Abstract
Anterior cruciate ligament (ACL) injuries are physically and emotionally debilitating for athletes, while motor and biomechanical deficits that contribute to ACL injury have been identified, limited knowledge about the relationship between the central nervous system (CNS) and biomechanical patterns of motion has impeded approaches to optimize ACL injury risk reduction strategies. In the current study it was hypothesized that high-risk athletes would exhibit altered temporal dynamics in their resting state electrocortical activity when compared to low-risk athletes. Thirty-eight female athletes performed a drop vertical jump (DVJ) to assess their biomechanical risk factors related to an ACL injury. The athletes' electrocortical activity was also recorded during resting state in the same visit as the DVJ assessment. Athletes were divided into low- and high-risk groups based on their performance of the DVJ. Recurrence quantification analysis was used to quantify the temporal dynamics of two frequency bands previously shown to relate to sensorimotor and attentional control. Results revealed that high-risk participants showed more deterministic electrocortical behavior than the low-risk group in the frontal theta and central/parietal alpha-2 frequency bands. The more deterministic resting state electrocortical dynamics for the high-risk group may reflect maladaptive neural behavior—excessively stable deterministic patterning that makes transitioning among functional task-specific networks more difficult—related to attentional control and sensorimotor processing neural regions.
Keywords: anterior cruciate ligament, drop vertical jump, electrocortical dynamics, electroencephalogram, recurrence quantification analysis
1 ∣. INTRODUCTION
Anterior cruciate ligament (ACL) injuries are physically and mentally debilitating. Athletes suffer a variety of short-term consequences from ACL injury—typically experiencing significant pain (Tripp, Stanish, Coady, & Reardon, 2004), depression (Garcia et al., 2016), lost athletic identity (Brewer, Cornelius, Stephan, & Van Raalte, 2010), and lower academic performance (Freedman, Glasgow, Glasgow, & Bernstein, 1998). There are also long-term consequences, such as increased likelihood of osteoarthritis (OA) and chronic pain (Deacon, Bennell, Kiss, Crossley, & Brukner, 1997; Fleming, Hulstyn, Oksendahl, & Fadale, 2005; Lohmander, Englund, Dahl, & Roos, 2007; Ruiz, Kelly, & Nutton, 2002). As a consequence, the National Public Health Agenda for OA has strongly recommended expansion and refinement of ACL injury prevention strategies (Center for Disease Control and Prevention, 2010). Nonetheless, injury rates continue to rise and there is an increasingly prevalent view that programs should specifically target those who are most likely to sustain an ACL injury in order to reduce the ACL injury rates (Mall et al., 2014; Myer, Ford, Brent, & Hewett, 2007). However, while numerous motor and biomechanical deficits that contribute to ACL injury have been identified, (Hewett et al., 2005; Myer et al., 2013; Pap, Machner, Nebelung, & Awiszus, 1999; Reider et al., 2003; Stroube et al., 2013; Zazulak, Hewett, Reeves, Goldberg, & Cholewicki, 2007) limited knowledge about the relationship between the central nervous system (CNS) and biomechanical patterns of motion has impeded attempts to optimize ACL injury risk prevention strategies (Grooms & Onate, 2016; Grooms et al., 2017). While CNS changes after ACL injury have been documented, much less is known regarding potential CNS contributors to the primary injury.
1.1 ∣. CNS alterations following injury and recovery
Following an ACL injury, knee mechanoreceptors are disrupted and a number of afferent pathways are lost contributing to significant sensorimotor reorganization (Kapreli & Athanasopoulos, 2006; Valeriani et al., 1996, 1999). It is well-documented that sensorimotor behavior is altered following the ACL reconstruction (ACLR) (Petersen, Taheri, Forkel, & Zantop, 2014; Stone, Roper, Herman, & Hass, 2018), and that functional performance on strength and biomechanical tests, such as the single-leg hop, commonly varies during the course of an athlete's recovery (Abrams et al., 2014; Undheim et al., 2015). However, an athlete's functional recovery depends on more than just biomechanical rehabilitation—successful sensorimotor reinnervation following an ACLR (Ochi, Iwasa, Uchio, Adachi, & Sumen, 1999) may also facilitate neural adaptation and motor skill reacquisition (Ingersoll, Grindstaff, Pietrosimone, & Hart, 2008; Kapreli & Athanasopoulos, 2006). Alterations to the CNS following a peripheral joint injury underlie neurological and sensorimotor differences between ACLR and never injured ACL control participants (Melnyk, Faist, Gothner, Claes, & Friemert, 2007). Specifically, electroencephalography (EEG) measurements made during a joint position task have revealed differences in the activity of the parietal alpha-2 (9.75–12.5 Hz) frequency band—contralateral to the ACLR knee—in ACLR participants when compared to their own unaffected limb and to healthy controls (Baumeister, Reinecke, & Weiss, 2008). Differences are also found in the frontal theta frequency band (4–7 Hz) between ACLR and control participants during a joint position task (Baumeister, Reinecke, & Weiss, 2008) and a force production task (Baumeister, Reinecke, Schubert, & Weiß, 2011). The observed alterations within the alpha-2 and theta frequency bands reveal that ACLR athletes required increased resources related to somatosensory processing, working memory, and attentional demand during performance of the sensorimotor tasks when compared to control participants (Baumeister et al., 2011; Baumeister, Reinecke, & Weiss, 2008; Gevins, Smith, McEvoy, & Yu, 1997; Niedermeyer & da Silva, 2005).
Moreover, the results of these studies are complemented by the outcome of a functional magnetic resonance imaging (fMRI) study which revealed altered brain activation patterns related to knee control in ACLR participants when compared to matched control participants (Grooms et al., 2017). Similar to the findings of the EEG studies, fMRI revealed increased activation in the secondary somatosensory area and lingual gyrus during a lower extremity movement task in ACLR individuals compared to controls. The combined EEG and fMRI results highlight the differences in brain functioning for areas responsible for the integration of sensory information, attention, and memory. While this evidence indicates that there are neurological differences present in athletes following an ACL injury, it cannot determine if the neurological differences contributed to, existed prior to, or arose during recovery from the ACL injury event. Prospective studies linking CNS activity to ACL injury risk are required to address those questions.
1.2 ∣. Prospective evidence of CNS differences prior to injury
Two recent longitudinal studies compared prospective brain functional connectivity using fMRI in high school athletes prior to an ACL injury relative to their non-injured peers (Diekfuss, Grooms, Nissen, et al., 2019; Diekfuss, Grooms, Yuan, et al., 2019). The preliminary investigations revealed decreased connectivity—or less co-activation—among brain areas implicated in processing sensory information and coordinating motor behavior for the ACL-injured athletes relative to their healthy teammates. While these studies highlight the presence of abnormal neural behavior prior to injury, they lack an immediate link to the athletes' motor behavior, and thus, it is unknown if the athletes who went on to tear their ACL exhibited both neural and biomechanical abnormalities that predisposed them to injury.
Other prospective investigations have demonstrated alterations in functional behavior prior to an ACL injury (e.g., ImPACT testing (Swanik, Covassin, Stearne, & Schatz, 2007) and/or muscle activation and strength (Grindstaff, Jackson, Garrison, Diduch, & Ingersoll, 2008)), but did not include any direct measurements of brain activity (i.e., EEG or fMRI). The results of these prospective studies must be cautiously interpreted as the samples sizes were extremely limited (excluding (Swanik et al., 2007)) with less than six total ACL injured participants in all three studies (n = 1 (Grindstaff et al., 2008) n = 2 (Diekfuss, Grooms, Yuan, et al., 2019); and n = 3 (Diekfuss, Grooms, Nissen, et al., 2019)). However, considering the combined evidence from EEG, fMRI, and functional behavior data in ACL injured participants compared to uninjured controls—both prior to and following recovery from injury—it is possible that dysfunctional neural behavior contributes significantly to ACL injury risk.
1.3 ∣. Current study
The purpose of the current study was to investigate the relationship between electrocortical activity and biomechanical performance related to ACL injury risk. Specifically, the study investigated the spatiotemporal dynamics of electrocortical behavior of participants who display low- and high-risk knee biomechanics predictive of ACL injuries. As the current study integrated several multidisciplinary topics (i.e., EEG, biomechanics, and nonlinear dynamical systems analyses) to accomplish this purpose, a brief description of each is presented below.
1.3.1 ∣. Dynamics of electrocortical oscillations
Oscillatory electrocortical activity—or more precisely, the periodic fluctuations of extracellular current in coherent groups of neurons (Buzsáki & Draguhn, 2004)—has been noted since Hans Berger's first description of EEG in humans nearly a century ago (Berger, 1929). Since then, the observed electrical oscillations have become a central focus of EEG research (Buzsaki, 2006; Buzsáki & Draguhn, 2004; Buzsáki & Watson, 2012; Helfrich et al., 2018; Helfrich & Knight, 2016; Kelso, 1995) with the functional correlates (i.e., behavior) of such oscillations becoming an increasingly important factor to consider (BaŞar & Güntekin, 2008; Buzsáki & Watson, 2012). Quantifying oscillations is often difficult due to electrocortical variations that are not obviously attributable to the measured phenomenon (Buzsáki & Draguhn, 2004), which is commonly considered noise and viewed as an adulteration of the true underlying signal (Stam, 2005). Traditionally, the global oscillations are broken down in to ranges of functionally relevant frequencies (e.g., theta, alpha-1, alpha-2, beta, and gamma) through a linear decomposition (e.g., a Fourier transform) (Cohen, 2014). The resultant power spectral distribution—a set of numbers proportionally related to the amplitude of each oscillation—is then utilized to determine the peak characterization (i.e., most prevalent frequency) of a brain state. While such methods have been highly informative, they do not completely characterize temporal variations in oscillatory electrocortical behavior. Rather than reflecting meaningless noise, these variations may contain information that is useful for understanding the neural activity.
The latter claim stems from an approach that considers the brain as a densely interconnected and complex dynamical system that maintains stable modes of activity through highly variable, multiscale activity (Buzsaki, 2006; Kelso, 1995). From this view, noise is seen not as a detriment to performance, but instead as a beneficial intrinsic property of neurons (Jackson, 2004; Pinneo, 1966) where noise interacting with deterministic activity supports probabilistic decision making (Deco, Jirsa, McIntosh, Sporns, & Kötter, 2009; Deco, Rolls, & Romo, 2009; Faisal, Selen, & Wolpert, 2008; Ward, 2003), improves the rates of information transfer (Benzi, Sutera, & Vulpiani, 1981; Butson & Clark, 2008; Lugo, Doti, & Faubert, 2008), and increases the network robustness (Basalyga & Salinas, 2006; Faisal et al., 2008; Li, von Oertzen, & Lindenberger, 2006) (see Garrett et al. for a review of noise's potential utility (Garrett et al., 2013)). In other words, electrocortical activity reflects the salubrious combination of deterministic (i.e., predictable network-based activity) and stochastic (i.e., random, spontaneous spiking of individual neurons) processes of the brain (Deco, Jirsa, et al., 2009). In terms of behavioral performance, the classical perspective assumes that the optimal ratio of signal-to-noise is traditionally one-to-zero, while from this current alternate view it can be thought of as an inverted U-shaped curve (McDonnell & Ward, 2011). Comparable to the classic Yerkes-Dodson law relating performance and arousal, performance (e.g., information transmission (Shew, Yang, Yu, Roy, & Plenz, 2011)) is optimized when there is a well-balanced mix of deterministic and stochastic processes—a “sweet spot” between the two extremes. Therefore, quantifying the spatiotemporal patterns—specifically by estimating the level of deterministic and stochastic behavior—can provide insight into how electrocortical variability is related to functional behavior.
