Abstract
Purpose of Review
We reviewed the effects of hypertension and the means to prevent and treat it across the spectrum of a woman’s lifespan and identified gaps in sex-specific mechanisms contributing to hypertension in women that need to be addressed.
Recent Findings
Hypertension continues to be an important public health problem for women across all life stages from adolescence through pregnancy, menopause, and older age. There remain racial, ethnic, and socioeconomic differences in hypertension rates not only overall but also between the sexes. Blood pressure cutoffs during pregnancy have not been updated to reflect the 2017 ACC/AHA changes due to a lack of data. Additionally, the mechanisms behind hypertension development in menopause, including sex hormones and genetic factors, are not well understood.
Summary
In the setting of increasing inactivity and obesity, along with an aging population, hypertension rates are increasing in women. Screening and management of hypertension throughout a women’s lifespan are necessary to reduce the burden of cardiovascular disease, and further research to understand sex-specific hypertension mechanisms is needed.
Keywords: Hypertension, Women, Sex differences, Life cycle
Introduction
Hypertension is the most common modifiable risk factor for cardiovascular disease (CVD) and is highly prevalent worldwide, affecting 1.3 billion people [1, 2]. In the USA, 116 million adults (47.5%) have hypertension, defined as having a systolic blood pressure (SBP) > 130 mmHg or diastolic BP (DBP) > 80 mmHg or taking any antihypertensive medication [3]. Furthermore, hypertension prevalence, control rates, and subsequent outcomes are not universal and differ based on several factors, such as social inequities, environmental factors, historical factors, access to healthcare, and sex [4–6]. Hypertension is less prevalent in women than in men until 60 years of age, after which this pattern is reversed [7–9]. The pattern could be related to the longer life expectancy among women, postmenopausal hypertension, and lower response rate to treatment [10, 11]. Understanding sex differences in BP across the life course is important for the future diagnosis and treatment of hypertension. This is especially true, as effectively treating hypertension in women was found to have the greatest effect on CVD mortality and saved lives of any present preventive strategy, as evidenced by a cross-sectional study of the National Health and Nutrition Examination Survey (NHANES) data [12].
This review focuses on providing the reader with a look at the effects of hypertension and the means to prevent and treat it across the spectrum of a woman’s life course, starting with adolescence and going through pregnancy, menopause, and ending with a look at its role in the elderly (Fig. 1). Attention will also be given to the pathophysiology and biological underpinnings of hypertension in women. The goal is to identify the most up-to-date, evidence-based recommendations for treating hypertension across these periods. This review will also elucidate areas of ongoing research and questions that have yet to be answered in the fight against hypertension in women. For the purposes of this review, we will focus on the effects of hypertension in individuals born biologically female. There are limitations in epidemiological data where the differences between sex and gender are not identified. The word “women” will be used, as epidemiological studies generally include individuals who self-identify as women.
Fig. 1.
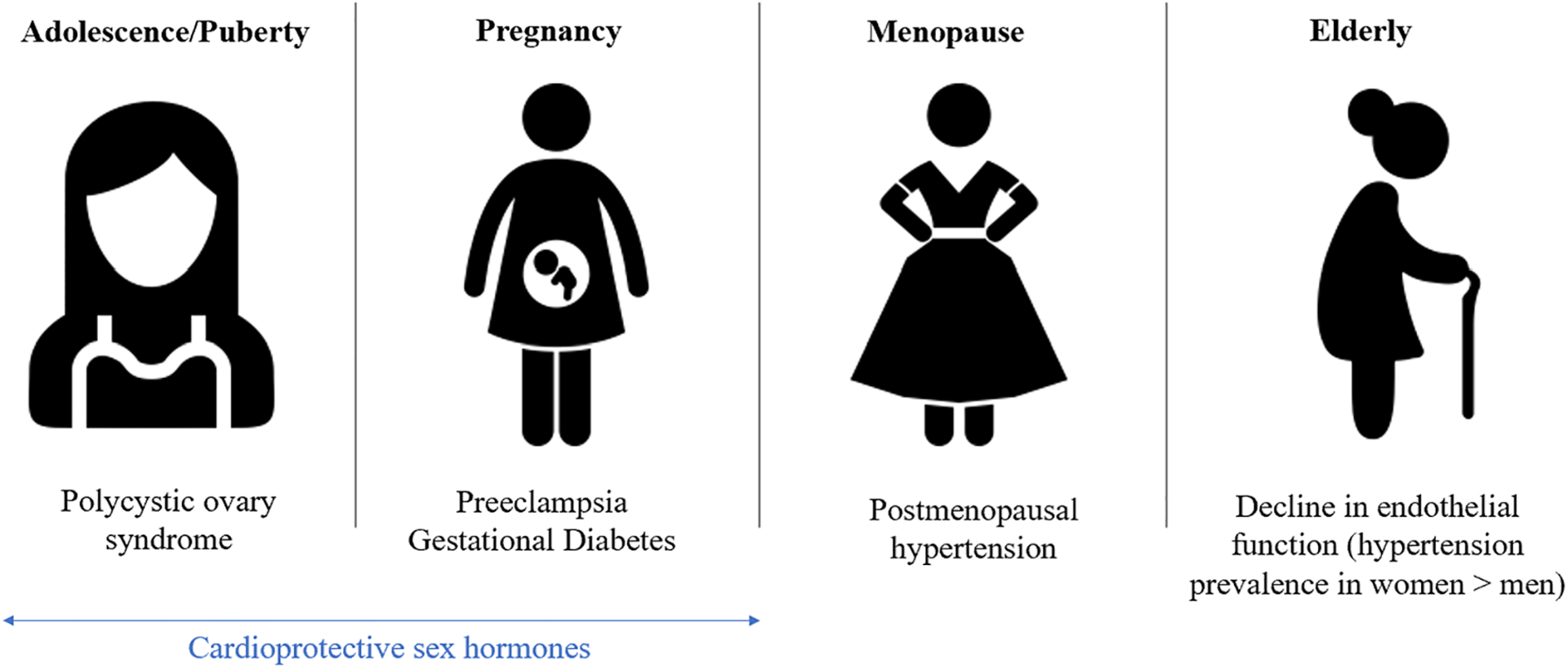
Main causes of blood pressure changes across a woman’s life cycle
Hypertension in Female Children
BP trends in childhood can lead to an increased risk of hypertension and CVD [13, 14]. The Centers for Disease Control and Prevention (CDC), using the updated 2017 American Academy of Pediatrics (AAP) clinical practice guideline, found that roughly 1.3 million or approximately 1 in 25 children between the ages of 10 and 19 have high BP [15, 16]. Obesity, which is known to be a significant risk factor for developing high BP, was a major driving force in the increasing prevalence of high BP in children and adolescents [17].
Risk factor modification is key to preventing the development of high BP in children and adolescents and thus preventing further CVD in adulthood. In addition to obesity, abdominal circumference has also been associated with an increased incidence of high BP in this demographic [18]. Disrupted sleep, especially obstructive sleep apnea and primary snoring disorders, has also been associated with increased hypertension rates [19]. Moreover, a family history of hypertension is a strong predictor of the development of hypertension in children [20–22]. Studies have shown that Hispanic and Black children have the highest overall prevalence of hypertension at 3.1% and 2.7%, respectively [23]. However, race and ethnicity are social, cultural, and political constructs and efforts to reduce disparities should also target the underlying structural differences between groups [24].
