Abstract
Background:
Anlotinib is used as a third-line treatment for advanced non-small-cell lung cancer (NSCLC), but has limited clinical benefits and several side effects, such as diarrhea and acneiform skin rash. Traditional Chinese Medicine (TCM) is commonly used to treat cancers in China. Chinese herbal medicines may have the potential as adjuvant therapies to reduce toxicity and improve the efficacy of treatments for NSCLC. Given the positive outcomes of basic research, we plan to evaluate whether the addition of the Chinese herbal medicine Yifei Sanjie formula (YFSJF) to anlotinib can improve the progression-free survival (PFS) of advanced NSCLC patients.
Methods:
A multicenter, randomized, double-blind, placebo-controlled parallel-group controlled pilot trial will be performed. Forty eligible patients will be randomized in a ratio of 1:1 to the intervention (YFSJF + anlotinib) and control (placebo + anlotinib) groups. Participants will be advised to take 12 mg/day of anlotinib on days 1 to 14 of each 21-day cycle. YFSJF or placebo will be administered (15 g twice daily) during each cycle until progression of disease (PD). The primary outcome will be progression-free survival (PFS), and the secondary outcomes will be overall survival (OS), the objective response rate (ORR), and patient-reported outcomes (PRO). Tumors will be assessed based on RECIST v. 1.1 after every 2 cycles of treatment. The M. D. Anderson Symptom Inventory-Lung Cancer (MDASI-LC) will be used to evaluate PRO at baseline and weekly thereafter until PD.
Discussion:
This will be the first trial to evaluate the effectiveness and safety of TCM combined with anlotinib for the treatment of NSCLC. The results of this randomized controlled trial will fill a gap in the research by showing whether YFSJF combined with anlotinib can improve PFS in NSCLC patients.
Trial Registration:
The study was registered on June 8th, 2021 on Chinese Clinical Registry; registration number ChiCTR2100047143. (https://www.chictr.org.cn/index.aspx).
Ethics and Dissemination:
The Ethics Committee of the First Affiliated Hospital of Guangzhou University of Traditional Chinese Medicine approved the study protocol (approval no.: K2020151, 2021/08/19). The study will also be supervised and managed by the Ethics Committee.
Keywords: NSCLC, TCM, anloinib, YFSJF, combination therapy
Background
Lung cancer has the highest fatality rate among all cancers.1 Non-small-cell lung cancer (NSCLC) is the most common lung cancer type, accounting for 85% of all lung cancers.2 Advanced NSCLC cannot be managed surgically and requires platinum-containing dual drug chemotherapy or targeted therapy as the first-line treatment. Based on tumor biological mechanisms, an increasing number of treatments are being developed for advanced NSCLC, including angiogenesis inhibitors. Bevacizumab was the first angiogenesis inhibitor approved for patients with advanced NSCLC.3 Multiple phase III trials showed that chemotherapy combined with bevacizumab significantly improved the median progression-free survival (PFS) of NSCLC patients and was well-tolerated.4-6 After bevacizumab, no other antiangiogenic drugs were shown to be suitable for antitumor therapy until anlotinib.
Anlotinib is a novel oral small-molecule tyrosine kinase inhibitor (TKI) with multiple targets.7 Anlotinib is used alone as a maintenance drug in the treatment of advanced NSCLC and provides a survival benefit to patients. The inhibition of VEGFR1–3, FGFR1–4, PDGFRα–β, c-Kit, and Ret reduces tumor angiogenesis, which plays a significant role in tumor genesis, development, and metastasis.8,9 In a phase III clinical trial (ALTER0303 and NCT02388919), the median overall survival (OS) time in the anlotinib and placebo groups was 9.6 months (95% CI, 8.2-10.6) and 6.3 months (95% CI, 5.0-8.1), respectively. In addition, the median PFS was significantly prolonged in the anlotinib group (5.4 months; 95% CI, 4.4-5.6) compared to the placebo group (1.4 months; 95% CI, 1.1-1.5).10 This trial led to the approval of anlotinib as a third-line treatment for NSCLC. However, anlotinib has several side effects, including hand-foot skin reaction, hypertension, and proteinuria. Among them, the main adverse events (AEs) of grade 3 and above are: hypertension (13.6%), hyponatremia (8.2%), and increased γ-transpeptidase (5.4%), which limit its clinical use.7