1.3.2 ∣. Quantification of electrocortical dynamics with recurrence quantification analysis
One method used to index the spatiotemporal dynamics of EEG activity is recurrence quantification analysis (RQA; Eckmann, Kamphorst, & Ruelle, 1987; Marwan, Romano, Thiel, & Kurths, 2007; Webber & Zbilut, 1994, 2005; Zbilut & Webber, 1992)). RQA is able to quantify the temporal dynamics of electrocortical activity (Bonnette et al., 2018; Rizzi, Frigerio, & Iori, 2016; Rizzi, Weissberg, Milikovsky, & Friedman, 2016) and is a commonly utilized method to analyze the inherently noisy data such as EEG time series (Romano, Thiel, Kurths, Kiss, & Hudson, 2005). Specifically, it is able to quantify the relative degree of determinism, or regularity (i.e., predictability), of a time series (see Table 1 for a brief description of dependent measures returned by RQA and Table 2 for helpful terms related to the analysis itself).The degree of determinism in a system's behavior is thought to reflect the ability of the system (in this case the CNS) to organize coherent, functional behavior. As just discussed, reduced deterministic behavior (or too much stochastic activity) may reflect the inability to maintain a stable behavioral state (Kiefer & Myer, 2015; Stergiou & Decker, 2011; Stergiou, Harbourne, & Cavanaugh, 2006).Moreover, the presence of excessively deterministic (i.e., rigid) electrocortical behavior during resting state may indicate an inability to react to external stimuli and functional demands (Kiefer & Myer, 2015). Previous applications of RQA to analyze EEG data have revealed that the spatiotemporal dynamics of electrocortical activity can distinguish physiological states that characterize conditions such as multiple sclerosis (Carrubba, Minagar, Chesson, Frilot, & Marino, 2012), exposure to explosive blasts (Bonnette et al., 2018), epilepsy (Thomasson, Hoeppner, Webber, & Zbilut, 2001; Zhang, Worrell, & He, 2008), anesthesia (Nicolaou & Georgiou, 2014), sleep stages (Song, Lee, & Kim, 2004), and states of consciousness (Becker et al., 2010). In the present study, we applied RQA to identify the patterns of electrocortical dynamics that related to high-risk movement biomechanics.
TABLE 1.
Description of dependent measures provided by recurrence quantification analysis
| Dependent measure | Description | EEG translation |
|---|---|---|
| Mean diagonal line length (MnDL) | The average length of recurrent points that form a diagonal line | A longer average diagonal line length indicates that the system robustly repeats itself for longer periods of time without interruption from noise or a perturbation |
| Max diagonal line length (MaxDL) | The longest period of recurrent points that contribute to a diagonal line structure | A longer maximum diagonal indicates that an EEG signal is exhibiting a longer single period of deterministic behavior |
| Percent determinism (DET) | The proportion of recurrent points that contribute to diagonal line structures | Higher percent determinism indicates that EEG is more regular and predictable over consecutive samples. The EEG signal is more likely to repeat a previously observed behavior |
| Shannon entropy (ShanEn) | Characterizes the complexity of the patterns exhibited by the system | Higher Shannon entropy values indicate that the distribution of diagonal line lengths—or of the lengths of the time periods of deterministic EEG states—became less regular, indicating that the system is repeating different subsets of its trajectory over time |
TABLE 2.
General description of recurrence quantification analysis parameters and terms
| Term | Description | References |
|---|---|---|
| Delay (τ) | The delay should be chosen so as to minimize the information among measured data points within the original time series—a longer delay reduces the interdependence of data points | Kennel and Abarbanel (2002), Marwan et al. (2007), Webber and Zbilut (2005) |
| Embedding dimension (m) | The number of dimensions (i.e., variables) that are utilized to recreate and describe the system's underlying dynamics within the phase space | Marwan et al. (2007), Webber and Zbilut (2005) |
| Radius (r) | The radius is the distance used to determine if two states or “points” within the recreated phase space are considered recurrent | Webber and Zbilut (2005) |
| Recurrence plot | A recurrence plot is a visualization of a two-dimensional matrix that compares all of the system's states. If the states are sufficiently similar (closer than r to each other in reconstructed phase space) the plot displays a point indicating recurrence, and if the states are not similar the plot is blank at that coordinate. See Figure 2d,e | Eckmann et al. (1987), Marwan et al. (2007), Webber and Zbilut (2005) |
| Recurrent point | Recurrent points occur in state space when different sections of reconstructed trajectories—of length m—reside within a distance less than r | Marwan et al. (2007), Webber and Zbilut (2005) |
| Phase space | Phase space is the mathematical space constituted by the system's state variables. Points within the phase space represent the trajectory of the system's states | Marwan et al. (2007), Webber and Zbilut (2005) |
1.3.3 ∣. Selection of frequency bands and electrode locations
The selection of frequency bands and electrode sites in the present study was based on previous literature investigating the relationship between biomechanical performance and electrocortical behavior (Baumeister, Reinecke, Liesen, & Weiss, 2008; Baumeister et al., 2011; Baumeister, Reinecke, & Weiss, 2008; Luchsinger, Sandbakk, Schubert, Ettema, & Baumeister, 2016). Nine common electrodes were selected (O1, O2, P3, P4, C3, C4, F3, F4, and Fz) and two frequency bands were selected (theta and alpha-2). For the frontal electrodes (i.e., F3, F4, and Fz), the theta frequency band (4.0–7.0 Hz) was of interest based on previous findings of differences between ACLR and uninjured participants (Baumeister et al., 2011; Baumeister, Reinecke, & Weiss, 2008) and also between athletes of different sport background (Luchsinger et al., 2016). The alpha-2 band (9.75–12.5 Hz) was of interest in the remaining electrodes (O1, O2, P3, P4, C3, and C4) due to similar findings of differences between ACLR and uninjured participants (Baumeister et al., 2011; Baumeister, Reinecke, & Weiss, 2008), experts (Baumeister, Reinecke, Liesen, et al., 2008; Hatfield, Haufler, Hung, & Spalding, 2004; Haufler, Spalding, Santa Maria, & Hatfield, 2000), and skill acquisition (Gevins et al., 1997; Smith, McEvoy, & Gevins, 1999).
1.3.4 ∣. Hypothesis
Based on past EEG findings comparing ACLR and control participants, we hypothesized that the activity of the frontal theta and the parietal, central, and occipital alpha-2 frequency bands during resting state EEG would exhibit different temporal dynamics in the low- and high-risk participants. Specifically, we hypothesized that the degree of deterministic electrocortical behavior would significantly differ between the low- and high-risk participants. High-risk participants were expected to exhibit a departure from the “sweet spot” of deterministic structure that marks the balance between behavioral coherence and adaptability. Due to the uniqueness and novelty of the data set, we did not have a basis for hypothesizing whether the deterministic electrocortical behavior of high-risk athletes would be lower (less coherent or stable behavior) or greater (rigid, less adaptive behavior) relative to the electrocortical behavior of low-risk athletes.
2 ∣. METHOD
2.1 ∣. Participants
Adolescent female athletes were recruited from a larger study investigating the effect of a neuromuscular training protocol on female ACL injury risk. Thirty-eight participants (weight = 59.46 ± 9.72 kg, height = 1.65 ± 0.05 m, age = 16.01 ± 1.30 years) were enrolled in the study and completed the EEG and DVJ data collection protocols. Participants were then divided into low-, average-, and high-risk groups depending on their performance on the DVJ (see Figure 1 and supporting literature for group classification and the DVJ procedure below). The average-risk group data (n = 16) was not included in the analysis as those participants did not meet the risk cutoffs for the DVJ (discussed below). Twelve participants were assigned to the low-risk group (weight = 57.32 ± 6.23 kg, height = 1.64 ± 0.04 m, age = 16.58 ± 0.80 years) and 10 participants were assigned to the high-risk group (weight = 66.84 ± 10.59 kg, height = 1.66 ± 0.04 m, age = 15.89 ± 1.25 years). There were no differences between groups in height or age (both p > .05). There was a significant difference between the weights of the two groups, t(20) = 2.50, p = .02, d = 1.07.
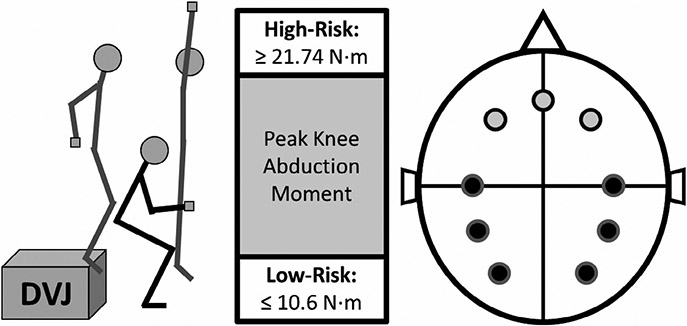
FIGURE 1.
The DVJ is shown in the left of the figure. The peak knee abduction moment is calculated at the position of the black stick figure. Based on that value, the participants were assigned to their respective groups using the cutoffs displayed in the middle panel. Finally, the electrode positions are displayed on the right side of the figure. The light gray circles indicate the locations of the frontal theta frequency band electrodes. In order from left to right they are F3, FZ, and F4. The black circles indicate the locations of the parietal, central, and occipital alpha-2 frequency band electrodes. Starting from the top-left midline electrode they are C3, P3, O1, O2, P4, and C4 in counterclockwise order
2.2 ∣. Procedure
2.2.1 ∣. Drop vertical jump and group assignment
For the DVJ assessment (Ford, Myer, & Hewett, 2003; Hewett et al., 2005; Myer, Ford, Khoury, & Hewett, 2011; Myer, Ford, Khoury, Succop, & Hewett, 2011), participants wore 31 retroreflective markers. The DVJ procedure began with participants standing still on top of a plyometric box (31 cm high) with their feet positioned 35 cm apart (see Figure 1). They were instructed to drop directly down off the box and upon landing immediately perform a maximum vertical jump while raising both arms toward an overhead target (i.e., a basketball). A successful trial required that participants leave the box and land simultaneously with both feet and that they initially dropped down from rather than jumped vertically from the plyometric box. Participants were required to perform three successful trials. Marker trajectories and ground reaction forces were recorded synchronously using a 44-camera, high-speed digital motion analysis system (Motion Analysis Corp.) sampled at 240 Hz and two embedded force platforms (AMTI, Watertown, MA) sampled at 1,200 Hz, respectively. The marker and force data were used to compute three-dimensional joint angular motions and moments of force using an inverse dynamics analysis in Visual3D (C-Motion, Inc., Germantown, MD). The joint angles and moments were normalized to the stance phase of the DVJ (i.e., the period of time when the subjects were on the ground) using custom MATLAB scripts (MathWorks, Inc, Natick, MA). Peak knee abduction moment (pKAM) during landing is associated with increased ACL injury risk and was used as the biomechanical dependent variable of interest (bilateral, averaged across the three DVJs). The participants' pKAM values were used to classify the high-risk and low-risk participants (see Figure 1). High-risk participants landed with ≥21.74 N m of peak knee abduction moment and the low-risk participants landed with ≤10.6 N·m of peak knee abduction moment. Average-risk participants landed with values between the low- and high-risk cut-off values. The risk cut-offs were selected based on prior work establishing knee abduction moment as a prospective predictor of ACL injury risk and other biomechanical studies that have further developed the thresholds for injury risk relative to knee loading (Hewett et al., 2005; Myer, Ford, Khoury, & Hewett, 2011; Myer, Ford, Khoury, Succop, et al., 2011).
2.2.2 ∣. EEG data collection and processing
Electrocortical activity was measured during a preseason visit prior to the competitive high-school soccer season and within 1 hr before the DVJ assessment. EEG data were recorded at the resting state with the participant's eyes closed at a sampling rate of 250 Hz for 2 min using the 10–20 international system as part of a larger EEG testing battery. Participants wore a 128 channel EEG cap (Electrical Geodesics Inc., OR, USA). Impedance was kept below 5 kΩ and recordings were removed if they did not meet this criteria. Only the middle 90 s of each time series was retained for analysis; the first and last 15s of data were removed to reduce computational demands during data analysis while preserving the largest period of artifact-free data. Artifacts were commonly observed during the beginning of the EEG recordings when participants may have been “settling” into resting state.
