Abstract
The cyclic AMP (cAMP)-dependent protein kinase, PKA, is dispensable for growth of Dictyostelium cells but plays a variety of crucial roles in development. The catalytic subunit of PKA is inhibited when associated with its regulatory subunit but is activated when cAMP binds to the regulatory subunit. Deletion of pkaR or overexpression of the gene encoding the catalytic subunit, pkaC, results in constitutive activity. Development is independent of cAMP in strains carrying these genetic alterations and proceeds rapidly to the formation of both spores and stalk cells. However, morphogenesis is aberrant in these mutants. In the wild type, PKA activity functions in a circuit that can spontaneously generate pulses of cAMP necessary for long-range aggregation. It is also essential for transcriptional activation of both prespore and prestalk genes during the slug stage. During culmination, PKA functions in both prespore and prestalk cells to regulate the relative timing of terminal differentiation. A positive feedback loop results in the rapid release of a signal peptide, SDF-2, when prestalk cells are exposed to low levels of SDF-2. The signal transduction pathway that mediates the response to SDF-2 in both prestalk and prespore cells involves the two-component system of DhkA and RegA. When the cAMP phosphodiesterase RegA is inhibited, cAMP accumulates and activates PKA, leading to vacuolation of stalk cells and encapsulation of spores. These studies indicate that multiple inputs regulate PKA activity to control the relative timing of differentiations in Dictyostelium.
Cellular differentiation does not happen all at once but goes through a series of stages that have to be carefully timed to result in the proper tissues and structures. In multicellular organisms where organs are generated from different cell types the processes have to be temporally coordinated among diverse cells. Often one cell type cannot proceed to the next stage in differentiation until a signal is received from adjacent cells. While there are multiple signal transduction pathways leading from surface receptors, many of them utilize the cyclic AMP (cAMP)-dependent protein kinase, PKA, as a central component. This highly conserved protein kinase is able to phosphorylate a variety of proteins and thereby affect their activity. However, PKA is held in an inactive form by association of the catalytic subunit (PKA-C) with its regulatory subunit (PKA-R). Only when the internal concentration of cAMP in the cell exceeds 100 nM is PKA-C active. The regulatory subunit then binds cAMP at two sites and dissociates from the catalytic subunit. The primary amino acid sequences of both the regulatory and the catalytic subunits are highly conserved among diverse organisms, and the basic enzymatic properties of PKA appear to be similar no matter where it is found (109). In metazoans the holoenzyme consists of two copies of both the catalytic subunit and the regulatory subunit, while in Dictyostelium PKA is found as a heterodimer with a single catalytic subunit bound to a regulatory subunit that lacks a dimerization domain (105, 109).
A wide range of developmental processes in Drosophila, vertebrates, and Dictyostelium are mediated by PKA. In both Drosophila and zebra fish, PKA functions in signal transduction pathways initiated by the hedgehog intercellular signalling protein (34, 45, 58, 62, 80). Moreover, PKA activity plays a central role in learning and memory in both flies and mammals (2, 106). Flies in which the structural gene for PKA (DC0) is mutated are learning impaired (21, 47, 106). Likewise, mutants with defects in either the gene responsible for the synthesis of cAMP (rutabaga) (60, 64) or that encoding a cAMP-specific phosphodiesterase (dunce) (9, 16, 22) have memory defects. Evidence that PKA is essential for long-term potentiation and memory in mammals came from studies with transgenic mice that express a modified gene, R(AB), that encodes a PKA-R protein which is unable to bind cAMP at either of its two sites as the result of site-directed mutations (2). Expression of this dominant-negative gene under the control of the Ca2+/calmodulin protein kinase IIa regulatory region in the hippocampus results in mice with defects in spatial and long-term memory. Although the pathways that establish long-term potentiation shortly after a neuron is exposed to a train of neurotransmitter pulses are not well understood, it appears that cAMP activation of PKA plays a central role. This is probably only the “tip of the iceberg” of roles that PKA plays in metazoans. In Dictyostelium, PKA plays multiple roles throughout development affecting chemotactic aggregation, prespore and prestalk differentiations, and terminal differentiation (35–37, 75, 81, 104).
PKA
Both PKA-C and PKA-R are present at low levels in exponentially growing cells of Dictyostelium (59, 82, 94). However, PKA is not needed for growth, as shown by the fact that strains carrying null mutations in the structural genes for either of the subunits, pkaC or pkaR, have been isolated and found to grow well in defined medium (72, 105). This greatly facilitates the analysis of PKA mutants since amoebae can grow indefinitely as single cells as long as there is an adequate source of food. Growth stops when the nutrients in the environment are exhausted and there is no further replication of the chromosomes until a fresh food source is found (101). If the cell density is sufficiently high, they aggregate into groups of up to 105 cells within about 10 h and then proceed to build a fruiting body in which spores are held up on a thin, tapering stalk, from where they may be better dispersed.
Both the PKA-R and PKA-C subunits accumulate at least fourfold during the first 12 h of development and then remain at that level until culmination (59). The level of the regulatory subunit was estimated from the amount of photoaffinity-labelled 41-kDa protein formed following addition of 8N3-cAMP to the soluble protein fraction, while the level of the catalytic subunit was measured by Kemptide phosphorylation activity after the addition of 1 μM cAMP to partially purified soluble proteins. The accumulation of the regulatory subunit during development was confirmed with specific antibodies to stain Western blots of electrophoretically separated proteins (82). The levels of mRNA from both pkaC and pkaR increase about fivefold during the first 6 h of development and then remain essentially constant throughout development (72, 78, 94). Thus, transcriptional control of these genes appears to be coordinate and can account for the accumulation of the subunits. If the catalytic subunit accumulated more than the regulatory subunit, some of the PKA activity would be expected to be cAMP independent. However, there is no evidence for differential accumulation of either subunit during aggregation or in the two major cell types at the slug stage (93, 112). On the other hand, cells of a strain transformed with a construct that results in artificially high levels of the regulatory subunit fail to aggregate, suggesting that the catalytic subunit is always inhibited in these cells (104). Likewise, cells of a strain carrying multiple copies of pkaC resulting in fivefold overaccumulation of PKA develop in the same abnormal manner as cells carrying null mutations in pkaR, suggesting that they have constitutively active PKA (5). Cells of these strains develop much more rapidly than wild-type cells, forming spores in as little as 16 h. However, the fruiting bodies are misshapen: the stalks are short and fat, and many of the spores are left at the base. Very little cAMP accumulates intracellularly during aggregation in these strains (1, 5, 105).
TIMING OF EARLY DEVELOPMENTAL EVENTS
Unlike metazoans that develop from a mass of cells generated from a fertilized egg, Dictyostelium has to bring dispersed single cells together before initiating multicellular development. A highly sophisticated network has evolved in Dictyostelium to result in intercellular signalling by pulses of cAMP coupled to a chemotactic response (19, 20, 25, 32, 55). The enzyme responsible for the synthesis of cAMP from ATP, adenylyl cyclase, rapidly accumulates during the first 8 h of development and is activated when cAMP binds to a specific cell surface receptor, CAR1 (53). acaA, the gene encoding adenylyl cyclase, can be induced within 2 h by artificially stimulating the cells with pulses of 20 nM cAMP (76, 83). The signal transduction pathway that leads from cell surface binding of cAMP to accumulation of acaA mRNA appears to act through PKA since acaA is not expressed at all in pkaC-null cells (76).
The 2-kb CAR1 mRNA is not present in growing cells but accumulates within the first few hours of development even in the absence of external cAMP pulses (53, 72, 91). Moreover, it accumulates in pkaC-null cells, indicating that PKA is not essential for induction of the gene, carA, encoding this cell surface cAMP receptor (72). Later in development, carA is transcribed from more-distal promoters, and the larger mRNAs accumulate to high levels only when the cells are exposed to pulses of cAMP. These later transcripts do not appear in pkaC-null cells whether or not they are treated with pulses of cAMP (72).
CAR1 is a serpentine seven-transmembrane protein that is coupled to heterotrimeric G proteins in a manner similar to the β-adrenergic receptor of vertebrate nerves (53, 91). When cAMP binds to its extracellular domain, the cytoplasmic domain is activated and facilitates the exchange of GTP for GDP bound to the Gα2 subunit (24, 81). The GTP form of Gα2 dissociates from the trimeric complex, liberating Gβγ, which can then, in conjunction with another protein, CRAC (44), activate adenylyl cyclase. Disruption of the gene encoding Gα2 results in cells that are unable to aggregate and fail to show many of the cAMP-mediated responses (57). Likewise, disruption of either of the genes encoding Gβ or CRAC results in cells that fail to activate adenylyl cyclase and, as a consequence, are unable to aggregate (44, 63). CRAC carries a PH domain that may mediate its interaction with phospholipids or other proteins. It is found in the soluble cytoplasm prior to stimulation of cells with cAMP but rapidly becomes associated with the membrane following ligand binding to CAR1 (63, 71, 81). Within a minute of addition of cAMP to the cells, the activity of adenylyl cyclase increases 5- to 10-fold to reach a peak at 90 s. Thereafter, the activity decreases with a half-life of about 2 min. This response to a pulse of cAMP results in a burst of cAMP that can relay the signal to adjacent cells. While the activation of adenylyl cyclase is fairly well understood, the subsequent inactivation is not yet fully elucidated.
The phenotype of mutants lacking the catalytic subunit or overexpressing a dominant inhibitory form of PKA-R suggests that PKA plays a central role in the timing of the burst of adenylyl cyclase activity (37, 76). Since pkaC-null cells do not transcribe the acaA gene, the consequences of the lack of PKA could be seen only if the mutant cells also carried a construct in which adenylyl cyclase was driven by a constitutive regulatory region (act15). The basal level of adenylyl cyclase activity is somewhat higher in these pkaC-null cells constitutively expressing acaA and increases to the wild-type peak level upon addition of cAMP (76). Once stimulated, adenylyl cyclase stays active for much longer in the mutant cells than in wild-type cells.
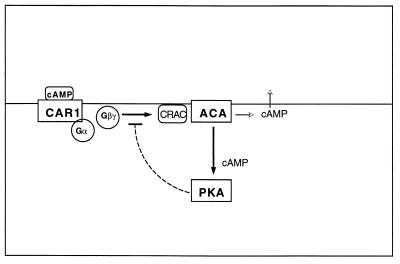
A burst of cAMP is synthesized when adenylyl cyclase is first activated. While some of the newly made cAMP is secreted to signal nearby cells, the remainder stays within the cell, where it can activate PKA by binding to the regulatory subunit (29, 31). The observations with the pkaC mutant cells indicate that, either directly or indirectly, PKA turns adenylyl cyclase off. Therefore, we would expect overexpression of pkaC or null mutations in pkaR to result in cells that accumulate little or no cAMP, and this is exactly what has been found (1, 5). PKA could regulate the enzymatic activity by phosphorylating adenylyl cyclase itself or any coupling component. By itself this feedback loop linking adenylyl cyclase and PKA can account for the timing of a single pulse of activity (Fig. 1). However, it does not explain how the system is reset such that cells can generate repeated pulses of cAMP.
