Abstract
Background
Acute isolated posterior cerebral artery (PCA) occlusions account for 5–10% of all ischemic events. Due to peculiar patient presentation, the potential benefit of mechanical thrombectomy (MT) remains controversial. We evaluated the safety, feasibility, and effectiveness of MT in our patients and compared our results with the literature review conducted.
Methods
Charts were reviewed retrospectively for consecutive patients diagnosed with acute PCA stroke who underwent MT. Demographics, procedural, and follow-up details were noted. For the literature review, a systematic search of PubMed, MEDLINE, and EMBASE databases was conducted for the keywords “posterior cerebral artery” and “thrombectomy” for articles published between January 1, 2010 and June 30, 2021. Estimated rates for recanalization, favorable outcomes (modified Rankin Scale [mRS] score 0–2), symptomatic intracerebral hemorrhage (sICH), and mortality were extracted.
Results
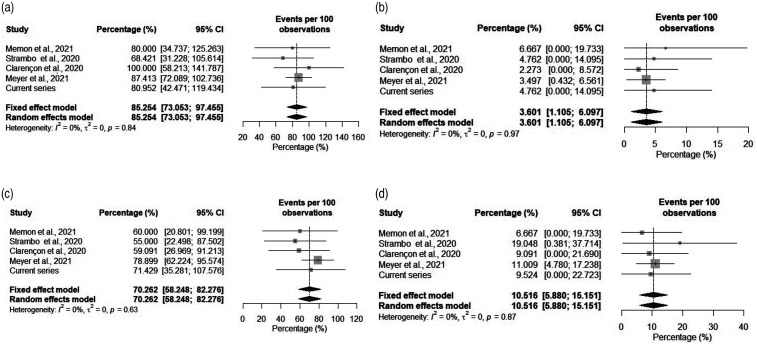
Our cohort included 21 patients. Mean age was 71.2 years (standard deviation [SD] ± 10.2). Median National Institutes of Health Stroke Scale (NIHSS) presentation score was 9 (interquartile range [IQR] 5–15), with visual symptoms reported in 12(57.1%) patients. Overall, final modified thrombolysis in cerebral infarction (mTICI) 2b-3 was achieved in 17 patients (80.9%) with first-pass mTICI 2b-3 attained in 8 (38.1%). Postprocedure sICH occurred in 1 (4.8%) patient. Fifteen (71.4%) patients had a 0–2 mRS score at 90 days. Visual symptoms resolved in 10 of 12(83.3%) patients. Mortality occurred in 2 (9.5%) patients. For the systematic review, cohorts from 4 articles plus ours were included, totaling 222 patients. The estimated rate of successful recanalization was 85.25% (95% confidence interval[CI], 73.05%–97.45%), sICH was 3.60% (95% CI, 1.11%–6.09%), and mortality was 10.51% (95% CI, 5.88%–15.15%).
Conclusion
The results of our series and systematic review indicate MT as a potentially safe and effective treatment modality for acute PCA stroke. These results also indicate that patient selection and assessment may be the key in obtaining favorable outcomes.
Keywords: Posterior cerebral artery occlusion, stroke, mechanical thrombectomy
Introduction
Posterior circulation strokes account for approximately 20% of all stroke cases with posterior cerebral artery (PCA) accounting for 5–10% of all ischemic events.1–3 PCA is responsible for suppling blood to a number of eloquent areas of the brain including the primary visual cortex in the occipital lobe, the inferomedial temporal lobe, and a major portion of the thalamus.4,5 This diverse vascular distribution makes a PCA territory infarct different from an anterior circulation stroke, in terms of not only the presenting symptoms but also the permanent and severe neurologic deficit that leads to substantial impact on the patient's quality of life and functional independence.6,7 Patients who suffer a PCA territory infarct often present with an array of uncommon and nonspecific neuropsychological symptoms that may result in a low presenting National Institute of Health Stroke Scale (NIHSS) score, leading to a delayed diagnosis and avoidance of mechanical thrombectomy (MT).2,5 Consequently, these patients often present outside the 4.5-h therapeutic window for tissue plasminogen activator (tPA) therapy and on the occasion when they present within that window, recanalization rates are inadequate at best 5
Advances in neurointervention device technology have allowed for safe access to more distal, medium to small intracranial vasculature.8–10 Reports of the safety and efficacy of thrombectomy for this subset of patients are scarce but show some encouraging results.6,11–13 In this study, we describe a series of isolated acute PCA occlusions treated with MT at our institution and additionally conduct a systematic review. Our objective was to evaluate the safety, feasibility, and effectiveness of MT in these patients and compare our results with those in the literature.
Methods
Clinical series and data collection
After receiving institutional review board approval, we retrospectively searched our prospectively maintained database for consecutive patients with an isolated, acute PCA occlusion (P1, P2, or P3 segment) who underwent MT between January 1, 2015 and July 31, 2020. Informed consent for these procedures had been provided by each patient or a legally authorized representative.
