Abstract
Background
The natural history and outcome of unruptured posterior circulation dissecting fusiform aneurysms is not fully understood. These have a high risk of morbidity and mortality, not only due to natural history but also due to the challenging and controversial treatment approaches currently available compared to other types of intracranial aneurysms.
Methods
We performed a retrospective study of a prospectively collected aneurysm database at a quaternary neurovascular hospital. We included consecutive patients with unruptured intradural vertebrobasilar dissecting aneurysms between January 2000 and July 2016 who were followed to 2020. Description of baseline, procedural, and outcomes data was performed. Comparisons of patient who had aneurysm rupture on follow-up, increase in 2 or more points of mRS in follow-up and progression of the aneurysm was performed.
Results
Seventy patients with 78 fusiform posterior circulation aneurysms were identified. Thirty-nine (55.7%) patients were male with a mean age of 51.7 years (SD ± 17.6). When multiple, aneurysms were more likely to be fusiform (60%) than saccular (40.0%). Baseline diameter (measured on CTA/MRA/DSA), length as well as symptomatic presentation were significantly higher in aneurysms which grew over time. Coronary disease, diabetes and growth were associated an >2 increase in mRS. Diabetes as well as initial symptomatic presentation were associated with rupture.
Conclusions
Unruptured dissecting/fusiform aneurysm are associated with a considerable rate of rupture during follow-up. Growth is associated with morbidity even in the absence of rupture. Initial large size, coronary disease, diabetes, and to a lesser extent female gender may merit closer follow-up and/or prophylactic treatment.
Keywords: Intracranial, dissecting aneurysm, fusiform aneurysm, natural history, outcome
Introduction
Dissecting fusiform aneurysms of the posterior circulation are a comparatively rare heterogenous spectrum of disease with different underlying pathology compared to those from the anterior circulation or other morphologies.1–4 The posterior intracranial circulation has a different embryological development and spectrum of risk and disease compared to the anterior circulation.5,6
The disease process leading to dissecting fusiform aneurysms involves the entire vessel wall. It relates to the disruption or degeneration of internal elastic lamina, smooth-muscle atrophy, endothelial injury, vasculitic or infectious processes, and rarely neoplastic infiltration.3,7 Vertebrobasilar dissecting fusiform aneurysms are contended to be strongly associated with atherosclerotic disease.8,9 Patients may present with symptoms secondary to compression on the adjacent structures or recurrent ischemic events due to perforators occlusion or distal emboli. 8
The natural history and outcome of the unruptured posterior circulation dissecting fusiform aneurysms is not fully understood. Furthermore, the progression of these specific aneurysms is poor and quite different from any other forms of intracranial aneurysms and fusiform lesions from the anterior circulation. The importance of understanding the natural history of posterior circulation fusiform aneurysms comes from the high risk of morbidity and mortality, and also the challenging and controversial treatment approaches currently available as compared to other types of intracranial aneurysms. 10
We aimed to understand the natural history, risk factors, and associated conditions of the unruptured posterior circulation dissecting fusiform aneurysms. We also assessed if the clinical course and outcome of the unruptured aneurysms should affect the current management approaches.
Methods
We performed a retrospective study of a prospectively collected database at a quaternary neurovascular Canadian hospital. All consecutive patients with unruptured intradural vertebrobasilar dissecting fusiform aneurysms between January 2000 and July 2016 with follow-up to 2020 were included. These were defined as non-saccular elongated dilatations of vessels from the vertebrobasilar circulation, w
hich were preceded immediately upstream with a proximal narrowing/stenosis (Figure 1). The aneurysm length was determined at the beginning of the transition zone between the parent vessel and the dilated segment. All excluded aneurysms were traumatic, inflammatory, infectious aneurysms and other morphologies (i.e. saccular or blister). Database records were verified, and all electronic charts were reviewed, inclusive of imaging and clinical information.
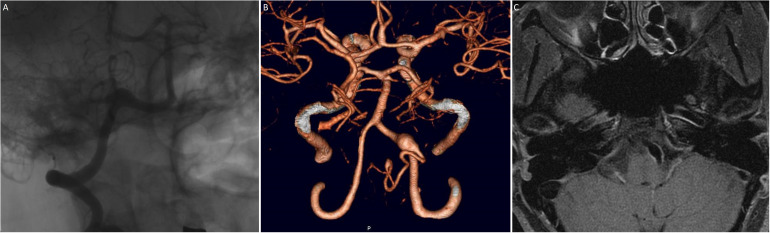
Figure 1.
