Abstract
In the gas extraction and utilization process of coal mines, gas (mainly containing methane) explosion accidents happen occasionally under high-temperature conditions, causing serious casualties and economic losses. To reveal the mechanism and risk evolution of methane explosion under high-temperature conditions and control such accidents, the explosive characteristics of methane at 25∼200 °C were experimentally investigated by establishing a test platform for gas explosion under high-temperature conditions. In the experiments, three conditions were considered: the concentration near the upper explosion limit (CNUEL) (15.47 vol %), stoichiometric concentration (SC), and concentration near the lower explosion limit (4.68 vol %). Furthermore, the explosion pressure of methane–air mixtures and sensitivity characteristics of key free radicals at different high temperatures were determined based on the GRI-Mech 3.0 reaction mechanism of methane and using software CHEMKIN-PRO. The results show that at SC, Pmax decreases, while (DP/DT)max remains unchanged as the temperature increases, indicating a gradual decrease in the explosion risk. Near the explosion limits, Pmax and (DP/DT)max both grow as an exponential function, which implies that the explosion risk gradually increases. The temperature rise exerts a greater effect in improving the risk of explosion overpressure of methane at CNUEL (15.47 vol %), and compared with Pmax, the temperature rise has a greater improvement effect on (DP/DT)max. In the early stage of consuming methane, methane at SC mainly has two chemical reaction paths: CH4 → CH3 → CH3O → CH2O → HCO → CO and CH4 → CH3 → HCO → CO. The former and the latter to some extent separately promote and inhibit the explosive reactions. As the temperature increases, the proportion of methane consumed by the former reduces, while that by the latter slightly increases. The temperature rise inhibits the increase in the explosion risk of methane at SC, which is consistent with the experimental results.
Introduction
A large amount of gas, as hydrocarbon gas (mainly comprising methane, namely, CH4), associated with coal seams can be released with the mining of coal. Once the gas is combusted or exploded, it will cause serious casualties and property losses. Ambient conditions have important influences on the explosive characteristics of combustible gas. Therein, the ambient pressure and ambient temperature exert the greatest influences.1−9 In China, high-temperature condition is inevitable in coal mines with the increase in mining depth and spontaneous ignition of coal seams.10,11 In the utilization of low-concentration oxygen-containing gas, processes including methane extraction by pressure swing adsorption,12 gas deoxygenation and concentration,13 and low-concentration gas regenerative oxidation14 also encounter high-temperature conditions, so the temperature rise poses a severe challenge for controlling gas explosion. Therefore, studying explosive characteristics of methane under high-temperature conditions can provide an important theoretical basis for the prevention of gas explosion accidents, design of bearing pressure devices, and explosion venting design.
To study the influences of temperature on the explosion limits of combustible gas, Kondo et al.15 used a 12 L spherical glass bottle as the reactor to test the explosion limits of combustible gas including CH4 at 5, 21, 35, 50, 75, and 100 °C. Both the limits shift almost linearly to temperatures within the range examined. Li and Cui16,17 experimentally analyzed the influences of different initial temperatures on the explosion limits of CH4–air mixtures and found that increasing the initial temperature significantly enlarges the range of explosion limits. Lin and Addai18,19 investigated the explosive characteristics of coal dust–air mixtures and revealed that when the coal dust is drawn into the combustible gas produced after the explosion of CH4, the lower explosion limit (LEL) decreases with the increasing mass concentration of the coal dust. Many scholars also have investigated the characteristics of explosion overpressure of CH4 under different initial conditions. Zhu et al.20 studied the propagation characteristics of explosive blasts induced by changes in the CH4 concentration and found that the decrease in the gas concentration reduces the total heat release and the maximum explosion overpressure (Pmax). By using a standard 20 L spherical explosion vessel, Li et al.21 explored the explosive characteristics of H2–CH4–air and CH4–coal dust–air mixtures and revealed that the generation of hydrogen in the spontaneous combustion of coal significantly improves Pmax and the maximum pressure rise rate (DP/DT)max of the mixtures. Luo et al.22 adopted a 20 L spherical explosion vessel to evaluate the influences of adding CO on parameters related to the CH4–air mixtures including Pmax, (DP/DT)max, and deflagration index (KG). Results show that CO accelerates flame propagation. Meanwhile, much experimental research has shown that the explosive characteristics of CH4 are significantly influenced by the ambient temperature.23,24 Pekalski and Gieras25,26 conducted experimental research into the explosive characteristics of CH4 at different initial temperatures and estimated changes in the explosion pressure of CH4 at different initial temperatures.
To study the kinetic mechanism underpinning a methane explosion, Nie et al.27 used a closed homogeneous zero-dimensional reactor in CHEMKIN III to study the kinetic behaviors of gas explosion and revealed the relationship between the final concentration of free radicals and the initial CH4 concentration. Based on the GRI-Mech3.0 mechanism, Luo et al.28 explored the mechanism of influence of C3H8 as an additive to the explosion of CH4. According to the results, the highest temperature and highest pressure in the explosion process as well as the production rates of CO and NOx increase after adding C3H8, and more H, O, and OH free radicals are consumed on the whole. By using the GRI-Mech3.0 mechanism, Wang et al.29 analyzed the influencing mechanism of adding C2H4 to the explosion of CH4, and the results show that the addition of C2H4 improves the explosion risk of lean–fuel mixtures while reducing that of fuel-rich mixtures. Based on the GRI-Mech3.0 mechanism, Ma and Lin30 established a simplified reaction mechanism of methane oxidation that contains 16 compositions and 31 steps. By verifying the mechanism using simulation data at temperatures of 1400∼2000 K and methane concentrations of 0.5∼5%, the mechanism was found to be mainly applicable to extremely lean combustion conditions with an equivalence ratio lower than 0.2. When the temperature is low and the methane concentration is high, the ignition delay time is shorter than the calculated value based on the detailed reaction mechanism. To clarify the chemical kinetic behaviors in the initial mixing stage of CH4, H2, and air, Su et al.31 used CAMB3LYP/6-31 G density functional theory and the GRdetailed3.0 mechanism to obtain the kinetic and thermodynamic parameters.
In summary, the ambient temperature is an important factor that affects the explosion limits and explosion risk of methane. At present, research into the explosive characteristics of methane mainly focuses on tests on explosion pressure within the explosion limits at high temperatures. However, there is little research on changes in the explosion pressure of methane–air mixtures beyond the explosion limits [concentrations near the upper explosion limit (CNUEL) and concentrations near the LEL (CNLEL)]. Therefore, it is necessary to enrich the sensitivity characteristics and reaction paths of methane at high temperatures. By using a 20 L test system for explosive characteristics in the special environment, the research explores the evolution of the risk of explosion overpressure of methane under conditions with temperatures of 25∼200 °C. Particularly, the research expounded the evolution characteristics of the explosion risk of methane at CNUEL and CNLEL and further unveiled the explosion mechanism under high-temperature conditions by using the chemical kinetic method. The research results provide important scientific guidance for the prevention of high-temperature gas explosion accidents in coal mines and the safe utilization of low-concentration oxygen-bearing coalbed methane.
