Abstract
Background:
Ankle hemiarthroplasty is a 1-piece implant system replacing the talar side of the tibiotalar joint. Hemiarthroplasty offers limited bone resection and may provide easier revision options than joint-ablating procedures.
Methods:
Prospective, multicenter, noncomparative, nonrandomized clinical study with short term follow-up on patients undergoing hemiarthroplasty of the ankle. Radiologic and functional outcomes (Foot and Ankle Outcome Score FAOS, Foot and Ankle Ability Measure [FAAM], Short Form–36 Health Survey [SF-36], Short Musculoskeletal Functional Assessment [SMFA], and visual analog scale [VAS] pain scores) were obtained at 3 and 12 months and the last follow-up (mean 31.9 months).
Results:
Ten patients met the inclusion criteria. Three were converted to total ankle replacement at 14, 16, and 18 months. Pain VAS scores improved on average from 6.8 to 4.8 (P = .044) of the remaining 7 at a mean of 31.9 months’ follow-up. For these 7 in the Survival Group, we found that SF-36 physical health component improved from 25.03 to 42.25 (P = .030), SMFA dysfunction and bother indexes improved from 46.36 to 32.28 (P = .001), and from 55.21 to 30.14 (P = .002) in the Survival Group, and FAAM sports improved from 12.5 to 34.5 (P = .023).
Conclusion:
Patients undergoing hemiarthroplasty of the ankle joint for talar-sided lesions had a 30% failure rate by 18 months. Those who did not have an early failure exhibited modest pain reduction, functional improvements, and better quality of life in short-term follow-up. This procedure offers a possible alternative for isolated talar ankle cartilage cases.
Level of Evidence:
Level IV, prospective case series.
Keywords: hemiarthroplasty, ankle arthritis, ankle spacer, osteochondral lesion
Introduction
Ankle arthritis can be a significant source of pain and disability, showing similar morbidity as arthritis in other joints, such as the hip and the knee.9 Nevertheless, the scenario is different as ankle arthritic patients are younger and have higher expectations and demands.22 This condition could be partially explained because the compromise of the ankle joint often results from posttraumatic chondrolysis and is not a degenerative condition as it is in the other joint counterparts. To complicate things more, after failing nonoperative treatment, patients have limited surgical options with no demonstrated superiority of one treatment modality over another.22
Treating young adults with ankle arthritis is possibly one of the most challenging controversies in modern orthopaedics. In this scope, physicians must select an appropriate procedure from a broad spectrum, comprising joint-sparing and ablative procedures, usually with limited information about the best treatment alternative.
After joint-sparing procedures fail to relieve symptoms, patients are usually limited to an ankle fusion or total ankle arthroplasty, in particular when extensive osteochondral lesions are associated with cystic degeneration.2,5,7 However, it is not infrequent to consider that these ablative procedures appear too aggressive to treat the underlying condition. As neither fusion nor replacement is not without complications, an interim option that does not burn any bridges may be desirable in this situation.
Ankle hemiarthroplasty replaces the talar side of the ankle joint using a metallic component with limited bone resection. The procedure involves debriding the talar cartilage and is technically less demanding than total ankle arthroplasty.
Our objective is to describe the short-term experience, PROMs (patient-reported outcome measures), and complications of performing ankle hemiarthroplasty. We hypothesize that the Ankle Spacer (Arthrex, Naples, FL) is an effective alternative for treating moderate ankle arthritis or more significant cartilage defects. We believe that failure of the procedure should not compromise revision surgery, either for fusion or replacement.
Material and Methods
A prospective, multicenter, nonrandomized investigation that evaluates clinical and radiologic results of the Ankle Spacer was performed between January 2018 and August 2020. Each patient gave informed consent, and each institutional review board approved the study. All patients failed conservative treatment with nonsteroidal anti-inflammatory drugs, modification of activities, attempted weight loss, smoking cessation, and physiotherapy.
The Ankle Spacer is a 1-piece implant system anatomically designed to replace the native talus surface to match the distal articular tibial surface.
The implant is a cobalt-chromium alloy, polished to a mirror effect on the articulating upper surface. It has a rough TPS (titanium plasma spray)–coated undersurface to enable secondary fixation by bony ingrowth. It is associated with 2 posts and spikes for implant fixation at the posterior part of the prosthesis.
