); in the amino terminus of pol in AIDS-associated retrovirus type 2 (ARV-2) (248); in the same reading frame as both gag and pol in Moloney murine leukemia virus (M-MuLV) (256); and as a separate reading frame in simian AIDS retrovirus type I (SRV-I) (231), human T-cell leukemia virus type 2 (HTLV-2) (255), bovine leukaemia virus (BLV) (245), and Mason-Pfizer monkey virus (MPMV) (268). Besides cloning and sequencing of the prt gene, there are a few reports on expression of the gene in E. coli (40, 105, 144). Significant inhibition of the expressed protease activity by pepstatin A confirmed that HTLV-1 protease is a member of the aspartyl protease group (246).
Human immunodeficiency virus (HIV), a causative agent of AIDS, is also a member of the family Retroviridae. The virus exhibits the same overall gag-pol-env genome organization as that of other retroviruses. The genome-size mRNA of HIV-1 is translated into two polyproteins: Pr55 (gag gene product) and Pr160 (gag-pol gene product). Cleavage of these polyproteins by the viral protease into smaller structural proteins and replication enzymes such as reverse transcriptase and integrase is necessary to produce infectious progeny virions from immature virus particles. The enzyme, a part of the polyprotein, has a highly conserved sequence, Asp-Thr-Gly, which is homologous to the active site of the aspartic proteases and is thought to belong to this enzyme family (216). The protease is essential for the retroviral life cycle, as indicated by the production of noninfectious, replication-deficient virions by Moloney murine leukemia virus variants mutated in the protease-encoding region (130). This suggests that HIV protease is a good target for chemotherapy and that specific inhibitors of this enzyme may have a significant function in the treatment of AIDS without interfering with the host cell physiology. To obtain sufficient quantities of the HIV protease for biochemical and structural analyses, several groups have described expression of the recombinant HIV-1 protease in E. coli (46, 78, 171). Pichuantes et al. have reported extracellular expression of HIV-1 aspartic protease in S. cerevisiae (222). The expressed enzyme was shown to exhibit a proteolytic activity, as has been shown to be associated with the purified HIV-1 virions (164). Debouck et al. expressed the HIV protease gene product in E. coli (46). The product was shown to autocatalyze its maturation from a larger precursor and to process an HIV Pr55 gag protein when coexpressed in E. coli. This allowed a structure-function analysis of the HIV protease and provided a simple assay for the development of potential therapeutic agents directed against the critical viral enzyme.
(iv) Picornaviruses.
Human rhinovirus is a member of the picornavirus (small RNA) family. Rhinovirus has commercial importance since it is the causative agent of about 15% of cases of the common cold. A cDNA encoding the viral protease from the 3C region of human rhinovirus type 14 was expressed in E. coli through the use of a periplasmic secretion vector (162). A comparison of the 3C protease regions of all the available picornavirus (foot-and-mouth disease virus, encephalomyocarditis virus, and poliovirus) sequences revealed two completely conserved residues, Cys147 and His161, which may be the reactive residues of the active sites of these cysteine proteases (6).
Plant viruses.
Potyviruses are a cause of serious losses of several major crop plants. In plants infected with the potyviruses, inclusions consisting of viral proteins are found in the cell nucleus. One of them, the nuclear inclusion protease (NIa), is the major viral protein responsible for the proteolytic maturation of the polyprotein encoded by the virus. The elucidation of the structure of such virus-encoded proteins could eventually facilitate the design of novel polypeptides which bind to them and inhibit their functions. With this objective, cDNAs for NIa proteases were cloned and sequenced from bean yellow mosaic virus (22) and zucchini yellow mosaic virus (Singapore isolate) (316).
The potential contributions of genetic engineering to mankind are enormous and will benefit agriculture, animal husbandry, environmental protection, food production and processing, human health care, manufacture of biochemicals and biofuels, etc. In general, the application of genetic engineering to proteases will facilitate their use in industry and enable the development of therapeutic agents against the proteases that are important in the life cycle of organisms which cause serious diseases.
PROTEIN ENGINEERING
Many industrial applications of proteases require enzymes with properties that are nonphysiological. Protein engineering allows the introduction of predesigned changes into the gene for the synthesis of a protein with an altered function that is desired for the application. Recent advances in recombinant DNA technology and the ability to selectively exchange amino acids by site-directed mutagenesis (SDM) have been responsible for the rapid progress of protein engineering. Identification of the gene and knowledge of the three-dimensional structure of the protein in question are the two main prerequisites for protein engineering. The X-ray crystallographic structures of several proteases have been determined (39, 143, 223, 267). Proteases from bacteria, fungi, and viruses have been engineered to improve their properties to suit their particular applications.
Bacteria
Subtilisin has been chosen as a model system for protein engineering since a lot of basic information about this commercially important enzyme is available. Its pH dependance (290), catalytic activity (278, 281), stability to heat or denaturing agents (112, 199), and substrate specificity (10, 14, 30, 59, 243) have been altered through SDM. A slightly reduced rate of thermal inactivation was observed for a subtilisin BPN′ variant containing two cysteine residues (Cys22, Cys87) (186, 214). Oxidation of Met222 adjacent to the Ser221 in the active site of subtilisin reduces the catalytic activity of subtilisin. The effect of substitution of Met222 with different amino acids revealed that small side chains yield the highest activity. The mutant enzymes Ser222, Ala222, and Leu222 were active and stable to peroxide for 1 h. Probing of the specificity of the S1 binding site of Met222 Cys/Ser mutants of subtilisin from Bacillus lentus with boronic acid inhibitors revealed similar binding trends for the mutant and the parent (269). The disulfide bonds introduced into subtilisin away from its catalytic center were shown to possess increased autolytic stability (312). Higher thermostability of subtilisin E as a result of introduction of a disulfide bond engineered on the basis of structural comparison with a thermophilic serine protease has been reported (280). Strausberg et al. have created the environment for stabilization of subtilisin by deleting the calcium-binding loop from the protein (273). Analysis of the structure and stability of the prototype with the loop deleted followed by SDM resulted in a mutant with native proteolytic activity and 1,000-fold-greater stability under strongly chelating conditions. SDM-mediated substitution of Asn241 buried in the neutral protease of B. stearothermophilus by leucine resulted in an increase in thermostability of 0.7 ± 0.1°C (55). The thermostability of the neutral protease from B. subtilis was increased by 0.3 and 1.0°C by replacing Lys with Ser at positions 249 and 290, respectively, whereas the Asp249 and Asp290 mutants exhibited an increased stabilization by 0.6 and 1.2°C, respectively (54).
A protein engineering study was undertaken by Bruinenberg et al. to determine the functions of one of the largest loop insertions (residues 205 to 219), predicted to be spatially close to the substrate-binding region of the SK11 protease from L. delbrueckii and susceptible to autoproteolysis (28). Deletion or modification of this loop was shown to affect the activity and autoprocessing of the protease. Graham et al. showed that random mutagenesis of the substrate-binding site of α-lytic protease, a serine protease secreted by the soil bacterium Lysobacter enzymogenes, generated enzymes with increased activities and altered primary specifities (77). Substitution of His120 by Ala in the LasA protease of P. aeruginosa yielded an enzyme devoid of staphylolytic activity. Thus, His120 was shown to be essential for LasA activity (82).
Fungi
Fungal aspartic proteases are able to cleave substrate with “Lys” in the P1 position. Sequencing and structural comparison suggest that two aspartic acid residues (Asp30 and Asp77) may be responsible for conferring this unique specificity. Lowther et al. engineered the substrate specificity of rhizopus-pepsin from Rhizopus niveus and demonstrated the role of Asp77 in the hydrolysis of the substrates with lysine in the P-1 position (173).
The primary structure of aspergillopepsin I from Aspergillus saitoi ATCC 14332 (now designated A. phoenicis) was deduced from the nucleotide sequence of the gene (257). To identify the residue responsible for determining the specificity of aspergillopepsin I toward the basic substrates in the substrate-binding pocket, Asp76 was replaced with a Ser residue by SDM. The striking feature of this mutation was that only the trypsinogen-activating activity of the enzyme was destroyed, suggesting the importance of the Asp76 residue in binding to basic substrates.
To elucidate whether the processing of the pro-region occurs by autoproteolysis or by involving a processing enzyme, Tatsumi et al. changed Ser228 to Ala by SDM (287). S. cerevisiae cells harboring a recombinant plasmid with mutant Alp did not secrete active Alp into the culture medium. The yeast cells accumulated a protein of 44 kDa, probably a precursor of Alp (the 34-kDa mature Alp plus the 10-kDa pro-peptide), suggesting that autoproteolytic processing of the pro-region was occurring.
Introduction of a disulfide bond by SDM is known to enhance the thermostability of a cysteine-free enzyme. Aqualysin I, a thermostable subtilisin-type protease from Thermus aquaticus YT-1, contains four Cys residues forming two disulfide bonds (149). The primary structure of Alp showed 44% homology to that of aqualysin I, and sites for Cys substitutions to form a disulfide bond were chosen in the Alp based on this homology. Ser69, Gly101, Gly169, and Val200 were replaced by Cys in the mutant Alp. Both Cys69-Cys101 and Cys169-Cys200 mutant Alps were expressed in S. cerevisiae, and the enzymes were purified to homogeneity. The Cys169-Cys200 disulfide bond was shown to increase the thermostability as well as the thermoresistance of Aspergillus oryzae Alp (110).
In vitro mutation of an aspartic acid residue predicted to be in the active site abolished the barrier activity of S. cerevisiae (177). BAR1 possesses a carboxyl-terminal domain of an unknown function, and deletion of 166 of 191 aa of this region had no significant effect on the barrier activity.
Viruses
The protease of HCMV was rendered stable by conversion of one of the three Val141, Val207, or Val254 residues to Gly by SDM (151). The resulting stable proteases are useful as screening tools for HCMV antiviral agents and as diagnostic tools for diseases resulting from HCMV infection.
Replacement of Asp64, a residue from the catalytic core sequence among aspartyl proteases, with Gly was shown to abolish the correct processing of the 53K gag precursor by HTLV-1 gag protease (87).
In poliovirus, the mutation of highly conserved residues, e.g., Cys147 or His161, produced an inactive enzyme while mutation of a nonconserved residue, Cys154, had only a negligible effect on the proteolytic activity (117).
The protein-engineering technique has been exploited successfully for obtaining proteases which show unique specificity and/or stability at high temperature and pH. It has also contributed substantially to our understanding of the structure-function relationship of proteases. In future, protein engineering will offer possibilities of generating proteases possessing entirely new functions.
SEQUENCE HOMOLOGY
Studies of DNA and protein sequence homology are important for a variety of purposes and have therefore become routine in computational molecular biology. They serve as a prelude to phylogenetic analysis of proteins and assist in predicting the secondary structure of DNA and proteins. Proteases are a complex group of enzymes and vary enormously in their physicochemical and catalytic properties. The nucleotide and amino acid sequences of a number of proteases have been determined, and their comparison is useful for elucidating the structure-function relationship (5). The homology of proteases with respect to the nature of the catalytic site has been studied (12, 13). Accordingly, the enzymes have been allocated to evolutionary families and clans. It has been suggested that there may be as many as 60 evolutionary lines of peptidases with separate origins. Some of these contain members with quite diverse peptidase activities, and yet there are some striking examples of convergence (236).
A number of reports on the homology of proteases are available. Takagi et al. found that the thermostable proteases of Bacillus stearothermophilus and B. thermoproteolyticus are 85% homologous and the thermolabile proteases of B. subtilis and B. amyloliquefaciens are 82% homologous, whereas the thermostable protease of B. stearothermophilus is only 30% homologous to the thermolabile protease of B. subtilis (279). However, an amino acid sequence of 17 residues, which also includes the active-site histidine residue, was found to be highly conserved in all four neutral proteases, suggesting that they have the same three-dimensional structure around the active site despite the difference in their source and physicochemical properties such as thermostability.
Koide et al. compared the amino acid sequences of intracellular serine proteases from B. subtilis with those of subtilisin Carlsberg and subtilisin BPN′ and showed that they were 45% homologous (138). The sequence around the catalytic triad of serine, aspartate, and histidine is highly conserved, suggesting that the genes for both the intracellular and extracellular proteases have evolved from a common ancestor by divergent evolution (200).
The amino acid sequence of an extracellular alkaline protease, subtilisin J, is highly homologous to that of subtilisin E and shows 69% identity to that of subtilisin Carlsberg, 89% identity to that of subtilisin BPN′, and 70% identity to that of subtilisin DY. The amino acid sequence of subtilisin J is completely identical to that of the protease from B. amylosacchariticus except for two amino acid substitutions, Thr130 to Ser130 and Thr162 to Ser162, in addition to one amino acid substitution in the signal peptide and two in the propeptide region. The probable active-site residues of subtilisin J, i.e., Asp32, His64, and Ser221, are identical to those of other subtilisins from Bacillus. Therefore, it was concluded that the alkaline protease from B. stearothermophilus is a subtilisin. Similarly, the various Bacillus serine alkaline proteases, such as bacillopeptidase F, subtilisin, Epr, and ISP-1, show considerable homology and conserved amino acids around the active-site residues, i.e., Ser, Asp, and His (265).
The extracellular proteases of B. subtilis are synthesized as prepro-enzymes. Four neutral proteases from bacilli with known pro-sequences were compared, and considerable homology within the pro-peptide region was observed (297). Since the pro-peptide region mediates the folding of the protease, it would be interesting to learn about the residues essential for folding and to determine whether the mechanism of folding is similar in these proteases. Sequences corresponding to the mature form of these enzymes were compared by using thermolysin sequence as a reference. The zinc-binding site (His142, His146, and Glu166) and the residues participating in the catalytic reaction and positioning of the substrate backbone in the active site (Asn112, Ala113, Glu143, Tyr157, and His231) were found to be conserved. Differences in these might lead to altered substrate specificities. Of the four calcium-binding sites in thermolysin, two sites, i.e., sites 3 and 4, are absent in the thermolabile neutral proteases of B. amyloliquefaciens and B. subtilis (NprA) whereas in NprB, Asn187 in site 3 is replaced by Arg. Such changes are responsible for the loss of thermostability and can be detected by sequence homology studies.
Alkaline proteases from various species of Aspergillus also show a high degree of homology (131). Alp from A. oryzae shows considerable homology (29 to 44%) to the members of the subtilisin family with conserved active-site residues (288). However, Alp exhibits little homology to mammalian serine proteases such as trypsin and chymotrypsin. The deduced structure of the KEX-1 protein, required for the production of the killer toxin of Kluyveromyces lactis contains an internal domain with a striking homology to the sequences of subtilisin-type proteases (242). Therefore it was deduced that the product of the KEX-1 gene of K. lactis is a protease involved in the processing of the toxin precursor.
The characteristic of trypsin-related enzymes is the presence of disulfide bonds, which are absent in all known subtilisins. Proteinase K from Tritirachium album Limber is a single chain protein of 277 aa with two disulfide bonds at positions 34-124 and positions 179-248 and a free -SH group at position 73. Sequences around the active-site residues correspond to those around the active-site residues of subtilisins. Comparison of the proteinase K sequence with known subtilisins shows 35% homology and 44% sequence identity to thermitase, which is indicative of a relationship between proteinase K and the subtilisin family. It is likely that these enzymes have evolved from a common ancestral precursor serine proteinase (122). However, there is a distinct difference between the typical subtilisins and proteinase K, since the latter has two disulfide bonds, which are lacking in subtilisins. Therefore, it has been assumed that the two progenitors diverged from an ancestral proteinase, separating the subtilisin-related enzymes into two subclasses: (i) cysteine-containing subtilisins e.g., proteinase K and thermitase, and (ii) cysteine-free subtilisins, e.g., subtilisin Novo, Carlsberg, or DY.
The proteasome or multicatalytic endopeptidase complex is a high-molecular-mass multisubunit complex that is ubiquitous in eukaryotes and also found in the archaebacterium Thermoplasma acidophilum (336). While eukaryotic proteasomes contain 15 to 20 different subunits, the archaebacterial proteasome is made of only two different subunits (α and β), yet the complexes are almost identical in size and shape. The α (233-aa) and β (211-aa) subunits of T. acidophilum have a sequence identity of 24% and an overall similarity of 47%, indicating that the genes encoding the two subunits arose from a common ancestor. All the sequences of proteasomal subunits from eukaryotes available to date can be related to either the α or β subunit of the T. acidophilum “urproteasome,” and they can be distinguished by the presence or absence of a highly conserved N-terminal extension which is characteristic of α-type subunits. In terms of evolution, the genes for these α and β subunits can be considered paralogous (genes resulting from duplication and divergence of one gene within one genome) and therefore are able to acquire different functions. The α subunit of the T. acidophilum proteasome shows sequence similarity to the S. cerevisiae wild-type suppressor gene scl1-encoded polypeptide, which is probably identical to the subunit YC7-α of the yeast proteasome. This lends support to a putative role of proteasomes in the regulation of gene expression (337). The amino acid sequence of Xenopus proteasome subunit XC3 is highly homologous (95.3%) to those of the rat RC3 and human HC3 subunits (66). The presence of an accessible nuclear targeting signal at the C terminus of the subunits suggests that it is probably involved in the regulation of the cellular distribution of the proteasome.
