Abstract
Introduction: Despite the convincing epidemiologic association between smoking and vascular disease, the pathophysiologic mechanisms by which smoking initiates and contributes to the progression of atherosclerosis remain incompletely understood. A precise dose-dependent correlation has never been demonstrated, suggesting that the biological relationship is complex and influenced by individual genetic and possibly environmental factors. Although endothelial dysfunction and intimal damage appear to be central to atherogenesis, how tobacco products cause this effect has not been established. The purpose of this review is to describe the current state of knowledge of the main pathophysiologic pathways of how tobacco smoking abets atherosclerosis
Constituents of Tobacco Smoke: Tobacco combustion produces a mixture of organic substances derived from burning organic materials. The predominant gaseous phase constituents include carbon monoxide, acetaldehyde, formaldehyde, acrolein, and other carbonyls, as well as nicotine and tobacco-specific nitrosamines.
Potential Pathophysiologic Mechanisms: Smoking-induced changes in coronary vasomotor tone, platelet activation, and endothelial integrity are major components of both the development of atherosclerosis and its clinical presentation. Smoking may initiate and accelerate the progression of atherosclerosis by injuring the vascular intima. Other potential mechanisms include intimal damage and endothelial dysfunction, oxidative stress and injury, thrombosis, lipid abnormalities, and inflammation.
Conclusion: Smoking tobacco products contributes measurably to the incidence of acute vascular events and chronic disease. The causative compound, the exact mechanism of injury, and whether the atherogenic effect is modifiable are not known.
Keywords: Smoking, tobacco, pathogenesis, atherosclerosis, nicotine, vascular disease
1. INTRODUCTION
Smoking is the most important modifiable risk factor associated with atherogenesis. Tobacco has been implicated in the origin and progression of the atherosclerotic process by numerous population-based studies [1-5]. Tobacco smoking is associated with atherosclerosis, most prominently by contributing as a risk factor for myocardial infarction and coronary artery disease (Table 1) [6]. Low-tar and nicotine cigarettes, smokeless tobacco, and passive smoking are also associated with an increased risk of vascular disease [7-11].
Table 1.
Vascular manifestations of tobacco-related atherosclerosis.
| • Stable ischemic heart disease (including exacerbation of angina pectoris) • Myocardial infarction • Stroke • Aortic aneurysm • Peripheral arterial disease (including intermittent claudication, vascular thrombosis and limb loss) • Erectile dysfunction • Vasospastic angina • Cardiac arrhythmia • Heart failure • Sudden cardiac death |
Despite the convincing epidemiologic association between smoking and vascular disease, the pathophysiologic mechanisms by which smoking initiates and contributes to the progression of atherosclerosis remain incompletely understood. A precise dose-dependent correlation has never been demonstrated [1, 4], suggesting that the biological relationship is complex and influenced by individual genetic and possibly environmental factors. Although endothelial dysfunction and intimal damage appear to be central to atherogenesis, the precise mechanism by which tobacco products cause this effect has not been established. The purpose of this review is to describe the current state of knowledge of the main pathophysiologic pathways of how tobacco smoking abets atherosclerosis.
2. COMPONENTS OF TOBACCO SMOKE
Over 7000 chemical constituents in tobacco smoke have been identified; of these, about 400 are detected routinely in mainstream and sidestream smoke [12-14]. Tobacco combustion produces a mixture of organic substances derived from burning organic materials. The predominant gaseous phase constituents include carbon monoxide, acetaldehyde, formaldehyde, acrolein, and other carbonyls, as well as nicotine and tobacco-specific nitrosamines [15]. These chemicals are further modified by detoxification systems, such as the cytochrome P450 system. Commercial tobacco products contain additional compounds, and their combustion chemistry is unstudied. However, the precise mechanisms of how particular substances contribute to the initiation, progression, and outcome of atherosclerotic disease have not been delineated. It is likely that the inciting constituents of cigarette smoke are not of one compound or class but rather are due to a mixture interacting with genetic and environmental influences [2, 3].
Cigarette smoke drawn through the tobacco into the mouth is termed mainstream smoke. Sidestream smoke is the smoke that emanates from the burning end of a cigarette. Mainstream cigarette smoke comprises 8% tar and 92% gaseous components. Tobacco smoke is the combination of sidestream smoke (85%) and exhaled mainstream smoke (15%). Sidestream cigarette smoke contains a higher concentration of the toxic gaseous component than mainstream cigarette smoke [5, 9, 16, 17].
The two best studied substances in smoke are nicotine and carbon monoxide (CO). While their physiologic effects have been studied for decades, no mechanism directly links these to the pathophysiology leading to atherosclerosis [5, 15]. Although other gaseous constituents have been linked generally to atherosclerosis, no mechanisms have been hypothesized or elucidated.
