Abstract
Chondromyxoid fibroma is one of the rarest benign cartilaginous tumors accounting for less than 0.5% of bone tumors and mostly found in the metaphysis of long bones. Diagnosis is by histology showing lobular pattern with stellate-shaped cells in a myxoid or chondroid background. Often they can be misdiagnosed as chondrosarcomas. Recommended treatment approach is surgically excision due to the high risk of malignancy. Although benign, local recurrence is common as presented from this case report.
Keywords: Chest, chondromyxoid fibroma, pediatric, recurrence
Background
Chondromyxoid fibroma (CMF) is a rare benign tumor of cartilaginous origin with occurrence of less than 0.5% of bone tumors and 2% of benign bone tumors.1 It usually affects the metaphysis of long bones, proximal tibia being the most common location.2 It is a slow-growing tumor of chondroblastic origin and has a raising concern for malignancy with characteristics of single lobular and eccentric lesion with expansion of the affected bone.3 CMF was first described in 1943 and said to be distinct from chondrosarcoma. They are usually asymptomatic at diagnosis but commonly cause local pain and rarely cause pathologic fractures.3 Herein, we present a case of recurrent CMF after multiple excision in a child.
Case presentation
A 11-year-old boy presented with a left breast mass for 3 years. The mass was reported to be painful, bled easily on touch with some pus discharge. This was accompanied with episodes of low-grade intermittent fevers. He had the same history 5 years ago of which the mass was resected but gradually recurred 2 years later, but unfortunately no histopathology analysis was done then.
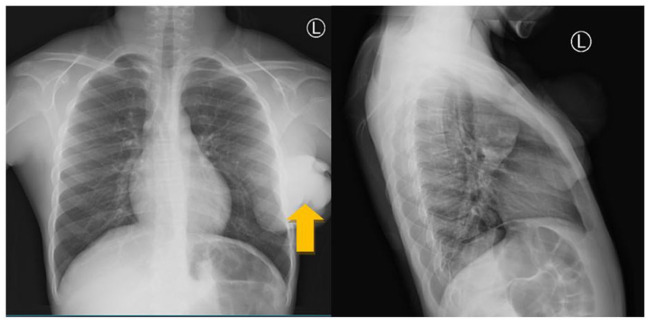
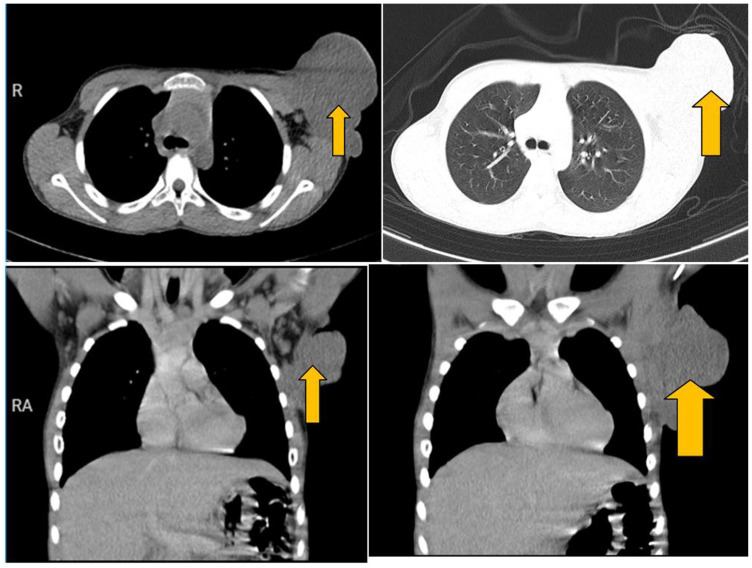
On examination, the child was fully conscious, alert, and oriented, mildly pale but not jaundiced, not febrile with stable vitals. Locally, a pedunculated mass on his left breast extending to his axillary region measuring about 10 × 10 × 6 cm3 in size with necrotic skin and some pus discharge. The mass was firm with a soft tip and mildly tender to touch. His complete blood count showed a hemoglobin of 9.0 g/dL, leukocyte count of 9.48 × 109/L, platelet count of 647 × 109/L, and erythrocyte sedimentation rate (ESR) of 80 mm/h. Chest X-ray showed large left chest wall soft tissue mass with axillary extension involving the inferior angle of the scapula (Figure 1). The abdominal pelvic ultrasound had normal findings. His chest computed tomography (CT)-scan showed left axillary soft tissue mass measuring 9.6 × 8.5 × 10.9 cm3 in size infiltrating the pectoralis muscle. Left axillary lymph nodes enlargement, the largest measuring 1.1 × 1.05 cm2 in size (Figure 2).
