TO THE EDITOR
In psoriasis, immune systemic activation and cardiometabolic abnormalities, including dyslipidemia, play a role in promoting impaired endothelial health and increased cardiovascular (CV) risk (Greb et al., 2016). Patients with psoriasis have elevated brachial vein endothelial proinflammatory transcript expression (impaired vascular endothelial health), with the degree of impairment directly correlated with skin activity and circulating biomarkers of systemic inflammation (Garshick et al., 2019). In psoriasis, TNF and IL-17A synergism promote inflammasome (IL-1β) signaling and downstream IL-6 production (driver of CRP) (Verma et al., 2021), which associates with vascular endothelial inflammatory transcript expression, suggesting a pathogenic (immune-mediated) mechanism of atherosclerosis development (Garshick et al., 2019).
In general (nonpsoriasis) populations, β-hydroxy β-methylglutaryl-CoA reductase inhibitors (e.g., statins) reduce cholesterol and CV risk, improve endothelial function, and have pleiotropic and anti-inflammatory effects (Adhyaru and Jacobson, 2018; Garshick and Underberg, 2017; Ridker et al., 2008). In psoriasis, although observational data suggest a benefit (Wu et al., 2012), randomized controlled trial data showing the efficacy of statins whether through lipid-mediated or antiinflammatory properties are yet to be established. We therefore conducted a randomized open-label controlled trial to investigate the effect of high-intensity atorvastatin on vascular endothelial health in the psoriatic population.
Patients with active psoriasis (≥1% body surface area of psoriasis or psoriatic arthritis ≥1 swollen/tender joint; consort diagram in Supplementary Figure S1) without clinical CV disease were recruited as part of ongoing studies (NCT03228017) to investigate vascular endothelial health in psoriasis at NYU Langone Health (New York City, NY) (Institutional Review Board approval i17–00692) (Garshick et al., 2019). To directly assess the endothelium and vascular endothelial cell proinflammatory activation, subjects underwent brachial vein endothelial harvesting and serum lipid and inflammatory biomarker assessment (Jelic et al., 2010). Participants with psoriasis were then randomized to 40 mg atorvastatin per day or to no treatment (Supplementary Figure S1), and a repeat assessment occurred at week 2. The primary study endpoint and outcome measure was a change in the composite brachial vein endothelial cell inflammatory transcriptomeedefined as the mean expression values from endothelial cell proinflammatory transcripts (i.e., mean expression of proinflammatory vascular endothelial transcript [meanEC]) (composite transcript expression of LTB, CCL3, CX3CL1, CCL2, CXCL1, ICAM1, IL-8, IL-1B, COX-2) (Garshick et al., 2020). Subjects provided written informed consent before participation, in line with the Declaration of Helsinki protocols (Supplementary Materials and Methods).
For sample size determination in this pilot study, 10 subjects (control) and 20 subjects (treatment) per group provided a margin of error (95% confidence interval half width) of 0.13 for the log2 fold change in vascular endothelial inflammation between treatment groups (Garshick et al., 2020; Kianifard and Islam, 2011; nQuery, 2017, Statistical Solutions, Cork, Ireland). For the primary outcome, log transformation with paired sample t-test or Wilcoxon test for changes between baseline and follow-up data points were also performed with multivariable modeling as appropriate. Statistical significance was determined using a two-tailed α < 0.05, with full study details in the Supplementary Materials and Methods. The datasets, including circulating and vascular endothelial inflammatory and lipid biomarkers, which support the findings of this study, are available from the corresponding author (MSG) on reasonable request.
Recruited patients with psoriasis were young and of low CV risk with moderate psoriasis disease severity, many (40%) of whom were on biologic therapy (Table 1 and Supplementary Table S1). At baseline, meanEC (mean expression of proinflammatory brachial vein endothelial cell transcripts) correlated with both PASI (r = 0.41, P = 0.02) and high-sensitivity CRP (hs-CRP) (r = 0.61, P < 0.01; Supplementary Figure S2a and b) but not with psoriasis disease duration (r = −0.09, P = 0.64), CV risk score (r = 0.01, P = 0.95), low-density lipoprotein cholesterol (LDL-C) (r = 0.18, P = 0.36), or total cholesterol (r = 0.15, P = 0.45). After 2 weeks of high-intensity statin therapy, the median reduction from baseline LDL-C was 44% (P < 0.001), and that of hs-CRP was 41% (P = 0.06), with no significant changes noted in the no-treatment group (Supplementary Table S2). Other measures, including IL-6, TNF-α, and IL-17A, were not significantly decreased in both groups (Supplementary Figure S3a–f and Supplementary Table S2).