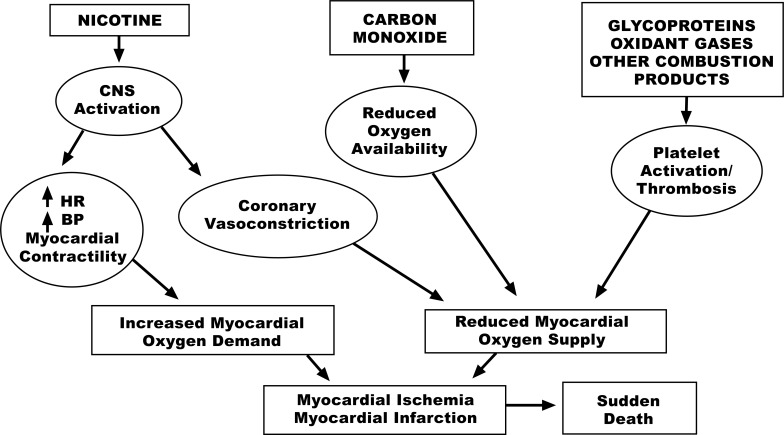
Carbon Monoxide (CO). Serum carbon monoxide levels are increased in smokers. When CO is inhaled, it combines with hemoglobin to form carboxyhemoglobin. CO binds to the same sites as oxygen but with 210 times more affinity. Carboxy-hemoglobin releases carbon monoxide slowly so that less hemoglobin is available to transport oxygen, which greatly diminishes hemoglobin’s oxygen-carrying capacity. As a result, small amounts of CO can substantially reduce hemoglobin’s ability to transport oxygen, resulting in diminished oxygen carrying capacity. This may be a critical concern in patients with severe coronary and peripheral artery disease or congestive heart failure (Fig. 1). Chronic hypoxia also leads to compensatory polycythemia, which increases blood viscosity and contributes to a prothrombotic state.
Fig. (1).
Figure reproduced from benowitz [1].
Nicotine. The most well-studied substance in cigarette smoke impacting the cardiovascular system is nicotine. Nicotine is a component of the tar phase. The addictive quality of tobacco is largely due to nicotine and related alkaloid content. Some chemical constituents of tobacco, such as ammonia, indirectly influence the toxicity of smoke by increasing the pH of inhaled smoke and, therefore, facilitate the absorption of nicotine. Nicotine inhaled from cigarette smoke is absorbed over a large surface area of the lungs and is transported to the brain in 10–20 seconds via pulmonary venous absorption directly to the left heart. A typical cigarette contains 20 mg of nicotine, of which about 2.5 mg is absorbed, with a half-life of about 2 hours. 80-90% is metabolized hepatically. About 80% of nicotine is broken down to cotinine by enzymes in the liver (e.g., CYP2A6). Nicotine is also metabolized in the lungs to cotinine and nicotine-N-oxide. Cotinine and the remaining nicotine are filtered from the blood by the kidneys and excreted in the urine.
Smokeless tobacco users likely take in as much nicotine per day as smokers, but the kinetics are much slower [11]. Nicotine derived from the gum, lozenges, inhalers, and nasal spray is absorbed through the oral or nasal mucosa, entering the venous circulation. These forms of intake cause nicotine levels to peak in the order of minutes, while transdermal patches release nicotine more gradually to peak concentrations within hours after application
Nicotine is a sympathomimetic that is a direct agonist of nicotinic acetylcholine receptors [α2-adrenergic receptor], located in the plasma membranes of certain neurons and on the postsynaptic side of the neuromuscular junction. These receptors are found in the central and peripheral nervous system, muscle, adrenal gland, and brain. They are the primary receptor at the neuromuscular junction mediating motor nerve-muscle communication that controls muscle contraction [5].
Nicotine is primarily responsible for increases in cardiac output, heart rate, and blood pressure during smoking [15]. The rapid increase in heart rate and elevation in blood pressure occurs as a result of direct stimulation by nicotine of peripheral postganglionic adrenergic receptors and carotid and aortic body chemoreceptors. Nicotine activates the sympathetic nervous system by increasing the release of adrenal catecholamines and by increasing peripheral sympathetic nerve activity. Adrenal hormones are released almost immediately with the onset of smoking and likely participate in the systemic hemodynamic response. Nicotine also has proven vasoconstrictive effects on coronary circulation [18-20]. The possibility of nicotine signaling in the vascular wall, with activation of the nicotinic acetylcholine receptors, increasing permeability, has been suggested [21].
Nicotine alters the shape of endothelial cells and reduces prostacyclin production. Increased endothelial cell proliferation and intimal hyperplasia have been observed in animal experimental studies. Interestingly, tobacco smoke has a much greater effect than nontobacco smoke on circulating endothelial cells and platelets, suggesting the involvement of components of tobacco smoke. However, the mechanism of this effect is complex: people exposed lifelong to smokeless tobacco and high levels of nicotine do not have accelerated atherosclerosis. Nicotine causes acute endothelial dysfunction, although to a significantly smaller extent than cigarette smoke. There is little doubt that nicotine and smoking cause endothelial dysfunction and damage the intimal layer. Free radicals contained in the tar and gas phase of cigarette smoke can damage the vascular endothelium, but a direct link to plaque rupture has not been established [20, 22-25].
Although no direct contribution of nicotine to smoking-related atherosclerosis has been demonstrated, it likely does contribute to the occurrence of acute cardiovascular events via its effects on increasing demand and limiting supply [15]. Current evidence suggests that the effects of nicotine are much less important than are the prothrombotic effects of cigarette smoking or the effects of CO. Nicotine itself does not appear to enhance thrombosis [1, 26]. In various models, although high doses of nicotine are associated with atherogenic changes, the majority of current evidence suggests that nicotine, at concentrations similar to a smoker’s blood level, has a minor effect on the initiation or propagation of atherosclerosis [5, 20].
