Abstract
Paget’s disease of the breast is a rare pathology resulting from abnormal proliferation of glandular epithelial cells in the nipple–areolar epidermis. The disease is named after James Paget, a pathologist and surgeon, who reported a relationship between a nipple rash and mammary gland tumors in 1874. Early diagnosis may be quite difficult. Histopathology can give the definitive diagnosis and the treatment depends upon the presence or absence of an associated breast lump. The treatment options include simple lumpectomy, mastectomy or radical mastectomy depending upon the lump (cancer) in the diseased breast. Herein, we present a case of Paget’s disease of the breast presented with a long history of nipple eczema.
Keywords: Breast cancer, diagnostic challenge, differential diagnosis, Paget’s disease
Introduction
Paget’s disease of the breast (PDB) is an uncommon histological breast cancer accounting for 1%–3% of female breast cancer. It affects mainly post-menopausal women with an average age of 62 years.1 It presents as an eroding ulcer of the nipple. Its appearance can sometimes be mistaken as a benign condition, hence delays in diagnosis.2 The most common treatment is complete mastectomy. The 5-year overall survival rate of patients with PDB is greater than 80% but is lower among the older and Black population.3 In this case, we report a case of Paget’s disease (PD) where the patient lived with the ulcer for 20 years.
Case presentation
A 45-year-old female, P3L3, presented with a left nipple lesion for more than 20 years. She reported to experience itchiness on her right nipple initially which was accompanied by skin peeling and a pricking sensation. The lesion gradually worsened involving the whole nipple and darkening of the skin surrounding the nipple that became ulcerative that was producing pus-like discharge that later progressed to total disruption of her nipple. During the course of her illness, she used various topical medications prescribed at peripheral health centers and over-the-counter, but no definitive diagnosis was yielded and no improvement could be seen. She denied a history of breast trauma. She has three children, all delivered by cesarean section, and has no history of contraceptive and tobacco use. Currently, she is 1 year post-menopause. She had her menarche at 14 years of age with a regular 28–30 days cycle. There is no family history of breast, ovarian or prostate cancer.
On initial examination, she was hemodynamically stable with vital signs within normal range. She had a body mass index of 30 kg/m2. Breast examination revealed an eczematous lesion confined to the right nipple associated with an ulcer on the areola (no nipple appreciated) measuring 2 × 4 cm and non-tender (Figure 1). There was a mass on the right lower outer breast. No lymph nodes appreciated on both axillas, and her other systemic examination was unremarkable, also confirmed by ultrasonography. Complete blood count (CBC) revealed a leukocyte count of 5.26 × 109/L, hemoglobin of 12.5 g/dL and a platelet count of 249 × 109/L. There were normal findings on the abdominal pelvic ultrasound, but her plain chest X-ray showed cannonball appearance suggestive of lung metastasis (Figure 2). The histology report of the breast tissue biopsy revealed PD of the nipple (Figure 3) and the core biopsy of the mass revealed infiltrating ductal carcinoma and Nottingham score 7/9, positive for ER (estrogen receptor) and PR (progesterone receptor) but negative for HER-2, and her metastasis stage was cT4N0M1.
Figure 1.
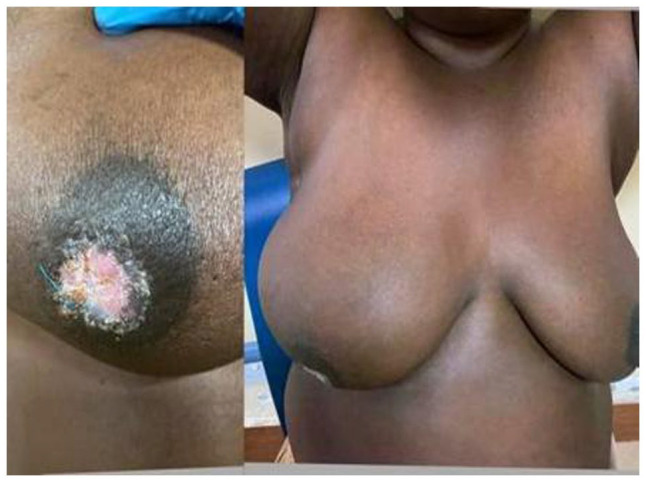
Clinical photograph showing nipple–areolar ulcer on right breast.
