Abstract
Cyanobacteria are ecologically important photosynthetic prokaryotes that also serve as popular model organisms for studies of photosynthesis and gene regulation. Both molecular and ecological studies of cyanobacteria benefit from real-time information on photosynthesis and acclimation. Monitoring in vivo chlorophyll fluorescence can provide noninvasive measures of photosynthetic physiology in a wide range of cyanobacteria and cyanolichens and requires only small samples. Cyanobacterial fluorescence patterns are distinct from those of plants, because of key structural and functional properties of cyanobacteria. These include significant fluorescence emission from the light-harvesting phycobiliproteins; large and rapid changes in fluorescence yield (state transitions) which depend on metabolic and environmental conditions; and flexible, overlapping respiratory and photosynthetic electron transport chains. The fluorescence parameters FV/FM, FV′/FM′,qp,qN, NPQ, and φPS II were originally developed to extract information from the fluorescence signals of higher plants. In this review, we consider how the special properties of cyanobacteria can be accommodated and used to extract biologically useful information from cyanobacterial in vivo chlorophyll fluorescence signals. We describe how the pattern of fluorescence yield versus light intensity can be used to predict the acclimated light level for a cyanobacterial population, giving information valuable for both laboratory and field studies of acclimation processes. The size of the change in fluorescence yield during dark-to-light transitions can provide information on respiration and the iron status of the cyanobacteria. Finally, fluorescence parameters can be used to estimate the electron transport rate at the acclimated growth light intensity.
Principles of Modulated Fluorescence Analysis
Chlorophyll fluorescence analysis allows noninvasive, near-instantaneous measurement of key aspects of photosynthetic light capture and electron transport. For natural samples, fluorescence signals are specific to photobionts and allow in situ measurements of small (61) or dilute (65, 130) mixed natural populations. For molecular studies, fluorescence signals can be used for rapid screening of mutant or transgenic colonies and cultures and for tracking physiological processes during gene regulation experiments. Rapid screening has become increasingly important with the advent of genomic sequencing and saturation mutagenesis. Therefore, applications of chlorophyll fluorescence are expanding in both field and laboratory settings.
In cyanobacteria, the photosynthetic system is tightly connected to the other principal metabolic paths and is in itself a major metabolic sink for iron, nitrogen, and carbon skeletons. Therefore, chlorophyll fluorescence signals can provide rapid, real-time information on both photosynthesis and the overall acclimation status of cyanobacteria. We and other groups have been adapting to cyanobacteria techniques of in vivo fluorescence analysis originally developed for plants (5, 20–28, 58, 61, 69, 74, 80, 87–91, 112, 116, 128, 133, 138, 141, 142, 150).
Fluorescence analysis depends on the phenomenon that when a pigment absorbs the energy of a photon and enters an excited electronic state, there are essentially four routes for the return to ground state: (i) photochemical reactions in which the excited electron leaves the pigment molecule and enters an electron transport chain, as occur in specific chlorophylls in photosynthetic reaction centers; (ii) heat dissipation, in which the excited electron returns to ground state by releasing heat; (iii) transfer of the excitation energy to an adjacent pigment, as occurs in the light-harvesting antenna systems of photosynthetic organisms; and (iv) emission of a fluorescence photon, of a wavelength longer than that of the photon initially absorbed. These four processes are in competition, and for a given excited molecule, the path with the largest first-order rate constant predominates. For biological systems, the overall chlorophyll fluorescence yield is usually low, and in vivo chlorophyll fluorescence from photosystem II (PS II) predominates (38, 66, 112). In cyanobacteria, phycobiliproteins also contribute fluorescence, which overlaps with the spectrum of chlorophyll emission.
Although PS II fluorescence is a minor pathway for excitation dissipation, it competes with the quantitatively more important energy dissipation routes of PS II photochemistry, exciton transfer to other pigment systems (such as PS I), and heat dissipation. Therefore, changes in photochemistry or in the two nonphotochemical routes (energy transfer and heat emission) cause changes in the fluorescence yield from PS II (13, 66, 112). When the potentials for photochemistry and nonphotochemical dissipation are minimal, the fluorescence yield is maximal. Quenching or lowering of the fluorescence yield below its maximum occurs when excitation flow increases to the competing photochemical or nonphotochemical pathways.
To deduce information on photosynthesis from analysis of fluorescence quenching, one assumes that changes in fluorescence yield reflect proportional changes in the competing deexcitation pathways of photochemistry, exciton transfer, and heat dissipation. This basic assumption is not strictly valid (53, 56, 144). Nevertheless, the fluorescence signal is rich in information, and in plants the parameters FV/FM, FV′/FM′, qP, qN, NPQ, and φPS II are empirically verifiable indices of photosynthetic performance and acclimation status (12, 36, 45, 66, 67, 82, 99, 102–104, 106, 107, 127, 129, 134, 145, 153).
For any pigment, the level of fluorescence emission depends on the pigment concentration, the excitation light intensity, and the fluorescence yield or efficiency of fluorescence emission. For fluorescence quenching analysis, the excitation intensity and pigment concentration must be constant, so that changes in fluorescence reflect the changes in fluorescence yield which result from the competing photochemical and nonphotochemical deexcitation pathways. Changes in pigment concentration are generally not a concern over the brief periods of fluorescence measurements.
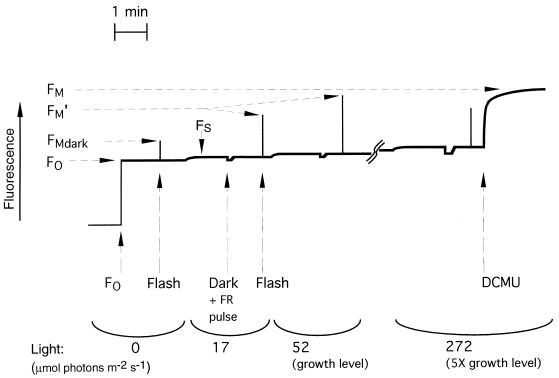
Modulated fluorometers are currently widely used to measure in vivo chlorophyll fluorescence from plants and increasingly from cyanobacteria in both the laboratory and the field (128, 130). This review concentrates on data obtained with modulated fluorometers, although other approaches are also used (40). Modulated fluorometers specifically detect and amplify only the fluorescence excited by a weak, constant measuring beam consisting of a train of low light pulses at a frequency of 1 to 100 kHz. Therefore, the excitation intensity is constant and changes in the fluorometer signal reflect changes in fluorescence yield. The modulated measuring beam is sufficiently weak that it drives essentially no photosynthesis, allowing determination of the fluorescence yield of dark-adapted samples. Furthermore, since the detection system ignores fluorescence excited by other light, it is possible to change the actinic light and provide multiple saturating pulses of light over the course of one measurement (Table 1; see Fig. 2). The fluorescence yield can therefore be measured under different levels of actinic light, and saturating flashes can be used at any point to momentarily close all PS II centers and drive photochemical quenching to zero (see Fig. 2).
TABLE 1.
Fluorescence levels and associated light treatments used for cyanobacterial fluorescence quenching analysisa
| Fluorescence level | Light treatment (weak modulated measuring beam throughout) |
|---|---|
| FO | Dark |
| FMdark′ | Dark + saturating flash |
| FS | Actinic light |
| FO′ | Dark or weak far-red |
| FM′ | Actinic light + saturating flash |
| FM | Actinic light + DCMU |
For further details see Appendix.
FIG. 2.
Fluorescence emission trace for cyanobacterial quenching analysis. This trace from Synechococcus sp. strain PCC 7942 shows a typical cyanobacterial response over a series of increasing light intensities. The brief pulses of saturating light result in a rapid increase in fluorescence as PS II centers close transiently. The measurement terminates with addition of DCMU, which closes PS II centers, causing a rapid rise in fluorescence followed by a slower fluorescence rise phase as the cells go to full state I. Modified from reference 23 with permission of the publisher.
Goals and Scope
In this review, we discuss pulse-amplitude modulated fluorescence as a rapid, noninvasive monitor of acclimation and photosynthesis in cyanobacteria and cyanolichens. We do not cover the biophysical mechanisms underlying chlorophyll fluorescence emission, which are well reviewed elsewhere (31, 38, 66, 112, 127, 137, 141, 144). Rather, we summarize some of the potentials and limitations of fluorescence analysis for extracting physiologically and ecologically useful information from cyanobacteria, whose photosynthetic physiology (see Fig. 1) and fluorescence patterns (see Fig. 2) differ in important respects from those of plants (20, 22, 91, 112, 128, 141). In particular, we demonstrate how characteristic changes in nonphotochemical quenching of fluorescence can be used to estimate the light level to which the sample is acclimated. This information can then be used in conjunction with the φPS II parameter to estimate electron transport under acclimated conditions.
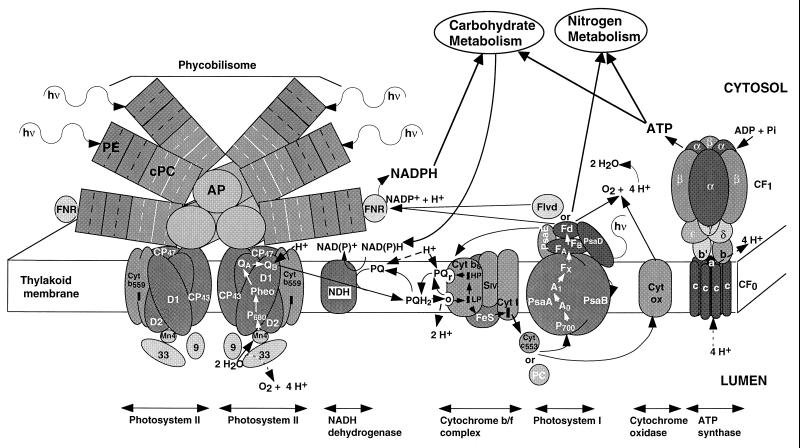
FIG. 1.
Cyanobacterial thylakoid electron transport. This schematic diagram is based on data primarily from Synechocystis sp. strain PCC 6701, which contains phycoerythrin, phycocyanin, and allophycocyanin pigment proteins in the phycobilisomes. The phycobilisomes move rapidly along the surface of the thylakoid membranes (96), so the phycobilisome-PS II dimer complex is transient. Excitation absorbed by the phycobilisome can reach either PS II or PS I, particularly in cells in state II. This excitation flow may involve specialized subunits of the phycobilisome core (not shown here). The composition and organization of the phycobilisome rods and core is variable in different cyanobacteria; the three-cylinder core and six-peripheral rod configuration is common, but in Synechococcus sp. strain PCC 7942 the core contains only two cylinders. The PS I-PS II stoichiometry is usually higher than 1:1; in the Synechococcus cells used for most experiments described in later figures, the ratio was 2 to 3 PS I/PS II. There are multiple interacting and flexible paths of electron flow including linear flow from water to NADPH; several possible cyclic pathways around subsections of the transport system; pseudocyclic flows from water with electron donation back to oxygen; and respiratory flows of electrons derived from reserve molecules. Some possible flows are indicated by black arrows. The donor-acceptor stoichiometries of electron transfers are not shown, but various redox centres carry different numbers of electrons, from 1 (e.g., PC, cytochrome c553, Fd, and Flvd), 2 [e.g., PQ and NAD(P)H] and even 4 (the Mn complex of PS II). The redox reactions are reversible depending upon the oxidation-reduction status of the acceptor-donor pair and the local proton concentration, so that in some cases the indicated direction of electron flow could be reversed. Proton uptake and transmembrane transport are indicated by dashed arrows; other proton translocation pathways may also exist. The plastoquinone-plastoquinol pool can be reduced by electrons from PS II, from an NAD(P)H dehydrogenase(s) (NDH) whose composition and substrate specifities vary between strains, and from the “r” site of the cytochrome bf complex. Plastoquinone reduction by NDH is the entry point for electrons derived from respiration. The various plastoquinone reductions involve proton uptake from the stroma, while oxidation of a plastoquinol at the ‘o’ site of the cytochrome bf complex releases two protons to the lumen. As indicated, electron and ATP flow to carbohydrate and nitrogen metabolism can have strong and rapid effects on thylakoid function, while electrons derived from carbohydrate reserves also enter the thylakoid system and influence photosynthetic function. During ATP synthesis, protons enter a channel from the lumen formed by the a subunit of Cf0, and their exit to the cytosol is coupled to rotation of the ring of c subunits through directed diffusion (37). The c-ring rotation drives rotation of the γ subunit of CF1 within the α3β3 ring (117), which in turn drives a sequence of conformational changes in three identical ATP/ADP binding sites. The changes in binding site lead to phosphorylation of ADP and expulsion of ATP from the site. The stoichiometry is 1 ATP/4H+ passing through the complex (146). Abbreviations: hν, photons of visible light; PE, phycoerythrin α3β3 trimers; CPC, phycocyanin α3β3 trimers; AP, allophycocyanin rods of the phycobilisome core, composed partly of α3β3 trimer disks along with other related phycobilin-binding proteins; D1 and D2, core polypeptide dimer of PS II which binds the redox cofactors; Cyt b559, cytochrome b559 in the PS II core; Mn4, manganese cluster of the oxygen evolving complex; 9 and 33, 9- and 33-kDa subunits of the oxygen evolving complex of PS II; CP43 and CP47, 43- and 47-kDa chlorophyll protein complexes associated with the PS II core; P680, dimeric chlorophyll center which is photooxidized in PS II; Pheo, pheophytin primary electron acceptor of PS II; QA, the quinone secondary electron acceptor of PS II; QB, a plastoquinone bound to PS II which accepts two electrons from QA and equilibrates with the thylakoidal plastoquinone-plastoquinol pool; NDH, NAD(P)H dehydrogenase (in various strains there are different forms of the complex with differing activities and specificities for NADH or NADPH); Cyt b6, a cytochrome containing both low- and high-potential heme centers which are involved in a Q-cycle electron flow from plastoquinol bound to the o site to plastoquinone bound to the “r” site (this cycle results in proton translocation); SIV, subunit IV of the cytochrome bf complex; FeS, an iron-sulfur redox center; PC, plastocyanin, a copper-containing luminal single-electron transport protein; Cyt c553, cytochrome c553, a heme-containing luminal single-electron transport protein (plastocyanin and cytochrome c553 can be reciprocally regulated in response to copper and iron availability); PsaA and PsaB, related chlorophyll binding proteins which form the core of PS I; P700, the chlorophyll which is photooxidized in PS I; A0, A1, FX, FA, FB, bound redox intermediates of PS I; Flvd, flavodoxin (a flavin protein which is a cytosolic mobile single electron carrier that can accept electrons from PS I and that can substitute for ferredoxin, particularly under low-iron conditions); Fd, ferredoxin (an iron protein which is a cytosolic mobile single-electron carrier that can accept electrons from PS I and can transfer the electrons to NADPH or participate directly in some biosynthetic reactions, particularly in nitrogen metabolism); FNR, ferredoxin/flavodoxin NADPH oxidoreductase; Cyt ox, the cytochrome oxidase complex involved in respiratory electron transport (it can also withdraw electrons from photosynthetic electron transport, particularly under excess light); α to γ, subunits of the CF1 complex of ATP synthase; a to c, subunits of the CF0 complex of ATP synthase. Modified and redrawn from reference 19 with permission of the publisher.
