Abstract
N 6 ‐methyladenosine (m6A) is one of the most abundant internal modifications in eukaryotic messenger RNAs (mRNAs) and non‐coding RNAs (ncRNAs). It is a reversible and dynamic RNA modification that has been observed in both internal coding segments and untranslated regions. Studies indicate that m6A modifications play important roles in translation, RNA splicing, export, degradation and ncRNA processing control. In this review, we focus on the profiles and biological functions of RNA m6A methylation on both mRNAs and ncRNAs. The dynamic modification of m6A and its potential roles in cancer development are discussed. Moreover, we discuss the possibility of m6A modifications serving as potential biomarkers for cancer diagnosis and targets for therapy.
Keywords: cancers, m6A, m6A modulators, mRNA, ncRNA, therapy
N6‐methyladenosine (m6A) is a widespread regulator not only in messenger RNAs but also in non‐coding RNAs. m6A can be reversibly methylated and unmethylated by “m6A writer” and “m6A eraser” proteins. The methylated RNA is recognized by m6A “readers” protein and then to modulate cancer progression. Target m6A regulators has been becoming a promising approach for cancer diagnosis and treatment.
Abbreviations
- ALKBH
AlkB homolog
- AML
acute myeloid leukemia
- APA
alternative polyadenylation
- BC
breast cancer
- BCA
bladder cancer
- carRNA
chromosome‐associated regulatory RNA
- CDS
protein‐coding sequence
- ceRNA
competitive endogenous RNA
- circRNA
circular RNA
- CRC
colorectal cancer
- eIF3
eukaryotic initiation factor 3
- EMT
epithelial–mesenchymal transition
- EOC
endometrioid cancer
- eRNA
enhancer RNA
- ESCC
esophageal squamous cell carcinoma
- FTO
obesity‐associated protein
- GBM
glioblastoma
- GC
gastric cancer
- HCC
hepatocellular carcinoma
- HDGF
hepatoma‐derived growth factor
- hESCs
human embryonic stem cells
- HMGA
high mobility group protein
- HNRNPs
heterogeneous nuclear ribonucleoproteins
- HMGA2
high mobility group protein 2
- IGF2BPs
insulin‐like growth factor 2 mRNA‐binding proteins
- KIAA1429/virilizer
virilizer like m6A methyltransferase associated protein
- LC
lung cancer
- lncRNA
long non‐coding RNA
- lincRNA
long intergenic non‐coding RNA
- LUAD
lung adenocarcinoma
- m6A
N6‐methyladenosine
- METTL3
methyltransferase‐like 3
- MTase
methyltransferase
- MTC
methyltransferase complex
- NPC
nasopharyngeal carcinoma
- NSCLC
non–small cell lung cancer
- OC
ovarian cancer
- OS
osteosarcoma
- paRNA
promoter‐associated RNA
- PAAD
pancreatic adenocarcinoma
- PPP
pentose phosphate pathway
- PRAD
prostate cancer
- Prrc2a
proline‐rich coiled‐coil 2 A
- RB
retinoblastoma
- RBM
RNA‐binding motif
- RBP
RNA‐binding protein
- RCC
renal cell carcinoma
- RNAP II
RNA polymerase II
- R‐2HG
R‐2‐hydroxyglutarate
- rRNA
ribosomal RNA
- SAM
S‐adenosylmethionine
- SJ
splice junction
- SRSF3
serine and arginine‐rich splicing factor 3
- TC
thyroid cancer
- tRNA
transfer RNA
- UTR
untranslated terminal region
- WTAP
Wilms tumor 1–associated protein
- XIST
X‐inactive specific transcript
- YTH
YT521‐B homology
- ZCCHC4
CCHC zinc finger‐containing protein
- ZC3H13
zinc finger CCCH‐type containing 13
1. Introduction
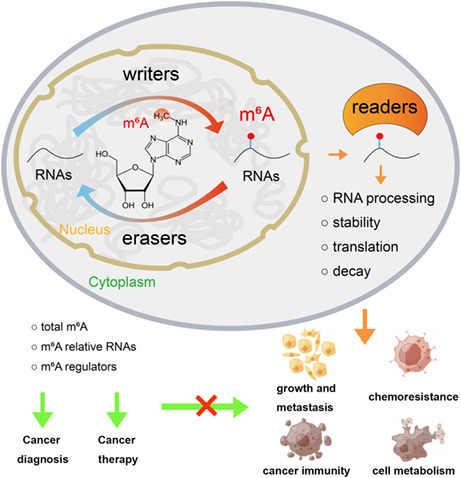
RNA modifications were discovered more than 50 years ago, and over 170 chemical modifications on RNA have so far been identified [1]. N 6‐methyladenosine (m6A) is the most prevalent internal modification on eukaryotic RNAs including messenger RNA (mRNA) and non‐coding RNA (ncRNA). The N6 position of adenosine can be reversibly methylated and unmethylated by ‘m6A writer’ and ‘m6A eraser’ proteins, respectively, and m6A RNA can be recognized and bound by ‘m6A reader’ proteins [2] (Fig. 1, Table 1, Box 1).
Fig. 1.
Molecular reaction for m6A methylation and its functions in cancer development. m6A writers (METTL3, METTL14, WTAP, VIRMA, RBM15, ZC3H13, METTL5, METTL16 and ZCCHC4) and m6A erasers (FTO and ALKBH5) mediate the m6A methylation/demethylation of RNAs, including mRNA, tRNA, rRNA, snRNA and pre‐miRNA. m6A readers (YTHDF1‐3, YTHDC1‐2, HNRNPs, IGF2BP1‐3 and eIF3), locating in either nucleus or cytoplasm, bind to RNA targets and play different roles in the regulation of RNA behaviors such as RNA processing and decay. All m6A modulators are involved in cancer growth and metastasis, cancer chemoresistance, cancer immunity and cell metabolism [3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20].
Table 1.
m6A writers, erasers and readers and their functions in cancers.
| Type | Protein | Role/effect | |
|---|---|---|---|
| Promote | Suppress | ||
| Writer | METTL3 |
Cancer progression
Cell differentiation and cell proliferation
|
Tumor metastasis
|
| METTL14 |
Leukemogenesis
Cancer progression
Tumor metastasis
Tumor malignancy
|
Tumor metastasis
Tumor malignancy
Cell self‐renewal and tumorigenesis
|
|
| METTL16 |
Cell proliferation
Translation and tumorigenesis
|
||
| METTL5 |
Cancer progression
Cell proliferation
|
||
| WTAP |
Cancer progression
|
||
| KIAA1429 |
Cancer progression
|
||
| RBM15 |
Cancer progression
|
||
| ZC3H13 |
Cell proliferation and invasion
|
||
| Eraser | FTO |
Leukemogenesis
Cancer progression
|
Stem cell self‐renewal
Tumor metastasis
|
| ALKBH5 |
Tumorigenesis
Cancer progression
|
Tumorigenesis
|
|
| Reader | YTHDF1 |
Tumorigenesis
Cancer progression
|
|
| YTHDF2 |
Stem cell self‐renewal
Cancer progression
Tumorigenesis
|
Cell proliferations
|
|
| YTHDF3 |
Tumorigenesis
Tumor metastasis
|
||
| YTHDC1 |
Cell proliferations
|
Tumorigenesis
|
|
| YTHDC2 |
Cancer progression
Tumor metastasis
|
Tumorigenesis
|
|
| IGF2BP1 |
Cancer progression
Stem cell stemness
Tumor metastasis
|
Cancer progression
|
|
| IGF2BP2 |
Cell proliferation
Tumor metastasis
|
||
| IGF2BP3 |
Cell proliferation
Angiogenesis
Tumor metastasis
|
||
| hnRNPR |
Cancer progression
|
||
Box 1. RNAs and m6A‐related proteins.
rRNA: ribosomal ribonucleic acid is the component of ribosomes to process protein synthesis. lncRNAs: are longer than 200 nucleotides that do not encode proteins, including both intergenic and genic non‐coding RNA. lincRNA: long intergenic non‐coding RNAs are longer than 200 nucleotides which constitute more than half of lncRNA transcripts in humans. LincRNAs are non‐coding RNA transcripts that make up most of the lncRNAs. miRNA: is a 21‐25nt single‐stranded non‐coding RNA. It plays a role in RNA silencing and post‐transcriptional regulation of gene expression. paRNA: promoter‐associated RNAs is a type of lncRNA, which could influence promoter activity of other genes. eRNA: enhancer RNA is a type of lncRNA transcribed from the DNA sequence of enhancer regions. circRNA: is a type of single‐stranded RNA formed into continuous loop. It also shows potential to code for proteins. m 6 A writer: is a methyltransferase complex (MTC), which catalyzes m6A deposition through transferring a methyl group from donor S‐adenosylmethionine (SAM) and includes METTL3, METTL14, WTAP, METTL16, METTL5, KIAA1429/Virilizer, RBM15, ZCCHC4 and ZC3H13; m 6 A eraser: is a demethylase which reverts m6A to adenosine on RNAs, including FTO and ALKBH5; m 6 A reader: is executer to exert functions of m6A and plays important roles in epigenetics, including YTH family proteins, HNRNPs, IGF2BPs, eIF3 and Prrc2a.
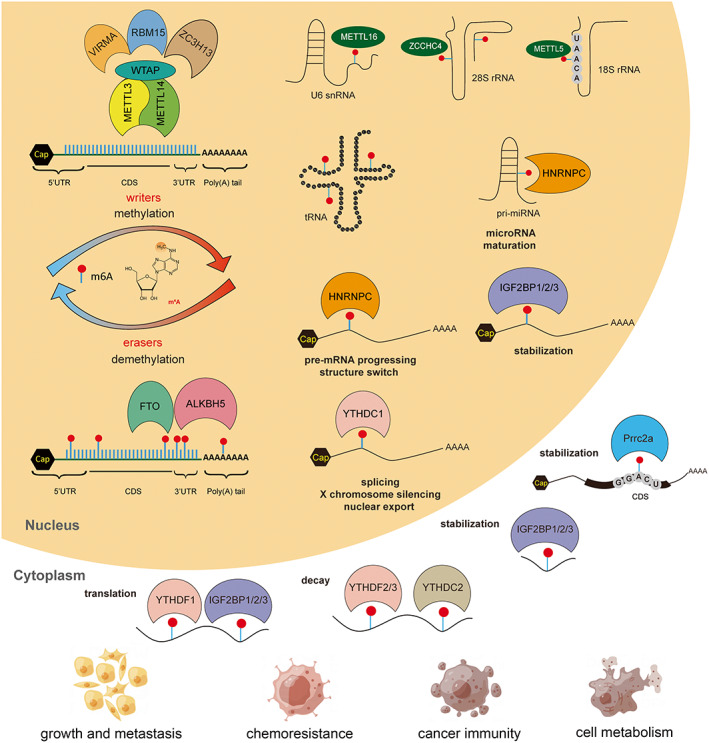
1.1. m6A writers, erasers and readers
The known m6A writers include METTL3 [3], METTL14 [3], WTAP [3], METTL16 [4], METTL5 [5], KIAA1429/Virilizer [6], RBM15 [6], ZCCHC4 [7] and ZC3H13 [8]. An m6A ‘writer’ is an MTase complex (MTC), which catalyzes m6A deposition by transferring a methyl group from donor S‐adenosylmethionine (SAM) [3]. METTL3 is a 70 kDa protein highly conserved in eukaryotic cells belonging to class I MTases, which contains a conserved SAM‐binding domain [3] to recognize the DRACH motif of RNA, whose consensus sequence is D = A/G/U, R = A/G and H = A/C/U [83]. METTL14 forms a heterodimer with METTL3, facilitating METTL3 binding with target RNA in MTC [3]. WTAP is indispensable to the MTC by binding with the N‐terminal helix of METTL3, acting as a regulatory subunit of MTC [84]. In the absence of WTAP, the RNA binding ability of the MTC is highly reduced [84]. KIAA1429, also known as VIRMA, tends to bind the 3′UTR, near mRNA stop codons, recruiting MTC to enhance region‐selective m6A methylation [6]. RBM15/15B, interacts with METTL3 in a WTAP‐dependent manner to support m6A modification and promote RNA splicing (Box 2) [6, 85]. ZC3H13 is required for the nuclear localization of the ZC3H13‐WTAP‐Virilizer‐Hakai complex to facilitate m6A methylation in 3′UTR of targets [8]. METTL5 is a newly discovered m6A writer of 18S ribosomal RNA (rRNA; Box 1), binding to a UAACA motif and catalyzing m6A 1832 in 18S rRNA [5]. METTL16 catalyzes m6A methylation on U6 spliceosomal snRNA, which is associated with the expression of SAM synthetase [4]; ZCCHC4 deposits m6A on a subset of mRNAs as well as 28S rRNA [7].
Box 2. Functional consequences of m6A modification on mRNA.
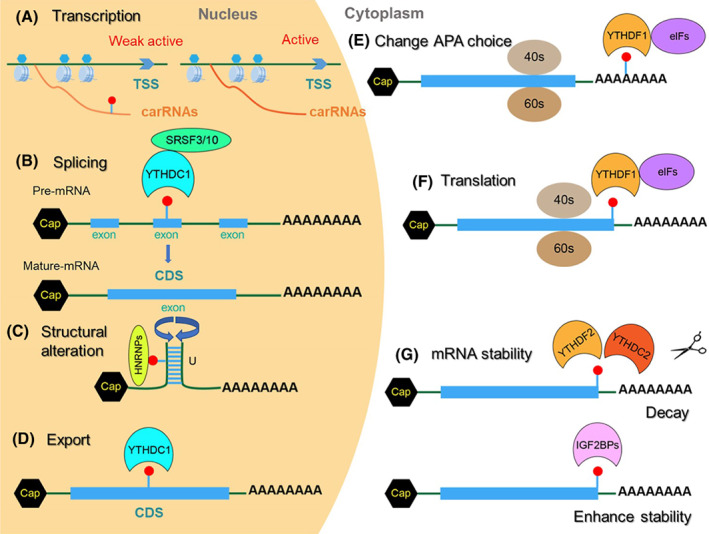
mRNA transcription: mRNA transcription can be regulated by chromosome‐associated regulatory RNAs (carRNAs). carRNAs can be modified by m6A methylation. Reduction of m6A in selected carRNAs elevates carRNAs levels and promotes an open chromatin state and downstream transcription [86]. Moreover, YTHDC1 recruits the H3K9me2 demethylase KDM3B to m6A‐associated chromatin region, where H3K9me2 demethylation initiates gene expression [87]. Finally, m6A methyltransferase complex promotes RNAP II pause release and affects nascent RNA transcription [88] (Fig. 2A).
Splicing: m6A participates in pre‐mRNA processing and regulation of alternative splicing [89]. Early m6A was deposited near the splice junctions (SJs) and introns of nascent RNA, whilst these signals disappeared in mature RNAs [90]. Early co‐transcriptional m6A deposition near SJs promotes fast splicing, and the presence of m6A modifications in introns is associated with long, slowly processed introns and alternative splicing events. In addition, YTHDC1 can recognize m6A on alternative exons, which recruits the splicing factor serine and arginine‐rich splicing factor 3 (SRSF3) but restricts binding with exon‐skipping factor SRSF10, resulting in exon inclusion during alternative splicing [14] (Fig. 2B).
mRNA structure: RNA secondary structure is formed by nucleotide bases paired within its sequence via hydrogen bonding, forming the scaffold and the folding of RNA three‐dimensional structures [91]. m6A can weaken the A/U pairings, leading to the alterations of RNA secondary structure and thermostability of RNA duplexes. These structural changes would influence the interaction of related regulatory proteins, such as hnRNP and HNRNPs, leading to the inhibition of RNA‐protein interactions [16] (Fig. 2C).
mRNA export: m6A might act as export signals for mRNAs. Treatment with methylase inhibitor S‐tubercidinylhomocysteine reduces m6A level and attenuates mRNA export [92]. ALKBH5 knockdown leads to m6A‐modified mRNA accumulation in cytoplasm [93], whereas YTHDC1 knockdown extends residence time for nuclear m6A‐containing mRNAs, with an accumulation of transcripts in the nucleus and accompanying depletion within the cytoplasm [94] (Fig. 2D).
Alternative polyadenylation (APA): APA is an important post‐transcriptional regulation mechanism that targets the 3′end of pre‐mRNA during mRNA maturation in eukaryotic cells. As a result of APA, there are multiple transcripts for over half of human genes [95]. Bioinformatic analysis suggests a possible connection of m6A to polyA site choices in mRNA: m6A is preferentially located within 3′UTRs containing multiple APA and regulates proximal APA choice [96]. As APA regulates the stability, translation and location of mRNAs, m6A might also regulate mRNA behaviors indirectly via modulation of APA choice (Fig. 2E).
Translation: m6A modulates translational dynamics by potentially influencing the progress of different stages. 5′UTR m6A promotes cap‐independent translation by directly binding to eIF3 [97]; CDS m6A acts as a barrier to tRNA accommodation to regulate translation‐elongation dynamics [98]; 3′UTR m6A facilitates the translation by METTL3‐eIF3h‐mediated mRNA circularization [99]. m6A might also play roles in both translation initiation and elongation: CDS m6A can enhance mRNA translation by relieving ribosome stalling [100] or trigger polysome‐mediated translation in the case of Snail mRNA [101]; Conversely, decrease m6A promoted eIF4E3‐mediated cap‐independent translation of β‐catenin [102]. Whilst m6A deposition in transcripts may regulate mRNA translation, a complete picture of how translation is regulated is currently lacking (Fig. 2F).
mRNA stability: m6A modification has been shown to regulate mRNA stability, dependent on its bound m6A readers. m6A‐containing mRNAs underwent two distinct pathways of rapid degradation: deadenylation via YTHDF2‐CCR4/NOT (deadenylase) complex or YTHDF2‐HRSP12‐RNase P/MRP (endoribonuclease) complex [103]. m6A‐modified mRNAs can also be targeted toward an opposite fate. For instance, IGF2BP proteins can increase the half‐lives of m6A‐containing mRNAs [17] (Fig. 2G).
Conversely, ‘erasers’ revert m6A to adenosine on RNAs. The identified m6A erasers are fat mass and obesity‐associated protein (FTO) [104] and AlkB Homolog 5 (ALKBH5) [93]. Both FTO and ALKBH5 require ferrum and α‐ketoglutaric acid as co‐factors to remove m6A in eukaryotic cells [105]. However, they demethylate different targets due to their different structural interaction. FTO contains a C‐terminal domain which is easy to engage in protein–RNA interaction, while the isolated N‐terminal domain is incompetent for catalysis [106]. Regarding m6A in mRNA, cap m6Am, m1A and m6Am in snRNA are the substrates of FTO in vivo [9]. ALKBH5, which is predominant in the nucleus, can directly bind to RNA substrates and be a part of the mRNA‐bound proteome [93, 107].
‘m6A readers’ are executers to exert functions of m6A and play important roles in epigenetics, including YTH family proteins, HNRNPs, IGF2BPs, eIF3 and Prrc2a [108]. Among them, YTH family proteins are the most studied m6A readers, including YTHDF1, YTHDF2, YTHDF3, YTHDC1 and YTHDC2 [109]. Among them, YTHDF1‐3 paralogs have been reported to mediate the major effects of m6A on RNA regulations [110]: YTHDF1 enhances mRNA translation [10]; YTHDF2 accelerates the decay of m6A‐modified transcripts [11]; YTHDF3 enhances both mRNA translation and degradation (Box 2, Fig. 2) [12]. Aside from YTH conserved domain, YTHDC1 and YTHDC2 are not related to paralogs proteins and play different roles in cells [13]: YTHDC1 is the only known m6A reader in the nucleus, regulating RNA splicing and translocation [14], while YTHDC2 enhances translation of target RNAs by recruiting other protein complexes [15]. Additional m6A reader proteins have been identified such as the HNRNP family containing hnRNPA2/B1, HNRNPC and HNRNPF involved in promoting primary microRNA processing [111], mRNA alternative splicing, processing of target transcripts and interaction of m6A‐rich long non‐coding RNA (lncRNA; Box 1) [16]. m6A readers in the IGF2BPs family include IGF2BP1, IGF2BP2 and IGF2BP3. The binding of m6A‐methylated mRNA with IGF2BPs protein resulted in the up‐regulation of mRNA stability (Box 2) [17]. Recently, Prrc2a was identified as a novel m6A reader binding to GGACU motif in the CDS region of mRNAs via an m6A‐dependent manner, which then stabilized m6A‐modified mRNAs [18].
Fig. 2.
Functions of m6A modification on mRNA. A schematic image of the roles of m6A on mRNA. m6A modification on mRNA plays different roles in nucleus and cytoplasm. (A) Regulation of transcription, (B) regulation of splicing, (C) alteration of RNA structure, (D) facilitation of mRNA export, (E) determination of APA, (F) regulation of translation and (G) regulation of mRNA stability.
1.2. m6A profiles of RNAs
m6A modifications can be found in mRNA, rRNA and various ncRNAs, such as lncRNA, long intergenic non‐coding RNA (lincRNA), microRNA (miRNA), promoter‐associated RNA (paRNA), enhancer RNA (eRNA) and circular RNA (circRNA) (Box 1, 2, 3, 4) [112]. The sites of m6A marks on an RNA molecule seem to affect RNA biogenesis, processing, localization, translation and metabolism [113] (Figs 1 and 2; Box 2, 3, 4).