Patient-reported outcomes measures (PROMs) were obtained on a preoperative basis (Foot and Ankle Ability Measure [FAAM], Short Musculoskeletal Functional Assessment [SMFA], Foot and Ankle Outcome Score [FAOS], Short Form–36 Health Survey [SF-36], and visual analog scale [VAS] pain scores) and at 3 months, 12 months, and at the last follow-up with a mean of 31.9 months (SD = 7.65) (range, 23-42).
Magnetic resonance imaging scans and anteroposterior and lateral weightbearing radiographs of the ankle joint were obtained to determine the degree of joint compromise and rule out articular malalignment. Postoperatively, loosening, subsidence, implant overhanging, cystic changes, progression to osteoarthritis, and congruence were assessed by obtaining weightbearing radiographs at 6, 12, and 24 weeks, and annually after that. A computed tomographic scan of the ankle joint was obtained in case of persistent pain.
Patients were offered to participate in this study if they were older than 18 years, presented unilateral disease, had talar osteochondritis deffect larger than 200 cm2 on magnetic resonance imaging scan, or had avascular necrosis less than 30% of the talar dome. Patients were excluded from the study if they had moderate/severe neuropathy, tibial arthritis on their preoperative study higher than grade 2 Kellgren-Lawrence score, severe ankle malalignment (more than 5 degrees varus/valgus), insufficient bone quantity or quality, previous infection, peripheral vascular disease, or body mass index higher than 30.
The implant’s durability (revision date), operation time, adverse events, length of hospital stay, and intra- or postoperative complications were noted. All surgeries were performed by the same surgical team with extensive experience in total ankle arthroplasty.
General data were analyzed for the entire cohort. Patients were divided into 2 groups for specific statistical analyses according to implant survival. Patients who remained with their Ankle Spacer implanted were allocated to the Survival Group (SG), and those whose device was explanted were allocated to the Revision Group (RG).
Surgical Technique
The patient was supine with a popliteal nerve block, sedation, and limb tourniquet applied.
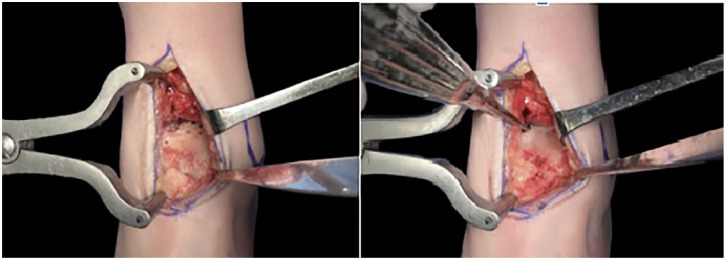
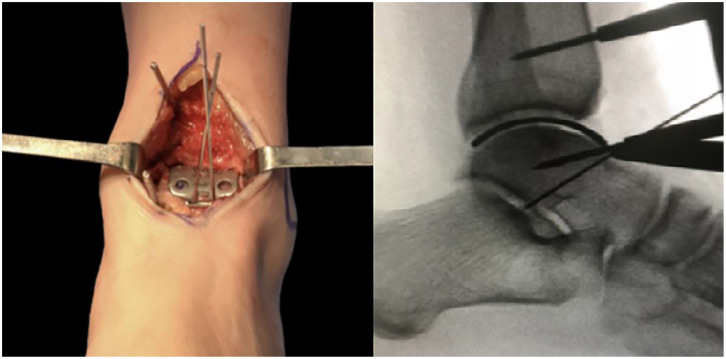
After an anterior ankle approach between the tibialis anterior and the extensor hallucis longus tendons, all remaining cartilage from the talar dome was debrided using chisels to expose the subchondral plate (Figure 1 (Left)). If there were any bone cysts, the defect was filled using a demineralized bone matrix and bone chips (Figure 1(Right)). The talar dome was sized using one of the 6 available trials from the “Ankle Spacer” (Arthrex) to obtain the best anteroposterior and mediolateral coverage without impinging any bony structure (Figure 2, Left and Right). In cases where 2 selected implants were desirable, undersizing was always favored. Once the desired trial was set and provisionally fixed to the talar dome, 2 holes were drilled in the anterior portion of the component to provide definitive fixation (Figure 3). Next, the trial component was removed, and the remaining talar dome was drilled using a 2.0-mm drill with holes separated by 3 mm to enhance fixation by bony ingrowth. The definitive prosthesis was then implanted by direct impaction (Figure 4) and the wound closed in a layered fashion. Patients were immobilized for 6 weeks in a removable boot and authorized to weightbear immediately.
