Abstract
The first months of life are critical for establishing neural connections relevant for social and cognitive development. Yet, the United States lacks a national policy of paid family leave during this important period of brain development. This study examined associations between paid leave and infant electroencephalography (EEG) at 3 months in a sociodemographically diverse sample of families from New York City (N = 80; 53 males; 48% Latine; data collection occurred 05/2018–12/2019). Variable-centered regression results indicate that paid leave status was related to differences in EEG power (ps < .02, R2s > .12). Convergent results from person-centered latent profile analyses demonstrate that mothers with paid leave were 7.39 times as likely to have infants with EEG profiles characterized by increased higher-Hz power (95% CI, 1.9–36.9), potentially reflecting more mature patterns of brain activity.
The period after birth is often a vulnerable time for mothers. Typical experiences for new mothers include post-birth complications, lactation difficulties, sleep deprivation, and chronic stress (Saxbe et al., 2018). Despite the many challenges occurring during this transition, an estimated 23% of working mothers in the United States who did not have access to paid leave returned to work within 10 days of giving birth (Klerman et al., 2012). One survey of New York City mothers even reported that 5% of working mothers interviewed took no leave at all (Slopen, 2020). This is in stark contrast to guidelines from the American College of Obstetricians and Gynecologists that mothers need 12 weeks to recover from the physical and psychological impact of childbirth (ACOG, 2018).
Virtually every country in the world provides new parents with paid family leave, with most countries providing at least 6–9 months of paid leave to new mothers on average (Khan, 2020; Nandi et al., 2018). The United States is the only high-income country to not have a federal policy mandating paid leave for working caregivers who give birth. As such, only 23% of all employed workers in the United States have access to paid parental leave through their employers (Bureau of Labor Statistics, U.S. Department of Labor, 2021). Although the Federal Medical Leave Act enables new caregivers to receive 12-weeks of job-protected unpaid leave, eligibility criteria severely restrict access to this policy for most working parents (Rossin-Slater & Uniat, 2019). Presently, caregivers only have access to paid leave through their employer, state-run family paid leave programs, or temporary disability insurance (TDI). This hodgepodge of available policies has resulted in inconsistencies in duration of paid leave and amount of wage replacement during leave (Van Niel et al., 2020). Nine states and the District of Columbia currently offer some form of paid leave that allows caregivers to have partially paid time off work to care for a new child (Kaiser Family Foundation, 2020). The introduction of a paid leave program at the state level doubled the length of leave taken, particularly among mothers from lower-income households (Brainerd, 2017).
Paid leave affords caregivers the time to attend postnatal healthcare visits for themselves and for their infants, which has been shown to translate into gains in a number of indicators of health. Studies have demonstrated that paid leave is associated with a 47% decrease in the odds of re-hospitalization for infants and a 51% decrease in odds of re-hospitalization for mothers (Jou et al., 2018), as well as reduced incidences of postpartum depression (Mandal, 2018). Additionally, a previous study found that children whose mothers returned to work within 12 weeks post-birth were 3.4 percentage points less likely to receive their required immunizations and 7.4 percentage points less likely to be breastfed (Berger & Waldfogel, 2004). Often, the overall benefits of paid leave are driven by families from lower-socioeconomic status (SES) households (Lichtman-Sadot & Bell, 2017), indicating that paid leave may have a bigger effect for caregivers who currently cannot afford to take unpaid leave after giving birth.
Impact of paid leave on infant development
The absence of a U.S. federal paid leave policy makes it difficult to study the intricate effects of paid leave on subsequent child outcomes beyond indicators of physical health. The potential benefits on developmental outcomes are more abundantly illustrated in international studies. For example, a study conducted in Chile examined outcomes after expanding the duration of paid leave (from 12 to 24 weeks). Researchers found increases in the probability of breastfeeding, decreases in maternal stress, and positive effects on children's cognitive abilities, particularly for families from lower-SES households (Albagli & Rau, 2018). Similarly, researchers in Korea reported positive associations between paid leave and a composite measure of infant development assessing motor, communication, problem-solving, and social skills (Hwang & Jung, 2016). On the other hand, Baker and Milligan (2010) found little impact of increasing the length of paid leave in Canada on assessments of parenting behaviors, child social skills, or motor development during toddlerhood. But this analysis excluded families from single-parent households and examined the length, rather than the initial implementation, of paid leave policies. Results from international studies indicate promising benefits of paid leave, but it is difficult to compare results across countries with varying levels of government support and social safety nets (i.e., childcare, medical insurance coverage; Burtle & Bezruchka, 2016).
Previous studies examining outcomes related to paid family leave in the United States have largely focused on maternal or infant physical health. There have been a few studies examining the impact of the length of leave on child developmental outcomes in the United States (i.e., Clark et al., 1997; Feldman et al., 2004; Plotka & Busch-Rossnagel, 2018), but these studies did not specifically examine differences in paid versus unpaid leave status. Kozak et al. (2021) examined parental self-report assessments of language, cognitive, and socioemotional outcomes for toddlers (24–36 months, N = 328) in relation to paid versus unpaid leave status for mothers. Results indicated that mothers who took paid leave after birth had toddlers with significantly higher language scores than children of mothers who took unpaid leave, even after accounting for differences in family income and length of leave. Furthermore, paid leave was also related to better socioemotional skills, but this effect was only present for mothers from lower-SES households. These findings suggest that expanding access to paid leave could be beneficial to all families, and may have an even larger benefit for under-resourced households (Kozak et al., 2021).
Factors linking paid leave to child outcomes
The first several months after birth are a sensitive period for an infant's socioemotional and cognitive development (Knudsen, 2004) as key neural connections within the brain are emerging in the context of consistent, warm, and contingent social interactions between the infant and caregiver (Shonkoff & Phillips, 2000b). There are multiple potential factors that could explain how paid leave could be beneficial for promoting healthy infant outcomes, including affording families the time to establish sensitive and responsive parent–child interactions and decreasing experiences of chronic maternal stress.
The quality of parent–child interactions has been found to scaffold children's subsequent cognitive and social-emotional development (Bigelow et al., 2010; Feldman & Eidelman, 2009). Quality is often measured within the child development literature using the constructs of maternal sensitivity and mother–infant reciprocity. Maternal sensitivity is typically defined as the ability to notice, interpret, and appropriately respond to infant cues (Beebe & Steele, 2013). Mother–infant reciprocity, sometimes referred to as dyadic reciprocity or synchrony, refers to the coordinated, back-and-forth exchanges of affective and behavioral cues (Feldman, 2007). Past research has also indicated that an absence of these behaviors may contribute to long-term negative outcomes for physiological and emotional development (Clark et al., 1997; Shonkoff & Phillips, 2000a). Both maternal sensitivity and mother–infant reciprocity have been linked to better emotion regulation and attachment security in children (Raikes et al., 2007) and the quality of parent–child interactions have also been shown to be predictive of infant brain function, assessed via electroencephalography (EEG), during the first 2 years of life (Bernier et al., 2016).
Early neurobehavioral development is sensitive to maternal mood (O'Sullivan & Monk, 2020). Although stress is a common experience for caregivers in the first several months after birth, high levels of chronic stress can impede a caregiver's ability to respond to their infant's cues in a sensitive and synchronous manner (Lutz et al., 2012; Tarullo et al., 2017). Very few studies have examined maternal stress in the context of paid leave, with only one study reporting that mothers who took some form of paid leave after childbirth were 1.78 times more likely to do better with stress management than mothers who had unpaid leave (Jou et al., 2018). In addition to self-reported measures of chronic stress, hair cortisol may be a useful indicator of chronic physiological stress reflecting cumulative cortisol exposure (Flom et al., 2017). For example, maternal hair cortisol concentration (HCC) has been associated with the quality of parent–child interactions, such that mothers with higher HCCs demonstrated less positive engagement synchrony with their infants (Tarullo et al., 2017).