40-year-old With fusiform dissection aneurysm who presented with subarachnoid hemorrhage. A) Catheter angiography, B) computed tomography 3D reconstruction and C) double inversion recovery magnetic resonance imaging demonstrates classic fusiform morphology. In a dissecting aneurysm there is usually a narrowing/stenosis at the beginning of the fusiform dilatation, most easily seen on DSA. Although the lesion incorporated the PICA origin, proximal occlusion was pursued using a flow diverter reconstruction.
These aneurysms were managed after discussion by a institutional multidisciplinary team consisting of interventional neuroradiologists and cerebrovascular neurosurgeons. Our institutional policy is to initially seek conservative management with observation for unruptured fusiform aneurysms in the posterior circulation. However, surgical or endovascular treatment may be offered in the case of rupture, growth, and new symptoms increasing the patient's baseline modified Rankin Score (mRS) of the patient. The treatment modality is decided collaboratively according to the presentation, anatomy, access, clinical status, and discussion with the patient and family as applicable. Clinical outcome was assessed using the mRS at the last clinical follow-up. Good clinical outcome was defined as mRS score of 0–2. Confounders for changes in mRS were ruled out by a thorough chart review.
Statistical methods
Results of categorical variables are reported as proportions. Continuous variables are reported as means (±standard deviation) or medians [Interquartile range (IQR)] based on the distribution of the data assessed by the Shapiro-Wilk test A univariate comparison of baseline patient and aneurysm characteristics was performed between the patients that presented a ruptured fusiform aneurysm and those who did not to identify potential factors associated with higher risk of this event. The Chi-square test was used for the analysis of categorical variables. The t-test or the Mann-Whitney test was used for comparing continuous variables according to the normality distribution. Statistical significance was defined as p < 0.05. Statistical analysis was performed using Stata-14 Software.
Results
Patient characteristics
During the data collection period, 86 aneurysms were identified in the database, of which 78 were included. Thirty-nine (55.7%) patients were male with a mean age of 51.7 years (SD ± 17.6) (Table 1); 30.9% were smokers, 10.9% were diabetics, 54.2% had chronic hypertension, and 6 (19.4%) had a family history of intracranial aneurysm. Twenty-one (30.0%) of patients had symptomatic aneurysms, and the rest were found incidentally. From the symptomatic patients (n = 21), eight patients (38.1%) had TIA/Stroke related symptoms, five (23.8%) mass effect symptoms, four (19.0%) presented as ruptured with subarachnoid hemorrhage, one (4.8%) with mass effect and hydrocephalus, one (4.8%) with TIA and mass effect symptoms, one (4.8%) with intraparenchymal hemorrhage and another one (4.8%) with blurred vision. Fourteen (20.0%) patients had multiple intracranial aneurysms, comprising a total of 20 additional ones. Of those additional aneurysms, 12 (60%) were fusiform and 8 (40.0%) saccular aneurysms. On admission, 43 (64.2%) patients had an mRS classification of 0–1, 16 (23.9%) an mRS of 2, and 8 (12.0%) an mRS of 3–4.
Table 1.
Baseline characteristics.
| Number of patients | n = 70 |
|---|---|
| Gender | |
| Male | 39 (55.7%) |
| Female | 31 (44.3%) |
| Age (Years) | 51.7 (±17.6) |
| Admission mRS* | |
| 0 | 5 (7.5%) |
| 1 | 38 (56.7%) |
| 2 | 16 (23.9%) |
| 3 | 6 (9.0%) |
| 4 | 2 (3.0%) |
| Multiple Intracranial Aneurysms | 14 (20.0%) |
| Total aneurysm count | |
| 1 | 56 (80.0%) |
| 2 | 10 (14.3%) |
| 3 | 3 (4.3%) |
| 4 | 1 (1.4%) |
| Chronic headaches* | 34 (50.8%) |
| Family history screening detection † | 1 (2.6%) |
| Incidental finding (Asymptomatic) | 49 (70.0%) |
| Neurological symptoms | 41 (58.6%) |
| Neurological symptoms related to the aneurysm§ | 16 (22.1%) |
| Previous TIA / Stroke II | 27 (39.1%) |
| Type of TIA / Stroke | |
| TIA | 5 (18.5%) |
| Ischemic stroke | 12 (44.4%) |
| SAH from prior aneurysm | 3 (11.1%) |
| ICH | 1 (3.7%) |
| Dissection | 2 (7.4%) |
| Unknown | 4 (14.8%) |
| TIA / Stroke related to aneurysm ¶ | 14 (60.9%) |
| Family history of aneurysm ‡ | 6 (19.4%) |
| Hypertension # | 32 (54.2%) |
| Smoker** | 17 (30.9%) |
| CAD*** | 9 (16.7%) |
| Hypercholesterolemia**** | 16 (36.4%) |
| Trauma*** | 6 (11.1%) |
| Diabetes mellitus** | 6 (10.9%) |
| Cancer***** | |
| No | 52 (78.8%) |
| Benign | 2 (3.0%) |
| Malignant | 12 (18.2%) |
* 3 Missing data.
