Abstract
Several Asian natricine snakes of the genus Rhabdophis feed on toads and sequester steroidal cardiac toxins known as bufadienolides (BDs) from them. A recent study revealed that species of the Rhabdophis nuchalis Group ingest lampyrine fireflies to sequester BDs. Although several species of fireflies are distributed in the habitat of the R. nuchalis Group, only lampyrine fireflies, which have BDs, are included in the diet of these snakes. Thus, we hypothesized that the R. nuchalis Group chemically distinguishes fireflies that have BDs from those that do not have BDs. We also predicted that the R. nuchalis Group detects BDs as the chemical cue of toxin source. To test these predictions, we conducted 3 behavioral experiments using Rhabdophis chiwen, which belongs to the R. nuchalis Group. In the first experiment, R. chiwen showed a moderate tongue flicking response to cinobufagin, a compound of BDs. On the other hand, the snake showed a higher response to the chemical stimuli of lampyrine fireflies (BD fireflies) than those of lucioline fireflies (non-BD fireflies). In the second experiment, in which we provided live BD and non-BD fireflies, the snake voluntarily consumed only the former. In the third, a Y-maze experiment, the snake tended to select the chemical trail of BD fireflies more frequently than that of non-BD fireflies. These results demonstrated that R. chiwen discriminates BD fireflies from non-BD fireflies, but the prediction that BDs are involved in this discrimination was not fully supported. To identify the proximate mechanisms of the recognition of novel toxic prey in the R. nuchalis Group, further investigation is necessary.
Keywords: Bufadienolides, chemical preference, fireflies, Rhabdophis, toxin sequestration
Many animals use toxic chemicals to defend themselves against predators. Chemically defended animals either synthesize their defensive toxins by themselves or sequester intact toxins from environmental sources, such as diet (Porto et al. 1972; Daly 1995; González et al. 1999; Opitz and Müller 2009; Savitzky et al. 2012). The latter phenomenon, toxin sequestration, is widespread among invertebrates and has been extensively studied in phytophagous insects, such as leaf beetles and butterflies (Dobler et al. 1996; de Castro et al. 2018). In contrast, examples of toxin sequestration among vertebrates are limited to a relatively small number of lineages, such as poison frogs and Asian natricine snakes of the genus Rhabdophis (Takada et al. 2005; Hutchinson et al. 2007; Saporito et al. 2007; Savitzky et al. 2012).
In animals that sequester toxins from the diet, they usually obtain toxins from their main diet. For example, the majority of phytophagous insects are specialized in, or even monophagous to the host plants used as a toxin source (Petschenka and Agrawal 2016). At least some species of poison frogs are “specialists” in the alkaloid-rich arthropods, such as ants and mites (e.g., Dendrobates pumilio; Donnelly 1991). Contrary to such animals that specialize in a certain toxic food, the toxic source of Rhabdophis does not comprise their main food. For example, Rhabdophis tigrinus , the most well-studied species in Rhabdophis, eats nontoxic frogs as their main food, and only infrequently eats toads for the toxin source (e.g., Fukada 1992; Mori and Vincent 2008).
Rhabdophis is widely distributed in Asia and consists of approximately 30 species (Takeuchi et al. 2018; Boundy 2020; Piao et al. 2020). Several lines of evidence indicate that at least 7 species of Rhabdophis sequester cardiotonic steroids known as bufadienolides (BDs) from toads (Bufonidae) consumed as prey (Hutchinson et al. 2007; Mori et al. 2012; Yoshida et al. 2020). Toads synthesize BDs from cholesterol and store BDs in their skin and parotoid glands (Porto et al. 1972). Rhabdophis stores the sequestered BDs in the nuchal glands, which are located under the dorsal skin of the neck region (Hutchinson et al. 2007; Mori et al. 2012). When a snake is attacked, the glands rupture and the stored toxins are released (Mori et al. 2012). Thus, the nuchal glands are presumed to be used for antipredator defense. Until now, the nuchal glands and similar organs, nucho-dorsal glands which extend the full length of the body, have been reported in 19 species of Rhabdophis (Takeuchi et al. 2018; Piao et al. 2020; Zhu et al. 2020).
Recently, based on comprehensive evidence, Yoshida et al. (2020) revealed that a derived clade of Rhabdophis, the Rhabdophisnuchalis Group (R. nuchalis, Rhabdophispentasupralabialis, and Rhabdophisleonardi), sequesters defensive BDs not from toads but from the larvae of fireflies (subfamily Lampyrinae). Shortly afterward, Piao et al. (2020) described Rhabdophischiwen as a new cryptic species, which was originally referred to as R. pentasupralabialis. Based on the molecular phylogenetic analysis and the locality of R. chiwen described in Piao et al. (2020), it is obvious that R. chiwen was included in the samples that Yoshida et al. (2020) referred to as R. pentasupralabialis. Therefore, it is clear that R. chiwen, as well as R. nuchalis, R. leonardi, and R.pentasupralabialis sensu stricto, feed on lampyrine fireflies and sequester BDs from them.
The limited number of literatures suggests that the diet of R. chiwen consists of earthworms, leeches, and larvae of lampyrine fireflies (Piao et al. 2020; Yoshida et al. 2020). In the habitat of R. chiwen (Sichuan Province), not only lampyrine fireflies but also several other fireflies (e.g., subfamily Luciolinae) are distributed (Fu 2014). Based on the molecular phylogenetic data of extant lampyrid species, Lampyrinae and Luciolinae are closely related (Martin et al. 2017). However, until now, no lucioline fireflies have been found in the stomach contents of R. chiwen. Because the possible difference between lampyrine and lucioline fireflies is the possession of BDs (Eisner et al. 1997; Yoshida et al. 2020; Berger et al. 2021), R. chiwen may utilize only a specific group of fireflies as diet. Generally, snakes use chemical cues to recognize prey as edible food (Arnold 1981; Cadle and Greene 1993). Thus, we assume that R. chiwen chemically distinguishes fireflies that have BDs (“BD fireflies”) from those that do not have BDs (“non-BD fireflies”), and only consumes BD fireflies. If the snake chemically discriminates these fireflies, it is plausible that BDs are chemical cues for the snake to detect toxic prey. To test this possibility, we examined chemical preference of the snake for fireflies. We also investigated the chemical response of the snake toward toads, which are presumed to be the toxic source in ancestral Rhabdophis.
