Abstract
Chronic inflammation exerts pleiotropic effects in the etiology and progression of chronic obstructive pulmonary disease (COPD). Glucosamine is widely used in many countries and may have anti-inflammatory properties. We aimed to prospectively evaluate the association of regular glucosamine use with incident COPD risk and explore whether such association could be modified by smoking in the UK Biobank cohort, which recruited more than half a million participants aged 40–69 years from across the UK between 2006 and 2010. Cox proportional hazards models with adjustment for potential confounding factors were used to calculate hazard ratios (HRs) as well as 95% confidence intervals (95% CIs) for the risk of incident COPD. During a median follow-up of 8.96 years (interquartile range 8.29 to 9.53 years), 9016 new-onset events of COPD were documented. We found that regular use of glucosamine was associated with a significantly lower risk of incident COPD with multivariable adjusted HR of 0.80 (95% CI, 0.75 to 0.85; P<0.001). When subgroup analyses were performed by smoking status, the adjusted HRs for the association of regular glucosamine use with incident COPD were 0.84 (0.73 to 0.96), 0.84 (0.77 to 0.92), and 0.71 (0.62 to 0.80) among never smokers, former smokers and current smokers, respectively. No significant interaction was observed between glucosamine use and smoking status (P for interaction=0.078). Incident COPD could be reduced by 14% to 84% through a combination of regular glucosamine use and smoking cessation.
Keywords: glucosamine use, chronic obstructive pulmonary disease, smoking status, smoking pack-years, prospective cohort study
Introduction
Chronic obstructive pulmonary disease (COPD) is a progressive life-threatening chronic respiratory disease that commonly causes breathlessness with recurrent exacerbations and serious illness(1; 2). According to the Global Burden of Disease Study, COPD affected about 251 million people worldwide as of 2016(3). An estimated 3.17 million deaths were caused by COPD in 2015 (accounting for 5% of all deaths globally in that year). The primary risk factor of COPD is exposure to tobacco smoke, which causes oxidative stress of lung parenchyma and peripheral airways and triggers chronic inflammatory responses(2; 4; 5; 6; 7; 8; 9). Thus, drugs or supplements with anti-inflammatory properties may be of potential benefit for reducing risk of COPD.
Glucosamine is a very popular non-vitamin, non-mineral dietary supplement in many countries(10; 11) and commonly taken for osteoarthritis and joint pain(12; 13; 14; 15). A number of laboratory(11; 16; 17; 18), animal(19; 20; 21), and human studies(22; 23; 24) have shown that glucosamine may have anti-inflammatory properties. Notably, different from other drugs with anti-inflammatory properties, glucosamine is considered relatively safe because it has no known serious adverse effects, such as intracerebral or gastrointestinal hemorrhage(25; 26; 27). Thus, there is a substantial interest in assessing whether regular use of glucosamine is inversely associated with the risk of COPD. Moreover, if an association exists between glucosamine use and COPD risk, it is clinically important to determine whether smoking is a potential confounding factor or an effect modifier in the association.
We therefore evaluated the association of regular glucosamine use with the risk of incident COPD using data from the UK Biobank, a large-scale cohort of more than half a million participants. Furthermore, we explored whether the association between glucosamine use and incident COPD risk varied by different smoking subgroups.
Methods
Study setting and participants
The UK Biobank is a valuable research resource with the aim of widely exploring the prevention, diagnosis, and treatment of the most common and life-threatening illnesses(28). As detailed elsewhere(29), this prospective cohort recruited approximately half a million community-based participants aged 40 to 69 years from across the UK between 2006 and 2010. At baseline, each participant completed a touchscreen self-reported questionnaire and a face-to-face oral interview at one of 22 assessment centers after signing an informed consent. Then, they had standardized anthropometric measurements taken and provided biological samples. Follow-up information was collected through linking to the national routine health-related data resources. We excluded participants who dropped out during the study (n=1329), those with missing information on glucosamine use (n=6156), those with history of COPD at baseline (n=9476), as well as those with missing values on smoking status before analyses (n=1860). Therefore, our analyses included 483 703 participants. Furthermore, participants with missing information on smoking pack-years (n=72 134) were also excluded (Figure 1). The research activities were approved by the North West Multi-Center Research Ethics Committee (London, UK). Additionally, ethics approvals were obtained from the National Information Governance Board for Health & Social Care in England and Wales, and the Community Health Index Advisory Group in Scotland.
Figure 1.
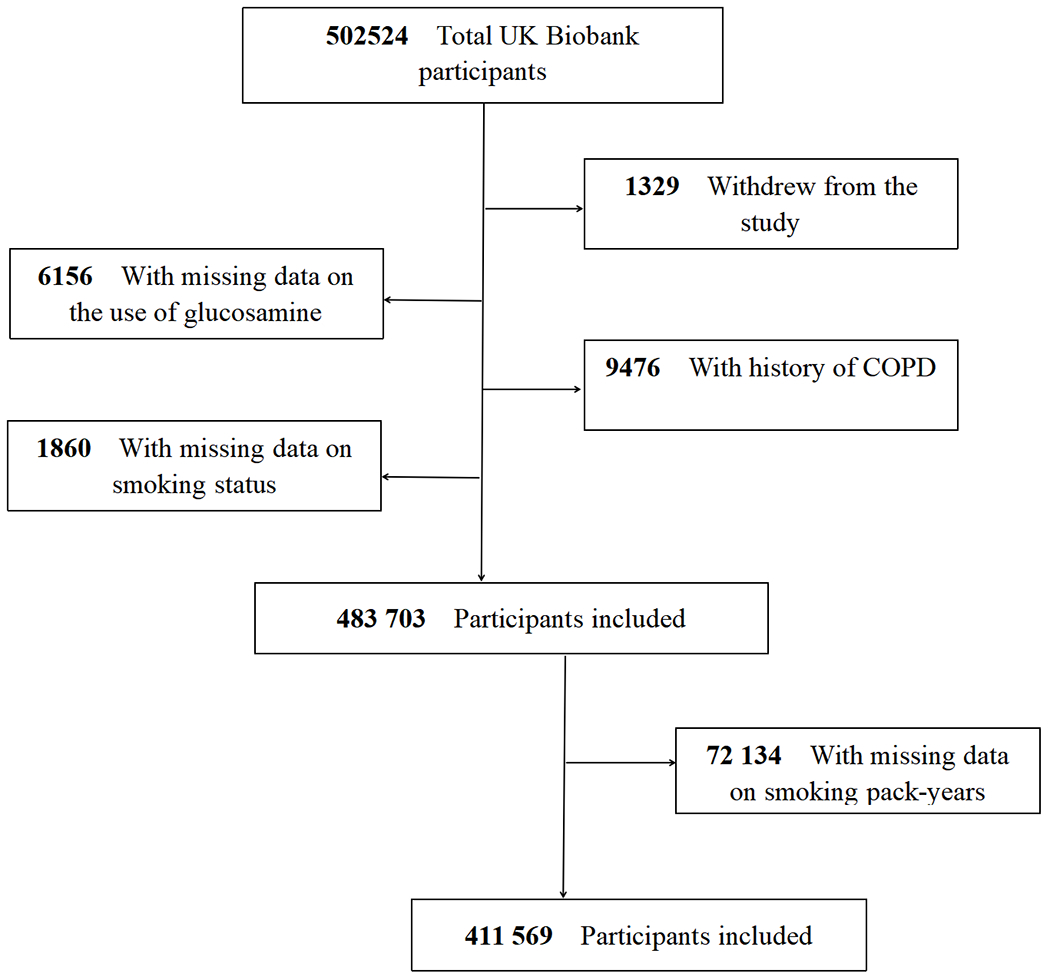
Flowchart of participant enrolment
Assessment of regular glucosamine use
One of the questions in the baseline electronic questionnaire was “Do you regularly take any of the following?”. Each participant could select answers from a list of supplements, including glucosamine, fish oil, selenium, iron, zinc, and calcium, or select a final option of “none of the above” indicating they took none of listed supplements. According to this information, we scored regular glucosamine supplement use as “1=yes” or “0=no”.