2. Experiments and Kinetic Model
2.1. Experimental System
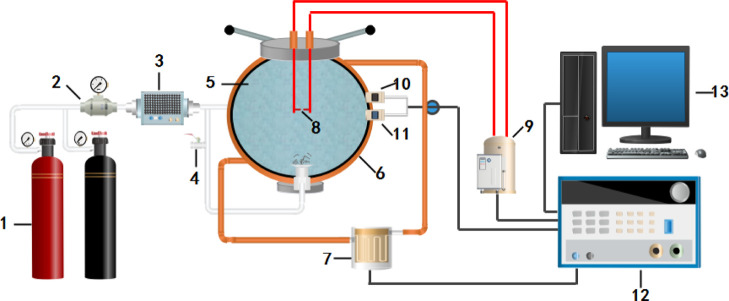
Experiments were conducted using a 20 L test system for explosive characteristics in the special environment. The system mainly consisted of a 20 L spherical explosion tank, a heating system, a gas distribution system, an ignition system, a control system, and a data acquisition system (Figure 1). Resembling the experimental equipment described by the German Society for Testing and Materials, the system could be used to conduct experiments on the explosive characteristics of combustible gas under special conditions. A pressure sensor and a temperature sensor were connected to the explosion tank and the control system. The control system controlled the heating system, gas distribution system, and ignition system through the sequential circuit and was connected to a high-frequency data acquisition system through a wireless transmitter. The heating system was composed of an oil bath heater, an insulating layer, and a temperature sensor. In the experiments, the target temperature was preset; thereafter, the heater was switched on and the temperature sensor was used to monitor the temperature inside the explosion tank in real time. Once the temperature inside the tank reached its preset target, the heater was automatically switched off for as long as was necessary to maintain the temperature inside the tank at the target temperature. The gas distribution system comprised a gas distribution controller, a feeding solenoid valve, a high-pressure cylinder, and a gas distribution pipeline. In the experiments, Dalton’s law of partial pressures was adopted for gas distribution, and the pressure sensor was used for real-time monitoring of the gas pressure in the tank. The ignition system consisted of an ignition controller and an ignition electrode.
Figure 1.
Working principle of the 20 L test system for measuring explosive characteristics. (1) Gas cylinder; (2) solenoid valve; (3) gas distribution system; (4) valve; (5) 20 L spherical explosion tank; (6) insulating layer; (7) heater; (8) ignition electrode; (9) ignition controller; (10) temperature sensor; (11) pressure sensor; (12) control system; (13) data analysis system.
2.2. Explosion Criterion and Experimental Conditions
In order to investigate the important effect of ambient temperature on the explosion limit and explosion risk of methane, experiments were conducted to determine the explosion limit and explosion overpressure characteristics of methane–air mixture under high-temperature conditions. In the experiments, the ignition energy, the initial pressure, and the initial temperature are separately set to be 10 J, 0.101 MPa, and 25 ∼ 200 °C.
Explosion limits of combustible gas at room temperature were mainly measured using the experimental methods recommended by the standard of the People’s Republic of China (GB/T12474-2008), standards of the American Society for Testing and Materials (ASTM E918-83 and ASTM E681-09), and those of the German DIN Institute for Standardization.32−36 Combining stipulations in various standards, a 7% increase in pressure after ignition was taken as the explosion criterion. The experimental process can be summarized as vacuolization, air inflation, ignition, judgment, and repetition after changing the concentration. That is, once the methane–air mixture at a certain concentration is exploded, the methane concentration is equivalently increased [the upper explosion limit (UEL)] or decreased (LEL) until the explosion does not recur. For the methane–air mixture at a certain concentration, if it is not exploded in three experiments under the same conditions, it is considered that the methane is not exploded at that concentration; if the mixture is exploded in one experiment, the mixture at that concentration is deemed explosive. The explosion limit is set to be the arithmetic mean of two concentrations. If the judging condition meeting the explosion criterion occurs after igniting methane at a certain concentration, the average between the concentration and the closest concentration in the experiment without explosion is taken as the explosion limit. After deriving the explosion limit, the experimental concentrations [stoichiometric concentration, (SC), CNLEL, and CNUEL] for conducting the explosion overpressure characteristics of methane air mixture are determined. After that, the explosion overpressure characteristics of the three concentrations of methane/air mixture under high-temperature conditions are measured, and the laws of explosion overpressure and explosion overpressure rise rate with ambient temperature are derived.
The specific experimental steps are described as follows: (1) The gas tightness of the pipeline and valve was checked before experiments, and a vacuum pump was adopted to depressurize the explosion tank. (2) A methane–air mixture at a certain concentration was prepared using the gas distribution system according to the pressure matching method, and the mixture was pumped into the explosion tank. A laser methane sensor was adopted to measure the methane concentration in the tank, which was appropriately adjusted. (3) The heating system was turned on to heat the tank to the experimental temperature. (4) The initial ambient pressure of gas in the tank was adjusted so that it was maintained at the normal pressure. (5) The ignition system was turned on to start the ignition and explosion experiments, and the data acquisition system was used to collect and store pressure data during the explosion. (6) The vacuum pump was adopted for ventilation of the explosion and to discharge the tail gas in the tank in readiness for the next experiment.
2.3. Kinetic Model
To reveal the mechanism of influence of the temperature on the explosive characteristics of methane, the CHEMKIN-Pro closed homogeneous batch reactor was used for kinetic analysis of the explosion process of methane at high temperatures. Current chemical reaction kinetic models for methane combustion include Ranzi, USC 2.0, GRI-Mech 3.0, Aramco 1.3, FFCM-1, and so forth. Among them, the GRI-Mech 3.0 mechanism has been widely validated37 and is the most recognized methane combustion mechanism model, which performs well in simulating the combustion characteristics of methane at high temperatures.38−45 The initial pressure in the simulation was set to 101 kPa, and the initial temperatures were set to 1000, 1050, 1100, and 1150 K. The volumetric concentration of methane was 10 vol %. It is worth noting that in the closed homogeneous batch reactor, 1000, 1050, 1100, and 1150 K are the compression temperatures at the moment of homogeneous explosion of the gas rather than ambient temperatures. The combustible gas is combusted at the normal pressure and these compression temperatures. If the compression temperature is too low, chemical reactions of combustible gas fail to occur in the closed homogeneous batch reactor. However, studying the influences of temperatures on the kinetic mechanism of the explosion of the combustible gas using compression temperatures is a common method adopted in explosion research.
In addition, the reaction path of the fuel differs in different transient states. The academic community commonly uses fuel consumption as an important index for studying the reaction paths. Considering the important influences of the chain reaction in the early stage of fuel combustion on the ignition, explosion, and chemical explosion suppression, the path analysis of fuel refers to that moment when the fuel consumption reaches 20%.