Box 3. Functional consequences of m6A for lncRNA.
Structure switch and RNA stability: m6A may alter the lncRNA structure switch via interfering with the base pairing and therefore affecting its stability [131]. m6A methylation of A2577 and A2515 in lncRNA MALAT1 promote its binding to HNRNPC and HNRNPG, and loss of METTL3 reduces the accessibility of MALAT1 to HNRNPC/HNRNPG [16, 132]. A high level of m6A modification increases the stability of the lncRNA FAM225A [133] and METTL3 increases the stability of LINC00958 [134] and lncRNA RMBP [135] via decreasing the RNA degradation rate. In addition, m6A modification of DIAPH1‐AS1 enhances its stability by relying on the IGF2BP2‐dependent pathway [136].
Regulation of competitive endogenous RNA (ceRNA): lncRNAs can act as miRNA sponges and mediate ceRNA to regulate the biological functions of miRNAs. On one hand, m6A increases the stability of lncRNAs to promote ‘sponging’ miRNAs to regulate their gene expression. For instance, LINC00958 sponges miR‐3619‐5p to increase hepatoma‐derived growth factor (HDGF) expression [134] and MALAT1 acts as ceRNA to abolish the gene silencing function of miR‐1914‐3p [137]. On the other hand, m6A affects RNA‐RNA interactions via RRACU m6A sequence motifs interfering binding efficiency. For instance, knockdown of METTL3 suppresses the binding between linc1281 and let‐7 miRNA, thus sequestering let‐7 functions and regulating the differentiation of hESCs [138].
Gene silencing and protein binding potential: Silencing of gene transcription on the X chromosome is mediated by the lncRNA X‐inactive specific transcript (XIST). m6A deposition has been identified in XIST, which is necessary for XIST‐mediated transcriptional repression of X‐linked genes, such as Gpc4 and Atrx, and X chromosome inactivation [139]. In addition, methylation of lncRNA Pvt1 transcripts stabilizes the MYC protein by enhancing the Pvt1‐MYC interaction in epidermal progenitor cells [140].
Subcellular localization: m6A modulates the subcellular localization of lncRNA. For instance, m6A methylation involves in the up‐regulation of RP11 by increasing its nuclear accumulation due to the m6A‐enhancing interaction of RP11 with hnRNPA2B1 [141].
Box 4. Functional consequences of m6A for circRNA.
Biogenesis: circRNA biogenesis requires the back splicing, which occurs at the m6A‐enriched sites for a subset of circRNAs. These m6A‐enriched sites are usually located around the start and stop codons in linear mRNAs [222]. A recent study also revealed that METTL3 and YTHDC1 could regulate the biogenesis of circ‐ZNF609 via regulating circ‐ZNF609 level [223].
Degradation and stability: Deposition of m6A on circRNA have dual effect on the regulation of circRNA stability: promotes degradation and enhances stability. m6A in circRNA can be recognized by YTHDF2, which recruits the RNase R/MRP complex to cleave circRNA, and therefore promotes the degradation of circRNA [224]. Conversely, m6A stabilizes the expression of circCUX1 [225] and circRNA‐SORE [226]. It is likely that the m6A‐regulated circRNA stability is dependent on the recognition of different m6A readers or the deposition location of m6A in circRNA.
Initiation of extensive translation: Most of the circRNAs are ncRNAs, which fail to recruit translation initiation complexes due to a lack of 5′UTR and m7G cap. However, some circRNAs can be m6A modified and recognized by YTHDF3, which therefore recruit the pre‐initiation complex to circRNAs. This m6A‐mediated extensive translation of circRNAs is cap‐independent. Nowadays, over a hundred peptides produced by circRNAs have been identified in germ cells [227]. YTHDF3 and eIF4G2 are physically associated with endogenous circ‐ZNF609 and are essential for its translation driven by m6A [223].
Cytoplasmic export: m6A‐modified circRNA, circNSUN2, could be recognized by YTHDC1 and facilitate its export to cytoplasm [213]. Cytoplasmic circNSUN2 can form an RNA‐protein ternary complex with IGF2BP2 and high mobility group protein 2 (HMGA2), which stabilizes HMGA mRNA and promotes metastasis of CRC [213].
Regulation of biological functions: circRNAs often act as miRNA ‘sponges’. m6A on circRNA influences the binding between circRNA and miRNA, thereby affecting the miRNA‐silencing functions on target mRNAs [123] or sequestering target miRNAs in the cytoplasm [228]. m6A depositions on circRNA can be used as markers to identify ‘self’ and ‘foreign’ circRNA during viral defense [229]. For instance, circE7 from the HPV virus can be modified by m6A and labeled as ‘self’ circRNA, which facilitates the virus's escape from the host antiviral immune response [229].
m6A is the most abundant internal modification in mammalian mRNA [114]. There are more than 7000 human transcripts that contain m6A [115, 116] and over 12 000 m6A sites are identified in the RRACH motif, with 70% and 30% frequency of ‐G‐m6A‐C and ‐A‐m6A‐C, respectively [117]. m6A has been widely observed in the CDS (~ 50%), 3′UTR (~ 40%) near the stop codons [116], 5′UTR (> 7%) and intronic regions (> 2%) [116]. The enriched m6A observed near the stop codon and in the 3′UTR suggests a definite functional role of m6A [116]. In addition, over 54% of mRNAs containing at least two m6A sites are frequently clustered in the adjacent regions of transcripts [116], suggesting a potential role of m6A in RNA processing.
In rRNA, two conserved m6A sites, m6A1832 in 18S rRNA and m6A4220 in 28S rRNA, have been identified in X. laevis and mammalians [118, 119]. Human rRNA modifications are introduced during ribosome biogenesis [7], where m6A 1832 in 18S rRNA is deposited in one of the last steps in 40S maturation. Both m6A modifications in rRNAs tend to localize in the functionally important regions of rRNAs, playing roles in the promotion of protein synthesis [7, 39, 120], but has no impact on overall processing or maturation of rRNA [5, 7].
m6A modifications have been identified in other ncRNAs. Over 700 lncRNAs with m6A methylation were identified [121], which is widespread in the entire body of lncRNAs and tends to be present in lncRNAs undergoing alternative splicing [122]. Over 1400 circRNAs, accounted for 54% of total circRNAs, contain m6A modification [123]. m6A is also isolated from tRNAVal [19]. Despite the consensus reports show that m6A methylation exists on tRNA, scientists usually hard to find m6A abundance details on tRNA [24]. In lincRNA, the most frequent consensus motif for m6A deposition is GG/A(m6A)CH, which is slightly different from that in mRNAs [124]. Compared with unmodified lincRNAs, m6A‐modified lincRNAs tend to be alternatively spliced [122]. In miRNA, m6A modification can influence the maturation of miRNAs [125, 126]. Our previous study indicated that METTL3 can increase the splicing of precursor miR‐143‐3p to facilitate its biogenesis [127]. In addition, m6A could indirectly regulate the biological functions of miRNAs [112]: (a) m6A interferes with miRNA‐mRNA interactions by altering the RNA secondary structure of alternative polyadenylation (APA) choice in 3′UTR of targets (Box 2) [128]; (b) m6A could stabilize lncRNAs to act as ceRNA to regulate the activity and function of miRISC, resulting in the modulation of gene expression (Box 3) [129] and (c) miRNAs can also affect the m6A of targets via occupying the 3′UTR m6A site of mRNAs [130].
As a result, m6A methylation is involved in various cellular functions [142]. Increasing evidence supports that m6A levels are often up‐regulated in RNA molecules isolated from various cancers, and this RNA modification appears to have roles in tumorigenesis and cancer progression [143, 144]. Therefore, targeting m6A methylation might act as a potential approach for cancer treatment. Meanwhile, alteration of m6A level is being considered as a predictive biomarker for cancer diagnosis [143, 145, 146].
In this review, we first review the changes of m6A methylation modification and the alteration of gene expression of m6A writers, erasers and readers in different types of cancers. Next, we examine how m6A methylation is associated with tumorigenesis and cancer progression, and the possible mechanisms through which m6A methylation of mRNA and ncRNA targets affects tumor cell proliferation, metastasis, chemoresistance, cancer microenvironment and cancer metabolism. In addition, we discuss the potential of targeting m6A modifications for cancer diagnosis and therapy and highlight future challenges. In addition, we have shown the functional consequences of m6A modification on mRNA in Box 2.
2. Regulation of m6A writers in cancers
2.1. METTL3
As the major RNA m6A writer, the expression of METTL3 is closely associated with the genesis and development of cancers. In TCGA datasets, METTL3 is overexpressed in a variety of cancers and shows high mutations in bladder cancer (BCA), endometrioid cancer (EOC) and colon cancer. In pancreatic adenocarcinoma (PAAD), cigarette smoke condensate induces hypomethylation of METTL3 promoter and excessively maturates miR‐25 to promote cancer progression [147]. In CRC, butyrate, a classical intestinal microbial metabolite, can down‐regulate the expression of METTL3 to inhibit CRC development [148]. In GC, P300‐mediated H3K27 acetylation activation in the promoter region of METTL3 induces its mRNA transcription (Box 2) to promote tumor angiogenesis [149]. In lung cancer (LC), SUMOylation of METTL3 significantly represses its m6A MTase activity, resulting in the enhancement of tumorigenesis [150]. We previously identified the TATA‐binding protein can transcriptionally increase the expression of METTL3 in cervical cancer cells via binding to its promoter [25]. In addition, miRNAs including miR‐186 [151], miR‐4429 [152], miR‐600 [153] and let‐7g [22], are proposed to bind with METTL3 mRNA to regulate its expression. METTL3 function in cancer is shown in Table 1.
2.2. METTL14
METTL14 expression is dysregulated in cancers through different mechanisms. In breast cancer (BC), METTL14 can be stabilized by AURKA by inhibiting proteasomal‐dependent degradation [154]. In AML, METTL14 expression is negatively regulated by SPI1 [28]. In CRC, KDM5C mediated demethylation of H3K4me3 in the promoter region of METTL14 to inhibit its transcription [33]. In addition to expression dysregulation, METTL14 can be directly recruited by LNC942 to promote cancer progression of BC [29]. Interestingly, Lang et al. [155] revealed that viral‐encoded latent oncoprotein EBNA3C activated transcription of METTL14 and directly interacted with METTL14 to enhance its stability in viral‐associated tumorigenesis. METTL14 function in cancer is shown in Table 1.
2.3. WTAP
WTAP, which is mainly regulated by ncRNAs in cancers, is commonly up‐regulated in many cancer types [156, 157]. In osteosarcoma, SNHG10 up‐regulates WTAP through decreasing miR‐141‐3p expression [158]. In BCA, circ0008399 binds to WTAP to promote the formation of MTC [159]. In diffuse large B‐cell lymphoma (DLBC), piRNA‐30473 up‐regulates WTAP to promote tumorigenesis [160]. Intriguingly, METTL3 regulates the homeostasis of WTAP protein via an m6A‐dependent manner [161]. Interestingly, m6A modification can stabilize WTAPP1 RNA, which further bound its protein‐coding counterpart WTAP mRNA and recruited more eIF3 translation initiation complex to promote WTAP translation [162], suggesting a close crosslink between m6A and WTAP. WTAP function in cancer is shown in Table 1.
2.4. Other m6A writers
Less research has been done on the regulation of other m6A writers in cancers. For instance, Wu et al. [163] reported that ZC3H13 could be down‐regulated by miR‐362‐3p/miR‐425‐5p in hepatocellular carcinoma (HCC). Tran et al. [5] showed that METTL5 formed a heterodimeric complex with TRMT112 to gain metabolic stability. Substantial efforts are required to promote our understanding of how other m6A writers are modulated in cancers. Other m6A writers function in cancer are shown in Table 1.
Dysregulation of m6A writers is widely observed in different types of cancers, which has been considered to be one of the most important factors for the development of cancers. Both mRNA and ncRNA are commonly targeted by m6A writers in cancers, and the effects of m6A writers seems complex, since it can act as either promoter or suppressor to modulate the development of cancers via various mechanisms.
3. Regulation of m6A erasers in cancers
3.1. FTO
As the first identified RNA m6A demethylase, FTO is the most studied and found to be frequently dysregulated in its expression, localization, post‐translational modification and functions in various types of cancers. In CRC, hypoxia could decrease FTO expression via increasing its ubiquitin‐mediated protein degradation [47]. In EOC, the nuclear localization of FTO increases and then enhances cancer progress via the mTOR signaling pathway [164]. As to the post‐translational of FTO, p62 negatively regulates FTO stability via directly binding with FTO to facilitate the degradation of FTO protein via autophagy [165]. In AML, FTO promotes the stability of MYC/CEBPA transcripts and leads to the enhancement of relevant pathways [166]. Additionally, a recent study discovered that zinc finger protein 217 [167] and nicotinamide adenine dinucleotide phosphate [168] uncovered roles in FTO‐dependent adipogenic regulation. FTO function in cancer is shown in Table 1.
3.2. ALKBH5
Increasing research has focused on exploring the mechanisms responsible for the dysregulation of ALKBH5 in cancers: Hypoxia: ALKBH5 is a direct target of HIF‐1α, indicating that ALKBH5 may be involved in the regulation of cellular responses to hypoxia [169]. In addition, ALKBH5 is significantly up‐regulated under hypoxic conditions, while knockdown of HIF‐1α and/or HIF‐2α abrogates this effect in human BC cells [170]. Histone modifications: Wang et al. [171] found that histone demethylase KDM4C regulated ALKBH5 expression via increasing chromatin accessibility of ALKBH5 locus, by reducing H3K9me3 levels and promoting the recruitment of MYB and Pol II in AML. Qu et al. [172] identified that the highly expressed ALKBH5 was induced by HBx‐mediated H3K4me3 modification of ALKBH5 promoter in a WDR5‐dependent manner after HBV infection. Hao et al. [173] showed that EP300‐induced H3K27 acetylation increased ALKBH5 expression in uveal melanoma (UVM). Transcription factors: Guo et al. [174] described that p53 interacted with the ALKBH5 promoter, transcriptionally activating ALKBH5 and indirectly reducing m6A amounts in PAAD. ncRNAs: The lncRNA FOXM1‐AS enhanced ALKBH5 binding to FOXM1 nascent mRNA in glioblastoma (GBM) cells [49]. CircRNA cIARS regulates ferroptosis in HCC cells through physically interacting with ALKBH5 [175]. ALKBH5 function in cancer is shown in Table 1.
The effect of m6A erasers on cancer development has been studied extensively. Similar to m6A writers, both m6A erasers play essential roles during cancer development. It's noteworthy that the expression of m6A erasers is sensitive to the extracellular environment such as hypoxia, hinting that m6A erasers might be a potential therapeutic target to increase the efficiency of novel cancer treatments such as hyperbaric oxygen therapy. In addition, expression of m6A erasers is commonly associated with the transcription of RNA targets and the transduction of cellular signaling, showing the global effect of m6A erasers in cells.
4. Regulation of m6A modification readers
4.1. YTH‐containing proteins
The expressions of YTH domain‐containing proteins in cancers are regulated by different mechanisms. Smoking and hypoxia conditions were demonstrated to closely correlate with the expression level of YTH proteins. YTHDC2 was significantly reduced in both LC cells and cigarette smoke‐exposed cells [176]. Hypoxia induces YTHDF2 overexpression via activation of the mTOR/AKT axis during the progression of lung squamous cell carcinoma [177]. Hypoxia can also induce a specific switch in the YTHDC1 expression pattern toward the two non‐protein coding mRNA variants [178]. HIF1α can on one hand promote the transcription activity of the YTHDF2, and on the other hand bind to the 5'UTR of YTHDF2 mRNA [179]. In ocular melanoma, transcription of YTHDF2 is activated by histone acetylation [57]. It has been reported that Musashi‐1 (MSI1) up‐regulated YTHDF1 by stabilizing YTHDF1 mRNA in GBM cells [180]. In addition, microRNAs including miR‐139‐5p [181], miR‐145 [182, 183], miR‐3436 [184], miR‐376c [185], miR‐454‐3p [186], miRNA‐495 [187] have been proposed to suppress YTH proteins by targeting their mRNAs in various cancers. YTHDF1‐3 and YTHDC1‐2 functions are shown in Table 1.
In addition, YTH proteins are also regulated by post‐translational modification. Fang et al. [188] showed that EGFR/SRC/ERK signaling phosphorylated YTHDF2 at Serine‐39 and Threonine‐381, therefore stabilizing YTHDF2 protein to promote cholesterol dysregulation and invasive growth of GBM. In contrast, Xu et al. [189] unveiled that FBW7 counteracted the tumor‐promoting effect of YTHDF2 by inducing proteasomal degradation of YTHDF2 in ovarian cancer (OV).
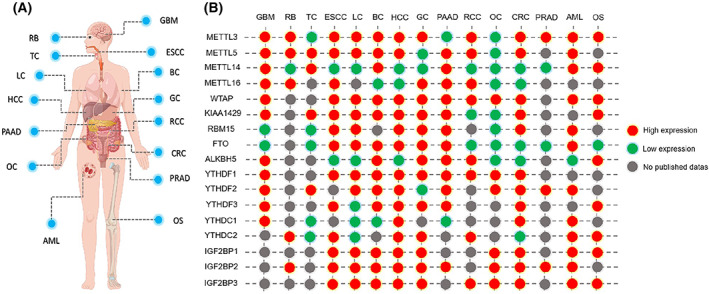
4.2. IGF2BPs
IGF2BP1: IGF2BP1 was found to be commonly and significantly up‐regulated in almost all cancer cell lines (Fig. 3) [190, 191, 192]. In HCC and GC, lncRNA HCG11 can interact with IGF2BP1 and enhance its physical interaction with c‐Myc mRNA to promote tumorigenesis [193, 194]. In human intrahepatic cholangiocarcinoma, miR‐885‐5p promotes the down‐regulation of IGF2BP1 to inhibit cell proliferation and metastasis [195]. IGF2BP2: HMGAs are crucial for the expression of IGF2BP2. HMGA1 suppressed the expression of IGF2BP2, which in turn bound and stabilized HMGA1 mRNA to promote cell proliferation [196]. HMGA2 can also promote IGF2BP2 transcription by binding to the AT‐rich region of the IGF2BP2 gene in cooperation with NF‐κB [197]. In addition, Lai et al. [198] unveiled that IGF2BP2 activity could be mediated by mTOR, a major effector downstream of PI3K/Akt signaling. IGF2BP3: Similar to IGF2BP1, a major mechanism of IGF2BP3 regulation is based on its complex interaction with the ncRNA machinery. For example, hsa_circ_0003258 is physically bound to IGF2BP3 in the cytoplasm to activate ERK signaling pathway in prostate cancer (PRAD) [76]. circIGHG directly binds with miR‐142‐5p and consequently elevates IGF2BP3 activity in oral squamous cell carcinoma [199]. IGF2BP1‐3 function are shown in Table 1.
Fig. 3.
Abundances of RNA modifiers in human cancers. Comparison of expression abundance among m6A modifiers in different types of cancers. (A) The construction of the human body considered in different types of tumors. (B) The gene expression levels of m6A modifiers. The expression levels of modifiers are compared between cancer and normal tissues. Differences of which over 1.5‐fold are marked. Red plots annotated modifiers are highly expressed in tumor compared with the normal tissues, whereas green plots annotated modifiers are low expressed in tumor compared with the normal tissues. Grey plots represent that there is not enough data to identify the expression in indicated cancers. The source of the data is from the GEPIA database [200].
4.3. hnRNPCs
hnRNPCs including hnRNPA2/B1, HNRNPC, HNRNPE and HNRNPH are found to be prevalently and significantly up‐regulated expression in a variety of tumors associated with cancer cells metastasis [77, 78, 79, 80]. hnRNPA2/B1 and HNRNPC: both hnRNPA2/B1 and HNRNPC are up‐regulated in tumors [201]. However, their up‐regulated mechanisms remain to be elucidated [80]; hNPNPCs could directly bind with oncogenes to control tumorigenesis, including regulating RNA splicing, RNA exportation, RNA expression, RNA stability and translation (Box 2) [78, 202, 203]. HNRNPE: For instance, Breege et al. [79] demonstrated that E3 ubiquitin ligase ARIH1 could regulate hnRNP‐E1 to promote BC cells invasion. HNRNPH: HNRNPH could interact with a broad of target to act as splicing factor in tumor progression. The functions of hnRNPRs are shown in Table 1.
m6A readers are the executors of m6A marks, leading to various regulatory effects on targets and, therefore, affecting the cellular events. It is worth to notice that the relationship between m6A readers and RNAs are not straightforward. On the one hand, m6A readers can modulate the expression and/or biological functions of RNAs such as via RNA‐RNA interaction. On the other hand, the activity or expression of m6A readers can be regulated by RNAs. Although increasing studies show the importance of m6A readers in the development of cancers, the detailed mechanisms of m6A readers and the cooperations among different m6A readers need to be further explored.