Before performing RQA, each EEG time series (all 128 electrodes) was preprocessed using EEGLAB (Delorme & Makeig, 2004) and custom written MATLAB scripts (The MathWorks, Inc.; Natick, MA). Artifacts arising from muscular origins (e.g., oculomotor activity, chewing, and muscle/head movements) were identified using independent component analysis (Cohen, 2014; Hoffmann & Falkenstein, 2008). Highly weighted components isolated primarily in the anterior (i.e., frontal) electrodes were subsequently subtracted from the raw EEG time series and the data were then additionally referenced to a common average reference following any data removal (Cohen, 2014). Finally, data from each of the selected electrodes were processed with a 1st-order Butterworth bandpass filter with cutoff frequencies of 4.0 and 7.0 Hz for the theta and of 9.75 and 12.5 Hz for the alpha-2 frequency bands, respectively (see Figure 2). Lastly, the time series were downsampled from 250 to 75 Hz in order to reduce the computational demands and to avoid “false strands” when performing phase space reconstruction and RQA (i.e., misclassifying sequential samples as instances of the system re-visiting a region in phase space) (Kennel & Abarbanel, 2002).
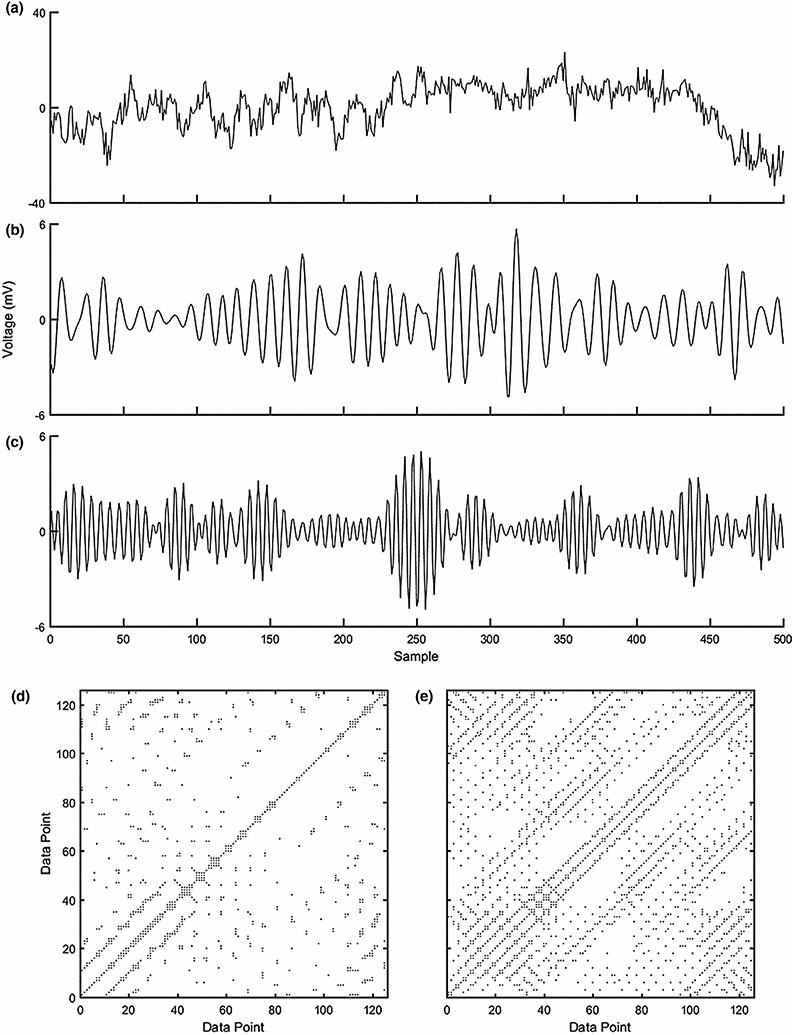
FIGURE 2.
Displayed above is the analysis process. First, in plot (a), 2 s of example raw and unfiltered EEG data from the Fz electrode is presented. The data were then filtered to retain only the theta frequency band (displayed in plot b) and the alpha-2 frequency band (displayed in plot c). Both plots (b) and (c) originated from the data presented in plot (a) and display the same 2 s of data (i.e., 500 samples). The last step, after downsampling, was to create the recurrence plots for each of the electrodes' two specific frequency-band time series. The recurrence plot displayed in (d) is of the theta frequency band and the approximately 120 data points equates to roughly two seconds of EEG data. In order to highlight the structure of the plots, only two seconds of the whole recurrence plots are shown because the whole plots are too large to easily observe the intricate structure within. The same procedure is displayed in plot (e), but with the alpha-2 frequency band data
2.3 ∣. Data analysis
Extensive methodological overviews of RQA have been published previously (Marwan et al., 2007; Marwan, Wessel, Meyerfeldt, Schirdewan, & Kurths, 2002; Webber & Zbilut, 1994, 2005). Tables 1 and 2 contain an overview of the analysis and associated outcome variables. Specifically, Table 2 outlines the following terms related to RQA. The analysis parameters of radius (r), delay (τ), and embedding dimension (m) were determined as follows: (a) a variable r value was utilized in order to ensure each recurrence plot maintained a fixed recurrence rate of 10.0%.1 Selecting a variable radius prevents the oversaturation of recurrence plots with recurrent points, which may influence the dependent measures derived from the plot (Marwan et al., 2007). (b) Using the mutual information approach (Roulston, 1999), an average τ of 12 samples was chosen for the purpose of creating time-delayed copies of the original EEG signals to serve as surrogate dimensions for reconstructing the original system's state (Takens, 1981). (3) False nearest neighbors analysis (Kennel, Brown, & Abarbanel, 1992) was used to select an m of four for dimensionality of the reconstructed state space. The distance matrix was rescaled using the maximum normalized distance.
While RQA can return numerous dependent measures (see Marwan et al., 2007 for an in-depth discussion on each measure), we chose to focus on a subset of four commonly used dependent variables based on the structure of the diagonal lines within the recurrence plot: percent determinism (DET), mean diagonal line length (MnDL), maximum diagonal line length (MaxDL), and Shannon entropy (ShanEn). The presence of diagonal lines indicate that the system is repeating sequences of previously observed behavioral states (i.e., a similar trajectory within the reconstructed state space)—the characteristic signature of deterministic dynamics— and the length of the diagonal lines indicate the duration of time the system maintains similar behavior, which is reflective of the stability of the system (i.e., its ability to withstand perturbations that would prevent the system from repeating the same sequences of states). See Table 1 for descriptions of RQA measures and their interpretations related to EEG activity. See Figure 2 for examples of recurrence plots.
Potential outliers in the RQA-dependent measures were identified by any value ± 2.698 SD from the median.2 If an outlier was statistically identified, the specific electrode's raw EEG time series was further inspected and if artifacts were identified (such as large voltage spikes) the data point was removed. Outliers were replaced by the median value to avoid reducing statistical power of the unique but already limited sample size. Following this procedure, less than 1.5% of all data points were replaced (9 out of 792 total RQA dependent measure values). RQA was performed using a combination of custom-written MATLAB (MathWorks, Inc, Natick, MA) scripts and an open source toolbox of functions related to RQA (Marwan et al., 2002) while statistical analyses were performed using JASP (JASP Team, 2018). An alpha level of .05 was selected a priori to assess significance for independent samples t-test analyses, which were utilized to conduct group comparisons for each electrode. Effect sizes are reported as the Cohen's d statistic.
3 ∣. RESULTS
3.1 ∣. Drop vertical jump
The high-risk participants (M = −30.44 ± 6.66 N·m) displayed significantly greater pKAM magnitudes, t(20) = 11.98, p < .0001, d = 5.13, than the low-risk participants (M = −5.08 ± 2.84 N·m).
3.2 ∣. Frontal theta frequency
See Table 3 for descriptive and inferential statistics. Of the three frontal electrodes investigated (i.e., F3, F4, and Fz), only electrode Fz exhibited differences in the dynamics of the theta frequency band between low- and high-risk participants. The electrocortical activity of the high-risk group was more deterministic overall (DET) and for a greater maximum time period (MaxDL) compared to that of the low-risk group.
TABLE 3.
Inferential and descriptive statistics
| Group |
Independent samples t-test |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Low-Risk |
High-Risk |
|||||||||
| Frequency band | Electrode | RQA measure | M | SD | M | SD | t | df | p | d |
| Frontal theta (4–7 Hz) | F3 | DET | 0.39 | 0.02 | 0.38 | 0.02 | – | – | – | – |
| MnDL | 2.61 | 0.03 | 2.60 | 0.05 | – | – | – | – | ||
| MaxDL | 100.50 | 23.11 | 107.50 | 25.60 | – | – | – | – | ||
| ShanEn | 0.80 | 0.02 | 0.80 | 0.04 | – | – | – | – | ||
| F4 | DET | 0.39 | 0.02 | 0.39 | 0.03 | – | – | – | – | |
| MnDL | 2.62 | 0.05 | 2.62 | 0.06 | – | – | – | – | ||
| MaxDL | 112.67 | 26.89 | 114.70 | 44.05 | – | – | – | – | ||
| ShanEn | 0.81 | 0.04 | 0.81 | 0.04 | – | – | – | – | ||
| Fz | DET | 0.38 | 0.01 | 0.40 | 0.04 | 2.17 | 20 | .042 | 0.93 | |
| MnDL | 2.60 | 0.02 | 2.65 | 0.07 | 1.98 | 20 | .061 | 0.85 | ||
| MaxDL | 100.50 | 17.23 | 139.00 | 52.70 | 2.39 | 20 | .027 | 1.02 | ||
| ShanEn | 0.81 | 0.04 | 0.83 | 0.02 | 1.93 | 20 | .068 | 0.83 | ||
| Parietal, central, and occipital alpha-2 (9.75–12.5 Hz) | C3 | DET | 0.18 | 0.03 | 0.18 | 0.02 | – | – | – | – |
| MnDL | 4.26 | 0.29 | 4.54 | 0.34 | 2.01 | 20 | .058 | 0.86 | ||
| MaxDL | 203.00 | 56.97 | 309.30 | 112.92 | 2.86 | 20 | .010 | 1.23 | ||
| ShanEn | 1.93 | 0.10 | 2.01 | 0.10 | 1.93 | 20 | .069 | 0.82 | ||
| P3 | DET | 0.18 | 0.03 | 0.19 | 0.03 | – | – | – | – | |
| MnDL | 4.30 | 0.16 | 4.85 | 0.50 | 3.53 | 20 | .002 | 1.51 | ||
| MaxDL | 221.50 | 57.74 | 312.00 | 105.06 | 2.56 | 20 | .019 | 1.10 | ||
| ShanEn | 1.974 | 0.12 | 2.08 | 0.14 | 3.21 | 20 | .004 | 1.37 | ||
| O1 | DET | 0.22 | 0.06 | 0.19 | 0.04 | – | – | – | – | |
| MnDL | 5.122 | 0.89 | 4.92 | 0.68 | – | – | – | – | ||
| MaxDL | 477.42 | 383.74 | 363.50 | 167.55 | – | – | – | – | ||
| ShanEn | 2.14 | 0.20 | 2.10 | 0.18 | – | – | – | – | ||
| O2 | DET | 0.21 | 0.07 | 0.19 | 0.04 | – | – | – | – | |
| MnDL | 5.039 | 0.92 | 5.07 | 0.73 | – | – | – | – | ||
| MaxDL | 357.08 | 209.19 | 447.20 | 233.54 | – | – | – | – | ||
| ShanEn | 2.116 | 0.20 | 2.14 | 0.19 | – | – | – | – | ||
| P4 | DET | 0.187 | 0.03 | 0.18 | 0.02 | – | – | – | – | |
| MnDL | 4.534 | 0.72 | 4.73 | 0.45 | – | – | – | – | ||
| MaxDL | 237.58 | 177.15 | 291.40 | 111.53 | – | – | – | – | ||
| ShanEn | 1.994 | 0.20 | 2.00 | 0.14 | – | – | – | – | ||
| C4 | DET | 0.17 | 0.02 | 0.18 | 0.02 | – | – | – | – | |
| MnDL | 4.35 | 0.25 | 4.55 | 0.49 | – | – | – | – | ||
| MaxDL | 253.08 | 89.55 | 285.90 | 130.56 | – | – | – | – | ||
| ShanEn | 1.96 | 0.09 | 2.01 | 0.14 | – | – | – | – | ||
Note: Cohen's d (d). Dashes (−) indicate that the p value for that specific electrode and measure was greater than .10 and, therefore, inferential statistics are not reported.