FIG. 1.
Control of activity. adenylyl cyclase (ACA) ACA is activated when extracellular cAMP binds to the serpentine receptor CAR1. Ligand-bound CAR1 facilitates exchange of GTP for GDP on the Gα subunit of trimeric G protein that results in release of the Gβγ subunits. In conjunction with the cytosolic regulator of ACA, CRAC, Gβγ stimulates ACA such that it catalyzes the formation of cAMP from ATP. Some of the newly made cAMP is secreted, while the remainder is free to interact with the regulatory subunit of PKA, thereby activating PKA. PKA activity then indirectly inhibits ACA activation. Since activation of PKA depends on accumulation of cAMP, it occurs somewhat after ACA is activated. The difference between the time of activation of ACA and that of PKA determines the length of the period during which cAMP is synthesized.
Following an exogenous pulse of cAMP the cells enter a refractory period of several minutes during which adenylyl cyclase cannot be reactivated by a second pulse of cAMP unless the concentration of cAMP increases significantly (19). Thereafter, the cells recover full excitability and can relay the next signal. If PKA keeps adenylyl cyclase off, then there must be a mechanism to turn PKA itself off a few minutes after it is activated. Reassociation of the catalytic subunit with the regulatory subunit is the most obvious mechanism, but biochemical studies on PKA-R have shown that the purified subunit has such a high affinity for cAMP that it will not be able to reassociate with the catalytic subunit for days (15, 17). A solution to this paradox has recently been found in the cytoplasmic cAMP phosphodiesterase, RegA (99, 103).
regA mRNA starts to accumulate at 4 h of development, increases to a maximum by 8 h, and stays at this high level throughout the remainder of development (102). It encodes a cAMP-specific phosphodiesterase that can reduce the cAMP available to PKA-R within cells and so controls in vivo PKA activity. regA-null mutants have the same precocious phenotype as pkaR-null mutants, indicating that in the absence of RegA, PKA-C is constitutively active (99, 103). RegA activity itself appears to be negatively regulated by the protein kinase encoded by erkB (67a). Mutants carrying erkB mutations are unable to aggregate or relay cAMP signals even after they have been artificially stimulated by pulses of 100 nM cAMP for 4 h and have accumulated adenylyl cyclase (97). However, double mutants in which both erkB and regA are inactivated are able to proceed further in development and form elongated mounds (67a). These genetic studies, together with biochemical studies showing that the erkB product, ERK2, can phosphorylate RegA, suggest that ERK2 is responsible for turning RegA off. ERK2 is a member of the MAP kinase family and is transiently activated by external pulses of cAMP acting through CAR1 (8, 54, 71, 97). The activity increases rapidly for a minute and then decreases with a half-life of about 3 min. In mutant cells that lack either adenylyl cyclase or CRAC, ERK2 stays active for much longer (8, 54). PKA activity would be expected to be low in these strains since intracellular cAMP does not increase in response to the extracellular pulse. Likewise, ERK2 stays active for much longer following addition of extracellular cAMP to cells of a pkaC-null strain, indicating that PKA is essential for turning ERK2 off, possibly working through RAS and its regulatory proteins, RAS-GEF and RAS-GAP (8).
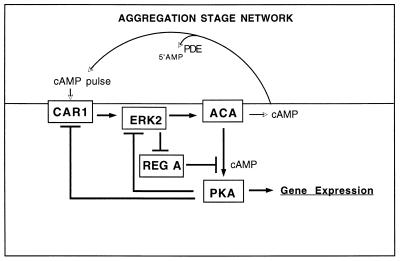
We can now consider how these interactions may control the timing of cellular responses to pulses of cAMP. For the sake of analysis, we can subsume the intermediates in each of the connections and focus on the end results (Fig. 2). External cAMP binds to CAR1 and rapidly activates ERK2, leading to the subsequent activation of adenylyl cyclase. ERK2 also inhibits RegA such that the cAMP generated by adenylyl cyclase can bind to the regulatory subunit of PKA, liberating it from the catalytic subunit. Over the next minute or so PKA activity increases and inhibits ERK2, thereby terminating the activation of adenylyl cyclase. When RegA is no longer inhibited by ERK2, it lowers the concentration of cAMP such that the regulatory subunit of PKA can reassociate with the catalytic subunit and inhibit it. The system is reset to respond to another pulse of cAMP from adjacent cells. While each of these proposed steps is supported by genetic evidence, several of the steps have yet to be demonstrated biochemically. It is also not clear whether activation of RegA that occurs when it is no longer inhibited by ERK2 requires a specialized phosphatase or a general protein phosphatase.
FIG. 2.
Signal relay stage network. Activation of CAR1 by extracellular cAMP results in a brief period during which adenylyl cyclase is active as described in the legend to Fig. 1. Either directly or indirectly, CAR1 also activates the MAP kinase, ERK2, when it binds extracellular cAMP. ERK2 inhibits the cAMP phosphodiesterase RegA while cAMP is accumulating in the cell. After PKA is activated by the cAMP produced by adenylyl cyclase, it inhibits ERK2. When RegA is no longer inhibited by ERK2, it can reduce the level of cAMP such that the regulatory subunit of PKA can associate with the catalytic subunit and inhibit it. cAMP secreted into the extracellular space is degraded by an extracellular phosphodiesterase, but some may survive to bind to CAR1. During the period when PKA is active, it can lead to an increase in the expression of genes such as acaA.
Stirred suspensions of aggregation-competent cells will spontaneously generate pulses of cAMP every 5 to 10 min as the result of oscillations in adenylyl cyclase activity (30, 31). It appears that when sufficient cAMP accumulates in the extracellular space to bind to CAR1, adenylyl cyclase is activated. Simultaneously, there is a loss of ligand binding activity as the result of phosphorylation of CAR1 (10, 11, 38, 51). An extracellular phosphodiesterase then reduces the external cAMP to a level below the affinity of the modified CAR1, and the cells are no longer stimulated. Over the next few minutes CAR1 gradually returns to the high-affinity state and can then be activated once again by the residual external cAMP (98). While it is not yet clear which protein kinase phosphorylates CAR1, PKA may be either directly or indirectly involved (Fig. 2).
Computer simulations of this circuitry show that it is sufficient to account for the spontaneous oscillations in adenylyl cyclase activity seen in stirred suspensions of cells that have developed for 4 h. The model is based on the measured peak values of adenylyl cyclase activity as well as the detailed timing of adenylyl cyclase and ERK2 activity. The output predicts a rapid rise in PKA activity shortly after the increase in adenylyl cyclase activity followed by an equally rapid decrease. The 8-min periodicity of the system is highly robust in that two- to fourfold changes in the parameters have negligible consequences to the output (58a). The model predicts that the phase will be delayed when an exogenous pulse of cAMP is added during the first half of the cycle and will be advanced when added later in the cycle. This is exactly what is observed experimentally (30, 31). This network appears to be sufficient to account for the spontaneous generation of cAMP pulses as well as the signal relay process that generates target patterns and spirals in cells spread on plates (3, 29, 33).
About 6 h after washed cells are spread on a thin agar layer, they form concentric rings of dark and light areas that can be seen with lateral lighting. The rings spread outwards at a rate (300 μm/min) consistent with the diffusion of a nondissipating relayed signal (19, 81). Gaps form in the rings when they encounter an inhomogeneity, and the pattern changes to spirals that form from the free ends. By directly measuring the spatial distribution of cAMP in a field of cells displaying spiral patterns, Tomchik and Devreotes (110) were able to show a superimposable pattern of cAMP. Thus, the dark-field images give a macroscopic indication of the underlying process of cAMP relay in the field. While the inhomogeneity that disrupts an outward propagation ring can be any physical barrier, such as a grain of sand, it can also be an intrinsic property of the cells such as the ability to relay the signal. Since there is a positive-feedback loop between the activation of PKA by cAMP and the stimulation of transcription of acaA such that more adenylyl cyclase accumulates when PKA is activated, cells that have slightly more adenylyl cyclase than others and so can produce more cAMP to activate PKA will rapidly accumulate significantly more adenylyl cyclase. This feedback loop on its own can lead to inhomogeneities in the population. Cells that are delayed in accumulation of adenylyl cyclase will not relay the wave of cAMP effectively and so will lead to a break in the propagating wave. As the ends of the wave continue to spread outward they will stimulate outlying cells, resulting in the establishment of an inwardly curling spiral. These behaviors can be predicted from mathematical analyses of excitable fields with the properties of Dictyostelium cells (50, 61).
As might be expected, cells lacking adenylyl cyclase due to disruption of acaA fail to aggregate or show any sign of morphogenesis (83). Surprisingly, development of acaA-null cells can be rescued by simply transforming them with a construct (act15::pkaC) that leads to the overproduction of the catalytic subunit of PKA (113). These mutant cells still fail to aggregate when spread at low cell densities but, when spread at a higher cell density, form mounds and proceed to construct normally proportioned fruiting bodies—all in the absence of detectable levels of cAMP. It appears that as long as the amoebae are sufficiently close to randomly bump into each other during the first few hours of development, chemotactic responses to cAMP are not necessary to form tight cell aggregates and external cAMP is not essential for subsequent events such as cell sorting or the relative cell movements seen during culmination. Moreover, the behavior of these cells indicates that all of the essential responses to cAMP as an internal second messenger are mediated by PKA.
Just because acaA mutants of Dictyostelium that have been engineered to have constitutively active PKA can develop in the absence of cAMP does not mean that wild-type cells do not normally use extracellular cAMP to coordinate developmental timing. Observations on the development of acaA-null act15::pkaC cells argue against a role for spatial gradients of extracellular cAMP in postaggregative morphogenesis but do not rule out potential roles of cAMP acting through surface receptors to control temporal changes in gene expression. These are events that occur over hours rather than minutes.
TIMING OF LATER DEVELOPMENTAL EVENTS
After relaying 10 to 20 pulses of cAMP, the cells start to move towards each other and stream into aggregates. Several genes that are expressed during this stage of development have been shown to be essential for subsequent morphogenesis. These include the genes encoding the small GTP-binding RAS homolog RasD, the DNA-binding factor GBF, and LagC (23, 25, 86, 96). These genes are induced by pulses of cAMP in both wild-type and pkaC-null strains, indicating that PKA activity is not necessary for their expression (76). On the other hand, overproduction of the catalytic subunit of PKA in the absence of cAMP pulses is sufficient for expression of all essential postaggregative genes, as demonstrated by the normal development of cells lacking adenylyl cyclase but carrying multiple copies of the act15::pkaC construct (113). The most likely explanation is that external cAMP uses two independent signal transduction pathways, one of which requires activation of PKA while the other is PKA independent. In fact, GBF and rasD are only partially induced in pkaC-null cells (72, 76). Overexpression of PKA appears to be sufficient to activate both pathways.