5. The m6A modification in cancer cell proliferation
5.1. Regulation via m6A on mRNAs
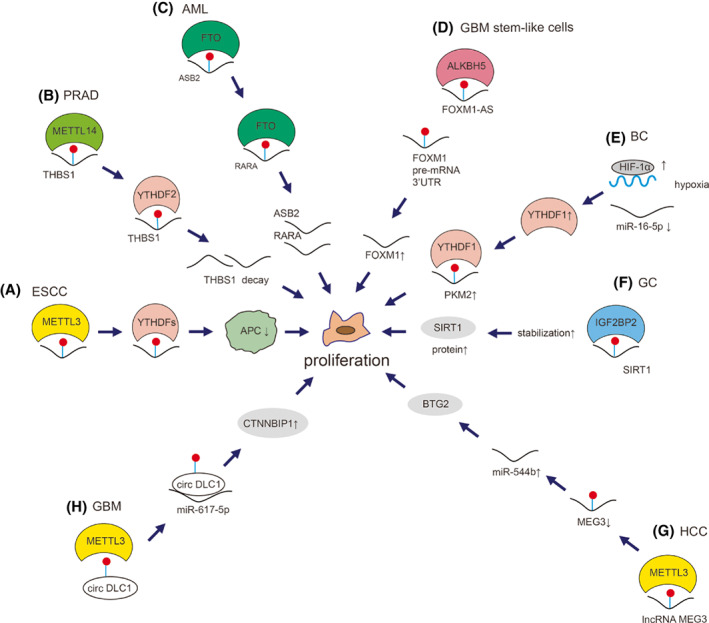
METTL3 can promote the cell proliferation of esophageal squamous cell carcinoma (ESCC) by decreasing APC expression mediated by APC mRNA m6A‐dependent YTHDFs binding (Fig. 4A) [204]. METTL14 can promote PRAD cell proliferation by inhibiting THBS1 via an m6A‐YTHDF2‐dependent mechanism (Fig. 4B) [205]. FTO targets and suppresses the expression of ASB2 and RARA mRNA to promote cell proliferation and viability in AML (Fig. 4C) [44]. ALKBH5 demethylates the nascent transcripts of FOXM1 mRNA to enhance its expression, leading to the promotion of proliferation and tumorigenesis of GBM stem‐like cells (Fig. 4D) [49]. YTHDF1 mediates cell growth and metastasis of BC through regulating PKM2 mRNA to affect glycolysis (Fig. 4E) [206]. IGF2BP2 regulates the proliferation/migration of GC by recognizing the m6A modification sites of SIRT1 mRNA (Fig. 4F) [207].
Fig. 4.
Mechanism of m6A on cancer proliferation. m6A modulates the proliferation via various mechanisms in cancers. (A) METTL3‐mediated deposition of m6A decreases APC expression with YTHDFs binding in ESCC cells [204]. (B) METTL14‐mediated m6A modification on THBS1 mRNA promotes YTHDF2‐mediated THBS1 decay in PRAD cells [205]. (C) FTO‐mediated m6A modification on both ASB2 and RARA mRNA suppresses their expression in AML [44]. (D) ALKBH5 removes m6A on lncRNA FOXM1‐AS facilitating the interaction between FOXM1 3′UTR and ALKBH5 to promote the expression of FOXM1 in GBM stem‐like cells [49]. (E) tumor hypoxia induces HIF‐1α and decreases miR‐16‐5p level, resulting in the up‐regulation of YTHDF1 to promote the YTHDF1‐mediated PKM2 expression in BC cells [206]. (F) IGF2BP2 recognizes m6A on SIRT1 mRNA and stabilizes SIRT1 in GC cells [207]. (G) METTL3 deposits m6A on lncRNA MEG3, down‐regulating MEG3 levels and up‐regulating miR‐544 and, therefore, regulates BTG2 expression to represses proliferation of HCC cells [208]. (H) METTL3‐mediated m6A upregulates circDLC1 expression and the interaction between circDLC1 and miR‐671‐5p and, therefore, promotes CTNNBIP1 expression in GBM cells [209].
5.2. Regulation via m6A on ncRNAs
Wu et al. [208] showed that m6A‐induced lncRNA MEG3 suppressed the proliferation, migration and invasion of HCC cells through miR‐544b/BTG2 signaling (Fig. 4G). Wu et al. [209] determined that METTL3‐mediated m6A modification up‐regulated circDLC1 expression and promoted CTNNBIP1 transcription by sponging miR‐671‐5p, thus repressing the malignant proliferation of GBM (Fig. 4H).
The relationship between m6A modification and cancer cell proliferation has been drawing attention in recent years. The regulation and/or role of m6A in cell proliferation appears to be cancer type‐dependent. Furthermore, the regulatory effects of m6A on cell proliferation can be achieved through different mRNAs or ncRNAs, which could be positive or negative, mainly dependent on the m6A targets. Nevertheless, YTHDFs play more essential roles in the regulation of cell proliferation than other m6A readers.
6. The m6A modification in metastasis
6.1. Regulation via m6A on mRNAs
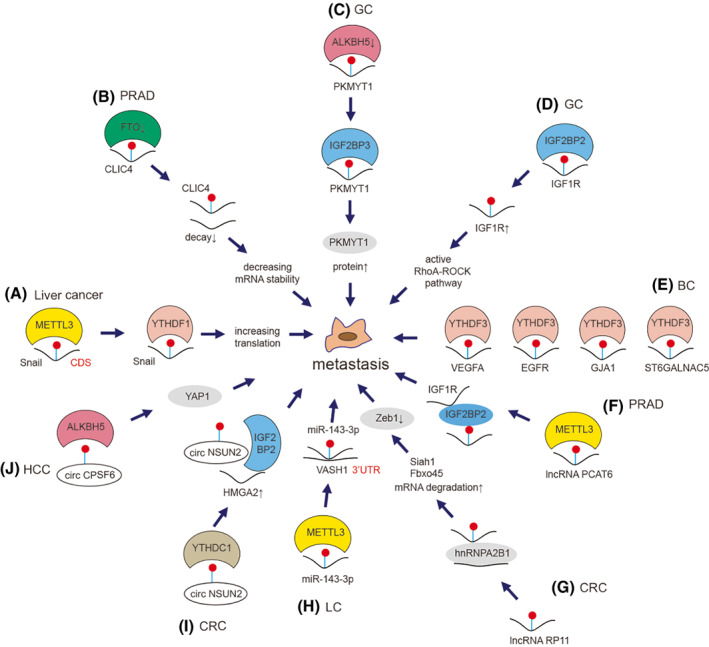
We previously highlighted that m6A was critical in the progress of epithelial–mesenchymal transition (EMT) since Snail could be modified by m6A in the CDS region and METTL3/YTHDF1 could mediate the expression and translation of Snail mRNA to regulate cancer cells growth and metastasis (Fig. 5A) [101]. Zou et al. [210] demonstrated that FTO suppressed PRAD cell proliferation and metastasis by reducing the degradation of CLIC4 mRNA in an m6A‐dependent manner (Fig. 5B). Hu et al. [211] found that ALKBH5 suppressed the invasion of GC via PKMYT1 m6A modification (Fig. 5C). IGF2BP2 increased the expression of IGF1R by identifying m6A modification sites in IGF1R mRNA, thus activating the RhoA‐ROCK pathway to promote GC metastasis (Fig. 5D) [212]. YTHDF3 induced the translation of m6A‐enriched gene transcripts such as ST6GALNAC5 and GJA1 to promote metastasis of BC in the brain (Fig. 5E) [60].
Fig. 5.
Mechanism of m6A on cancer metastasis. m6A modulates metastasis via various mechanisms in cancers. (A) METTL3 deposits m6A on CDS of Snail mRNA and then targeted by YTHDF1 to increase its translation to mediate metastasis in liver cancer [101]. (B) FTO‐mediated demethylation of m6A on CLIC4 mRNA decreases its stability, resulting in the repression of metastasis in PRAD cells [210]. (C) IGF2BP3 helps stabilize the mRNA stability of PKMYT1 via an ALKBH5‐dependent manner to regulate metastasis in GC cells [211]. (D) IGF2BP2 recognize m6A on IGF1R mRNA and increase its expression to activate RhoA‐ROCK pathway and therefore promote metastasis in GC cells [212]. (E) YTHDF3 promotes metastasis by inducing the translation of ST6GALNAC5, GJA1, EGFR and VEGFA mRNAs in BC cells [60]. (F) METTL3 promotes metastasis by methylating lncRNA PCAT6, which recognized by IGF2BP2 to stabilize IGF2BP2/IGF1R interaction in PRAD cells [73]. (G) m6A‐modificed lncRNA RP11 forms complex with hnRNPA2B1, accelerating the mRNA degradation of Siah1 and Fbxo45 to mediate metastasis by targeting of Zeb1 in CRC cells [141]. (H) m6A‐modificed miR‐143‐3p binds to the 3′UTR of VASH1 to promote metastasis in LC cells [127]. (I) YTHDC1 recognizes m6A‐modified circNSUN2 to enhance the circNSUN2/ HMGA2/IGF2BP2 interaction to promote metastasis in CRC cells [213]. (J) ALKBH5‐mediated demethylation of circCPSF6 promotes metastasis by activating YAP1 in HCC cells [214].
6.2. Regulation via m6A on ncRNAs
Lang et al. [73] showed that m6A‐modified lncRNA PCAT6 stabilized IGF2BP2/IGF1R to promote PRAD bone metastasis and tumor growth (Fig. 5F). We previously identified that m6A‐induced lncRNA RP11 triggered the dissemination of CRC cells via up‐regulation of Zeb1 (Fig. 5G) [141]. We found that m6A‐induced miR‐143‐3p promoted the brain metastasis of LC via regulation of VASH1 (Fig. 5H) [127]. Chen et al. [213] elucidated that m6A modification of circNSUN2 modulated the cytoplasmic export and stabilized HMGA2 to promote liver metastasis of CRC (Fig. 5I, Box 4). Furthermore, m6A‐modified circCPSF6 triggered the metastasis of HCC cells via activation of YAP1 (Fig. 5J) [214] (Table 2).
Table 2.
Non‐coding RNA influenced by m6A and its function in cancers.
| Type | Name | Effect | Mechanisms |
|---|---|---|---|
| circRNA | circ0008399 | Promotes cell cisplatin resistance (BCA) | Up‐regulation of TNFAIP3 [159] |
| circ_104075 | Stimulates YAP‐dependent tumorigenesis (HCC) | Up‐regulation of YAP by absorbing miR‐582‐3p [215] | |
| circDLC1 | Inhibits MMP1‐mediated cancer progression (LC) | Interaction with HuR and down‐regulation of MMP1 [41] | |
| miRNA | miR‐25‐3p | Promotes cancer progression (PRAD) | Activation of AKT‐p70S6K signaling [147] |
| miR‐96 | Promotes cancer occurrence and progression (CRC) | Regulation of AMPKα2‐FTO‐m6A/MYC axis [216] | |
| miR‐143‐3p | Promotes lung cancer brain metastasis (LC) | Inhibition of VASH1 [127] | |
| miR‐320b | Inhibits cancer angiogenesis and tumor growth (LC) | Inhibition of HNF4G, IGF2BP2 and TK1 [217] | |
| miR‐135 | Inhibits cell epithelial–mesenchymal transition (BC) | Regulation of miR‐135/ZNF217/METTL3/NANOG axis [218] | |
| lncRNA | FAM225A | Promotes tumorigenesis and metastasis (NPC) | Adsorption of miR‐590‐3p and miR‐1275 and up‐regulation of ITGB3 [133] |
| LCAT3 | Promotes tumorigenesis (LC) | Activation of c‐MYC [219] | |
| LINC00278 | Inhibits cell apoptosis (ESCC) | Down‐regulation of YY1BM [220] | |
| GAS5 | Inhibits cancer progression (CRC) | Phosphorylation and degradation of YAP [221] | |
| rRNA | 28S | Inhibits cell proliferation (HCC) | Reduction of global translation [7] |
| 18S | Promotes cell proliferation (BC) | Promotion of translation initiation [39] |
Metastasis is a major cause of cancer mortality, but its molecular mechanisms are severely understudied. Increasing research reveals the link between m6A and metastasis, showing that m6A may help modulate metastasis in cancer progression via different mechanisms. Among them, promotion of translation seems to be the major effect of m6A on the metastasis process, since YTHDF1/3 and IGF2BP2/3 are commonly involved. Despite mRNA, ncRNA including circRNA, lncRNA and miRNA are contributed to the regulation of metastasis, most of them are related to the up‐regulation of targets that promote metastasis.
7. The m6A modification in chemoresistance
7.1. Regulation via m6A on mRNAs
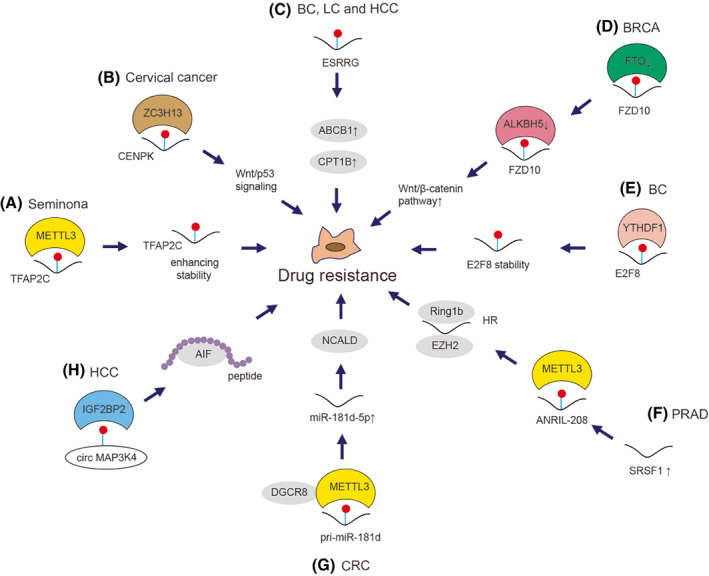
Wei et al. [230] showed that METTL3 enhanced the stability of TFAP2C mRNA by m6A modification in seminoma to potentiate resistance to cisplatin (Fig. 6A). Lin et al. [231] found that ZC3H13‐mediated m6A modification of CENPK mRNA promoted cervical cancer stemness and chemoresistance (Fig. 6B). We previously found that m6A can trigger the splicing of precursor ESRRG mRNA to confer chemoresistance of cancer cells through up‐regulation of ABCB1 and CPT1B (Fig. 6C) [232]. Fukumoto et al. [233] elucidated that down‐regulation of ALKBH5 and FTO increased m6A modified of FZD10 mRNA contributed to PARP inhibitors resistance in BRCA‐deficient epithelial ovarian cancers cells via up‐regulation of Wnt/β‐catenin pathway (Fig. 6D). YTHDF1 modulates E2F8 mRNA stability to promote BC cell growth, DNA damage repair and chemoresistance (Fig. 6E) [234].
Fig. 6.
Mechanism of m6A on cancer cell drug resistance. m6A modulates cancer cell drug resistance via various mechanisms in cancers. (A) METTL3 methylates TFAP2C, which enhances the stability of TFAP2C to increase chemoresistance in seminoma [230]. (B) ZC3H13 targets m6A on CENPK to activates Wnt/p53 signaling and therefore enhances chemoresistance in cervical cancer [231]. (C) m6A‐modified ESRRG mRNA upregulates protein expression of both ABCB1 and CPT1B to enhance chemoresistance in BC, LC and HCC cells [232]. (D) Down‐regulation of either ALKBH5 or FTO promotes m6A deposition on FZD10 mRNA, which activates Wnt/β‐catenin pathway to enhance chemoresistance in BRCA cells [233]. (E) YTHDF1 recognize m6A on E2F8, modulating E2F8 mRNA stability to enhance chemoresistance in BC cells [234]. (F) Upregulation of SRSF3 promotes ANRIL splicing and m6A modification of ANRIL in PRAD cells. ANRIL‐208 (one of the ANRIL spliceosomes) can enhance DNA homologous recombination repair (HR) capacity by forming a complex with Ring1b and EZH2, which enhances chemoresistance [235]. (G) METTL3‐dependent m6A modification of pri‐miR‐181d promotes miR‐181b‐5p process by DiGeorge Syndrome Critical Region 8 (DGCR8). miR‐181b‐5p directly targets neurocalcin δ (NCALD) to enhance chemoresistance in CRC cells [236]. (H) IGF2BP1 recognized the circMAP3K4 m6A modification and promotes its translation into a novel peptide, which interacts with AIF to prevent cisplatin‐induced apoptosis in HCC [237].
7.2. Regulation via m6A on ncRNAs
Wang et al. [235] found that the lncRNA ANRIL splicing is m6A modification‐related, which is mediated by SRSF3 and leads to the gemcitabine‐resistance of PRAD (Fig. 6F). Pan et al. [236] reported that METTL3‐dependent m6A methylation increased miR‐181d‐5p expression, then inhibited the 5‐Fluorouracil sensitivity of CRC cells by targeting neurocalcin δ (Fig. 6G). Duan et al. [237] demonstrated that m6A‐modified circMAP3K4 could encode a novel peptide to prevent apoptosis in HCC (Fig. 6H; Table 2)
Cancer cells gradually develop resistance to progressive chemotherapy, resulting in treatment failure that has become a serious clinical problem in cancer therapy. m6A modification has been reported to be involved in cancer cells developing drug resistance by regulating target either transcript level or translation. Unlike the dual effect of m6A modification on cell proliferation, m6A commonly promote the chemoresistance of cancer cells, since up‐regulation of METTL3 and down‐regulation of FTO/ALKBH5 are frequently observed in drug resistance cancer cells, hinting that targeting m6A might be a feasible direction for drug resistant cancer therapy.
8. The m6A modification and the tumor microenvironment
8.1. Regulation via m6A on mRNAs in immune cells
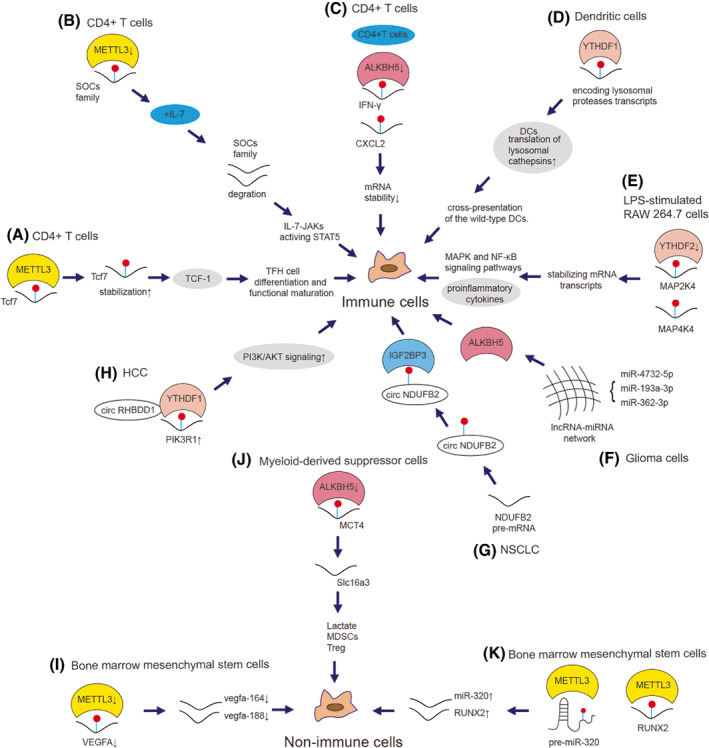
METTL3 in CD4+ T cells stabilizes Tcf7 mRNA to prevent their differentiation and functional maturation, further inhibiting the antibody response of B cells (Fig. 7A) [238]. METTL3 can also inhibit T‐cell homeostatic proliferation and differentiation by stabilization of the mRNAs of SOCS pLfamily, which are the STAT signaling inhibitory proteins (Fig. 7B) [239]. During the induced neuroinflammation, ALKBH5 deficiency in CD4+ T cells decreases the mRNA stability of IFN‐γ and CXCL2, thereby alleviating experimental autoimmune encephalomyelitis (Fig. 7C) [240]. YTHDF1 enhances the translation of mRNAs that encode lysosomal proteases, which can degrade antigens in lysosomes to down‐regulate the anti‐tumor immune responses of dendritic cells (Fig. 7D) [241]. YTHDF2 knockdown increases MAP2K4 and MAP4K4 expression levels via stabilizing mRNA transcripts, which activates MAPK and NF‐κB signaling pathways to promote the expression of proinflammatory cytokines (Fig. 7E) [242]. On the other hand, when it comes to non‐immune cells, METTL3 knockdown inhibits osteogenic differentiation and alternative splicing of VEGFA in bone marrow mesenchymal stem cells (Fig. 7I) [243]. ALKBH5 can modulate Mct4/Slc16a3 expression and lactate content of the tumor microenvironment to regulate the composition of tumor‐infiltrating Treg and myeloid‐derived suppressor cells (Fig. 7J) [244].
Fig. 7.
