Abstract
Background
Technetium-99m-labeled Tilmanocept, a multivalent mannose, is readily internalized by the CD206 surface receptor on macrophages and dendritic cells which are abundantly present in lymph nodes. We want to examine the drainage patterns of Technetium-99m-labeled Tilmanocept to sentinel lymph nodes (SLNs) in melanoma patients following the 10% rule.
Methods
Multi-center retrospective review of patients with cutaneous melanoma undergoing SLN biopsy using Technetium-99m-labeled Tilmanocept between 2008 and 2014 was conducted. Statistical methods were used for data analyses.
Results
Of the 564 patients (mean age of 60.3 and 62% male) with preoperative lymphoscintigraphy showing at least one SLN, several primary tumor sites were included: 27% head/neck, 33% trunk, 21% upper extremity and 19% lower extremity. For the head/neck primary site, 36.5% of patients had multiple draining basins; for the trunk site, 36.4% of patients; for the upper extremity site, 13% of patients; and for the lower extremity, 27.4% of patients. A median of 3 (range 1-18) SLNs were identified and resected. Overall, 78% of patients had >1 SLN identified by Technetium-99m-labeled Tilmanocept. In a multivariate model, patients with >1 SLN were significantly associated with age, Breslow depth, tumor location and higher AJCC tumor stage. A total of 17.7% of patients (100/564) had a positive SLN identified. A total of 145 positive SLNs were identified out of 1,812 SLNs with a positive SLN rate of 8%. Positive SLN status was significantly associated with younger age, greater Breslow depth, mitosis rate, higher AJCC tumor stage, presence of ulceration and angiolymphatic invasion.
Conclusions
Using the 10% rule, Technetium-99m-labeled Tilmanocept detects multiple SLNs in most melanoma patients.
Keywords: tilmanocept (lymphoseek), melanoma lymphatic drainage, melanoma sentinel lymph nodes
Highlights
What do we already know about this topic?
It is well known that melanoma occurs in different parts of the body and via lymphatic spread drains to different regional nodal basins. Therefore, lymphoscintigraphy is a prerequisite to identify the appropriate nodal basins at risk for metastatic disease. Technetium sulfur colloid has been traditionally used as a radiotracer to identify melanoma SLNs. Recently, Tilmanocept has been approved by the FDA as a radiotracer used to detect SLNs in melanoma, breast cancer and head and neck cancer based on evidence obtained from multicenter clinical trials. Based on the literature, the average number of melanoma SLN in each regional nodal basin is about 3. We hypothesized that technetium-99m-labeled Tilmanocept would confirm similar drainage and uptake patterns as historical agents used.
How does your research contribute to the Cancer Control field?
Technetium-99m-labeled Tilmanocept, a multivalent mannose, has been shown to be readily internalized by the CD206 surface receptor on macrophages and dendritic cells in lymph nodes. As a radiotracer when injected dermally around the melanoma primary biopsy site, it has been shown to identify the SLN in the regional nodal basin. Taking advantage of the 2 large melanoma centers at California Pacific Medical Center in San Francisco and Moffitt Cancer Center in Tampa, we want to study the drainage patterns of primary melanoma using the agent technetium-99m-labeled Tilmanocept.
In this multi-center retrospective investigation of 564 patients with cutaneous melanoma, SLN biopsy was performed using technetium-99m-labeled Tilmanocept, The goal of this study was to identify SLNs in melanoma patients using technetium-99m-labeled Tilmanocept. Using the 10% rule, we have demonstrated that multiple SLNs exist in the majority of melanoma patients. Moreover, in this cohort of patients, we have found that >1 SLN being removed is significantly associated with age, Breslow depth, tumor location and higher AJCC tumor stage. We feel that the potential for increased false negative SLN biopsy could result from just removing the “hottest” SLN. This study has established the fact that melanoma SLNs are multiple in the majority of the cases.
What are your research’s implications towards theory, practice, or policy
Technetium-99m-labeled Tilmanocept appears to be a reliable radiotracer to identify melanoma SLN. Preoperative lymphoscintigraphy is mandatory to identify lymphatic drainage from primary melanoma. Intraoperative identification of SLNs with technetium-99m-labeled Tilmanocept was accomplished by a gamma probe. For those patients with a positive SLN biopsy, adjuvant therapy including targeted therapy or check point inhibition immunotherapy may be instituted to achieve a better survival outcome.
Introduction
In the early 1990s, Morton introduced the concept of SLN detection in an effort to accurately stage nodal basins downstream of melanoma1,2 while mitigating potential complications associated with complete lymphadenectomy.3-5 Since that time, minimally invasive SLN biopsy has become widely adopted in the management of melanoma6 and breast cancer7 being associated with accurate prognostic information.6-10
Melanoma accounts for less than one percent of all dermatological cancer diagnoses in the United States but causes the vast majority of skin cancer deaths.11,12 The single most important determinant of patient survival in early-stage melanoma is nodal status.13,14 Therefore, SLN biopsy has become an essential component of staging the nodal basin for melanoma patients.
Several methods have been used to identify the SLN in melanoma patients. Localization of SLN is achieved by injection of tracer agents into the dermis surrounding the primary skin lesion. The tracers are taken up by lymphatic vessels and concentrated in regional lymph nodes downstream, akin to the lymphatic spread of tumor cells. Vital blue dyes, such as isosulfan blue, provide a visual roadmap for the surgeon intraoperatively.2 However, it has a one percent associated risk of causing anaphylaxis15 and, when used alone, extensive soft tissue dissections are often needed to locate the relevant nodes within the nodal basin. The introduction of radiocolloid allows surgeons to perform preoperative lymphoscintigraphy to help guide surgical planning.16,17 Moreover, it enables surgeons to employ intraoperative handheld gamma probe with or without an intraoperative portable gamma camera to locate lymph nodes with precision.18-20
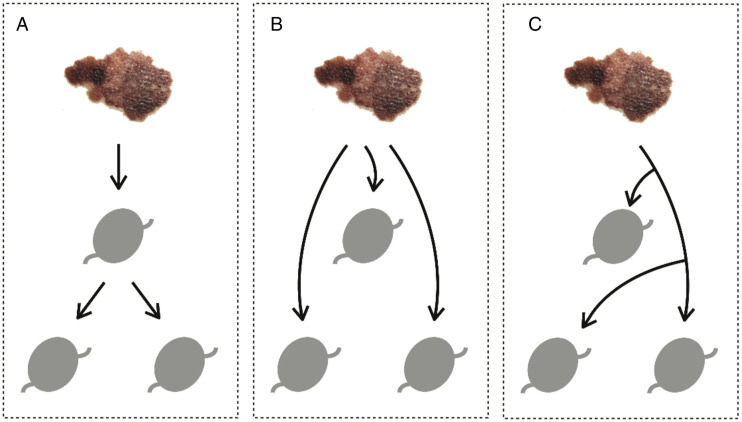
Earlier studies have shown that although radiocolloid tracers alone have an excellent rate of detecting SLNs, a combination with vital blue dye may increase the detection rate slightly.21-23 While vital blue dye and radiocolloid tracers are highly effective in locating the regional lymph nodes, when multiple SLNs are identified such tracers cannot discriminate whether the primary lesion has drained first to a single SLN and then continued to travel downstream to additional nodes, so called second-echelon nodes (single channel, multiple nodes) or whether the primary site drained to several nodes independently (parallel channels, multiple nodes). (Figure 1)
Figure 1.
