Abstract
Current guidelines recommend two-drug cytotoxic chemotherapy with a fluoropyrimidine (fluorouracil or capecitabine) and a platinum-based agent (oxaliplatin or cisplatin) as first-line treatment for advanced gastric cancer. Pembrolizumab monotherapy has demonstrated durable antitumor activity in patients with advanced programmed death ligand 1-positive (combined positive score ≥1) gastric/gastroesophageal junction adenocarcinoma. Accumulating evidence indicates that combining pembrolizumab with standard-of-care chemotherapy for the treatment of advanced or metastatic cancer improves clinical outcomes. We describe the rationale for and the design of the randomized, double-blind, placebo-controlled, Phase III KEYNOTE-859 study, which is investigating pembrolizumab in combination with chemotherapy as first-line treatment for patients with human epidermal growth factor receptor 2-negative advanced unresectable or metastatic gastric/gastroesophageal junction adenocarcinoma. The planned sample size is 1542 patients, and the primary end point is overall survival.
Clinical trial registration: NCT03675737 (ClinicalTrials.gov)
Keywords: : adenocarcinoma, chemotherapy, first-line therapy, gastric cancer, gastroesophageal junction cancer, HER2-negative, immunotherapy, pembrolizumab
Gastric cancer is the fifth most diagnosed cancer worldwide (∼1 million new cases in 2018) and the third most common cause of cancer-related deaths (∼783,000 in 2018) [1]. Age-standardized incidence rates are twofold higher in men than in women and vary across regions of the world, with elevated incidence rates in Eastern Asia (e.g., Mongolia, Japan and South Korea) [1]. Similarly, mortality rates are highest in eastern Asia and lowest in North America [2].
Gastric cancers are generally classified into two topographical categories: cardia gastric cancer arises in areas of the upper stomach and the gastroesophageal junction (GEJ) and noncardia gastric cancer occurs in more distal regions of the stomach [3]. These two categories of gastric cancer display distinct characteristics; cardia gastric cancer is frequently associated with obesity and gastroesophageal reflux disease, whereas noncardia gastric cancer is strongly associated with Helicobacter pylori infection [1,3]. The rate of cardia gastric cancer has been increasing, particularly in high-income countries, whereas noncardia gastric cancer rates have been declining in most populations [1]. Further classifications of gastric cancer have been developed based on a comprehensive integrative analysis of the genome and proteome of gastric cancer tissue and the discovery of four molecular subtypes: Epstein–Barr virus subtype, microsatellite instability (MSI) subtype, genomically stable subtype and chromosomal instability subtype [4].
Complete surgical resection is the only curative therapeutic option for gastric cancer and is the primary treatment option for patients with localized disease [5,6]. Combined modality therapies, such as perioperative chemotherapy and adjuvant chemotherapy or chemoradiotherapy, have been used in the treatment of locoregional disease [5,6]. Prognosis is poor for patients with inoperable locally advanced or metastatic disease; however, those with a good performance status may receive systemic chemotherapy [5,6]. Standard first-line therapy for patients with unresectable locally advanced recurrent or metastatic HER2-negative gastric cancer is a two-drug cytotoxic regimen that includes a fluoropyrimidine (fluorouracil or capecitabine) combined with a platinum-based agent (oxaliplatin or cisplatin) [5,6]. The addition of docetaxel improves overall survival (OS), but it is associated with increased toxicity and is reserved for patients with good performance status scores and good organ function [5,7]. Recently, immune checkpoint inhibitors were added to chemotherapy regimens in an attempt to improve clinical outcomes [8–12].
KEYNOTE-859
Here we describe the design and rationale for the multicenter, randomized, double-blind, placebo-controlled, Phase III KEYNOTE-859 study of pembrolizumab plus standard-of-care chemotherapy compared with placebo plus standard-of-care chemotherapy as first-line treatment of patients with advanced gastric or GEJ cancer (ClinicalTrials.gov; NCT03675737).
Background & rationale
Pembrolizumab is a high-affinity, highly selective, humanized immunoglobulin G4 kappa monoclonal antibody that binds programmed death 1 and blocks its interaction with its ligands, PD-L1 and PD-L2 [13]. Pembrolizumab has demonstrated robust, durable antitumor activity and a manageable safety profile in patients with multiple tumor types and is approved in >80 countries for the treatment of ≥1 advanced cancers [13]. Preclinical and clinical evidence indicates that chemotherapy can enhance tumor immunogenicity and increase susceptibility to immune attack, potentially increasing the efficacy of immune checkpoint inhibitors [14]. Combining pembrolizumab with chemotherapy has demonstrated efficacy and manageable safety in multiple tumor types, including gastric cancer [13,15–17].