Synthetic therapy or multimodality therapy, which means treating cancer in more than 1 way, has become a research hotspot in the field of cancer in recent days. Traditional Chinese Medicine (TCM) is a common choice to improve patients’ quality of life as a comprehensive therapy in China. Traditional Chinese medicine (TCM) believes that the occurrence of lung cancer is closely related to the loss of vital qi and the invasion of pathogenic factors. Phlegm, blood stasis, poison, and deficiency of vital qi are the basic pathogenesis of lung cancer, among which phlegm and deficiency are the most critical. Yifei Sanjie Formula is a pharmaceutical preparation endorsed by the First Affiliated Hospital of Guangzhou University of Chinese Medicine (Canton Province Drug: Z20190015000). This formula was developed by the TCM oncologist Prof. Lizhu Lin based on the primary pathogenesis of lung cancer, that is, spleen deficiency and phlegm dampness. It is derived from Yiqi Chutan Formula (YQCTF), which is widely used in The First Affiliated Hospital of Guangzhou University of Chinese Medicine and consists of 8 Chinese herbal medicines: catclaw buttercup root (Mao Zhua Cao), stir-fried stiff silkworm (Chao Jiang Can), Sarcandra (Zhong Jie Feng), appendiculate Cremastra pseudobulb (Shan Ci Gu), Thunberg fritillary bulb (Zhe Bei Mu), processed Pinellia tuber (Fa Ban Xia), glossy Ganoderma (Ling Zhi), and American ginseng (Xi Yang Shen). YFSJF also includes several other similar medicines. Basic biological research suggests that a comprehensive TCM treatment plan based on YQCTF effectively induces tumor cell apoptosis, delays tumor metastasis, and stabilizes the tumor body.11-17 Among the ingredients, Mao Zhua Cao, Shan Ci Gu, and Ling Zhi have been proven to have the effect of inhibiting tumor angiogenesis, and Mao Zhua Cao exerts anti-tumor effect through the epidermal growth factor receptor (EGFR) target.18-20 In addition, these 8 herbs also exert anti-tumor effects by promoting cell apoptosis and inhibiting cell proliferation. In clinical practice, we also found that YFSJF can enhance the constitution of patients and reduce the side effects of antitumor therapy. Therefore, we believe that YFSJF can enhance the vital qi of lung cancer patients, and assist the anti-tumor efficacy of anlotinib, so that the overall treatment effect can be better. In the present study, we propose to evaluate whether YFSJF and anlotinib could improve NSCLC treatment effectiveness and safety.
Methods
Trial Design
This will be a multicenter, randomized, double-blind, placebo-controlled, parallel-group pilot study conforming to the CONSORT (Consolidated Standards of Reporting Trials)21 and 2013 SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) guidelines. Up to 40 participants will be enrolled from 7 research centers: The First Affiliated Hospital of Guangzhou University of Chinese Medicine, China Academy of Chinese Medical Science Guang’anmen Hospital, Foshan Hospital of Traditional Chinese Medicine, Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine, Hainan Cancer Hospital, The First Affiliated Hospital of Guangxi University of Traditional Chinese Medicine, and Shunde Hospital Guangzhou University of Chinese Medicine. Written informed consent will be obtained from the participants.
Eligible participants will be randomly assigned in a 1:1 ratio to anlotinib combined with YFSJF (A + YFSJF) and anlotinib combined with placebo (A + P) groups. The placebo is a pellet made of dextrin, starch, honey concentrate, and caramel coloring. The placebo has the same smell, color, and appearance as the trial drug, but no obvious drug treatment effect.
The participants will be provided with anlotinib and the investigational product or placebo; they will undergo an imaging examination every 2 cycles (42 days), and clinical observation and safety assessments every treatment cycle (21 days), during the study period. In addition, the participants will complete the PRO questionnaire every week. During the follow-up phase of the study, the patients will undergo imaging examinations, clinical observation, and safety assessments every 3 months until death (Figure 1).
Figure 1.