Cutaneous melanoma lymphatic drainage. Graphical depiction of hypothetical lymphatic drainage patterns from cutaneous melanoma to downstream lymph nodes. (A) Primary lesion drains first to a single SLN and then to additional nodes downstream (single channel, multiple nodes). (B, C) Cutaneous melanoma drains to several nodes through independent pathways (parallel channels, multiple nodes).
99m-labeled Tilmanocept is a multivalent mannose-containing radiopharmaceutical designed for SLN detection.24 The mannose moieties serve as ligands for the CD206 receptor expressed on the surface of nodal macrophages and dendritic cells. Once injected around the primary tumor, the Technetium-99m-labeled Tilmanocept enters the lymphatics and travels to the SLN. The radioactive ligand and the mannose-CD206 receptor are internalized into the macrophages and dendritic cells resulting in SLN radioactivity. This biological process is thought to impede transit of Technetium-99m-labeled Tilmanocept to downstream second-echelon nodes.24-32 When Technetium-99m-labeled Tilmanocept is internalized to a SLN, it is assumed that Tilmanocept will remain within these cells. However, it cannot be ruled out that the radiotracer does not leak out of the cell and migrate to other adjacent lymph nodes. Isolated cases of comparing immediate and delayed (less than 24 hours) imaging suggested that Technetium-99m-labeled Tilmanocept did not go to the second echelon lymph node.32 In a group of 617 node-negative breast cancer patients undergoing SLN biopsy, 550 patients were injected with Technetium-99m-labeled Tilmanocept on day 1 and 67 patients were injected on day 2, the authors have found no significance in the number of removed SLNs between the “one-day” vs “two-day” groups of patients.33
We sought to capitalize on the biological properties of this novel tracer to delineate the patterns of melanoma lymphatic drainage more precisely to SLNs in a large number of patients from 2 large melanoma centers with the application of 10% rule34. To determine the number of SLNs in patients with cutaneous melanoma, we characterized a multicenter cohort of patients who had undergone melanoma surgery with SLN detection by Technetium-99m-labeled Tilmanocept. We believe that this is the largest number of melanoma patients undergoing SLN biopsy with Technetium-99m-labeled Tilmanocept and, therefore, the SLN identification data may represent a reliable pattern of melanoma SLN identification by Technetium-99m-labeled Tilmanocept.
It has been acknowledged in the literature that melanoma SLNs are often multiple.34 One study has reported that the average number of melanoma SLNs is 2.28 (range 1-6).35 Therefore, our hypothesis is that melanoma SLNs are multiple. Taking advantage of the property of Technetium-99m-labeled Tilmanocept as mentioned above and with a large multicenter database using Technetium-99m-labeled Tilmanocept as the radiotracer, we sought to identify the drainage of primary melanoma to the SLNs and verify the fact that melanoma SLNs are mostly multiple.
Methods
A retrospective study was conducted with a continuous cohort of patients with cutaneous melanoma who had undergone SLN biopsy with Technetium-99m-labeled Tilmanocept at the California Pacific Medical Center (San Francisco, CA) and Moffitt Cancer Center (Tampa, FL) between January 2008 and August 2014. No patients were excluded. Demographic information, tumor site, tumor characteristics and lymph node characteristics were collected. This study was approved by the institutional review boards at both participating centers that this was a retrospective review of the existing patient data in each institution with the IRB allowing -no additional oral or written consents from individual patients. The data from each institution was encrypted according to the HIPAA regulations and analyzed by a statistician in its encrypted format.
Technetium-99m-labeled Tilmanocept was used for SLN biopsy in a standardized manner. Patients received a fixed dose of 50 μg of [99mTc] Tilmanocept (∼2.7 nmol) with a varying amount of radioactivity. It was administered by intradermal injection to the area surrounding the primary cutaneous melanoma. Same day surgery patients received .6 mCi of 99 mTc, while next day surgery patients received 2.0 mCi (timing of injection was at the surgeon’s discretion). Preoperative lymphoscintigraphy was performed for each patient to identify the location of SLNs. Intraoperatively, a handheld gamma probe was used to locate the relevant SLNs. A SLN was defined as any node that exceeds the background count plus three times the standard deviation of the background (“3-sigma rule”)36 or whose radioactivity exceeds 10% of the most radioactive node identified (“10% rule”).34,37 Blue dye injection was as the discretion of the surgeon. Comparison between blue dye and Technetium-99m-labeled Tilmanocept identification of SLN was discussed in a previously published study.23 In this study, analysis was performed only in the Technetium-99m-labeled Tilmanocept-identified SLNs. Histological evaluation of SLNs has been published previously in detail.20
Continuous variables were summarized with mean, median and range, and categorical variables using frequencies and percentages. Univariate analyses examining the relationship between tumor characteristics and SLN biopsy status were performed using t-tests, Chi-square tests and Fisher’s exact tests. A multivariate logistic regression model was developed to further assess characteristics independently associated with having a single vs multiple SLNs. All tumor characteristics (except Clark level) were included initially; variables that did not reach a significance of P < .1 were removed sequentially. All statistical analyses were conducted using STATA version 13 (StataCorp, College Station, TX).
Results
A total of 564 patients with cutaneous melanoma who underwent Technetium-99m-labeled Tilmanocept-mediated SLN biopsy during the study period were identified. A patient example is illustrated in Figure 2. All the patients in this study were Caucasian. Mean cohort age was 60.3 ± 16.5 years and 62% of patients were male. Median follow-up duration was 16 (interquartile range 6-29) months. Primary tumor sites included head/neck (151, 27%), trunk (188, 33%), lower extremity (105, 19%) and upper extremity (119, 21%). Melanoma from each anatomical site demonstrated occasional drainage to multiple nodal basins as shown in Table 1. More multiple drainage basins are seen in head/neck and trunk melanomas than the upper and lower extremity. In the head/neck, additional drainage may include contralateral neck, parotid and supraclavicular basins. In the trunk, additional drainage may include in-transit, contralateral axillary, contralateral inguinal and pelvic basins. In the upper extremity, additional drainage may include in-transit, epitrochlear and supraclavicular basins. In the lower extremity, additional drainage may include in-transit, popliteal and pelvic basins. Detailed drainage from each melanoma site will not be included in this study.
