Abstract
Background:
We explored the use of a novel smart phone-based application (APP) for delivery and monitoring of meditation to treat mood symptoms experienced by cancer patients
Methods:
We assessed the feasibility of using a meditation delivery and tracking APP over 2-weeks and its impact on cancer patients’ self-reported anxiety and depression. Outpatients reporting depression and/or anxiety were recruited and randomized to the APP or waitlist control group. Assessments included an expectancy scale, exit survey, mood rating before and after each meditation, and the Edmonton Symptom Assessment Scale (ESAS-FS), Hospital Anxiety and Depression Scale (HADS), and Pittsburgh Sleep Quality Index (PSQI) at baseline and after 2-weeks. The primary aim was to assess feasibility; secondary aims included satisfaction with the APP, association between meditation frequency and length with self-reported symptoms, and change in symptom measures (symptoms, anxiety, depression, and sleep).
Results:
Our study included 35 participants (17 meditation group; 18 controls) who were primarily female (94%) with breast cancer (60%). The 61% enrollment rate and 71% adherence rate met pre-specified feasibility criteria. Most meditation group participants described the APP as “Useful” to “Very Useful” and would “Probably” or “Definitely” recommend its use. Mixed model analysis revealed a statistically significant association between meditation length (5, 10, or 15 minutes) and change in anxiety, with 15-minute sessions associated with greater reductions in anxiety. In the exit survey, more meditation group vs. control group participants reported improved focus, mood, and sleep. Study groups differed significantly by ESAS fatigue score change; the meditation group decreased a median of 1.5 pts (IQR 2.5) and the control group increased a median of 0.5 points (IQR 2). The meditation group, but not the control group, experienced statistically significant improvement in ESAS fatigue, depression, anxiety, appetite, and physical, psychological, and global distress. Change in PSQI and HADS anxiety and depression scores did not reveal any statistically significant between-group differences.
Conclusions:
This pilot study demonstrated the feasibility and acceptability of a meditation APP for cancer patients. Meditation APP users reported improvement in several measures of symptom distress. Future studies should explore ways to enhance the APP’s usability and clinical benefit.
Keywords: integrative oncology, meditation, mobile application, anxiety, depression
Introduction
Anxiety and depression are prevalent from the time of a cancer diagnosis, throughout treatment, and into survivorship. With a prevalence of up to 54% for anxiety (or worry) and 39% for depression (or sadness), the National Cancer Institute Symptom Management and Quality of Life Steering Committee considers these 2 of the 12 core symptoms for which better management strategies are needed.1 Challenges associated with use of medications for mood management include undesirable side effects, such as drowsiness, loss of appetite, insomnia, agitation, and more. Some anxiolytic medications also have a potential for abuse. Self- administered, non-pharmaceutical approaches to help patients improve mood symptoms such as anxiety and depression are lacking and poorly understood and is an area in need of well-designed clinical trials.
Meditation is a mind-body technique that uses breath, and/or sound, and/or visualizations, and extensive research has revealed reductions in distress and improved health and quality of life.2,3 Mindfulness based therapies have shown promise in providing relief for anxiety and other mood symptoms.4 Mindfulness Based Stress Reduction (MBSR) programs can reduce mood disturbance and stress symptoms, including tension-anxiety, in the cancer patient population.5 A challenge with MBSR-type programs is their time intensiveness, which can include weekly sessions for up to 8-weeks in addition to prescribed “homework.” Prior pilot research examining the “pain, fatigue, and sleep” symptom cluster in cancer patients has shown that behavioral interventions such as relaxation, guided imagery and distraction exercises delivered via a mp3 player over a 2-week period were feasible and showed initial signs of efficacy.6,7 In a study by Wahbeh et al,8 a custom software application was developed to monitor objective adherence to mind-body interventions in combat veterans. They concluded that such electronic systems can be useful to mind-body researchers looking to effectively deliver meditation remotely and examine home practice adherence in future clinical trials.
Our own research supports the use of meditation for reducing anxiety and improving quality of life.9-11 As part of MD Anderson’s Integrative Medicine Center clinical services, we offer one-on-one meditation consultations and meditation group classes to cancer patients and their caregivers. All patients receiving an individual meditation consultation are asked to complete the Edmonton Symptom Assessment Scale (ESAS) before and after their visit, and overall, patients have reported clinically significant improvements in anxiety, fatigue, sleep, and depression symptoms after meditation sessions.9
Prior research on the benefits of meditation combined with our own clinical experience suggests that novel, non-pharmaceutical strategies, such as meditation, could prove beneficial for improving mood in cancer patients. To date, there is no information on the ideal frequency and dosing of meditation as a bio-behavioral strategy to help improve mood symptoms. Effectively delivering and tracking meditation use is critical to the success of measuring the impact of meditation on mood symptoms. According to the literature on development of health behavior change applications for smart devices, users are looking for programs that are reliable, easy to use, require little effort, and are able to support delivery, monitoring, tracking, and reviewing of behavior.12 As part of our pilot study, we developed a meditation delivery and tracking program in the form of a portable, computer-based application (APP) to track the patient’s frequency and length of meditation practice and to assess feasibility, acceptability/patient compliance, and effect size estimates to inform future research in this area.
Materials and Methods
Study Design
We assessed the impact of meditation using a portable, computer-based meditation delivery and tracking program (APP) over a 2-week period on cancer patients’ self-reported anxiety, depression, and other symptoms (ClinicalTrials.gov identifier: NCT02988271). Study goals included: (1) determining the feasibility of conducting the proposed study and prospect for a larger trial (primary aim); and secondary outcomes of (2) assessing changes in self-reported anxiety, depression, and other symptoms, (3) evaluation of participant satisfaction with a meditation delivery and tracking program, and (4) examining the association between the frequency and length of practice and patient self-reported outcomes. The study was approved by the MD Anderson Cancer Center, Internal Review Board (ID# 2016-0491). Recruitment for this 2-week intervention took place between 4/25/2019 and 8/30/2019.