Demographic characteristics (age, sex, and comorbidities), clinical presentation, administration of intravenous (IV) alteplase (tPA), and time from symptom onset to groin or wrist puncture were recorded. Occlusion location, devices used, number of total passes performed, intraprocedural, and postprocedural complications, recanalization grades, and clinical outcomes were also recorded. The NIHSS was used to measure initial symptom severity. The modified Rankin Scale score (mRS) was used to define pre-stroke functional status and clinical outcome at 90 days. Determination of the arterial occlusion site was based on Fischer's segmentation (from P1 to P4) on digital subtraction angiography (DSA). 14 Successful recanalization was defined as modified thrombolysis in cerebral infarction (mTICI) score of 2b-3 at the time of the conclusion of the thrombectomy. Symptomatic intracerebral hemorrhage (sICH) was defined according to the European Cooperative Acute Stroke Study (ECASS) guidelines as symptomatic if a parenchymal hematoma type II was accompanied by a ≥4-point increase in the NIHSS score or if it led to death. 15
Literature review
A systematic search of the PubMed, MEDLINE, and EMBASE databases was conducted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Keywords (“posterior cerebral artery” and “thrombectomy”) were used with Boolean operators to increase specificity and sensitivity. Screening and initial study selection for the systematic review was conducted using Rayyan, a web-based application for reviews and meta-analyses. 16 We included articles published in the English language between January 1, 2010 and June 30, 2021 that reported 5 or more patients with acute isolated PCA occlusion treated with MT. Background articles and animal and cadaveric studies were excluded. Studies in which data specific to acute PCA occlusion could not be separately extracted were also excluded. Full-text articles were assessed regarding study center and time period, and those with overlapping patient population were excluded while retaining the most recent and/or complete study. Data extracted included sample size; rates of successful recanalization, sICH, and mortality; and mRS 0–2 scores at 90-days.
Statistical analysis
Continuous variables were reported as means or medians and respective standard deviations (SD) and interquartile ranges (IQR) according to data normality. Categorical variables were reported as frequencies. For our systematic review analysis, we included our study's results and estimated rates of sICH, recanalization, favorable outcomes, and mortality at 90-days were generated with weighting for each study sample size. The extent of heterogeneity among studies was assessed with an I 2 test. Pooled analyses were performed with fixed effect, and DerSimonian and Laird random effects models. All analyses were completed using RStudio version 1.4.1106 and the R General Package for Meta-Analysis (https://www.rstudio.com).
Results
Patient characteristics
Twenty-one patients were included in our cohort. Mean age was 71.2 years (SD ± 10.2), and 12 (57.1%) were women. Hypertension was the most common comorbidity (52.3%). Median NIHSS score at presentation was 9 (IQR 5–15). Visual symptoms such as hemianopia or complete monocular blindness were reported in 12 patients (57.1%). Ten patients (47.6%) received IV-tPA. The median time from symptom onset to groin or wrist puncture was 189 min (IQR 110–255) for the 13 patients for whom time last known well was available (unknown for 8 patients [38.1%]). These characteristics are summarized on Table 1.
Table 1.
Demographics and clinical characteristics at presentation.
| Case no. | Age (years) | Sex | Comorbidities | NIHSS score at presentation | Visual symptoms at presentation | IV-tPA administration | Time of onset to MT (minutes) |
|---|---|---|---|---|---|---|---|
| 1 | 84 | Male | None | 9 | None | No | 226 |
| 2 | 68 | Female | None | 5 | Right hemianopia and partial gaze palsy | No | Unknown |
| 3 | 69 | Female | DM, Hypertension, Hyperlipidemia | 8 | Right hemianopia | No | Unknown |
| 4 | 88 | Female | Hypertension | 7 | Right hemianopia | No | 189 |
| 5 | 71 | Female | Hypertension, Afib | 24 | Left quadrant hemianopia | No | 85 |
| 6 | 70 | Female | COPD | 9 | Right temporal field deficit | No | 105 |
| 7 | 68 | Female | DM, Hypertension, Hyperlipidemia, CAD | 17 | Right, partial hemianopia | No | 1080 |
| 8 | 79 | Male | Hypertension, Afib | 5 | Right-sided complete vision loss | No | 390 |
| 9 | 76 | Male | Afib | 7 | None | Yes | 158 |
| 10 | 78 | Male | None | 24 | None | Yes | 115 |
| 11 | 63 | Male | DM, Hypertension | 1 | Right upper quadrant hemianopia | Yes | 283 |
| 12 | 88 | Female | Hypertension, Afib, DVT, Hyperlipidemia | 14 | Left sided complete vision loss | No | Unknown |
| 13 | 74 | Female | Hypertension, Afib | 3 | Right sided complete vision loss | No | 94 |
| 14 | 54 | Male | None | 11 | Right hemianopia | No | 197 |
| 15 | 58 | Male | Afib | 8 | Left sided complete vision loss | Yes | 205 |
| 16 | 80 | Female | DM | 4 | None | Yes | Unknown |
| 17 | 53 | Male | Hyperlipidemia | 3 | None | Yes | Unknown |
| 18 | 56 | Female | Hyperlipidemia | 10 | None | Yes | Unknown |
| 19 | 82 | Female | Hypertension, Afib | 25 | None | Yes | Unknown |
| 20 | 67 | Male | DM, Hypertension, Hyperlipidemia | 11 | None | Yes | 186 |
| 21 | 69 | Female | Hypertension, Hyperlipidemia, CAD, COPD | 22 | None | Yes | Unknown |
Afib: atrial fibrillation; CAD: coronary artery disease; COPD: chronic obstructive pulmonary disease; DM: diabetes mellitus; DVT: deep vein thrombosis; IV-tPA: intravenous tissue plasminogen activator; MT: mechanical thrombectomy; NIHSS: National Institutes of Health Stroke Scale; no.: number.