Figure 1.
Left. After an anterior ankle approach between the tibialis anterior and extensor hallucis longus tendon, the tibiotalar joint is distracted using a Hintermann distractor. Right. All remaining cartilage over the talar dome is mechanically debrided to expose the subchondral bone and all necrotic bone at the osteochondral lesion of the talus is removed using a curette. The subchondral bone at the talar dome is drilled to enhance bony ingrowth into the Ankle Spacer. The defect is filled with autogenous bone until a smooth and congruent surface is checked.
Figure 2.
Clinical and radiologic view of trial size. Left. The Ankle Spacer is trialed until the desired size is selected. The goal is to achieve the best mediolateral (left picture) an anteroposterior (right picture, fluoroscope) coverage that avoids impingement on bony structures. Right. Intraoperative radiographs assess the correct position of the desirable size of the device.
Figure 3.

Holes are used to perform 2 drills of the anterior surface of the talus for the correct implant adaptation.
Figure 4.

Final clinical view of the Ankle Spacer.
After 1 week, patients were encouraged to perform active and passive movements of the ankle. The stitches were removed after 3 weeks. Six weeks after surgery, the splint was removed, and physiotherapy was started until full weightbearing was achieved without a boot or crutches. All patients received antibiotic prophylaxis for 3 days and thromboprophylaxis with dabigatran for 30 days.
Statistical Analysis
We performed a Student t test to analyze differences in PROMs within groups, comparing baseline against 3 months, 12 months, and last follow-up for each group.
After a Bonferroni post hoc validity analysis, multifactorial analysis of variance (ANOVA) compared the studied variables at different time frames. A P value of <.05 was considered statistically significant, with a CI of 95%.
A post hoc analysis was conducted to establish the achieved power sample size, resulting in 0.42. To achieve a power of 0.8, we would need a sample size of at least 27 patients.
All data were analyzed with SPSS v.27 and the Kaplan-Meier analysis with the Statistics Kingdom program.
Results
Ten patients were included in the study after meeting the selection criteria. No patient was lost in the follow-up. The mean surgical time was 66 minutes (SD = 12.7), and the mean hospital stay was 1 day (SD = 0.3). Demographic data are summarized in Table 1.
Table 1.
Demographic Data.
| Characteristics | Mean or n (%) |
|---|---|
| Age, y, mean | 45.3 |
| Sex | |
| Male | 8 (80) |
| Female | 2 (20) |
| Side | |
| Left | 5 (50) |
| Right | 5 (50) |
| BMI | 30.23 |
| Duration of symptoms, wk | 30.6 |
| Smoker | 4 (40) |
| Previous surgery | |
| None | 2 (20) |
| BMS | 6 (60) |
| OATS | 2 (20) |
| Etiology | |
| OCDs | 7 (70) |
| AVN of the talus | 3 (30) |
| Size, mm2, mean | 242.1 |
Abbreviations: BMI, body mass index; BMS, bone marrow stimulation; AVN, avascular necrosis of the talus; OATS, Osteochondral autologous tissue transfer; OCD, osteochondritis dissecans.
At a mean of 31.9 months (last follow-up) (SD = 7.65), 7 patients (70%) remain with their Ankle Spacer implanted with a survival rate of 62.5% at this time. A Kaplan-Meier curve is presented for the complete cohort with 95% CI, P = .05, effect size 0.3. Survival analysis represented in Figure 6.
Figure 6.
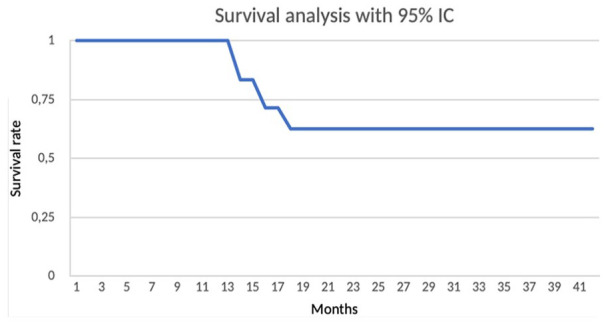
Kaplan-Meier survival analysis with 100% survivorship at 13 months, 83% at 14 months, 71% at 16 months, and 62.5% at 18 months.