Participants
Study participants were identified by physicians in the Integrative Medicine Center, Supportive Care Center, and other clinical centers at the MD Anderson Cancer Center. Patients were included if they met the following criteria: cancer survivors age ≥18 years (patients in active treatment or who have completed treatment); could understand and read English, sign a written informed consent, and follow protocol requirements; and had a self-reported ESAS psychological scale score (sum of anxiety and depression scores) ≥4 and <11 and/or individual anxiety or depression score ≥4 and <8 on a 0 to 10 numeric scale. If patients were on medication for anxiety or depression, they needed to be on a stable dose for at least 6 weeks prior to enrollment with no plans to change medications in the subsequent 4 weeks. Increases or decreases were allowed within drug class, but no changes in drug class were permitted. Patients were excluded if they had a diagnosis of a formal thought disorder (eg, schizophrenia) or known history of a neurological and/or psychological disorder that in the physician’s opinion could interfere with the patient’s ability to cooperate with study procedures.
Procedures
Targeted recruitment was 30 participants; we expected a 20% drop out for a final enrollment of 24. A research coordinator was responsible for enrollment, randomization, and administration of assessments (in-person on paper or via computer using REDCap). Participants were recruited based on their responses to the ESAS questionnaire completed as part of the standard of care prior to each clinical encounter. Patients who consented to participate in the study completed baseline data collection. After a patient was enrolled and completed baseline assessments, the research coordinator used a random number generator to assign the first participant to either the meditation group or a waitlist control group with subsequent patients being randomized using a form of adaptive randomization to reduce imbalances between groups. We used minimization, which is a dynamic randomization algorithm, based on age, baseline anxiety and/or depression, sex, time since end of treatment (greater than 6 months, less than 6 months), with an allocation probability of 1.0.13 The research coordinator was unblinded to group assignment. The waitlist control group completed all questionnaires at similar time intervals as the meditation group.
Intervention
Participants in the meditation group listened to a recorded audio track introducing the practice of meditation and were encouraged to meditate at least once daily. A software application to track and deliver meditation was specially developed for this study in compliance with institutional and patient privacy concerns. The application was pre-installed on an iPod touch for study participants to use during the study period. Participants had access to the APP to self-administer a 5-, 10-, or 15-minute audio recording of a meditation session depending on their preference. See Figure 1 for meditation APP program functionality. The meditations directed participants to align their body posture, breathe deeper, focus their mind’s attention, and connect to inner qualities like loving-kindness, compassion, joy, equanimity, and peace of mind. Meditation content was developed by Dr. Alejandro Chaoul who has been conducting mind-body research for more than 20 years and has trained with Tibetan lamas since 1989.
Figure 1.
Meditation application program functionality.
Assessment Measures
At enrollment, participants completed an expectancy questionnaire and other patient-reported outcomes; listened to an audio track introducing them to meditation as a practice; and received instruction on how to use the meditation software program. During the 2-week study period, the APP tracked participant use of the meditation recordings. The APP also prompted participants to rate their mood on a continuous Likert scale from 0 = “worst mood” to 8 = “best mood” before and after each meditation session. At the end of the study period, participants were again asked (via REDCap) to complete the patient reported outcomes (ESAS, HADS, and PSQI) and for those in the meditation group an exit survey was completed regarding their satisfaction with the APP, the meditation practice, and perceptions of benefit. Data collected by the meditation tracking program was uploaded to a secured database for analysis.
Questionnaires
The baseline expectancy questionnaire and satisfaction with the APP, the meditation practice, and perceptions of benefit were adapted from measures previously used by our research group for study purposes.14 The expectancy questionnaire, completed at baseline by all participants, asked 5 questions on a 5-point scale from strongly disagree (0) to strongly agree (5) about the degree to which they thought meditation would help with: well-being, focus mood, energy, and sleep. The satisfaction questionnaire asked 10 questions related to using the APP, recommending the APP to others, and continuing practice. Participants were also asked about their perceived benefit from the meditation using the same questions from the expectancy questionnaire.
The Edmonton Symptom Assessment Scale - Financial Spiritual (ESAS-FS) includes 10 core symptoms (pain, fatigue, nausea, depression, anxiety, drowsiness, appetite, well-being, shortness of breath, and sleep) and additional items of spiritual pain and financial distress rated on a numerical scale of 0 to 10 (10 = worst possible expression of that symptom).15 ESAS subscale scores included global distress (GDS, 0-90), physical distress (PHS, 0-60), and psychological distress (PSS, 0-20). The GDS is the sum of pain, fatigue, nausea, drowsiness, appetite, shortness of breath, anxiety, depression, and well-being scores. The PHS is a sum of pain, fatigue, nausea, drowsiness, appetite, and shortness of breath. The PSS is a sum of anxiety and depression. We defined a clinically significant change for a symptom as a change of ≥1 point on an individual symptom item score.16 A clinically significant change for a subscale score was defined as a change of ≥3 points for the GDS, ≥3 points for the PHS, and ≥2 points for the PSS.17 The Hospital Anxiety and Depression Scale (HADS) is a 14-item scale specifically developed for use with medically ill patients and focuses on cognitive symptoms. HADS scores range from 0 to 21 for anxiety or depression, with higher scores indicating worse symptoms and a score of ≥8 considered clinically significant anxiety or depression.18 The Pittsburgh Sleep Quality Index (PSQI) is an 18-item self-rated questionnaire that assesses sleep quality and sleep disturbances over 1 month. Scores range from 0 to 21, with higher scores signifying worse sleep quality and scores ≥5 considered clinically significant sleep disturbance.19
Statistical Analyses
To assess feasibility and acceptability, we calculated frequencies and rates with 95% confidence intervals (CIs) for the accrual rate among patients approached for study participation and the adherence rate in the meditation group. We defined adherence as practicing at least 2 meditation sessions for each of the 2 study weeks. Demographics, clinical characteristics, baseline expectations, intervention satisfaction (meditation group only), and the exit survey were described overall and by study arm using frequencies and proportions. We compared baseline group differences using Fisher’s exact test.