Hypertension in Teenage and Young Adult Women
Unlike in adults where 90 to 95% of hypertension cases are deemed primary or essential, hypertension in young women is often due to a secondary cause [25, 26]. Most secondary causes are related to intrinsic renal disease. Congenital malformations of the kidneys, such as autosomal recessive polycystic kidney disease, can cause hypertension in children [27]. Fibromuscular dysplasia (FMD) is a well-known cause of secondary hypertension in teenage girls. FMD is a non-inflammatory, non-atherosclerotic arteriopathy that most often affects the renal, vertebral, and, extracranial carotid arteries and may lead to hypertension [28]. The exact prevalence of this condition is unknown, as estimates suggest a prevalence of 12 per 100,000, but the prevailing belief is that it is underdiagnosed [29]. While the underlying pathophysiology has not been elucidated, there is a strong genetic predominance; one referral center estimates that 10% of cases are inherited [30].
There are conditions other than FMD that can cause hypertension in this demographic. Menarche, for instance, plays a role in the development of hypertension. A systematic review and meta-analysis of 17 studies showed that early menarche (defined as the onset of menses before age 12) was associated with an increased likelihood of developing hypertension in adulthood [31]. Polycystic ovarian syndrome (PCOS) has been thought to be associated with the development of hypertension in young women, though the results to date are inconclusive. A recent population-based cohort study from Taiwan showed that young women, most notably those of reproductive age, with PCOS had an increased risk of developing hypertension [32]. Hypertensive patients with hypokalemia who are not on diuretics may have primary aldosteronism or Cushing’s syndrome due to excess endogenous glucocorticoid secretion. Pheochromocytoma is a rare neuroendocrine tumor in the adrenal medulla capable of secreting large amounts of catecholamines, resulting in periodic episodes of tachycardia diaphoresis, and hypertension [33, 34]. Congenital vascular abnormalities, such as aortic coarctation, can also result in hypertension [35, 36]. Coarctation of the aorta is the most common cardiac lesion found in Turner syndrome [37]. Other secondary causes of hypertension include endocrine disorders (e.g., hyperthyroidism, diabetes), psychological disorders (e.g., mental stress, anxiety), and pharmacological causes (e.g., corticosteroids, antidepressants) [38–42].
Hormonal Contraception and Hypertension in Reproductive Aged Women
Hormonal contraceptives have been associated with hypertension [43]. This association is of particular importance, as many women of reproductive age who are on contraceptives have hypertension or are at risk of developing the condition. The American College of Obstetricians and Gynecologists (ACOG) has noted that hormonal contraceptives increase SBP and DBP by as much as 8 mm Hg and 6 mm Hg, respectively [44]. Per a 2019 ACOG Practice Bulletin, women with a BP < 140/90 mm Hg can use any form of hormonal contraception. In women who have hypertension with SBP 140–159 mm Hg or DBP 90–99 mm Hg, ACOG recommends that combined hormonal contraceptives not be used unless there are absolutely no other appropriate or acceptable options. Finally, for women with SBP ≥ 160 mm Hg or DBP ≥ 100 mm Hg or with concomitant vascular disease, no combined hormonal contraceptives should be used. The use of progestin-only contraceptives appears to be safe as they do not appear to have a significant effect on BP [44].
Hypertension in Pregnancy
Hypertension in pregnancy can be deleterious for both the mother and the developing fetus. Therefore, it is of the utmost importance for clinicians and caregivers to promptly recognize, diagnose, and treat hypertension early in pregnancy to prevent the development of any lasting complications. The prevalence of hypertension in pregnancy is significant and is increasing. A recent epidemiological study estimated the annual global incidence of hypertensive disorders in pregnancy as 18.08 million globally, a 10.92% increase since 1990 [45]. Hypertension in pregnancy is currently defined as SBP ≥ 140 mmHg and DBP ≥ 90 mmHg. This definition comes from the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy from 2000 and the more recent ACOG guidelines [46, 47]. Hypertension in pregnancy is further broken down into two categories: (1) non-severe hypertension, SBP between 140 and 159 mmHg and/or a DBP between 90 and 109 mmHg [48]; and (2) severe hypertension, SBP ≥ 160 mmHg and/or DBP ≥ 110 mmHg [49].
The latest recommendations from the ACOG concerning the diagnostic criteria for hypertension in pregnancy differ from the most recent updates from the American College of Cardiology and American Heart Association (ACC/AHA) [47, 50]. The ACC/AHA guidelines define hypertension into two stages. Stage 1 hypertension is defined as a SBP between 130 and 139 mmHg and/or a DBP between 80 and 89 mmHg. Stage 2 hypertension is defined as a SBP ≥ 140 mmHg and/or a DBP ≥ 90 mmHg [51]. Other international guidelines also have different criteria regarding hypertension in pregnancy [52, 53]. Specific types of hypertensive disorders of pregnancy, including chronic hypertension, gestational, preeclampsia/eclampsia, and chronic hypertension with superimposed preeclampsia/eclampsia, have been expanded upon by ACOG and are beyond the scope of this review [47, 50, 54]. Regarding the treatment of hypertension in pregnancy, it should be noted that all antihypertensive medications cross the placenta to some degree.
To further complicate things, there are no large-scale, randomized controlled trials comparing the efficacy and safety of antihypertensive drug classes to one another in pregnancy hypertension [55, 56]. However, based on clinical data, certain antihypertensives have been determined to be efficacious and safe to use during pregnancy. ACOG and the 2017 ACC/AHA Hypertension guidelines recommend that the following antihypertensives be used as first-line agents for patients with non-severe hypertension during pregnancy: labetalol, nifedipine extended release, and methyldopa. Hypertensive women who are treated for hypertension and are planning to become pregnant should be transitioned to one of these first-line agents. Some antihypertensive agents, most notably angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs), are contraindicated during pregnancy due to their teratogenic effects, especially when women are exposed to them during the second or third trimester [57].
Treatment of preeclampsia is primarily based on prevention. Low-dose aspirin has been associated with a 1 to 5% reduction in the incidence of preeclampsia [58]. The United States Preventive Services Task Force (USPSTF) recommends initiating low-dose aspirin at 12 weeks of pregnancy in women deemed high risk for developing preeclampsia [59].
In eclampsia, intravenous magnesium sulfate has been shown to effectively prevent convulsions [60]. Furthermore, lowering BP with parenteral labetalol, parenteral hydralazine, or oral nifedipine is also a mainstay of treatment. However, the only definitive treatment for both preeclampsia and eclampsia is delivery of the fetus.
The Chronic Hypertension and Pregnancy (CHAP) study was a recent open-label, multicenter, randomized trial that sought to determine whether a strategy of targeting a BP < 140/90 mm Hg in pregnant women with mild chronic hypertension (< 160/100 mm Hg) decreased adverse pregnancy outcomes (composite of preeclampsia with severe features, medically indicated preterm birth at less than 35 weeks of gestation, placental abruption, or fetal or neonatal death) more than treating severe hypertension in pregnancy (SBP ≥ 160 mmHg and/or a DBP ≥ 105 mmHg). The primary outcome of the trial was a composite outcome of fetal or neonatal death, preeclampsia with severe features, medically indicated preterm birth, and placental abruption. CHAP randomized 2408 women and showed that a strategy of targeting a BP of < 140/90 mm Hg resulted in a statistically significant decrease in the primary outcome (30.2% vs. 37.0%). Achieving the lower treatment target also significantly reduced the incidence of preeclampsia. Overall, the results of CHAP show that treatment of mild chronic hypertension in pregnancy is associated with better outcomes then reserving treatment for severe hypertension [61••].
Long-Term Implications of Hypertensive Disorders of Pregnancy
Hypertension in pregnancy results in an increased lifetime risk of adverse cardiovascular events in women. Guidelines now include a history of hypertension in pregnancy and other adverse pregnancy outcomes as risk enhancers that should be taken into account in the healthcare of women [62, 63]. It has been shown that women with a history of severe preeclampsia are at increased risk of cardiovascular death within the first 10 years after their pregnancy [64]. Endothelial dysfunction plays a central role in the pathogenesis of preeclampsia, and may persist for years after the affected pregnancy, conferring increased lifetime risk for CVD.
