Abstract
Rationale
Gastroesophageal reflux disease (GERD) is commonly associated with atopic disorders, but cause–effect relationships remain unclear.
Objectives
We applied Mendelian randomization analysis to explore whether GERD is causally related to atopic disorders of the lung (asthma) and/or skin (atopic dermatitis [AD]).
Methods
We conducted two-sample bidirectional Mendelian randomization to infer the magnitude and direction of causality between asthma and GERD, using summary statistics from the largest genome-wide association studies conducted on asthma (Ncases = 56,167) and GERD (Ncases = 71,522). In addition, we generated instrumental variables for AD from the latest population-level genome-wide association study meta-analysis (Ncases = 22,474) and assessed their fidelity and confidence of predicting the likely causal pathway(s) leading to asthma and/or GERD.
Measurements and Main Results
Applying three different methods, each method revealed similar magnitude of causal estimates that were directionally consistent across the sensitivity analyses. Using an inverse variance–weighted method, the largest effect size was detected for asthma predisposition to AD (odds ratio [OR], 1.46; 95% confidence interval [CI], 1.34–1.59), followed by AD to asthma (OR, 1.34; 95% CI, 1.24–1.45). A significant association was detected for genetically determined asthma on risk of GERD (OR, 1.06; 95% CI, 1.03–1.09) but not genetically determined AD on GERD. In contrast, GERD equally increased risks of asthma (OR, 1.21; 95% CI, 1.09–1.35) and AD (OR, 1.21; 95% CI, 1.07–1.37).
Conclusions
This study uncovers previously unrecognized causal pathways that have clinical implications in European-ancestry populations: 1) asthma is a causal risk for AD, and 2) the predisposition to AD, including asthma, can arise from specific pathogenic mechanisms manifested by GERD.
Keywords: human genetics, epidemiological approach, causal pathways, risk factors
At a Glance Commentary
Scientific Knowledge on the Subject
Numerous clinical and epidemiological studies have reported an association between gastroesophageal reflux disease (GERD) and asthma. However, as these observational studies are often limited to cross-sectional design and susceptible to confounding and reverse causation, whether the presence of GERD causally increases the risk for asthma remains unclear.
What This Study Adds to the Field
A two-sample bidirectional Mendelian randomization analysis establishes a causal effect of GERD on asthma and identifies a complex genetic interplay between atopic disorders of the skin and lung with GERD in European-ancestry populations. Specifically, our findings uncover previously unrecognized causal pathways that have clinical implications: 1) asthma is a causal risk for atopic dermatitis (AD), and 2) the predisposition to AD, including increased risk of asthma, can arise from specific pathogenic mechanisms manifested by GERD, a disease of the gastrointestinal tract. Furthermore, our results show that the effect of asthma on GERD is nominal and hence provides a basis for reexamining current therapeutic approaches to the management of patients with asthma and GERD.
Gastroesophageal reflux disease (GERD), a condition caused by persistent regurgitation of gastric contents leading to the sequelae of esophageal and/or extraesophageal complications (1), is highly prevalent and occurs in ∼75% of patients with asthma (2). The frequent coexistence of traits, or GERD comorbidity in patients with asthma (3, 4), may underlie common genetic etiology and/or pathogenic mechanisms shared between the two diseases. Of note, microaspiration of endogenous acids can reduce the pH of the airways and, via either reflex mechanisms (5) or direct effects on the airways (6), has been proposed to contribute to the pathophysiology of obstructive lung diseases (7–9).
Subsequently, it was determined that GERD can trigger asthma exacerbation (10). In addition, numerous clinical and epidemiological studies have reported association between asthma and GERD, and a large body of evidence suggests that GERD increases the risk of asthma (11, 12). However, as these observational studies are often limited to cross-sectional design and susceptible to confounding and reverse causation, whether the presence of GERD causally increases the risk for asthma remains unclear.