Based on data from the Phase II KEYNOTE-059 trial, pembrolizumab has been approved in the USA as third-line or later therapy for patients with recurrent locally advanced or metastatic gastric cancer or GEJ cancer whose tumors express PD-L1 (combined positive score [CPS] ≥1) [13]. In cohort 2 of the KEYNOTE-059 study, pembrolizumab plus chemotherapy (cisplatin plus 5-fluorouracil or capecitabine) demonstrated manageable safety and promising antitumor activity with an objective response rate (ORR) of 60.0% (95% CI: 38.7–78.9) as first-line therapy in 25 patients with PD-L1-positive (CPS ≥1) advanced gastric or GEJ cancer [9]. Pembrolizumab has also been approved in USA for the treatment of patients who have refractory cancers, including gastric and GEJ adenocarcinomas, that are MSI-high or tumor mutational burden-high (≥ten mutations/megabase) and who have no satisfactory treatment alternative [13]. The KEYNOTE-062 study was a randomized, active-controlled, partially blinded, Phase III trial investigating pembrolizumab as monotherapy or in combination with standard-of-care cytotoxic chemotherapy (cisplatin plus fluorouracil or capecitabine) in the first-line treatment setting of PD-L1-positive (CPS ≥1) advanced/metastatic gastric and GEJ adenocarcinoma. Clinically meaningful improvements in ORR and favorable trends in progression-free survival (PFS) and OS were observed in select patients treated with pembrolizumab plus chemotherapy [8].
Other Phase II and III trials have investigated programmed death 1 blockade in combination with chemotherapy in gastric cancer. Interim results from the randomized Phase II/III ATTRACTION-4 study (NCT02746796) showed that first-line nivolumab plus chemotherapy (S-1 plus oxaliplatin or capecitabine plus oxaliplatin) led to an ORR of 65.8% (95% CI: 48.6–80.4) in 38 Asian patients with unresectable advanced or recurrent gastric or GEJ cancer [10]. In the second part of this study, nivolumab plus chemotherapy significantly improved PFS over chemotherapy at the interim analysis (median PFS: 10.5 vs 8.3; hazard ratio [HR]: 0.68; 98.51% CI: 0.51–0.90; p = 0.0007); a significant difference in OS was not observed at the final analysis (HR: 0.90; 95% CI: 0.75–1.08; p = 0.257) [11]. Recently, the Phase III Checkmate-649 study (n = 1581; NCT02872116) evaluating nivolumab plus chemotherapy (capecitabine and oxaliplatin or 5-fluorouracil and leucovorin calcium and oxaliplatin) compared with chemotherapy alone as first-line treatment of gastric and esophageal cancers reported encouraging results from a prespecified interim analysis [12,18]. Nivolumab plus chemotherapy showed statistically significant improvement compared with chemotherapy in patients whose tumors expressed CPS ≥5 for the dual primary end points, OS (median OS: 14.4 vs 11.1 months; HR: 0.71; 98.4% CI: 0.59–0.86; p < 0.0001) and PFS (median PFS: 7.7 vs 6.1 months; HR: 0.68; 98.0% CI: 0.56–0.81; p < 0.0001) [12]. Taken together, data from these studies suggest that combining immune checkpoint inhibitors with chemotherapy may be a promising first-line treatment for patients with advanced gastric or GEJ cancer.
