Abstract
Purpose of Review: To summarise and discuss the implications of recent technological advances in heart failure care.
Recent Findings: Heart failure remains a significant source of morbidity and mortality in the US population despite multiple classes of approved pharmacological treatments. Novel cardiac devices and technologies may offer an opportunity to improve outcomes. Baroreflex Activation Therapy and Cardiac Contractility Remodelling may improve myocardial contractility by altering neurohormonal stimulation of the heart. Implantable Pulmonary Artery Monitors and Biatrial Shunts may prevent heart failure admissions by altering the trajectory of progressive congestion. Phrenic Nerve Stimulation offers potentially effective treatment for comorbid conditions. Smartphone applications offer an intriguing strategy for improving medication adherence.
Summary: Novel heart failure technologies offer promise for reducing this public health burden. Randomized controlled studies are indicated for assessing the future role of these novel therapies.
Keywords: Heart failure, technology, cardiac device, pulmonary artery pressure monitoring, phrenic nerve stimulation, baroreflex activation therapy, biatrial shunts, cardiac contractility, modulation healthcare mobile phone applications
1. INTRODUCTION
Heart failure is a chronic health condition that is projected to affect 12% of US citizens aged over 70 [1] and is associated with a significant burden of morbidity and mortality. In the US, the number of primary admissions for heart failure is reducing, yet remainsat over 1 million/year [2]. HF-associated mortality remains high at 14-21% at 2 years [3, 4], despite the availability of 9 pharmacological classes approved for HF treatment [5]. It is hoped that similar technological innovations in the non-pharmacological sphere could complement the pharmaceutical armamentarium to improve patient outcomes [6].
The heart is unique amongst organs in that its mechanical structure powering unidirectional flow through sequential chambers permits innovative technological changes to augment function. Impressive advances have been made recently for valvular heart disease (e.g. TAVR and Mitraclip) and coronary disease (e.g. coronary stents). Heart failure already has several devices with class I guideline recommendations, including ICDs and CRT and LVADs in well-defined subpopulations. This paper will not discuss these well-established technologies, although interested readers are referred to other publications [7]. This chapter will instead focus onnovel technologies, particularly those that are in later stages of development under the FDA’s breakthrough device program.
2. PULMONARY ARTERY PRESSURE MONITORS
The natural trajectory of heart failure is significantly and adversely altered by hospital admission requiring intravenous diuresis, with a subsequent 5.9-fold increased risk of death [8]. Because subclinical congestion frequently commences weeks to months prior to the onset of symptoms, earlier detection and outpatient treatment of congestion may offer an opportunity to prevent HF admission [9].
Indwelling Pulmonary Artery Monitors (PAM) allow for real-time assessment of right-sided cardiac pressures. A PAM system usually comprises adelivery catheter that implants a hermetically sealed implantable wireless sensor into the main pulmonary artery. A patient electronic system then regularly uploads the measurement data to a central database, where it is available for viewing by the patient’s physician. The Cardio MEMs system is a PAM system that currently has FDA approval for specific patient subgroups and has been assessed in both HFrEF and HFpEF (Fig. 1).
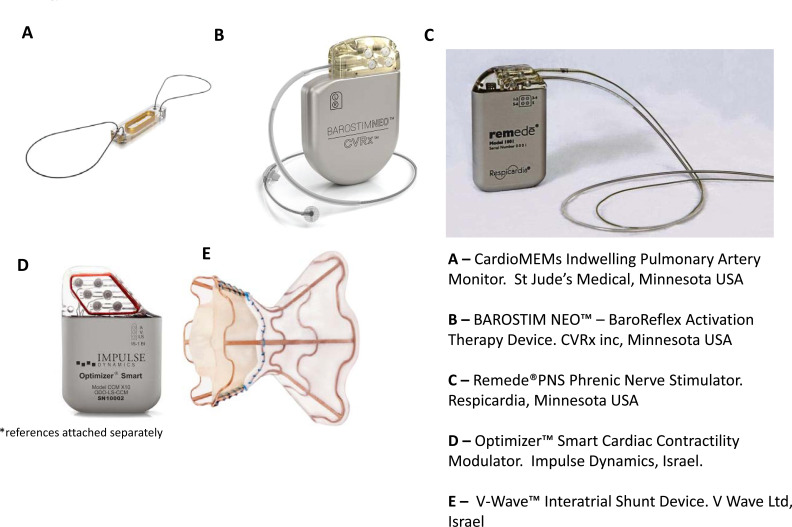
Fig. (1).
Images demonstrating the appearance and physical characteristics of the novel heart failure devices discussed in main text. The devices are not shown to scale. And include: A) CardioMEMs implantable pulmonary artery monitor (Abbott Inc., Atlanta USA); B) BarostimNEO baroreflex activation therapy device (CVRx Inc., Minneapolis USA); C) Remede phrenic nerve stimulator (Respicardia Inc., Minnetonka USA); D) Optimizer Smart (Impulse Dynamics Inc., New York USA); E) V-Wave interatrial shunt (V Wave Inc., Caesarea Israel)
In Champion, the largest randomized, placebo-controlled trial of PAM to date, 456 HFrEF patients underwent Cardio MEMs device implantation and were subsequently randomized into two groups with clinicians being blinded to (control) or having access to PA pressures (treatment), with a target PA diastolic pressure < 20 mmHg in the latter [10]. Participants had mean baseline characteristics of age 60 years, LVEF 25% and PCWP 18 mm Hg. Exclusion criteria included active infection, prior venous thromboembolism and severe renal dysfunction (CrCl< 25 mL/m2). A significant 28% reduction in HF admissions (p=0.001) was seen over 18 months with a non-significant 32% reduction in mortality (p = 0.06). Procedural adverse events were similar to those of standard right-heart catheterization. For a separate subgroup of 119 HFpEF patients, HF hospitalization was 46% lower in the treatment group (p < 0.0001) [11].