The control equations of explosive reactions of gas in the reactor include46
The composition equation is written as
| 1 |
The energy equations are
| 2 |
| 3 |
| 4 |
where t represents time (s); Yk is the mass fraction of component k (%); Wk is
the relative molecular mass of component k (kg/mol); 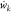 is the chemical reaction rate of component k [mol/(L·s)]; v represents the specific
volume of the mixed gas (m3/kg); Vki = Vki″ – Vki, in which Vki′ and Vki are the positive and reverse
chemical equivalent coefficients of the component k in the reaction of the ith step; R is the gas constant
of the mixed gas [J/(mol·K)]; cv denotes the specific heat capacity at a constant
volume of the mixed gas [J/(kg·K)]; T is the
temperature of the mixed gas (K); ek is the internal energy of the component and represents the
total number of components and reaction steps; kfi is the forward rate constant in reaction
step i; the pre-exponential factor and temperature
index in reaction step is the activation energy of real action in
reaction step i (kJ/mol); [Xk] denotes the concentration of component k (mol/L).
is the chemical reaction rate of component k [mol/(L·s)]; v represents the specific
volume of the mixed gas (m3/kg); Vki = Vki″ – Vki, in which Vki′ and Vki are the positive and reverse
chemical equivalent coefficients of the component k in the reaction of the ith step; R is the gas constant
of the mixed gas [J/(mol·K)]; cv denotes the specific heat capacity at a constant
volume of the mixed gas [J/(kg·K)]; T is the
temperature of the mixed gas (K); ek is the internal energy of the component and represents the
total number of components and reaction steps; kfi is the forward rate constant in reaction
step i; the pre-exponential factor and temperature
index in reaction step is the activation energy of real action in
reaction step i (kJ/mol); [Xk] denotes the concentration of component k (mol/L).
3. Results and Discussion
3.1. Explosion Limits
Only when combustible materials (combustible gas, vapor, and dust) and air (or oxygen) are uniformly mixed in a certain concentration range to form the premixed gas can an explosion occur after exposure to an ignition source. The concentration range is the explosion limits, which are basic parameters used to characterize the explosion risk of materials. By conducting these experiments, changes in the explosion limits of methane at different ambient temperatures were obtained (Figure 2).
Figure 2.
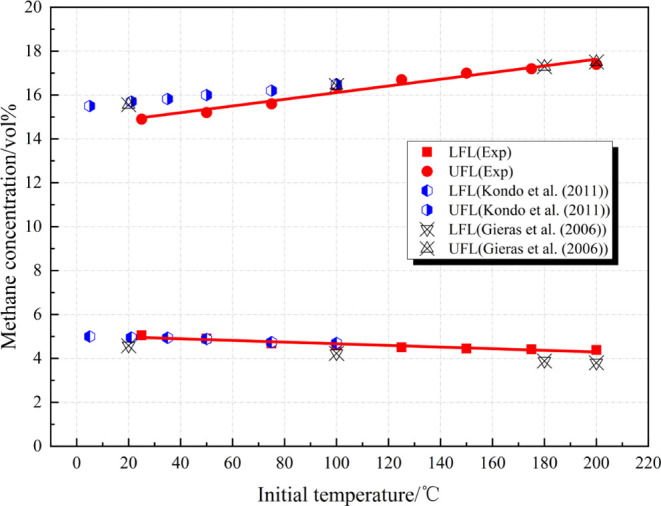
Explosion limits of the methane–air mixture at the SC at different initial temperatures.
The following two equations are attained through function fitting of the explosion limits obtained in the experiments
| 5 |
| 6 |
According to Figure 2 and the expected values in eqs 5 and 6, the UEL of methane increases linearly while the LEL decreases linearly with the increase in the ambient temperature, that is, the range of explosion limits widens. As a result, the methane–air mixture that is inexplosive under conditions of room temperature and normal pressure becomes explosive. When the ambient temperature is increased from 25 to 200 °C, the range of explosion limits of methane widens from 5.05∼14.9 to 4.38∼17.4 vol %. The UEL increases by 1.9% and the LEL decreases by 0.84%, so the range of explosion limits is increased by 2.74%. This is consistent with the research results of Kondo15 and Gieras,23 and the initial temperature in the experiments conducted by Kondo only reaches 100 °C; although Gieras conducted experiments at 200 °C, only four conditions are considered, so the data are too few to be genuinely representative. The UEL changes more with the temperature rise mainly because the temperature rise enhances the activities between molecules and increases the kinetic energy of gaseous molecules. As a result, the thermal mother ion of molecules becomes more active, collisions occur more frequently, and activating groups that can take part in reactions in a constant volume increase. Meanwhile, the LEL varies slightly with the temperature rise mainly because a large amount of combustion-supporting gas (oxygen) is present near the LEL of the methane–air mixture. However, due to the low concentration of combustible gas, the molecular motion between reactants is so slow that collision happens at a low frequency. Meanwhile, the excess air also has a cooling effect, which hinders the further progress of combustion or explosion.
3.2. Explosion Overpressure
Explosion overpressure refers to the pressure that is higher than the standard atmospheric pressure and formed by blast waves of explosion.47,48 Based on the above experimental results, selection of the CNUEL (15.47 vol %), SC, and CNLEL (4.68 vol %) of three cases is carried out for experimental studies of the explosion characteristics.
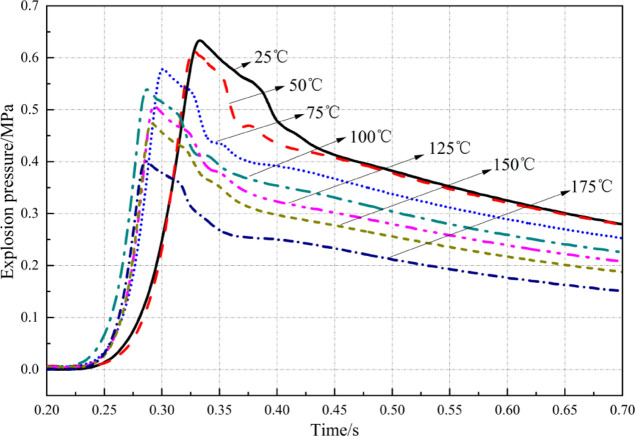
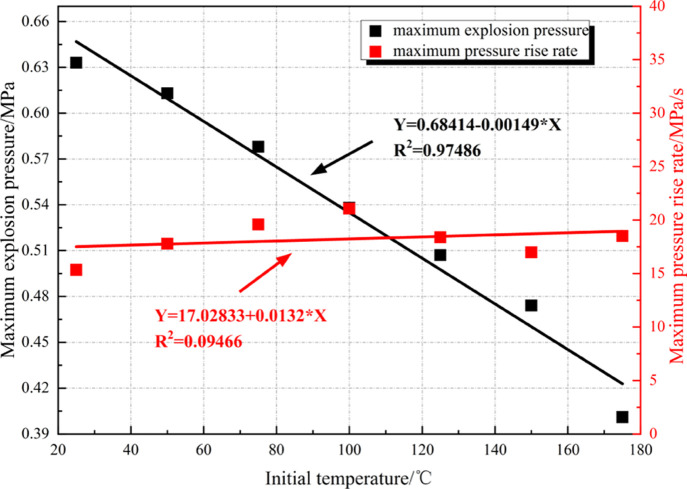
Figure 3 shows the historical evolution process of overpressure of the methane–air mixture at the SC at different initial temperatures. The mixture is ignited 0.2 s after beginning data acquisition. Figure 4 illustrates the change curves of the maximum explosion pressure (Pmax) and the maximum rate of change of pressure DP[(DP/DT)max] of the methane–air mixture at the SC with the initial temperature.
Figure 3.
Evolution of explosion overpressure at SC.
Figure 4.
Changes in Pmax and (DP/DT)max at SC with initial temperatures.