Assessment of smoking
Information on smoking was collected by touchscreen electronic questionnaire at baseline. All eligible participants were classified as the following groups: never smokers, former smokers, or current smokers based on their smoking status; or none smokers (0 pack-years), hardly ever smokers (0.1–10.0 pack-years), light smokers (10.1–20.0 pack-years), moderate smokers (20.1–30.0 pack-years), heavy smokers (>30 pack-years), or the group of no available data according to their smoking pack-years. Smoking pack-years is a composite index of smoking based on number of cigarettes per day, age stopped smoking and age start smoking. Detailed definitions of smoking status and smoking pack-years were provided in Table S1 in the Supplement.
Outcome ascertainment
Incident COPD in this cohort was determined based on having a diagnosis in hospital admission electronic records or in death register databases. Death information was obtained via linking to national death registries. Causes of death and diseases diagnoses in the UK Biobank cohort were coded using the International Classification of Diseases, 9th Revision (ICD-9) and 10th Revision (ICD-10). COPD was defined as ICD-9 codes 492, 492.0, 492.8, 496.X or ICD-10 codes J43, J43.0, J43.1, J43.2, J43.8, J43.9, J44, J44.0, J44.1, J44.8, J44.9. We calculated follow-up person-years of included participants from the date of conducting the baseline survey until the date of the first COPD diagnosis, date of death, or the date of the end of follow-up (February 28, 2017 for Scotland, and February 25, 2018 for England and Wales), whichever was earliest.
Ascertainment of covariates
We collected information on risk factors of COPD and correlates of glucosamine use at baseline to assess several potential confounders: sociodemographic characteristics (age, sex, ethnicity, education, and household income), lifestyle and health-related behavioral factors (body mass index [BMI], physical activity, alcohol consumption, fruit consumption, vegetable consumption, passive smoking, and occupational exposure), drug use (cholesterol lowering medication, anti-hypertensive drug, insulin, aspirin, non-aspirin non-steroidal anti-inflammatory drugs [NSAIDs], chondroitin, and cortisone), vitamin supplementation (vitamin A, vitamin B, vitamin C, vitamin D, vitamin E, folic acid, and multivitamin), mineral or other dietary supplementation (fish oil, selenium, zinc, iron, and calcium), and disease history (cardiovascular disease [CVD], hypertension, diabetes, cancer, chronic pulmonary infections, rheumatoid arthritis, osteoarthritis, joint pain, arthritis, and asthma). BMI was calculated as the weight divided by the square of the height (kg/m2). According to the validated International Physical Activity Questionnaire embedded in the touchscreen electronic questionnaire, the intensity and duration of physical activity were ascertained(30). Passive smoking was defined as being exposed to other people’s tobacco smoking for more than one hour per week in the home or other relatively closed space. Occupational exposure was classified based on the self-reported frequency of exposure to diesel exhaust, paints, thinners, glues, pesticides, asbestos, or other chemical smog in daily work. Further details on covariates are available on the UK Biobank website (www.ukbiobank.ac.uk).
Statistical analysis
The distribution of participants’ baseline characteristics was summarized by habitual glucosamine use as mean (standard deviation [SD]) for normally distributed continuous variables, median (interquartile range) for skewed distributed continuous variables, or as number (percentage [%]) for categorical variables. Correspondingly, t test, Wilcoxon rank sum test, or Chi-square test were used to examine the difference of participant characteristics. We conducted multiple imputation with chained equations to assigned missing values (all missing values <3%), thus minimizing the possibility for inferential bias(31).
Cox proportional hazards models with progressive adjustment for potential confounders were performed to calculate hazard ratios (HRs) along with 95% confidence intervals (95% CIs) for associations of habitual glucosamine use or smoking with incident COPD risk, respectively. Model 1 was adjusted for age (numerical variable), sex (male or female), ethnicity (white or others), education (lower qualification or higher qualification), household income (<£18 000, £18 000–£30 999, £31 000–£51 999, £52 000–£100000, or >£100 000), BMI (numerical variable), alcohol consumption (never, 1–2, 3–4, or ≥5 times/week), physical activity (regular physical activity, some physical activity, or no regular physical activity), fruit consumption (<1, 1–3, 3–4, or ≥4 servings/day), vegetable consumption (<3, 3–4, 4–6, or ≥6 servings/day), passive smoking (yes or no), occupational exposure (rarely/never, sometimes, or often), CVD (yes or no), hypertension (yes or no), diabetes (yes or no), cancer (yes or no), chronic pulmonary infections (yes or no), rheumatoid arthritis (yes or no), osteoarthritis (yes or no), joint pain(yes or no), arthritis (yes or no), and asthma (yes or no), aspirin use (yes or no), and Non-aspirin NSAIDs use (yes or no). In model 2, we adjusted for not only the same confounding factors as model 1 but also for the following variables: smoking status (former, current, or never), cholesterol lowering medication use (yes or no), anti-hypertensive drug use (yes or no), insulin use (yes or no), vitamin supplementation (yes or no), mineral or other dietary supplementation (yes or no), glucosamine use (yes or no), chondroitin use (yes or no), and cortisone use (yes or no). In model 3, smoking status was replaced by smoking pack-years (numerical variable), which represented participants’ total active smoking exposure. It is worth noting that glucosamine use and smoking status/pack-years were adjusted for each other. We used a Schoenfeld residuals plot to evaluate the proportional hazards assumption; no violation of this assumption was observed in our study. The linear trend test was performed by treating each smoking category as a continuous variable. Additionally, incidence rates of COPD per 1000 person-years were calculated.
Multivariable adjusted stratified analyses were conducted by smoking status (never, former, or current), or smoking pack-years (none, hardly ever, light, moderate, or heavy) to explore the association between glucosamine use and incident COPD. Additionally, we also conducted stratified analysis by sex (male or female), age (<60 or ≥60 years), obesity (BMI<30 or ≥ 30kg/m2), CVD (yes or no), hypertension (yes or no), diabetes (yes or no), cancer (yes or no), osteoarthritis (yes or no), joint pain (yes or no), asthma (yes or no), vitamin use (yes or no), minerals and other dietary supplements use (yes or no), aspirin use (yes or no), and Non-aspirin NSAIDs use (yes or no) to assess the potential modification effect. The statistical interaction was evaluated by adding the cross-product term of the stratifying variable with glucosamine use to fully adjusted Cox regression models.
We performed several sensitivity analyses to examine the robustness of the results. First, we categorized all eligible participants as glucosamine/chondroitin users (who taken glucosamine alone, or chondroitin alone, or taken both of them), or glucosamine/chondroitin nonusers (who taken neither glucosamine nor chondroitin) to explore the association between glucosamine/chondroitin use and incident COPD. Second, we excluded participants who used chondroitin. Third, participants who developed COPD within the first two years of follow-up were removed in order to minimize the possibility of reverse causation. Final, given the poor health of NSAIDs users, we excluding participants who taken aspirin or non-aspirin NSAIDs at baseline.
The population-attributable fraction (PAF), an estimated fraction of all COPD cases that would not have occurred if all individuals would have been in the less smoking category and/or have taken glucosamine(32), was calculated according to Miettinen’s formula(33). We used R software version 3.6.1 (R Development Core Team, Vienna, Austria) to conduct all statistical analyses; all tests in our study were 2-sided and P <0.05 was considered statistically significant.
Results
Baseline characteristics of participants
Table 1 shows the baseline features of the eligible participants stratified by glucosamine use status (users versus nonusers). Of the 483 703 participants (mean [SD] age, 56.5 [8.1] years), 263 992 (54.6%) were male. At baseline, a total of 92 593 (19.1%) participants self-reported habitual glucosamine use. Compared with nonusers, glucosamine users were older, more likely to be female, white, current non-smokers, and passive smokers. They were also likely to have a lower education qualification, lower household income, more physically activity, more alcohol consumption, and more frequent occupational exposure. They also had a higher prevalence of cancer, osteoarthritis, joint pain, and arthritis, but a lower prevalence of diabetes, hypertension, and CVD. Additionally, glucosamine users more frequently took NSAIDs, vitamins, chondroitin, and minerals and other dietary supplements than nonusers. Of note, glucosamine users have a lower C-reactive protein (CRP) concentration than nonusers.
Table 1.