The secretable acid protease of the yeast Saccharomycopsis fibuligera carries a hydrophobic amino-terminal segment of about 20 aa which resembles signal sequences found in a wide variety of secretory protein precursors (95). Alignment of this sequence with the aspartyl protease family showed significant homologies, especially in the regions surrounding the two active-site aspartate residues. These results suggest that the PEP1 gene is a structural gene for the secretable acid protease of S. fibuligera. The aspartic protease from Rhizopus niveus (RNAP) shows 76% homology to rhizopuspepsin, 42% homology to penicillopepsin, and 41% homology to human pepsin (100, 101). The homology between RNAP and rhizopuspepsin is found throughout their structures. Based on this homology, an intron within the coding region and a prepro-enzyme sequence of 66 aa upstream of the mature enzyme were detected in RNAP. Studies of the homology of proteases have shown that the residues involved in the substrate and metal ion binding, catalysis, disulfide bond formation and active-site formation are conserved. Analysis of sequence homology is used in deciphering the structure-function relationship of proteases.
EVOLUTIONARY RELATIONSHIP OF PROTEASES
Proteases are present in all living organisms and are considered to have arisen in the earliest phases of biological evolution, some 1 billion years ago. Comparisons of amino acid sequences, three-dimensional structures, and mechanism of action of proteases assist in deciphering of their course of evolution. Changes in molecular structure have accompanied the demands for altered functions of proteases during evolution. We have compiled the amino acid sequences of proteases from diverse origins such as microbes, plants, and animals and have arranged them in three different groups based on the pH of their action. These sequences, which have been selected from SWISS-PROT and PIR entries, are of comparable length and have been aligned with CLUSTAL W software for multiple alignments (291) (Fig. 4).
FIG. 4.
Homology alignment of the protease sequences. The protease sequences have been selected from the SWISS-PROT and PIR entries, and some have been deduced from the nucleotide sequences obtained from the EMBL database. These are aligned by using CLUSTAL W software for multiple alignment (291). (A) Acidic proteases. (B) Neutral proteases. (C) Alkaline proteases. The key to the sequences is given in Table 5. Numbering of the amino acid residues is based on the first sequence in the list. Identical (○) and conserved (•) residues are boxed; those involved in the active site are indicated by ∗.
Acidic Proteases
The proteases selected here for comparison of amino acid sequences are active between pH 2 and 6. They include mostly aspartic proteases and also some of the cysteine proteases and metalloproteases. They are about 380 to 420 aa long and have different amino acid residues constituting the active site, as shown in Table 6. The homology between these acidic proteases is shown in Fig. 4A. The sequences belonging to pepsin family (A1) are grouped and are aligned below the other sequences. As expected, there is considerable homology among these five acidic proteases. The sequences around the two aspartic residues (D97 and D258, residues numbered according to the Bajra protease) constituting the active site are conserved. Among these five proteases, the rat and monkey proteases show maximum homology (68.4%) and are related to the mosquito lysosomal aspartic protease. When four monkey pepsinogens which show development-dependent expression were compared, a very high homology was observed (126). Pepsinogens A-1 and A-2/3 differed in seven amino acids and only in five amino acids when the pepsin moiety alone was examined. The mosquito lysosomal protease is very closely related to human cathepsin D, exhibiting 92% homology (37).
TABLE 6.
Proteases selected for multiple alignmenta
| SWISS-PROT/PIR entry | No. of amino acid residues | Type of protease | Residue(s) at active site |
|---|---|---|---|
| Acidic proteases | |||
| CYS2_HORVU | 373 | C1/papain (cysteine) | C158 H297, N318 |
| HRTD_CROAT | 414 | M12B (metallo) | E311 |
| GPR_BACME | 371 | U3 (aspartic) | D89, D258 |
| ASPP_AEDAE | 395 | Lysosomal (aspartic) | D96, D258 |
| CARP_CANTR | 390 | Candidapepsin (aspartic) | D96, D258 |
| CARP_SACFI | 390 | Saccharopepsin (aspartic) | D96, D258 |
| PEPC_RAT | 382 | Gastricsin (aspartic) | D96, D258 |
| PEP2_MACFU | 378 | Pepsin A (aspartic) | D96, D258 |
| Neutral proteases | |||
| PRCA_THEAC | 233 | PS | U |
| PRC3_YEAST | 250 | PS | U |
| PRC6_SCHPO | 259 | PS | U |
| PRC2_ORYSA | 270 | PS | U |
| PRC6_ARATH | 250 | PS | U |
| PRC6_DICDI | 250 | PS | U |
| PRC8_CAEEL | 259 | PS | U |
| PRC6_DROME | 249 | PS | U |
| PRC3_XENLA | 233 | PS | U |
| CANS_BOVIN | 263 | C2/calpain (cysteine) | U |
| CANS_PIG | 266 | C2/calpain (cysteine) | U |
| TRYT_CANFA | 275 | S1/trypsin (serine) | H74, D121, S191 |
| TRYB_HUMAN | 275 | S1/trypsin (serine) | H74, D121, S191 |
| SNPA_STRLI | 237 | M7 (metallo) | E64 |
| Alkaline proteases | |||
| JC6052 | 355 | Trypsin-like protease | H91, D126, S201 |
| EYLA_BACAO | 380 | S8/subtilase | D120, H150, S302 |
| SUBT_BACST | 381 | S8/subtilase | D120, H150, S302 |
| PRTK_TRIAL | 384 | S8/subtilase | D120, H150, S302 |
| ALP_TRIHA | 409 | S8/subtilase | D120, H150, S302 |
| ALP_CEPAC | 402 | S8/subtilase | D120, H150, S302 |
| ORYZ_ASPFL | 403 | S8/subtilase | D120, H150, S302 |
| ORYZ_ASPFU | 403 | S8/subtilase | D120, H150, S302 |
| I50494 | 410 | Serine protease inhibitor | U |
Key to the entry names of acidic proteases: CYS2_HORVU, Hordeum vulgare; HRTD_CROAT, Crotalus atrox; GPR_BACME, Bacillus megaterium; ASPP_ AEDAE, Aedes aegyptii; CARP_CANTR, Candida tropicalis; CARP_SACFI, Saccharomycopsis fibuligera; PEPC_RAT, Rattus norvegicus; PEP2_MACFU, Macaca fuscata. Sequences are numbered according to the Hordeum vulgare cysteine protease. Key to the neutral protease sequences: PRCA_THEAC, Thermoplasma acidophilum; PRC3_YEAST, Saccharomycopsis fibuligera; PRC6_SCHPO, Schizosaccharomyces pombe; PRC2_ORYSA, Oryza sativa; PRC6_ARATH, Arabidopsis thaliana; PRC6_DICDI, Dictyostellium discoideum; PRC8_CAEEL, Caenorhabditis elegans; PRC6_DROME, Drosophila melanogaster; PRC3_XENLA, Xenopus laevis; CANS_BOVIN, Bos taurus; CANS_PIG, Sus scrofa; TRYT_CANFA, Canis familiaris; TRYB_HUMAN, Homo sapiens; SNPA_STRLI, Streptomyces lividans. Sequences are numbered according to the Thermoplasma protease. PS, proteasome subunit; U, unknown. Key to the alkaline protease sequences: JC6052, Escherichia coli; ELYA_BACAO, Bacillus amyloliquefaciens; SUBT_BACST, Bacillus subtilis; PRTK_TRIAL, Tritirachium album Limber; ALP_TRIHA, Tritirachium harzianum; ALP_CEPAC, Cephalosporium acremonium; ORYZ_ASPFL, Aspergillus flavus; ORYZ_ASPFU, Aspergillus fumigatus; I50494, Cyprinus carpio. Residues are numbered according to the E. coli protease.
The amino acid sequences of C. tropicalis and Saccharomycopsis fibuligera show considerable homology (42.6%). High similarity scores were obtained when the acid protease from C. tropicalis was compared with Rhizopus aspartic proteases, human pepsinogen A precursor, protease A from yeast, the barrier protein from S. cerevisiae, and an acidic protease from S. fibuligera (294).
The cysteine protease from Hordeum vulgare shows some homology to the snake venom metalloprotease from Crotalus atrox, which is not statistically significant, whereas the Gpr protease from Bacillus megaterium, which plays a vital role in spore germination, shows least homology to all other acidic proteases but shares one of the active-site aspartate residues (D258) with them. The Gpr acidic proteases of B. subtilis and B. megaterium showed 68% identity in their sequences, but comparison of the B. subtilis Gpr amino acid sequence with that of its serine protease or metalloprotease revealed no significant homologies (274), which supports our observations. This suggests that the genes encoding these proteases do not have a common ancestor or that if they do so, they have undergone much divergence. The lack of homology between the spore protease and other B. subtilis proteases can be explained by differences in their properties such as the number of subunits and sequence specificity for the substrate. Thus, our results, in agreement with previous reports, indicate that the extent of homology is greater if the proteases belong to the same family and that in the same family the homology is greater if the phylogenetic distance is shorter.
A pairwise computer comparison also provides more information about the evolutionary relationships between the members of the different families. The dendrograms generated by this analysis, using the TreeView package (213), demonstrate the relationship among the proteins based on the similarity of the amino acid sequences (Fig. 5a).
FIG. 5.
Dendrogram showing the relationships among the proteases, created by the TreeView package (213). The proteases are grouped as acidic proteases (a), neutral proteases (b), and alkaline proteases (c). Abbreviations of the species described are those used in Table 6. The differences between the sequences are proportional to the length along the horizontal axis.
Neutral Proteases
The neutral proteases, which are active at neutral or weakly alkaline or weakly acidic pH, include cysteine proteases, metalloproteases, and some of the serine proteases. Brenner (25) has pointed out that the two codons for serine TCN and AGY cannot be interconverted by single nucleotide mutations but can be connected by two other codons, ACN for threonine and TGY for cysteine. Thus, there can be at least two different lines of descent for the active-site sequences of the serine proteases. The simplest pathway for this convergent evolution is by the divergence of each line from a precursor which was itself catalytically active and had much the same sequence. It was further demonstrated that modern serine enzymes are likely to have arisen from cysteine precursors. These findings encourage the search for evidence to connect the presumed and existing cysteine sequences with their postulated metalloenzyme predecessors. For this search and construction of phylogenetic trees, gene structure is important. Thus, multiple lines of descent can be realistically considered in situations with sequence similarity but with differences in gene structure.
The neutral proteases selected for sequence analysis in the present study are in the size range of 225 to 275 aa (Table 6). The homology between them is shown in Fig. 4B. Of 14 proteases, 9 belong to the T1A or proteasome A family of the multicatalytic endopeptidase complex. The sequences of the proteasomal subunits aligned here can be related to the α subunit of the Thermoplasma proteasome and show considerable homology. It is still not clear to which family of proteases the proteasomes belong (93). As in the cysteine family of proteases, all nine proteasome subunits show a conserved proline residue (P-17), which may serve to prevent unwanted N-terminal proteolysis (12). Many residues at the N terminus are highly conserved, which is a characteristic of the α-type subunits. The similarity decreases toward the C terminus which appears to be rather variable (337). Although the β subunit shows no sequence motif characteristic of serine proteases, it contains all the essential amino acid residues forming the catalytic triad or the “charge relay system” (Ser, Asp, and His). These residues are found to be conserved (Ser16, His73, and Asp84), except for the histidine in the α subunits of Thermoplasma, yeast (S. cerevisiae), and Caenorhabditis elegans (residues are numbered according to the Thermoplasma α-subunit sequence). Therefore, it is possible that the active site is shared between the α and β subunits (336). The tyrosine autophosphorylation site at Tyr123 is conserved in six of the nine sequences. The cAMP/cGMP-dependent phosphorylation sites between aa 31 and 37 are found only in Thermoplasma and Drosophila spp. (84), as reported by Zwickl et al. (337). A consensus nuclear localization signal between aa 49 and 56 (240) and a region complementary to the nuclear localization signal consensus sequence (326) between aa 201 and 212 can be identified in these sequences. Thus, the sequence comparison of various α proteasome subunits from archaebacteria to mammals shows high homology.
The bovine and porcine proteases which belong to the calpain or C2 family of cysteine proteases differ from each other in only six amino acid residues and thus show almost 99% homology to highly conserved calcium-binding domains and the N-terminal glycine-rich hydrophobic region. The region rich in proline residues (aa 76 to 81, numbered as in the Thermoplasma protease) is also conserved except at position 79, where proline is replaced by valine.
Tryptase precursors from humans and dogs (301), which belong to the S1 or trypsin family of serine proteases, show 76% sequence identity. The signal sequence from residues 1 to 30 is 60% identical; the difference is only in the site of glycosylation, which is Asn132 in the canine sequence and Asn233 in the human sequence. The sequences for active-site and disulfide bond formation are highly conserved and correspond to those of chymotrypsinogen (302).
The relationship between these neutral proteases is evident from the dendrogram shown in Fig. 5b.
Alkaline Proteases
The alkaline proteases selected here are active in the pH range of 8 to 13 and are about 420 to 480 residues in length. Six of them belong to the S8 or subtilase family of serine proteases (Table 6). They are aligned in their phylogenetic order, as shown in Fig. 4C. Considerable homology within the same genus is observed for Bacillus and Aspergillus proteases and three other fungal proteases. However, these proteases show comparatively lower homology among themselves. The active-site residues, as well as the residues around the active site, are highly conserved, suggesting that they may have evolved from a common ancestor. The sequences of E. coli and Cyprinus carpio seem to be homologous to some extent, but they do not have common active-site residues and therefore do not have a common ancestor. These two, in turn, show no significant homology to the other seven alkaline proteases. The overall homology among all these sequences can be represented by the dendrogram in Fig. 5c.
The results of our analysis of the amino acid sequences of the acidic, neutral, and alkaline proteases indicate that the members of the pepsin family of acidic proteases may have evolved from a common ancestor by convergent evolution. High homology between the sequences of the α subunits of proteasomes provides evidence for the presence of an evolutionarily conserved gene family. No amino acid residues are conserved in all the acidic or neutral proteases, except glycine. The alkaline serine proteases seem to have evolved from a common ancestor by divergent evolution. In general, the sequences belonging to the same family show more homology or are more closely related. This criterion is currently used to assign a particular sequence to a particular family, i.e., the serine protease, cysteine protease, aspartate protease, or metalloprotease family. Within a family, the extent of homology is inversely proportional to the phylogenetic distance. The proteases from distantly related organisms show less homology or more diversity. However, this needs extensive sequence analysis of proteases, since the homology depends on many parameters or factors such as structure, function, source, and nature of the catalytic or active site. Thus, proteases are highly diverse enzymes having different active sites and metal-binding regions. The residues involved in disulfide bond formation and their positions, which vary in different proteases, can be detected by multiple alignments.
CURRENT PROBLEMS AND POTENTIAL SOLUTIONS
Proteases are a complex group of enzymes which differ in their properties such as substrate specificity, active site, and catalytic mechanism. Their exquisite specificities provide a basis for their numerous physiological and commercial applications. Despite the extensive research on several aspects of proteases from ancient times, there are several gaps in our knowledge of these enzymes and there is tremendous scope for improving their properties to suit projected applications. The future lines of development would include (i) genetic approaches to generate microbial strains for hyperproduction of the enzymes, (ii) application of SDM to design proteases with unique specificity and increased resistance to heat and alkaline pH, (iii) synthesis of peptides (synzymes) to mimic proteases, (iv) production of abzymes (catalytic antibodies) with specific protease activity, and (v) understanding of the structure-function relationship of the enzymes. Although the section on protein engineering describes in detail how SDM has been used to alter the properties and functions of proteases of bacterial, fungal, and viral origins, some of the important problems faced in their desired usages and the possible solutions to overcome these hurdles are discussed below.