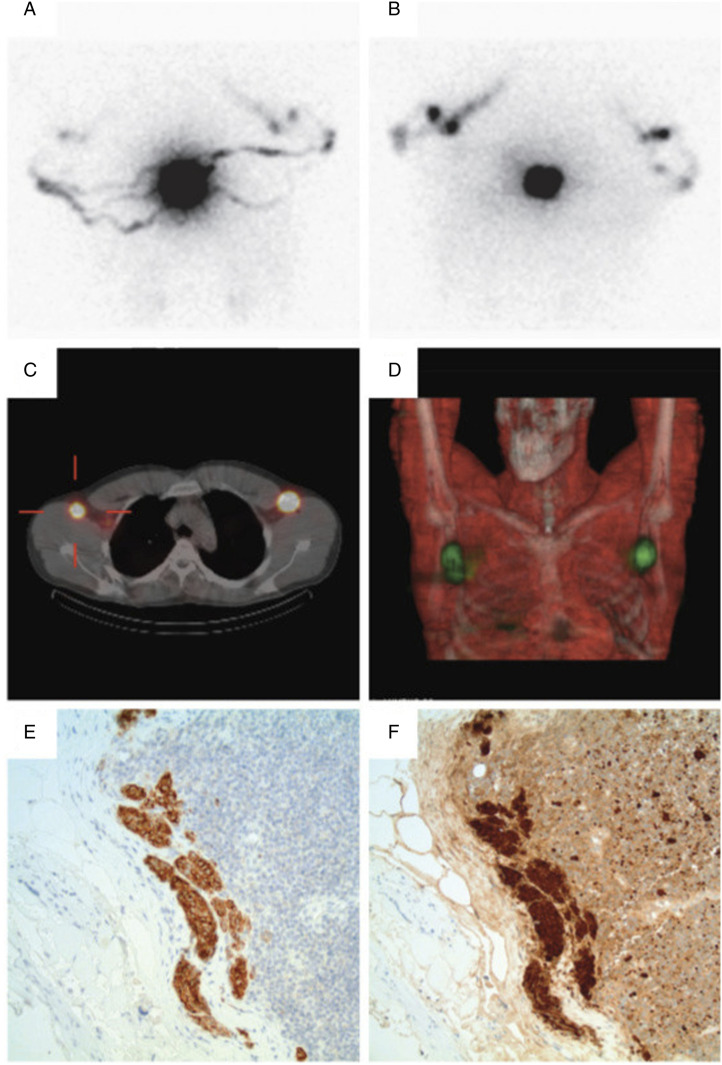
Figure 2.
Preoperative lymphoscintigraphy: Patient illustration. Patient is a 32-year-old Caucasian who presented with an irregular mole on the upper midline back at T6/T7. On 05/28/2013, biopsy revealed .6-mm thick melanoma, Clark level II, non-ulcerated with one mitosis per square millimeter. Subsequently, the patient underwent a wide local re-excision followed by SLN mapping with Technetium-99m-labeled Tilmanocept and selective SLN biopsy. Preoperatively, four intradermal injections of Technetium-99m-labeled Tilmanocept were performed surrounding the primary site on the back. Standard preoperative planar SLN lymphoscintigraphy revealed expected intense activity at the injection site with multiple visualized channels extending bilaterally to the axillae (A, posterior; B, anterior, flow images). SPECT was performed with low-dose CT for attenuation correction of the SPECT data and anatomic correlation (C, axial SPECT/CT; D, coronal volumetric SPECT/CT). Preoperative mapping demonstrated one and two foci of radiotracer accumulation in the left and right axilla, respectively. Intraoperatively, three and four SLNs were biopsied from the left and right axilla, respectively. On pathological assessment, a single left-sided axillary lymph node was positive for a subcapsular deposit of metastatic melanoma measuring at least .3-mm in diameter (E-F, representative 20x images of anti-Melan A and anti-S100 immunohistochemical stains of the subcapsular micrometastasis, respectively).
Table 1.
Multiple Nodal Basins from Melanoma of Different Anatomical Sites.a.
| Anatomical Site | Total | Number with Multiple Basins | Total with 2 Basins | Total with 3 Basins | Total with 4 Basins | Total with 5 Basins | % with Multiple Basins |
|---|---|---|---|---|---|---|---|
| Head/Neck | 158 | 58 | 47 | 11 | 0 | 0 | 36.7 |
| Trunk | 188 | 68 | 53 | 14 | 0 | 1 | 36.1 |
| Upper extremity | 92 | 12 | 11 | 1 | 0 | 0 | 13.0 |
| Lower extremity | 95 | 26 | 20 | 3 | 3 | 0 | 27.4 |
aThe anatomical sites are from the patients from California Pacific Medical Center only.
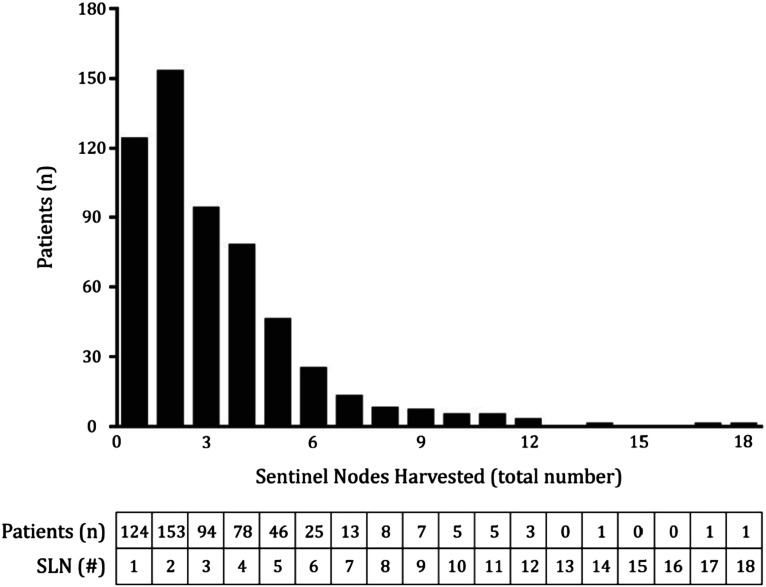
Patients were found to have a median of 3 (range 1-18) SLNs identified and excised for pathological assessment. Overall, 78% of patients (440) had more than 1 SLN identified by Technetium-99m-labeled Tilmanocept. The distribution of SLNs harvested in the patient cohort is depicted in Figure 3.
Figure 3.
Distribution of Number of Sentinel Nodes Harvested (Top) Number of SLN3 harvested (x-axis) plotted alongside frequency of patients (y-axis) in the studied population; (Bottom) Tabular format of data.
Patient and tumor characteristics were compared based on SLN status (positive vs negative) (Table 2). A total of 17.7% of patients (100/564) had a positive SLN identified. A total of 145 positive SLNs were identified out of 1812. Thus, Technetium-99m-labeled Tilmanocept-identified SLNs with a positive rate of 8.0%. More specifically, among the 100 patients in this study with positive SLNs, the 145 positive SLNs were identified out of 349 Technetium-99m-labeled Tilmanocept-identified SLNs (41.5%). Patients with positive SLNs were younger (mean age 56.7 as compared to 61.1, P = .013) and Breslow tumor depth varied significantly with SLN status (P < .0001). Increasing Breslow depth was associated with increasing SLN positivity (Table 2). In addition, mitotic rate (P = .002) as well as AJCC tumor stage (P < .0001) varied significantly between those patients with positive vs negative SLNs. Furthermore, patients with a positive SLN biopsy were significantly more likely to have ulcerated primary tumors (P < .0001) and angiolymphatic invasion (P = .0002). No significant difference in SLN status was noted based on primary tumor site (P = .54) or Clark’s level (P = .1).