Baseline Characteristics of Study Participants by Glucosamine Use
| Characteristics | Overall (N=483 703) |
Glucosamine nonuser (N=391 110) |
Glucosamine user (N=92 593) |
P Value |
|---|---|---|---|---|
| Age, mean (SD), y | 56.5 (8.1) | 55.9 (8.2) | 59.0 (7.1) | <0.001 |
| Sex | ||||
| Female | 263 992 (54.6) | 205 951 (52.7) | 58 041 (62.7) | <0.001 |
| Male | 219 711 (45.4) | 185 159 (47.3) | 34 552 (37.3) | |
| Ethnicity | ||||
| White | 457 744 (94.6) | 368 704 (94.3) | 89 040 (96.2) | <0.001 |
| Others | 25 959 (5.4) | 22 406 (5.7) | 3553 (3.8) | |
| Education | ||||
| Lower qualification | 248 915 (51.5) | 200 742 (51.3) | 48 173 (52.0) | <0.001 |
| Higher qualification | 234 788 (48.5) | 190 368 (48.7) | 44 420 (48.0) | |
| Household income (£) | ||||
| <18 000 | 111 453 (23.0) | 91 246 (23.3) | 20 207 (21.8) | <0.001 |
| 18 000–30 999 | 124 574 (25.8) | 98 090 (25.1) | 26 484 (28.6) | |
| 31 000–51 999 | 125 990 (26.0) | 101 522 (26.0) | 24 468 (26.4) | |
| 52 000–100 000 | 96 332 (19.9) | 79 116 (20.2) | 17 216 (18.6) | |
| >100 000 | 25 354 (5.2) | 21 136 (5.4) | 4218 (4.6) | |
| Body mass index (kg/m2) | ||||
| Mean (SD) | 27.4 (4.8) | 27.4 (4.8) | 27.3 (4.6) | <0.001 |
| <18.5 | 2406 (0.5) | 2080 (0.5) | 326 (0.4) | <0.001 |
| 18.5–24.9 | 158 040 (32.7) | 127 522 (32.6) | 30 518 (33.0) | |
| 25–29.9 | 206 028 (42.6) | 165 756 (42.4) | 40 272 (43.5) | |
| ≥30 | 117 229 (24.2) | 95 752 (24.5) | 21 477 (23.2) | |
| Physical activity (min/week) | ||||
| Regular physical activity | 281 573 (58.2) | 222 403 (56.9) | 59 170 (63.9) | <0.001 |
| Some physical activity | 147 517 (30.5) | 121 952 (31.2) | 25 565 (27.6) | |
| No regular physical activity | 54 613 (11.3) | 46 755 (12.0) | 7858 (8.5) | |
| Smoking status | ||||
| Never | 267 855 (55.4) | 216 421 (55.3) | 51 434 (55.5) | <0.001 |
| Former | 166 271 (34.4) | 131 021 (33.5) | 35 250 (38.1) | |
| Current | 49 577 (10.2) | 43 668 (11.2) | 5909 (6.4) | |
| Pack-years of smoking | ||||
| Not available | 72 134 (14.9) | 56 966 (14.6) | 15 168 (16.4) | <0.001 |
| None (0) | 268989 (55.6) | 217369 (55.6) | 51620 (55.7) | |
| Hardly ever (0.1–10.0) | 37329 (7.7) | 29610 (7.6) | 7719 (8.3) | |
| Light (10.1–20.0) | 39141 (8.1) | 31668 (8.1) | 7473 (8.1) | |
| Moderate (20.1–30.0) | 28268 (5.8) | 23367 (6.0) | 4901 (5.3) | |
| Heavy (>30.0) | 37842 (7.8) | 32130 (8.2) | 5712 (6.2) | |
| Alcohol consumption(times/week) | ||||
| Never | 147 743 (30.5) | 122 315 (31.3) | 25 428 (27.5) | <0.001 |
| 1–2 | 125 267 (25.9) | 10 1949 (26.1) | 23 318 (25.2) | |
| 3–4 | 112 265 (23.2) | 89 169 (22.8) | 23 096 (24.9) | |
| ≥5 | 98 428 (20.3) | 77 677 (19.9) | 20 751 (22.4) | |
| Vegetable consumption (servings/day) | ||||
| <3.0 | 84 629 (17.5) | 72 761 (18.6) | 11 868 (12.8) | <0.001 |
| ≥3–4 | 82 273 (17.0) | 67 606 (17.3) | 14 667 (15.8) | |
| ≥4–6 | 163 740 (33.9) | 130 554 (33.4) | 33 186 (35.8) | |
| ≥6 | 153 061 (31.6) | 120 189 (30.7) | 32 872 (35.5) | |
| Fruit consumption (servings/day) | ||||
| <1 | 38 700 (8.0) | 34 646 (8.9) | 4054 (4.4) | <0.001 |
| ≥1–3 | 200 799 (41.5) | 168 333 (43.0) | 32 466 (35.1) | |
| ≥3–4 | 89 974 (18.6) | 71 507 (18.3) | 18 467 (19.9) | |
| ≥4 | 154 230 (31.9) | 116 624 (29.8) | 37 606 (40.6) | |
| Passive smoking | ||||
| No | 381 755 (78.9) | 306 575 (78.4) | 75 180 (81.2) | <0.001 |
| Yes | 101 948 (21.1) | 84 535 (21.6) | 17 413 (18.8) | |
| Occupational exposure | ||||
| Rarely/never | 383 788 (79.3) | 312 651 (79.9) | 71 137 (76.8) | <0.001 |
| Sometimes | 62 019 (12.8) | 48 443 (12.4) | 13 576 (14.7) | |
| Often | 37 896 (7.8) | 30 016 (7.7) | 7880 (8.5) | |
| C-reactive protein, median (interquartile range), mg/L | 1.31 (0.65-2.72) | 1.32 (0.65-2.74) | 1.29 (0.65-2.63) | <0.001 |
| Supplement or drug use | ||||
| Cholesterol lowering medication | 82 813 (17.1) | 67 287 (17.2) | 15 526 (16.8) | 0.002 |
| Anti-hypertensive drug | 99 607 (20.6) | 81 162 (20.8) | 18 445 (19.9) | <0.001 |
| Insulin | 5260 (1.1) | 4636 (1.2) | 624 (0.7) | <0.001 |
| Aspirin | 66 487 (13.7) | 53 587 (13.7) | 12 900 (13.9) | 0.068 |
| Non-aspirin NSAIDs | 143 970 (29.8) | 112 175 (28.7) | 31 795 (34.3) | <0.001 |
| Chondroitin | 5973 (1.2) | 147 (0.0) | 5826 (6.3) | <0.001 |
| Cortisone | 4323 (0.9) | 3662 (0.9) | 661 (0.7) | <0.001 |
| Vitamin | 153 292 (31.7) | 101 927 (26.1) | 51 365 (55.5) | <0.001 |
| Minerals and other dietary supplements | 60 439 (12.5) | 38 629 (9.9) | 21 810 (23.6) | <0.001 |
| Disease history | ||||
| CVD | 27 524 (5.7) | 23 457 (6.0) | 4067 (4.4) | <0.001 |
| Hypertension | 126 914 (26.2) | 103 039 (26.3) | 23 875 (25.8) | <0.001 |
| Diabetes | 23 827 (4.9) | 20626 (5.3) | 3201 (3.5) | <0.001 |
| Cancer | 39 838 (8.2) | 31391 (8.0) | 8447 (9.1) | <0.001 |
| Chronic Pulmonary Infections | 4373 (0.9) | 3419 (0.9) | 954 (1.0) | <0.001 |
| Rheumatoid arthritis | 5269 (1.1) | 4131 (1.1) | 1138 (1.2) | <0.001 |
| Osteoarthritis | 38 656 (8.0) | 23 060 (5.9) | 15 596 (16.8) | <0.001 |
| Joint pain | 4685 (1.0) | 3237 (0.8) | 1448 (1.6) | <0.001 |
| Arthritis | 3754 (0.8) | 2371 (0.6) | 1383 (1.5) | <0.001 |
| Asthma | 53 433 (11.0) | 42 912 (11.0) | 10 521 (11.4) | <0.001 |
Values are numbers (%) unless stated otherwise.
Abbreviations: CVD, cardiovascular disease; NSAID, nonsteroidal anti-inflammatory drug; SD, standard deviation.