Thiocyanate and Aromatic Amines. The potential roles of these compounds have been discussed for decades, but no definitive evidence exists to implicate them directly in smoking-induced arterial disease [27]. Thiocyanate is a known combustion product of tobacco in small quantities and is metabolized to hydrogen cyanide, which is toxic to endothelial cells. Aromatic amines certainly are carcinogenic and may raise blood pressure, but any link to the pathophysiology of smoking is conjectural.
3. CLINICAL AND PATHOPHYSIOLOGIC EFFECTS OF SMOKING
Hemodynamic Response to Smoking. The systemic hemodynamic response to smoking results from direct effects as well as influences on autonomic and peripheral ganglia, systemic hormonal release, myocardial wall stress, and contractility. Cigarette smoking increases heart rate, systolic blood pressure, myocardial contractility, and cardiac output at 1- 2.5 minutes after the start of smoking, with a peak hemodynamic response after 5 minutes. These effects start within seconds, preceding catecholamine release from the adrenal cortex [15, 20, 28-32].
Myocardial wall stress increases consequent to increased afterload produced by elevated blood pressure. Systemic catecholamine release causes increased contractility and further increases in heart rate beyond nicotine's direct positive chronotropic effect. Thus, cardiac output increases chiefly as a result of increased heart rate, with variable changes in stroke volume. All of these physiologic responses to smoking increase myocardial oxygen demand. Under these circumstances, increased coronary blood flow must occur to maintain the balance between supply and demand, as oxygen extraction by the myocardium is maximal at rest [15, 20].
The neural and hormonal mechanisms that mediate this response are well described [18]. Sympathetic ganglionic stimulation and activation of alpha-adrenergic neural reflexes result in peripheral vasoconstriction. The increased peripheral resistance elevates blood pressure. Direct chronotropic effects and adrenal catecholamine secretion raise the heart rate. Myocardial contractility increases because of the catecholamine effect, but changes in stroke volume vary considerably. Cardiac output increases primarily as a consequence of the increased heart rate.; stroke volume changes are variable.
Smoking induces the release of catecholamines from the adrenal medulla and adrenergic terminal nerve endings. An early increase in serum norepinephrine levels is followed by an increase in serum epinephrine levels [15, 21]. These cardiovascular effects are also due to the stimulation of sympathetic neurotransmission by nicotine, which stimulates catecholamine release by activation of nicotinic acetylcholine receptors localized on peripheral postganglionic sympathetic nerve endings.
Vasomotor Dysfunction in Peripheral and Coronary Arteries. Smoking impairs limb flow-mediated vasodilation [FMD] of the brachial artery in a dose-dependent manner, demonstrating vascular dysfunction. The reduction of endothelium-dependent dilatation by smoking is reversible. Smoking low nicotine cigarettes impairs FMD as much as smoking regular cigarettes, suggesting that nicotine is not the only factor mediating vasoconstriction. FMD impairment is due to a significant reduction of NO bioavailability in the vasculature [21, 33-35].
Smoking is associated with diminished coronary vasodilator reserve [36] and has a demonstrative vasoconstrictive effect, especially notable in the presence of severe proximal stenosis [28]. Diminished endothelium dependent coronary vasodilation has also been demonstrated [37]. Smoking causes vasoconstriction of proximal and distal epicardial coronary arteries and an increase in coronary resistance and vessel tone. The diminished local flow despite an increase in myocardial oxygen demand may produce transient mild ischemia [15, 32].
Smoking-induced changes in coronary vasomotor tone, platelet activation, and endothelial integrity are major components of both the development of atherosclerosis and its clinical presentation. Smoking may initiate and accelerate the progression of atherosclerosis by injuring the vascular intima. Mechanical damage, possibly as a result of persistently increased coronary artery tone and frequent episodes of hemodynamic stress, has been hypothesized, similar to that postulated for hypertension [38]. Thus, smoking-induced vasoconstriction and enhanced platelet aggregation at the site of a severe coronary lesion may partially explain the increased incidence of acute cardiac events in chronic smokers and the improved prognosis of those who quit.
Plaque rupture. Although it is highly likely that increased vasomotor tone contributes to plaque rupture, no definite mechanistic connection between the physiologic effects of tobacco products and plaque rupture has been proven. Nevertheless, smoking is strongly associated with acute myocardial infarction. A relationship between cigarette smoking and the development of vulnerable coronary artery plaques using virtual histology intravascular ultrasound [VH-IVUS] has been shown [39]. Cigarette smoking is associated with a higher burden of necrosis in the core of plaques (20.7 vs. 17.2%, p=0.04) and is independently associated with a 4.54% increase in the quantity of necrotic core (p=0.01). This compositional difference may partially explain the clinical sequelae of smoking since the burden of necrotic atherosclerotic plaque predicts vulnerability and the likelihood of plaque rupture.
The “smoker's paradox” is the unexplained lower mortality and morbidity in smokers vs. non-smokers with myocardial infarction. It is most likely an epiphenomenon caused by different baseline characteristics of smokers and nonsmokers [40, 41]. Younger MI patients have more acute plaque rupture but less extensive coronary atherosclerosis and fewer risk factors than older patients. The critical point is that smoking causes myocardial infarction at an earlier age in smokers compared to non-smokers.