Cell-type-specific genes are expressed in subpopulations of cells soon after they have entered into mounds (27, 65, 102, 117). Not surprisingly, none of these genes are expressed in pkaC-null cells, since these cells cannot even aggregate, let alone form mounds. Molecular genetic techniques have been used to knock out PKA activity in prespore or prestalk cells once they have entered into mounds. Site-directed mutagenesis was used to change the amino acid sequence in the two cAMP binding sites of the regulatory subunit such that it would inhibit the catalytic subunit even in the presence of cAMP (36). This construct was ligated to the regulatory region of the prespore-specific gene pspA so that the mutated PKA-R protein, Rm, accumulates specifically in prespore cells when their pattern of transcriptional activity diverges from that in prestalk cells. Cells carrying this construct transcribe the spore coat genes for an hour or so but then stop due to the buildup of Rm (39, 40). Transcription of pspA is not dependent on PKA, and so the mRNA encoding Rm continues to be synthesized long after PKA is inhibited. All other prespore genes that have been studied have been found to depend on PKA activity. These include the spore coat genes cotA, cotB, cotC, cotD, pspB, and spiA (26, 39, 72, 73). As might be expected, cells carrying the pspA::Rm construct fail to form spores.
Cells transformed with a prestalk-specific construct, ecmA::Rm, in which the regulatory region of the prestalk gene, ecmA, drives the modified version of the pkaR gene that encodes a dominant negative PKA regulatory subunit form small, outwardly normal slugs that fail to culminate (36, 39, 119). Expression of ecmA is reduced in these cells, suggesting that PKA plays a role during the slug stage in the induction of prestalk-specific genes (36, 119). However, the situation is complicated by the fact that there is a period when prestalk cells are beginning to differentiate before Rm accumulates to inhibitory levels and once PKA activity is inhibited both the endogenous ecmA gene and the ecmA::Rm construct are no longer transcribed and their mRNAs will decay. Nevertheless, the dominant PKA-inhibitory protein appears to be sufficiently stable to completely block culmination.
Studies on expression of another prestalk gene, ecmB, indicate that PKA is responsible for overcoming repression of this gene at culmination. In wild-type strains ecmB is hardly expressed at all prior to culmination but is then expressed in the upper and lower cup cells that cradle the ball of spores as well as in terminally differentiating stalk cells. Mutants in which PKA activity is blocked in prestalk cells as a consequence of carrying the ecmA::Rm construct fail to culminate or express a marker gene (lacZ) driven by the ecmB regulatory region (36). Repression during the slug stage is mediated by two promoter domains, either of which is sufficient to keep ecmB inactive. Modification of the palindromic consensus sequence present in these domains, TTGnCAA, results in unrepressed expression in prestalk cells during the slug stage (37). These results have been interpreted to indicate that PKA activity at culmination releases the repression that keeps ecmB off during the slug stage.
Mutations in several different genes have been found to result in rapid development (1, 49, 79, 107). Spores appear in strains carrying these mutations in as little as 16 h; however, the spores do not appear to be completely normal and lose their ability to germinate fairly rapidly (79, 103). Strains carrying mutations in the rdeA locus have been shown to accumulate high levels of cAMP, those carrying mutations in rdeC lack functional PKA-R, while those with mutations in regA lack the phosphodiesterase such that PKA will be activated (1, 99, 103, 105). Each of these mutations should result in constitutive PKA activity. Simply overexpressing the catalytic subunit of PKA in prespore cells by introducing multiple copies of a pspA::pkaC construct also leads to precocious sporulation. Thus, control of PKA activity in prespore cells appears to be the mechanism by which premature encapsulation is avoided. Mutations in several other genes result in rapid development. One of these, yelA, encodes a protein of unknown function that interacts with a member of the highly conserved cullin family of proteins. Mutations in the gene encoding the Dictyostelium cullin, culA, result in a block following aggregation before the expression of cell-type-specific genes (77a). Mutations in the closest homolog in Caenorhabditis elegans, cul-1, have abnormal cell divisions during the larval stages (52). A related protein in yeast, CDC53, directs the ubiquination of phosphorylated forms of cyclin 2 leading to their degradation in proteosomes (116). While it is not yet known what CulA might do in Dictyostelium, it is conceivable that it facilitates the breakdown of YelA or PKA-R.
TIMING OF EVENTS DURING CULMINATION
Constitutive PKA not only results in premature encapsulation but makes the cells sporogenous (5, 75, 105). Cells of sporogenous strains form spores within 16 h after being washed and incubated as monolayers submerged in buffer containing high levels of cAMP. Cells of wild-type strains make no spores even after several days under these conditions. Each of the mutations and constructs that result in constitutive PKA including rdeA, regA, pkaR, pspA::pkaC, and pkaC::pkaC result in sporogeny. However, there is a dramatic density dependence for sporogeny of cells that overexpress PKA-C as a consequence of carrying multiple copies of a construct in which the structural gene pkaC is driven by its own regulatory region. At 2 × 104 cells/cm2 about 30% of the cells encapsulate, while at 5 × 102 cells/cm2 only 5% encapsulate (6). A phosphopeptide has been isolated from high-density pkaC::pkaC cells and shown to stimulate the frequency of encapsulation of low-density pkaC::pkaC test cells. Treatment of the factor, referred to as SDF-1, with alkaline phosphatase inactivates it, but such material can be reactivated by phosphorylating it with PKA (6). When test cells that have been incubated at low cell density for 24 h are stimulated with a partially purified phosphopeptide, the number of spores starts to increase within an hour and reaches the maximum level within 2 h (6). Protein synthesis appears to be required during this induction since the number of spores does not increase if the cells are treated with the protein synthesis inhibitor cycloheximide prior to addition of the phosphopeptide (6). It is not clear exactly which proteins must be synthesized in prespore cells in response to SDF-1, but PKA itself is a good candidate.
When the regulatory region used to drive pkaC is ligated to lacZ, most of the X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside)-stained cells are found at the anterior during the slug stage, indicating that pkaC is preferentially expressed in prestalk cells (74). However, following the initiation of culmination, cells in the sorus are much more strongly stained than those in the tip, indicating that expression of the construct has been rapidly activated in prespore cells. Likewise, PKA-R accumulates in spores to much higher levels than in stalk cells of terminally differentiated fruiting bodies (82). Something must be inducing PKA in prespore cells during culmination.
A wave of gene expression has been seen to pass down the sorus from the end nearest prestalk cells to the bottom (89). When the regulatory region of the spore-specific gene spiA is used to drive lacZ, stained cells are first seen when the sorus is about halfway up the stalk. Cells near the top stain first followed by those further down the sorus until all the presore cells are stained (89). spiA encodes one of the inner spore coat proteins that is necessary for stability of the spores (87, 88). Its pattern of expression suggested that a signal might be emanating from prestalk cells to trigger sporulation. Another hint came from analyzing the prestalk genes tagB and tagC (100). Mutants in which either of these genes is disrupted are blocked at the mound stage and fail to make either spores or stalk cells. However, if they are allowed to develop in chimeric mixtures with wild-type cells, they efficiently form spores (97). It appears that Tag− prespore cells are fully capable of encapsulating but require a signal that Tag− prestalk cells cannot release but that can be provided by wild-type prestalk cells. The tag genes encode highly similar membrane proteins with two distinct domains in each that appear to act as a heterodimer. One domain is similar to serine proteases and appears to be on the outside of the plasma membrane. The other domain has multiple transmembrane stretches and is homologous to the ABC family of ATP-driven transporters (100). It certainly looks like the sort of gene product that might be involved in the secretion of a peptide signal. Both tagB and tagC are first expressed at 8 h of development but only in prestalk cells (100).
Another gene in this pathway, dhkA, is also expressed at 8 h of development, but its mRNA is found in both prespore and prestalk cells (114). It has the correct properties to encode the receptor for the signal released by TagB/C. At the N terminus of DhkA there are two transmembrane domains flanking a 300-amino-acid region that has been directly shown to be on the outside of the cell, where it could bind a specific ligand (115). The cytoplasmic portion of DhkA is a histidine kinase that is a member of the two-component family of signal-transducing systems (66, 114, 115). These protein kinases autophosphorylate a histidine moiety when activated and relay the phosphate to an aspartate (4, 108). Strains in which dhkA is deleted have a cell-autonomous block to sporulation, as would be expected if its product functioned in the response to the signal emanating from prestalk cells during culmination (114). Site-directed mutagenesis of either the histidine that is autophosphorylated or the aspartate to which the phosphate is passed renders DhkA nonfunctional (115).
Suppressor mutations that permitted sporulation in a tagB-null background were isolated following saturation mutagenesis (99). One of the mutated genes turned out to be regA, which encodes the cAMP phosphodiesterase that regulates PKA activity during aggregation as described above (103). The N terminus of this enzyme carries a domain similar to those of the response regulators of two-component systems and thus is likely to have a phosphate relayed from a histidine kinase. Moreover, it can accept a phosphate from acetylphosphate, another characteristic of response regulators (68). Disruption of regA not only suppresses the block to sporulation resulting from mutations in tagB or tagC but also suppresses the block resulting from mutations in dhkA (115). Thus, RegA appears to have a negative role downstream of both of these genes. The most likely pathway leads from TagB/C in prestalk cells to DhkA in prespore cells via an extracellular signal, resulting in the activation of DhkA such that it can lead to the inactivation of RegA just prior to encapsulation (66). When RegA is inactive, cAMP would no longer be degraded and could activate PKA. The observations that pkaR mutations can suppress both tagB and dhkA mutations and that pspA::Rm is epistatic to the regA deletion provide further genetic support for this pathway (Fig. 3). Biochemical support for this model is provided by the measured spike in cAMP concentration that has been observed during culmination (1, 77).
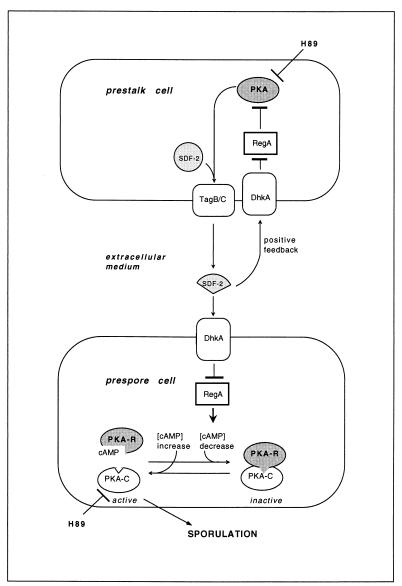
FIG. 3.