Menopause and Hypertension
Postmenopausal women have a higher prevalence of hypertension than aged matched men [65]. Sex differences in hypertension, specifically with aging, have led to increased interest in studying the role of hormones on hypertension development over the life course [66]. The evidence supporting a positive relationship between menopause and the development of hypertension is unclear. A study from the 1990s that followed women prospectively for 5 years found that peri- and postmenopausal women had a rise in SBP compared to premenopausal women and men. Importantly, postmenopausal women had higher SBPs at baseline, and these women saw a statistically significant increase in their SBPs throughout the 5-year follow-up period [67]. Although the above study used body mass index (BMI)-matched women, other studies have posited that the rise in SBP and the development of hypertension with menopause are better explained by BMI and age. A cross-sectional Italian study that spanned 16 years saw no difference in the rates of hypertension between pre- and postmenopausal women when data were corrected for age [68]. Other factors such as stiffening of arterial walls, obesity, and genetics affect BP in menopausal women [69]. Importantly, these discordant findings do not mean that sex hormones and the changes seen in the menopausal period do not affect BP [70].
Factors Influencing HTN in Menopause
While genetics appear to play a role in the development of hypertension in postmenopausal women, the precise genetic contribution is not well understood [71]. Menopause per se appears to activate a cluster of genes that lead to hypertension [72], while polymorphisms in certain adrenergic receptors appear to play a role in development of hypertension in both genders [73].
Menopause is associated with decreases in sex hormones, particularly estrogens, and these reductions are associated with vascular endothelial dysfunction [74]. These hormonal changes also result in upregulation of the renin–angiotensin–aldosterone system, leading to increased vasoconstriction [75] and increased salt sensitivity [76]. It has also been hypothesized that a continuation of androgen production in postmenopausal women may result in increased arterial stiffness and vascular inflammation, resulting in endothelial dysfunction and resultant hypertension [77, 78]. However, this association between menopause and subsequent hormonal changes with endothelial dysfunction leading to hypertension is far from definitive. Menopausal women with normal BP also experience endothelial dysfunction that appears to be age-related [74]. Therefore, the association between BP elevation and increased BMI and aging may better explain BP elevation in menopausal women [79, 80].
Menopausal Hormone Therapy
Menopausal hormonal therapy (MHT) or hormone replacement therapy is used to treat the signs and symptoms of menopause [81]. Evidence of the effect of MHT on BP is mixed. An observational cohort study of 43,405 previously normotensive, postmenopausal women in Australia found that MHT was associated with significantly higher odds of elevated BP. These odds increased with the duration of MHT use [82]. A recent French observational study showed a slight but significant increase in hypertension risk in postmenopausal women using oral estrogen and progesterone combination therapies [83•]. However, the Kronos Early Estrogen Prevention Study (KEEPS) showed that MHT use, regardless of formulation (oral conjugated estrogen or weekly transdermal estradiol, each with intermittent progesterone administration) did not affect BP in normotensive postmenopausal women. Other studies have shown that estrogen replacement therapy is associated with a decrease in ambulatory BP [84•]. Thus, women starting MHT should be counseled on its varied effects on BP with the understanding that there is no consensus to the underlying association between the two, and that MHT has not been shown to reduce CVD risk [85, 86].
Hypertension in Elderly Women
Hypertension occurs in up to 80% of older adults, and hypertension control rates are lower in older patients [51, 87, 88]. Moreover, hypertension rates in women are greater than in men that are 65 years of age or older [89]. Among US adults ≥ 75 years of age, 81.2% of women and 73.4% of men have hypertension. This trend may be due to a greater decline in endothelial function later in life in women than men due to decreased nitric oxide synthesis after menopause [90, 91]. Furthermore, older women, compared to middle aged and young women, have more severe and uncontrolled hypertension [89]. BP increase is associated with cognitive decline in both younger and older women [92, 93]. Therefore, it is important to control hypertension in order to reduce both mortality and incident dementia [94, 95].
Current guidelines recommend a BP goal of < 130/80 mmHg for all non-pregnant adults [51]. In the USA, 76% and 82% of adults aged 65–74 and ≥ 75 years had a SBP/DBP ≥ 130/80 mmHg, respectively [51]. Additionally, only 46% and 33% of US adults taking antihypertensives aged 65–74 and ≥ 75 had a SBP/DBP < 130/80 mmHg. The cardiovascular benefit of intensive BP control in older adults has been shown in major clinical trials [96–99]. In SPRINT (36% women), the cardiovascular benefit of a lower BP target was shown among those 75 years or older regardless of sex [97, 100]. Intensive BP lowering did not result in a greater number of injurious falls or prevalence of orthostatic hypotension. Additionally, in a recent randomized controlled trial (STEP: Strategy of Blood Pressure Intervention in the Elderly Hypertensive Patients) of Chinese patients 60–80 years of age (53% women), the SBP target 110 to < 130 mmHg resulted in a lower incidence of cardiovascular events compared to an SBP target of 130 to 150 mmHg [99]. The incidence of hypotension was higher in the intensive treatment group; but, there was no difference in the incidence of syncope, fractures, or dizziness. Most trials included only fit non-frail adults; therefore, future studies are needed to assess the effect of intensive BP lowering in older frail adults and whether sex differences exist in this population.
Disparities in Hypertension
Racial and Ethnic Disparities by Sex
BP prevalence and control rates vary by race, ethnicity, and sex [87, 101]. Non-Hispanic Black individuals have higher hypertension rates and lower control rates than non-Hispanic White individuals. In Northern California, a survey found that hypertension rates varied from 59.9 in Filipino men to 30% in Chinese women. They also found that Asian Americans and non-Hispanic Black individuals compared to non-Hispanic White individuals were more likely to be treated for hypertension. However, control rates were lowest for Filipino women and non-Hispanic Black men [5]. Socioeconomic factors, such as poor insurance coverage and limited healthcare access, contribute to disparities in hypertension control, with lower use of antihypertensive therapies observed in Hispanic individuals compared to non-Hispanic White individuals [102]. These differences are in part driven by cultural and historical underpinnings, including genetic and social differences in potassium and sodium intake, nocturnal diuresis, and fat distribution [103–107]. Future interventions should consider sex, racial, and ethnic differences to quantify risk and improve healthcare delivery. For instance, in Europe, unlike the USA, the atherosclerotic cardiovascular risk calculator accounts for ethnic group-based differences (SCORE or QRISK 2–2017). While the prevalence of hypertension is greater among aging women than men and an increase in the age-related decline in hypertension control has been shown among women, hypertension control rates differ between women of different ethnicities and races, and there have been no sex or race/ethnic-specific efforts to address these differences [89, 101, 108–110]. Importantly, race is a sociopolitical construct, and race per se should not be used to direct treatment. Rather, the underpinnings of these apparent racial differences, including socioeconomic differences, should be addressed in future studies aimed at improving hypertension control [111].
Socioeconomic Disparities
Low socioeconomic status, including low educational status and low income, are risk factors for hypertension [51, 112, 113]. Adults with an income > 400% above the US government poverty line, i.e., have higher socioeconomic status, have better BP control than adults below this line (43.2% vs. 30.2% respectively) [114]. Low socioeconomic status makes it challenging for people to adopt a healthy lifestyle (e.g., exercise, healthy food) and access healthcare and medications [89, 101, 115]. Providing BP treatment initiatives and free medications has been shown to improve BP control [100, 116, 117]. Additional barriers affecting BP control include poor language proficiency and social support. People with limited English proficiency have lower hypertension control rates than those with adequate English proficiency [118]. Providers are also less likely to engage in participatory decision-making and to be more verbally dominant with Black patients [119]. This translates into shorter visit times and less biomedical and psychosocial support provided to Black patients with uncontrolled hypertension than Whites with controlled hypertension [120]. A study of Black patients with uncontrolled hypertension found that most were not receiving a diuretic, even though it was recommended [121]. Area-level socioeconomic status also affects hypertension rates. Findings from the 2011 Behavioral Risk Factor Surveillance System showed that states with low median household income and high percentages of the population living below the poverty line had a greater prevalence of hypertension, irrespective of individual socioeconomic status [122]. In sum, the socioeconomic and racial/ethnic disparities in hypertension reflect deeper structural issues that have long persisted in the USA [123].