INTERPRETING CYANOBACTERIAL FLUORESCENCE SIGNALS
A Distinct Photosynthetic System Yields Distinct Fluorescence Signals
The central PS II and PS I photosynthetic complexes are very similar in plants and cyanobacteria, as are many elements of the light capture, electron transport, and carbon dioxide fixation systems. Nevertheless, cyanobacteria are metabolically flexible prokaryotic organisms, with several key structural and metabolic distinctions which strongly influence the nature and interpretation of their fluorescence signals (Fig. 1).
In cyanobacteria, the principal light-harvesting complexes are phycobilisomes peripheral to the thylakoid membranes, rather than the integral membrane chlorophyll-a/b binding proteins which capture light in plants. Cyanobacterial phycobilisomes diffuse along the surface of the thylakoids, at a rate sufficient to allow movement from PS II to PS I within 100 ms (96). This distinction in light capture structures between plants and cyanobacteria has many metabolic and functional consequences (7). In particular, the cellular phycobiliprotein content influences cellular fluorescence yield. Furthermore, cyanobacteria have high and variable ratios between PS I and PS II complexes (98, 112), so that in comparison with plants, PS II accounts for relatively little of the cellular chlorophyll. This can also influence the interpretation of fluorescence signals, since the variable fluorescence component arises from PS II while the constant or F0 fluorescence component contains emissions from PS II, phycobiliproteins, and possibly also PS I chlorophyll (112).
Photosynthetic and respiratory electron flow both occur in cyanobacterial thylakoid membranes (62, 121), sometimes simultaneously, and they share numerous electron transport intermediates (Fig. 1). Under illumination, there is net input of electrons into the transport system from the water-splitting activity of PS II. Under light or dark conditions, there are variable electron fluxes from NAD(P)H, which is oxidized by one or more thylakoid-bound dehydrogenases (11, 54, 85, 86, 121, 139). Electrons from ferredoxin can also enter the transport system, possibly passing via the same complex(es) that catalyze NAD(P)H oxidation (86). In a photoautotroph, electrons derived from NAD(P)H or ferredoxin are clearly not a net input of reductant into the system; rather, they represent some form of cyclic flow, since the electrons used to reduce NAD(P)H or ferredoxin derive ultimately from the water-splitting activity of PS II. Typically, cyclic electron flow is used to describe flow from PS I via ferredoxin and/or NADPH (54, 86) directly back to the intersystem transport chain. This cyclic flow can drive proton translocation through localized reductions of plastoquinone at the cytosolic side of the thylakoid, with concomitant proton uptake, and plastoquinol oxidation near the luminal side, with proton release to the lumen (55) (Fig. 1). More generally, electrons derived from the oxidation of carbohydrates or other reserve molecules can be carried by NAD(P)H into the thylakoid intersystem transport chain via the thylakoid-bound dehydrogenase(s). These electrons from the reserve molecules are derived ultimately from PS II water splitting and, upon reentry to the thylakoid system, can pass to oxygen under light or dark or to PS I (139) under illumination. The reserves thus act as an electron bank so that the flow into the thylakoid system can be offset in time from the original photosynthetic production of reductant, with important regulatory consequences (34, 84, 94).
In all known cases, electrons from these various inputs come together at the cytochrome bf complex, which is a plastoquinol oxidoreductase (68) (Fig. 1). There are two plastoquinone binding sites in the complex, which allow for a Q cycle, in which some of the electrons removed from plastoquinol at the luminal side of the membrane are cycled within the complex and passed back to plastoquinone bound near the cytoplasmic side of the membrane. This branch of the transport chain allows the cyanobacteria to increase the number of protons translocated per net electron passing through the transport chain. Since the reduction involves proton uptake from the cytosol and the oxidation releases protons to the lumen, electron flux through the Q cycle must respond sensitively to the magnitude of the proton gradient across the membrane.
The primary electron flux through the cytochrome bf complex is from plastoquinone to luminal electron carriers, primarily plastocyanin or cytochrome c553 (81, 113), which transport the electrons either to PS I or to a cytochrome oxidase complex which may include cytochrome c(m) (81). Plastocyanin and cytochrome c553 can each fulfill transport roles to PS I or the cytochrome oxidase (81). Double-inactivation mutants mutated in both proteins are inviable in some (81) but not all (155) strains, so that some strains must have an alternate route for electron flow away from cytochrome bf. Although single-inactivation mutants mutated in one or the other protein are viable (26, 71, 81), the loss of one protein can lower the capacity for electron flux away from PS II, particularly under conditions of excess excitation (26). Conversely, overexpression of heterologous plastocyanin in Synechococcus can increase the electron transport capacity (44). Thus, although partially complementary, the two proteins may play somewhat distinct functional roles. Furthermore, plastocyanin contains a copper redox cofactor while cytochrome c553 contains an iron redox cofactor, and in some strains they are differentially regulated in response to copper and iron availability (15, 16, 119, 120, 154). Another example of alternate electron carriers is the iron-sulfur protein ferredoxin, which accepts electrons from PS I but which can be replaced under conditions of iron stress by flavodoxin (70, 75).
The flow of electrons to oxygen as a final acceptor responds rapidly to environmental and metabolic conditions and can be an important element in preventing overreduction of PS II and the intersystem transport chain under excess illumination (22a, 88, 147). This flow to oxygen can be mediated by cytochrome oxidase activity (131) or by photoreduction of oxygen by electrons from PS I, either directly (3, 79) or via ferredoxin (43, 50).
Carbon metabolism and nitrogen metabolism in the prokaryotic cyanobacteria occur in close proximity to the cytosolic surface of the thylakoids and so have strong and direct influences on electron transport and hence on fluorescence (63, 83, 84, 92, 116, 122), both as sinks for ATP and electrons and as sources of electrons extracted from reserve molecules.
In summary, this system forms a web of electron sources and sinks, linked by interconnected redox intermediates, that allows for flexible and rapid shifts in electron fluxes in response to environmental or metabolic changes (5, 54, 55, 74, 80, 81, 85, 86, 131, 135, 147). Furthermore, through shared electron transport carriers, respiration directly influences the photosynthetic regulatory status and vice versa (34, 84, 94). Several of the characteristic properties of cyanobacterial fluorescence signals result from these respiration/photosynthesis interactions, including their distinct patterns of photochemical and nonphotochemical quenching.
In plants, a cycle of conversions of xanthophyll carotenoids is driven by the trans-thylakoid ΔpH gradient and is implicated in regulating nonphotochemical dissipation of excess light energy (1, 46, 47, 57). Cyanobacteria lack this cycle (33) but have alternate strategies to cope with excess excitation (105, 147). Finally, cyanobacteria show changes in the functional organization of the light capture system, termed state transitions, which can result in large changes in the PS II fluorescence yield depending upon the level of illumination (7, 14, 97, 111, 112). In contrast, in higher plants, state transitions have relatively minor influences on PS II fluorescence (66). This review deals now with how the distinct organization and function of cyanobacterial photosynthesis lead to opportunities and limitations for chlorophyll fluorescence analysis of cyanobacteria.
FO, FV/FM, and FO′ in Cyanobacteria versus Plants
Ting and Owens (141) have shown that for any chlorophyll-containing suspension, the values of FO, FM and FV/FM measured with a modulated fluorometer vary somewhat with pigment concentration. Therefore, within a set of experiments, the chlorophyll concentration should be standardized if precision is required or, alternately, a small correction could be introduced to compensate for variation in the chlorophyll concentration. In our experiments, chlorophyll concentrations from 1 to 3 μg/ml gave results sufficiently consistent for quenching analysis. The use of alternate cuvettes and detectors can greatly extend this concentration range (125).
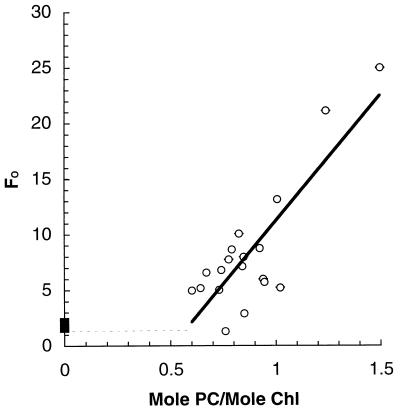
A more fundamental problem with the measurement of FO in cyanobacteria is that FO fluorescence varies considerably depending on the cellular phycobiliprotein concentration. Figure 3 illustrates that as the phycocyanin/chlorophyll ratio of wild-type Synechococcus sp. strain PCC 7942 rises, FO fluorescence also increases, particularly once the phycocyanin content is increased above a threshold level. This phycobiliprotein contribution to FO fluorescence is not influenced by changes in the redox state of PS II (51, 72, 111). It could be a low-yield fluorescence emission from coupled phycobilisomes or a high-yield emission from a small population of uncoupled phycobilisomes (96) or free phycobiliproteins. The exact source(s) of this phycobiliprotein fluorescence could be addressed by studies using cyanobacteria with a range of phycobiliprotein contents analyzed with the range of different color light-emitting diodes now available as modulated fluorescence excitation sources (65). In a Synechococcus sp. strain PCC 7942 mutant lacking phycocyanin, FO is low (Fig. 3), which confirms the FO/phycobiliprotein correlation observed in the wild type. Furthermore, in this mutant strain, FV/FM under acclimated growth is about 0.75 (157), as opposed to values of 0.4 to 0.6, typical of wild-type Synechococcus grown under the same conditions.
FIG. 3.
FO fluorescence increases with the phycocyanin content in Synechococcus sp. strain PCC 7942. FO fluorescence is normalized to the molar chlorophyll concentration to allow for different culture concentrations and plotted against the molar ratio of phycocyanin (PC) to chlorophyll (Chl). Each point is a single determination on an independently grown culture. ○, wild-type cells; ■, mutant strain which lacks phycobilisome rods and contains no phycocyanin. Note that at low phycocyanin contents, FO in the wild type falls toward the level in the rodless mutant. Modified from reference 23 with permission of the publisher.
In plants FV/FM [(FM − FO)/FM] (Fig. 2) is well verified as an index of the maximal photochemical efficiency of PS II (12), but this interpretation depends on both FO and FV originating predominantly from PS II. This assumption is not valid for cyanobacteria (20, 111, 112, 128), since phycobiliprotein fluorescence contributes to FO and PS II accounts for only a small proportion of total chlorophyll. In higher plants under ideal conditions, FV/FM is near 0.8 and lower values reflect inhibition of PS II function (12). In cyanobacteria, changes in FV/FM under conditions of constant pigment content correlate well with changes in independent measurements of PS II function such as oxygen evolution (24, 27, 28, 80), but the absolute level of FV/FM is not a reliable indicator of PS II function. The distortion of FO fluorescence is pronounced only at high cellular concentrations of phycocyanin (Fig. 3), which may be achieved primarily under nutrient-rich artificial culture conditions, as used in our experiments to date. When interpreted with caution, FV/FM is still a useful parameter, particularly if the same sample is monitored repeatedly over time and if the cellular pigment content is constant. As expected, the prochlorophyte Prochloron shows a high value of FV/FM (126), consistent with the lack of phycobilisomes and the chlorophyll-based antenna system in this group of cyanobacterial relatives.
FO′ is the minimal fluorescence level with all PS II reaction centers open. It is measured with cells under a given light acclimation status but transferred briefly to darkness or far-red light. The determination of FO′ is a problematic aspect of quenching analysis in cyanobacteria, since under moderate light intensities FO′ is often very close to the steady-state FS fluorescence level (Fig. 2). Furthermore, unlike in plants, FO′ in cyanobacteria is usually higher than the FO fluorescence as a result of the dark-to-light increase in PS II fluorescence yield, i.e., the state transition (see below). It is therefore difficult to distinguish the initial small but rapid drop in fluorescence yield as PS II centers open, from the slower state transition-dependent decline to the dark FO fluorescence level. Computerized data logging might alleviate this problem by resolving the fluorescence relaxation kinetic phases.
In higher plants, FV′/FM′, defined as (FM′ − FO′)/FM′ (Fig. 2), reflects the photochemical efficiency of open PS II centers under a given light acclimation status (45). FV′/FM′ generally varies inversely with qN (see below), since nonphotochemical energy dissipation lowers the photochemical efficiency of PS II below the maximum levels reflected by FV/FM. A drop in FV/FM, as occurs during photoinhibition of PS II activity, also feeds through and results in a drop in FV′/FM′. Thus, in a plant, changes in the FV′/FM′ parameter reflect the combined regulation of PS II through both reversible nonphotochemical quenching and photoinhibitory inactivation of PS II.
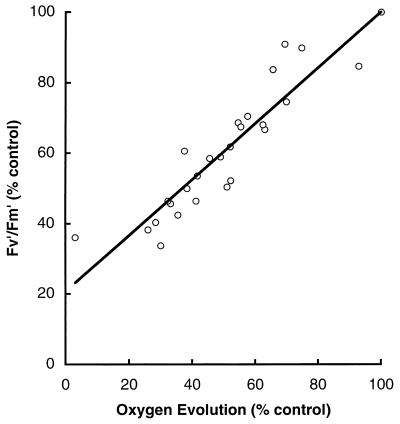
In cyanobacteria, changes in FV′/FM′ also combine nonphotochemical influences on PS II function and photoinhibitory inactivation of PS II. As described below, nonphotochemical quenching of cyanobacterial PS II fluorescence results primarily from changes in excitation distribution between the two photosystems rather than from excitation dissipation. Therefore, in a cyanobacterium, a drop in FV′/FM′ can result from a photoinhibitory drop in FV/FM or from a regulatory redistribution of excitation from PS II to PS I. In plant fluorescence analysis, a common implicit assumption is that down-regulation of PS II reflects overall down-regulation of photosynthetic electron transport. This assumption is not applicable to cyanobacteria, which have more flexible excitation distribution and electron transport systems. Furthermore, cyanobacterial FV′/FM′ suffers the same limitations as described above for FV/FM, which are further compounded by the difficulty of measuring the FO′ fluorescence level. Nevertheless, as shown in Fig. 4, changes in measured FV′/FM′ correlate well with changes in oxygen evolution during a photoinhibitory treatment (24, 27). Cyanobacterial FV′/FM′ is a useful integrated measure of PS II activity, even though various mechanisms may underlie the changes in PS II function.
FIG. 4.
Photoinhibition of oxygen evolution correlates with declines in FV′/FM′ in Synechococcus sp. strain PCC 7942. Cultures were subjected to a photoinhibitory decrease in growth temperature from 37 to 25°C. Oxygen evolution and FV′/FM′ were monitored and expressed as a percentages of the pretreatment control values. Four cultures were used. y = 0.79x + 21; R2 = 0.89.