Procedural information and outcomes
The occlusions were located in the P1 segment in 13 patients (61.9%) and P2 segment in 8 (38.1%). No P3 or P4 occlusions were reported in our cohort. The access site was the femoral artery in 13 patients (61.9%) and the radial artery in 8 cases (38.1%). One of these patients required switching the access site from the radial artery to the femoral artery. The primary technique for MT was the direct aspiration first pass technique (ADAPT) in 9 (42.9%) cases, stent-retriever assisted aspiration (SRA) in 7 (33.3%) cases, and stent-retriever alone or with manual aspiration in 5 cases (23.8%). The median number of passes to achieve complete recanalization was 2 (IRQ 1–2).
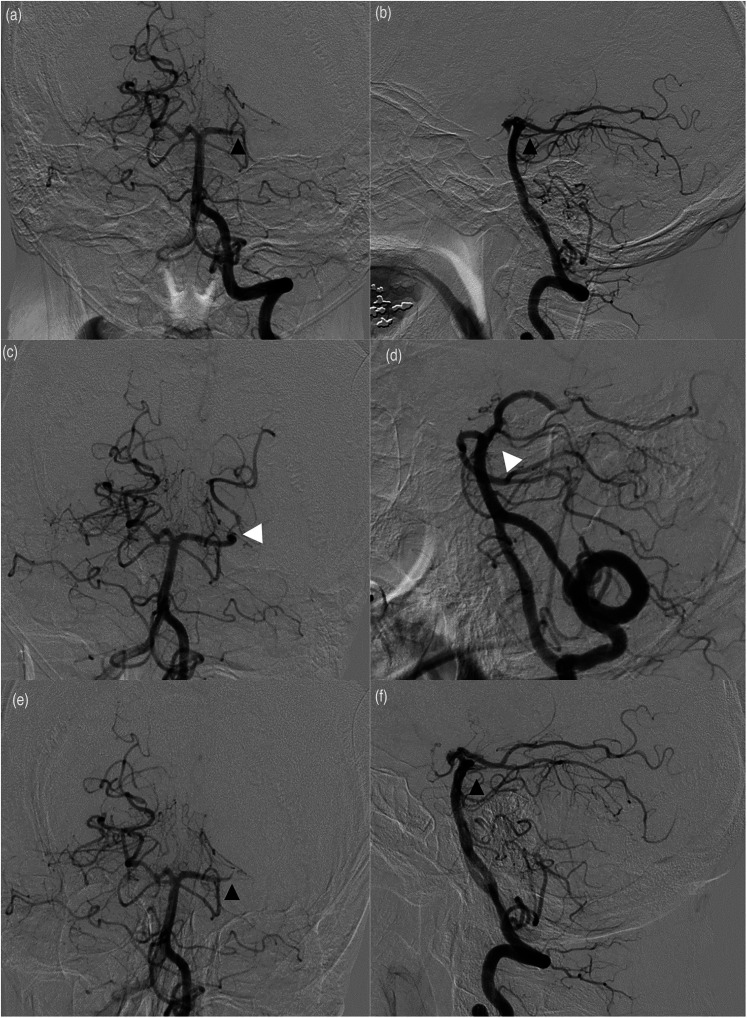
An intraprocedural complication occurred in 1 case (4.8%). This patient had an immediate reocclusion following recanalization (Figure 1(a) to (f)). Initially, we were successful at crossing the occlusion at P1-P2 junction and obtaining a run distal to the thrombus. A triple-axial system using a .041in aspiration catheter (Penumbra Inc., Alameda, CA) advanced over a Velocity microcatheter (Penumbra, Inc.) over a Synchro-2 microwire (Stryker Neurovascular, Fremont, CA) was used to access the left PCA. The Velocity and Synchro-2 were then used to cross through the area of occlusion, which turned out to be a very hard, calcified thrombus that was difficult to cross. A Solitaire stent-retreiver device (Medtronic) measuring 4 mm × 15 mm was then used to attempt MT and was partially successful. We then attempted to fragment the thrombus with a .041in separator (Separator Flex, Penumbra Inc.) in combination with a 014 in aspiration catheter (Penumbra Inc.). There was partial reopening of the left PCA initially; however, further imaging demonstrated reocclusion of the vessel. Half of a loading dose of intra-arterial eptifibatide (90 μg/kg of body weight) was administered in an attempt to break up the clot, but this was not felt to be successful and the procedure was eventually aborted. Of note, the patient had been given tPA prior to this intervention and was also fully heparinized for an activated coagulation time of >250 s
Figure 1.
(a) anteroposterior (AP) and lateral (b) views of a left vertebral artery selective catheterization demonstrating an occlusion of the left posterior cerebral artery (PCA) at the P1-P2 junction (black arrows). After stent retriever-assisted aspiration, partial reopening of the PCA (white arrows) was witnessed, as shown in the AP (c) and lateral (d) views. However, despite administering eptifibatide, further imaging demonstrated reocclusion of the vessels (E, AP; F, lateral) (black arrows).