Enhancement of Thermostability
The industrial use of proteases in detergents or for leather processing requires that the enzyme be stable at higher temperatures. One of the common strategies to enhance the thermostability of the enzyme is to introduce disulfide bonds into the protease by SDM. Introduction of a disulfide bond into subtilisin E from Bacillus subtilis resulted in an increase of 4.5°C in the Tm of the mutant enzyme without causing any change in its catalytic efficiency (280). However, the properties of the mutant enzyme were found to revert to those of the wild-type enzyme. Enhanced stability of subtilisin was observed as a result of mutations of Asn109 and Asn218 to Ser. The analog containing both the mutations showed an additive effect on thermal stability. Thermostability of the alkaline protease from Aspergillus oryzae is important because of its extensive use in the manufacture of soy sauce. The optimal temperature of the wild-type enzyme was enhanced from 51 to 56°C by the introduction of a disulfide (Cys 169-Cys 200) bond. Another strategy for improving the stability of the protease was by replacing the polar amino acid groups by hydrophobic groups. The presence of positively charged amino acids in the N-terminal turn of an α-helix is undesirable in view of the possibility of an occurrence of the repulsive interactions with the helix dipole. Replacement of Lys by Ser or Asp resulted in an increase in the thermostability of the neutral protease from B. subtilis in the range of 0.3 to 1.2°C (54). Although these approaches result in an increased stability of proteases, the difference in the thermostabilities of the parent and the mutant enzymes is only marginal, and further research involving cassette mutagenesis, etc., is necessary to yield an enzyme with substantially enhanced thermostability.
Prevention of Autoproteolytic Inactivation
Subtilisin, an extensively studied protease, is widely used in detergent formulations due to its stability at alkaline pH. However, its autolytic digestion presents a major problem for its use in industry. It was deduced that there is a correlation between the autolytic and conformational stabilities. Computer modelling followed by introduction of a Cys24 or Cys87 mutation resulted in destabilization of subtilisin from Bacillus amyloliquefaciens (312). Introduction of a disulfide bond increased the stability of the mutant to a level less than or equal to that of the wild-type enzyme. It appears logical that mutations in the amino acids involved at the site of autoproteolysis may prevent the protease inactivation caused by self-digestion.
Alteration of pH Optimum
Different applications of proteases require specific optimal pHs for the best performance of the enzyme; e.g., the use of proteases in the leather and detergent industries requires an enzyme with an alkaline pH optimum, whereas the use in the cheesemaking industry requires an acidic protease. Protein engineering enables us to tailor the pH dependence of the enzyme catalysis to optimize the industrial processes. Modifications in the overall surface charge of the proteins are known to alter the optimal pH of the enzyme. A change of Asp99 to Ser in subtilisin from Bacillus amyloliquefaciens has demonstrated the potential of altering the optimal pH of the enzyme by systematic multiple mutations on the surface of the protein (290).
Changing of Substrate Specificity
The properties needed for industrial applications of proteases differ from their physiological properties. The natural substrates of the enzyme are usually different from those desired for their industrial applications. Despite extensive research on proteases, relatively little is known about the factors that control their specificities toward nonphysiological substrates. Strategies involving SDM are being explored to tailor these specificities at will. A combinatorial random-mutagenesis approach has been used to generate mutants that secrete proteases with functional properties different from those of the parent enzyme (77). Introduction of several point mutations into the substrate-binding site of α-lytic protease was shown to affect its specificity in a predictable manner. The protease preferentially cleaves on the C-terminal side of small uncharged residues such as Ala, mainly because the pocket that accommodates the substrate P-1 residue is shallow due to the presence of two bulky methionine residues (Met190 and Met213) at the subsite. Replacement of Met213 with a His residue had a beneficial effect on its substrate specificity.
Improvement of Yield
The cost of enzyme production is a major obstacle in the successful application of proteases in industry. Protease yields have been improved by screening for hyperproducing strains and/or by optimization of the fermentation medium. Strain improvement by either conventional mutagenesis or recombinant-DNA technology have been useful in improving the production of proteases. Hyperexpression by genetic manipulation of microbes is described in the section on genetic engineering. Increases in the yield of viral proteases are particularly important for developing therapeutic agents against devastating diseases such as malaria, cancer, and AIDS.
There are many major problems in the commercialization of proteases. Although they are being addressed by both conventional and novel methods of genetic manipulation, there are no entirely satisfactory solutions and many of these problems remain unanswered.
FUTURE SCOPE
Proteases are a unique class of enzymes, since they are of immense physiological as well as commercial importance. They possess both degradative and synthetic properties. Since proteases are physiologically necessary, they occur ubiquitously in animals, plants, and microbes. However, microbes are a goldmine of proteases and represent the preferred source of enzymes in view of their rapid growth, limited space required for cultivation, and ready accessibility to genetic manipulation. Microbial proteases have been extensively used in the food, dairy and detergent industries since ancient times. There is a renewed interest in proteases as targets for developing therapeutic agents against relentlessly spreading fatal diseases such as cancer, malaria, and AIDS. Advances in genetic manipulation of microorganisms by SDM of the cloned gene opens new possibilities for the introduction of predesigned changes, resulting in the production of tailor-made proteases with novel and desirable properties. The development of recombinant rennin and its commercialization by Pfizer and Genencor is an excellent example of the successful application of modern biology to biotechnology. The advent of techniques for rapid sequencing of cloned DNA has yielded an explosive increase in protease sequence information. Analysis of sequences for acidic, alkaline, and neutral proteases has provided new insights into the evolutionary relationships of proteases.
Despite the systematic application of recombinant technology and protein engineering to alter the properties of proteases, it has not been possible to obtain microbial proteases that are ideal for their biotechnological applications. Industrial applications of proteases have posed several problems and challenges for their further improvements. The biodiversity represents an invaluable resource for biotechnological innovations and plays an important role in the search for improved strains of microorganisms used in the industry. A recent trend has involved conducting industrial reactions with enzymes reaped from exotic microorganisms that inhabit hot waters, freezing Arctic waters, saline waters, or extremely acidic or alkaline habitats. The proteases isolated from extremophilic organisms are likely to mimic some of the unnatural properties of the enzymes that are desirable for their commercial applications. Exploitation of biodiversity to provide microorganisms that produce proteases well suited for their diverse applications is considered to be one of the most promising future alternatives. Introduction of extremophilic proteases into industrial processes is hampered by the difficulties encountered in growing the extremophiles as laboratory cultures. Revolutionary robotic approaches such as DNA shuffling are being developed to rationalize the use of enzymes from extremophiles. The existing knowledge about the structure-function relationship of proteases, coupled with gene-shuffling techniques, promises a fair chance of success, in the near future, in evolving proteases that were never made in nature and that would meet the requirements of the multitude of protease applications.
A century after the pioneering work of Louis Pasteur, the science of microbiology has reached its pinnacle. In a relatively short time, modern biotechnology has grown dramatically from a laboratory curiosity to a commercial activity. Advances in microbiology and biotechnology have created a favorable niche for the development of proteases and will continue to facilitate their applications to provide a sustainable environment for mankind and to improve the quality of human life.
ACKNOWLEDGMENTS
We thank M. C. Srinivasan, S. U. Phadtare, K. R. Bandivadekar, S. H. Bhosale, and D. Nath for providing some of the literature information. We are grateful to A. S. Kolaskar, P. B. Vidyasagar, and S. Jagtap, Bioinformatics Centre, University of Pune, for their help in analyzing the protease sequences.
Financial support to M. S. Ghatge and A. M. Tanksale from the Council of Scientific and Industrial Research is gratefully acknowledged.
Footnotes
National Chemical Laboratory communication 6440.
REFERENCES
- 1.Akimkina T V, Nosovskaya E A, Kostrov S V. Cloning and expression of the neutral proteinase gene of Bacillus cereus in Bacillus subtiliscells. Mol Biol. 1992;26:418–423. [PubMed] [Google Scholar]
- 2.Allen C, Stromer V K, Smith F D, Lacy G H, Mount M S. Complementation of an Erwinia carotovora subsp. carotovoraprotease mutant with a protease encoding cosmid. Mol Gen Genet. 1986;202:276–279. [Google Scholar]
- 3.Amerik A, Chistyakova L G, Ostroumova N I, Gurevich A I, Antonov V K. Cloning, expression and structure of the truncated functionally active lon gene of Escherichia coli. Bioorg Khim. 1988;14:408–411. [PubMed] [Google Scholar]
- 4.Ammerer G, Hunter C P, Rothman J H, Saari G C, Valls L A, Stevens T M. PEP4 gene of Saccharomyces cerevisiaeencodes proteinase A, a vacuolar enzyme required for processing of vacuolar precursors. Mol Cell Biol. 1986;6:2490–2497. doi: 10.1128/mcb.6.7.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Argos P. A sensitive procedure to compare amino acid sequences. J Mol Biol. 1987;193:385–396. doi: 10.1016/0022-2836(87)90226-9. [DOI] [PubMed] [Google Scholar]
- 6.Argos P, Kamer G, Nicklin M J H, Wimmer E. Similarity in gene organization and homology between proteins of animal picornaviruses and a plant comovirus suggest common ancestry of these virus families. Nucleic Acids Res. 1984;12:7251–7267. doi: 10.1093/nar/12.18.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atsumi Y, Yamamoto S, Morihara K, Fukushima J, Takeuchi H, Mizuki N, Kawamoto S, Okuda K. Cloning and expression of the alkaline proteinase gene from Pseudomonas aeruginosaIFO 3455. J Bacteriol. 1989;171:5173–5175. doi: 10.1128/jb.171.9.5173-5175.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Austew B M, Smith E L. Action of staphylococcal proteinase on peptides of varying chain length and composition. Biochem Biophys Res Commun. 1976;72:411–417. doi: 10.1016/s0006-291x(76)80058-7. [DOI] [PubMed] [Google Scholar]
- 9.Avakov A S, Bolotin A P, Sorokin S V. The structure of Bacillus brevismetalloprotease gene. Mol Biol. 1990;24:1363–1372. [PubMed] [Google Scholar]
- 10.Ballinger M D, Tom J, Wells J A. Furilisin: a variant of subtilisin BPN′ engineered for cleaving tribasic substrates. Biochemistry. 1996;35:13579–13585. doi: 10.1021/bi961543h. [DOI] [PubMed] [Google Scholar]
- 11.Baker E N, Drenth J. Active sites of enzymes. In: Jurnak F A, McPherson A, editors. Biological macromolecules and assemblies. 3. Active sites of enzymes. New York, N.Y: John Wiley & Sons, Inc.; 1987. p. 314. [Google Scholar]
- 12.Barett A J. Proteolytic enzymes: serine and cysteine peptidases. Methods Enzymol. 1994;244:1–15. [Google Scholar]
- 13.Barett A J. Proteolytic enzymes: aspartic and metallopeptidases. Methods Enzymol. 1995;248:183. [Google Scholar]
- 14.Berglund P, Stabile M R, Gold M, Jones J B, Mitchinson C, Bolt R R, Graycar T P. Altering the specificity of subtilisin B. lentusby combining site-directed mutagenesis and chemical modification. Bioorg Med Chem Lett. 1996;6:2507–2512. [Google Scholar]
- 15.Berka R M, Ward M, Wilson L J, Hayenga K J, Kodama K H, Carlomagno L P, Thompson S A. Molecular cloning and deletion of the gene encoding aspergillopepsin A from Aspergillus awamori. Gene. 1990;86:153–162. doi: 10.1016/0378-1119(90)90274-u. [DOI] [PubMed] [Google Scholar]
- 16.Bhosale S H, Rao M B, Deshpande V V, Srinivasan M C. Thermostability of high activity alkaline protease from Conidiobolus coronatus(NCL 86.8.20) Enzyme Microb Technol. 1995;17:136–139. [Google Scholar]
- 17.Binnie C, Liao L, Walczyk E, Malek L T. Isolation and characterization of a gene encoding a chymotrypsin-like serine protease from Streptomyces lividans66. Can J Microbiol. 1996;42:284–288. doi: 10.1139/m96-041. [DOI] [PubMed] [Google Scholar]
- 18.Binnie C, Butler M J, Aphale J S, Bourgault R, Dizonno M A, Krygsman P, Liao L, Walczyk E, Malek L T. Isolation and characterization of 2 genes encoding proteases associated with the mycelium of Streptomyces lividans66. J Bacteriol. 1995;177:6033–6040. doi: 10.1128/jb.177.21.6033-6040.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blobel G, Dobberstein B. Transfer of proteins across membranes. J Cell Biol. 1975;67:835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blundell T L, Cooper J B, Sali A, Zhu Z. Comparisons of the sequences, 3-D structures and mechanisms of pepsin-like and retroviral aspartic proteinases. Adv Exp Med Biol. 1991;306:443–453. doi: 10.1007/978-1-4684-6012-4_57. [DOI] [PubMed] [Google Scholar]
- 21.Boguslawski G, Shultz J L, Yehle C O. Purification and characterization of an extracellular protease from Flavobacterium arborescens. Anal Biochem. 1983;132:41–49. doi: 10.1016/0003-2697(83)90423-2. [DOI] [PubMed] [Google Scholar]
- 22.Boye K, Stummann B M, Henningsen K W. cDNA cloning and sequencing of the bean yellow mosaic virus nuclear inclusion protein genes. Plant Mol Biol. 1992;18:1203–1205. doi: 10.1007/BF00047728. [DOI] [PubMed] [Google Scholar]
- 23.Boyer P D. The enzymes. 3rd ed. New York, N.Y: Academic Press, Inc.; 1971. [Google Scholar]
- 24.Braunagel S C, Benedik M J. The metalloprotease gene of Serratia marcescensstrain SM6. Mol Gen Genet. 1990;222:446–451. doi: 10.1007/BF00633854. [DOI] [PubMed] [Google Scholar]
- 25.Brenner S. The molecular evolution of genes and proteins: a tale of two serines. Nature. 1988;334:528–530. doi: 10.1038/334528a0. [DOI] [PubMed] [Google Scholar]
- 26.Browner M F, Smith W W, Castelhano A L. Matrilysin-inhibitor complexes: common themes among metalloproteinases. Biochemistry. 1995;34:6601–6610. doi: 10.1021/bi00020a004. [DOI] [PubMed] [Google Scholar]
- 27.Brueckner R, Shoseyor O, Doi R H. Multiple active forms of a novel serine protease from Bacillus subtilisin. Mol Gen Genet. 1990;221:486–490. doi: 10.1007/BF00259415. [DOI] [PubMed] [Google Scholar]