Associations between smoking and incident COPD risk
Table 2 shows the associations between smoking and incident COPD. During a median follow-up of 8.96 years (interquartile range 8.29 to 9.53 years), a total of 9016 participants developed incident COPD. Incidence rates (IRs) and HRs (P for trend <0.001) of incident COPD were increased in association with smoking status and increases of smoking pack-years. Compared with never smokers, the multivariable adjusted HRs of former smokers and current smokers was 3.09 (95% CI, 2.91 to 3.28) and 10.61 (95% CI, 9.96 to 11.29)]; similarly, the multivariable adjusted HRs of hardly ever smokers, light smokers, moderate smokers, and heavy smokers was 1.92 (95% CI, 1.72 to 2.14), 3.24 (95% CI, 2.99 to 3.53), 5.58 (95% CI, 5.17 to 6.01), and 10.42 (95% CI, 9.79 to 11.09), respectively (Table 2).
Table 2.
Risk of COPD According to Smoking Categories
| Smoking | Total No. of participants | No. of COPD cases (%) | Person-years | IRa | Model 1b |
Model 2c |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | P for trend | HR (95% CI) | P value | P for trend | |||||
| Smoking status | ||||||||||
| Never | 267 855 | 1586 (0.59) | 2 377 156 | 0.67 | 1.00 (reference) | - | <0.001 | 1.00 (reference) | - | <0.001 |
| Former | 166 271 | 4000 (2.41) | 1 454 015 | 2.75 | 3.10 (2.92 to 3.29) | <0.001 | 3.09 (2.91 to 3.28) | <0.001 | ||
| Current | 49 577 | 3430 (6.92) | 423 520 | 8.10 | 10.67 (10.03 to 11.36) | <0.001 | 10.61 (9.96 to 11.29) | <0.001 | ||
| Smoking pack-years | ||||||||||
| Never (0) | 268 989 | 1662 (0.62) | 2 386 656 | 0.70 | 1.00 (reference) | - | <0.001 | 1.00 (reference) | <0.001 | |
| Hardly ever (0.1–10.0) | 37 329 | 415 (1.11) | 330 209 | 1.26 | 1.92 (1.72 to 2.14) | < 0.001 | 1.92 (1.72 to 2.14) | <0.001 | ||
| Light (10.1–20.0) | 39 141 | 855 (2.18) | 343 461 | 2.49 | 3.25 (2.99 to 3.53) | < 0.001 | 3.24 (2.99 to 3.53) | < 0.001 | ||
| Moderate (20.1–30.0) | 28 268 | 1213 (4.29) | 245 238 | 4.95 | 5.60 (5.20 to 6.04) | < 0.001 | 5.58 (5.17 to 6.01) | < 0.001 | ||
| Heavy (>30.0) | 37 842 | 3811 (10.07) | 3 143 608 | 12.12 | 10.51 (9.88 to 11.18) | < 0.001 | 10.42 (9.79 to 11.09) | <0.001 | ||
Abbreviations: CI, confidence interval; COPD, Chronic Obstructive Pulmonary Disease; HR, hazard ratio; IR, incidence rate.
Incidence rates are provided per 1000 person-years;
Model 1: Cox proportional hazards regression adjusted for age and sex, ethnicity, education, household income, body mass index, physical activity, alcohol consumption, fruit consumption, vegetable consumption, passive smoking, occupational exposure, CVD, hypertension, diabetes, cancer, chronic pulmonary infections, rheumatoid arthritis, osteoarthritis, joint pain, asthma, arthritis, aspirin use, and Non-aspirin NSAIDs use.
Model 2: Cox proportional hazards regression adjusted for Model 1 and cholesterol lowering medication use, anti-hypertensive drug use, insulin use, vitamin use, minerals and other dietary supplements use, chondroitin use, glucosamine use, and cortisone use.
Inverse associations between regular glucosamine use and incident COPD risk
Table 3 shows the associations between habitual glucosamine use and incident COPD. In model 1, we found a significant inverse association between regular use of glucosamine and risk of incident COPD (HR=0.73, 95% CI, 0.69 to 0.78; P<0.001). Regular glucosamine use was significantly associated with a reduced risk of incident COPD with the multivariable adjusted HRs of 0.80 (95% CI, 0.75 to 0.85; P<0.001) and 0.78 (95% CI, 0.73 to 0.84; P<0.001) in model 2 and model 3, respectively.
Table 3.
Risk of Incident COPD According to Glucosamine Use
| glucosamine/chondroitin use | Total No. of participants | No. of COPD cases (%) | Person-years | IRa | Model 1b |
Model 2c |
Model 3d |
Model 4e |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |||||
| Non-user | 391 110 | 7638 (1.95) | 3437363 | 2.22 | 1.00 (reference) | - | 1.00 (reference) | - | 1.00 (reference) | - | 1.00 (reference) | - |
| User | 92 593 | 1378 (1.49) | 81 7327 | 1.69 | 0.73 (0.69 to 0.78) | <0.001 | 0.80 (0.75 to 0.85) | <0.001 | 0.78 (0.73 to 0.84) | <0.001 | 0.83 (0.78 to 0.89) | <0.001 |
Abbreviations: CI, confidence interval; COPD, Chronic Obstructive Pulmonary Disease; HR, hazard ratio; IR, incidence rate.
Incidence rates are provided per 1000 person-years;
Model 1: Cox proportional hazards regression adjusted for age and sex, ethnicity, education, household income, body mass index, physical activity, alcohol consumption, fruit consumption, vegetable consumption, passive smoking, occupational exposure, CVD, hypertension, diabetes, cancer, chronic pulmonary infections, rheumatoid arthritis, osteoarthritis, joint pain, asthma, arthritis, aspirin use, and Non-aspirin NSAIDs use;
Model 2: Cox proportional hazards regression adjusted for Model 1 and smoking status, cholesterol lowering medication use, anti-hypertensive drug use, insulin use, vitamin use, minerals and other dietary supplements use, chondroitin use, and cortisone use;
Model 3: Cox proportional hazards regression adjusted for Model 1 and smoking pack-years (numerical variable), cholesterol lowering medication use, anti-hypertensive drug use, insulin use, vitamin use, minerals and other dietary supplements use, chondroitin use, and cortisone use.
Model 4: Cox proportional hazards regression adjusted for Model 2 and smoking pack-years.
Joint associations of glucosamine use and smoking with incident COPD
We conducted multivariable adjusted stratified analyses by smoking to explore whether smoking status or smoking pack-years could modify the association between habitual glucosamine use and incident COPD risk (Table 4). We found that glucosamine use was associated with a lower risk on incident COPD with the adjusted HRs of 0.84 (95% CI, 0.73 to 0.96; P=0.009), 0.84 (95% CI, 0.77 to 0.92; P<0.001), and 0.71 (95% CI; 0.63 to 0.80; P<0.001) among never, former, and current smokers. The hazard ratio of incident COPD associated with glucosamine use was 0.82 (95% CI 0.72 to 0.94; P=0.003) among none smokers, 0.78 (95% CI 0.68 to 1.02; P=0.065) among hardly ever smokers, 0.70 (95% CI 0.57 to 0.85; P<0.001) among light smokers, 0.66 (95% CI 0.55 to 0.79; P<0.001) among moderate smokers, and 0.85 (95% CI 0.77 to 0.94; P=0.002) among heavy smokers. We observed a significant interaction between glucosamine use and smoking pack-years on the risk of incident COPD (P for interaction=0.019). A similar interaction pattern was not found in the analyses stratified by smoking status (P for interaction=0.078).
Table 4.