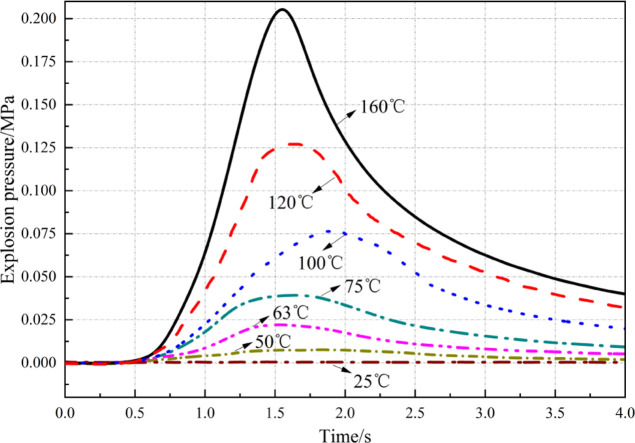
Figure 3 demonstrates that at different initial temperatures, the historical curves of explosion overpressure of the methane–air mixture at SC show a similar change trend: they all rapidly ascend at first, then slowly descend, and finally flatten. At an initial temperature of 25 °C, it takes 130 ms to reach the peak overpressure (explosion time); at 75 °C, the explosion time is 101 ms; as the temperature further rises to 175 °C, the explosion time decreases to 86 ms. Throughout the process, the explosion time decreases and the energy release rate also grows constantly. The energy release rate is increased by 33.85% at most. Figure 4 shows that with the increase in the ambient temperature, Pmax decreases linearly, while (DP/DT)max fluctuates around 17.5 MPa/s. As the ambient temperature rises from 25 to 175 °C, the peak explosion overpressure decreases from 0.633 to 0.401 MPa, indicating a decrease of 36.65% (0.232 MPa). According to the state equation of ideal gas, the mass of reactants per unit of volume reduces substantially, the total heat release of reactants decreases, and therefore the final explosion pressure drops as the ambient temperature rises under conditions of the initial ambient pressure and volume being unchanged. In general, the temperature rise mainly weakens the explosion risk for the methane–air mixture at SC.
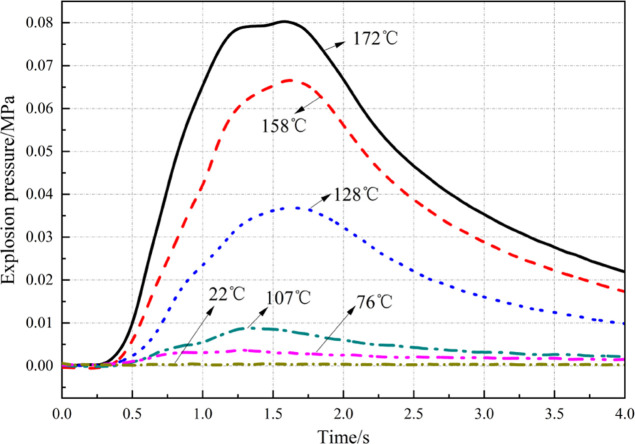
Figure 5 displays the historical evolution of overpressure of the methane–air mixture at CNLEL (4.68 vol %) at different initial temperatures. Figure 6 shows the changes in the maximum explosion pressure (Pmax) and the maximum pressure rise rate [(DP/DT)max] of the methane–air mixture at CNLEL (4.68 vol %) with the initial temperature.
Figure 5.
Evolution of explosion overpressure at CNLEL (4.68 vol %).
Figure 6.
Changes in Pmax and (DP/DT)max at CNLEL (4.68 vol %) with initial temperatures.
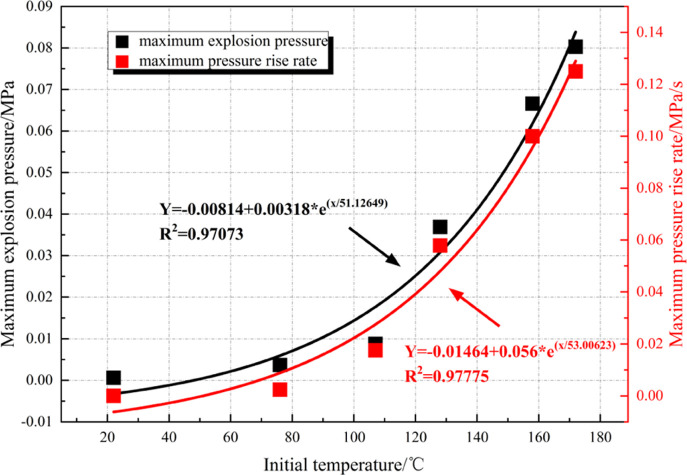
As shown in Figure 5, the overpressure curve of the methane–air mixture at CNLEL (4.68 vol %) does not fluctuate at room temperature (22 °C), which means the mixture under the condition is not explosive. With the constant rising of the ambient temperature, the historical evolution of overpressure in the mixture begins to show slight fluctuations. Once the temperature reaches 128 °C, the mixture has shown high overpressure risk. If the temperature further rises to 158 °C and above, the overpressure of the mixture exceeds 60 kPa, which is enough to cause death and severe damage to buildings. In summary, the temperature rise not only increases the probability of explosion of the methane–air mixture at CNLEL (4.68 vol %) but also greatly increases the risk of explosion overpressure, warranting close attention.
Figure 6 indicates that as the ambient temperature rises, the Pmax ADP (DP/DT)max of the methane–air mixture grows as an exponential function at CNLEL (4.68 vol %). Pmax is 0.004 MPa at 76 °C; when the temperature rises to 172 °C, Pmax reaches 0.08 MPa, as the overpressure increases by 1900%. FDP (DP/DT)max value is 0.002 MPa/s at 76 °C; (DP/DT)max reaches 0.125 MPa/s when the temperature rises to 172 °C, with the pressure rise rate growing by 6150%. The temperature rise exerts more significant influences on the maximum pressure rise rate than on the explosion overpressure.
Figure 7 depicts the historical evolution process of overpressure of the methane–air mixture at CNUEL (15.47 vol %) at different initial temperatures. The changes in the maximum explosion pressure (Pmax) and the maximum pressure rise rate [(DP/DT)max] of the methane–air mixture at CNUEL (15.47 vol %) with the initial temperatures are illustrated in Figure 8.
Figure 7.
Evolution of explosion overpressure at CNUEL (15.47 vol %).
Figure 8.
Changes in Pmax and (DP/DT)max at CNUEL (15.47 vol %) with initial temperatures.
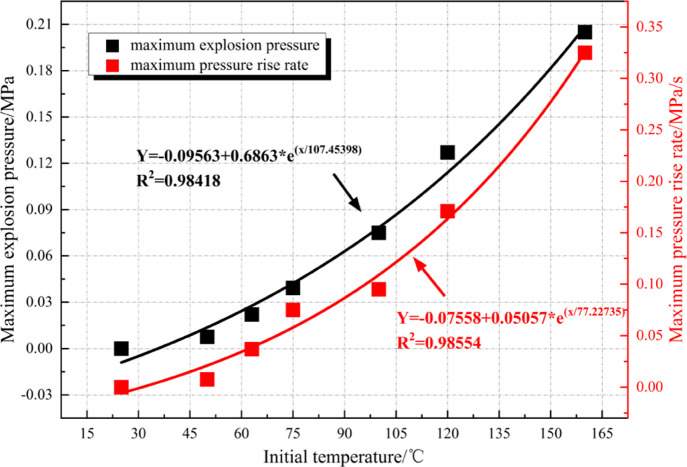
Figure 7 shows that the overpressure curve of the methane–air mixture at the CNUEL (15.47 vol %) does not have any fluctuation at room temperature (25 °C), implying the mixture is inexplosive under the condition. As the ambient temperature constantly rises, the historical curves of the pressure of the mixture begin to show slight fluctuations. When the temperature rises to 75 °C, the mixture already has a high overpressure risk. When further increasing the temperature to 100 °C and above, the overpressure exceeds 75 kPa, which is enough to cause death and complete collapse of buildings. In summary, the temperature rise not only elevates the probability of explosion of the methane–air mixture at CNUEL (15.47 vol %) but also significantly increases the overpressure risk of explosion. Compared with the mixture at CNLEL (4.68 vol %), the temperature rise has a greater effect on improving the risk of explosion overpressure of the methane–air mixture at CNUEL (15.47 vol %).