Primary Prevention of Hypertension in Women
The prevalence of hypertension varies across a woman’s life cycle and cardiovascular risk profiles differ between hypertensive women and men [124]. Hypertensive women are older and have more non-traditional risk factors such as kidney disease and abdominal obesity. However, there are no sex-specific strategies to screen or prevent hypertension in women.
Screening
The 2021 Unites States Preventive Task Force (USPSTF) recommends screening for hypertension in the office in adults ≥ 18 years with confirmation by an out-of-office BP measurement (home BP monitor or ambulatory BP monitor). For adults ≥ 40 years and those with risk factors for hypertension, yearly evaluation is recommended. BP evaluation is recommended every 3 to 5 years for younger adults with no risk factors and previously normal BP. Risk factors for hypertension include older age, Black race, family history, obesity or excess weight, dietary factors, and other lifestyle factors [89, 125]. The 2017 ACC/AHA guidelines, 2018 European Society of Cardiology, European Society of Hypertension (ECC/ESH) guidelines, and the Canadian Hypertension Education Program (CHEP) recommend that all adults ≥ 18 years should be evaluated with appropriate technique in the office and/or other clinical setting [51, 126–128]. Specifically, screening for hypertension should occur in pregnancy, as discussed in the previous “Hypertension in Pregnancy” section.
Irrespective of sex or age, the following are modifiable risk factors for hypertension: diabetes mellitus, dyslipidemia/hypercholesterolemia, excess weight or obesity, lack of physical fitness or low fitness activities, unhealthy diet (diet high in sodium, diet low in potassium, excessive alcohol intake), cigarette smoking or secondhand smoking [51, 89, 125]. Treating these risk factors might reduce BP and decrease the global risk factor burden. These include weight loss, following the DASH (Dietary Approaches to Stop Hypertension) diet, sodium reduction, potassium supplementation, increasing physical activity, and decreasing alcohol consumption [51, 129–136]. Other modifiable risk factors though challenging to change include chronic kidney disease, obstructive sleep apnea, and low socioeconomic and/or educational status. Additionally, the following medications can affect BP: over-the-counter medications (nonsteroidal anti-inflammatory drugs, decongestants), recreational drugs, prescription medications (e.g., corticosteroids, atypical antipsychotics), herbal supplements (e.g., St. John’s wort). Specifically, in women, combined oral hormonal contraceptives may contribute to an increase in BP [137–140]. These drugs, when possible, should be reduced, discontinued, or replaced with an alternative agent. For pregnant women, ACOG recommends that in women with a medical history of early-onset preeclampsia and preterm delivery (<34 weeks of gestation) or preeclampsia in more than one prior pregnancy to begin taking aspirin (60–80 mg) in the third trimester to prevent recurrence of preeclampsia [141].
Women in Clinical Trials of Hypertension
The National Institute of Health (NIH) encourages the inclusion of women and minorities as research participants to be consistent with the epidemiology of the disease being studied [142]. In addition, as of 2019, the NIH has an Inclusion Across the Lifespan policy to ensure the broadest age range to be included in clinical trials. Specifically, the National Heart, Lung, and Blood Institute (NHLBI) are committed to these decisions and funding studies to improve all patients. Effective strategies to ensure women’s representation in hypertension and cardiovascular clinical trials are important to improve women’s health. Women’s representation in hypertension trials is comparable to men’s, with a participation prevalence ratio of 0.82 (42.4% out of 136 trials). In the latest hypertension clinical trial, SPRINT, of those randomized to the intensive (SBP < 120 mmHg) and standard (SBP < 140 mmHg) treatment arm, 36% and 35.2% were women, respectively [143]. Women are also well represented in trials for hypertension drugs with a participation prevalence ratio of 0.9 [144]. However, among cardiovascular clinical trials between 2010 and 2017, women represented only 38.2% of trial participants despite making up 51% of the population [145].
Key Knowledge Gaps
Several areas would benefit from further research: (1) studying the effect of lower BP targets in women with gestational hypertension and those that develop preeclampsia; (2) development of new therapies to treat hypertension in pregnancy; (3) understanding the roles of genetic factors and sex hormones on hypertension development throughout a women’s lifespan; and (4) further research aimed at developing actionable interventions to reduce racial, ethnic, and social disparities in hypertension control and management in women.
Conclusion
Hypertension affects women at all stages of life and contributes to cardiovascular morbidity and mortality. Several knowledge gaps exist in understanding sex-specific prevention, detection, and management of hypertension across the life stages, including menarche, pregnancy, menopause, and old age. It is critical to include women at all stages of life in future research to guide individualized care and improve quality of life.
Conflict of Interest
Lama Ghazi receives grant funding from American Heart Association Postdoctoral Fellowship award (829804). Natalie A. Bello receives grant funding from the NIH/NHLBI (K23-HL136853, R01-HL153382) and is a member of a CEC for a GSK trial. Bharathi Upadhya reported receiving honoraria from Novartis. Rahul V. Annabathula, Li Zhou, and Richard Brandon Stacey declare that they have no conflict of interest.
Footnotes
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Hypertension. Key facts. 13 September 2019. [website]. https://www.who.int/news-room/fact-sheets/detail/hypertension. Accessed 15 Sept 2022.