Risk of COPD According to Glucosamine Use Within Each Smoking Category
| Subgroup | Total No. of participants | No. of COPD cases (%) | Person-years | IRa | HR (95% CI) b | P value | P for interaction |
|---|---|---|---|---|---|---|---|
| Smoking status and glucosamine | 0.078 | ||||||
| Never smoking | |||||||
| Non-user | 216 421 | 1275 (0.59) | 1 920 589 | 0.66 | 1.00 (reference) | - | |
| User | 51 434 | 311 (0.60) | 456 567 | 0.68 | 0.84 (0.73 to 0.96) | 0.009 | |
| Former smoking | |||||||
| Non-user | 131 021 | 3258 (2.49) | 1 144 262 | 2.85 | 1.00 (reference) | - | |
| User | 35 250 | 742 (2.10) | 309 753 | 2.40 | 0.84 (0.77 to 0.92) | <0.001 | |
| Current smoking | |||||||
| Non-user | 43 668 | 3105 (7.11) | 372 512 | 8.34 | 1.00 (reference) | - | |
| User | 5909 | 325 (5.50) | 51 008 | 6.37 | 0.71 (0.63 to 0.80) | <0.001 | |
| Smoking pack-years and glucosamine | 0.019 | ||||||
| None smoking (0) | |||||||
| Non-user | 217 369 | 1343 (0.62) | 1 928 471 | 0.70 | 1.00 (reference) | - | |
| User | 51 620 | 319 (0.62) | 458 184 | 0.70 | 0.82 (0.72 to 0.94) | 0.003 | |
| Hardly ever smoking (0.1–10.0) | |||||||
| Non-user | 29 610 | 335 (1.13) | 261 763 | 1.28 | 1.00 (reference) | - | |
| User | 7719 | 80 (1.04) | 68 446 | 1.17 | 0.78 (0.68 to 1.02) | 0.065 | |
| Light smoking (10.1–20.0) | |||||||
| Non-user | 31 668 | 727 (2.30) | 277 697 | 2.62 | 1.00 (reference) | - | |
| User | 7473 | 128 (1.71) | 65 765 | 1.95 | 0.70 (0.57 to 0.85) | <0.001 | |
| Moderate smoking (20.1–30.0) | |||||||
| Non-user | 23 367 | 1059 (4.53) | 202 325 | 5.23 | 1.00 (reference) | - | |
| User | 4901 | 154 (3.14) | 42 912 | 3.59 | 0.66 (0.55 to 0.79) | <0.001 | |
| Heavy smoking (>30.0) | |||||||
| Non-user | 32 130 | 3315 (10.32) | 266 394 | 12.44 | 1.00 (reference) | - | |
| User | 5712 | 496 (8.68) | 47 966 | 10.34 | 0.85 (0.77 to 0.94) | 0.002 | |
Abbreviations: CI, confidence interval; COPD, Chronic Obstructive Pulmonary Disease; HR, hazard ratio; IR, incidence rate.
Incidence rates are provided per 1000 person-years;
Cox proportional hazards regression adjusted for age, sex, ethnicity, education, household income, body mass index, physical activity, alcohol consumption, fruit consumption, vegetable consumption, passive smoking, occupational exposure, CVD, hypertension, diabetes, cancer, chronic pulmonary infections, rheumatoid arthritis, osteoarthritis, joint pain, asthma, arthritis, cholesterol lowering medication use, anti-hypertensive drug use, insulin use, aspirin use, Non-aspirin NSAIDs use, vitamin use, minerals and other dietary supplements use, chondroitin use, and cortisone use.
Other subgroup analyses
We conducted stratified analyses for the association of glucosamine use with incident COPD risk according to other potential risk factors using the fully adjusted model (Figure 2). The association between the use of glucosamine use and the risk of incident COPD was seemed not to be significantly modified by sex, age, obesity, CVD, hypertension, diabetes, cancer, osteoarthritis, joint pain, asthma, vitamin use, minerals and other dietary supplements use, aspirin use, or Non-aspirin NSAIDs (all p for interaction>0.05).
Figure 2. Association between glucosamine use and incident COPD risk stratified by other potential risk factors.
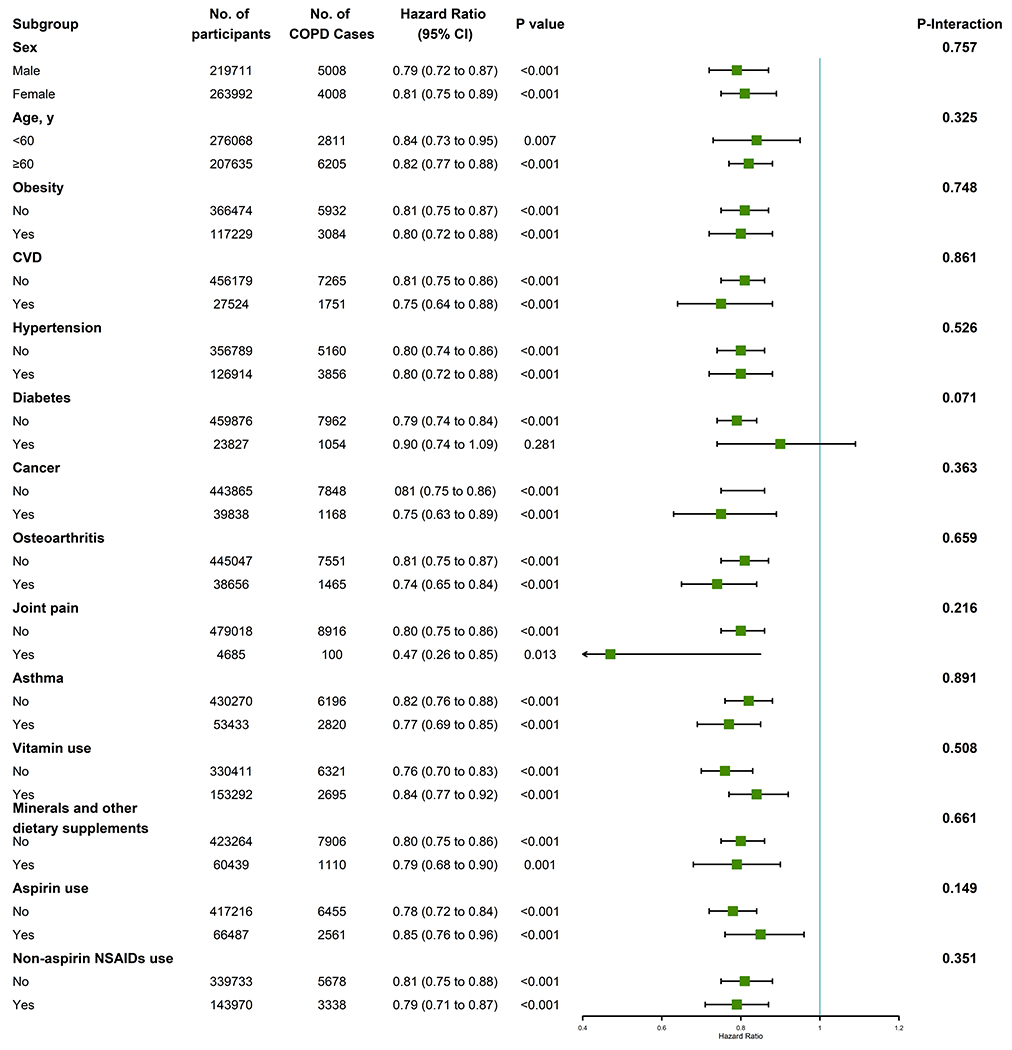
Abbreviations:COPD, chronic obstructive pulmonary disease; CI, confidence interval.
Results were adjusted for age, sex, ethnicity, education, household income, body mass index, physical activity, smoking status, alcohol consumption, fruit consumption, vegetable consumption, passive smoking, occupational exposure, CVD, hypertension, diabetes, cancer, chronic pulmonary infections, rheumatoid arthritis, osteoarthritis, joint pain, asthma, arthritis, cholesterol lowering medication use, anti-hypertensive drug use, insulin use, aspirin use, Non-aspirin NSAIDs use, vitamin use, minerals and other dietary supplements use, chondroitin use, and cortisone use.
Sensitivity analyses
When all eligible participants were categorized as glucosamine/chondroitin users or nonusers, no substantial changes of results were observed, whether or not stratified by smoking (Table S2 and S3). Likewise, when we removed participants who regularly took chondroitin (Table S4), or who reported COPD events within the first two years of follow-up (Table S5), there was no significant change on the association between glucosamine use and incident COPD. When the analyses were restricted NSAIDs nonusers, the result still shown significant inverse associations between the glucosamine use and the new-onset COPD events risk (Table S6).
Population Attributable Fractions
We calculated the population-attributable fraction (PAF). If current smokers who were glucosamine nonusers regularly took glucosamine supplements before the baseline evaluation, new-onset COPD cases could be reduced by 25.53% (95% CI, 17.49 to 32.70). If all individuals who currently smoke actively and did not regularly take glucosamine quit smoking during follow-up and regularly took glucosamine before baseline, 61.74% (95% CI, 59.98 to 63.30) of incident COPD could be prevented. If they had never smoked and regularly took glucosamine before baseline, the new-onset COPD events could be reduced by 83.72% (95% CI, 82.79 to 84.52).