Table 1.
Baseline Characteristics
| Characteristics | No Treatment (n = 10) | Atorvastatin (n = 20) | P-Value |
|---|---|---|---|
| Age, y, median (IQR) | 35 (28–37) | 44 (32–52) | 0.16 |
| Male sex, n (%) | 5 (50) | 9 (45) | 0.80 |
| Body mass index, kg/m2 | 28 ± 6 | 28 ± 7 | 0.97 |
| Caucasian, n (%) | 6 (60) | 15 (75) | 0.40 |
| Systolic blood pressure (mm Hg) | 121 ± 10 | 127 ± 17 | 0.40 |
| Diastolic blood pressure (mm Hg) | 75 | 76 ± 12 | 0.87 |
| ACC/AHA ASCVD Risk Score, % | 2.7 ± 3.5 | 3.3 ± 5.4 | 0.77 |
| Psoriasis | |||
| PASI score, median (IQR) | 5.6 (3.2–19) | 4.0 (3.6–6.9) | 0.69 |
| Psoriasis duration, y, median (IQR) | 8 (3–10) | 20 (12–29) | 0.01 |
| Psoriatic arthritis, n (%) | 3 (15) | 4 (40) | 0.13 |
| Biologic therapy, n (%) | 6 (60) | 6 (30) | 0.11 |
| Laboratory studies | |||
| WBC, ×103 cells/mm3, median (IQR) | 7.9 (6.1–9.9) | 6.2 (5.3–7.1) | 0.11 |
| hs-CRP, mg/l, median (IQR) | 2.1 (1.0–4.2) | 1.7 (0.8–2.0) | 0.45 |
| IL-17A, NPX, median (IQR) | 3.5 (1.8–6.1) | 2.4 (1.8–3.6) | 0.43 |
| IL-6, NPX, median (IQR) | 3.8 (3.4–4.6) | 3.3 (3.1–4.2) | 0.20 |
| TNF-α, NPX, median (IQR) | 3.9 (3.7–4.7) | 3.6 (3.3–3.9) | 0.22 |
| Lipids | |||
| Total cholesterol, mg/dl, median (IQR) | 179 (178–186) | 182 (162–216) | 0.79 |
| Triglyercides, mg/dl, median (IQR) | 83 (58–150) | 85 (61–104) | 0.98 |
| LDL-C, mg/dl, median (IQR) | 111 (110–121) | 108 (83–131) | 0.20 |
| HDL-C, mg/dl, median (IQR) | 45 (43–52) | 56 (46–63) | 0.07 |
| meanEC, median (IQR) | 5.9 (2.8–13) | 4.7 (3.2–7.4) | 0.96 |
Abbreviations: ACC/AHA, American College of Cardiology/American Heart Association; ASCVD, atherosclerotic cardiovascular disease; HDL-C, high-density lipoprotein cholesterol; hs-CRP, high-sensitivity CRP; IQR, interquartile range; LDL-C, low-density lipoprotein cholesterol; meanEC, mean expression of proinflammatory vascular endothelial transcript; NPX, normalized eXpression unit; WBC, white blood cell.
Data are presented as mean ± SD or n (%) unless otherwise stated. meanEC indicates mean vascular endothelial transcript inflammation (arbitrary units standardized to the housekeeping gene hARP).
For the primary outcome, after 2 weeks of high-intensity statin therapy, meanEC was reduced (log2fold change = −0.10, 95% confidence interval = −0.003 to −0.21) in the atorvastatin group compared with the meanEC change in the no-treatment group (log2fold change = 0.10, 95% confidence interval = −0.04 to 0.25; P = 0.01; Figure 1a). This difference between no-treatment and statin randomized participants remained after adjusting for potential confounders, including age, sex, biologic use, psoriasis severity (PASI), and psoriasis duration (β = −0.49, P = 0.03), showing that atorvastatin reduced vascular endothelial inflammation. The reduction in meanEC correlated with the degree of LDL-C lowering (Figure 1b) but not with a reduction in hs-CRP or IL-6 (Supplementary Figure S4a and b). In line with these observations, participants with a more significant reduction in LDL-C (Figure 1c and d) or in a follow-up LDL-C <70 mg/dl (Supplementary Figure S4c) displayed the largest reductions in vascular endothelial inflammatory transcript expression from baseline.