On the basis of these findings, the Cardio MEMs system was approved by the FDA in 2014 for HF patients that had NYHA III dyspnoea and a HF admission in the past 12 months. It has also been given a class II-b recommendation in the 2016 European Heart Failure guidelines [12]. It has not to date been discussed in North American guidelines. Cost-effectiveness studies havecalculated an Incremental Cost-Effectiveness Ratio of US$44,832 in 2013 US dollars, suggesting that the system may be cost-effective, although these findings were contingent on whether observed health benefits were maintained long-term [13]. The GUIDE-HF RCT (NCT03387813) of 1022 HF patients with expanded indications, has recently been published. A suggested schema for patient selection for PAM and other cardiac devices is presented (Fig. 2).
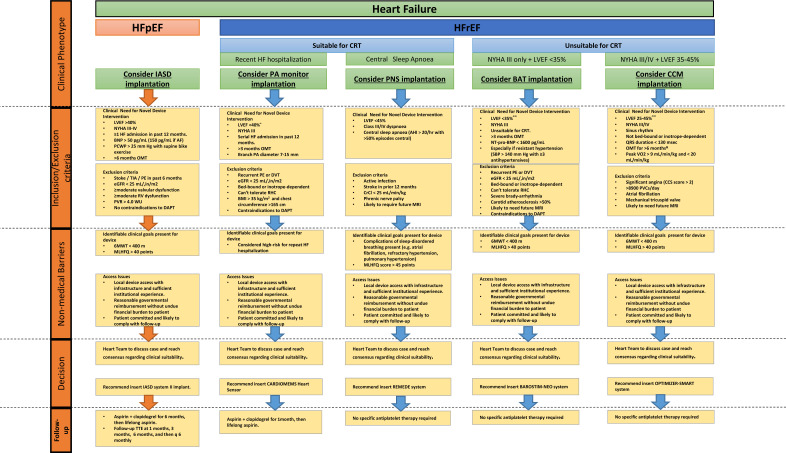
Fig. (2).
Suggested scheme for identifying patients in clinical practice that might benefit from utilization of a novel cardiac device to improve their heart failure symptoms and trajectory, and may assist in matching patient subgroups to devices with demonstrated benefit to that subgroup
3. PHRENIC NERVE STIMULATION
Central sleep apnoea has been estimated to occur in 30-50% of HF patients [14]. It is characterized by periodic breathing (i.e. Cheyne-Stokes breathing) followed by withdrawal of brainstem-driven respiratory drive resulting in apnoeic episodes. This causes a cumulative increase in sympathetic nervous system activity with elevated nocturnal noradrenaline levels [15]. CSA presence is associated with 53% increase in HF hospitalizations and 6% increase in mortality [16]. Servo-ventilation CSA treatment was initially considered as a potential therapeutic target in HF patients with CSA, but the SERVE-HF study showed servo-ventilation was associated with a 34% increase in cardiovascular mortality (p = 0.006), mostly due to an increase in sudden cardiac death [17].
An alternative approach to treating CSA in HF is phrenic nerve stimulation. This system includes a neurostimulator which is typically implanted in the right pectoral region (to allow for possible ICD/CRT insertion on left side), with a stimulator lead placed in the right brachiocephalic vein adjacent to the diaphragm. The system uses an in-built motion and position sensor to identify periods of supine inactivity as sleep, at which point the phrenic nerve stimulation (PNS) is switched on. Unlike CRT, PNS cannot cause a “hiccup-like” sensation because the amplitude and duration of the stimulation areconfigured for respiratory rather than cardiac capture [18].
Phrenic nerve stimulation has been demonstrated to reduce apnoeic-hyponoeic episodes by 60% [19]. Although to date, there have not been any RCTs restricted to the HF population, one RCT of 151 pts included a subset of 96 HFrEF patients, 64% of total participants [18]. For all 151 participants, PNS was associated with a 48% reduction in Apnoea-Hypopnoea Index and also improved respiration, sleep quality and quality of life. For the subset of 96 HFrEF pts, PNS was associated with a 7-point improvement in the Minnesota-Living-With-Heart-Failure-Questionaire(MLWHFQ), 4% absolute improvement in LVEF (p = 0.004) and numerically fewer HF admission in the PNS group (4.7% vs 15%, p = 0.15), although the study was underpowered for this outcome [20].
In October 2017, the RemedePNS system was approved by the FDA for treatment of CSA in adults. It has not been discussed in contemporary HF guidelines and is unlikely to be so until further RCTs are conducted. A post-approval prospective longitudinal single-arm cohort study of PNS is ongoing (NCT03884660). There is contemporary guideline support for screening for sleep disordered breathing in HF patients with recognized cardiac complications or respiratory disease (e.g. atrial fibrillation, refractory hypertension, pulmonary hypertension) [21]. For these patients, PNS may be considered as a therapeutic option if logistical and cost barriers are not prohibitive.