Signal transduction during culmination. Genetic studies suggest that the prestalk-specific membrane complex TagB/C is essential for release of the signal that triggers encapsulation of prespore cells and that the signal is recognized by the extracellular domain of the histidine kinase DhkA. DhkA can then inhibit the cAMP phosphodiesterase RegA that had been regulating PKA activity by facilitating the reversible interaction of the regulatory and catalytic subunits. The resulting increase in PKA activity leads to rapid encapsulation of prespore cells and stimulates SDF-2 release from prestalk cells. H89, the specific inhibitor of PKA activity, blocks the ability of prestalk cells to respond to SDF-2 by releasing more SDF-2 and blocks the ability of prespore cells to respond to SDF-2 by sporulating.
If this signal transduction pathway functions in the response to the phosphopeptide that induces sporulation in low-density monolayer preparations of pkaC::pkaC cells, then SDF-1 should be ineffective in dhkA mutant cells. However, it was found that the phosphopeptide induces sporulation effectively in such cells and so appears to act in a DhkA-independent manner (7). Subsequently, a different peptide was found that has all the characteristics expected of the DhkA ligand (7). This peptide, referred to as SDF-2, also induces sporulation in low-density monolayer preparations of pkaC::pkaC cells but does so much more rapidly and in a manner that is independent of protein synthesis. Within 10 min of addition of the peptide, the number of spores starts to increase and the maximum is reached in half an hour even if the test cells had been treated with cycloheximide prior to addition of the peptide (7). Evidence that the SDF-2 signal is mediated by the two-component system leading to activation of PKA came from studies of pkaC::pkaC cells in which dhkA was deleted as well as studies on the specific inhibition of PKA with the drug H89. In both cases, SDF-2 was completely ineffectual in stimulating the frequency of sporulation (7). Since regA mutants sporulate in an SDF-2 independent manner, it appears that SDF-2 triggers rapid encapsulation in wild-type cells by activating DhkA such that it inhibits RegA, leading to activation of PKA (Fig. 3).
SDF-2 can be recovered from the buffer in which regA-null cells developed but not from the buffer in which either wild-type or pkaC::pkaC cells developed (7). However, pkaC::pkaC cells can be induced to release high levels of SDF-2 if they are stimulated with SDF-2. Within a few minutes the level of SDF-2 in the buffer increases several hundredfold. Release of SDF-2 does not occur in tagC mutant cells even if they carry the pkaC::pkaC construct (7). Since only prestalk cells express tagC, they must be the ones that are responsible for releasing SDF-2 into the environment. The structure of the TagB/C complex raises the possibility that the extracellular serine protease domain may process a precursor exported by this ABC transporter so as to generate active SDF-2. The signal transduction pathway by which SDF-2 stimulates prestalk cells appears to be the same two-component system that leads to PKA in prespore cells. Cells in which dhkA is disrupted do not amplify a SDF-2 signal even when they carry the pkaC::pkaC construct, while regA mutants constitutively release SDF-2 (7). Moreover, cells carrying the ecmA::Rm construct that blocks PKA activity in prestalk cells fail to release the signal for encapsulation (100). When SDF-2 activates DhkA, RegA is inhibited and PKA activity increases. Thus, it is likely that PKA activity is also involved in the release of SDF-2. This conclusion is further supported by the fact that addition of the PKA-specific inhibitor H89 can also block release of SDF-2 (Fig. 3). Since H89 can be expected to inhibit even the basal levels of PKA activity, it is possible that it is the basal activity rather than the stimulated level of PKA activity that is essential for SDF-2 release.
Analysis of the peptide signals indicates that there is a continuing conversation between prestalk cells and prespore cells during culmination. SDF-1 first prepares prespore cells for encapsulation by inducing the synthesis of essential proteins that may include PKA, and subsequently SDF-2 activates PKA and triggers sporulation. Such a two-step interaction attests to the selective pressure to avoid premature sporulation that would result in encapsulated cells that are unable to reach the top of the rising stalk as well as the need to encapsulate when the prespore cells are in place. It is all in the timing.
DORMANCY
Encapsulated spores lie dormant as long as they remain together in a ball held on the top of a stalk. Dormancy is ensured by the high osmotic conditions found in the sorus and the presence of an inhibitor of germination, discadenine, that accumulates during culmination (42, 111). An adenylyl cyclase specialized for function within spores is activated at high osmolarity and is responsible for the high levels of cAMP and PKA activity in dormant spores. Spores in which the gene encoding this adenylyl cyclase, acgA, is disrupted germinate even under conditions of high osmolarity (111). Conversely, cells with constitutively high PKA activity due to mutations in pkaR or overexpression of pkaC germinate poorly even when the osmotic pressure is dropped. It appears that inhibition of PKA activity is necessary for efficient germination.
The cAMP levels that control PKA activity are kept high in spores not only by stimulation of ACG but also by inhibition of the cAMP phosphodiesterase RegA (103, 120). When spores are dispersed from the tops of fruiting bodies, ACG activity falls as a result of the reduced osmotic pressure and RegA activity rises; the cAMP levels rapidly drop and the regulatory subunit of PKA associates with the catalytic subunit to inhibit its activity. The spores can then germinate. Mutants in which the cytoplasmic phosphodiesterase is missing due to deletion of regA germinate poorly (103).
When prespore cells are about halfway up the rising stalk, RegA activity is inhibited by DhkA, the histidine kinase that appears to be activated by SDF-2. As a consequence, cAMP increases and encapsulation rapidly ensues. The job of inhibiting RegA is then taken over by a separate but related histidine kinase, DhkB, that appears to be activated by the germination inhibitor discadenine (120). If DhkB is inactivated by mutation, the spores germinate almost as soon as they encapsulate. However, this phenotype can be overcome by expressing pkaC at high levels (120). The concentration of cAMP in dhkB-null spores is less than half of that in wild-type spores as the result of failure to inhibit the phosphodiesterase. If regA is also inactivated in a dhkB-null strain, spores of the double mutants not only remain dormant but germinate slowly when spread out.
Further evidence that DhkB is normally active during dormancy and inactive when spores are dispersed from the sorus comes from observations on strains transformed with a construct that directs the synthesis of the protein kinase domain free of regulatory domains. The predicted constitutive DhkB activity in these cells leads to difficulties in germination (105a). Thus, RegA appears to be controlled by two sensor kinases such that its activity can be modulated both before and after culmination. When SDF-2 activates DhkA, it inhibits RegA from hydrolyzing cAMP such that PKA activity can trigger encapsulation. Shortly thereafter, discadenine activates DhkB, which also inhibits RegA. When the contents of sori are diluted, DhkB is no longer activated and RegA reduces the cAMP and PKA levels to those compatible with germination.
TEMPORAL REGULATION OF GENETIC NETWORKS
Some very early developmental genes including carA, pdsA, cadA, manA, nagA, and dscA are expressed when the cell density gets high even if the cells are still growing (12–14, 85). Throughout their growth period cells secrete a 68-kDa protein, referred to as PSF, that activates transcription of these genes when it accumulates above a certain threshold. The threshold is higher if there are bacteria remaining in the environment, but PSF will nonetheless induce cells to start their developmental cycle when it exceeds the threshold. Adenylyl cyclase is then induced but reaches maximal levels only after 8 h. Expression of acaA is under the control of PKA, which is itself stimulated by the cAMP that is produced by adenylyl cyclase. This feedback loop amplifies initial differences among the cells such that some start producing extracellular cAMP before others. Once the loop is functioning, extracellular cAMP, acting through the surface receptor CAR1 in a PKA-independent signal transduction pathway, leads to the accumulation of other components of the signal transduction mechanism including CAR1 itself, Gα2, CRAC, and the extracellular cAMP phosphodiesterase that reduces the level of cAMP around cells after each pulse is secreted (65, 81). As pulses of cAMP are relayed from one cell to the next, the entire population becomes aggregation competent. The time it takes to reach aggregation competence can vary significantly and is sensitive to environmental conditions. At low cell densities or low temperatures or in the presence of patches of food, cells may take a day before they start to stream into aggregates, while at high cell densities in optimally buffered salt solutions at 22°C, cells form aggregates within 8 h.
When all of the signal transduction components are in place, pulses of cAMP can be generated every 5 min or so. As described above, adenylyl cyclase activity is stimulated by CRAC together with Gβγ released from the trimeric G protein when cAMP binds to CAR1. However, adenylyl cyclase activity is short-lived because some of the cAMP it makes binds to the regulatory subunit of PKA activating the catalytic subunit that inhibits ERK2 and results in loss of ligand binding. Initially, the intracellular phosphodiesterase, RegA, is inhibited by the MAP kinase, ERK2, that is activated when cAMP binds to CAR1. PKA-R will not inhibit PKA-C again until RegA is no longer inhibited and can then reduce the internal cAMP concentration. During this period the cells are refractory to further pulses of cAMP. The circuit is completed when PKA inhibits ERK2 such that it can no longer inhibit RegA and PKA itself is inhibited. The detailed enzymatic characteristics of these components set the timing of these early developmental events.
As cells enter into mounds, they accumulate a cAMP receptor, CAR3, that is activated by the higher, sustained levels of cAMP that are found at that stage (46). Like CAR1, CAR3 is coupled to activation of adenylyl cyclase and so would be expected to activate PKA (84). PKA, in turn, is necessary for transcription of the spore coat genes (40). The transcription factor GBF is also essential for expression of these prespore genes and binds to its cognate sites on DNA only when phosphorylated (40, 95, 96). Thus, the internal cAMP sets the stage for the expression of prespore genes. PKA also seems to modulate expression of the prestalk-specific gene ecmA, since the level of mRNA from this gene is reduced in cells carrying the ecmA::Rm construct (36, 41, 119).
There is evidence suggesting that the circuit in which ERK2 and RegA control the level of PKA activity continues to function at the mound stage. Cells of an erkB-null strain carrying a construct that generates temperature-sensitive ERK2 were developed at the permissive temperature (20°C) for 12 h before being shifted to the nonpermissive temperature (25°C). Under these conditions the cells formed mounds but did not accumulate mRNA from the prespore gene cotC (28). On the other hand, mRNA from the prestalk-specific gene ecmA accumulated to essentially normal levels. When ERK2 is inactivated, the phosphodiesterase activity of RegA would be expected to remove cAMP from PKA-R, leading to inhibition of PKA activity. These results are consistent with having the aggregation stage circuit continuing to work in prespore cells.