Figure 1. A 2-week lipid-lowering therapy with atorvastatin reduces vascular endothelial inflammation.
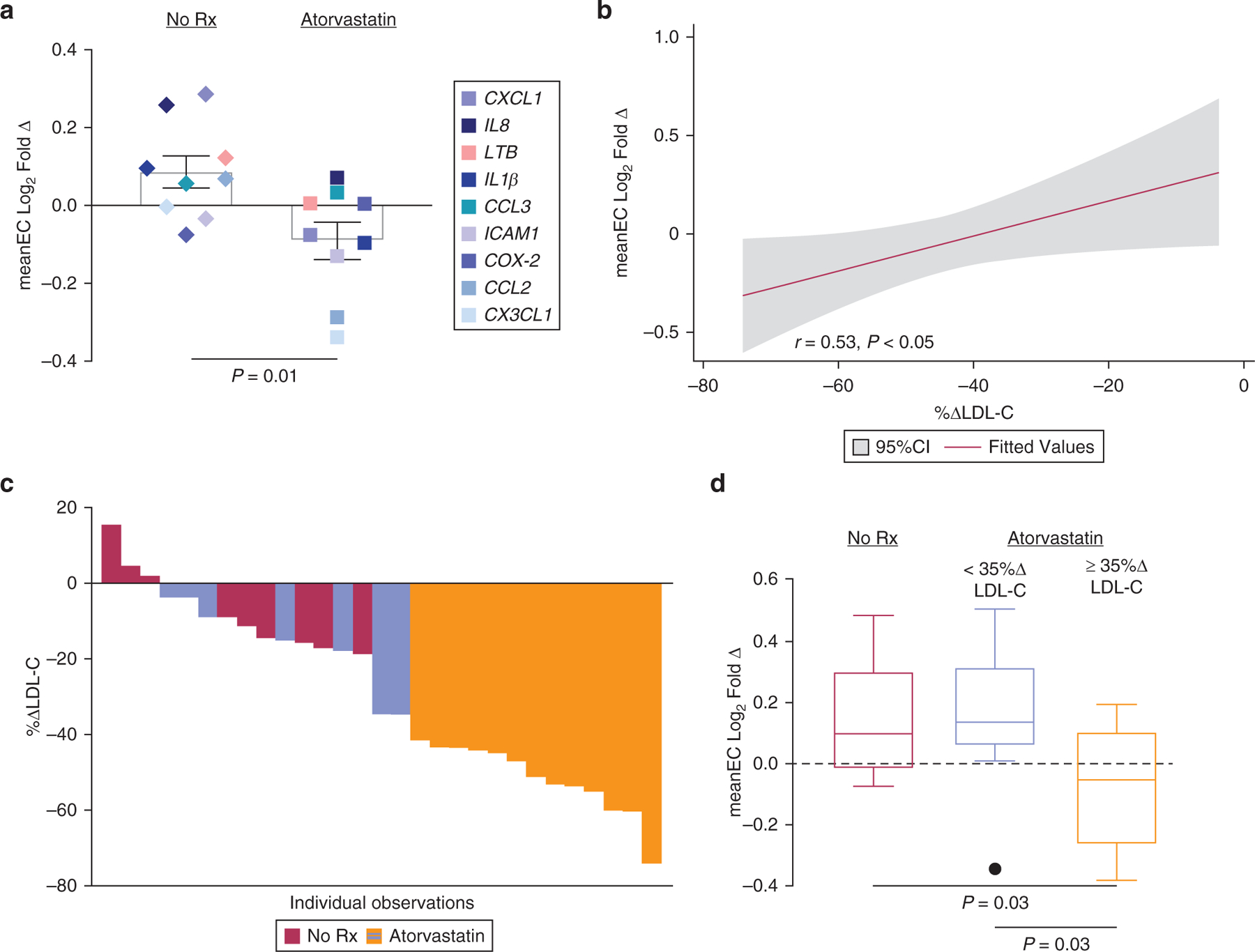
(a) Composite log2 fold change in brachial vein endothelial inflammatory transcript expression in those randomized to atorvastatin (n = 181) or no treatment (n = 10) for 2 weeks. (b) Correlation between atorvastatin-treated patients and composite log2 fold change in brachial vein endothelial inflammatory transcript expression. (c) Percentage change in LDL-C over 2 weeks1; colors correspond to <35% or ≥35% reduction in LDL-C. (d) Composite log2 fold change in brachial vein endothelial inflammatory transcript expression stratified by no treatment (n = 10), <35% reduction in LDL-C (n = 6), and ≥35% reduction in LDL-C (n = 121) after atorvastatin treatment.