Higher levels of maternal stress have also been associated with alterations in infant brain function (Pierce et al., 2019; St. John et al., 2017; Troller-Renfree et al., 2020). Previous research has reported a developmental pattern of brain activation whereby children demonstrate decreases in lower-frequency resting EEG power, along-side increases in higher-frequency resting EEG power as children develop (Bell, 1998; John et al., 1980; Marshall et al., 2004). This pattern of brain activity has been suggested to reflect the organization and maturation of cortical brain regions (Corning et al., 1982; Thatcher et al., 2008). Higher levels of maternal perceived stress (Pierce et al., 2019) and increased concentration of cortisol in samples of maternal hair (Troller-Renfree et al., 2020) have been associated with heightened levels of lower-frequency (i.e., theta) EEG power and reductions in higher-frequency (i.e., alpha, beta, gamma) EEG power during the first year of life. In addition to links to maternal stress, this pattern of brain activation has also been associated with disparities in language, cognitive, and socioemotional skills (Brito et al., 2016, 2019; Corning et al., 1986; Maguire & Schneider, 2019).
Current study
The literature clearly illustrates that paid leave contributes to the physical and mental well-being of both mothers and infants (Berger & Waldfogel, 2004; Jou et al., 2018; Mandal, 2018) and paid leave may support early child cognitive and socioemotional development (Kozak et al., 2021). Time off after childbirth that is financially supported may afford mothers the ability to establish and engage in sensitive and synchronous interactions in the first few months of life. Additionally, instances of unpaid leave may heighten levels of stress within the home during this already stressful perinatal period. The present exploratory study aims to examine whether experiences of paid versus unpaid maternity leave are associated with differences in infant brain activation patterns early in life, and if paid leave is associated with maternal stress or the quality of parent–child interactions. We hypothesize that infants of mothers with paid leave will demonstrate decreases in lower-frequency EEG power and increases in higher-frequency EEG power compared to infants whose mothers took unpaid leave. Furthermore, we hypothesize that paid leave will be associated with decreased maternal stress (both perceived and HCCs) and higher levels of maternal sensitivity and dyadic reciprocity. Finally, within exploratory person-centered analyses we will conduct a latent profile analysis (LPA) to isolate (1) groups of infants with similar patterns of EEG activity, and (2) characteristics that may predict different infant EEG profiles. The aims of these exploratory analyses are to corroborate our variable-centered regression analyses, and to determine the probability that specific maternal characteristics, such as paid leave, predict distinct patterns of EEG activity.
METHOD
Participants
The initial sample included 100 infants (63 males; age M = 3.46 months, SD = 0.38) recruited from community events, family services, and health care providers around New York City. Exclusion criteria included birth before 36 weeks’ gestation, multiple births, or presence of developmental disorders. Data collection took place at a research lab within NYU between May 2018 and December 2019. Only mothers who reported that they took time off work after childbirth and either returned to work or intended to return to work within the child's first year of life were included in the final sample (n = 80; 53 males). There were no mothers who reported taking no leave within the sample. The racial breakdown of infants in the final sample was as follows: 44% two or more races, 31% White, 18% Black, 6% Asian, and 1% unreported. The sample also comprised 48% of mothers identifying as Hispanic or Latino. Annual household income ranged from $3,000 to $500,000 (Median = $100,000), see Table 1 for additional sociodemographic information. All research procedures were approved by the NYU IRB.
TABLE 1.
Descriptive statistics
| Paid leave (n = 47) |
Unpaid leave (n = 33) |
Paid versus unpaid | |||
|---|---|---|---|---|---|
| Variable | M or N | SD or % | M or N | SD or % | Statistical results |
| Infant age (days) | 104.86 | 11.96 | 105.23 | 11.36 | t(78) = 0.14, p = .89 |
| Infant sex (male) | 18 | 38% | 9 | 27% | χ2(1) = 0.62, p = .43 |
| Gestational age (weeks) | 39.41 | 1.17 | 39.06 | 1.37 | t(78) = −1.23, p = .22 |
| Ethnicity (% Hispanic/Latin) | 20 | 43% | 18 | 56% | χ2(1) = 0.93, p = .33 |
| Race | |||||
| Asian | 4 | 9% | 1 | 3% | χ2(1) = 0.28, p = .60 |
| Black | 5 | 11% | 9 | 27% | χ2(1) = 2.65, p = .10 |
| Two or more | 19 | 40% | 16 | 49% | χ2(1) = 2.62, p = .11 |
| White | 19 | 40% | 7 | 21% | χ2(1) = 2.45, p = .12 |
| Number of children | 1.49 | 0.84 | 2.22 | 1.26 | t(75) = 3.04, p = <.01 |
| Relationship status (% partnered) | 45 | 96% | 22 | 67% | χ2(1) = 10.00, p = .001 |
| Income-to-needs (log-transformed) | 1.69 | 0.91 | 0.66 | 1.07 | t(78) = −4.44, p < .001 |
| Maternal education (years) | 17.14 | 3.3 | 14.45 | 3.42 | t(78) = −3.55, p = <.001 |
| Occupational prestige (O*Net zone) | 3.91 | 0.97 | 2.91 | 1.17 | t(77) = −4.16, p = <.001 |
| Maternal leave duration (weeks) | 12.44 | 3.37 | 12.04 | 4.1 | t(78) = −0.40, p = .69 |
| Maternal cortisol (log-transformed) | 0.39 | 1.72 | 1.72 | 1.9 | t(61) = 2.88, p < .01 |
| Maternal perceived stress | 12.87 | 5.48 | 14.32 | 7.49 | t(74) = 0.98, p = .33 |
| Maternal sensitivity | 0.1 | 0.29 | −0.12 | 0.39 | t(63) = −2.55, p = .01 |
| Dyadic reciprocity | 0.1 | 0.27 | −0.12 | 0.39 | t(63) = −2.70, p = .01 |
| Parent-led interaction | 3.86 | 0.77 | 4.21 | 0.54 | t(63) = 2.04, p = .04 |
Bold indicate significant results.
Measures
Demographic Questionnaire
Mothers provided demographic information including maternal and infant age, educational attainment, occupational prestige, household income, relationship status (partnered vs. single), number of children in the household, race, and ethnicity. Maternal occupational prestige was coded on a scale from 1 to 5 based on the O*NET job zone for the occupation. O*NET is a national database developed by the US Department of Labor, which categorizes occupations by the level of education, experience, and training needed for a given job title (Table S1). Family income-to-needs ratio (ITN) is the total household income divided by the federal poverty line for the corresponding number of adults and children in the home. Family ITN was log-transformed to reflect the nonlinear relation between ITN and neural measures; extreme values >2 SDs above the median (equivalent to incomes > $400,000 for a family of three; n = 6; 7%) were winsorized by replacing these values with the value representing 2 SDs above the median.
Family Leave Questionnaire
To measure the experiences of paid leave, mothers were asked (1) if they took time off work after giving birth, (2) if this time off was compensated monetarily, and (3) how long they took off before returning to work. For mothers who were still on leave at the 3-month visit, they were asked for an estimate of when they would return to work. If mothers reported any monetary compensation during their leave, they were categorized as having paid leave (e.g., paid vs. unpaid leave). Mothers were also asked what percentage of their previous pay was compensated during their time off (0%–100%), and this was used as the continuous variable of wage-replacement during their leave.