- 28.Bruinenberg P G, De Vos W M, Siezen R J. Prevention of C-terminal autoprocessing of Lactococcus lactisSK11 cell-envelope proteinase by engineering of an essential surface loop. Biochem J. 1994;302:957–963. doi: 10.1042/bj3020957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carmona C, Gray G L. Nucleotide sequence of the serine protease gene of Staphylococcus aureus, strain V8. Nucleic Acids Res. 1987;15:6757. doi: 10.1093/nar/15.16.6757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carter P, Wells J A. Engineering substrate specificity by substrate assisted catalysis. Science. 1987;237:394–399. doi: 10.1126/science.3299704. [DOI] [PubMed] [Google Scholar]
- 31.Cerny G. Studies on the aminopeptidase test for the distinction of gram-negative from gram-positive bacteria. Eur J Appl Microbiol Biotechnol. 1978;5:113–122. [Google Scholar]
- 32.Chang P C, Kuo T-C, Tsugita A, Lee Y-H W. Extracellular metalloprotease gene of Streptomyces cacaoi: structure, nucleotide sequence and characterization of the cloned gene product. Gene. 1990;85:87–95. doi: 10.1016/0378-1119(90)90063-w. [DOI] [PubMed] [Google Scholar]
- 33.Cheevadhanarak S, Renno D V, Saunders G, Holt G. Cloning and selective overexpression of an alkaline protease-encoding gene from Aspergillus oryzae. Gene. 1991;108:151–155. doi: 10.1016/0378-1119(91)90501-2. [DOI] [PubMed] [Google Scholar]
- 34.Cheng J-C, Shao C-P, Hor L-I. Cloning and nucleotide sequencing of the protease gene of Vibrio vulnificus. Gene. 1996;183:255–257. doi: 10.1016/s0378-1119(96)00488-x. [DOI] [PubMed] [Google Scholar]
- 35.Chin D T, Goff S A, Webster T, Smith T, Goldberg A L. Sequence of the lon gene in Escherichia coli. A heat-shock gene which encodes the ATP-dependent protease La. J Biol Chem. 1988;263:11718–11728. [PubMed] [Google Scholar]
- 36.Chiplonkar J M, Gangodkar S V, Wagh U V, Ghadge G D, Rele M V, Srinivasan M C. Applications of alkaline protease from Conidiobolusin animal cell culture. Biotechnol Lett. 1985;7:665–668. [Google Scholar]
- 37.Cho W-L, Raikhel A S. Cloning of cDNA for mosquito lysosomal aspartic protease. J Biol Chem. 1992;267:21823–21829. [PubMed] [Google Scholar]
- 38.Chung C H, Goldberg A L. The product of the lon (capR) gene in Escherichia coliis the ATP dependent protease, protease La. Proc Natl Acad Sci USA. 1981;78:4931–4935. doi: 10.1073/pnas.78.8.4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cooper J B, Khan G, Taylor G, Yickle I J, Blundell T L. X-ray analyses of aspartic proteinases. II. Three dimensional structure of the hexagonal crystal form of porcine pepsin at 2.3 A resolution. J Mol Biol. 1990;214:199–222. doi: 10.1016/0022-2836(90)90156-G. [DOI] [PubMed] [Google Scholar]
- 40.Corr B R, Richardson M, Wilderspin A F. Cloning, expression and mutagenesis of SIVmac proteinase in E. coli. Biochem Soc Trans. 1992;20:1605. doi: 10.1042/bst020160s. [DOI] [PubMed] [Google Scholar]
- 41.Dancer B N, Mandelstam J. Production and possible function of serine protease during sporulation of Bacillus subtilis. J Bacteriol. 1975;121:406–410. doi: 10.1128/jb.121.2.406-410.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Date T, Wickner W. Isolation of the Escherichia coli leader peptidase gene and effects of leader peptidase overproduction in vitro. Proc Natl Acad Sci USA. 1981;78:6106–6110. doi: 10.1073/pnas.78.10.6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.David V A, Deutch A H, Sloma A, Pawlyk D, Ally A, Durham D R. Cloning, sequencing and expression of the gene encoding the extracellular neutral protease, vibriolysin, of Vibrio proteolyticus. Gene. 1992;112:107–112. doi: 10.1016/0378-1119(92)90310-l. [DOI] [PubMed] [Google Scholar]
- 44.Davidow L S, O’Donnell M M, Kaczmaerk F S, Pereira D A, DeZeeuw J R, Franke A E. Cloning and sequencing of the alkaline extracellular protease gene of Yarrowia lipolytica. J Bacteriol. 1987;169:4621–4629. doi: 10.1128/jb.169.10.4621-4629.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deane S M, Robb F T, Robb S M, Wood D R. Nucleotide sequence of the Vibrio anginolyticuscalcium-dependent, detergent-resistant alkaline serine exoprotease A. Gene. 1989;76:281–288. doi: 10.1016/0378-1119(89)90168-6. [DOI] [PubMed] [Google Scholar]
- 46.Debouck C, Gorniak J G, Strickler J E, Meek T D, Metcalf B W, Rosenberg M. Human immunodeficiency virus protease expressed in Escherichia coliexhibits autoprocessing and specific maturation of the gag precursor. Proc Natl Acad Sci USA. 1987;84:8903–8906. doi: 10.1073/pnas.84.24.8903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deckman I C, Hagen M, McCann P J. Herpes simplex virus type 1 protease expressed in Escherichia coliexhibits autoprocessing and specific cleavage of the ICP 35 assembly protein. J Virol. 1992;66:7362–7367. doi: 10.1128/jvi.66.12.7362-7367.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Marco A C, Dick A J. Aminopeptidase I activities in several microorganisms. Can J Biochem. 1978;56:66–71. doi: 10.1139/o78-010. [DOI] [PubMed] [Google Scholar]
- 49.De Vos W M. Gene cloning and expression in lactic streptococci. FEMS Microbiol Rev. 1987;46:281–295. [Google Scholar]
- 50.De Vos W M, Vos P, deHaard H, Boerrigter I. Cloning and expression of the Lactococcus lactis subsp. cremorisSk11 gene encoding an extracellular serine proteinase. Gene. 1989;85:169–176. doi: 10.1016/0378-1119(89)90477-0. [DOI] [PubMed] [Google Scholar]
- 51.Dickinson L, Harboe M, Van Heeswijck R, Stroeman P, Jepsen L P. Expression of active Mucor miehei aspartic protease in Mucor circinelloides. Carlsberg Res Commun. 1987;52:243–252. [Google Scholar]
- 52.Drapeau G R, Boily Y, Houmard J. Purification and properties of an extracellular protease of Staphylococcus aureus. J Biol Chem. 1972;247:6720–6726. [PubMed] [Google Scholar]
- 53.Egel-Mitani M, Flygering H P, Hansen M T. A novel aspartyl protease allowing KEX-2 independent MFα propheromone processing in yeast. Yeast. 1990;6:127–137. doi: 10.1002/yea.320060206. [DOI] [PubMed] [Google Scholar]
- 54.Eijsink V G H, Vriend G, vanden Burg B, vander Zee J R, Venema G. Increasing the thermostability of a neutral protease by replacing positively charged amino acids in the N-terminal turn of α-helices. Protein Eng. 1992;5:165–170. doi: 10.1093/protein/5.2.165. [DOI] [PubMed] [Google Scholar]
- 55.Eijsink V G H, vander Zee J K, vanden Burg B, Vriend G, Venema G. Improving the thermostability of the neutral protease of Bacillus stearothermophilusby replacing a burried aspargine by leucine. FEBS Lett. 1991;282:13–16. doi: 10.1016/0014-5793(91)80434-5. [DOI] [PubMed] [Google Scholar]
- 56.Reference deleted.
- 57.Epstein D M, Wensink P C. The α-lytic protease gene of Lysobacter enzymogenes. J Biol Chem. 1988;263:16586–16590. [PubMed] [Google Scholar]
- 58.Esposito R E, Klapholz S. Meiosis and ascospore development. In: Strathern J N, Jones E W, Broach J R, editors. The molecular biology of the yeast Saccharomyces. Life cycle and inheritance. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1981. pp. 211–287. [Google Scholar]
- 59.Estell D A, Gyaycar T P, Miller J V, Powers D B, Burnier J P, Ng P G, Wells J A. Probing steric and hydrophobic effects on enzyme substrate interactions by protein engineering. Science. 1986;233:659–663. doi: 10.1126/science.233.4764.659. [DOI] [PubMed] [Google Scholar]
- 60.Fastrez J, Fersht A R. Demonstration of the acyl-enzyme mechanism for the hydrolysis of peptides and anilides by chymotrypsin. Biochemistry. 1973;12:2025–2034. doi: 10.1021/bi00735a001. [DOI] [PubMed] [Google Scholar]
- 61.Felix F, Brouillet N. Purification and properties of two peptidases from brewer’s yeast. Biochim Biophys Acta. 1966;122:127–144. [PubMed] [Google Scholar]
- 62.Fishman Y, Bricker J, Gilbert J V, Plant A G, Wright A. Cloning of the type 1 immunoglobulin A1 protease from Neisseria gonorrhoeae and secretion of the enzyme from Escherichia coli. In: Schoolnik G K, editor. Proceedings of the Fourth International Symposium. Washington, D.C: American Society for Microbiology; 1985. pp. 164–168. [Google Scholar]
- 63.Fitzgerald P M D, McKeever B M, Van Middlesworth J F, Springer J P, Heimbach J C, Leu Chih-Tai, Herber W K, Dixon R A F, Darke P L. Crystallographic analysis of a complex between human immunodeficiency virus type 1 protease and acetyl-pepstatin at 2.0A resolution. J Biol Chem. 1990;265:14209–14219. [PubMed] [Google Scholar]
- 64.Fox J W, Shannon J D, Bjarnason J B. Proteinases and their inhibitors in biotechnology. Enzymes in biomass conversion. ACS Symp Ser. 1991;460:62–79. [Google Scholar]
- 65.Friedman H, Goebel M, Synder M. A homolog of the proteasome-related RING10 gene is essential for yeast cell growth. Gene. 1992;122:203–206. doi: 10.1016/0378-1119(92)90051-p. [DOI] [PubMed] [Google Scholar]
- 66.Fujii G, Tashiro K, Emori Y, Saigo K, Tanaka K, Shiokawa K. Deduced primary structure of a Xenopusproteasome subunit XC3 and expression of its mRNA during early development. Biochem Biophys Res Commun. 1991;178:1233–1239. doi: 10.1016/0006-291x(91)91025-8. [DOI] [PubMed] [Google Scholar]
- 67.Fujii M, Takagi M, Imanaka T, Aiba S. Molecular cloning of a thermostable neutral protease gene from Bacillus stearothermophilus in a vector plasmid and its expression in Bacillus stearothermophilus and Bacillus subtilis. J Bacteriol. 1983;154:831–837. doi: 10.1128/jb.154.2.831-837.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Geisen R. Cloning of a protease gene from Pseudomonas nalgiovense by expression in Escherichia coli. Lett Appl Microbiol. 1993;16:303–306. [Google Scholar]
- 69.Gilbert C, Atlan D, Blane B, Portalier R, Germond J E, Lapierre L, Mollet B. A new cell surface proteinase: sequencing and analysis of the prtB gene from Lactobacillus delbrueckii subsp. bulgaricus. J Bacteriol. 1996;178:3059–3065. doi: 10.1128/jb.178.11.3059-3065.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gilbert J V, Plant A G, Fishman Y, Wright A. Cloning of the gene encoding streptococcal immunoglobulin A1 protease and its expression in Escherichia coli. Infect Immun. 1988;56:1961–1966. doi: 10.1128/iai.56.8.1961-1966.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gilles A M, Imhoff J M, Keil B. α-Clostripain. Chemical characterization, activity and thiol content of the highly active form of clostripain. J Biol Chem. 1979;254:1462–1468. [PubMed] [Google Scholar]
- 72.Godfrey T, West S. Industrial enzymology. 2nd ed. New York, N.Y: Macmillan Publishers Inc.; 1996. p. 3. [Google Scholar]
- 73.Goldberg A L, Shreedharaswamy K H, Chung C H, Larimore F S. Proteases of Escherichia coli. Methods Enzymol. 1983;80:680–702. doi: 10.1016/s0076-6879(81)80052-3. [DOI] [PubMed] [Google Scholar]
- 74.Gomi K, Arikawa K, Kamiya N, Kitamoto K, Kumagai C. Cloning and nucleotide sequence of the acid protease encoding gene (pep A) from Aspergillus oryzae. Biosci Biotechnol Biochem. 1993;57:1095–1100. doi: 10.1271/bbb.57.1095. [DOI] [PubMed] [Google Scholar]
- 75.Gottesman S, Maurizi M R. Regulation by proteolysis: energy-dependent proteases and their targets. Microbiol Rev. 1992;56:592–621. doi: 10.1128/mr.56.4.592-621.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Govind N S, Mehta B, Sharma M, Modi V V. Protease and carotenogenesis in Blakeslea trispora. Phytochemistry. 1981;20:2483–2485. [Google Scholar]
- 77.Graham L D, Haggett K D, Jennings P A, Brocque D S, Whittaker R G. Random mutagenesis of the substrate binding site of a serine protease can generate enzymes with increased activities and altered primary specificities. Biochemistry. 1993;32:6250–6258. doi: 10.1021/bi00075a019. [DOI] [PubMed] [Google Scholar]
- 78.Graves M C, Lim J J, Heimer E P, Kramer R A. An 11-kDa form of human immunodeficiency virus protease expressed in Escherichia coliis sufficient for enzymatic activity. Proc Natl Acad Sci USA. 1988;85:2449–2453. doi: 10.1073/pnas.85.8.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gray G L, Hayenga K, Cullen D, Wilson L J, Norton S. Primary structure of Mucor mieheiaspartyl protease: evidence for a zymogen intermediate. Gene. 1986;48:41–53. doi: 10.1016/0378-1119(86)90350-1. [DOI] [PubMed] [Google Scholar]
- 80.Grodberg J, Lundrigan M D, Toledo D L, Mangel W F, Dunn J J. Complete nucleotide sequence and deduced amino acid sequence of the omp T gene of Escherichia coliK-12. Nucleic Acids Res. 1988;16:1209. doi: 10.1093/nar/16.3.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gunkel P A, Gassen H G. Proteinase K from Tritirachium album Limber: characterization of the chromosomal gene and expression of the cDNA in Escherichia coli. Eur J Biochem. 1989;179:185–194. doi: 10.1111/j.1432-1033.1989.tb14539.x. [DOI] [PubMed] [Google Scholar]
- 82.Gustin J K, Kessler E, Ohman D E. A substitution at His-120 in the LasA protease of Pseudomonas aeruginosablocks enzyme activity without affecting propeptide processing or extracellular secretion. J Bacteriol. 1996;178:6608–6617. doi: 10.1128/jb.178.22.6608-6617.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guzzo J, Murgier M, Filloux A, Lazdunski A. Cloning of the Pseudomonas aeruginosa alkaline protease gene and secretion of the protease into the medium by Escherichia coli. J Bacteriol. 1990;172:942–948. doi: 10.1128/jb.172.2.942-948.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Haass C, Pesold-Hurt B, Multhaup G, Beyreuther K, Kloetzel P-M. The DrosophilaPROS-28.1 gene is a member of the proteasome gene family. Gene. 1990;90:235–241. doi: 10.1016/0378-1119(90)90185-t. [DOI] [PubMed] [Google Scholar]
- 85.Hartley B S. Proteolytic enzymes. Annu Rev Biochem. 1960;29:45–72. doi: 10.1146/annurev.bi.29.070160.000401. [DOI] [PubMed] [Google Scholar]
- 86.Hase C C, Finkelstein R A. Cloning and nucleotide sequence of the Vibrio choleraehemagglutinin/protease (HA/protease) gene and construction of an HA/protease negative strain. J Bacteriol. 1991;173:3311–3317. doi: 10.1128/jb.173.11.3311-3317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hatanaka M, Nam S H. Identification of HTLV-I gag protease fits sequential processing of the gag gene product. J Cell Biochem. 1989;40:15–30. doi: 10.1002/jcb.240400103. [DOI] [PubMed] [Google Scholar]
- 88.He C, Xu M, Jin J, Reu D. Cloning and expression of an alkaline protease gene from Bacillus licheniformis in Bacillus subtilis. Gongye Weishengwu. 1988;18:1–5. [Google Scholar]
- 89.Henderson G, Krygsman P, Liu C J, Davey C C, Malek L T. Characterization and structure of genes for proteases A and B from Streptomyces griseus. J Bacteriol. 1987;169:3778–3784. doi: 10.1128/jb.169.8.3778-3784.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Henner D J, Yang M, Band L, Wells J A. Proceedings of the 9th International Spore Conference. 1985. Expression of cloned protease genes in Bacillus subtilis; pp. 95–103. [Google Scholar]
- 91.Hershko A, Leshinsky E, Ganoth D, Heller H. ATP-dependent degradation of ubiquitin protein conjugates. Proc Natl Acad Sci USA. 1984;81:1619–1623. doi: 10.1073/pnas.81.6.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hibbs M S, Hasty K A, Seyer J M, Kang A H, Mainardi C L. Biochemical and immunological characterization of the secreted forms of human neutrophil gelatinase. J Biol Chem. 1985;260:2493–2500. [PubMed] [Google Scholar]
- 93.Hilt W, Wolf D H. Proteasomes: destruction as a programme. Trends Biochem Sci. 1996;21:81–119. [PubMed] [Google Scholar]
- 94.Hiramatsu R, Aikawa J, Horinouchi S, Beppu T. Secretion by yeast of the zymogen form of Mucor rennin, an aspartic proteinase of Mucor pusillus, and its conversion to the mature form. J Biol Chem. 1989;264:16862–16866. [PubMed] [Google Scholar]
- 95.Hirata D, Fukui S, Yamashita I. Nucleotide sequences of the secretable acid protease gene PEP1 in the yeast Saccharomycopsis fubiligera. Agric Biol Chem. 1988;52:2647–2649. [Google Scholar]
- 96.Hodgson J. The changing bulk catalysis market: recombinant DNA techniques have changed bulk enzyme production dramatically. Bio/Technology. 1994;12:789–790. [Google Scholar]
- 97.Hoffman T. Food related enzymes. Adv Chem Ser. 1974;136:146–185. [Google Scholar]