When the heavy smokers and glucosamine nonusers were defined as the reference group, COPD events could be reduced by 13.83% (95% CI, 6.35 to 20.62), 61.45% (95% CI, 455.37 to 66.63), 75.29% (95% CI, 71.14 to 78.75), 83.19% (95% CI, 79.52 to 86.08), and 83.83% (95% CI, 82.91 to 84.72) for groups who i) regularly took glucosamine, ii) were moderate smokers as well as regularly took glucosamine, iii) were light smokers as well as regularly took glucosamine, iv) were hardly ever smokers as well as regularly took glucosamine, and v) were none smokers as well as regularly took glucosamine before baseline, respectively (Table 5).
Table 5.
Population Etiologic Fraction According to Smoking Category and Glucosamine Use
| Subgroup | HR (95% CI)a | PAF (%) | 95% CI (%) | P value |
|---|---|---|---|---|
| Smoking status and glucosamine | ||||
| Current smoking | ||||
| Non-user | 1.00 (reference) | - | ||
| User | 0.72 (0.70 to 0.81) | 25.53 | 17.49 to 32.70 | <0.001 |
| Former smoking | ||||
| Non-user | 0.29 (0.27 to 0.30) | 34.79 | 34.02 to 35.49 | <0.001 |
| User | 0.23 (0.22 to 0.26) | 61.74 | 59.98 to 63.30 | <0.001 |
| Never smoking | ||||
| Non-user | 0.09 (0.09 to 0.10) | 64.37 | 63.91 to 64.77 | <0.001 |
| User | 0.08 (0.07 to 0.09) | 83.72 | 82.79 to 84.52 | <0.001 |
| Smoking pack-years and glucosamine | ||||
| Heavy smoking (>30.0) | ||||
| Non-user | 1.00 (reference) | - | ||
| User | 0.84 (0.76 to 0.93) | 13.83 | 6.35 to 20.62 | 0.001 |
| Moderate smoking (20.1–30.0) | ||||
| Non-user | 0.55 (0.52 to 0.59) | 33.73 | 30.63 to 36.55 | <0.001 |
| User | 0.36 (0.30 to0.42) | 61.45 | 55.37 to 66.63 | <0.001 |
| Light smoking (10.1–20.0) | ||||
| Non-user | 0.32 (0.30 to 0.35) | 55.61 | 53.29 to 57.65 | <0.001 |
| User | 0.22 (0.18 to0.26) | 75.29 | 71.14 to 78.75 | <0.001 |
| Hardly ever smoking (0.1–10.0) | ||||
| Non-user | 0.19 (0.17 to 0.21) | 74.02 | 71.98 to 75.88 | <0.001 |
| User | 0.15 (0.12 to 0.19) | 83.19 | 79.52 to 86.08 | <0.001 |
| None smoking (0) | ||||
| Non-user | 0.10 (0.09 to 0.10) | 64.41 | 63.95 to 64.87 | <0.001 |
| User | 0.08 (0.07 to 0.09) | 83.83 | 82.91 to 84.72 | <0.001 |
Abbreviations: CI, confidence interval; HR, hazard ratio; PAF, population etiologic fraction.
Cox proportional hazards regression adjusted for age, sex, ethnicity, education, household income, body mass index, physical activity, alcohol consumption, fruit consumption, vegetable consumption, passive smoking, occupational exposure, CVD, hypertension, diabetes, cancer, chronic pulmonary infections, rheumatoid arthritis, osteoarthritis, joint pain, asthma, arthritis, cholesterol lowering medication use, anti-hypertensive drug use, insulin use, aspirin use, Non-aspirin NSAIDs use, vitamin use, minerals and other dietary supplements use, chondroitin use, and cortisone use.
Discussion
In this large-scale prospective cohort study involving 483 703 individuals, we found a significant inverse association of regular glucosamine use with incident COPD risk. This association was independent of potential confounders, including socioeconomics characteristics, lifestyle and health-related behavioral factors, other dietary supplementation consumption, health conditions, and drugs use. Furthermore, we observed a prominent interaction between glucosamine use and smoking pack-years on the risk of incident COPD.
In our study, 19.1% of participants reported regular glucosamine use. Similarly, regular glucosamine use has been reported by 22.0% of Australians aged 45+ years(11), and 16.7% of Americans aged 50+ years(10). To our knowledge, this is first study exploring the relationship between regular glucosamine use and incident COPD risk in human populations; thus, it is challenging to contextualize our finding with respect to the current knowledge-base. Of note, several previous epidemiological studies have demonstrated glucosamine supplementation use was associated with a lower risk of incident diseases(34; 35; 36; 37; 38) and mortality(38; 39; 40). For instance, the VITamins And Lifestyle (VITAL) cohort study suggested negative associations of glucosamine use with incident lung cancer and colorectal cancer(34; 35). A recent study including 43 163 individuals from the Health Professionals Follow-up Study (HPFS), Nurses’ Health Study (NHS), and NHS II indicated that glucosamine use may have a protective effect on new-onset colorectal carcinogenesis events in older adults(36). The results from a surveillance, epidemiology and end results (SEER) cancer registry suggested that glucosamine use was associated with a lower total mortality and with reductions of some broad causes of death in adults aged 50–76 years(39). Based on the UK Biobank cohort, Ma and colleagues found that habitual use of glucosamine was associated with lower risk of multiple conditions including, 17% for incident type 2 diabetes (T2D)(37), 18% for incident coronary heart disease (CHD), 18% for incident stroke, and 22% for cardiovascular disease (CVD) death(38); Li and colleagues also reported that regular use of glucosamine was associated lower risk of multiple conditions including, 15% for all-cause mortality, 27% for respiratory mortality, 26% for digestive mortality, 18% for CVD mortality, and 6% for cancer mortality.(40)
Although the precise biological mechanisms underlying the inverse association between regular use of glucosamine and risk of COPD remain to be determined, a wealth of emerging evidence provides various plausible explanations for the inverse association. Given the detrimental roles of inflammation in the development of COPD, we assumed that glucosamine supplementation might reduce the incident COPD risk at least partly through the anti-inflammatory effect. First, glucosamine may achieve its beneficial effect by reducing the translocation of nuclear factor kappa B [NF-kB] and inhibiting the activation of NF-kB, a well-characterized transcription factor involved in inflammatory response, and thus suppress the subsequent cascade of related events(41; 42). An animal study in which mice received an injection of lipopolysaccharide to induce endotoxic shock and systemic inflammation has demonstrated that glucosamine decreased the production of inflammatory cytokines related to NF-kB activation(43). A number of vitro and vivo studies suggested glucosamine use decreases levels of various proinflammatory cytokines(16; 17; 18; 19; 20; 44; 45; 46; 47; 48), such as IL-1β, PGE2, COX-2, and TNF-α, which lies downstream stream of NF-kB(49; 50). Second, some evidence, even if limited, indicated a potential mechanism by which glucosamine exerts an anti-inflammatory effect by regulating the metabolic, composition, or immunological activities of gut microbiota(51; 52). Additionally, glucosamine, a significant component of intestinal mucin, could potentially affect intestinal immune responses and gut permeability(53; 54).
Several previous human studies(22; 23; 24; 55) also have shown that circulating levels of CRP, a marker of low-grade systemic inflammation, were significantly lower in glucosamine users compared with nonusers; a small randomized controlled cross-over clinical trial also suggested that glucosamine use may significantly reduce CRP concentrations compared with the placebo group(23). Interestingly, we found that the CRP level at baseline was significantly lower in glucosamine users than in nonusers.
Although some previous studies suggested the positive effect of aspirin use among patients with COPD(56; 57), results of our study (Table S7) and other studies showed that the use of either aspirin or ibuprofen was not associated with COPD or lung function(58; 59). The potential benefit of glucosamine supplementation for incident COPD was significant and was independent of a series of potential confounding factors. Additionally, glucosamine is considered relatively safe because no known serious adverse effects related to it have been reported(21). Furthermore, even though we observed a significantly positive association between cigarette smoking and new-onset COPD events, regular glucosamine use could reverse this relationship to a certain extent. Glucosamine seems promising as a recommended protective agent for prevention of COPD. It should be noted that, given the limitations in this study, including potential residual confounding, and a sparse dose and duration information, PARs may provide an overestimation and misrepresentation of the potential preventive effect glucosamine has on COPD.