3.3 ∣. Parietal, central, and occipital alpha-2 frequencies
See Table 3 for descriptive and inferential statistics. The C3 (central) and P3 (parietal) electrodes exhibited differences in the dynamics of the alpha-2 frequency band between low- and high-risk participants. Both electrodes showed differences in the MaxDL measure with the high-risk group having a longer MaxDL than the low-risk group. This particular outcome for the C3 and P3 electrodes mirrors the results of the Fz electrode for the MaxDL measure. The electrocortical activity of the P3 electrode also revealed differences for MnDL and ShanEn. On average, the high-risk group had significantly longer periods of deterministic electrocortical behavior (MnDL), and the distribution of the time periods displaying deterministic electrocortical behavior was less regular (ShanEn). There were no other significant comparisons in any other electrode (all p > .05; C4, P4, O1, and O2).
4 ∣. DISCUSSION
The low- and high-risk groups exhibited differences in resting-state electrocortical activity as assessed by RQA. Specific measures indicated altered electrocortical dynamics of the frontal theta (Fz) and the parietal and central alpha-2 (C3 and P3) frequency bands in the high-risk group compared to the low-risk group. Findings of higher diagonal line measures (DET for Fz; MaxDL for Fz, C3, and P3; and MnDL for P3) indicate greater regularity and predictability (more deterministic behavior) of resting state electrocortical dynamics for the high-risk group, which may be a signature of loss of adaptability (Glass, 2015; Stergiou & Decker, 2011).
4.1 ∣. Frontal theta frequency band
Baumeister and colleagues (Baumeister et al., 2011; Baumeister, Reinecke, & Weiss, 2008) found that ACLR participants displayed higher mean power in the theta-band during a joint position and a force production task. Because the midline frontal theta frequency band (~4–7 Hz) is widely associated with mental effort and attention, where higher theta-band power is commonly observed during periods of increased mental demand relative to resting state and less demanding tasks (Aftanas & Golocheikine, 2001; Gevins et al., 1997; Jensen & Tesche, 2002; Onton, Delorme, & Makeig, 2005), they interpreted the increased theta-band power as reflecting the higher attentional demands required of ACLR participants to perform the sensorimotor tasks. Similarly, the current study found differences in the dynamics of electrocortical activity within the frontal theta-band between low- and high-risk groups. Specifically, the frontal theta-band frequency—measured in electrode Fz—was more deterministic (i.e., DET) and maintained a period of recurrent electrocortical behavior for a greater maximum duration (i.e., MaxDL) in the high-risk group compared to the low-risk group. Despite the differences in methodology and populations, the combined findings of Baumeister and colleagues27,28 with the current study offer converging evidence that the activity of neuronal populations generating the frontal theta frequency band might be an important neurological factor for ACL injury recovery and also a potential contributor to primary ACL injury risk as well (Baumeister et al., 2011; Baumeister, Reinecke, & Weiss, 2008).
Exactly how the activity of the frontal theta-band precisely contributes to potential ACL injury and recovery is unknown. However, it is likely related to the reciprocal electrocortical behavior of the default mode network (DMN) (Scheeringa et al., 2008). Frontal theta-band activity has been shown to negatively correlate with activity of the DMN in resting state (Scheeringa et al., 2008). The DMN—also called the task-negative network—is a large-scale brain network that is principally active during the resting state. Altered DMN activity has been associated with multiple pathological conditions including schizophrenia (Broyd et al., 2009; Greicius, 2008), autism spectrum disorder (Broyd et al., 2009), multiple sclerosis (MS) (Lowe et al., 2002), Parkinson's disease (Tinaz, Schendan, & Stern, 2008), and chronic pain (Baliki, Geha, Apkarian, & Chialvo, 2008). In addition, as DMN activity is inversely related to cognitive control (Seeley et al., 2007) and attentional demands (Hayden, Smith, & Platt, 2009; Raichle et al., 2001; Weissman, Roberts, Visscher, & Woldorff, 2006), altered DMN activity has been shown to predict the severity of attentional deficits following mild traumatic brain injury (Bonnelle et al., 2011), to discriminate between cognitively normal Alzheimer and dementia patients (Galvin, Price, Yan, Morris, & Sheline, 2011), and to index the severity of cognitive impairment in MS (Hawellek, Hipp, Lewis, Corbetta, & Engel, 2011). Through the investigation of DMN activity, the neural architecture that supports and underlies functional task-based human behavior may be better understood. Therefore, the increased determinism of electrocortical behavior in the high-risk group—specifically the altered theta-band behavior observed in the Fz electrode—may reflect one or both of two possible scenarios from which the abnormal electrocortical activity may have arisen.
This first possible scenario is that the high-risk participants exhibited alterations in the dynamics of frontal theta-band activity as a result of underlying differences in the DMN. The ability to transition from the DMN (resting state) to specific functional networks in order to facilitate behavior is important (Raichle, 2010), especially in the highly demanding and dynamic sport environments where the risk for injury is increased. More strongly deterministic electrocortical activity may place an athlete at higher risk for injury during competitive environments where dynamically evolving demands place extreme stress on an athlete's ability to transition among behavioral modes (e.g., transitioning from offense to defense or from running to jumping), because a stronger, more stable deterministic pattern is harder to perturb than a weaker, less stable pattern of activity.
The second possible scenario is that the observed differences in the frontal theta-band reflect neurological compensation for less adaptive activity in sensorimotor regions through the increased rigidity of electrocortical behavior in areas related to cognitive and attentional demands (i.e., higher DET and MaxDL in the Fz electrode). In terms of functional biomechanical behavior, this increased rigidity may result in the reduction of an athlete's ability to properly detect and organize sensorimotor-related information during action. In fact, following a 10 week targeted neuromuscular training program, participants exhibited decreased DET (similar to the low-risk group in the current study) in muscle tonus compared to control participants (Kiefer & Myer, 2015).
4.2 ∣. Parietal, central, and occipital alpha-2 frequency band
Past results have revealed that the alpha-2 frequency band (~9.75—12.5 Hz) reflects the task-specific demands of the somatosensory cortex (Baumeister, Reinecke, Liesen, et al., 2008; Hatfield et al., 2004). The power of the alpha-band is inversely related to the amount of neural recruitment during cognitive and motor processes (Gevins et al., 1997; Niedermeyer & da Silva, 2005). In other words, observations of increased alpha-band power indicate a reduction in neural recruitment. This reduction in neural recruitment is known to accompany expert motor performance (e.g., golfing (Baumeister, Reinecke, Liesen, et al., 2008), shooting (Haufler et al., 2000), karate (Babiloni et al., 2010)) and may be interpreted as more efficient neural control by experts compared to novices (Hatfield et al., 2004; Nakata, Yoshie, Miura, & Kudo, 2010). Although those studies did not compare brain activation among various injury-risk cohorts related to their performance (indexed by biomechanical variables), they do provide insight into the relationship between electrocortical behavior and motor control. Likewise, the current results reveal differences in the dynamics of electrocortical activity within the parietal and central alpha-2-band between low- and high-risk groups. The high-risk group displayed more deterministic electrocortical behavior in the P3 (i.e., MaxDL, MnDL, and ShanEn) and C3 (i.e., MaxDL) electrodes.
Like the theta-band, the alpha-band's relation to the DMN may be explained by transitions from the DMN to separate the networks that support functional behavior. The alpha band has been implicated in DMN function where decreased alpha power commonly accompanies increased DMN activity (Laufs et al., 2006; Laufs, Kleinschmidt, et al., 2003; Mantini, Perrucci, Del Gratta, Romani, & Corbetta, 2007). Specifically, it has been hypothesized that the alpha frequency band may act as a baseline for brain structures associated with attentional control (Laufs, Krakow, et al., 2003), that alpha phase-synchronization is a communication mechanism in the brain (Engel, Gerloff, Hilgetag, & Nolte, 2013; Palva, Palva, & Kaila, 2005), and that the alpha frequency is also related to a posterior network dedicated to visual processing (Mantini et al., 2007). The observed increase in the deterministic temporal dynamics of the C3 and P3 electrodes for the high-risk group could indicate a deficiency in DMN functioning associated with changing from baseline, task-free networks to more demanding sensorimotor networks.
4.3 ∣. DMN and the salience network
The potential role of the DMN may be further refined when considering the intricate influence of the salience network (SN). The SN is responsible for transitions between then DMN and the central executive network (Sridharan, Levitin, & Menon, 2008). Transitions from resting state to task-specific networks occur through the inhibition of the DMN by the SN, and the efficient regulation of the DMN may be integral to adaptive and efficient motor control (Bonnelle et al., 2012; Duann, Ide, Luo, & Li, 2009). For instance, the presupplementary motor area (preSMA) of the SN plays a key role in response inhibition and action control and selection (Duann et al., 2009; Nachev, Kennard, & Husain, 2008). Furthermore, damage to white matter of the SN tract, such as the preSMA, following injury predicts the level of abnormality of DMN functioning (Bonnelle et al., 2012), with less-inhibited DMN activity resulting in less functional behavior performance (Bonnelle et al., 2011). As a result, it is unknown if the interpretations of the role of the DMN in the current results arise from the DMN itself or from its interactions with other networks (i.e., SN). Although this particular study was not designed to investigate if differences in biomechanical patterns of motion are related to brain network deficiencies, future studies should attempt to determine if resting state and/or task-positive networks are related to functional biomechanical patterns of motion. Specifically, to investigate the relationship between the DMN, the SN, and biomechanical patterns of motion, an EEG-based behavioral task, such as go/no-go or oddball paradigms, would be beneficial to include in future investigations.
4.4 ∣. Relating variability in other physiological systems
While quantifying variability in electrocortical behavior is a relatively novel analysis technique, similar methods have been used to quantify the structure of variability in several other physiological signals. For example, the regularity of postural sway (i.e., center of pressure) increases following a concussion (Cavanaugh et al., 2006; Gao, Hu, Buckley, White, & Hass, 2011; Quatman-Yates et al., 2015), throughout adolescence (Quatman-Yates et al., 2018), and again in the later years of life (Lamoth & van Heuvelen, 2012). Likewise the regularity of neonatal heartbeat variability increases prior to sepsis or sepsis-like event (Lake, Richman, Griffin, & Moorman, 2002). Although these particular examples highlight how variable physiological signals—a hallmark of biological processes—can be quantified to improve understanding of how development, disease, or illness can affect behavior, there is still much work to be done in order to uncover the specific relationship between the structure of physiological variability and its implications for behavior.