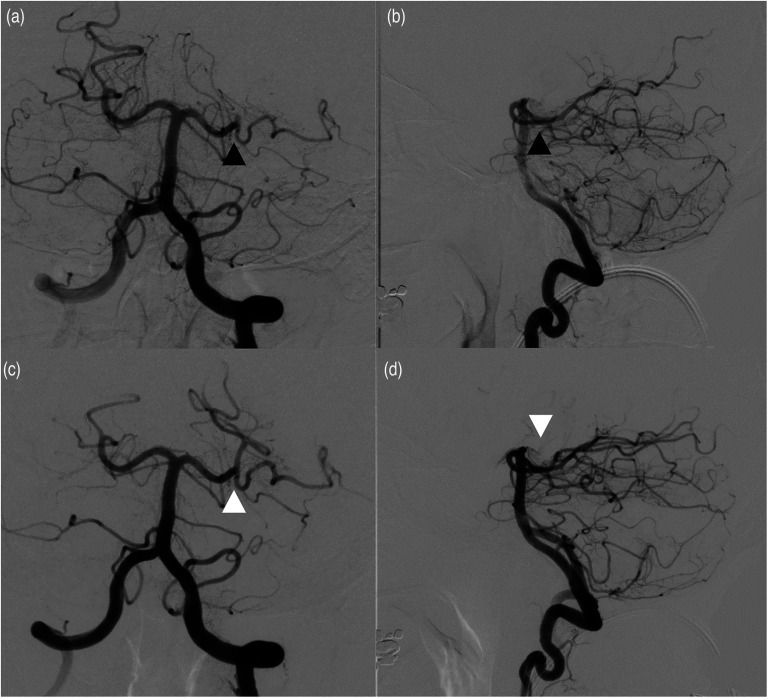
A final mTICI 2b-3 score was achieved in 17 patients (80.9%) with first-pass mTICI 2b-3 attained in 8 cases (38.1%) (Figure 2(a) to (d)). Postprocedure sICH occurred in 1 patient (4.8%). These data are summarized in Table 2.
Figure 2.
(a) AP and lateral (b) views demonstrating normal filling of the left P1 segment of the PCA with a cut off (occlusion) of the artery in the P2 segment (black arrows). Complete revascularization (modified thrombolysis in cerebral infarction [mTICI] score of 3) was obtained with a single pass using stent retriever-assisted aspiration technique as shown in AP (c) and lateral (d) views.
Table 2.
Periprocedural details.
| Case no. | Occlusion location | Arterial access site | Primary thrombectomy technique | Passes (no.) | Final mTICI score | Intraprocedural complications | sICH |
|---|---|---|---|---|---|---|---|
| 1 | P1 | Femoral | Stent retriever | 2 | 2c | None | No |
| 2 | P1 | Radial | ADAPT | 2 | 3 | None | Yes |
| 3 | P2 | Femoral | ADAPT | 2 | 3 | None | No |
| 4 | P2 | Femoral | ADAPT | 2 | 2b | None | No |
| 5 | P1 | Femoral | Stent retriever | 2 | 2a | None | No |
| 6 | P1 | Femoral | Stent retriever | 1 | 3 | None | No |
| 7 | P2 | Radial | ADAPT | 1 | 3 | None | No |
| 8 | P1 | Radial | SRA | 2 | 2b | None | No |
| 9 | P2 | Radial | ADAPT | 2 | 1 | None | No |
| 10 | P1 | Femoral | Stent retriever | 2 | 2b | None | No |
| 11 | P2 | Radial | ADAPT | 1 | 3 | None | No |
| 12 | P1 | Femoral (after unsuccess-ful radial attempt) | ADAPT | 3 | 0 | None | No |
| 13 | P2 | Radial | SRA | 1 | 3 | None | No |
| 14 | P1 | Femoral | SRA | 1 | 3 | None | No |
| 15 | P1 | Radial | SRA | 3 | 3 | None | No |
| 16 | P2 | Radial | Stent retriever | 2 | 2c | None | No |
| 17 | P1 | Femoral | SRA | 3 | 0 | Reocclusion | No |
| 18 | P1 | Femoral | SRA | 1 | 3 | None | No |
| 19 | P1 | Femoral | ADAPT | 1 | 3 | None | No |
| 20 | P2 | Femoral | SRA | 2 | 3 | None | No |
| 21 | P1 | Femoral | ADAPT | 1 | 2b | None | No |
ADAPT: a direct aspiration first pass technique; mTICI: modified thrombolysis in cerebral infarction; sICH: symptomatic intracranial hemorrhage; no.: number; SRA: stent-retriever assisted aspiration.
In follow up at 90 days, 15 patients (71.4%) had an mRS score of 0–2. Mortality occurred in 2 patients (9.5%). Visual symptoms persisted in only 2 of 12 patients (16.7%), which included mild decrease in visual acuity in 1 patient and homonymous hemianopia in the other.