- 2.Collaborators GRF. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the global burden of disease study 2017. Lancet. 2018;392:1923–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Hypertension cascade: hypertension prevalence, treatment and control estimates among U.S. adults aged 18 years and older applying the criteria from the American College of Cardiology and American Heart Association’s 2017 Hypertension Guideline—NHANES 2015–2018. Atlanta, GA: U.S. Department of Health and Human Services; 2021. www.cdc.gov/bloodpressure/facts.htm. Accessed 15 Sept 2022. [Google Scholar]
- 4.Bennett A, Parto P, Krim SR. Hypertension and ethnicity. Curr Opin Cardiol. 2016;31:381–6. [DOI] [PubMed] [Google Scholar]
- 5.Zhao B, Jose PO, Pu J, Chung S, Ancheta IB, Fortmann SP, et al. Racial/ethnic differences in hypertension prevalence, treatment, and control for outpatients in northern california 2010–2012. Am J Hypertens. 2015;28:631–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Munter JS, Agyemang C, van Valkengoed IG, Bhopal R, Stronks K. Sex difference in blood pressure among South Asian diaspora in Europe and North America and the role of BMI: a meta-analysis. J Hum Hypertens. 2011;25:407–17. [DOI] [PubMed] [Google Scholar]
- 7.Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, et al. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation. 2016;134:441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu J, Lu Y, Wang X, Li X, Linderman GC, Wu C, et al. Prevalence, awareness, treatment, and control of hypertension in china: data from 1·7 million adults in a population-based screening study (china peace million persons project). Lancet. 2017;390:2549–58. [DOI] [PubMed] [Google Scholar]
- 9.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Subcommittee AHASCaSS: Heart disease and stroke statistics–2015 update: a report from the american heart association. Circulation. 2015;131:e29–322. [DOI] [PubMed] [Google Scholar]
- 10.Gerdts E, Okin PM, de Simone G, Cramariuc D, Wachtell K, Boman K, et al. Gender differences in left ventricular structure and function during antihypertensive treatment: the losartan intervention for endpoint reduction in hypertension study. Hypertension. 2008;51:1109–14. [DOI] [PubMed] [Google Scholar]
- 11.Gerdts E, Izzo R, Mancusi C, Losi MA, Manzi MV, Canciello G, et al. Left ventricular hypertrophy offsets the sex difference in cardiovascular risk (the campania salute network). Int J Cardiol. 2018;258:257–61. [DOI] [PubMed] [Google Scholar]
- 12.Patel SA, Winkel M, Ali MK, Narayan KM, Mehta NK. Cardiovascular mortality associated with 5 leading risk factors: national and state preventable fractions estimated from survey data. Ann Intern Med. 2015;163:245–53. [DOI] [PubMed] [Google Scholar]
- 13.Falkstedt D, Koupil I, Hemmingsson T. Blood pressure in late adolescence and early incidence of coronary heart disease and stroke in the Swedish 1969 conscription cohort. J Hypertens. 2008;26:1313–20. [DOI] [PubMed] [Google Scholar]
- 14.Högström G, Nordström A, Eriksson M, Nordström P. Risk factors assessed in adolescence and the later risk of stroke in men: a 33-year follow-up study. Cerebrovasc Dis. 2015;39:63–71. [DOI] [PubMed] [Google Scholar]
- 15.Dost A, Bechtold S, Fink K, Bonfig W, Wiemann D, Kapellen TM, et al. Center iD-SatGDR: 2017 American Academy Of Pediatrics Clinical Practice guideline: impact on prevalence of arterial hypertension in children and adolescents with type 1 diabetes. Diabetes Care. 2020;43:1311–8. [DOI] [PubMed] [Google Scholar]
- 16.Al Kibria GM, Swasey K, Sharmeen A, Day B. Estimated change in prevalence and trends of childhood blood pressure levels in the United States after application of the 2017 AAP guideline. Prev Chronic Dis. 2019;16:E12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sorof J, Daniels S. Obesity hypertension in children: a problem of epidemic proportions. Hypertension. 2002;40:441–7. [DOI] [PubMed] [Google Scholar]
- 18.Falkner B, Gidding SS, Ramirez-Garnica G, Wiltrout SA, West D, Rappaport EB. The relationship of body mass index and blood pressure in primary care pediatric patients. J Pediatr. 2006;148:195–200. [DOI] [PubMed] [Google Scholar]
- 19.Archbold KH, Vasquez MM, Goodwin JL, Quan SF. Effects of sleep patterns and obesity on increases in blood pressure in a 5-year period: report from the Tucson children’s assessment of sleep apnea study. J Pediatr. 2012;161:26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grandi AM, Gaudio G, Fachinetti A, Bianchi L, Nardo B, Zanzi P, et al. Hyperinsulinemia, family history of hypertension, and essential hypertension. Am J Hypertens. 1996;9:732–8. [DOI] [PubMed] [Google Scholar]
- 21.von Eiff AW, Gogolin E, Jacobs U, Neus H. Ambulatory blood pressure in children followed for 3 years. Influence of sex and family history of hypertension. Clin Exp Hypertens A. 1986;8:577–81. [DOI] [PubMed] [Google Scholar]
- 22.Munger RG, Prineas RJ, Gomez-Marin O. Persistent elevation of blood pressure among children with a family history of hypertension: the Minneapolis children’s blood pressure study. J hypertens. 1988;6:647–53. [DOI] [PubMed] [Google Scholar]
- 23.Cheung EL, Bell CS, Samuel JP, Poffenbarger T, Redwine KM, Samuels JA. Race and obesity in adolescent hypertension. Pediatrics. 2017;139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogunniyi MO, Commodore-Mensah Y, Ferdinand KC. Race, ethnicity, hypertension, and heart disease: Jacc focus seminar 1/9. J Am Coll Cardiol. 2021;78:2460–70. [DOI] [PubMed] [Google Scholar]
- 25.Bolívar JJ. Essential hypertension: an approach to its etiology and neurogenic pathophysiology. Int J Hypertens. 2013;2013:547809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chrysaidou K, Chainoglou A, Karava V, Dotis J, Printza N, Stabouli S. Secondary hypertension in children and adolescents: novel insights. Curr Hypertens Rev. 2020;16:37–44. [DOI] [PubMed] [Google Scholar]
- 27.Hartung EA, Guay-Woodford LM. Autosomal recessive polycystic kidney disease: a hepatorenal fibrocystic disorder with pleiotropic effects. Pediatrics. 2014;134:e833–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Narula N, Kadian-Dodov D, Olin JW. Fibromuscular dysplasia: contemporary concepts and future directions. Prog Cardiovasc Dis. 2018;60:580–5. [DOI] [PubMed] [Google Scholar]
- 29.Hendricks NJ, Matsumoto AH, Angle JF, Baheti A, Sabri SS, Park AW, et al. Is fibromuscular dysplasia underdiagnosed? A comparison of the prevalence of FMD seen in coral trial participants versus a single institution population of renal donor candidates. Vasc Med. 2014;19:363–7. [DOI] [PubMed] [Google Scholar]
- 30.Poloskey SL, Olin JW, Mace P, Gornik HL. Fibromuscular dysplasia. Circulation. 2012;125:e636–639. [DOI] [PubMed] [Google Scholar]
- 31.Bubach S, De Mola CL, Hardy R, Dreyfus J, Santos AC, Horta BL. Early menarche and blood pressure in adulthood: systematic review and meta-analysis. J Public Health (Oxf). 2018;40:476–84. [DOI] [PubMed] [Google Scholar]
- 32.Wu CH, Chiu LT, Chang YJ, Lee CI, Lee MS, Lee TH, et al. Hypertension risk in young women with polycystic ovary syndrome: a nationwide population-based cohort study. Front Med (Lausanne). 2020;7:574651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma Lancet. 2005;366:665–75. [DOI] [PubMed] [Google Scholar]
- 34.Lenders JW, Pacak K, Walther MM, Linehan WM, Mannelli M, Friberg P, et al. Biochemical diagnosis of pheochromocytoma: which test is best? JAMA. 2002;287:1427–34. [DOI] [PubMed] [Google Scholar]
- 35.Sybert VP, McCauley E. Turner’s syndrome. N Engl J Med. 2004;351:1227–38. [DOI] [PubMed] [Google Scholar]
- 36.Lin AE, Prakash SK, Andersen NH, Viuff MH, Levitsky LL, Rivera-Davila M, et al. Recognition and management of adults with turner syndrome: from the transition of adolescence through the senior years. Am J Med Genet A. 2019;179:1987–2033. [DOI] [PubMed] [Google Scholar]
- 37.Lacro RV, Jones KL, Benirschke K. Coarctation of the aorta in turner syndrome: a pathologic study of fetuses with nuchal cystic hygromas, hydrops fetalis, and female genitalia. Pediatrics. 1988;81:445–51. [PubMed] [Google Scholar]
- 38.Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation. 2008;117:3171–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Colafella KMM, Denton KM. Sex-specific differences in hypertension and associated cardiovascular disease. Nat Rev Nephrol. 2018;14:185–201. [DOI] [PubMed] [Google Scholar]
- 40.Viera AJ, Neutze DM. Diagnosis of secondary hypertension: an age-based approach. Am Fam Physician. 2010;82:1471–8. [PubMed] [Google Scholar]
- 41.Wenger NK, Arnold A, Bairey Merz CN, Cooper-DeHoff RM, Ferdinand KC, Fleg JL, et al. Hypertension across a woman’s life cycle. J Am Coll Cardiol. 2018;71:1797–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pappadis SL, Somers MJ. Hypertension in adolescents: a review of diagnosis and management. Curr Opin Pediatr. 2003;15:370–8. [DOI] [PubMed] [Google Scholar]
- 43.Oparil S. Hypertension and oral contraceptives. J Cardiovasc Med. 1981;6(381):384–7. [PubMed] [Google Scholar]
