Abstract
Lactobacilli are gram-positive bacteria usually found in the normal flora and are commonly used as probiotic treatments for vaginal candidiasis. Lactobacilli are normally considered non-pathogenic; however, certain risk factors can make a patient susceptible to severe infections. This case describes an immunocompetent 61-year-old female with an automated intracardiac defibrillator who presented with a 10-day history of nausea and vomiting. Furthermore, diagnostic tests, including a transesophageal echocardiography, revealed a large vegetation, and blood cultures were consistently positive for Lactobacillus. The patient was treated with intravenous penicillin and gentamicin, along with removal of the automated intracardiac defibrillator. In patients with significant underlying conditions, physicians should consider Lactobacillus as a causative organism to avoid delays in treatment.
Keywords: Lactobacillus endocarditis, probiotic, endocarditis
Introduction
Lactobacilli are facultative, anaerobic, and non-spore-forming gram-positive rods that ferment to yield lactic acid.1 They are part of the normal flora of the oropharynx, gastrointestinal, and genitourinary tracts. Lactobacillus strains are commonly sold in supplements and probiotics for a theoretical effect on the stimulation of the immune system and for their ability to prevent colonization of pathogenic organisms in the colon and genitourinary tracts.2,3 Most of the time, Lactobacillus is considered a commensal organism that has limited clinical significance. Risk factors such as diabetes mellitus, implantable heart devices, structural heart disease, and immunosuppression can make a patient susceptible to severe infections.2,4
Endocarditis associated with Lactobacillus is rare, responsible for less than 0.05% of all endocarditis cases, and is associated with a 30% mortality rate.5,6 Lactobacillus endocarditis is usually associated with immunodeficiency and severe comorbidities.7 Identifying Lactobacillus species and its sensitivity profile is crucial to provide the most effective therapeutic treatment regimen for favorable patient outcomes. However, identifying the species and sensitivity profile can be difficult. In practice, Lactobacillus species isolated from blood cultures are not further characterized due to their perceived low virulence.3
Here, we describe a case of Lactobacillus endocarditis in a patient with no immunodeficiencies and who reported taking a Lactobacillus probiotic. The sensitivity profile revealed that the Lactobacillus was resistant to meropenem. Given the rising prevalence of diabetes mellitus and the increased use of probiotics, it is expected that there will be a rise in infections due to Lactobacillus species. This case emphasizes the importance of early identification and prompt treatment of Lactobacillus endocarditis.
Case
A 61-year-old Caucasian immunocompetent female with a past medical history of uncontrolled diabetes mellitus, nonischemic cardiomyopathy (left ventricular ejection fraction 20%–25%), ventricular tachycardia, and ventricular fibrillation status post biventricular automated intracardiac defibrillator (AICD) presented to our hospital with nausea and emesis. She was recently admitted to an outside facility and blood cultures from that admission were significant for Lactobacillus. Her examination was significant for systolic murmur and extensive bilateral redness around the upper thigh. The rest of the physical examination was within normal limits.
Laboratory data on arrival were significant for a white blood cell count of 20.57 k/μL, total bilirubin of 2.3 mg/dL, alkaline phosphatase of 177 U/L, and lactic acid of 2.20 mmol/L. The patient was afebrile on admission and throughout her hospital stay. A computed tomography (CT) scan of the abdomen showed pericholecystic fluid, but a hepatobiliary iminodiacetic acid scan did not show biliary obstruction. She was initially started on ciprofloxacin 400 mg intravenously (IV) twice daily and metronidazole IV 500 mg every 8 h. Over the next 24 h, she became hypotensive, tachycardic, and tachypneic. Three sets of blood cultures grew Lactobacillus, and final speciation showed Lactobacillus casei, Lactobacillus paracasei, and Lactobacillus zeae. Ciprofloxacin and metronidazole were discontinued, and meropenem 1 g IV every 8 h was initiated before susceptibility reports were released. Blood cultures were repeated after 2 days and remained positive for Lactobacillus. A transesophageal echocardiography (TEE), shown in Figure 1, was performed, revealing 2 × 3 cm vegetations on the tricuspid valve and the right atrial lead, as shown in Figure 2.