We reported medians and interquartile ranges when score distributions departed significantly from a normal distribution. For normally distributed scores and score changes, we reported frequencies, means, standard deviations, and 95% CIs. For normally distributed change scores, we reported effect sizes as Cohen’s d, with verbal interpretations based on Cohen’s guidelines.20 For change score distributions that departed significantly from a normal distribution, we presented the effect size as epsilon-squared (ε2) and interpreted effect size using the guidelines proposed by Rea and Parker.21
This pilot study focused on feasibility and acceptability. However, as an exploratory analysis, we used statistical testing to further examine between-group differences and within-group changes. For normally distributed changes, within-group change was tested using the paired t-test, and between-group differences were tested using the independent samples t-test. For non-normally distributed change scores, we used the Wilcoxon signed-rank test to evaluate pre- to post-intervention changes and the Wilcoxon rank-sum test to assess between-group differences in changes. To account for participants who may have selected a meditation yet did not complete it, we included in the analysis only those meditation sessions that were at least within 1 minute of the total session length (eg, ≥4 minutes of a 5-minute session). To explore the effect of the meditation length and meditation time of day on change in pre- to post-session anxiety, we used a linear mixed effect model (SAS PROC MIXED) with repeated and random effects to account for within-subject and between-subject variability. The model outcome was anxiety change and covariates included the pre-session anxiety rating, meditation session length (short, medium, long), meditation time of day (early morning, late morning, afternoon, evening), and session number (sequential number 1-33) as fixed effects, allowing for a random intercept by subject. Based on the Akaike Information Criterion (AIC), we selected an autoregressive (AR(1)) covariance structure to model the within-subject serial correlation and compound symmetry covariance structure (corresponding to random intercepts) to model the variation between subjects. Model parameters were calculated using the restricted maximum likelihood method. We also reran these same analyses adjusted for the number of meditation sessions in which each patient was engaged and the results remained similar. Statistical test results for this pilot study were not adjusted for multiple comparisons. P-values < .05 were considered statistically significant, and all analyses were conducted in SAS 9.4 (Cary, NC, USA).
Results
Demographics, Recruitment Rate, Adherence
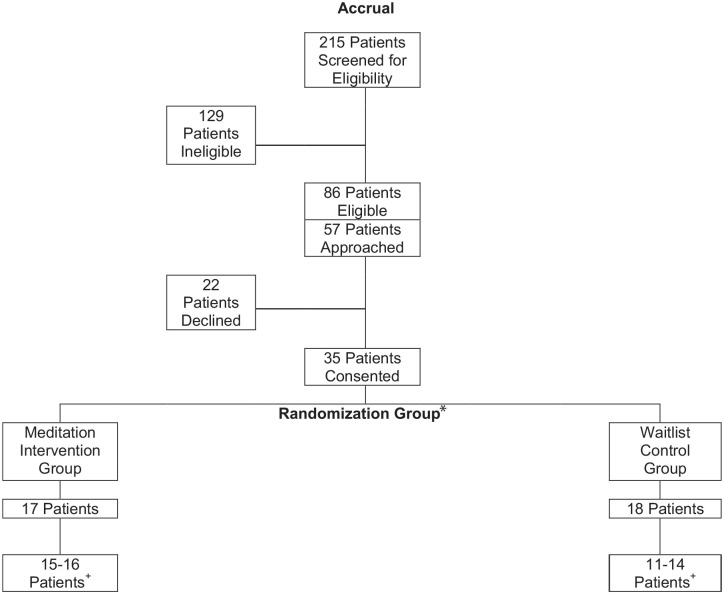
Two hundred fifteen patients were screened for study eligibility; 86 patients met eligibility criteria of which 57 were referred to the study by their physicians; 22 declined participation and 35 were consented. The majority of patients screened (90%, n = 191) were recruited from the Integrative Medicine Center. Our recruitment rate of 35/57 [61.4% (95% CI: 47.6%, 74.0%)] included 17 participants randomized to the meditation group and 18 randomized to the waitlist group (Figure 2). The majority of participants were women (94%), had a diagnosis of breast cancer (60%), were married (79%), and had a college education (65%) (Table 1). There were no significant group differences in medical, demographic, or questionnaire data at baseline. At baseline, 13 (76%) in the meditation group and 14 (78%) in the control group reported clinically significant psychological distress (PSS ESAS scores ≥4). A clinically relevant baseline score (each ≥8) for HADS anxiety and depression, respectively, was reported for 8 (47%) and 10 (59%) in the meditation group and 9 (50%) and 12 (67%) in the control group. In the meditation group, 12/17 [70.6% (95% CI: 44.0%, 89.7%)] adhered to the recommended 2 meditation sessions per week during the 2-week study period. Adherence status was not significantly associated with baseline demographic and clinical characteristics.
Figure 2.
Outpatient meditation study consort diagram.
*All patients approached and randomized are considered evaluable for protocol primary objective.