4. BAROREFLEX ACTIVATION THERAPY
Heart failure is characterized by an imbalance of autonomic nervous system input. Chronic exaggerated sympathetic nervous system activity is accompanied by down-regulation of parasympathetic tone, leading to β-adrenoreceptor desensitization and cardiomyocyte apoptosis and culminates in adverse cardiac remodelling. Guideline-directed medical therapy may ameliorate the down-stream complications but does not restore healthy autonomic balance [22].
Initial trials studied the benefit of increased parasympathetic nervous system activity via vagal nerve stimulation. However, a large RCT of vagal nerve stimulation in 707 HFrEF patients was stopped early due to futility [23]. The alternative concept of Baroreflex Activation Therapy [BAT] via carotid stimulation has the advantage of simultaneously down-regulating the sympathetic nervous system and upregulating the parasympathetic nervous system. Animal studies have observed an association of blood pressure responses with physiological down-stream effects of BAT, including up to 35% decrease in plasma noradrenaline levels [24], inhibition of renin secretion [24], significantly reduced muscle sympathetic nerve activity [25] and up to 45/% reduction in noradrenaline spillover [26]. Each of these pathway alterations could plausibly culminate in improved arterial and venous compliance and reduced peripheral vascular resistance, which could in turn improve myocardial contractility.
The BAROSTIM NEO system (CVRx Inc., Minneapolis, USA) is currently the most tested BAT system. It comprises a single carotid sinus lead and a generator with a battery life of 3 years. Implantation requires a vascular surgical procedure where the carotid is exposed and mapped for the optimal stimulation point with the lead subsequently sutured and covered subcutaneously, with no endovascular approach. An open-label RCT of 146 HFrEF patients followed for 6 months found that BAT was associated with improved 6-minute-walk test (+59.6 ±14.0 metres vs +1.52 ±13.2 metres, p = 0.0004), improved quality of life (MLWHFQ score -17.42 ± 2.8 vs +2.1 ± 3.1, p < 0.0001) and reduced NT-pro-NBP (-69.0 pg/mL vs +129.5, p = 0.02) [27]. There was a non-significant trend towards fewer HF hospitalized days in BAT group (p = 0.08). 14.1% had procedural related complications, the majority of which were minor.
BAROSTIM NEO system is currently approved for use in Europe [28], and recently received pre-market FDA approval in the US. A preliminary study suggested that the BAROSTIM NEO system has a 59% probability of being cost-effective [29], but further information is needed. The BeAT-HF study is an ongoing larger open-label RCT that aims to recruit at least 480 patients (NCT02627196) and is due to be completed in 2021. It is hoped that this study may pave the way for this additional therapeutic option for a challenging disease.
5. BI-ATRIAL SHUNTS
Heart Failure with preserved Ejection Fraction (HFpEF) accounts fornearly half of HF admissions and is associated with significantly increased morbidity and mortality [30]. Unlike HFrEF, HFpEF currently lacks a pharmacological armamentarium demonstrated to alter the natural trajectory, and the majority of interventions are aimed towards risk factor management and congestion control [12]. It has been speculated that one reason for multiple RCTs failing to demonstrate clinical benefit is that HFpEF may represent a cluster of different pathophysiological processes to different degrees, including myocardial fibrosis, endothelial dysfunction and autonomic dysfunction, each culminating in chronically elevated left atrial pressures [31, 32]. An intervention that acts at the common bottleneck of relieving left atrial pressure could be an effective treatment for all subgroups.
Percutaneous insertion of an interatrial shunt could offload chronically elevated left atrial pressures. The two most assessed biatrial shunts (BAT) are the IASD II system (Corvia Medical, Tewkohury, MA, USA) and the V-Wave system (V-Wave Caesarea, Israel). The IASD II system comprises a self-expanding, double disc-metal cage that surrounds an 8 mm lumen and is delivered via a 16-French sheath. The 8 mm lumen has been calculated to create a Qp:Qs of approximately 1.2 to 1.3 to prevent volume overloading of right-sided chambers. The REDUCE LAP I study was a phase 2, randomized, blinded multicentre study of 94 HFpEF pts (all had LVEF >40%, patients with right ventricular dysfunction excluded) [33]. Implantation of IASD II shunt was associated with significant PCWP reduction at rest (-5.0 +/- 5.7 mm Hg vs 0 +/- 0.6 mm Hg at rest, p = 0.002) and a nonsignificant peak PCWP reduction of 3.0 mm Hg during exercise (p = 0.14). Pulmonary artery oxygenation increased at 6 months from IASD insertion from 69% to 75% associated with a small stable increase in RV end-diastolic volume at 12 months, consistent with a small left- to-right shunt [34]. REDUCE LAP-II trial (NCT 030880332) is currently actively recruiting HFpEF patients and may be completed by 2021.
The V-Wave differs in that itis an hourglass-shaped shunt containing a porcine trileaflet bioprosthetic valve, so only left-to-right shunting can occur. A phase 1 study of 38 patients with either HFrEF or HFpEF for 12 months saw a -5.3 mm Hg reduction in PCWP [35]. Concerningly, approximately 50% of patients had partial or complete reduction in shunt luminal patency by 12 months. The ongoing RELIEVE-HF study of the V-Wave (NCT 03499236) aims to recruit 500 HF patients (either HFpEF or HFrEF). In the interim, the FDA has given the V-Wave a breakthrough device designation, which is designed to accelerate development by spreading the burden of demonstrating device safety and effectiveness over the product life-cycle.