We did not collect information on how mothers were receiving wage-replacement during their paid leave (i.e., via employer, TDI, or state-run paid leave program). State-sponsored paid family leave was first launched in New York State in 2018 through a 4-year phase-in mechanism, and reached target-level benefits (i.e., 12-weeks of paid time off at 67% of average weekly wage; max weekly benefit at 67% of Statewide Average Weekly Wage) in 2021. Additionally, in the first year of the program, less than 2% of the covered workforce applied for paid leave claims (Office of Governor Cuomo, 2019). Therefore, as data collection took place from May 2018 to December 2019, it is not very likely that mothers in the current study would have taken paid leave through the state-run paid leave program.
Perceived Stress Scale
The Perceived Stress Scale (PSS; Cohen et al., 1983) was used to assess self-reported maternal stress. The PSS includes 14-questions that assess the degree to which the respondent has perceived situations as stressful within the last month. Items are rated on a five-point Likert scale ranging from 0 (never) to 4 (very often). A total score is the sum of scores from all items (after four items are reverse scored), with scores ranging from 0 to 40.
Physiological stress (hair cortisol)
A small hair sample was collected from caregivers, with each sample weighing at least 15 mg. Each hair sample was trimmed to be approximately 3-cm long (measured from the end closest to the root). As human hair grows approximately 1 cm per month, each sample contains cortisol deposited during roughly the first 3 months postpartum. The samples were stored at −40°C until sent for analysis. Each sample was weighed, washed twice in isopropanol to remove external contaminants, ground to a fine powder, and extracted with methanol. The methanol extract was evaporated, re-dissolved in an assay buffer, and analyzed along with standards and quality controls by a sensitive and specific enzyme-linked immunosorbent assay. Assay readout was converted to pg cortisol per mg dry hair weight. Intra- and inter-assay coefficients of variation for this assay are <10%. Hair cortisol values were log-transformed to correct for skew.
Parent–child interaction
Each dyad was given a standardized set of toys including a book, stacking cups, and a rattling ball, and was instructed to play like they normally would at home for 5 min. The quality of mother–infant interactions was assessed using the Coding Interactive Behavior (CIB) measure (Feldman, 1998). The CIB is a global rating scale of 22 parent behaviors, 16 child behaviors, and five dyadic behaviors. Each behavior was coded on a 5-point scale, where 1 denotes a minimal level of the specific behavior and 5 denotes a maximal level across the 5-min interaction. The interactions were also coded for the extent that they were parent-led (i.e., the extent the parent was preoccupied with “teaching” the infant, re-directed the infant's attention, or directed the infant to parent-led activities). Inter-rater reliability was established between four independent coders on 30% of the data and Fleiss’ κ ranged from .71 to 1.0.
Confirmatory factor analysis (CFA) was used to derive a composite measure of maternal sensitivity using the following CIB behavioral codes: maternal positive affect, gaze toward the child, acknowledgment of the child's cues, imitation, appropriate range of affect, resourcefulness, and supportive presence. All variables loaded significantly (ps < .001) and model fit was excellent, RMSEA = .05, 90% CI (0, 0.14), CFI = .98, χ2 = 16.25, p = .30. Similarly, CFA was also used to derive a global composite measure of dyadic reciprocity, indexed by CIB codes for dyadic reciprocity, adaptation-regulation, and fluency, which showed model convergence with all variables loading significantly (ps < .01). The resulting factor score estimates for composite measures of maternal sensitivity and dyadic reciprocity were used in subsequent analyses.
EEG data acquisition and processing
Resting EEG data were acquired while infants watched a video of engaging, nonsocial stimuli (e.g., bubbles, spinning wheel) while seated on their caregivers’ laps. EEG was recorded using a 64-channel HydroCel Geodesic Sensor Net (Electrical Geodesic, Inc.) and amplifier (Electrical Geodesic, Inc.; EB NEURO S.p.A.). Electrode impedances were kept below 100 KΩ and the sampling rate was recorded at 1000 Hz. Among the 80 infants in the final sample, 25% (n = 20) were missing EEG data (n = 12 or 26% of the paid leave group and n = 8 or 24% of the unpaid leave group). Reasons for missing EEG data were given as follows: participant refusal (n = 1), computer/technical issues (n = 4), infant fussiness during acquisition (n = 3), and poor data quality (n = 12). Infants who were missing EEG data did not differ from those who had EEG data on any of the study variables or covariates (ps > .18).
All EEG files were processed in batch EEG automated processing platform (BEAPP) software to ensure standardization in data processing and cleaning across all files (Levin et al., 2018). Data preprocessing was carried out using the Harvard Automated Processing Pipeline for EEG (HAPPE), an automated preprocessing pipeline designed for infant EEG data (Gabard-Durnam et al., 2018). First, a 1 Hz high-pass and 100 Hz low-pass filter were applied to each EEG dataset. Second, the data, which were originally sampled at 1000 Hz were resampled with interpolation to 250 Hz, following the guidelines for further HAPPE processing. The third step involved artifact removal and included CleanLine's multitaper approach to removing 60 Hz electrical noise, bad channel rejection, and wavelet-enhanced ICA for artifact rejection with automated component rejection through the Multiple Artifact Rejection Algorithm in EEGLAB. A subset of spatially distributed electrodes was selected for analysis with MARA: 2 3 5 6 8 9 10 11 12 13 14 18 20 24 25 28 30 31 34 35 39 40 42 44 48 50 52 57 58 59 60 (NetStation Geodesic 64- Channel Net). Bad channels that were initially rejected were repopulated using spherical interpolation to reduce bias in re-referencing and the signal was mean detrended. Finally, each EEG file was segmented into 2-s windows and each segment was assessed for remaining artifacts. Segment rejection thresholds were determined according to HAPPE’s automated rejection criteria, which uses amplitude thresholding and assessment of segment likelihood using joint probability calculations.
Electroencephalography power decomposition was accomplished using Fast Fourier transformation using a multi-taper windowing (3 windows) to decompose power into 2-s segments for each channel. Consistent with other infant studies (Tomalski et al., 2013; Troller-Renfree et al., 2020), spectral power was computed for the frequency bands theta (4–6 Hz), low alpha (6–9 Hz), high alpha (9–13 Hz), beta (13–19 Hz), low gamma (21-30Hz), high gamma (31–45 Hz). At each channel, mean power within each frequency band was calculated across all segments and normalized by a log base 10 transformation. Power was then averaged across electrodes in each region of interest: frontal cortex (2, 3, 5, 9, 10, 11, 12, 13, 14, 18, 57, 58, 59, 60), temporal cortex (20, 24, 25, 48, 50, 52), parietal cortex (28, 30, 31, 40, 44, 42), and occipital cortex (35, 39). Relative power (i.e., absolute power in one frequency band divided by total absolute power from all frequency bands) was computed and analyzed below.
Analysis plan
Both continuous (percentage of previous pay that was paid during leave) and categorical (paid vs. unpaid leave status) variables were used in subsequent analyses. There were no significant differences between the paid and unpaid leave groups in terms of leave duration, gestational age at birth, infant age, infant sex, maternal race or ethnicity, but there were significant differences in family ITNs ratio, number of children in the household, maternal education, relationship status, and maternal occupational prestige (see Table 1). In addition to significant variables, gestational age at birth, infant sex, and infant age were also included as covariates in primary analyses as these variables have been previously found to impact early brain and cognitive development (McCarton et al., 1996; Prior et al., 1993).