- 44.Bulletins—Gynecology. Acog practice bulletin no. 206: Use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol. 2019;133:e128–50. [DOI] [PubMed] [Google Scholar]
- 45.Wang W, Xie X, Yuan T, Wang Y, Zhao F, Zhou Z, et al. Epidemiological trends of maternal hypertensive disorders of pregnancy at the global, regional, and national levels: a population-based study. BMC Pregnancy Childbirth. 2021;21:364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Program NHBPE. Report of the national high blood pressure education program working group on high blood pressure in pregnancy. Am J Bbstet Gynecol. 2000;183(1):s1–s22. [PubMed] [Google Scholar]
- 47.American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 202: gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133(1);e1–e25. [DOI] [PubMed] [Google Scholar]
- 48.Visintin C, Mugglestone MA, Almerie MQ, Nherera LM, James D, Walkinshaw S, et al. Management of hypertensive disorders during pregnancy: summary of nice guidance. BMJ. 2010;341:c2207. [DOI] [PubMed] [Google Scholar]
- 49.Bernstein PS, Martin JN, Barton JR, Shields LE, Druzin ML, Scavone BM, et al. Consensus bundle on severe hypertension during pregnancy and the postpartum period. J Obstet Gynecol Neonatal Nurs. 2017;46:776–87. [DOI] [PubMed] [Google Scholar]
- 50.Bulletins Obstetrics ACoOaGCoP. Acog practice bulletin no. 203: Chronic hypertension in pregnancy. Obstet Gynecol. 2019;133:e26–50. [DOI] [PubMed] [Google Scholar]
- 51.Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, et al. 2017 acc/aha/aapa/abc/acpm/ags/apha/ash/aspc/nma/pcna guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College Of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2018;138:e426–83. [DOI] [PubMed] [Google Scholar]
- 52.Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, Blomström-Lundqvist C, Cífková R, De Bonis M, et al. 2018 esc guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018;39:3165–241. [DOI] [PubMed] [Google Scholar]
- 53.Butalia S, Audibert F, Côté AM, Firoz T, Logan AG, Magee LA, et al. Hypertension Canada’s 2018 guidelines for the management of hypertension in pregnancy. Can J Cardiol. 2018;34:526–31. [DOI] [PubMed] [Google Scholar]
- 54.Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P. Group CHDoPW: Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy: Executive summary. J Obstet Gynaecol Can. 2014;36:416–41. [DOI] [PubMed] [Google Scholar]
- 55.Boesen EI. Consequences of in-utero exposure to antihypertensive medication: the search for definitive answers continues. J Hypertens. 2017;35:2161–4. [DOI] [PubMed] [Google Scholar]
- 56.Fitton CA, Steiner MFC, Aucott L, Pell JP, Mackay DF, Fleming M, et al. In-utero exposure to antihypertensive medication and neonatal and child health outcomes: a systematic review. J Hypertens. 2017;35:2123–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Magee LA, Ornstein MP, von Dadelszen P. Fortnightly review: management of hypertension in pregnancy. BMJ. 1999;318:1332–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duley L, Meher S, Hunter KE, Seidler AL, Askie LM. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2019;2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roberge S, Nicolaides K, Demers S, Hyett J, Chaillet N, Bujold E. The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: systematic review and meta-analysis. Am J Obstet Gynecol. 2017;216:110–120.e116. [DOI] [PubMed] [Google Scholar]
- 60.Euser AG, Cipolla MJ. Magnesium sulfate for the treatment of eclampsia: a brief review. Stroke. 2009;40:1169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.••.Tita AT, Szychowski JM, Boggess K, Dugoff L, Sibai B, Lawrence K, et al. Treatment for mild chronic hypertension during pregnancy. N Engl J Med. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article reports the results of a randomized controlled study on the effectiveness of treatment of mild chronic hypertension in pregnant women.
- 62.Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women–2011 update: a guideline from the American Heart Association. J Am Coll Cardiol. 2011;57:1404–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bushnell C, McCullough LD, Awad IA, Chireau MV, Fedder WN, Furie KL, et al. Nursing CoCaS, Cardiology CoC, Prevention CoEa, Research CfHBP: guidelines for the prevention of stroke in women: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:1545–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mongraw-Chaffin ML, Cirillo PM, Cohn BA. Preeclampsia and cardiovascular disease death: prospective evidence from the child health and development studies cohort. Hypertension. 2010;56:166–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Subcommittee AHASCaSS: Heart Disease and Stroke Statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martins D, Nelson K, Pan D, Tareen N, Norris K. The effect of gender on age-related blood pressure changes and the prevalence of isolated systolic hypertension among older adults: data from NHANES III. J Gend Specif Med. 2001;4(10–13):20. [PubMed] [Google Scholar]
- 67.Staessen JA, Ginocchio G, Thijs L, Fagard R. Conventional and ambulatory blood pressure and menopause in a prospective population study. J Hum Hypertens. 1997;11:507–14. [DOI] [PubMed] [Google Scholar]
- 68.Casiglia E, d’Este D, Ginocchio G, Colangeli G, Onesto C, Tramontin P, et al. Lack of influence of menopause on blood pressure and cardiovascular risk profile: a 16-year longitudinal study concerning a cohort of 568 women. J Hypertens. 1996;14:729–36. [DOI] [PubMed] [Google Scholar]
- 69.Clarkson TB. Estrogen effects on arteries vary with stage of reproductive life and extent of subclinical atherosclerosis progression. Menopause. 2007;14:373–84. [DOI] [PubMed] [Google Scholar]
- 70.Meyer MR, Haas E, Barton M. Gender differences of cardiovascular disease: new perspectives for estrogen receptor signaling. Hypertension. 2006;47:1019–26. [DOI] [PubMed] [Google Scholar]
- 71.Levy D, DeStefano AL, Larson MG, O’Donnell CJ, Lifton RP, Gavras H, et al. Evidence for a gene influencing blood pressure on chromosome 17. Genome scan linkage results for longitudinal blood pressure phenotypes in subjects from the Framingham Heart Study. Hypertension. 2000;36:477–83. [DOI] [PubMed] [Google Scholar]
- 72.Coylewright M, Reckelhoff JF, Ouyang P. Menopause and hypertension: an age-old debate. Hypertension. 2008;51:952–9. [DOI] [PubMed] [Google Scholar]
- 73.Rana BK, Insel PA, Payne SH, Abel K, Beutler E, Ziegler MG, et al. Population-based sample reveals gene-gender interactions in blood pressure in White Americans. Hypertension. 2007;49:96–106. [DOI] [PubMed] [Google Scholar]
- 74.Taddei S, Virdis A, Ghiadoni L, Mattei P, Sudano I, Bernini G, et al. Menopause is associated with endothelial dysfunction in women. Hypertension. 1996;28:576–82. [DOI] [PubMed] [Google Scholar]
- 75.Rossi R, Chiurlia E, Nuzzo A, Cioni E, Origliani G, Modena MG. Flow-mediated vasodilation and the risk of developing hypertension in healthy postmenopausal women. J Am Coll Cardiol. 2004;44:1636–40. [DOI] [PubMed] [Google Scholar]
- 76.Pechère-Bertschi A, Burnier M. Female sex hormones, salt, and blood pressure regulation. Am J Hypertens. 2004;17:994–1001. [DOI] [PubMed] [Google Scholar]
- 77.Moreau KL, Babcock MC, Hildreth KL. Sex differences in vascular aging in response to testosterone. Biol Sex Differ. 2020;11:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.DuPont JJ, Kenney RM, Patel AR, Jaffe IZ. Sex differences in mechanisms of arterial stiffness. Br J Pharmacol. 2019;176:4208–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gierach GL, Johnson BD, Bairey Merz CN, Kelsey SF, Bittner V, Olson MB, et al. Hypertension, menopause, and coronary artery disease risk in the women’s ischemia syndrome evaluation (wise) study. J Am Coll Cardiol. 2006;47:S50–58. [DOI] [PubMed] [Google Scholar]
- 80.Oparil S. Women and hypertension: what did we learn from the women’s health initiative? Cardiol Rev. 2006;14:267–75. [DOI] [PubMed] [Google Scholar]
- 81.Stuenkel CA, Davis SR, Gompel A, Lumsden MA, Murad MH, Pinkerton JV, et al. Treatment of symptoms of the menopause: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:3975–4011. [DOI] [PubMed] [Google Scholar]
- 82.Chiu CL, Lujic S, Thornton C, O’Loughlin A, Makris A, Hennessy A, et al. Menopausal hormone therapy is associated with having high blood pressure in postmenopausal women: Observational cohort study. PLoS ONE. 2012;7:e40260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.•.Madika AL, MacDonald CJ, Fournier A, Mounier-Vehier C, Béraud G, Boutron-Ruault MC. Menopausal hormone therapy and risk of incident hypertension: role of the route of estrogen administration and progestogens in the e3n cohort. Menopause. 2021;28:1204–8. [DOI] [PubMed] [Google Scholar]; This is an observational cohort study that assessed effect of different formulations of menopausal hormone therapy on incident hypertension among menopausal women.