Mechanism of m6A on immune cells and non‐immune cells. m6A is involved in immunity via various mechanisms in cancers. (A) METTL3 methylates and stabilizes Tcf7 mRNA to enhance TCF‐1 level, which promotes TFH cell differentiation/function maturation to inhibit the antibody response of B cells in CD4+ T cells [238]. (B) Deficiency of METTL3 stabilizes SOCS family expression, which inhibits IL‐7‐mediated STAT5 activation and T‐cell homeostatic proliferation and differentiation in CD4+ T cells [239]. (C) ALKBH5 deficiency decreases IFN‐γ and CXCL2 expression to alleviate experimental autoimmune encephalomyelitis in CD4+ T cells [240]. (D) YTHDF1 downregulates the anti‐tumor immune responses by enhancing the translation of lysosomal proteases related mRNA in Dendritic cells [241]. (E) YTHDF2 knockdown increases the expression and stability of MAP2K4 and MAP4K4 mRNAs and, therefore, activates MAPK and NF‐κB signaling pathways to promote the expression of proinflammatory cytokines and aggravate the inflammatory response in LPS‐stimulated RAW 264.7 cells [242]. (F) lncRNA‐miRNA network such as miR‐4732, miR‐193a‐3p and miR‐362‐3p regulates ALKBH5 expression to recruit the M2 macrophage to glioma cells [245]. (G) m6A‐modified circNDUFB2 inhibits the progression of NSCLC via destabilizing IGF2BPs to activate anti‐tumor immunity [246]. (H) circRHBDD1 recruits YTHDF1 to the m6A‐modifed PIK3R1 mRNA and accelerates its translation to restrict anti‐PD‐1 therapy via activation of PI3K/AKT signaling in HCC [247]. (I) METTL3 knockdown decrease VEGFA expression especially two transcripts vegfa‐164 and vegfa‐188 to inhibit osteogenic differentiation in bone marrow mesenchymal stem cells [243]. (J) ALKBH5 targets MCT4 to modulate Mct4/Slc16a3 expression to regulate the composition of tumor‐infiltrating Treg, level of lactate and myeloid‐derived suppressor cells [244]. (K) METTL3 targets both RUNX2 and precursor‐miR‐320 to increase their expression, which controls the osteogenic potential of bone marrow–derived mesenchymal stem cells [248].
8.2. Regulation via m6A on ncRNAs in immune cells
Expression of ALKBH5 can be regulated by lncRNA‐miRNA network containing miR‐4732‐5p, miR‐193a‐3p and miR‐362‐3p, which can recruit the M2 macrophage to glioma cells (Fig. 7F) [245]. circNDUFB2 inhibits the progression of NSCLC via destabilizing IGF2BPs to activate anti‐tumor immunity (Fig. 7G, Box 4) [246]. Cai et al. [247] found that CircRHBDD1 restricted PD‐L1 immunotherapy efficacy via m6A modification in HCC (Fig. 7H). In terms of non‐immune cell m6A regulation such as bone marrow mesenchymal stem cells, Yan et al. [248] demonstrated that METTL3 controlled the osteogenic potential of bone marrow‐derived mesenchymal stem cells by m6A methylation of precursor‐miR‐320/RUNX2 (Fig. 7K). The underlying effects of regulation of m6A on ncRNAs in the TME should be further explored. (Table 2)
The tumor microenvironment consists mainly of an immune microenvironment dominated by immune cells and a non‐immune microenvironment dominated by fibroblasts, formed by the combined action of malignant tumor cells and non‐transformed cells [249]. Roles of the m6A modification in both immune cells and non‐immune cells in the cancer microenvironment have been studied. However, the regulatory effects of m6A on cancer microenvironment is controversial, especially for the roles of METTL3 and ALKBH5 in immune cells and non‐immune cells. Since cancer microenvironment is special and complex, the multiple effect/roles of m6A modification requires further exploration.
9. The m6A modification and cancer metabolism
9.1. Regulation via m6A on mRNAs
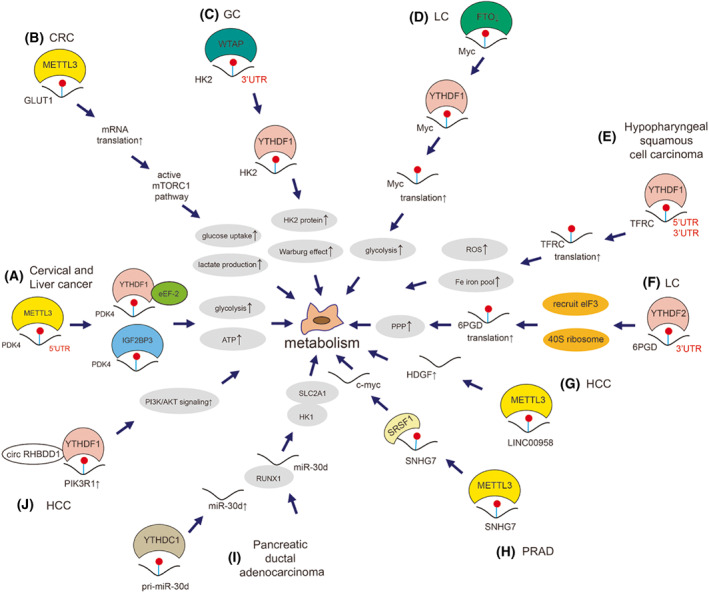
We previously showed that METTL3‐modified 5′UTR of PDK4 mRNA could positively regulate the glycolysis and ATP generation in cervical and liver cancer cells (Fig. 8A) [25]. METTL3 enhanced GLUT1 mRNA translation in an m6A‐dependent manner to promote glucose uptake and lactate production in CRC (Fig. 8B) [250]. WTAP enhances the stability of HK2 mRNA through binding with its 3′UTR m6A site, leading to the promotion of GC cell proliferation and glycolytic capacity (Warburg effect) (Fig. 8C) [251]. Down‐regulated FTO in LC cells promoted the translation of MYC mRNA and increased glycolysis and cancer progression (Fig. 8D) [252]. YTHDF1 could regulate the translation of TFRC mRNA by binding its 3′ and 5'UTR to enhance iron metabolism in hypopharyngeal squamous cell carcinoma (Fig. 8E) [53]. YTHDF2 could directly bind to the 3′UTR of 6PGD mRNA to promote its translation, therefore enhancing the activity of the pentose phosphate pathway (PPP) flux in LC cells (Fig. 8F) [253].
Fig. 8.
Mechanism of m6A on cancer cell metabolism. m6A modulates metabolism via various mechanisms in cancers. (A) METTL3‐modified m6A on 5′UTR of PDK4 is recognized by YTHDF1/eEF‐2 and IGF2BP3 to promote the glycolysis and ATP generation in both cervical and liver cancer cells [25]. (B) METTL3 targets GLUT1 to increase its mRNA, which actives mTORC1 pathway to promote glucose uptake and lactate production in CRC cells [250]. (C) WTAP targets 3′UTR of HK2 to enhance its stability, which is recognized by YTHDF1 to promote glycolytic capacity in GC cells [251]. (D) FTO downregulation promotes the YTHDF1‐medicated translation of MYC, which increases glycolysis in LC cells [252]. (E) YTHDF1 recognizes both 5′UTR an 3′ UTR m6A of TFRC, promoting its translation to enhance iron metabolism in hypopharyngeal squamous cell carcinoma [53]. (F) YTHDF2 binds to 6PGD mRNA to facilitate its translation, which enhances the activity of the PPP flux in LC cells [253]. (G) METTL3 increases HDGF‐involved lipogenesis by upregulating lncRNA LINC00958 in HCC cells [134]. (H) METTL3 targets lncRNA SNHG7 to increase its mRNA level. SNHG7 regulated c‐Myc via interacting with SRSF1 to promote glycolysis via SRSF1/c‐Myc axis in PRAD cells [254]. (I) YTHDC1 promotes the maturation of miR‐30d to increase its expression, which further regulates the expression of RUNX1, SLC2A1 and HK1 and therefore attenuates the Warburg effect in pancreatic ductal adenocarcinoma cells [62]. (J) circRHBDD1 recruits YTHDF1 to the m6A‐modifed PIK3R1 mRNA and accelerates its translation to augment aerobic glycolysis via activation of PI3K/AKT signaling in HCC [247].
9.2. Regulation via m6A on ncRNAs
METTL3 mediated the up‐regulation of lncRNA LINC00958 through stabilizing its transcript and increasing lipogenesis, which could act as a nanotherapeutic target in HCC (Fig. 8G, Box 3) [134]. Liu et al. [254] found that METTL3‐stabilized lncRNA SNHG7 accelerated glycolysis in PRAD via the SRSF1/c‐Myc axis (Fig. 8H). YTHDC1 promoted the maturation of miR‐30d to suppress aerobic glycolysis by binding RUNX1, regulating SLC2A1 and HK1 expression, thus attenuating the Warburg effect to inhibit tumor progression in pancreatic ductal adenocarcinoma (Fig. 8I) [62]. circRHBDD1 was revealed to augment aerobic glycolysis in HCC (Fig. 8K) [247].
Recently, the relationship between m6A modification and cancer metabolism has received attention. Increasing reports suggest that m6A modification is extensively involved in the metabolic regulation of tumors. Compared with m6A erasers, m6A writers, especially METTL3, plays more critical roles in the regulation of cancer metabolism. In addition, m6A‐promoted translation is important for the glycolysis of cancer cells. On one hand, it hints that glycolysis of cancer cells could be regulated by multiple pathways. On the other hand, targeting the m6A‐modifed translation may be a potential approach to inhibit cancer metabolism, and therefore achieving efficient treatment of cancers.
10. m6A modifications as diagnostic and therapeutic targets
m6A is commonly up‐regulated in several cancers and promotes tumorigenesis. Targeting m6A is emerging as a new trend for cancer diagnosis and therapy due to the specific induction of m6A by cancer tissues and the critical effects of m6A on cancer development. Here, we summarized the development of potential cancer diagnosis and therapy methods by targeting m6A.
10.1. m6A as biomarkers for cancer diagnosis
10.1.1. Total m6A
m6A level in blood/serum could be measured as simply noninvasive biomarkers for cancers. For instance, Pei et al. [145] found that leukocyte m6A was significantly elevated in non–small cell lung cancer (NSCLC) patients, which was suitable for NSCLC monitoring and diagnosis. In GC patients, we found that the level of m6A in peripheral blood RNA increased significantly. The sensitivity of for m6A, estimated by the value of area under the curve (AUC), in the GC group was 0.929 (95% confidence interval (CI), 0.88–0.96), which was markedly greater than the AUCs for carcinoembryonic antigen (CEA; 0.694) and carbohydrate antigen 199 (CA199; 0.603). It indicated that the level of m6A in peripheral blood RNA was a promising noninvasive diagnostic biomarker for GC [143]. Similarly, the m6A levels in peripheral blood leukocytes could be a noninvasive biomarker for both NSCLC [145] and CRC [146].
10.1.2. m6A‐related RNAs
Over 138 m6A‐related transcripts were identified to be potential prognostic biomarkers so far, such as NMPM1 in lung adenocarcinoma [255], SNRPC in HCC [256], GLUT1 in esophageal cancer [257], BATF2 in GC [258], PGM1 and ENO1 in BCA [259], NUF2/CDCA3/KIF14 in clear cell renal cell carcinoma [260]. m6A‐associated miRNAs are also used for developing new cancer biomarkers. Zhang et al. [261] demonstrated that the m6A‐miRNA signatures showed superior sensitivities in each cancer type and presented a satisfactory AUC in identifying LC, GC and HCC; m6A‐related lncRNAs have also been identified as cancer biomarkers. For instance, 12 m6A‐related lncRNAs in lung adenocarcinoma (LUAD) [262] and 6 m6A‐related lncRNAs in BC [263] were identified as promising predictive biomarkers. In addition, specific lncRNAs including circ3823 and circ1662 in CRC [264, 265], LINC00022 in ESCC [266], circRNA_104075 in HCC [215] and MIR497HG/FENDRR/RP1‐199J3 in LUAD [267] were suggested for diagnosis.
10.1.3. m6A regulators
The abundance of m6A‐related writers, erasers and readers could be candidates for tumor diagnosis. For instance, METTL3 is suggested to be a prognostic and immune‐related biomarker in BCA [268], while METTL14 is correlated with prognosis in rectal cancer patients and immune infiltration level [269]. Demethylase ALKBH5 is up‐regulated in several solid tumors and can be a biomarker for some malignant tumor prognosis, such as NSCLC and CRC [245]. Similarly, FTO [270], WTAP [271], KIAA1429 [272], RBM15 [273], ZC3H13 [274], METTL5 [275], METTL16 [274], ZCCHC4 [276], HNRNPC [276] YTHDF1 [277], YTHDF2 [278, 279], YTHDF3 [280, 281], YTHDC1 [282], YTHDC2 [56], IGF2BP1 [283, 284], IGF2BP2 [285, 286], IGF2BP3 [287] have been reported to be potential biomarkers for prognosis in different cancers.
These studies indicate that the m6A level in blood/serum reflects the abnormal RNA methylation in the body, which may have potential to be a specific and sensitive biomarker for cancer diagnosis. Total m6A levels in blood samples, m6A‐related RNAs and m6A modifiers can be associated with tumor development and may constitute promising approaches in cancer prognosis.
10.2. m6A as targets for cancer therapy
10.2.1. Targeting m6A‐associated regulators
In the past decades, small molecule chemicals were the most explored as inhibitors to target m6A‐related proteins. As the first identified demethylase, inhibitors for FTO were most studied. Over ten FTO‐targeted small molecule inhibitors were developed against cancers, such as Rhein [288], meclofenamic acid [289], quercetin [290], entacapone [291], FB23 and FB23‐2 [292]. We recently developed two FTO inhibitors named 18077 and 18097, which can significantly suppress in vivo growth and lung colonization of BC cells [293]. Regarding FTO, inhibitors targeting other m6A‐related enzymes were being explored. For example, Yankova et al. [294] described that a catalytic inhibitor of METTL3, named STM2457, could be a potential therapeutic drug against AML due to its oral activity. Sabnis et al. [295] developed new compounds as ALKBH5 inhibitors (IC50 = 0.84 μm) for cancer treatment. In addition, a number of natural inhibitors are being discovered continuously, including quercetin for METTL3 [296], betaine for METTL14 [297], clausine for FTO [298], curcumin for ALKBH5 [299] and fusaric acid for YTHs [300, 301]. A list of candidate compounds targeting m6A regulators for cancer therapy is presented in Table 3.
Table 3.
Candidate compounds targeting m6A regulators for cancer therapy.
| Target | Compound | IC50 (μm) | Functions |
|---|---|---|---|
| METTL3 | Adenosine 2 | 8.7 | METTL3 inhibitor [302] |
| METTL3 | UZH1a | 7 | METTL3 inhibitor, reduces the m6A/A ratio in mRNAs of three AML cell lines [303] |
| METTL3 | STM2457 | 0.0169 | METTL3 inhibitor, reduces AML growth and increases differentiation and apoptosis [294] |
| FTO | Rhein | 21 | FTO inhibitor, exhibits good inhibitory activity on m6A demethylation inside cells [288] |
| FTO | MO‐I‐500 | 8.7 | FTO inhibitor, shows anti‐convulsant activity [304] |
| FTO | Meclofenamic acid | 8 | FTO inhibitor [289] |
| FTO | CHTB | 39.24 | FTO inhibitor [305] |
| FTO | R‐2HG | 133.3 | FTO inhibitor, exerts a broad anti‐leukemic activity in vitro and in vivo [166] |
| FTO | FB23‐2 | 2.6 | FTO inhibitor, suppresses proliferation and promotes the differentiation/apoptosis of human AML cell lines [292] |
| FTO | Entacapone | 3.5 | FTO inhibitor, mediates metabolic regulation through FOXO1 [291] |
| FTO | CS1 | 0.14 | FTO inhibitor, suppresses cancer stem cell maintenance and immune evasion [306] |
| FTO | CS2 | 2.6 | FTO inhibitor, suppresses cancer stem cell maintenance and immune evasion [306] |
| FTO | Saikosaponin‐d | 0.46 | FTO inhibitor, shows a broadly suppressed AML cell proliferation and promoted apoptosis and cell‐cycle arrest both in vitro and in vivo [307] |
| FTO | Dac51 | 0.4 | FTO inhibitor, blocks FTO‐mediated immune evasion, and synergizes with checkpoint blockade for better tumor control [308] |
| FTO | FTO‐4 | 3.4 | FTO inhibitor, prevents neurosphere formation in patient‐derived GBM stem cells [309] |
| FTO | 18097 | 0.64 | FTO inhibitor, shows anti‐cancer activities both in vitro and in vivo [310] |
| ALKBH5 | MV1035 | / | ALKBH5 inhibitor, shows an inhibitory effect on GBM [311] |
| ALKBH5 | ALK‐04 | / | ALKBH5 inhibitor, enhances the efficacy of cancer immunotherapy [244] |
| ALKBH5 | 2‐[(1‐hydroxy‐2‐oxo‐2‐phenylethyl)sulfanyl]acetic acid | 0.84 | ALKBH5 inhibitor, suppresses cell proliferation at low micromolar concentrations in AML [312] |
| ALKBH5 | 4‐[(furan‐2‐yl)methyl]amino‐1,2‐diazinane‐3,6‐dione | 1.79 | ALKBH5 inhibitor, suppresses cell proliferation at low micromolar concentrations in AML [312] |
| ALKBH5 | Compound 20m | 0.021 | ALKBH5 inhibitor [313] |
| IGF2BP1 | BTYNB | 5 | IGF2BP1 inhibitor, targes c‐Myc and inhibits melanoma and ovarian cancer cell proliferation [314] |
| IGF2BP1 | 7773 | 30.45 | IGF2BP1 inhibitor, represses Kras and a pro‐oncogenic phenotype in LUAD [315] |
| IGF2BP2 | Benzamidobenzoic acid class and ureidothiophene clas | / | IGF2BP2 inhibitors, show anti‐cancer activities both in vitro and in vivo [316] |
Targeting the expression of m6A‐related proteins is another strategy for cancer therapy. RNA interference and CRISPR/Cas9 are techniques that target m6A‐related proteins to suppress their expression. The CRISPR system can also be used to identify potential targets that modulate the expression of m6A‐related proteins through a genome‐wide CRISPR screen [317].
10.2.2. Single‐site editing of m6A‐modified RNAs
Given specific m6A modifications on particular RNA molecules can have different effects, modulating single‐site m6A on transcript targets may affect the expression of target genes such as oncogenes. We have developed a PspCas13b‐ALKBH5‐based tool named dm6ACRISPR for the targeted demethylation of specific mRNAs [318]. Targeting m6A modifications of oncogenes such as EGFR and MYC can significantly suppress their expression and the proliferation of cancer cells [318]; demethylating metabolic gene PDK4 can reduce its expression and glycolysis of cancer cells [25]. Similarly, Qian's lab has devised an RNA‐targeting‐dCas9 system for site‐specific methylation or demethylation via fusion with a truncated METTL3‐METTL14 heterodimer or full‐length ALKBH5/FTO, respectively [319]. The m6A site‐specific manipulation has been summarized recently [320]. The discovery of more potent Cas derivatives, such as Cas13bt, Cas13X, Cas13Y and ABE8, will further improve the CRISPR‐based RNA editing systems and have great potential for applications in various genetic diseases including cancers [320].
Since the oncogenic roles of m6A modification have been identified in various types of cancers, studies investigating the potential roles of m6A as biomarkers for cancer diagnosis have been performed. In general, levels of total m6A, m6A‐related RNAs and m6A regulators can be used as diagnostic biomarkers for multiple cancers. The relationship between m6A/m6A‐related markers and cancer progression is satisfactory. Nevertheless, combining m6A and clinical used biomarkers can further increase the diagnostic sensitivity of cancer [142], showing a potential application of m6A in cancer diagnosis. In addition to the application in diagnosis, targeting m6A may serve as a novel direction for cancer therapy due to its effect on tumorigenesis. Nowadays, therapeutic strategies targeting m6A mainly include inhibition of enzyme activity and/or expression, and targeted inhibition based on m6A editing of specific RNAs. Both in vitro and in vivo trials show satisfactory results of cancer cell inhibition via either inhibitors or single‐site editing tools. It suggests that targeting m6A is a potential and powerful approach for cancer therapy.
11. Challenges and perspectives
m6A modification is widely distributed in almost all RNA species and has a far‐reaching biological impact. Increasing evidence shows that m6A has important regulatory roles in the process of tumorigenesis and cancer development, which can be achieved by the changes in m6A‐related protein expression, reader protein activity or the biological functions of m6A related‐mRNA and/or ncRNAs. As a matter of fact, m6A is expected to become a potential biomarker for cancer diagnosis by monitoring overall m6A, m6A‐related RNAs and m6A modifiers. Since total m6A in peripheral blood shows great potential as a biomarker for gastric [143], lung [145] and colorectal [146] cancers, its specific roles in cancer diagnosis warrant further investigation. Moreover, whether m6A can be used as a biomarker to distinguish the early stage of cancer patients and healthy people, and whether the levels of m6A can be used as a biomarker for prediction or monitoring therapy efficiency remains unclear. In addition, it is reasonable to hypothesize that m6A‐methylated transcripts such as mRNAs, ncRNAs and even the RNA fragments may be associated with tumorigenesis and cancer development [321]. However, the potential roles of specific m6A‐methylated transcripts in cancer diagnosis need further investigation.