Almost as soon as the cell types arise, they sort out such that the prestalk cells are at the apex. From there they distend the extracellular matrix to form a finger-shaped structure that will fall over and migrate away under some conditions. When developed on low ionic buffer and exposed to unilateral light, slugs generated by wild-type strains will embark on extended phototactic migrations. However, those in which PKA is constitutive due to disruption of pkaR rapidly undergo terminal differentiation and form simple balls of spores (1, 104). On the other hand, slugs formed from cells in which PKA is constitutive due to disruption of regA migrate for a considerable amount of time before forming spores and stalks (103). One of the few differences between cells of these two strains is that cAMP is very low throughout development in pkaR-null cells due to the inhibition of adenylyl cyclase as a consequence of the constitutive PKA activity but is higher in regA-null cells than in wild-type cells due to the loss of the phosphodiesterase (67a). If high levels of intracellular cAMP are necessary for slugs to enter the migratory stage, then it appears that in this situation cAMP is affecting PKA-independent pathways. In any case, it is interesting that the conditions that favor migration seem to be able to override the PKA-mediated signals that result in precocious terminal differentiation. The spore coat genes are expressed in prespore cells during migration, and their products, the spore coat proteins, are stockpiled in prespore vesicles for rapid release during culmination (18).
Prestalk cells accumulate two low-affinity cAMP receptors, CAR2 and CAR4, that mediate slug behavior (67, 90). The primary sequences of these receptors are 40% identical to those of the other members of the CAR family and are likely to be G protein coupled to adenylyl cyclase activation. CAR2 may mediate the effects of the high levels of extracellular cAMP that induce postaggregative genes such as gbfA and rasD. The gene encoding this surface receptor, carB, is first expressed in tight mounds and is subsequently preferentially expressed at the very front in slugs, where the PST-A cells are found (92). Expression is induced by high levels of cAMP and is repressed by DIF-1 (92). Mutant strains lacking CAR2 are arrested at the mound stage, although some form tips after more than 20 h of development (90). Prestalk cells sort out to the top of the mounds but seem to be unable to distend the extracellular matrix normally. Prespore genes are overexpressed in these strains, suggesting that CAR2 also plays a role in regulating these genes.
CAR4 accumulates somewhat after CAR2 and is found predominantly in prestalk cells but also at a low level in prespore cells (32, 67). Although slugs are formed normally in strains lacking CAR4 due to mutations in its structural gene, carD, they are not able to migrate well and form squat fruiting bodies with short stalks (67). Expression of the prestalk gene ecmA is greatly reduced in carD-null strains, suggesting that CAR4 plays a role in regulating this prestalk gene. Moreover, prespore cells can be seen in slug anteriors, suggesting that CAR4 is involved in maintaining the separation of the cell types (67). Thus, it appears that extracellular cAMP signals are used in various ways to integrate the cell types into an optimally functional multicellular organism but that these signals are dispensable under certain conditions as long as PKA is constitutively active (113).
Throughout the slug stage, pkaC is expressed at considerably higher levels in prestalk cells than in prespore cells (74). Assuming that the enzyme continuously accumulates in these cells, it may prepare them for terminal differentiation. Secretion of SDF-1 may potentiate this process by further inducing PKA in both prestalk and prespore cells. Since this factor is phosphorylated by PKA, a positive feedback loop will soon be established (6). However, until prestalk cells secrete SDF-2, most of the PKA will be in an inactive form due to association of the catalytic and regulatory subunits. Prespore cells rapidly respond to SDF-2 by a DhkA-dependent signal transduction pathway that activates PKA and leads to encapsulation. This signalling pathway can be bypassed by directly activating PKA with 8-Br–cAMP (48, 56, 69, 70). The spore-specific gene spiA is induced in less than an hour following addition of 8-Br–cAMP to cells dissociated from early culminants, suggesting that, once PKA is activated in prespore cells, they proceed directly to spore formation (87). Moreover, addition of 8-Br–cAMP to dhkB-null cells can suppress the premature germination usually seen in these spores (120). As long as PKA activity stays high, spores remain dormant, but when it drops, they germinate.
CONCLUSIONS AND FUTURE DIRECTIONS
Maybe PKA is controlled at so many different levels because it is used in so many different developmental processes (Fig. 4). Maximal activity is dependent on the amount of the catalytic subunit, which is a function of the rate of transcription, as well as the stability of the mRNA and the protein in the cell types. However, most of the catalytic subunit appears to be in an inactive form, complexed with the regulatory subunit, until activated by cAMP. The internal levels of cAMP are set by the relative rates of synthesis and hydrolysis of cAMP determined by adenylyl cyclase and phosphodiesterase, enzymes that are themselves subject to several control systems. Moreover, PKA is found in both the cytoplasm and the nucleus (118), so that we have to also consider subcellular localization of the enzyme. It would not be surprising if microfilament- or microtubule-based motors modulated PKA activity as well.
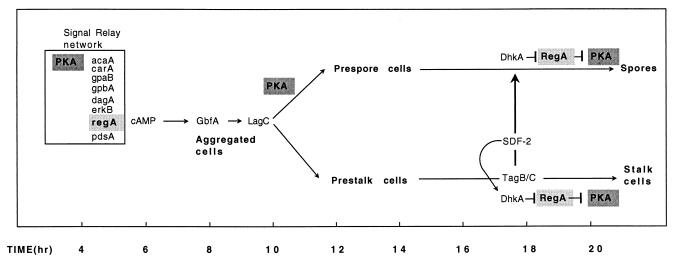
FIG. 4.
Multiple roles for RegA and PKA during development. The signal relay network is presented in Fig. 1 and 2. When aggregated cells are exposed to high levels of cAMP, genes dependent on the DNA-binding protein GBF are activated and cell type divergence proceeds. PKA is essential for prespore-specific gene expression and may also be involved in modulating prestalk genes. PKA activity during this stage of development is likely to be a function of RegA activity and the relative rates of transcription of pkaC and pkaR. During culmination pkaC is expressed at high levels in prespore cells, perhaps as a response to SDF-1. The autocrine circuit that results in rapid release of SDF-2 from prestalk cells exposes prespore cells to this sporulation inducer. The signal is transduced to PKA activity via the two-component system of DhkA and RegA.
PKA appears to play an integral role in the relay of extracellular pulses of cAMP in preparation for aggregation. Activation of adenylyl cyclase is terminated within minutes when ERK2 activity is inhibited by increased PKA activity that results from an increase in internal cAMP. The circuit returns to its initial state when RegA reduces cAMP to basal levels. This feedback circuit appears to be sufficient unto itself to generate a train of cAMP pulses with the observed time constants. Similar circuits may control neuronal activities.
Although it is clear that PKA activity is essential for accumulation of mRNAs from a variety of genes including acaA, prespore genes, and culmination genes, the direct targets in these pathways are not known. PKA could phosphorylate a cytoplasmic transcription factor, leading to its nuclear localization, or it could directly activate a nuclear factor. Studies with cells carrying the pspA::Rm construct indicate that transcription of the spore coat genes requires continuous PKA activity (40), suggesting that activation is relatively short-lived in this pathway.
The response of prespore cells to PKA activation with 8-Br–cAMP is so fast that the pathway leading from PKA to encapsulation must be very short. Within an hour of addition of the membrane-permeable derivative of cAMP about half the cells can be seen to have encapsulated. Normally, cells are not exposed to 8-Br–cAMP and the responses are more drawn out (Fig. 4). Both pkaC and pkaR may be induced in prespore cells by SDF-1, preparing them for subsequent activation of PKA activity by SDF-2 (6, 7). Release of SDF-2 is dependent on the prestalk ABC transporter, TagB/C, and activates DhkA in prespore cells, leading to a rapid increase in PKA activity. Moreover, a feedback loop connecting extracellular SDF-2 to release of more SDF-2 uses the same system in prestalk cells, most likely to activate PKA. Thus, this pathway is used in both prespore and prestalk cells, although it leads to quite different end results. When the natural substrates for PKA are known and their phosphorylation can be directly assayed in vivo, it is likely that PKA will be found to function in many other pathways that control when and where specific differentiations occur.
ACKNOWLEDGMENTS
I am grateful to Christophe Anjard, Rick Firtel, Robert Insall, Adam Kuspa, Michael Laub, Gadi Shaulsky, Fredrik Söderbom, and Jeff Williams for detailed comments on this review and for providing unpublished results.
This work was supported by a grant from the NIH (HD30892).
