Abstract
Background
The overall poor prognosis in pancreatic cancer is related to late clinical detection. Early diagnosis remains a considerable challenge in pancreatic cancer. Unfortunately, the onset of clinical symptoms in patients usually indicate advanced disease or presence of metastasis.
Analysis and Results
Currently, there are no designated diagnostic or screening tests for pancreatic cancer in clinical use. Thus, identifying risk groups, preclinical risk factors or surveillance strategies to facilitate early detection is a target for ongoing research. Hereditary genetic syndromes are a obvious, but small group at risk, and warrants close surveillance as suggested by society guidelines. Screening for pancreatic cancer in asymptomatic individuals is currently associated with the risk of false positive tests and, thus, risk of harms that outweigh benefits. The promise of cancer biomarkers and use of ‘omics’ technology (genomic, transcriptomics, metabolomics etc.) has yet to see a clinical breakthrough. Several proposed biomarker studies for early cancer detection lack external validation or, when externally validated, have shown considerably lower accuracy than in the original data. Biopsies or tissues are often taken at the time of diagnosis in research studies, hence invalidating the value of a time-dependent lag of the biomarker to detect a pre-clinical, asymptomatic yet operable cancer. New technologies will be essential for early diagnosis, with emerging data from image-based radiomics approaches, artificial intelligence and machine learning suggesting avenues for improved detection.
Conclusions
Early detection may come from analytics of various body fluids (eg ‘liquid biopsies’ from blood or urine). In this review we present some the technological platforms that are explored for their ability to detect pancreatic cancer, some of which may eventually change the prospects and outcomes of patients with pancreatic cancer.
Keywords: screening, early detection, biomarker, radiology, diagnosis, curative surgery, prevention, early diagnosis, liquid biopsy
Introduction
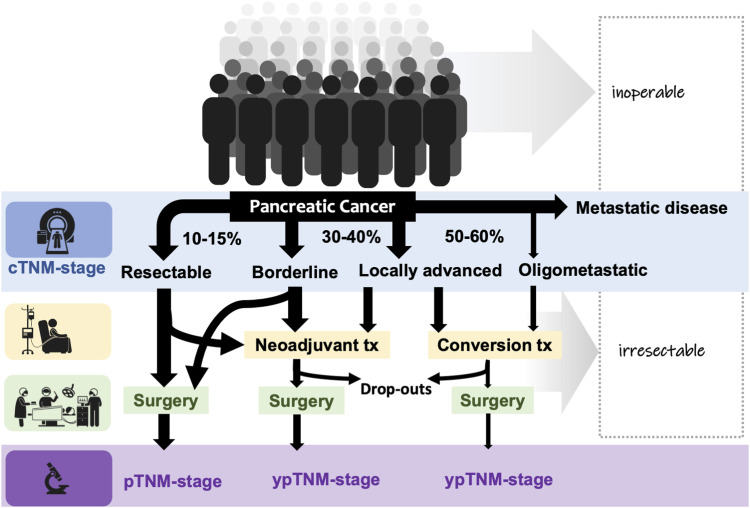
Pancreatic cancer is increasing in incidence and will soon become a major cause of cancer-related deaths in several parts of the world.1 Pancreatic cancers are typically diagnosed at a time when the patients have developed symptoms, usually indicating locally unresectable disease and/or metastasis.2,3 Currently, only 15-20% are diagnosed at a stage when curative surgery may be considered (Figure 1). Unfortunately, symptoms are in general vague and unspecific in most patients. Of concern is an increase in the early-onset rates of pancreatic cancers reported from several countries.4-7 Predictions suggest that pancreatic cancer will become 1 of the most common causes to cancer-related deaths in most Western countries within a few years. Hence, a more timely diagnosis and more efficient therapy is urgently needed.8
Figure 1.
Proportion of patients presenting for potential curative treatment. Legend: Any given patient may be deemed inoperable at time of diagnosis or irresectable through clinical (image-based) staging. Definitions for borderline/locally advanced cancers are floating, with variation in management. More effective systemic therapy (eg FOLFIRINOX) is increasingly introduced in the pre-operative setting, with more resections offered after therapy, possibly influencing the pathological TNM-staging and interpretation of its prognostic role. Better predictive and prognostic biomarkers of cancer biology are needed. Reproduced with permission from Roalsø et al. Copyright © 2020 The Author(s). Published by Elsevier Ltd. All rights reserved.
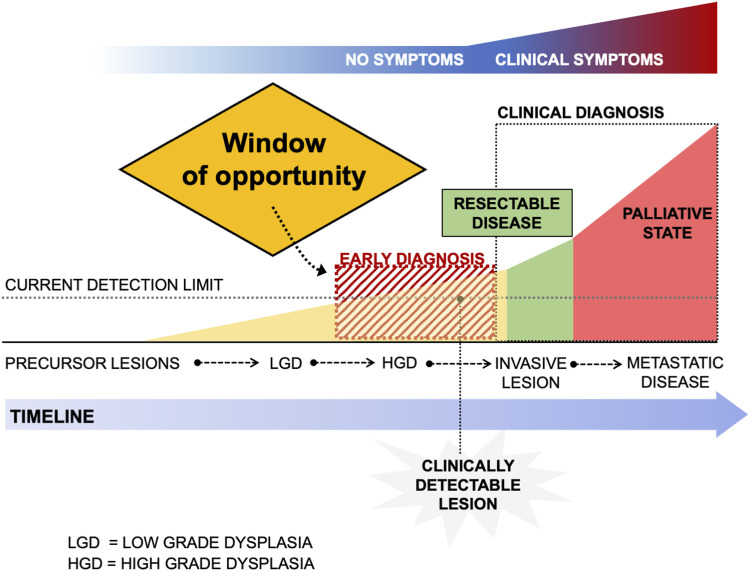
The idea behind the effect of screening is that early detection of disease in an asymptomatic or precursor stage will allow for timely treatment and hence improve prognosis.9 The criteria and principles set out by the World Health Organization10 (WHO) for justifying public screening programmes include a list of 10 points, including the need to address an important health problem; availability of accepted treatment for the condition; recognizable latent or symptomatic stages of the disease; suitable test or examinations to detect the disease; the test should be acceptable to the population; proper understanding of the natural history of the disease should be available; cost-efficiency of test and economic burden to medical care should be available; agreed policy to treatment, to mention some of the scientific principles.9,10 Unfortunately, pancreatic cancer is not suited for population screening given the overall low incidence of the disease and the current lack of accurate, inexpensive and non-invasive screening tests. Hence, population-based screening for pancreatic cancer is currently not recommended and should be avoided. However, there is a dire need to identify groups in the general population of asymptomatic individuals that are at a higher risk for developing pancreatic cancer. The precursor stages have been defined and should allow intervening with preventive strategies or early surgery by early detection of pre-symptomatic, non-invasive disease in a “window of opportunity” (Figure 2).
Figure 2.
Window of opportunity for early detection of pancreatic cancer. Legend: The early detection of resectable disease or precursor lesions requires earlier detection at a time when no symptoms are present, yet biological signals (eg imaging, blood tests, biomarkers) are present for detection. Detection and treatment of high-grade dysplasia (HGD) before invasive cancer may provide cure (yellow zone); detection of early-stage cancer (green zone) may improve survival and cure rates.
In the future, defining at-risk groups may be needed for cohort studies of screening, for studies of early diagnosis or, for preventive intervention strategies. In this article, we will discuss some emerging areas raising an opportunity for earlier detection of pancreatic cancer.