Targeting regulators of DNA and histone methylation have been proven as clinically applicable and important therapeutic strategies [322]. Increasing evidence shows that RNA methylation is a new target for cancer therapy. Developing inhibitors/activators of m6A‐related proteins has become a hot spot in the field of anti‐cancer epigenetic drugs. At present, the small molecule candidate drug STM2457 targeting METTL3 is expected to enter the clinical trial stage, which has a significant possibility to become the first RNA epigenetic drug for cancer therapy. However, whether the global methylation/demethylation effect induced by inhibitors/activators of m6A‐related proteins would cause unexpected side effects or toxic effects remains up to further investigation. In addition to global demethylation, m6A site‐specific editing to target‐specific RNA has gradually become a novel direction of cancer treatment. Similar to CRISPR/Cas9 system targeting DNA, CRISPR proteins targeting RNA (such as Cas13b, CasRx) combined with m6A‐related proteins can achieve site‐specific deposition and demethylation of m6A, leading to the degradation, translation and other effects of specific targets [319]. Compared with CRISPR/Cas9, CRISPR targeting RNA does not affect the DNA, which can circumvent mutations caused by off‐target effects being passed down to the next generation. Therefore, a site‐targeting m6A‐editing method would be a promising direction for tumor treatment. Remarkably, numerous challenges need to be overcome before the clinical application of a targeted m6A‐editing method, such as ways to achieve sufficient delivery in vivo, approaches to target tumor cells specifically, means to reduce off‐target effects, and more. An in‐depth study of m6A distribution, functions and biological impact will broaden our understanding of RNA epigenetic regulation of tumor development. We therefore believe that an increasing number of novel, specific, effective and promising methods targeting m6A modifications could be developed, being a new direction for both cancer diagnosis and targeted therapy.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
Conception and design: JL, HW. Writing, review and/or revision of the manuscript: ZW, JZ, JL, HW. Collation of information: HZ, LG.
Acknowledgements
This research was supported by the National Key Research and Development Program of China (No. 2022YFC2601800), the National Natural Science Foundation of China (Grant Nos. 32161143017, 82173833, 82173126 and 81973343), the International Cooperation Project of the Science and Technology Planning Project of Guangdong Province, China (No. 2021A0505030029), the Open Program of Shenzhen Bay Laboratory (No. SZBL202009051006), the Guangdong Provincial Key Laboratory of Chiral Molecule and Drug Discovery (2019B030301005), the Guangdong Basic and Applied Basic Research Foundation (No. 2020A1515010290 and 2021A1515111161) and Shenzhen Bay Scholars Program.
Zhaotong Wang and Jiawang Zhou contributed equally to this article
Contributor Information
Jiexin Li, Email: lijiexin3@mail.sysu.edu.cn.
Hongsheng Wang, Email: whongsh@mail.sysu.edu.cn.
Data availability statement
Data openly available in a public repository.
References
- 1. Wei CM, Gershowitz A, Moss B. Methylated nucleotides block 5' terminus of HeLa cell messenger RNA. Cell. 1975;4:379–86. 10.1016/0092-8674(75)90158-0 [DOI] [PubMed] [Google Scholar]
- 2. Fu Y, Dominissini D, Rechavi G, He C. Gene expression regulation mediated through reversible m(6)A RNA methylation. Nat Rev Genet. 2014;15:293–306. 10.1038/nrg3724 [DOI] [PubMed] [Google Scholar]
- 3. Liu JZ, Yue Y, Han D, Wang X, Fu Y, Zhang L, et al. A METTL3‐METTL14 complex mediates mammalian nuclear RNA N‐6‐adenosine methylation. Nat Chem Biol. 2014;10:93–5. 10.1038/nchembio.1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pendleton KE, Chen B, Liu K, Hunter OV, Xie Y, Tu BP, et al. The U6 snRNA m(6)A methyltransferase METTL16 regulates SAM synthetase intron retention. Cell. 2017;169:824–835 e814. 10.1016/j.cell.2017.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Tran N, Ernst FGM, Hawley BR, Zorbas C, Ulryck N, Hackert P, et al. The human 18S rRNA m6A methyltransferase METTL5 is stabilized by TRMT112. Nucleic Acids Res. 2019;47:7719–33. 10.1093/nar/gkz619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Horiuchi K, Kawamura T, Iwanari H, Ohashi R, Naito M, Kodama T, et al. Identification of Wilms' tumor 1‐associating protein complex and its role in alternative splicing and the cell cycle. J Biol Chem. 2013;288:33292–302. 10.1074/jbc.M113.500397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ma H, Wang X, Cai J, Dai Q, Natchiar SK, Lv R, et al. N(6‐)Methyladenosine methyltransferase ZCCHC4 mediates ribosomal RNA methylation. Nat Chem Biol. 2019;15:88–94. 10.1038/s41589-018-0184-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guo J, Tang HW, Li J, Perrimon N, Yan D. Xio is a component of the Drosophila sex determination pathway and RNA N‐6‐methyladenosine methyltransferase complex. Proc Natl Acad Sci USA. 2018;115:3674–9. 10.1073/pnas.1720945115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wei J, Liu F, Lu Z, Fei Q, Ai Y, He PC, et al. Differential m(6)A, m(6)A(m), and m(1)A demethylation mediated by FTO in the cell nucleus and cytoplasm. Mol Cell. 2018;71:973–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang X, Zhao BS, Roundtree IA, Lu Z, Han D, Ma H, et al. N(6)‐methyladenosine modulates messenger RNA translation efficiency. Cell. 2015;161:1388–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang X, Lu Z, Gomez A, Hon GC, Yue Y, Han D, et al. N6‐methyladenosine‐dependent regulation of messenger RNA stability. Nature. 2014;505:117–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shi H, Wang X, Lu Z, Zhao BS, Ma H, Hsu PJ, et al. YTHDF3 facilitates translation and decay of N(6)‐methyladenosine‐modified RNA. Cell Res. 2017;27:315–28. 10.1038/cr.2017.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ma C, Liao S, Zhu Z. Crystal structure of human YTHDC2 YTH domain. Biochem Biophys Res Commun. 2019;518:678–84. [DOI] [PubMed] [Google Scholar]
- 14. Roundtree IA, He C. Nuclear m(6)A reader YTHDC1 regulates mRNA splicing. Trends Genet. 2016;32:320–1. 10.1016/j.tig.2016.03.006 [DOI] [PubMed] [Google Scholar]
- 15. Patil DP, Pickering BF, Jaffrey SR. Reading m(6)A in the transcriptome: m(6)A‐binding proteins. Trends Cell Biol. 2018;28:113–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu N, Dai Q, Zheng G, He C, Parisien M, Pan T. N(6)‐methyladenosine‐dependent RNA structural switches regulate RNA‐protein interactions. Nature. 2015;518:560–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang H, Weng H, Sun W, Qin X, Shi H, Wu H, et al. Recognition of RNA N(6)‐methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat Cell Biol. 2018;20:285–95. 10.1038/s41556-018-0045-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu R, Li A, Sun B, Sun JG, Zhang J, Zhang T, et al. A novel m(6)A reader Prrc2a controls oligodendroglial specification and myelination. Cell Res. 2019;29:23–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Saneyoshi M, Harada F, Nishimura S. Isolation and characterization of N6‐methyladenosine from Escherichia coli valine transfer RNA. Biochim Biophys Acta. 1969;190:264–73. [DOI] [PubMed] [Google Scholar]
- 20. Zaccara S, Ries RJ, Jaffrey SR. Reading, writing and erasing mRNA methylation. Nat Rev Mol Cell Biol. 2019;20:608–24. [DOI] [PubMed] [Google Scholar]
- 21. Shen C, Xuan B, Yan T, Ma Y, Xu P, Tian X, et al. m(6)A‐dependent glycolysis enhances colorectal cancer progression. Mol Cancer. 2020;19:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cai X, Wang X, Cao C, Gao Y, Zhang S, Yang Z, et al. HBXIP‐elevated methyltransferase METTL3 promotes the progression of breast cancer via inhibiting tumor suppressor let‐7g. Cancer Lett. 2018;415:11–9. [DOI] [PubMed] [Google Scholar]
- 23. Han H, Yang C, Zhang S, Cheng M, Guo S, Zhu Y, et al. METTL3‐mediated m(6)A mRNA modification promotes esophageal cancer initiation and progression via Notch signaling pathway. Mol Ther Nucleic Acids. 2021;26:333–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vu LP, Pickering BF, Cheng Y, Zaccara S, Nguyen D, Minuesa G, et al. The N‐6‐methyladenosine (m(6)A)‐forming enzyme METTL3 controls myeloid differentiation of normal hematopoietic and leukemia cells. Nat Med. 2017;23:1369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li Z, Peng Y, Li J, Chen Z, Chen F, Tu J, et al. N‐6‐methyladenosine regulates glycolysis of cancer cells through PDK4. Nat Commun. 2020;11:2578. 10.1038/s41467-020-16306-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ma L, Xue X, Zhang X, Yu K, Xu X, Tian X, et al. The essential roles of m(6)A RNA modification to stimulate ENO1‐dependent glycolysis and tumorigenesis in lung adenocarcinoma. J Exp Clin Cancer Res. 2022;41:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shi Y, Zheng C, Jin Y, Bao B, Wang D, Hou K, et al. Reduced expression of METTL3 promotes metastasis of triple‐negative breast cancer by m6A methylation‐mediated COL3A1 up‐regulation. Front Oncol. 2020;10:1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weng H, Huang H, Wu H, Qin X, Zhao BS, Dong L, et al. METTL14 inhibits hematopoietic stem/progenitor differentiation and promotes leukemogenesis via mRNA m(6)A modification. Cell Stem Cell. 2018;22:191–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sun T, Wu Z, Wang X, Wang Y, Hu X, Qin W, et al. LNC942 promoting METTL14‐mediated m(6)A methylation in breast cancer cell proliferation and progression. Oncogene. 2020;39:5358–72. [DOI] [PubMed] [Google Scholar]
- 30. Liu J, Eckert MA, Harada BT, Liu SM, Lu Z, Yu K, et al. m(6)A mRNA methylation regulates AKT activity to promote the proliferation and tumorigenicity of endometrial cancer. Nat Cell Biol. 2018;20:1074–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang M, Liu J, Zhao Y, He R, Xu X, Guo X, et al. Upregulation of METTL14 mediates the elevation of PERP mRNA N(6) adenosine methylation promoting the growth and metastasis of pancreatic cancer. Mol Cancer. 2020;19:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xie Q, Li Z, Luo X, Wang D, Zhou Y, Zhao J, et al. piRNA‐14633 promotes cervical cancer cell malignancy in a METTL14‐dependent m6A RNA methylation manner. J Transl Med. 2022;20:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen X, Xu M, Xu X, Zeng K, Liu X, Pan B, et al. METTL14‐mediated N6‐methyladenosine modification of SOX4 mRNA inhibits tumor metastasis in colorectal cancer. Mol Cancer. 2020;19:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Du L, Li Y, Kang M, Feng M, Ren Y, Dai H, et al. USP48 Is upregulated by Mettl14 to attenuate hepatocellular carcinoma via regulating SIRT6 stabilization. Cancer Res. 2021;81:3822–34. [DOI] [PubMed] [Google Scholar]
- 35. Gu C, Wang Z, Zhou N, Li G, Kou Y, Luo Y, et al. Mettl14 inhibits bladder TIC self‐renewal and bladder tumorigenesis through N(6)‐methyladenosine of Notch1. Mol Cancer. 2019;18:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang XK, Zhang YW, Wang CM, Li B, Zhang TZ, Zhou WJ, et al. METTL16 promotes cell proliferation by up‐regulating cyclin D1 expression in gastric cancer. J Cell Mol Med. 2021;25:6602–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Su R, Dong L, Li Y, Gao M, He PC, Liu W, et al. METTL16 exerts an m(6)A‐independent function to facilitate translation and tumorigenesis. Nat Cell Biol. 2022;24:205–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Huang H, Li H, Pan R, Wang S, Khan AA, Zhao Y, et al. Ribosome 18S m(6)A methyltransferase METTL5 promotes pancreatic cancer progression by modulating c‐Myc translation. Int J Oncol. 2022;60:31. [DOI] [PubMed] [Google Scholar]
- 39. Rong B, Zhang Q, Wan J, Xing S, Dai R, Li Y, et al. Ribosome 18S m(6)A methyltransferase METTL5 promotes translation initiation and breast cancer cell growth. Cell Rep. 2020;33:108544. 10.1016/j.celrep.2020.108544 [DOI] [PubMed] [Google Scholar]
- 40. Chen Y, Peng C, Chen J, Chen D, Yang B, He B, et al. WTAP facilitates progression of hepatocellular carcinoma via m6A‐HuR‐dependent epigenetic silencing of ETS1. Mol Cancer. 2019;18:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu H, Lan T, Li H, Xu L, Chen X, Liao H, et al. Circular RNA circDLC1 inhibits MMP1‐mediated liver cancer progression via interaction with HuR. Theranostics. 2021;11:1396–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang X, Tian L, Li Y, Wang J, Yan B, Yang L, et al. RBM15 facilitates laryngeal squamous cell carcinoma progression by regulating TMBIM6 stability through IGF2BP3 dependent. J Exp Clin Cancer Res. 2021;40:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhu D, Zhou J, Zhao J, Jiang G, Zhang X, Zhang Y, et al. ZC3H13 suppresses colorectal cancer proliferation and invasion via inactivating Ras‐ERK signaling. J Cell Physiol. 2019;234:8899–907. [DOI] [PubMed] [Google Scholar]
- 44. Li Z, Weng H, Su R, Weng X, Zuo Z, Li C, et al. FTO plays an oncogenic role in acute myeloid leukemia as a N(6)‐methyladenosine RNA demethylase. Cancer Cell. 2017;31:127–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Niu Y, Lin Z, Wan A, Chen H, Liang H, Sun L, et al. RNA N6‐methyladenosine demethylase FTO promotes breast tumor progression through inhibiting BNIP3. Mol Cancer. 2019;18:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Huang H, Wang Y, Kandpal M, Zhao G, Cardenas H, Ji Y, et al. FTO‐dependent N (6)‐methyladenosine modifications inhibit ovarian cancer stem cell self‐renewal by blocking cAMP signaling. Cancer Res. 2020;80:3200–14. 10.1158/0008-5472.CAN-19-4044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ruan DY, Li T, Wang YN, Meng Q, Li Y, Yu K, et al. FTO downregulation mediated by hypoxia facilitates colorectal cancer metastasis. Oncogene. 2021;40:5168–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Qu J, Hou Y, Chen Q, Chen J, Li Y, Zhang E, et al. RNA demethylase ALKBH5 promotes tumorigenesis in multiple myeloma via TRAF1‐mediated activation of NF‐κB and MAPK signaling pathways. Oncogene. 2022;41:400–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang S, Zhao BS, Zhou A, Lin K, Zheng S, Lu Z, et al. m(6)A demethylase ALKBH5 maintains tumorigenicity of glioblastoma stem‐like cells by sustaining FOXM1 expression and cell proliferation program. Cancer Cell. 2017;31:591–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tang B, Yang Y, Kang M, Wang Y, Wang Y, Bi Y, et al. m(6)A demethylase ALKBH5 inhibits pancreatic cancer tumorigenesis by decreasing WIF‐1 RNA methylation and mediating Wnt signaling. Mol Cancer. 2020;19:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang S, Gao S, Zeng Y, Zhu L, Mo Y, Wong CC, et al. N6‐methyladenosine reader YTHDF1 promotes ARHGEF2 translation and RhoA signaling in colorectal cancer. Gastroenterology. 2022;162:1183–96. [DOI] [PubMed] [Google Scholar]
- 52. Pi J, Wang W, Ji M, Wang X, Wei X, Jin J, et al. YTHDF1 promotes gastric carcinogenesis by controlling translation of FZD7. Cancer Res. 2021;81:2651–65. [DOI] [PubMed] [Google Scholar]
- 53. Ye J, Wang Z, Chen X, Jiang X, Dong Z, Hu S, et al. YTHDF1‐enhanced iron metabolism depends on TFRC m(6)A methylation. Theranostics. 2020;10:12072–89. 10.7150/thno.51231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Liu T, Wei Q, Jin J, Luo Q, Liu Y, Yang Y, et al. The m6A reader YTHDF1 promotes ovarian cancer progression via augmenting EIF3C translation. Nucleic Acids Res. 2020;48:3816–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Paris J, Morgan M, Campos J, Spencer GJ, Shmakova A, Ivanova I, et al. Targeting the RNA m(6)A reader YTHDF2 selectively compromises cancer stem cells in acute myeloid leukemia. Cell Stem Cell. 2019;25:137–148 e136. 10.1016/j.stem.2019.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dixit D, Prager BC, Gimple RC, Poh HX, Wang Y, Wu Q, et al. The RNA m6A reader YTHDF2 maintains oncogene expression and is a targetable dependency in glioblastoma stem cells. Cancer Discov. 2021;11:480–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yu J, Chai P, Xie M, Ge S, Ruan J, Fan X, et al. Histone lactylation drives oncogenesis by facilitating m(6)A reader protein YTHDF2 expression in ocular melanoma. Genome Biol. 2021;22:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chen J, Sun Y, Xu X, Wang D, He J, Zhou H, et al. YTH domain family 2 orchestrates epithelial‐mesenchymal transition/proliferation dichotomy in pancreatic cancer cells. Cell Cycle. 2017;16:2259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Xu Y, He X, Wang S, Sun B, Jia R, Chai P, et al. The m(6)A reading protein YTHDF3 potentiates tumorigenicity of cancer stem‐like cells in ocular melanoma through facilitating CTNNB1 translation. Oncogene. 2022;41:1281–97. [DOI] [PubMed] [Google Scholar]
- 60. Chang G, Shi L, Ye Y, Shi H, Zeng L, Tiwary S, et al. YTHDF3 induces the translation of m(6)A‐enriched gene transcripts to promote breast cancer brain metastasis. Cancer Cell. 2020;38:857–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sheng Y, Wei J, Yu F, Xu H, Yu C, Wu Q, et al. A critical role of nuclear m6A reader YTHDC1 in leukemogenesis by regulating MCM complex‐mediated DNA replication. Blood. 2021;138:2838–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hou Y, Zhang Q, Pang W, Hou L, Liang Y, Han X, et al. YTHDC1‐mediated augmentation of miR‐30d in repressing pancreatic tumorigenesis via attenuation of RUNX1‐induced transcriptional activation of Warburg effect. Cell Death Differ. 2021;28:3105–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yuan W, Chen S, Li B, Han X, Meng B, Zou Y, et al. The N6‐methyladenosine reader protein YTHDC2 promotes gastric cancer progression via enhancing YAP mRNA translation. Transl Oncol. 2022;16:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tanabe A, Tanikawa K, Tsunetomi M, Takai K, Ikeda H, Konno J, et al. RNA helicase YTHDC2 promotes cancer metastasis via the enhancement of the efficiency by which HIF‐1α mRNA is translated. Cancer Lett. 2016;376:34–42. [DOI] [PubMed] [Google Scholar]
- 65. Ma L, Chen T, Zhang X, Miao Y, Tian X, Yu K, et al. The m(6)A reader YTHDC2 inhibits lung adenocarcinoma tumorigenesis by suppressing SLC7A11‐dependent antioxidant function. Redox Biol. 2021;38:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhang L, Wan Y, Zhang Z, Jiang Y, Gu Z, Ma X, et al. IGF2BP1 overexpression stabilizes PEG10 mRNA in an m6A‐dependent manner and promotes endometrial cancer progression. Theranostics. 2021;11:1100–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhu P, He F, Hou Y, Tu G, Li Q, Jin T, et al. A novel hypoxic long noncoding RNA KB‐1980E6.3 maintains breast cancer stem cell stemness via interacting with IGF2BP1 to facilitate c‐Myc mRNA stability. Oncogene. 2021;40:1609–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chen F, Chen Z, Guan T, Zhou Y, Ge L, Zhang H, et al. N(6) ‐methyladenosine regulates mRNA stability and translation efficiency of KRT7 to promote breast cancer lung metastasis. Cancer Res. 2021;81:2847–60. 10.1158/0008-5472.CAN-20-3779 [DOI] [PubMed] [Google Scholar]