4.5 ∣. Implications for injury prevention
Numerous prevention programs have been implemented to reduce ACL injury incidence. Some of these have shown effectiveness in reducing ACL injury risk (Gagnier, Morgenstern, & Chess, 2013; Gilchrist et al., 2008; Hewett, Lindenfeld, Riccobene, & Noyes, 1999; LaBella et al., 2011; Mandelbaum et al., 2005; Waldén, Atroshi, Magnusson, Wagner, & Hägglund, 2012), while others have not (Pfeiffer, Shea, Roberts, Grandstrand, & Bond, 2006; Söderman, Werner, Pietilä, Engström, & Alfredson, 2000; Sugimoto, Myer, McKeon, & Hewett, 2012). The totality of evidence regarding the effectiveness of training interventions to reduce ACL injury risk unfortunately reveals that, while these interventions can reduce injury risk factors in the lab, their efficacy is not consistently transferred to the field of play, as injury rates have not declined despite the introduction of ACL injury prevention programs (Hootman, Dick, & Agel, 2007; Myer et al., 2007; Myer, Ford, Brent, & Hewett, 2012; Sugimoto et al., 2012). One method that shows promise in reducing ACL injury rates is augmented neuromuscular training with a real-time, interactive biofeedback stimulus (Bonnette et al., 2019).
Current biomechanical analyses and biofeedback approaches are limited in that they can only quantify resultant or symptoms of poor neuromuscular control. Understanding the neural correlates of maladaptive sensorimotor control associated with biomechanical predictors of ACL injuries will improve the effectiveness of current ACL injury interventions through the introduction of neurotherapeutic targets. For example, the theta-band is thought to originate in the region of the anterior cingulate cortex (ACC) (Gevins et al., 1997; Sauseng, Griesmayr, Freunberger, & Klimesch, 2010; Sauseng, Klimesch, Schabus, & Doppelmayr, 2005), and its activity is suggested to be an indication of attentional demands and error detection (Smith et al., 1999; Tana, Montin, Cerutti, & Bianchi, 2010). In the case of participants with altered theta-band dynamics, a biofeedback stimulus could be made more effective by modulating participants’ interaction with the stimulus—through online cortical load evaluation—so that the task is less demanding and the CNS responds more efficiently. Likewise, differences in the dynamics of the alpha-2 band can be corrected by tailoring a neurofeedback stimulus that “pushes” participants toward a functional level—the sweet spot—of electrocortical deterministic dynamics. Neurofeedback has been used effectively in the past to modulate symptoms of attention deficit/hyperactivity disorder (Monastra, Monastra, & George, 2002) and other psychiatric orders such as anxiety, depression, and psychosis (Cheon et al., 2015; Schoenberg & David, 2014). Moreover, an exercise-based game and balance training regime has been shown to be effective in altering both biomechanics and brain activity (Schättin, Arner, Gennaro, & de Bruin, 2016). Conceptually similar paradigms can be used to implicitly improve sensorimotor control related to ACL injury risk, as prior work has demonstrated (Bonnette et al., 2019).
4.6 ∣. Limitations
There are several limitations of the current study that must be addressed. One is that the DVJ and the EEG were not collected concurrently and, as a result, direct associations relating to the behavior of the DVJ and cortical activity cannot be made. There has been work demonstrating differences in electrocortical activity directly related to the execution of a DVJ (Baumeister et al., 2013). This result combined with the numerous studies demonstrating the linkage among the frontal theta and parietal alpha-2-bands during the performance of competitive sport behavior (Babiloni et al., 2010; Baumeister, Reinecke, Liesen, et al., 2008; Hatfield et al., 2004; Haufler et al., 2000; Nakata et al., 2010) provides grounding for the current study's results and interpretations. In addition, the inference of a relationship between the BOLD signal fluctuations in resting-state DMN (as measured by fMRI) and cortical activity (as measured by EEG) cannot be directly addressed as the EEG data were not concurrently collected with MRI data. However, there is strong evidence in the literature supporting the relationship between DMN and EEG function (He, Snyder, Zempel, Smyth, & Raichle, 2008; Jorge, Van der Zwaag, & Figueiredo, 2014; Laufs et al., 2006; Laufs, Kleinschmidt, et al., 2003; Laufs, Krakow, et al., 2003). Future studies are warranted to directly test the relationship among biomechanical ACL injury risk variables and concurrently collected EEG and fMRI data.
4.7 ∣. Conclusion
The current results highlight the potential for dynamical analyses of EEG data to inform the current understanding of CNS functioning and biomechanical performance. High-risk participants showed more deterministic electrocortical behavior than the low-risk group in the frontal theta (i.e., Fz) and central/parietal alpha-2 (C3 and P3) frequency bands. The more deterministic resting state electrocortical dynamics for the high-risk group may reflect maladaptive neural behavior—excessively stable deterministic patterning that makes transitioning among functional task-specific networks more difficult. Interventions may capitalize on this potential injury-risk factor by addressing attentional control and sensorimotor processing neural regions during training. Biofeedback systems could be developed to integrate neural feedback to enhance current strategies aimed at reducing ACL injury risk by targeting both biomechanical control and the supporting neural architecture. Future investigations that explicitly investigate electrocortical activity, concurrently recorded DMN behavior, can further delineate their relationships to sensorimotor and biomechanical performance.
ACKNOWLEDGEMENTS
The authors would like to thank the athletes, parents, and coaching staff from Madeira and Seton High Schools for their participation in this study. We would also like to thank the SPORT Center staff and interns.
Footnotes
CONFLICT OF INTEREST
The authors report no conflicts of interest.
The value of 10.0% was selected after close visual inspection of the recurrence plot structure at 3 different percentages (i.e., 2.5%, 5.0%, and 10.0%). Increasing the percentage of the recurrent points to 10.0% resulted in the most “defined” diagonal line structure; however, it did not substantially affect the overall pattern of results for each percentage.
2.698 SD from the median corresponds the 1st & 3rd quartiles ± 1.5 × interquartile range, which includes 99.3% of all data between these values. The values are used to create the “whiskers” of a boxplot that are commonly used to identify outliers.
REFERENCES
- Abrams GD, Harris JD, Gupta AK, McCormick FM, Bush-Joseph CA, Verma NN, … Bach BR (2014). Functional performance testing after anterior cruciate ligament reconstruction: A systematic review. Orthopaedic Journal of Sports Medicine, 2(1). 10.1177/2325967113518305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aftanas L, & Golocheikine S (2001). Human anterior and frontal midline theta and lower alpha reflect emotionally positive state and internalized attention: High-resolution EEG investigation of meditation. Neuroscience Letters, 310(1), 57–60. 10.1016/S0304-3940(01)02094-8 [DOI] [PubMed] [Google Scholar]
- Babiloni C, Marzano N, Infarinato F, Iacoboni M, Rizza G, Aschieri P, … Del Percio C (2010). “Neural efficiency” of experts' brain during judgment of actions: A high-resolution EEG study in elite and amateur karate athletes. Behavioural Brain Research, 207(2), 466–475. 10.1016/j.bbr.2009.10.034 [DOI] [PubMed] [Google Scholar]
- Baliki MN, Geha PY, Apkarian AV, & Chialvo DR (2008). Beyond feeling: Chronic pain hurts the brain, disrupting the default-mode network dynamics. Journal of Neuroscience, 28(6), 1398–1403. 10.1523/JNEUROSCI.4123-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basalyga G, & Salinas E (2006). When response variability increases neural network robustness to synaptic noise. Neural Computation, 18(6), 1349–1379. 10.1162/neco.2006.18.6.1349 [DOI] [PubMed] [Google Scholar]
- Başar E, & Güntekin B (2008). A review of brain oscillations in cognitive disorders and the role of neurotransmitters. Brain Research, 1235, 172–193. 10.1016/j.brainres.2008.06.103 [DOI] [PubMed] [Google Scholar]
- Baumeister J, Reinecke K, Liesen H, & Weiss M (2008). Cortical activity of skilled performance in a complex sports related motor task. European Journal of Applied Physiology, 104(4), 625. 10.1007/s00421-008-0811-x [DOI] [PubMed] [Google Scholar]
- Baumeister J, Reinecke K, Schubert M, & Weiß M (2011). Altered electrocortical brain activity after ACL reconstruction during force control. Journal of Orthopaedic Research, 29(9), 1383–1389. 10.1002/jor.21380 [DOI] [PubMed] [Google Scholar]
- Baumeister J, Reinecke K, & Weiss M (2008). Changed cortical activity after anterior cruciate ligament reconstruction in a joint position paradigm: An EEG study. Scandinavian Journal of Medicine & Science in Sports, 18(4), 473–484. 10.1111/j.1600-0838.2007.00702.x [DOI] [PubMed] [Google Scholar]
- Baumeister J, Von Detten S, van Niekerk S-M, Schubert M, Ageberg E, & Louw Q (2013). Brain activity in predictive sensorimotor control for landings: An EEG pilot study. International Journal of Sports Medicine, 34(12), 1106–1111. 10.1055/s-0033-1341437 [DOI] [PubMed] [Google Scholar]
- Becker K, Schneider G, Eder M, Ranft A, Kochs EF, Zieglgänsberger W, & Dodt H-U (2010). Anaesthesia monitoring by recurrence quantification analysis of EEG data. PLoS ONE, 5(1), e8876. 10.1371/journal.pone.0008876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzi R, Sutera A, & Vulpiani A (1981). The mechanism of stochastic resonance. Journal of Physics A: Mathematical and General, 14(11), L453–L457. [Google Scholar]
- Berger H (1929). Über das elektrenkephalogramm des menschen. Archiv für Psychiatrie und Nervenkrankheiten, 87(1), 527–570. 10.1007/BF01797193 [DOI] [Google Scholar]
- Bonnelle V, Ham TE, Leech R, Kinnunen KM, Mehta MA, Greenwood RJ, & Sharp DJ (2012). Salience network integrity predicts default mode network function after traumatic brain injury. Proceedings of the National Academy of Sciences, 109(12), 4690–4695. 10.1073/pnas.1113455109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnelle V, Leech R, Kinnunen KM, Ham TE, Beckmann CF, De Boissezon X, … Sharp DJ (2011). Default mode network connectivity predicts sustained attention deficits after traumatic brain injury. Journal of Neuroscience, 31(38), 13442–13451. 10.1523/JNEUROSCI.1163-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnette S, DiCesare CA, Kiefer AW, Riley MA, Barber Foss KD, Thomas S, … Myer GD (2019). Injury risk factors integrated into self-guided real-time biofeedback improves high-risk biomechanics. Journal of Sport Rehabilitation, 28(8), 831–839. 10.1123/jsr.2017-0391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnette S, Diekfuss JA, Kiefer AW, Riley MA, Barber Foss KD, Thomas S, … Myer GD (2018). A jugular vein compression collar prevents alterations of endogenous electrocortical dynamics following blast exposure during special weapons and tactical (SWAT) breacher training. Experimental Brain Research, 236(10), 2691–2701. 10.1007/s00221-018-5328-x [DOI] [PubMed] [Google Scholar]