The Challenge With Early Cancer Detection and Early Cancer Stages
All cancers have risk factors attributed to lifetime exposures that may trigger tumorigenesis and enhance malignant progression. Pancreatic cancer develops through defined cellular and molecular pathways, with well-described precursors such as Pancreatic Intraepithelial Neoplasia (PanIN) and cystic mucinous precursors Mucinous Cystic Neoplasms (MCNs) and Intraductal Papillary Mucinous Neoplasms (IPMNs), harbouring specific associated genetic characteristics.11 PanIN lesions are microscopic, typically found in resected specimens, often for other reasons and generally cannot be detected on preoperative imaging. PanIN lesions precede any development of clinically detectable disease. They represent part of a multistep tumor progression model to invasive ductal adenocarcinoma in which increasing morphological grades of dysplasia are accompanied by accumulation of various genetic alterations.12 Given its microscopic nature, PanIN is currently not a target for screening as it is mostly a finding on histopathology. Hence, these premalignant lesions are usually identified either through intense surveillance of populations at particularly high risk, such as those in surveillance programs.13 In high-risk individuals, high grade PanIN is frequently multifocal and often associated with lobulocentric atrophy that has been suggested for possible detection on EUS, suggesting indirectly a potential screening tool in this particular group of patients.14,15 However, most premalignant lesions are detected as incidental pancreatic cysts on conventional imaging.16
Early-onset cancers has been called an emerging global epidemic, also for pancreatic cancer.17 Also, more patients are diagnosed with early-stage cancers (stage IA), suggesting that closer surveillance of high-risk groups may contribute to an earlier diagnosis.5 However, the relative contribution is small, with less than 1% being diagnosed as “early cancers” in the beginning of the study period only to rise to less than 3% at the end.5 This is in parallel to a study from England, showing that stage I made up less than 1% of all resected pancreatic cancers, and stage II made up less than 2%.6 A similar rate was corroborated in a multi-center Japanese cohort, with less than 1% and 3% being stage I and II, respectively.7 As such, early-stage cancers make up a very little part of all pancreatic cancers. Further, a screening test would require a very high diagnostic specificity (>95%) to avoid generating too many false-positive tests.18 Therefore, pancreatic cancer is not included for screening in the general population in most countries.19-21
The Challenge With Cancer Screening
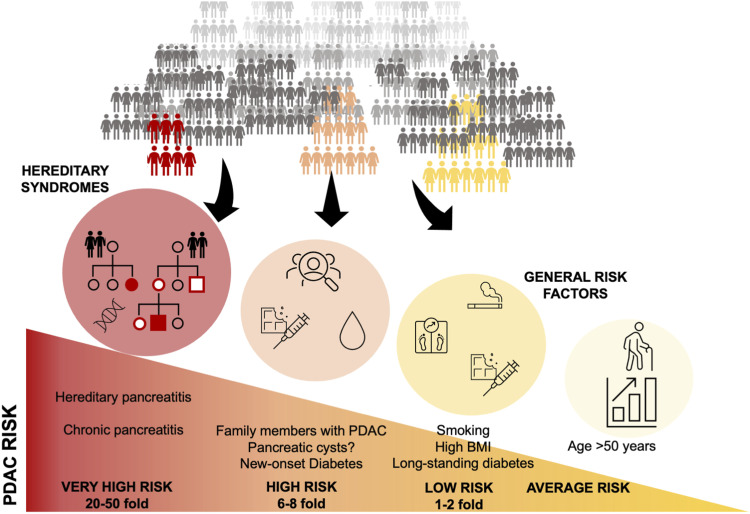
For screening of a disease to be effective, the disease should be diagnosed at an early, asymptomatic stage when cure is possible, but for pancreatic cancer this is a rare event in clinical practice.22 The prerequisite for any screening program,9,10 is having a patient population with a high enough prevalence of the disease that 1 is looking for, as even a good test will suffer from a low prevalence and result in low yield, ie low positive predictive value. As such, the first challenge should be identifying high-risk groups (Figure 3).
Figure 3.
Relative risk for pancreatic cancer in the population. Legend: Highest risk is found in hereditary genetic syndromes, yet the majority present without specific risk factors.
Even in early-stage cancers, only about 1 in 5 may present without any symptoms.7 The consensus is that widespread population-based screening for pancreatic cancer in the general population is neither practicable nor indicated in most countries.20,21,23,24 One report concluded that screening for pancreatic cancer would not improve disease-specific survival based on the rapid progression of the disease; the overall benefits was estimated to be small at best; and, that screening would be associated by a modest risk of harms.25,26 Consequently, screening is not supported in most guidelines.27,28
An ideal test for early detection (and, prevention) would include a sensitive, accurate serum marker to detect asymptomatic cancers that are otherwise clinically and radiographically undetectable. Additionally, the marker should allow isolation of the organ involved and, since the lesion is too small to detect, be able to be treated with natural products (eg dietary compounds, or food products) to prevent growth and for the marker to become undetectable. The sensitivity of a biomarker-based screening test will need to be much higher for cancers with a modest public health burden than for those with larger burdens.29 One important reason for this is that small changes in the sensitivity of any biomarker (alone; as a panel, or; as an imaging modality) applied for screening purposes can have modest or enormous impacts on system-wide costs per cancer detected,30 depending on the prevalence of the disease being screened.31,32 Currently, such a screening test that satisfies all criteria is not available for pancreatic cancer.
Screening of High-Risk Individuals
Patients with high risk (>5% life-time risk) of PDAC are currently offered screening in certain programmes. Certain risk-groups with hereditary syndromes (Table 1) and familial pancreatic cancer are included in ongoing programs for early detection.33 Persons with pancreatic cystic lesions is another risk group,16 for which some need surveillance while others may need resection.
Table 1.
Hereditary Genetic or Cancer Syndroms and PDAC Risk.
| Syndrome | Mutation | Lifetime risk % |
|---|---|---|
| Peutz-Jeghers syndrome | STK11 (LKB1) | 11-32 |
| Hereditary pancreatitis | PRSS1 | 25-40 |
| FAMMM | P16INK4A/CDKN2A | 17 |
| Lynch syndrome (HNPCC) | MLH1, MSH2, MSH6, PMS2, EPCAM | 8.6 |
| FAP | APC | 1.7 |
| Cystic fibrosis | CFTR | <5 |
| HBOC syndrome | BRCA1, BRCA2/FANCD1, PALB2/FANCN, FANCC, FANCG | Increased |
| Ataxia telangiectasia | ATM | Increased |
FAMMM, Familial atypical multiple mole melanoma syndrome; FAP, familial adenomatous polyposis; FDR, first degree relative; HBOC, hereditary breast and ovarian cancer; HNPCC, hereditary nonpolyposis colorectal cancer; PDAC, pancreatic ductal adenocarcinoma.
A systematic review34 of prospective cohort studies (including those with more than 20 patients) of asymptomatic adults determined to be at high-risk of pancreatic cancer (lifetime risk >5%, including specific genetic-associated conditions) who were screened by endoscopic ultrasound (EUS) and/or magnetic resonance imaging (MRI) to detect pancreatic lesions. The investigators34 found 19 studies with a total of 7085 individuals at high risk for pancreatic cancer. Of these, 1660 patients were evaluated by EUS and/or MRI. Fifty-nine high-risk lesions were identified (43 adenocarcinomas, of which 28 during the initial exam and 15 during follow-up surveillance) and 257 patients had pancreatic surgery. Based on the meta-analysis,34 the overall diagnostic yield screening for high-risk pancreatic lesions was .74 (95% CI, .33-1.14), with moderate heterogeneity among studies. The ‘number needed to screen’ to identify 1 patient with a high-risk lesion was 135 persons (95% CI, 88-303) per detected high-risk lesion. The diagnostic yield was similar for patients with different genetic features that increased risk, and whether patients were screened by EUS or MRI.34 Hence, the screening yield, even in high-risk populations, is currently labour-intensive with a modest outcome on early detection rates and opportunity for intervention. However, it is expected to see improvements as technology and tools develop and population at-risk definitions are refined.
Population at Risk for Pancreatic Cancer: Emerging Data
Most patients with pancreatic cancer are diagnosed after presentation of symptoms (Figure 1 and 2) with some higher-risk groups undergoing surveillance.35,36 Unfortunately, the clinical symptoms occur late. Weight loss and/or silent jaundice may be robust indicators for an underlying cancer that warrant referral and work up,37 but are usually associated with already advanced disease or metastases. Notably, the most common risk factors (including age, smoking, obesity) are too generic and do not warrant screening per se. Hence, most patients are unfortunately diagnosed when cure is no longer possible (Figure 1).
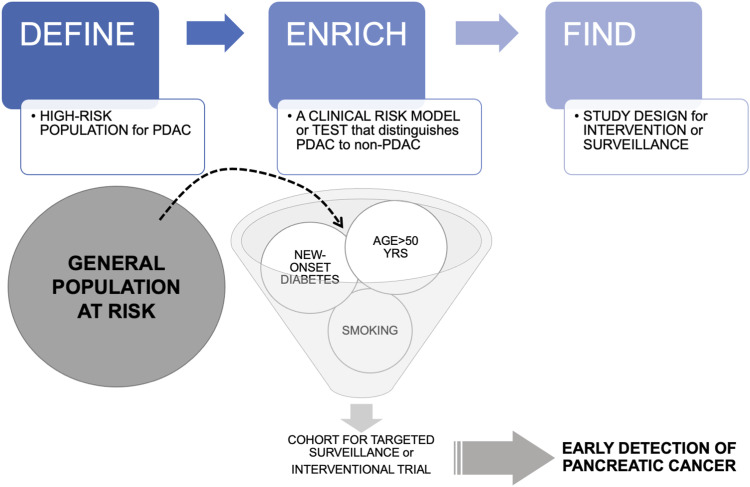
Thus, there is a need to narrow the sieve through which subjects with a particular risk are enriched (Figure 4), so as to increase screening accuracy and cost-effectiveness. One way would be to narrow down the population at-risk going through the screening system (Figure 3). A specific risk group of increasing attention is subjects >50 years of age with new-onset diabetes – a population with the highest risk for sporadic PDAC.38 However, even in this scenario with an estimated pancreatic cancer prevalence of .8% the risk-benefit scenario is complex even with an assumed very sensitive and specific test.18 Indeed, identifying robust, valid risk factors for appropriate screening and early detection of pancreatic cancer is challenging, as demonstrated in several epidemiological models.39-42
Figure 4.