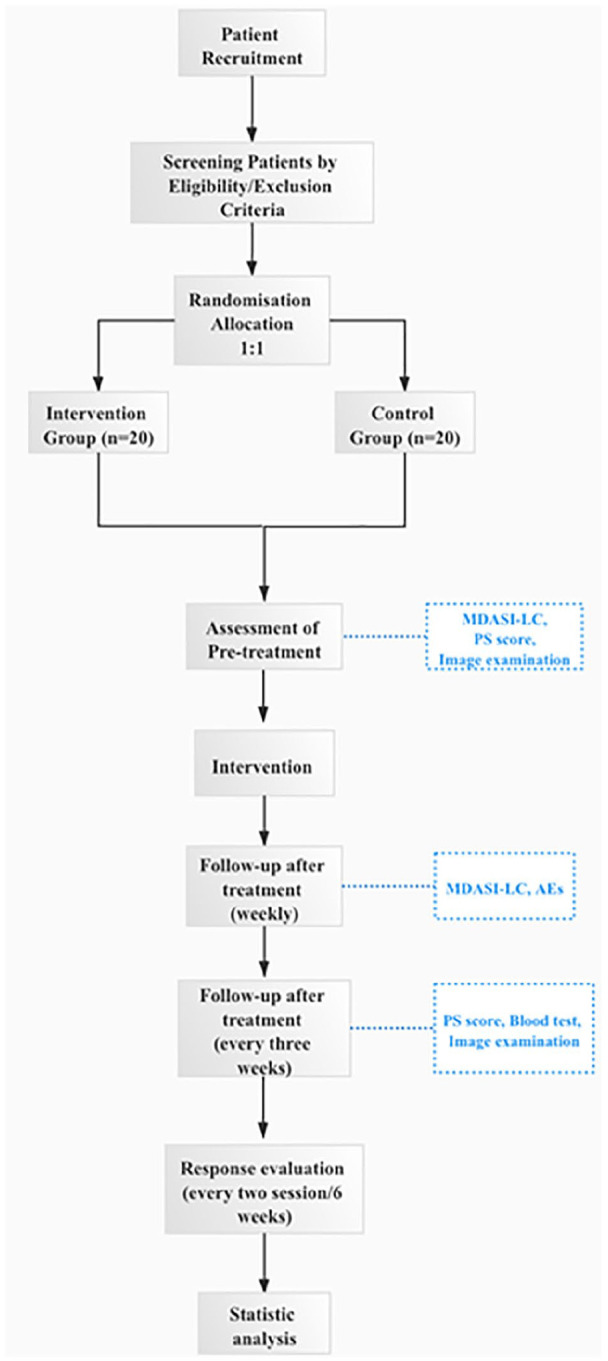
Study procedure.
Study Population and Eligibility Criteria
Patients who present to the oncology outpatient or inpatient department (between March 2021 and June 2023) of the study centers will be screened according to the inclusion and exclusion criteria (Table 1).
Table 1.
Study Inclusion and Exclusion Criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
| 1. Pathologically and/or cytologically diagnosed with IIIB–IVB NSCLC according to AJCC Cancer Staging Manual (8th edition).22
2. Patients without driver gene mutations who have received at least first-line systemic chemotherapy (immune checkpoint inhibitors or anti-vascular drug therapy) and developed disease progression, relapse, or intolerance 3. Patients with driver gene mutations (EGFR mutation or ALK fusion-positive) who have developed disease progression, relapse, or intolerance after receiving the corresponding standard targeted drug therapy 4. Men or women aged 18 to 75 y 5. ECOG PS score of 0 to 2 and Expected survival time ≥24 wk 6. Measurable lesion based on RECIST v 1.123 7. Adequate liver and kidney function (Cr ≥ 60 mL/min; TB ≤ 1.5 × ULN; ALT and AST ≤ 2.5 × ULN); 8. Hb ≥ 100 g/L; NEU ≥ 1.5 × 109/L; PLT ≥ 100 × 109/L 9. Agree to use contraception during the trial and for 6 mo thereafter 10. Informed consent provided by the patient or family member. |
1. Unstable brain metastases or bleeding risk 2. Previously used anlotinib or apatinib 3. Other types of concurrent malignant tumors 4. Unable to take oral medications due to dysphagia, chronic diarrhea, or intestinal obstruction 5. Pregnant or breastfeeding women 6. Mental illness or intellectual disability 7. Allergic to or intolerant of the study drug |
Abbreviations: AJCC, American Joint Committee on Cancer; NSCLC, non-small-cell lung cancer; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; ECOG PS, Eastern Cooperative Oncology Group performance status; RECIST, Response Evaluation Criteria in Solid Tumors; Cr, creatinine clearance rate; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TB, total bilirubin; Hb, hemoglobin; NEU, neutrophils; PLT, platelet; hCG, human chorionic gonadotropin
Concurrent Treatment
1. Other anti-tumor therapies, including traditional Chinese medicine decoction and proprietary Chinese medicine, are prohibited during treatment. Symptomatic drug treatment should be recorded truthfully in the electronic Case Report Form (eCRF). 2. Palliative radiotherapy can be used for pain relief or other non-radical purposes. The target lesion should not be treated with radiotherapy, and the radiotherapy site should not be used as a parameter to evaluate efficacy. 3. During the experimental treatment, drug symptomatic treatment such as bleeding, myelosuppression, nausea and vomiting, diarrhea, abnormal liver and kidney function, and infection can be used in combination.