+Patients evaluable for protocol secondary objectives: number of patients with pre/post data for symptoms, depression, anxiety, and sleep.
Table 1.
Summary of Demographic and Clinical Characteristics.
| Characteristic | Total (n = 35) (%) | Randomization group | ||
|---|---|---|---|---|
| Meditation (%) | Waitlist (%) | |||
| Sex | Female | 33 (94.3) | 16 (94.1) | 17 (94.4) |
| Male | 2 (5.7) | 1 (5.9) | 1 (5.6) | |
| Race | Asian | 1 (3.0) | 1 (6.7) | 0 (0) |
| Black | 6 (18.2) | 3 (20) | 3 (16.7) | |
| Hispanic | 6 (18.2) | 2 (13.3) | 4 (22.2) | |
| Other | 1 (3.0) | 1 (6.7) | 0 (0) | |
| White | 19 (57.6) | 8 (53.3) | 11 (61.1) | |
| Cancer Category | Breast | 21 (60.0) | 10 (58.8) | 11 (61.1) |
| Gastrointestinal | 4 (11.4) | 4 (23.5) | 0 (0) | |
| Genitourinary | 2 (5.7) | 1 (5.9) | 1 (5.6) | |
| Gynecologic | 2 (5.7) | 0 (0) | 2 (11.1) | |
| Head and neck | 1 (2.9) | 0 (0) | 1 (5.6) | |
| Lung | 1 (2.9) | 0 (0) | 1 (5.6) | |
| Sarcoma | 2 (5.7) | 1 (5.9) | 1 (5.6) | |
| Thoracic head and neck | 2 (5.7) | 1 (5.9) | 1 (5.6) | |
| Employment status | No | 9 (26.4) | 5 (31.3) | 4 (22.2) |
| Retired | 12 (35.3) | 5 (31.3) | 7 (38.9) | |
| Yes, full-time | 7 (20.6) | 3 (18.8) | 4 (22.2) | |
| Yes, part-time | 2 (5.9) | 2 (12.5) | 0 (0) | |
| Yes, taking time off work for treatment. | 4 (11.8) | 1 (6.3) | 3 (16.7) | |
| Marital status | Divorced | 1 (3.0) | 1 (6.3) | 0 (0) |
| Married | 26 (78.8) | 14 (87.5) | 12 (70.6) | |
| Never married and not now living with a partner | 2 (6.1) | 1 (6.3) | 1 (5.9) | |
| Widowed | 4 (12.1) | 0 (0) | 4 (23.5) | |
| Highest level of education | Other | 2 (5.8) | 0 (0) | 2 (11.2) |
| High school/GED | 3 (8.8) | 2 (12.5) | 1 (5.6) | |
| College graduate (4-year degree) | 8 (23.5) | 4 (25) | 4 (22.2) | |
| Graduate or professional degree | 14 (41.2) | 6 (37.5) | 8 (44.4) | |
| Annual income | Less than $30 000 | 4 (12.9) | 2 (14.2) | 2 (11.8) |
| $30 001-$50 000 | 4 (12.9) | 1 (7.1) | 3 (17.6) | |
| $50 001-$75 000 | 5 (16.1) | 3 (21.4) | 2 (11.8) | |
| $75 001-$100 000 | 6 (19.4) | 5 (35.7) | 1 (5.9) | |
| Greater than $100 000 | 12 (38.7) | 3 (21.4) | 9 (52.9) | |
| Religious preference | Protestant | 12 (37.6) | 5 (33.3) | 7 (41.1) |
| Catholic | 7 (21.9) | 4 (26.7) | 3 (17.6) | |
| Hindu | 2 (6.3) | 2 (13.3) | 0 (0) | |
| Jewish | 2 (6.3) | 1 (6.7) | 1 (5.9) | |
| None | 5 (15.6) | 2 (13.3) | 3 (17.6) | |
| Other | 4 (12.5) | 1 (6.7) | 3 (17.6) | |
ε2 = Epsilon-squared effect size: 0.01 to <0.04, Weak; 0.04 to <0.16, Moderate; 0.16 to <0.36, Relatively Strong; 0.36 < 0.64, Strong.
d = Cohen’s d effect size: 0.20, small; 0.50, medium; 0.80, large.
Abbreviation: CI = confidence interval.
Expectancy, Satisfaction
At baseline, there were no significant group differences in expectancy regarding study participation. At the study exit assessment, a higher proportion of participants in the meditation versus control group reported that they “Strongly Agree” or “Agree” that they felt improved well-being (50.0% vs 8.3%, P = .039), focus (56.3% vs 0%, P = .003), mood (68.8% vs 0%, P = .0003), energy (31.3% vs 0%, P = .053), and sleep (50.0% vs 0%, P = .008).
At the end of the study, 16 of the 17 meditation group participants completed a meditation APP satisfaction questionnaire. A high proportion reported “Strongly Agree” or “Agree” to having received adequate instruction on the use of the APP (87.5%) and handheld device (87.5%) and indicated that the APP was “Useful” to “Very Useful” (93.8%) and that they were “Probably” or “Definitely” likely to recommend the APP to a friend (70.0%). Most participants (81.3%) stated they “Probably” or “Definitely” plan to continue meditating after the study period and that they “Strongly Agree” or “Agree” that they would use the meditation APP to continue their home-based practice (68.8%).
Meditation Application Use (as Tracked by the APP)
Total number of sessions and meditation minutes
Not all meditation sessions started were completed. The 17 participants in the meditation group completed 0 to 33 meditation sessions (Median = 7, IQR = 10, Q1 = 3, Q3 = 13) during the 2-week intervention, totaling from 0 to 370 minutes of meditation per person. Median time spent meditating with the application was 91 minutes with the middle half of participants spending from half an hour to 2 hours using the meditation application over the 2 weeks (IQR = 97, Q1 = 34, Q3 = 131).