This study has several notable strengths, including the large sample size, the prospective cohort study design, and detailed information on socioeconomic characteristics, lifestyle and health-related behavioral factors, supplementation use, drugs use, health status, and other covariates.
Nevertheless, there are several limitations of our study. First, information on regular dietary supplements intake was obtained via self-reported baseline questionnaire; and detailed information on the formulation of supplements was not collected. Some participants who take compound preparation containing glucosamine and chondroitin might only reported the glucosamine use. Second, dosage, duration and frequency of glucosamine use was not collected; further studies are needed to explore those associations. Third, glucosamine users were likely to have a healthier lifestyle. However, it is difficult to distinguish the effects of a healthy lifestyle from habitual glucosamine use in this observational study. Although we carefully adjusted for a great many potential confounding factors, the observed inverse associations might have been driven by some unmeasured health-related lifestyles. Additionally, the possibility of residual confounders due to other unknown factors or imprecise measurements cannot be eliminated in our study.
Conclusions
In summary, this large-scale prospective cohort study showed that a considerable proportion (19.1%) of the UK population reported regular glucosamine use. Our study suggests that regular glucosamine use is inversely associated with incident COPD. The inverse association was modified by smoking pack-years. Functional studies and clinical trials are needed to enhance our understanding of potential benefits of glucosamine supplementation for incident COPD.
Supplementary Material
Acknowledgements:
The authors would like to thank the UK Biobank participants. This research has been conducted using the UK Biobank resource under application number 43795.
Funding:
This work was supported by the National Natural Science Foundation of China (81973109), the Project Supported by Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme (2019), Chang Jiang Scholars Program ( 2020), the Guangdong Basic and Applied Basic Research Foundation (2021A1515011629), the National Key Research and Development Program of China (2018YFC2000400), the Construction of High-level University of Guangdong (G820332010, G618339167 and G618339164), the Guangzhou Science and Technology Project (202002030255), and the National Institutes of Health / National Institute on Aging of USA (P30-AG028716). The funders played no role in the study design or implementation; data collection, management, analysis or interpretation; manuscript preparation, review or approval or the decision to submit the manuscript for publication.
Footnotes
Code availability Code is available upon request to the corresponding author.
Conflicts of Interest: The authors declare that they have no conflict of interest.
Patient and public involvement Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Patient consent for publication Not required.
Ethics approval The UK Biobank received ethical approval from the research ethics committee (REC reference for UK Biobank 11/NW/0382) and participants provided written informed consent.
Informed consent to participate All participants gave written informed consent to participate in the study.
Data availability statement
Data are available in a public, open access repository. The UK Biobank data are available from the UK Biobank on request (www.ukbiobank.ac.uk/).
References
- 1.Labaki WW, Rosenberg SR (2020) Chronic Obstructive Pulmonary Disease. Annals of internal medicine 173, Itc17–itc32. [DOI] [PubMed] [Google Scholar]
- 2.Barnes PJ (2016) Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. The Journal of allergy and clinical immunology 138, 16–27. [DOI] [PubMed] [Google Scholar]
- 3.Mathers CD, Loncar D (2006) Projections of global mortality and burden of disease from 2002 to 2030. PLoS medicine 3, e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Celli BR, Decramer M, Wedzicha JA et al. (2015) An Official American Thoracic Society/European Respiratory Society Statement: Research questions in chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine 191, e4–e27. [DOI] [PubMed] [Google Scholar]
- 5.Ferrera MC, Labaki WW, Han MK (2021) Advances in Chronic Obstructive Pulmonary Disease. Annual review of medicine 72, 119–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Su YC, Jalalvand F, Thegerström J et al. (2018) The Interplay Between Immune Response and Bacterial Infection in COPD: Focus Upon Non-typeable Haemophilus influenzae. Frontiers in immunology 9, 2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu S, Jørgensen JT, Ljungman P et al. (2021) Long-term exposure to low-level air pollution and incidence of chronic obstructive pulmonary disease: The ELAPSE project. Environment international 146, 106267. [DOI] [PubMed] [Google Scholar]
- 8.Zhu T, Li S, Wang J et al. (2020) Induced sputum metabolomic profiles and oxidative stress are associated with chronic obstructive pulmonary disease (COPD) severity: potential use for predictive, preventive, and personalized medicine. The EPMA journal 11, 645–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacNee W (2000) Oxidants/antioxidants and COPD. Chest 117, 303s–317s. [DOI] [PubMed] [Google Scholar]
- 10.Qato DM, Wilder J, Schumm LP et al. (2016) Changes in Prescription and Over-the-Counter Medication and Dietary Supplement Use Among Older Adults in the United States, 2005 vs 2011. JAMA internal medicine 176, 473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sibbritt D, Adams J, Lui CW et al. (2012) Who uses glucosamine and why? A study of 266,848 Australians aged 45 years and older. PloS one 7, e41540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jordan KM, Arden NK, Doherty M et al. (2003) EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: Report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Annals of the rheumatic diseases 62, 1145–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blanco FJ, Camacho-Encina M, González-Rodríguez L et al. (2019) Predictive modeling of therapeutic response to chondroitin sulfate/glucosamine hydrochloride in knee osteoarthritis. Therapeutic advances in chronic disease 10, 2040622319870013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reginster JL, Bruyere O, Cooper C (2018) Different glucosamine sulfate products generate different outcomes on osteoarthritis symptoms. Annals of the rheumatic diseases 77, e39. [DOI] [PubMed] [Google Scholar]
- 15.Runhaar J, Rozendaal RM, van Middelkoop M et al. (2017) Subgroup analyses of the effectiveness of oral glucosamine for knee and hip osteoarthritis: a systematic review and individual patient data meta-analysis from the OA trial bank. Annals of the rheumatic diseases 76, 1862–1869. [DOI] [PubMed] [Google Scholar]
- 16.Largo R, Alvarez-Soria MA, Díez-Ortego I et al. (2003) Glucosamine inhibits IL-1beta-induced NFkappaB activation in human osteoarthritic chondrocytes. Osteoarthritis and cartilage 11, 290–298. [DOI] [PubMed] [Google Scholar]
- 17.Chan PS, Caron JP, Rosa GJ et al. (2005) Glucosamine and chondroitin sulfate regulate gene expression and synthesis of nitric oxide and prostaglandin E(2) in articular cartilage explants. Osteoarthritis and cartilage 13, 387–394. [DOI] [PubMed] [Google Scholar]
- 18.Rajapakse N, Kim MM, Mendis E et al. (2008) Inhibition of inducible nitric oxide synthase and cyclooxygenase-2 in lipopolysaccharide-stimulated RAW264.7 cells by carboxybutyrylated glucosamine takes place via down-regulation of mitogen-activated protein kinase-mediated nuclear factor-kappaB signaling. Immunology 123, 348–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campo GM, Avenoso A, Campo S et al. (2003) Efficacy of treatment with glycosaminoglycans on experimental collagen-induced arthritis in rats. Arthritis research & therapy 5, R122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chou MM, Vergnolle N, McDougall JJ et al. (2005) Effects of chondroitin and glucosamine sulfate in a dietary bar formulation on inflammation, interleukin-1beta, matrix metalloprotease-9, and cartilage damage in arthritis. Experimental biology and medicine (Maywood, NJ) 230, 255–262. [DOI] [PubMed] [Google Scholar]
- 21.Wen ZH, Tang CC, Chang YC et al. (2010) Glucosamine sulfate reduces experimental osteoarthritis and nociception in rats: association with changes of mitogen-activated protein kinase in chondrocytes. Osteoarthritis and cartilage 18, 1192–1202. [DOI] [PubMed] [Google Scholar]