Specifically, we tested the following 3 questions: (1) whether R. chiwen distinguishes firefly larvae that have BDs from those that do not have BDs; (2) whether R. chiwen detects BDs as a cue of edible prey; and (3) whether R. chiwen chemically detects toads. Because the current knowledge of toxicity of Asian fireflies is limited, we first conducted chemical analysis to investigate which species of fireflies possess BDs.
Materials and Methods
Chemical analysis of fireflies
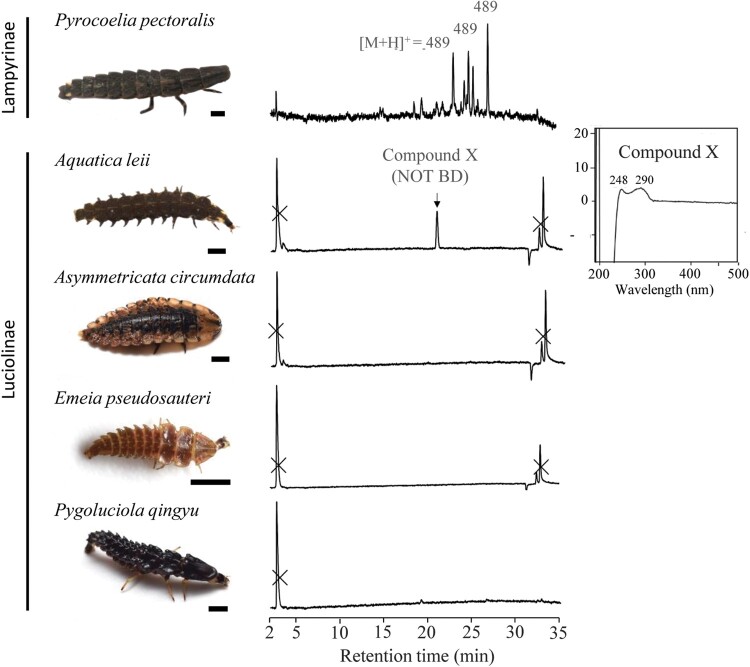
Larvae of a lampyrine firefly Pyrocoelia pectoralis and larvae of 4 species of lucioline fireflies (Aquatica leii, Pygoluciola qingyu, Asymmetricata circumdata, and Emeia pseudosauteri) were chemically analyzed (Table 1). All of these species are sympatric with R. chiwen. Pyrocoelia pectoralis, As. circumdata, and E. pseudosauteri are terrestrial species (Wang et al. 2007; Fu et al. 2012; Fu 2014). Pygoluciola qingyu and Aq. leii are semiaquatic and aquatic species, respectively (Fu et al. 2012; Fu 2014). All larvae of fireflies were obtained from breeding colonies in the laboratory of Leshan Normal University. The species were identified based on the external morphology of adults.
Table 1.
Sample size of each species of fireflies used in the chemical analysis and each behavioral experiment, and the result of chemical analysis (presence of BDs)
| Species | Subfamily | Chemical analysis | Existence of BDs | Feeding test | Y-maze test | Chemical response test |
|---|---|---|---|---|---|---|
| Pyr. pectoralis (Pp) | Lampyrinae | 12 | Present | 13 | 3 | 5 |
| Aq. leii (Al) | Luciolinae | 57 | Absent | 0 | 0 | 5 |
| As. circumdata (Ac) | Luciolinae | 6 | Absent | 10 | 0 | 0 |
| E. pseudosauteri (Ep) | Luciolinae | 13 | Absent | 10 | 0 | 5 |
| Pyg. qingyu (Pq) | Luciolinae | 15 | Absent | 10 | 3 | 5 |
Abbreviations of each firefly are shown in parentheses.
Two to about 20 individuals of firefly larvae were immersed in ∼3 mL of methanol within a glass vial with a Teflon-lined cap and were stored at –20°C in the dark. The total sample size of each firefly species is shown in Table 1. The firefly larvae were removed into a 2 mL screw-cap tube (Watson Co., Ltd., Tokyo, Japan) with a small amount of methanol and 2 stainless steel balls (5 mm in diameter). The samples were then crushed and extracted (3,200 rpm, 1 min) by a bead crusher µT-12 (Tietech Co., Ltd., Saitama, Japan). The crushed solution was centrifuged (6,000 rpm, 5 min), and the supernatant was obtained. Methanol was added again to the pellet, and the operation of crushing and centrifuging was repeated a total of 3 times to collect the supernatant, resulting in a crushed extract of ∼10 mL/sample.
The crushed extract (hereafter ext.) was concentrated to dryness under reduced pressure. The extract was weighed, dissolved in methanol at a concentration of 1 mg ext./mL, and filtered with a syringe filter (DISMIC-13HP, pore diameter, 0.45 µm; Roshi Kaisha Ltd., Tokyo, Japan). Then, 5 µL of digitoxigenin (as an internal standard) methanol solution (0.5 mg/mL) was added to 40 µL of this filtrate (1 mg ext./mL), and 1 µL of this solution was analyzed by liquid chromatography–mass spectrometry (LC–MS).
LC–MS was performed with a prominence high-performance LC system coupled with LCMS-2010 (Shimadzu Co., Kyoto, Japan). A reversed-phase column (Mightysil RP-18 GP 50 × 2.0 mm internal diameter, 5 µm particle size; Kanto Chemical Co., Inc., Tokyo, Japan) was eluted (0.2 mL/min) with a gradient of 20% (0–2 min), 20–55% (2–20 min), 55–100% (20–35 min), and 100% (5 min) methanol in H2O containing 0.1% formic acid. The column temperature was maintained at 40 °C. The MS was manipulated in atmospheric pressure chemical ionization (APCI) positive ion mode with nebulizer gas flow of 2.5 L/min, APCI voltage of 1.9 kV, temperature of 400 °C, curved desolvation line temperature of 250 °C, and heat block temperature of 200 °C. The scan range for m/z values was 350–1,000. BDs were characterized by UV absorption spectroscopy, which showed a maximum absorbance at 290–300 nm by the common moiety of a 6 membered pyrone ring (Green et al. 1985).
Behavioral tests
We collected a total of 20 adult R. chiwen (10 males and 10 females; mean snout–vent length [SVL] = 442 mm) from Xingou Village, Ya’an City, Sichuan, China in June 2018. These snakes were housed individually in transparent plastic cages (360 × 200 × 110 mm) with a paper substrate and a water dish at a temperature between 25 and 28°C. We fed megascolid earthworms to the snakes every day.