The Define-Enrich-Find strategy for early detection of pancreatic cancer. Legend: Screening for sporadic PDAC in the average risk general population is considered unrealistic because of the low incidence. An alternative to screening is a proposed DEF (Define, Enrich, Find) strategy that allows PDAC surveillance in a subset of higher risk asymptomatic patients where it might be most beneficial. New-onset diabetes or pancreatic cysts may be such targeted populations.
Cross-Section Imaging for Detection
Imaging is the current diagnostic reference standard for pancreatic lesions. Imaging consists of endoscopic ultrasound (EUS), computed tomography (CT) and magnetic resonance imaging (MRI). Each have benefits and disadvantages, and all are equally accurate in diagnosing pancreatic cancer,43 together with transabdominal ultrasound and contrast-enhanced ultrasound.44 However, no imaging modality is practical as stand-alone screening tool in individuals at regular risk for pancreatic cancer. Notably, imaging has detection limits regarding size. Cysts are the only visible precursor lesion, as PanINs are not detected on standard imaging. However, pancreatic cystic precursors such as IPMNs or premalignant mucinous cystic lesions are detectable with imaging studies.16,45 An increasing number of individuals are diagnosed with incidental pancreatic cysts.16,46 Notably, the guidelines for surveillance or resection are conflicting, with considerable variation in the recommendations of observation vs resection.16,46-50 However, individuals with cystic lesions represent a defined risk population for exploring biomarkers to assess risk and define progression from precursor to invasive cancer.51,52
Biomarkers for Early Detection of PDAC
Biomarkers have yet to make an impact on early diagnosis for pancreatic cancer, even if there is no lack of suggested candidate markers in the available literature.53-62 The Alliance of Pancreatic Cancer Consortia for Biomarkers for Early Detection provided a common platform, listed the resources necessary for validation and, named the available markers felt to be promising for further pursuit.63 None of the biomarkers were ready for a large-scale trial for biomarker validation.
In systematic review reports, several markers have been labelled as promising, yet remain under investigation for clinical utility. Extracellular vesicle (including microRNAs and others) as biomarkers have been scrutinized,60 yet technological difficulties and standardization needs to be overcome before translation into clinical use.
One study has explored the utility of early elevation of CA 19-9 as an “anchor test” together with other biomarkers, to identify risk of early pancreatic cancer up to 5 years prior to diagnosis.64,65 This is promising, giving the ubiquitous use of CA 19-9. However, about 10% in the population will be Lewis-antibody negative, and hence not express CA 19-9 at all even if cancer is present. Thus, a more universally expressed marker with sufficient sensitivity may be needed.
New-Onset Diabetes, Glucose Intolerance and Metabolic Alterations
A strong correlation to risk of developing PDAC is associated with reduced blood glucose tolerance and new onset diabetes.66 In 1 meta-analysis, with every .56 mmol/L increase in fasting blood glucose there was an associated with a 14% increase in the rate of PDAC.67 In a model (Enriching New-Onset Diabetes for Pancreatic Cancer; ENPAC) based on changes in weight, change in blood glucose, and age at onset of diabetes, the investigators found persons with a score ≥3 to have 80% sensitivity and specificity for developing PDAC.42 While needing validation, such risk scores could improve risk-stratification to improve the diagnostic yield by use of a screening test or modality.
Blood glucose alteration is but 1 among several metabolic changes that may follow the progression or even be caused by pancreatic cancer.35 While fasting blood glucose may be a target based on the PDAC specific mechanisms to increased blood glucose, several other metabolic alterations occur in PDAC, involving muscle mass, lipids and protein synthesis.68-73
Higher levels of branched-chain amino acids have been found to occur years before diagnosis of PDAC in several studies, suggesting these to be metabolomic biomarkers for future PDAC risk.72,74,75 In 1 study,75 elevated plasma levels of branched-chain amino acids (BCAAs) are associated with a greater than 2-fold increased risk of future pancreatic cancer diagnosis. This increased risk was independent of other, known predisposing factors. The strongest association was observed among subjects with samples collected 2 to 5 years before diagnosis of PDAC.
In an attempt at validation of the findings, a European cohort data (from Norway, Finland, Estonia and the Netherlands) did not support the branched-chain amino acids identified earlier in several US cohorts as potential biomarkers for pancreatic cancer.76 The European cohorts rather identified glutamine and histidine as potential biomarkers of interest. However, they investigators concluded that the study did not yield metabolomic biomarkers with sufficient predictive value to be clinically useful as a prognostic biomarkers.
One general problem with several of the proposed biomarkers is that the sample is collected at the time of PDAC diagnosis (or, even later after diagnosis) which may not correctly reflect the metabolomic profile year before a diagnosis is made. Similar experience has been made with other types of serum markers, including microRNA in serum.77
Further Developments and Novel Technology
Novel approaches are investigating non-invasive biomarkers that can be easily accessed or monitored, of which some will be briefly mentioned here. The attractive principle for most biomarkers for early detection would be a test that is a non-invasive, repeatable test which would allow for early detection of resectable PDAC with potential for cure, or better, prevention by operation of high-grade dysplasia not yet transformed into invasive cancer.36 Some tests have been proposed for use by sampling saliva59,78-81 or urine52,61,82-85 for detection of pancreatic cancer. However, these technologies and their accuracy needs further refinement before being introduced as useful clinical tests. Also, the various use of biosensors,86,87 although attractive, have yet to see a development that is near clinical implication. Hence, we have focused on the role of test already in routine use, such as the expanded use of conventional imaging information (radiomics), the evolving role of sampling pancreatic juice and analyses, and the emerging role of liquid biopsies and markers in blood.
Cross-Sectional Abdominal Imaging Tools and Radiomics
Radiomics is a sub-field of computer vision analysis. The core premise of radiomics is that the differences in size, shape, texture, and greyness of a tumor contoured from a radiological image can reflect the variations in histological phenotype and genotype of the tumor.88 Briefly explained, various radiological images (typically CT or MRI scans) can be converted into mineable data through which high-throughput extraction of quantitative features can be done by computers. The extracted data can then be combined with clinical features to generate a diagnostic or prognostic model for cancer or, even by means of adding artificial intelligence or machine learning allow for early detection of cancer.89-93 However, there is a need to harmonize data towards a common standard.94
Current studies on quantitative imaging biomarkers in pancreatic cancer are hampered by small sample sizes, together with a lack of standardization in image pre-processing and acquisition protocols, external validation, and the substantial heterogeneity in features analyzed, making it hard to compare data sets.95 Thus, radiomics is currently not recommended for routine clinical practice. No commercially available radiomics solution exist for pancreatic tumors, albeit progress is being made in automating image segmentation, lesion characterization and cancer detection.96 Machine learning is a technique for analyzing and predicting by learning from sample data, finding patterns in it, and applying it to new data.90 Early detection of pancreatic cancer is challenging due to overlapping imaging features with benign lesions, though quantitative imaging has helped differentiate autoimmune pancreatitis from PDAC with an accuracy of 95.2%,97 and all PDAC cases were correctly classified when compared to healthy pancreata,98 both studies showcasing radiomics as useful in differentiating pancreatic disease states. In addition, a proof-of-concept study utilizing clinical data, miRNAs and radiomics in 38 surgically resected, pathologically confirmed IPMN cases, managed to predict malignant IPMNs superior to conventional models.99 Of significance, here radiomics helped identify the true negatives from otherwise false positives, and correctly classified a true positive, which was false negative using conventional imaging results; findings which can simultaneously lead to a reduction in pancreatic resections, and correctly identify patients in need of surgery. Further, non-invasive insights have been found utilizing radiomics regarding drug sensitivity, tumor subtypes, treatment response and clinical stratification, which ultimately can help guide patient care.100-102 Radiomics with machine learning has proposed to be able to detect PDAC up to 2 years prior to diagnois in 1 study.103
Pancreatic Juice and Cyst Fluids
Detection of biomarkers in pancreatic juice have been explored across several clinical settings, including for high-risk subjects with familial risk or for patients with pancreatic cystic lesions. Both genomic, metabolomic and proteomic biomarkers have been explored.88,104-107 Studies on early cancer detection through analysis of pancreatic juice, including brush cytology during ERCP, show sensitivity ranging from 21.3% to 63.6% and specificity of 94% to 100%.108 However, pancreatic juice analysis could be affected by the position and size of the catheter and in addition, patients may suffer from frequent complications, such as ERCP-associated pancreatitis.109,110
Sometimes it is not possible to obtain a diagnostic sample on EUS or ERCP, even if imaging suggests a lesion or suspicious finding. In this setting, some investigators have used a technique called serial pancreatic juice aspiration cytologic examination (SPACE) for the diagnosis.111-114 The approach is not universally available, and early experience comes mainly from Japan, but may represent a useful diagnostic method in select cases for early-stage pancreatic cancers, such as carcinoma in situ that are difficult to diagnose by endoscopic ultrasound-guided fine needle aspiration (EUS-FNA).113 The method is initiated via ERCP, whereby an endoscopically placed nasopancreatic drainage allows for serial measurements of pancreatic juice for cytology, or ‘liquid biopsy’.114 Of note, the method carries the risk of post-ERCP pancreatitis.