- 22.Kantor ED, Lampe JW, Navarro SL et al. (2014) Associations between glucosamine and chondroitin supplement use and biomarkers of systemic inflammation. Journal of alternative and complementary medicine (New York, NY) 20, 479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Navarro SL, White E, Kantor ED et al. (2015) Randomized trial of glucosamine and chondroitin supplementation on inflammation and oxidative stress biomarkers and plasma proteomics profiles in healthy humans. PloS one 10, e0117534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kantor ED, O’Connell K, Du M et al. (2020) Glucosamine and Chondroitin Use in Relation to C-Reactive Protein Concentration: Results by Supplement Form, Formulation, and Dose. Journal of alternative and complementary medicine (New York, NY). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thorat MA, Cuzick J (2015) Prophylactic use of aspirin: systematic review of harms and approaches to mitigation in the general population. European journal of epidemiology 30, 5–18. [DOI] [PubMed] [Google Scholar]
- 26.Wolfe MM, Lichtenstein DR, Singh G (1999) Gastrointestinal toxicity of nonsteroidal antiinflammatory drugs. The New England journal of medicine 340, 1888–1899. [DOI] [PubMed] [Google Scholar]
- 27.Solomon SD, Wittes J, Finn PV et al. (2008) Cardiovascular risk of celecoxib in 6 randomized placebo-controlled trials: the cross trial safety analysis. Circulation 117, 2104–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sudlow C, Gallacher J, Allen N et al. (2015) UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 12, e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fry A, Littlejohns TJ, Sudlow C et al. (2017) Comparison of Sociodemographic and Health-Related Characteristics of UK Biobank Participants With Those of the General Population. American journal of epidemiology 186, 1026–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bassett DR Jr. (2003) International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 35, 1396. [DOI] [PubMed] [Google Scholar]
- 31.Sv Buuren, Groothuis-Oudshoorn K (2010) mice: Multivariate imputation by chained equations in R. J Stat Softw 45, 1–68. [Google Scholar]
- 32.Mansournia MA, Altman DG (2018) Population attributable fraction. BMJ (Clinical research ed) 360, k757. [DOI] [PubMed] [Google Scholar]
- 33.Miettinen OS (1974) Proportion of disease caused or prevented by a given exposure, trait or intervention. American journal of epidemiology 99, 325–332. [DOI] [PubMed] [Google Scholar]
- 34.Satia JA, Littman A, Slatore CG et al. (2009) Associations of herbal and specialty supplements with lung and colorectal cancer risk in the VITamins and Lifestyle study. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 18, 1419–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brasky TM, Lampe JW, Slatore CG et al. (2011) Use of glucosamine and chondroitin and lung cancer risk in the VITamins And Lifestyle (VITAL) cohort. Cancer causes & control : CCC 22, 1333–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee DH, Cao C, Zong X et al. (2020) Glucosamine and Chondroitin Supplements and Risk of Colorectal Adenoma and Serrated Polyp. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 29, 2693–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma H, Li X, Zhou T et al. (2020) Glucosamine Use, Inflammation, and Genetic Susceptibility, and Incidence of Type 2 Diabetes: A Prospective Study in UK Biobank. Diabetes care 43, 719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma H, Li X, Sun D et al. (2019) Association of habitual glucosamine use with risk of cardiovascular disease: prospective study in UK Biobank. BMJ (Clinical research ed) 365, l1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bell GA, Kantor ED, Lampe JW et al. (2012) Use of glucosamine and chondroitin in relation to mortality. European journal of epidemiology 27, 593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li ZH, Gao X, Chung VC et al. (2020) Associations of regular glucosamine use with all-cause and cause-specific mortality: a large prospective cohort study. Annals of the rheumatic diseases 79, 829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.du Souich P (2014) Absorption, distribution and mechanism of action of SYSADOAS. Pharmacology & therapeutics 142, 362–374. [DOI] [PubMed] [Google Scholar]
- 42.Zahedipour F, Dalirfardouei R, Karimi G et al. (2017) Molecular mechanisms of anticancer effects of Glucosamine. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 95, 1051–1058. [DOI] [PubMed] [Google Scholar]
- 43.Silva JF, Olivon VC, Mestriner F et al. (2019) Acute Increase in O-GlcNAc Improves Survival in Mice With LPS-Induced Systemic Inflammatory Response Syndrome. Frontiers in physiology 10, 1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hong H, Park YK, Choi MS et al. (2009) Differential down-regulation of COX-2 and MMP-13 in human skin fibroblasts by glucosamine-hydrochloride. Journal of dermatological science 56, 43–50. [DOI] [PubMed] [Google Scholar]
- 45.Yomogida S, Hua J, Sakamoto K et al. (2008) Glucosamine suppresses interleukin-8 production and ICAM-1 expression by TNF-alpha-stimulated human colonic epithelial HT-29 cells. International journal of molecular medicine 22, 205–211. [PubMed] [Google Scholar]
- 46.Nakamura H, Shibakawa A, Tanaka M et al. (2004) Effects of glucosamine hydrochloride on the production of prostaglandin E2, nitric oxide and metalloproteases by chondrocytes and synoviocytes in osteoarthritis. Clinical and experimental rheumatology 22, 293–299. [PubMed] [Google Scholar]
- 47.Gouze JN, Bordji K, Gulberti S et al. (2001) Interleukin-1beta down-regulates the expression of glucuronosyltransferase I, a key enzyme priming glycosaminoglycan biosynthesis: influence of glucosamine on interleukin-1beta-mediated effects in rat chondrocytes. Arthritis and rheumatism 44, 351–360. [DOI] [PubMed] [Google Scholar]
- 48.Zhang T, Chen S, Dou H et al. (2021) Novel glucosamine-loaded thermosensitive hydrogels based on poloxamers for osteoarthritis therapy by intra-articular injection. Materials science & engineering C, Materials for biological applications 118, 111352. [DOI] [PubMed] [Google Scholar]
- 49.Romano S, Mallardo M, Romano MF (2011) FKBP51 and the NF-κB regulatory pathway in cancer. Current opinion in pharmacology 11, 288–293. [DOI] [PubMed] [Google Scholar]
- 50.Li Q, Withoff S, Verma IM (2005) Inflammation-associated cancer: NF-kappaB is the lynchpin. Trends in immunology 26, 318–325. [DOI] [PubMed] [Google Scholar]
- 51.Hao X, Shang X, Liu J et al. (2021) The gut microbiota in osteoarthritis: where do we stand and what can we do? Arthritis research & therapy 23, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coulson S, Butt H, Vecchio P et al. (2013) Green-lipped mussel extract (Perna canaliculus) and glucosamine sulphate in patients with knee osteoarthritis: therapeutic efficacy and effects on gastrointestinal microbiota profiles. Inflammopharmacology 21, 79–90. [DOI] [PubMed] [Google Scholar]
- 53.Lee HS, Han SY, Ryu KY et al. (2009) The degradation of glycosaminoglycans by intestinal microflora deteriorates colitis in mice. Inflammation 32, 27–36. [DOI] [PubMed] [Google Scholar]
- 54.Sicard JF, Vogeleer P, Le Bihan G et al. (2018) N-Acetyl-glucosamine influences the biofilm formation of Escherichia coli. Gut pathogens 10, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kantor ED, Lampe JW, Vaughan TL et al. (2012) Association between use of specialty dietary supplements and C-reactive protein concentrations. American journal of epidemiology 176, 1002–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goto T, Faridi MK, Camargo CA et al. (2018) The association of aspirin use with severity of acute exacerbation of chronic obstructive pulmonary disease: a retrospective cohort study. NPJ primary care respiratory medicine 28, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fawzy A, Putcha N, Aaron CP et al. (2019) Aspirin Use and Respiratory Morbidity in COPD: A Propensity Score-Matched Analysis in Subpopulations and Intermediate Outcome Measures in COPD Study. Chest 155, 519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McKeever TM, Lewis SA, Smit HA et al. (2005) The association of acetaminophen, aspirin, and ibuprofen with respiratory disease and lung function. American journal of respiratory and critical care medicine 171, 966–971. [DOI] [PubMed] [Google Scholar]
- 59.Aaron CP, Schwartz JE, Hoffman EA et al. (2018) A Longitudinal Cohort Study of Aspirin Use and Progression of Emphysema-like Lung Characteristics on CT Imaging: The MESA Lung Study. Chest 154, 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available in a public, open access repository. The UK Biobank data are available from the UK Biobank on request (www.ukbiobank.ac.uk/).