Figure 1.
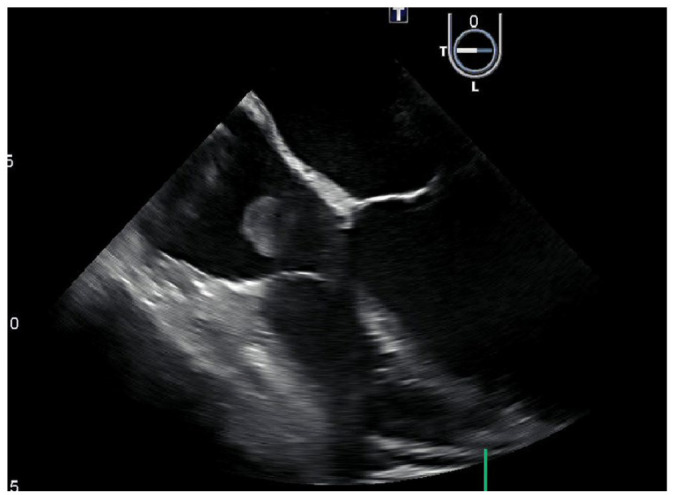
The transesophageal echocardiography showed a 2 × 3 cm vegetation on the anterior leaflet of the tricuspid valve.
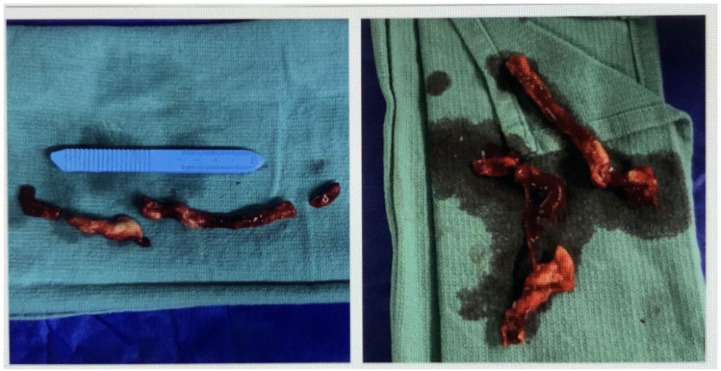
Figure 2.
Post-surgical vegetations.
The patient was transferred to a tertiary care facility for a cardiothoracic surgery evaluation. As she was considered high risk for open surgical intervention, she underwent catheter-based large-bore aspiration thrombectomy and AICD removal. The removed material was sent for a bacterial culture test, and the presence of Lactobacilli was confirmed. Antibiotics were changed to ampicillin 2 g IV every 4 h and gentamicin 80 mg IV daily which she was discharged home on. The patient completed 6 weeks of antibiotic therapy with multiple negative blood cultures, and repeat echocardiogram did not show any vegetations.
Minimal inhibitory concentrations (MICs) were determined by use of the agar microdilution method. The cutoffs used for penicillin susceptibility were as follows: less than or equal to 8 μg/mL was considered susceptible and greater than 8 μg/mL was considered resistant. The cutoffs used for meropenem susceptibility were as follows: less than 1 μg/mL was considered susceptible and greater than 2 μg/mL was considered resistant. Susceptibilities of the Lactobacilli isolated from the blood cultures and removed material from AICD were listed as penicillin MIC 1 μg/mL (susceptible) and meropenem MIC 4 μg/mL (resistant).