Lipids. Smokers have significantly higher serum cholesterol, triglyceride, and low-density lipoprotein [LDL] levels compared to nonsmokers. Smoking alters lipoprotein metabolism, particularly increasing the generation of oxidized LDL [2, 42-47]. High-density lipoprotein and apolipoprotein A1 levels are decreased in smokers. There is also a significant association between secondhand smoke inhalation and abnormal serum lipids [45].
Apart from modulating lipid quantities, smoking also impacts lipids qualitatively. Free radicals and oxidants present in cigarette smoke contribute to lipid oxidation and to an increase in oxidative metabolism. An increased presence of lipid peroxidation products in the serum of smokers has been reported, as have increased levels of circulating autoantibodies against oxidized LDL [2, 48, 49]. Peroxynitrite, which is generated by a reaction between NO and superoxide, has been implicated in the oxidation of LDL in smokers [50, 51]. Smokers demonstrate significant evidence of increased lipid oxidation, signs of oxidative stress, and impairment of antioxidant systems [2], but it has not been demonstrated whether this represents the specific mechanism of endothelial damage.
Interaction with other risk factors. Cigarette smoke aggravates other risk factors for atherosclerosis. Smoking raises systolic and diastolic blood pressure, worsening hypertension and requiring more drugs for control, but there is a paucity of data suggesting that smoking actually causes hypertension. Similarly, smoking is associated with, but not necessarily a cause of hyperlipidemia, hyperglycemia, and insulin resistance [52].
4. EFFECTS ON INITIATION AND PROGRESSION OF VASCULAR DISEASE
Despite the overwhelming epidemiological evidence linking cigarette smoking with cardiovascular disease, the precise components of cigarette smoke responsible for its causation and the mechanism by which they exert their effects have not been elucidated [2, 22]. Mechanisms by which smoking may contribute to acute vascular events are summarized in Table 2.
Table 2.
Mechanisms of tobacco related physiological effects.
| • Carbon monoxide–mediated reduced oxygen-carrying capacity of the blood oxygen transfer and dissociation from hemoglobin • Hemodynamic stress & increased myocardial work • Stimulates the sympathetic nervous system & catecholamine release • Increases coronary artery tone/vasoconstriction • Endothelial cell damage, Damage cells that line the blood vessels & vascular dysfunction • Vasomotor dysfunction • Oxidative stress, & injury • Plaque Rupture • Vascular inflammation • Induction of a hypercoagulable state platelet activation & Coagulation: Prothrombotic and more likely to clot, which can block blood flow to the heart and brain • Increased fibrinogen and blood viscosity, increased stickiness • Worsens Lipid profile • Increased insulin resistance & synergism with other CAD risk factors |
Intimal damage and endothelial dysfunction. Endothelial dysfunction is produced by reactive oxygen species [ROS] that leads to endothelial cell loss by apoptosis or necrosis [2, 22]. Smoking damages the endothelium leading to measurable vascular dysfunction. Endothelial dysfunction, including an increase in permeability and decreased nitric oxide [NO] production, along with increased expression of adhesion molecules and adherence of leukocytes to the vessel wall, is a common mediator of many CAD risk factors. Endothelial injury is generally the initiating event in the pathogenesis of atherosclerosis and it has been the “working hypothesis” for decades that components of cigarette smoke damage the endothelium [22, 52]. It must be emphasized, however, that, perhaps surprisingly, no definitive evidence exists to prove the concept, despite consistent in vitro studies.
Cigarette smoke damages the vascular endothelium mechanically. Endothelial cells contract, mediated by oxidation and collapse of the tubulin system. This response may be reversible and may be the cause of the reduction of FMD, but perhaps results in endothelial cell death. In addition, smoking induces tissue remodeling, with the functional consequence of increased permeability of the endothelial cell layer [2]. A simple but attractive proposition is that chronic smoking predisposes to accelerated coronary atherosclerosis, at least in part, as a consequence of a cumulation of its acute effects on coronary vasomotor tone [38]. Frequent episodes of acute vascular stress may lead to recurrent coronary vasoconstriction, creating intimal damage.
It is likely that although, the substances in the smoke are causing the damage. All forms of cell death (apoptosis, necrosis, programmed necrosis, and autophagy) are induced by cigarette smoke combustion products. Endothelial cells release inflammatory and proatherogenic cytokines in response to smoke. Endothelial cell morphology and function were altered by CO exposure. CO exerts growth arrest in smooth muscle cells in the vascular endothelium by inhibiting the cell cycle transition from G0/G1 phase to the S phase and has a regulatory effect on cell apoptosis through the expression of apoptosis-associated genes [2, 22, 52].
Oxidative Stress & Injury. Cigarette smoke contains a large number of oxidants, which raises the possibility that its atherogenic effects are a consequence of oxidative damage to the vascular endothelium. Supportive evidence is that antioxidants such as ascorbic acid reverse flow mediated endothelial dysfunction [53]. For these reasons, it has been hypothesized that free radicals in smoke mediate oxidative stress, contributing to the initiation and progression of atherosclerotic disease [5, 52, 54). The main free radical in the tar phase is the quinone/hydroquinone complex, which is a redox system that reduces molecular oxygen to produce superoxide. The second free radical of note is in the Gas phase: small oxygen and carbon centered radicals produced by the oxidation of nitric oxide (NO) [22]. It has also been shown that aldehydes in smoke increase reactive oxygen species production by activating NADPH oxidase. Moreover, compounds in tobacco smoke increase eNOS acetylation and expression and decrease and uncouple eNOS activity [54-57].