Prior to analyses, we first checked for multivariate outliers across the EEG and sociodemographic variables using Mahalanobis distance, which indicated no multivariate outliers within or across groups. Prior to testing our primary hypotheses, we first ensured that there were no region- or hemisphere-specific differences in EEG power between paid leave groups using a series of repeated measures ANOVAs. We examined the frequency bands separately. Region (frontal, temporal, parietal, occipital) and hemisphere (left, right) were used as within-subject factors, paid leave group (paid, unpaid) was a between-subject-factor, and log-10 transformed absolute EEG power was the dependent variable. Across all frequency bands, there were no significant interactions between paid leave group and brain region or hemisphere (all ps > .07, all Fs < 3.37), indicating no region- or hemisphere-specific differences between groups. Thus, we collapsed across brain regions and hemispheres for subsequent analyses.
Multiple linear regressions were used to test primary hypotheses regarding the association between maternal paid leave and relative EEG power. Full information maximum likelihood (FIML) was used to account for missing data in all analyses, as FIML produces unbiased parameter estimates. Exploratory analyses examined how experiences of paid leave, maternal stress, and parent–child interactions contributed to patterns of infant brain activation.
RESULTS
Means and standard deviations, as well as comparisons between groups, for all study variables are reported in Table 1. Correlations between all variables are reported in Table 2.
TABLE 2.
Bivariate correlations
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Theta | 1 | ||||||||||||||||||||
| 2. Low alpha | .07 | 1 | |||||||||||||||||||
| 3. High alpha | −.91** | .02 | 1 | ||||||||||||||||||
| 4. Beta | −.89** | −.40** | .80** | 1 | |||||||||||||||||
| 5. Low gamma | −.68** | −.59** | .43** | .69** | 1 | ||||||||||||||||
| 6. High gamma | −.61** | −.50** | .38** | .60** | .69** | 1 | |||||||||||||||
| 7. Infant age | .27* | −.22 | −.35** | −.14 | .06 | −.10 | 1.00 | ||||||||||||||
| 8. Infant sex | −.04 | .00 | −.03 | .03 | .08 | .05 | −.09 | 1 | |||||||||||||
| 9. Gestational age | −.19 | −.11 | .09 | .10 | .28* | .36** | −.29** | .23* | 1 | ||||||||||||
| 10. Number of children | −.09 | .12 | .05 | .06 | .06 | −.10 | .03 | −.02 | −.10 | 1 | |||||||||||
| 11. Relationship status | .09 | −.01 | −.14 | −.09 | −.04 | .07 | .12 | −.04 | −.01 | −.41** | 1 | ||||||||||
| 12. Cortisol level | .06 | .14 | −.08 | −.14 | −.08 | −.02 | .12 | −.08 | −.33** | .18 | −.08 | 1 | |||||||||
| 13. Perceived stress | −.06 | −.15 | .09 | .05 | .15 | .08 | .08 | −.11 | .06 | .07 | −.17 | .11 | 1 | ||||||||
| 14. Maternal sensitivity | .07 | −.15 | −.08 | −.02 | −.02 | .14 | −.02 | .03 | −.10 | −.33** | .50** | −.06 | −.12 | 1 | |||||||
| 15. Dyadic reciprocity | −.01 | −.23 | .02 | .08 | .07 | .22 | .15 | .20 | −.11 | −0.21 | .27* | .00 | −.09 | .73** | 1 | ||||||
| 16. Parent-led interaction | −.10 | .12 | .03 | .02 | .05 | .13 | −.04 | −.09 | −.03 | .26* | −0.16 | .06 | −.14 | −.40** | −.40** | 1 | |||||
| 17. Income-to-needs | .04 | .04 | −.08 | −.04 | −.07 | .07 | −.06 | .10 | −.08 | −.59** | −.63** | −.22 | −.31** | .47** | .35** | −.09 | 1 | ||||
| 18. Maternal education | −.09 | −.10 | .03 | .08 | .10 | .26* | −.09 | .10 | .22 | −.48** | .32** | −.32* | −.27* | .33** | .23 | .06 | .67** | 1 | |||
| 19. Occupational prestige | −.01 | −.17 | −.03 | .04 | .07 | .23 | −.09 | .18 | .13 | −.51** | .34** | −.31* | −.29* | .42** | .34** | −.10 | .73** | .84** | 1 | ||
| 20. % Paid leave | −.25 | −.07 | .24 | .23 | .20 | .16 | −.01 | .13 | .14 | −.26* | .37** | −.34** | −.07 | .40** | .36** | −.40** | .43** | .33** | .40** | 1 | |
| 21. Paid leave status | −.29* | −.11 | .27* | .27* | .27* | .21 | −.02 | .11 | .14 | −.33** | .39** | −.35** | −.11 | .31* | .32** | −.25* | .47** | .37** | .43** | .91** | 1 |
p < .05.
p < .01.
Paid leave is associated with infant EEG power
We first examined paid leave as a continuous variable (percentage previous pay, with no paid leave coded as zero), controlling for infant age, infant sex, infant gestational age, number of children in the home, maternal relationship status (single vs. married or cohabitating), ITN, maternal education, and occupational prestige. Results indicated that greater percent paid leave was associated with increased high alpha EEG power (β = .39, p = .003, R2 = .27) and increased beta power (β = .32, p = .02, R2 = .12), as well as reduced theta power (β = −.33, p = .01, R2 = .22), see Table S2. There were no other significant effects of percent paid leave on infant EEG power (ps > .15).
Using categorical groups of paid versus unpaid leave, infants of mothers who had some level of paid leave after birth demonstrated a distinct pattern of relative EEG activation from infants of mothers who took unpaid leave. Notably, paid leave was associated with increased EEG power across higher frequency bands (high alpha, beta, and low gamma) and reduced EEG power across lower frequency bands (theta). Full regression results are reported in Table 3.
TABLE 3.
Multiple linear regression results predicting electroencephalography brain activity
| 4–6 Hz | 6–9 Hz | 9–13 Hz | 13–19 Hz | 21–30 Hz | 31–45 Hz | |
|---|---|---|---|---|---|---|
| Overall R2 | .23 | .19 | .27 | .14 | .22 | .21 |
| Beta coefficients | ||||||
| Infant age (days) | .23 | −.30 | −.37* | −.10 | .17 | .00 |
| Infant sex | .03 | .06 | −.05 | −.03 | −.03 | −.07 |
| Gestational age | −.07 | −.07 | −.11 | −.03 | .27 | .31 |
| Number of children | −.13 | .10 | −.03 | .05 | .17 | .11 |
| Relationship status | .09 | .04 | −.11 | −.16 | −.05 | .08 |
| Income-to-needs | .11 | .34 | −.23 | −.14 | −.19 | −.22 |
| Maternal education | −.28 | .03 | .21 | .22 | .19 | .22 |
| Occupational prestige | .23 | −.39 | −.19 | −.10 | .01 | .12 |
| Maternal paid leave | −.39* | −.11 | .42** | .38* | .31* | .11 |
FDR-adjusted p < .05.
FDR-adjusted p < .01.
We conducted sensitivity analyses to examine the robustness of the observed effects of paid leave on relative infant EEG power to potential bias from unobserved confounding variables. We used the coefficient of proportionality method (Dearing & Zachrisson, 2019; Oster, 2019), which indexes how large the impact of omitted variables would need to be, relative to observed confounders, to invalidate a result. Assuming conservative maximum R2 values ranging from .8 to 1, we found that omitted variables would need to be 60%–990% as powerful as the observed socioeconomic and demographic covariates to nullify the results (Table 4). Notably, the majority of these values are well above recommended conservative thresholds for robustness (coefficients > or = of 1.00 [100%]), with three of the four values under 1.00 within the low gamma band.
TABLE 4.