Study design
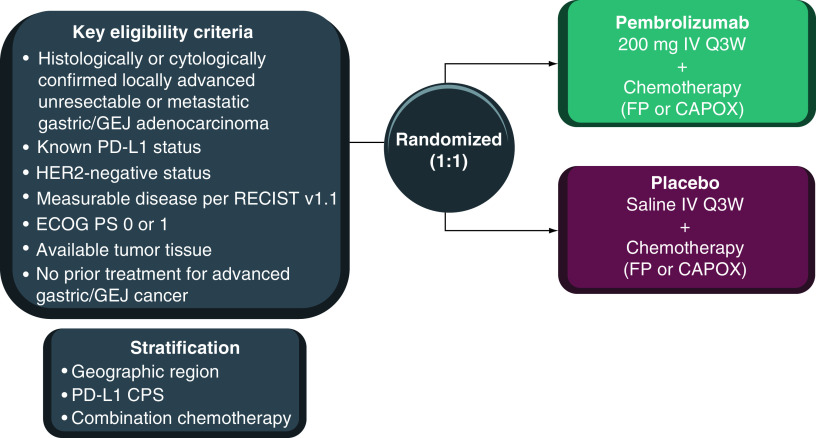
KEYNOTE-895 is an international, multicenter, randomized, double-blind, placebo-controlled Phase III study (Figure 1). Eligible patients will be randomly assigned in a 1:1 ratio to receive pembrolizumab 200 mg or placebo intravenously (iv.) every 3 weeks (Q3W) in combination with investigator’s choice of FP (continuous infusion of 5-fluorouracil [800 mg/m2/day on days 1–5 of each cycle] + iv. cisplatin [80 mg/m2] Q3W) or CAPOX (oral capecitabine [1000 mg/m2 twice daily on days 1–14 of each cycle] + iv. oxaliplatin [130 mg/m2 on day 1 of each cycle] Q3W). Treatment will continue until confirmed disease progression, unacceptable toxicity, investigator or patient decision to withdraw from the study, noncompliance with treatment or trial procedures, or completion of 35 cycles of treatment. Cisplatin or oxaliplatin treatment may be capped at six cycles per local standard; however, treatment with 5-fluorouracil or capecitabine may continue per protocol. Patients who complete 35 cycles of pembrolizumab treatment or who achieve a complete response but progress after discontinuation of treatment can initiate a second course of pembrolizumab for up to 17 cycles.
Figure 1. . KEYNOTE-859 study design.
CAPOX: Capecitabine plus oxaliplatin; CPS: Combined positive score; ECOG PS: Eastern Cooperative Oncology Group performance status; FP: 5-Fluorouracil plus cisplatin; GEJ: Gastroesophageal junction; iv.: Intravenously; Q3W: Every 3 weeks.
Randomization will be performed centrally using an interactive system. Stratification will be based on geographic location (Europe/Israel/North America/Australia vs Asia vs rest of world), PD-L1 tumor expression status (CPS <1 vs ≥1), and chemotherapy regimen (FP vs CAPOX). Pembrolizumab or placebo assignment will be masked to patients and investigators.
Key eligibility criteria
Eligibility criteria are described in Table 1. Briefly, adults are eligible if they had previously untreated HER2-negative advanced gastric or GEJ adenocarcinoma, measurable disease per RECIST v1.1, adequate tumor tissue sample for biomarker analysis, and Eastern Cooperative Oncology Group performance status 0 or 1.
Table 1. . Eligibility criteria for KEYNOTE-859.
| Inclusion criteria | Exclusion criteria |
|---|---|
| • Age ≥18 years • Histologically or cytologically confirmed locally advanced unresectable or metastatic gastric or GEJ adenocarcinoma • Known PD-L1 status • HER2-negative cancer • Measurable disease per RECIST 1.1 as assessed by investigator • Archival tumor tissue sample or newly obtained core or excisional biopsy for PD-L1 expression and MSI biomarker analysis • ECOG PS 0 or 1 • Adequate hematologic function, defined as ANC ≥1500/μl, platelet count ≥100,000/μl and hemoglobin count ≥9.0 g/dl or ≥5.6 mmol/l • Adequate renal function, defined as creatinine ≤1.5× ULN or measured or calculated creatinine clearance ≥60 ml/min for those with creatinine levels >1.5× ULN • Adequate hepatic function, defined as total bilirubin ≤1.5× ULN or direct bilirubin ≤ULN for those with total bilirubin >1.5× ULN, ALT/AST levels ≤2.5× ULN (≥5× ULN for participants with liver metastasis) and albumin ≥2.5 g/dl • Adequate coagulation function, defined as INR ≤1.5× ULN unless the patient is receiving anticoagulant therapy as long as PT or aPTT is within the therapeutic range • Willing to use an adequate method of contraception throughout the study and for 120 days after the last dose of pembrolizumab and up to 180 days after the last dose of chemotherapy • Negative urine or serum pregnancy test results within 72 h before the first dose of study intervention • Written informed consent |
• Squamous cell or undifferentiated gastric cancer • Major surgery, open biopsy or significant traumatic injury within 28 days before randomization or anticipated need for major surgery during the study treatment period • Pre-existing peripheral neuropathy grade >1 • Any prior therapy for locally advanced or metastatic gastric or GEJ cancer • Prior therapy with an anti-PD-1, anti-PD-L1 or anti-PD-L2 agent or with any other agent directed to stimulatory or co-inhibitory T-cell receptor (e.g., CTLA-4, OX40, CD137) • Prior radiotherapy within 2 weeks of study intervention • Systemic anticancer therapy, including investigational agents, ≤4 weeks before randomization • History of live vaccine within 30 days before the first dose of study intervention • Known additional malignancy that is progressing or has required active treatment within the past 5 years (except