Pre- and Post-session Anxiety Ratings
Fourteen meditation group participants rated pre- and post-session anxiety from 0 (low anxiety) to 8 (high anxiety) for 1 to 26 meditation sessions per participant, resulting in a total of 100 pairs of anxiety ratings. We detected no significant interaction between baseline anxiety and choice of session length. Type 3 tests of fixed effects indicated that both pre-session anxiety (F1, 83 = 79.01, P < .0001) and session length (F2, 83 = 3.46, P < .0361) had a statistically significant effect on the pre- to post-session change in anxiety rating. The long session showed a greater effect on anxiety change than did the medium or short sessions (Table 2). Medium and short sessions did not produce a statistically significant difference in their effect on anxiety change. The results remained similar when the model controlled for the total number of sessions in which each patient was engaged, a potential surrogate of the individual mean efficacy of the meditation, due to the self-chosen nature of the number of sessions (see the footnote Table 2). Controlling for baseline anxiety, the least squares means change in anxiety for the long sessions was −1.57 points (95% CI −2.45, −0.69) compared to −0.95 (95% CI-1.86, −0.05) for the medium length sessions and −1.07 (−1.95, −0.20) for the short sessions. Using this same method, we observed no significant association between anxiety score change and time of day meditating (12 am-6 am, 6 am-12 pm, 12 pm-6 pm, 6 pm-12 am).
Table 2.
Linear Mixed Model Estimates of Fixed Effects to Predict Pre- to Post-Session Change in Anxiety.*.
| Effect | β | SE | df | t | P | β 95% CI | ||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Intercept | 1.03 | 0.50 | 13 | 2.08 | .0582 | −0.04 | 2.10 | |
| Pre-session anxiety | −0.52 | 0.06 | 83 | −8.89 | <.0001 | −0.63 | −0.40 | |
| Session length | Long (15 min) | −0.49 | 0.24 | 83 | −2.04 | .0446 | −0.98 | −0.01 |
| Medium (10 min) | .12 | 0.27 | 83 | 0.44 | .6600 | −0.42 | 0.66 | |
| Short (5 min) | Reference | |||||||
Results remained similar when the linear mixed model was adjusted for the number of meditation sessions in which each patient was engaged. Specifically, the P-value became 0.0412 and 0.7438, respectively, for the effects of long and medium session lengths compared to the short session length.
ESAS
Between-group differences
Between-group differences in the change in ESAS composite subscale scores did not reach statistical significance but represented medium effect size point estimates (physical distress d = −0.33, 95% CI −0.97, 0.32; psychological distress d = −0.33, 95% CI −1.14, 0.48; and global distress d = −0.36, 95% CI −1.12, 0.40) (Table 3). Unlike subscale scores, individual ESAS item scores departed significantly from a normal distribution and were evaluated using nonparametric measures. Between-group analyses revealed change in fatigue as the only item that reached statistical significance. The median fatigue score decreased 1.5 points (IQR 2.5) in the meditation group and increased 0.5 points (IQR 2.0) in the control group, P = .016. This between group difference in fatigue score change represents a relatively large effect size point estimate (ε2 = 0.19; 95% CI 0.02, 0.45). For other individual ESAS items, effect size point estimates for between-group differences ranged from moderate (pain, depression, and spiritual pain) to weak (anxiety, appetite, sleep, and financial distress) to negligible (nausea, drowsiness, shortness of breath, and feelings of well-being) (Table 3).
Table 3.
ESAS Item and Subscale Pre- to Post-Intervention Score Change and Between-Group Effect Sizes.
| ESAS item | Group | n | Median | IQR | Effect size | |
|---|---|---|---|---|---|---|
| ε2 | 95% CI | |||||
| Pain | Meditation | 16 | −0.5 | 2 | 0.07 | (0.00, 0.28) |
| Control | 14 | 0 | 2 | |||
| Fatigue | Meditation | 16 | −1.5 | 2.5 | 0.19 | (0.02, 0.45) |
| Control | 14 | 0.5 | 2 | |||
| Nausea | Meditation | 16 | 0 | 1 | 0.00 | (0.00, 0.16) |
| Control | 14 | 0 | 1 | |||
| Depression | Meditation | 16 | −1.5 | 2.5 | 0.04 | (0.00, 0.25) |
| Control | 14 | 0 | 3 | |||
| Anxiety | Meditation | 16 | −2 | 4 | 0.01 | (0.00, 0.17) |
| Control | 14 | −1.5 | 2 | |||
| Drowsiness | Meditation | 16 | 0 | 4 | 0.0003 | (0.00, 0.03) |
| Control | 14 | 0 | 3 | |||
| Shortness of breath | Meditation | 16 | 0 | 0 | 0.0001 | (0.00, 0.13) |
| Control | 14 | 0 | 2 | |||
| Appetite | Meditation | 16 | −1 | 2.5 | 0.02 | (0.00, 0.24) |
| Control | 14 | 0 | 3 | |||
| Wellbeing | Meditation | 16 | −0.5 | 2.5 | 0.0002 | (0.00, 0.13) |
| Control | 14 | −0.5 | 4 | |||
| Sleep | Meditation | 16 | −0.5 | 5 | 0.01 | (0.00, 0.19) |
| Control | 14 | −1 | 3 | |||
| Financial distress | Meditation | 16 | 0 | 3 | 0.01 | (0.00, 0.17) |
| Control | 14 | −0.5 | 4 | |||
| Spiritual pain | Meditation | 16 | 0 | 1 | 0.04 | (0.00, 0.25) |
| Control | 14 | 0 | 2 | |||
| ESAS subscale | Group | n | Mean | SD | Effect size | |
| d | (95% CI) | |||||
| Physical Distressa (PHS) | Meditation | 16 | −4.94 | 7.40 | −0.33 | (−0.97, 0.32) |
| Control | 14 | −0.43 | 8.55 | |||
| Psychological Distressa (PSS) | Meditation | 16 | −3.06 | 4.28 | −0.33 | (−1.14, 0.48) |
| Control | 14 | −2.07 | 4.48 | |||
| Global Distressa (GDS) | Meditation | 16 | −8.69 | 11.73 | −0.36 | (−1.12, 0.40) |
| Control | 14 | −3.14 | 12.75 | |||
ε2 = Epsilon-squared effect size: 0.01 to <0.04, Weak; 0.04 to <0.16, Moderate; 0.16 to <0.36, Relatively Strong; 0.36 < 0.64, Strong.