Figure 2.
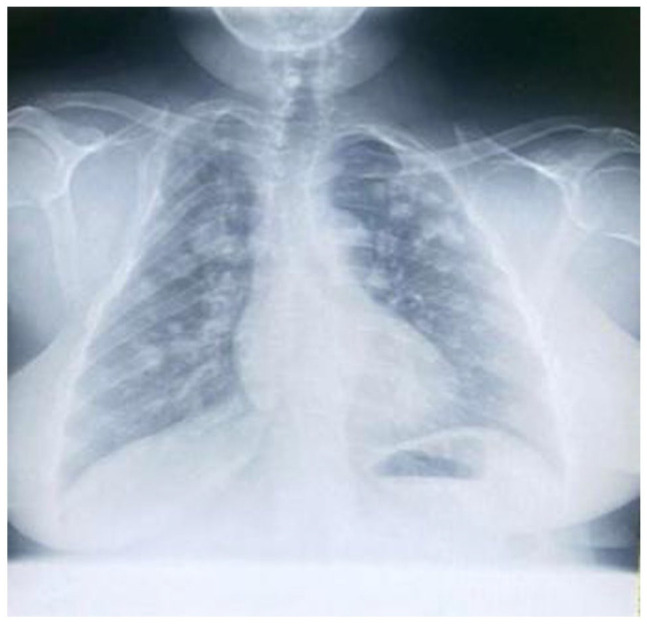
Plain chest X-ray showing “cannon-ball” lesions.
Figure 3.
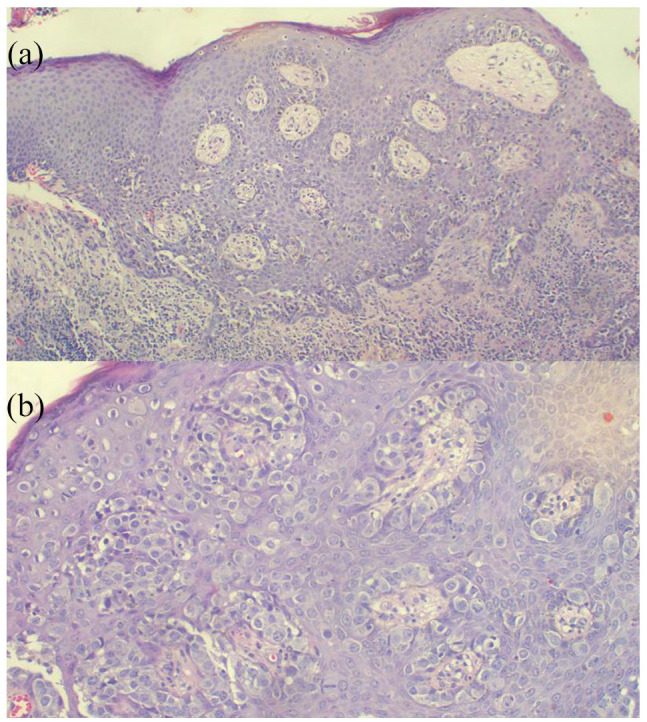
(a) Histopathology of Paget’s disease of the breast displaying single or clusters of cells spread throughout the epidermis; underlying dermis has chronic inflammation (H&E 100 × original magnification). (b) Photomicroscopy highlighting high power of the Paget cells with abundant pale cytoplasm, large irregular nuclei with prominent nucleoli (H&E 200 × original magnification).
The patient was referred to our oncology unit for further evaluation whereby she was planned for palliative chemotherapy (Flourouracil, Epirubicin, Cyclophosphamide × 6 cycles). Her baseline blood workup prior to chemotherapy showed a normal CBC with a hemoglobin of 13.2 g/dL, alanine transaminase of 19 U/L, aspartate transaminase of 19 U/L and serum creatinine of 62.15 µmol/L. Echocardiogram was also normal with an ejection fraction of 56%. She has received her first chemotherapy cycle, and it is currently doing well under oncology management.