6. CARDIAC CONTRACTION MODULATION
Whilst Cardiac Resynchronization Therapy (CRT) is as sociated with significant improvement in morbidity and mor tality in HF patients, only 16% of HF-hospitalized patients meet class I or Class IIA criteria for CRT implantation [36]. This leaves 84% currently without a cardiac device option to improve LVEF.
HF causes down-regulation of genes involved in calcium handling, such as phospholamban and sarcoplasmic-reticulum ATP-dependent calcium pump [37], which can reduce contractile strength. In animal models, delivery of current during cardiomyocyte refractory period leads to up-regulation of L-type calcium channels, increased intracellular calcium uptake and release and has been demonstrated to improve contractile strength [38]. This intervention is described as cardiac contraction modulation (CCM). Exploratory data has also suggested the possibility that CCM may cause beneficial LV remodelling [37].
Currently, the only CCM device to have undergone an RCT is the Optimizer Smart device (Impulse Dynamics, Orangeburg, New York). The system comprises of a stimulator inserted into the right pectoral region with transvenous leads inserting into the right ventricular septum that send high strength signals (approximately 7.5 mV for 20 msec) for one-hour periods up to seven times per day. It has been most studied in sinus rhythm, although itcan be programmed to detect the refractory period in AF, datain this subgroup is limited.
The FIX-HF-5 is the largest CCM RCT to date, having enrolled 428 NYHA III/IV HF patients, all with LVEF < 35% and QRS < 130 msec [39]. The study was unblinded and conducted prospectively over 12 months. The primary endpoint of the increased anaerobic threshold was not reached (≥20% in VO2 achieved in 17.9% vs. 11.8%, p = 0.09). However, significant differences in secondary endpoints were seen, including improved quality of life with absolute -11.8-point reduction in MLWHFQ score. Non-inferiority of adverse events was also seen (p = 0.035).
As there was a statistically significant interaction demonstrated between LVEF and increased anaerobic threshold, this suggests a clinical benefit of CCM may be to patients confined to those with LVEF >25% [40]. Whilst a sham controlled study was not performed in FIX-HF-5 [39], it should be noted that the original feasibility study (FIX-HF-4) included a sham-controlled arm randomizing 164 patients to either CCM or sham device for 3 months [37], and a vice-versa crossover, and achieved better quality of life scores in the CCM group.
A subsequent unblinded RCT, FIX-HF-5C, enrolled 160 patients with LVEF 25-45% and found a significant increase in anaerobic threshold (VO2 increased by 0.84 mL/kg.min with Bayesian Credible Interval 0.132 to 0.155) [41]. Secondary outcomes included significant increase in 6MWT 9.3 ± 87.4 m (p = 0.009) and an absolute -11.7-point drop in MLWHFQ (p < 0.0001). The greatest improvements were seen in patients with LVEF in 35-45% range. Additionally, additional data from two registry-based studies of 81 and 106 patients have suggested that LVEF is improved by 2.5 to 5 percentage points in the CCM group compared to optimal medical therapy [42, 43] over a 2 year follow-up period.
On the basis of these findings, FDA granted the Optimizer Smart breakthrough device designation, and may be considered for patients in sinus rhythm, NYHA II-IV, LVEF 25-45% and not a candidate for CRT therapy. Cost is likely to be a barrier, as the currently listed 2020 Medicare average payment for CCM insertion procedure is US$23,155. Preliminary data suggest CCM could be cost-effective in carefully selected patients [44]. An pilot open-label study of CCM in 60 HFpEF patients is currently recruiting (NCT03240237).
7. SMARTPHONE APPLICATIONS
Although less invasive, smartphone applications are also a novel technological means of enhancing heart failure care that may yet prove to have substantial impact. Medication non-adherence has been demonstrated to be a source of HF morbidity, with partial non-adherence rates estimated to be as high as 50% [45]. Medication adherence to < 80% of doses is associated with increased risk (HR 2.07, 95% CI 0.62 - 2.64) for all-cause mortality or HF hospitalization. Mobile phone applications (or “apps” for short) have been identified in a recent ACC/AHA expert consensus document as a potential strategy for improving adherence [5]. The majority of HF patients are likely to be eligible for this approach, as 81% of US citizens have a smartphone [46].
A recent systematic review of 27 studies of mobile health interventions in multiple diseases found that apps improved medication adherence (OR 4.51, p<0.0001) [47], supporting the concept of mobile phone app as a health invention. There currently have not been any RCTs of mobile phone apps restricted to the HF population. A technological review of 35 heart-failure related healthcare apps found that the majority did not meet high standards either technologically or clinically [48]. Two apps (Heart Failure Health Storylines and Symple) of the 34 were found to be associated both with the greatest degree of behavioural change and greatest technological sophistication. Of the two, Heart Failure Health Storylines was the only app that addressed all 8 behaviour-modification targets specified by the Heart Failure Society of America (HFSA), likely owing to its origin as a collaboration project with HFSA [49]. Heart Failure Health sends timed reminders to take medications, can track multiple symptoms simultaneously with color-coding to assist in communicating severity. It is available as a free download of 21.9 Mb size on either iTunes of Google app store and has a printed privacy policy. It is hoped that in the future an RCT might be conducted of novel software like this to delineate clinical impact.