REFERENCES
- 1.Abe K, Yanagisawa K. A new class of rapid developing mutants in Dictyostelium discoideum: implications for cyclic AMP metabolism and cell differentiation. Dev Biol. 1983;95:200–210. doi: 10.1016/0012-1606(83)90018-0. [DOI] [PubMed] [Google Scholar]
- 2.Abel T, Nguyen P V, Barad M, Deuel T A, Kandel E R, Bourtchouladze R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997;88:615–626. doi: 10.1016/s0092-8674(00)81904-2. [DOI] [PubMed] [Google Scholar]
- 3.Alcantara F, Monk M. Signal propagation during aggregation in the slime mould Dictyostelium discoideum. J Gen Microbiol. 1974;85:321–334. doi: 10.1099/00221287-85-2-321. [DOI] [PubMed] [Google Scholar]
- 4.Alex L, Simon M. Protein histidine kinases and signal transduction in prokaryotes and eukaryotes. Trends Genet. 1994;10:133–138. doi: 10.1016/0168-9525(94)90215-1. [DOI] [PubMed] [Google Scholar]
- 5.Anjard C, Pinaud S, Kay R R, Reymond C D. Overexpression of DdPK2 protein kinase causes rapid development and affects the intracellular cAMP pathway of Dictyostelium discoideum. Development. 1992;115:785–790. doi: 10.1242/dev.115.3.785. [DOI] [PubMed] [Google Scholar]
- 6.Anjard C, van Bemmelen M, Véron M, Reymond C D. SDF, a new spore differentiation factor secreted by Dictyostelium cells, is phosphorylated by the cAMP dependent protein kinase. Differentiation. 1997;62:43–49. doi: 10.1046/j.1432-0436.1997.6210043.x. [DOI] [PubMed] [Google Scholar]
- 7.Anjard C, Zeng C, Loomis W F, Nellen W. Signal transduction pathways leading to spore differentiation in Dictyostelium discoideum. Dev Biol. 1998;193:146–155. doi: 10.1006/dbio.1997.8804. [DOI] [PubMed] [Google Scholar]
- 8.Aubry L, Maeda M, Insall R, Devreotes P N, Firtel R A. The Dictyostelium mitogen-activated protein kinase ERK2 is regulated by ras and cAMP-dependent protein kinase (PKA) and mediates PKA function. J Biol Chem. 1997;272:3883–3886. doi: 10.1074/jbc.272.7.3883. [DOI] [PubMed] [Google Scholar]
- 9.Bellen H J, Kiger J A. Sexual hyperactivity and reduced longevity of dunce females of Drosophila melanogaster. Genetics. 1987;115:153–160. doi: 10.1093/genetics/115.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caterina M J, Devreotes P N, Borleis J, Hereld D. Agonist-induced loss of ligand binding is correlated with phosphorylation of cAR1, a G protein-coupled chemoattractant receptor from Dictyostelium. J Biol Chem. 1995;270:8667–8672. doi: 10.1074/jbc.270.15.8667. [DOI] [PubMed] [Google Scholar]
- 11.Caterina M J, Hereld D, Devreotes P N. Occupancy of the Dictyostelium cAMP receptor, cAR1, induces a reduction in affinity which depends upon COOH-terminal serine residues. J Biol Chem. 1995;270:4418–4423. doi: 10.1074/jbc.270.9.4418. [DOI] [PubMed] [Google Scholar]
- 12.Clarke M, Gomer R H. PSF and CMF, autocrine factors that regulate gene expression during growth and early development of Dictyostelium. Experientia. 1995;51:1124–1134. doi: 10.1007/BF01944730. [DOI] [PubMed] [Google Scholar]
- 13.Clarke M, Kayman S C, Riley K. Density-dependent induction of discoidin-I synthesis in exponentially growing cells of Dictyostelium discoideum. Differentiation. 1987;34:79–87. doi: 10.1111/j.1432-0436.1987.tb00053.x. [DOI] [PubMed] [Google Scholar]
- 14.Clarke M, Yang J, Kayman S. Analysis of the prestarvation response in growing cells of Dictyostelium discoideum. Dev Genet. 1988;9:315–326. doi: 10.1002/dvg.1020090413. [DOI] [PubMed] [Google Scholar]
- 15.Corbin J D, Sugden P H, West L, Flockhart D A, Lincoln T M, McCarthy D. Studies on the properties and mode of action of the purified regulatory subunit of bovine heart adenosine 3′:5′-monophosphate-dependent protein kinase. J Biol Chem. 1978;253:3997–4003. [PubMed] [Google Scholar]
- 16.Davis R L, Takayasu H, Eberwine M, Myres J. Cloning and characterization of mammalian homologs of the Drosophila dunce+ gene. Proc Natl Acad Sci USA. 1989;86:3604–3608. doi: 10.1073/pnas.86.10.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Gunzburg J, Veron M. A cAMP-dependent protein kinase is present in differentiating Dictyostelium discoideum cells. EMBO J. 1982;1:1063–1068. doi: 10.1002/j.1460-2075.1982.tb01297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devine K M, Bergmann J E, Loomis W F. Spore coat proteins of Dictyostelium discoideum are packaged in prespore vesicles. Dev Biol. 1983;99:437–446. doi: 10.1016/0012-1606(83)90293-2. [DOI] [PubMed] [Google Scholar]
- 19.Devreotes P N. Chemotaxis. In: Loomis W F, editor. The development of Dictyostelium discoideum. New York, N.Y: Academic Press; 1982. pp. 117–168. [Google Scholar]
- 20.Devreotes P N. G-protein-linked signaling pathways control the developmental program of Dictyostelium. Neuron. 1994;12:235–241. doi: 10.1016/0896-6273(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 21.Drain P, Folkers E, Quinn W G. cAMP-dependent protein kinase and the disruption of learning in transgenic flies. Neuron. 1991;6:71–82. doi: 10.1016/0896-6273(91)90123-h. [DOI] [PubMed] [Google Scholar]
- 22.Dudai Y, Jan Y-N, Byers D, Quinn W, Benzer S. dunce, a mutant of Drosophila deficient in learning. Proc Natl Acad Sci USA. 1976;73:1684–1688. doi: 10.1073/pnas.73.5.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dynes J L, Clark A M, Shaulsky G, Kuspa A, Loomis W F, Firtel R A. LagC is required for cell-cell interactions that are essential for cell-type differentiation in Dictyostelium. Genes Dev. 1994;8:948–958. doi: 10.1101/gad.8.8.948. [DOI] [PubMed] [Google Scholar]
- 24.Firtel R A. Signal transduction pathways controlling multicellular development in Dictyostelium. Trends Genet. 1991;7:381–388. doi: 10.1016/0168-9525(91)90260-w. [DOI] [PubMed] [Google Scholar]
- 25.Firtel R A. Integration of signaling information in controlling cell-fate decisions in Dictyostelium. Genes Dev. 1995;9:1427–1444. doi: 10.1101/gad.9.12.1427. [DOI] [PubMed] [Google Scholar]
- 26.Fosnaugh K L, Loomis W F. Coordinate regulation of the spore coat genes in Dictyostelium discoideum. Dev Genet. 1991;12:123–132. doi: 10.1002/dvg.1020120120. [DOI] [PubMed] [Google Scholar]
- 27.Fosnaugh K L, Loomis W F. Enhancer regions responsible for temporal and cell-type-specific expression of a spore coat gene in Dictyostelium. Dev Biol. 1993;157:38–48. doi: 10.1006/dbio.1993.1110. [DOI] [PubMed] [Google Scholar]
- 28.Gaskins C, Clark A M, Aubry L, Segall J E, Firtel R A. The Dictyostelium MAP kinase ERK2 regulates multiple, independent developmental pathways. Genes Dev. 1996;10:118–128. doi: 10.1101/gad.10.1.118. [DOI] [PubMed] [Google Scholar]
- 29.Gerisch G, Hess B. Cyclic-AMP-controlled oscillations in suspended Dictyostelium cells: their relation to morphogenetic cell interactions. Proc Natl Acad Sci USA. 1974;71:2118–2122. doi: 10.1073/pnas.71.5.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerisch G, Maeda Y, Malchow D, Roos W, Wick U, Wurster B. Cyclic AMP signals and the control of cell aggregation in Dictyostelium discoideum. In: Cappuccinelli P, Ashworth J M, editors. Development and differentiation in the cellular slime moulds. Amsterdam, The Netherlands: Elsevier/North-Holland; 1977. pp. 105–124. [Google Scholar]
- 31.Gerisch G, Malchow D, Roos W, Wick U. Oscillations of cyclic nucleotide concentrations in relation to the excitability of Dictyostelium cells. J Exp Biol. 1979;81:33–47. doi: 10.1242/jeb.81.1.33. [DOI] [PubMed] [Google Scholar]
- 32.Ginsburg G T, Gollop R, Yu Y M, Louis J M, Saxe C L, Kimmel A R. The regulation of Dictyostelium development by transmembrane signalling. J Eukaryot Microbiol. 1995;42:200–205. doi: 10.1111/j.1550-7408.1995.tb01565.x. [DOI] [PubMed] [Google Scholar]
- 33.Gross J D, Peacey M J, Trevan D J. Signal emission and signal propagation during early aggregation in Dictyostelium discoideum. J Cell Sci. 1976;22:645–656. doi: 10.1242/jcs.22.3.645. [DOI] [PubMed] [Google Scholar]
- 34.Hammerschmidt M, Bitgood M J, McMahon A P. Protein kinase A is a common negative regulator of Hedgehog signaling in the vertebrate embryo. Genes Dev. 1996;10:647–668. doi: 10.1101/gad.10.6.647. [DOI] [PubMed] [Google Scholar]
- 35.Harwood A, Plyte S, Woodgett J, Strutt H, Kay R. Glycogen synthetase kinase 3 (GSK-3) regulates cell fate in Dictyostelium. Cell. 1995;80:139–148. doi: 10.1016/0092-8674(95)90458-1. [DOI] [PubMed] [Google Scholar]
- 36.Harwood A J, Hopper N A, Simon M N, Bouzid S, Veron M, Williams J G. Multiple roles for cAMP-dependent protein kinase during Dictyostelium development. Dev Biol. 1992;149:90–99. doi: 10.1016/0012-1606(92)90266-j. [DOI] [PubMed] [Google Scholar]
- 37.Harwood A J, Hopper N A, Simon M N, Driscoll D M, Veron M, Williams J G. Culmination in Dictyostelium is regulated by the cAMP-dependent protein kinase. Cell. 1992;69:615–624. doi: 10.1016/0092-8674(92)90225-2. [DOI] [PubMed] [Google Scholar]
- 38.Hereld D, Vaughan R, Kim J Y, Borleis J, Devreotes P. Localization of ligand-induced phosphorylation sites to serine clusters in the C-terminal domain of the Dictyostelium cAMP receptor, car1. J Biol Chem. 1994;269:7036–7044. [PubMed] [Google Scholar]
- 39.Hopper N A, Anjard C, Reymond C D, Williams J G. Induction of terminal differentiation of Dictyostelium by cAMP-dependent protein kinase and opposing effects of intracellular and extracellular cAMP on stalk cell differentiation. Development. 1993;119:147–154. doi: 10.1242/dev.119.1.147. [DOI] [PubMed] [Google Scholar]
- 40.Hopper N A, Sanders G, Fosnaugh K, Williams J, Loomis W F. Protein kinase A is a positive regulator of spore coat gene transcription in Dictyostelium. Differentiation. 1995;58:183–188. doi: 10.1046/j.1432-0436.1995.5830183.x. [DOI] [PubMed] [Google Scholar]
- 41.Hopper N A, Williams J. A role for cAMP-dependent protein kinase in determining the stability of prespore cell differentiation in Dictyostelium. Dev Biol. 1994;163:285–287. doi: 10.1006/dbio.1994.1145. [DOI] [PubMed] [Google Scholar]
- 42.Ihara M, Taya Y, Nishimura S. Developmental regulation of cytokinin, spore germination inhibitor discadenine and related enzymes in Dictyostelium discoideum. Exp Cell Res. 1980;126:273–278. doi: 10.1016/0014-4827(80)90265-7. [DOI] [PubMed] [Google Scholar]
- 43.Insall R, Kuspa A, Lilly P J, Shaulsky G, Levin L R, Loomis W F, Devreotes P. CRAC, a cytosolic protein containing a pleckstrin homology domain, is required for receptor and G protein-mediated activation of adenylyl cyclase in Dictyostelium. J Cell Biol. 1994;126:1537–1545. doi: 10.1083/jcb.126.6.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Insall R H, Soede R D M, Schaap P, Devreotes P N. Two cAMP receptors activate common signaling pathways in Dictyostelium. Mol Biol Cell. 1994;5:703–711. doi: 10.1091/mbc.5.6.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang J, Struhl G. Protein kinase A and hedgehog signaling in Drosophila limb development. Cell. 1995;80:183–188. doi: 10.1016/0092-8674(95)90510-3. [DOI] [PubMed] [Google Scholar]
- 46.Johnson R L, Saxe III C L, Gollop R, Kimmel A R, Devreotes P N. Identification and targeted gene disruption of cAR3, a cAMP receptor subtype expressed during multicellular stages of Dictyostelium development. Genes Dev. 1993;7:273–282. doi: 10.1101/gad.7.2.273. [DOI] [PubMed] [Google Scholar]
- 47.Kalderon D, Rubin G M. Isolation and characterization of Drosophila cAMP-dependent protein kinase genes. Genes Dev. 1988;2:1539–1556. doi: 10.1101/gad.2.12a.1539. [DOI] [PubMed] [Google Scholar]
- 48.Kay R R. Evidence that elevated intracellular cyclic AMP triggers spore maturation in Dictyostelium. Development. 1989;105:753–759. [Google Scholar]
- 49.Kessin R. Mutation causing rapid development of Dictyostelium discoideum. Cell. 1977;10:703–708. doi: 10.1016/0092-8674(77)90104-0. [DOI] [PubMed] [Google Scholar]
- 50.Kessler D A, Levine H. Pattern formation in Dictyostelium via the dynamics of cooperative biological entities. Phys Rev E. 1993;48:4801–4804. doi: 10.1103/physreve.48.4801. [DOI] [PubMed] [Google Scholar]
- 51.Kim J Y, Soede R, Valkema R, Borleis J A, VanHaastert P J M, Devreotes P N, Hereld D. Phosphorylation of chemoattractant receptors is not essential for chemotaxis or termination of G-protein-mediated responses. J Biol Chem. 1997;272:27313–27318. doi: 10.1074/jbc.272.43.27313. [DOI] [PubMed] [Google Scholar]
- 52.Kipreos E T, Lander L E, Wing J P, He W W, Hedgecock E M. cul-1 is required for cell cycle exit in C. elegans and identifies a novel gene family. Cell. 1996;85:829–839. doi: 10.1016/s0092-8674(00)81267-2. [DOI] [PubMed] [Google Scholar]
- 53.Klein P S, Sun T J, Saxe III C L, Kimmel A R, Johnson R L, Devreotes P N. A chemoattractant receptor controls development in Dictyostelium discoideum. Science. 1988;241:1467–1472. doi: 10.1126/science.3047871. [DOI] [PubMed] [Google Scholar]
- 54.Knetsch M L W, Epskamp S J P, Schenk P W, Wang Y W, Segall J E, Snaar-Jagalska B E. Dual role of cAMP and involvement of both G-proteins and ras in regulation of ERK2 in Dictyostelium discoideum. EMBO J. 1996;15:3361–3368. [PMC free article] [PubMed] [Google Scholar]
- 55.Konijn T M, van de Meene J G C, Bonner J T, Barkley D S. The acrasin activity of adenosine-3′,5′-cyclic phosphate. Proc Natl Acad Sci USA. 1967;58:1152–1154. doi: 10.1073/pnas.58.3.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kubohara, Y., and M. Maeda. Efficient induction by DIF-1 and 8-bromo cyclic AMP of prespore-to-stalk conversion in Dictyostelium discoideum. Comp. Biochem. Physiol., press.