† 31 Missing data.
§ 2 Missing data.
II 1 Missing data.
¶ 5 Missing data.
‡ 39 Missing data.
# 11 Missing data.
** 15 Missing data.
*** 16 Missing data.
**** 26 Missing data.
***** 4 Missing data.
Aneurysm characteristics and treatment
The median maximal aneurysm diameter was 7 mm with a median length of 12 mm (Table 2). The vertebral artery in 38.5% and the basilar artery in 30.8% of the aneurysms were the most common locations. There were 20 (25.6%) aneurysms treated (Table 2). A total of 12 aneurysms (15.4%) underwent endovascular treatment, and 8 (10.3%) had surgical treatment.
Table 2.
Aneurysm, procedural and follow up characteristics.
| Aneurysm | n = 78 |
|---|---|
| Parent vessel location | |
| Vertebral | 30 (38.5%) |
| Basilar | 24 (30.8%) |
| PCA | 15 (19.2%) |
| PICA | 8 (10.3%) |
| AICA | 1 (1.3%) |
| Side | |
| Left | 24 (30.8%) |
| Right | 36 (46.1%) |
| Midline | 18 (23.1%) |
| Maximal diameter, mm | 7 (IQR 5–9.9) |
| Length, mm | 12 (IQR 9–18.9) |
| Ratio | 0.59 (IQR 0.42–0.75) |
| Imaging modality | |
| CTA | 58 (74.4%) |
| MRA | 68 (87.2%) |
| DSA | 38 (48.7%) |
| Vessel wall MRI | 21 (26.9%) |
| Procedures per aneurysm | n = 78 |
| Aneurysm treated | |
| Yes | 20 (25.6%) |
| No | 58 (74.4%) |
| mRS before treatment | n = 20 |
| 1 | 10 (50.0%) |
| 2 | 6 (30.0%) |
| 3 | 4 (20.0%) |
| Endovascular treatment | 12 (15.4%) |
| Stent Assisted Coiling | 4 (33.3%) |
| Flow diverter | 4 (33.3%) |
| Primary vessel occlusion (PVO) | 4 (33.3%) |
| Endovascular re-treatment | 3 (3.8%) |
| Flow diverter | 1 (33.3%) |
| PVO | 2 (66.7%) |
| Surgery | 8 (10.3%) |
| Clipping | 2 (25.0%) |
| Bypass ICIC | 4 (50.0%) |
| Bypass ECIC | 2 (25.0%) |
| Other surgeries | 5 (8.2%) |
| EVD placement/Revision | 3 (60.0%) |
| Decompressive craniectomy | 2 (40.0%) |
| Intraprocedural aneurysm rupture | 6 (7.7%) |
| Stent Assisted Coiling | 2 (33.3%) |
| Flow diversion | 1 (16.7%) |
| PVO | 2 (33.3%) |
| Clipping | 1 (16.7%) |
| Outcomes | n = 70 |
| mRS at last follow-up* | |
| 0 | 5 (8.5%) |
| 1 | 36 (61.0%) |
| 2 | 11 (18.6%) |
| 3 | 3 (5.1%) |
| 4 | 1 (1.7%) |
| 6 | 3 (5.1%) |
| Length of clinical follow-up (Months) § | 15 (IQR 2–49) |
| Length of imaging follow-up (Months) | 36 (IQR 12–73) |
| Progression † | 9 (13.9%) |
*11 Missing data.
§ 7 Missing data.
† 5 Missing data.