Patients with psoriasis have a heightened prevalence of dyslipidemia and an elevated risk of CV disease compared with those without psoriasis (Greb et al., 2016). A posthoc analysis of two secondary prevention lipid-lowering trials identified ~500 (of ~19,000) patients with psoriasis (Ports et al., 2017). Patients with psoriasis on high-intensity statins displayed a similar reduction in lipids and CV events to those in patients without psoriasis. Our findings support and expand on these analyses, whereby after atorvastatin therapy, the lower the LDL-C (<70 mg/dl) achieved, the greater the improvement in vascular health. Although specific LDL-C goals are no longer firmly recommended in the primary prevention of CV disease (Arnett et al., 2019), our findings are similar to those of other high CV risk groups, whereby aggressive lowering of LDL-C (<70 mg/dl) correlates with reduced CV risk.
To explore the mechanisms of CV risk in psoriasis, we employed a surrogate of vascular health, brachial vein endothelial analysis, and cannot definitively state that statins improve CV outcomes in psoriasis. However, analysis of directly obtained venous endothelium is an innovative technique, and in other studies, reductions in brachial vein endothelial inflammation correlate with improvement in endothelial flow–mediated dilation, which itself is predictive of CV events (Jelic et al., 2010; Michelson et al., 2000). Other limitations include a high proportion of participants on biologic therapy and some randomization imbalance between psoriasis groups, but these were adjusted for in multivariable analyses. Finally, we did not observe a reduction in inflammatory biomarkers (IL-6, TNF-α, or IL-17A) after statin therapy nor a significant correlation between changes in hs-CRP and vascular endothelial transcriptome expression. Whether this was due to the short, pilot nature of our study with limited sample size or other factors intrinsic to psoriasis itself requires larger, longer-duration investigations.
In summary, in a pilot randomized clinical trial of patients with psoriasis without clinical CV disease, 40 mg of atorvastatin reduced vascular endothelial proinflammatory transcript expression, a surrogate of CV risk. The degree of improvement correlated with the degree of LDL-C reduction and not with hs-CRP, with those observing the most benefit achieving an LDL-C below 70 mg/dl. Our findings highlight the need for larger clinical trials to investigate the clinical benefit of statin therapy in psoriasis.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by a National Institutes of Health (Bethesda, MD) training grant T32HL098129; National Institutes of Health Clinical and Translational Science Award at New York University Awards UL1TR001445, KL2TR001446, and TL1TR001447; American Heart Association Career Development Grant 18CDA34080540; Dermatology Foundation Research Grant; and National Psoriasis Foundation Bridge Grant (all awarded to MSG) and American Heart Association Career Development Grant 18CDA34110203AHA and American Society of Hematology (18-A0-00-1001884) to TJB. JUS was supported, in part, by the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases R01AR074500, the Riley Family Foundation, the Beatriz Snyder Foundation, the Rheumatology Research Foundation, and the National Psoriasis Foundation Diagnostic Challenge Grant. JSB was supported, in part, by the National Institutes of Health grants R01HL139909 and R35HL144993.
Abbreviations:
- CV
cardiovascular
- hs-CRP
high-sensitivity CRP
- LDL-C
low-density lipoprotein cholesterol
- meanEC
mean expression of proinflammatory vascular endothelial transcript
Footnotes
ETHICS STATEMENT
The study protocol was approved by the New York University School of Medicine Institutional Review Board (i17-00692). All subjects provided written informed consent before participation, in line with the Declaration of Helsinki protocols, which were followed.