- 98.Holmes M A, Matthews B W. Binding of hydroxamic acid inhibitors to crystalline thermolysine suggests a pentacoordinate zinc intermediate in catalysis. Biochemistry. 1981;20:6912–6920. doi: 10.1021/bi00527a026. [DOI] [PubMed] [Google Scholar]
- 99.Honjo M, Manabe K, Shimada H, Mita I, Nakayama A, Furutani Y. Cloning and expression of the gene for neutral protease of Bacillus amyloliquefaciens in Bacillus subtilis. J Biotechnol. 1984;1:265–77. doi: 10.1016/0168-1656(92)90099-u. [DOI] [PubMed] [Google Scholar]
- 100.Horiuchi H, Yanai K, Okazaki T, Takagi M, Yano K. Isolation and sequencing of a genomic clone encoding the aspartic proteinase of Rhizopus niveus. J Bacteriol. 1988;170:272–278. doi: 10.1128/jb.170.1.272-278.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Horiuchi H, Ashikari T, Amachi T, Yoshizumi H, Takagi M, Yano K. High level secretion of a Rhizopus niveus aspartic proteinase in Saccharomyces cerevisiae. Agric Biol Chem. 1990;54:1771–1779. [PubMed] [Google Scholar]
- 102.Houde A, Weber J M. Sequence of the protease of human subgroup E adenovirus type 4. Gene. 1987;54:51–56. doi: 10.1016/0378-1119(87)90346-5. [DOI] [PubMed] [Google Scholar]
- 103.Houde A, Weber J M. The primary structure of human adenovirus type 12 protease. Nucleic Acids Res. 1988;16:7195. doi: 10.1093/nar/16.14.7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Houde A, Weber J M. Sequence of the human adenovirus type 3 protease. Nucleic Acids Res. 1988;16:11374. doi: 10.1093/nar/16.23.11374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hruskova-Heidingsfeldova O, Andreansky M, Fabry M, Blaha I, Strop P, Hunter E. Cloning, bacterial expression, and characterization of the Mason-Pfizer monkey virus proteinase. J Biol Chem. 1995;270:15053–15058. doi: 10.1074/jbc.270.25.15053. [DOI] [PubMed] [Google Scholar]
- 106.Hube B, Turver C J, Odds F C, Eiffert H, Boulnois G J, Kochel H, Ruchel R. Sequence of the Candida albicansgene encoding the secretory aspartate protease. J Med Vet Mycol. 1991;29:129. [PubMed] [Google Scholar]
- 107.Hwang D H, Kim J C, Ko J H, Chun M J, Byun S M. Molecular cloning and nucleotide sequence of the protease B gene from Streptomyces griseusATCC 10137. Korean Biochem J. 1993;26:383–390. [Google Scholar]
- 108.Ichihara S, Suzuki T, Suzuki M, Mizushima S. Molecular cloning and sequencing of the sppA gene and characterization of the encoded protease IV, a signal peptide peptidase, of Escherichia coli. J Biol Chem. 1989;261:9405–9411. [PubMed] [Google Scholar]
- 109.Igarashi H, Morita T, Iwanaga S. A new method for purification of staphylocoagulase by a bovine prothrombin-Sepharose column. J Biochem. 1979;86:1615–1618. doi: 10.1093/oxfordjournals.jbchem.a132679. [DOI] [PubMed] [Google Scholar]
- 110.Ikegaya K, Ishida Y, Murakami K, Masaki A, Sugio N, Takechi K, Murakami S, Tatsumi H, Ogawa Y, Nakano E, Motai H, Kawabe H. Enhancement of the thermostability of the alkaline protease from Aspergillus oryzaeby introduction of a disulfide bond. Biosci Biotechnol Biochem. 1992;56:326–327. doi: 10.1271/bbb.56.326. [DOI] [PubMed] [Google Scholar]
- 111.Ikemura H, Takagi H, Inouye M. Requirement of pro-sequence for the production of active subtilisin E in Escherichia coli. J Biol Chem. 1987;242:7859–7864. [PubMed] [Google Scholar]
- 112.Imanaka T, Shibazaki M, Takagi M. A new way of enhancing the thermostability of proteases. Nature. 1986;324:695–697. doi: 10.1038/324695a0. [DOI] [PubMed] [Google Scholar]
- 113.Inoue H, Lu J, Athanda S B P, Kong K, Hayashi T, Kimura T, Makabe O, Takahashi K. Aspergillus niger var. macrosporusproteinase B. cDNA cloning, expression and activation of the proenzyme. Adv Exp Med Biol. 1995;362:581–587. doi: 10.1007/978-1-4615-1871-6_78. [DOI] [PubMed] [Google Scholar]
- 114.Inoue H, Kimura T, Makabe O, Takahashi K. The gene and deduced protein sequences of the zymogen of Aspergillus nigeracid proteinase A. J Biol Chem. 1991;266:19484–19489. [PubMed] [Google Scholar]
- 114a.International Union of Biochemistry. Enzyme nomenclature. Orlando, Fla: Academic Press, Inc.; 1992. [Google Scholar]
- 115.Isogai T, Fukagawa M, Kojo H, Kohsaka M, Aoki H, Imanaka H. Cloning and nucleotide sequences of the complementary and genomic DNAs for the alkaline protease from Acremonium chrysogenum. Agric Biol Chem. 1991;55:471–477. [PubMed] [Google Scholar]
- 116.Ito K, Sakakibara S-I, Izaki K. Cloning of two protease genes from Rhodocyclus gelatinosa APR 3-2 and their expression in Escherichia coli. Curr Microbiol. 1989;18:41–45. [Google Scholar]
- 117.Ivanoff L A, Towatari T, Ray J, Korant B D, Patteway S R., Jr Expression and site specific mutagenesis of the poliovirus 3C protease in Escherichia coli. Proc Natl Acad Sci USA. 1986;83:5392–5396. doi: 10.1073/pnas.83.15.5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jackson D P, Cotter D A. Expression of proteolytic enzymes during Dictyostelium discoideumspore germination. Arch Microbiol. 1984;137:205–208. [Google Scholar]
- 119.Jacobs M, Eliasson M, Uhlen M, Flock J-I. Cloning, sequencing and expression of subtilisin Carlsberg from Bacillus licheniformis. Nucleic Acids Res. 1985;13:8913–8926. doi: 10.1093/nar/13.24.8913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.James M N G, Sielecki A R, Hayakawa K, Gelb M H. Crystallographic analysis of transition state mimics bound to penicillopepsin: difluorostatine- and difluorostatone-containing peptides. Biochemistry. 1992;31:3872–3886. doi: 10.1021/bi00130a019. [DOI] [PubMed] [Google Scholar]
- 121.James M N G, Hsu I N, Delbaere L T J. Mechanism of acid protease catalysis based on the crystal structure of penicillopepsin. Nature. 1977;267:808–813. doi: 10.1038/267808a0. [DOI] [PubMed] [Google Scholar]
- 122.Jany K D, Lederer G, Mayer B. Amino acid sequence of proteinase K from the mold Tritirachium albumLimber. FEBS Lett. 1986;199:139–144. [Google Scholar]
- 123.Jaton-Ogay K, Suter M, Crameri R, Falchetto R, Faitu A, Monod M. Nucleotide sequence of a genomic and cDNA clone encoding an extracellular alkaline protease of Aspergillus fumigatus. FEMS Microbiol Lett. 1992;92:162–168. doi: 10.1016/0378-1097(92)90506-j. [DOI] [PubMed] [Google Scholar]
- 124.Jaton-Ogay K, Paris S, Huerre M, Quadroni M, Falchetto R, Togri G, Latge J, Monod M. Cloning and disruption of the gene encoding an extracellular metalloprotease of Aspergillus fumigatus. Mol Microbiol. 1994;14:917–928. doi: 10.1111/j.1365-2958.1994.tb01327.x. [DOI] [PubMed] [Google Scholar]
- 125.Kagani N, Inone H, Kimura T, Makabe O, Takahashi K. Expression in Escherichia coli of Aspergillus niger var. macrosporusproteinase A, a non-pepsin type acid proteinase. Adv Exp Med Biol. 1995;362:597–603. doi: 10.1007/978-1-4615-1871-6_80. [DOI] [PubMed] [Google Scholar]
- 126.Kageyama T, Tanabe K, Koiwai O. Development dependent expression of isozymogens of monkey pepsinogens and structural differences between them. Eur J Biochem. 1991;202:205–215. doi: 10.1111/j.1432-1033.1991.tb16364.x. [DOI] [PubMed] [Google Scholar]
- 127.Kakudo S, Kikuchi N, Kitadokoro K, Fujiwara T, Nakamura E, Okamoto H, Shin M, Tamaki M, Teraoka H, Tsuzuki H, Yoshida N. Purification, characterization, cloning and expression of a glutamic acid-specific protease from Bacillus licheniformisATCC 14580. J Biol Chem. 1992;33:23782–23788. [PubMed] [Google Scholar]
- 128.Kalisz M H. Microbial proteinases. Adv Biochem Eng Biotechnol. 1988;36:17–55. doi: 10.1007/BFb0047944. [DOI] [PubMed] [Google Scholar]
- 129.Kaneko R, Koyama N, Tsai Y-C, Juang R-Y, Yoda K, Yamasaki M. Molecular cloning of the structural gene for alkaline elastase YaB, a new subtilisin produced by an alkalophilic Bacillusstrain. J Bacteriol. 1989;171:5232–5236. doi: 10.1128/jb.171.9.5232-5236.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Katoh I, Yashinaka Y, Rein A, Shibuya M, Okada T, Oroszlan S. Murine leukemia virus maturation: protease region required for conversion from ‘immature’ to ‘mature’ core form and for virus infectivity. Virology. 1985;145:280–292. doi: 10.1016/0042-6822(85)90161-8. [DOI] [PubMed] [Google Scholar]
- 131.Katz M E, Rice R N, Cheetham B F. Isolation and characterization of an Aspergillus nidulansgene encoding an alkaline protease. Gene. 1994;150:287–292. doi: 10.1016/0378-1119(94)90439-1. [DOI] [PubMed] [Google Scholar]
- 132.Keil B. Specificity of proteolysis. Berlin, Germany: Springer-Verlag KG; 1992. [Google Scholar]
- 133.Kikuchi, M., K. Nakahama, and K. Yoshimura. July 1985. Molecular cloning of Bacillus amyloliquefaciens neutral proteinase gene in Bacillus subtilis. Japanese patent 60,145,090.
- 134.Kim M H, Choi S K, Koo B T, Shin B S, Sohn C B, Kim J. Cloning and expression of Serratia marcescens protease gene in Escherichia coli. J Microbiol Biotechnol. 1992;2:231–236. [Google Scholar]
- 135.Kitadokora K, Nakamura E, Tamaki M, Horii T, Okamoto H, Shin M, Sato T, Fujiwara T, Tsuzuki H, Yoshida N, Teraoka H. Purification, characterization and molecular cloning of an acid amino acid-specific proteinase from Streptomyces fradiaeATCC 14544. Biochim Biophys Acta. 1993;1163:149–157. doi: 10.1016/0167-4838(93)90176-r. [DOI] [PubMed] [Google Scholar]
- 136.Kitano, K., S. Morita, M. Kuriyama, and K. Maejima. December 1992. Cloning, expression, and use of alkaline protease gene from Fusarium. European patent 519,229.
- 137.Kiwaki M, Ikemura H, Shimizu-Kadoba M, Hirashima A. Molecular characterization of a cell wall-associated proteinase gene from Streptococcus lactisNCDO 763. Mol Microbiol. 1989;3:359–369. doi: 10.1111/j.1365-2958.1989.tb00181.x. [DOI] [PubMed] [Google Scholar]
- 138.Koide Y, Nakamura A, Uozumi T, Beppu T. Cloning and sequencing of the major intracellular serine protease gene of Bacillus subtilis. J Bacteriol. 1986;167:110–116. doi: 10.1128/jb.167.1.110-116.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kok J, Hill D, Haandrikman A J, Reuver M, Laan H, Venema G. Deletion analysis of the proteinase gene of Streptococcus cremorisWg2. Appl Environ Microbiol. 1988;54:239–244. doi: 10.1128/aem.54.1.239-244.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kok J, Van Dijl J M, Van Der Vossen J, Venema G. Cloning and expression of a Streptococcus cremoris proteinase in Bacillus subtilis and Streptococcus lactis. Appl Environ Microbiol. 1985;50:94–101. doi: 10.1128/aem.50.1.94-101.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kok J, Leenhouts K J, Haandrikman A J, Ledeboer A M, Venema G. Nucleotide sequence of the cell wall proteinase gene of Streptococcus cremorisWg2. Appl Environ Microbiol. 1988;54:231–238. doi: 10.1128/aem.54.1.231-238.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kornberg A, Spudich J A, Nelson D L, Deutscher M P. Origin of proteins in sporulation. Annu Rev Biochem. 1968;37:51–78. doi: 10.1146/annurev.bi.37.070168.000411. [DOI] [PubMed] [Google Scholar]
- 143.Koszelak S, Ng J D, Day J, Ko T P, Greenwood A, McPherson A. The crystallographic structure of the subtilisin protease from Penicillium cyclopium. Biochemistry. 1997;36:6597–6604. doi: 10.1021/bi963189t. [DOI] [PubMed] [Google Scholar]
- 144.Kotler M, Katz R A, Skalka A M. Activity of avian retroviral protease expressed in Escherichia coli. J Virol. 1988;62:2696–2700. doi: 10.1128/jvi.62.8.2696-2700.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kubo M, Imanaka T. Cloning and nucleotide sequence of the highly thermostable neutral protease gene from Bacillus stearothermophilus. J Gen Microbiol. 1988;134:1883–1891. doi: 10.1099/00221287-134-7-1883. [DOI] [PubMed] [Google Scholar]
- 146.Kubo M, Higo Y. Expression and genetic analysis of the highly thermostable neutral protease (aspartame synthetic enzyme) gene in Bacillus subtilis. Toso Kenkyu Hokoku. 1989;33:101–112. [Google Scholar]
- 147.Kuo L C, Shafer J A. Retroviral proteases. Methods Enzymol. 1994;241:3–178. [Google Scholar]
- 148.Kwon S T, Terada I, Matsuzawa H, Ohta T. Nucleotide sequence of the gene for aqualysin I (a thermophilic alkaline serine protease) of Thermus aquaticusYT-1 and characteristics of the deduced primary structure of the enzyme. Eur J Biochem. 1988;173:491–497. doi: 10.1111/j.1432-1033.1988.tb14025.x. [DOI] [PubMed] [Google Scholar]
- 149.Kwon S T, Matsuzawa H, Ohta T. Determination of the positions of the disulfide bonds in aqualysin I (a thermophilic alkaline serine protease) of Thermus aquaticusYT-1. J Biochem. 1988;104:557–559. doi: 10.1093/oxfordjournals.jbchem.a122509. [DOI] [PubMed] [Google Scholar]
- 150.Labbe J P, Rebegrotte P, Turpine M. Demonstrating extracellular leucine aminopeptidase (EC 3.4.1.1) of Aspergillus oryzae(IP 410): leucine aminopeptidase 2 fraction. C R Acad Sci (Paris) 1974;278D:2699. [PubMed] [Google Scholar]
- 151.Lafemina, R. L., V. V. Sardana, and C. A. Veloski. April 1996. Stable recombinant protease from human cytomegalovirus by site-specific mutagenesis. US patent 323,953.
- 152.Lao G, Wilson D B. Cloning, sequencing and expression of a Thermomonospora fusca protease gene in Streptomyces lividans. Appl Environ Microbiol. 1996;62:4256–4259. doi: 10.1128/aem.62.11.4256-4259.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Law J, Vos P, Hayes F, Daly C, de Vos W, Fitzgerald G. Cloning and partial sequencing of the proteinase gene complex from Lactococcus lactis subsp. lactisUC317. J Gen Microbiol. 1992;138:709–718. doi: 10.1099/00221287-138-4-709. [DOI] [PubMed] [Google Scholar]
- 154.Lecadet M M, Lescourret M, Klier A. Characterization of an intracellular protease isolated from Bacillus thuringiensissporulating cells and able to modify homologous RNA polymerase. Eur J Biochem. 1977;79:329–338. doi: 10.1111/j.1432-1033.1977.tb11813.x. [DOI] [PubMed] [Google Scholar]
- 155.Lee C, Su S, Liaw R. Molecular analysis of an extracellular protease gene from Vibrio parahaemolyticus. Microbiology. 1995;141:2569–2576. doi: 10.1099/13500872-141-10-2569. [DOI] [PubMed] [Google Scholar]
- 156.Lee J D, Kolattukudy P E. Molecular cloning of the cDNA and gene for an elastinolytic aspartic proteinase from Aspergillus fumigatusand evidence of its secretion by the fungus during invasion of the host lung. Infect Immun. 1995;63:3796–3803. doi: 10.1128/iai.63.10.3796-3803.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lee S, Yoon K, Nam H, Oh T, Lee S, Chae K. Cloning and molecular characterization of a nprx gene of B. subtilisN515-4 encoding a neutral protease. J Microbiol. 1996;34:63–73. [Google Scholar]
- 158.Leenhouts K J, Gietema J, Kok J, Venema G. Chromosomal stabilization of the proteinase genes in Lactococcus lactis. Appl Environ Microbiol. 1991;57:2568–2575. doi: 10.1128/aem.57.9.2568-2575.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Leighton T J, Stock J J. Biochemical changes during fungal sporulation and spore germination. I. Phenyl methyl sulfonyl fluoride inhibition of macroconidial germination in Microsporum gypseum. J Bacteriol. 1970;101:931–940. doi: 10.1128/jb.101.3.931-940.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Leung K Y, Stevenson R M. Characteristics and distribution of extracellular proteases from Aeromonas hydrophila. J Gen Microbiol. 1988;134:151–160. [Google Scholar]
- 161.Li S L, Norioka S, Sakiyama F. Molecular cloning and nucleotide sequence of the B-lytic protease gene from Achromobacter lyticus. J Bacteriol. 1990;172:6506–6511. doi: 10.1128/jb.172.11.6506-6511.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Libby R T, Cosman D, Cooney M K, Merriam J E, March C J, Hopp T P. Human rhinovirus 3C protease: cloning and expression of an active form in Escherichia coli. Biochemistry. 1988;27:6262–6268. doi: 10.1021/bi00417a010. [DOI] [PubMed] [Google Scholar]
- 163.Lichenstein, H. January 1993. Isolation and characterization of a novel protease from Streptomyces lividans. U.S. patent 724,721.