Sample Size
The sample size has not been estimated for this exploratory pilot study of drug effectiveness and safety. A sample size of 20 patients per group meets the minimum statistical requirements.
Subject Withdrawal
Participants will be free to withdraw from the study at any stage, without any consequences. Additionally, participants will be excluded if they do not complete the PRO questionnaire during treatment.
Compliance
We will record the quantity of medicines returned by the participants to calculate the use rate. We will use various measures to improve compliance, including requiring them to complete a declaration as part of the informed consent process, emphasizing the importance of completing the study, and asking them to use medication log cards.
Trial Procedures
Eligible participants will be randomized in a 1:1 ratio to the A + YFSJF and A + P groups using a central randomization and drug administration system (http://iwrs.cltinc.org/dist/#/login). This system was designed by data administrators who will not participate in the group allocation process. Researchers will use this system to generate a unique random number for each participant. None of the patients, medical staff, or paramedics will be informed about the group allocations. Any serious adverse events (SAEs) will be reported by the physicians to the principal investigators, and blinding will be broken after approval.
The participants will receive anlotinib (12 mg per day on days 1-14 of a 21-day course) until progression of disease (PD). In cases of anlotinib toxicity, alternative doses of 8 or 10 mg may be used. When patients experience adverse effects from anlotinib, investigators can manage them with symptomatic treatment, drug discontinuation, and/or dose adjustment. The first dose adjustment is 10 mg QD D1-D14 Q21D; the second dose adjustment is 8 mg QD D1-D14 Q21D. National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events version 5.0 (CTCAE V5.0) will be used to assess and grade adverse events (AEs).24 The general principles of dose adjustment according to the level of adverse reactions are shown in the Supplemental Appendix. During each cycle until PD, patients in the A + YFSJF group will receive 15 g of YFSJF twice daily, whereas those in the A + P group will receive 15 g of placebo twice daily (Tables 2 and 3).
Table 2.
Details of Interventions.
| Intervention group | Control group | |
|---|---|---|
| Standard treatment | Anlotinib (12 mg) once daily on days 1 to 14 of a 21-day course | |
| Variable | YFSJF | Placebo (YFSJF simulant) |
| Frequency and duration of a treatment session | 15 g twice daily until PD | 15 g twice daily until PD |
Abbreviations: YFSJF, Yifei Sanjie formula; PD, progression of disease.
Table 3.
Data Collected at Each Visit.
| Timepoint | Baseline-1 cycle | Treatment phase | Follow-up phase | ||
|---|---|---|---|---|---|
| ~D0 | Cycle 1 | Cycle 2 | Cycle n | Every 3 mo | |
| D1 ± 3 | D21 ± 3 | ||||
| Eligibility screening | × | ||||
| Informed consent | × | ||||
| Medical history | × | ||||
| Clinical observation | × | × | × | × | × |
| MDASI–LC | × | Once a week | |||
| Imaging examination | × | Every two treatment cycles | × | ||
| Tumor marker assessment (optional) | × | × | × | × | |
| Safety observation | × | × | × | × | × |
| NCI AEs | × | × | × | × | |
| Peripheral blood examination | × | × | |||
| Survival status | × | ||||
Abbreviations: MDASI-LC, M.D. Anderson Symptom Inventory-Lung Cancer; NCI, National Cancer Institute; AEs, adverse events.
AEs are defined as aggravation of pre-existing disease or symptoms, and serious adverse events (SAEs) are defined as death, life-threatening, requiring or prolonging hospitalization. All AEs should be recorded in the eCRF and graded according to CTCAE 5.0. In the event of serious life-threatening adverse events (grade 4), the trial should be suspended, and at the same time, the unit undertaking the clinical research must take immediate measures to protect the safety of the subjects.
Discontinuation Criteria
Participants will be withdrawn from the study if they have an endpoint event (death), intolerable AEs, Eastern Cooperative Oncology Group performance status (ECOG PS) >3, or voluntarily withdraw from the study.
Data Collection and Management
An electronic data capture (EDC) system will be used to record the study data. The EDC system was designed by ePRO-vision (Beijing) Health Technology Co., Ltd.
The raw data will be stored in the EDC system according to standard operating procedures for the collection, storage, and analysis of electronic case report forms (eCRF). Data will be accessible only to the authorized study personnel through input of a confidential username and password. Electronic communication between the server and the user’s personal computer will be encrypted and no protected data (eg, patient name, address, or ID number) will be transmitted. A complete audit trail will be created for changes to the study data. Paper-based materials will be managed by specialized personnel. Quality control personnel will ensure the quality of the inputted data, promptly report and log missing data, and review and address data errors.