- Brewer BW, Cornelius AE, Stephan Y, & Van Raalte J (2010). Self-protective changes in athletic identity following anterior cruciate ligament reconstruction. Psychology of Sport and Exercise, 11(1), 1–5. 10.1016/j.psychsport.2009.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, & Sonuga-Barke EJ (2009). Default-mode brain dysfunction in mental disorders: A systematic review. Neuroscience & Biobehavioral Reviews, 33(3), 279–296. 10.1016/j.neubiorev.2008.09.002 [DOI] [PubMed] [Google Scholar]
- Butson CR, & Clark GA (2008). Random noise paradoxically improves light-intensity encoding in Hermissenda photoreceptor network. Journal of Neurophysiology, 99(1), 146–154. 10.1152/jn.01247.2006 [DOI] [PubMed] [Google Scholar]
- Buzsaki G (2006). Rhythms of the brain. New York, NY: Oxford University Press. [Google Scholar]
- Buzsaki G, & Draguhn A (2004). Neuronal oscillations in cortical networks. Science, 304(5679), 1926–1929. 10.1126/science.1099745 [DOI] [PubMed] [Google Scholar]
- Buzsáki G, & Watson BO (2012). Brain rhythms and neural syntax: Implications for efficient coding of cognitive content and neuropsychiatric disease. Dialogues in Clinical Neuroscience, 14(4), 345–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrubba S, Minagar A, Chesson AL, Frilot C, & Marino AA (2012). Increased determinism in brain electrical activity occurs in association with multiple sclerosis. Neurological Research, 34(3), 286–290. 10.1179/1743132812Y.0000000010 [DOI] [PubMed] [Google Scholar]
- Cavanaugh JT, Guskiewicz KM, Giuliani C, Marshall S, Mercer VS, & Stergiou N (2006). Recovery of postural control after cerebral concussion: New insights using approximate entropy. Journal of Athletic Training, 41(3), 305–313. [PMC free article] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention. (2010). A national public health agenda for osteoarthritis. Retrieved from http://www.cdc.gov/arthritis/docs/oaagenda.pdf
- Cheon E-J, Koo B-H, Seo W-S, Lee J-Y, Choi J-H, & Song S-H (2015). Effects of neurofeedback on adult patients with psychiatric disorders in a naturalistic setting. Applied Psychophysiology and Biofeedback, 40(1), 17–24. 10.1007/s10484-015-9269-x [DOI] [PubMed] [Google Scholar]
- Cohen MX (2014). Analyzing neural time series data: Theory and practice. Cambridge, MA: MIT Press. [Google Scholar]
- Deacon A, Bennell K, Kiss ZS, Crossley K, & Brukner P (1997). Osteoarthritis of the knee in retired, elite Australian Rules footballers. The Medical Journal of Australia, 166(4), 187–190. 10.5694/j.1326-5377.1997.tb140072.x [DOI] [PubMed] [Google Scholar]
- Deco G, Jirsa V, McIntosh AR, Sporns O, & Kötter R (2009). Key role of coupling, delay, and noise in resting brain fluctuations. Proceedings of the National Academy of Sciences, 106(25), 10302–10307. 10.1073/pnas.0901831106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco G, Rolls ET, & Romo R (2009). Stochastic dynamics as a principle of brain function. Progress in Neurobiology, 88(1), 1–16. 10.1016/j.pneurobio.2009.01.006 [DOI] [PubMed] [Google Scholar]
- Delorme A, & Makeig S (2004). EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134(1), 9–21. 10.1016/j.jneumeth.2003.10.009 [DOI] [PubMed] [Google Scholar]
- Diekfuss JA, Grooms DR, Nissen KS, Schneider DK, Foss KDB, Thomas S, … Myer GD (2019). Alterations in knee sensorimotor brain functional connectivity contributes to ACL injury in male high-school football players: A prospective neuroimaging analysis. Brazilian Journal of Physical Therapy. 10.1016/j.bjpt.2019.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekfuss JA, Grooms DR, Yuan W, Dudley J, Barber Foss KD, Thomas S, … Myer GD (2019). Does brain functional connectivity contribute to musculoskeletal injury? A preliminary prospective analysis of a neural biomarker of ACL injury risk. Journal of Science and Medicine in Sport, 22(2), 169–174. 10.1016/j.jsams.2018.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duann J-R, Ide JS, Luo X, & Li C-S-R (2009). Functional connectivity delineates distinct roles of the inferior frontal cortex and presupplementary motor area in stop signal inhibition. Journal of Neuroscience, 29(32), 10171–10179. 10.1523/JNEUROSCI.1300-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckmann J, Kamphorst S, & Ruelle D (1987). Recurrence plots of dynamical systems. Europhysics Letters, 4(9), 973–977. 10.1209/0295-5075/4/9/004 [DOI] [Google Scholar]
- Engel AK, Gerloff C, Hilgetag CC, & Nolte G (2013). Intrinsic coupling modes: Multiscale interactions in ongoing brain activity. Neuron, 80(4), 867–886. 10.1016/j.neuron.2013.09.038 [DOI] [PubMed] [Google Scholar]
- Faisal AA, Selen LP, & Wolpert DM (2008). Noise in the nervous system. Nature Reviews Neuroscience, 9(4), 292. 10.1038/nrn2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming BC, Hulstyn MJ, Oksendahl HL, & Fadale PD (2005). Ligament injury, reconstruction and osteoarthritis. Current Opinion in Orthopaedics, 16(5), 354. 10.1097/01.bco.0000176423.07865.d2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford KR, Myer GD, & Hewett TE (2003). Valgus knee motion during landing in high school female and male basketball players. Medicine & Science in Sports & Exercise, 35(10), 1745–1750. 10.1249/01.MSS.0000089346.85744.D9 [DOI] [PubMed] [Google Scholar]
- Freedman KB, Glasgow MT, Glasgow SG, & Bernstein J (1998). Anterior cruciate ligament injury and reconstruction among university students. Clinical Orthopaedics and Related Research, 356, 208–212. 10.1097/00003086-199811000-00028 [DOI] [PubMed] [Google Scholar]
- Gagnier JJ, Morgenstern H, & Chess L (2013). Interventions designed to prevent anterior cruciate ligament injuries in adolescents and adults: A systematic review and meta-analysis. The American Journal of Sports Medicine, 41(8), 1952–1962. 10.1177/0363546512458227 [DOI] [PubMed] [Google Scholar]
- Galvin J, Price J, Yan Z, Morris J, & Sheline Y (2011). Resting bold fMRI differentiates dementia with Lewy bodies vs Alzheimer disease. Neurology, 76(21), 1797–1803. 10.1212/WNL.0b013e31821ccc83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Hu J, Buckley T, White K, & Hass C (2011). Shannon and Renyi entropies to classify effects of mild traumatic brain injury on postural sway. PLoS ONE, 6(9), e24446. 10.1371/journal.pone.0024446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia GH, Wu H-H, Park MJ, Tjoumakaris FP, Tucker BS, Kelly JD IV, & Sennett BJ (2016). Depression symptomatology and anterior cruciate ligament injury: Incidence and effect on functional outcome—A prospective cohort study. The American Journal of Sports Medicine, 44(3), 572–579. 10.1177/0363546515612466 [DOI] [PubMed] [Google Scholar]
- Garrett DD, Samanez-Larkin GR, MacDonald SW, Lindenberger U, McIntosh AR, & Grady CL (2013). Moment-to-moment brain signal variability: A next frontier in human brain mapping? Neuroscience & Biobehavioral Reviews, 37(4), 610–624. 10.1016/j.neubiorev.2013.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevins A, Smith ME, McEvoy L, & Yu D (1997). High-resolution EEG mapping of cortical activation related to working memory: Effects of task difficulty, type of processing, and practice. Cerebral Cortex (New York, NY: 1991), 7(4), 374–385. 10.1093/cercor/7.4.374 [DOI] [PubMed] [Google Scholar]
- Gilchrist J, Mandelbaum BR, Melancon H, Ryan GW, Silvers HJ, Griffin LY, … Dvorak J (2008). A randomized controlled trial to prevent noncontact anterior cruciate ligament injury in female collegiate soccer players. The American Journal of Sports Medicine, 36(8), 1476–1483. 10.1177/0363546508318188 [DOI] [PubMed] [Google Scholar]
- Glass L (2015). Dynamical disease: Challenges for nonlinear dynamics and medicine. Chaos: An Interdisciplinary Journal of Nonlinear Science, 25(9), 097603. 10.1063/E4915529 [DOI] [PubMed] [Google Scholar]
- Greicius M (2008). Resting-state functional connectivity in neuropsychiatric disorders. Current Opinion in Neurology, 21(4), 424–430. 10.1097/WCO.0b013e328306f2c5 [DOI] [PubMed] [Google Scholar]
- Grindstaff TL, Jackson KR, Garrison JC, Diduch DR, & Ingersoll CD (2008). Decreased quadriceps activation measured hours prior to a noncontact anterior cruciate ligament tear. Journal of Orthopaedic & Sports Physical Therapy, 38(8), 502–507. 10.2519/jospt.2008.2761 [DOI] [PubMed] [Google Scholar]
- Grooms DR, & Onate JA (2016). Neuroscience application to noncontact anterior cruciate ligament injury prevention. Sports Health, 8(2), 149–152. 10.1177/1941738115619164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grooms DR, Page SJ, Nichols-Larsen DS, Chaudhari AM, White SE, & Onate JA (2017). Neuroplasticity associated with anterior cruciate ligament reconstruction. Journal of Orthopaedic & Sports Physical Therapy, 47(3), 180–189. 10.2519/jospt.2017.7003 [DOI] [PubMed] [Google Scholar]
- Hatfield BD, Haufler AJ, Hung T-M, & Spalding TW (2004). Electroencephalographic studies of skilled psychomotor performance. Journal of Clinical Neurophysiology, 21(3), 144–156. 10.1097/00004691-200405000-00003 [DOI] [PubMed] [Google Scholar]
- Haufler AJ, Spalding TW, Santa Maria D, & Hatfield BD (2000). Neuro-cognitive activity during a self-paced visuospatial task: Comparative EEG profiles in marksmen and novice shooters. Biological Psychology, 53(2–3), 131–160. 10.1016/s0301-0511(00)00047-8 [DOI] [PubMed] [Google Scholar]
- Hawellek DJ, Hipp JF, Lewis CM, Corbetta M, & Engel AK (2011). Increased functional connectivity indicates the severity of cognitive impairment in multiple sclerosis. Proceedings of the National Academy of Sciences, 108(47), 19066–19071. 10.1073/pnas.1110024108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden BY, Smith DV, & Platt ML (2009). Electrophysiological correlates of default-mode processing in macaque posterior cingulate cortex. Proceedings of the National Academy of Sciences, 106(14), 5948–5953. 10.1073/pnas.0812035106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He BJ, Snyder AZ, Zempel JM, Smyth MD, & Raichle ME (2008). Electrophysiological correlates of the brain's intrinsic large-scale functional architecture. Proceedings of the National Academy of Sciences, 105(41), 16039–16044. 10.1073/pnas.0807010105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich RF, Fiebelkorn IC, Szczepanski SM, Lin JJ, Parvizi J, Knight RT, & Kastner S (2018). Neural mechanisms of sustained attention are rhythmic. Neuron, 99(4), 854–865.e855. 10.1016/j.neuron.2018.07.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich RF, & Knight RT (2016). Oscillatory dynamics of prefrontal cognitive control. Trends in Cognitive Sciences, 20(12), 916–930. 10.1016/j.tics.2016.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewett TE, Lindenfeld TN, Riccobene JV, & Noyes FR (1999). The effect of neuromuscular training on the incidence of knee injury in female athletes. The American Journal of Sports Medicine, 27(6), 699–706. 10.1177/03635465990270060301 [DOI] [PubMed] [Google Scholar]
- Hewett TE, Myer GD, Ford KR, Heidt RS Jr., Colosimo AJ, McLean SG, … Succop P (2005). Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: A prospective study. The American Journal of Sports Medicine, 33(4), 492–501. 10.1177/03635465990270060301 [DOI] [PubMed] [Google Scholar]
- Hoffmann S, & Falkenstein M (2008). The correction of eye blink artefacts in the EEG: A comparison of two prominent methods. PLoS ONE, 3(8), e3004. 10.1371/journal.pone.0003004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hootman JM, Dick R, & Agel J (2007). Epidemiology of collegiate injuries for 15 sports: Summary and recommendations for injury prevention initiatives. Journal of Athletic Training, 42(2), 311–319. [PMC free article] [PubMed] [Google Scholar]