for BCC or SCC of the skin or for carcinoma in situ [e.g., breast carcinoma, cervical cancer in situ] that has undergone potentially curative treatment) • Active autoimmune disease that has necessitated systemic treatment (other than replacement therapy) in the past 2 years or history of solid organ/allogeneic stem cell transplant • Diagnosis of immunodeficiency, receiving chronic systemic steroid therapy (>10 mg daily prednisone equivalent) or receiving any other form of immunosuppressive therapy within 7 days before the first dose of study treatment • History or current evidence of any condition, therapy or laboratory abnormality that might confound the study results or interfere with study participation • Active infection necessitating systemic therapy • Active CNS metastases and/or carcinomatous meningitis • Known psychiatric or substance abuse disorder that would interfere with cooperation with study requirements • Pregnant or breastfeeding or expecting to conceive within the projected study duration • Known severe hypersensitivity (grade ≥3) to any of the study drugs or their excipients • Known history of HIV, HBV or HCV infection • Known history of active tuberculosis • History of noninfectious pneumonitis treated with steroids or current pneumonitis |
ANC: Absolute neutrophil count; aPTT: Activated partial thromboplastin time; BCC: Basal cell carcinoma; ECOG PS: Eastern Cooperative Oncology Group performance status; GEJ: Gastroesophageal junction; HBV: Hepatitis B virus; HCV: Hepatitis C virus; HIV: Human immunodeficiency virus; INR: International normalized ratio; MSI: Microsatellite instability; PT: Prothrombin time; SCC: Squamous cell carcinoma: ULN: Upper limit of normal.
Planned sample size & study period
The planned sample size is 1542 patients. The study started on 8 November 2018, and the estimated study completion date is 28 September 2024.
Outcome measures/end points
The primary end point is OS (defined as the time from randomization to death from any cause). Secondary end points are PFS (defined as the time from randomization to the first documented disease progression per RECIST v1.1 as assessed by blinded independent central review [BICR] or death from any cause, whichever comes first), ORR (defined as the proportion of patients who achieve a complete or partial response per RECIST v1.1 as assessed by BICR), duration of response (defined as the time from first response [complete response or partial response] to subsequent disease progression per RECIST v1.1 as assessed by BICR or death from any cause, whichever occurs first), safety and tolerability. Exploratory end points include health-related quality of life (assessed using the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30 [QLQ-C30], European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire–Stomach [QLQ-STO22] and EuroQoL 5D, 5-level questionnaire [EQ-5D-5L]), PFS and ORR using RECIST v1.1 modified for immune-based therapeutics (iRECIST) [19], and molecular biomarkers (genomic, metabolic and/or proteomic) that may be indicative of clinical response or resistance (may include analysis of patients with PD-L1 CPS or MSI status).
Study procedures
Tumor response and disease progression will be assessed using computed tomography or magnetic resonance imaging if computed tomography with contrast is contraindicated. Initial tumor imaging will be performed during screening (within 28 days before randomization). The same imaging technique and the use of contrast will be used for each patient throughout the study. The first on-study imaging will be performed at week 6 from randomization. Subsequent tumor imaging will be performed every 6 weeks (or more frequently if clinically indicated) until disease progression (confirmed by central imaging; unless the investigator continues treatment and follows iRECIST), start of new anticancer treatment, withdrawal of consent or death, whichever occurs first.
Safety will be monitored throughout the study and for 30 days after the end of treatment (90 days for serious events), including the incidence, causality and outcome of adverse events and serious adverse events and changes in vital signs and laboratory values. Adverse events will be graded and recorded per the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
Patient-reported outcome assessments will be completed electronically before treatment administration at cycle 1 through cycle 5 and every two cycles thereafter, at the treatment discontinuation visit, and at the 30-day safety follow-up visit.