Outcomes
PFS will be the primary endpoint of the study, whereas OS, the objective response rate (ORR), and patient-reported outcomes (PRO) will be the secondary outcomes. The participants will receive an imaging examination every 2 cycles (42 ± 3 days) to evaluate the treatment response. PRO are to be reported by patients without any intervention or instructions from investigators.25 We will collect the PRO data by instructing patients to complete the relevant questionnaire on WeChat (the most popular social media application in China), at baseline and weekly thereafter, using the modified M. D. Anderson Symptom Inventory-Lung Cancer (MDASI-LC).26 This scale is concise, comprehensive, easy to complete, and strongly supported by psychometric data.27
Safety Assessments
Patient safety will be monitored throughout the study. Participants will be required to measure and report their blood pressure daily during the intervention. Upon enrollment in the study, participants will be issued with log cards and will be asked to record medication, blood pressure, and adverse events daily. The log card should be returned to the researcher at each visit. The patients will be asked to have their blood pressure measured every morning after they get up. The sphygmomanometer is purchased by the patient. Electronic blood pressure monitoring is recommended. The ECOG PS and laboratory test results of the participants will be recorded before each intervention cycle and after the completion of all interventions. AEs will be classified according to the CTCAE 5.0. Participants with grade 3 to 4 AEs will be excluded from the study; in addition, the interventions will be discontinued and the AEs will be managed accordingly. All AEs will be reported to the Ethics Committee and posted on the official website of the National Center for Drug Evaluation (www.cde.org.cn).
Statistical Analysis
SAS software (v. 9.4 or later) will be used for the statistical analysis.
The data will be analyzed according to intention-to-treat (ITT), modified intention-to-treat (mITT), per-protocol set (PPS), and safety set (SS) protocols. ITT analysis of the results is based on the initial treatment assignment and not on the treatment eventually received, it does not require observation of compliance status for units assigned to different treatments or incorporation of compliance into the analysis. PP analysis is a comparison of treatment groups that include only those patients who completed the treatment originally allocated. The mITT set excludes the participants who never receive treatment and includes the one who drop out in the process of trial but generate baseline data and at least 1 follow-up data. SS is used for safety analysis, and includes patients who receive treatment. This set of patients is called the Safety population, and patients are grouped for analysis according to the treatment they received, as opposed to the treatment they were allocated to receive at randomization.
Quantitative data will be presented as number of cases, mean, standard deviation, median, and minimum and maximum values (along with 25th quantile [Q1]–75th quantile [Q3] as necessary). The classification index data will be presented as cases and percentages.
The Kaplan-Meier method will be used to estimate the median PFS, median OS, and 95% confidence intervals (CIs) for each group. The log-rank test will be used to compare the groups. Hazard ratios (HRs) and 95% CIs will be calculated using a Cox proportional hazard model. Repeated measurements will be analyzed using a generalized additive mixed model (GAMM) to compare PRO data between the 2 groups over time. Fisher’s exact test and χ2 will be used to compare the incidence of AEs between groups. Researchers will contact participants through regular text messages and phone calls to avoid data loss, and missing data will not be recorded.
Discussion
This study aims to evaluate the efficacy and safety of anlotinib combined with YFSJF for the treatment of patients with stage IIIB–IVB NSCLC who have previously received first- or second-line treatment. We will evaluate the efficacy of comprehensive treatment based on YFSJF using objective (PFS, OS, and ORR) and subjective markers (PRO). The objective and subjective markers will provide evidence of efficacy in both physiological and psychological terms, in line with the biological-psychological-sociological approach to medicine.
Increased angiogenesis is essential for tumor genesis, development, and metastasis.9 Therefore, drugs that prevent tumor vascular growth may be useful as antitumor therapy.28 Inhibiting endothelial cell proliferation and blood vessel growth can lead to hypoxia and nutrient deprivation of tumor cells, in turn leading to dormancy. Anlotinib is a multi-target angiogenesis inhibitor on the basis of this concept. The anti-angiogenic effects of TCM extracts have received significant attention from researchers. Many studies have evaluated the effects of Chinese medicine monomers and their biologically active extracts. For example, a flavonoid isolated from Sophora flavescens (Kushen) blocked the cell cycle without inducing apoptosis in the G0/G1 phase, and downregulated the expression of vascular endothelial growth factor (VEGF), thereby exerting antiangiogenic effects.29 Cantharidin (CTD) suppresses VEGF-induced activation of STAT3 in a dose-dependent manner, and also inhibits the phosphorylation of JAK1 and ERK, thereby exerting antiangiogenic effects.30 Curcumin inhibits the proliferation of human hepatocellular carcinoma cells in vitro and in vivo by reducing VEGF expression.31 Other antitumor effects of Chinese herbs, such as cytotoxicity and endonucleolytic DNA cleavage, have also been reported. Moreover, network pharmacology studies have confirmed antitumor effects of TCM compounds.32,33 Therefore, the synergistic antitumor effects of TCM compounds should be evaluated in clinical studies.