Figure 1.
Left chest wall mass extending to axilla (orange arrow).
Figure 2.
CT-chest shows left axillary soft tissue mass measuring 9.6 × 8.5 × 10.9 cm3 in size infiltrating the pectoralis muscle (orange arrow).
Left axillary lymph nodes enlargement, the largest measuring 1.1 × 1.05 cm2 in size.
The patient was admitted and transfused with a unit of blood and planned for mass excision (wide local excision). Intraoperatively, the tumor was found to infiltrate the pectoralis major and minor muscles, hence excision was done (R1); part of the tumor was left attached to the involved muscle. Post-operatively, the patient was kept on analgesics and antibiotics. The excised tumor was taken for histopathology which concluded it to be CMF (Figure 3) with differential diagnosis of inflammatory myofibroblastic tumor. Immunohistochemical testing for the tumor revealed non-specific soft tissue entity. However, 1 month after the surgery, the child was seen and reviewed at the surgical clinic doing well clinically with granulating wound with normal range of movement of the left arm. He was referred to the oncology unit but was lost to follow-up unfortunately.
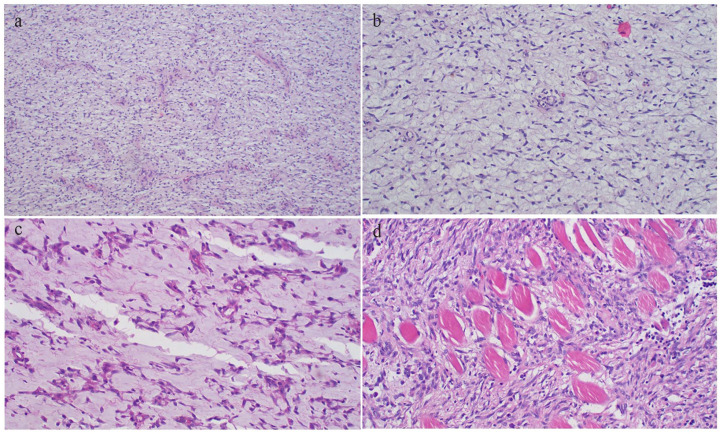
Figure 3.
(a) Diffuse proliferation of mononuclear spindle cells admixed with occasional multinucleated giant cells; with variably myxoid to chondroid stroma, representing various stages of cartilaginous development (H&E stained 40× original magnification). (b) Photomicrograph of the tumor displaying myofibroblastic-like spindle cell proliferation with loosely arranged myxoid stroma, minimal cellular atypia, and rare mitoses together with storiform or fascicular pattern (H&E stained 200× original magnification).