Discussion
Infective endocarditis should be suspected in patients with fever and certain risk factors, with or without bacteremia. Cardiac risk factors include history of infective endocarditis, the presence of a prosthetic valve or cardiac device, or history of valvular or congenital heart disease. Noncardiac risk factors include intravenous drug use, immunosuppression, or recent surgery. The modified Duke criteria include both major and minor criteria and are used as a guide for diagnosing endocarditis. Diagnosis must be made promptly to ensure the initiation of appropriate empiric antibiotic regimens and identify high-risk patients who may benefit from early surgical intervention. The selection of empiric antibiotic regimens is based on patient factors, prior antimicrobial exposures, and epidemiology. Duration of therapy is to begin on the first day on which blood cultures are negative.8
Lactobacilli are part of the normal commensals of the oropharynx, gastrointestinal tract, and the female genital tract.1 These areas are the most common portals of entry for a Lactobacillus bacteremia. Lactobacilli have low virulence and rarely cause infection in immunocompetent patients.9 When Lactobacilli grow in a culture, they are usually regarded as contaminants and antibiotic therapy is withheld. Infections with Lactobacilli are uncommon and poorly defined. Lactobacilli are estimated to cause only 0.05% of all infective endocarditis cases.10 Careful analysis of the patient’s signs, symptoms, and laboratory workup is essential for diagnosis of a clinical infection. In most cases, Lactobacillus bacteremia was associated with clear signs of clinical illness, including fever, elevated leukocyte counts, and elevated C-reactive protein values.10
Infection with Lactobacillus can be a complication in patients who are already chronically ill and debilitated. Risk factors for Lactobacillus bacteremia include immunosuppression and underlying comorbidities, including cancer, recent abdominal surgery, implantable heart devices, and diabetes mellitus. Prior prolonged antibiotic therapy ineffective for Lactobacilli has also been identified as a risk factor.11 When Lactobacillus bacteremia is identified, it serves as an indicator of serious underlying illness and poor long-term prognosis. Case reports that result in patient death following Lactobacillus bacteremia are usually found to be from other underlying conditions and not the infection itself.4 Our patient has a past medical history of uncontrolled diabetes mellitus and an AICD, but does not have any other immunosuppressive-related comorbidities that would put her at risk for Lactobacillus bacteremia.
Lactobacillus bacteremia may be polymicrobial. Polymicrobial isolates were identified in between 39% and 60% of cases of Lactobacillus bacteremia. Concomitant bloodstream isolates usually include streptococci, Candida species, and/or enteric gram-negative bacilli.4 In our patient, Lactobacillus was the only organism isolated from the total of five positive blood cultures.
In a review of 45 cases of Lactobacillus bacteremia, 44% of patients died during hospitalization, and mortality 1 year following the bacteremia was 60%.4 This emphasizes the importance of prompt recognition, organism isolation, and treatment for Lactobacillus bacteremia. An immunocompetent patient is described in a published case study of a bioprosthetic aortic valve Lactobacillus endocarditis, presenting with severe aortic regurgitation, which responded to conventional medical and surgical treatment.12 This may suggest Lactobacilli could target intracardiac devices more than native valves.
Molecular studies suggest that Lactobacillus species may facilitate the breakdown of glycoproteins and the synthesis and lysis of fibrin clots, which may aid in the colonization of a valve and survival of bacteria.13 Antibiotic susceptibility of Lactobacilli is variable. In a retrospective study of 200 cases of Lactobacillus infections, the most commonly used regimens included penicillin monotherapy (n = 35), penicillin therapy combined with aminoglycoside (n = 20), and cephalosporins in monotherapy (n = 16).14 Lactobacillus is typically susceptible to β-lactam antibiotics, yet resistant strains have been reported.9 Clindamycin and penicillin are the most active agents in vitro, and vancomycin resistance is common, dependent on the strain of Lactobacillus.4 Previous data demonstrate generally low MICs to imipenem, piperacillin-tazobactam, erythromycin, and clindamycin.15 Although no prospective trials have been conducted, combination therapy with a penicillin and an aminoglycoside has been successful in treatment for Lactobacillus bacteremia and is the recommended regimen for this type of infection.16
Conclusion
This case report demonstrates the need to use caution and clinical judgment in patients who have positive blood cultures for Lactobacillus and risk factors such as implantable heart devices, especially with the rising incidence of diabetes mellitus and overall use of probiotics. Our patient, who reports taking probiotics, had a positive culture with Lactobacillus at an outside facility which was left untreated. The patient required multiple surgical interventions and a prolonged antibiotic course as a result. We believe that more data are required to guide assessment and treatment in patients with infective endocarditis secondary to Lactobacillus.