Discussion
PDB was first reported in 1874 by Sir James Paget and extramammary PD was reported 15 years later by Radcliffe Cocker.4 Both mammary and extramammary PD have identical morphological and histological findings.5 It is a rare type of breast carcinoma with a high rate of misdiagnosis or delayed diagnosis.6 It usually presents as an eczematous lesion of the nipple, hence the name eczematoid carcinoma.6 They present similar to eczematous dermatitis causing delay in diagnosis; therefore, PD should be suspected in those who present with chronic, cutaneous changes of the nipple–areolar tissue with no relief from topical treatment.7,8 This is evident in the index case that the patient had an eczematous lesion for 20 years that was not relieved by various over-the-counter topical agents.
PDB is reported in women aged 24–90 years but is most common in post-menopausal group. The pathology has also been reported in men which is even rarer.9 The initial clinical presentation is non-specific, presenting with itch, burning and paresthesia of nipple–areolar complex. Later, it progresses to crusting of the skin and gives a “Psoriatic” picture with raised borders and irregular contours.10 In advanced stages, the nipple–areolar texture is disrupted with an ulcer with an associated deep breast mass/nodule.10
A majority of the patients with PD have an associated underlying neoplasm of breast and up to half present with a palpable mass as in our case.11 It is characterized by epidermal invasion by malignant glandular cells, which are large, foamy and may contain mucin as in the index case (Figure 3), and when stained with hematoxylin and eosin have pale cytoplasm and hyperchromatic nuclei.11 It is necessary to confirm by histology and immunohistochemistry as clinically can mimic other benign skin conditions. Paget cells show positive staining to HER-2, cytokeratin 7 (CK7), Mucin1 (MUC1) and CAM5.2 antibody.9 Differential diagnoses include basal cell carcinoma, melanoma, sebaceous carcinoma, allergic eczema, mastitis, candidiasis and tinea corporis.12
There are two proposed theories for the origin of PDB. One is the epidermotropic theory that describes the migration of ductal cancer cells along the basement membrane due to the expression of HER-2 and an association of a breast lump.4 Second hypothesis/theory states that there in an in situ malignant transformation of cells within the keratinocytes, not associated with a breast lump and/or a negative mammography.4,8
Nipple masses pose a diagnostic challenge for radiologists due to their location or symptoms, resulting in difficulties in imaging and biopsy access. The nipple–areolar complex is said to be a “blind-spot” for radiologists due to poor imaging resolution, confluence of multiple structures and lack of specific guidelines of imaging targeting this area.13 Nevertheless, mammography is still required in all biopsy-positive cases because of the high association between PD and underlying breast carcinoma, although it will be normal in most cases limited to nipple–areolar lesions. Magnetic resonance imaging (MRI) can augment to detect underlying carcinoma in those with normal mammogram; however, normal images cannot rule out Paget’s in those with cutaneous lesions; hence, confirmation should be done with biopsy.7,8
Recently in the time of esthetical requirements, mastectomy has become controversial, whereas conservative management and use of sentinel lymph node biopsy (SLNB) has increased.14 Breast conserving surgery (BCS) is an effective method in selective cases, with SLNB being a safe alternative for axillary lesions.5 To enhance survival, radiotherapy is given to those receiving BCS.15 In addition, those with PD alone or PD-ductal carcinoma in situ (DCIS) should not receive systemic chemotherapy; furthermore, those with hormone positive receptor should be managed with endocrine therapy alone.5 Radical surgery gives a local control rate of 90%–98%; however, BCS with radiotherapy has become suitable in selected cases. Recurrences are frequently invasive with poor prognosis; therefore, free histological margins should be confirmed before starting radiotherapy; otherwise, mastectomy should be done.14
Prognostic factors particularly leading to unfavorable outcomes include presence of a palpable breast mass, enlarged lymph nodes, invasive carcinoma by histology and those who are below 60 years. When there is a palpable mass, there are higher rates of lymph node metastasis according to Lopes Filho et al.16 The 5-year survival of PD in men is only 20%–30%.
Conclusion
PDB has an uncommon clinical presentation that often resembles an eczematous/erythematous disease of the skin. Diagnosis in the early stage may cause a diagnostic dilemma due its clinical resemblance of eczema. It is mostly accompanied by an underlying breast malignancy. Cutaneous manifestation is due to tumor cells involving the epidermis and disrupting intercellular junctions with underlying high-grade DCIS or invasive carcinoma in >95% of patients. It is still a controversial issue that PD is a locally advanced carcinoma.