Statistical analysis
Efficacy will be assessed in the intention-to-treat population and analyzed between randomized groups. Safety will be analyzed in all randomly assigned patients who received ≥1 dose of study medication according to their treatment intervention. The primary hypothesis for OS will be evaluated by comparing pembrolizumab plus chemotherapy with placebo plus chemotherapy using a stratified log-rank test. HRs will be estimated using a stratified Cox proportional hazards model with Efron’s method of tie handling. The stratification factors used for randomization will be applied to both the stratified log-rank test and the stratified Cox model. Event rates over time will be estimated using the Kaplan–Meier method. The overall type I error for the primary and secondary end points will be strongly controlled at 2.5% (one-sided).
Conclusion
Pembrolizumab has demonstrated antitumor activity in metastatic gastric cancer and is approved in USA for third-line or later treatment of patients with advanced metastatic gastric and GEJ adenocarcinoma. Here we have described the methodology of the KEYNOTE-859 study, which will investigate pembrolizumab plus chemotherapy compared with placebo plus chemotherapy (FP or CAPOX) as treatment of patients with previously untreated, HER2-negative, unresectable or metastatic gastric cancer or GEJ cancer. To ensure inclusion of the entire spectrum of first-line gastric cancers, patients will be eligible regardless of PD-L1 expression status. KEYNOTE-859 will build on the knowledge learned from KEYNOTE-062 and will further define the clinical benefit to be derived from the addition of pembrolizumab to standard-of-care chemotherapy (FP or CAPOX) as first-line treatment of patients with advanced unresectable and/or metastatic gastric or GEJ cancer. In so doing, KEYNOTE-859 will use a different standard-of-care chemotherapy backbone than KEYNOTE-062 and a modified statistical design to better define and elucidate the underlying clinical benefit. The results of this study will help define the role of immune checkpoint inhibitors in combination with chemotherapy in the first-line setting for patients with gastric cancer or GEJ cancer.
Executive summary.
Prognosis is poor for patients with unresectable locally advanced or metastatic gastric cancer, and standard first-line chemotherapy offers limited survival.
Background & rationale
Accumulating evidence demonstrates that combining immunotherapy with chemotherapy for the treatment of advanced unresectable and/or metastatic cancer improves clinical outcomes.
Data from the KEYNOTE-059 trial in patients with advanced HER2-negative gastric or gastroesophageal junction cancer support further investigation into the use of pembrolizumab plus chemotherapy as first-line therapy in this population.
KEYNOTE-859 study design & eligibility criteria
KEYNOTE-859 is a double-blind, randomized, placebo-controlled, Phase III trial that will evaluate the efficacy and safety of pembrolizumab plus chemotherapy compared with placebo plus chemotherapy as first-line treatment of patients with gastric or gastroesophageal adenocarcinoma.
An estimated 1542 patients with HER2-negative, previously untreated, unresectable or metastatic gastric or gastroesophageal junction adenocarcinoma will be enrolled.
Eligible patients will be randomly assigned 1:1 to receive pembrolizumab or placebo in combination with chemotherapy.
Outcomes
The primary end point is overall survival.
Conclusion
The results of KEYNOTE-859 will help define the role of pembrolizumab plus chemotherapy as a first-line treatment option for patients with HER2-negative advanced gastric or gastroesophageal junction adenocarcinoma.
Supplementary Material
Acknowledgments
The authors thank the patients and their families and caregivers, as well as all investigators and site personnel, for participating in this trial.
Footnotes
Supplementary data
An infographic accompanies this paper and is included at the end of the references section in the PDF version. To view or download this infographic in your browser please click here: www.futuremedicine.com/doi/suppl/10.2217/fon-2021-0176
Author contributions
J Tabernero, YJ Bang, EV Cutsem, CS Fuchs, P Bhagia, D Adelberg, SK Qin contributed in conception, design or planning of the study. YJ Bang, EV Cutsem, SK Qin contributed in acquisition of the data. CS Fuchs, YY Janjigian, K Li contributed in analysis of the data. EV Cutsem, CS Fuchs, K Li contributed in interpretation of the results. J Tabernero assisted in drafting of the manuscript. J Tabernero, YJ Bang, EV Cutsem, CS Fuchs, YY Janjigian, P Bhagia, K Li, D Adelberg, SK Qin contributed in critically reviewing or revising the manuscript for important intellectual content. Final approval was given by all authors.