As displayed in Figure 8, Pmax and (DP/DT)max of the methane–air mixture at CNUEL (15.47 vol %) grow as an exponential function with the increase of the ambient temperature. For Pmax, it is 0.008 MPa at 50 °C; when the temperature rises to 160 °C, Pmax reaches 0.205 MPa and the overpressure rises by 2462%. (DP/DT)max is 0.008 MPa/s at 50 °C; (DP/DT)max reaches 0.325 MPa/s as the temperature rises to 160 °C, with the pressure rise rate growing by 3962%. The temperature rise exerts a greater influence on the maximum pressure rise rate than the explosion overpressure, which is also applicable to the situation at CNLEL.
In summary, the increase in temperature has different effects on the likelihood of explosion and severity of methane/air mixtures for SCs, CNLEL (4.68 vol %), and CNUEL (15.47 vol %). For methane/air mixtures with SCs, the increase in temperature weakens the explosion risk, while for methane/air mixtures with CNLEL (4.68 vol %) and CNUEL (15.47 vol %), the increase in temperature increases the likelihood of explosion of the mixture as well as the hazard.
3.3. Mechanism Analysis of Chemical Kinetics
To reveal the mechanism of influence of the ambient temperature on the methane explosion, the reaction kinetic process of methane explosion at different ambient temperatures was simulated based on the GRI-Mech 3.0 mechanism.
3.3.1. Sensitivity Analysis of Explosion Pressure
Main elementary reactions that affect the explosion pressure were obtained according to the value of the sensitivity coefficient, as shown in Figure 9. A positive sensitivity coefficient indicates that an elementary reaction exerts a promotion effect on the explosion pressure, while the negative one inhibits the increase in the explosion pressure.
Figure 9.
Sensitivity analysis of explosion pressure at different ambient temperatures.
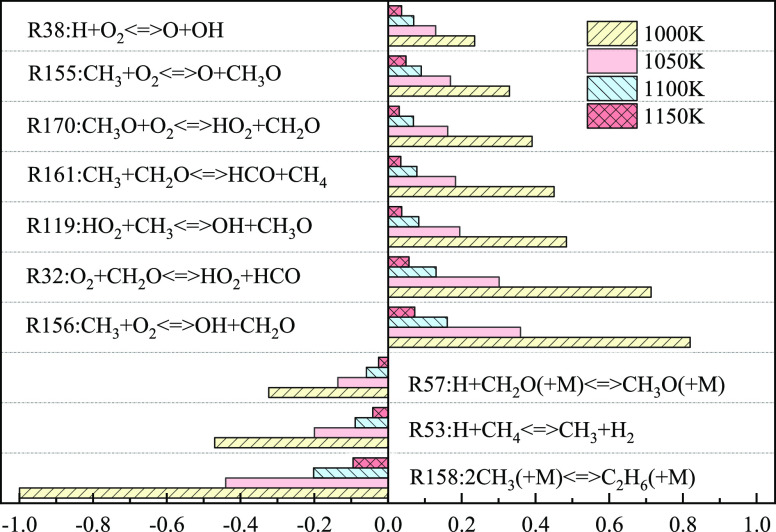
As shown in Figure 9, the elementary reactions that promote the increase in explosion pressure at the four temperatures are R156, R32, R119, R161, R170, R155, and R38, and those reactions inhibiting the increase in explosion pressure include R158, R53, and R57, all listed in rank order according to their influences. For reactions R156 and R32, they both consume the reactant oxygen and produce active free radicals that play a main effect in promoting the explosion, such as OH and HO2. For R158, it consumes many methyl radicals and generates ethane molecules that are more nonpyrolytic and nonoxidizing, thus inhibiting the forward progress of chemical reactions. At an initial temperature of 1000 K, the sensitivity coefficients (or absolute values) of various elementary reactions are the greatest. As the initial temperature increases, the sensitivity coefficients (or absolute values) of various reactions gradually decrease, while the relative influences of various reactions do not change to any significant extent; the temperature rise weakens the forward and backward progress of reactions at the same time.
3.3.2. Sensitivity Analysis of Key Free Radicals
In the methane explosion process, the presence of free radicals ensures the smooth completion of the whole explosive reaction. Therein, H, O, and OH play an essential role in the chain reaction that promotes the explosion.49 According to the value of sensitivity coefficients, the main elementary reactions that influence key free radicals such as H, O, and OH are attained (Figure 10). Therein, a positive sensitivity coefficient means that the elementary reactions promote the increases in key free radicals, while a negative coefficient inhibits the increase in free radicals.
Figure 10.
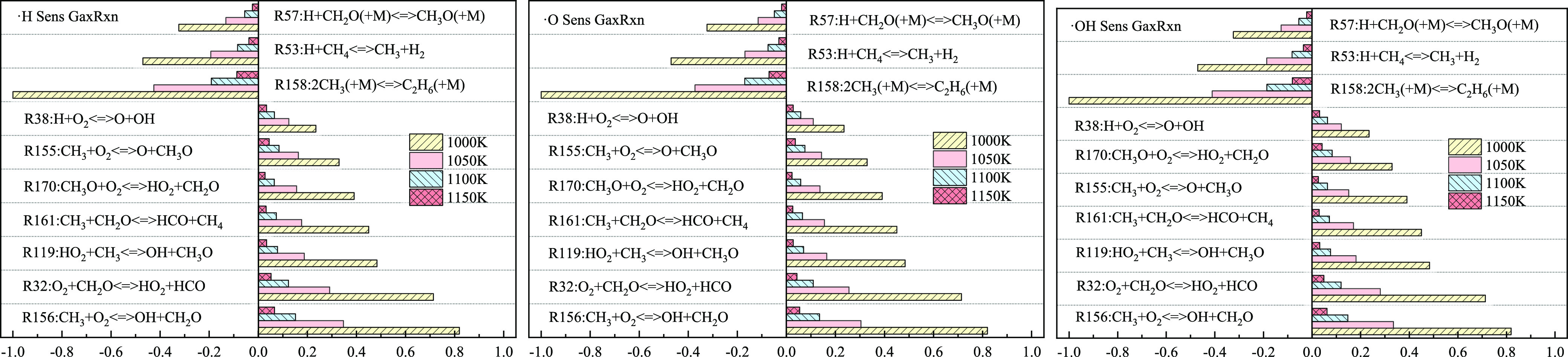
Sensitivity analysis of key free radicals at different ambient temperatures.