Free radicals such as superoxide and NO decrease NO availability and generateperoxynitrite, which further enhances cellular oxidative stress [16]. Antioxidants and agents that increase NO availability have been shown to either improve or reverse the proatherogenic, proinflammatory, and prothrombotic attributes associated with CS [5, 54, 58-62]. Chemicals in smoke chemicals lead to adhesion molecule expression on the surface of endothelial cells and induce the release of proatherogenic cytokines, such as interleukin-6 and interleukin-8. The core of these processes is the activation of the NFkB cascade [2, 5].
Inflammation. Smoking activates the immune system both systemically and locally in the arterial milieu. Smokers have significantly increased serum levels of cytokines that enhance inflammation, including tumor necrosis factor-α, interleukin-1β, and serum C-reactive protein. Interestingly, pro-inflammatory markers are increased in both active smokers and those exposed to secondhand smoke [2]. Cigarette smoking increases thromboxane and decreases prostacyclin production, causing vasoconstriction and platelet aggregation. Moreover, soluble VCAM-1, ICAM-1, E-selectin levels are higher in smokers. Plaque formation and the development of vulnerable plaques may also result from smoke enhancing the activation of matrix metalloproteases [52]. The recent MESA trial showed an association between high levels of high sensitivity C-reactive protein in smokers to be associated with atherosclerosis [63].
Platelet Activation and Thrombosis. Smoking increases the activation, adherence, and aggregation of platelets, further favoring the development of a procoagulant and inflammatory environment. Smoke also increases the number of platelets, which likely increases the risk of thrombosis [2]. Smoking also has been shown to increase fibrinogen levels and blood viscosity [64]. Not only does this affect flow dynamics, but also the increased stickiness predisposes to thrombosis. Perhaps these mechanisms contribute to the risk of MI and stroke, which are acute thrombotic processes.
CONCLUSION
Smoking tobacco products contributes measurably to the incidence of acute vascular events and chronic disease. The precise compound, the exact mechanism of injury, and the question of whether the response is modifiable are all unknown.
ACKNOWLEDGEMENTS
Declared none.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Benowitz N.L. The role of nicotine in smoking-related cardiovascular disease. Prev. Med. 1997;26(4):412–417. doi: 10.1006/pmed.1997.0175. [DOI] [PubMed] [Google Scholar]
- 2.Messner B., Bernhard D. Smoking and cardiovascular disease: Mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2014;34(3):509–515. doi: 10.1161/ATVBAHA.113.300156. [DOI] [PubMed] [Google Scholar]
- 3.Siasos G., Tsigkou V., Kokkou E., et al. Smoking and atherosclerosis: Mechanisms of disease and new therapeutic approaches. Curr. Med. Chem. 2014;21(34):3936–3948. doi: 10.2174/092986732134141015161539. [DOI] [PubMed] [Google Scholar]
- 4.Benowitz N.L., Gourlay S.G. Cardiovascular toxicity of nicotine: Implications for nicotine replacement therapy. J. Am. Coll. Cardiol. 1997;29(7):1422–1431. doi: 10.1016/S0735-1097(97)00079-X. [DOI] [PubMed] [Google Scholar]
- 5.Ambrose J.A., Barua R.S. The pathophysiology of cigarette smoking and cardiovascular disease: An update. J. Am. Coll. Cardiol. 2004;43(10):1731–1737. doi: 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 6.United States Department of Health, Education and Welfare, Public Health Service Smoking and Health: Report of the Advisory Committee to the Surgeon General of the Public Health Service (PHS Publication No 103) Washington, DC: Government Printing Office; 1964. [Google Scholar]
- 7.Negri E., Franzosi M.G., La Vecchia C., Santoro L., Nobili A., Tognoni G. Tar yield of cigarettes and risk of acute myocardial infarction. BMJ. 1993;306(6892):1567–1570. doi: 10.1136/bmj.306.6892.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolinder G., Alfredsson L., Englund A., de Faire U. Smokeless tobacco use and increased cardiovascular mortality among Swedish construction workers. Am. J. Public Health. 1994;84(3):399–404. doi: 10.2105/AJPH.84.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glantz S.A., Parmley W.W. Passive smoking and heart disease. Epidemiology, physiology, and biochemistry. Circulation. 1991;83(1):1–12. doi: 10.1161/01.CIR.83.1.1. [DOI] [PubMed] [Google Scholar]