CONCLUSION
The pace of pharmacological advancements in HF has not kept up with the increasing proportion population affected. It is noteworthy that the two classes cited as the backbone of HFrEF treatment, ACE inhibitors and beta-blockers, were both discovered over 40 years ago. The novel technological treatments listed here may assist in advancing HF care [6], and it is encouraging that the majority have ongoing RCTs that are actively recruiting. Publication of successful RCTs is a prerequisite step before international guideline recommendationscan occur.
An ongoing challenge unique to technological treatments is non-uniform regulatory requirements globally, which can lead to uneven implementation and costs. For example, the Watchman left atrial appendage exclusion device was approved for use in Europe a decade before it was approved in the US [50]. Another unique challenge is the higher cost, as the cost of a trial device with several hundred patients can exceed US$100 million [51], which is much higher than for pharmacological treatments. A recent step in the US to facilitate clinical device trials is the creation of FDA’s breakthrough device program. This program uses sequential trial and endpoint assessment so that research cost is distributed over the product lifespan. This novel approach necessitates the use of Bayesian analysis so that predicting future trial data can be based on accumulating partial trial data (Fig. 3). This statistical approach is expected to become more prominent in future cardiac device trials.
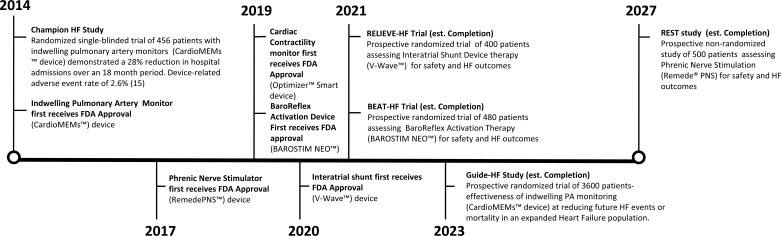
Fig. (3).
Timeline of large clinical trials of novel cardiac devices for heart failure, including important studies scheduled for publication in the upcoming years.
The future prospects of HF technological advancements are promising, and it is to be hoped that they may improve outcomes for our HF patients.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.
REFERENCES
- 1.Roger V.L., Go A.S., Lloyd-Jones D.M., Benjamin E.J., Berry J.D., Borden W.B., Bravata D.M., Dai S., Ford E.S., Fox C.S., Fullerton H.J., Gillespie C., Hailpern S.M., Heit J.A., Howard V.J., Kissela B.M., Kittner S.J., Lackland D.T., Lichtman J.H., Lisabeth L.D., Makuc D.M., Marcus G.M., Marelli A., Matchar D.B., Moy C.S., Mozaffarian D., Mussolino M.E., Nichol G., Paynter N.P., Soliman E.Z., Sorlie P.D., Sotoodehnia N., Turan T.N., Virani S.S., Wong N.D., Woo D., Turner M.B. Heart disease and stroke statistics-2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blecker S., Paul M., Taksler G., Ogedegbe G., Katz S. Heart failure–associated hospitalizations in the United States. J. Am. Coll. Cardiol. 2013;61(12):1259–1267. doi: 10.1016/j.jacc.2012.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong P.W., Pieske B., Anstrom K.J., Ezekowitz J., Hernandez A.F., Butler J., Lam C.S.P., Ponikowski P., Voors A.A., Jia G., McNulty S.E., Patel M.J., Roessig L., Koglin J., O’Connor C.M. Vericiguat in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2020;382(20):1883–1893. doi: 10.1056/NEJMoa1915928. [DOI] [PubMed] [Google Scholar]
- 4.McMurray J.J., Packer M., Desai A.S., Gong J., Lefkowitz M.P., Rizkala A.R., Rouleau J.L., Shi V.C., Solomon S.D., Swedberg K., Zile M.R. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014;371(11):993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 5.Yancy C.W., Januzzi J.L., Jr, Allen L.A., Butler J., Davis L.L., Fonarow G.C., Ibrahim N.E., Jessup M., Lindenfeld J., Maddox T.M., Masoudi F.A., Motiwala S.R., Patterson J.H., Walsh M.N., Wasserman A. 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the american college of cardiology task force on expert consensus decision pathways. J. Am. Coll. Cardiol. 2018;71(2):201–230. doi: 10.1016/j.jacc.2017.11.025. [DOI] [PubMed] [Google Scholar]
- 6.Lam C.S.P., Voors A.A., de Boer R.A., Solomon S.D., van Veldhuisen D.J. Heart failure with preserved ejection fraction: from mechanisms to therapies. Eur. Heart J. 2018;39(30):2780–2792. doi: 10.1093/eurheartj/ehy301. [DOI] [PubMed] [Google Scholar]
- 7.Hussein A.A., Wilkoff B.L. Cardiac implantable electronic device therapy in heart failure. Circ. Res. 2019;124(11):1584–1597. doi: 10.1161/CIRCRESAHA.118.313571. [DOI] [PubMed] [Google Scholar]
- 8.Okumura N., Jhund P.S., Gong J., Lefkowitz M.P., Rizkala A.R., Rouleau J.L., Shi V.C., Swedberg K., Zile M.R., Solomon S.D., Packer M., McMurray J.J. Importance of clinical worsening of heart failure treated in the outpatient setting: evidence from the prospective comparison of arni with acei to determine impact on global mortality and morbidity in heart failure trial (PARADIGM-HF). Circulation. 2016;133(23):2254–2262. doi: 10.1161/CIRCULATIONAHA.115.020729. [DOI] [PubMed] [Google Scholar]