CONFLICT OF INTEREST
MSG has received personal fees from Abbvie. JUS has served as a consultant for Janssen, Abbvie, Novartis, Sanofi, UCB, and BMS. JK has received grants from Novartis, Pfizer, Amgen, Lilly, Boehringer, Innovaderm, BMS, Janssen, Abbvie, Paraxel, Leo Pharma, Vitae, Akros, Regeneron, Allergan, Novan, Biogen MA, Sienna, UCB, Celgene, Botanix, Incyte, Avillion, and Exicure. He has received personal fees from Novartis, Pfizer, Amgen, Lilly, Boehringer, BiogenIdec, Abbvie, Leo Pharma, Escalier, Valeant, Aurigne, Allergan, Asana, UCB, Sienna, Celgene, Nimbus, Menlo, Aristea, Sanofi, Sun Pharma, Almirall, Arena, and BMS. JSB has grants from the National Institutes of Health/National Heart, Lung, and Blood Institute related to the topic and has served on the advisory board for Amgen and Jannsen.
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at www.jidonline.org, and at https://doi.org/10.1016/j.jid.2021.07.190.
One patient in the No Rx group did not have a follow-up LDL-C, whereas two atorvastatin-treated patients with psoriasis did not have adequate follow-up brachial vein endothelial collection. meanEC of LTB, CCL3, CX3CL1, CCL2, CXCL1, ICAM1, iNOS, IL-8, IL-1B, COX-2 is shown. CI, confidence interval; meanEC, mean expression of proinflammatory vascular endothelial transcript; LDL-C, low-density lipoprotein cholesterol; No Rx, no treatment.
REFERENCES
- Adhyaru BB, Jacobson TA. Safety and efficacy of statin therapy. Nat Rev Cardiol 2018;15: 757–69. [DOI] [PubMed] [Google Scholar]
- Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice guidelines [published correction appears in J Am Coll Cardiol 2019;74:1428–9] [published correction appears in J Am Coll Cardiol 2020;75:840]. J Am Coll Cardiol 2019;74:1376–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garshick M, Underberg JA. The use of primary prevention statin therapy in those predisposed to atherosclerosis. Curr Atheroscler Rep 2017;19:48. [DOI] [PubMed] [Google Scholar]
- Garshick MS, Barrett TJ, Wechter T, Azarchi S, Scher JU, Neimann A, et al. Inflammasome signaling and impaired vascular health in psoriasis. Arterioscler Thromb Vasc Biol 2019;39: 787–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garshick MS, Tawil M, Barrett TJ, Salud-Gnilo CM, Eppler M, Lee A, et al. Activated platelets induce endothelial cell inflammatory response in psoriasis via COX-1. Arterioscler Thromb Vasc Biol 2020;40:1340–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greb JE, Goldminz AM, Elder JT, Lebwohl MG, Gladman DD, Wu JJ, et al. Psoriasis. Nat Rev Dis Primers 2016;2:16082. [DOI] [PubMed] [Google Scholar]
- Jelic S, Lederer DJ, Adams T, Padeletti M, Colombo PC, Factor PH, et al. Vascular inflammation in obesity and sleep apnea. Circulation 2010;121:1014–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kianifard F, Islam MZ. A guide to the design and analysis of small clinical studies. Pharm Stat 2011;10:363–8. [DOI] [PubMed] [Google Scholar]
- Michelson AD, Barnard MR, Krueger LA, Frelinger AL 3rd, Furman MI. Evaluation of platelet function by flow cytometry. Methods 2000;21:259–70. [DOI] [PubMed] [Google Scholar]
- Ports WC, Fayyad R, DeMicco DA, Laskey R, Wolk R. Effectiveness of lipid-lowering statin therapy in patients with and without psoriasis. Clin Drug Investig 2017;37:775–85. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008;359:2195–207. [DOI] [PubMed] [Google Scholar]
- Verma D, Fekri SZ, Sigurdardottir G, Bivik Eding C, Sandin C, Enerbäck C. Enhanced inflammasome activity in patients with psoriasis promotes systemic inflammation. J Invest Dermatol 2021;141:586–95.e5. [DOI] [PubMed] [Google Scholar]
- Wu JJ, Poon KY, Channual JC, Shen AY. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis [published correction appears in Arch Dermatol 2013;149:158]. Arch Dermatol 2012;148:1244–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