d = Cohen’s d effect size: 0.20, small; 0.50, medium; 0.80, large.
Abbreviation: CI = confidence interval.
GDS equals sum of pain, fatigue, nausea, depression, anxiety, drowsiness, appetite, well-being, and shortness of breath (total score 0-90); PHS equals sum of pain, fatigue, nausea, drowsiness, appetite, and shortness of breath (total 0-60); and PSS equals sum of depression and anxiety. For each individual symptom item, a change score ≥1 is considered clinically significant. For the PSS, ≥2 is considered clinically significant, and ≥3 for the PHS and GDS.
Within-Group Changes
For all 3 ESAS subscales, pre- to post-intervention change scores demonstrated statistically and clinically significant improvement (ie, decreased score) within the meditation group (physical distress: Mean −4.94, SD 7.40, P = .018; psychological distress: Mean −3.06, SD 4.28, P = .012; global distress: Mean −8.69, SD 11.73, P = .010). Within the meditation group, effect size point estimates for all subscales were large. Within the control group, no statistically significant baseline to follow-up change for the any of the ESAS subscale scores was evident. However, on average, ESAS subscale scores for the control group demonstrated a clinically meaningful improvement from baseline for the psychological distress (Mean −2.07, SD 4.48) and global distress subscales (Mean −3.14, SD 12.75) but not the physical distress subscale (Mean −0.43, SD 8.55). Control group effect size point estimates for the within-group change in subscale scores ranged from small (physical distress) to medium (psychological distress and global distress).
For individual ESAS items, within-group change showed statistically significant and clinically relevant improvements in the meditation group for fatigue (Median −1.5, IQR 2.5, P = .013), depression (Median −1.5, IQR 2.5, P = .041), anxiety (Median −2, IQR 4, P = .016), and appetite (Median −1.0, IQR 2.5, P = .031). No statistically significant within-group changes were observed in the meditation group for pain, nausea, drowsiness, shortness of breath, wellbeing, sleep, financial distress, or spiritual pain. For the control group, none of the 12 ESAS individual items showed a statistically significant within-group change from baseline to end of the study, but median decreases in anxiety (Median −1.5, IQR 2) and sleep (Median −1, IQR 3) were clinically meaningful.
HADS and PSQI
The between-group differences in HADS depression score change (−0.69; 95% CI −2.22, 0.84, P = .36) and HADS anxiety score change (−1.33, 95% CI −3.46, 0.81, P = .21) were not statistically significant (Table 4). Effect sizes for the depression score change (d = 0.11, 95% CI −0.32, 0.54;) and anxiety score change (d = 0.27; 95% CI −0.36, 0.90,) represented small effect size point estimates. Within-group changes for both study groups had statistically significant decreases in HADS depression scores from baseline to the end of the study. In the meditation group, the HADS depression score decreased by a mean of 1.69 points (95% CI −3.10, −0.27, P = .02), representing a medium effect size (d = −0.64; 95% CI −1.02, −0.25). In the control group, the HADS depression score decreased by a mean of 1.00 point (95% CI −1.69, −0.31, P = .008), representing a large effect size (d = −0.80, 95% CI −1.15, −0.44). Within-group changes in anxiety were non-significant for both meditation and control groups; scores decreased from baseline in the meditation group, with a medium effect size (difference = −1.06 points, d = −0.29; 95% CI −0.78, 0.20) and increased from baseline in the control group with a small effect size (difference = 0.27 points, d = −0.15; 95% CI −0.24, 0.54).
Table 4.
HADS Depression and Anxiety Subscale Scores and the PSQI Global Score Within-Group Pre- to Post-Intervention Change and Effect Size and Between-Group Difference and Effect Size.