- 164.Lillehoj E P, Salazar F H R, Mervis R J, Raum M G, Chan H W, Ahmad N, Venkatesan S. Purification and structural characterization of the putative gag-polprotease of human immunodeficiency virus. J Virol. 1988;62:3053–3058. doi: 10.1128/jvi.62.8.3053-3058.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lindberg R A, Eirich L D, Price J S, Wolfinbarger L, Jr, Drucker H. Alkaline protease from Neurospora crassa. J Biol Chem. 1981;256:811–814. [PubMed] [Google Scholar]
- 166.Liu H, Muller-Plathe F, Van Gunsteren W F. A combined quantum/classical molecular dynamics study of the catalytic mechanism of HIV protease. J Mol Biol. 1996;261:454–469. doi: 10.1006/jmbi.1996.0476. [DOI] [PubMed] [Google Scholar]
- 167.Liu P V. Extracellular toxins of Pseudomonas aeruginosa. J Infect Dis. 1974;130:94–99. doi: 10.1093/infdis/130.supplement.s94. [DOI] [PubMed] [Google Scholar]
- 168.Liu Y-N, Tang J-L, Clark B R, Dow J M, Danniels M J. A multipurpose broad host range cloning vector and its use to characterize an extracellular protease gene of Xanthomonas campestris pathovar. campestris. Mol Gen Genet. 1990;220:433–440. doi: 10.1007/BF00391750. [DOI] [PubMed] [Google Scholar]
- 169.Lomholt H, Poulsen K, Kilian M. Comparative characterization of the iga gene encoding IgA1 protease in Neisseria meningitidis, N. gonorrhoeae and Haemophilus influenzae. Mol Microbiol. 1995;15:495–506. doi: 10.1111/j.1365-2958.1995.tb02263.x. [DOI] [PubMed] [Google Scholar]
- 170.Lott T J, Page L S, Boiron P, Benson J, Reiss E. Nucleotide sequence of the Candida albicansaspartyl proteinase gene. Nucleic Acids Res. 1989;17:1779. doi: 10.1093/nar/17.4.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Louis J M, Wondrak E M, Copeland T D, Dale Smith C A, Mora P T, Oroszlan S. Chemical synthesis and expression of the HIV-1 protease gene in E. coli. Biochem Biophys Res Commun. 1989;159:87–94. doi: 10.1016/0006-291x(89)92408-x. [DOI] [PubMed] [Google Scholar]
- 172.Loutsch J M, Galvin N J, Bryant M L, Holwerda B C. Cloning and sequence analysis of murine cytomegalovirus protease and capsid assembly protein genes. Biochem Biophys Res Commun. 1994;203:472–478. doi: 10.1006/bbrc.1994.2206. [DOI] [PubMed] [Google Scholar]
- 173.Lowther W T, Majer P, Duhn B M. Engineering the substrate specificity of rhizopuspepsin: the role of Asp77 of fungal aspartic proteinases in facilitating the cleavage of oligopeptide substrates with lysine in P1. Protein Sci. 1995;4:689–702. doi: 10.1002/pro.5560040409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lu A Y H, Junk K W, Coon M J. Resolution of the cytochrome P-450 containing W-Hydroxylation system of liver microsomes into three components. J Biol Chem. 1969;244:3714–3721. [PubMed] [Google Scholar]
- 175.Lu J, Inoue H, Kimura T, Makabe O, Takashi K. Molecular cloning of a cDNA for proctase B from Aspergillus niger var. macrosporusand sequence comparison with other aspergillopepsins I. Biosci Biotechnol Biochem. 1995;59:954–955. doi: 10.1271/bbb.59.954. [DOI] [PubMed] [Google Scholar]
- 176.MacDougall J H, Beighton D, Russell R R B. Cloning and expression of protease genes from Treponema denticola in Escherichia coli. Oral Microbiol Immunol. 1991;6:270–274. doi: 10.1111/j.1399-302x.1991.tb00490.x. [DOI] [PubMed] [Google Scholar]
- 177.MacKay V L, Welch S K, Insley M Y, Manney T R, Holly J, Saari G C, Parker M L. The Saccharomyces cerevisiaeBAR1 gene encodes an exported protein with homology to pepsin. Proc Natl Acad Sci USA. 1988;85:55–59. doi: 10.1073/pnas.85.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Manabe K, Morh M, Honjo M, Ohoka M, Fushimi K, Sawakura A, Furutani Y. N-terminal amino acid sequences of neutral proteases from Bacillus amyloliquefaciens and Bacillus subtilis: identification of a neutral protease gene cloned in Bacillus subtilis. Agric Biol Chem. 1985;49:2261–2267. [Google Scholar]
- 179.Masaki T, Nakamura K, Isono M, Soejima M. A new proteolytic enzyme from Achromobacter lyticusM497-1. Agric Biol Chem. 1978;42:1443–1445. [Google Scholar]
- 180.Maurizi M R, Trisler P, Gottesman S. Insertional mutagenesis of the lon gene in Escherichia coli: lonis dispensable. J Bacteriol. 1985;164:1124–1135. doi: 10.1128/jb.164.3.1124-1135.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Maurizi M R, Clark W P, Katayama Y, Rudikoff S, Pumphrey J, Bowers B, Gottesman S. Sequences and structure of ClpP, the proteolytic component of the ATP-dependent Clp protease of Escherichia coli. J Biol Chem. 1990;265:12536–12544. [PubMed] [Google Scholar]
- 182.Maurizi M R, Clark W P, Kim S-H, Gottesman S. ClpP represents a unique family of serine proteases. J Biol Chem. 1990;265:12546–12552. [PubMed] [Google Scholar]
- 183.Menon A S, Goldberg A L. Protein substrates activate the ATP-dependent protease La by promoting nucleotide binding and release of bound ADP. J Biol Chem. 1987;262:14929–14934. [PubMed] [Google Scholar]
- 184.Miller M, Jasko′lski M, Rao J K M, Leis J, Wlodawer A. Crystal structure of a retroviral protease proves relationship to aspartic protease family. Nature. 1989;337:576–579. doi: 10.1038/337576a0. [DOI] [PubMed] [Google Scholar]
- 185.Milton D L, Norqvist A, Wolf-Watz H. Cloning of a metalloprotease gene involved in the virulence mechanism of Vibrio anguillarum. J Bacteriol. 1992;174:7235–7244. doi: 10.1128/jb.174.22.7235-7244.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mitchinson C, Wells J A. Protein engineering of disulfide bonds in subtilisin BPN′. Biochemistry. 1989;28:4807–4815. doi: 10.1021/bi00437a043. [DOI] [PubMed] [Google Scholar]
- 187.Miyazaki H, Yanagida N, Horinouchi S, Beppu T. Characterization of the precursor of Serratia marcescens serine protease and COOH-terminal processing of the precursor during its excretion through the outer membrane of Escherichia coli. J Bacteriol. 1989;171:6566–6572. doi: 10.1128/jb.171.12.6566-6572.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mizaeno K, Nakamura T, Oshima T, Tanaka S, Matsuo H. Yeast KEX2 gene encodes an endopeptidase homologous to subtilisin-like serine proteases. Biochem Biophys Res Commun. 1988;156:246–254. doi: 10.1016/s0006-291x(88)80832-5. [DOI] [PubMed] [Google Scholar]
- 189.Mizuno K, Matsuo H. A novel protease from yeast with specificity towards paired basic residues. Nature. 1984;309:558–560. doi: 10.1038/309558a0. [DOI] [PubMed] [Google Scholar]
- 190.Moehle C M, Aynardi M W, Kolodny M R, Park F J, Jones E W. Protease B of Saccharomyces cerevisiae: isolation and regulation of the PRB1 structural gene. Genetics. 1987;115:255–263. doi: 10.1093/genetics/115.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Monod M, Togni G, Hube B, Sanglard D. Multiplicity of genes encoding secreted aspartic proteinases in Candidasp. Mol Microbiol. 1994;13:357–368. doi: 10.1111/j.1365-2958.1994.tb00429.x. [DOI] [PubMed] [Google Scholar]
- 192.Morihara K, Oda K. Microbial degradation of proteins. In: Guenther W, editor. Microbial degradation of natural products. Weinheim, Germany: VCH Publishers; 1993. pp. 293–364. [Google Scholar]
- 193.Morita S, Kuriyama M, Nakatsu M, Kitano K. High level expression of Fusarium alkaline protease gene in Acremonium chrysogenum. Biosci Biotechnol Biochem. 1994;58:627–630. doi: 10.1271/bbb.58.627. [DOI] [PubMed] [Google Scholar]
- 194.Moses E K, Rood J I, Yong W K, Riffkin G G. Molecular analysis of one of multiple protease-encoding genes from the prototype virulent strain of Bacteroides nodosus. Gene. 1989;77:219–228. doi: 10.1016/0378-1119(89)90070-x. [DOI] [PubMed] [Google Scholar]
- 195.Murakami K, Ishida Y, Masaki A, Tatsumi H, Murakami S, Nakano E, Motai H, Kawabe H, Arimura H. Isolation and characterization of the alkaline protease gene of Aspergillus oryzae. Agric Biol Chem. 1991;55:2807–2812. [PubMed] [Google Scholar]
- 196.Murayama S Y, Takeda O, Mukai H, Takesako K, Sano E, Kato I, Yamaguchi H. Candida albicansaspartic proteinase: cDNA cloning and comparison among strains. Adv Exp Med Biol. 1995;362:573–576. doi: 10.1007/978-1-4615-1871-6_76. [DOI] [PubMed] [Google Scholar]
- 197.Nagami, Y., and T. Tanaka. December 1986. Expression plasmid of Bacillus natto protease. Japanese patent 61,282,400.
- 198.Nakahama K, Yoshimuza K, Maramoto R, Kikuchi M, Lee I, Hase T, Matsubara H. Cloning and sequencing of Serratiaprotease gene. Nucleic Acids Res. 1986;14:5843–5855. doi: 10.1093/nar/14.14.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Narhi O L, Stabinsky Y, Levitt M, Miller L, Sachdev R, Finley S, Park S, Kolvenbach C, Arakawa T, Zukowski M. Enhanced stability of subtilisin by three point mutations. Biotechnol Appl Biochem. 1991;13:12–24. [PubMed] [Google Scholar]
- 200.Neurath H. Evolution of proteolytic enzymes. Science. 1984;224:350–357. doi: 10.1126/science.6369538. [DOI] [PubMed] [Google Scholar]
- 201.Nicaud J M, Fabre E, Beckerich J M, Fournier P, Gaillardin C. Cloning, sequencing, and amplification of the alkaline extracellular protease (XPR2) gene of the yeast Yarrowia lipolytica. J Biotechnol. 1989;12:285–297. [Google Scholar]
- 202.Nielsen T L, Holmberg S, Petersen J G L. Regulated overproduction and secretion of yeast carboxypeptidase Y. Appl Microbiol Biotechnol. 1990;33:307–312. doi: 10.1007/BF00164527. [DOI] [PubMed] [Google Scholar]
- 203.Nishiya Y, Imanaka T. Cloning and nucleotide sequences of the Bacillus stearothermophilusneutral protease gene and its transcriptional activator gene. J Bacteriol. 1990;172:4861–4869. doi: 10.1128/jb.172.9.4861-4869.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Nissen-Meyer J, Lillehaug D, Nes I F. The plasmid-encoded lactococcal envelope-associated proteinase is encoded by a chromosomal gene in Lactococcus lactis subsp. cremorisBC101. Appl Environ Microbiol. 1992;58:750–753. doi: 10.1128/aem.58.2.750-753.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.North M J. Comparative biochemistry of the proteinases of eukaryotic microorganisms. Microbiol Rev. 1982;46:308–340. doi: 10.1128/mr.46.3.308-340.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.O’Day D H. Acid protease activity during germination of microcysts of the cellular slime mold Polysphodylium pallidum. J Bacteriol. 1976;125:8–13. doi: 10.1128/jb.125.1.8-13.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ogawa Y, Tatsumi H, Murakami S, Ishida Y, Murakami K, Masaki A, Kawabe H, Arimura H, Nakano E, Motai H, Toh-e A. Secretion of Aspergillus oryzae alkaline protease in an osmophilic yeast, Zygosaccharomyces rouxii. Agric Biol Chem. 1990;54:2521–2529. [PubMed] [Google Scholar]
- 208.Ohara T, Makino K, Shinagawa H, Nakata A, Norioka S, Sakiyama F. Cloning, nucleotide sequence, and expression of Achromobacterprotease I gene. J Biol Chem. 1989;264:20625–20631. [PubMed] [Google Scholar]
- 209.Okada, J., H. Shimogaki, K. Murata, H. Kimura, and K. Oba. January 1986. Genetically engineered Bacillus subtilis for extracellular protease manufacture. Japanese patent 61,12,287.
- 210.Okada Y, Nagase H, Harris E D. A metalloproteinase from human rheumatoid synovial fibroblasts that digests connective tissue matrix components. Purification and characterization. J Biol Chem. 1986;261:14245–14255. [PubMed] [Google Scholar]
- 211.Osawa, M., O. Hatamoto, Y. Hanya, S. Ushijima, and K. Uchida. 1992. Cloning of alkaline protease gene of Aspergillus. Japanese patent 04,148,684.
- 212.Oshima, T., and K. Mizuno. 1989. Manufacture of KEX-2 endoproteinase and soluble derivatives using expression vectors. European patent 327,377.