- 57.Kumagai A, Hadwiger J A, Pupillo M, Firtel R A. Molecular genetic analysis of two Galpha protein subunits in Dictyostelium. J Biol Chem. 1991;266:1220–1228. [PubMed] [Google Scholar]
- 58.Lane M E, Kalderon D. Genetic investigation of cAMP-dependent protein kinase function in Drosophila development. Genes Dev. 1993;7:1229–1243. doi: 10.1101/gad.7.7a.1229. [DOI] [PubMed] [Google Scholar]
- 58a.Laub, M. T., and W. F. Loomis. Unpublished data.
- 59.Leichtling B H, Majerfeld I H, Spitz E, Schaller K L, Woffendin C, Kakinuma S, Rickenberg H V. A cytosolic cAMP-dependent protein kinase in Dictyostelium discoideum. II. Developmental regulation. J Biol Chem. 1984;259:662–668. [PubMed] [Google Scholar]
- 60.Levin L R, Han P L, Hwang P M, Feinstein P G, Davis R L, Reed R R. The Drosophila learning and memory gene rutabaga encodes a Ca2+/calmodulin-responsive adenylyl cyclase. Cell. 1992;68:479–489. doi: 10.1016/0092-8674(92)90185-f. [DOI] [PubMed] [Google Scholar]
- 61.Levine H, Aranson I, Tsimring L, Truong T V. Positive genetic feedback governs cAMP spiral wave formation in Dictyostelium. Proc Natl Acad Sci USA. 1996;93:6382–6386. doi: 10.1073/pnas.93.13.6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li W, Ohlmeyer J T, Lane M E, Kalderon D. Function of protein kinase A in hedgehog signal transduction and Drosophila imaginal disc development. Cell. 1995;80:553–562. doi: 10.1016/0092-8674(95)90509-x. [DOI] [PubMed] [Google Scholar]
- 63.Lilly P, Wu L J, Welker D L, Devreotes P N. A G-protein beta-subunit is essential for Dictyostelium development. Genes Dev. 1993;7:986–995. doi: 10.1101/gad.7.6.986. [DOI] [PubMed] [Google Scholar]
- 64.Livingstone M S, Sziber P P, Quinn W G. Loss of the calcium/calmodulin responsiveness in adenylate cyclase of rutabaga, a Drosophila learning mutant. Cell. 1984;37:205–215. doi: 10.1016/0092-8674(84)90316-7. [DOI] [PubMed] [Google Scholar]
- 65.Loomis W F. Genetic networks that regulate development in Dictyostelium cells. Microbiol Rev. 1996;60:135–150. doi: 10.1128/mr.60.1.135-150.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Loomis W F, Shaulsky G, Wang N. Histidine kinases in signal transduction pathways of eukaryotes. J Cell Sci. 1997;110:1141–1145. doi: 10.1242/jcs.110.10.1141. [DOI] [PubMed] [Google Scholar]
- 67.Louis J M, Ginsburg G T, Kimmel A R. The cAMP receptor CAR4 regulates axial patterning and cellular differentiation during late development of Dictyostelium. Genes Dev. 1994;8:2086–2096. doi: 10.1101/gad.8.17.2086. [DOI] [PubMed] [Google Scholar]
- 67a.Lu, S., and A. Kuspa. Unpublished data.
- 68.Lukat G S, McCleary W R, Stock A M, Stock J B. Phosphorylation of bacterial response regulator proteins by low molecular weight phospho-donors. J Cell Biochem. 1992;51:41–46. doi: 10.1073/pnas.89.2.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maeda M. Dual effects of cAMP on the stability of prespore vesicles and 8-bromo-cAMP enhanced maturation of spore and stalk cells of Dictyostelium discoideum. Dev Growth Differ. 1988;30:573–588. doi: 10.1111/j.1440-169X.1988.00573.x. [DOI] [PubMed] [Google Scholar]
- 70.Maeda M. Efficient induction of sporulation of Dictyostelium prespore cells by 8-bromocyclic AMP under both submerged- and shaken-culture conditions and involvement of protein kinase(s) in its action. Dev Growth Differ. 1992;34:263–275. doi: 10.1111/j.1440-169X.1992.tb00015.x. [DOI] [PubMed] [Google Scholar]
- 71.Maeda M, Aubry L, Insall R, Gaskins C, Devreotes P N, Firtel R A. Seven helix chemoattractant receptors transiently stimulate mitogen-activated protein kinase in Dictyostelium—role of heterotrimeric G proteins. J Biol Chem. 1996;271:3351–3354. doi: 10.1074/jbc.271.7.3351. [DOI] [PubMed] [Google Scholar]
- 72.Mann S K O, Firtel R A. A developmentally regulated, putative serine/threonine protein kinase is essential for development in Dictyostelium. Mech Dev. 1991;35:89–101. doi: 10.1016/0925-4773(91)90060-j. [DOI] [PubMed] [Google Scholar]
- 73.Mann S K O, Firtel R A. cAMP-dependent protein kinase differentially regulates prestalk and prespore differentiation during Dictyostelium development. Development. 1993;119:135–146. doi: 10.1242/dev.119.1.135. [DOI] [PubMed] [Google Scholar]
- 74.Mann S K O, Richardson D L, Lee S, Kimmel A R, Firtel R A. Expression of cAMP-dependent protein kinase in prespore cells is sufficient to induce spore cell differentiation in Dictyostelium. Proc Natl Acad Sci USA. 1994;91:10561–10565. doi: 10.1073/pnas.91.22.10561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mann S K O, Yonemoto W M, Taylor S S, Firtel R A. DdPK3, which plays essential roles during Dictyostelium development, encodes the catalytic subunit of cAMP-dependent protein kinase. Proc Natl Acad Sci USA. 1992;89:10701–10705. doi: 10.1073/pnas.89.22.10701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mann S K O, Brown J M, Briscoe C, Parent C, Pitt G, Devreotes P N, Firtel R A. Role of cAMP-dependent protein kinase in controlling aggregation and postaggregative development in Dictyostelium. Dev Biol. 1997;183:208–221. doi: 10.1006/dbio.1996.8499. [DOI] [PubMed] [Google Scholar]
- 77.Merkle R K, Cooper K K, Rutherford C L. Localization and levels of cyclic AMP during development of Dictyostelium discoideum. Cell Differ. 1984;14:257–266. doi: 10.1016/0045-6039(84)90014-9. [DOI] [PubMed] [Google Scholar]
- 77a.Mohanty, S., and R. A. Firtel. Unpublished data.