- 10.Law M.R., Morris J.K., Wald N.J. Environmental tobacco smoke exposure and ischaemic heart disease: An evaluation of the evidence. BMJ. 1997;315(7114):973–980. doi: 10.1136/bmj.315.7114.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piano M.R., Benowitz N.L., Fitzgerald G.A., et al. Impact of smokeless tobacco products on cardiovascular disease: Implications for policy, prevention, and treatment: A policy statement from the American Heart Association. Circulation. 2010;122(15):1520–1544. doi: 10.1161/CIR.0b013e3181f432c3. [DOI] [PubMed] [Google Scholar]
- 12.U.S. Surgeon General Report, 1988: The Health Consequences of Smoking: Nicotine Addiction. U.S. Department of Health and Human Services. [Google Scholar]
- 13.The chemical constituents in cigarettes and cigarette smoke: Potential for harm reduction. A Report to the New Zealand Ministry of Health; 2000. [Google Scholar]
- 14.California Environmental Protection Agency (Cal/EPA) Health effects of exposure to environmental tobacco smoke. Final report. 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein LW. Systemic and Coronary Hemodynamic Effects of Tobacco Products on the Cardiovascular System and Potential Pathophysiologic Mechanisms. Cardiology. 2021 doi: 10.1097/CRD.0000000000000395. Publish Ahead of Print. [DOI] [PubMed] [Google Scholar]
- 16.Schick S., Glantz S. Philip Morris toxicological experiments with fresh sidestream smoke: More toxic than mainstream smoke. Tob. Control. 2005;14(6):396–404. doi: 10.1136/tc.2005.011288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith C.J., Fischer T.H. Particulate and vapor phase constituents of cigarette mainstream smoke and risk of myocardial infarction. Atherosclerosis. 2001;158(2):257–267. doi: 10.1016/S0021-9150(01)00570-6. [DOI] [PubMed] [Google Scholar]
- 18.Cryer P.E., Haymond M.W., Santiago J.V., Shah S.D. Norepinephrine and epinephrine release and adrenergic mediation of smoking-associated hemodynamic and metabolic events. N. Engl. J. Med. 1976;295(11):573–577. doi: 10.1056/NEJM197609092951101. [DOI] [PubMed] [Google Scholar]
- 19.West J.W., Guzman S.V., Bellett S. Cardiac effects of intracoronary arterial injections of nicotine. Circ. Res. 1958;6(3):389–395. doi: 10.1161/01.RES.6.3.389. [DOI] [PubMed] [Google Scholar]
- 20.Czernin J., Waldherr C. Cigarette smoking and coronary blood flow. Prog. Cardiovasc. Dis. 2003;45(5):395–404. doi: 10.1016/S0033-0620(03)80003-8. [DOI] [PubMed] [Google Scholar]
- 21.Lee J., Cooke J.P. The role of nicotine in the pathogenesis of atherosclerosis. Atherosclerosis. 2011;215(2):281–283. doi: 10.1016/j.atherosclerosis.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michael Pittilo R. Cigarette smoking, endothelial injury and cardiovascular disease. Int. J. Exp. Pathol. 2000;81(4):219–230. doi: 10.1046/j.1365-2613.2000.00162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamasaki H., Sato J., Masuda H., et al. Effect of nicotine on the intimal hyperplasia after endothelial removal of the rabbit carotid artery. Gen. Pharmacol. 1997;28(5):653–659. doi: 10.1016/S0306-3623(96)00369-2. [DOI] [PubMed] [Google Scholar]
- 24.Davis J.W., Shelton L., Eigenberg D.A., Hignite C.E., Watanabe I.S. Effects of tobacco and non-tobacco cigarette smoking on endothelium and platelets. Clin. Pharmacol. Ther. 1985;37(5):529–533. doi: 10.1038/clpt.1985.83. [DOI] [PubMed] [Google Scholar]
- 25.Neunteufl T., Heher S., Kostner K., et al. Contribution of nicotine to acute endothelial dysfunction in long-term smokers. J. Am. Coll. Cardiol. 2002;39(2):251–256. doi: 10.1016/S0735-1097(01)01732-6. [DOI] [PubMed] [Google Scholar]
- 26.Barua R.S., Ambrose J.A. Mechanisms of coronary thrombosis in cigarette smoke exposure. Arterioscler. Thromb. Vasc. Biol. 2013;33(7):1460–1467. doi: 10.1161/ATVBAHA.112.300154. [DOI] [PubMed] [Google Scholar]
- 27.Leone A. How and why chemicals from tobacco smoke can induce a rise in blood pressure. World J. Pharmacol. 2012;1(1):10–20. doi: 10.5497/wjp.v1.i1.10. [DOI] [Google Scholar]
- 28.Klein L.W., Ambrose J., Pichard A., Holt J., Gorlin R., Teichholz L.E. Acute coronary hemodynamic response to cigarette smoking in patients with coronary artery disease. J. Am. Coll. Cardiol. 1984;3(4):879–886. doi: 10.1016/S0735-1097(84)80344-7. [DOI] [PubMed] [Google Scholar]
- 29.Nicod P., Rehr R., Winniford M.D., Campbell W.B., Firth B.G., Hillis L.D. Acute systemic and coronary hemodynamic and serologic responses to cigarette smoking in long-term smokers with atherosclerotic coronary artery disease. J. Am. Coll. Cardiol. 1984;4(5):964–971. doi: 10.1016/S0735-1097(84)80058-3. [DOI] [PubMed] [Google Scholar]
- 30.Winniford M.D., Wheelan K.R., Kremers M.S., et al. Smoking-induced coronary vasoconstriction in patients with atherosclerotic coronary artery disease: Evidence for adrenergically mediated alterations in coronary artery tone. Circulation. 1986;73(4):662–667. doi: 10.1161/01.CIR.73.4.662. [DOI] [PubMed] [Google Scholar]