As shown in Figure 10, at the four temperatures, the elementary reactions that promote the generation of free radicals H, O, and OH are R156, R32, R119, R161, R155, R170, and R38, and those inhibiting generation of key free radicals are R158, R53, and R57, all listed according to their influences. This is consistent with the sensitivity analysis results of the explosion pressure, indicating that key free radicals H, O, and OH are positively correlated with the explosion pressure, and main elementary reactions exert consistent influences on the explosion pressure and key free radicals. At the initial temperature of 1000 K, the sensitivity coefficients (or absolute values) of various elementary reactions are the greatest; with increasing initial temperature, the sensitivity coefficients (or absolute values) of various reactions gradually decrease, while their relative influences do not change substantially. The effects that promote and inhibit generate the notion of key free radicals H, O, and OH, and both diminish with the increasing temperature.
3.3.3. Reaction Paths
To evaluate the influences of changes in high temperatures on the chemical reaction paths of methane explosion, the fuel flow in the chemical reactions of methane at SC was obtained at temperatures T of 1000, 1050, 1100, and 1150 K (Figure 11).
Figure 11.
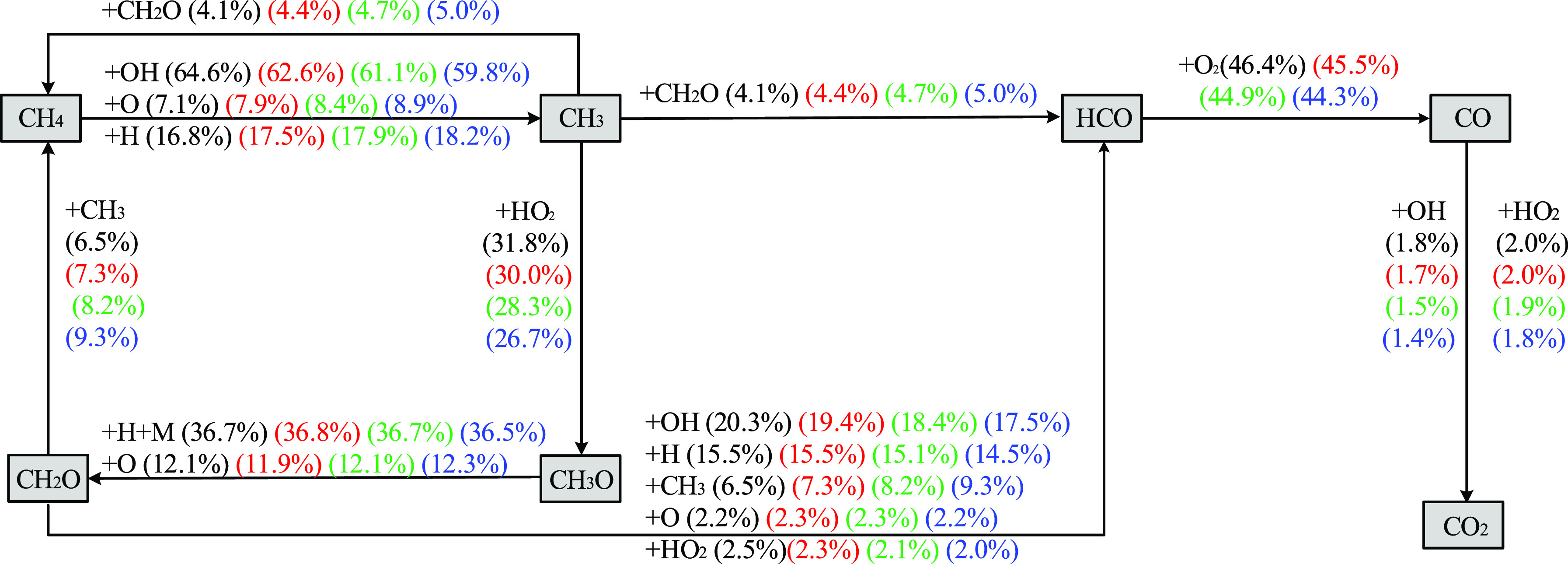
Reaction path of methane explosion at different initial temperatures (black, red, green, and blue separately represent temperatures T of 1000, 1050, 1100, and 1150 K).
Figure 11 shows that in the early stage of consuming methane, the main chemical reaction paths of methane can be determined as CH4 → CH3 → CH3O → CH2O → HCO → CO and CH4 → CH3 → HCO → CO according to the consumption proportion of each free radical. At first, most of the methane (>88%) generates methyl radicals (CH3) through the hydrogen abstraction reaction. In the process, OH and H play the dominant role, particularly OH, which has the most significant contribution (about 60%), and free radical O also contributes to some extent to the generation of CH3. Afterward, CH3 is consumed via two paths. In reaction path 1, about 26.7∼31.8% of CH3 undergoes an oxidation reaction with HO2 to generate OH and CH3O. The elementary reaction can promote the increased explosion risk. After the successive hydrogen abstraction reaction, CH3O further generates CH2O and HCO. In the reaction CH3 (about 4.1∼5.0%) undergoes a redox reaction with CH2O to generate HCO and CH4. It is these reactants that inhibit the increase in explosion risk. As the temperature gradually grows, the proportion of reaction path 1 gradually reduces, while that of reaction path 2 grows slightly. This means the temperature rise at high temperatures inhibits the increase in explosion risk, which proves the experimental phenomenon of reduced explosion risk of methane at SC.
4. Conclusions
-
(1)
As the ambient temperature increases from 25 to 200 °C, the range of explosion limits of methane widens from 5.05∼14.9 vol % to 4.38∼17.4 vol %. The UEL of gas is increased by 1.9% and the LEL is decreased by 0.84%, so the range of explosion limits is increased by 2.74%. The rising ambient temperature increases the probability of methane explosion.
-
(2)
When the temperature of the methane–air mixture at SC increases, Pmax decreases while (DP/DT)max remains unchanged, indicative of gradual reduction of explosion risk, whereas both Pmax and (DP/DT)max increase as an exponential function for the methane–air mixture near the explosion limits, which implies that the explosion risk gradually rises. The temperature rise causes distinct evolution patterns of explosion risk for methane at different concentrations.
-
(3)
Compared with the methane–air mixture at CNLEL (4.68 vol %), the temperature rise exerts a more obvious promotional effect on the risk of explosion overpressure of the methane–air mixture at CNUEL (15.47 vol %). Meanwhile, the temperature rise exerts a more significant effect in increasing (DP/DT)max than Pmax for the methane–air mixture near its explosion limits.
-
(4)
The temperature rise weakens the forward and backward progress of combustion reactions of methane simultaneously. Meanwhile, the effects that promote and inhibit the generation of key free radicals H, O, and OH are both weakened with the increase of temperature. In the early stage of consuming methane at high temperatures, methane mainly undergoes two chemical reaction paths, namely, CH4 → CH3 → CH3O → CH2O → HCO → CO and CH4 → CH3 → HCO → CO. The former promotes the explosive reactions due to the generation of large amounts of active free radicals, while the latter to some extent inhibits the explosive reactions due to the generation of some products of methane. As the temperature increases, the consumption proportion of methane by the former reduces, while that by the latter increases slightly, which means that the temperature rise exerts an inhibitory effect on the explosion risk of methane at SC, which matches the experimental results.