High concentration of carcinoembryonic antigen (CEA) in pancreatic cyst fluid is reflective of a mucinous cystic precursor and associated with 57-79% sensitivity. Cytologic examination of cyst fluid regarded as an enhancement of EUS’ utility in identifying cystic neoplastic precursors, reaches high specificity rates, but the technique is hampered by low cellularity and therefore the sensitivity varies from 25 to 88%. Genomic alterations revealed by next generation sequencing (NGS) of exfoliated epithelium in cyst fluid correlate with mutational profiles of the major mucinous pancreatic cysts and those that progressed to invasive carcinoma. For example, the detection of KRAS mutations in pancreatic cyst fluid by NGS shows a 76%-89% sensitivity and 96%-100% specificity for IPMNs and MCNs. Mutational analysis of pancreatic cyst fluid is becoming widespread clinically available with the increased availability of NGS and reduced costs.36,115-117 Recently, a 22-gene NGS panel (PancreaSeq) was examined in a multicenter cohort of over 1800 patients with pancreatic cysts.118 In this study,118 the PancreaSeq was not sensitive and specific for various pancreatic cyst types and advanced neoplasia arising from mucinous cysts and, also, had better diagnostic performance than comparative clinical cyst guidelines. In addition, the PancreaSeq also revealed the diversity of genomic alterations seen in pancreatic cysts and their clinical relevance.118
Variations in expression of glycosylated, high-molecular-weight glycoproteins, like MUCs have been highlighted as novel biomarkers for early detection of IPMN-associated invasive cancer and differentiation of mucinous from non-mucinous pancreatic cysts.119 Other promising biomarker results that may be analysed in cyst fluid, like differentially methylated DNA, telomerase activity, protease expression have not been vigorously validated in diverse cohorts of pancreatic cysts.
Nonetheless, acquiring pancreatic cyst fluid and juices require invasive procedures, challenging operational systems and are highly investigator-dependent. Although some of the aforementioned biomarkers are currently in use in some centres, high costs, reduced availability and variable method sensitivity, advocate for a multimodal approach, rather than identifying a single optimal biomarker.
Biomarkers in Stool and the Role of Faecal Microbiome in Early Detection of Pancreatic Cancer
In theory, pancreatic juice with exfoliated cancer or pre-cancerous cells harbouring tumour-specific mutations may be secreted into the intestines and hence be discovered as mutations in the stool. This has indeed been investigated in a few studies, as reviewed by Sammallathi et al,120 but has yet to make it into clinical practice. Related to this, is the specific microbiome and patterns related to alteration sin healthy compared to patients with cancer.121-123 The current field is too premature to allow for any diagnostic or screening measures to be clinically meaningful,55,124 but improved understanding of this field may facility better methods in the future. Indeed, a recent study81 using 2 cohorts from Spain and Germany demonstrated very promising data using shotgun metagenomic and 16S rRNA amplicon sequencing of fecal microbiota. The microbial pattern together with serum levels of CA 19-9 provided very high accuracy for detection of pancreatic cancer. The study suggests that such specific fecal microbiota-based screening for the early detection of PDAC may become feasible in the future.
Liquid Biopsies and Circulating Biomarkers
Several metabolic alterations follow the progression of pancreatic cancer.35,62,125 Thus, circulating elements that may be derived from precursor lesions or pancreatic cancers are of interest as genomic and proteomic biomarkers,8,126-128 as well as circulating cancer cells (CTCs) and exosomes,58 and cell free DNA (cfDNA).129-136 Such biomarkers have been used to demonstrate the ability to detect several cancer types,137 with ability to diagnose at an early stage for when resection is possible.138 One such biomarker test called CancerSEEK was designed detect early-stage cancers across anatomical locations (not specific for pancreatic cancer, but including PDAC), through assessment of the levels of circulating proteins and mutations in cell-free DNA138 and demonstrated ability for early detection. Others have looked into multi-biomarker panels, adding CA 19-9 to the panel of markers in order to increasing the diagnostic accuracy.139 While promising, none have reached a routine clinical implementation as it stands. A large meta-analysis140 of all available studies included 19 studies at the time, with some 1872 individuals. The studies were designed to explore liquid biopsies for diagnosis of PDAC.140 Seven of the cohorts found were studies on ctDNA, 7 were on CTCs and 6 were investigating exosomes. The overall sensitivity, specificity and AUC of the sROC curve for overall liquid biopsy in detecting PDAC were .80 (95% CI 0.77-.82), .89 (95% CI 0.87-.91) and .95, respectively.140 The AUC is not very good and, better diagnostic accuracy is clearly needed.141,142 The meta-analysis140 confirmed that liquid biopsy had high diagnostic value in detecting PDAC, with exosomes showed highest sensitivity and specificity.140 Possibly, such biomarkers and improved technology may have the potential to change early cancer detection in the future, yet further work is needed before implementation as a routine screening or diagnostic tool.
Conclusion
Several barriers to early detection of PDAC remain but developments in novel technology and new fields of research are providing opportunities for improvement. Some of the reported biomarkers, technology and reported accuracy for detection may see translation routine clinical use if validated and robust data on clinical cohort can confirm the diagnostic performance in average-risk or specific high-risk populations. In order to reduce the number of untimely deaths from this dreaded disease, more effective and specific biomarkers for patients having early‐stage pancreatic cancer is needed. Only then may we experience earlier diagnosis and detection at a curable stage allowing for appropriate surgery and multimodal management.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Helse Vest RHF, Western Norway Regional Health Authority F-12625.
ORCID iD
Kjetil Søreide https://orcid.org/0000-0001-7594-4354
References
- 1.Pourshams A, Sepanlou SG, Ikuta KS, et al. The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2019;4:934-947. doi: 10.1016/s2468-1253(19)30347-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roalsø M, Aunan JR, Søreide K. Refined TNM-staging for pancreatic adenocarcinoma - real progress or much ado about nothing? Eur J Surg Oncol. 2020;46:1554-1557. doi: 10.1016/j.ejso.2020.02.014 [DOI] [PubMed] [Google Scholar]
- 3.Søreide K. Early diagnosis of sporadic pancreatic cancer. In: Søreide K, Stättner S, eds. Textbook of Pancreatic Cancer: Principles and Practice of Surgical Oncology. Cham: Springer International Publishing; 2021:339-356. [Google Scholar]
- 4.Huang BZ, Liu L, Zhang J, et al. Rising incidence and racial disparities of early-onset pancreatic cancer in the United States, 1995-2018. Gastroenterology. 2022;163:310-312. doi: 10.1053/j.gastro.2022.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blackford AL, Canto MI, Klein AP, Hruban RH, Goggins M. Recent trends in the incidence and survival of stage 1A pancreatic cancer: A surveillance, epidemiology, and end results analysis. J Natl Cancer Inst. 2020;112:1162-1169. doi: 10.1093/jnci/djaa004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Exarchakou A, Papacleovoulou G, Rous B, et al. Pancreatic Cancer Incidence and Survival and the Role of Specialist Centres in Resection Rates in England, 2000 to 2014: A Population-Based Study. Pancreatology. 2020;20(3):454-461. doi: 10.1016/j.pan.2020.01.012 [DOI] [PubMed] [Google Scholar]
- 7.Kanno A, Masamune A, Hanada K, et al. Multicenter study of early pancreatic cancer in Japan. Pancreatology. 2018;18:61-67. doi: 10.1016/j.pan.2017.11.007 [DOI] [PubMed] [Google Scholar]