Table 2.
Factors Associated with Sentinel Lymph Node Status.
| SLN Negative (464) | SLN Positive (100) | P-value | |
|---|---|---|---|
| Age, years | 61.1 +/− 16.5 | 56.7 +/− 16.1 | .013a |
| Follow-up months, median (IQR) | 14 (15-26) | 23 (10-36) | <.0001a |
| Primary tumor location (% by row) | .54b | ||
| Trunk | 150 (80) | 38 (20) | |
| Head/neck | 128 (85) | 23 (15) | |
| Lower extremity | 84 (80) | 21 (20) | |
| Upper extremity | 102 (86) | 17 (14) | |
| Unknown | 0 (0) | 1 (100) | |
| Breslow tumor depth (% by row) | <.0001b | ||
| 0-1 mm | 152 (96) | 7 (4) | |
| 1-2 mm | 174 (87) | 25 (13) | |
| 2-4 mm | 80 (67) | 39 (33) | |
| >4 mm | 44 (63) | 26 (37) | |
| Unknown | 13 (93) | 1 (7) | |
| Highest clark level (% by row) | 0.1b | ||
| II | 29 (90) | 3 (9) | |
| III | 124 (87) | 18 (13) | |
| IV | 239 (80) | 60 (20) | |
| V | 18 (67) | 9 (303) | |
| Unknown | 53 (84) | 10 (16) | |
| Ulceration (% by row) | <.0001c | ||
| Probable | 4 (100) | 0 (0) | |
| Focal | 3 (75) | 1 (25) | |
| Yes | 82 (66) | 42 (34) | |
| Mitosis rate (% by row) | .002b | ||
| 0 | 60 (95) | 3 (5) | |
| 1 | 113 (87) | 17 (13) | |
| 2+ | 238 (78) | 68 (22) | |
| Microsatellite lesion (% by row) | 7 (58) | 5 (42) | .14c |
| Angiolymphatic invasion (% by row) | 21 (50) | 21 (50) | .0002c |
| AJCC tumor stage (% by row) | <.0001b | ||
| Microscopic | 6 (75) | 2 (25) | |
| I | 254 (99) | 1 (1) | |
| II | 128 (98) | 3 (2) | |
| III | 30 (30) | 71 (70) | |
| IV | 11 (41) | 16 (59) | |
| Unknown | 35 (83) | 7 (17) |
Abbreviations: SLN (sentinel lymph node); SD (standard deviation); IQR (interquartile range); AJCC (American Joint Committee on Cancer); Statistical tests: (a) T‐test; (b) Chi‐ square test; and (c) Fisher’s exact test.
Additionally, patients with 1 SLN vs >1 SLN were compared (Table 3). Patients with 1 vs >1 SLN was significantly different by primary tumor site (P < .0001). Patients with head/neck melanomas were the least likely to have >1 SLN identified (58%) compared to greater than 80% of patients with melanomas elsewhere. Patients with >1 SLN were younger (mean age 59.2 as compared to 64.4, P = .0018), had a higher pathologic American Joint Committee on Cancer (AJCC) tumor stage (P < .0001) and a higher Clark level (P = .015) and Breslow tumor depth (P = .003). No significant differences were noted with respect to microsatellite lesion, mitosis rate, presence of ulceration, or lymphatic or vascular invasion. Subsequently, a multivariate logistic regression model was developed to assess factors that remained significantly associated with >1 SLN vs 1 SLN. In the initial model, all tumor characteristics excluding Clark level were included, and variables failing to reach a significance of P < .1 were removed. In the final model, age and tumor location remained independently associated with >1 SLN. Compared to patients with head/neck melanomas, those with lesions elsewhere were significantly more likely to have >1 SLN: trunk (OR 1.68, 95% CI 1.41-2.01; P < .0001); upper extremity (OR 5.06, 95% CI 2.70-9.50; P < .0001); and lower extremity (OR 1.71, 95% CI 1.28-2.28; P = .0003).
Table 3.
Factors Associated with Single or Multiple Sentinel Lymph Nodes.
| 1 SLN (124) | >1 SLN (440) | P-value | |
|---|---|---|---|
| Age, years | 64.4 +/− 17.7 | 59.2 +/− 16.0 | .0018a |
| Follow-up months, median (IQR) | 14 (5-25) | 16 (7-30) | .045a |
| Primary tumor location (% by row) | <.0001b | ||
| Trunk | 25 (13) | 163 (87) | |
| Head/neck | 63 (42) | 88 (58) | |
| Lower extremity | 21 (20) | 84 (80) | |
| Upper extremity | 15 (13) | 104 (87) | |
| Unknown | 0 | 1 (100) | |
| Breslow tumor depth (% by row) | .003b | ||
| 0-1 mm | 37 (23) | 122 (77) | |
| 1-2 mm | 41 (20) | 158 (80) | |
| 2-4 mm | 25 (21) | 94 (79) | |
| >4 mm | 16 (22) | 56 (78) | |
| Unknown | 5 (36) | 9 (64) | |
| Highest clark level (% by row) | .015b | ||
| II | 8 (25) | 24 (75) | |
| III | 27 (19) | 115 (81) | |
| IV | 57 (19) | 242 (81) | |
| V | 8 (30) | 19 (70) | |
| Unknown | 24 (38) | 39 (62) | |
| Ulceration (% by row) | .72c | ||
| Probable | 0 (0) | 3 (1) | |
| Focal | 1 (20) | 4 (80) | |
| Yes | 29 (23) | 95 (77) | |
| Mitosis rate (% by row) | .82b | ||
| 0 | 13 (21) | 50 (79) | |
| 1 | 24 (18) | 106 (82) | |
| 2+ | 64 (21) | 242 (79) | |
| Microsatellite lesion (% by row) | 3 (25) | 9 (75) | .74c |
| Angiolymphatic invasion (% by row) | 5 (12) | 37 (88) | .44c |
| AJCC tumor stage (% by row) | <.0001b | ||
| Microscopic | 0 (0) | 8 (1) | |
| I | 66 (26) | 189 (74) | |
| II | 22 (17) | 109 (83) | |
| III | 21 (21) | 80 (79) | |
| IV | 4 (15) | 23 (85) | |
| Unknown | 11 (26) | 31 (74) | |
| Positive SLN identified (% by column) | 17 (13) | 83 (19) | 0.1c |
Abbreviations: SLN: sentinel lymph node; SD: standard deviation; IQR: interquartile range; AJCC: American Joint Committee on Cancer; Statistical tests: (a) T‐test; (b) Chi‐square test; and (c) Fisher’s exact test.
Discussion
In cutaneous melanoma, SLN biopsy has proven highly accurate in the identification of patients who may benefit from early lymphadenectomy and/or adjuvant therapy. Patients with a negative SLN biopsy will be spared a lymph node dissection, thus, avoiding potential major lifelong complications such as lymphedema.