The species used as prey subjects were larvae of a lampyrine firefly Pyr. pectoralis, larvae of lucioline fireflies (As. circumdata, Aq. leii, E. pseudosauteri, and Pyg. qingyu), a megascolid earthworm (Amynthas sp.), and a Chinese toad Bufo gargarizans. All these species are sympatric with R. chiwen. The larvae of lampyrine fireflies are considered as a potential prey of R. chiwen based on the recent studies (Piao et al. 2020; Yoshida et al. 2020). Earthworms are the main diet of R. chiwen (Piao et al. 2020) and were used as positive control. All fireflies were obtained from breeding colonies in the laboratory of Leshan Normal University. Earthworms were purchased at pet shops. Toads were collected in the field and were frozen until the behavioral tests.
We conducted 3 behavioral tests: chemical response test, feeding test, and Y-maze test. All tests were conducted in the laboratory of Chengdu Institute of Biology in 2018. The chemical response, feeding, and Y-maze tests were conducted on 11–12 June, 15–22 June, and 20–24 June, respectively. We used the same individuals repeatedly in each behavioral test with at least 3 days intervals between tests (see below for details). Before each test, we stopped feeding for at least 3 days to increase snakes’ feeding motivation. In each test, we recorded the behavior of snakes with a video camera (Nikon D5300) for later analysis.
Chemical response test
Snakes were exposed to 8 types of odors presented on a cotton swab: distilled water, a megascolid earthworm (Amynthas sp.), cinobufagin (a BD), a Chinese toad B. gargarizans, larvae of a lampyrine firefly Pyr. pectoralis, and larvae of 3 species of lucioline fireflies (E. pseudosauteri, Pyg. qingyu, and Aq. leii). We prepared samples from 2 individuals of toads and earthworms and 6 individuals of each firefly species. Toads were kept frozen and were thawed before the test. Immediately before each trial, we collected odors with a cotton swab by rolling it over the external surface of each animal (for details, see Cooper and van Wyk 1994; Takeuchi and Mori 2012; Fukuda and Mori 2021). For toad and earthworm stimuli, each individual was alternately used as a source of odors. For each species of fireflies, we divided the 6 individuals equally into 2 groups, and each group was used alternately as a source of odors. For preparation for water control, we dipped the cotton swab into the vial filled with distilled water. Cinobufagin is a purified nonvolatile odorant and is one of the BDs contained in the skin secretions of toads (e.g., B. gargarizans) (Shimada et al. 1985; Qi et al. 2011). We purchased the reagent of cinobufagin from Wako Pure Chemical Corporation. Cinobufagin solution (MeOH) was prepared at the concentration of 1.0 mg/mL. This concentration was selected as a standard value of chemicals obtained from a toad, based on LC–MS analysis of the cotton swab that was rolled over the external surface of Bufojaponicus and then dipped into methanol (see Fukuda and Mori 2021). In case of the presentation of cinobufagin to snakes, we put the cotton swab into the solution and then dried the swab to ensure that the solvent evaporated so as to avoid a snake’s behavioral reaction toward the solvent.
Nineteen adult R. chiwen (10 males and 9 females; mean SVL = 458 mm) were used in the chemical response test. All arenas were visually isolated from each other by cardboard. We performed the tests from 10:00 to 18:00 h at the temperature between 24 and 26 °C. We tested each snake once for each chemical stimulus. The procedure of the experiment is based on Burghardt (1970) and Cooper (1998). Each snake was removed from its home cage, introduced into a transparent plastic arena (360 × 200 × 100 mm) covered with an opaque plastic board, and left undisturbed for acclimation for 12 h before the experiment. In the trial, we removed the ceiling and presented a swab 1 cm from the snout of a snake for 60 s and recorded the number of tongue flicks and strikes (bites) toward the swab. Each stimulus was presented in a random order. We maintained an interval of >15 min between the presentations of each stimulus. If a snake did not exhibit any tongue flick for 30 s, we gently touched the snake with the tip of the cotton swab. We scored 0 points if snakes did not show any tongue flicks for another 30 s (in total 60 s). We considered that snakes have a greater preference for stimuli when snakes attempted to strike or bite the cotton swab. Thus, we applied the tongue-flick attack score (TFAS) developed by Burghardt (1970) and Cooper and Burghardt (1990). The TFAS was calculated as:
where TFmax is the maximum number of tongue flicks emitted by any snake in any of the trials, test duration is 60 s, and attack latency is the latency from first tongue flick to strike or bite, in seconds. If a snake escaped from the cage, we stopped the trial and retested with the same stimulus after 15 min.
The effects of chemical stimulus on TFAS were examined using the Friedman test, followed by pairwise multiple comparisons using a Wilcoxon signed-rank test. In multiple comparisons, we did not use Bonferroni correction because of the conservative nature of this correction (Perneger 1998; Moran 2003; Nakagawa 2004). Instead, we showed the results with the levels of P < 0.01, P < 0.005, and P < 0.001 for multiple comparisons. All statistical analyses were conducted using R version 3.4.2.
Feeding test
We used 18 adult R. chiwen (9 males and 9 females; mean SVL = 457 mm) for the feeding test of Pyr. pectoralis, Pyg. qingyu, and As. circumdata and 10 adult R. chiwen (5 males and 5 females; mean SVL = 449 mm) for the feeding test of E. pseudosauteri. The experimental arena was a round steel box (52 cm in diameter, 50 cm in depth) with a round plastic board (same diameter as the steel strainer) as a substrate. A glass dish (11 × 11 × 4 cm) with a sheet of laboratory paper in it (Kimwipe, Kimberly Clark; folded to 10 × 10 cm) was set at the rim of a round box. We performed the tests from 12:00 to 18:00 h at a temperature between 25 and 26 °C. We tested each snake once for each prey species in a random order, and we maintained an interval of >24 h between the presentations of each prey animal to the same individual. Prior to the trial, a larva of a firefly was gently placed onto the wet laboratory paper in the dish. At the beginning of each trial, a snake was gently introduced to the center of the box and kept undisturbed for 20 min. If the snake fed on the firefly within 20 min, we stopped the test and returned the snake to its home cage. If a snake did not feed on the firefly in 20 min, we removed the firefly, introduced an earthworm, and left the snake undisturbed for another 20 min. If the snake fed on the earthworm within 20 min, we considered that the snake had a feeding motivation. If the snake did not feed on the earthworm within 20 min, we considered that the snake did not have a feeding motivation, discarded the trial, and conducted the trial again with the same individual ∼2 days later. After each trial, we cleaned the substrate to remove all odor cues.