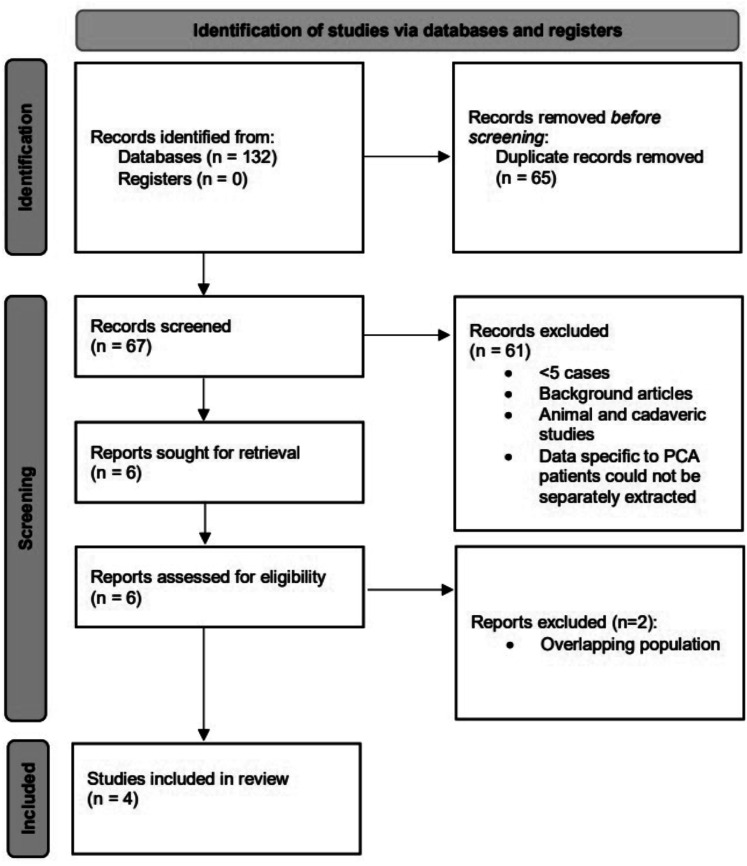
Systematic literature review
Our search resulted in 132 articles, of which 65 were duplicates. From 67 unique articles, 61 were excluded with reasons (Figure 3). The remaining 6 studies were assessed in full-text, and 2 of these were excluded for overlap in patient populations among the studies. A total of 4 articles plus our cohort were included in final analysis for a total of 222 patients.6,11–13 The estimated rate of successful recanalization (mTICI 2b-3 score) was 85.25% (95% confidence interval [CI], 73.05%–97.45%), of sICH was 3.60% (95% CI, 1.11%–6.09%), of favorable clinical outcome (mRS 0–2 score) at 90 days was 70.26% (95% CI, 58.24%–82.27%) and of mortality was 10.51% (95% CI, 5.88%–15.15%). These rates are plotted in Figure 4(a) to (d).
Figure 3.
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow chart showing the selection process.
Figure 4.
Plotted rates of successful recanalization (final mTICI score of 2b-3) (a), symptomatic intracranial hemorrhage (b), favorable outcome (modified Rankin Scale 0–2 score) at 90 days (c), and mortality (d).
Discussion
In this study, we reported the experience at our own institution and presented a systematic review of the literature on acute PCA strokes treated with MT. We obtained high rates of successful recanalization (80.9%) and favorable clinical outcomes (71.4%), with a low incidence of postprocedural sICH (4.8%). Our systematic review showed similar results in terms of final mTICI and postprocedure sICH.
Treatment and management of PCA strokes is often challenging due not only to the atypical nature of patient symptomatology and low presenting NIHSS scores (≤5) but also to the lack of consensus and specific guidelines detailing the management of these patients.2,5,17,18 This lack of consensus is mostly because all five major thrombectomy trials evaluating the efficacy of MT for LVO failed to include posterior circulation strokes in either the MT arm or the IV-tPA group. 19 For this reason, most reports highlighting these cases are either small case series that include concomitant occlusions of the basilar or vertebral arteries or subanalyses of a handful of postmarketing LVO registries that include PCA strokes.11,20,21 One reason for excluding these patients is the lack of consensus regarding an agreed upon definition for the classification of PCA into large and distal, medium vessel occlusions (DMVO) with P2 and P3 occlusions being generally considered as DMVO and P1 as an LVO. 22 In our cohort, both P1 and P2 occlusions were included (P1 in 13 patients [61.9%] and P2 in 8 [38.1%]), representing both LVO and DMVO subgroups. This is in contrast to Thrombectomy for Primary Distal Posterior Cerebral Artery Occlusion Stroke (TOPMOST), the largest retrospective, multicenter, case-control study assessing the safety and efficacy of MT for PCA strokes, in which only DMVO cases of P2 and P3 occlusion were included, with the former comprising more than 80% of cases. 6 MT did prove to be significantly beneficial for a subgroup of patients who had an initial NIHSS score of ≥10 points (mean difference, − 5.6; 95%CI, − 10.9 to − 0.2; p = 0.04), although no significant difference for the mean NIHSS score decrease at discharge between standard medical therapy and MT was noted (−1.5 points; 95% CI, 3.2 to −0.8; p = 0.06). TOPMOST also reported a postprocedure sICH complication rate of 4.3%, similar to ours of 4.8%. The risk of postprocedure sICH appeared to be similar in the MT and standard medical therapy groups (3.5 vs. 3%), highlighting the comparable safety of MT for PCA strokes. 6 Other studies included in our review reported sICH rates in a similar range for patients undergoing MT (0–6.7%) (Table 3).
Table 3.
Studies included in the systematic review.
| Study (Year) | Design | Sample size | Presenting NIHSS score (median [IQR]) | Successful recanalization (%) | Good outcome (%) | Mortality at 3-months (%) | sICH (%) |
|---|---|---|---|---|---|---|---|
| Strambo et al. 13 2020 | Retrospective | 21 | 7 [5–8.3] | 68.4 | 55 | 19.1 | 4.8 |
| Memon et al. 12 2021 | Retrospective | 15 | 9 [5–15] | 80 | 60 | 6.7 | 6.7 |
| Clarençon et al. 11 2020 | Retrospective | 22 | 14 [8–16] | 100 | 59 | 9.1 | 0 |
| Meyer et al. 6 2021 (TOPMOST) | Retrospective | 143 | 7 [4–11] | 87.4 | 78.9 | 13.3 | 4.3 |
| Current Series | Retrospective | 21 | 9 [5–15.5] | 80.9 | 71.4 | 9.5 | 4.8 |
IQR: interquartile range; NIHSSA: National Institutes of Health Stroke Scale; sICH: symptomatic intracranial hemorrhage; TOPMOST: Thrombectomy for Primary Distal Posterior Cerebral Artery Occlusion Stroke.