- 213.Page R D M. TreeView: an application to display phylogenetic trees on personal computers. Comput Appl Biosci. 1996;12:357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- 214.Pantoliano M W, Ladner R C, Bryan P N, Rollence M L, Wood J F, Poulos T L. Protein engineering of subtilisin BPN′: enhanced stabilization through the introduction of two cysteines to form a disulfide bond. Biochemistry. 1987;26:2077–2082. doi: 10.1021/bi00382a002. [DOI] [PubMed] [Google Scholar]
- 215.Pearl L H. The catalytic mechanism of aspartic proteinases. FEBS Lett. 1987;214:8–12. doi: 10.1016/0014-5793(87)80003-0. [DOI] [PubMed] [Google Scholar]
- 216.Pearl L H, Taylor W R. A structural model for the retroviral proteases. Nature. 1987;329:351–354. doi: 10.1038/329351a0. [DOI] [PubMed] [Google Scholar]
- 217.Perlmann G E, Lorand L. Proteolytic enzymes. Methods Enzymol. 1970;19:732–735. [Google Scholar]
- 218.Phadatare S, Rao M, Deshpande V. A serine alkaline protease from the fungus Conidiobolus coronatuswith a distinctly different structure than the serine protease subtilisin Carlsberg. Arch Microbiol. 1997;166:414–417. doi: 10.1007/BF01682989. [DOI] [PubMed] [Google Scholar]
- 219.Phadatare S U, Srinivasan M C, Deshpande V V. High activity alkaline protease from Conidiobolus coronatus(NCL 86.8.20): enzyme production and compatibility with commercial detergents. Enzyme Microb Technol. 1993;15:72–76. [Google Scholar]
- 220.Phadatare S U, Srinivasan M C, Deshpande V V. Evidence for controlled autoproteolysis of alkaline protease: a mechanism for physiological regulation of conidial discharge in Conidiobolus coronatus. Eur J Biochem. 1992;205:679–686. doi: 10.1111/j.1432-1033.1992.tb16829.x. [DOI] [PubMed] [Google Scholar]
- 221.Phadatare S U, Srinivasan M C, Deshpande M V. Evidence for the involvement of serine protease in the conidial discharge of Conidiobolus coronatus. Arch Microbiol. 1989;153:47–49. [Google Scholar]
- 222.Pichuantes S, Babe L M, Barr P J, Craik C S. Recombinant HIV-1 protease secreted by Saccharomyces cerevisiaecorrectly processes myristylated gag polyprotein. Proteins Struct Funct Genet. 1989;6:324–337. doi: 10.1002/prot.340060315. [DOI] [PubMed] [Google Scholar]
- 223.Pickersgill R W, Sumner I G, Goodenough P W. Preliminary crystallographic data for protease a. Eur J Biochem. 1990;190:443–444. doi: 10.1111/j.1432-1033.1990.tb15594.x. [DOI] [PubMed] [Google Scholar]
- 224.Pohlner J, Halter R, Beyreuther K, Meyer T F. Gene structure and extracellular secretion of Neisseria gonorrhoeaeIgA protease. Nature. 1986;325:458–462. doi: 10.1038/325458a0. [DOI] [PubMed] [Google Scholar]
- 225.Poldermans B. Proteolytic enzymes. In: Gerhartz W, editor. Proteolytic enzymes in industry: production and applications. Weinheim, Germany: VCH Publishers; 1990. pp. 108–123. [Google Scholar]
- 226.Polgar L. Common feature of the four types of protease mechanisms. Biol Chem Hoppe-Seyler. 1990;371:327–331. [PubMed] [Google Scholar]
- 227.Postemsky C J, Dignam S S, Setlow P. Isolation and characterization of Bacillus megateriummutants containing decreased levels of spore protease. J Bacteriol. 1978;135:841–850. doi: 10.1128/jb.135.3.841-850.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Poulsen K, Brandt J, Hjorth J P, Thoegersen H C, Kilian M. Cloning and sequencing of the immunoglobulin A1 protease gene (iga) of Haemophilus influenzaeserotype 6. Infect Immun. 1989;57:3097–3105. doi: 10.1128/iai.57.10.3097-3105.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Poulsen K, Reinholdt J, Kilian M. Characterization of Streptococcus pneumoniae immunoglobulin A1 protease gene (iga) and its translation product. Infect Immun. 1996;64:3957–3966. doi: 10.1128/iai.64.10.3957-3966.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Poulsen K, Hjorth K, Peter J, Kilian M. Limited diversity of the immunoglobulin A1 protease gene (iga) among Haemophilus influenzaeserotype 6 strains. Infect Immun. 1988;56:987–992. doi: 10.1128/iai.56.4.987-992.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Power M D, Marx P A, Bryant M L, Gardner M D, Barr P J, Luciw P A. Nucleotide sequence of SRV-1, a type D simian acquired immune deficiency syndrome retrovirus. Science. 1986;231:1567–1572. doi: 10.1126/science.3006247. [DOI] [PubMed] [Google Scholar]
- 232.Rahr S, Halter R, Mueller H, Pohlner J, Meyer T F. Genetic analysis of neisserial immunoglobulin A1 proteases. In: Schoolnik G K, editor. Proceedings of the Fourth International Symposium. Washington, D.C: American Society for Microbiology; 1985. pp. 157–163. [Google Scholar]
- 233.Ramesh M V, Sirakova T D, Kolattukudy P E. Isolation, characterization and cloning of the cDNA and the gene for an elastinolytic serine proteinase from Aspergillus flavus. Infect Immun. 1994;62:79–85. doi: 10.1128/iai.62.1.79-85.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Ramesh M V, Sirakova T D, Kolattukudy P E. Cloning and characterization of the cDNAs and genes (mep20) encoding homologous metalloproteinases from Aspergillus flavus and Aspergillus fumigatus. Gene. 1995;165:121–125. doi: 10.1016/0378-1119(95)00434-8. [DOI] [PubMed] [Google Scholar]
- 235.Rao, M. B., and V. V. Deshpande. Proteases and their applications in biotechnology. In A. Varna (ed.), Microbes: for health, wealth and sustainable environment, in press. Malhotra Publishing House, New Delhi, India.
- 236.Rawlings N D, Barrett A J. Evolutionary families of peptidases. Biochem J. 1993;290:205–218. doi: 10.1042/bj2900205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Reichard U, Monod M, Ruechel R. Molecular cloning and sequencing of the gene encoding an extracellular aspartic proteinase from Aspergillus fumigatus. FEMS Microbiol Lett. 1995;130:69–74. doi: 10.1016/0378-1097(95)00185-8. [DOI] [PubMed] [Google Scholar]
- 238.Rivero O, Anguita J, Paniagua C, Naharro G. Molecular cloning and characterization of an extracellular protease gene from Aeromonas hydrophila. J Bacteriol. 1990;172:3905–3908. doi: 10.1128/jb.172.7.3905-3908.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Rivero O, Anguita J, Mateos D, Paniagua C, Naharro G. Cloning and characterization of an extracellular temperature-labile serine protease gene from Aeromonas hydrophila. FEMS Microbiol Lett. 1991;81:1–8. doi: 10.1016/0378-1097(91)90461-i. [DOI] [PubMed] [Google Scholar]
- 240.Roberts B. Nuclear location signal-mediated protein transport. Biochim Biophys Acta. 1989;1008:263–280. doi: 10.1016/0167-4781(89)90016-x. [DOI] [PubMed] [Google Scholar]
- 241.Roberts J W, Roberts C W, Mount D W. Inactivation and proteolytic cleavage of phage repressor in vitroin an ATP-dependent reaction. Proc Natl Acad Sci USA. 1977;74:2283–2287. doi: 10.1073/pnas.74.6.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Rougeau C T, Lauvel M W, Fukuhara H. The Kluyveromyces lactisKEX1 gene encodes a subtilisin-type serine protease. FEBS Lett. 1988;234:464–470. doi: 10.1016/0014-5793(88)80139-x. [DOI] [PubMed] [Google Scholar]
- 243.Russel A J, Ferhst A R. Rational modification of enzyme catalysis by engineering surface charge. Nature. 1987;328:496–500. doi: 10.1038/328496a0. [DOI] [PubMed] [Google Scholar]
- 244.Russel A J, Thomas P G, Fersht A R. Electrostatic effects on modification of charged groups in the active site cleft of subtilisin by protein engineering. J Mol Biol. 1987;193:803–813. doi: 10.1016/0022-2836(87)90360-3. [DOI] [PubMed] [Google Scholar]
- 245.Sagata N, Yasunaga T, Ogawa Y, Tsuzuku-Kawamara J, Ikawa Y. Bovine leukemia virus. Unique structural features of its long terminal repeats and evolutionary relationship to human T-cell leukemia virus. Proc Natl Acad Sci USA. 1984;81:4741–4745. doi: 10.1073/pnas.81.15.4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Saiga A, Tanaka T, Orita S, Sato A, Sato S, Hachisu T, Abe K, Kimura Y, Kondo Y, et al. Human T-cell leukemia virus type I protease protein expressed in Escherichia colipossesses aspartic proteinase activity. Arch Virol. 1993;128:195–210. doi: 10.1007/BF01309434. [DOI] [PubMed] [Google Scholar]
- 247.Samal B, Karan B, Boone T C, Chen K K, Rahde M F, Stabinsky Y. Cloning and expression of the gene encoding a novel proteinase from Tritirachium albumLimber. Gene. 1989;85:329–333. doi: 10.1016/0378-1119(89)90425-3. [DOI] [PubMed] [Google Scholar]
- 248.Sanchez-Pescador R, Power M D, Borr P J, Steimer K S, Stempien M M, Brown-Shimer S L, Gee W W, Renar A, Randoph A, Levy J A, Dina D, Luciw P A. Nucleotide sequence and expression of an AIDS-associated retrovirus (ARV-2) Science. 1985;227:484–492. doi: 10.1126/science.2578227. [DOI] [PubMed] [Google Scholar]
- 249.Saul D J, Williams L C, Toogood H S, Daniel R M, Bergquist P L. Sequence of the gene encoding a highly thermostable neutral proteinase from Bacillus sp. strain EA1: expression in Escherichia coliand characterization. Biochim Biophys Acta. 1996;1308:74–80. doi: 10.1016/0167-4781(96)00074-7. [DOI] [PubMed] [Google Scholar]
- 250.Schechler I, Berger A. On the size of the active site in proteases I papain. Biochem Biophys Res Commun. 1967;27:157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- 251.Schmidt B F, Woodhouse L, Adams R M, Ward T, Mainzer S E, Lad P J. Alkalophilic Bacillussp. strain LG12 has a series of serine protease genes. Appl Environ Microbiol. 1995;61:4490–4493. doi: 10.1128/aem.61.12.4490-4493.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Schwartz D E, Tizard R, Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983;32:853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- 253.Shannon J D, Baramova E N, Bjarnason J B, Fox J W. Amino acid sequence of a Crotalus atroxvenom metalloprotease which cleaves type IV collagen and gelatin. J Biol Chem. 1989;264:11575–11583. [PubMed] [Google Scholar]
- 254.Shibano, Y., K. Morihara, K. Okuda, and A. Fukushima. December 1991. Molecular cloning of alkaline protease gene of Pseudomonas aeruginosa. Japanese patent 03,277,278.
- 255.Shimotohno K, Takahashi Y, Shimizu N, Gojobori T, Golde D W, Chen I S Y, Miwa M, Sugimura T. Complete nucleotide sequence of an infectious clone of human T-cell leukemia virus type II: an open reading frame for the protease gene. Proc Natl Acad Sci USA. 1985;82:3101–3105. doi: 10.1073/pnas.82.10.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Shinick T M, Lerner R A, Sutcliffe J G. Nucleotide sequence of Moloney murine leukaemia virus. Nature. 1981;293:543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- 257.Shintani T, Ichishima E. Primary structure of aspergillopepsin I deduced from nucleotide sequence of the gene and aspartic acid-76 is an essential active site of the enzyme for trypsinogen activation. Biochim Biophys Acta. 1994;1204:257–264. doi: 10.1016/0167-4838(94)90016-7. [DOI] [PubMed] [Google Scholar]
- 258.Sidorenkov I N, Nosovskaya E A, Kostrov S V. Cloning and expression of Bacillus stearothermophilus-thermostable metallo-protease gene in Bacillus subtilis. Mol Biol. 1995;29:507–511. [PubMed] [Google Scholar]
- 259.Sielecki A R, Fujinaga M, Read R J, James M N G. Refined structure of porcine pepsinogen at 1.8A resolution. J Mol Biol. 1991;219:671–692. doi: 10.1016/0022-2836(91)90664-r. [DOI] [PubMed] [Google Scholar]
- 260.Silen J L, McGrath C N, Smith K R, Agard D A. Molecular analysis of the gene encoding α-lytic protease: evidence for a preproenzyme. Gene. 1988;69:237–244. doi: 10.1016/0378-1119(88)90434-9. [DOI] [PubMed] [Google Scholar]
- 261.Silen J L, Frank D, Fujishige A, Bone R, Agard D A. Analysis of prepro-α-lytic proteases expression in Escherichia colireveals that the proregion is required for activity. J Bacteriol. 1989;171:1320–1325. doi: 10.1128/jb.171.3.1320-1325.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Sirakova T D, Markaryan A, Kolattukudy P E. Molecular cloning and sequencing of the cDNA and gene for a novel elastinolytic metalloproteinase from Aspergillus fumigatus and its expression in Escherichia coli. Infect Immun. 1994;62:4208–4218. doi: 10.1128/iai.62.10.4208-4218.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Sloma A, Ally A, Ally D, Pero J. Gene encoding a minor extracellular protease in Bacillus subtilis. J Bacteriol. 1988;170:5557–5563. doi: 10.1128/jb.170.12.5557-5563.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Sloma A, Rudolph C F, Rufo Jr G A, Sullivan B J, Theriault K A, Ally D, Pero J. Gene encoding a novel extracellular metalloprotease in Bacillus subtilis. J Bacteriol. 1990;172:1024–1029. doi: 10.1128/jb.172.2.1024-1029.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Sloma A, Rufo G A, Jr, Rudolph C F, Sullivan B J, Theriault K A, Pero J. Bacillopeptidase F of Bacillus subtilis: purification of the protein and cloning of the gene. J Bacteriol. 1990;172:1470–1477. doi: 10.1128/jb.172.3.1470-1477.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Smeekens S P. Processing of protein precursors by a novel family of subtilisin-related mammalian endoproteases. Bio/Technology. 1993;11:182–186. doi: 10.1038/nbt0293-182. [DOI] [PubMed] [Google Scholar]
- 267.Sobek H, Hecht H J, Hoffman B, Aeble W, Schomburg D. Crystal structure of an alkaline protease from Bacillus alkalophilusat 2.4 A resolution. FEBS Lett. 1990;274:57–66. doi: 10.1016/0014-5793(90)81328-l. [DOI] [PubMed] [Google Scholar]
- 268.Sonigo P, Barker C, Hunter E, Wain-Hobson S. Nucleotide sequence of Mason-Pfizer monkey virus: an immunosuppressive D-type retrovirus. Cell. 1986;45:375–385. doi: 10.1016/0092-8674(86)90323-5. [DOI] [PubMed] [Google Scholar]
- 269.Stabile M R, Lai W G, DeSantis G, Gold M, Jones J B. Probing the specificity of the S1 binding site of M222 mutants of subtilisin B. lentuswith boronic acid inhibitors. Bioorg Med Chem Lett. 1996;6:2501–2506. [Google Scholar]
- 270.Stahl M L, Ferrari E. Replacement of the Bacillus subtilissubtilisin structural gene with an in vitro-derived deletion mutation. J Bacteriol. 1984;158:411–418. doi: 10.1128/jb.158.2.411-418.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Steffy K R, Schoen S, Chen C. Nucleotide sequence of the herpes simplex virus type 2 gene encoding the protease and capsid protein ICP35. J Gen Virol. 1995;76:1069–1072. doi: 10.1099/0022-1317-76-4-1069. [DOI] [PubMed] [Google Scholar]
- 272.Stoll E, Weder H G, Zuber H. Aminopeptidase II from Bacillus sterothermophilus. Biochim Biophys Acta. 1976;438:212–220. [PubMed] [Google Scholar]
- 273.Strausberg S L, Alexander P A, Gallagher D T, Gilliland G L, Barnett B L, Bryan P B. Directed evolution of a subtilisin with calcium-independent stability. Bio/Technology. 1995;13:669–673. doi: 10.1038/nbt0795-669. [DOI] [PubMed] [Google Scholar]
- 274.Sussman M D, Setlow P. Cloning, nucleotide sequence and regulation of the Bacillus subtilis gprgene, which codes for the protease that initiates degradation of small, acid-soluble proteins during spore germination. J Bacteriol. 1991;173:291–300. doi: 10.1128/jb.173.1.291-300.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Suzuki C K, Rep M, Van Dijl J M, Suda K, Grivell L A, Schatz G. ATP-dependent proteases that also chaperone protein biogenesis. Trends Biochem Sci. 1997;22:118–123. doi: 10.1016/s0968-0004(97)01020-7. [DOI] [PubMed] [Google Scholar]
- 276.Suzuki T, Itoh A, Ichihara S, Mizushima S. Characterization of the sppA gene coding for protease IV, a signal peptide peptidase of Escherichia coli. J Bacteriol. 1987;169:2523–2528. doi: 10.1128/jb.169.6.2523-2528.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Svendsen I, Jensen M R, Breddman K. The primary structure of the glutamic acid specific protease of Streptomyces griseus. FEBS Lett. 1991;292:165–167. doi: 10.1016/0014-5793(91)80859-2. [DOI] [PubMed] [Google Scholar]
- 278.Takagi H, Morinaga T, Ikemuar H, Inouye M. The role of pro-239 in the catalysis and heat stability of subtilisin E. Eur J Biochem. 1989;105:953–956. doi: 10.1093/oxfordjournals.jbchem.a122786. [DOI] [PubMed] [Google Scholar]
- 279.Takagi M, Imanaka T, Aiba S. Nucleotide sequence and promoter region for the neutral protease gene from Bacillus stearothermophilus. J Bacteriol. 1985;163:824–831. doi: 10.1128/jb.163.3.824-831.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Takagi H, Takahashi T, Momose H, Inouye M, Maeda Y, Matsuzawa H, Ohta T. Enhancement of the thermostability of subtilisin E by introduction of a disulfide bond engineered on the basis of structural comparison with a thermophilic serine protease. J Biol Chem. 1990;265:6874–6878. [PubMed] [Google Scholar]
- 281.Takagi H, Morinaga Y, Ikemura H, Inouye M. Mutant subtilisin E with enhanced protease activity obtained by site directed mutagenesis. J Biol Chem. 1988;263:19592–19596. [PubMed] [Google Scholar]
- 282.Takahashi K. The amino acid sequence of rhizopuspepsin, an aspartic proteinase from Rhizopus chinensis. J Biol Chem. 1987;262:1468–1478. [PubMed] [Google Scholar]
- 283.Takahashi, K., H. Inoue, T. Kimura, C. Nojiri, and O. Makabe. March 1992. Cloning and expression of acid protease A gene of Aspergillus. Japanese patent 04,75,586.