Sensitivity to omitted variable bias
| Assumed max R2 | Theta | High alpha |
Beta | Low gamma |
|---|---|---|---|---|
| 1 | 1.78 | 7.32 | 0.95 | .61 |
| 0.9 | 2.04 | 8.40 | 1.08 | .69 |
| 0.8 | 2.39 | 9.86 | 1.24 | .80 |
Note: The absolute values of the coefficient of proportionality, which indicate how strong unobserved confounders would need to be relative to measured covariates to nullify the effect of paid leave on infant brain function.
Examining potential factors explaining links between paid leave and infant EEG
We next explored associations between paid leave and maternal indicators of stress. Controlling for ITN, maternal education, and occupational prestige, paid leave status was associated with maternal cortisol concentration levels (β = −.27, p = .05), with experiences of paid leave being linked to lower cortisol levels. Importantly, ITN, maternal education, and occupational prestige did not predict maternal cortisol levels (ps > .34), indicating that elevated maternal cortisol is specific to differences in paid leave status and do not merely reflect SES differences. Contrary to our hypothesis, maternal perceived stress was not associated with experiences of paid leave (categorical: p = .68; continuous: p = .43). Multivariate linear regressions were used to estimate associations between maternal physiological stress and EEG power across each frequency band. Results indicated that maternal cortisol in and of itself was not associated with infant EEG power (ps > .13), nor any interactions between paid leave and maternal cortisol on relative EEG power (ps > .14). In addition, the indirect effects of paid leave on infant EEG power through maternal cortisol levels were not significant (ps > .65).
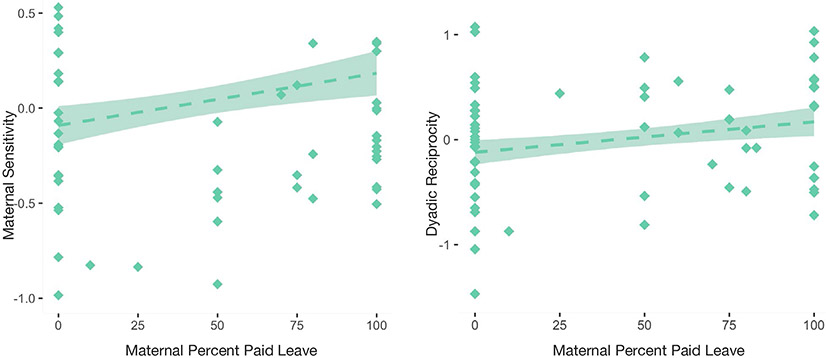
We then examined associations between paid leave and parent–child interactions. Controlling for ITN, maternal education, occupational prestige, infant age, infant sex, and maternal cortisol, both higher levels of sensitivity (β = .27, p = .03) and reciprocity (β = .24, p = .06) were associated with a greater percent paid leave (Figure 1). The extent that interactions were parent-led was also associated with lower percent paid leave (β = −.40, p = .002). There were no associations between PCI behaviors and infant EEG power (ps > .07), nor any interactions between paid leave and PCI behaviors on EEG power values (ps > .13). In addition, there were no significant indirect effects of paid leave on infant EEG power through parent–child interactions (ps > .54).
FIGURE 1.
Associations between percentage of income provided during leave and mother–infant interaction quality
Exploratory analyses examining profiles of brain function in relation to mother–infant interactions and paid leave
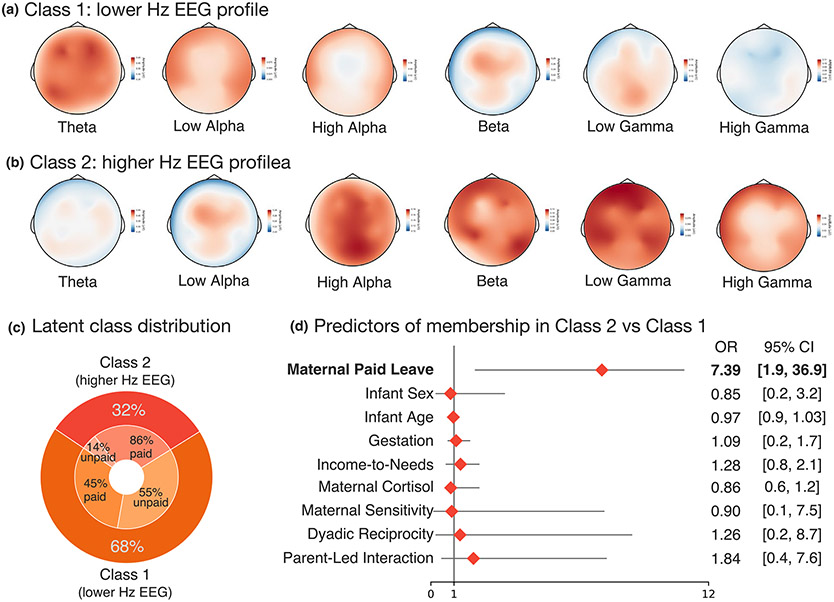
Finally, we used LPA to isolate unique profiles of whole-brain infant EEG patterns to further explore maternal characteristics that are associated with global differences in brain function. Mplus version 8.1 was used to fit latent profile models to infant EEG power across all frequency bands. Each LPA was initialized 2000 times, with 100 iterations for the final stage of optimization. A2-class model was selected as the best fit, based on the Bayesian information criterion (BIC = 986.62), a high entropy value (.89, indicating low classification error), and high posterior probabilities (indicating high confidence that an individual assigned to a given profile actually belongs to that profile; class 1 = .98, class 2 = .96).
The first latent class accounted for 68% of the sample (n = 41) and the second accounted for 32% (n = 19; Figure 2). Class 1 was characterized by relatively higher EEG power across lower frequencies (theta; 4–9 Hz) and relatively reduced EEG power across higher frequencies (9–45 Hz). In contrast, Class 2 was characterized by relatively reduced power across low-frequency bands (4–9 Hz) and relatively increased power across high-frequency bands (9–45 Hz). Based on most likely class membership, 86% of infants in class 2 had mothers with paid leave and 14% had unpaid leave, whereas 45% of infants in class 1 had paid leave while 55% had unpaid leave.
FIGURE 2.
Two profile model of electroencephalography (EEG) relative activation patterns. (a) Class 1 had higher relative EEG power across low frequency bands (theta). (b) Class 2 was characterized by increased relative EEG power across higher frequency bands, a profile typically reflecting greater maturational development. (c) Classification of infants based on most-likely profile membership. (d) Mothers with paid leave were over 7 times as likely to have infants classified in the higher Hz EEG profile. No other variables predicted profile membership
We then examined predictors of EEG class membership using the three-step procedure to account for measurement error associated with most-likely class membership (Asparouhov & Muthén, 2014). The full results are shown in Figure 2d. Notably, paid leave was the only significant predictor of class membership (p = .01), with infants in the higher Hz EEG class being over 7 times more likely to have mothers with paid leave relative to infants in the lower Hz EEG. Infant age, infant sex, gestational age at birth, ITN, maternal sensitivity, and mother–infant reciprocity were not significant predictors of latent profile membership (ps > .14).
These results are consistent with the primary analyses and indicate that widespread differences in infant relative EEG profiles are predicted by experiences of paid leave, over and above the effects of sociodemographic and mother–infant characteristics. However, paid and unpaid leave may likely contribute to infant EEG patterns through a variety of potential pathways. In addition, paid leave is likely associated with a number of confounding characteristics, over and beyond the sociodemographic factors that we controlled for in all analyses. To further probe potential explanations and control for confounding variables, we conducted exploratory post hoc multiple linear regressions directly comparing infants of mothers with paid leave who were classified into the lower Hz EEG profile (paid/low Hz group), infants of mothers without paid leave who were classified into the lower Hz EEG profile (unpaid/low Hz group), and infants in the higher Hz EEG profile (high Hz group). These direct comparisons afford a more precise examination of the proximal variables that may contribute to brain development in infants of mothers with paid or unpaid leave.