Clinical and imaging follow-up
A total of 3 (5.1%) patients died during the follow-up. Causes of death include occlusion of ECIC bypass graft in 1 patient, another patient died from a pulmonary embolism, and one patient had an unclear cause of death.
Median follow-up imaging for all the aneurysms was 38 (12–74) months. An increase from preadmission mRS to last follow-up mRS of ≥ 2 was evidenced in 6 (10.3%) patients (Table 3). Multiple intracranial aneurysms (p < 0.01), coronary artery disease (p = 0.02) and aneurysm progression (p < 0.01) were factors that had significant higher rates in the patients with the increase in 2 or more points of mRS (Table 3). Interestingly, overall initial presentation symptoms related to the aneurysm and those specifically related to mass effect, TIA/Stroke, and SAH/ICH occurrence were not significantly different (p = 0.34, p = 0.23, p = 0.47, and p = 0.50, respectively). There was also no difference in terms of intraprocedural rupture occurrence (p = 0.68). Extension to an mRS of 3 or more points yielded no further significance.
Table 3.
Worsening neurological outcome on follow-up.
| Variable | mRS increase of 2 or more points from admission to last f/u | p-Value | |
|---|---|---|---|
| No | Yes | ||
| 57 (89.7%) | 6 (10.3%) | ||
| Age at time of treatment | |||
| Years (median; IQR) | 52.5 (39–62.5) | 46.5 (40–69) | 0.68 |
| Female Gender | 21 (40.4%) | 3 (50.0%) | 0.65 |
| Multiple intracranial aneurysms | 9 (17.3%) | 4 (66.7%) | <0.01 |
| Family history of aneurysm* | 5 (20.0%) | 0 (0.0%) | 0.33 |
| Hypertension † | 20 (45.5%) | 5 (83.3%) | 0.08 |
| Smoker § | 13 (30.3%) | 2 (40.0%) | 0.66 |
| CAD II | 5 (12.2%) | 3 (50.0%) | 0.02 |
| Hypercholesterolemia ¶ | 12 (35.3%) | 1 (25.0%) | 0.68 |
| Trauma ‡ | 5 (12.2%) | 1 (20.0%) | 0.62 |
| Diabetes mellitus II | 4 (9.5%) | 0 (0.0%) | 0.47 |
| Cancer # | |||
| No | 38 (77.6%) | 5 (83.3%) | 0.87 |
| Benign | 2 (4.1%) | 0 (0.0%) | |
| Malignant | 9 (18.4%) | 1 (16.7%) | |
| Parent vessel location | |||
| Vertebral | 20 (38.5%) | 1 (16.7%) | 0.14 |
| Basilar | 19 (36.5%) | 1 (16.7%) | |
| PCA | 9 (17.3%) | 2 (33.3%) | |
| PICA | 3 (5.8%) | 2 (33.3%) | |
| AICA | 1 (1.9%) | 0 (0.0%) | |
| Aneurysm symptomatic on presentation | 16 (28.6%) | 4 (44.4%) | 0.34 |
| Symptoms related to aneurysm mass effect on presentation | 5 (8.9%) | 2 (22.2%) | 0.23 |
| Symptoms related to aneurysm TIA/Stroke on presentation | 12 (21.4%) | 1 (11.1%) | 0.47 |
| Symptoms related to aneurysm SAH/ICH on presentation | 3 (5.4%) | 1 (11.1%) | 0.50 |
| Maximal diameter, mm | 6.6 (IQR 5.25–9) | 11 (IQR 3.6–15) | 0.32 |
| Length, mm | 12 (IQR 9.5–19) | 14.05 (IQR 6.8–28) | 0.92 |
| Ratio | 0.58 (IQR 0.42–0.75) | 0.55 (IQR 0.5–0.92) | 0.66 |
| Progression** | 4 (8.7%) | 3 (75.0%) | <0.01 |
| Intraprocedural rupture | 4 (7.1%) | 1 (11.1%) | 0.68 |
Bold values: p-Value <0.05; n (%); p-Value: Chi2 test Median (IQR).
p-Value: Mann-Whitney test.
* 29 Missing data.
†8 Missing data.
§10 Missing data.
II11 Missing data.
¶20 Missing data.
‡12 Missing data.
#3 Missing data.