- 78.Mutzel R, Simon M, Lacombe M, Veron M. Expression and properties of the regulatory subunit of Dictyostelium cAMP-dependent protein kinase encoded by lambda gt11 cDNA clones. Biochemistry. 1988;27:481–486. doi: 10.1021/bi00401a069. [DOI] [PubMed] [Google Scholar]
- 79.Osherov N, Wang N, Loomis W F. Precocious sporulation and developmental lethality in yelA null mutants of Dictyostelium. Dev Genet. 1997;20:307–319. doi: 10.1002/(SICI)1520-6408(1997)20:4<307::AID-DVG2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 80.Pan D, Rubin G M. Protein kinase and hedgehog act antagonistically in regulating decapentaplegic transcription in Drosophila imaginal discs. Cell. 1995;80:543–552. doi: 10.1016/0092-8674(95)90508-1. [DOI] [PubMed] [Google Scholar]
- 81.Parent C A, Devreotes P N. Molecular genetics of signal transduction in Dictyostelium. Annu Rev Biochem. 1996;65:411–440. doi: 10.1146/annurev.bi.65.070196.002211. [DOI] [PubMed] [Google Scholar]
- 82.Part D, De Gunzburg J, Veron M. The regulatory subunit of cAMP-dependent protein kinase from Dictyostelium discoideum: cellular localization and developmental regulation analyzed by immunoblotting. Cell Differ. 1985;17:221–227. doi: 10.1016/0045-6039(85)90496-8. [DOI] [PubMed] [Google Scholar]
- 83.Pitt G S, Milona N, Borleis J, Lin K C, Reed R R, Devreotes P N. Structurally distinct and stage-specific adenylyl cyclase genes play different roles in Dictyostelium development. Cell. 1992;69:305–315. doi: 10.1016/0092-8674(92)90411-5. [DOI] [PubMed] [Google Scholar]
- 84.Pupillo M, Insall R, Pitt G S, Devreotes P N. Multiple cyclic AMP receptors are linked to adenylyl cyclase in Dictyostelium. Mol Biol Cell. 1992;3:1229–1234. doi: 10.1091/mbc.3.11.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rathi A, Kayman S C, Clarke M. Induction of gene expression in Dictyostelium by prestarvation factor, a factor secreted by growing cells. Dev Genet. 1991;12:82–87. doi: 10.1002/dvg.1020120115. [DOI] [PubMed] [Google Scholar]
- 86.Reymond C D, Gomer R H, Nellen W, Theibert A, Devreotes P, Firtel R. Phenotypic changes induced by a mutated Ras gene during the development of Dictyostelium transformants. Nature. 1986;323:340–343. doi: 10.1038/323340a0. [DOI] [PubMed] [Google Scholar]
- 87.Richardson D L, Hong C B, Loomis W F. A prespore gene, Dd31, expressed during culmination of Dictyostelium discoideum. Dev Biol. 1991;144:269–280. doi: 10.1016/0012-1606(91)90421-x. [DOI] [PubMed] [Google Scholar]
- 88.Richardson D L, Loomis W F. Disruption of the sporulation-specific gene spiA in Dictyostelium discoideum leads to spore instability. Genes Dev. 1992;6:1058–1070. doi: 10.1101/gad.6.6.1058. [DOI] [PubMed] [Google Scholar]
- 89.Richardson D L, Loomis W F, Kimmel A R. Progression of an inductive signal activates sporulation in Dictyostelium discoideum. Development. 1994;120:2891–2900. doi: 10.1242/dev.120.10.2891. [DOI] [PubMed] [Google Scholar]
- 90.Saxe C L, Ginsburg G T, Louis J M, Johnson R, Devreotes P N, Kimmel A R. CAR2, a prestalk cAMP receptor required for normal tip formation and late development of Dictyostelium discoideum. Genes Dev. 1993;7:262–272. doi: 10.1101/gad.7.2.262. [DOI] [PubMed] [Google Scholar]
- 91.Saxe C L, III, Johnson R L, Devreotes P N, Kimmel A R. Expression of a cAMP receptor gene of Dictyostelium and evidence for a multigene family. Genes Dev. 1991;5:1–8. doi: 10.1101/gad.5.1.1. [DOI] [PubMed] [Google Scholar]
- 92.Saxe C L, III, Yu Y M, Jones C, Bauman A, Haynes C. The cAMP receptor subtype cAR2 is restricted to a subset of prestalk cells during Dictyostelium development and displays unexpected DIF-1 responsiveness. Dev Biol. 1996;174:202–213. doi: 10.1006/dbio.1996.0066. [DOI] [PubMed] [Google Scholar]
- 93.Schaller K L, Leichtling B H, Majerfeld I H, Woffendin C, Spitz E, Kakinuma S, Rickenberg H V. Differential cellular distribution of cAMP-dependent protein kinase during development of Dictyostelium discoideum. Proc Natl Acad Sci USA. 1984;81:2127–2131. doi: 10.1073/pnas.81.7.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schaller K L, Leichtling B H, Rickenberg H V. Isolation of a clone for the regulatory subunit of the cyclic cAMP-dependent protein kinase of D. discoideum. In: Firtel R A, Davidson E H, editors. Molecular approaches to developmental biology. New York, N.Y: Alan R. Liss; 1987. pp. 351–360. [Google Scholar]
- 95.Schnitzler G R, Fischer W H, Firtel R A. Cloning and characterization of the G-box binding factor, an essential component of the developmental switch between early and late development in Dictyostelium. Genes Dev. 1994;8:502–514. doi: 10.1101/gad.8.4.502. [DOI] [PubMed] [Google Scholar]
- 96.Schnitzler G R, Briscoe C, Brown J M, Firtel R A. Serpentine cAMP receptors may act through a G protein-independent pathway to induce postaggregative development in Dictyostelium. Cell. 1995;81:737–745. doi: 10.1016/0092-8674(95)90535-9. [DOI] [PubMed] [Google Scholar]
- 97.Segall J E, Kuspa A, Shaulsky G, Ecke M, Maeda M, Gaskins C, Firtel R A, Loomis W F. A MAP kinase necessary for receptor-mediated activation of adenylyl cyclase in Dictyostelium. J Cell Biol. 1995;128:405–413. doi: 10.1083/jcb.128.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Segel L A, Goldbeter A, Devreotes P N, Knox B E. A mechanism for exact sensory adaptation based on receptor modification. J Theor Biol. 1986;120:151–179. doi: 10.1016/s0022-5193(86)80171-0. [DOI] [PubMed] [Google Scholar]
- 99.Shaulsky G, Escalante R, Loomis W F. Developmental signal transduction pathways uncovered by genetic suppressors. Proc Natl Acad Sci USA. 1996;93:15260–15265. doi: 10.1073/pnas.93.26.15260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shaulsky G, Kuspa A, Loomis W F. A multidrug resistance transporter serine protease gene is required for prestalk specialization in Dictyostelium. Genes Dev. 1995;9:1111–1122. doi: 10.1101/gad.9.9.1111. [DOI] [PubMed] [Google Scholar]
- 101.Shaulsky G, Loomis W F. Mitochondrial DNA replication but no nuclear DNA replication during development of Dictyostelium. Proc Natl Acad Sci USA. 1995;92:5660–5663. doi: 10.1073/pnas.92.12.5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shaulsky G, Loomis W F. Initial cell type divergence in Dictyostelium is independent of DIF-1. Dev Biol. 1996;174:214–220. doi: 10.1006/dbio.1996.0067. [DOI] [PubMed] [Google Scholar]
- 103.Shaulsky G, Fuller D, Loomis W F. A cAMP-phosphodiesterase controls PKA-dependent differentiation. Development. 1998;125:691–699. doi: 10.1242/dev.125.4.691. [DOI] [PubMed] [Google Scholar]
- 104.Simon M N, Driscoll D, Mutzel R, Part D, Williams J, Veron M. Overproduction of the regulatory subunit of the cAMP-dependent protein kinase blocks the differentiation of Dictyostelium discoideum. EMBO J. 1989;8:2039–2044. doi: 10.1002/j.1460-2075.1989.tb03612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Simon M N, Pelegrini O, Veron M, Kay R R. Mutation of protein kinase-A causes heterochronic development of Dictyostelium. Nature. 1992;356:171–172. doi: 10.1038/356171a0. [DOI] [PubMed] [Google Scholar]
- 105a.Singleton, C. Personal communication.
- 106.Skoulakis E M, Kalderon D, Davis R L. Preferential expression in mushroom bodies of the catalytic subunit of protein kinase A and its role in learning. Neuron. 1993;11:197–208. doi: 10.1016/0896-6273(93)90178-t. [DOI] [PubMed] [Google Scholar]
- 107.Sonneborn D R, White G J, Sussman M. A mutation affecting both rate and pattern of morphogenesis in Dictyostelium discoideum. Dev Biol. 1963;7:79–93. doi: 10.1016/0012-1606(63)90108-8. [DOI] [PubMed] [Google Scholar]
- 108.Stout V, Gottesman S. RcsB and RcsC: a two-component regulator of capsule synthesis in Escherichia coli. J Bacteriol. 1990;172:659–669. doi: 10.1128/jb.172.2.659-669.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Taylor S S, Buechler J A, Yonemoto W. cAMP-dependent protein kinase: framework for a diverse family of regulatory enzymes. Annu Rev Biochem. 1990;9:971–1005. doi: 10.1146/annurev.bi.59.070190.004543. [DOI] [PubMed] [Google Scholar]
- 110.Tomchik K J, Devreotes P N. Adenosine 3′,5′-monophosphate waves in Dictyostelium discoideum: a demonstration by isotope dilution-fluorography technique. Science. 1981;212:443–446. doi: 10.1126/science.6259734. [DOI] [PubMed] [Google Scholar]
- 111.van Es S, Virdy K J, Pitt G S, Meima M, Sands T W, Devreotes P N, Cotter D A, Schaap P. Adenylyl cyclase G, an osmosensor controlling germination of Dictyostelium spores. J Biol Chem. 1996;271:23623–23625. doi: 10.1074/jbc.271.39.23623. [DOI] [PubMed] [Google Scholar]
- 112.Vaughan R A, Rutherford C L. Distribution of cAMP-dependent protein kinase during development in Dictyostelium discoideum. Dev Biol. 1987;121:359–367. doi: 10.1016/0012-1606(87)90172-2. [DOI] [PubMed] [Google Scholar]
- 113.Wang B, Kuspa A. Dictyostelium development in the absence of cAMP. Science. 1997;277:251–254. doi: 10.1126/science.277.5323.251. [DOI] [PubMed] [Google Scholar]
- 114.Wang N, Shaulsky G, Escalante R, Loomis W F. A two-component histidine kinase gene that functions in Dictyostelium development. EMBO J. 1996;15:3890–3898. [PMC free article] [PubMed] [Google Scholar]
- 115.Wang, N., F. Söderbom, G. Shaulsky, and W. F. Loomis. The histidine kinase DhkA regulates terminal differentiation of Dictyostelium. Unpublished data. [DOI] [PMC free article] [PubMed]
- 116.Willems A R, Lanker S, Patton E E, Craig K L, Nason T F, Mathias N, Kobayashi R, Wittenberg C, Tyers M. Cdc53 targets phosphorylated G1 cyclins for degradation by the ubiquitin proteolytic pathway. Cell. 1996;86:453–463. doi: 10.1016/s0092-8674(00)80118-x. [DOI] [PubMed] [Google Scholar]
- 117.Williams J G, Duffy K T, Lane D P, McRobbie S J, Harwood A J, Traynor D, Kay R R, Jermyn K A. Origins of the prestalk-prespore pattern in Dictyostelium development. Cell. 1989;59:1157–1163. doi: 10.1016/0092-8674(89)90771-x. [DOI] [PubMed] [Google Scholar]
- 118.Woffendin C, Chambers T C, Schaller K L, Leichtling B H, Rickenberg H V. Translocation of cAMP-dependent protein kinase to the nucleus during development of Dictyostelium discoideum. Dev Biol. 1986;115:1–8. doi: 10.1016/0012-1606(86)90221-6. [DOI] [PubMed] [Google Scholar]
- 119.Zhukovskaya N, Early A, Kawata T, Abe T, Williams J. cAMP-dependent protein kinase is required for the expression of a gene specifically expressed in Dictyostelium prestalk cells. Dev Biol. 1996;179:27–40. doi: 10.1006/dbio.1996.0239. [DOI] [PubMed] [Google Scholar]
- 120.Zinda M J, Singleton C K. The hybrid histidine kinase dhkB regulates spore germination in Dictyostelium discoideum. Dev Biol. 1998;196:171–183. doi: 10.1006/dbio.1998.8854. [DOI] [PubMed] [Google Scholar]