- 8.Tonini V, Zanni M. Early diagnosis of pancreatic cancer: What strategies to avoid a foretold catastrophe. World J Gastroenterol. 2022;28:4235-4248. doi: 10.3748/wjg.v28.i31.4235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bretthauer M, Kalager M. Principles, effectiveness and caveats in screening for cancer. Br J Surg. 2013;100:55-65. doi: 10.1002/bjs.8995 [DOI] [PubMed] [Google Scholar]
- 10.Andermann A, Blancquaert I, Beauchamp S, et al. Revisiting Wilson and Jungner in the genomic age: A review of screening criteria over the past 40 years. Bull World Health Organ. 2008;86:317-319. doi: 10.2471/blt.07.050112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basturk O, Hong SM, Wood LD, et al. A revised classification system and recommendations from the baltimore consensus meeting for neoplastic precursor lesions in the pancreas. Am J Surg Pathol. 2015;39:1730-1741. doi: 10.1097/pas.0000000000000533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hata T, Suenaga M, Marchionni L, et al. Genome-wide somatic copy number alterations and mutations in high-grade pancreatic intraepithelial neoplasia. Am J Pathol. 2018;188:1723-1733. doi: 10.1016/j.ajpath.2018.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Overbeek KA, Goggins MG, Dbouk M, et al. Timeline of development of pancreatic cancer and implications for successful early detection in high-risk individuals. Gastroenterology. 2022;162:772-785.e774. doi: 10.1053/j.gastro.2021.10.014 [DOI] [PubMed] [Google Scholar]
- 14.Canto MI, Goggins M, Hruban RH, et al. Screening for early pancreatic neoplasia in high-risk individuals: A prospective controlled study. Clin Gastroenterol Hepatol. 2006;4:766-781. quiz 665. 20060506. doi: 10.1016/j.cgh.2006.02.005 [DOI] [PubMed] [Google Scholar]
- 15.Brune K, Abe T, Canto M, et al. Multifocal neoplastic precursor lesions associated with lobular atrophy of the pancreas in patients having a strong family history of pancreatic cancer. Am J Surg Pathol. 2006;30:1067-1076. [PMC free article] [PubMed] [Google Scholar]
- 16.Søreide K, Marchegiani G. Clinical management of pancreatic premalignant lesions. Gastroenterology. 2022;162:379-384. doi: 10.1053/j.gastro.2021.09.073 [DOI] [PubMed] [Google Scholar]
- 17.Ugai T, Sasamoto N, Lee HY, et al. Is early-onset cancer an emerging global epidemic? Current evidence and future implications. Nat Rev Clin Oncol. 2022;19:65620220906-65620221673. doi: 10.1038/s41571-022-00672-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goggins M. Circulating biomarkers to identify patients with resectable pancreatic cancer. J Natl Cancer Inst. 2017;109:djx004. doi: 10.1093/jnci/djx004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waleleng BJ, Adiwinata R, Wenas NT, et al. Screening of pancreatic cancer: Target population, optimal timing and how? Ann Med Surg (Lond). 2022;84:10481420221105. doi: 10.1016/j.amsu.2022.104814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koopmann BDM, Omidvari AH, Lansdorp-Vogelaar I, Cahen DL, Bruno MJ, de Kok IMCM. The impact of pancreatic cancer screening on life expectancy: A systematic review of modeling studies. Int J Cancer 2022. doi: 10.1002/ijc.34379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Draus T, Ansari D, Andersson R. Model-based screening for pancreatic cancer in Sweden. Scand J Gastroenterol 2022;120221128. doi: 10.1080/00365521.2022.2142481 [DOI] [PubMed] [Google Scholar]
- 22.Takeda Y, Saiura A, Takahashi Y, et al. Asymptomatic pancreatic cancer: Does incidental detection impact long-term outcomes? J Gastrointest Surg. 2017;21:1287-1295. doi: 10.1007/s11605-017-3421-2 [DOI] [PubMed] [Google Scholar]
- 23.Moutinho-Ribeiro P, Coelho R, Giovannini M, Macedo G. Pancreatic cancer screening: Still a delusion? Pancreatology. 2017;17:754-765. doi: 10.1016/j.pan.2017.07.001 [DOI] [PubMed] [Google Scholar]
- 24.Torphy RJ, Schulick RD. Screening of patients at risk for familial pancreatic cancer: What is bene fi cial? Surg Clin North Am. 2018;98:25-35. doi: 10.1016/j.suc.2017.09.003 [DOI] [PubMed] [Google Scholar]
- 25.Henrikson NB, Aiello Bowles EJ, Blasi PR, et al. Screening for pancreatic cancer: Updated evidence report and systematic review for the US preventive services task force. JAMA. 2019;322:445-454. doi: 10.1001/jama.2019.6190 [DOI] [PubMed] [Google Scholar]
- 26.Owens DK, Davidson KW, Krist AH, et al. Screening for pancreatic cancer: US preventive services task force reaffirmation recommendation statement. JAMA. 2019;322:438-444. doi: 10.1001/jama.2019.10232 [DOI] [PubMed] [Google Scholar]
- 27.Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic adenocarcinoma, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15:1028-1061. doi: 10.6004/jnccn.2017.0131 [DOI] [PubMed] [Google Scholar]
- 28.Ducreux M, Cuhna AS, Caramella C, et al. Cancer of the pancreas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(suppl 5):v56-v68. doi: 10.1093/annonc/mdv295 [DOI] [PubMed] [Google Scholar]
- 29.Hartwell L, Mankoff D, Paulovich A, Ramsey S, Swisher E. Cancer biomarkers: A systems approach. Nat Biotechnol. 2006;24:905-908. doi: 10.1038/nbt0806-905 [DOI] [PubMed] [Google Scholar]
- 30.Ghatnekar O, Andersson R, Svensson M, et al. Modelling the benefits of early diagnosis of pancreatic cancer using a biomarker signature. Int J Cancer. 2013;133:2392-2397. doi: 10.1002/ijc.28256 [DOI] [PubMed] [Google Scholar]
- 31.Joergensen MT, Gerdes AM, Sorensen J, Schaffalitzky de Muckadell O, Mortensen MB. Is screening for pancreatic cancer in high-risk groups cost-effective? - experience from a Danish national screening program. Pancreatology. 2016;16:584-592. doi: 10.1016/j.pan.2016.03.013 [DOI] [PubMed] [Google Scholar]
- 32.Corral JE, Das A, Bruno MJ, Wallace MB. Cost-effectiveness of pancreatic cancer surveillance in high-risk individuals: An economic analysis. Pancreas. 2019;48:526-536. doi: 10.1097/mpa.0000000000001268 [DOI] [PubMed] [Google Scholar]
- 33.Lu C, Xu CF, Wan XY, et al. Screening for pancreatic cancer in familial high-risk individuals: A systematic review. World J Gastroenterol. 2015;21:8678-8686. doi: 10.3748/wjg.v21.i28.8678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corral JE, Mareth KF, Riegert-Johnson DL, Das A, Wallace MB. Diagnostic yield from screening asymptomatic individuals at high risk for pancreatic cancer: A meta-analysis of cohort studies. Clin Gastroenterol Hepatol. 2019;17:41-53. doi: 10.1016/j.cgh.2018.04.065 [DOI] [PubMed] [Google Scholar]
- 35.Soreide K. Sweet predictions speak volumes for early detection of pancreatic cancer. Gastroenterology. 2018;155:265-268. doi: 10.1053/j.gastro.2018.06.054 [DOI] [PubMed] [Google Scholar]
- 36.Singhi AD, Koay EJ, Chari ST, Maitra A. Early detection of pancreatic cancer: Opportunities and challenges. Gastroenterology. 2019;156:202420190202-202420192040. doi: 10.1053/j.gastro.2019.01.259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nicholson BD, Hamilton W, O’Sullivan J, Aveyard P, Hobbs FR. Weight loss as a predictor of cancer in primary care: A systematic review and meta-analysis. Br J Gen Pract. 2018;68:e311-e322. doi: 10.3399/bjgp18X695801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharma A, Chari ST. Pancreatic cancer and diabetes mellitus. Curr Treat Options Gastroenterol. 2018;16:466-478. doi: 10.1007/s11938-018-0197-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Risch HA, Yu H, Lu L, Kidd MS. Detectable symptomatology preceding the diagnosis of pancreatic cancer and absolute risk of pancreatic cancer diagnosis. Am J Epidemiol. 2015;182:26-34. doi: 10.1093/aje/kwv026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klein AP, Lindstrom S, Mendelsohn JB, et al. An absolute risk model to identify individuals at elevated risk for pancreatic cancer in the general population. PLoS One. 2013;8:e72311. doi: 10.1371/journal.pone.0072311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boursi B, Finkelman B, Giantonio BJ, et al. A clinical prediction model to assess risk for pancreatic cancer among patients with new-onset diabetes. Gastroenterology. 2017;152:840-850. doi: 10.1053/j.gastro.2016.11.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sharma A, Kandlakunta H, Nagpal SJS, et al. Model to determine risk of pancreatic cancer in patients with new-onset diabetes. Gastroenterology. 2018;155:730-739. doi: 10.1053/j.gastro.2018.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toft J, Hadden WJ, Laurence JM, et al. Imaging modalities in the diagnosis of pancreatic adenocarcinoma: A systematic review and meta-analysis of sensitivity, specificity and diagnostic accuracy. Eur J Radiol. 2017;92:17-23. doi: 10.1016/j.ejrad.2017.04.009 [DOI] [PubMed] [Google Scholar]