As described in the Introduction, [99 mTc] Tilmanocept is a unique multivalent mannose-containing radiopharmaceutical designed for SLN detection with its mannose ligands bound to the CD206 receptor on the surface of nodal macrophages and dendritic cells to be internalized, which is thought to prevent the transit of Technetium-99m-labeled Tilmanocept to second-echelon nodes.24-32 As noted earlier in the Introduction, Unkart et al have found no significance in the number of removed SLNs between the “one-day” vs “two-day” groups of patients in a cohort of 617 node-negative breast cancer patients undergoing SLN biopsy.33
Prior to the use of Technetium-99m-labeled Tilmanocept filtered Technetium-99m sulfur colloid has been extensively used to identify sentinel lymph nodes. It is difficult to compare Technetium-99m-labeled Tilmanocept and sulfur colloid simultaneously as when these two radiotracers are injected at the same time, it is not possible to determine which sentinel lymph node contains Technetium-99m-labeled Tilmanocept vs sulfur colloid. However, comparison has been made between groups with Technetium-99m sulfur colloid vs Technetium-99m-labeled Tilmanocept. Previous reports from the University of California San Deigo showed that in breast cancer SLN identification Technetium-99m-labeled Tilmanocept demonstrated more rapid injection site clearance times, lower mean number of SLNs found and an increased concordance of SLNs than Technetium-99m sulfur colloid.38 The authors from the University of California San Diego have found that Technetium-99m sulfur colloid injection resulted in more pain during the first 3 minute postinjection period as compared to Technetium-99m-labeled Tilmanocept injection. However, a subsequent study from the Mayo Clinic showed no significant difference between Technetium-99m-labeled Tilmanocept and Technetium-99m sulfur colloid regarding the above-mentioned characteristics in breast cancer SLN identification.39
In another preliminary report for melanoma SLN identification in 62 patients, the authors have found no significant difference between Technetium-99m-labeled Tilmanocept and Technetium-99m sulfur colloid.40 In a separate study, melanoma SLN biopsy for Technetium-99m-labeled Tilmanocept was compared to Technetium-99m sulfur colloid by retrospective review in 370 consecutive patients with 185 patients in each group. Technetium-99m-labeled Tilmanocept has been found to require lower radiation dosages and shorter mapping times. Also, the number of SLNs removed was less with the Technetium-99m-labeled Tilmanocept, but the number of patients with positive SLNs showed no difference. With less number of lymph nodes removed and yet the sensitivity was the same, the authors suggest that unnecessary removal of nodes may lessen the complications such as lymphedema.41
One potential problem with Technetium-99m sulfur colloid is the difference of filtered and unfiltered radioactive sulfur colloid where different particle sizes whereas Technetium-99m-labeled Tilmanocept is a homogeneous molecule of 7 nm.32 In a pig model, variability in the lymphatic mapping of sentinel lymph nodes has been demonstrated between filtered and unfiltered Technetium-99m sulfur colloid.42
In a pilot study, a comparison between [68 Ga]Ga-tilmanocept PET/CT lymphoscintigraphy and [99m Tc]Tc-tilmanocept lymphoscintigraphy for sentinel lymph node identification in oral squamous cell cancer showed that [68Ga]Ga-tilmanocept PET/CT lymphoscintigraphy yielded more accurate identification of SLNs with improved visualization of lymphatic vessels as these characteristics were compared to those being generated by [99mTc]Tc-tilmanocept lymphoscintigraphy. When simultaneous peritumoral injection of [99mTc]Tc-tilmanocept, SLNs detected by [68Ga]Ga-tilmanocept PET/CT lymphoscintigraphy can be reliably identified during surgery using conventional gamma-probe.43 It should be noted that [68 Ga]Ga-tilmanocept has been rarely used in the literature.
In another pilot study, [99 mTc]Tc-tilmanocept was compared to [99mTc]Tc-nanocolloid in oral squamous cancer. The authors could not reach a conclusion for the utility of [99mTc]Tc-tilmanocept for SLN biopsy in early stage oral squamous cell carcinoma.44 It should be noted that nanocolloid has been rarely used in the United States.
In a recent study by Ooms et al, [99mTc]Tc-tilmanocept (TcTM) was compared with [99mTc]Tc-sulphur colloid (TcSC) and [99mTc]Tc-albumin colloid (TcAC) for detection of head and neck cancer sentinel lymph nodes. Although the study population was relatively small of 62 patients, the authors concluded that TcTM showed comparable overall performance to TcSC and TcAC.45
Based on the safety and ability of Technetium-99m-labeled Tilmanocept to identify SLNs reliably, it has been approved by the FDA as a radioitracer for preoperative lymphoscintigraphy for detect SLNs in melanoma, breast cancer and head and neck squamous cell carcinoma. Further, the FDA has also granted approval of Technetium-99m-labeled Tilmanocept for draining lymph node mapping in pediatric patients.32
It is known in the literature that SLN biopsy in melanoma often yields multiple SLNs.34 In this multi-center retrospective cohort study, we have also shown that melanoma sentinel lymph nodes are multiple in the majority of cases. We employed Technetium-99m-labeled Tilmanocept to identify SLNs in patients with cutaneous melanoma. Overall, 78.0% of 564 patients had more than one SLN identified. Therefore, based on the unique biological attributes of Technetium-99m-labeled Tilmanocept, we surmise that cutaneous melanoma may have multiple primary lymphatic channels draining to multiple SLNs. Moreover, characterization of our patient cohort revealed that patients with more than one SLN varied significantly from those with a single SLN in numerous ways, including younger age, higher AJCC tumor stage, greater Breslow depth and greater Clark level. In addition, single vs more than one SLN varied significantly with primary tumor site. For example, 87% of trunk melanomas had greater than one SLN, whereas this was true of only 58% head/neck melanomas (P = <.0001).
It should be stated that we have found no correlation between sentinel lymph node positivity and the number of sentinel lymph nodes 1 vs greater than 1. SLNs greater than 1 may be a reflection of multiple lymphatic channels whereas 1 SLN most likely indicates a single channel. Although there is no correlation between SLN positivity and the number of SLNs, SLN greater than 1 is significantly correlated with primary sites, younger age, higher pathologic AJCC tumor stage, higher Clark level and deeper Breslow depth. Perhaps, multiple lymphatic channels being associated with primary sites may indicate that the lymphatic channels may differ in different primary anatomical sites. In a previous study, we have found that multiple lymphatic channels are associated with a poorer prognosis.46
Younger age group is known to have more robust lymphatic system and, thus, more SLNs and lymphatic channels may be found.47 The fact that greater than 1 SLN group being associated with multiple channels may potentially be associated with more aggressive melanoma as shown by higher pathologic AJCC tumor stage, higher Clark level and deeper Breslow depth. Recent study has shown that Stage IIB and IIC and more so with Stage IIC may have a shorter survival than Stage IIIA,48 with the Keynote 716 adjuvant study showing benefit of treating melanoma patients with Stage IIB and IIC.49 In fact, Stage IIIA patients were not even included in the study. Thus, SLN positivity group is a heterogeneous group, some may have an excellent prognosis especially when the tumor burden in the SLN is low.50 In this study, we have not examined the tumor burden in the positive SLNs.