PHOTOCHEMICAL QUENCHING AND EXCITATION PRESSURE
Photochemical and nonphotochemical quenching measure changes in variable fluorescence, which derives from PS II; they are therefore less susceptible to distortion from non-PS II contributions to FO fluorescence. They involve minimal mechanistic assumptions (145), although the terms qP and qN often carry mechanistic associations which are not applicable to cyanobacteria.
Photochemical quenching reflects a lowering of fluorescence below maximal levels through photochemical competition with fluorescence emission. Thus, when all PS II reaction centers are open and the potential for photochemistry is maximal, photochemical quenching of fluorescence is also maximal and fluorescence yield is low (FO or FO′). Conversely, when all PS II centers are closed and no photochemistry can occur, photochemical quenching is zero and the fluorescence yield is maximal (FM or FM′). In practice, photochemical quenching is quantified by the photochemical quenching coefficient (145):
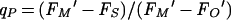 |
This parameter gives the position of steady-state fluorescence, FS, on the scale from FO′ (all PS II centers open) to FM′ (all PS II centers closed) (Fig. 2). Thus, if steady-state fluorescence is equal to FO′, qP = 1, indicating that all PS II centers are open. If steady-state fluorescence equals FM′, qP = 0, indicating that all PS II centers are closed. Between these extremes, progressive reaction center closure is reflected by a declining qP, although the relationship is not strictly linear (20) because of excitation migration from closed to open reaction centers (66) and fluorescence quenching by oxidized plastoquinone (148). The absolute level of FO or FO′ does not distort the calculation, since it is scaled to variable fluorescence, FV′ = FM′ − FO′, under a given condition.
qP reflects the balance between excitation of PS II centers, which closes them, and removal of electrons from PS II by the electron transport chain, which reopens the centers. This balance, or excitation pressure on PS II, responds not only to incident light intensity (27) but also to factors influencing electron flow away from PS II, such as temperature (24, 59, 82, 101, 102, 106, 107) and the availability of terminal electron acceptors such as CO2 or O2 (88, 147). Indeed, the pivotal position of PS II in photosynthetic electron transport means that environmental and metabolic signals are integrated into qP, which is thus a general index of the balance between energy capture and consumption.
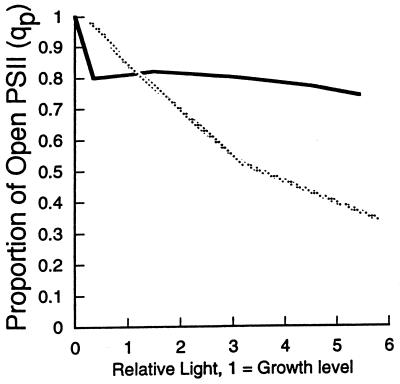
As shown in Fig. 5, in cyanobacteria and cyanolichens, qP typically stays high over a broad range of incident light intensity, up to 10 times higher than the growth light intensity (21, 23, 27, 28, 80, 138). This contrasts sharply with the pattern typical of higher plants, where qP falls progressively as the light intensity exceeds the growth light. This cyanobacterial capacity to maintain PS II centers open under excess light reflects a complex and flexible electron transport system (5, 44, 55, 85, 86, 131, 135), as well as a generally high PS I/PS II ratio (23, 42, 98, 112). In particular, cyanobacteria have a very high and flexible capacity to remove electrons from PS II, with oxygen as a terminal acceptor for electron flow from water (21a, 88, 91, 128, 147). This flow is low under low light, variable but significant at the growth light intensity, and large under excess light (see below). It thus serves to buffer PS II from excess excitation by removing electrons as required. At least part of this flow is sensitive to cyanide inhibition, suggesting a contribution from respiratory electron flow through cytochrome oxidase (131).
FIG. 5.
Photochemical quenching of fluorescence plotted against light intensity. Typical light response curves of photochemical quenching in a cyanobacterium, Synechococcus sp. strain) PCC 7942 (solid line), and in rye for a plant-type pattern (dotted line) are shown. For comparison, light intensity is expressed relative to the growth light intensity; 1 = growth level. The actual growth light intensities were 50 μmol of photons m−2 s−1 for the cyanobacterium and 250 μmol of photons m−2 s−1 for the rye.
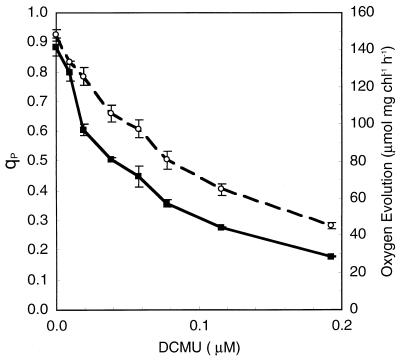
In spite of the different patterns of qP in cyanobacteria and plants, the parameter does successfully measure PS II closure in cyanobacteria, as shown by titration with 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU). This inhibitor binds to the QB binding site of PS II, blocking electron flow and causing reaction center closure. Figure 6 shows that in Synechococcus sp. strain PCC 7942, as expected, qP and oxygen evolution drop in parallel upon progressive DCMU inhibition of PS II. Furthermore, our work on Synechococcus sp. strain PCC 7942 showed that although this strain has a strong capacity to maintain PS II open, even fractional closure of the reaction centers can lead to photoinhibition and large changes in gene expression, indicative of active acclimation processes (24, 27, 28) in response to relatively small drops in the proportion of open reaction centers.
FIG. 6.
Photochemical quenching of fluorescence under DCMU treatment compared with oxygen evolution in Synechococcus sp. strain PCC 7942. DCMU inhibits oxygen evolution (○), closing PS II centers in parallel (qP) (■). Results are means and standard errors of measurements on the same culture at 37°C (n = 3). Modified from reference 24 with permission of the publisher.
PREDICTING LIGHT ACCLIMATION STATUS
State Transitions Dominate Nonphotochemical Quenching of Cyanobacterial Fluorescence
Nonphotochemical quenching reflects any process other than photochemistry which lowers the yield of variable fluorescence. It can be quantified by using the coefficient qN (145):
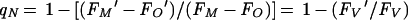 |
qN compares the span of variable fluorescence under a given condition, FV′ = (FM − FO′), with the maximum potential variable fluorescence, FV = (FM − FO) (Fig. 2). In cyanobacteria, variable fluorescence appears to arise essentially from PS II, while as discussed, the FO′ and FO fluorescence levels arise only partly from PS II. The cyanobacterial FO′ and FO signals detected by a modulated fluorometer with a red-modulated light-emitting diode (LED) each contain a contribution from phycocyanin fluorescence, which we believe is fairly constant over the course of a measurement. This underlying background fluorescence is subtracted out as an equivalent component of both FO′ and FO and so does not seriously distort the calculation of qN.
Significant drops in FO fluorescence during illumination can distort qN in higher plants. This FO quenching is quantified as follows (9):
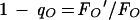 |
A modified calculation of the qN coefficient compensates for qO (9):
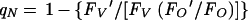 |
In cyanobacteria, FO′ is usually close to or somewhat higher than FO (‘negative’ qO), and both qN expressions give similar results.
An alternate quantification of nonphotochemical fluorescence quenching is as follows (67):
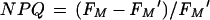 |
This is a Stern-Volmer formulation that measures the ratio of quenched to remaining fluorescence. The absolute value of NPQ does suffer from distortion by the underlying phycobiliprotein fluorescence, which contributes to both FM and FM′. This potential problem may be addressed by fluorescence measurements with alternate modulated light sources, such as a blue LED (65), which allow excitation of chlorophyll fluorescence without interference from phycobiliprotein emissions. The use of NPQ also avoids the problematic measurement of FO′ and is therefore possibly a preferable measure of nonphotochemical quenching in cyanobacteria. Plots of qN and NPQ derived from the same fluorescence traces generally show similar although not identical patterns.
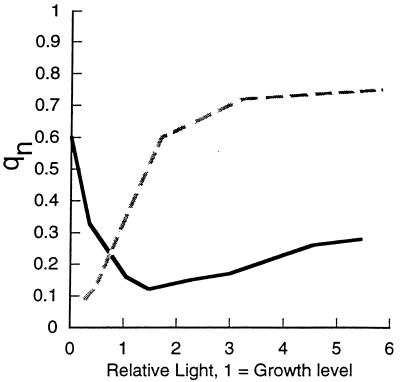
Figure 7 presents typical light response curves of nonphotochemical quenching in the cyanobacterium Synechococcus sp. strain PCC 7942, along with a curve from a rye plant for comparison. In the cyanobacterium, qN (or NPQ) is high in the dark and drops to a minimum near the growth light intensity. In the plant, qN climbs steadily as the light intensity surpasses the growth level. These differing patterns reflect a fundamental difference in the predominant processes contributing to nonphotochemical quenching. In plants nonphotochemical quenching is dominated by a mechanism(s) for excitation-dependent thermal dissipation of energy from PS II and its antennae, in competition with fluorescence and photochemistry (1, 46, 47, 57, 66, 67, 153).
FIG. 7.
Nonphotochemical quenching of fluorescence plotted against light. Typical light response curves of nonphotochemical quenching in a cyanobacterium, Synechococcus sp. strain PCC 7942 (solid line), and in rye for a plant-type pattern (dotted line) are shown. For comparison, light intensity is expressed relative to the growth light intensity; 1 = growth level. The actual growth light intensities were 50 μmol of photons m−2 s−1 for the cyanobacterium and 250 μmol of photons m−2 s−1 for the rye.
In contrast, nonphotochemical quenching in cyanobacteria largely reflects changes in the PS II fluorescence yield as a result of the state transition mechanism (7, 14, 17, 18, 21, 22, 33, 97, 111, 112), which regulates the distribution of excitation energy between PS II and PS I. The biophysical basis of the energy redistributions remains incompletely understood (2, 17, 18, 118, 156), but it is clear that the relative distribution of excitation energy from the phycobilisome to the two photosystems changes (7, 42, 136). An allophycocyanin-B protein in the phycobilisome core serves as a regulated secondary terminal emitter, which receives about 25% of the excitation energy captured by the phycobilisome. Under state I, this portion of the captured excitation is directed largely to PS II, but under state II, most of it is redirected to PS I, thereby lowering the yield of PS II fluorescence and photochemistry (48, 49, 112, 156). A recent model proposes that reversible changes in the oligomerization of PS II and PS I underpins the state transition mechanism (7). Furthermore, the phycobilisomes diffuse along the surface of the thylakoids (96) sufficiently rapidly that movement of phycobilisomes could be involved in the state transitions.
State transitions in cyanobacteria are regulated by the redox status of the electron transport chain joining PS II and PS I (34, 41, 94, 95, 97, 128, 149). If the chain is reduced, cells tend to state II, with a low yield of PS II fluorescence and a distribution of excitation energy to PS I, which extracts electrons from the chain. If the chain becomes more oxidized the cells shift toward state I, with a higher yield of PS II fluorescence and more distribution of excitation energy to PS II. Although the evidence for redox control of state transitions is quite strong, it is possible to observe conditions of low qP, indicating PS II reduction, while cells maintain low qN, presumed to reflect state I and an oxidized intersystem transport chain (86a). To accommodate these observations within the framework of redox regulation of state transitions, there must be partial decoupling of PS II and the intersystem redox status under some conditions, or some as yet unknown subtlety to the redox sensing mechanism(s).
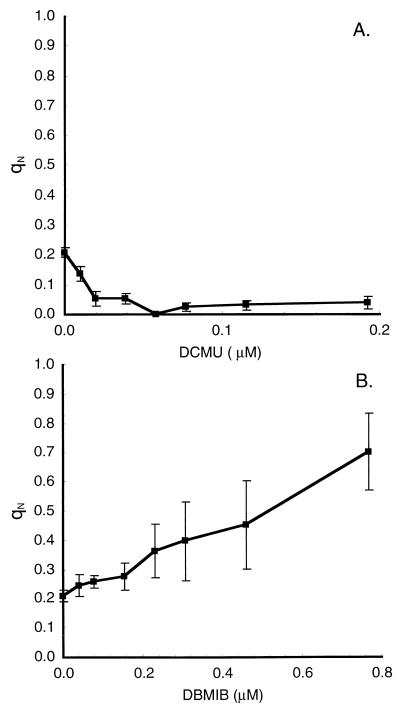
Respiratory and photosynthetic electron flow occur via the same electron transport intermediates in cyanobacteria (5, 44, 55, 85, 86, 131, 135, 147) (Fig. 1). Respiratory electron flow in the dark generally poises the electron transport chain toward a reduced state; therefore, in the dark or under very low light, cyanobacteria are in state II (41, 94, 95). This is reflected in low variable fluorescence and high nonphotochemical quenching, as shown in Fig. 7. As light is applied, PS I activity partially oxidizes the electron transport chain and the cells shift toward state I, with higher PS II fluorescence yield (Fig. 2) and lower nonphotochemical quenching (Fig. 7). As light exceeds the growth level, the PS II variable fluorescence yield remains high or drops somewhat (Fig. 2) and nonphotochemical quenching may increase, although not to the levels achieved in darkness (Fig. 7). Finally, if DCMU is added (Fig. 2), PS II centers close, resulting in a rapid rise in fluorescence as photochemical quenching is lost. This rapid rise is equivalent to the fluorescence peak under a brief saturating light pulse, which also closes reaction centers (Fig. 2). The DCMU inhibition of PS II substantially lowers electron input to the transport chain, and the electron transport chain becomes oxidized by continuing PS I activity. This results in a second, slower fluorescence rise phase (Fig. 2) as the cells enter a full state I with maximum PS II fluorescence (FM) (Fig. 2) and minimal nonphotochemical quenching. This redox dependence of the state transition is illustrated in Fig. 8. Titration with the inhibitor DCMU (143) under the growth light intensity progressively blocks PS II activity (Fig. 6), resulting in net oxidation of the transport chain and state I with low qN (Fig. 8A). Titration with DBMIB (143) also inhibits electron transport, but PS II itself remains active, resulting in reduction of the portion of the transport chain preceding the DBMIB binding site on the cytochrome bf complex. The state transition mechanism senses this change as an apparent overexcitation of PS II, and the cells enter state II with high qN (Fig. 8B).
FIG. 8.
Nonphotochemical quenching of fluorescence under DCMU (A) and DBMIB (B) treatments. Modulated fluorescence traces (Fig. 2) were measured under the growth light intensity of 50 μmol of photons m−2 s−1. Results are means and standard errors of measurements on the same culture at 37°C (n = 3). Modified from reference 22 with permission of the publisher.