Y-maze test
The maze was constructed with pieces of wood and consisted of a base arm (length × wide × height: 41 × 9 × 10 cm) and 2 diverging arms (40 × 9 × 10 cm) connected to the base arm at a 40° angle. A box (20 × 28 × 16 cm) was attached at the end of the base arm and at the end of each diverging arm. We conducted 2 tests: water–water trails (control test) and Pyrocoelia–Pygoluciola trails (BD vs. non-BD test). In the control test, we prepared a piece of Kimwipe that was dampened with distilled water and folded to 20 × 1 cm. In the BD versus non-BD test, we prepared chemical cues of larvae of a lampyrine firefly Pyr. pectoralis and a larvae of lucioline firefly Pyg. qingyu by gently scrubbing their bodies with a piece of Kimwipe (folded to 20 × 1 cm and dampened with distilled water) at a standardized pressure. We made trails by placing 4 pieces of the treated paper on the substrate of the craft paper, extending continuously from the beginning of the base arm to the ends of the divergent arms. Each paper treated with the chemical stimuli of fireflies was put separately on each divergent arm (e.g., stimulus A on the right arm and stimulus B on the left arm, or in the reverse side). On the base arm, the papers from the 2 stimuli were placed side by side, on the same side each as the corresponding divergent arm (Kojima and Mori 2015). Assignment of the larvae of fireflies to the right and left arms was balanced throughout the trials.
We used 12 adult R. chiwen (5 males and 7 females; mean SVL = 483 mm) for the control test and 11 adult R. chiwen (5 males and 6 females; mean SVL = 497 mm) for the BD versus non-BD test. We used each snake once for each test. We conducted the control test first, and then conducted the BD versus non-BD test ∼2 days later. The trials were conducted between 12:00 and 18:00 h. We placed a snake in the starting box, which was partitioned from the maze by a removable plastic board. After 20 min acclimation, we removed the partition. The snake typically proceeded from the base arm into the end box on the left or right arm, while emitting tongue flicks frequently. After each trial, we removed all paper strips and craft paper substrates. Snakes that did not choose either end box within 1 h were returned to their home cage and were tested again ∼2 days later. A video camera was set above the arena to record behaviors of the snake. The video records were analyzed to quantify behaviors of snakes: arm choice, tracing time, and tongue-flick rate (TFR; tongue-flicks per minute). Arm choice was determined when snake’s snout entered one of the end boxes. Tracing time was measured from when a snake exited the starting box to when snake’s snout entered one of the end boxes. TFR was calculated by the total number of tongue flicks divided by the tracing time in minutes. The effects of chemical stimulus on the trail choice were examined using binomial test.
Results
Chemical analysis of fireflies
BDs were detected in Pyr. pectoralis, but were not detected in any of the 4 species of lucioline fireflies (Figure 1). The UV absorption spectra of Compound X detected in Aq. leii show absorption maxima at 248 and 290 nm, and the expected m/z of protonated ions ([M + H]+) of Compound X is less than 300 or greater than 1,000. Because all BDs hitherto reported from animals have a molecular weight between 350 and 1,000, we concluded that compound X is not a BD.
Figure 1.
Chromatograms of Pyr. pectoralis (Lampyrinae) and 4 species of lucioline fireflies were detected at 300 nm UV in LC–MS analysis. The peaks for which MS spectra were available are shown as the m/z of the predicted protonated ion ([M + H]+). Insert: UV absorption spectra of Compound X detected in Aq. leii. Bold bars under each photograph represent 2 mm scale.
Chemical response test
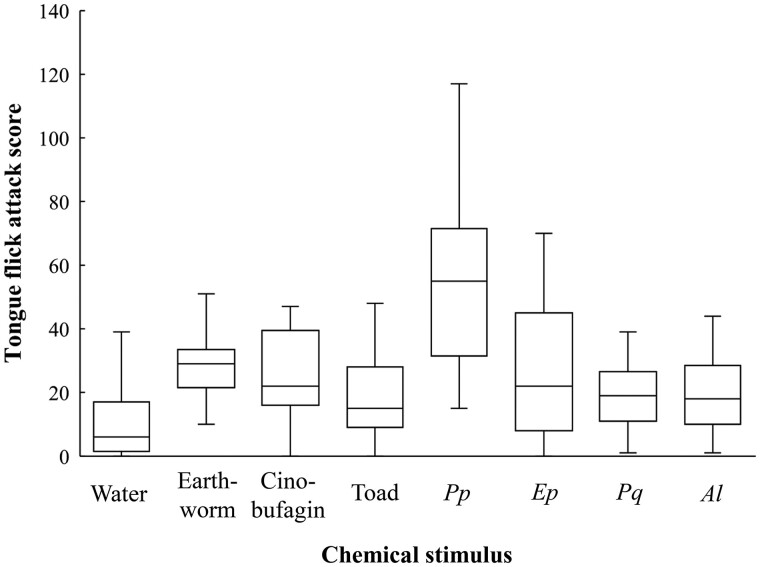
There were significant effects of chemical stimulus on TFAS (Friedman test, χ2 = 49.726, P < 0.0001; Figure 2). Snakes showed the highest score toward Pyr. pectoralis, following earthworms. Bites to the cotton swab were observed only in the stimulus of Pyr. pectoralis (3 of the 19 individuals). Multiple comparisons showed that TFAS to Pyr. pectoralis was higher than that to water in P < 0.001 level. TFAS to Pyr. pectoralis was significantly higher than that to cinobufagin, toads, and the 3 species of lucioline fireflies in P < 0.005 level and that to earthworms in P < 0.01 level (Figure 2 and Table 2). TFAS to earthworms was significantly higher than that to water in P < 0.001 level, and that to toads and to Pyg. qingyu in P < 0.01 level. TFAS to cinobufagin and the 3 species of lucioline fireflies was significantly higher than that to water in P < 0.01 level. There was no significant difference between toads and water.
Figure 2.
TFAS in the chemical prey preference test of R. chiwen. Interval between 25% and 75% quartiles is represented by boxes, and range is represented by whiskers. Median is represented by the middle horizontal line in the box plot. Pp, Ep, Pq, and Al in the chemical stimulus represents fireflies. See Tables 1 and 2 for the abbreviations of firefly species and the result of statistical comparisons, respectively.
Table 2.