In both univariate and multivariate analyses, younger patients were significantly more likely to have more than one SLN identified. Various studies have demonstrated that the natural history of melanoma and survival outcomes are different for younger patients.51-53 In fact, the characteristics of primary melanomas from younger patients under the age of 20 compared to individuals in the subsequent few decades of life are more advanced (increased tumor thickness and incidence of ulceration). Despite an increase of highrisk features as well as SLN involvement in younger patients, the mortality rate, compared to older age groups, is diminished.54,55 This discrepancy may represent an area worthy of future investigation. While it may reflect genetic or age-related differences in tumor behavior, ie, invasiveness, migration, it may also represent differences in age-related melanoma-host interaction such as immune system, lymphatic flow competency and existence of comorbid illnesses.47 Understanding better this age-related discrepancy in tumor behavior and outcomes may lend insight into future investigation and therapy.
In this patient cohort, the median number of identified nodes was 3, using the 10% rule as stated above, although a few patients had many more nodes removed at the time of surgery (range 1-18). Some may argue that the removal of multiple lymph nodes has drawbacks, not only in the length of surgery but also in the potential for increased morbidity such as lymphedema. We have previously found that 20% of the positive SLNs do not have the highest radioactivity.56 Kroon et al37 has further shown the value of 10% rule34 and they have validated the significance of the 10% rule that these additional lymph nodes should be removed in addition to the hottest lymph node to avoid a false negative rate up to 11%.46 If fewer lymph nodes were removed to reduce potential morbidity, positive lymph nodes may have been missed.37 Furthermore, a missed SLN with occult micrometastasis may negatively impact patient’s prognosis and survival.6 Thus, it is critical to identify melanoma SLNs as accurately as possible to reduce the false negative rate. With recent positive clinical trials either for targeted therapy or immunotherapy, nodal staging is critical for patients to get adjuvant therapy if needed.57 For these various reasons, we have adopted the use of intraoperative gamma camera in addition to gamma probe to aid in the intraoperative identification of SLNs.20,58
We sought to identify factors associated with SLN status in this patient cohort (Table 2). Not surprisingly, positive SLN status was associated with widely accepted aggressive tumor characteristics, including greater Breslow depth, mitosis rate, presence of ulceration, as well as lymphatic and vascular invasion. In addition, SLN positivity was associated with higher AJCC tumor stage.
Conclusion
Technetium-99m-labeled Tilmanocept identifies multiple primary SLNs in the majority of melanoma SLN basins. Using the 10% rule,34,37 we have resected 1812 SLNs being identified by Technetium-99m-labeled Tilmanocept. Of these SLNs, 145 were positive, thus, the overall positive SLN rate was 8%. Since these 145 positive SLNs were derived from 100 patients, the patient positivity rate was 17.7% (100/564) from a total number of 564 patients in this study.
This study supports the findings in the literature that melanoma SLNs being identified by a radiotracer are usually multiple. Multiple SLNs are found to be associated with younger age and more aggressive melanoma as shown by higher pathologic AJCC tumor stage, higher Clark level and deeper Breslow depth.
Appendix.
Abbreviations
- SLN
Sentinel Lymph Node
- CD206
Cluster Designation 206
- AJCC
American Joint Committee on Cancer
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MKS-Consulting to Merck, Cepheid, Myriad Genetics, Melanoma Diagnostics. SPL-Consulting to Cardinal Health, Castle Biosciences. JSZ-Consulting to Castle Biosciences, Philogen, Merck, Delcath Systems. Grant/Research Support–Castle Biosciences, NeraCare, Philogen, Provectus.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was partially supported by the Cardinal Health.
Ethical Approval: This study was approved on January 26, 2016 by the institutional review board of California Pacific Medical Center (approval number approval number is 2016.011EXP; San Francisco, CA), and on February 09, 2016 by Moffitt Cancer Center (University of South Florida IRB approval number Pro00025281 Tampa, FL).
Statement of Human Rights: All procedures in this study were conducted in accordance with the institutional review boards of California Pacific Medical Center (San Francisco, CA) and Moffitt Cancer Center (Tampa, FL) approved protocols.
Informed Consent: Informed consent for patient information to be published in this article was not obtained because this was a retrospective review on the melanoma database from California Pacific Medical Center and Moffitt Cancer Center. The Internal Review Boards of both medical centers have approved this study without consenting the patients. The data being collected has been encrypted according to HIPAA.
Data Availability: Due to the nature of this research as mentioned above, participants of this study did not agree for their data to be shared publicly, so supporting data is not available.
ORCID iD
Stanley P. Leong https://orcid.org/0000-0002-0047-0112
References
- 1.Wong JH, Cagle LA, Morton DL. Lymphatic drainage of skin to a sentinel lymph node in a feline model. Ann Surg. 1991;214(5):637-641. doi: 10.1097/00000658-199111000-00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morton DL, Wen DR, Wong JH, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127(4):392-399. doi: 10.1001/archsurg.1992.01420040034005 [DOI] [PubMed] [Google Scholar]
- 3.Chang SB, Askew RL, Xing Y, et al. Prospective assessment of postoperative complications and associated costs following inguinal lymph node dissection (ILND) in melanoma patients. Ann Surg Oncol. 2010;17(10):2764-2772. doi: 10.1245/s10434-010-1026-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silberman AW, McVay C, Cohen JS, et al. Comparative morbidity of axillary lymph node dissection and the sentinel lymph node technique: Implications for patients with breast cancer. Ann Surg. 2004;240(1):1-6. doi: 10.1097/01.sla.0000129358.80798.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Starritt EC, Joseph D, McKinnon JG, Lo SK, de Wilt JH, Thompson JF. Lymphedema after complete axillary node dissection for melanoma: Assessment using a new, objective definition. Ann Surg. 2004;240(5):866-874. doi: 10.1097/01.sla.0000143271.32568.2b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faries MB, Thompson JF, Cochran AJ, et al. Completion dissection or observation for sentinel-node metastasis in melanoma. N Engl J Med. 2017;376(23):2211-2222. doi: 10.1056/NEJMoa1613210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giuliano AE. The evolution of sentinel node biopsy for breast cancer: Personal experience. Breast J. 2020;26(1):17-21. doi: 10.1111/tbj.13729 [DOI] [PubMed] [Google Scholar]