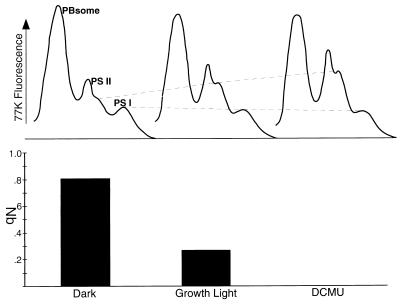
The origin of cyanobacterial nonphotochemical quenching in the state transition mechanism is illustrated in Fig. 9, which compares the 77K fluorescence emission spectra and qN values of a cyanobacterial sample in the darkness, under growth light intensity, and after addition of DCMU. The 77K emission spectra, measured with excitation of the phycobilisome at 574 nm, show the changing distribution of energy. In the dark, PS II fluorescence is low, reflecting the high nonphotochemical quenching. Upon illumination, qN drops as the excitation energy is redistributed in favour of PS II. This process continues to an extreme upon addition of DCMU.
FIG. 9.
77K Fluorescence emission spectra show that changing qN reflects state transitions. The dashed lines emphasize increasing PS II fluorescence and decreasing PS I fluorescence in parallel with the drop in nonphotochemical quenching, measured in the same cultures. A Synechococcus sp. strain PCC 7942 culture sample was incubated in the PAM cuvette in darkness, under the growth light intensity (50 μmol of photons m−2 s−1), and after addition of DCMU under continuing illumination. For each condition, qN was measured and a small sample was taken for 77K fluorescence emission spectral analysis. Excitation of 77K fluorescence was carried out with 574 nm light absorbed by the phycobilisome (PBsome). Fluorescence spectra are from samples of equal chlorophyll content but are not otherwise normalized.
In plants, excitation-dependent quenching driven by the trans-thylakoid ΔpH gradient is the major component of qN (1, 46, 47, 57), with only minor contributions from state transitions and other mechanisms (66). In plants, nonphotochemical quenching drops upon collapse of the trans-thylakoid pH gradient (67, 153). In cyanobacteria, nonphotochemical quenching does not collapse upon addition of carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP), an ionophore uncoupler (22, 86a). We therefore concluded that energy-dependent quenching is not a significant contributor to qN in cyanobacteria. Interestingly, Delphin et al. (32) present evidence that in red algae, whose chloroplasts also contain phycobilisomes, a plant-type ΔpH-dependent nonphotochemical quenching occurs even under low light levels, in contrast to the state transitions observed in cyanobacteria, which do not depend on ΔpH.
Nonphotochemical quenching reflects changes in PS II photochemistry but not necessarily net energy dissipation from the photosynthetic system, if energy is redirected from PS II to PS I. In cyanobacteria, therefore, a high qN does not necessarily mean a low overall photosynthetic efficiency (21, 138). Indeed, redistribution of energy from the PS II-phycobilisome supracomplex to PS I is an important regulatory mechanism in cyanobacteria, to accommodate changing excitation (21, 132, 133) or requirements for ATP to accumulate CO2 and nutrients (5, 91, 116). In contrast to this work, nonphotochemical quenching in a Microcystis strain has been interpreted as reflecting thermal energy dissipation of excitation. These cyanobacteria had a sustained content of the carotenoid zeaxanthin (60). Furthermore, some cyanobacterial strains such as Synechocystis sp. strain PCC 6803 do not display high nonphotochemical quenching after dark adaptation, indicating that they do not enter state II in the dark (51).
The diversity of cyanobacterial pigment and photosynthetic systems requires further characterization of the various origins and regulation of nonphotochemical quenching, particularly under natural growth conditions and limiting nutrient conditions, which can also lead to the loss or alteration of the state transition response (29, 39).
Recent evidence suggests that state II in dark-adapted cyanobacteria might involve an inactivation of the water-splitting complex (83). This limits the electron supply to the P680 chl of PSII and favors recombination in the reaction center, lowering the fluorescence yield (112a). This interpretation suggests that state transitions involve several different mechanisms, since there is clear evidence that under some conditions redirection of excitation energy is responsible for the changes in fluorescence yield observed as state transitions (see, e.g., reference 21).
A methodological problem in measuring light response curves of qN is the potential photoinhibitory loss of variable fluorescence as the actinic light is increased above the acclimated growth level. This can be detected if the FM level measured with DCMU is lower than a previous FM′ level, usually that measured around the growth light intensity. In this case, the uninhibited FM level for the sample is unknown, and therefore absolute values of qN cannot be determined. Nevertheless, if a relative qN is calculated by using the highest FM′ level achieved, the pattern with respect to light intensity is valid. Photoinhibition during measurement can be avoided by minimizing saturating flashes, keeping exposure to high actinic light brief, and terminating light response curves at 5 to 10 times the growth light intensity.
Predicting the Acclimated Light Intensity from qN Light Response
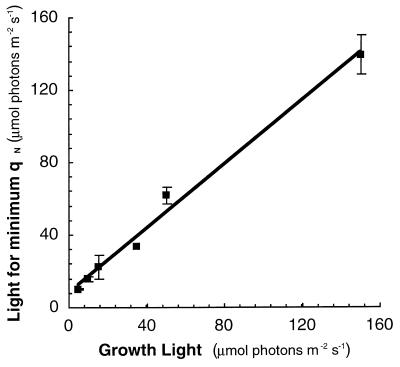
Figure 10 shows that in a wide range of cyanobacteria with different pigment contents and morphologies, grown under different conditions and light histories, qN reaches a minimum near the acclimated growth light intensity (22). Therefore, the light intensity to which the population is photosynthetically acclimated can be predicted from a readily measured light response curve of qN (or NPQ). Although ambient light is readily measured under many circumstances, this relation between qN and growth light shows the range of the overall light regime which is exploited for acclimated growth. In many cases, the natural light regime is highly variable and cyanobacteria must integrate light information over time to regulate synthesis of the abundant proteins of the photosynthetic system. We hope that the qN light response curve will prove useful with samples where the past light regime is unknown or samples from variable light regimes in which the optimal light intensities for acclimated growth are unknown (132, 133). For controlled light acclimation studies, tracking the qN minimum over time may show the point at which a population completes acclimation after a light shift. Furthermore, for prediction of electron transport in cyanobacteria from fluorescence parameters, it is essential to measure fluorescence under approximately the acclimated growth light intensity (see below).
FIG. 10.
Near the growth light intensity, qN reaches a minimum for a wide range of cyanobacterial strains and culture conditions. Mean values are plotted for strains grown at 5 (n = 3), 10 (n = 5), 15 (n = 6), 35 (n = 1), 50 (n = 20) and 150 (n = 3) μmol of photons m−2 s−1. The strains were Anabaena/Nostoc sp. strain 7120, Calothrix sp. strain PCC 7601, Nostoc sp., Pseudanabaena sp. strain PCC 6901, Synechococcus sp. strain PCC 7942, Synechococcus sp. strain PCC 6301, and Synechocystis sp. strain PCC 6701. Modified from reference 22 with permission of the publisher.
Note that this relation does not involve the absolute levels of qN or NPQ but simply their pattern in response to light intensity. Determination of actual qN or NPQ levels requires the measurement of FM by destructive DCMU treatment. If a nondestructive measurement is required and the absolute levels of the parameters are not critical, a simple plot of FM′ or FV′/FM′ against light intensity will suffice; the light intensity at which maximum values are achieved coincides approximately with the acclimated light intensity.
Inorganic Carbon Accumulation and Fluorescence Quenching
Cyanobacteria and some algae accumulate an intracellular pool of inorganic carbon to limit photorespiration (4, 109). The size of the pool influences both photochemical and nonphotochemical fluorescence quenching (5, 87–91). These fluorescence effects can be used to monitor the transport and accumulation of inorganic carbon noninvasively and in real time (30, 90). One mechanism for the fluorescence effects appears to be a bicarbonate-dependent stimulation of linear electron flow from PS I to O2, CO2, or nitrite in some strains, which increases photochemical quenching (5, 51, 77, 78, 88, 91, 92).
Carbon accumulation can also influence nonphotochemical quenching, possibly by driving a transition to state II to increase PS I cyclic electron transport to fulfill the need for ATP (93) to drive the accumulation pump.
Miller et al. (89) also describe a form of nonphotochemical quenching which depends on inorganic carbon accumulation in Synechococcus sp. strain PCC 7942 cells grown at high light intensities but which does not occur in cells grown at low light intensities. The modulated fluorescence trace from the cells grown at high light intensities resembles a transition to state II upon carbon accumulation, with a drop in FM′ fluorescence yield and an increase in qN. This increase in qN was not, however, reflected in a comparable change in the cellular fluorescence emission spectra at 77K. This is in contrast to a large drop in 77K PS II fluorescence emission upon dark adaptation of the same cells, similar to that presented in Fig. 9. This component of carbon accumulation-dependent nonphotochemical quenching in cells grown at high light intensities thus appears distinct from the state transition mechanism. It might ultimately relate to bicarbonate-dependent changes in the PS II water splitting-complex (112a).
State Transitions Can Be Measured Nondestructively
The change in fluorescence yield during state transitions is strongly influenced by the excitation light, the rate of respiration, the cellular iron supply (39), and the circadian status of the cell (83). Therefore, determining the size of state transitions can provide information about these factors. The magnitude of state transitions can be measured by comparing qN or NPQ values under different conditions, but explicit calculation of these parameters requires a lethal DCMU treatment to determine FM. This precludes repeated measures on a single sample over time. Alternately, changes in qN between different conditions, for example cells in the dark and under illumination, can be quantified by a nondestructive method that does not require explicit measurement of FM:
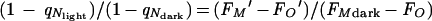 |
In general, for the strains we have studied, this ratio is maximal for a given sample if measured by using the growth light intensity. During prolonged dark incubation, this ratio declines toward 1, as the FMdark level approaches but does not reach FM′. Thus, during dark incubation, the state transition gradually disappears. This probably reflects the progressive consumption of reserves used to support electron transport in the dark (34, 94, 123). The presence or size of state transitions can also be influenced by iron or nitrogen stress (29, 39), opening the possibility of using the state transition as a noninvasive monitor of these aspects of physiology.
PREDICTING PHOTOSYNTHESIS FROM FLUORESCENCE
For field measurements, a fluorescence-based estimate of electron transport and carbon dioxide fixation is very valuable (104, 134). Fluorescence measurements are possible with dilute samples; unlike gas exchange, they are specific to photobionts and so do not detect interference from heterotrophic respiratory activity in mixed samples or lichens. The fluorescence transients arise largely from PS II, and so calculations based on fluorescence reflect PS II activity and electron transport through PS II. In extrapolating from fluorescence signals to photosynthesis, we therefore rely on a congruence between PS II activity, net electron transport, and overall photosynthesis. Cyanobacteria and cyanolichens have carbon-concentrating mechanisms which suppress the oxygenase reaction of the ribulose-1,5-bisphospate oxygenase-carboxylase enzyme (4, 5, 87, 90, 91, 108, 110). This simplifies the empirical relation between PS II activity, reflected in fluorescence signals, and gas exchange, as is also the case in C4 plants (36).
Sundberg et al. (138) have simultaneously measured fluorescence quenching parameters and CO2 exchange in cyano-lichens to develop a model to predict gross photosynthesis from fluorescence parameters. They found the empirical relation
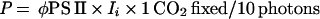 |
where P = micromoles of CO2 fixed per square meter per hour, φPS II = (FM′ − FS)/FM′ = (FV′/FM′)qP (a fluorescence estimate of the photochemical yield of PS II), Ii = number of incident photons per square meter per hour, and 1 CO2 fixed/10 photons is an empirical conversion factor.
This predictor gave good estimates of actual CO2 fixation near the acclimated growth light intensity. Under higher light, the predictor, which is based on light-driven electron flow through PS II, progressively overestimated actual CO2 fixation. The overestimation probably reflects electron flow back to O2 under excess light (22a, 88, 131, 147), which maintains PS II centers open but does not contribute to CO2 CO2 fixation. Other workers have found that this flexible electron transport to O2 interferes with estimates of CO2 fixation from fluorescence measures (74). In our experiments, we could estimate the acclimated growth light intensity from the light response curve of nonphotochemical quenching. With this light intensity, we made reasonable predictions of the acclimated rate of CO2 fixation from fluorescence parameters. The general applicability of this approach must, however, be further tested. The 1 CO2 fixed/10 photons is an empirical conversion factor that reflects the (unknown) quantum yield of CO2 fixation and also compensates for the downward distortion of FV′/FM′ by phycobilisome fluorescence.
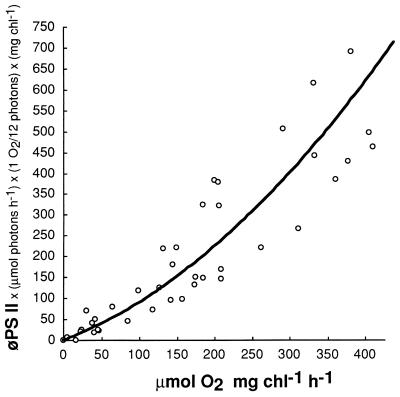
We developed a similar empirical relation to predict gross oxygen evolution from φPS II in liquid cyanobacterial cultures:
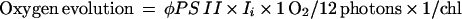 |
where oxygen evolution is expressed as micromoles of O2 per milligram of chlorophyll per hour, φPS II = (FM′ − FS)/FM′ = (FV′/FM′)qP, Ii = number of micromoles of photons incident per hour; 1 O2/12 photons is an empirical conversion factor, and chl is the chlorophyll content in milligrams.
Figure 11 shows that this relation gives a good approximation of measured oxygen evolution at or near the growth light intensity. At higher light intensities, the predictor increasingly overestimates measured oxygen evolution, again because of pseudocyclic electron flow, with electrons extracted from water by PS II ultimately reaching oxygen as a terminal acceptor. The empirical conversion factor of 1 O2/12 photons again combines the unknown quantum yield of O2 evolution and compensation for the low FV′/FM′ values in cyanobacteria.
FIG. 11.
φPS II reflects O2 evolution. Synechococcus sp. strain PCC 7942 was grown under 50 μmol of photons m−2 s−1 at 37°C and then incubated under a range of light intensities. Gross oxygen evolution (micromoles of O2 per milligram of chlorophyll per hour) was estimated as light-dependent oxygen evolution minus dark uptake. φPS II = (FM′ − FS)/FM′ = (FV′/FM′)qP. Ii = micromoles of photons incident on the sample per hour.
In summary, a two-step process gives reasonable estimates of oxygen evolution or carbon fixation under the acclimated growth light intensity. First, a light response curve of nonphotochemical quenching shows the acclimated light intensity. Then oxygen evolution or carbon fixation is estimated from the fluorescence parameter φPS II, the acclimated light level, and an empirical conversion factor calibrated against gas exchange measurements. Failure to measure near the growth light intensity, or an inappropriate empirical calibration factor, can lead to a significant overestimation of actual photosynthesis.