On the basis of Mendel’s laws of inheritance (random segregation and independent assortment of genes), Mendelian randomization (MR) collects genetic variants (i.e., SNPs, which are invariant to measured and unmeasured confounding factors or reverse causation) for use in an instrumental variable (IV) analysis to estimate a potential causal effect of a modifiable exposure on a risk outcome (13). Recently, MR methods have been used to clarify our understanding of multiple risk factors (biomarkers) that may simply be correlated from those that are causally related to various health outcomes (14–19). For instance, with respect to asthma, studies have established a positive causal effect of obesity and related traits on disease susceptibility (20–23). Conversely, Freuer and colleagues (24) reported, using one-sample MR within one study cohort, that genetically determined childhood-onset asthma, and not adult-onset asthma, is on the causal pathway leading to a number of gastrointestinal disorders, including GERD. The latter study, however, is prone to sample overlap (25), which may limit power and induce some biases, thus motivating additional work to quantify the causal relationship between GERD and asthma.
Here, we used summary-level data sets from the two largest genome-wide association studies (GWASs) conducted on asthma (26) and GERD (27) in European-ancestry populations and performed two-sample bidirectional MR analysis to infer the magnitude and direction of causality between the two diseases. In addition, we generated independent genetic IVs for atopic dermatitis (AD) from the latest population-level GWAS meta-analysis (28) and assessed their exposure commonality with genetically determined asthma on the predisposition to GERD. We conducted these studies because AD, an inflammatory disease of the skin, is frequently present in patients with asthma (29), shares a strong genetic etiology with asthma (30), and is believed to be an important early causal factor in the ultimate development of atopic asthma (“atopic march”) and asthma severity (31, 32).
Methods
Data Sources
The genetic variants were, all or partially, identified from the UK Biobank. Genetic IVs for asthma were obtained from GWASs in the UK Biobank on a broad asthma definition (56,167 cases and 352,255 control subjects) (26). The GWAS summary data included results from association tests of 35,270,583 SNPs with asthma in a cohort with White British ancestry. Genetic IVs for GERD and AD were obtained from two of the latest and largest population-level GWAS meta-analyses on GERD (71,522 cases and 261,079 control subjects) (27) and AD (22,474 cases and 774,187 control subjects) (28), respectively. The participants were primarily of White European ancestry from the United Kingdom and Australia for GERD and from the United Kingdom, Finland, and Estonia for AD. Detailed information on data sources is shown in Table E1 in the online supplement.
Genetic Correlations
The shared genetic architectures of the study traits (asthma, GERD, and AD) were calculated in a pairwise comparison using a linkage disequilibrium score regression method on HapMap 3 SNPs (33). Computed genetic correlations were corrected for multiple testing on the basis of the total number of correlations by applying a Bonferroni-corrected threshold of P = 0.017 (0.05/3 traits).
Genetic IV Selection
First, we extracted SNPs associated with each trait at a genome-wide degree of significance (P < 5 × 10−8) in the respective studies (26–28). To ensure that SNPs were independent, IVs were clumped using a stringent linkage disequilibrium threshold of r2 = 0.001 within a genetic window of 10 Mb on the basis of the 1,000 Genomes European reference panel. The effect estimates of both exposure and outcome variants were harmonized and expressed per effect allele increase, and possible palindromic SNPs were excluded. F statistics were calculated to assess the strength of genetically determined IVs (F > 10 is sufficient for the first MR assumption and does not suffer from weak instrument bias) (13, 34). Table E2 summarizes F statistics of final instruments.
MR Analyses
Figure 1 depicts the work flow of our IV analyses. As the primary analysis, we used a random-effect inverse variance–weighted (IVW) method, which allows for heterogeneity for the SNPs used in the instruments (35). Before running the IVW method, we first conducted sensitivity analyses to assess the directional pleiotropy (horizontal pleiotropy) using MR-Egger regression (36). Furthermore, we applied MR pleiotropy residual sum and outlier to detect any horizontal pleiotropic outlier (37). Table E3 shows the final number of IVs used in the IVW method. To ascertain the robustness of the primary analysis, we performed IV analyses using the MR-Egger method and the weighted median regression method (Figure 1). Finally, we performed leave-one-out analysis to test if the effect estimates were influenced by any one variant.
Figure 1.
The work flow of instrumental variable analysis to estimate a potential causal effect of a modifiable exposure on a risk outcome. GWAS = genome-wide association study; IVW = inverse variance weighted; MR = Mendelian randomization.
Statistical Analysis
All statistical analyses were performed using the TwoSampleMR (38) and MR-PRESSO (37) packages in R version 4.1.0 (https://www.r-project.org/). To correct for multiple testing, we applied Bonferroni correction, imposing a significance threshold of 0.017 (i.e., 0.05/3 tests).