- Ingersoll CD, Grindstaff TL, Pietrosimone BG, & Hart JM (2008). Neuromuscular consequences of anterior cruciate ligament injury. Clinics in Sports Medicine, 27(3), 383–404. 10.1016/j.csm.2008.03.004 [DOI] [PubMed] [Google Scholar]
- Jackson BS (2004). Including long-range dependence in integrate-and-fire models of the high interspike-interval variability of cortical neurons. Neural Computation, 16(10), 2125–2195. 10.1162/0899766041732413 [DOI] [PubMed] [Google Scholar]
- JASP Team.(2018). JASP (Version 0.9). Retrieved from https://jasp-stats.org/
- Jensen O, & Tesche CD (2002). Frontal theta activity in humans increases with memory load in a working memory task. European Journal of Neuroscience, 15(8), 1395–1399. 10.1046/j.1460-9568.2002.01975.x [DOI] [PubMed] [Google Scholar]
- Jorge J, Van der Zwaag W, & Figueiredo P (2014). EEG–fMRI integration for the study of human brain function. NeuroImage, 102, 24–34. 10.1016/jneuroimage.2013.05.114 [DOI] [PubMed] [Google Scholar]
- Kapreli E, & Athanasopoulos S (2006). The anterior cruciate ligament deficiency as a model of brain plasticity. Medical Hypotheses, 67(3), 645–650. 10.1016/j.mehy.2006.01.063 [DOI] [PubMed] [Google Scholar]
- Kelso J (1995). Dynamic patterns: The self-organization of brain and behavior. Cambridge, MA: MIT Press. [Google Scholar]
- Kennel MB, & Abarbanel HD (2002). False neighbors and false strands: A reliable minimum embedding dimension algorithm. Physical Review E, 66(2), 026209. 10.1103/PhysRevE.66.026209 [DOI] [PubMed] [Google Scholar]
- Kennel MB, Brown R, & Abarbanel HD (1992). Determining embedding dimension for phase-space reconstruction using a geometrical construction. Physical Review A, 45(6), 3403. 10.1103/PhysRevA.45.3403 [DOI] [PubMed] [Google Scholar]
- Kiefer A, & Myer GD (2015). Training the antifragile athlete: A preliminary analysis of neuromuscular training effects on muscle activation dynamics. Nonlinear Dynamics, Psychology, and Life Sciences, 19(4), 489–510. [PubMed] [Google Scholar]
- LaBella CR, Huxford MR, Grissom J, Kim K-Y, Peng J, & Christoffel KK (2011). Effect of neuromuscular warm-up on injuries in female soccer and basketball athletes in urban public high schools: Cluster randomized controlled trial. Archives of Pediatrics & Adolescent Medicine, 165(11), 1033–1040. 10.1001/archpediatrics.2011.168 [DOI] [PubMed] [Google Scholar]
- Lake DE, Richman JS, Griffin MP, & Moorman JR (2002). Sample entropy analysis of neonatal heart rate variability. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 283(3), R789–R797. 10.1152/ajpregu.00069.2002 [DOI] [PubMed] [Google Scholar]
- Lamoth CJ, & van Heuvelen MJ (2012). Sports activities are reflected in the local stability and regularity of body sway: Older ice-skaters have better postural control than inactive elderly. Gait & Posture, 35(3), 489–493. 10.1016/j.gaitpost.2011.11.014 [DOI] [PubMed] [Google Scholar]
- Laufs H, Holt JL, Elfont R, Krams M, Paul JS, Krakow K, & Kleinschmidt A (2006). Where the BOLD signal goes when alpha EEG leaves. NeuroImage, 31(4), 1408–1418. 10.1016/j.neuroimage.2006.02.002 [DOI] [PubMed] [Google Scholar]
- Laufs H, Kleinschmidt A, Beyerle A, Eger E, Salek-Haddadi A, Preibisch C, & Krakow K (2003). EEG-correlated fMRI of human alpha activity. NeuroImage, 19(4), 1463–1476. 10.1016/S1053-8119(03)00286-6 [DOI] [PubMed] [Google Scholar]
- Laufs H, Krakow K, Sterzer P, Eger E, Beyerle A, Salek-Haddadi A, & Kleinschmidt A (2003). Electroencephalographic signatures of attentional and cognitive default modes in spontaneous brain activity fluctuations at rest. Proceedings of the National Academy of Sciences, 100(19), 11053–11058. 10.1073/pnas.1831638100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S-C, von Oertzen T, & Lindenberger U (2006). A neurocomputational model of stochastic resonance and aging. Neurocomputing, 69(13–15), 1553–1560. 10.1016/jneucom.2005.06.015 [DOI] [Google Scholar]
- Lohmander LS, Englund PM, Dahl LL, & Roos EM (2007). The long-term consequence of anterior cruciate ligament and meniscus injuries. The American Journal of Sports Medicine, 35(10), 1756–1769. 10.1177/0363546507307396 [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Phillips MD, Lurito JT, Mattson D, Dzemidzic M, & Mathews VP (2002). Multiple sclerosis: Low-frequency temporal blood oxygen level-dependent fluctuations indicate reduced functional connectivity—Initial results. Radiology, 224(1), 184–192. 10.1148/radiol.2241011005 [DOI] [PubMed] [Google Scholar]
- Luchsinger H, Sandbakk Ø, Schubert M, Ettema G, & Baumeister J (2016). A comparison of frontal theta activity during shooting among biathletes and cross-country skiers before and after vigorous exercise. PLoS One, 11(3), e0150461. 10.1371/journal.pone.0150461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugo E, Doti R, & Faubert J (2008). Ubiquitous crossmodal stochastic resonance in humans: Auditory noise facilitates tactile, visual and proprioceptive sensations. PLoS ONE, 3(8), e2860. 10.1371/journal.pone.0002860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mall NA, Chalmers PN, Moric M, Tanaka MJ, Cole BJ, Bach BR Jr., & Paletta GA Jr. (2014). Incidence and trends of anterior cruciate ligament reconstruction in the United States. The American Journal of Sports Medicine, 42(10), 2363–2370. 10.1177/0363546514542796 [DOI] [PubMed] [Google Scholar]
- Mandelbaum BR, Silvers HJ, Watanabe DS, Knarr JF, Thomas SD, Griffin LY, … Garrett W (2005). Effectiveness of a neuromuscular and proprioceptive training program in preventing anterior cruciate ligament injuries in female athletes. The American Journal of Sports Medicine, 33(7), 1003–1010. 10.1177/0363546504272261 [DOI] [PubMed] [Google Scholar]
- Mantini D, Perrucci MG, Del Gratta C, Romani GL, & Corbetta M (2007). Electrophysiological signatures of resting state networks in the human brain. Proceedings of the National Academy of Sciences, 104(32), 13170–13175. 10.1073/pnas.0700668104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marwan N, Romano CM, Thiel M, & Kurths J (2007). Recurrence plots for the analysis of complex systems. Physics Reports, 438(5), 237–329. 10.1016/j.physrep.2006.11.001 [DOI] [Google Scholar]
- Marwan N, Wessel N, Meyerfeldt U, Schirdewan A, & Kurths J (2002). Recurrence-plot-based measures of complexity and their application to heart-rate-variability data. Physical Review E, 66(2), 026702. 10.1103/PhysRevE.66.026702 [DOI] [PubMed] [Google Scholar]
- McDonnell MD, & Ward LM (2011). The benefits of noise in neural systems: Bridging theory and experiment. Nature Reviews Neuroscience, 12(7), 415. 10.1038/nrn3061 [DOI] [PubMed] [Google Scholar]
- Melnyk M, Faist M, Gothner M, Claes L, & Friemert B (2007). Changes in stretch reflex excitability are related to “giving way” symptoms in patients with anterior cruciate ligament rupture. Journal of Neurophysiology, 97(1), 474–480. 10.1152/jn.00529.2006 [DOI] [PubMed] [Google Scholar]
- Monastra VJ, Monastra DM, & George S (2002). The effects of stimulant therapy, EEG biofeedback, and parenting style on the primary symptoms of attention-deficit/hyperactivity disorder. Applied Psychophysiology and Biofeedback, 27(4), 231–249. 10.1023/A:1021018700609 [DOI] [PubMed] [Google Scholar]
- Myer GD, Ford KR, Brent JL, & Hewett TE (2007). Differential neuromuscular training effects onACL injury risk factors in “high-risk” versus “low-risk” athletes. BMC Musculoskeletal Disorders, 8(1), 39. 10.1186/1471-2474-8-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myer GD, Ford KR, Brent JL, & Hewett TE (2012). An integrated approach to change the outcome part I: Neuromuscular screening methods to identify high ACL injury risk athletes. Journal of Strength and Conditioning research/National Strength & Conditioning Association, 26(8), 2265. 10.1519/JSC.0b013e31825c2b8f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myer GD, Ford KR, Khoury J, & Hewett TE (2011). Three-dimensional motion analysis validation of a clinic-based nomogram designed to identify high ACL injury risk in female athletes. The Physician and Sportsmedicine, 39(1), 19–28. 10.3810/psm.2011.02.1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myer GD, Ford KR, Khoury J, Succop P, & Hewett TE (2011). Biomechanics laboratory-based prediction algorithm to identify female athletes with high knee loads that increase risk of ACL injury. British Journal of Sports Medicine, 45(4), 245–252. 10.1136/bjsm.2009.069351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myer GD, Stroube BW, DiCesare CA, Brent JL, Ford KR Heidt RS Jr., & Hewett TE (2013). Augmented feedback supports skill transfer and reduces high-risk injury landing mechanics: A double-blind, randomized controlled laboratory study. The American Journal of Sports Medicine, 41(3), 669–677. 10.1177/0363546512472977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachev P, Kennard C, & Husain M (2008). Functional role of the supplementary and pre-supplementary motor areas. Nature Reviews Neuroscience, 9(11), 856. 10.1038/nrn2478 [DOI] [PubMed] [Google Scholar]
- Nakata H, Yoshie M, Miura A, & Kudo K (2010). Characteristics of the athletes' brain: Evidence from neurophysiology and neuroimaging. Brain Research Reviews, 62(2), 197–211. 10.1016/j.brainresrev.2009.11.006 [DOI] [PubMed] [Google Scholar]
- Nicolaou N, & Georgiou J (2014). The study of EEG dynamics during anesthesia with cross-recurrence rate. Cureus, 6(8), e195. 10.7759/cureus.195 [DOI] [Google Scholar]
- Niedermeyer E, & da Silva FL (2005). Electroencephalography: Basic principles, clinical applications, and related fields. Philadelphia, PA: Lippincott Williams & Wilkins. [Google Scholar]
- Ochi M, Iwasa J, Uchio Y, Adachi N, & Sumen Y (1999). The regeneration of sensory neurones in the reconstruction of the anterior cruciate ligament. The Journal of Bone and Joint surgery-British, 81(5), 902–906. 10.1302/0301-620x.81b5.9202 [DOI] [PubMed] [Google Scholar]
- Onton J, Delorme A, & Makeig S (2005). Frontal midline EEG dynamics during working memory. NeuroImage, 27(2), 341–356. 10.1016/j.neuroimage.2005.04.014 [DOI] [PubMed] [Google Scholar]
- Palva JM, Palva S, & Kaila K (2005). Phase synchrony among neuronal oscillations in the human cortex. Journal of Neuroscience, 25(15), 3962–3972. 10.1523/JNEUROSCI.4250-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pap G, Machner A, Nebelung W, & Awiszus F (1999). Detailed analysis of proprioception in normal and ACL-deficient knees. The Journal of Bone and Joint surgery-British, 81(5), 764–768. 10.1302/0301-620x.81b5.9352 [DOI] [PubMed] [Google Scholar]
- Petersen W, Taheri P, Forkel P, & Zantop T (2014). Return to play following ACL reconstruction: A systematic review about strength deficits. Archives of Orthopaedic and Trauma Surgery, 134(10), 1417–1428. 10.1007/s00402-014-1992-x [DOI] [PubMed] [Google Scholar]
- Pfeiffer RP, Shea KG, Roberts D, Grandstrand S, & Bond L (2006). Lack of effect of a knee ligament injury prevention program on the incidence of noncontact anterior cruciate ligament injury. The Journal of Bone and Joint Surgery, 88(8), 1769–1774. 10.2106/JBJS.E.00616 [DOI] [PubMed] [Google Scholar]
- Pinneo LR (1966). On noise in the nervous system. Psychological Review, 73(3), 242. 10.1037/h0023240 [DOI] [PubMed] [Google Scholar]