In terms of patient presentation and selection for endovascular intervention, PCA strokes present a unique challenge by manifesting a broad range of visual and neuropsychological symptoms that not only delay the diagnosis but also lead to a low NIHSS score (≤5).2–5 Our review revealed that the median NIHSS score at presentation for patients who underwent MT was 7.9. This low NIHSS score highlights the apparent mild nature of these strokes despite debilitating visual and neuropsychological symptoms. This presents a unique challenge in terms of patient assessment and outcome measurement because the scores tend to be less reliable in prognosticating the impact of this kind of neurologic disability. The NIHSS score is tailored for anterior circulation more so than PCA strokes and does not reflect the entire spectrum of posterior circulation stroke symptoms leading to a low score and subsequent patient exclusion from potential endovascular intervention. 23 Likewise, mRS scores are also tailored to assess functional neurologic status, reflecting motor functions more so than visual or cognitive capabilities. Strambo et al. showed that attention and executive function were the two most commonly impaired cognitive domains seen in 67% and 50%, respectively, of their cohort of patients with isolated PCA occlusion. 13 This finding is consistent with earlier studies on the general stroke population and reflects the extent of PCA territory infarcts throughout the cerebral hemispheres. Notably, of the 4 studies included in our systematic review, only Strambo et al. reported visual symptoms at presentation and the rate of resolution for those symptoms. 13 A visual field defect was present in 83 of 106 patients (78.3%), with resolution at follow up seen in 25 of the 83 patients (30.1%). 13 Similar results were seen in our cohort whereby visual deficits were the presenting symptoms that were documented in 12 cases (57.1%), ranging in severity from mild or partial hemianopia to complete blindness. The visual symptom resolution rate at 90 days was 83.3% with symptoms persistence in 2 cases (16.7%). These results, although limited, point to not only the pitfalls in terms of patient selection and assessment but also those for proper outcome measurements.
Despite the late and peculiar presentation, thrombectomy for PCA has shown some promising results.6,11–13,17 In their subanalysis of patients from the Trevo retriever (Stryker Neurovascular) registry, Clarenҁon et al. showed a high first-pass effect (mTICI ≥2b) of 65% and an even more impressive final mTICI ≥2b of 100%. 11 Unfortunately, this high recanalization rate did not translate clinically because good outcomes (mRS scores of 0–2) at 90-days postprocedure were reported in only 59.1% of cases. 11 In another study, Strambo et al. compared MT with best medical therapy and demonstrated significantly higher and more frequent complete recanalization in the MT arm when compared with best medical therapy (68% vs. 34%; OR [95% CI] = 4.11 [1.35–12.53]). The MT group also had a higher proportion of functional independence, visual, and cognitive outcomes compared with the best medical therapy group (adjORs [95% CI] = 1.44 [0.51 − 4.10], 4.28 [1.00 − 18.29] 4.37 [0.72 − 26.53], respectively). 13 Similarly, our systematic review showed an estimated rate of good outcomes at 70.2% (95% CI, 58.2–82.3). Our cohort also showed similar results in terms of an acceptable first-pass effect attained in 8 cases (38.1%) and an overall final mTICI 2b-3 achieved in 17 patients (80.9%). Interestingly, for our cohort, this high initial recanalization rate translated to favorable clinical outcomes (mRS 0–2) at 90-days as well, with 15 patients (71.4%) having no or little disability. This finding may be explained by the initial presentation consisting of mostly visual symptoms in the majority of patients in our cohort. However, these results point towards an encouraging response to timely endovascular therapy and highlight the potential benefit of intervention, particularly in patients who presented solely with visual symptoms.
Our study has limitations that include the retrospective and observational nature of the patient data. The systematic review also includes retrospective case series that evaluated small populations and may potentially be affected by publication bias. Yet, we highlight some key points regarding patient presentation and assessment with proper selection for endovascular management of these cases.
Conclusion
In our study, we report MT as a potentially safe and effective treatment modality for patients presenting with a PCA stroke. The results of our series and the systematic review indicate that despite the scarcity of data on these cases, patient selection and assessment may be the key in obtaining favorable long-term clinical outcomes.
Acknowledgements
The authors thank Paul H. Dressel BFA for formatting the illustration and Debra J. Zimmer for editorial assistance.
Abbreviations and acronyms
- ADAPT
direct aspiration first pass technique
- adjORs
adjusted odds ratios
- CI
confidence interval
- DMVO
distal, medium vessel occlusions
- DSA
digital subtraction angiography
- ECASS
European Cooperative Acute Stroke Study
- IQR
interquartile range
- IV
intravenous
- LVO
large vessel occlusion
- mRS
modified Rankin scale
- mTICI
modified thrombolysis in cerebral infarction
- NIHSS
National Institutes of Health Stroke Scale Score
- OR
odds ratio
- PCA
posterior cerebral artery
- sICH
symptomatic intracranial hemorrhage
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- SD
standard deviation
- SRA
stent-retriever assisted aspiration
- TOPMOST
Thrombectomy for Primary Distal Posterior Cerebral Artery Occlusion Stroke
- tPA
tissue plasminogen activator.
Footnotes
Contributorship: Conception and design: AAB, AM. Acquisition of the data: AM, AAB, MS. Analysis and interpretation of the data: all authors. Drafting the manuscript: AAB, AM. Critically revising the manuscript: all authors. Reviewed submitted version of manuscript: all authors.