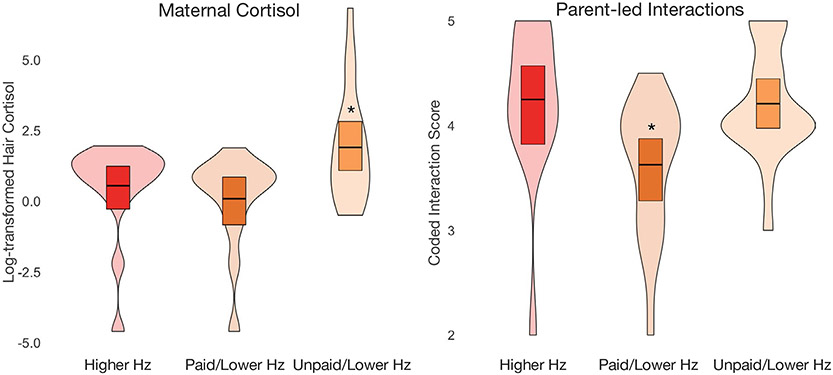
Results indicated significant differences between groups in levels of maternal cortisol and the extent of parent-led interactions (Figure 3). Notably, the unpaid/low Hz group showed higher maternal cortisol levels than both the paid/low Hz group (β = −.38, p = .02) and the higher Hz group (β = −.31, p = .05), specifically controlling for ITN. There were no differences in maternal cortisol levels between the high Hz and paid/low Hz groups (p = .60). Dyads in the paid/low Hz group also exhibited lower levels of parent-led interactions than dyads in both the higher Hz group (β = −.39, p = .04) and unpaid/low Hz group (β = −.50, p < .01), specifically controlling for ITN, maternal cortisol, infant age, and infant sex. Dyads in the unpaid/low Hz group did not differ from dyads in the higher Hz group (p = .78). There were no differences in maternal sensitivity or dyadic reciprocity between groups (ps > .07). Taken together, these results suggest that elevated maternal physiological stress specifically associated with unpaid leave, and not simply SES, may have impacts on infant neural functioning that are observable as early as 3 months of age.
FIGURE 3.
Within the low Hz electroencephalography (EEG) profile, mothers with unpaid leave had significantly greater cortisol levels than mothers of infants in the low Hz paid leave profile and the higher Hz profile. In addition, dyads in the paid leave/low Hz EEG profile showed significantly lower levels of parent-led interactions. Cross bars represent group means with 95% bootstrapped confidence intervals
DISCUSSION
A recent systematic review concluded that paid leave results in improved maternal and child health, and that experiences of unpaid leave do not have the same effect on economic or health indicators (Nandi et al., 2018). Results from the current study extend previous findings into the domain of infant brain function and suggest associations between experiences of paid leave and patterns of EEG activation that may potentially reflect a more mature profile of brain function. This financially stable, job-protected time may increase the period of connection needed for new dyads to foster sensitive, reciprocal interactions (Rossin-Slater et al., 2013), and could reduce the stress and worry over finances that may potentially hinder these experiences (McKelvey et al., 2002).
One experience, multiple pathways
There are many possible pathways through which paid time off after childbirth could be beneficial for early brain development and child outcomes. First, this financially supported time off may substantially decrease maternal tension resulting from reduced income or the pressure to return to work. Mothers often cope with the many stressors of returning to work before feeling physically or mentally ready, hassles of needing to find childcare, or juggling the countless challenges of both working and caring for a newborn, including but not limited to demands associated with breastfeeding and exhaustion from sleep deprivation. When given the opportunity, mothers who take paid leave are more likely to spend time at home with their newborns (Rossin-Slater et al., 2013), allowing for a consistent and predictable home environment where the mother–infant dyad can develop routines and practice learning cues from each other through repeated interactions (Zigler & Hall, 2000). Within the first few months of life, these sensitive and contingent behavioral responses form the basis of vital developmental capacities related to regulation and attachment.
Our main finding indicated that, compared to infants whose mothers had unpaid leave, infants whose mothers took paid leave after giving birth were more likely to have increased EEG power across higher frequency bands, and reduced EEG power across lower frequency bands. This profile of early brain function has been demonstrated in previous studies as reflecting a more mature pattern of brain activation during childhood (Corning et al., 1982; Thatcher et al., 2008). Surprisingly, associations between paid leave and infant EEG remained significant even after accounting for factors like family income and parent–child interactions, which have previously been shown to predict early brain development (Brito & Noble, 2014; Hanson et al., 2013). Within exploratory analyses, both maternal physiological stress and parent–child interactions were associated with experiences of paid leave. When examining variation in paid leave status within distinct profiles of EEG, we observed differences in both maternal physiological stress and dyadic interactions, independent of SES. Thus, it may be that this distal experience of paid leave, or lack thereof, may act upon infant brain development through multiple overlapping pathways that may take into account individualized family interactions and stressors.
Limitations and conclusions
While the present study is the first to examine if experiences of maternal paid leave are associated with differences in early brain function in a relatively sociodemographically diverse sample of families, causal inferences cannot be drawn from the study, but rather results contribute to the nonexperimental literature on child outcomes of paid leave. Results also need to be replicated with a larger sample size. We calculated the robustness of the results to omitted-variables bias, and the large majority of associations were robust to consensus thresholds, with the exception of the results pertaining to the low gamma (21–30 Hz) band. There are other limitations and considerations to also keep in mind. First, we focus on the experiences of the mother and do not include information about support from secondary caregivers or extended family members. More information about the role of specific household (e.g., wage-replacement during leave, government assistance) or family (e.g., childcare, emotional support, availability of paid/unpaid leave by partners) resources, as well as qualitative data on mothers’ decisions regarding type and length of leave, are needed in future studies to better understand the context in which paid leave may be most beneficial. Second, although results indicate that paid leave is a robust predictor of early brain function, it is important to note that these brain activation profiles are not diagnostic in any way and do not necessarily reflect atypical or deficient neural development. The first few years of life are an important period of neural plasticity and the brain is shaped to reflect the child's ongoing experiences through a path-dependent process. Instead of characterizing these differential patterns of EEG activation as “good” or “poor” brain development, these differences likely illustrate adjustments to unique early environmental contexts, which may or may not be adaptive as the child's unique environmental context continually changes as they grow and develop (Werchan & Amso, 2017). The role adaptation to the early environment may have on brain development and subsequent outcomes needs to be examined by incorporating longitudinal behavioral outcomes that could be associated with infant's adaptive processing.
There is an abundance of evidence across an array of fields (e.g., economics, public health, and now neuroscience) suggesting that increasing access to paid leave is related to a wide range of benefits for both mother and child. Nonetheless, there is still resistance to this globally supported public policy within legislatures across the United States. Without a national policy of paid leave, inequities created by existing family leave policies and societal norms will continue to escalate—contributing to even greater disparities in maternal and infant outcomes, particularly for families of color. Black mothers, single mothers, and mothers with lower levels of educational attainment have been reported to have significantly less access to both paid and unpaid leave than the national average (Houser & Vartanian, 2012).