| Measure | Group | Pre-test |
Post-test |
Difference (Posttest – Pretest) |
Effect Size |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | 95% CI | n | Mean | 95% CI | n | Mean | 95% CI | d a | 95% CI | ||
| HADS Depression | Meditation | 17 | 8.88 | (6.80, 10.96) | 16 | 6.94 | (4.68, 9.19) | 16 | −1.69 | (−3.10, –0.27) | −0.64 | (−1.02, –0.25) |
| Control | 18 | 9.78 | (8.10, 11.45) | 15 | 8.67 | (7.18, 10.16) | 15 | −1.00 | (−1.69, –0.31) | −0.80 | (−1.15, –0.44) | |
| Between-Group Difference | −0.90 | (−3.45, 1.66) | −1.73 | (−4.36, 0.90) | −0.69 | (−2.22, 0.84) | 0.11* | (−0.32,0.54) | ||||
| HADS Anxiety | Meditation | 17 | 8.41 | (6.39, 10.44) | 16 | 7.00 | (5.03, 8.97) | 16 | −1.06 | (−3.02, 0.90) | −0.29 | (−0.78, 0.20) |
| Control | 18 | 7.94 | (6.37, 9.52) | 15 | 7.67 | (6.45, 8.88) | 15 | −0.27 | (−0.73, 1.26) | −0.15 | (−0.24, 0.54) | |
| Between-Group Difference | 0.47 | (−1.98, 2.92) | −0.67 | (−2.92, 1.58) | −1.33 | (−3.46, 0.81) | 0.27* | (−0.36, 0.90) | ||||
| PSQI Global | Meditation | 16 | 8.63 | (6.58, 10.67) | 16 | 8.68 | (6.72, 10.66) | 15 | 0.40 | (−1.79, 2.59) | 0.10 | (−0.41, 0.61) |
| Control | 18 | 10.83 | (8.89, 12.78) | 14 | 11.21 | (9.24, 13.19) | 14 | 0.00 | (−2.05, 2.05) | 0.00 | (−0.51, 0.51) | |
| Between-Group Difference | −2.21 | (−4.93, 0.51) | −2.53 | (−5.21, 0.15) | 0.40 | (−2.47, 3.27) | −0.09* | (−0.81, 0.64) | ||||
Between-group effect size; Effect sizes are within group unless otherwise noted.
d = Cohen’s d effect size: 0.20, small; 0.50, medium; 0.80, large.
For PSQI global scores, we detected no statistically significant between-group differences (Mean 0.40; 95% CI −2.47, 3.27, P = .78) or within-group differences for the meditation group (Mean 0.40; 95% CI −1.79, 2.59; P = .70) and control group (Mean 0; 95% CI −0.51, 0.51; P = 1.00). Effect size point estimates for both the between group change difference and the within-group differences were small (Table 4). Overall, sleep quality was poor (PSQI global score of 5 or higher) among study participants. In the 15 meditation group participants with both pre- and post-intervention PSQI global scores, 12 (80%) at baseline and 13 (87%) at post-intervention had poor sleep quality. In the 14 control group participants with pre- and post-intervention PSQI global scores, 13 (93%) of participants at baseline and all 14 (100%) participants at post-intervention had poor sleep quality.
Discussion
Our study explored the use of a meditation APP to help with the delivery of a meditation intervention to cancer patients experiencing moderate symptoms of depression and/or anxiety. The APP was well received by participants and the recruitment rate and adherence rate met the a priori feasibility criteria (50% recruited and 70% of meditation group adherent to 2 sessions per week). Satisfaction regarding use of the meditation application was high, with the majority of participants reporting moderate to high levels of satisfaction. As part of an exploratory analysis, we examined pre- and post- anxiety score change for individual sessions of different lengths (short, medium, and long), with an observed trend toward greater reduction in anxiety with a longer meditation session. We also observed clinically and statistically significant within-group improvement in multiple symptoms and group differences for fatigue.
Limitations include recruitment at a single clinical center. Although our sample included mostly women with breast cancer, it may not be representative of findings with other cancer types. As a possible confounding factor, we did not account for cancer stage as part of study recruitment or randomization. Based on our study design, it will be difficult to separate how much of the observed effects with regard to symptom change were due to the delivery system (the APP) or the intervention (meditation). Another limitation concerns meditation length tracking; a participant may have selected a short or a long meditation but could have spent the same amount of time meditating (eg, they only completed 5 minutes of a total 15-minute long meditation). Another limitation is the sample size. This trial was designed as a pilot study to gain insight into feasibility and acceptability and to estimate effect sizes. It was not powered to detect clinically or statistically significant differences in clinical outcome measures. Therefore, the results of exploratory outcome analyses need to be interpreted with caution. Effect sizes can be of value in planning futures studies, but the width of confidence intervals should be taken into consideration along with point estimates, as the values from small studies may not be replicated in future trials. Of note, patients scoring >3 on the ESAS were included as part of this study, based on literature suggesting that an ESAS anxiety or depression score >3 is considered a useful screening tool for detecting anxiety or depression in patients with non-advanced cancer.22 However, on the more clinically relevant HADS measure, few patients reported clinically significant depression or anxiety at baseline.
A number of longer (8 weeks) programs already exist to help with management of cancer related symptoms, including Mindfulness Based Cancer Recovery and MBSR.23 When compared to longer interventions such as MBSR for the management of cancer related symptoms, we would expect a lower magnitude of effect with our less intensive 2-week intervention (see Tables 3 and 4). In a review of clinical studies exploring the MBSR intervention in cancer care, observed effect sizes were 0.42 (95% CI 0.26-0.58; P < .0001) for mood, 0.58 (95% CI 0.45-0.72; P < .0001) for distress, and 0.29 (95% confidence interval (CI) 0.17-0.40; P ≤ .00005) for quality of life.24 Future studies with the APP should explore changes in the magnitude of effect if participants are exposed to a longer intervention period.
In conclusion, we found that we could successfully recruit people to this study and that most adhered, with a majority generally liking the experience of meditating using the APP. Exploratory results suggested that longer sessions may more effectively reduce symptoms; however, further research with a larger sample size is needed to optimize session duration as well as the recommended number of sessions per week. Future research is also warranted to identify how to improve adherence with the meditation intervention, which may lead to greater effects on self-reported symptoms. Strategies to improve adherence could include modifications to the APP itself to improve usability and development of built-in meditation reminders and other incentives.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Lorenzo Cohen is the co-author of the book Anticancer Living: Transform Your Life and Health with the Mix of Six for which he receives royalties. Other authors declare no conflicts of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Supported by an MD Anderson Cancer Center the Duncan Family Institute for Cancer Prevention and Research. Partial funding for Lorenzo Cohen provided by the Richard E. Haynes Distinguished Professorship for Clinical Cancer Prevention at The University of Texas MD Anderson Cancer Center. The Cancer Center Support Grant (NCI Grant P30 CA016672) provided part of the support for this work.