** 8 Missing data.
Aneurysm's growth (progression) on follow-up imaging was evident in 9 (13.9%) aneurysms (Table 4). Aneurysms were mainly located in the basilar (44.4%) and vertebral (33.3%) arteries. The median baseline size was 15 mm, and length of 27 mm. Baseline diameter and length were significantly higher in aneurysms that grew compared to those aneurysms that did not progress over time. Males were predominant in the sample of patients with aneurysm growth (77.7%), which trended towards significance (p = 0.30). Aneurysm related presentation symptoms rates were also higher compared to patients with aneurysms that progressed (88.9% vs. 25.0%, p < 0.01).
Table 4.
Associations with progression.
| Variable | Aneurysm progression | p-Value | |
|---|---|---|---|
| No | Yes | ||
| 56 (86.2%) | 9 (13.8%) | ||
| Age at time of treatment | |||
| Years (median; IQR) | 53 (42.5–64.5) | 53 (44–68) | 0.93 |
| Female Gender | 29 (51.8%) | 3 (33.3%) | 0.30 |
| Multiple Intracranial Aneurysms | 13 (23.2%) | 2 (22.2%) | 0.95 |
| Family history of aneurysm* | 5 (20.8%) | 0 (0.0%) | 0.26 |
| Hypertension † | 24 (50.0%) | 5 (71.4%) | 0.29 |
| Smoker § | 12 (26.1%) | 2 (33.3%) | 0.71 |
| CAD II | 7 (16.3%) | 2 (28.6%) | 0.43 |
| Hypercholesterolemia ¶ | 15 (41.7%) | 3 (50.0%) | 0.70 |
| Trauma ‡ | 5 (12.5%) | 0 (0.0%) | 0.29 |
| Diabetes mellitus II | 5 (11.6%) | 1 (14.3%) | 0.84 |
| Cancer # | |||
| No | 39 (75.0%) | 9 (100.0%) | 0.24 |
| Benign | 2 (3.8%) | 0 (0.0%) | |
| Malignant | 11 (21.2%) | 0 (0.0%) | |
| Parent vessel location | |||
| Vertebral | 24 (42.9%) | 3 (33.3%) | 0.75 |
| Basilar | 18 (32.1%) | 4 (44.4%) | |
| PCA | 11 (19.6%) | 1 (11.1%) | |
| PICA | 2 (3.6%) | 1 (11.1%) | |
| AICA | 1 (1.8%) | 0 (0.0%) | |
| Aneurysm symptomatic on presentation | 14 (25.0%) | 8 (88.9%) | <0.01 |
| Maximal diameter, mm | 6 (IQR 5–8.5) | 15 (IQR 11–23) | <0.01 |
| Length, mm | 12 (IQR 8.95–15) | 27 (IQR 18.9–36) | <0.01 |
| Ratio | 0.57 (IQR 0.42–0.72) | 0.59 (IQR 0.49–0.82) | 0.45 |
Bold values: p-Value <0.05; n (%); p-Value: Chi2 test.
Median (IQR); p-Value: Mann-Whitney test.
*36 Missing data.
†10 Missing data.
§13 Missing data.
II15 Missing data.
¶23 Missing data.
‡17 Missing data.
#4 Missing data.
Ruptured posterior circulation fusiform aneurysm
Six (7.7%) patients presented aneurysm rupture during follow-up. Two aneurysms were of the vertebral artery, three of the basilar artery, and 1 in the posterior cerebral artery. Aneurysm rupture occurred predominantly in females (n = 4; 66.7%). The median maximal diameter was 7 mm, and the median length was 13.5 mm. Progression of the aneurysm was evidenced in only one case during follow-up.
The univariate analysis showed higher rates of diabetes mellitus associated with the occurrence of rupture (p = 0.04). Aneurysm-related presentation symptoms rates were also higher compared to patients with aneurysms that ruptured (83.3% vs. 25.0%, p < 0.01). Factors that were not significantly associated with rupture were aneurysm location (p = 0.81), size (p = 0.68), length (p = 0.65), ratio (p = 0.55), progression (p = 0.50), smoking (p = 0.55) and chronic hypertension (p = 0.50) (Table 5).
Table 5.