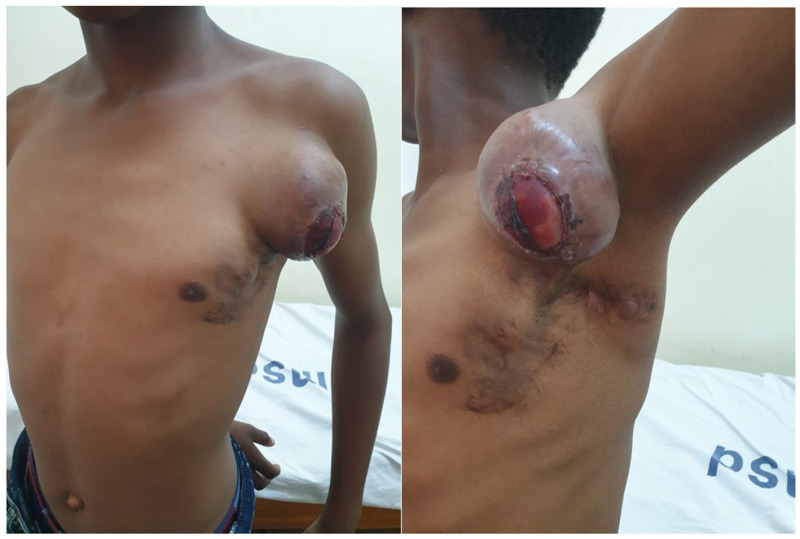
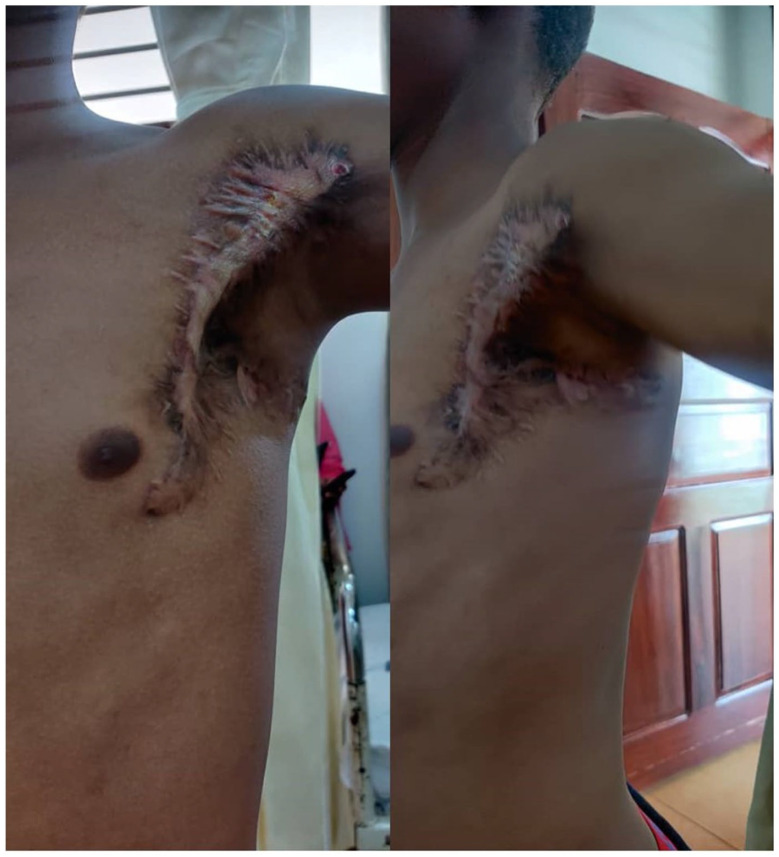
The child presented again after 7 months with recurrence for the second time at the same site (Figure 4). He was clinically stable with no clinical features of metastasis with good range of movement of his left arm. He was admitted and underwent a wide local excision whereby the tumor was resected whole including the part of the pectoralis major muscle (R1). He faired well post-operatively and was discharged through the pediatric oncology unit. He was reviewed after 1 month, whereby the wound has healed with a hypertrophic scar, has no upper limb edema, and has a full range of motion of his shoulder joint (Figure 5). Histologically, the resected specimen demonstrated zonal architecture morphology comprising lobules of myxoid to chondroid tissue with intervening spindle or stellate and myxoid stromal degeneration. Areas with infiltrative pattern, necrosis, and pleomorphic cells suggesting malignant transformation were associated. The presence of infiltrative pattern and necrosis gave the differential diagnosis of low-grade myxofibrosarcoma, myxoid dermatofibrosarcoma, proliferative fasciitis, and solitary fibrous tumor (Figure 6).
Figure 4.
Photograph showing recurrent left axillary mass with previous surgery scars.
Figure 5.
Post-operative clinical photograph showing no recurrence and healed hypertrophic scar.
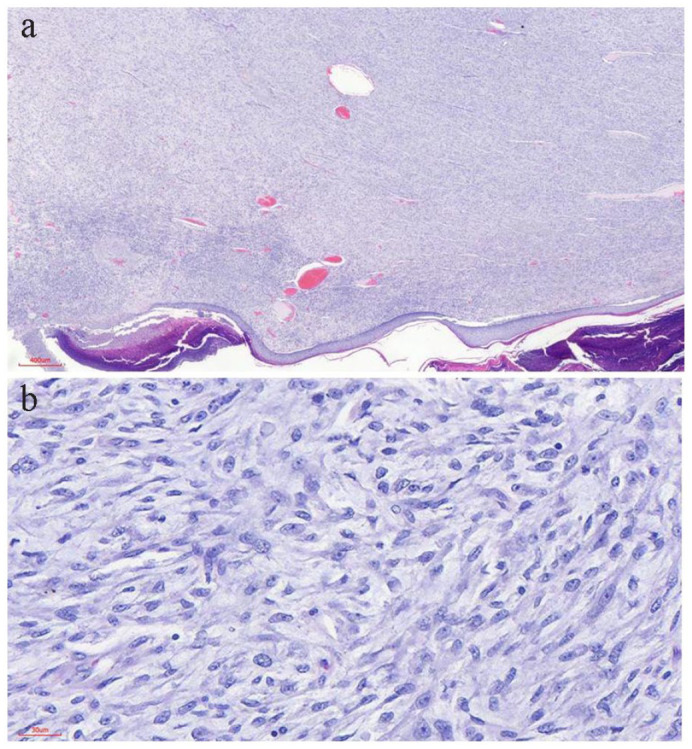
Figure 6.