APPLYING FLUORESCENCE ANALYSIS TO DIFFERENT CYANOBACTERIA
We have tested the methods described in nine strains of cyanobacteria representing a wide range of pigment contents, phycobilisome structures, and physiological properties, as well as six strains of cyanobacterial lichens, as outlined in Table 2. The methods have been extensively validated with Synechococcus sp. strain PCC 7942 (Anacystis nidulans R2) grown under a range of light intensities, CO2 levels, and temperatures (22–24, 26–28; see above). Furthermore, a mutant of this strain lacking phycobilisome rods and containing no PC proved amenable to quenching analysis (Fig. 3) (158). The closely related strain Synechococcus sp. strain PCC 6301 (Anacystis nidulans) displays very similar fluorescence properties (21a). Both these strains have a somewhat unusual phycobilisome with a core composed of only two cylinders.
TABLE 2.
Cyanobacteria and cyanolichens used for the modulated fluorescence analyses described in this review
| Species | Growth light | Phycobiliproteina |
|---|---|---|
| Cyanobacteria | ||
| Anabaena/Nostoc sp. strain PCC 7120 | Fluorescent (50 μmol of photons m−2 s−1) | APC, PC, PEC |
| Calothrix/Tolypothrix/Fremyella sp. strain PCC 7601 | Fluorescent (10 μmol of photons m−2 s−1) | APC, PC, PE |
| Calothrix/Tolypothrix/Fremyella sp. strain PCC 7601 | Green (15 μmol of photons m−2 s−1) | APC, PC, PE |
| Calothrix/Tolypothrix/Fremyella sp. strain PCC 7601 | Red (15 and 35 μmol of photons m−2 s−1) | APC, PC |
| Gloeobacter violaceus PCC 7421 | Fluorescent (10 μmol of photons m−2 s−1) | APC, PC, PE, PUB |
| Pseudanabaena sp. strain PCC 6901 | Fluorescent (50 μmol of photons m−2 s−1) | APC, PC |
| Synechococcus sp. strain PCC 7942 (Anacystis nidulans R2) | Incandescent (10, 15, 50, and 150 μmol of photons m−2 s−1) | APC, PC |
| Synechococcus sp. strain PCC 7942 phycobilisome-minus | Incandescent (50 μmol of photons m−2 s−1) | APC |
| Synechococcus sp. strain PCC 6301 (Anacystis nidulans) | Fluorescent (10 and 50 μmol of photons m−2 s−1) | APC, PC |
| Synechocystis sp. strain PCC 6701 | Fluorescent (50 μmol of photons m−2 s−1) | APC, PC, PE |
| Nostoc from lichen Peltigera canina | Fluorescent (5 μmol of photons m−2 s−1) | APC, PC, (PE, PEC?) |
| Cyanolichens (Nostoc) | ||
| Leptogium coralloideum | Natural | APC, PC, (PE, PEC?) |
| Lobaria scrobiculata | Natural | APC, PC, (PE, PEC?) |
| Nephroma bellum | Natural | APC, PC, (PE, PEC?) |
| Peltigera malacea | Natural | APC, PC, (PE, PEC?) |
| Peltigera neopolydactyla | Natural | APC, PC, (PE, PEC?) |
| Nephroma arcticum (Nostoc and green algae Coccomyxa) | Natural | APC, PC, (PE, PEC?) |
APC, allophycocyanin; PC, phycocyanin; PE, phycoerythrin; PEC, phycoerythrocyanin; PUB, phycoerythrin containing phycourobilin chromophores.
Calothrix sp. strain PCC 7601 is a heterocystous, filamentous strain which strongly regulates its content of phycoerythrin and phycocyanin according to the relative supply of green and red light. Quenching analysis has shown that when this strain is grown under green light, energy captured by phycoerythrin is transferred from the phycobilisome to PS I to maintain balanced electron transport. This transfer is reflected in a high qN and serves as a good demonstration that in a cyanobacterium a high qN does not usually reflect excitation dissipation but, rather, reflects the transfer of excitation to PS I at the expense of PS II. Upon transfer to red light or prolonged growth under red light, this excitation transfer stops, the PS II fluorescence yield increases, and qN drops (21).
Four other diverse cyanobacterial strains have also shown responses similar to that of Synechococcus sp. strain PCC 7942 (21a): Anabaena/Nostoc sp. strain PCC 7120 is a heterocystous, filamentous strain with another atypical phycobilisome structure (35), Nostoc sp. is a strain originally isolated from the lichen Peltigera canina, Synechocystis sp. strain PCC 6701 is a unicellular strain which contains phycoerythrin; and Pseudanabaena sp. strain PCC 6901 is a gas-vacuolated strain forming short filaments.
Six lichens with Nostoc strains as symbionts have also proved amenable to quenching analysis by using a Hansatech cuvette designed for leaf discs; Leptogium coralloideum, Lobaria scrobiculata, Nephroma bellum, Peltigera malacea, Peltigera neopolydactyla, and the cephalodia regions of Nephroma arcticum, a tri-partite lichen with the green algae Coccomyxa and Nostoc. In lichens, the dark-light state II-state I transition tends to be small, probably reflecting fungus-cyanobacterium interactions in respiration and carbohydrate consumption (138).
Goosney and Miller (51) found that the widely studied facultative heterotrophic strain Synechocystis sp. strain PCC 6803 shows little or no increase in FM′ upon illumination under most conditions. The fluorescence induction trace in this strain is rather plant-like in that FM′ measured in dark-adapted cells is close to FM. This distinct pattern might reflect differences in this strain in the redox balance of intersystem electron transport in the dark, such that the cells do not enter state II in the dark. This plant-like induction pattern upon illumination can occur in other strains, particularly when dark respiration is slow as under conditions of low carbohydrate reserves or under nutrient stress (29, 39). Alternatively, there may be more fundamental distinctions in the organization of light capture and electron transport between various strains.
Interestingly, the unusual cyanobacteria Gloeobacter violaceus PCC 7421 was the only strain surveyed for which quenching analysis proved impossible. This strain lacks thylakoids (115) and instead of typical phycobilisomes contains simpler rod-like phycobiliprotein structures (52) associated with the plasma membrane. In our measurements, this strain showed almost no variable fluorescence even when growth and oxygen evolution were readily measurable (21a). 77K fluorescence spectra from this strain also lack the expected long-wavelength PS I emission, even though the presence of PS I was verified functionally (64). Clearly, the unusual photosynthetic system of this cyanobacterium results in distinct fluorescence properties.
CONCLUSIONS AND PROSPECTS
Tracking Acclimation Status in the Laboratory and Field
Fluorescence analysis is an integral part of studies of photosynthesis in cyanobacteria and other organisms. In recent years, advances in instrumentation and interpretation have greatly expanded the applications of fluorescence to ecophysiological and molecular studies (6, 13, 40, 99).
For cyanobacteria, estimating the acclimated light in a population from the light response of nonphotochemical quenching (22) will allow rapid tracking of acclimation in laboratory experiments or field studies. Cells must integrate changing environmental signals (132, 133) to regulate the expression of abundant proteins such as ribulose-1,5-bisphosphate carboxylase/oxygenase or phycobilisomes, in order to produce appropriate long-term levels of protein. The use of fluorescence to monitor acclimation (10, 61, 73, 74, 130) may show how cyanobacteria and cyanolichens set their targets for gene expression and metabolic acclimation in the face of changing light, environmental factors, and circadian status.
The size of the dark-to-light state transition is strongly influenced by cellular respiration, which poises the electron transport chain (34, 55, 84–86, 94, 95, 131, 135). Therefore, the state transition may provide estimates of respiration and indirectly of the reserves available to support respiration. Further work in this area might lead to rapid measures of the levels of reserves in cyanobacteria or cyanolichens, as they fluctuate diurnally or seasonally (123, 124). Estimates of reserves are valuable in understanding physiological and differentiation responses of cyanobacteria and also in determining production available for export from the community.
The size of the dark-to-light state transition is also strongly influenced by cellular iron and nitrogen status (29, 39). Under iron limitation, which is widespread in nature (8), cyanobacteria produce alternate chlorophyll-protein complexes associated with PS II (70, 75, 76), which leads to suppression of the state transition (39). Therefore, the size of state transition may be useful as a measure of iron limitation.
The iron example illustrates that as we apply fluorescence analysis to natural cyanobacteria, nutrient or reserve limitations may greatly alter fluorescence signals from those observed with laboratory cultures. Thus, further work is needed on measuring and interpreting fluorescence signals under nonoptimal and natural conditions, where pigment composition and cellular organization may be very different from typical laboratory cultures. Simultaneous parallel detection of fluorescence transients at several excitation and emission wavelengths (61, 65) holds great promise for the study of mixed populations or strains with complex pigment compositions.
Conclusions
Chlorophyll fluorescence signals from cyanobacteria and cyanolichens show patterns very distinct from those of green plants. Therefore, the fluorescence measurements and analyses originally developed for green plants must be modified, but cyanobacterial fluorescence also yields information not accessible from plant fluorescence signals. The cellular phycobiliprotein content influences the FO level fluorescence, particularly when phycobiliprotein levels are high. This leads in some cases to downward distortion of the parameter FV/FM, which is widely used as an index of PS II activity. The photochemical quenching coefficient, qP, provides a robust index of the balance between excitation of PS II and electron transport and shows that cyanobacteria have a high and flexible capacity to remove electrons from PS II. Nonphotochemical quenching of PS II variable fluorescence in cyanobacteria reflects the state transition mechanism for distribution of excitation between the photosystems. A characteristic decline of nonphotochemical quenching during a shift from dark to increasing light provides a valuable means of estimating the light level to which a cyanobacterial population is photosynthetically acclimated. Gross oxygen evolution or carbon fixation can be estimated in at least some cases (138; but see reference 74)) from the fluorescence parameter φPS II, the acclimated growth light level, and an empirically verified apparent quantum yield.
ACKNOWLEDGMENTS
We thank our collaborators Doug Bruce, Amanda Cockshutt, Kristin Palmqvist, Bodil Sundberg, and Guoqing Zhou for valuable discussions. Anthony Miller, Darwyn Coxson, Henrik Schubert, Nicole Tandeau de Marsac, and Jean Houmard have also contributed generously to discussions of this work.
This work was supported by the Swedish Natural Science Research Council grants to G.Ö.
Appendix
Cyanobacterial Cultures and Pigment Measurements
Cyanobacteria were grown in BG-11 inorganic medium (114), supplementally buffered with 10 mM 3-(N-morpholino)propanesulfonic acid (MOPS) to a final pH of 7.5. Synechococcus sp. strain PCC 7942 cultures (300 ml) were grown in flat flasks bubbled with 5% CO2 in air (about 1 ml s−1) at 37 or 25°C with continuous, even illumination of 10, 15, 50, or 150 μmol of photons m−2 s−1. For Synechococcus sp. strain PCC 7942, the chlorophyll and phycocyanin contents were determined by using whole-cell spectra as described by Myers et al. (98), corrected for scattering by subtracting the absorbance at 750 nm from the chlorophyll and phycocyanin peaks. Whole-cell spectra are less useful for filamentous strains, since significant and variable light scattering distorts spectra. For these strains, chlorophyll was extracted in methanol and measured by the method of Tandeau de Marsac and Houmard (140). For the other strains used in the qN measurements shown in Fig. 9, the growth conditions were generally similar but the temperature ranged from 18 to 37°C, the light intensity ranged from 5 to 150 μmol of photons m−2 s−1, either fluorescent or incandescent, and the CO2 supply ranged from ambient to 5% in air.
Modulated Fluorometer Configuration and Measurement Procedure
In our experiments, chlorophyll a fluorescence induction was measured with a pulse amplitude modulated fluorometer (128, 129) (PAM chlorophyll fluorometer; Walz, Effeltrich, Germany) with the PAM 103 accessory and a Schott KL1500 lamp (Schott, Mainz, Germany) to provide saturating flashes. The recording device, in our case a chart recorder, should respond to changes within less than 100 ms (128). A PAM-compatible system of cuvette, magnetic stirrer, oxygen electrode, and halogen incandescent actinic lamp were used for the simultaneous measurement of fluorescence and oxygen evolution (Hansatech, King’s Lynn, United Kingdom) (151). Simultaneous measurement strengthens the conclusions which can be drawn, since the two techniques are complementary. This cuvette system allows measurement of liquid samples down to concentrations of about 0.2 μg of chlorophyll/ml, suitable for laboratory cultures or somewhat concentrated natural samples. An alternate cuvette (Walz ED-101 US) (125) uses a smaller vessel and light guides to extend the functional concentration range to less than 1 μg of chlorophyll per liter, allowing direct measurement of many natural water samples. The actinic beam and fluorescence detector are set at 90° in this system, rather than at 180° as in the Hansatech cuvette. This alternate geometry may result in changes in some measurements (60a), and so the influences of cuvette geometry on signals are an issue for further research. A further innovation is a system involving light-emitting diodes of different wavelengths, for preferential excitation of specific pigments (65). This technique shows promise for resolution of fluorescence signals from different photobionts in mixed samples. Comparable modulated fluorometers, with variations in the nature and wavelength of the measuring and actinic lights and in the cuvettes, are available from several companies.
Culture samples, generally at around 2 μg of chlorophyll per ml, were dark adapted for 5 min in the Hansatech cuvette. The analysis procedure is outlined in Fig. 2. Minimum fluorescence, FO, was determined by illuminating the dark-adapted cells with a low-intensity light modulated at 1.6 kHz (average intensity, 0.14 μmol of photons m−2 s−1) from a light-emitting diode (peak emission, 655 nm). Fluorescence was detected at wavelengths greater than 700 nm. The intensity of the measuring beam should be checked to ensure that it is sufficiently weak to avoid electron transport, detected as changes in the oxygen electrode response (20). A 1-s flash of saturating white light (8,000 μmol of photons m−2 s−1) was then given to determine the maximal fluorescence in the dark-adapted state, FMdark, with all PS II centers closed by the saturating flash. In cyanobacteria, FMdark is generally significantly lower than the maximal fluorescence, FM (20, 22, 23, 27, 28, 91), although this is not the case in all strains under all conditions (see, e.g., reference 51). After a further 30 s, the actinic light was activated. Steady-state fluorescence, FS, was reached within 2 min. Minimum fluorescence in the light-adapted state, FO′, was then measured by briefly interrupting the actinic beam and either leaving culture in darkness for ca. 5 s or applying weak far-red light (ca. 5 μmol of photons m−2 s−1; >700 nm) to excite PS I activity and extract electrons from the transport chain. Both procedures for measuring FO′ gave similar results in cyanobacterial measurements. The actinic light was then resumed, and after FS was reestablished, a saturating light pulse was given to again close all PS II centers, driving photochemical quenching to zero for determination of maximal fluorescence in the light-adapted state, FM′. The actinic light intensity was then increased, and the process was repeated sequentially to generate a light response curve.