Financial & competing interests disclosure
Funding for this study was provided by Merck Sharp and Dohme Corp., a subsidiary of Merck & Co., Inc., NJ, USA. J Tabernero served in a scientific consultancy role for Array Biopharma, AstraZeneca, Bayer, Boehringer Ingelheim, Chugai, Daiichi Sankyo, F. Hoffmann-La Roche Ltd, Genentech, Inc, HalioDX SAS, Ikena Oncology, IQVIA, Imedex, Lilly, Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Menarini, Merck Serono, Mirati, Novartis, Peptomyc, Pfizer, Pierre Fabre, Samsung Bioepis, Sanofi, Seattle Genetics, Servier, Taiho, Tessa Therapeutics and TheraMyc. YJ Bang served in a consulting/advisory role for AstraZeneca, Novartis, Genentech/Roche, Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Merck Serono, Bayer, Bristol Myers Squibb, Eli Lilly, Taiho, Daiichi Sankyo, Astellas, BeiGene, GreenCross, Samyang Biopharm, Hanmi and Genexine and reports grants (to the institution for clinical trials) from AstraZeneca, Novartis, Genentech/Roche, Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Merck Serono, Bayer, Bristol Myers Squibb, GSK, Pfizer, Eli Lilly, Boehringer Ingelheim, MacroGenics, Boston Biomedical, FivePrime, Curis, Taiho, Takeda, Ono, Daiichi Sankyo, Astellas, BeiGene, Green Cross, CKD Pharma and Genexine. EV Cutsem served in an advisory/consultancy role for Array, Astellas, AstraZeneca, Bayer, Biocartis, Bristol Myers Squibb, Celgene, Daiichi Sankyo, Halozyme, GSK, Pierre Fabre, Incyte, Ipsen, Lilly, Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Merck KGaA, Novartis, Roche, Servier, Sirtex, and Taiho and reports grants (to his institution) from Amgen, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Ipsen, Lilly, Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Merck KGaA, Novartis, Roche and Servier. CS Fuchs served in an advisory/consultancy role for Agios, Amylin Pharmaceuticals, AstraZeneca, Bain Capital, CytomX Therapeutics, Daiichi Sankyo, Eli Lilly, Entrinsic Health, EvolveImmune Therapeutics, Genentech, Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Taiho, and Unum Therapeutics. He also serves as a director for CytomX Therapeutics and owns unexercised stock options for CytomX and Entrinsic Health; is a cofounder of EvolveImmune Therapeutics and has equity in this private company; and has provided expert testimony for Amylin Pharmaceuticals and Eli Lilly. YY Janjigian has received advisory fees from Merck Serono, Pfizer, Daiichi Sankyo, Imugene, Zymeworks Inc., Basilea Pharmaceutica and AstraZeneca. P Bhagia is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc. K Li is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc. D Adelberg is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc. SK Qin reports no conflict of interest. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Medical writing and/or editorial assistance was provided by Kathleen Richards, PhD, and Holly C. Cappelli, PhD, CMPP, of ApotheCom (PA, USA). This assistance was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.
Ethical conduct of research
The authors attest that the study protocol was approved by the appropriate ethics committee or institutional review board at each participating center. The study was conducted in accordance with the standards of Good Clinical Practice and the Declaration of Helsinki. All participants will provide written informed consent before enrollment.
Data sharing statement
Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., (MSD) is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company’s clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at: http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the US and EU or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68(6), 394–424 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Colombet M, Soerjomataram I et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 144(8), 1941–1953 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Colquhoun A, Arnold M, Ferlay J, Goodman KJ, Forman D, Soerjomataram I. Global patterns of cardia and non-cardia gastric cancer incidence in 2012. Gut 64(12), 1881–1888 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 513(7517), 202–209 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN guidelines): gastric cancer (version 4.2020). (2020). https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf [DOI] [PubMed]
- 6.Smyth EC, Verheij M, Allum W et al. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 27(Suppl. 5), v38–v49 (2016). [DOI] [PubMed] [Google Scholar]
- 7.van Cutsem E, Moiseyenko VM, Tjulandin S et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J. Clin. Oncol. 24(31), 4991–4997 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Shitara K, van Cutsem E, Bang YJ et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 Phase 3 randomized clinical trial. JAMA Oncol. 6(10), 1571–1580 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; • The efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy versus chemotherapy alone were investigated in first-line therapy for patients with advanced gastric cancer in the randomized Phase III KEYNOTE-062 study.