Tumor recurrence and metastasis are serious challenges for clinical tumor treatment. In China, TCM is widely used for the comprehensive treatment of cancer.
Wu et al34 found that treating advanced lung cancer patients with kanglaite injection, containing anticancer components extracted from a Chinese herbal, can significantly down their expression of miRNA-21, an indicator relate to the prognosis of lung cancer. Xiao et al35 verified that the efficacy and safety of TCM, including kang’ai injection, herbal decoction, and zhenqifuzheng capsules, in advanced NSCLC patients underwent chemotherapy through a randomized controlled trial. And a single-arm phase II study also showed that the comprehensive TCM treatment (shenqi injection) combined with a dendritic cell vaccination for lung cancer (DCVAC/LuCa) and standard of care chemotherapy exhibited good benefit in recurrent metastatic or advanced NSCLC.36
Although TCM shows excellent efficacy in clinical practice, the scientific evidence supporting its use is weak. Our study should provide data supporting the use of anlotinib combined with YFSJF as second-line or above treatment for stage IIIB–IVB NSCLC. The study results will help to determine whether anlotinib combined with YFSJF can effectively prolong the PFS of patients. In addition, our trial results will fill the research gap regarding the efficacy of Chinese herbal compounds as elements of comprehensive antitumor therapies.
Acknowledgments
We are grateful to Xu D for his expert assistance with the writing of the study protocol.
Footnotes
List of Abbreviation: NSCLC, non-small-cell lung cancer
TCM, Traditional Chinese Medicine
YFSJF, Yifei Sanjie formula
PFS, progression-free survival
PD, progression of disease
OS, overall survival
ORR, objective response rate
PRO, patient-reported outcomes
TKI, tyrosine kinase inhibitor
MDASI-LC, M. D. Anderson Symptom Inventory-Lung Cancer
YQCTF, Yiqi Chutan Formula
CONSORT, Consolidated Standards of Reporting Trials
EGFR, Epidermal growth factor receptor
SAEs, serious adverse events
NCI, National Cancer Institute
CTCAE V5.0, Common Terminology Criteria for Adverse Events version 5.0
AEs, adverse events
ECOG PS, Eastern Cooperative Oncology Group performance status
EDC, electronic data capture
MITT, intention-to-treat
PPS, per-protocol set
MITT, modified intention-to-treat
SS, safety set
CIs, confidence intervals
HRs, Hazard ratios
GAMM, generalized additive mixed model
DCVAC/LuCa, dendritic cell vaccination for lung cancer
AJCC, American Joint Committee on Cancer;
Author Contributions: Study concept and design: Lizhu L, Yang C, Jietao L, Wenmin C, and Lanting T. Study conduct: Jietao L, Wenmin C, Lingling S, Zhiwei X, Hanrui C, and Lanting T. Data management and statistical analysis: Ting Y, Zhiwei X, Zexing Z, and Xiangjun Q. Drafting of the manuscript: Wenmin C, Jietao L, and Ting Y. Critical review and revision of the manuscript for intellectual content: Jietao L, Wenmin C, Ting Y, Yang C, Lingling S, Zhiwei X, Lanting T, Hanrui C, Zexin Z, Xiangjun Q, and Lizhu L.
Availability of Data and Material: The results of our study will be presented at relevant scientific conferences and/or magazines. The raw trial data will be published on the China Clinical Trial Registration website within 6 months of trial completion.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Administration of Traditional Chinese Medicine: 2019 Project of building evidence-based practice capacity for TCM (No.2019XZZX-ZL001).
Ethics Approval and Consent to Participate: The trial will be conducted in accordance with Good Clinical Practice (GCP) guidelines; it will be supervised and managed by the Ethics Committee of the First Affiliated Hospital of Guangzhou University of Traditional Chinese Medicine. The trial was registered at www.chictr.org.cn/index.aspx (ChiCTR2100047143), and the investigational plan was approved by the First Affiliated Hospital of Guangzhou University of Traditional Chinese Medicine (approval no.: K2020151). All participants would sign the informed consent before joining the trial.