- 44.Li XZ, Song J, Sun ZX, Yang YY, Wang H. Diagnostic performance of contrast-enhanced ultrasound for pancreatic neoplasms: A systematic review and meta-analysis. Dig Liver Dis. 2018;50:132-138. doi: 10.1016/j.dld.2017.10.012 [DOI] [PubMed] [Google Scholar]
- 45.Canto MI, Almario JA, Schulick RD, et al. Risk of neoplastic progression in individuals at high risk for pancreatic cancer undergoing long-term surveillance. Gastroenterology. 2018;155:740-751. doi: 10.1053/j.gastro.2018.05.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aunan JR, Al-Saiddi MS, Stutchfield B, et al. Pancreatic cystic lesions and risk of cancer. In: Søreide K, Stättner S, eds. Textbook of Pancreatic Cancer: Principles and Practice of Surgical Oncology. Cham: Springer International Publishing; 2021:777-797. [Google Scholar]
- 47.Aunan JR, Jamieson NB, Søreide K. Observation or resection of pancreatic intraductal papillary mucinous neoplasm: An ongoing tug of war. World J Gastrointest Oncol. 2019;11:1092-1100. doi: 10.4251/wjgo.v11.i12.1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marchegiani G, Salvia R, Stefano A, et al. Guidelines on pancreatic cystic neoplasms: Major inconsistencies with available evidence and clinical practice- results from an international survey. Gastroenterology. 2021;160:2234-2238. doi: 10.1053/j.gastro.2021.02.026 [DOI] [PubMed] [Google Scholar]
- 49.Coban S, Basar O, Brugge WR. Pancreatic cystic neoplasms. Gastroenterol Clin North Am. 2022;51:537-559. doi: 10.1016/j.gtc.2022.06.008 [DOI] [PubMed] [Google Scholar]
- 50.Aziz H, Acher AW, Krishna SG, Cloyd JM, Pawlik TM. Comparison of society guidelines for the management and surveillance of pancreatic cysts: A review. JAMA Surg. 2022;157:723-730. doi: 10.1001/jamasurg.2022.2232 [DOI] [PubMed] [Google Scholar]
- 51.Gaiser RA, Pessia A, Ateeb Z, et al. Integrated targeted metabolomic and lipidomic analysis: A novel approach to classifying early cystic precursors to invasive pancreatic cancer. Sci Rep. 2019;9:10208. doi: 10.1038/s41598-019-46634-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yip-Schneider MT, Soufi M, Carr RA, et al. Performance of candidate urinary biomarkers for pancreatic cancer - correlation with pancreatic cyst malignant progression? Am J Surg. 2019;219:492-495. doi: 10.1016/j.amjsurg.2019.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boyd LNC, Ali M, Leeflang MMG, et al. Diagnostic accuracy and added value of blood-based protein biomarkers for pancreatic cancer: A meta-analysis of aggregate and individual participant data. EClinicalMedicine. 2023;55:10174720221124. doi: 10.1016/j.eclinm.2022.101747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu H, Ou S, Zhang H, et al. Advances in biomarkers and techniques for pancreatic cancer diagnosis. Cancer Cell Int. 2022;22:220-2022. doi: 10.1186/s12935-022-02640-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wheatley RC, Valle JW, McNamara MG. The microbiome as a potential diagnostic biomarker for pancreatic ductal adenocarcinoma (PDAC). Hepatobiliary Surg Nutr. 2022;11:752-754. doi: 10.21037/hbsn-22-380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roalsø MTT, Hald Ø H, Alexeeva M, Soreide K. Emerging role of epigenetic alterations as biomarkers and novel targets for treatments in pancreatic ductal adenocarcinoma. Cancers. 2022;14:546. doi: 10.3390/cancers14030546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nesteruk K, Levink IJM, de Vries E, et al. Extracellular vesicle-derived microRNAs in pancreatic juice as biomarkers for detection of pancreatic ductal adenocarcinoma. Pancreatology. 2022;22:626-635. doi: 10.1016/j.pan.2022.04.010 [DOI] [PubMed] [Google Scholar]
- 58.Nakamura K, Zhu Z, Roy S, et al. An exosome-based transcriptomic signature for noninvasive, early detection of patients with pancreatic ductal adenocarcinoma: A multicenter cohort study. Gastroenterology. 2022;163:1252-1266. doi: 10.1053/j.gastro.2022.06.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koopaie M, Kolahdooz S, Fatahzadeh M, Aleedawi Z. Salivary noncoding RNA in the diagnosis of pancreatic cancer: Systematic review and meta-analysis. Eur J Clin Invest. 2022;52:e1384820220812. doi: 10.1111/eci.13848 [DOI] [PubMed] [Google Scholar]
- 60.Jia E, Ren N, Shi X, et al. Extracellular vesicle biomarkers for pancreatic cancer diagnosis: A systematic review and meta-analysis. BMC Cancer. 2022;22:573-2022. doi: 10.1186/s12885-022-09463-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Debernardi S, Blyuss O, Rycyk D, et al. Urine biomarkers enable pancreatic cancer detection up to 2 years before diagnosis. Int J Cancer. 2022;152:769-780. doi: 10.1002/ijc.34287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hanna-Sawires RG, Schiphuis JH, Wuhrer M, et al. Clinical perspective on proteomic and glycomic biomarkers for diagnosis, prognosis, and prediction of pancreatic cancer. Int J Mol Sci. 2021;22:22. doi: 10.3390/ijms22052655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Young MR, Wagner PD, Ghosh S, et al. Validation of biomarkers for early detection of pancreatic cancer: Summary of the alliance of pancreatic cancer consortia for biomarkers for early detection workshop. Pancreas. 2018;47:135-141. doi: 10.1097/mpa.0000000000000973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fahrmann JF, Schmidt CM, Mao X, et al. Lead-time trajectory of CA19-9 as an anchor marker for pancreatic cancer early detection. Gastroenterology. 2021;160:1373-1383. doi: 10.1053/j.gastro.2020.11.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Modi S, Kir D, Saluja AK. Old dog, new tricks: Use of CA 19-9 for early diagnosis of pancreatic cancer. Gastroenterology. 2021;160:1019-1021. doi: 10.1053/j.gastro.2021.01.001 [DOI] [PubMed] [Google Scholar]
- 66.Sharma A, Smyrk TC, Levy MJ, Topazian MA, Chari ST. Fasting blood glucose levels provide estimate of duration and progression of pancreatic cancer before diagnosis. Gastroenterology. 2018;155:490-500. doi: 10.1053/j.gastro.2018.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liao WC, Tu YK, Wu MS, Lin JT, Wang HP, Chien KL. Blood glucose concentration and risk of pancreatic cancer: Systematic review and dose-response meta-analysis. Bmj. 2015;350:g7371. doi: 10.1136/bmj.g7371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moore HB, Culp-Hill R, Reisz JA, et al. The metabolic time line of pancreatic cancer: Opportunities to improve early detection of adenocarcinoma. Am J Surg. 2019;218:1206-1212. doi: 10.1016/j.amjsurg.2019.08.015 [DOI] [PubMed] [Google Scholar]
- 69.Khalaf N, Wolpin BM. Metabolic alterations as a signpost to early pancreatic cancer. Gastroenterology. 2019;156:1560-1563. doi: 10.1053/j.gastro.2019.03.028 [DOI] [PubMed] [Google Scholar]
- 70.Sah RP, Sharma A, Nagpal S, et al. Phases of metabolic and soft tissue changes in months preceding a diagnosis of pancreatic ductal adenocarcinoma. Gastroenterology. 2019;156:1742-1752. doi: 10.1053/j.gastro.2019.01.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Danai LV, Babic A, Rosenthal MH, et al. Altered exocrine function can drive adipose wasting in early pancreatic cancer. Nature. 2018;558:600-604. doi: 10.1038/s41586-018-0235-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mayers JR. Metabolic markers as cancer clues. Science. 2017;358:1265-2017. doi: 10.1126/science.aar2001 [DOI] [PubMed] [Google Scholar]
- 73.Mehta KY, Wu HJ, Menon SS, et al. Metabolomic biomarkers of pancreatic cancer: A meta-analysis study. Oncotarget. 2017;8:68899-68915. doi: 10.18632/oncotarget.20324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Katagiri R, Goto A, Nakagawa T, et al. Increased levels of branched-chain amino acid associated with increased risk of pancreatic cancer in a prospective case-control study of a large cohort. Gastroenterology. 2018;155:1474-1482. e1471. doi: 10.1053/j.gastro.2018.07.033 [DOI] [PubMed] [Google Scholar]