(a) CMF demonstrating diffuse population of mononuclear spindle cells and admixed multinucleated giant cells (H&E 40× original magnification). (b) CMF highlighting the presence of lobules of stellate cells with variably myxoid to chondroid stroma, representing various stages of cartilaginous development (H&E 100× original magnification). (c) Photomicrograph of CMF showing cells having variable pink cytoplasm, bipolar to multipolar cytoplasmic extensions and oval to spindled nuclei with myxoid stroma (H&E 200× original magnification). (d) Infiltration of skeletal muscle by the tumor cells with moderate nuclear pleomorphism (H&E 200× original magnification).
Discussion
CMF was first described in 1948 by Jaffe and Lichtenstein, and is usually diagnosed in the second decade of life with a slightly male predominance unlike in the index case, the tumor first occurred in the first decade.4 Due to its rarity and overlapping of characteristics with other bone tumors, diagnosis is not always easy.4 The prognosis is promising with the risk of malignant transformation in 1%–2%, though a high recurrence rate of up to 80%, as seen in our case.4
Rib presentation is unusual with CMF, and to our knowledge, only a few have been reported.5 Other unusual locations reported are temporal bone causing facial nerve palsy and zygoma.6,7 Commonest sites are long bones, particularly of the lower extremity; proximal tibia and distal femur have the highest incidence.8 According to Zeinoddini et al.,7 only one case of CMF has been reported in the pediatric age group and the index being second. Due to its rarity and unspecific presentation, it is also misdiagnosed using radiology alone the authors added; hence, biopsy is needed for definitive diagnosis. Histologically, CMF is characterized by pseudolobulated architecture of spindle or stellate cells in a myxoid or chondroid stroma, which typically stain-positive for S-100, Sox 9, and collagen Type-II, as seen in our case.9,10
The histogenesis remains unclear for CMF, as there are no specific characteristic abnormality or chromosomal breaking point, however, has myofibroblastic differentiation in its “fibrous” areas driven by transforming growth factor β-1.11 Similar to chondroblastoma, CMF poses Sox9 gene responsible for chondrocytic differentiation and regulation of the expression of cartilage-specific genes in mature chondrocytes, especially the synthesis of collagen type-II.11
CMF can be confused with chondrosarcoma as both can have a low predilection for low 18 F-FDG uptake and the presence of pleomorphic cells with hyperchromatic nuclei.12 However, radiologic characteristics of CMF include the presence of a single lobular and eccentric lesion with expansion of the affected bone toward local soft tissue, cortical thinning and destruction, and a sclerotic rim.13 Like chondrosarcoma, both are aggressive but calcifications in CMF are much less.
Management is challenging due to its recurrence but options range from simple curettage to curettage with phenol application, but en bloc resection with negative surgical margins is preferred because of the low recurrence rate compared to curette alone having 80%.5 Recurrence is more common in younger age groups and often associated with higher quantities of myxoid stroma and cytological atypia as in the index case.14,15 Although Jaffe and Lichtenstein in their initial description stated that recurrences were not common after incomplete removal, other series reported a 25% recurrence rate with curate and bone grafting which may be higher in young children.16 This warrants a longer follow-up but it was unfortunate that our patient was lost to follow-up. En bloc resection is usually curative, depending on the site, but was not possible due to the extension of the tumor into the muscles of the chest wall as it would expose the ribs causing more morbidity. Radiation is not recommended due to the fear of radiation-induced malignancy.8,15,16
Conclusion
CMF is a rare benign tumor and can recur following excision. The diagnosis and management can be challenging due to its rarity, including atypical locations. A high index of suspicion, adequate treatment, and follow-up are critical for the successful management of these uncommon benign tumors. Due to the diagnostic dilemma and high recurrence rates, CMF may be better managed in specialist centers.