The VAS improved in the survival group from 6.8 preoperatively to 5 at 3 months (P = .002), 4.5 at 12 months (P = .005), and remained at 4.8 during the last follow-up (P = .044). Regarding the SF-36 score, the SG increased their physical health component from 25.03 preoperatively to 42.25 at 12 months (P = .030) (Figure 5, A and B).
Figure 5.

(A) VAS improvements with statistically significant differences only in the survival group between preoperation and 3 months, 12 months, and last follow-up. (B) SF-36 improvements with statistically significant differences only in the survival group between preoperation and last follow-up. VAS, visual analog scale for pain; SF-36, Short Form–36 Health Survey. *Statistical difference, p < 0.05.
The SMFA dysfunction index reported an improvement from 46.36 to 39.35 at 3 months (P = .042), 36.05 at 12 months (P = .008), and 32.28 at last follow-up (P = .001). The SMFA bother index subscale improved from 55.21 to 39.51 at 12 months(P = .012) and 30.14 at the last follow-up(P = .002) in the SG. Regarding the FAOS, improvements were observed in the SG, in the function of daily living activity subscale, from 65.45 to 46.53 at 12 months (P = .037), sports recreation from 95 to 75.83 at 3 months (P = .001) and 73.33 at 12 months (P = .001), and quality of life subscale from 94.78 to 83.36 at 3 months (P = .046).
Concerning the FAAM scale, the sports component improved from 12.5 to 34.5 in the last follow-up (P = .023), and in the functional component, it improved from 30.28 to 46.15 at 3 months (P = .014) in the SG.
Intergroup analysis is summarized in Table 2.
Table 2.
Intergroup analysis: patients with ankle failiure, tend to decrease their functional outcomes one year after surgery.
| PROMs | Survival Group | Revision Group | P Value |
|---|---|---|---|
| VAS pain, 12 mo | 4.50 | 8.00 | .037 |
| SF-36 physical health component, 12 mo | 42.25 | 20.87 | .03 |
| SF-36 mental health component, 12 mo | 43.78 | 29.25 | .072 |
| SMFA functional index, 12 mo | 36.05 | 48.85 | .057 |
| SMFA bother index, 12 mo | 39.51 | 67.7 | .001 |
| FAOS sports recreation, 12 mo | 73.33 | 97.5 | .014 |
| FAAM sports, 3 mo | 8.75 | 21.90 | .034 |
Abbreviations: FAAM, Foot and Ankle Ability Measure; FAOS, Foot and Ankle Outcome Score; PROMs, patient-reported outcome measures; SF-36, Short Form–36 Health Survey; SMFA, Short Musculoskeletal Functional Assessment; VAS, visual analog scale.
We did not observe loosening, subsidence, or adjacent joint degeneration under radiographic evaluation in the SG. Figure 7 demonstrates a patient after 3 years of ankle hemiarthroplasty.
Figure 7.

Radiograph of a patient at 3 years of follow-up, where no loosening, subsidence, or progression of osteoarthritis is observed in adjacent joints.
Three patients were converted to a total ankle prosthesis because of persistent pain and failing conservative treatment at 14, 16, and 18 months. All 3 patients developed severe ankylosis of the ankle joint without implant interphase radiolucency under computed tomographic examination.
Discussion
We describe our short-term experience with the Ankle Spacer as an intermediate alternative to reduce pain for patients with talar-sided ankle arthritis or massive osteochondral lesion of the talus. When addressing these issues, the scarcity of clinical success has led to a lack of consensus on approaching this clinical situation, representing a true challenge for orthopaedic surgeons.20
The treatment armamentarium for these lesions ranges from joint-sparing to joint-ablating procedures. Joint-preserving procedures include osteochondral autograft/allografts, corticoperiosteal grafts, Hemi-CAP, autologous chondrocyte implantation, and supramalleolar osteotomies.3,4,10,16 Osteochondral autografts have the theoretical advantage of providing viable chondrocytes, an intact hyaline chondral surface, and inherent stability based on the bone-to-bone union.11 Conversely, the procedure presents disadvantages such as donor site morbidity, limited availability, and potential fibrocartilage formation with the native talus interphase. Moreover, cartilage mismatch may lead to articular overstress and early degeneration.13,18,24 To overcome these limitations, Hintermann et al12 described the transplantation of a vascularized corticoperiosteal graft from the ipsilateral femoral condyle in 14 patients, decreasing their VAS from 5.8 to 1.8 (P = .001) and increasing American Orthopaedic Foot & Ankle Society (AOFAS) ankle-hindfoot score from 65 to 81 (P = .003) at 4.1 years of follow-up.