Comparisons of TFAS in R. chiwen for each pair of 8 stimuli
| Stimulus | Water | Earthworm | Cinobufagin | Toad | Pyr. pectoralis | E. pseudosauteri | Pyg. qingyu |
|---|---|---|---|---|---|---|---|
| Earthworm | 0.0002*** | — | — | — | — | — | — |
| Cinobufagin | 0.0053* | 0.1416 | — | — | — | — | — |
| Toad | 0.0606 | 0.0079* | 0.1215 | — | — | — | — |
| Pyr. pectoralis | 0.0001*** | 0.0074* | 0.0027** | 0.0005*** | — | — | — |
| E. pseudosauteri | 0.0037** | 0.4206 | 0.6163 | 0.0584 | 0.0027** | — | — |
| Pyg. qingyu | 0.0074* | 0.0040** | 0.1362 | 0.7602 | 0.0003*** | 0.0670 | — |
| Aq. Leii | 0.0065* | 0.0400 | 0.7763 | 0.5859 | 0.0008*** | 0.2509 | 0.8276 |
P-values obtained by Wilcoxon signed-rank tests are shown. See Table 1 for the abbreviation of fireflies.
P < 0.01,
P < 0.005,
P < 0.001.
Feeding test
Thirteen out of the 18 R. chiwen consumed Pyr. pectoralis. The other 5 individuals did not eat Pyr. pectoralis, but they consumed earthworms immediately after the trial. None of R. chiwen consumed lucioline fireflies, but 16 of the 18, 16 of the 18, and 9 of the 10 individuals consumed earthworms after the trials of As. circumdata, Pyg. qingyu, and E. pseudosauteri, respectively.
Y-maze test
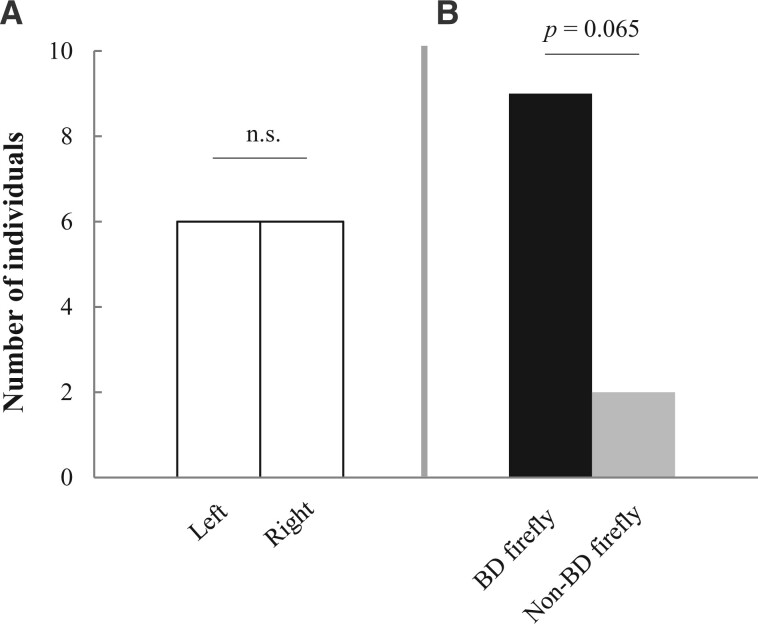
In all experiments, snakes frequently exhibited tongue flicks while moving in the maze (mean TFR ± standard deviation = 52.66 ± 16.46 in the control test and 70.81 ± 22.68 in the BD versus non-BD test). In the control test, R. chiwen showed no preference for 1 arm over the other (binomial test, P = 1.00; Figure 3A), with the same number of snakes choosing the right or left arm. In the BD versus non-BD test, 9 of the 11 individuals followed the trail of Pyr. pectoralis although this bias fell short of statistical significance (binomial test, P = 0.065; Figure 3B).
Figure 3.
Results of Y-maze test in R. chiwen. The number of snakes that followed each trail is shown. (A) Control test. (B) BD versus non-BD test. BD firefly: Pyr. pectoralis, non-BD firefly: Pyg. qingyu. ns, not significant.
Discussion
All behavioral experiments revealed that R. chiwen has a strong preference for Pyr. pectoralis (hereafter referred to as BD firefly): snakes voluntarily consumed BD fireflies and showed significantly higher TFAS to them than any other stimuli, including lucioline fireflies (hereafter referred to as non-BD fireflies). Cooper (1998) considered that significant differences in TFAS between stimuli A and B indicated that snakes “discriminate” the stimulus A from B. Thus, our results indicate that R. chiwen discriminates BD fireflies from non-BD fireflies by their odors.
In this study, we used only 1 species, Pyr. pectoralis, as BD fireflies in the behavioral tests. It is known that several species of lampyrine fireflies, such as Diaphanes, Ellychinia, Photinus, and Lampyris, as well as Pyrocoelia, possess BDs in their body (Tyler et al. 2008; Yoshida et al. 2020; Berger et al. 2021). Although studies on the natural diet of R. chiwen are quite limited, Yoshida et al. (2020) recovered larvae of Diaphanes sp. from the stomach contents of R. chiwen. This observation, along with our finding, supports the presumption that R. chiwen selectively eats BD fireflies. To confirm its selective consumption of BD fireflies, we need to conduct behavioral experiments using other genera of lampyrine fireflies.
Rhabdophis chiwen showed significantly lower chemical preference for lucioline fireflies than for its natural diet (lampyrine fireflies and earthworms), and no individuals fed on lucioline fireflies. One possible reason for the lower preference is that lucioline fireflies possess deterrents or repellents other than BDs. Generally, many species of lucioline fireflies are known to be distasteful and possess chemical substances used as repellents (Day 2011). For example, Aq. leii secretes 2 types of terpenoides, that is, terpinolene and γ-terpinene (Fu et al. 2007), which are well known as toxic, deterrent, or repellant agents in defensive secretions of many invertebrates (e.g., termite soldiers: Moore 1968; stink bugs: Aldrich 1988; Krall et al. 1997). Thus, it is possible that lucioline fireflies have some chemical substances that work as deterrent or repellent agents for predators including snakes, and these substances may lower the feeding and chemical responses of R. chiwen.
Even so, R. chiwen showed significantly higher TFAS to non-BD fireflies than to water. Cooper (1998) considered that higher TFAS to stimuli than to a control indicates that snakes “detect” the stimuli. Thus, our results indicate that R. chiwen detected the chemicals of non-BD fireflies. A possible reason that may enable the detection of non-BD fireflies is that a chemical similarity may exist among lampyrid fireflies (Table 3). Because of such a similarity, the snakes may have shown a higher reaction to non-BD fireflies than to water. A precedent for this is the finding that hatchling Elaphequadrivirgata, a generalist snake that feeds on a variety of anuran species, showed a bite response to chemical cues from Glandirana rugosa (Mori 1989), which adult E. quadrivirgata refuses to eat because of the existence of unpalatable skin secretions (Yoshimura and Kasuya 2013). Our study, as well as that of Mori (1989), suggests that snakes show a moderate tongue flick response even to unpalatable prey if a chemical similarity with related palatable prey species exists.