- 84.•.Yoon BK, Sung J, Song YM, Kim SM, Son KA, Yoo JH, et al. Effects of menopausal hormone therapy on ambulatory blood pressure and arterial stiffness in postmenopausal Korean women with grade 1 hypertension: a randomized, placebo-controlled trial. Clin Hypertens. 2021;27:18. [DOI] [PMC free article] [PubMed] [Google Scholar]; This was a randomized placebo controlled trial that assessed the impact of micronized progesterone combined estradiol gel on hemodynamics in postmenopausal women with grade 1 hypertension.
- 85.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Investigators WGftWsHI: risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the women’s health initiative randomized controlled trial. JAMA. 2002;288:321–33. [DOI] [PubMed] [Google Scholar]
- 86.Mosca L, Collins P, Herrington DM, Mendelsohn ME, Pasternak RC, Robertson RM, et al. Hormone replacement therapy and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 2001;104:499–503. [DOI] [PubMed] [Google Scholar]
- 87.Muntner P, Carey RM, Gidding S, Jones DW, Taler SJ, Wright JT, et al. Potential us population impact of the 2017 acc/aha high blood pressure guideline. Circulation. 2018;137:109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bundy JD, Mills KT, Chen J, Li C, Greenland P, He J. Estimating the association of the 2017 and 2014 hypertension guidelines with cardiovascular events and deaths in us adults: an analysis of national data. JAMA Cardiol. 2018;3:572–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Subcommittee AHACoEaPSCaSS: Heart Disease and Stroke Statistics-2019 update: a report from the American Heart Association. Circulation. 2019;139:e56–528. [DOI] [PubMed] [Google Scholar]
- 90.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24:471–6. [DOI] [PubMed] [Google Scholar]
- 91.Sandberg K, Ji H. Sex differences in primary hypertension. Biol Sex Differ. 2012;3:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Elias PK, Elias MF, Robbins MA, Budge MM. Blood pressure-related cognitive decline: does age make a difference? Hypertension. 2004;44:631–6. [DOI] [PubMed] [Google Scholar]
- 93.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4:487–99. [DOI] [PubMed] [Google Scholar]
- 94.de Bruijn RF, Bos MJ, Portegies ML, Hofman A, Franco OH, Koudstaal PJ, et al. The potential for prevention of dementia across two decades: the prospective, population-based rotterdam study. BMC Med. 2015;13:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Danaei G, Ding EL, Mozaffarian D, Taylor B, Rehm J, Murray CJ, et al. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009;6:e1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shep Cooperative Research Group. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the systolic hypertension in the elderly program (shep). JAMA. 1991;265:3255–64. [PubMed] [Google Scholar]
- 97.Lewis CE, Fine LJ, Beddhu S, Cheung AK, Cushman WC, Cutler JA, et al. Final report of a trial of intensive versus standard blood-pressure control. N Engl J Med. 2021;384:1921–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Staessen JA, Fagard R, Thijs L, Celis H, Arabidze GG, Birkenhäger WH, et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The systolic hypertension in Europe (Syst-Eur) trial investigators. Lancet. 1997;350:757–64. [DOI] [PubMed] [Google Scholar]
- 99.Zhang W, Zhang S, Deng Y, Wu S, Ren J, Sun G, et al. Trial of intensive blood-pressure control in older patients with hypertension. N Engl J Med. 2021;385:1268–79. [DOI] [PubMed] [Google Scholar]
- 100.Williamson JD, Supiano MA, Applegate WB, Berlowitz DR, Campbell RC, Chertow GM, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: a randomized clinical trial. JAMA. 2016;315:2673–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yoon SS, Carroll MD, Fryar CD. Hypertension prevalence and control among adults: United States, 2011–2014. NCHS Data Brief. 2015:1–8. [PubMed] [Google Scholar]
- 102.Gu A, Yue Y, Desai RP, Argulian E. Racial and ethnic differences in antihypertensive medication use and blood pressure control among us adults with hypertension: the national health and nutrition examination survey, 2003 to 2012. Circ Cardiovasc Qual Outcomes. 2017;10. [DOI] [PubMed] [Google Scholar]
- 103.Wang X, Poole JC, Treiber FA, Harshfield GA, Hanevold CD, Snieder H. Ethnic and gender differences in ambulatory blood pressure trajectories: results from a 15-year longitudinal study in youth and young adults. Circulation. 2006;114:2780–7. [DOI] [PubMed] [Google Scholar]
- 104.Fumo MT, Teeger S, Lang RM, Bednarz J, Sareli P, Murphy MB. Diurnal blood pressure variation and cardiac mass in American Blacks and Whites and South African Blacks. Am J Hypertens. 1992;5:111–6. [DOI] [PubMed] [Google Scholar]
- 105.Stevens J, Truesdale KP, Katz EG, Cai J. Impact of body mass index on incident hypertension and diabetes in Chinese Asians, American Whites, and American Blacks: the People’s Republic of China study and the atherosclerosis risk in communities study. Am J Epidemiol. 2008;167:1365–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Andrew ME, Jones DW, Wofford MR, Wyatt SB, Schreiner PJ, Brown CA, et al. Ethnicity and unprovoked hypokalemia in the atherosclerosis risk in communities study. Am J Hypertens. 2002;15:594–9. [DOI] [PubMed] [Google Scholar]
- 107.Wright JT, Rahman M, Scarpa A, Fatholahi M, Griffin V, Jean-Baptiste R, et al. Determinants of salt sensitivity in black and white normotensive and hypertensive women. Hypertension. 2003;42:1087–92. [DOI] [PubMed] [Google Scholar]
- 108.Sorlie PD, Allison MA, Avilés-Santa ML, Cai J, Daviglus ML, Howard AG, et al. Prevalence of hypertension, awareness, treatment, and control in the Hispanic Community Health Study/Study of Latinos. Am J Hypertens. 2014;27:793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fryar CD, Ostchega Y, Hales CM, Zhang G, Kruszon-Moran D. Hypertension prevalence and control among adults: United States, 2015–2016. NCHS Data Brief. 2017:1–8. [PubMed] [Google Scholar]
- 110.Foti K, Wang D, Appel LJ, Selvin E. Hypertension awareness, treatment, and control in us adults: trends in the hypertension control cascade by population subgroup (national health and nutrition examination survey, 1999–2016). Am J Epidemiol. 2019;188:2165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fuentes A, Ackermann RR, Athreya S, Bolnick D, Lasisi T, Lee SH, et al. Aapa statement on race and racism. Am J Phys Anthropol. 2019;169:400–2. [DOI] [PubMed] [Google Scholar]
- 112.Leng B, Jin Y, Li G, Chen L, Jin N. Socioeconomic status and hypertension: a meta-analysis. J Hypertens. 2015;33:221–9. [DOI] [PubMed] [Google Scholar]
- 113.Nachman D, Gilan A, Goldstein N, Constantini K, Littman R, Eisenkraft A, et al. 24-hour ambulatory blood pressure measurement using a novel non-invasive, cuff-less, wireless device. Am J Hypertens. 2021. [DOI] [PubMed] [Google Scholar]
- 114.National Center for Health Statistics (U.S.). Health US, 2013: With special feature on, prescription drugs. Hyattsville MNCfHS, (U.S.), 2014. [PubMed] [Google Scholar]