- 69. Xie F, Huang C, Liu F, Zhang H, Xiao X, Sun J, et al. CircPTPRA blocks the recognition of RNA N(6)‐methyladenosine through interacting with IGF2BP1 to suppress bladder cancer progression. Mol Cancer. 2021;20:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hou P, Meng S, Li M, Lin T, Chu S, Li Z, et al. LINC00460/DHX9/IGF2BP2 complex promotes colorectal cancer proliferation and metastasis by mediating HMGA1 mRNA stability depending on m6A modification. J Exp Clin Cancer Res. 2021;40:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Pu J, Wang J, Qin Z, Wang A, Zhang Y, Wu X, et al. IGF2BP2 promotes liver cancer growth through an m6A‐FEN1‐dependent mechanism. Front Oncol. 2020;10:578816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Xu X, Yu Y, Zong K, Lv P, Gu Y. Up‐regulation of IGF2BP2 by multiple mechanisms in pancreatic cancer promotes cancer proliferation by activating the PI3K/Akt signaling pathway. J Exp Clin Cancer Res. 2019;38:497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lang C, Yin C, Lin K, Li Y, Yang Q, Wu Z, et al. m(6)A modification of lncRNA PCAT6 promotes bone metastasis in prostate cancer through IGF2BP2‐mediated IGF1R mRNA stabilization. Clin Transl Med. 2021;11:426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Huang W, Li Y, Zhang C, Zha H, Zhou X, Fu B, et al. IGF2BP3 facilitates cell proliferation and tumorigenesis via modulation of JAK/STAT signalling pathway in human bladder cancer. J Cell Mol Med. 2020;24:13949–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yang Z, Wang T, Wu D, Min Z, Tan J, Yu B. RNA N6‐methyladenosine reader IGF2BP3 regulates cell cycle and angiogenesis in colon cancer. J Exp Clin Cancer Res. 2020;39:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Yu YZ, Lv DJ, Wang C, Song XL, Xie T, Wang T, et al. Hsa_circ_0003258 promotes prostate cancer metastasis by complexing with IGF2BP3 and sponging miR‐653‐5p. Mol Cancer. 2022;21:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Liu X, Zhou Y, Lou Y, Zhong H. Knockdown of HNRNPA1 inhibits lung adenocarcinoma cell proliferation through cell cycle arrest at G0/G1 phase. Gene. 2016;576:791–7. [DOI] [PubMed] [Google Scholar]
- 78. Wu Y, Zhao W, Liu Y, Tan X, Li X, Zou Q, et al. Function of HNRNPC in breast cancer cells by controlling the dsRNA‐induced interferon response. EMBO J. 2018;37:e99017. 10.15252/embj.201899017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Howley BV, Mohanty B, Dalton A, Grelet S, Karam J, Dincman T, et al. The ubiquitin E3 ligase ARIH1 regulates hnRNP E1 protein stability, EMT and breast cancer progression. Oncogene. 2022;41:1679–90. 10.1038/s41388-022-02199-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lefave CV, Squatrito M, Vorlova S, Rocco GL, Brennan CW, Holland EC, et al. Splicing factor hnRNPH drives an oncogenic splicing switch in gliomas. EMBO J. 2011;30:4084–97. 10.1038/emboj.2011.259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Chen EB, Qin X, Peng K, Li Q, Tang C, Wei YC, et al. HnRNPR‐CCNB1/CENPF axis contributes to gastric cancer proliferation and metastasis. Aging. 2019;11:7473–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yan Q, Zeng P, Zhou X, Zhao X, Chen R, Qiao J, et al. RBMX suppresses tumorigenicity and progression of bladder cancer by interacting with the hnRNP A1 protein to regulate PKM alternative splicing. Oncogene. 2021;40:2635–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wang J, Wang L. Deep analysis of RNA N(6)‐adenosine methylation (m(6)A) patterns in human cells. NAR Genom Bioinform. 2020;2:lqaa007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ping XL, Sun BF, Wang L, Xiao W, Yang X, Wang WJ, et al. Mammalian WTAP is a regulatory subunit of the RNA N6‐methyladenosine methyltransferase. Cell Res. 2014;24:177–89. 10.1038/cr.2014.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Patil DP, Chen CK, Pickering BF, Chow A, Jackson C, Guttman M, et al. m6A RNA methylation promotes XIST‐mediated transcriptional repression. Nature. 2016;537:369–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Liu J, Dou X, Chen C, Chen C, Liu C, Xu MM, et al. N (6)‐methyladenosine of chromosome‐associated regulatory RNA regulates chromatin state and transcription. Science. 2020;367:580–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Li Y, Xia L, Tan K, Ye X, Zuo Z, Li M, et al. N(6)‐Methyladenosine co‐transcriptionally directs the demethylation of histone H3K9me2. Nat Genet. 2020;52:870–7. [DOI] [PubMed] [Google Scholar]
- 88. Akhtar J, Renaud Y, Albrecht S, Ghavi‐Helm Y, Roignant JY, Silies M, et al. m(6)A RNA methylation regulates promoter‐ proximal pausing of RNA polymerase II. Mol Cell. 2021;81:3356–67. [DOI] [PubMed] [Google Scholar]
- 89. Lence T, Akhtar J, Bayer M, Schmid K, Spindler L, Ho CH, et al. m(6)A modulates neuronal functions and sex determination in Drosophila . Nature. 2016;540:242–7. 10.1038/nature20568 [DOI] [PubMed] [Google Scholar]
- 90. Louloupi A, Ntini E, Conrad T, Ørom UAV. Transient N‐6‐methyladenosine transcriptome sequencing reveals a regulatory role of m6A in splicing efficiency. Cell Rep. 2018;23:3429–37. [DOI] [PubMed] [Google Scholar]
- 91. Singh J, Hanson J, Paliwal K, Zhou Y. RNA secondary structure prediction using an ensemble of two‐dimensional deep neural networks and transfer learning. Nat Commun. 2019;10:5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Camper SA, Albers RJ, Coward JK, Rottman FM. Effect of undermethylation on mRNA cytoplasmic appearance and half‐life. Mol Cell Biol. 1984;4:538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zheng G, Dahl JA, Niu Y, Fedorcsak P, Huang CM, Li CJ, et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell. 2013;49:18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Roundtree IA, Luo GZ, Zhang Z, Wang X, Zhou T, Cui Y, et al. YTHDC1 mediates nuclear export of N(6)‐methyladenosine methylated mRNAs. Elife. 2017;6:31311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Chen CA, Shyu AB. Emerging themes in regulation of global mRNA turnover in cis. Trends Biochem Sci. 2017;42:16–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ke S, Alemu EA, Mertens C, Gantman EC, Fak JJ, Mele A, et al. A majority of m6A residues are in the last exons, allowing the potential for 3′ UTR regulation. Genes Dev. 2015;29:2037–53. 10.1101/gad.269415.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Meyer KD, Patil DP, Zhou J, Zinoviev A, Skabkin MA, Elemento O, et al. 5' UTR m(6)A promotes cap‐independent translation. Cell. 2015;163:999–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Choi J, Ieong KW, Demirci H, Chen J, Petrov A, Prabhakar A, et al. N(6)‐methyladenosine in mRNA disrupts tRNA selection and translation‐elongation dynamics. Nat Struct Mol Biol. 2016;23:110–5. 10.1038/nsmb.3148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Choe J, Lin S, Zhang W, Liu Q, Wang L, Ramirez‐Moya J, et al. mRNA circularization by METTL3‐eIF3h enhances translation and promotes oncogenesis. Nature. 2018;561:556–60. 10.1038/s41586-018-0538-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Barbieri I, Tzelepis K, Pandolfini L, Shi J, Millán‐Zambrano G, Robson SC, et al. Promoter‐bound METTL3 maintains myeloid leukaemia by m(6)A‐dependent translation control. Nature. 2017;552:126–31. 10.1038/nature24678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Lin X, Chai G, Wu Y, Li J, Chen F, Liu J, et al. RNA m(6)A methylation regulates the epithelial mesenchymal transition of cancer cells and translation of Snail. Nat Commun. 2019;10:2065. 10.1038/s41467-019-09865-9 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 102. Li J, Xie G, Tian Y, Li W, Wu Y, Chen F, et al. RNA m(6)A methylation regulates dissemination of cancer cells by modulating expression and membrane localization of β‐catenin. Mol Ther. 2022;30:1578–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lee Y, Choe J, Park OH, Kim YK. Molecular mechanisms driving mRNA degradation by m(6)A modification. Trends Genet. 2020;36:177–88. [DOI] [PubMed] [Google Scholar]
- 104. Jia G, Fu Y, Zhao X, Dai Q, Zheng G, Yang Y, et al. N6‐methyladenosine in nuclear RNA is a major substrate of the obesity‐associated FTO. Nature Chem Biol. 2011;7:885–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kurowski MA, Bhagwat AS, Papaj G, Bujnicki JM. Phylogenomic identification of five new human homologs of the DNA repair enzyme AlkB. BMC Genomics. 2003;4:48. 10.1186/1471-2164-4-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Khatiwada B, Nguyen TT, Purslow JA, Venditti V. Solution structure ensemble of human obesity‐associated protein FTO reveals druggable surface pockets at the interface between the N‐ and C‐terminal domain. J Biol Chem. 2022;298:101907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Baltz AG, Munschauer M, Schwanhäusser B, Vasile A, Murakawa Y, Schueler M, et al. The mRNA‐bound proteome and its global occupancy profile on protein‐coding transcripts. Mol Cell. 2012;46:674–90. 10.1016/j.molcel.2012.05.021 [DOI] [PubMed] [Google Scholar]
- 108. Zhen D, Wu Y, Zhang Y, Chen K, Song B, Xu H, et al. m(6)A reader: epitranscriptome target prediction and functional characterization of N (6)‐methyladenosine (m(6)A) readers. Front Cell Dev Biol. 2020;8:741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Liao S, Sun H, Xu C. YTH domain: a family of N(6)‐methyladenosine (m(6)A) readers. Genomics Proteomics Bioinformatics. 2018;16:99–107. 10.1016/j.gpb.2018.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Zaccara S, Jaffrey SR. A Unified model for the function of YTHDF proteins in regulating m(6)A‐modified mRNA. Cell. 2020;181:1582–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Wu B, Su S, Patil DP, Liu H, Gan J, Jaffrey SR, et al. Molecular basis for the specific and multivariant recognitions of RNA substrates by human hnRNP A2/B1. Nat Commun. 2018;9:420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Huang H, Weng H, Chen J. m(6)A modification in coding and non‐coding RNAs: roles and therapeutic implications in cancer. Cancer Cell. 2020;37:270–88. 10.1016/j.ccell.2020.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Roundtree IA, Evans ME, Pan T, He C. Dynamic RNA modifications in gene expression regulation. Cell. 2017;169:1187–200. 10.1016/j.cell.2017.05.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Wu B, Li L, Huang Y, Ma J, Min J. Readers, writers and erasers of N(6)‐methylated adenosine modification. Curr Opin Struct Biol. 2017;47:67–76. 10.1016/j.sbi.2017.05.011 [DOI] [PubMed] [Google Scholar]
- 115. Dominissini D, Moshitch‐Moshkovitz S, Schwartz S, Salmon‐Divon M, Ungar L, Osenberg S, et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A‐seq. Nature. 2012;485:201–6. 10.1038/nature11112 [DOI] [PubMed] [Google Scholar]
- 116. Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR. Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell. 2012;149:1635–46. 10.1016/j.cell.2012.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Arribas‐Hernández L, Rennie S, Köster T, Porcelli C, Lewinski M, Staiger D, et al. Principles of mRNA targeting via the Arabidopsis m(6)A‐binding protein ECT2. Elife. 2021;30:e72375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Maden BE. Identification of the locations of the methyl groups in 18 S ribosomal RNA from Xenopus laevis and man. J Mol Biol. 1986;189:681–99. 10.1016/0022-2836(86)90498-5 [DOI] [PubMed] [Google Scholar]
- 119. Maden BE. Locations of methyl groups in 28 S rRNA of Xenopus laevis and man. Clustering in the conserved core of molecule. J Mol Biol. 1988;201:289–314. [DOI] [PubMed] [Google Scholar]
- 120. Ignatova VV, Stolz P, Kaiser S, Gustafsson TH, Lastres PR, Sanz‐Moreno A, et al. The rRNA m(6)A methyltransferase METTL5 is involved in pluripotency and developmental programs. Genes Dev. 2020;34:715–29. 10.1101/gad.333369.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Lv Z, Sun L, Xu Q, Xing C, Yuan Y. Joint analysis of lncRNA m(6)A methylome and lncRNA/mRNA expression profiles in gastric cancer. Cancer Cell Int. 2020;20:464. 10.1186/s12935-020-01554-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Xiao S, Cao S, Huang Q, Xia L, Deng M, Yang M, et al. The RNA N(6)‐methyladenosine modification landscape of human fetal tissues. Nat Cell Biol. 2019;21:651–61. [DOI] [PubMed] [Google Scholar]
- 123. Zhou C, Molinie B, Daneshvar K, Pondick JV, Wang J, Van Wittenberghe N, et al. Genome‐wide maps of m6A circRNAs identify widespread and cell‐type‐specific methylation patterns that are distinct from mRNAs. Cell Rep. 2017;20:2262–76. 10.1016/j.celrep.2017.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Liu H, Xu Y, Yao B, Sui T, Lai L, Li Z. A novel N6‐methyladenosine (m6A)‐dependent fate decision for the lncRNA THOR. Cell Death Dis. 2020;11:613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Erson‐Bensan AE, Begik O. m6A modification and implications for microRNAs. Microrna. 2017;6:97–101. [DOI] [PubMed] [Google Scholar]
- 126. Han X, Guo J, Fan Z. Interactions between m6A modification and miRNAs in malignant tumors. Cell Death Dis. 2021;12:598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Wang H, Deng Q, Lv Z, Ling Y, Hou X, Chen Z, et al. N6‐methyladenosine induced miR‐143‐3p promotes the brain metastasis of lung cancer via regulation of VASH1. Mol Cancer. 2019;18:181. 10.1186/s12943-019-1108-x [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 128. Bhat SS, Bielewicz D, Gulanicz T, Bodi Z, Yu X, Anderson SJ, et al. mRNA adenosine methylase (MTA) deposits m(6)A on pri‐miRNAs to modulate miRNA biogenesis in Arabidopsis thaliana . Proc Natl Acad Sci USA. 2020;117:21785–95. 10.1073/pnas.2003733117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Chen Y, Lin Y, Shu Y, He J, Gao W. Interaction between N(6)‐methyladenosine (m(6)A) modification and noncoding RNAs in cancer. Mol Cancer. 2020;19:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Song P, Feng L, Li J, Dai D, Zhu L, Wang C, et al. β‐catenin represses miR455‐3p to stimulate m6A modification of HSF1 mRNA and promote its translation in colorectal cancer. Mol Cancer. 2020;19:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Coker H, Wei G, Brockdorff N. m6A modification of non‐coding RNA and the control of mammalian gene expression. Biochim Biophys Acta Gene Regul Mech. 2018;1862:310–8. [DOI] [PubMed] [Google Scholar]
- 132. Liu N, Zhou KI, Parisien M, Dai Q, Diatchenko L, Pan T. N6‐methyladenosine alters RNA structure to regulate binding of a low‐complexity protein. Nucleic Acids Res. 2017;45:6051–63. 10.1093/nar/gkx141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Zheng ZQ, Li ZX, Zhou GQ, Lin L, Zhang LL, Lv JW, et al. Long noncoding RNA FAM225A promotes nasopharyngeal carcinoma tumorigenesis and metastasis by acting as ceRNA to sponge miR‐590‐3p/miR‐1275 and upregulate ITGB3. Cancer Res. 2019;79:4612–26. [DOI] [PubMed] [Google Scholar]
- 134. Zuo X, Chen Z, Gao W, Zhang Y, Wang J, Wang J, et al. m6A‐mediated upregulation of LINC00958 increases lipogenesis and acts as a nanotherapeutic target in hepatocellular carcinoma. J Hematol Oncol. 2020;13:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Yin H, Chen L, Piao S, Wang Y, Li Z, Lin Y, et al. m6A RNA methylation‐mediated RMRP stability renders proliferation and progression of non‐small cell lung cancer through regulating TGFBR1/SMAD2/SMAD3 pathway. Cell Death Differ. 2021;Online ahead of print. 10.1038/s41418-021-00888-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Li ZX, Zheng ZQ, Yang PY, Lin L, Zhou GQ, Lv JW, et al. WTAP‐mediated m(6)A modification of lncRNA DIAPH1‐AS1 enhances its stability to facilitate nasopharyngeal carcinoma growth and metastasis. Cell Death Differ. 2022;29:1137–51. 10.1038/s41418-021-00905-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Jin D, Guo J, Wu Y, Du J, Yang L, Wang X, et al. m(6)A mRNA methylation initiated by METTL3 directly promotes YAP translation and increases YAP activity by regulating the MALAT1‐miR‐1914‐3p‐YAP axis to induce NSCLC drug resistance and metastasis. J Hematol Oncol. 2019;12:12. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 138. Yang D, Qiao J, Wang G, Lan Y, Li G, Guo X, et al. N6‐Methyladenosine modification of lincRNA 1281 is critically required for mESC differentiation potential. Nucleic Acids Res. 2018;46:3906–20. 10.1093/nar/gky130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Patil DP, Chen CK, Pickering BF, Chow A, Jackson C, Guttman M, et al. m(6)A RNA methylation promotes XIST‐mediated transcriptional repression. Nature. 2016;537:369–73. 10.1038/nature19342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Lee J, Wu Y, Harada BT, Li Y, Zhao J, He C, et al. N(6) ‐methyladenosine modification of lncRNA Pvt1 governs epidermal stemness. EMBO J. 2021;40:e106276. 10.15252/embj.2020106276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Wu Y, Yang X, Chen Z, Tian L, Jiang G, Chen F, et al. m(6)A‐induced lncRNA RP11 triggers the dissemination of colorectal cancer cells via upregulation of Zeb1. Mol Cancer. 2019;18:87. 10.1186/s12943-019-1014-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Roignant JY, Soller M. m(6)A in mRNA: an ancient mechanism for fine‐tuning gene expression. Trends Genet. 2017;33:380–90. 10.1016/j.tig.2017.04.003 [DOI] [PubMed] [Google Scholar]
- 143. Ge L, Zhang N, Chen Z, Song J, Wu Y, Li Z, et al. Level of N6‐methyladenosine in peripheral blood RNA: a novel predictive biomarker for gastric cancer. Clin Chem. 2020;66:342–51. [DOI] [PubMed] [Google Scholar]
- 144. Wu Q, Xie X, Huang Y, Meng S, Hu Y. N6‐methyladenosine RNA methylation regulators contribute to the progression of prostate cancer. J Cancer. 2018;12:682–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Pei Y, Lou X, Li K, Xu X, Guo Y, Xu D, et al. Peripheral blood leukocyte N6‐methyladenosine is a noninvasive biomarker for non‐small‐cell lung carcinoma. Onco Targets Ther. 2020;13:11913–21. 10.2147/OTT.S267344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Xie J, Huang Z, Jiang P, Wu R, Jiang H, Luo C, et al. Elevated N6‐methyladenosine RNA levels in peripheral blood immune cells: a novel predictive biomarker and therapeutic target for colorectal cancer. Front Immunol. 2021;12:760747. 10.3389/fimmu.2021.760747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Zhang J, Bai R, Li M, Ye H, Wu C, Wang C, et al. Excessive miR‐25‐3p maturation via N(6)‐methyladenosine stimulated by cigarette smoke promotes pancreatic cancer progression. Nat Commun. 2019;10:1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Zhu W, Si Y, Xu J, Lin Y, Wang JZ, Cao M, et al. Methyltransferase like 3 promotes colorectal cancer proliferation by stabilizing CCNE1 mRNA in an m6A‐dependent manner. J Cell Mol Med. 2020;24:3521–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Wang Q, Chen C, Ding Q, Zhao Y, Wang Z, Chen J, et al. METTL3‐mediated m(6)A modification of HDGF mRNA promotes gastric cancer progression and has prognostic significance. Gut. 2020;69:1193–205. [DOI] [PubMed] [Google Scholar]
- 150. Du Y, Hou G, Zhang H, Dou J, He J, Guo Y, et al. SUMOylation of the m6A‐RNA methyltransferase METTL3 modulates its function. Nucleic Acids Res. 2018;46:5195–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Cui X, Wang Z, Li J, Zhu J, Ren Z, Zhang D, et al. Cross talk between RNA N6‐methyladenosine methyltransferase‐like 3 and miR‐186 regulates hepatoblastoma progression through Wnt/β‐catenin signalling pathway. Cell Prolif. 2020;53:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. He H, Wu W, Sun Z, Chai L. MiR‐4429 prevented gastric cancer progression through targeting METTL3 to inhibit m(6)A‐caused stabilization of SEC62. Biochem Biophys Res Commun. 2019;517:581–7. [DOI] [PubMed] [Google Scholar]
- 153. Wei W, Huo B, Shi X. miR‐600 inhibits lung cancer via downregulating the expression of METTL3. Cancer Manag Res. 2019;11:1177–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Peng F, Xu J, Cui B, Liang Q, Zeng S, He B, et al. Oncogenic AURKA‐enhanced N(6)‐methyladenosine modification increases DROSHA mRNA stability to transactivate STC1 in breast cancer stem‐like cells. Cell Res. 2021;31:345–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Lang F, Singh RK, Pei Y, Zhang S, Sun K, Robertson ES. EBV epitranscriptome reprogramming by METTL14 is critical for viral‐associated tumorigenesis. PLoS Pathog. 2019;15:e1007796. 10.1371/journal.ppat.1007796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Han H, Fan G, Song S, Jiang Y, Li B. piRNA‐30473 contributes to tumorigenesis and poor prognosis by regulating m6A RNA methylation in DLBCL. Blood. 2020;137:1603–14. [DOI] [PubMed] [Google Scholar]