Table 3.
Occurrence of possible chemical cues that may be involved in the active response of R. chiwen to the stimuli used in the chemical test
| Chemical cue | Stimulus |
|||
|---|---|---|---|---|
| Purified BD (Moderate) | Toad (Weak) | BD firefly (High) | Non-BD firefly (Moderate) | |
| Firefly-type BDs | – | – | + | – |
| Multiple compounds of BDs | – | + | + | – |
| Sufficient amount of BDs | + | – | + | – |
| Chemical substances other than BDs that are common to lampyrid fireflies | – | – | + | – |
The response of the snake toward each stimulus is shown in parentheses. +: absent, –: present.
Our prediction that R. chiwen recognizes BDs as a cue of toxic prey was not fully supported: R. chiwen showed only a medium preference for cinobufagin. One possibility that may account for the lower response to cinobufagin is the structural difference between toad-derived and firefly-derived BDs (Table 3). Cinobufagin is a BD found in the skin of several species of toads, including B. gargarizans (Qi et al. 2011). It has been revealed that the chemical component of BDs extracted from toads and lampyrine fireflies are different in acetylated place, the structure of A-B ring system (trans-fused ring unique to fireflies), and the compound of a side chain at the C-3 position (Steyn and van Heerden 1998; Nogawa et al. 2001; Hutchinson et al. 2007; Yoshida et al. 2020). In this study, we used toad-derived BD in the chemical response test because we considered that the ancient R. nuchalis Group might react to the chemical component from toads, which is the ancestral toxin source in this group. From our results, however, it is likely that R. chiwen, which sequesters BDs from fireflies, would have lost the reactivity to the toad-derived BDs. In the future study, it would be important to examine whether R. chiwen reacts to BDs purified from lampyrine fireflies.
Another possibility for the lower response to cinobufagin is that a single compound of BDs may not be sufficient to elicit the response of snakes (Table 3). It has been reported that an individual toad or lampyrine firefly possesses multiple compounds of BDs (Hutchinson et al. 2007; Qi et al. 2011; Yoshida et al. 2020; Berger et al. 2021). When R. chiwen encounters a lampyrine firefly in the wild, the chemical cues that the snake recognizes would not be a single type of BDs but the mixture of several BDs, or a mixture of BD and other chemical substances of the prey. A precedent for this is the finding that Zodarion rubidum, a specialized ant-eating zodariid spider, responds well to a mixture of 2 compounds (undecane and decyl acetate), but does not respond to each of the single compound (Cárdenas et al. 2012).
In spite of the medium preference for cinobufagin, R. chiwen did not show any chemical preference for B. gargarizans, which possesses cinobufagin in its skin secretion (Qi et al. 2011). One possible reason for this contradiction is that because we used frozen and thawed toads as the source of the chemical stimulus, enough amount of cinobufagin may not have been secreted on the skin of the toad (Table 3). Toads store BDs in the concentrated granular skin glands and paired parotoid glands (Porto et al. 1972; Cannon and Hostetler 1976). BDs are secreted from these glands to the surface of the skin only when a toad is disturbed (Hutchinson and Savitzky 2004; Barbosa et al. 2009). Because we used dead specimens to prepare the chemical stimulus, we did not observe any apparent fluid secreted on the skin surface of the toads. Thus, we may not have collected a sufficient amount of BDs to induce the natural response of the snake. It is also possible that the lack of firefly-type BDs, particularly those with trans-fused A-B rings, would be reflected in the lower response of the snake toward toads (Table 3). As mentioned above, the chemical component of BDs extracted from toads and lampyrine fireflies are different in acetylation and in the structure of A-B ring system. Rhabdophis chiwen, which relies on fireflies as the toxin source, may have high reactivity only to firefly-type BDs.
Our ultimate goal is to clarify the factors that have induced the ancient species of the R. nuchalis Group to exploit larvae of lampyrine fireflies as the toxin source. In this study, we hypothesized that R. chiwen chemically distinguishes BD fireflies from non-BD fireflies. Our behavioral tests supported this hypothesis. We also predicted that extant R. chiwen detects BDs as the chemical cue of toxin source. However, our results did not fully support this prediction: R. chiwen showed only a medium chemical preference for a single BD compound (cinobufagin), but showed a strong preference for BD fireflies. Thus, we presume that the possible chemical cues that may be involved in the active response of R. chiwen would be multiple compounds of BDs. It is also possible that the ancestral species of the R. nuchalis Group recognized chemical substances other than BDs that are common to toads and lampyrine fireflies, and the presence of those common substances may have facilitated the new exploitation of lampyrine fireflies as the toxin source. In this case, as implied by the low response to the toad stimulus, R. chiwen, which relies on fireflies as the toxin source, would subsequently have lost the response to such chemical substances. Another possibility is that the firefly-eating snakes have evolved preference to some other surface chemicals that are not present in toads. However, we think this possibility is unlikely considering that toads and fireflies are the only animals that are presently known or suspected to possess BDs (Yoshida et al. 2020), and thus the chance of a coincidental shift between them would be extremely low. Future studies of the chemical response of species in the R. nuchalis Group to multiple BDs and investigation of chemical substances other than BDs that may be common to toads and lampyrine fireflies are necessary.
Acknowledgments
We are grateful to 3 anonymous reviewers for a number of valuable comments on the manuscript, and to G. M. Burghardt and A. H. Savitzky for a number of valuable comments on the experiment. We also would like to thank K. Eto for helping with identifying fireflies. The experiment was conducted under the permission of the Animal Care and Use Committee of Chengdu Institute of Biology, Chinese Academy of Sciences (CIBDWLL2021022).
Funding
This study was supported in part by Japan Society for the Promotion of Science Grants-in-Aid for Scientific Research (17H03719, 18KK0205, and 21H02551).
Authors’ Contributions
M.F. and A.M. designed research. M.F., Q.C., C.C., and L.D. collected and prepared animals. M.F. and Q.C. conducted the behavioral experiments. R.U. and N.M. conducted the chemical analysis. M.F. and T.I. wrote the paper. A.M., C.C., N.M., and Q.C. reviewed and edited the paper. A.M. supervised the research.
Conflicts of Interest
The authors declare no conflict of interest.
Contributor Information
Masaya Fukuda, Department of Zoology, Graduate School of Science, Kyoto University, Kyoto 606-8502, Japan.
Rinako Ujiie, Department of Applied Life Science, Graduate School of Agriculture, Kyoto University, Kyoto 606-8502, Japan.