Results
Genetic Correlations
Before performing MR analysis, we first evaluated the genetic correlation between the study traits in the GWAS summary statistics (26–28). Using linkage disequilibrium score regression (33), we detected the highest genetic correlation between AD and asthma (rg = 0.710, SE = 0.165; P = 1.72 × 10−5), followed by the correlation between asthma and GERD (rg = 0.362, SE = 0.051; P = 2.04 × 10−12) and then between GERD and AD (rg = 0.2, SE = 0.049; P = 3.90 × 10−5).
Asthma and AD
On the basis of the ranked order in genetic correlations, and the well-recognized link between atopic disorders and asthma (29, 30), we first conducted MR analysis of AD and asthma. We conducted these studies because AD is believed to be on the causal pathway along the progression of asthma (atopic march) (31, 32).
Using the GWAS summary statistics conducted on asthma (Ncases = 56,167) (26) and AD (Ncases = 22,474) (28), we derived 48 genetic variants for asthma and 11 genetic variants for AD. Of note, 11 AD-associated SNPs (F statistics of SNPs range from 32 to 62) were equally strong instruments as that of 48 asthma-associated SNPs (F statistics of SNPs range from 30 to 247) (see Table E2).
As expected, genetically determined AD was associated with increased risk of asthma (Figure 2), increasing the risk by 34% using the IVW method (odds ratio [OR], 1.34; 95% confidence interval [CI], 1.24–1.45; PIVW = 1.32 × 10−14). Similar effect estimates of AD on risk of asthma were detected using the weighted median regression method (OR, 1.32; 95% CI, 1.21–1.44; P = 5.77 × 10−10) as well as the MR-Egger method (OR, 1.54; 95% CI, 1.08–2.20; P = 4.14 × 10−2) (see Table E4). Leave-one-out analysis showed that the effect estimates were not influenced by any one variant (see Figure E1). In addition, the MR-Egger regression intercept did not significantly deviate from zero (see Table E5), suggesting no evidence of “horizontal pleiotropy” or violation of the second MR assumption (37). These results collectively established that AD is a causal risk factor for the development of asthma.
Figure 2.
Causal relationships between asthma and AD. The inverse variance–weighted method was used to estimate the magnitude and direction of effect sizes, presented as ORs and 95% CIs. AD = atopic dermatitis; CI = confidence interval; OR = odds ratios.
Surprisingly, when exposure and outcome were reversed, 48 genetic variants for asthma were also causally associated with increased risk of AD, increasing the risk by 46% (OR, 1.46; 95% CI, 1.34–1.59; PIVW = 1.67 × 10−18) (Figure 2). The effect estimates were directionally consistent across the sensitivity analyses (see Table E4) with no evidence of horizontal pleiotropy (see Table E5). The reciprocal direction of causality was not driven by a single outlying variant (see Figure E2).
As our study entailed partially overlapping sets of participants that can affect the causal estimates, we calculated the magnitude of potential bias, including type 1 error rate inflation due to sample overlap, using an online tool described by Burgess and colleagues (39). Across the entire range of possible sample overlap (see Table E6), we did not detect evidence for sample overlap in GWAS summary statistics (26–28). Hence, two-sample bidirectional MR analyses not only confirmed a well-recognized causal pathway in the progression of asthma but also identified a previously unrecognized association that is suggestive of asthma as a plausible risk factor for developing AD.
Asthma and GERD
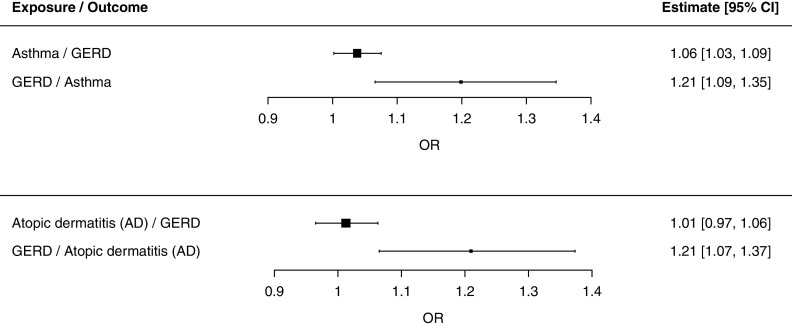
For MR analysis of asthma and GERD, we identified 62 asthma-associated SNPs and 21 GERD-associated SNPs in the GWAS summary statistics (26, 27) that were sufficiently strong independent genetic IVs (see Tables E2 and E3). On one hand, we detected a small but significant effect estimate of genetically determined asthma on increased risk of GERD (OR, 1.06; 95% CI, 1.03–1.09; PIVW = 4.94 × 10−4) (Figure 3; see Table E4 and Figure E3). On the other hand, we found strong and large causal effect estimate of genetically determined GERD on increased risk of asthma (Figure 3; see Table E4 and Figure E4), increasing the risk by 21% (OR, 1.21; 95% CI, 1.09–1.35; PIVW = 5.63 × 10−4).