- 75.Mayers JR, Wu C, Clish CB, et al. Elevation of circulating branched-chain amino acids is an early event in human pancreatic adenocarcinoma development. Nat Med. 2014;20:1193-1198. doi: 10.1038/nm.3686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fest J, Vijfhuizen LS, Goeman JJ, et al. Search for early pancreatic cancer blood biomarkers in five European prospective population biobanks using metabolomics. Endocrinology. 2019;160:1731-1742. doi: 10.1210/en.2019-00165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Franklin O, Jonsson P, Billing O, et al. Plasma micro-RNA alterations appear late in pancreatic cancer. Ann Surg. 2018;267:775-781. doi: 10.1097/sla.0000000000002124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sturque J, Berquet A, Loison-Robert LS, Ahossi V, Zwetyenga N. Interest of studying the saliva metabolome, transcriptome and microbiome in screening for pancreatic cancer. J Stomatol Oral Maxillofac Surg. 2019;120:554-558. doi: 10.1016/j.jormas.2019.04.013 [DOI] [PubMed] [Google Scholar]
- 79.Asai Y, Itoi T, Sugimoto M, et al. Elevated polyamines in saliva of pancreatic cancer. Cancers. 2018;10:43. doi: 10.3390/cancers10020043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Setti G, Pezzi ME, Viani MV, et al. Salivary MicroRNA for diagnosis of cancer and systemic diseases: A systematic review. Int J Mol Sci. 2020;21:21. doi: 10.3390/ijms21030907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kartal E, Schmidt TSB, Molina-Montes E, et al. A faecal microbiota signature with high specificity for pancreatic cancer. Gut. 2022;71:135920220308-135920221372. doi: 10.1136/gutjnl-2021-324755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bax C, Lotesoriere BJ, Sironi S, Capelli. Review and comparison of cancer biomarker trends in urine as a basis for new diagnostic pathways. Cancers. 2019;11:11. doi: 10.3390/cancers11091244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nissinen SI, Roine A, Hokkinen L, et al. Detection of pancreatic cancer by urine volatile organic compound analysis. Anticancer Res. 2019;39:73-79. doi: 10.21873/anticanres.13081 [DOI] [PubMed] [Google Scholar]
- 84.Blyuss O, Zaikin A, Cherepanova V, et al. Development of pancRISK, a urine biomarker-based risk score for stratified screening of pancreatic cancer patients. Br J Cancer. 2020;122:692-696. doi: 10.1038/s41416-019-0694-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Radon TP, Massat NJ, Jones R, et al. Identification of a three-biomarker panel in urine for early detection of pancreatic adenocarcinoma. Clin Cancer Res. 2015;21:3512-3521. doi: 10.1158/1078-0432.Ccr-14-2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kalubowilage M, Covarrubias-Zambrano O, Malalasekera AP, et al. Early detection of pancreatic cancers in liquid biopsies by ultrasensitive fluorescence nanobiosensors. Nanomedicine. 2018;14:1823-1832. doi: 10.1016/j.nano.2018.04.020 [DOI] [PubMed] [Google Scholar]
- 87.Qian L, Li Q, Baryeh K, et al. Biosensors for early diagnosis of pancreatic cancer: A review. Transl Res. 2019;213:67-89. doi: 10.1016/j.trsl.2019.08.002 [DOI] [PubMed] [Google Scholar]
- 88.Carmicheal J, Patel A, Dalal V, et al. Elevating pancreatic cystic lesion stratification: Current and future pancreatic cancer biomarker(s). Biochim Biophys Acta Rev Cancer. 2020;1873:188318. doi: 10.1016/j.bbcan.2019.188318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Korn RL, Rahmanuddin S, Borazanci E. Use of precision imaging in the evaluation of pancreas cancer. Cancer Treat Res. 2019;178:209-236. doi: 10.1007/978-3-030-16391-4_8 [DOI] [PubMed] [Google Scholar]
- 90.Tabari A, Chan SM, Omar OMF, Iqbal SI, Gee MS, Daye D. Role of machine learning in precision oncology: Applications in gastrointestinal cancers. Cancers. 2022;15:20221222. doi: 10.3390/cancers15010063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang S, Lin C, Kolomaya A, et al. Compute tomography radiomics analysis on whole pancreas between healthy individual and pancreatic ductal adenocarcinoma patients: Uncertainty analysis and predictive modeling. Technol Cancer Res Treat. 2022;21:15330338221126869. doi: 10.1177/15330338221126869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rosenthal MH, Schawkat K. Beyond the AJR: CT radiomic features of the pancreas predict development of pancreatic cancer. AJR Am J Roentgenol 2022. doi: 10.2214/ajr.22.28582 [DOI] [PubMed] [Google Scholar]
- 93.Laino ME, Ammirabile A, Lofino L, et al. Artificial intelligence applied to pancreatic imaging: A narrative review. Healthcare (Basel). 2022;10:20220811. doi: 10.3390/healthcare10081511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Soliman MAS, Kelahan LC, Magnetta M, et al. A framework for harmonization of radiomics data for multicenter studies and clinical trials. JCO Clin Cancer Inform. 2022;6:e2200023. doi: 10.1200/cci.22.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Abunahel BM, Pontre B, Kumar H, Petrov MS. Pancreas image mining: A systematic review of radiomics. Eur Radiol. 2021;31:344720201105-344720203467. doi: 10.1007/s00330-020-07376-6 [DOI] [PubMed] [Google Scholar]
- 96.Barat M, Chassagnon G, Dohan A, et al. Artificial intelligence: A critical review of current applications in pancreatic imaging. Jpn J Radiol. 2021;39:51420210206-51420210523. doi: 10.1007/s11604-021-01098-5 [DOI] [PubMed] [Google Scholar]
- 97.Park S, Chu LC, Hruban RH, et al. Differentiating autoimmune pancreatitis from pancreatic ductal adenocarcinoma with CT radiomics features. Diagn Interv Imaging. 2020;101:55520200408-55520200564. doi: 10.1016/j.diii.2020.03.002 [DOI] [PubMed] [Google Scholar]
- 98.Chu LC, Park S, Kawamoto S, et al. Utility of CT radiomics features in differentiation of pancreatic ductal adenocarcinoma from normal pancreatic tissue. AJR Am J Roentgenol. 2019;213:349-357. doi: 10.2214/AJR.18.20901 [DOI] [PubMed] [Google Scholar]
- 99.Permuth JB, Choi J, Balarunathan Y, et al. Combining radiomic features with a miRNA classifier may improve prediction of malignant pathology for pancreatic intraductal papillary mucinous neoplasms. Oncotarget. 2016;7:85785-85797. doi: 10.18632/oncotarget.11768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Koay EJ, Truty MJ, Cristini V, et al. Transport properties of pancreatic cancer describe gemcitabine delivery and response. J Clin Invest. 2014;124:1525-1536. doi: 10.1172/JCI73455.20140310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Koay EJ, Lee Y, Cristini V, et al. A visually apparent and quantifiable CT imaging feature identifies biophysical subtypes of pancreatic ductal adenocarcinoma. Clin Cancer Res. 2018;24:588320180806-588320185894. doi: 10.1158/1078-0432.CCR-17-3668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nasief H, Zheng C, Schott D, et al. A machine learning based delta-radiomics process for early prediction of treatment response of pancreatic cancer. NPJ Precis Oncol. 2019;3:25-20191004. doi: 10.1038/s41698-019-0096-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mukherjee S, Patra A, Khasawneh H, et al. Radiomics-based machine-learning models can detect pancreatic cancer on prediagnostic CTs at a substantial lead time prior to clinical diagnosis. Gastroenterology. 2022;163(5):1435-1446. 10.1053/j.gastro.2022.06.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Okada T, Iwano H, Ono Y, et al. Utility of “liquid biopsy” using pancreatic juice for early detection of pancreatic cancer. Endosc Int Open. 2018;6:E1454-e1461. doi: 10.1055/a-0721-1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Takeda Y, Matsumoto K, Kurumi H, et al. Efficacy and safety of pancreatic juice cytology by using synthetic secretin in the diagnosis of pancreatic ductal adenocarcinoma. Dig Endosc. 2018;30:771-776. doi: 10.1111/den.13203 [DOI] [PubMed] [Google Scholar]
- 106.Choi MH, Mejlaender-Andersen E, Manueldas S, et al. Mutation analysis by deep sequencing of pancreatic juice from patients with pancreatic ductal adenocarcinoma. BMC Cancer. 2019;19:11. doi: 10.1186/s12885-018-5195-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nakamura S, Sadakari Y, Ohtsuka T, et al. Pancreatic juice exosomal microRNAs as biomarkers for detection of pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2019;26:2104-2111. doi: 10.1245/s10434-019-07269-z [DOI] [PubMed] [Google Scholar]
- 108.Kuwatani M, Sakamoto N. Pathological and molecular diagnoses of early cancer with bile and pancreatic juice. Dig Endosc. 2022;34:1340-1355. doi: 10.1111/den.14348 [DOI] [PubMed] [Google Scholar]