- 9.Klein L. (Re)discovering the neurohormonal and hemodynamic duality of heart failure. J. Am. Coll. Cardiol. 2017;70(15):1887–1889. doi: 10.1016/j.jacc.2017.08.058. [DOI] [PubMed] [Google Scholar]
- 10.Givertz M.M., Stevenson L.W., Costanzo M.R., Bourge R.C., Bauman J.G., Ginn G., Abraham W.T. Pulmonary artery pressure-guided management of patients with heart failure and reduced ejection fraction. J. Am. Coll. Cardiol. 2017;70(15):1875–1886. doi: 10.1016/j.jacc.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Adamson P.B., Abraham W.T., Bourge R.C., Costanzo M.R., Hasan A., Yadav C., Henderson J., Cowart P., Stevenson L.W. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ. Heart Fail. 2014;7(6):935–944. doi: 10.1161/CIRCHEARTFAILURE.113.001229. [DOI] [PubMed] [Google Scholar]
- 12.Ponikowski P., Voors A.A., Anker S.D., Bueno H., Cleland J.G.F., Coats A.J.S., Falk V., González-Juanatey J.R., Harjola V.P., Jankowska E.A., Jessup M., Linde C., Nihoyannopoulos P., Parissis J.T., Pieske B., Riley J.P., Rosano G.M.C., Ruilope L.M., Ruschitzka F., Rutten F.H., van der Meer P. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016;37(27):2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 13.Schmier J.K., Ong K.L., Fonarow G.C. Cost-effectiveness of remote cardiac monitoring with the CardioMEMS heart failure system. Clin. Cardiol. 2017;40(7):430–436. doi: 10.1002/clc.22696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khayat R., Jarjoura D., Porter K., Sow A., Wannemacher J., Dohar R., Pleister A., Abraham W.T. Sleep disordered breathing and post-discharge mortality in patients with acute heart failure. Eur. Heart J. 2015;36(23):1463–1469. doi: 10.1093/eurheartj/ehu522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bekfani T., Abraham W.T. Current and future developments in the field of central sleep apnoea. Europace. 2016;18(8):1123–1134. doi: 10.1093/europace/euv435. [DOI] [PubMed] [Google Scholar]
- 16.Khayat R., Abraham W., Patt B., Brinkman V., Wannemacher J., Porter K., Jarjoura D. Central sleep apnea is a predictor of cardiac readmission in hospitalized patients with systolic heart failure. J. Card. Fail. 2012;18(7):534–540. doi: 10.1016/j.cardfail.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cowie M.R., Woehrle H., Wegscheider K., Angermann C., d’Ortho M.P., Erdmann E., Levy P., Simonds A.K., Somers V.K., Zannad F., Teschler H. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N. Engl. J. Med. 2015;373(12):1095–1105. doi: 10.1056/NEJMoa1506459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Costanzo M.R., Ponikowski P., Javaheri S., Augostini R., Goldberg L., Holcomb R., Kao A., Khayat R.N., Oldenburg O., Stellbrink C., Abraham W.T. Transvenous neurostimulation for central sleep apnoea: a randomised controlled trial. Lancet. 2016;388(10048):974–982. doi: 10.1016/S0140-6736(16)30961-8. [DOI] [PubMed] [Google Scholar]
- 19.Costanzo M.R., Ponikowski P., Javaheri S., Augostini R., Goldberg L.R., Holcomb R., Kao A., Khayat R.N., Oldenburg O., Stellbrink C., Abraham W.T. Sustained 12 month benefit of phrenic nerve stimulation for central sleep apnea. Am. J. Cardiol. 2018;121(11):1400–1408. doi: 10.1016/j.amjcard.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 20.Costanzo M.R., Ponikowski P., Coats A., Javaheri S., Augostini R., Goldberg L.R., Holcomb R., Kao A., Khayat R.N., Oldenburg O., Stellbrink C., McKane S., Abraham W.T. Phrenic nerve stimulation to treat patients with central sleep apnoea and heart failure. Eur. J. Heart Fail. 2018;20(12):1746–1754. doi: 10.1002/ejhf.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKelvie R.S., Moe G.W., Cheung A., Costigan J., Ducharme A., Estrella-Holder E., Ezekowitz J.A., Floras J., Giannetti N., Grzeslo A., Harkness K., Heckman G.A., Howlett J.G., Kouz S., Leblanc K., Mann E., O’Meara E., Rajda M., Rao V., Simon J., Swiggum E., Zieroth S., Arnold J.M., Ashton T., D’Astous M., Dorian P., Haddad H., Isaac D.L., Leblanc M.H., Liu P., Sussex B., Ross H.J. The 2011 Canadian Cardiovascular Society heart failure management guidelines update: focus on sleep apnea, renal dysfunction, mechanical circulatory support, and palliative care. Can. J. Cardiol. 2011;27(3):319–338. doi: 10.1016/j.cjca.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz P.J., La Rovere M.T., De Ferrari G.M., Mann D.L. Autonomic modulation for the management of patients with chronic heart failure. Circ. Heart Fail. 2015;8(3):619–628. doi: 10.1161/CIRCHEARTFAILURE.114.001964. [DOI] [PubMed] [Google Scholar]