Another option is to replace the lesion with a hemitalar allograft and provide a better bone stock for subsequent procedures. Adams et al1 performed transplantation of geometrically contoured fresh talar allograft in 8 patients, with a significant decrease in pain and functional improvement. No patients underwent subsequent arthrodesis or arthroplasty at the final follow-up of 48 months. Despite exhibiting some improvements in SMFA, FAAM, and FAOS scores in our survival cohort, these remained higher than those reported by Adams et al. However, these results should be considered in light of the cost and availability of the fresh allografts, and the simplicity and reproducibility of the Ankle Spacer technique.
Another alternative is the Hemi-CAP, a metallic resurfacing device that replaces a portion of the joint and has been used with some success in other articulations such as the shoulder, hip, and metatarsal heads.4,15 Vuurberg et al23 reported satisfactory results in 38 patients at 5-year follow-ups, with only 2 undergoing subsequent ankle arthrodesis. These numbers compare favorably with our survival rate of 62.5% at 31.9 months, reflecting the relatively short life span of the Ankle Spacer. However, considering that the alternative is an ablative procedure, consideration can be given to the procedure in selected cases. In any case, comparing the results of the Hemi-CAP with hemiarthroplasty is unfair because of the comparison of focal vs global disease.
On the other hand, ankle arthrodesis or total ankle replacement represents the spectrum of joint ablation procedures. Ankle arthrodesis is a reasonable option for young patients with ankle arthritis, mainly focusing on pain reduction. Recently, arthroscopic tibiotalar arthrodesis has gained popularity after demonstrating similar pain reduction, patient outcomes, and complications when compared to total ankle replacement, in selected cases.14 However, the functional impairment produced after eliminating the joint limits its indications, jeopardizing patients’ satisfaction and expectations.3 Furthermore, sacrificing the entire joint because of an osteochondral injury may seem to be an excessive surgery.8 Conversely, total ankle replacement is equally effective in reducing pain compared with ankle arthrodesis but offers the advantage of preserving mobility and maintaining function, theoretically diminishing hindfoot joint degeneration.6,7 However, because the affected patients are often very young, there is a greater risk of additional/revision surgery and implant failure.7
A potential intermediate surgical option that can allow buying time and, in theory, should not compromise future surgeries is desirable in such a scenario.
Replacing one side of a joint is not new in orthopaedics, particularly when considering patients on which age or the need for future procedures is the concerning issue.21 Replacing the talar portion of the ankle has the putative advantage of limited bone resection (if any), leading to an easier revision to fusion or replacement. Moreover, subchondral bone preservation offers a strong underlayer for the resurfacing device to prevent loosening or subsidence while enabling integration.19 There are 2 previous investigations regarding this “Ankle Spacer,” the first one performed by Lerch et al described 10 patients at 3 months, with improvements in AOFAS ankle-hindfoot score from 55.5 to 79.5, European Foot and Ankle Society score from 5.6 to 13.5, and the VAS pain score decreasing from 3 to 1.1. Although they report no signs of component loosening, and no progression toward osteoarthritis, drawing definitive conclusions is not possible given their extremely short follow-up period.17 The other one, despite having a longer follow-up than the first one, only has 2 patients in its cohort. They reported an improvement from 6 and 7 preoperatively to 2 and 2 postoperatively in the numeric rating scales during walking and substantial improvement in other PROMs such as American Orthopaedic Foot & Ankle Society ankle-hindfoot score, FAOS, and SF-36.5 In our experience, the majority of our patients improved their functional outcomes. And those patients who remain with their Ankle Spacer implanted maintained their functional outcomes (SF-36, FAAM, FAOS, and SMFA) improvement while sustaining a moderate amount of pain.