- Quatman-Yates C, Bonnette S, Gupta R, Hugentobler JA, Wade SL, Glauser TA, … Riley MA (2018). Spatial and temporal analysis center of pressure displacement during adolescence: Clinical implications of developmental changes. Human Movement Science, 58, 148–154. 10.1016/j.humov.2018.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quatman-Yates CC, Bonnette S, Hugentobler JA, Médé MB, Kiefer AW, Kurowski BG, & Riley MA (2015). Postconcussion postural sway variability changes in youth: The benefit of structural variability analyses. Pediatric Physical Therapy, 27(4), 316. 10.1097/PEP.0000000000000193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME (2010). Two views of brain function. Trends in Cognitive Sciences, 14(4), 180–190. 10.1016/j.tics.2010.01.008 [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, & Shulman GL (2001). A default mode of brain function. Proceedings of the National Academy of Sciences, 98(2), 676–682. 10.1073/pnas.98.2.676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reider B, Arcand MA, Diehl LH, Mroczek K, Abulencia A, Stroud CC, … Staszak P (2003). Proprioception of the knee before and after anterior cruciate ligament reconstruction. Arthroscopy: The Journal of Arthroscopic & Related Surgery, 19(1), 2–12. 10.1053/jars.2003.50006 [DOI] [PubMed] [Google Scholar]
- Rizzi M, Frigerio F, & Iori V (2016). The early phases of epileptogenesis induced by status epilepticus are characterized by persistent dynamical regime of intermittency type. In Webber CL Jr., Ioana C, & Marwan N (Eds.), Recurrence plots and their quantifications: Expanding horizons (pp. 185–208). Cham, Switzerland: Springer International Publishing. [Google Scholar]
- Rizzi M, Weissberg I, Milikovsky DZ, & Friedman A (2016). Following a potential epileptogenic insult, prolonged high rates of nonlinear dynamical regimes of intermittency type is the hallmark of epileptogenesis. Scientific Reports, 6. 10.1038/srep31129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano MC, Thiel M, Kurths J, Kiss IZ, & Hudson J (2005). Detection of synchronization for non-phase-coherent and non-stationary data. Europhysics Letters, 71(3), 466. 10.1209/epl/i2005-10095-1 [DOI] [Google Scholar]
- Roulston MS (1999). Estimating the errors on measured entropy and mutual information. Physica D: Nonlinear Phenomena, 125(3), 285–294. 10.1016/S0167-2789(98)00269-3 [DOI] [Google Scholar]
- Ruiz A, Kelly M, & Nutton R (2002). Arthroscopic ACL reconstruction: A 5–9 year follow-up. The Knee, 9(3), 197–200. 10.1016/S0968-0160(02)00019-4 [DOI] [PubMed] [Google Scholar]
- Sauseng P, Griesmayr B, Freunberger R, & Klimesch W (2010). Control mechanisms in working memory: A possible function of EEG theta oscillations. Neuroscience & Biobehavioral Reviews, 34(7), 1015–1022. 10.1016/jmeubiorev.2009.12.006 [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Schabus M, & Doppelmayr M (2005). Fronto-parietal EEG coherence in theta and upper alpha reflect central executive functions of working memory. International Journal of Psychophysiology, 57(2), 97–103. 10.1016/j.ijpsycho.2005.03.018 [DOI] [PubMed] [Google Scholar]
- Schättin A, Arner R, Gennaro F, & de Bruin ED (2016). Adaptations of prefrontal brain activity, executive functions, and gait in healthy elderly following exergame and balance training: A randomized-controlled study. Frontiers in Aging Neuroscience, 8, 278. 10.3389/fnagi.2016.00278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheeringa R, Bastiaansen MC, Petersson KM, Oostenveld R, Norris DG, & Hagoort P (2008). Frontal theta EEG activity correlates negatively with the default mode network in resting state. International Journal of Psychophysiology, 67(3), 242–251. 10.1016/j.ijpsycho.2007.05.017 [DOI] [PubMed] [Google Scholar]
- Schoenberg PL, & David AS (2014). Biofeedback for psychiatric disorders: A systematic review. Applied Psychophysiology and Biofeedback, 39(2), 109–135. 10.1007/s10484-014-9246-9 [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, … Greicius MD (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience, 27(9), 2349–2356. 10.1523/JNEUROSCI.5587-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shew WL, Yang H, Yu S, Roy R, & Plenz D (2011). Information capacity and transmission are maximized in balanced cortical networks with neuronal avalanches. Journal of Neuroscience, 31(1), 55–63. 10.1523/JNEUROSa.4637-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ME, McEvoy LK, & Gevins A (1999). Neurophysiological indices of strategy development and skill acquisition. Cognitive Brain Research, 7(3), 389–404. 10.1016/S0926-6410(98)00043-3 [DOI] [PubMed] [Google Scholar]
- Söderman K, Werner S, Pietilä T, Engström B, & Alfredson H (2000). Balance board training: Prevention of traumatic injuries of the lower extremities in female soccer players? Knee Surgery, Sports Traumatology, Arthroscopy, 8(6), 356–363. 10.1007/s001670000147 [DOI] [PubMed] [Google Scholar]
- Song I-H, Lee D-S, & Kim SI (2004). Recurrence quantification analysis of sleep electoencephalogram in sleep apnea syndrome in humans. Neuroscience Letters, 366(2), 148–153. 10.1016/j.neulet.2004.05.025 [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, & Menon V (2008). A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proceedings of the National Academy of Sciences, 105(34), 12569–12574. 10.1073/pnas.0800005105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stam CJ (2005). Nonlinear dynamical analysis of EEG and MEG: Review of an emerging field. Clinical Neurophysiology, 116(10), 2266–2301. 10.1016/j.clinph.2005.06.011 [DOI] [PubMed] [Google Scholar]
- Stergiou N, & Decker LM (2011). Human movement variability, nonlinear dynamics, and pathology: Is there a connection? Human Movement Science, 30(5), 869–888. 10.1016/j.humov.2011.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stergiou N, Harbourne RT, & Cavanaugh JT (2006). Optimal movement variability: A new theoretical perspective for neurologic physical therapy. Journal of Neurologic Physical Therapy, 30(3), 120–129. 10.1097/01.NPT.0000281949.48193.d9 [DOI] [PubMed] [Google Scholar]
- Stone AE, Roper JA, Herman DC, & Hass CJ (2018). Cognitive performance and locomotor adaptation in persons with anterior cruciate ligament reconstruction. Neurorehabilitation and Neural Repair, 32(6–7), 568–577. 10.1177/1545968318776372 [DOI] [PubMed] [Google Scholar]
- Stroube BW, Myer GD, Brent JL, Ford KR, Heidt RS Jr., & Hewett TE (2013). Effects of task-specific augmented feedback on deficit modification during performance of the tuck-jump exercise. Journal of Sport Rehabilitation, 22(1), 7–18. 10.1123/jsr.22.1.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto D, Myer GD, McKeon JM, & Hewett TE (2012). Evaluation of the effectiveness of neuromuscular training to reduce anterior cruciate ligament injury in female athletes: A critical review of relative risk reduction and numbers-needed-to-treat analyses. British Journal of Sports Medicine, 46, 979–988. 10.1136/bjsports-2011-090895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanik CB, Covassin T, Stearne DJ, & Schatz P (2007). The relationship between neurocognitive function and noncontact anterior cruciate ligament injuries. The American Journal of Sports Medicine, 35(6), 943–948. 10.1177/0363546507299532 [DOI] [PubMed] [Google Scholar]
- Takens F (1981). Detecting strange attractors in turbulence. Lecture Notes in Mathematics, 898(1), 366–381. 10.1007/BFb0091924 [DOI] [Google Scholar]
- Tana MG, Montin E, Cerutti S, & Bianchi AM (2010). Exploring cortical attentional system by using fMRI during a continuous perfomance test. Computational Intelligence and Neuroscience, 2010, 3. 10.1155/2010/329213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomasson N, Hoeppner TJ, Webber CL, & Zbilut JP (2001). Recurrence quantification in epileptic EEGs. Physics Letters A, 279(1), 94–101. 10.1016/S0375-9601(00)00815-X [DOI] [Google Scholar]
- Tinaz S, Schendan HE, & Stern CE (2008). Fronto-striatal deficit in Parkinson's disease during semantic event sequencing. Neurobiology of Aging, 29(3), 397–407. 10.1016/j.neurobiolaging.2006.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp DA, Stanish WD, Coady C, & Reardon G (2004). The subjective pain experience of athletes following anterior cruciate ligament surgery. Psychology of Sport and Exercise, 5(3), 339–354. 10.1016/S1469-0292(03)00022-0 [DOI] [Google Scholar]
- Undheim MB, Cosgrave C, King E, Strike S, Marshall B, Falvey É, & Franklyn-Miller A (2015). Isokinetic muscle strength and readiness to return to sport following anterior cruciate ligament reconstruction: Is there an association? A systematic review and a protocol recommendation. British Journal of Sports Medicine, 49(20), 1305–1310. 10.1136/bjsports-2014-093962 [DOI] [PubMed] [Google Scholar]
- Valeriani M, Restuccia D, Di Lazzaro V, Franceschi F, Fabbriciani C, & Tonali P (1999). Clinical and neurophysiological abnormalities before and after reconstruction of the anterior cruciate ligament of the knee. Acta Neurologica Scandinavica, 99(5), 303–307. 10.1111/j.1600-0404.1999.tb00680.x [DOI] [PubMed] [Google Scholar]
- Valeriani M, Restuccia D, Lazzaro VD, Franceschi F, Fabbriciani C, & Tonali P (1996). Central nervous system modifications in patients with lesion of the anterior cruciate ligament of the knee. Brain, 119(5), 1751–1762. 10.1093/brain/119.5.1751 [DOI] [PubMed] [Google Scholar]
- Waldén M, Atroshi I, Magnusson H, Wagner P, & Hägglund M (2012). Prevention of acute knee injuries in adolescent female football players: Cluster randomised controlled trial. The BMJ, 344, e3042. 10.1136/bmj.e3042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward LM (2003). Synchronous neural oscillations and cognitive processes. Trends in Cognitive Sciences, 7(12), 553–559. 10.1016/j.tics.2003.10.012 [DOI] [PubMed] [Google Scholar]
- Webber CL Jr., & Zbilut JP (1994). Dynamical assessment of physiological systems and states using recurrence plot strategies. Journal of Applied Physiology, 76(2), 965–973. 10.1152/jappl.1994.76.2.965 [DOI] [PubMed] [Google Scholar]
- Webber CL Jr, & Zbilut JP (2005). Recurrence quantification analysis of nonlinear dynamical systems. In Riley MA, & Van Orden GC (Eds.), Tutorials in contemporary nonlinear methods for the behavioral sciences (pp. 33–101). Retrieved from https://www.nsf.gov/pubs/2005/nsf05057/nmbs/nmbs.pdf. [Google Scholar]
- Weissman DH, Roberts K, Visscher K, & Woldorff M (2006). The neural bases of momentary lapses in attention. Nature Neuroscience, 9(7), 971. 10.1038/nn1727 [DOI] [PubMed] [Google Scholar]
- Zazulak BT, Hewett TE, Reeves NP, Goldberg B, & Cholewicki J (2007). Deficits in neuromuscular control of the trunk predict knee injury risk: Prospective biomechanical-epidemiologic study. The American Journal of Sports Medicine, 35(7), 1123–1130. 10.1177/0363546507301585 [DOI] [PubMed] [Google Scholar]
- Zbilut JP, & Webber CL Jr (1992). Embeddings and delays as derived from quantification of recurrence plots. Physics Letters A, 171 (3–4), 199–203. 10.1016/0375-9601(92)90426-M [DOI] [Google Scholar]
- Zhang W, Worrell G, & He B (2008). Recurrence based deterministic trends in EEG records of epilepsy patients. Paper presented at the ITAB 2008. International Conference on Information Technology and Applications in Biomedicine, 2008, Shenzhen, China. 10.1109/ITAB.2008.4570657 [DOI] [Google Scholar]