Acknowledgments
We would like to thank the patient who entrusted us to be part of her care and permitted us to share her case.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed consent: Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
ORCID iD: Elizabeth DeMarco  https://orcid.org/0000-0002-8038-3640
https://orcid.org/0000-0002-8038-3640
References
- 1. Husni RN, Gordon SM, Washington JA, et al. Lactobacillus bacteremia and endocarditis: review of 45 cases. Clin Infect Dis 1997; 25(5): 1048–1055. [DOI] [PubMed] [Google Scholar]
- 2. Franko B, Fournier P, Jouve T, et al. Lactobacillus bacteremia: pathogen or prognostic marker? Med Mal Infect 2017; 47(1): 18–25. [DOI] [PubMed] [Google Scholar]
- 3. Ozer M, Goksu SY, Shahverdiani A, et al. Lactobacillus acidophilus-induced endocarditis and associated splenic abscess. Case Rep Infect Dis 2020; 2020: 1382709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ramos-Coria D, Canto-Losa J, Carrillo-Vázquez D, et al. Lactobacillus gasseri liver abscess and bacteremia: a case report. BMC Infect Dis 2021; 21(1): 518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Encarnacion CO, Loranger AM, Bharatkumar AG, et al. Bacterial endocarditis caused by Lactobacillus acidophilus leading to rupture of sinus of Valsalva Aneurysm. Tex Heart Inst J 2016; 43(2): 161–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Prendergast BD. The changing face of infective endocarditis. Heart 2006; 92(7): 879–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Salvana EMT, Frank M. Lactobacillus endocarditis: case report and review of cases reported since 1992. J Infect 2006; 53(1): e5–e10. [DOI] [PubMed] [Google Scholar]
- 8. Baddour LN, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications. Circulation 2015; 132: 1435–1486. [DOI] [PubMed] [Google Scholar]
- 9. Vanichanan J, Chávez V, Wanger A, et al. Carbapenem-resistant Lactobacillus intra-abdominal infection in a renal transplant recipient with a history of probiotic consumption. Infection 2016; 44(6): 793–796. [DOI] [PubMed] [Google Scholar]
- 10. Borriello SP, Hammes WP, Holzapfel W, et al. Safety of probiotics that contain lactobacilli or bifidobacteria. Clin Infect Dis 2003; 36(6): 775–780. [DOI] [PubMed] [Google Scholar]
- 11. Antony SJ, Stratton CW, Dummer JS. Lactobacillus bacteremia: description of the clinical course in adult patients without endocarditis. Clin Infect Dis 1996; 23(4): 773–778. [DOI] [PubMed] [Google Scholar]
- 12. Tavernese A, Stelitano M, Mauceri A, et al. Progression of Lactobacillus plantarum prosthetic valve endocarditis followed by transesophageal echocardiogram. Int J Infect Dis 2020; 97: 160–161. [DOI] [PubMed] [Google Scholar]
- 13. Oakey HJ, Harty DW, Knox KW. Enzyme production by lactobacilli and the potential link with infective endocarditis. J Appl Bacteriol 1995; 78(2): 142–148. [DOI] [PubMed] [Google Scholar]
- 14. Cannon JP, Lee TA, Bolanos JT, et al. Pathogenic relevance of Lactobacillus: a retrospective review of over 200 cases. Eur J Clin Microbiol Infect Dis 2005; 24(1): 31–40. [DOI] [PubMed] [Google Scholar]
- 15. Salminen MK, Rautelin H, Tynkkynen S, et al. Lactobacillus bacteremia, species identification, and antimicrobial susceptibility of 85 blood isolates. Clin Infect Dis 2006; 42(5): e35–e44. [DOI] [PubMed] [Google Scholar]
- 16. Salminen MK, Rautelin H, Tynkkynen S, et al. Lactobacillus bacteremia, clinical significance, and patient outcome, with special focus on probiotic L. rhamnosus GG. Clin Infect Dis 2004; 38(1): 62–69. [DOI] [PubMed] [Google Scholar]