Cyanobacterial cells have CO2-concentrating mechanisms (CCM) (4, 87), and when this system is induced the cells do not usually become CO2 limited during short measurements. Laboratory cyanobacterial cultures are often supplemented with high CO2 levels, and under these growth conditions the CCM activity is repressed. In these cases, photosynthesis may deplete the dissolved inorganic carbon supply of the cuvette volume, resulting in CO2 limitation of photosynthesis. Even in cells grown under low inorganic carbon, prolonged photosynthesis in a closed cuvette can deplete the inorganic carbon sufficiently to cause carbon limitation. Furthermore, electron flow to oxygen is often dependent on the presence of an intracellular biocarbonate pool, so CO2 limitation can also limit electron flow to oxygen as a terminal acceptor (88). The onset of CO2 limitation results in steady-state FS fluorescence increasing toward FM′, in parallel with a decline in the oxygen evolution rate. Cultures grown under CO2 supplementation were therefore routinely supplemented with 7 mM NaH2CO3 at the start of the measurement.
Finally, DCMU (final concentration, 0.5 μM) was injected into the cuvette to bind to PS II centers (143). This leads to a rapid rise in fluorescence to a level similar to FM′, as PS II closes, photochemistry is blocked, and photochemical quenching is lost (Fig. 2). The loss of PS II activity leads to oxidation of the plastoquinone pool, which in turn drives a slower fluorescence rise phase (Fig. 2) as the cells go to full state I with maximal fluorescence, FM. The concentration of DCMU required for rapid PS II closure is somewhat dependent on the strain and growth conditions and should be verified empirically. The parameters FO, FMdark′ FS, FO′, FM′, and FM were used for the calculation of photochemical (qP) and nonphotochemical (qN) quenching (145), and the apparent efficiency of excitation energy capture by open PS II reaction centers (FV′/FM′) (45).
Figure 2 is a fluorescence trace from our usual protocol for measuring room temperature fluorescence in Synechococcus sp. strain PCC 7942, showing a typical cyanobacterial response over a series of increasing light intensities. The number of saturating flashes is kept to a minimum to avoid photoinhibition or sustained induction of electron transport. Schreiber et al. (128) described the use of much shorter flashes (50 ms) which can be applied to measure FM′ more frequently with a reduced risk of photoinhibition or perturbation of electron transport.
77K Fluorescence Emission Spectra
A clear acrylic rod sample holder for 77K fluorescence was dipped briefly into the Hansatech PAM cuvette to collect about 100 μl; this subsample was plunged directly into liquid nitrogen. The sample holder was attached to a fiberoptic fluorometer, and 77K fluorescence emission spectra were collected (100), with excitation at 574 nm. The 77K spectra are therefore from cells under the same conditions as those used for room temperature fluorescence quenching and oxygen measurements.
Alternate Fluorescence Quenching Parameters
Havaux et al. (53) and Walters and Horton (152) have proposed the parameter (1/FO) − (1/FM) as a measure of the rate constant of excitation trapping by the PS II reaction center and 1/FM as a measure of nonphotochemical loss of excitation energy from the light-harvesting antennae. In our experiments, plots of (1/FO) − (1/FM) closely parallel FV/FM. The 1/FM parameter shows some discrepancy from cyanobacterial qN and NPQ, possibly reflecting the distinct nature of nonphotochemical quenching in cyanobacteria and plants.
An alternate approach to extracting information from fluorescence signals is the pump and probe method reviewed by Falkowski and Kolber (40). For methodological and theoretical reasons, these authors define an alternate quantum efficiency term,
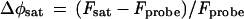 |
as the difference in fluorescence yield driven by a weak probe light before and immediately following a saturating flash, divided by the fluorescence yield before the flash. This alternate parameter can be related to FV/FM as
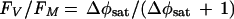 |
Another approach developed particularly for oceanographic purposes is fast repetition rate fluorescence (40), which uses a rapid train of 20 to 60 brief (5-μs), nonsaturating pulses applied at frequencies up to 200 kHz. Fluorescence excited by the first flash gives the FO level fluorescence with all reaction centers open. The rapid succession of flashes then progressively closes PS II centers, driving fluorescence toward FM. The rate of saturation is proportional to the effective absorption cross-section of PS II, ςPS II, a useful parameter which is not determined by methods using single saturating pulses. The FM measured with this train of pulses is not equivalent to the FM determined with a single saturating pulse used in the modulated fluorometer (127, 128) or the pump and probe methods. Therefore, data from fast repetition rate fluorescence are not directly comparable or convertible to data from the other methods. Nevertheless, the results generate conclusions which are qualitatively similar to those obtained from quenching analysis involving modulated fluorescence (127).
REFERENCES
- 1.Adams W W, Demmig-Adams B. Energy dissipation and photoprotection in leaves of higher plants. In: Yamamoto H Y, Smith C M, editors. Photosynthetic responses to the environment. Rockville, Md: American Society of Plant Physiologists; 1993. pp. 27–36. [Google Scholar]
- 2.Allen J F, Sanders C E, Holmes N G. Correlation of membrane protein phosphorylation with excitation energy distribution in the cyanobacterium Synechococcus 6301. FEBS Lett. 1985;193:271–275. [Google Scholar]
- 3.Asada K, Kiso K, Yoshikawa K. Univalent reduction of molecular oxygen by spinach chloroplasts on illumination. J Biol Chem. 1974;249:2175–2181. [PubMed] [Google Scholar]
- 4.Badger M R, Price G D. The CO2 concentrating mechanism in cyanobacteria and green algae. Physiol Plant. 1992;84:606–615. [Google Scholar]
- 5.Badger M R, Schreiber U. Effects of inorganic carbon accumulation on photosynthetic oxygen reduction and cyclic electron flow in the cyanobacterium Synechococcus PCC 7942. Photosynth Res. 1993;37:177–191. doi: 10.1007/BF00032822. [DOI] [PubMed] [Google Scholar]
- 6.Baker N R, Farage P K, Stirling C M, Long S P. Photoinhibition of crop photosynthesis in the field at low temperatures. In: Baker N R, Bowyer J R, editors. Photoinhibition of photosynthesis: from molecular mechanisms to the field. Oxford, United Kingdom: Bios Scientific Publishers; 1994. pp. 349–363. [Google Scholar]
- 7.Bald D, Kruip J, Rögner M. Supramolecular architecture of cyanobacterial thylakoid membranes: how is the phycobilisome connected with the photosystems? Photosynth Res. 1996;49:103–118. doi: 10.1007/BF00117661. [DOI] [PubMed] [Google Scholar]
- 8.Behrenfeld M J, Bale A J, Kolber Z S, Aiken J, Falkowski P G. Confirmation of iron limitation of phytoplankton photosynthesis in the equatorial pacific-ocean. Nature. 1996;383:508–511. [Google Scholar]
- 9.Bilger W, Schreiber U. Energy-dependent quenching of dark-level chlorophyll fluorescence in intact leaves. Photosynth Res. 1986;10:303–308. doi: 10.1007/BF00118295. [DOI] [PubMed] [Google Scholar]
- 10.Bilger W, Budel B, Mollenhauer R, Mollenhauer D. Photosynthetic activity of 2 developmental stages of a Nostoc strain (cyanobacteria) isolated from Geosiphon pyriforme (mycota) J Phycol. 1994;30:225–230. [Google Scholar]
- 11.Binder A, Hauser R, Krogmann D. Respiration in energy transducing membranes of the thermophilic cyanobacterium Mastigocladus laminosus. I. Relation of the respiratory and photosynthetic electron transport. Biochim Biophys Acta. 1984;765:241–246. [Google Scholar]
- 12.Björkman O, Demmig B. Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77K among vascular plants of diverse origins. Planta. 1987;170:489–504. doi: 10.1007/BF00402983. [DOI] [PubMed] [Google Scholar]
- 13.Bolhàr-Nordenkampf H R, Öquist G. Chlorophyll fluorescence as a tool in photosynthesis research. In: Hall D O, Scurlock J M O, Bolhàr-Nordenkampf H R, Leegood R C, Long S P, editors. Photosynthesis and production in a changing environment: a field and laboratory manual. London, United Kingdom: Chapman & Hall; 1993. pp. 193–206. [Google Scholar]
- 14.Bonaventura C, Myers J. Fluorescence and oxygen evolution for Chlorella pyrenoidosa. Biochim Biophys Acta. 1969;189:366–383. doi: 10.1016/0005-2728(69)90168-6. [DOI] [PubMed] [Google Scholar]
- 15.Bovy A, de Vrieze G, Borrias M, Weisbeek P. Transcriptional regulation of the plastocyanin and cytochrome c553 genes from the cyanobacterium Anabaena species PCC 7937. Mol Microbiol. 1992;6:1507–1513. doi: 10.1111/j.1365-2958.1992.tb00871.x. [DOI] [PubMed] [Google Scholar]
- 16.Briggs L M, Pecoraro V L, McIntosh L. Copper-induced expression, cloning, and regulatory studies of the plastocyanin gene from the cyanobacterium Synechocystis sp. PCC 6803. Plant Mol Biol. 1990;15:633–542. doi: 10.1007/BF00017837. [DOI] [PubMed] [Google Scholar]
- 17.Bruce D, Biggins J. Mechanism of the light-state transition in photosynthesis. V. 77K linear dichroism of Anacystis nidulans in State 1 and State 2. Biochim Biophys Acta. 1985;810:295–301. [Google Scholar]
- 18.Bruce D, Biggins J, Steiner T, Thewalt M. Mechanism of the light-state transition in photosynthesis. IV. Picosecond fluorescence spectroscopy of Anacystis nidulans and Porphyridium cruentum in State 1 and State 2. Biochim Biophys Acta. 1985;810:237–246. [Google Scholar]
- 19.Bryant D A, editor. The molecular biology of cyanobacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. [Google Scholar]
- 20.Büchel C, Wilhem C. In vivo analysis of slow chlorohyll fluorescence induction kinetics in algae: progress, problems and perspectives. Photochem Photobiol. 1993;58:137–148. [Google Scholar]
- 21.Campbell D. Complementary chromatic adaptation alters photosynthetic strategies in the cyanobacterium Calothrix. Microbiology. 1996;142:1255–1263. doi: 10.1099/13500872-142-5-1255. [DOI] [PubMed] [Google Scholar]
- 21a.Campbell, D. Unpublished data.
- 22.Campbell D, Öquist G. Predicting light acclimation in cyanobacteria from non-photochemical quenching of PSII fluorescence, which reflects state transitions in these organisms. Plant Physiol. 1996;111:1293–1298. doi: 10.1104/pp.111.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22a.Campbell, D., and G. Öquist. Unpublished data.