Associations with rupture.
| Variable | Aneurysm rupture | p-Value | |
|---|---|---|---|
| No 72 (92.3%) | Yes 6 (7.7%) | ||
| Age at time of treatment | |||
| Years (median; IQR) | 51 (40–59.5) | 60.5 (18–76) | 0.65 |
| Female gender | 33 (45.8%) | 4 (66.7%) | 0.33 |
| Multiple Intracranial Aneurysms | 19 (26.4%) | 3 (50.0%) | 0.22 |
| Family history of aneurysm* | 6 (18.2%) | 0 (0.0%) | 0.51 |
| Hypertension † | 32 (52.5%) | 4 (66.7%) | 0.50 |
| Smoker § | 16 (27.6%) | 2 (40.0%) | 0.55 |
| CAD II | 7 (12.5%) | 2 (33.3%) | 0.17 |
| Hypercholesterolemia ¶ | 17 (36.2%) | 1 (25.0%) | 0.65 |
| Trauma § | 7 (12.5%) | 0 (0.0%) | 0.40 |
| Diabetes mellitus II | 4 (7.0%) | 2 (33.3%) | 0.04 |
| Cancer ¶ | |||
| No | 55 (80.9%) | 5 (83.3%) | 0.91 |
| Benign | 2 (2.9%) | 0 (0.0%) | |
| Malignant | 11 (16.2%) | 1 (16.7%) | |
| Parent vessel location | |||
| Vertebral | 28 (38.9%) | 2 (33.3%) | 0.81 |
| Basilar | 21 (29.2%) | 3 (50.0%) | |
| PCA | 14 (19.4%) | 1 (16.7%) | |
| PICA | 8 (11.1%) | 0 (0.0%) | |
| AICA | 1 (1.4%) | 0 (0.0%) | |
| Aneurysm symptomatic on presentation | 18 (25.0%) | 5 (83.3%) | <0.01 |
| Maximal diameter, mm | 7 (IQR 5.25–9.95) | 7 (IQR 5–8) | 0.68 |
| Length, mm | 12 (IQR 9–18.9) | 13.5 (IQR 12–15) | 0.65 |
| Ratio | 0.60 (IQR 0.42–0.75) | 0.51 (IQR 0.5–0.66) | 0.55 |
| Progression ‡ | 8 (13.1%) | 1 (25.0%) | 0.50 |
Bold values: p-Value <0.05.
n (%); p-Value: Chi2 test.
*43 Missing data.
†11 Missing data.
§15 Missing data.
II16 Missing data.
¶27 Missing data.
‡13 Missing data.
Discussion
Medical literature on intracranial fusiform aneurysms is limited in comparison to the extensive research on saccular aneurysms. These two types of intracranial aneurysms have different pathophysiological features, hemodynamics, natural histories, risks of bleeding and complications.3,5,11 Reported series from the literature have studied the natural history of both anterior and posterior circulation fusiform aneurysms, 12 or only described the basilar fusiform aneurysms including ruptured and saccular aneurysms. 13
Fusiform aneurysms in the posterior circulation may be found incidentally in 30% of cases (in our series asymptomatic presentation reached up to 75.7%). However, when symptomatic they can present with serious neurological symptoms such as ischemic stroke (due to distal embolic or local thrombotic events), mass effect to the brainstem, and subarachnoid hemorrhage due to aneurysm rupture. 8 The natural course and outcome of these aneurysms have been shown to depend on the initial clinical presentation. 14 Currently, little is known about the long-term risk and factors predicting rupture of fusiform aneurysms in the vertebrobasilar circulation. The dissecting type of fusiform aneurysm has been implicated more commonly in the development of subarachnoid hemorrhage, which has a reported mortality rate of approximately 55%. 8 Intracranial hemorrhage after fusiform aneurysm rupture, although devastating, is reported in only 7% of the case series reported among patients treated for fusiform aneurysms in tertiary neurological centres. 8
Due to the small case series reported, there is still uncertainty regarding which are considered risks factors for aneurysm rupture in intradural fusiform aneurysms. Overall, there is a higher prevalence of intracranial fusiform aneurysms and particularly dissecting type aneurysms, reported in males, which we also observed. However, higher rates of rupture among females trended towards significance. Regarding clinical risk factors for aneurysm rupture, we found a higher association with diabetes mellitus (33.3% vs. 7.0%, p = 0.04). In contrast to the saccular aneurysm literature, 15 other factors evaluated, such as aneurysm maximal diameter, patient age, smoking status, hypertension, and parent artery location within the posterior circulation were not found to be significantly different. Interestingly, we did not find a significantly higher degree of aneurysm rupture in those that had grown (progressed) during the follow-up. As regards growth itself, large initial size was the sole predictor of growth. Furthermore, while growth did not necessarily predict rupture, it did predict an increase of >2 over baseline on follow-up, which could relate to nearby mass effect and ischemic sequelae. While diabetes and coronary disease also affected follow-up mRS, these relationships may be related to the overall health status rather than a relation to specific entities.