Takato Inoue, Department of Applied Life Science, Graduate School of Agriculture, Kyoto University, Kyoto 606-8502, Japan.
Qin Chen, Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu, Sichuan 610041, China.
Chengquan Cao, College of Life Sciences, Leshan Normal University, Leshan, Sichuan 614000, China.
Li Ding, Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu, Sichuan 610041, China.
Naoki Mori, Department of Applied Life Science, Graduate School of Agriculture, Kyoto University, Kyoto 606-8502, Japan.
Akira Mori, Department of Zoology, Graduate School of Science, Kyoto University, Kyoto 606-8502, Japan.
References
- Aldrich JR, 1988. Chemical ecology of the Heteroptera. Annu Rev Entomol 33:211–238. [Google Scholar]
- Arnold SJ, 1981. Behavioral variation in natural populations. I. Phynotypic, genetic and environmental correlations between chemoreceptive responses to prey in the garter snake Thamnophis elegans. Evolution 35:489–509. [DOI] [PubMed] [Google Scholar]
- Barbosa CM, Medeiros MS, Riani Costa CCM, Camplesi AC, Sakate M, 2009. Toad poisoning in three dogs: case reports. J Venom Anim Toxins Incl Trop Dis 15:789–798. [Google Scholar]
- Berger A, Petschenka G, Degenkolb T, Geisthardt M, Vilcinskas A, 2021. Insect collections as an untapped source of bioactive compounds: fireflies (Coleoptera: Lampyridae) and cardiotonic steroids as a proof of concept. Insects 12:689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boundy J, 2020. Snakes of the World: A Supplement. Boca Raton (FL: ): CRC Press. [Google Scholar]
- Burghardt GM, 1970. Intraspecific geographical variation in chemical food cue preferences of newborn garter snakes Thamnophis sirtalis. Behaviour 36: 246–257. [DOI] [PubMed] [Google Scholar]
- Cadle JE, Greene HW, 1993. Phylogenetic patterns, biogeography, and the ecological structure of Neotropical snake assemblages. In: Ricklefs RE, Schluter D, editors. Species Diversity in Ecological Communities: Historical and Geographical Perspectives .Chicago (IL: ): University of Chicago Press, 281–293. [Google Scholar]
- Cárdenas M, Jiroš P, Pekár S, 2012. Selective olfactory attention of a specialised predator to intraspecific chemical signals of its prey. Naturwissenschaften 99:597–605. [DOI] [PubMed] [Google Scholar]
- Cannon MS, Hostetler JR, 1976. The anatomy of the parotoid gland in Bufonidae with some histochemical findings. II. Bufo alvarius. J Morphol 148:137–159. [DOI] [PubMed] [Google Scholar]
- Cooper WE, 1998. Evaluation of swab and related tests as a bioassay for assessing responses by squamate reptiles to chemical stimuli. J Chem Ecol 24:841–866. [Google Scholar]
- Cooper WE, Burghardt GM, 1990. A comparative analysis of scoring methods for chemical discrimination of prey by squamate reptiles. J Chem Ecol 16:45–65. [DOI] [PubMed] [Google Scholar]
- Cooper Jr. WE, van , WykJH, 1994. Absence of prey chemical discrimination by tongue-flicking in an ambush-foraging lizard having activity foraging ancestors. Ethology 97: 317–328. [Google Scholar]
- Daly JW, 1995. The chemistry of poisons in amphibian skin. Proc Natl Acad Sci USA 92:9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JC, 2011. Parasites, predators and defence of fireflies and glow-worms. Lampyrid 1:70–102. [Google Scholar]
- de Castro ÉCP, Zagrobelny M, Cardoso MZ, Bak S, 2018. The arms race between heliconiine butterflies and Passiflora plants: new insights on an ancient subject. Biol Rev 93:555–573. [DOI] [PubMed] [Google Scholar]
- Dobler S, Mardulyn P, Pasteels JM, Rowell-Rahier M, 1996. Host-plant switches and the evolution of chemical defense and life history in the leaf beetle genus Oreina. Evolution 50:2373–2386. [DOI] [PubMed] [Google Scholar]
- Donnelly MA, 1991. Feeding patterns of the strawberry poison frog, Dendrobates pumilio (Anura: Dendrobatidae). Copeia 1991: 723–730. [Google Scholar]
- Eisner T, Goetz MA, Hill DE, Smedley SR, Meinwald J, 1997. Firefly “femmes fatales” acquire defensive steroids (lucibufagins) from their firefly prey. Proc Natl Acad Sci USA 94:9723–9728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, 2014. Ecological Illustrated Handbook of Fireflies in China. Beijing, China: The Commercial Press. [Google Scholar]
- Fu X, Ballantyne L, Lambkin C, 2012. The external larval morphology of aquatic and terrestrial Luciolinae fireflies (Coleoptera: Lampyridae). Zootaxa 3405:1–34. [Google Scholar]
- Fu X, Vencl FV, Ohba N, Meyer-Rochow VB, Lei C, Zhang Z, 2007. Structure and function of the eversible glands of the aquatic firefly Luciola leii (Coleoptera: Lampyridae). Chemoecology 17:117–124. [Google Scholar]
- Fukada H, 1992. Snake Life History in Kyoto. Tokyo, Japan: Impact Shuppankai. [Google Scholar]
- Fukuda M, Mori A, 2021. Does an Asian natricine snake Rhabdophis tigrinus have chemical preference for a skin toxin of toads? Curr Herpetol 40:1–9. [Google Scholar]
- González A, Schroeder FC, Attygalle AB, Svatoš A, Meinwald J, Eisner T, 1999. Metabolic transformations of acquired lucibufagins by firefly “femmes fatales.” Chemoecol 9:105–112. [Google Scholar]
- Green B, Crane RI, Khaidem IS, Leighton RS, Newaz SS, Smyser TE, 1985. Synthesis of steroidal 16, 17-fused unsaturated δ–lactones. J Org Chem 50:640–644. [Google Scholar]
- Hutchinson DA, Mori A, Savitzky AH, Burghardt GM, Wu X. et al. 2007. Dietary sequestration of defensive steroids in nuchal glands of the Asian snake Rhabdophis tigrinus. Proc Natl Acad Sci USA 104:2265–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson DA, Savitzky AH, 2004. Vasculature of the parotoid glands of four species of toads (Bufonidae: Bufo). J Morphol 260:247–254. [DOI] [PubMed] [Google Scholar]