- 31.Martin J.L., Wilson J.R., Ferraro N., Laskey W.K., Kleaveland J.P., Hirshfeld J.W., Jr Acute coronary vasoconstrictive effects of cigarette smoking in coronary heart disease. Am. J. Cardiol. 1984;54(1):56–60. doi: 10.1016/0002-9149(84)90303-5. [DOI] [PubMed] [Google Scholar]
- 32.Quillen J.E., Rossen J.D., Oskarsson H.J., Minor R.L., Jr, Lopez A.G., Winniford M.D. Acute effect of cigarette smoking on the coronary circulation: Constriction of epicardial and resistance vessels. J. Am. Coll. Cardiol. 1993;22(3):642–647. doi: 10.1016/0735-1097(93)90170-6. [DOI] [PubMed] [Google Scholar]
- 33.Celermajer D.S., Sorensen K.E., Georgakopoulos D., et al. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation. 1993;88(5 Pt 1):2149–2155. doi: 10.1161/01.CIR.88.5.2149. [DOI] [PubMed] [Google Scholar]
- 34.Barua R.S., Ambrose J.A., Eales-Reynolds L.J., DeVoe M.C., Zervas J.G., Saha D.C. Dysfunctional endothelial nitric oxide biosynthesis in healthy smokers with impaired endothelium-dependent vasodilatation. Circulation. 2001;104(16):1905–1910. doi: 10.1161/hc4101.097525. [DOI] [PubMed] [Google Scholar]
- 35.Celermajer D.S., Adams M.R., Clarkson P., et al. Passive smoking and impaired endothelium-dependent arterial dilatation in healthy young adults. N. Engl. J. Med. 1996;334(3):150–154. doi: 10.1056/NEJM199601183340303. [DOI] [PubMed] [Google Scholar]
- 36.Klein L.W., Pichard A.D., Holt J., Smith H., Gorlin R., Teichholz L.E. Effects of chronic tobacco smoking on the coronary circulation. J. Am. Coll. Cardiol. 1983;1(2 Pt 1):421–426. doi: 10.1016/S0735-1097(83)80069-2. [DOI] [PubMed] [Google Scholar]
- 37.Zeiher A.M., Schächinger V., Minners J. Long-term cigarette smoking impairs endothelium-dependent coronary arterial vasodilator function. Circulation. 1995;92(5):1094–1100. doi: 10.1161/01.CIR.92.5.1094. [DOI] [PubMed] [Google Scholar]
- 38.Klein L.W. Cigarette smoking, atherosclerosis and the coronary hemodynamic response: A unifying hypothesis. J. Am. Coll. Cardiol. 1984;4(5):972–974. doi: 10.1016/S0735-1097(84)80059-5. [DOI] [PubMed] [Google Scholar]
- 39.Bolorunduro O., Cushman C., Kapoor D., et al. Comparison of coronary atherosclerotic plaque burden and composition of culprit lesions between cigarette smokers and non-smokers by in vivo virtual histology intravascular ultrasound. J. Invasive Cardiol. 2015;27(8):354–358. [PubMed] [Google Scholar]
- 40.Gupta T., Kolte D., Khera S., et al. Smoker’s paradox in patients with st-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. J. Am. Heart Assoc. 2016;5(4):e003370. doi: 10.1161/JAHA.116.003370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yadav M., Mintz G.S., Généreux P., et al. The Smoker’s Paradox Revisited: A Patient-Level Pooled Analysis of 18 Randomized Controlled Trials. JACC Cardiovasc. Interv. 2019;12(19):1941–1950. doi: 10.1016/j.jcin.2019.06.034. [DOI] [PubMed] [Google Scholar]
- 42.Craig W.Y., Palomaki G.E., Haddow J.E. Cigarette smoking and serum lipid and lipoprotein concentrations: An analysis of published data. BMJ. 1989;298(6676):784–788. doi: 10.1136/bmj.298.6676.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Freedman D.S., Srinivasan S.R., Shear C.L., et al. Cigarette smoking initiation and longitudinal changes in serum lipids and lipoproteins in early adulthood: The Bogalusa Heart Study. Am. J. Epidemiol. 1986;124(2):207–219. doi: 10.1093/oxfordjournals.aje.a114379. [DOI] [PubMed] [Google Scholar]
- 44.Nakamura K., Barzi F., Huxley R., et al. Does cigarette smoking exacerbate the effect of total cholesterol and high-density lipoprotein cholesterol on the risk of cardiovascular diseases? Heart. 2009;95(11):909–916. doi: 10.1136/hrt.2008.147066. [DOI] [PubMed] [Google Scholar]
- 45.Neufeld E.J., Mietus-Snyder M., Beiser A.S., Baker A.L., Newburger J.W. Passive cigarette smoking and reduced HDL cholesterol levels in children with high-risk lipid profiles. Circulation. 1997;96(5):1403–1407. doi: 10.1161/01.CIR.96.5.1403. [DOI] [PubMed] [Google Scholar]
- 46.Gepner A.D., Piper M.E., Johnson H.M., Fiore M.C., Baker T.B., Stein J.H. Effects of smoking and smoking cessation on lipids and lipoproteins: Outcomes from a randomized clinical trial. Am. Heart J. 2011;161(1):145–151. doi: 10.1016/j.ahj.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maeda K., Noguchi Y., Fukui T. The effects of cessation from cigarette smoking on the lipid and lipoprotein profiles: A meta-analysis. Prev. Med. 2003;37(4):283–290. doi: 10.1016/S0091-7435(03)00110-5. [DOI] [PubMed] [Google Scholar]