- 23.Gold M.R., Van Veldhuisen D.J., Hauptman P.J., Borggrefe M., Kubo S.H., Lieberman R.A., Milasinovic G., Berman B.J., Djordjevic S., Neelagaru S., Schwartz P.J., Starling R.C., Mann D.L. Vagus Nerve Stimulation for the Treatment of Heart Failure: The INOVATE-HF Trial. J. Am. Coll. Cardiol. 2016;68(2):149–158. doi: 10.1016/j.jacc.2016.03.525. [DOI] [PubMed] [Google Scholar]
- 24.Lohmeier T.E., Irwin E.D., Rossing M.A., Serdar D.J., Kieval R.S. Prolonged activation of the baroreflex produces sustained hypotension. Hypertension. 2004;43(2):306–311. doi: 10.1161/01.HYP.0000111837.73693.9b. [DOI] [PubMed] [Google Scholar]
- 25.Heusser K., Tank J., Engeli S., Diedrich A., Menne J., Eckert S., Peters T., Sweep F.C., Haller H., Pichlmaier A.M., Luft F.C., Jordan J. Carotid baroreceptor stimulation, sympathetic activity, baroreflex function, and blood pressure in hypertensive patients. Hypertension. 2010;55(3):619–626. doi: 10.1161/HYPERTENSIONAHA.109.140665. [DOI] [PubMed] [Google Scholar]
- 26.Lohmeier T.E., Iliescu R., Dwyer T.M., Irwin E.D., Cates A.W., Rossing M.A. Sustained suppression of sympathetic activity and arterial pressure during chronic activation of the carotid baroreflex. Am. J. Physiol. Heart Circ. Physiol. 2010;299(2):H402–H409. doi: 10.1152/ajpheart.00372.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abraham W.T., Zile M.R., Weaver F.A., Butter C., Ducharme A., Halbach M., Klug D., Lovett E.G., Müller-Ehmsen J., Schafer J.E., Senni M., Swarup V., Wachter R., Little W.C. Baroreflex activation therapy for the treatment of heart failure with a reduced ejection fraction. JACC Heart Fail. 2015;3(6):487–496. doi: 10.1016/j.jchf.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Mann J.A., Abraham W.T. Cardiac contractility modulation and baroreflex activation therapy in heart failure patients. Curr. Heart Fail. Rep. 2019;16(1):38–46. doi: 10.1007/s11897-019-0422-3. [DOI] [PubMed] [Google Scholar]
- 29.Borisenko O., Müller-Ehmsen J., Lindenfeld J., Rafflenbeul E., Hamm C. An early analysis of cost-utility of baroreflex activation therapy in advanced chronic heart failure in Germany. BMC Cardiovasc. Disord. 2018;18(1):163. doi: 10.1186/s12872-018-0898-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfeffer M.A., Shah A.M., Borlaug B.A. Heart failure with preserved ejection fraction in perspective. Circ. Res. 2019;124(11):1598–1617. doi: 10.1161/CIRCRESAHA.119.313572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shah S.J., Katz D.H., Selvaraj S., Burke M.A., Yancy C.W., Gheorghiade M., Bonow R.O., Huang C.C., Deo R.C. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation. 2015;131(3):269–279. doi: 10.1161/CIRCULATIONAHA.114.010637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaye D.M., Nanayakkara S. Interatrial shunt device for heart failure with preserved ejection fraction. Front. Cardiovasc. Med. 2019;6:143. doi: 10.3389/fcvm.2019.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feldman T., Mauri L., Kahwash R., Litwin S., Ricciardi M.J., van der Harst P., Penicka M., Fail P.S., Kaye D.M., Petrie M.C., Basuray A., Hummel S.L., Forde-McLean R., Nielsen C.D., Lilly S., Massaro J.M., Burkhoff D., Shah S.J. Transcatheter interatrial shunt device for the treatment of heart failure with preserved ejection fraction (REDUCE LAP-HF I [reduce elevated left atrial pressure in patients with heart failure]): a phase 2, randomized, sham-controlled trial. Circulation. 2018;137(4):364–375. doi: 10.1161/CIRCULATIONAHA.117.032094. [DOI] [PubMed] [Google Scholar]
- 34.Kaye D.M., Hasenfuß G., Neuzil P., Post M.C., Doughty R., Trochu J.N., Kolodziej A., Westenfeld R., Penicka M., Rosenberg M., Walton A., Muller D., Walters D., Hausleiter J., Raake P., Petrie M.C., Bergmann M., Jondeau G., Feldman T., Veldhuisen D.J., Ponikowski P., Silvestry F.E., Burkhoff D., Hayward C. One-year outcomes after transcatheter insertion of an interatrial shunt device for the management of heart failure with preserved ejection fraction. Circ. Heart Fail. 2016;9(12):9. doi: 10.1161/CIRCHEARTFAILURE.116.003662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodés-Cabau J., Bernier M., Amat-Santos I.J., Ben Gal T., Nombela-Franco L., García Del Blanco B., Kerner A., Bergeron S., Del Trigo M., Pibarot P., Shkurovich S., Eigler N., Abraham W.T. Interatrial shunting for heart failure: early and late results from the first-in-human experience with the v-wave system. JACC Cardiovasc. Interv. 2018;11(22):2300–2310. doi: 10.1016/j.jcin.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 36.Osmanska J., Hawkins N.M., Toma M., Ignaszewski A., Virani S.A. Eligibility for cardiac resynchronization therapy in patients hospitalized with heart failure. ESC Heart Fail. 2018;5(4):668–674. doi: 10.1002/ehf2.12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borggrefe M., Mann D.L. Cardiac contractility modulation in 2018. Circulation. 2018;138(24):2738–2740. doi: 10.1161/CIRCULATIONAHA.118.036460. [DOI] [PubMed] [Google Scholar]