- 23.Campbell D, Bruce D, Carpenter C, Gustafsson P, Öquist G. Two forms of the photosystem II D1 protein alter energy dissipation and state transitions in the cyanobacterium Synechococcus sp. PCC 7942. Photosynth Res. 1996;47:131–144. doi: 10.1007/BF00016176. [DOI] [PubMed] [Google Scholar]
- 24.Campbell D, Zhou G, Gustafsson P, Öquist G, Clarke A K. Electron transport regulates exchange of two forms of Photosystem II D1 protein in the cyanobacterium Synechococcus. EMBO J. 1995;14:5457–5466. doi: 10.1002/j.1460-2075.1995.tb00232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campbell D, Houmard J, Tandeau de Marsac N. Electron transport regulates cellular differentiation in the filamentous cyanobacterium Calothrix. Plant Cell. 1993;5:451–463. doi: 10.1105/tpc.5.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clarke A K, Campbell D. Inactivation of the petE gene for plastocyanin lowers photosynthetic capacity and exacerbates chilling-induced photoinhibition in the cyanobacterium Synechococcus. Plant Physiol. 1996;112:1551–1561. doi: 10.1104/pp.112.4.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clarke A K, Campbell D, Gustafsson P, Öquist G. Dynamic responses of photosystem II and phycobilisomes to changing light in the cyanobacterium Synechococcus sp. PCC 7942. Planta. 1995;197:553–562. [Google Scholar]
- 28.Clarke A K, Hurry V M, Gustafsson P, Öquist G. Two functionally distinct forms of the PS II reaction-center protein D1 in the cyanobacterium Synechococcus sp. PCC 7942. Proc Natl Acad Sci USA. 1993;90:11985–11989. doi: 10.1073/pnas.90.24.11985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collier J L, Herbert S K, Fork D C, Grossman A R. Changes in the cyanobacterial photosynthetic apparatus during acclimation to macronutrient deprivation. Photosynth Res. 1994;42:173–183. doi: 10.1007/BF00018260. [DOI] [PubMed] [Google Scholar]
- 30.Crotty C M, Tyrrell P N, Espie G S. Quenching of chlorophyll-a fluorescence in response to Na+-dependent HCO3− transport-mediated accumulation of inorganic carbon in the cyanobacterium Synechococcus UTEX-625. Plant Physiol. 1994;104:785–791. doi: 10.1104/pp.104.2.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dau H. Molecular mechanisms and quantitative models of variable photosystem II fluorescence. Photochem Photobiol. 1994;60:1–23. [Google Scholar]
- 32.Delphin E, Duval J-C, Etienne A-L, Kirilovsky D. State transitions or ΔpH-dependent quenching of photosystem II fluorescence in red algae. Biochemistry. 1996;35:9435–9445. doi: 10.1021/bi960528+. [DOI] [PubMed] [Google Scholar]
- 33.Demmig-Adams B, Adams III W W, Czygan F-C, Schreiber U, Lange O L. Differences in the capacity for radiationless energy dissipation in gree and blue-green algal lichens associated with differences in carotenoid composition. Planta. 1990;180:582–589. doi: 10.1007/BF02411457. [DOI] [PubMed] [Google Scholar]
- 34.Dominy P J, Williams W P. The role of respiratory electron flow in the control of excitation energy distribution in blue-green algae. Biochim Biophys Acta. 1987;892:264–274. [Google Scholar]
- 35.Ducret A, Sidler W, Zuber H. 1993 Westcoast Cyanobacterial Workshop. 1993. Structural studies on the atypical phycobilisome core complex of the cyanobacterium Anabaena sp. PCC 7120; p. 37. [Google Scholar]
- 36.Edwards G E, Baker N R. Can CO2 assimilation in maize leaves be predicted accurately from chlorophyll fluorescence analysis? Photosynth Res. 1993;37:89–102. doi: 10.1007/BF02187468. [DOI] [PubMed] [Google Scholar]
- 37.Elston T, Wang H, Oster G. Energy transduction in ATP synthase. Nature. 1998;391:510–513. doi: 10.1038/35185. [DOI] [PubMed] [Google Scholar]
- 38.Evans E H, Brown R G. An appraisal of photosynthetic fluorescence kinetics as a probe of plant function. J Photochem Photobiol B. 1994;22:95–104. [Google Scholar]
- 39.Falk S, Samson G, Bruce D, Huner N P A, Laudenbach D E. Functional analysis of the iron-stress induced CP-43′-polypeptide of PS-II in the cyanobacterium Synechococcus sp. PCC-7942. Photosynth Res. 1995;45:51–60. doi: 10.1007/BF00032235. [DOI] [PubMed] [Google Scholar]
- 40.Falkowski P G, Kolber Z. Variations in the chlorophyll fluorescence yields in phytoplankton in the world oceans. Aust J Plant Physiol. 1995;22:341–355. [Google Scholar]
- 41.Fork D C, Satoh K. State I-State II transitions in the thermophilic blue-green alga (cyanobacterium) Synechococcus lividus. Photochem Photobiol. 1983;37:421–427. [Google Scholar]
- 42.Fujita Y, Murakami A, Aizawa K, Ohki K. Short-term and long-term adaptation of the photosynthetic apparatus: homeostatic properties of thylakoids. In: Bryant D A, editor. The molecular biology of Cyanobacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 677–692. [Google Scholar]
- 43.Furbank R T, Badger M R. Oxygen exchange associated with electron transport and photophosphorylation in spinach thylakoids. Biochim Biophys Acta. 1983;723:400–409. [Google Scholar]
- 44.Geerts D, Schubert H, de Vrieze G, Borrias M, Matthijs H C P, Weisbeek P J. Expression of Anabaena PCC 7937 plastocyanin in Synechococcus PCC 7942 enhances photosynthetic electron transfer and alters the electron distribution between photosystem I and cytochrome-c oxidase. J Biol Chem. 1994;269:28068–28075. [PubMed] [Google Scholar]
- 45.Genty B, Briantais J M, Baker N R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta. 1989;990:87–92. [Google Scholar]
- 46.Gilmore A M. Mechanistic aspects of xanthophyll cycle dependent photoprotection in higher plant chloroplasts and leaves. Physiol Plant. 1997;99:197–209. [Google Scholar]
- 47.Gilmore A M, Yamamoto H Y. Biochemistry of xanthophyll-dependent nonradiative energy dissipation. In: Yamamoto H Y, Smith C M, editors. Photosynthetic responses to the environment. Rockville, Md: American Society of Plant Physiologists; 1993. pp. 160–165. [Google Scholar]
- 48.Gindt Y M, Zhou J H, Bryant D A, Sauer K. Spectroscopic studies of phycobilisome subcore preparations lacking key core chromophores—assignment of excited-state energies to the L(CM), beta(18) and alpha (AP-B) chromophores. Biochim Biophys Acta. 1994;1186:153–162. doi: 10.1016/0005-2728(94)90174-0. [DOI] [PubMed] [Google Scholar]
- 49.Glazer A N, Chan C, Williams R C, Yeh S W, Clark J H. Kinetics of energy flow in the phycobilisome core. Science. 1985;230:1051–1053. doi: 10.1126/science.230.4729.1051. [DOI] [PubMed] [Google Scholar]
- 50.Goetze D C, Carpentier R. Ferredoxin-NADP+ reductase is the site of oxygen reduction in pseudocyclic electron transport. Can J Bot. 1994;72:256–260. [Google Scholar]
- 51.Goosney D L, Miller A G. High rates of O2 photoreduction by the unicellular cyanobacterium Synechocystis PCC 6803 as determined by the quenching of chlorophyll fluorescence. Can J Bot. 1997;75:394–401. [Google Scholar]
- 52.Guglielmi G, Cohen-Bazire G, Bryant D A. The structure of Gloeobacter violaceus and its phycobilisomes. Arch Microbiol. 1981;129:181–189. [Google Scholar]
- 53.Havaux M, Straser R J, Greppin H. A theoretical and experimental analysis of the qP and qN coefficients of chlorophyll fluorescence quenching and their relation to photochemical and nonphotochemical events. Photosynth Res. 1991;27:41–55. doi: 10.1007/BF00029975. [DOI] [PubMed] [Google Scholar]
- 54.Herbert S K, Martin R E, Fork D C. Light adaptation of cyclic electron-transport through photosystem-I in the cyanobacterium Synechococcus sp. PCC7942. Photosynth Res. 1995;46:277–285. doi: 10.1007/BF00020441. [DOI] [PubMed] [Google Scholar]
- 55.Hirano M, Satoh K, Katoh S. Plastoquinone as a common link between photosynthesis and respiration in blue-green alga. Photosynth Res. 1980;1:149–162. doi: 10.1007/BF00020594. [DOI] [PubMed] [Google Scholar]
- 56.Holzwarth A R. Is it time to throw away your apparatus for chlorophyll fluorescence induction? Biophys J. 1993;64:1280–1281. doi: 10.1016/S0006-3495(93)81461-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Horton P, Ruban A V, Walters R G. Regulation of light harvesting in green plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:655–684. doi: 10.1146/annurev.arplant.47.1.655. [DOI] [PubMed] [Google Scholar]
- 58.Hovenden M J, Seppelt R D. Utility of modulated fluorescence in measuring photosynthetic activity of Antarctic plants: field and laboratory studies. Aust J Plant Physiol. 1995;22:321–330. [Google Scholar]
- 59.Huner N P A, Öquist G, Hurry V M, Krol M, Falk S, Griffith M. Photosynthesis, photoinhibition and low temperature acclimation in cold tolerant plants. Photosynth Res. 1993;37:19–39. doi: 10.1007/BF02185436. [DOI] [PubMed] [Google Scholar]
- 60.Ibelings B W, Kroon B M A, Mur L R. Acclimation of Photosystem-II in a cyanobacterium and a eukaryotic green-alga to high and fluctuating photosynthetic photon flux densities, simulating light regimes induced by mixing in lakes. New Phytol. 1994;128:407–424. doi: 10.1111/j.1469-8137.1994.tb02987.x. [DOI] [PubMed] [Google Scholar]
- 60a.Ivanov, A., and N. Huner. Personal communication.
- 61.Jensen M, Siebke K. Fluorescence imaging of lichens in the macro scale. Symbiosis. 1997;23:183–195. [Google Scholar]
- 62.Jones L W, Myers J. A common link between photosynthesis and respiration in a blue-green alga. Nature. 1963;199:670–672. doi: 10.1038/199670a0. [DOI] [PubMed] [Google Scholar]
- 63.Kana T M. Rapid oxygen cycling in Trichodesmium thiebautii. Limnol Oceanogr. 1993;38:18–24. [Google Scholar]
- 64.Koenig F, Schmidt M. Gloeobacter violaceus—investigation of an unusual photosynthetic apparatus—absence of the long-wavelength emission of photosystem-I in 77 K fluorescence-spectra. Physiol Plant. 1995;94:621–628. [Google Scholar]
- 65.Kolbowski J, Schreiber U. Computer controlled phytoplankton analyser based on chlorophyll fluorescence analysis using 4 different wavelengths. Xth International Photosynthesis Congress. Photosynth Res. 1995;S1:10–013. [Google Scholar]
- 66.Krause G H, Weis E. Chlorophyll fluorescence and photosynthesis: the basics. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:313–349. [Google Scholar]
- 67.Krause G H, Vernotte C, Briantais J-M. Photoinduced quenching of chlorophyll fluorescence in intact chloroplasts and algae. Resolution into two components. Biochim Biophys Acta. 1982;679:116–124. [Google Scholar]
- 68.Krinner M, Hauska G, Hurt E, Lockau W. A cytochrome f-b6 complex with plastoquinol-cytochrome c oxidoreductase activity from Anabaena variabilis. Biochim Biophys Acta. 1982;681:110–117. [Google Scholar]
- 69.Lange O L, Bilger W, Rimke S, Schreiber U. Chlorophyll fluorescence of lichens containing green and blue-green algae during hydration by water vapor uptake and by addition of liquid water. Bot Acta. 1989;102:306–313. [Google Scholar]
- 70.Laudenbach D E, Straus N A. Characterization of a cyanobacterial iron stress-induced gene similar to psbC. J Bacteriol. 1988;170:5018–5026. doi: 10.1128/jb.170.11.5018-5026.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Laudenbach D E, Herbert S K, McDowell C, Fork D C, Grossman A, Straus N A. Cytochrome c-553 is not required for photosynthetic activity in the cyanobacterium Synechococcus. Plant Cell. 1990;2:913–924. doi: 10.1105/tpc.2.9.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee T, Tsuzuki M, Yokoyama K, Karube I. In-Vivo fluorometric method for early detection of cyanobacterial waterblooms. J App Phycol. 1994;6:489–495. [Google Scholar]
- 73.Leisner J M R, Bilger W, Lange O L. Chlorophyll fluorescence characteristics of the cyanobacterial lichen Peltigera rufescens under field conditions. 1. Seasonal patterns of photochemical activity and the occurrence of Photosystem II inhibition. Flora. 1996;191:261–273. [Google Scholar]
- 74.Leisner J M R, Green T G A, Lange O L. Photobiont activity of a temperate crustose lichen—long-term chlorophyll fluorescence and CO2 exchange measurements in the field. Symbiosis. 1997;23:165–182. [Google Scholar]
- 75.Leonhardt K G, Straus N A. An iron stress operon involved in photosynthetic electron transport in the marine cyanobacterium Synechococcus sp. PCC 7002. J Gen Microbiol. 1992;138:1613–1621. doi: 10.1099/00221287-138-8-1613. [DOI] [PubMed] [Google Scholar]
- 76.Leonhardt K G, Straus N A. Photosystem II genes isiA, psbD1 and psbC in Anabaena sp. PCC 7120: cloning, sequencing and the transcriptional regulation in iron-stressed and iron-repleted cells. Plant Mol Biol. 1994;24:63–73. doi: 10.1007/BF00040574. [DOI] [PubMed] [Google Scholar]
- 77.Li Q L, Canvin D T. Inorganic carbon accumulation stimulates linear electron flow to artificial electron-acceptors of Photosystem-I in air-grown cells of the cyanobacterium Synechococcus UTEX-625. Plant Physiol. 1997;114:1273–1281. doi: 10.1104/pp.114.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li Q L, Canvin D T. Effect of the intracellular inorganic carbon pool on chlorophyll-a fluorescence quenching and O2 photoreduction in air-grown cells of the cyanobacterium Synechococcus UTEX-625. Can J Bot. 1997;75:946–954. [Google Scholar]
- 79.Lien S, San Pietro A. On the reactivity of oxygen with photosystem I electron acceptors. FEBS Lett. 1979;99:189–193. [Google Scholar]
- 80.Lüttge U, Büdel B, Ball E, Strube F, Weber P. Photosynthesis of terrestrial cyanobacteria under light and desiccation stress as expressed by chlorophyll fluorescence and gas exchange. J Exp Bot. 1995;46:309–319. [Google Scholar]
- 81.Manna P, Vermass W. Lumenal proteins involved in respiratory electron-transport in the cyanobacterium Synechocystis sp. PCC 6803. Plant Mol Biol. 1997;35:407–416. doi: 10.1023/a:1005875124387. [DOI] [PubMed] [Google Scholar]
- 82.Maxwell D P, Falk S, Huner N P A. Photosystem II excitation and development of resistance to photoinhibition. Plant Physiol. 1995;107:687–694. doi: 10.1104/pp.107.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meunier P C, Watters J W, Colón-López M S, Sherman L A. Regulation of the O2-evolving mechanism during N2 fixation in the diazotrophic cyanobacterium Cyanothece sp. ATCC 51142. In: Mathis P, editor. Research in photosynthesis. II. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 389–392. [Google Scholar]
- 84.Meunier P C, Burnap R L, Sherman L A. Interaction of the photosynthetic and respiratory electron transport chains producing slow O2 signals under flashing light in Synechocystis sp. PCC 6803. Photosynth Res. 1995;45:31–40. doi: 10.1007/BF00032233. [DOI] [PubMed] [Google Scholar]
- 85.Mi H L, Endo T, Schreiber U, Ogawa T, Asada K. Electron donation from cyclic and respiratory flows to the photosynthetic interesystem chain is mediated by pyridine nucleotide dehydrogenase in the cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. 1992;33:1233–1237. [Google Scholar]
- 86.Mi H L, Endo T, Ogawa T, Asada K. Thylakoid membrane-bound, NADPH-specific pyridine nucleotide dehydrogenase complex mediates cyclic electron transport in the cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. 1995;36:661–668. [Google Scholar]
- 86a.Miller, A. Personal communication.