Data availability: Data that support the findings of this study are available from the corresponding author on reasonable request
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AAB, AM, MW, JMC, MS, JD, RHD, FA, WIK: None. JMD Research grants: NIH NINDS, NSF SBIR; Consulting fees: Medtronic; Honoraria: Medtronic; Support for attending meetings and/or travel: Medtronic; Patents planned, issued or pending: QAS.ai; Participation on a Data Safety Monitoring Board or Advisory Board: NIH NIHDS Strokenet; Stock or stock options: Synchron, Cerebrotech, QAS.ai, RIST.KVS--Consulting Fees: Boston Scientific, Canon Medical Systems USA, Inc., MicroVention, Medtronic, Stryker Neurovascular. Payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational event: Canon Medical Systems USA Inc. Stock or stock options: Boston Scientific, Access Closure Inc, Niagara Gorge Medical.EIL--Consulting fees: Claret Medical, GLG Consulting, Guidepoint Global, Imperial Care, Medtronic, Rebound, StimMed, Misionix, Mosiac, Clarion, IRRAS. Payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events: Medtronic; Payment for expert testimony: for rendering medical/legal opinions as an expert. Support for attending meetings and/or travel: Reimbursement for travel and food for some meetings with the CNS and ABNS. Stock or stock options: NeXtGen Biologics, RAPID Medical, Claret Medical, Cognition Medical, Imperative Care, Rebound Therapeutics, StimMed, Three Rivers Medical.AHS—Consulting fees: Amnis Therapeutics, Apellis Pharmaceuticals, Inc., Boston Scientific, Canon Medical Systems USA, Inc., Cardinal Health 200, LLC, Cerebrotech Medical Systems, Inc., Cerenovus, Cerevatech Medical, Inc., Cordis, Corindus, Inc., Endostream Medical, Ltd, Imperative Care, Integra, IRRAS AB, Medtronic, MicroVention, Minnetronix Neuro, Inc., Penumbra, Q’Apel Medical, Inc., Rapid Medical, Serenity Medical, Inc., Silk Road Medical, StimMed, LLC, Stryker Neurovascular, Three Rivers Medical, Inc., VasSol, Viz.ai, Inc., W.L. Gore & Associates. Leadership or fiduciary role in other board, society, committee or advocacy group: Secretary of the Board of the Society of NeuroInterventional Surgery, Chair of the Cerebrovascular Section of the AANS/CNS. Stock or stock options: Adona Medical, Inc., Amnis Therapeutics, Bend IT Technologies, Ltd., BlinkTBI, Inc, Buffalo Technology Partners, Inc., Cardinal Consultants, LLC, Cerebrotech Medical Systems, Inc, Cerevatech Medical, Inc., Cognition Medical, CVAID Ltd., E8, Inc., Endostream Medical, Ltd, Imperative Care, Inc., Instylla, Inc., International Medical Distribution Partners, Launch NY, Inc., NeuroRadial Technologies, Inc., Neurotechnology Investors, Neurovascular Diagnostics, Inc., PerFlow Medical, Ltd., Q’Apel Medical, Inc., QAS.ai, Inc., Radical Catheter Technologies, Inc., Rebound Therapeutics Corp. (Purchased 2019 by Integra Lifesciences, Corp), Rist Neurovascular, Inc. (Purchased 2020 by Medtronic), Sense Diagnostics, Inc., Serenity Medical, Inc., Silk Road Medical, Adona Medical, Inc., Amnis Therapeutics, Bend IT Technologies, Ltd., BlinkTBI, Inc, Buffalo Technology Partners, Inc., Cardinal Consultants, LLC, Cerebrotech Medical Systems, Inc, Cerevatech Medical, Inc., Cognition Medical, CVAID Ltd., E8, Inc., Endostream Medical, Ltd, Imperative Care, Inc., Instylla, Inc., International Medical Distribution Partners, Launch NY, Inc., NeuroRadial Technologies, Inc., Neurotechnology Investors, Neurovascular Diagnostics, Inc., PerFlow Medical, Ltd., Q’Apel Medical, Inc., QAS.ai, Inc., Radical Catheter Technologies, Inc., Rebound Therapeutics Corp. (Purchased 2019 by Integra Lifesciences, Corp), Rist Neurovascular, Inc. (Purchased 2020 by Medtronic), Sense Diagnostics, Inc., Serenity Medical, Inc., Silk Road Medical, SongBird Therapy, Spinnaker Medical, Inc., StimMed, LLC, Synchron, Inc., Three Rivers Medical, Inc., Truvic Medical, Inc., Tulavi Therapeutics, Inc., Vastrax, LLC, VICIS, Inc., Viseon, Inc. Other financial or non-financial interests: National PI/Steering Committees: Cerenovus EXCELLENT and ARISE II Trial; Medtronic SWIFT PRIME, VANTAGE, EMBOLISE and SWIFT DIRECT Trials; MicroVention FRED Trial & CONFIDENCE Study; MUSC POSITIVE Trial; Penumbra 3D Separator Trial, COMPASS Trial, INVEST Trial, MIVI neuroscience EVAQ Trial; Rapid Medical SUCCESS Trial; InspireMD C-GUARDIANS IDE Pivotal Trial.
Ethical approval statement: At the time of admission, informed consent for procedures and for publication of patient data was provided by each patient or a legally authorized representative.
The institutional review board at the University at Buffalo approved this study (STUDY00005357).
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
Previous presentation: E-poster presentation at the Congress of Neurological Surgeons (CNS) Annual Meeting – October 16–20, 2021 – Austin, Texas, USA.