Paid leave may aid in mitigating maternal and infant mortality and hospitalization rates (Berger & Waldfogel, 2004; Jou et al., 2018), incidences of maternal mental health diagnoses (Mandal, 2018), and disparities in child developmental outcomes (Kozak et al., 2021). If paid leave is ultimately implemented as federal policy in the United States, eligibility criteria for this leave need to be lenient so that the majority of the population can qualify, particularly the most under-resourced families, with wage replacement benefits high enough that households can maintain economic stability. As a policy intervention, paid leave may permit mothers to physically and mentally recover from the many perinatal and postpartum challenges faced, as well as provide the opportunity to spend quality time with their new infant, potentially leading to reductions in sociodemographic health disparities and setting the stage for healthy neurocognitive development.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to all the families who participated in the Stress, Home Environment, Language and Learning (SHELL) study and to Karina Kozak, Alejandra Lemus, Stephen Braren, Sarah Vogel, and Kaia Rastinehad for their invaluable help in collecting data. This publication was supported by NICHD grant (R00HD086255) and the New York University Research Challenge Fund Program awarded to NHB.
Abbreviations:
- BIC
Bayesian information criterion
- CFA
Confirmatory factor analysis
- CI
confidence interval
- CIB
Coding Interactive Behavior
- EEG
electroencephalography
- FIML
full information maximum likelihood
- HAPPE
Harvard Automated Processing Pipeline for EEG
- HCC
hair cortisol concentration
- ITN
income-to-need
- LPA
latent profile analysis
- PSS
Perceived Stress Scale
- SES
socioeconomic status
- TDI
temporary disability insurance
Footnotes
CONFLICT OF INTEREST
The authors declare no competing interests.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
REFERENCES
- ACOG. (2018). Committee opinion no. 736: Optimizing postpartum care. Obstetrics and Gynecology, 131(5), e140–e150. 10.1097/AOG.0000000000002633 [DOI] [PubMed] [Google Scholar]
- Albagli P, & Rau T (2018). The effects of a maternity leave reform on children’s abilities and maternal outcomes in Chile. The Economic Journal, 129, 1015–1047. [Google Scholar]
- Asparouhov T, & Muthén B (2014). Auxiliary variables in mixture modeling: Three-step approaches using Mplus. Structural Equation Modeling: A Multidisciplinary Journal, 21, 329–341. [Google Scholar]
- Baker M, & Milligan K (2010). Evidence from maternity leave expansions of the impact of maternal care on early child development. The Journal of Human Resources, 45, 1–32. [Google Scholar]
- Beebe B, & Steele M (2013). How does microanalysis of mother–infant communication inform maternal sensitivity and infant attachment? Attachment & Human Development, 15, 583–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MA (1998). The ontogeny of the EEG during infancy and childhood: Implications for cognitive development. In Garreau B (Ed.), Neuroimaging in child neuropsychiatric disorders (pp. 97–111). Springer. 10.1007/978-3-642-95848-9_9 [DOI] [Google Scholar]
- Berger LM, & Waldfogel J (2004). Maternity leave and the employment of new mothers in the United States. Journal of Population Economics, 17, 331–349. [Google Scholar]
- Bernier A, Calkins SD, & Bell MA (2016). Longitudinal associations between the quality of mother-infant interactions and brain development across infancy. Child Development, 87, 1159–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigelow AE, MacLean K, Proctor J, Myatt T, Gillis R, & Power M (2010). Maternal sensitivity throughout infancy: Continuity and relation to attachment security. Infant Behavior and Development, 33, 50–60. [DOI] [PubMed] [Google Scholar]
- Brainerd J (2017). Paid family leave in the states. Legis Brief, 25, 31. [Google Scholar]
- Brito NH, Elliott AJ, Isler JR, Rodriguez C, Friedrich C, Shuffrey LC, & Fifer WP (2019). Neonatal EEG linked to individual differences in socioemotional outcomes and autism risk in toddlers. Developmental Psychobiology. 10.1002/dev.21870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito NH, Fifer WP, Myers MM, Elliott AJ, & Noble KG (2016). Associations among family socioeconomic status, EEG power at birth, and cognitive skills during infancy. Developmental Cognitive Neuroscience, 19, 144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito NH, & Noble KG (2014). Socioeconomic status and structural brain development. Frontiers in Neuroscience, 8, 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau of Labor Statistics, U.S. Department of Labor. (2021). Family leave factsheet. https://www.bls.gov/ncs/ebs/factsheet/family-leave-benefits-fact-sheet.htm
- Burtle A, & Bezruchka S (2016). Population health and paid parental leave: What the United States can learn from two decades of research. Healthcare, 4(2), 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R, Hyde JS, Essex MJ, & Klein MH (1997). Length of maternity leave and quality of mother-infant interactions. Child Development, 68, 364–383. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, & Mermelstein R (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24, 385–396. [PubMed] [Google Scholar]
- Corning WC, Steffy RA, Anderson E, & Bowers P (1986). EEG “maturational lag” profiles: Follow-up analyses. Journal of Abnormal Child Psychology, 14, 235–249. [DOI] [PubMed] [Google Scholar]
- Corning WC, Steffy RA, & Chaprin IC (1982). EEG slow frequency and WISC-R correlates. Journal of Abnormal Child Psychology, 10, 511–530. [DOI] [PubMed] [Google Scholar]
- Dearing E, & Zachrisson HD (2019). Taking selection seriously in correlational studies of child development: A call for sensitivity analyses. Child Development Perspectives, 13, 267–273. [Google Scholar]
- Feldman R (1998). Coding Interactive Behavior (CIB): Version 4. Bar-Ilan University. [Google Scholar]
- Feldman R (2007). Parent-infant synchrony and the construction of shared timing; physiological precursors, developmental outcomes, and risk conditions. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 48, 329–354. [DOI] [PubMed] [Google Scholar]
- Feldman R, & Eidelman AI (2009). Biological and environmental initial conditions shape the trajectories of cognitive and social-emotional development across the first years of life. Developmental Science, 12, 194–200. [DOI] [PubMed] [Google Scholar]
- Feldman R, Sussman AL, & Zigler E (2004). Parental leave and work adaptation at the transition to parenthood: Individual, marital, and social correlates. Journal of Applied Developmental Psychology, 25, 459–479. [Google Scholar]
- Flom M, St. John AM, Meyer JS, & Tarullo AR (2017). Infant hair cortisol: Associations with salivary cortisol and environmental context. Developmental Psychobiology, 59, 26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabard-Durnam LJ, Mendez Leal AS, Wilkinson CL, & Levin AR (2018). The Harvard Automated Processing Pipeline for Electroencephalography (HAPPE): Standardized processing software for developmental and high-artifact data. Frontiers in Neuroscience, 12, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Hair N, Shen DG, Shi F, Gilmore JH, Wolfe BL, & Pollak SD (2013). Family poverty affects the rate of human infant brain growth. PLoS One, 8, e80954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser L, & Vartanian TP (2012). Pay matters. Report of the Center for Women and Work, New Brunswick, NJ. www.thestranger.com/images/blogimages/2016/08/10/1470864540-cww_paid_leave_brief_jan_2012.pdf [Google Scholar]
- Hwang W, & Jung E (2016). Does paid maternity leave affect infant development and second-birth intentions? Family Relations, 65, 562–575. [Google Scholar]
- John ER, Ahn H, Prichep L, Trepetin M, Brown D, & Kaye H (1980). Development equations for the EEG. Science, 210, 1255–1258. [DOI] [PubMed] [Google Scholar]