- 48.Garbin U., Fratta Pasini A., Stranieri C., et al. Cigarette smoking blocks the protective expression of Nrf2/ARE pathway in peripheral mononuclear cells of young heavy smokers favouring inflammation. PLoS One. 2009;4(12):e8225. doi: 10.1371/journal.pone.0008225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morrow J.D., Frei B., Longmire A.W., et al. Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. Smoking as a cause of oxidative damage. N. Engl. J. Med. 1995;332(18):1198–1203. doi: 10.1056/NEJM199505043321804. [DOI] [PubMed] [Google Scholar]
- 50.Pryor WA, Stone K. Oxidants in cigarette smoke. Radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite. Ann N Y Acad Sci. 1993;686(1 Tobacco Smoki):12–27. doi: 10.1111/j.1749-6632.1993.tb39148.x. [DOI] [PubMed] [Google Scholar]
- 51.Yamaguchi Y., Matsuno S., Kagota S., Haginaka J., Kunitomo M. Peroxynitrite-mediated oxidative modification of low-density lipoprotein by aqueous extracts of cigarette smoke and the preventive effect of fluvastatin. Atherosclerosis. 2004;172(2):259–265. doi: 10.1016/j.atherosclerosis.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 52.Klein L.W., Gopalakrishnan M. The correlation between cigarette smoking and other risk factors with coronary stenosis composition. J. Invasive Cardiol. 2015;27(8):359–361. [PubMed] [Google Scholar]
- 53.Kaufmann P.A., Gnecchi-Ruscone T., di Terlizzi M., Schäfers K.P., Lüscher T.F., Camici P.G. Coronary heart disease in smokers: Vitamin C restores coronary microcirculatory function. Circulation. 2000;102(11):1233–1238. doi: 10.1161/01.CIR.102.11.1233. [DOI] [PubMed] [Google Scholar]
- 54.Barua R.S., Ambrose J.A., Srivastava S., DeVoe M.C., Eales-Reynolds L.J. Reactive oxygen species are involved in smoking-induced dysfunction of nitric oxide biosynthesis and upregulation of endothelial nitric oxide synthase: An in vitro demonstration in human coronary artery endothelial cells. Circulation. 2003;107(18):2342–2347. doi: 10.1161/01.CIR.0000066691.52789.BE. [DOI] [PubMed] [Google Scholar]
- 55.Bernhard D., Wang X.L. Smoking, oxidative stress and cardiovascular diseases--do anti-oxidative therapies fail? Curr. Med. Chem. 2007;14(16):1703–1712. doi: 10.2174/092986707781058959. [DOI] [PubMed] [Google Scholar]
- 56.Abu-Hayyeh S., Sian M., Jones K.G., Manuel A., Powell J.T. Cadmium accumulation in aortas of smokers. Arterioscler. Thromb. Vasc. Biol. 2001;21(5):863–867. doi: 10.1161/01.ATV.21.5.863. [DOI] [PubMed] [Google Scholar]
- 57.Jaimes E.A., DeMaster E.G., Tian R.X., Raij L. Stable compounds of cigarette smoke induce endothelial superoxide anion production via NADPH oxidase activation. Arterioscler. Thromb. Vasc. Biol. 2004;24(6):1031–1036. doi: 10.1161/01.ATV.0000127083.88549.58. [DOI] [PubMed] [Google Scholar]
- 58.Kojda G., Harrison D. Interactions between NO and reactive oxygen species: Pathophysiological importance in atherosclerosis, hypertension, diabetes and heart failure. Cardiovasc. Res. 1999;43(3):562–571. doi: 10.1016/S0008-6363(99)00169-8. [DOI] [PubMed] [Google Scholar]
- 59.Nedeljkovic Z.S., Gokce N., Loscalzo J. Mechanisms of oxidative stress and vascular dysfunction. Postgrad. Med. J. 2003;79(930):195–199. doi: 10.1136/pmj.79.930.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heitzer T., Brockhoff C., Mayer B., et al. Tetrahydrobiopterin improves endothelium-dependent vasodilation in chronic smokers: Evidence for a dysfunctional nitric oxide synthase. Circ. Res. 2000;86(2):E36–E41. doi: 10.1161/01.RES.86.2.e36. [DOI] [PubMed] [Google Scholar]
- 61.Kayyali U.S., Budhiraja R., Pennella C.M., et al. Upregulation of xanthine oxidase by tobacco smoke condensate in pulmonary endothelial cells. Toxicol. Appl. Pharmacol. 2003;188(1):59–68. doi: 10.1016/S0041-008X(02)00076-5. [DOI] [PubMed] [Google Scholar]
- 62.Guthikonda S., Sinkey C., Barenz T., Haynes W.G. Xanthine oxidase inhibition reverses endothelial dysfunction in heavy smokers. Circulation. 2003;107(3):416–421. doi: 10.1161/01.CIR.0000046448.26751.58. [DOI] [PubMed] [Google Scholar]
- 63.McEvoy J.W., Blaha M.J., DeFilippis A.P., et al. Cigarette smoking and cardiovascular events: Role of inflammation and subclinical atherosclerosis from the MultiEthnic Study of Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2015;35(3):700–709. doi: 10.1161/ATVBAHA.114.304562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shimada S., Hasegawa K., Wada H., et al. High blood viscosity is closely associated with cigarette smoking and markedly reduced by smoking cessation. Circ. J. 2011;75(1):185–189. doi: 10.1253/circj.CJ-10-0335. [DOI] [PubMed] [Google Scholar]