Acknowledgments
The authors thank the child’s mother for permission to share her child’s medical history for educational purposes and publication.
Footnotes
Author contributions: J.L., G.G., and A.M. conceptualized and drafted the article; P.A. and A.M. reviewed the medical records and reported histology films. All authors have read and approved the final article.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed consent: Written informed consent was obtained from the patient’s mother for publication for this case report; additionally, accompanying images have been censored to ensure that the patient cannot be identified. A copy of the consent is available on record.
ORCID iD: Jay Lodhia  https://orcid.org/0000-0002-3373-5762
https://orcid.org/0000-0002-3373-5762
References
- 1. Soni R, Kapoor C, Shah M, et al. Chondromyxoid fibroma: a rare case report and review of literature. Cureus 2016; 8(9): e803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen C, Huang X, Chen M, et al. Surgical management of a giant sternal chondromyxoid fibroma: a case report. J Cardiothorac Surg 2015; 10(1): 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Long KL, Absher KJ, Draus JM., Jr. Chondromyxoid fibroma of the second rib. J Pediatr Surg 2013; 48(6): 1442–1444. [DOI] [PubMed] [Google Scholar]
- 4. Cappelle S, Pans S, Sciot R. Imaging features of chondromyxoid fibroma: report of 15 cases and literature review. Br J Radiol 2016; 89(1064): 20160088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Basak B, Haragan A, Shackcloth M, et al. Chondromyxoid fibroma of the rib: a rare benign tumor with potential for local recurrence. Cureus 2021; 13(10): e19172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chrysouli K, Papanikolaou V, Chrysovergis A, et al. A rare cause of peripheral facial nerve palsy: chondromyxoid fibroma of the temporal bone. J Craniofac Surg 2022; 33(3): e276–e279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zeinoddini A, Bezold A, Ezzeldin O, et al. Radiological manifestations of chondromyxoid fibroma in the zygoma: a case report and literature review. BJR Case Rep 2021; 7(4): 20210008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bush JB, Sweeney JP, Robison JE, et al. Chondromyxoid fibroma of the radial shaft treated with nonvascularized fibular autograft. Am J Orthop 2010; 39(1): 30–34. [PubMed] [Google Scholar]
- 9. Wangsiricharoen S, Wakely PE, Jr, Siddiqui MT, et al. Cytopathology of chondromyxoid fibroma: a case series and review of the literature. J Am Soc Cytopathol 2021; 10(4): 366–381. [DOI] [PubMed] [Google Scholar]
- 10. Minasian T, Claus C, Hariri OR, et al. Chondromyxoid fibroma of the sacrum: a case report and literature review. Surg Neurol Int 2016; 7(Suppl. 13): S370–S374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. De Mattos CB, Angsanuntsukh C, Arkader A, et al. Chondroblastoma and chondromyxoid fibroma. J Am Acad Orthop Surg 2013; 21(4): 225–233. [DOI] [PubMed] [Google Scholar]
- 12. Kilic D, Findikcioglu A, Tepeoglu M, et al. Chondromyxoid fibroma of the sternum in a 63-year-old woman. Tex Heart Inst J 2015; 42(4): 400–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Makis W, Ciarallo A, Lisbona R. Chondromyxoid fibroma of the rib mimics a chondrosarcoma on 18F-FDG PET/CT. Acta Radiologica 2011; 52(5): 554–556. [DOI] [PubMed] [Google Scholar]
- 14. Jamshidi K, Mazhar FN, Yahyazadeh H. Chondromyxoid fibroma of calcaneus. Foot Ankle Surg 2013; 19(1): 48–52. [DOI] [PubMed] [Google Scholar]
- 15. Chowdary PB, Patil MD, Govindarajan AK. Chon-dromyxoid fibroma: an unusual tumour at an atypical location. J Clin Diagn Res 2015; 9(7): XD04–XD05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. HemanthaKumar G, Sathish M. Diagnosis and literature review of chondromyxoid fibroma —a pathological puzzle. J Orthop Case Rep 2019; 9(4): 101. [DOI] [PMC free article] [PubMed] [Google Scholar]