- 109.Suzuki R, Thosani N, Annangi S, et al. Diagnostic yield of endoscopic retrograde cholangiopancreatography-based cytology for distinguishing malignant and benign intraductal papillary mucinous neoplasm: Systematic review and meta-analysis. Dig Endosc. 2014;26:586-593. doi: 10.1111/den.12230 [DOI] [PubMed] [Google Scholar]
- 110.Glomsaker T, Hoff G, Kvaløy JT, Soreide K, Aabakken L, Soreide JA. Patterns and predictive factors of complications after endoscopic retrograde cholangiopancreatography. Br J Surg. 2013;100:373-380. doi: 10.1002/bjs.8992 [DOI] [PubMed] [Google Scholar]
- 111.Hanada K, Minami T, Shimizu A, et al. Roles of ERCP in the early diagnosis of pancreatic cancer. Diagnostics (Basel). 2019;9:20190307. doi: 10.3390/diagnostics9010030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ishikawa-Kakiya Y, Maruyama H, Kinoshita Y, et al. The usefulness of serial pancreatic juice aspiration cytological examination for pancreatic cancer not diagnosed by EUS-FNAB. Clin J Gastroenterol. 2020;13:136720200629-136720201372. doi: 10.1007/s12328-020-01167-8 [DOI] [PubMed] [Google Scholar]
- 113.Hanada K, Shimizu A, Kurihara K, et al. Endoscopic approach in the diagnosis of high-grade pancreatic intraepithelial neoplasia. Dig Endosc. 2022;34:92720220214-92720220937. doi: 10.1111/den.14240 [DOI] [PubMed] [Google Scholar]
- 114.Kitagawa K, Mitoro A, Tomooka F, et al. Diagnostic yield of liquid-based cytology in serial pancreatic juice aspiration cytological examination. DEN Open. 2023;3:e177. doi: 10.1002/deo2.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Haeberle L, Schramm M, Goering W, et al. Molecular analysis of cyst fluids improves the diagnostic accuracy of pre-operative assessment of pancreatic cystic lesions. Sci Rep. 2021;11:2901-20210203. doi: 10.1038/s41598-021-81065-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rift CV, Melchior LC, Kovacevic B, et al. Targeted next-generation sequencing of EUS-guided through-the-needle-biopsy sampling from pancreatic cystic lesions. Gastrointest Endosc. 2023;97:50-58. e54. 20220812. doi: 10.1016/j.gie.2022.08.008 [DOI] [PubMed] [Google Scholar]
- 117.Singhi AD, McGrath K, Brand RE, et al. Preoperative next-generation sequencing of pancreatic cyst fluid is highly accurate in cyst classification and detection of advanced neoplasia. Gut. 2018;67:213120170928-213120172141. doi: 10.1136/gutjnl-2016-313586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Paniccia A, Polanco PM, Boone BA, et al. Prospective, multi-institutional, real-time next-generation sequencing of pancreatic cyst fluid reveals diverse genomic alterations that improve the clinical management of pancreatic cysts. Gastroenterology. 2023;164:117-133. e117. doi: 10.1053/j.gastro.2022.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sinha J, Cao Z, Dai J, et al. A gastric glycoform of MUC5AC Is a biomarker of mucinous cysts of the pancreas. PLoS One. 2016;11:e016707020161219. doi: 10.1371/journal.pone.0167070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sammallahti H, Sarhadi VK, Kokkola A, et al. Oncogenomic changes in pancreatic cancer and their detection in stool. Biomolecules. 2022;12:20220429. doi: 10.3390/biom12050652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Half E, Keren N, Reshef L, et al. Fecal microbiome signatures of pancreatic cancer patients. Sci Rep. 2019;9:16801-20191114. doi: 10.1038/s41598-019-53041-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mendez R, Kesh K, Arora N, et al. Microbial dysbiosis and polyamine metabolism as predictive markers for early detection of pancreatic cancer. Carcinogenesis. 2020;41:561-570. doi: 10.1093/carcin/bgz116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sammallahti H, Kokkola A, Rezasoltani S, et al. Microbiota alterations and their association with oncogenomic changes in pancreatic cancer patients. Int J Mol Sci. 2021;22:22. doi: 10.3390/ijms222312978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Newsome R, Jobin C. Finding clues in unexpected places: detection of pancreatic cancer through the faecal microbiome. Gut. 2022;71:124720220308-124720221248. doi: 10.1136/gutjnl-2021-326710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hu T, Shukla SK, Vernucci E, et al. Metabolic rewiring by loss of sirt5 promotes kras-induced pancreatic cancer progression. Gastroenterology. 2021;161:1584-1600. doi: 10.1053/j.gastro.2021.06.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Watanabe F, Suzuki K, Noda H, Rikiyama T. Liquid biopsy leads to a paradigm shift in the treatment of pancreatic cancer. World J Gastroenterol. 2022;28:6478-6496. doi: 10.3748/wjg.v28.i46.6478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Agarwal D, Covarrubias-Zambrano O, Bossmann SH, Natarajan B. Early detection of pancreatic cancers using liquid biopsies and hierarchical decision structure. IEEE J Transl Eng Health Med. 2022;10:4300208-4302022. doi: 10.1109/jtehm.2022.3186836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kaur S, Jain M, Batra SK. Liquid biopsy for identification of high-risk cystic lesions of pancreas. Gastroenterology. 2021;160:1016-1018. doi: 10.1053/j.gastro.2020.12.039 [DOI] [PubMed] [Google Scholar]
- 129.Miranda-Castro R, de-Los-Santos-Alvarez N, Lobo-Castanon MJ. Long noncoding RNAs: From genomic junk to rising stars in the early detection of cancer. Anal Bioanal Chem. 2019;411:4265-4275. doi: 10.1007/s00216-019-01607-6 [DOI] [PubMed] [Google Scholar]
- 130.Gao Z, Jiang W, Zhang S, Li P. The state of the art on blood microRNAs in pancreatic ductal adenocarcinoma. Anal Cell Pathol (Amst). 2019;2019:1-7. doi: 10.1155/2019/9419072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li G, Tang W, Yang F. Cancer liquid biopsy using integrated microfluidic exosome analysis platforms. Biotechnol J. 2020;15:e1900225. doi: 10.1002/biot.201900225 [DOI] [PubMed] [Google Scholar]
- 132.Nordgard O, Tjensvoll K, Gilje B, Soreide K. Circulating tumour cells and DNA as liquid biopsies in gastrointestinal cancer. Br J Surg. 2018;105:e110-e120. doi: 10.1002/bjs.10782 [DOI] [PubMed] [Google Scholar]
- 133.Kamyabi N, Bernard V, Maitra A. Liquid biopsies in pancreatic cancer. Expert Rev Anticancer Ther. 2019;19:869-878. doi: 10.1080/14737140.2019.1670063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Locke WJ, Guanzon D, Ma C, et al. DNA methylation cancer biomarkers: Translation to the clinic. Front Genet. 2019;10:1150. doi: 10.3389/fgene.2019.01150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Loft M, Lee B, Tie J, Gibbs P. Clinical applications of circulating tumour DNA in pancreatic adenocarcinoma. J Pers Med. 2019;9:37. doi: 10.3390/jpm9030037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lee JS, Park SS, Lee YK, Norton JA, Jeffrey SS. Liquid biopsy in pancreatic ductal adenocarcinoma: Current status of circulating tumor cells and circulating tumor DNA. Mol Oncol. 2019;13:1623-1650. doi: 10.1002/1878-0261.12537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shen SY, Singhania R, Fehringer G, et al. Sensitive tumour detection and classification using plasma cell-free DNA methylomes. Nature. 2018;563:579-583. doi: 10.1038/s41586-018-0703-0 [DOI] [PubMed] [Google Scholar]
- 138.Cohen JD, Li L, Wang Y, et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science. 2018;359:926-930. doi: 10.1126/science.aar3247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Berger AW, Schwerdel D, Reinacher-Schick A, et al. A blood-based multi marker assay supports the differential diagnosis of early-stage pancreatic cancer. Theranostics. 2019;9:1280-1287. doi: 10.7150/thno.29247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhu Y, Zhang H, Chen N, Hao J, Jin H, Ma X. Diagnostic value of various liquid biopsy methods for pancreatic cancer: A systematic review and meta-analysis. Medicine (Baltimore). 2020;99:e18581. doi: 10.1097/md.0000000000018581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Søreide K. Receiver-operating characteristic curve analysis in diagnostic, prognostic and predictive biomarker research. J Clin Pathol. 2009;62:1-5. doi: 10.1136/jcp.2008.061010 [DOI] [PubMed] [Google Scholar]
- 142.Søreide K, Kørner H, Søreide JA. Diagnostic accuracy and receiver-operating characteristics curve analysis in surgical research and decision making. Ann Surg. 2011;253:27-34. doi: 10.1097/sla.0b013e318204a892 [DOI] [PubMed] [Google Scholar]