Acknowledgments
The research presented in this paper was supported by the Natural Science Foundation of Chongqing, China (cstc2020jcyj-msxmX0618), the opening project of the State Key Laboratory of Explosion Science and Technology, Beijing Institute of Technology (KFJJ21-13M), and the Qingdao Science and Technology Plan project (no. 19-3-2-6 sec).
The authors declare no competing financial interest.
References
- Bai C.; Gong G.; Liu Q.; Chen Y.; Niu G. The explosion overpressure field and flame propagation of methane/air and methane/coal dust/air mixtures. J. Saf. Sci. 2011, 49, 1349–1354. 10.1016/j.ssci.2011.05.005. [DOI] [Google Scholar]
- Faghih M.; Gou X.; Chen Z. The explosion characteristics of methane, hydrogen and their mixtures: A computational study. J. Loss Prev. Process Ind. 2016, 40, 131–138. 10.1016/j.jlp.2015.12.015. [DOI] [Google Scholar]
- Ren C.; Zhang X.; Zhang Y. Overview of the characteristics of explosion limits of gases and gas mixtures. J. Fire Sci. Technol. 2017, 36, 1500–1503. [Google Scholar]
- Cammarota F.; Di Benedetto A.; Di Sarli V.; Salzano E. Influence of initial temperature and pressure on the explosion behavior of n-dodecane/air mixtures. J. Loss Prev. Process 2019, 62, 103920. 10.1016/j.jlp.2019.103920. [DOI] [Google Scholar]
- Huang L.; Wang Y.; Pei S.; Cui G.; Zhang L.; Ren S.; Zhang Z.; Wang N. Effect of elevated pressure on the explosion and flammability limits of methane-air mixtures. Energy 2019, 186, 115840. 10.1016/j.energy.2019.07.170. [DOI] [Google Scholar]
- Pio G.; Salzano E. The effect of ultra-low temperature on the flammability limits of methane/air/diluent mixtures. J. Hazard. Mater. 2019, 362, 224–229. 10.1016/j.jhazmat.2018.09.018. [DOI] [PubMed] [Google Scholar]
- Luo Z.; Li R.; Wang T.; Cheng F.; Liu Y.; Yu Z.; Fan S.; Zhu X. Explosion pressure and flame characteristics of CO/CH4/air mixtures at elevated initial temperatures. Fuel 2020, 268, 117377. 10.1016/j.fuel.2020.117377. [DOI] [Google Scholar]
- Mitu M.; Giurcan V.; Razus D.; Prodan M.; Oancea D. Propagation indices of methane-air explosions in closed vessels. J. Loss Prev. Process 2017, 47, 110–119. 10.1016/j.jlp.2017.03.001. [DOI] [Google Scholar]
- Huang L.; Pei S.; Wang Y.; Zhang L.; Ren S.; Zhang Z.; Xiao Y. Assessment of flammability and explosion risks of natural gas-air mixtures at high pressure and high temperature. Fuel 2019, 247, 47–56. 10.1016/j.fuel.2019.03.023. [DOI] [Google Scholar]
- Zhang Y.; Niu K.; Du W.; Zhang J.; Wang H.; Zhang J. A method to identify coal spontaneous combustion-prone regions based on goaf flow field under dynamic porosity. Fuel 2021, 288, 119690. 10.1016/j.fuel.2020.119690. [DOI] [Google Scholar]
- Zhang Y.; Wang H.; Du W.; Niu K.; Wei X. Temperature-Programmed Oxidation Experiments on Typical Bituminous Coal Under Inert Conditions. J. Energy Resour. 2020, 143, 032102. 10.1115/1.4048941. [DOI] [Google Scholar]
- Zhang J.; Qu S.; Wang P.; Li X.; Li L.; Che Y.; Li X. Research progress on the recovery of methane from coalbed methane by pressure swing adsorption. Clean Coal Technol. 2019, 25, 78–87. [Google Scholar]
- Qu S. J.; Dong W.; Li X.; Chen Y. Research and application of the low concentrated coalbed methane upgrading technique. J. China Coal Soc. 2014, 39, 1539–1544. [Google Scholar]
- Li X.; Chen H.; Li H.; Chen J. Change Law of Lower Limit of Gas Explosion at Ultra-High Temperatures. ACS Omega 2021, 6, 35112–35123. 10.1021/acsomega.1c05942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S.; Takizawa K.; Takahashi A.; Tokuhashi K. On the temperature dependence of flammability limits of gases. J. Hazard. Mater. 2011, 187, 585–590. 10.1016/j.jhazmat.2011.01.037. [DOI] [PubMed] [Google Scholar]
- Li Z.; Gong M.; Sun E.; Wu J.; Zhou Y. Effect of low temperature on the flammability limits of methane/nitrogen mixtures. Energy 2011, 36, 5521–5524. 10.1016/j.energy.2011.07.023. [DOI] [Google Scholar]
- Cui G.; Li Z.; Yang C. Experimental study of flammability limits of methane/air mixtures at low temperatures and elevated pressures. Fuel 2016, 181, 1074–1080. 10.1016/j.fuel.2016.04.116. [DOI] [Google Scholar]
- Lin S.; Liu Z.; Qian J.; Li X.; Zhang Q. Flammability and Explosion Risk of Post-explosion CH4/air and CH4/coal dust/air Mixtures. Combust. Sci. Technol. 2019, 193, 1279–1292. 10.1080/00102202.2019.1688313. [DOI] [Google Scholar]
- Addai E. K.; Gabel D.; Krause U. Lower explosion limit/minimum explosible concentration testing for hybrid mixtures in the Godbert-Greenwald furnace. Process Saf. Prog. 2017, 36, 81–94. 10.1002/prs.11825. [DOI] [Google Scholar]
- Zhu C.; Lu Z.; Lin B.; Jiang B. Effect of variation in gas distribution on explosion propagation characteristics in coal mines. Min. Sci. Technol. 2010, 20, 516–519. 10.1016/s1674-5264(09)60235-0. [DOI] [Google Scholar]
- Li R.; Luo Z.; Wang T.; Cheng F.; Lin H.; Zhu X. Effect of initial temperature and H2 addition on explosion characteristics of H2-poor/CH4 /air mixtures. Energy 2020, 213, 118979. 10.1016/j.energy.2020.118979. [DOI] [Google Scholar]
- Luo Z.; Liu L.; Cheng F.; Wang T.; Su B.; Zhang J.; Gao S.; Wang C. Effects of a carbon monoxide-dominant gas mixture on the explosion and flame propagation behaviors of methane in the air. J. Loss Prev. Process 2019, 58, 8–16. 10.1016/j.jlp.2019.01.004. [DOI] [Google Scholar]
- Li R.-z.; Huang Z.; Si R. Influence of environmental temperature on gas explosion pressure and its rise rate. Combust., Explos. Shock 2013, 33, 415–419. [Google Scholar]
- Li Q.; Lin B.; Dai H.; Zhao S. Explosion characteristics of H2/CH4/air and CH4/coal dust/air mixtures. Powder Technol. 2012, 229, 222–228. 10.1016/j.powtec.2012.06.036. [DOI] [Google Scholar]
- Pekalski A. A.; Schildberg H. P.; Smallegange P. S. D.; Lemkowitz S. M.; Zevenbergen J. F.; Braithwaite M.; Pasman H. J. Determination of the explosion behavior of methane and propene in air or oxygen at standard and elevated conditions. Process Saf. Environ. 2005, 83, 21–429. 10.1205/psep.04211. [DOI] [Google Scholar]