- 284.Takeuchi M, Ogura K, Hamamoto T, Kobayashi Y. Molecular cloning and sequence analysis of a gene encoding an aspartic proteinase from Aspergillus oryzae. Adv Exp Med Biol. 1995;362:577–580. doi: 10.1007/978-1-4615-1871-6_77. [DOI] [PubMed] [Google Scholar]
- 285.Tanguy-Rangean C, Wesolowski-Louvel M, Fukuhare H. The Kluyveromyces lactisKEX-1 gene encodes a subtilisin-type serine proteinase. FEBS Lett. 1988;234:464–470. doi: 10.1016/0014-5793(88)80139-x. [DOI] [PubMed] [Google Scholar]
- 286.Tatsumi H, Ohsawa M, Tsuji R F, Murakami S, Nakano E, Motai H, Masaki A, Ishida Y, Murakami K, Kawabe H, Arimura H. Cloning and sequencing of the alkaline protease cDNA from Aspergillus oryzae. Agric Biol Chem. 1988;52:1887–1888. [Google Scholar]
- 287.Tatsumi H, Murakami S, Ogawa Y, Masaki A, Ishida Y, Murakami K, Kuwabe H, Arimura H, Nakano E, Motai H. Autoproteolytic processing and processing dependent secretion of Aspergillus oryzaeprotease in yeast. Agric Biol Chem. 1991;55:3099–3101. [Google Scholar]
- 288.Tatsumi H, Ogawa Y, Murakami S, Ishida Y, Murakami K, Masaki A, Kawabe H, Arimura H, Nakano E, Motai H. A full length cDNA clone for the alkaline protease from Aspergillus oryzae: structural analysis and expression in Saccharomyces cerevisiae. Mol Gen Genet. 1989;219:33–38. doi: 10.1007/BF00261154. [DOI] [PubMed] [Google Scholar]
- 289.Tawara, T., S. Nanba, and J. Nagai. April 1989. Alkaline proteinase, its cloning and recombinant manufacture with Bacillus. Japanese patent 01,101,886.
- 290.Thomas P G, Russell A J, Fersht A R. Tailoring the pH dependence of enzyme catalysis using protein engineering. Nature. 1985;28:375–376. [Google Scholar]
- 291.Thompson J D, Higgins D G, Gibson T J. Clustal W: improving sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Tigue N J, Matharu P J, Roberts N A, Mills J S, Kay J, Jupp R. Cloning, expression and characterization of the proteinase from human herpesvirus 6. J Virol. 1996;70:4136–4141. doi: 10.1128/jvi.70.6.4136-4141.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Tobe, S., M. Odera, and Y. Asai. July 1992. Cloning and expression of gene for alkaline protease Ya of Bacillus. Japanese patent 04,197,182.
- 294.Togni G, Sanglard D, Falchetto R, Monod M. Isolation and nucleotide sequence of the extracellular acid protease gene (ACP) from yeast Candida tropicalis. FEBS Lett. 1991;286:181–185. doi: 10.1016/0014-5793(91)80969-a. [DOI] [PubMed] [Google Scholar]
- 295.Toma S, Delbue M, Pirola A, Grandi G. nprR1 and nprR2 regulatory regions for neutral protease expression in Bacillus subtilis. J Bacteriol. 1986;167:740–743. doi: 10.1128/jb.167.2.740-743.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Tonouchi N, Shoun H, Uozumi T, Beppu T. Cloning and sequencing of a gene for Mucor rennin, an aspartate protease from Mucor pusillus. Nucleic Acids Res. 1986;14:7557–7568. doi: 10.1093/nar/14.19.7557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Tran L, Wu X, Wong S. Cloning and expression of a novel protease gene encoding an extracellular neutral protease from Bacillus subtilis. J Bacteriol. 1991;173:6364–6372. doi: 10.1128/jb.173.20.6364-6372.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Tsujibo H, Miyamoto K, Tanaka K, Kawai M, Tainaka T, Imada C, Okami Y, Inamori Y. Cloning and sequence of an alkaline serine protease-encoding gene from the marine bacterium Alteromonassp. strain O-7. Gene. 1993;136:247–251. doi: 10.1016/0378-1119(93)90473-g. [DOI] [PubMed] [Google Scholar]
- 299.Tsujibo H, Miyamoto K, Tanaka K, Kaidzu Y, Imada C, Okami Y, Inamori Y. Cloning and sequence analysis of a protease encoding gene from the marine bacterium Alteromonassp. strain O-7. Biosci Biotechnol Biochem. 1996;60:1284–1288. doi: 10.1271/bbb.60.1284. [DOI] [PubMed] [Google Scholar]
- 300.Van der Laan J C, Gerritse G, Mulleners L, Van der Hoek R, Quax W J. Cloning, characterization and multiple chromosomal integration of a Bacillusalkaline protease gene. Appl Environ Microbiol. 1991;57:901–909. doi: 10.1128/aem.57.4.901-909.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Vanderslice P, Craik C S, Nadel J A, Caughey G H. Molecular cloning of dog mast cell tryptase and a related protease: structural evidence of a unique mode of serine protease activation. Biochemistry. 1989;28:4148–4155. doi: 10.1021/bi00436a004. [DOI] [PubMed] [Google Scholar]
- 302.Vanderslice P, Ballinger S M, Tam E K, Goldstein S M, Kraik C S, Caughey G H. Human mast cell tryptase: multiple cDNAs and genes reveal a multigene serine protease family. Proc Natl Acad Sci USA. 1990;87:3811–3815. doi: 10.1073/pnas.87.10.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Van Melderen L, Thi M, Leechi P, Gottesman S, Couturier M, Maurizi M L. ATP-dependent degradation of Ccd A by Lon protease. J Biol Chem. 1996;271:27730–27738. doi: 10.1074/jbc.271.44.27730. [DOI] [PubMed] [Google Scholar]
- 304.Vasantha N, Thompson L D, Rhodes C, Banner C, Nagle J, Filpula D. Genes for alkaline protease and neutral protease from Bacillus amyloliquefacienscontain a large open reading frame between the regions coding for signal sequence and mature protein. J Bacteriol. 1984;159:811–819. doi: 10.1128/jb.159.3.811-819.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Visser S, Exterkate F A, Slangen C J, De Veer G J. Comparative study of action of cell wall proteinases from various strains of Streptococcus cremorison bovine α- and κ-casein. Appl Environ Microbiol. 1986;52:1162–1166. doi: 10.1128/aem.52.5.1162-1166.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Vos H L, Van der Lee F M, Reemst A, Van Loon A E, Sussenbach J S. The genes encoding the DNA binding protein and the 23 K protease of adenovirus type 40 and 41. Virology. 1988;163:1–10. doi: 10.1016/0042-6822(88)90227-9. [DOI] [PubMed] [Google Scholar]
- 307.Wandersman C, Delepelaire P, Letoffe S, Schwartz M. Characterization of Erwinia chrysanthemi extracellular proteases: cloning and expression of the protease genes in Escherichia coli. J Bacteriol. 1987;169:5046–5053. doi: 10.1128/jb.169.11.5046-5053.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Wani J H, Gilbert J V, Plant A G, Weiser J N. Identification, cloning and sequencing of the immunoglobulin A1 protease gene of Streptococcus pneumoniae. Infect Immun. 1996;64:3967–3974. doi: 10.1128/iai.64.10.3967-3974.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Ward O P. Proteinases. In: Fogarty W M, editor. Microbial enzymes and biotechnology. London, England: Applied Science Publishers; 1983. pp. 251–305. [Google Scholar]
- 310.Watson R R. Substrate specificities of aminopeptidases: a specific method for microbial differentiation. Methods Microbiol. 1976;9:1–14. [Google Scholar]
- 311.Weaver L H, Kester W R, Matthews B W. A crystallographic study of the complex of phosphoramidon with thermolysin. A model for the presumed catalytic transition state and for the binding of structures. J Mol Biol. 1977;114:119–132. doi: 10.1016/0022-2836(77)90286-8. [DOI] [PubMed] [Google Scholar]
- 312.Wells J A, Powers D B. In vivoformation and stability of engineered disulfide bonds in subtilisin. J Biol Chem. 1986;261:6564–6570. [PubMed] [Google Scholar]
- 313.Wells J M, Ferrari E, Henner D J, Estell D A, Chen E Y. Cloning, sequencing and secretion of Bacillus amyloliquefaciens subtilisin in Bacillus subtilis. Nucleic Acids Res. 1983;11:7911–7925. doi: 10.1093/nar/11.22.7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Wilhelm S M, Collier I E, Kronberger A, Eisen A Z, Marmer B L, Grant G A, Bauer E A, Goldberg G I. Human skin fibroblast stromelysin: structure, glycosylation, substrate specificity and differential expression in normal and tumorigenic cells. Proc Natl Acad Sci USA. 1987;84:6725–6729. doi: 10.1073/pnas.84.19.6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Woolford C A, Daniels L B, Park F J, Jones E W, Van Arsdell J N, Innis M A. The PEP4 gene encodes an aspartyl protease implicated in the post-translational regulation of Saccharomyces cerevisiaevacuolar hydrolases. Mol Cell Biol. 1989;6:2500–2510. doi: 10.1128/mcb.6.7.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Wu M, Yeong C Y, Lee S C, Wong S M. Nucleotide sequence of the 3′ half of zucchini yellow mosaic virus (Singapore isolate) genome encoding the 4 k protein, protease, polymerase and coat protein. Nucleic Acids Res. 1993;21:1317. doi: 10.1093/nar/21.5.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Xu F-F, Pearce L E, Yu P-L. Molecular cloning and expression of a proteinase gene from Lactococcus lactis subsp. cremorisH2 and construction of a new lactococcal vector pFX1. Arch Microbiol. 1990;154:99–104. doi: 10.1007/BF00249185. [DOI] [PubMed] [Google Scholar]
- 318.Yamagata Y, Ichishima E. A new alkaline serine protease from alkalophilic Bacillussp.: cloning, sequencing and characterization of an intracellular protease. Curr Microbiol. 1995;30:357–366. doi: 10.1007/BF00369863. [DOI] [PubMed] [Google Scholar]
- 319.Yamagata Y, Abe R, Fujita Y, Ichishima E. Molecular cloning and nucleotide sequence of the 90K serine protease gene, hsp K, from Bacillus subtilis(natto) no. 16. Curr Microbiol. 1995;31:340–344. doi: 10.1007/BF00294696. [DOI] [PubMed] [Google Scholar]
- 320.Yamashita I, Hirata D, Machida M, Fukui S. Cloning and expression in Saccharomyces cerevisiae of the secretable acid protease gene from Saccharomycopsis fibuligera. Agric Biol Chem. 1986;50:109–113. [Google Scholar]
- 321.Yamashita T, Tonouchi N, Vozami T, Beppu T. Secretion of mucor rennin, a fungal aspartic protease of Mucor pusillus, by recombinant yeast cells. Mol Gen Genet. 1987;210:462–467. doi: 10.1007/BF00327198. [DOI] [PubMed] [Google Scholar]
- 322.Yanagida N, Vozumi T, Beppu T. Specific excretion of Serratia marcescens protease through the outer membrane of Escherichia coli. J Bacteriol. 1986;166:937–944. doi: 10.1128/jb.166.3.937-944.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Yang M Y, Ferrari E, Henner D J. Cloning of the neutral protease gene of Bacillus subtilisand the use of the cloned gene to create an in vitro-derived deletion mutation. J Bacteriol. 1984;160:15–21. doi: 10.1128/jb.160.1.15-21.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Yang Q, Jiang X, Wu L, Cui Y, Yang S. Cloning of a thermostable neutral protease gene from Bacillus stearothermophilus in Bacillus subtilis. Shengwu Gongcheng Xuebao. 1991;7:207–212. [Google Scholar]
- 325.Yang S, Jin C, Bao D, Zhang S. Cloning and expression of thermostable protease gene from Bacillus stearothermophilus HY-69 in Bacillus subtilis. Ann N Y Acad Sci. 1996;782:219–225. [Google Scholar]
- 326.Yoneda Y, Imamoto-Sonobe N, Matsuoka Y, Iwamoto R, Kiho Y, Uchida T. Antibodies to Asp-Asp-Glu-Asp can inhibit transport of nuclear proteins into the nucleus. Science. 1988;242:275–278. doi: 10.1126/science.3051382. [DOI] [PubMed] [Google Scholar]
- 327.Yoshimoto T, Oyama H, Honda T, Tone H, Takeshita T, Kamiyama T, Tsuru D. Cloning and expression of subtilisin amylosacchariticus gene. J Biochem. 1988;103:1060–1065. doi: 10.1093/oxfordjournals.jbchem.a122380. [DOI] [PubMed] [Google Scholar]
- 328.Yoshimoto T, Oyama H, Takeshita T, Higashi H, Xu S L, Tsura D. Nucleotide sequence of the neutral protease gene from Bacillus subtilis var. amylosacchariticus. J Ferment Bioeng. 1990;70:370–375. [Google Scholar]
- 329.Yoshimoto T, Walter R, Tsuru D. Proline-specific endopeptidase from Flavobacterium: purification and properties. J Biol Chem. 1980;225:4786–4792. [PubMed] [Google Scholar]
- 330.Yoshimura K, Miyazaki T, Nakahama K, Kikuchi M. Cloning and partial nucleotide sequence of a neutral protease gene from Bacillus amyloliquefaciens. Takeda Kenkyusho Ho. 1985;44:42–50. [Google Scholar]
- 331.Young T W, Wadeson A, Glover D J, Quincey R V, Butlin M J, Kamei E A. The extracellular acid protease gene of Yarrowia lipolytica: sequences and pH regulated transcription. Microbiology. 1996;142:2913–2921. doi: 10.1099/13500872-142-10-2913. [DOI] [PubMed] [Google Scholar]
- 332.Young T W, Hosford E A. Proceedings of the 21st Congr. -European Brewers Convention. 1987. Genetic manipulation of Saccharomyces cerevisiae to produce extracellular protease; pp. 521–528. [Google Scholar]
- 333.Yu F, Yamada H, Daishima K, Mizushima S. Nucleotide sequence of the lspA gene, the structural gene for lipoprotein signal peptidase of Escherichia coli. FEBS Lett. 1983;173:264–268. doi: 10.1016/0014-5793(84)81060-1. [DOI] [PubMed] [Google Scholar]
- 334.Zchnbauer B A, Markovitz A. Cloning of gene lon (CapR) in Escherichia coliK-12 and identification of polypeptide specified by the cloned deoxyribonucleic acid fragment. J Bacteriol. 1980;143:852–863. doi: 10.1128/jb.143.2.852-863.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Zen-Yoji H, Terayama T, Benoshi M. Studies on staphylococcal coagulase I. Antigenic difference of coagulase and distribution of the anticoagulase in human sera. Jpn J Microbiol. 1961;5:237–247. doi: 10.1111/j.1348-0421.1961.tb00204.x. [DOI] [PubMed] [Google Scholar]
- 336.Zwickl P, Grziwa A, Puhler G, Dahlmann B, Lottspeich F, Baumeister W. Primary structure of the Thermoplasmaproteasome and its implications for the structure, function and evolution of the multicatalytic proteinase. Biochemistry. 1992;31:964–972. doi: 10.1021/bi00119a004. [DOI] [PubMed] [Google Scholar]
- 337.Zwickl P, Lottspeich F, Dahlmann B, Baumeister W. Cloning and sequencing of the gene encoding the large (α-) subunit of the proteasome from Thermoplasma acidophilum. FEBS Lett. 1991;278:217–221. doi: 10.1016/0014-5793(91)80120-r. [DOI] [PubMed] [Google Scholar]