- 9.Bang YJ, Kang YK, Catenacci DV et al. Pembrolizumab alone or in combination with chemotherapy as first-line therapy for patients with advanced gastric or gastroesophageal junction adenocarcinoma: results from the Phase II nonrandomized KEYNOTE-059 study. Gastric Cancer 22(4), 828–837 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; • In cohorts 2 and 3 of the nonrandomized Phase II KEYNOTE-059 study, pembrolizumab alone or with chemotherapy was evaluated as first-line therapy for patients with advanced gastric or gastroesophageal junction (GEJ) adenocarcinoma.
- 10.Boku N, Ryu MH, Kato K et al. Safety and efficacy of nivolumab in combination with S-1/capecitabine plus oxaliplatin in patients with previously untreated, unresectable, advanced, or recurrent gastric/gastroesophageal junction cancer: interim results of a randomized, Phase II trial (ATTRACTION-4). Ann. Oncol. 30(2), 250–258 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; • The safety and efficacy of nivolumab with chemotherapy were investigated in patients with previously untreated unresectable advanced or recurrent gastric/GEJ cancer in part 1 of the randomized Phase III ATTRACTION-4 study.
- 11.Boku N, Ryu MH, Oh DY et al. Nivolumab plus chemotherapy versus chemotherapy alone in patients with previously untreated advanced or recurrent gastric/gastroesophageal junction (G/GEJ) cancer: ATTRACTION-4 (ONO-4538-37) study. Ann. Oncol. 31(Suppl. 4), S1192 (2020). [Google Scholar]; • In part 2 of the double-blind, randomized Phase III ATTRACTION-4 study, the efficacy of nivolumab plus chemotherapy versus placebo plus chemotherapy was evaluated as first-line therapy for patients with HER2 negative advanced or recurrent gastric/gastroesophageal cancer.
- 12.Moehler M, Shitara K, Garrido M et al. Nivolumab (nivo) plus chemotherapy (chemo) versus chemo as first-line (1L) treatment for advanced gastric cancer/gastroesophageal junction cancer (GC/GEJC)/esophageal adenocarcinoma (EAC): first results of the CheckMate 649 study. Ann. Oncol. 31(Suppl. 4), S1191 (2020). [Google Scholar]; • In the first results of the randomized Phase III CheckMate 649 study, nivolumab plus chemotherapy versus chemotherapy was investigated as first-line therapy for patients with advanced gastric cancer/GEJ cancer/esophageal adenocarcinoma.
- 13.Merck Sharp & Dohme Corp. KEYTRUDA® (pembrolizumab) injection, for intravenous use. 11/2020. Merck Sharp & Dohme Corp, Whitehouse Station, NJ, USA: (2020). [Google Scholar]
- 14.Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity 39(1), 74–88 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Burtness B, Harrington KJ, Greil R et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, Phase 3 study. Lancet 394(10212), 1915–1928 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Lara P, Beckett L, Li Y et al. Combination checkpoint immunotherapy and cytotoxic chemotherapy: pembrolizumab (pembro) plus either docetaxel or gemcitabine in patients with advanced or metastatic urothelial cancer. J. Clin. Oncol. 35(Suppl. 6), (2017). [Google Scholar]
- 17.Langer CJ, Gadgeel SM, Borghaei H et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, Phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 17(11), 1497–1508 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.CheckMate-649, a Phase 3 trial evaluating opdivo (nivolumab) plus chemotherapy vs. chemotherapy, meets primary endpoints demonstrating superior overall survival and progression-free survival in first-line treatment of gastric and esophageal cancers [news release]. Bristol Myers Squibb, Princeton, NJ, USA: (2020). https://news.bms.com/news/details/2020/CheckMate--577-a-Phase-3-Trial-Evaluating-Opdivo-nivolumab-as-Adjuvant-Therapy-for-Patients-with-Resected-Esophageal-or-Gastroesophageal-Junction-Cancer-Meets-Primary-Endpoint-of-Disease-Free-Survival/default.aspx [Google Scholar]
- 19.Seymour L, Bogaerts J, Perrone A et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 18(3), e143–e152 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.