- Gieras M.; Klemens R.; Rarata G.; Wolański P. Determination of explosion parameters of methane-air mixtures in the chamber of 40 dm at normal and elevated temperature. J. Loss Prev. Process 2006, 19, 263–270. 10.1016/j.jlp.2005.05.004. [DOI] [Google Scholar]
- Nie B.; Yang L.; Ge B.; Wang J.; Li X. Chemical kinetic characteristics of methane/air mixture explosion and its affecting factors. J. Loss Prev. Process 2017, 49, 675–682. 10.1016/j.jlp.2017.02.021. [DOI] [Google Scholar]
- Luo Z.; Su B.; Wang T.; Cheng F.; Wang Y.; Liu B.; Xie C. Effects of propane on the flammability limits and chemical kinetics of methane-air explosions. Combust. Sci. Technol. 2019, 192, 1785–1801. 10.1080/00102202.2019.1625041. [DOI] [Google Scholar]
- Wang T.; Luo Z.; Wen H.; Zhang J.; Mao W.; Cheng F.; Zhao J.; Su B.; Li R.; Deng J. Experimental study on the explosion and flame emission behaviors of methane-ethylene-air mixtures. J. Loss Prev. Process 2019, 60, 183–194. 10.1016/j.jlp.2019.04.018. [DOI] [Google Scholar]
- Ma C.; Lin Q. The reduced mechanism for lean-fuel methane oxidation through sensitivity analysis. J. Natl. Univ. Def. Technol. 2017, 39, 164–170. [Google Scholar]
- Su B.; Luo Z.; Wang T.; Xie C.; Cheng F. Chemical kinetic behaviors at the chain initiation stage of CH4/H2/air mixture. J. Hazard. Mater. 2021, 403, 123680. 10.1016/j.jhazmat.2020.123680. [DOI] [PubMed] [Google Scholar]
- General Administration of Quality Supervision . Inspection and Quarantine of the People’s Republic of China. Standardization Administration of the P.R.C. GB/T 12474-2008 Method of Test for Explosion Limits of Combustible Gases in the airv; China Standards Press: Beijing, 2008. [Google Scholar]
- American Society for Testing and Materials . Standard Test Method for Concentration Limits of Flammability of Chemicals (Vapors and Gases): ASTM E681-09 (2015) ; American Society for Testing and Materials: West Conshohocken, 2009. [Google Scholar]
- American Society for Testing and Materials . Standard Practice for Determining Limits of Flammability of Chemicals at Elevated Temperature and Pressure: ASTM E918-83(2011) ; American Society for Testing and Materials: West Conshohocken, 2011. [Google Scholar]
- German Institute for Standardization . Determination of the Explosion Limits and the Limiting Oxygen Concentration (LOC) for Flammable Gases and Vapors: DIN EN 1839:2017-04; CEN-CENELEC Management Centre: German Institute for Standardization, 2017. [Google Scholar]
- American Society for Testing and Materials . Standard Test Methods for Limiting Oxygen (Oxidant) Concentration in Gases and Vapors: ASTM E2079-07; American Society for Testing and Materials, 2013. [Google Scholar]
- Dors M.; Nowakowska H.; Jasiński M.; Mizeraczyk J. Chemical kinetics of methane pyrolysis in microwave plasma at atmospheric pressure. Plasma Chem. Plasma Process. 2014, 34, 313–326. 10.1007/s11090-013-9510-4. [DOI] [Google Scholar]
- Song Y.; Zou C.; He Y.; Zheng C. The chemical mechanism of the effect of CO2 on the temperature in methane oxy-fuel combustion. Int. J. Heat Mass Tran. 2015, 86, 622–628. 10.1016/j.ijheatmasstransfer.2015.03.008. [DOI] [Google Scholar]
- Paykani A. Comparative Study on Chemical Kinetics Mechanisms for Methane-Based Fuel Mixtures under Engine-Relevant Conditions. Energies 2021, 14, 2834. 10.3390/en14102834. [DOI] [Google Scholar]
- Hu E.; Jiang X.; Huang Z.; Iida N. Numerical study on the effects of diluents on the laminar burning velocity of methane-air mixtures. Energy Fuel. 2012, 26, 4242–4252. 10.1021/ef300535s. [DOI] [Google Scholar]
- Hu E.; Li X.; Meng X.; Chen Y.; Cheng Y.; Xie Y.; Huang Z. Laminar flame speeds and ignition delay times of methane–air mixtures at elevated temperatures and pressures. Fuel 2015, 158, 1–10. 10.1016/j.fuel.2015.05.010. [DOI] [Google Scholar]
- Mansha M.; Saleemi A. R.; Ghauri B. M.; Ramzan N. Development and testing of a detailed kinetic mechanism of natural gas combustion in internal combustion engine. J. Nat. Gas Chem. 2010, 19, 97–106. 10.1016/s1003-9953(09)60039-6. [DOI] [Google Scholar]
- Hu X.; Yu Q.; Liu J. Chemical effect of CO2 on the laminar flame speeds of oxy-methane mixtures in the condition of various equivalence ratios and oxygen concentrations. Int. J. Hydrogen Energy 2016, 41, 15068–15077. 10.1016/j.ijhydene.2016.05.276. [DOI] [Google Scholar]
- Hu X.; Yu Q.; Liu J.; Sun N. Investigation of laminar flame speeds of CH4/O2/CO2 mixtures at ordinary pressure and kinetic simulation. Energy 2014, 70, 626–634. 10.1016/j.energy.2014.04.029. [DOI] [Google Scholar]
- Xie Y.; Wang J.; Zhang M.; Gong J.; Jin W.; Huang Z. Experimental and Numerical Study on Laminar Flame Characteristics of Methane Oxy-fuel Mixtures Highly Diluted with CO2. Energy Fuel. 2013, 27, 6231–6237. 10.1021/ef401220h. [DOI] [Google Scholar]
- Lutz A.; Kee R.; Miller J.. SKIN: A Fortran program for predicting homogeneous gas phase chemical kinetics with sensitivity analysis; Sandia National Labs.: Livermore, CA (USA), 1988, p 31. [Google Scholar]
- Zhang Q.; Qian X.; Li R.; Zhou G.; Sun Y.; Ma Y.; Kong Y. Explosion characteristics and chemical kinetics of blended LPG/DME clean fuel based on pyrolysis and oxidation mechanism model. Fuel 2022, 320, 123896. 10.1016/j.fuel.2022.123896. [DOI] [Google Scholar]
- Zhang Q.; Qian X.; Fu L.; Yuan M.; Chen Y. Shock wave evolution and overpressure hazards in partly premixed gas deflagration of DME/LPG blended multi-clean fuel. Fuel 2020, 268, 117368. 10.1016/j.fuel.2020.117368. [DOI] [Google Scholar]
- He Z.; Li X. B.; Liu L. M.; Zhu W. The intrinsic mechanism of methane oxidation under explosion condition: A combined Reax FF and DFT study. Fuel 2014, 124, 85–90. 10.1016/j.fuel.2014.01.070. [DOI] [Google Scholar]