- 157. Ding L, Wang R, Zheng Q, Shen D, Wang H, Lu Z, et al. circPDE5A regulates prostate cancer metastasis via controlling WTAP‐dependent N6‐methyladenisine methylation of EIF3C mRNA. J Exp Clin Cancer Res. 2022;41:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Ge J, Liu M, Zhang Y, Xie L, Shi Z, Wang G. SNHG10/miR‐141‐3p/WTAP axis promotes osteosarcoma proliferation and migration. J Biochem Mol Toxicol. 2022;10:23031. [DOI] [PubMed] [Google Scholar]
- 159. Wei W, Sun J, Zhang H, Xiao X, Huang C, Wang L, et al. Circ0008399 interaction with WTAP promotes assembly and activity of the m(6)A methyltransferase complex and promotes cisplatin resistance in bladder cancer. Cancer Res. 2021;81:6142–56. [DOI] [PubMed] [Google Scholar]
- 160. Han H, Fan G, Song S, Jiang Y, Qian C, Zhang W, et al. piRNA‐30473 contributes to tumorigenesis and poor prognosis by regulating m6A RNA methylation in DLBCL. Blood. 2021;137:1603–14. [DOI] [PubMed] [Google Scholar]
- 161. Sorci M, Ianniello Z, Cruciani S, Larivera S, Ginistrelli LC, Capuano E, et al. METTL3 regulates WTAP protein homeostasis. Cell Death Dis. 2018;9:796. 10.1038/s41419-018-0843-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Deng J, Zhang J, Ye Y, Liu K, Zeng L, Huang J, et al. N(6) ‐methyladenosine‐mediated upregulation of WTAPP1 promotes WTAP translation and Wnt signaling to facilitate pancreatic cancer progression. Cancer Res. 2021;81:5268–83. 10.1158/0008-5472.CAN-21-0494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Wu S, Liu S, Cao Y, Chao G, Wang P, Pan H. Downregulation of ZC3H13 by miR‐362‐3p/miR‐425‐5p is associated with a poor prognosis and adverse outcomes in hepatocellular carcinoma. Aging. 2022;14:2304–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Zhu Y, Shen J, Gao L, Feng Y. Estrogen promotes fat mass and obesity‐associated protein nuclear localization and enhances endometrial cancer cell proliferation via the mTOR signaling pathway. Oncol Rep. 2016;35:2391–7. [DOI] [PubMed] [Google Scholar]
- 165. Cui YH, Yang S, Wei J, Shea CR, Zhong W, Wang F, et al. Autophagy of the m(6)A mRNA demethylase FTO is impaired by low‐level arsenic exposure to promote tumorigenesis. Nat Commun. 2021;12:2183. 10.1038/s41467-021-22469-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Su R, Dong L, Li C, Nachtergaele S, Wunderlich M, Qing Y, et al. R‐2HG exhibits anti‐tumor activity by targeting FTO/m(6)A/MYC/CEBPA signaling. Cell. 2018;172:90–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Song T, Yang Y, Wei H, Xie X, Lu J, Zeng Q, et al. Zfp217 mediates m6A mRNA methylation to orchestrate transcriptional and post‐transcriptional regulation to promote adipogenic differentiation. Nucleic Acids Res. 2019;47:6130–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Wang L, Song C, Wang N, Li S, Liu Q, Sun Z, et al. NADP modulates RNA m(6)A methylation and adipogenesis via enhancing FTO activity. Nat Chem Biol. 2020;16:1394–402. [DOI] [PubMed] [Google Scholar]
- 169. Thalhammer A, Bencokova Z, Poole R, Loenarz C, Adam J, O'Flaherty L, et al. Human AlkB homologue 5 is a nuclear 2‐oxoglutarate dependent oxygenase and a direct target of hypoxia‐inducible factor 1α (HIF‐1α). PLoS One. 2011;6:e16210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Zhang C, Samanta D, Lu H, Bullen JW, Zhang H, Chen I, et al. Hypoxia induces the breast cancer stem cell phenotype by HIF‐dependent and ALKBH5‐mediated m6A‐demethylation of NANOG mRNA. Proc Natl Acad Sci USA. 2016;113:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Wang J, Li Y, Wang P, Han G, Zhang T, Chang J, et al. Leukemogenic chromatin alterations promote AML leukemia stem cells via a KDM4C‐ALKBH5‐AXL signaling axis. Cell Stem Cell. 2020;27:81–97. [DOI] [PubMed] [Google Scholar]
- 172. Qu S, Jin L, Huang H, Lin J, Gao W, Zeng Z. A positive‐feedback loop between HBx and ALKBH5 promotes hepatocellular carcinogenesis. BMC Cancer. 2021;21:686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Hao L, Yin J, Yang H, Li C, Zhu L, Liu L, et al. ALKBH5‐mediated m(6)A demethylation of FOXM1 mRNA promotes progression of uveal melanoma. Aging. 2021;13:4045–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Guo X, Li K, Jiang W, Hu Y, Xiao W, Huang Y, et al. RNA demethylase ALKBH5 prevents pancreatic cancer progression by posttranscriptional activation of PER1 in an m6A‐YTHDF2‐dependent manner. Mol Cancer. 2020;19:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Liu Z, Wang Q, Wang X, Xu Z, Wei X, Li J. Circular RNA cIARS regulates ferroptosis in HCC cells through interacting with RNA binding protein ALKBH5. Cell Death Discov. 2020;6:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Wang J, Tan L, Jia B, Yu X, Yao R, OUYang N, et al. Downregulation of m(6)A reader YTHDC2 promotes the proliferation and migration of malignant lung cells via CYLD/NF‐κB pathway. Int J Biol Sci. 2021;17:2633–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Xu P, Hu K, Zhang P, Sun ZG, Zhang N. Hypoxia‐mediated YTHDF2 overexpression promotes lung squamous cell carcinoma progression by activation of the mTOR/AKT axis. Cancer Cell Int. 2022;22:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Hirschfeld M, Zhang B, Jaeger M, Stamm S, Erbes T, Mayer S, et al. Hypoxia‐dependent mRNA expression pattern of splicing factor YT521 and its impact on oncological important target gene expression. Mol Carcinog. 2014;53:883–92. [DOI] [PubMed] [Google Scholar]
- 179. Chen Z, Shao YL, Wang LL, Lin J, Zhang JB, Ding Y, et al. YTHDF2 is a potential target of AML1/ETO‐HIF1α loop‐mediated cell proliferation in t(8;21) AML. Oncogene. 2021;40:3786–98. [DOI] [PubMed] [Google Scholar]
- 180. Yarmishyn AA, Yang YP, Lu KH, Chen YC, Chien Y, Chou SJ, et al. Musashi‐1 promotes cancer stem cell properties of glioblastoma cells via upregulation of YTHDF1. Cancer Cell Int. 2020;20:507–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Chi F, Cao Y, Chen Y. Analysis and validation of circRNA‐miRNA network in regulating m(6)A RNA methylation modulators reveals CircMAP2K4/miR‐139‐5p/YTHDF1 axis involving the proliferation of hepatocellular carcinoma. Front Oncol. 2021;11:560506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Yang Z, Li J, Feng G, Gao S, Wang Y, Zhang S, et al. MicroRNA‐145 modulates N(6)‐methyladenosine levels by targeting the 3′‐untranslated mRNA region of the N(6)‐methyladenosine binding YTH domain family 2 protein. J Biol Chem. 2017;292:3614–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Li J, Wu L, Pei M, Zhang Y. YTHDF2, a protein repressed by miR‐145, regulates proliferation, apoptosis, and migration in ovarian cancer cells. J Ovarian Res. 2020;13:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Xu C, Yuan B, He T, Ding B, Li S. Prognostic values of YTHDF1 regulated negatively by mir‐3436 in Glioma. J Cell Mol Med. 2020;24:7538–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Zhou J, Xiao D, Qiu T, Li J, Liu Z. Loading microRNA‐376c in extracellular vesicles inhibits properties of non‐small cell lung cancer cells by targeting YTHDF1. Technol Cancer Res Treat. 2020;19:7538–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Qi B, Yang C, Zhu Z, Chen H. EZH2‐inhibited microRNA‐454‐3p promotes M2 macrophage polarization in glioma. Front Cell Dev Biol. 2020;8:574940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Du C, Lv C, Feng Y, Yu S. Activation of the KDM5A/miRNA‐495/YTHDF2/m6A‐MOB3B axis facilitates prostate cancer progression. J Exp Clin Cancer Res. 2020;39:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Fang R, Chen X, Zhang S, Shi H, Ye Y, Shi H, et al. EGFR/SRC/ERK‐stabilized YTHDF2 promotes cholesterol dysregulation and invasive growth of glioblastoma. Nat Commun. 2021;12:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Xu F, Li J, Ni M, Cheng J, Zhao H, Wang S, et al. FBW7 suppresses ovarian cancer development by targeting the N(6)‐methyladenosine binding protein YTHDF2. Mol Cancer. 2021;20:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Zhang X, Zhong L, Zou Z, Liang G, Zhu X. Clinical and prognostic pan‐cancer analysis of N6‐methyladenosine regulators in two types of hematological malignancies: a retrospective study based on TCGA and GTEx databases. Front Oncol. 2021;11:623170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Huang H, Wang D, Guo W, Zhuang X, He Y. Correlated low IGF2BP1 and FOXM1 expression predicts a good prognosis in lung adenocarcinoma. Pathol Res Pract. 2019;215:152433. [DOI] [PubMed] [Google Scholar]
- 192. Xiao W, Adhikari S, Dahal U, Chen YS, Hao YJ, Sun BF, et al. Nuclear m(6)A reader YTHDC1 regulates mRNA splicing. Mol Cell. 2016;61:507–19. 10.1016/j.molcel.2016.01.012 [DOI] [PubMed] [Google Scholar]
- 193. Xu Y, Zheng Y, Liu H, Li T. Modulation of IGF2BP1 by long non‐coding RNA HCG11 suppresses apoptosis of hepatocellular carcinoma cells via MAPK signaling transduction. Int J Oncol. 2017;51:791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Yang F, Xue X, Zheng L, Bi J, Zhou Y, Zhi K, et al. Long non‐coding RNA GHET1 promotes gastric carcinoma cell proliferation by increasing c‐Myc mRNA stability. FEBS J. 2014;281:802–13. [DOI] [PubMed] [Google Scholar]
- 195. Lixin S, Wei S, Haibin S, Qingfu L, Tiemin P. miR‐885‐5p inhibits proliferation and metastasis by targeting IGF2BP1 and GALNT3 in human intrahepatic cholangiocarcinoma. Mol Carcinog. 2020;59:1371–81. [DOI] [PubMed] [Google Scholar]
- 196. Dai N, Ji F, Wright J, Minichiello L, Sadreyev R, Avruch J. IGF2 mRNA binding protein‐2 is a tumor promoter that drives cancer proliferation through its client mRNAs IGF2 and HMGA1. Elife. 2017;28:e27155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Cleynen I, Brants JR, Peeters K, Deckers R, Debiec‐Rychter M, Sciot R, et al. HMGA2 regulates transcription of the Imp2 gene via an intronic regulatory element in cooperation with nuclear factor‐kappaB. Mol Cancer Res. 2007;5:363–72. [DOI] [PubMed] [Google Scholar]
- 198. Lai BQ, Che MT, Du BL, Zeng X, Ma YH, Feng B, et al. Transplantation of tissue engineering neural network and formation of neuronal relay into the transected rat spinal cord. Biomaterials. 2016;109:40–54. [DOI] [PubMed] [Google Scholar]
- 199. Liu J, Jiang X, Zou A, Mai Z, Huang Z, Sun L, et al. circIGHG‐induced epithelial‐to‐mesenchymal transition promotes oral squamous cell carcinoma progression via miR‐142‐5p/IGF2BP3 signaling. Cancer Res. 2021;81:344–55. [DOI] [PubMed] [Google Scholar]
- 200. Tang Z, Kang B, Li C, Chen T, Zhang Z. GEPIA2: an enhanced web server for large‐scale expression profiling and interactive analysis. Nucleic Acids Res. 2019;47:W556–60. 10.1093/nar/gkz430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Liu Y, Shi S‐L. The roles of hnRNP A2/B1 in RNA biology and disease. Wiley Interdiscip Rev RNA. 2021;12:e1612. 10.1002/wrna.1612 [DOI] [PubMed] [Google Scholar]
- 202. Fischl H, Neve J, Wang Z, Patel R, Louey A, Tian B, et al. hnRNPC regulates cancer‐specific alternative cleavage and polyadenylation profiles. Nucleic Acids Res. 2019;47:7580–91. 10.1093/nar/gkz461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. McCloskey A, Taniguchi I, Shinmyozu K, Ohno M. hnRNP C tetramer measures RNA length to classify RNA polymerase II transcripts for export. Science. 2012;335:1643–6. 10.1126/science.1218469 [DOI] [PubMed] [Google Scholar]
- 204. Wang W, Shao F, Yang X, Wang J, Zhu R, Yang Y, et al. METTL3 promotes tumour development by decreasing APC expression mediated by APC mRNA N(6)‐methyladenosine‐dependent YTHDF binding. Nat Commun. 2021;12:3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Wang Y, Chen J, Gao WQ, Yang R. METTL14 promotes prostate tumorigenesis by inhibiting THBS1 via an m6A‐YTHDF2‐dependent mechanism. Cell Death Discov. 2022;8:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Yao X, Li W, Li L, Li M, Zhao Y, Fang D, et al. YTHDF1 upregulation mediates hypoxia‐dependent breast cancer growth and metastasis through regulating PKM2 to affect glycolysis. Cell Death Dis. 2022;13:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Zhang Z, Xing Y, Gao W, Yang L, Shi J, Song W, et al. N(6)‐methyladenosine (m(6)A) reader IGF2BP2 promotes gastric cancer progression via targeting SIRT1. Bioengineered. 2022;13:11541–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Wu J, Pang R, Li M, Chen B, Huang J, Zhu Y. m6A‐induced LncRNA MEG3 suppresses the proliferation, migration and invasion of hepatocellular carcinoma cell through miR‐544b/BTG2 signaling. Onco Targets Ther. 2021;14:3745–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Wu Q, Yin X, Zhao W, Xu W, Chen L. Molecular mechanism of m(6)A methylation of circDLC1 mediated by RNA methyltransferase METTL3 in the malignant proliferation of glioma cells. Cell Death Discov. 2022;8:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Zou L, Chen W, Zhou X, Yang T, Luo J, Long Z, et al. N6‐methyladenosine demethylase FTO suppressed prostate cancer progression by maintaining CLIC4 mRNA stability. Cell Death Discov. 2022;8:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Hu Y, Gong C, Li Z, Liu J, Chen Y, Huang Y, et al. Demethylase ALKBH5 suppresses invasion of gastric cancer via PKMYT1 m6A modification. Mol Cancer. 2022;21:34. 10.1186/s12943-022-01522-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Liu D, Xia AD, Wu LP, Li S, Zhang K, Chen D. IGF2BP2 promotes gastric cancer progression by regulating the IGF1R‐RhoA‐ROCK signaling pathway. Cell Signal. 2022;94:16. [DOI] [PubMed] [Google Scholar]
- 213. Chen RX, Chen X, Xia LP, Zhang JX, Pan ZZ, Ma XD, et al. N(6)‐methyladenosine modification of circNSUN2 facilitates cytoplasmic export and stabilizes HMGA2 to promote colorectal liver metastasis. Nat Commun. 2019;10:4695. 10.1038/s41467-019-12651-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Chen Y, Ling Z, Cai X, Xu Y, Lv Z, Man D, et al. Activation of YAP1 by N6‐methyladenosine‐modified circCPSF6 drives malignancy in hepatocellular carcinoma. Cancer Res. 2022;82:599–614. 10.1158/0008-5472.CAN-21-1628 [DOI] [PubMed] [Google Scholar]
- 215. Zhang X, Xu Y, Qian Z, Zheng W, Wu Q, Chen Y, et al. circRNA_104075 stimulates YAP‐dependent tumorigenesis through the regulation of HNF4a and may serve as a diagnostic marker in hepatocellular carcinoma. Cell Death Dis. 2018;9:1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Yue C, Chen J, Li Z, Li L, Guo Y. microRNA‐96 promotes occurrence and progression of colorectal cancer via regulation of the AMPKα2‐FTO‐m6A/MYC axis. J Exp Clin Cancer Res. 2020;39:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Ma YS, Shi BW, Guo JH, Liu JB, Yang XL, Xin R, et al. microRNA‐320b suppresses HNF4G and IGF2BP2 expression to inhibit angiogenesis and tumor growth of lung cancer. Carcinogenesis. 2021;42:762–71. [DOI] [PubMed] [Google Scholar]
- 218. Xu LM, Zhang J, Ma Y, Yuan YJ, Yu H, Wang J, et al. MicroRNA‐135 inhibits initiation of epithelial‐mesenchymal transition in breast cancer by targeting ZNF217 and promoting m6A modification of NANOG. Oncogene. 2022;41:1742–51. [DOI] [PubMed] [Google Scholar]
- 219. Qian X, Yang J, Qiu Q, Li X, Jiang C, Li J, et al. LCAT3, a novel m6A‐regulated long non‐coding RNA, plays an oncogenic role in lung cancer via binding with FUBP1 to activate c‐MYC. J Hematol Oncol. 2021;14:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Wu S, Zhang L, Deng J, Guo B, Li F, Wang Y, et al. A novel micropeptide encoded by Y‐linked LINC00278 links cigarette smoking and AR signaling in male esophageal squamous cell carcinoma. Cancer Res. 2020;80:2790–803. [DOI] [PubMed] [Google Scholar]
- 221. Ni W, Yao S, Zhou Y, Liu Y, Huang P, Zhou A, et al. Long noncoding RNA GAS5 inhibits progression of colorectal cancer by interacting with and triggering YAP phosphorylation and degradation and is negatively regulated by the m(6)A reader YTHDF3. Mol Cancer. 2019;18:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Tang C, Xie Y, Yu T, Liu N, Wang Z, Woolsey RJ, et al. m(6)A‐dependent biogenesis of circular RNAs in male germ cells. Cell Res. 2020;30:211–28. 10.1038/s41422-020-0279-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Di Timoteo G, Dattilo D, Centrón‐Broco A, Colantoni A, Guarnacci M, Rossi F, et al. Modulation of circRNA metabolism by m(6)A modification. Cell Rep. 2020;31:107641. 10.1016/j.celrep.2020.107641 [DOI] [PubMed] [Google Scholar]
- 224. Park OH, Ha H, Lee Y, Boo SH, Kwon DH, Song HK, et al. Endoribonucleolytic cleavage of m(6)A‐containing RNAs by RNase P/MRP complex. Mol Cell. 2019;74:494–507. [DOI] [PubMed] [Google Scholar]
- 225. Wu P, Fang X, Liu Y, Tang Y, Wang W, Li X, et al. N6‐methyladenosine modification of circCUX1 confers radioresistance of hypopharyngeal squamous cell carcinoma through caspase1 pathway. Cell Death Dis. 2021;12:298. 10.1038/s41419-021-03558-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Xu J, Wan Z, Tang M, Lin Z, Jiang S, Ji L, et al. N(6)‐methyladenosine‐modified CircRNA‐SORE sustains sorafenib resistance in hepatocellular carcinoma by regulating beta‐catenin signaling. Mol Cancer. 2020;19:163. 10.1186/s12943-020-01281-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Yang Y, Fan X, Mao M, Song X, Wu P, Zhang Y, et al. Extensive translation of circular RNAs driven by N(6)‐methyladenosine. Cell Res. 2017;27:626–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Zhang D, Ni N, Wang Y, Tang Z, Gao H, Ju Y, et al. CircRNA‐vgll3 promotes osteogenic differentiation of adipose‐derived mesenchymal stem cells via modulating miRNA‐dependent integrin α5 expression. Cell Death Differ. 2021;28:283–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Chen YG, Chen R, Ahmad S, Verma R, Kasturi SP, Amaya L, et al. N6‐methyladenosine modification controls circular RNA immunity. Mol Cell. 2019;76:96–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Wei J, Yin Y, Zhou J, Chen H, Peng J, Yang J, et al. METTL3 potentiates resistance to cisplatin through m(6) A modification of TFAP2C in seminoma. J Cell Mol Med. 2020;24:11366–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Lin X, Wang F, Chen J, Liu J, Lin YB, Li L, et al. N(6)‐methyladenosine modification of CENPK mRNA by ZC3H13 promotes cervical cancer stemness and chemoresistance. Mil Med Res. 2022;9:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Chen Z, Wu L, Zhou J, Lin X, Peng Y, Ge L, et al. N6‐methyladenosine‐induced ERRγ triggers chemoresistance of cancer cells through upregulation of ABCB1 and metabolic reprogramming. Theranostics. 2020;10:3382–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Fukumoto T, Zhu H, Nacarelli T, Karakashev S, Fatkhutdinov N, Wu S, et al. N(6)‐methylation of adenosine of FZD10 mRNA contributes to PARP inhibitor resistance. Cancer Res. 2019;79:2812–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Sun Y, Dong D, Xia Y, Hao L, Wang W, Zhao C. YTHDF1 promotes breast cancer cell growth, DNA damage repair and chemoresistance. Cell Death Dis. 2022;13:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Wang ZW, Pan JJ, Hu JF, Zhang JQ, Huang L, Huang Y, et al. SRSF3‐mediated regulation of N6‐methyladenosine modification‐related lncRNA ANRIL splicing promotes resistance of pancreatic cancer to gemcitabine. Cell Rep. 2022;39:110813. 10.1016/j.celrep.2022.110813 [DOI] [PubMed] [Google Scholar]