This investigation is not without limitations. First, we acknowledge that the small nature of our patient cohort leaves our study underpowered for more comprehensive statistical analysis. Although prospective in design, the absence of a control group precludes drawing any conclusion regarding superiority to other treatments. However, considering the ideal candidate for ankle hemiarthroplasty is scarce, recruiting more patients can be difficult. Second, we did not include computed tomographic scan to evaluate implant integration in every patient, but only in those cases where the patients presented persisting pain.
Our results suggest that ankle hemiarthroplasty is a reasonable intermediate step to treat patients with ankle arthritis solely affecting the talar side of the joint, such as massive osteochondral lesions of the talus. This strategy appears not to jeopardize future surgeries shall they be needed, although there is always the risk of infection and bone loss with the use of a hemiarthroplasty in any joint.
Conclusion
In our small initial series, we had a relatively high rate of early failure. Among those who did not fail in the first 18 months, by an average of 32 months on average patients reported modest pain reduction, improvement in functional scores, and better general quality of life in patients after ankle hemiarthroplasty. This hemiarthroplasty approach may be an intermediate option for treating these difficult conditions where ankle arthrodesis or total arthroplasty is considered for talar-only-sided ankle cartilage loss.
Footnotes
Ethical Approval: Ethical approval for this study was obtained from North Metropolitan Health Service Research Ethics Committee (no. 063/2017).
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Manuel J. Pellegrini, MD, reports consulting fees from Arthrex. Giovanni Carcuro, MD, reports consulting fees and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Arthrex. ICMJE forms for all authors are available online.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study received funding from Arthrex.
ORCID iDs: Manuel J. Pellegrini, MD,  https://orcid.org/0000-0002-2820-5337
https://orcid.org/0000-0002-2820-5337
Franco Mombello, MD,  https://orcid.org/0000-0002-6520-9775
https://orcid.org/0000-0002-6520-9775
Felipe Chaparro, MD,  https://orcid.org/0000-0002-3524-0624
https://orcid.org/0000-0002-3524-0624
References
- 1. Adams SB, Viens NA, Easley ME, Stinnett SS, Nunley JA. Midterm results of osteochondral lesions of the talar shoulder treated with fresh osteochondral allograft transplantation. J Bone Joint Surg Am. 2011;93(7):648-654. [DOI] [PubMed] [Google Scholar]
- 2. Ahmad J, Raikin SM. Ankle arthrodesis: the simple and the complex. Foot Ankle Clin. 2008;13(3):381-400. doi: 10.1016/j.fcl.2008.04.007 [DOI] [PubMed] [Google Scholar]
- 3. Barg A, Pagenstert GI, Horisberger M, et al. Supramalleolar osteotomies for degenerative joint disease of the ankle joint: indication, technique and results. Int Orthop. 2013;37(9):1683-1695. doi: 10.1007/s00264-013-2030-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bilge O, Doral MN, Yel M, Karalezli N, Miniaci A. Treatment of osteonecrosis of the femoral head with focal anatomic-resurfacing implantation (HemiCAP): preliminary results of an alternative option. J Orthop Surg Res. 2015;10(1):56. doi: 10.1186/s13018-015-0199-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dahmen J, Altink JN, Vuurberg G, Wijdicks CA, Stufkens SA, Kerkhoffs GM. Clinical efficacy of the Ankle Spacer for the treatment of multiple secondary osteochondral lesions of the talus. World J Orthop. 2022;13(2):178-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Daniels TR, Younger ASE, Penner M, et al. Intermediate-term results of total ankle replacement and ankle arthrodesis: a COFAS multicenter study. J Bone Joint Surg Am. 2014;96(2):135-142. [DOI] [PubMed] [Google Scholar]