ORCID iDs: Wenmin Chen  https://orcid.org/0000-0002-2126-3112
https://orcid.org/0000-0002-2126-3112
Jietao Lin  https://orcid.org/0000-0002-5507-3499
https://orcid.org/0000-0002-5507-3499
Xiangjun Qi  https://orcid.org/0000-0003-1616-5400
https://orcid.org/0000-0003-1616-5400
Lizhu Lin  https://orcid.org/0000-0002-5396-3033
https://orcid.org/0000-0002-5396-3033
References
- 1. Arbour KC, Riely GJ. Systemic therapy for locally advanced and metastatic non-small cell lung cancer: a review. JAMA. 2019;322:764-774. doi: 10.1001/jama.2019.11058 [DOI] [PubMed] [Google Scholar]
- 2. Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359:1367-1380. doi: 10.1056/NEJMra0802714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Manzo A, Montanino A, Carillio G, et al. Angiogenesis inhibitors in NSCLC. Int J Mol Sci. 2017;18:1-17. doi: 10.3390/ijms18102021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sandler A, Gray R, Perry MC, et al. Paclitaxel–carboplatin alone or with bevacizumab for non–small-cell lung cancer. N Engl J Med. 2006;355:2542-2550. doi: 10.1056/nejmoa061884 [DOI] [PubMed] [Google Scholar]
- 5. Reck M, Von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAiL. J Clin Oncol. 2009;27:1227-1234. doi: 10.1200/JCO.2007.14.5466 [DOI] [PubMed] [Google Scholar]
- 6. Zhou C, Wu YL, Chen G, et al. BEYOND: a randomized, double-blind, placebo-controlled, multicenter, phase III study of first-line carboplatin/paclitaxel plus bevacizumab or placebo in Chinese patients with advanced or recurrent nonsquamous non-small-cell lung cancer. J Clin Oncol. 2015;33:2197-2204. doi: 10.1200/JCO.2014.59.4424 [DOI] [PubMed] [Google Scholar]
- 7. Shen G, Zheng F, Ren D, et al. Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol. 2018;11:1-11. doi: 10.1186/s13045-018-0664-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Syed YY. Anlotinib: first global approval. Drugs. 2018;78:1057-1062. doi: 10.1007/s40265-018-0939-x [DOI] [PubMed] [Google Scholar]
- 9. Wang HY, Chu JF, Zhang P, et al. Safety and efficacy of chemotherapy combined with anlotinib plus anlotinib maintenance in Chinese patients with advanced/metastatic soft tissue sarcoma. Onco Targets Ther. 2020;13:1561-1568. doi: 10.2147/OTT.S235349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Han B, Li K, Zhao Y, et al. Anlotinib as a third-line therapy in patients with refractory advanced non-small-cell lung cancer: a multicentre, randomised phase II trial (ALTER0302). Br J Cancer. 2018;118:654-661. doi: 10.1038/bjc.2017.478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang S, Lin L, Zhou J, Xiong S, Zhou D. Effects of yiqi chutan tang on the proteome in Lewis lung cancer in mice. Asian Pac J Cancer Prev. 2011;12:1665-1669. [PubMed] [Google Scholar]
- 12. Lin LZ, Wang SM, Zhou JX. Effects of yiqi chutan recipe on tumor growth, survival time and expressions of PRDX-1 and PRDX-6 in Lewis lung carcinoma model mice with pi-deficiency syndrome. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2011;31:99-103. [PubMed] [Google Scholar]
- 13. Zheng XT, Lin LZ. Efficacy observation of modified yiqi chutan recipe treating mid-late stage NSCLC patients by CT perfusion. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2016;36:155-159. [PubMed] [Google Scholar]
- 14. Sun LL, Lin LZ, Zhou JX, Chen ZZ, Tao WH. Correlation analysis of efficacy of yiqi chutan recipe in treating NSCLC and P4HB expression. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2015;35:184-187. [PubMed] [Google Scholar]
- 15. Chen CM, Sun LL, Fang RM, Lin LZ. YiQi ChuTan recipe inhibits epithelial mesenchymal transition of A549 cells under hypoxia. Cell Mol Biol. 2016;62:10-15. [PubMed] [Google Scholar]
- 16. Wang SM, Lin LZ, Zhou DH, Zhou JX, Xiong SQ. Expression of prolyl 4-hydroxylase beta-polypeptide in non-small cell lung cancer treated with Chinese medicines. Chin J Integr Med. 2015;21:689-696. doi: 10.1007/s11655-013-1535-2 [DOI] [PubMed] [Google Scholar]