Presentation with subarachnoid hemorrhage carries a high incidence of re-bleeding (22–58%), particularly in the first 24 h. 14 Due to this reason is imperative to secure a ruptured fusiform aneurysm within the hospital course. There are two main groups of treatment strategies for dissecting fusiform aneurysms, reconstructive techniques (preserving blood flow through the parent vessel) and deconstructive techniques (sacrifice of the parent artery).10,16,17 For each of these strategies, there are microsurgical 10 or endovascular 14 approaches as well. Recently, endovascular approaches have emerged and become more widely used for the treatment of intracranial aneurysms. 18 Particularly, flow diverters have become a favoured treatment strategy as it is a reconstructive technique.17,19 Nevertheless, a procedural rupture rate of 7.7% is considerable and must be weighed against natural history.20,21
Treatment of posterior circulation fusiform and dissecting aneurysms remains challenging and there is currently no consensus on what treatment would be the best, however there is a trend towards treating with endovascular approaches. Features that make these lesions difficult to treat with endovascular or open surgical approaches include the absence of an aneurysm neck and the presence of critical brainstem perforators. In our series, the majority of cases (74.4%) underwent conservative management with observation. A total of 12 cases (15.4%) underwent endovascular treatment and 3 required ret-treatment. Eight cases (10.3%) underwent open surgical intervention for treatment. Endovascular treatment is gaining popularity in recent years, in particular stent assisted coiling (SAC) and flow diversion (FD), especially with the development of new devices and the increasing experience. In a metanalysis performed by Domingo et al. 20 that evaluated the performance of FD compared to SAC, there was no difference in terms of complete/near-complete occlusion rates (83% vs. 84%; p = 0.95, respectively) but it did show increase risk of overall complications in the FD group (18% vs. 6%; p = 0.008), particularly stroke (13% vs. 5%; p = 0.04). A systematic review performed by Alwakeal et al. 21 that focused only in FD as treatment of these type of aneurysms confirmed these findings as completed occlusion rate was reported to be 75.2% and overall major complication rate of 19.6%. The main factors associated with complications and poor outcome in that study were increasing age and the use of multiple flow diverting stents. 21
Limitations
This study is limited by its retrospective, non-randomized nature with all the inherent bias of this study design. Data were obtained from a review of clinical images and electronic medical records. There was no available information in some baseline observations, which is reflected in the missing data in the tables. Missing data did not appear to inform the statistical significance of our results, including those of the small number of ruptured cases. Another limiting factor is the relatively short follow-up of the overall sample of 15 and 36 months for clinical and imaging follow-up. It would be ideal if we reached an overall follow-up of 5 years. Although this is one of the largest series of posterior circulation fusiform aneurysms, the sample size of this study was relatively small, which limits its ability to detect differences between subgroups. Operators chose to intervene at their discretion which precludes direct comparison with natural history.