- 7. Dekker TJ, Walton D, Vinson EN, et al. Hindfoot arthritis progression and arthrodesis risk after total ankle replacement. Foot Ankle Int. 2017;38(11):1183-1187. [DOI] [PubMed] [Google Scholar]
- 8. Flavin R, Coleman SC, Tenenbaum S, Brodsky JW. Comparison of gait after total ankle arthroplasty and ankle arthrodesis. Foot Ankle Int. 2013;34(10):1340-1348. [DOI] [PubMed] [Google Scholar]
- 9. Glazebrook M, Daniels T, Younger A, et al. Comparison of health-related quality of life between patients with end-stage ankle and hip arthrosis. J Bone Joint Surg Am. 2008;90(3):499-505. [DOI] [PubMed] [Google Scholar]
- 10. Gross CE, Adams SB, Easley ME, Nunley JA. Role of fresh osteochondral allografts for large talar osteochondral lesions. J Am Acad Orthop Surg. 2016;24(1):e9-e17. doi: 10.5435/jaaos-d-15-00302 [DOI] [PubMed] [Google Scholar]
- 11. Haene R, Qamirani E, Story RA, Pinsker E, Daniels TR. Intermediate outcomes of fresh talar osteochondral allografts for treatment of large osteochondral lesions of the talus. J Bone Joint Surg Am. 2012;94(12):1105-1110. [DOI] [PubMed] [Google Scholar]
- 12. Hintermann B, Wagener J, Knupp M, Schweizer C, Schaefer DJ. Treatment of extended osteochondral lesions of the talus with a free vascularised bone graft from the medial condyle of the femur. Bone Joint J. 2015;97-B(9):1242-1249. [DOI] [PubMed] [Google Scholar]
- 13. Hurley ET, Murawski CD, Paul J, et al. Osteochondral autograft: proceedings of the international consensus meeting on cartilage repair of the ankle. Foot Ankle Int. 2018;39(1 suppl):28S-34S. [DOI] [PubMed] [Google Scholar]
- 14. Jones CR, Wong E, Applegate GR, Ferkel RD. Arthroscopic ankle arthrodesis: a 2-15 year follow-up study. Arthroscopy. 2018;34(5):1641-1649. [DOI] [PubMed] [Google Scholar]
- 15. Kline AJ, Hasselman CT. Resurfacing of the metatarsal head to treat advanced hallux rigidus. Foot Ankle Clin. 2015;20(3):451-463. [DOI] [PubMed] [Google Scholar]
- 16. Kwak SK, Kern BS, Ferkel RD, Chan KW, Kasraeian S, Applegate GR. Autologous chondrocyte implantation of the ankle: 2- to 10-year results. Am J Sports Med. 2014;42(9):2156-2164. [DOI] [PubMed] [Google Scholar]
- 17. Lerch M, Yao D, Ettinger S, Claassen L, Plaass C, Stukenborg-Colsman C. The ankle spacer—a hemiarthroplasty for treatment of severe osteochondral defects of the talus. Oper Orthop Traumatol. 2022;34(1):79-88. [DOI] [PubMed] [Google Scholar]
- 18. Looze CA, Capo J, Ryan MK, et al. Evaluation and management of osteochondral lesions of the talus. Cartilage. 2017;8(1):19-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Martinez-Carranza N, Ryd L, Hultenby K, et al. Treatment of full thickness focal cartilage lesions with a metallic resurfacing implant in a sheep animal model, 1 year evaluation. Osteoarthritis Cartilage. 2016;24(3):484-493. doi: 10.1016/j.joca.2015.09.009 [DOI] [PubMed] [Google Scholar]
- 20. Raikin SM. Stage VI: massive osteochondral defects of the talus. Foot Ankle Clin. 2004;9(4):737-744, vi. [DOI] [PubMed] [Google Scholar]
- 21. Rasmussen JV, Polk A, Sorensen AK, Olsen BS, Brorson S. Outcome, revision rate and indication for revision following resurfacing hemiarthroplasty for osteoarthritis of the shoulder: 837 operations reported to the Danish Shoulder Arthroplasty Registry. Bone Joint J. 2014;96-B(4):519-525. [DOI] [PubMed] [Google Scholar]
- 22. Valderrabano V, Horisberger M, Russell I, Dougall H, Hintermann B. Etiology of ankle osteoarthritis. Clin Orthop Relat Res. 2009;467(7):1800-1806. doi: 10.1007/s11999-008-0543-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vuurberg G, Reilingh ML, van Bergen CJA, van Eekeren ICM, Gerards RM, van Dijk CN. Metal resurfacing inlay implant for osteochondral talar defects after failed previous surgery: a midterm prospective follow-up study: response. Am J Sports Med. 2019;47(2):NP19-NP20. [DOI] [PubMed] [Google Scholar]
- 24. Zanon G, Di Vico G, Marullo M. Osteochondritis dissecans of the talus. Joints. 2014;2(3):115-123. [DOI] [PMC free article] [PubMed] [Google Scholar]