- 38.Lawo T., Borggrefe M., Butter C., Hindricks G., Schmidinger H., Mika Y., Burkhoff D., Pappone C., Sabbah H.N. Electrical signals applied during the absolute refractory period: an investigational treatment for advanced heart failure in patients with normal QRS duration. J. Am. Coll. Cardiol. 2005;46(12):2229–2236. doi: 10.1016/j.jacc.2005.05.093. [DOI] [PubMed] [Google Scholar]
- 39.Kadish A, Nademanee K, Volosin K, et al. A randomized controlled trial evaluating the safety and efficacy of cardiac contractility modulation in advanced heart failure. Am Heart J. 2011;161:329–337. doi: 10.1016/j.ahj.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 40.Abraham W.T., Nademanee K., Volosin K., Krueger S., Neelagaru S., Raval N., Obel O., Weiner S., Wish M., Carson P., Ellenbogen K., Bourge R., Parides M., Chiacchierini R.P., Goldsmith R., Goldstein S., Mika Y., Burkhoff D., Kadish A. Subgroup analysis of a randomized controlled trial evaluating the safety and efficacy of cardiac contractility modulation in advanced heart failure. J. Card. Fail. 2011;17(9):710–717. doi: 10.1016/j.cardfail.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 41.Abraham W.T., Kuck K.H., Goldsmith R.L., Lindenfeld J., Reddy V.Y., Carson P.E., Mann D.L., Saville B., Parise H., Chan R., Wiegn P., Hastings J.L., Kaplan A.J., Edelmann F., Luthje L., Kahwash R., Tomassoni G.F., Gutterman D.D., Stagg A., Burkhoff D., Hasenfuß G. A randomized controlled trial to evaluate the safety and efficacy of cardiac contractility modulation. JACC Heart Fail. 2018;6(10):874–883. doi: 10.1016/j.jchf.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 42.Kuschyk J., Roeger S., Schneider R., Streitner F., Stach K., Rudic B., Weiß C., Schimpf R., Papavasilliu T., Rousso B., Burkhoff D., Borggrefe M. Efficacy and survival in patients with cardiac contractility modulation: long-term single center experience in 81 patients. Int. J. Cardiol. 2015;183:76–81. doi: 10.1016/j.ijcard.2014.12.178. [DOI] [PubMed] [Google Scholar]
- 43.Müller D., Remppis A., Schauerte P., Schmidt-Schweda S., Burkhoff D., Rousso B., Gutterman D., Senges J., Hindricks G., Kuck K.H. Clinical effects of long-term cardiac contractility modulation (CCM) in subjects with heart failure caused by left ventricular systolic dysfunction. Clin. Res. Cardiol. 2017;106(11):893–904. doi: 10.1007/s00392-017-1135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maniadakis N.F.V., Mylonas C., Sharma R., Stewart Coats A.J. Economic Evaluation of Cardiac Contractility Modulation (CCM) therapy with the optimizer IVs in the management of heart failure patients. International Cardiovascular Forum Journal. 2015;4:43–52. [Google Scholar]
- 45.Fitzgerald A.A., Powers J.D., Ho P.M., Maddox T.M., Peterson P.N., Allen L.A., Masoudi F.A., Magid D.J., Havranek E.P. Impact of medication nonadherence on hospitalizations and mortality in heart failure. J. Card. Fail. 2011;17(8):664–669. doi: 10.1016/j.cardfail.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 46.Pew research centre. Demographics of Mobile Device Ownership and Adoption in the United States. 2019 [Google Scholar]
- 47.Gandhi S., Chen S., Hong L., Sun K., Gong E., Li C., Yan L.L., Schwalm J.D. Effect of mobile health interventions on the secondary prevention of cardiovascular disease: systematic review and meta-analysis. Can. J. Cardiol. 2017;33(2):219–231. doi: 10.1016/j.cjca.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 48.Masterson Creber R.M., Maurer M.S., Reading M., Hiraldo G., Hickey K.T., Iribarren S. Review and analysis of existing mobile phone apps to support heart failure symptom monitoring and self-care management using the mobile application rating scale (MARS). JMIR Mhealth Uhealth. 2016;4(2):e74. doi: 10.2196/mhealth.5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.HFSA's Heart Failure Storylines App ranks Amopng the top 4 HF Apps. 2016. Available from:(Accessed 16th May 2020). https://hfsaorg/hfsas-heart-failure-health-storylines-app-ranks-among-top-4-hf-apps.
- 50.Stein K.M. The long and winding road after fda approval: a medical device industry perspective. Circulation. 2017;135(20):1877–1878. doi: 10.1161/CIRCULATIONAHA.117.024633. [DOI] [PubMed] [Google Scholar]
- 51.Zile M.R., Abraham W.T., Lindenfeld J., Weaver F.A., Zannad F., Graves T., Rogers T., Galle E.G. First granted example of novel FDA trial design under Expedited Access Pathway for premarket approval: BeAT-HF. Am. Heart J. 2018;204:139–150. doi: 10.1016/j.ahj.2018.07.011. [DOI] [PubMed] [Google Scholar]