- Jou J, Kozhimannil KB, Abraham JM, Blewett LA, & McGovern PM (2018). Paid maternity leave in the United States: Associations with maternal and infant health. Maternal and Child Health Journal, 22, 216–225. [DOI] [PubMed] [Google Scholar]
- Kaiser Family Foundation. (2020). Paid family and sick leave in the U.S. www.kff.org/womens-health-policy/fact-sheet/paid-family-leave-and-sick-days-in-the-u-s/
- Khan MS (2020). Paid family leave and children health outcomes in OECD countries. Children and Youth Services Review, 116, 105259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klerman JA, Daley K, & Pozniak A (2012). Family and medical leave in 2012: Technical report. Abt Associates Inc. [Google Scholar]
- Knudsen EI (2004). Sensitive periods in the development of the brain and behavior. Journal of Cognitive Neuroscience, 16, 1412–1425. [DOI] [PubMed] [Google Scholar]
- Kozak K, Greaves A, Waldfogel J, Angal J, Elliott AJ, Fifier WP, & Brito NH (2021). Paid maternal leave is associated with better language and socioemotional outcomes during toddlerhood. Infancy, 26, 536–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin AR, Méndez Leal AS, Gabard-Durnam LJ, & O’Leary HM (2018). BEAPP: The batch electroencephalography automated processing platform. Frontiers in Neuroscience, 12, 513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman-Sadot S, & Bell NP (2017). Child health in elementary school following California's paid family leave program. Journal of Policy Analysis and Management, 36, 790–827. [DOI] [PubMed] [Google Scholar]
- Lutz KF, Burnson C, Hane A, Samuelson A, Maleck S, & Poehlmann J (2012). Parenting stress, social support, and mother-child interactions in families of multiple and singleton preterm toddlers. Family Relations, 61, 642–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire MJ, & Schneider JM (2019). Socioeconomic status related differences in resting state EEG activity correspond to differences in vocabulary and working memory in grade school. Brain and Cognition, 137, 103619. [DOI] [PubMed] [Google Scholar]
- Mandal B (2018). The effect of paid leave on maternal mental health. Maternal and Child Health Journal, 22, 1470–1476. [DOI] [PubMed] [Google Scholar]
- Marshall PJ, Fox NA; Bucharest Early Intervention Project Core Group. (2004). A comparison of the electroencephalogram between institutionalized and community children in Romania. Journal of Cognitive Neuroscience, 16, 1327–1338. [DOI] [PubMed] [Google Scholar]
- McCarton CM, Wallace IF, Divon M, & Vaughan HG Jr. (1996). Cognitive and neurologic development of the premature, small for gestational age infant through age 6: Comparison by birth weight and gestational age. Pediatrics, 98, 1167–1178. [PubMed] [Google Scholar]
- McKelvey LM, Fitzgerald HE, Schiffman RF, & Von Eye A (2002). Family stress and parent-infant interaction: The mediating role of coping. Infant Mental Health Journal, 23, 164–181. [Google Scholar]
- Nandi A, Jahagirdar D, Dimitris MC, Labrecque JA, Strumpf EC, Kaufman JS, Vincent I, Atabay E, Harper S, Earle A, & Heymann SJ (2018). The impact of parental and medical leave policies on socioeconomic and health outcomes in OECD countries: A systematic review of the empirical literature. The Milbank Quarterly, 96, 434–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office of Governor Cuomo. (2019, August 13). New York state paid family leave: 2018 Year in review. Retrieved January 7, 2022, from https://www.governor.ny.gov/sites/governor.ny.gov/files/atoms/files/PFL_EOYReport_2018_FINAL.pdf
- Oster E (2019). Unobservable selection and coefficient stability: Theory and evidence. Journal of Business & Economic Statistics, 37, 187–204. [Google Scholar]
- O'Sullivan A, & Monk C (2020). Maternal and environmental influences on perinatal and infant development. Future of Children, 30, 11–34. [Google Scholar]
- Pierce LJ, Thompson BL, Gharib A, Schlueter L, Reilly E, Valdes V, Roberts S, Conroy K, Levitt P, & Nelson CA (2019). Association of perceived maternal stress during the perinatal period with electroencephalography patterns in 2-month-old infants. JAMA Pediatrics, 173, 561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotka R, & Busch-Rossnagel NA (2018). The role of length of maternity leave in supporting mother–child interactions and attachment security among American mothers and their infants. International Journal of Child Care and Education Policy, 12, 1–18. [Google Scholar]
- Prior M, Smart D, Sanson A, & Oberklaid F (1993). Sex differences in psychological adjustment from infancy to 8 years. Journal of the American Academy of Child and Adolescent Psychiatry, 32, 291–304; discussion 305. [DOI] [PubMed] [Google Scholar]
- Raikes HA, Robinson JL, Bradley RH, Raikes HH, & Ayoub CC (2007). Developmental trends in self-regulation among low-income toddlers. Social Development, 16, 128–149. [Google Scholar]
- Rossin-Slater M, Ruhm CJ, & Waldfogel J (2013). The effects of California's paid family leave program on mothers’ leave-taking and subsequent labor market outcomes. Journal of Policy Analysis and Management, 32, 224–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossin-Slater M, & Uniat L (2019). Paid family leave policies and population health. In Health affairs health policy brief. https://www.healthaffairs.org/do/10.1377/hpb20190301.484936/full/?utm_source=the%20longmont%20leader&utm_campaign=the%20longmont%20leader&utm_medium=referral [Google Scholar]
- Saxbe D, Rossin-Slater M, & Goldenberg D (2018). The transition to parenthood as a critical window for adult health. The American Psychologist, 73, 1190–1200. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, & Phillips DA (Eds.). (2000a). Nurturing relationships. National Academies Press. [PubMed] [Google Scholar]
- Shonkoff JP, & Phillips DA (Eds.). (2000b). The developing brain. National Academies Press. [PubMed] [Google Scholar]
- Slopen M (2020). Type and lengths of family leave among New York city women: Exploring the composition of paid and unpaid leave. Maternal and Child Health Journal, 24, 514–523. [DOI] [PubMed] [Google Scholar]
- St. John AM, Kao K, Liederman J, Grieve PG, & Tarullo AR (2017). Maternal cortisol slope at 6 months predicts infant cortisol slope and EEG power at 12 months. Developmental Psychobiology, 59, 787–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarullo AR, John AMS, & Meyer JS (2017). Chronic stress in the mother-infant dyad: Maternal hair cortisol, infant salivary cortisol and interactional synchrony. Infant Behavior and Development, 47, 92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatcher RW, North DM, & Biver CJ (2008). Development of cortical connections as measured by EEG coherence and phase delays. Human Brain Mapping, 29, 1400–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomalski P, Moore DG, Ribeiro H, Axelsson EL, Murphy E, Karmiloff-Smith A, Johnson MH, & Kushnerenko E (2013). Socioeconomic status and functional brain development–associations in early infancy. Developmental Science, 16, 676–687. [DOI] [PubMed] [Google Scholar]
- Troller-Renfree SV, Brito NH, Desai PM, Leon-Santos AG, Wiltshire CA, Motton SN, Meyer JS, Isler J, Fifer WP, & Noble KG (2020). Infants of mothers with higher physiological stress show alterations in brain function. Developmental Science, 23, e12976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Niel MS, Bhatia R, Riano NS, de Faria L, Catapano-Friedman L, Ravven S, Weissman B, Nzoodom C, Alexander A, Budde K, & Mangurian C (2020). The impact of paid maternity leave on the mental and physical health of mothers and children: A review of the literature and policy implications. Harvard Review of Psychiatry, 28, 113–126. [DOI] [PubMed] [Google Scholar]
- Werchan DM, & Amso D (2017). A novel ecological account of prefrontal cortex functional development. Psychological Review, 124, 720–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigler EF, & Hall NW (2000). Child development and social policy: Theory and applications. Series in developmental psychology. McGraw-Hill. https://psycnet.apa.org/fulltext/1999-04005-000.pdf [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.