- 236. Pan S, Deng Y, Fu J, Zhang Y, Zhang Z, Qin X. N6‐methyladenosine upregulates miR‐181d‐5p in exosomes derived from cancer‐associated fibroblasts to inhibit 5‐FU sensitivity by targeting NCALD in colorectal cancer. Int J Oncol. 2022;60:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Duan JL, Chen W, Xie JJ, Zhang ML, Nie RC, Liang H, et al. A novel peptide encoded by N6‐methyladenosine modified circMAP3K4 prevents apoptosis in hepatocellular carcinoma. Mol Cancer. 2022;21:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Yao Y, Yang Y, Guo W, Xu L, You M, Zhang YC, et al. METTL3‐dependent m(6)A modification programs T follicular helper cell differentiation. Nat Commun. 2021;12:1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Li HB, Tong J, Zhu S, Batista PJ, Duffy EE, Zhao J, et al. m(6)A mRNA methylation controls T cell homeostasis by targeting the IL‐7/STAT5/SOCS pathways. Nature. 2017;548:338–42. 10.1038/nature23450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Zhou J, Zhang X, Hu J, Qu R, Yu Z, Xu H, et al. m(6)A demethylase ALKBH5 controls CD4(+) T cell pathogenicity and promotes autoimmunity. Sci Adv. 2021;7:eabg0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Han D, Liu J, Chen C, Dong L, Liu Y, Chang R, et al. Anti‐tumour immunity controlled through mRNA m(6)A methylation and YTHDF1 in dendritic cells. Nature. 2019;566:270–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Yu R, Li Q, Feng Z, Cai L, Xu Q. m6A reader YTHDF2 regulates LPS‐induced inflammatory response. Int J Mol Sci. 2019;20:1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Tian C, Huang Y, Li Q, Feng Z, Xu Q. Mettl3 regulates osteogenic differentiation and alternative splicing of Vegfa in bone marrow mesenchymal stem cells. Int J Mol Sci. 2019;20:551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Li N, Kang Y, Wang L, Huff S, Tang R, Hui H, et al. ALKBH5 regulates anti‐PD‐1 therapy response by modulating lactate and suppressive immune cell accumulation in tumor microenvironment. Proc Natl Acad Sci USA. 2020;117:20159–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Wei C, Wang B, Peng D, Zhang X, Li Z, Luo L, et al. Pan‐cancer analysis shows that ALKBH5 is a potential prognostic and immunotherapeutic biomarker for multiple cancer types including gliomas. Front Immunol. 2022;13:849592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Li B, Zhu L, Lu C, Wang C, Wang H, Jin H, et al. circNDUFB2 inhibits non‐small cell lung cancer progression via destabilizing IGF2BPs and activating anti‐tumor immunity. Nat Commun. 2021;12:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Cai J, Chen Z, Zhang Y, Wang J, Zhang Z, Wu J, et al. CircRHBDD1 augments metabolic rewiring and restricts immunotherapy efficacy via m(6)A modification in hepatocellular carcinoma. Mol Ther Oncolytics. 2022;24:755–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Yan G, Yuan Y, He M, Gong R, Lei H, Zhou H, et al. m(6)A methylation of precursor‐miR‐320/RUNX2 controls osteogenic potential of bone marrow‐derived mesenchymal stem cells. Mol Ther Nucleic Acids. 2020;19:421–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Baghban R, Roshangar L, Jahanban‐Esfahlan R, Seidi K, Ebrahimi‐Kalan A, Jaymand M, et al. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun Signal. 2020;18:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Chen H, Gao S, Liu W, Wong CC, Wu J, Wu J, et al. RNA N(6)‐methyladenosine methyltransferase METTL3 facilitates colorectal cancer by activating the m(6)A‐GLUT1‐mTORC1 axis and is a therapeutic target. Gastroenterology. 2021;160:1284–300. [DOI] [PubMed] [Google Scholar]
- 251. Yu H, Zhao K, Zeng H, Li Z, Chen K, Zhang Z, et al. N(6)‐methyladenosine (m(6)A) methyltransferase WTAP accelerates the Warburg effect of gastric cancer through regulating HK2 stability. Biomed Pharmacother. 2021;133:9. [DOI] [PubMed] [Google Scholar]
- 252. Yang X, Shao F, Guo D, Wang W, Wang J, Zhu R, et al. WNT/β‐catenin‐suppressed FTO expression increases m(6)A of c‐Myc mRNA to promote tumor cell glycolysis and tumorigenesis. Cell Death Dis. 2021;12:462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Sheng H, Li Z, Su S, Sun W, Zhang X, Li L, et al. YTH domain family 2 promotes lung cancer cell growth by facilitating 6‐phosphogluconate dehydrogenase mRNA translation. Carcinogenesis. 2020;41:541–50. [DOI] [PubMed] [Google Scholar]
- 254. Liu J, Yuan JF, Wang YZ. METTL3‐stabilized lncRNA SNHG7 accelerates glycolysis in prostate cancer via SRSF1/c‐Myc axis. Exp Cell Res. 2022;416:9. [DOI] [PubMed] [Google Scholar]
- 255. Liu XS, Zhou LM, Yuan LL, Gao Y, Kui XY, Liu XY, et al. NPM1 is a prognostic biomarker involved in immune infiltration of lung adenocarcinoma and associated with m6A modification and glycolysis. Front Immunol. 2021;12:724741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Cai J, Zhou M, Xu J. N6‐methyladenosine (m6A) RNA methylation regulator SNRPC is a prognostic biomarker and is correlated with immunotherapy in hepatocellular carcinoma. World J Surg Oncol. 2021;19:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Liu XS, Gao Y, Wu LB, Wan HB, Yan P, Jin Y, et al. Comprehensive analysis of GLUT1 immune infiltrates and ceRNA network in human esophageal carcinoma. Front Oncol. 2021;11:665388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Xie JW, Huang XB, Chen QY, Ma YB, Zhao YJ, Liu LC, et al. m(6)A modification‐mediated BATF2 acts as a tumor suppressor in gastric cancer through inhibition of ERK signaling. Mol Cancer. 2020;19:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Zhao J, Huang S, Tan D, Yang K, Chen M, Jia X, et al. PGM1 and ENO1 promote the malignant progression of bladder cancer via comprehensive analysis of the m6A signature and tumor immune infiltration. J Oncol. 2022;24:8581805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Yang Z, Peng B, Pan Y, Gu Y. Analysis and verification of N(6)‐methyladenosine‐modified genes as novel biomarkers for clear cell renal cell carcinoma. Bioengineered. 2021;12:9473–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Zhang B, Chen Z, Tao B, Yi C, Lin Z, Li Y, et al. m(6)A target microRNAs in serum for cancer detection. Mol Cancer. 2021;20:170. 10.1186/s12943-021-01477-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Xu F, Huang X, Li Y, Chen Y, Lin L. m(6)A‐related lncRNAs are potential biomarkers for predicting prognoses and immune responses in patients with LUAD. Mol Ther Nucleic Acids. 2021;24:780–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Lv W, Wang Y, Zhao C, Tan Y, Xiong M, Yi Y, et al. Identification and validation of m6A‐related lncRNA signature as potential predictive biomarkers in breast cancer. Front Oncol. 2021;11:745719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Guo Y, Guo Y, Chen C, Fan D, Wu X, Zhao L, et al. Circ3823 contributes to growth, metastasis and angiogenesis of colorectal cancer: involvement of miR‐30c‐5p/TCF7 axis. Mol Cancer. 2021;20:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Chen C, Yuan W, Zhou Q, Shao B, Guo Y, Wang W, et al. N6‐methyladenosine‐induced circ1662 promotes metastasis of colorectal cancer by accelerating YAP1 nuclear localization. Theranostics. 2021;11:4298–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Cui Y, Zhang C, Ma S, Li Z, Wang W, Li Y, et al. RNA m6A demethylase FTO‐mediated epigenetic up‐regulation of LINC00022 promotes tumorigenesis in esophageal squamous cell carcinoma. J Exp Clin Cancer Res. 2021;40:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Zhou W, Bai C, Long C, Hu L, Zheng Y. Construction and Characterization of Long Non‐Coding RNA‐Associated Networks to Reveal Potential Prognostic Biomarkers in Human Lung Adenocarcinoma. Front Oncol. 2021;11:720400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Wang X, Yu J, Chen J, Hou Y, Du Z, Huang H, et al. Copy number variation analysis of m(6) A regulators identified METTL3 as a prognostic and immune‐related biomarker in bladder cancer. Cancer Med. 2021;10:7804–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Cai C, Long J, Huang Q, Han Y, Peng Y, Guo C, et al. m6A "Writer" gene METTL14: a favorable prognostic biomarker and correlated with immune infiltrates in rectal cancer. Front Oncol. 2021;11:615296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Zhao C, Liu Y, Ju S, Wang X. Pan‐cancer analysis of the N6‐methyladenosine eraser FTO as a potential prognostic and immunological biomarker. Int J Gen Med. 2021;14:7411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Feng ZY, Wang T, Su X, Guo S. Identification of the m(6)A RNA methylation regulators WTAP as a novel prognostic biomarker and genomic alterations in cutaneous melanoma. Front Mol Biosci. 2021;8:665222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Miao R, Dai CC, Mei L, Xu J, Sun SW, Xing YL, et al. KIAA1429 regulates cell proliferation by targeting c‐Jun messenger RNA directly in gastric cancer. J Cell Physiol. 2020;235:7420–32. [DOI] [PubMed] [Google Scholar]
- 273. Jiang H, Ning G, Wang Y, Lv W. Identification of an m6A‐related signature as biomarker for hepatocellular carcinoma prognosis and correlates with sorafenib and Anti‐PD‐1 immunotherapy treatment response. Dis Markers. 2021;10:5576683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Zhang B, Gu Y, Jiang G. Expression and prognostic characteristics of m(6) A RNA methylation regulators in breast cancer. Front Genet. 2020;11:604597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Wang Z, Liu J, Yang Y, Xing C, Jing J, Yuan Y. Expression and prognostic potential of ribosome 18S RNA m(6)A methyltransferase METTL5 in gastric cancer. Cancer Cell Int. 2021;21:569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Zhang Z, Zhang C, Yang Z, Zhang G, Wu P, Luo Y, et al. m(6)A regulators as predictive biomarkers for chemotherapy benefit and potential therapeutic targets for overcoming chemotherapy resistance in small‐cell lung cancer. J Hematol Oncol. 2021;14(1):190. 10.1186/s13045-021-01173-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Hu J, Qiu D, Yu A, Hu J, Deng H, Li H, et al. YTHDF1 is a potential pan‐cancer biomarker for prognosis and immunotherapy. Front Oncol. 2021;11:607224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Su G, Liu T, Han X, Sun H, Che W, Hu K, et al. YTHDF2 is a potential biomarker and associated with immune infiltration in kidney renal clear cell carcinoma. Front Pharmacol. 2021;12:709548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Liu M, Zhao Z, Cai Y, Bi P, Liang Q, Yan Y, et al. YTH domain family: potential prognostic targets and immune‐associated biomarkers in hepatocellular carcinoma. Aging. 2021;13:24205–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Demircan T, Yavuz M, Akgül S. m(6)A pathway regulators are frequently mutated in breast invasive carcinoma and may play an important role in disease pathogenesis. Omics. 2021;25:660–78. [DOI] [PubMed] [Google Scholar]
- 281. Lin Y, Jin X, Nie Q, Chen M, Guo W, Chen L, et al. YTHDF3 facilitates triple‐negative breast cancer progression and metastasis by stabilizing ZEB1 mRNA in an m(6)A‐dependent manner. Ann Transl Med. 2022;10:21–6857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Liu S, Li G, Li Q, Zhang Q, Zhuo L, Chen X, et al. The roles and mechanisms of YTH domain‐containing proteins in cancer development and progression. Am J Cancer Res. 2020;10:1068–84. [PMC free article] [PubMed] [Google Scholar]
- 283. Chen HM, Lin CC, Chen WS, Jiang JK, Yang SH, Chang SC, et al. Insulin‐like growth factor 2 mRNA‐binding protein 1 (IGF2BP1) is a prognostic biomarker and associated with chemotherapy responsiveness in colorectal cancer. Int J Mol Sci. 2021;22:6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Kuai D, Zhu S, Shi H, Yang R, Liu T, Liu H, et al. Aberrant expression of m(6)A mRNA methylation regulators in colorectal adenoma and adenocarcinoma. Life Sci. 2021;273:119258. [DOI] [PubMed] [Google Scholar]
- 285. Jia M, Shi Y, Xie Y, Li W, Deng J, Fu D, et al. WT1‐AS/IGF2BP2 axis is a potential diagnostic and prognostic biomarker for lung adenocarcinoma according to ceRNA network comprehensive analysis combined with experiments. Cells. 2021;11:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Wang X, Xu H, Zhou Z, Guo S, Chen R. IGF2BP2 maybe a novel prognostic biomarker in oral squamous cell carcinoma. Biosci Rep. 2022;42:BSR20212119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Sun C, Zheng X, Sun Y, Yu J, Sheng M, Yan S, et al. Identification of IGF2BP3 as an adverse prognostic biomarker of gliomas. Front Genet. 2021;12:743738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Chen B, Ye F, Yu L, Jia G, Huang X, Zhang X, et al. Development of cell‐active N6‐methyladenosine RNA demethylase FTO inhibitor. J Am Chem Soc. 2012;134:17963–71. 10.1021/ja3064149 [DOI] [PubMed] [Google Scholar]
- 289. Huang Y, Yan J, Li Q, Li J, Gong S, Zhou H, et al. Meclofenamic acid selectively inhibits FTO demethylation of m6A over ALKBH5. Nucleic Acids Res. 2015;43:373–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Zhang L, Ren T, Wang Z, Wang R, Chang J. Comparative study of the binding of 3 flavonoids to the fat mass and obesity‐associated protein by spectroscopy and molecular modeling. J Mol Recognit. 2017;30. 10.1002/jmr.2606 [DOI] [PubMed] [Google Scholar]
- 291. Peng S, Xiao W, Ju D, Sun B, Hou N, Liu Q, et al. Identification of entacapone as a chemical inhibitor of FTO mediating metabolic regulation through FOXO1. Sci Transl Med. 2019;11:eaau7116. 10.1126/scitranslmed.aau7116 [DOI] [PubMed] [Google Scholar]
- 292. Huang Y, Su R, Sheng Y, Dong L, Dong Z, Xu H, et al. Small‐molecule targeting of oncogenic FTO demethylase in acute myeloid leukemia. Cancer Cell. 2019;35:677–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Xie G, Wu XN, Ling Y, Rui Y, Wu D, Zhou J, et al. A novel inhibitor of N6‐methyladenosine demethylase FTO induces mRNA methylation and shows anti‐cancer activities. Acta Pharm Sin B. 2022;12:853–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Yankova E, Blackaby W, Albertella M, Rak J, De Braekeleer E, Tsagkogeorga G, et al. Small‐molecule inhibition of METTL3 as a strategy against myeloid leukaemia. Nature. 2021;593:597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Sabnis RW. Novel small molecule RNA m6A demethylase AlkBH5 inhibitors for treating cancer. ACS Med Chem Lett. 2021;12:856–7. 10.1021/acsmedchemlett.1c00102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Xu W, Xie S, Chen X, Pan S, Qian H, Zhu X. Effects of quercetin on the efficacy of various chemotherapeutic drugs in cervical cancer cells. Drug Des Devel Ther. 2021;15:577–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Zhang L, Qi Y, Aluo Z, Liu S, Zhang Z, Zhou L. Betaine increases mitochondrial content and improves hepatic lipid metabolism. Food Funct. 2019;10:216–23. [DOI] [PubMed] [Google Scholar]
- 298. Wang Y, Li J, Han X, Wang N, Song C, Wang R, et al. Identification of Clausine E as an inhibitor of fat mass and obesity‐associated protein (FTO) demethylase activity. J Mol Recognit. 2019;32:19. [DOI] [PubMed] [Google Scholar]
- 299. Chen Y, Wu R, Chen W, Liu Y, Liao X, Zeng B, et al. Curcumin prevents obesity by targeting TRAF4‐induced ubiquitylation in m(6) A‐dependent manner. EMBO Rep. 2021;22:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Ghazi T, Nagiah S, Chuturgoon AA. Fusaric acid decreases p53 expression by altering promoter methylation and m6A RNA methylation in human hepatocellular carcinoma (HepG2) cells. Epigenetics. 2021;16:79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Deng LJ, Deng WQ, Fan SR, Chen MF, Qi M, Lyu WY, et al. m6A modification: recent advances, anticancer targeted drug discovery and beyond. Mol Cancer. 2022;21:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Bedi RK, Huang D, Eberle SA, Wiedmer L, Śledź P, Caflisch A. Small‐molecule inhibitors of METTL3, the major human epitranscriptomic writer. ChemMedChem. 2020;15:744–8. [DOI] [PubMed] [Google Scholar]
- 303. Moroz‐Omori EV, Huang D, Kumar Bedi R, Cheriyamkunnel SJ, Bochenkova E, Dolbois A, et al. METTL3 inhibitors for epitranscriptomic modulation of cellular processes. ChemMedChem. 2021;16:3035–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Zheng G, Cox T, Tribbey L, Wang GZ, Iacoban P, Booher ME, et al. Synthesis of a FTO inhibitor with anticonvulsant activity. ACS Chem Neurosci. 2014;5:658–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Qiao Y, Zhou B, Zhang M, Liu W, Han Z, Song C, et al. A novel inhibitor of the obesity‐related protein FTO. Biochemistry. 2016;55:1516–22. 10.1021/acs.biochem.6b00023 [DOI] [PubMed] [Google Scholar]
- 306. Su R, Dong L, Li Y, Gao M, Han L, Wunderlich M, et al. Targeting FTO suppresses cancer stem cell maintenance and immune evasion. Cancer Cell. 2020;38:79–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Sun K, Du Y, Hou Y, Zhao M, Li J, Du Y, et al. Saikosaponin D exhibits anti‐leukemic activity by targeting FTO/m(6)A signaling. Theranostics. 2021;11:5831–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Liu Y, Liang G, Xu H, Dong W, Dong Z, Qiu Z, et al. Tumors exploit FTO‐mediated regulation of glycolytic metabolism to evade immune surveillance. Cell Metab. 2021;33:1221–33. [DOI] [PubMed] [Google Scholar]
- 309. Huff S, Tiwari SK, Gonzalez GM, Wang Y, Rana TM. m(6)A‐RNA demethylase FTO inhibitors impair self‐renewal in glioblastoma stem cells. ACS Chem Biol. 2021;16:324–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Xie G, Wu XN, Ling Y, Rui Y, Wu D, Zhou J, et al. A novel inhibitor of N (6)‐methyladenosine demethylase FTO induces mRNA methylation and shows anti‐cancer activities. Acta Pharm Sin B. 2022;12:853–66. 10.1016/j.apsb.2021.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Malacrida A, Rivara M, Di Domizio A, Cislaghi G, Miloso M, Zuliani V, et al. 3D proteome‐wide scale screening and activity evaluation of a new ALKBH5 inhibitor in U87 glioblastoma cell line. Bioorg Med Chem. 2020;28:30. [DOI] [PubMed] [Google Scholar]
- 312. Selberg S, Seli N, Kankuri E, Karelson M. Rational design of novel anticancer small‐molecule RNA m6A demethylase ALKBH5 inhibitors. ACS Omega. 2021;6:13310–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Fang Z, Mu B, Liu Y, Guo N, Xiong L, Guo Y, et al. Discovery of a potent, selective and cell active inhibitor of m(6)A demethylase ALKBH5. Eur J Med Chem. 2022;238:11. [DOI] [PubMed] [Google Scholar]
- 314. Mahapatra L, Andruska N, Mao C, Le J, Shapiro DJ. A novel IMP1 inhibitor, BTYNB, targets c‐Myc and inhibits melanoma and ovarian cancer cell proliferation. Transl Oncol. 2017;10:818–27. 10.1016/j.tranon.2017.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Wallis N, Oberman F, Shurrush K, Germain N, Greenwald G, Gershon T, et al. Small molecule inhibitor of Igf2bp1 represses Kras and a pro‐oncogenic phenotype in cancer cells. RNA Biol. 2022;19:26–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Dahlem C, Abuhaliema A, Kessler SM, Kröhler T, Zoller BGE, Chanda S, et al. First small‐molecule inhibitors targeting the RNA‐binding protein IGF2BP2/IMP2 for cancer therapy. ACS Chem Biol. 2022;17:361–75. [DOI] [PubMed] [Google Scholar]
- 317. Sun HL, Zhu AC, Gao Y, Terajima H, Fei Q, Liu S, et al. Stabilization of ERK‐phosphorylated METTL3 by USP5 increases m(6)A methylation. Mol Cell. 2020;80:633–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Li J, Chen Z, Chen F, Xie G, Ling Y, Peng Y, et al. Targeted mRNA demethylation using an engineered dCas13b‐ALKBH5 fusion protein. Nucleic Acids Res. 2020;48:5684–94. 10.1093/nar/gkaa269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Liu XM, Zhou J, Mao Y, Ji Q, Qian SB. Programmable RNA N(6)‐methyladenosine editing by CRISPR‐Cas9 conjugates. Nat Chem Biol. 2019;15:865–71. 10.1038/s41589-019-0327-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Sun X, Wang DO, Wang J. Targeted manipulation of m(6)A RNA modification through CRISPR‐Cas‐based strategies. Methods. 2022;203:56–61. 10.1016/j.ymeth.2022.03.006 [DOI] [PubMed] [Google Scholar]
- 321. Wu Y, Yang X, Jiang G, Zhang H, Wang H. 5′‐tRF‐GlyGCC: a tRNA‐derived small RNA as a novel biomarker for colorectal cancer diagnosis. Genome Med. 2021;13:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Mohammad HP, Barbash O, Creasy CL. Targeting epigenetic modifications in cancer therapy: erasing the roadmap to cancer. Nat Med. 2019;25:403–18. 10.1038/s41591-019-0376-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data openly available in a public repository.