- 115.Rodriguez CJ, Allison M, Daviglus ML, Isasi CR, Keller C, Leira EC, et al. Prevention AHACoEa, Cardiology AHACoC, Nursing AHACoCaS: Status of cardiovascular disease and stroke in Hispanics/Latinos in the United States: a science advisory from the American Heart Association. Circulation. 2014;130:593–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cushman WC, Evans GW, Byington RP, Goff DC, Grimm RH, Cutler JA, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Trial PHA. Major outcomes in high-Risk hypertensive patients. Jama. 2002;288(23):2981–97. [DOI] [PubMed] [Google Scholar]
- 118.Kim EJ, Kim T, Paasche-Orlow MK, Rose AJ, Hanchate AD. Disparities in hypertension associated with limited English proficiency. J Gen Intern Med. 2017;32:632–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Johnson RL, Roter D, Powe NR, Cooper LA. Patient race/ethnicity and quality of patient-physician communication during medical visits. Am J Public Health. 2004;94:2084–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cené CW, Roter D, Carson KA, Miller ER, Cooper LA. The effect of patient race and blood pressure control on patient-physician communication. J Gen Intern Med. 2009;24:1057–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gerber LM, Mann SJ, McDonald MV, Chiu YL, Sridharan S, Feldman PH. Diuretic use in black patients with uncontrolled hypertension. Am J Hypertens. 2013;26:174–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fan AZ, Strasser SM, Zhang X, Fang J, Crawford CG. State socioeconomic indicators and self-reported hypertension among us adults, 2011 behavioral risk factor surveillance system. Prev Chronic Dis. 2015;12:E27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mahajan S, Caraballo C, Lu Y, Valero-Elizondo J, Massey D, Annapureddy AR, et al. Trends in differences in health status and health care access and affordability by race and ethnicity in the united states, 1999–2018. JAMA. 2021;326:637–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tziomalos K, Giampatzis V, Baltatzi M, Efthymiou E, Psianou K, Papastergiou N, et al. Sex-specific differences in cardiovascular risk factors and blood pressure control in hypertensive patients. J Clin Hypertens (Greenwich). 2014;16:309–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Guirguis-Blake JM, Evans CV, Webber EM, Coppola EL, Perdue LA, Weyrich MS. Screening for hypertension in adults: updated evidence report and systematic review for the us preventive services task force. JAMA. 2021;325:1657–69. [DOI] [PubMed] [Google Scholar]
- 126.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. Members: ATF: 2018 ESC/ESH guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European society of cardiology and the European society of hypertension. J hypertens. 2018;36:1953–2041. [DOI] [PubMed] [Google Scholar]
- 127.Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. 2020 international society of hypertension global hypertension practice guidelines. Hypertension. 2020;75:1334–57. [DOI] [PubMed] [Google Scholar]
- 128.Nerenberg KA, Zarnke KB, Leung AA, Dasgupta K, Butalia S, McBrien K, et al. Hypertension Canada’s 2018 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults and children. Can J Cardiol. 2018;34:506–25. [DOI] [PubMed] [Google Scholar]
- 129.Whelton PK, Appel L, Charleston J, Dalcin AT, Ewart C, Fried L, Kaidy D, Klag MJ, Kumanyika S, Steffen L, Walker WG. The effects of nonpharmacologic interventions on blood pressure of persons with high normal levels: results of the Trials of Hypertension Prevention, phase I. Jama. 1992;267(9):1213–20. [DOI] [PubMed] [Google Scholar]
- 130.The Trials Of Hypertension Prevention Collaborative Research Group. Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in overweight people with high-normal blood pressure. The trials of hypertension prevention, phase ii. Arch Intern Med. 1997;157:657–67. [PubMed] [Google Scholar]
- 131.Whelton PK, Appel LJ, Espeland MA, Applegate WB, Ettinger WH, Kostis JB, et al. Sodium reduction and weight loss in the treatment of hypertension in older persons: a randomized controlled trial of nonpharmacologic interventions in the elderly (tone). JAMA. 1998;279:839–46. [DOI] [PubMed] [Google Scholar]
- 132.Forman JP, Stampfer MJ, Curhan GC. Diet and lifestyle risk factors associated with incident hypertension in women. JAMA. 2009;302:401–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.He J, Whelton PK, Appel LJ, Charleston J, Klag MJ. Long-term effects of weight loss and dietary sodium reduction on incidence of hypertension. Hypertension. 2000;35:544–9. [DOI] [PubMed] [Google Scholar]
- 134.Stamler R, Stamler J, Gosch FC, Civinelli J, Fishman J, McKeever P, et al. Primary prevention of hypertension by nutritional-hygienic means. Final report of a randomized, controlled trial. JAMA. 1989;262:1801–7. [PubMed] [Google Scholar]
- 135.Takahashi Y, Sasaki S, Okubo S, Hayashi M, Tsugane S. Blood pressure change in a free-living population-based dietary modification study in Japan. J Hypertens. 2006;24:451–8. [DOI] [PubMed] [Google Scholar]
- 136.Whelton PK, He J, Appel LJ, Cutler JA, Havas S, Kotchen TA, et al. Committee NHBPEPC: Primary prevention of hypertension: clinical and public health advisory from the national high blood pressure education program. JAMA. 2002;288:1882–8. [DOI] [PubMed] [Google Scholar]
- 137.Fisch IR, Frank J. Oral contraceptives and blood pressure. JAMA. 1977;237:2499–503. [PubMed] [Google Scholar]
- 138.Meade TW, Haines AP, North WR, Chakrabarti R, Howarth DJ, Stirling Y. Haemostatic, lipid, and blood-pressure profiles of women on oral contraceptives containing 50 microgram or 30 microgram oestrogen. Lancet. 1977;2:948–51. [DOI] [PubMed] [Google Scholar]
- 139.Weir RJ, Briggs E, Mack A, Naismith L, Taylor L, Wilson E. Blood pressure in women taking oral contraceptives. Br Med J. 1974;1:533–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liu H, Yao J, Wang W, Zhang D. Association between duration of oral contraceptive use and risk of hypertension: a meta-analysis. J Clin Hypertens (Greenwich). 2017;19:1032–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.American College of Obstetricians and Gynecologists. hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ task force on hypertension in pregnancy. Obstet Gynecol. 2013;122(5):1122–31. [DOI] [PubMed] [Google Scholar]
- 142.National Institutes of Health. Amendment. NIH policy and guidelines on the inclusion of women and minorities as subjects in clinical research. 2017. https://grants-nih-gov.yale.idm.oclc.org/grants/guide/notice-files/NOT-OD-18-014.html. Accessed 6 Jan 2022.
- 143.Wright JT, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Scott PE, Unger EF, Jenkins MR, Southworth MR, McDowell TY, Geller RJ, et al. Participation of women in clinical trials supporting FDA approval of cardiovascular drugs. J Am Coll Cardiol. 2018;71:1960–9. [DOI] [PubMed] [Google Scholar]
- 145.Jin X, Chandramouli C, Allocco B, Gong E, Lam CSP, Yan LL. Women’s participation in cardiovascular clinical trials from 2010 to 2017. Circulation. 2020;141:540–8. [DOI] [PubMed] [Google Scholar]