- 8.Scoggins CR, Chagpar AB, Martin RC, McMasters KM. Should sentinel lymph-node biopsy be used routinely for staging melanoma and breast cancers? Nat Clin Pract Oncol. 2005;2(9):448-455. doi: 10.1038/ncponc0293 [DOI] [PubMed] [Google Scholar]
- 9.Morton DL, Thompson JF, Cochran AJ, et al. Sentinel-node biopsy or nodal observation in melanoma. N Engl J Med 2006;355(13):1307-1317. doi: 10.1056/NEJMoa060992 [DOI] [PubMed] [Google Scholar]
- 10.Han D, Thomas DC, Zager JS, Pockaj B, White RL, Leong SP. Clinical utilities and biological characteristics of melanoma sentinel lymph nodes. World J Clin Oncol. 2016;7(2):174-188. doi: 10.5306/wjco.v7.i2.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howlader N, Noone A, Krapcho M, et al. SEER cancer statistics review, 1975–2013. Bethesda, MD: National Cancer Institute; Surveillance, Epidemiology, and End Results Program; 2016;19. [Google Scholar]
- 12.Society AC. Cancer Facts and Figures. Atlanta, GA: American Cancer Society; 2016. [Google Scholar]
- 13.Gershenwald JE, Thompson W, Mansfield PF, et al. Multi-institutional melanoma lymphatic mapping experience: The prognostic value of sentinel lymph node status in 612 stage I or II melanoma patients. J Clin Oncol. 1999;17(3):976-983. doi: 10.1200/JCO.1999.17.3.976 [DOI] [PubMed] [Google Scholar]
- 14.Balch CM, Soong SJ, Gershenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: Validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19(16):3622-3634. doi: 10.1200/jco.2001.19.16.3622 [DOI] [PubMed] [Google Scholar]
- 15.Leong SP, Donegan E, Heffernon W, Dean S, Katz JA. Adverse reactions to isosulfan blue during selective sentinel lymph node dissection in melanoma. Ann Surg Oncol. 2000;7(5):361-366. doi: 10.1007/s10434-000-0361-x [DOI] [PubMed] [Google Scholar]
- 16.Fee HJ, Robinson DS, Sample WF, Graham LS, Holmes EC, Morton DL. The determination of lymph shed by colloidal gold scanning in patients with malignant melanoma: A preliminary study. Surgery. 1978;84(5):626-632. [PubMed] [Google Scholar]
- 17.Balch CM. Detection of melanoma metastases with the sentinel node biopsy: The legacy of Donald L. Morton, MD 1934-2014. Clin Exp Metastasis. 2018;35(5-6):425-429. doi: 10.1007/s10585-018-9908-8 [DOI] [PubMed] [Google Scholar]
- 18.Alex JC, Krag DN. Gamma-probe guided localization of lymph nodes. Surg Oncol. 1993;2(3):137-143. doi: 10.1016/0960-7404(93)90001-f [DOI] [PubMed] [Google Scholar]
- 19.Alex JC, Weaver DL, Fairbank JT, Rankin BS, Krag DN. Gamma-probe-guided lymph node localization in malignant melanoma. Surg Oncol. 1993;2(5):303-308. doi: 10.1016/s0960-7404(06)80006-x [DOI] [PubMed] [Google Scholar]
- 20.Leong SP, Wu M, Lu Y, et al. Intraoperative imaging with a portable gamma camera may reduce the false-negative rate for melanoma sentinel lymph node surgery. Ann Surg Oncol. 2018;25(11):3326-3333. doi: 10.1245/s10434-018-6685-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leong S. The role of sentinel lymph node in human solid cancer. In: Devita V, Hellman S, Rosenberg S, eds. PPO Updates. Lippincott-Raven; 1998. [Google Scholar]
- 22.Hu Y, Melmer PD, Slingluff CL, Jr. Localization of the sentinel lymph node in melanoma without blue dye. Ann Surg. 2016;263(3):588-592. doi: 10.1097/SLA.0000000000001187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sondak VK, King DW, Zager JS, et al. Combined analysis of phase III trials evaluating [(9)(9)mTc]tilmanocept and vital blue dye for identification of sentinel lymph nodes in clinically node-negative cutaneous melanoma. Ann Surg Oncol. 2013;20(2):680-688. doi: 10.1245/s10434-012-2612-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vera DR, Wallace AM, Hoh CK, Mattrey RF. A synthetic macromolecule for sentinel node detection: (99m)Tc-DTPA-mannosyl-dextran. J Nucl Med. 2001;42(6):951-959. [PubMed] [Google Scholar]
- 25.Ezekowitz RA, Sastry K, Bailly P, Warner A. Molecular characterization of the human macrophage mannose receptor: Demonstration of multiple carbohydrate recognition-like domains and phagocytosis of yeasts in Cos-1 cells. J Exp Med. 1990;172(6):1785-1794. doi: 10.1084/jem.172.6.1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vera DR, Wisner ER, Stadalnik RC. Sentinel node imaging via a nonparticulate receptor-binding radiotracer. J Nucl Med. 1997;38(4):530-535. [PubMed] [Google Scholar]
- 27.Wallace AM, Hoh CK, Vera DR, Darrah DD, Lymphoseek SG. A molecular radiopharmaceutical for sentinel node detection. Ann Surg Oncol. 2003;10(5):531-538. doi: 10.1245/aso.2003.07.012 [DOI] [PubMed] [Google Scholar]
- 28.Wallace AM, Hoh CK, Darrah DD, Schulteis G, Vera DR. Sentinel lymph node mapping of breast cancer via intradermal administration of Lymphoseek. Nucl Med Biol. 2007;34(7):849-853. doi: 10.1016/j.nucmedbio.2007.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wallace AM, Hoh CK, Ellner SJ, Darrah DD, Schulteis G, Vera DR. Lymphoseek: A molecular imaging agent for melanoma sentinel lymph node mapping. Ann Surg Oncol. 2007;14(2):913-921. doi: 10.1245/s10434-006-9099-4 [DOI] [PubMed] [Google Scholar]
- 30.Leong SP, Kim J, Ross M, et al. A phase 2 study of (99m)Tc-tilmanocept in the detection of sentinel lymph nodes in melanoma and breast cancer. Ann Surg Oncol. 2011;18(4):961-969. doi: 10.1245/s10434-010-1524-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lorenzoni A, Santinami M, Maccauro M. Clinical applications of receptor-binding radiopharmaceutical 99mTc-Tilmanocept: Sentinel node biopsy and beyond. Clin Transl Imaging. 2020;8(6):413-418. doi: 10.1007/s40336-020-00399-5 [DOI] [Google Scholar]
- 32.Leong SP. Detection of melanoma, breast cancer and head and neck squamous cell cancer sentinel lymph nodes by Tc-99m Tilmanocept (Lymphoseek(R)). Clin Exp Metastasis. 2021;39:39-50. doi: 10.1007/s10585-021-10137-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Unkart JT, Proudfoot J, Wallace AM. Outcomes of “one-day” vs “two-day” injection protocols using Tc-99m tilmanocept for sentinel lymph node biopsy in breast cancer. Breast J. 2018;24(4):526-530. doi: 10.1111/tbj.13002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McMasters KM, Reintgen DS, Ross MI, et al. Sentinel lymph node biopsy for melanoma: How many radioactive nodes should be removed? Ann Surg Oncol. 2001;8(3):192-197. doi: 10.1007/s10434-001-0192-4 [DOI] [PubMed] [Google Scholar]