- 87.Miller A G, Canvin D T. The quenching of chlorophyll a fluorescence as a consequence of the transport of inorganic carbon by the cyanobacterium Synechococcus UTEX 625. Biochim Biophys Acta. 1987;894:407–413. [Google Scholar]
- 88.Miller A G, Espie G S, Canvin D T. Active transport of inorganic carbon increases the rate of O2 photo-reduction by the cyanobacterium Synechococcus UTEX 625. Plant Physiol. 1990;88:6–9. doi: 10.1104/pp.88.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Miller A G, Espie G S, Bruce D. Characterization of the non-photochemical quenching of chlorophyll fluorescence that occurs during the active accumulation of inorganic carbon in the cyanobacterium Synechococcus PCC 7942. Photosynth Res. 1996;49:251–262. doi: 10.1007/BF00034786. [DOI] [PubMed] [Google Scholar]
- 90.Miller A G, Espie G S, Canvin D T. Chlorophyll a fluorescence yield as a monitor of both active CO2 and HCO3− transport by the cyanobacterium Synechococcus UTEX 6251. Plant Physiol. 1988;86:655–658. doi: 10.1104/pp.86.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Miller A G, Espie G S, Canvin D T. The effects of inorganic carbon and oxygen on fluorescence in the cyanobacterium Synechococcus UTEX 625. Can J Bot. 1991;69:1151–1160. [Google Scholar]
- 92.Mir N A, Salon C, Canvin D T. Photosynthetic nitrite reduction as influenced by the internal inorganic carbon pool in air-grown cells of Synechococcus UTEX-625. Plant Physiol. 1995;108:313–318. doi: 10.1104/pp.108.1.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Miyachi S, Kotzabasis K, Thielmann J, Senger H, Burger J. Photosynthetic characteristics of 3 strains of cyanobacteria grown under low-CO2 or high-CO2 conditions. Z Naturforsch Sect C. 1996;51:40–46. [Google Scholar]
- 94.Mullineaux C W, Allen J F. The state 2 transition in the cyanobacterium Synechococcus 6301 can be driven by respiratory electron flow into the plastoquinone pool. FEBS Lett. 1986;205:155–160. [Google Scholar]
- 95.Mullineaux C W, Allen J F. State 1-state 2 transitions in the cyanobacterium Synechococcus 6301 are controlled by the redox state of electron carriers between Photosystems I and II. Photosynth Res. 1990;23:297–311. doi: 10.1007/BF00034860. [DOI] [PubMed] [Google Scholar]
- 96.Mullineaux C W, Tobin M J, Jones G R. Mobility of photosynthetic complexes in thylakoid membranes. Nature. 1997;390:421–424. [Google Scholar]
- 97.Murata N. Control of excitation transfer in photosynthesis. I. Light-induced change of chlorophyll a fluorescence in Porphyridium cruentum. Biochim Biophys Acta. 1969;172:242–251. doi: 10.1016/0005-2728(69)90067-x. [DOI] [PubMed] [Google Scholar]
- 98.Myers J, Graham J R, Wang R T. Light harvesting in Anacystis nidulans studied in pigment mutants. Plant Physiol. 1980;66:1144–1149. doi: 10.1104/pp.66.6.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ögren E. The significance of photoinhibition for photosynthetic productivity. In: Baker N R, Bowyer J R, editors. Photoinhibition of photosynthesis: from molecular mechanisms to the field. Oxford, United Kingdom: Bios Scientific Publishers; 1994. pp. 433–447. [Google Scholar]
- 100.Ögren E, Öquist G. Photoinhibition of photosynthesis in Lemna gibba as induced by the interaction between light and temperature. II. Photosynthetic electron transport. Physiol Plant. 1984;62:187–192. [Google Scholar]
- 101.Ögren E, Rosenqvist E. On the significance of photoinhibition of photosynthesis in the field and its generality among species. Photosynth Res. 1992;33:63–71. doi: 10.1007/BF00032983. [DOI] [PubMed] [Google Scholar]
- 102.Öquist G, Anderson J M, McCaffery S, Chow W S. Mechanistic differences in photoinhibition of sun and shade plants. Planta. 1992;188:422–431. doi: 10.1007/BF00192810. [DOI] [PubMed] [Google Scholar]
- 103.Öquist G, Huner N P A. Cold-hardening-induced resistance to photoinhibition of photosynthesis in winter rye is dependent upon an increased capacity for photosynthesis. Planta. 1993;189:150–156. [Google Scholar]
- 104.Öquist G, Chow W S. On the relationship between the quantum yield of Photosystem II electron transport, as determined by chlorophyll fluorescence and the quantum yield of CO2-dependent O2 evolution. Photosynth Res. 1992;33:51–62. doi: 10.1007/BF00032982. [DOI] [PubMed] [Google Scholar]
- 105.Öquist G, Campbell D, Clarke A K, Gustafsson P. The cyanobacterium Synechococcus modulates PS II function in response to excitation stress through D1 exchange. Photosynth Res. 1995;46:151–158. doi: 10.1007/BF00020425. [DOI] [PubMed] [Google Scholar]
- 106.Öquist G, Hurry V M, Huner N P A. Low-temperature effects on photosynthesis and correlation with freezing tolerance in spring and winter cultivars of wheat and rye. Plant Physiol. 1993;101:245–250. doi: 10.1104/pp.101.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Öquist G, Hurry V M, Huner N P A. The temperature dependence of the redox state of QA and susceptibility of photosynthesis to photoinhibition. Plant Physiol Biochem. 1993;31:683–691. [Google Scholar]
- 108.Palmqvist K. Uptake and fixation of CO2 in lichen photobionts. Symbiosis. 1995;18:95–109. [Google Scholar]
- 109.Palmqvist K. Photosynthetic CO2-use efficiency in lichens and their isolated photobionts: the possible role of a CO2-concentrating mechanism. Planta. 1993;191:48–56. [Google Scholar]
- 110.Palmqvist K, Samuelsson G, Badger M R. Photobiont-related differences in carbon acquisition among green-algal lichens. Planta. 1994;195:70–79. [Google Scholar]
- 111.Papageorgiou G, Govindjee Light-induced changes in the fluorescence yield of chlorophyll a in vivo. 1. Anacystis nidulans. Biophys J. 1968;8:1299–1315. doi: 10.1016/S0006-3495(68)86557-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Papageorgiou G C. The photosynthesis of cyanobacteria (blue bacteria) from the perspective of signal analysis of chlorophyll a fluorescence. J Sci Ind Res. 1996;55:596–617. [Google Scholar]
- 112a.Park, Y.-I., G. Samuelsson, and G. Öquist. Unpublished data.
- 113.Peschek G A, Schmetterer G. Evidence for plastoquinol-cytochrome f/b-563 oxidoreductase as a common electron donor to P-700 and cytochrome oxidase in cyanobacteria. Biochem Biophys Res Commun. 1982;108:1188–1195. doi: 10.1016/0006-291x(82)92126-x. [DOI] [PubMed] [Google Scholar]
- 114.Rippka R, Deruelles J, Waterbury J B, Herdman M, Stanier R Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol. 1979;111:1–61. [Google Scholar]
- 115.Rippka R, Waterbury J, Cohen-Bazire G. A cyanobacterium which lacks thylakoids. Arch Microbiol. 1974;100:419–436. [Google Scholar]
- 116.Romero J M, Lara C, Sivak M N. Effect of carbon and nitrogen assimilation on chlorophyll fluorescence emission by the cyanobacterium Anacystis nidulans. Physiol Plant. 1992;85:433–438. [Google Scholar]
- 117.Sabbert D, Engelbrecht S, Junge W. Intersubunit rotation in active F-ATPase. Nature. 1996;381:623–625. doi: 10.1038/381623a0. [DOI] [PubMed] [Google Scholar]
- 118.Salehian O, Bruce D. Distribution of excitation energy in photosynthesis, quantification of fluorescence yields form intact cyanobacteria. J Lumin. 1992;51:91–98. [Google Scholar]
- 119.Sandmann G. Formation of plastocyanin and cytochrome c-553 in different species of blue-green algae. Arch Microbiol. 1986;145:76–79. [Google Scholar]
- 120.Sandmann G, Böger P. Copper induced exchange of plastocyanin and cytochrome c-553 in cultures of Anabaena variabilis and Plectonema borianum. Plant Sci. 1980;17:417–424. [Google Scholar]
- 121.Scherer S, Stürzl E, Böger P. Interaction of respiratory and photosynthetic electron transport in Anabaena variabilis Kütz. Arch Microbiol. 1982;132:333–337. [Google Scholar]
- 122.Scherer S, Almon H, Böger P. Interaction of photosynthesis, respiration and nitrogen fixation in cyanobacteria. Photosynth Res. 1988;15:95–114. doi: 10.1007/BF00035255. [DOI] [PubMed] [Google Scholar]
- 123.Schneegurt M, Sherman D M, Nayar S, Sherman L M. Oscillating behaviour of carbohydrate granule formation and dinitrogen fixation in the cyanobacterium Cyanothece sp. strain ATCC 51142. J Bacteriol. 1994;176:1586–1597. doi: 10.1128/jb.176.6.1586-1597.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schneegurt M A, Sherman D M, Sherman L M. Composition of the carbohydrate granules of the cyanobacterium, Cyanothece sp. strain ATCC 51142. Arch Microbiol. 1997;167:89–98. [PubMed] [Google Scholar]
- 125.Schreiber U. New emitter-detector-cuvette assembly for measuring modulated chlorophyll fluorescence of highly diluted suspensions in conjunction with the standard PAM fluorometer. Z Naturforsch C. 1994;49:646–656. [Google Scholar]
- 126.Schreiber U, Gademann R, Ralph P J, Larkum A W D. Assessment of photosynthetic performance of Prochloron in Lissoclinum patella in hospite by chlorophyll fluorescence measurements. Plant Cell Physiol. 1997;38:945–951. [Google Scholar]
- 127.Schreiber U, Hormann H, Neubauer C, Klughammer C. Assessment of photosystem II photochemical quantum yield by chlorophyll fluorescence quenching analysis. Aust J Plant Physiol. 1995;22:209–220. [Google Scholar]
- 128.Schreiber U, Endo T, Mi H, Asada K. Quenching analysis of chlorophyll fluorescence by the saturation pulse method: particular aspects relating to the study of eukaryotic algae and cyanobacteria. Plant Cell Physiol. 1995;36:873–882. [Google Scholar]
- 129.Schreiber U, Schliwa U, Bilger W. Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth Res. 1986;10:51–62. doi: 10.1007/BF00024185. [DOI] [PubMed] [Google Scholar]
- 130.Schroeter B. In-situ photosynthetic differentiation of the green algal and the cyanobacterial photobiont in the crustose lichen Placopsis contortuplicata. Oecologia. 1994;98:212–220. doi: 10.1007/BF00341474. [DOI] [PubMed] [Google Scholar]
- 131.Schubert H, Matthijs H, Mur L. In vivo assay of P700 redox changes in the cyanobacterium Fremyella diplosiphon and the role of cytochrome-c-oxidase in regulation of photosynthetic electron transfer. Photosythetica. 1995;31:517–527. [Google Scholar]
- 132.Schubert H, Matthijs H C P, Mur L R, Schiewer U. Blooming of cyanobacteria in turbulent water with steep light gradients: the effect of intermittent light and dark periods on the oxygen evolution capacity of Synechocystis sp. PCC 6803. FEMS Microbiol Ecol. 1995;18:237–245. [Google Scholar]
- 133.Schubert H, Forster R, Sager S. In situ measurement of state transition in cyanobacterial blooms: kinetics and extent of the state change in relation to underwater light and vertical mixing. Mar Ecol Prog Ser. 1995;128:99–108. [Google Scholar]
- 134.Seaton G R, Walker D A. Chlorophyll fluorescence as a measure of photosynthetic carbon metabolism. Proc R Soc London Ser B. 1990;242:29–35. [Google Scholar]
- 135.Shyam R, Raghavendra A S, Sane P V. Role of dark respiration in photoinhibition of photosynthesis and its reactivation in the cyanobacterium Anacystis nidulans. Physiol Plant. 1993;88:446–452. [Google Scholar]
- 136.Sidler W. Phycobilisome and phycobiliprotein structures. In: Bryant D A, editor. The molecular biology of cyanobacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 139–216. [Google Scholar]
- 137.Strasser R J, Srivastava A, Govindjee Polyphasic chlorophyll-alpha fluorescence transient in plants and cyanobacteria. Photochem Photobiol. 1995;61:32–42. [Google Scholar]
- 138.Sundberg B, Campbell D, Palmqvist K. Predicting CO2 gain and photosynthetic light acclimation from fluorescence yield and quenching in cyano-lichens. Planta. 1997;201:138–145. [Google Scholar]
- 139.Tanaka Y, Katada S, Ishikawa H, Ogawa T, Takabe T. Electron flow front NAD(P)H dehydrogenase to Photosystem-I is required for adaptation to salt shock in the cyanobacterium-Synechocystis sp. PCC 6803. Plant Cell Physiol. 1997;38:1311–1318. [Google Scholar]
- 140.Tandeau de Marsac N, Houmard J. Complementary chromatic adaptation: physiological conditions and action spectra. Methods Enzymol. 1988;167:318–328. [Google Scholar]
- 141.Ting C S, Owens T G. Limitations of the pulse-modulated technique for measuring the fluorescence characteristics of algae. Plant Physiol. 1992;100:367–373. doi: 10.1104/pp.100.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Torzillo G, Accolla P, Pinzani E, Masojidek J. In-situ monitoring of chlorophyll fluorescence to assess the synergistic effect of low-temperature and high irradiance stresses in Spirulina cultures grown outdoors in photobioreactors. J Appl Phycol. 1996;8:283–291. [Google Scholar]
- 143.Trebst A. Inhibitors in electron flow. Methods Enzymol. 1980;69:675–715. [Google Scholar]
- 144.Trissl H-W, Lavergne J. Fluorescence induction from photosystem II: analytical equations for the yields of photochemistry and fluorescence derived from analysis of a model including exciton-radical pair equilibrium and restricted energy transfer between photosynthetic units. Aust J Plant Physiol. 1995;22:183–193. doi: 10.1016/S0006-3495(95)80429-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.van Kooten O, Snel J F H. The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth Res. 1990;25:147–150. doi: 10.1007/BF00033156. [DOI] [PubMed] [Google Scholar]
- 146.van Walraven H S, Strotman H, Schwarz O, Rumberg B. The H+/ATP coupling ratio of the ATP synthase from thiol-modulated chloroplasts and two cyanobacterial strains is four. FEBS Lett. 1996;379:309–313. doi: 10.1016/0014-5793(95)01536-1. [DOI] [PubMed] [Google Scholar]
- 147.Vermaas W F J, Shen G, Styring S. Electrons generated by photosystem II are utilized by an oxidase in the absence of photosystem I in the cyanobacterium Synechocystis sp. PCC 6803. FEBS Lett. 1994;337:103–108. doi: 10.1016/0014-5793(94)80638-1. [DOI] [PubMed] [Google Scholar]
- 148.Vernotte C, Etienne A L, Briantais J M. Quenching of the system II chlorophyll fluorescence by the plastoquinone pool. Biochim Biophys Acta. 1979;545:519–527. doi: 10.1016/0005-2728(79)90160-9. [DOI] [PubMed] [Google Scholar]
- 149.Vernotte C, Astier C, Olive J. State 1-state 2 adaptation in the cyanobacteria Synechocystis PCC 6714 wild type and Synechocystis PCC 6803 wild type and phycocyanin-less mutant. Photosynth Res. 1990;26:203–212. doi: 10.1007/BF00033133. [DOI] [PubMed] [Google Scholar]
- 150.Vonshak A, Torzillo G, Accolla P, Tomaselli L. Light and oxygen stress in Spirulina platensis (cyanobacteria) grown outdoors in tubular reactors. Physiol Plant. 1996;97:175–179. [Google Scholar]
- 151.Walker D. The use of the oxygen electrode and fluorescence probes in simple measurements of photosynthesis. Kings Lynn, United Kingdom: Hansatech Instruments Ltd.; 1987. [Google Scholar]
- 152.Walters R G, Horton P. Theoretical assessment of alternative mechanisms for non-photochemical quenching of PS II fluorescence in barley leaves. Photosynth Res. 1993;36:119–139. doi: 10.1007/BF00016277. [DOI] [PubMed] [Google Scholar]
- 153.Weis E, Berry J A. Quantum efficiency of photosystem II in relation to energy dependent quenching of chlorophyll fluorescence. Biochim Biophys Acta. 1987;894:198–208. [Google Scholar]
- 154.Zhang L, McSpadden B, Pakarasi H B, Whitmarsh J. Copper mediated regulation of cytochrome c553 and plastocyanin in the cyanobacterium Synechocystis 6803. J Biol Chem. 1992;267:19054–19059. [PubMed] [Google Scholar]
- 155.Zhang L, Pakrasi H B, Whitmarsh J. Photoautotrophic growth of the cyanobacterium Synechocystis sp. PCC 6803 in the absence of cytochrome c553 and plastocyanin. J Biol Chem. 1994;269:5036–5042. [PubMed] [Google Scholar]
- 156.Zhao J, Zhou J, Bryant D A. Energy transfer processes in phycobilisomes as deduced from analyses of mutants of Synechococcus sp. PCC 7002. In: Murata N, editor. Research in photosynthesis. Vol. 1. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1992. pp. 25–32. [Google Scholar]
- 157.Zhou, G., and P. Gustafsson. Unpublished data.
- 158.Zhou, G. Unpublished data.