- Kojima Y, Mori A, 2015. Active foraging for toxic prey during gestation in a snake with maternal provisioning of sequestered chemical defences. Proc R Soc London B Biol Sci 282:20142137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krall BS, Zilkowski BW, Kight SL, Bartelt RJ, Whitman DW, 1997. Chemistry and defensive efficacy of secretion of burrowing bug Sehirus cinctus cinctus. J Chem Ecol 23:1951–1962. [Google Scholar]
- Martin GJ, Branham MA, Whiting MF, Bybee SM, 2017. Total evidence phylogeny and the evolution of adult bioluminescence in fireflies (Coleoptera: Lampyridae). Mol Phylogenet Evol 107:564–575. [DOI] [PubMed] [Google Scholar]
- Moore BP, 1968. Studies on the chemical composition and function of the cephalic gland secretion in Australian termites. J Insect Physiol 14:33–39. [Google Scholar]
- Moran MD, 2003. Arguments for rejecting the sequential Bonferroni in ecological studies. Oikos 100:403–405. [Google Scholar]
- Mori A, 1989. Behavioral responses to an unpalatable prey Rana rugosa (Anura: Amphibia) by newborn Japanese striped snakes Elaphe quadrivirgata. In: Matsui M, Hikida T, Goris RC, editors. Current Herpetology in East Asia. Kyoto, Japan: Herpetological Society of Japan, 459–471. [Google Scholar]
- Mori A, Burghardt GM, Savitzky AH, Roberts KA, Hutchinson DA. et al. , 2012. Nuchal glands: a novel defensive system in snakes. Chemoecology 22:187–198. [Google Scholar]
- Mori A, Vincent SE, 2008. An integrative approach to specialization: Relationships among feeding morphology, mechanics, behaviour, performance and diet in two syntopic snakes. J Zool 275:47–56. [Google Scholar]
- Nakagawa S, 2004. A farewell to Bonferroni: the problems of low statistical power and publication bias. Behav Ecol 15:1044–1045. [Google Scholar]
- Nogawa T, Kamano Y, Yamashita A, Pettit GR, 2001. Isolation and structure of five new cancer cell growth inhibitory bufadienolides from the Chinese traditional drug Ch’an Su. J Nat Prod 64:1148–1152. [DOI] [PubMed] [Google Scholar]
- Opitz SEW, Müller C, 2009. Plant chemistry and insect sequestration. Chemoecology 19:117–154. [Google Scholar]
- Perneger TV, 1998. What’s wrong with Bonferroni adjustments. BMJ 316:1236–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petschenka G, Agrawal AA, 2016. How herbivores coopt plant defenses: natural selection, specialization, and sequestration. Curr Opin Insect Sci 14:17–24. [DOI] [PubMed] [Google Scholar]
- Piao Y, Chen Z, Wu Y, Shi S, Takeuchi H, et al. 2020. A new species of the genus Rhabdophis fitzinger, 1843 (Squamata: Colubridae) in southwestern Sichuan, China. Asian Herpetol Res 11:95–107. [Google Scholar]
- Porto AM, Baralle FE, Gros EG, 1972. Biosynthesis of bufadienolides in toads. III: Experiments with [2-14C]mevalonic acid, [20-14C]3β-hydroxy-5-pregnen-20-one and [20-14C]cholesterol. J Steroid Biochem 3:11–17. [DOI] [PubMed] [Google Scholar]
- Qi F, Li A, Inagaki Y, Kokudo N, Tamura S. et al. , 2011. Antitumor activity of extracts and compounds from the skin of the toad Bufo bufo gargarizans Cantor. Int Immunopharmacol 11:342–349. [DOI] [PubMed] [Google Scholar]
- Saporito RA, Donnelly MA, Jain P, Martin Garraffo H, Spande TF. et al. , 2007. Spatial and temporal patterns of alkaloid variation in the poison frog Oophaga pumilio in Costa Rica and Panama over 30 years. Toxicon 50:757–778. [DOI] [PubMed] [Google Scholar]
- Savitzky AH, Mori A, Hutchinson DA, Saporito RA, Burghardt GM. et al. , 2012. Sequestered defensive toxins in tetrapod vertebrates: Principles, patterns, and prospects for future studies. Chemoecol 22:141–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K, Ro JS, Ohishi K, Nambara T, 1985. Isolation and characterization of cinobufagin 3-Glutaroyl-L-arginine ester from Bufo bufo gargarizans Cantor. Chem Pharmaceut Bull 33:2767–2771. [Google Scholar]
- Steyn PS, van Heerden FR, 1998. Bufadienolides of plant and animal origin. Nat Prod Rep 15:397–413. [DOI] [PubMed] [Google Scholar]
- Takada W, Sakata T, Shimano S, Enami Y, Mori N. et al. , 2005. Scheloribatid mites as the source of pumiliotoxins in dendrobatid frogs. J Chem Ecol 31:2403–2415. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Mori A, 2012. Antipredator displays and prey chemical preference of an Asian natricine snake Macropisthodon rudis (Squamata: Colubridae). Curr Herpetol 31:47–53. [Google Scholar]
- Takeuchi H, Savitzky AH, Ding L, de Silva A, Das I. et al. , 2018. Evolution of nuchal glands, unusual defensive organs of Asian natricine snakes (Serpentes: Colubridae), inferred from a molecular phylogeny. Ecol Evol 8:10219–10232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler J, Mckinnon W, Lord GA, Hilton PJ, 2008. A defensive steroidal pyrone in the glow-worm Lampyris noctiluca L. (Coleoptera; Lampyridae). Physiol Entomol 33:167–170. [Google Scholar]
- Wang Y, Fu X, Lei C, Jeng ML, Ohba N, 2007. Biological characteristics of the terrestrial firefly Pyrocoelia pectoralis (Coleoptera: Lampyridae). Coleopt Bull 61:85–93. [Google Scholar]
- Yoshida T, Ujiie R, Savitzky AH, Jono T, Inoue T. et al. , 2020. Dramatic dietary shift maintains sequestered toxins in chemically defended snakes. Proc Natl Acad Sci USA 117:5964–5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura Y, Kasuya E, 2013. Odorous and non-fatal skin secretion of adult wrinkled frog Rana rugosa is effective in avoiding predation by snakes. PLoS ONE 8:e81280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu GX, Yang S, Savitzky AH, Zhang L, Cheng Y. et al. , 2020. The nucho-dorsal glands of Rhabdophis guangdongensis (Squamata: Colubridae: Natricinae), with notes on morphological varitaion and phylogeny based on additional specimens. Curr Herpetol 39:108–119. [Google Scholar]