ORCID iDs: Muhammad Waqas https://orcid.org/0000-0003-4500-7954
Wasiq I Khawar https://orcid.org/0000-0001-7966-6005
Jason M Davies https://orcid.org/0000-0002-5225-3072
Elad I Levy https://orcid.org/0000-0002-6208-3724
Adnan H Siddiqui https://orcid.org/0000-0002-9519-0059
References
- 1.Merwick Á, Werring D. Posterior circulation ischaemic stroke. BMJ 2014; 348: g3175. [DOI] [PubMed] [Google Scholar]
- 2.Arboix A, Arbe G, García-Eroles L, et al. Infarctions in the vascular territory of the posterior cerebral artery: clinical features in 232 patients. BMC Res Notes 2011; 4: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ntaios G, Spengos K, Vemmou AM, et al. Long-term outcome in posterior cerebral artery stroke. Eur J Neurol 2011; 18: 1074–1080. [DOI] [PubMed] [Google Scholar]
- 4.Schmahmann JD. Vascular syndromes of the thalamus. Stroke 2003; 34: 2264–2278. [DOI] [PubMed] [Google Scholar]
- 5.Sand KM, Wilhelmsen G, Næss H, et al. Vision problems in ischaemic stroke patients: effects on life quality and disability. Eur J Neurol 2016; 23: –7. [DOI] [PubMed] [Google Scholar]
- 6.Meyer L, Stracke CP, Jungi N, et al. Thrombectomy for primary distal posterior cerebral artery occlusion stroke: the TOPMOST study. JAMA Neurol 2021; 78: 434–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ng YS, Stein J, Salles SS, et al. Clinical characteristics and rehabilitation outcomes of patients with posterior cerebral artery stroke. Arch Phys Med Rehabil 2005; 86: 2138–2143. [DOI] [PubMed] [Google Scholar]
- 8.Goyal M, Ospel JM, Menon BK, et al. MeVO: the next frontier? J Neurointerv Surg 2020; 12: 545–547. [DOI] [PubMed] [Google Scholar]
- 9.Saver JL, Chapot R, Agid R, et al. Thrombectomy for distal, Medium vessel occlusions: a consensus statement on present knowledge and promising directions. Stroke 2020; 51: 2872–2884. [DOI] [PubMed] [Google Scholar]
- 10.Waqas M, Kuo CC, Dossani RH, et al. Mechanical thrombectomy versus intravenous thrombolysis for distal large-vessel occlusion: a systematic review and meta-analysis of observational studies. Neurosurg Focus 2021; 51: E5. [DOI] [PubMed] [Google Scholar]
- 11.Clarençon F, Baronnet F, Shotar E, et al. Should posterior cerebral artery occlusions be recanalized? Insights from the trevo registry. Eur J Neurol 2020; 27: 787–792. [DOI] [PubMed] [Google Scholar]
- 12.Memon MZ, Kushnirsky M, Brunet MC, et al. Mechanical thrombectomy in isolated large vessel posterior cerebral artery occlusions. Neuroradiology 2021; 63: 111–116. [DOI] [PubMed] [Google Scholar]
- 13.Strambo D, Bartolini B, Beaud V, et al. Thrombectomy and thrombolysis of isolated posterior cerebral artery occlusion: cognitive, visual, and disability outcomes. Stroke 2020; 51: 254–261. [DOI] [PubMed] [Google Scholar]
- 14.Fischer E. Die lageabweichungen der vorderen hirnarterie im gefassbild. zbl. Neurochir 1938; 3: 300–312. [Google Scholar]
- 15.Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). second european-australasian acute stroke study investigators. Lancet 1998; 352: 1245–1251. [DOI] [PubMed] [Google Scholar]
- 16.Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan-a web and mobile app for systematic reviews. Syst Rev 2016; 5: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyer L, Papanagiotou P, Politi M, et al. Feasibility and safety of thrombectomy for isolated occlusions of the posterior cerebral artery: a multicenter experience and systematic literature review. J Neurointerv Surg 2021; 13: 217–220. [DOI] [PubMed] [Google Scholar]
- 18.Kayan Y, Meyers PM, Prestigiacomo CJ, et al. Current endovascular strategies for posterior circulation large vessel occlusion stroke: report of the society of NeuroInterventional surgery standards and guidelines committee. J Neurointerv Surg 2019; 11: 1055–1062. [DOI] [PubMed] [Google Scholar]
- 19.Goyal M, Menon BK, van Zwam WH, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016; 387: 1723–1731. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto T, Ohshima T, Sato M, et al. A case of acute isolated posterior cerebral artery occlusion successfully treated with endovascular clot aspiration. NMC Case Rep J 2017; 4: 55–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weber R, Minnerup J, Nordmeyer H, et al. Thrombectomy in posterior circulation stroke: differences in procedures and outcome compared to anterior circulation stroke in the prospective multicentre REVASK registry. Eur J Neurol 2019; 26: 299–305. [DOI] [PubMed] [Google Scholar]
- 22.Waqas M, Rai AT, Vakharia K, et al. Effect of definition and methods on estimates of prevalence of large vessel occlusion in acute ischemic stroke: a systematic review and meta-analysis. J Neurointerv Surg 2020; 12: 260–265. [DOI] [PubMed] [Google Scholar]
- 23.Sato S, Toyoda K, Uehara T, et al. Baseline NIH stroke scale score predicting outcome in anterior and posterior circulation strokes. Neurology 2008; 70: 2371–2377. [DOI] [PubMed] [Google Scholar]