Acknowledgments
The authors would like to thank the patient for permission to share her medical history for educational purposes and publication.
Footnotes
Authors’ contributions: J.L. and A.M. conceptualized and drafted the manuscript. J.L. and E.U. reviewed the medical records and A.M. performed histopathology investigations. All authors have read and approved the final script.
Availability of data and material: We have not shared patient’s hospital records as they contain personal identification information.
The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
Ethics approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed consent: Written informed consent was obtained from the patient for publication for this case report; additionally, accompanying images have been censored to ensure patient confidentiality. A copy of the consent is available on record.
ORCID iD: Jay Lodhia  https://orcid.org/0000-0002-3373-5762
https://orcid.org/0000-0002-3373-5762
References
- 1. Dubar S, Boukrid M, Bouquet de, Joliniere J, et al. Paget’s breast disease: a case report and review of the literature. Front Surg 2017; 4: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Merrill AY, White A, Howard-McNatt M. Paget’s disease of the breast: an institutional review and surgical management. Am Surg 2017; 83(3): 96–98. [PubMed] [Google Scholar]
- 3. Sisti A, Huayllani MT, Restrepo DJ, et al. Paget disease of the breast: a national retrospective analysis of the US population. Breast Dis 2020; 39(3–4): 119–126. [DOI] [PubMed] [Google Scholar]
- 4. Challa VR, Deshmane V. Challenges in diagnosis and management of Paget’s disease of the breast—a retrospective study. Indian J Surg 2015; 77(Suppl. 3): 1083–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yao Y, Sun L, Meng Y, et al. Breast-conserving surgery in patients with mammary Paget’s disease. J Surg Res 2019; 241: 178–187. [DOI] [PubMed] [Google Scholar]
- 6. Song Q, Jin Y, Huang T, et al. Diagnosis and treatment of Paget’s disease of the breast: an analysis of 72 cases. Int J Clin Exp Med 2015; 8(10): 19616–19620. [PMC free article] [PubMed] [Google Scholar]
- 7. Brickley SA, Mercurio MG. Paget’s disease of the breast presenting as nipple ulceration with normal mammogram. J Dermatol Nurses Assoc 2020; 12(3): 121–123. [Google Scholar]
- 8. Gaurav A, Gupta V, Koul R, et al. Practical consensus recommendations for Paget’s disease in breast cancer. South Asian J Cancer 2018; 7(2): 83–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sandoval-Leon AC, Drews-Elger K, Gomez-Fernandez CR, et al. Paget’s disease of the nipple. Breast Cancer Res Treat 2013; 141(1): 1–2. [DOI] [PubMed] [Google Scholar]
- 10. Marques-Costa JC, Cuzzi T, Carneiro S, et al. Paget’s disease of the breast. Skinmed 2012; 10(3): 160–165. [PubMed] [Google Scholar]
- 11. Trebska-McGowan K, Terracina KP, Takabe K. Update on the surgical management of Paget’s disease. Gland Surgery 2013; 2(3): 1371–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jimenez RE, Hieken TJ, Peters MS, et al. Paget disease of the breast. In: Bland KI, Copeland EM, Klimberg VS, et al. (eds) The Breast. 5th ed, Amsterdam: Elsevier, 2018, pp. 169–176. [Google Scholar]
- 13. Omofoye TS, Scoggins ME, Dogan BE. Imaging approach to nipple masses: what a radiologist should know. Contemp Diagn Radiol 2015; 38(25): 1–7. [Google Scholar]
- 14. Bouzaiene H, Mezghani B, Slimane M, et al. Paget’s disease of the female breast: clinical findings and management in 53 cases at a single institution. Middle East J Cancer 2016; 7(3): 145–159. [Google Scholar]
- 15. Wong SM, Freedman RA, Stamell E, et al. Modern trends in the surgical management of Paget’s disease. Ann Surg Oncol 2015; 22(10): 3308–3316. [DOI] [PubMed] [Google Scholar]
- 16. Lopes Filho LL, Lopes IM, Lopes LR, et al. Mammary and extramammary Paget’s disease. An Bras Dermatol 2015; 90: 225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]