Conclusion
Unruptured dissecting/fusiform aneurysms are associated with a considerable rate of rupture during follow-up. Growth is associated with morbidity even in the absence of rupture. An initial aneurysm large size, coronary disease, diabetes, symptoms on initial presentation, and to a lesser extent, female gender may merit treatment versus closer clinical and radiological follow-up.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
ORCID iDs: Adam Andrew Dmytriw https://orcid.org/0000-0003-0131-5699
Gorky Mehdi https://orcid.org/0000-0002-0571-2205
References
- 1.Debette S, Compter A, Labeyrie M-A, et al. Epidemiology, pathophysiology, diagnosis, and management of intracranial artery dissection. Lancet Neurol 2015; 14: 640–654. [DOI] [PubMed] [Google Scholar]
- 2.van Gijn J, Rinkel GJ. Subarachnoid haemorrhage: diagnosis, causes and management. Brain J Neurol 2001; 124: 249–278. [DOI] [PubMed] [Google Scholar]
- 3.Mizutani T, Miki Y, Kojima H, et al. Proposed classification of nonatherosclerotic cerebral fusiform and dissecting aneurysms. Neurosurgery 1999; 45: 253–259; discussion 259–260. [DOI] [PubMed] [Google Scholar]
- 4.Pereira VM, Brina O, Gonzalez AM, et al. Biology and hemodynamics of aneurismal vasculopathies. Eur J Radiol 2013; 82: 1606–1617. [DOI] [PubMed] [Google Scholar]
- 5.Mizutani T. Natural course of intracranial arterial dissections. J Neurosurg 2011; 114: 1037–1044. [DOI] [PubMed] [Google Scholar]
- 6.Sasaki O, Ogawa H, Koike T, et al. A clinicopathological study of dissecting aneurysms of the intracranial vertebral artery. J Neurosurg 1991; 75: 874–882. [DOI] [PubMed] [Google Scholar]
- 7.Krings T, Geibprasert S, terBrugge KG. Pathomechanisms and treatment of pediatric aneurysms. Childs Nerv Syst ChNS Off J Int Soc Pediatr Neurosurg 2010; 26: 1309–1318. [DOI] [PubMed] [Google Scholar]
- 8.Shapiro M, Becske T, Riina HA, et al. Non-saccular vertebrobasilar aneurysms and dolichoectasia: a systematic literature review. J Neurointerventional Surg 2014; 6: 389–393. [DOI] [PubMed] [Google Scholar]
- 9.Flemming KD, Wiebers DO, Brown RD, et al. The natural history of radiographically defined vertebrobasilar nonsaccular intracranial aneurysms. Cerebrovasc Dis Basel Switz 2005; 20: 270–279. [DOI] [PubMed] [Google Scholar]
- 10.Safavi-Abbasi S, Kalani MYS, Frock B, et al. Techniques and outcomes of microsurgical management of ruptured and unruptured fusiform cerebral aneurysms. J Neurosurg 2017; 127: 1353–1360. [DOI] [PubMed] [Google Scholar]
- 11.Kinoshita M, Kida S, Hasegawa M, et al. Pathological examination of a ruptured fusiform aneurysm of the middle cerebral artery. Surg Neurol Int 2014; 5: S465–S468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sacho RH, Saliou G, Kostynskyy A, et al. Natural history and outcome after treatment of unruptured intradural fusiform aneurysms. Stroke 2014; 45: 3251–3256. [DOI] [PubMed] [Google Scholar]
- 13.Saliou G, Sacho RH, Power S, et al. Natural history and management of basilar trunk artery neurysms. Stroke 2015; 46: 948–953. [DOI] [PubMed] [Google Scholar]
- 14.Sikkema T, Uyttenboogaart M, Eshghi O, et al. Intracranial artery dissection. Eur J Neurol 2014; 21: 820–826. [DOI] [PubMed] [Google Scholar]
- 15.Hackenberg KAM, Hänggi D, Etminan N. Unruptured intracranial aneurysms. Stroke 2018. [Epub ahead of print]. [DOI] [PubMed]
- 16.Balik V, Yamada Y, Talari S, et al. State-of-art in surgical treatment of dissecting posterior circulation intracranial aneurysms. Neurosurg Rev 2018; 41: 31–45. [DOI] [PubMed] [Google Scholar]
- 17.Fang Y-B, Lin A, Kostynskyy A, et al. Endovascular treatment of intracranial vertebrobasilar artery dissecting aneurysms: parent artery occlusion versus flow diverter. Eur J Radiol 2018; 99: 68–75. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Sui B, Liu J, et al. Aneurysm wall enhancement on magnetic resonance imaging as a risk factor for progression of unruptured vertebrobasilar dissecting aneurysms after reconstructive endovascular treatment. J Neurosurg 2018; 128: 747–755. [DOI] [PubMed] [Google Scholar]
- 19.Maus V, Mpotsaris A, Dorn F, et al. The use of flow diverter in ruptured, dissecting intracranial aneurysms of the posterior circulation. World Neurosurg 2018; 111: e424–e433. [DOI] [PubMed] [Google Scholar]
- 20.Domingo RA, Tripathi S, Perez-Vega C, et al. Treatment of posterior circulation non-saccular aneurysms with flow diversion versus stent-assisted coiling: a systematic review and meta-analysis. J Neurointerventional Surg 2021; 13: 159–163. [DOI] [PubMed] [Google Scholar]
- 21.Alwakeal A, Shlobin NA, Golnari P, et al. Flow diversion of posterior circulation aneurysms: systematic review of disaggregated individual patient data. AJNR Am J Neuroradiol 2021; 42: 1827–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]