- 35.Sánchez JL, Medina MS, Duque OG, Pérez MF, Hernández GC, Palácios JF. Sentinel lymph node biopsy for cutaneous melanoma: A 6 years study. Indian J Plast Surg. 2013;46(01):092-097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wallace AM, Han LK, Povoski SP, et al. Comparative evaluation of [(99m)tc]tilmanocept for sentinel lymph node mapping in breast cancer patients: Results of two phase 3 trials. Ann Surg Oncol. 2013;20(8):2590-2599. doi: 10.1245/s10434-013-2887-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kroon HM, Lowe L, Wong S, et al. What is a sentinel node? Re-evaluating the 10% rule for sentinel lymph node biopsy in melanoma. J Surg Oncol. 2007;95(8):623-628. doi: 10.1002/jso.20729 [DOI] [PubMed] [Google Scholar]
- 38.Baker JL, Pu M, Tokin CA, et al. Comparison of [99mTc] tilmanocept and filtered [99mTc] sulfur colloid for identification of SLNs in breast cancer patients. Ann Surg Oncol. 2015;22(1):40-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murphy BL, Woodwick AR, Murphy KM, et al. 99mTc-Tilmanocept versus 99mTc-sulfur colloid in lymphoscintigraphy: Sentinel lymph node identification and patient-reported pain. J Nucl Med Technol. 2019;47(4):300-304. [DOI] [PubMed] [Google Scholar]
- 40.Pollard J, Zaidi B, Graham M. Comparative analysis of 99mTc-Tilmanocept (Lymphoseek) vs. 99mTc-sulfur colloid sentinel node lymphoscintigraphy and biopsy. Soc Nuclear Med. 2016;57:411. [Google Scholar]
- 41.Silvestri C, Christopher A, Intenzo C, et al. Consecutive case series of melanoma sentinel node biopsy for Lymphoseek compared to sulfur colloids. J Surg Res. 2019;233:149-153. doi: 10.1016/j.jss.2018.07.042. [DOI] [PubMed] [Google Scholar]
- 42.Tafra L, Chua AN, Ng PC, Aycock D, Swanson M, Lannin D. Filtered versus unfiltered technetium sulfur colloid in lymphatic mapping: A significant variable in a pig model. Ann Surg Oncol. 1999;6(1):83-87. doi: 10.1007/s10434-999-0083-7 [DOI] [PubMed] [Google Scholar]
- 43.Mahieu R, Donders DNV, Krijger GC, et al. Within-patient comparison between [(68)Ga]Ga-tilmanocept PET/CT lymphoscintigraphy and [(99m)Tc]Tc-tilmanocept lymphoscintigraphy for sentinel lymph node detection in oral cancer: A pilot study. Eur J Nucl Med Mol Imaging. 2022;49(6):2023-2036. doi: 10.1007/s00259-021-05645-0 [DOI] [PubMed] [Google Scholar]
- 44.Mahieu R, den Toom IJ, van Rooij R, Es RJJ, Hobbelink MGG, Krijger GC, et al. Diagnostic accuracy of [(99m) Tc]Tc-tilmanocept compared to [(99m) Tc]Tc-nanocolloid for sentinel lymph node identification in early-stage oral cancer. Clin Otolaryngol. 2021;46(6):1383-1388. doi: 10.1111/coa.13798 [DOI] [PubMed] [Google Scholar]
- 45.Ooms M, von Mallek D, Kaiser HJ, Holzle F, Mottaghy FM, Modabber A. Comparison of [(99m)Tc]Tc-tilmanocept with [(99m)Tc]Tc-sulphur colloids and [(99m)Tc]Tc-albumin colloids for sentinel lymph node detection in patients with cutaneous malignancies of the head. Eur J Nucl Med Mol Imaging. 2022. In press. doi: 10.1007/s00259-022-06017-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wall JK, Florero M, Accortt NA, et al. Impact of multiple lymphatic channel drainage to a single nodal basin on outcomes in melanoma. Arch Surg. 2007;142(8):753-758. [DOI] [PubMed] [Google Scholar]
- 47.Cakala-Jakimowicz M, Kolodziej-Wojnar P, Puzianowska-Kuznicka M. Aging-Related Cellular, Structural and Functional Changes in the Lymph Nodes: A Significant Component of Immunosenescence? An Overview. Cells. 2021;10(11):3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bajaj S, Donnelly D, Call M, et al. Melanoma prognosis: Accuracy of the American Joint Committee on cancer staging manual eighth edition. JNCI: J Natl Cancer Inst. 2020;112(9):921-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luke JJ, Rutkowski P, Queirolo P, et al. Pembrolizumab versus placebo as adjuvant therapy in completely resected stage IIB or IIC melanoma (KEYNOTE-716): A randomised, double-blind, phase 3 trial. Lancet. 2022;399(10336):1718-1729. doi: 10.1016/S0140-6736(22)00562-1 [DOI] [PubMed] [Google Scholar]
- 50.van Akkooi AC, de Wilt JH, Verhoef C, et al. Clinical relevance of melanoma micrometastases (<0.1 mm) in sentinel nodes: Are these nodes to be considered negative? Ann Oncol. 2006;17(10):1578-1585. doi: 10.1093/annonc/mdl176 [DOI] [PubMed] [Google Scholar]
- 51.Chagpar RB, Ross MI, Reintgen DS, et al. Factors associated with improved survival among young adult melanoma patients despite a greater incidence of sentinel lymph node metastasis. J Surg Res. 2007;143(1):164-168. doi: 10.1016/j.jss.2007.04.004 [DOI] [PubMed] [Google Scholar]
- 52.Moore-Olufemi S, Herzog C, Warneke C, et al. Outcomes in pediatric melanoma: Comparing prepubertal to adolescent pediatric patients. Ann Surg. 2011;253(6):1211-1215. doi: 10.1097/SLA.0b013e318217e852 [DOI] [PubMed] [Google Scholar]
- 53.Sassen S, Shaw HM, Colman MH, Scolyer RA, Thompson JF. The complex relationships between sentinel node positivity, patient age, and primary tumor desmoplasia: Analysis of 2303 melanoma patients treated at a single center. Ann Surg Oncol. 2008;15(2):630-637. doi: 10.1245/s10434-007-9684-1 [DOI] [PubMed] [Google Scholar]
- 54.Balch CM, Soong SJ, Gershenwald JE, et al. Age as a prognostic factor in patients with localized melanoma and regional metastases. Ann Surg Oncol. 2013;20(12):3961-3968. doi: 10.1245/s10434-013-3100-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Balch CM, Thompson JF, Gershenwald JE, et al. Age as a predictor of sentinel node metastasis among patients with localized melanoma: An inverse correlation of melanoma mortality and incidence of sentinel node metastasis among young and old patients. Ann Surg Oncol. 2014;21(4):1075-1081. doi: 10.1245/s10434-013-3464-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu LC, Parrett BM, Jenkins T, et al. Selective sentinel lymph node dissection for melanoma: Importance of harvesting nodes with lower radioactive counts without the need for blue dye. Ann Surg Oncol. 2011;18(10):2919-2924. doi: 10.1245/s10434-011-1689-0 [DOI] [PubMed] [Google Scholar]
- 57.Han D, van Akkooi ACJ, Straker RJ, 3rd, et al. Current management of melanoma patients with nodal metastases. Clin Exp Metastasis. 2021;39:181-199. doi: 10.1007/s10585-021-10099-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leong SP. The intraoperative portable gamma camera is an important adjunct to the gamma probe in identifying melanoma sentinel lymph nodes. Ann Surg Oncol. 2018;25(suppl 3):902-903. doi: 10.1245/s10434-018-6755-4 [DOI] [PubMed] [Google Scholar]