- 17. Zhang J, Sun L, Cui J, et al. Yiqi Chutan tang reduces gefitinib-induced drug resistance in non-small-cell lung cancer by targeting apoptosis and autophagy. Cytometry A. 2020;97:70-77. doi: 10.1002/cyto.a.23869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ji Y, Wu M. Research progress of pseudobulbus cremastrae seu pleiones on chemical composition and antitumor mechanisms. Chin Arch Tradit Chin Med. 2018;36:596-598. [Google Scholar]
- 19. Huang ZM, Peng HT, Lin XT. Study on the mechanism of ranunculi ternati radix in treatment of lung cancer based on network pharmacology pharmacology. J Guangdong Pharm Univ. 2021;37:90-97. doi: 10.16809/j.cnki.2096 [DOI] [Google Scholar]
- 20. Ma C, Zhang Z, Yan M, Wang S, Jian Q. Research status of bio-active components and anti-tumor of Ganoderma lucidum. Edible Med Mushrooms. 2022;30:114-118. [Google Scholar]
- 21. Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:698-702. doi: 10.1136/bmj.c332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Amin MB, Greene FL, Edge SB, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67:93-99. doi: 10.3322/caac.21388 [DOI] [PubMed] [Google Scholar]
- 23. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 24. NCI. Common terminology criteria for adverse events (CTCAE) version 5; 2017. Accessed November 27, 2017. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_archive
- 25. Bottomley A, Pe M, Sloan J, et al. Analysing data from patient-reported outcome and quality of life endpoints for cancer clinical trials: a start in setting international standards. Lancet Oncol. 2016;17:e510-e514. doi: 10.1016/S1470-2045(16)30510-1 [DOI] [PubMed] [Google Scholar]
- 26. Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: the M. D. Anderson symptom inventory. Cancer. 2000;89:1634-1646. doi: 10.1002/1097-0142(20001001)89:7<1634::AID-CNCR29>3.0.CO;2-V [DOI] [PubMed] [Google Scholar]
- 27. Bouazza YB, Chiairi I, El Kharbouchi O, et al. Patient-reported outcome measures (PROMs) in the management of lung cancer: a systematic review. Lung Cancer. 2017;113:140-151. doi: 10.1016/j.lungcan.2017.09.011 [DOI] [PubMed] [Google Scholar]
- 28. Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer. 2007;7:834-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang XL, Cao MA, Pu LP, et al. A novel flavonoid isolated from Sophora flavescens exhibited anti-angiogenesis activity, decreased VEGF expression and caused G0/G1 cell cycle arrest in vitro. Pharmazie. 2013;68:369-375. [PubMed] [Google Scholar]
- 30. Wang T, Liu J, Xiao XQ. Cantharidin inhibits angiogenesis by suppressing VEGF- induced JAK1/STAT3, ERK and AKT signaling pathways. Arch Pharm Res. 2015;38:282-289. [DOI] [PubMed] [Google Scholar]
- 31. Pan Z, Zhuang J, Ji C, Cai Z, Liao W, Huang Z. Curcumin inhibits hepatocellular carcinoma growth by targeting VEGF expression. Oncol Lett. 2018;15:4821-4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lin S, Fujii M, Hou DX. Rhein induces apoptosis in HL-60 cells via reactive oxygen species-independent mitochondrial death pathway. Arch Biochem Biophys. 2003;418:99-107. doi: 10.1016/j.abb.2003.08.004 [DOI] [PubMed] [Google Scholar]
- 33. Zhang Y, Zhou T, Wang H, Cui Z, Cheng F, Wang KP. Structural characterization and in vitro antitumor activity of an acidic polysaccharide from Angelica sinensis (Oliv.) diels. Carbohydr Polym. 2016;147:401-408. doi: 10.1016/j.carbpol.2016.04.002 [DOI] [PubMed] [Google Scholar]
- 34. Wu Y, Zhang J, Hong Y, Wang X. Effects of kanglaite injection on serum miRNA-21 in patients with advanced lung cancer. Med Sci Monit. 2018;24:2901-2906. doi: 10.12659/MSM.909719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xiao Z, Chen Z, Han R, et al. Comprehensive TCM treatments combined with chemotherapy for advanced non-small cell lung cancer: a randomized, controlled trial. Medicine (Baltimore). 2021;100:e25690. doi: 10.1097/MD.0000000000025690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu Q, Lou Y, Li L, et al. A single-arm phase II study to evaluate efficacy and safety of first-line treatment with DCVAC/LuCa, standard of care chemotherapy and shenqi fuzheng injection in advanced (Stage IIIB/IV) non-small cell lung cancer patients. Integr Cancer Ther. 2022;21. doi: 10.1177/15347354221083968 [DOI] [PMC free article] [PubMed] [Google Scholar]