ORCID iDs: Gabriel Lopez  https://orcid.org/0000-0002-3685-0280
https://orcid.org/0000-0002-3685-0280
Wenli Liu  https://orcid.org/0000-0003-1036-4564
https://orcid.org/0000-0003-1036-4564
Santhosshi Narayanan  https://orcid.org/0000-0003-0591-1500
https://orcid.org/0000-0003-0591-1500
References
- 1. Reeve BB, Mitchell SA, Dueck AC, et al. Recommended patient-reported core set of symptoms to measure in adult cancer treatment trials. J Natl Cancer Inst. 2014;106:dju129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Biegler KA, Chaoul MA, Cohen L. Cancer, cognitive impairment, and meditation. Acta Oncol. 2009;48:18-26. [DOI] [PubMed] [Google Scholar]
- 3. Goyal M, Singh S, Sibinga EM, et al. Meditation programs for psychological stress and well-being: a systematic review and meta-analysis. JAMA Intern Med. 2014;174:357-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hofmann SG, Sawyer AT, Witt AA, Oh D. The effect of mindfulness-based therapy on anxiety and depression: A meta-analytic review. J Consult Clin Psychol. 2010;78:169-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Garland SN, Tamagawa R, Todd SC, Speca M, Carlson LE. Increased mindfulness is related to improved stress and mood following participation in a mindfulness-based stress reduction program in individuals with cancer. Integr Cancer Ther. 2013;12:31-40. [DOI] [PubMed] [Google Scholar]
- 6. Kwekkeboom KL, Abbott-Anderson K, Wanta B. Feasibility of a patient-controlled cognitive-behavioral intervention for pain, fatigue, and sleep disturbance in cancer. Oncol Nurs Forum. 2010;37:E151-E159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kwekkeboom KL, Abbott-Anderson K, Cherwin C, Roiland R, Serlin RC, Ward SE. Pilot randomized controlled trial of a patient-controlled cognitive-behavioral intervention for the pain, fatigue, and sleep disturbance symptom cluster in cancer. J Pain Symptom Manag. 2012;44:810-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wahbeh H, Zwickey H, Oken B. One method for objective adherence measurement in mind-body medicine. J Altern Complement Med. 2011;17:175-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chaoul A, Lopez G, Lee R, et al. An analysis of meditation consultations in an integrative oncology outpatient clinic. J Altern Complement Med. 2014;20:A86-A86. [Google Scholar]
- 10. Milbury K, Chaoul A, Biegler K, et al. Tibetan sound meditation for cognitive dysfunction: results of a randomized controlled pilot trial. Psycho-oncology. 2013;22:2354-2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Narayanan S, Reddy A, Lopez G, et al. Randomized feasibility study of meditative practices in hospitalized cancer patients. Integr Cancer Ther. 2020;19:1534735420909903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dennison L, Morrison L, Conway G, Yardley L. Opportunities and challenges for Smartphone applications in supporting Health Behavior Change: Qualitative Study. J Med Internet Res. 2013;15:e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31:103-115. [PubMed] [Google Scholar]
- 14. Ratcliff CG, Prinsloo S, Chaoul A, et al. A randomized controlled trial of brief mindfulness meditation for women undergoing stereotactic breast biopsy. J Am Coll Radiol. 2019;16:691-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K. The Edmonton symptom assessment system (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care. 1991;7:6-9. [PubMed] [Google Scholar]
- 16. Hui D, Shamieh O, Paiva CE, et al. Minimal clinically important differences in the Edmonton Symptom Assessment Scale in cancer patients: a prospective, multicenter study. Cancer. 2015;121:3027-3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hui D, Shamieh O, Paiva CE, et al. Minimal clinically important difference in the physical, emotional, and total symptom distress scores of the Edmonton Symptom Assessment System. J Pain Symptom Manag. 2016;51:262-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361-370. [DOI] [PubMed] [Google Scholar]
- 19. Buysse DJ, Reynolds Cf, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193-213. [DOI] [PubMed] [Google Scholar]
- 20. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Lawrence Erlbaum Associates, Publishers; 1988. [Google Scholar]
- 21. Rea LM, Parker RA. Designing and Conducting Survey Research: A Comprehensive Guide. Jossey-Bass Publishers; 1992. [Google Scholar]
- 22. Ripamonti CI, Bandieri E, Pessi MA, Maruelli A, Buonaccorso L, Miccinesi G. The Edmonton Symptom Assessment System (ESAS) as a screening tool for depression and anxiety in non-advanced patients with solid or haematological malignancies on cure or follow-up. Support Care Cancer. 2014;22:783-793. [DOI] [PubMed] [Google Scholar]
- 23. Carlson LE, Doll R, Stephen J, et al. Randomized controlled trial of Mindfulness-based cancer recovery versus supportive expressive group therapy for distressed survivors of Breast Cancer (MINDSET). J Clin Oncol. 2013;31:3119-3126. [DOI] [PubMed] [Google Scholar]
- 24. Musial F, Büssing A, Heusser P, Choi KE, Ostermann T. Mindfulness-based stress reduction for Integrative Cancer Care – a summary of evidence. Forsch Komplementmed. 2011;18:192-202. [DOI] [PubMed] [Google Scholar]