Figure 3.
Causal relationships between GERD and atopic disorders (asthma or AD). The inverse variance–weighted method was used to estimate the magnitude and direction of effect sizes, presented as ORs and 95% CIs. AD = atopic dermatitis; CI = confidence interval; GERD = gastroesophageal reflux disease; OR = odds ratios.
Interestingly, 21 genetic variants associated with GERD also increased risk of AD (OR, 1.21; 95% CI, 1.07–1.37; PIVW = 3.32 × 10−3); the effect size of GERD on AD was similar to that of GERD on asthma (Figure 3; see Table E4 and Figure E5). There was no evidence of a causal relationship in the opposite direction (i.e., AD on risk of GERD) (see Table E4 and Figure E6). These results, taken together, established a complex genetic interplay between the inflammatory diseases of the lung and skin with GERD in European-ancestry populations and suggested that the predisposition to asthma, including AD, can arise from specific pathogenic mechanisms manifested by GERD.
Discussion
In this study, we set out to test whether asthma, an inflammatory disorder of the airways, is causally related to GERD, a condition that is perpetuated by chronic regurgitation of gastric contents. Applying three different MR methods, we found a similar magnitude of causal estimates, with an appreciably larger effect size for genetically determined GERD predisposing to asthma (increasing asthma risk by 21%) than genetically determined asthma on risk of GERD (6% increase) in European-ancestry populations. Furthermore, consistent with computed genetic correlations and a widely held notion that AD, an inflammatory disease of the skin, is an important early risk for the subsequent development and progression to asthma, genetically determined AD increased risk of asthma by 34%. Of note, the largest effect size was detected for asthma predisposition to AD (increase AD risk by 46%). Interestingly, whereas AD did not increase GERD, GERD increased risk of AD (21% increase), as it did for asthma. Thus, our studies reveal a complex genetic interplay between inflammatory diseases of the lung and skin with GERD and, moreover, suggest that the predisposition to asthma, including AD, can arise from specific pathogenic mechanisms manifested by GERD.
The frequent coexistence of traits, or GERD comorbidity in patients with asthma, may underlie common genetic etiology and/or pathogenic mechanisms shared between the two diseases. Of note, the lung and gut arise from the foregut, which, in time and space, undergoes a divergent developmental program that is requisite to each organogenesis and permissive for distinct physiological functions (40, 41). Anatomically, the upper respiratory and gastrointestinal tracts are in close proximity, separated by a short-lived tracheoesophageal septum, and the stimulatory and inhibitory reflex mechanisms of the upper gastrointestinal tract (i.e., esophagus) are designed to ensure protection against aspiration of ingested and gastric contents (reflux) into the airways (42, 43). Microaspiration of endogenous acids, due to altered reflex and/or reflux mechanisms (5, 6), can reduce the pH of the airways and has been proposed to contribute to the pathophysiology of obstructive lung diseases (7–9). Although numerous studies have suggested that GERD increases the risk of asthma (11, 12), most, if not all, are limited to retrospective clinical and epidemiological observations. Hence, whether the presence of GERD causally increases the risk for asthma and/or whether patients with asthma are genetically susceptible to develop GERD remain unclear. Of note, randomized controlled trials of acid-suppressive therapies in patients with asthma and GERD (adults and children) have shown limited therapeutic reductions in asthma symptoms and/or improvements in pulmonary function (44–46). These issues have led us to explore whether there exists a true cause–effect relationship between these two diseases.
Similar to randomized controlled trials, MR applies a universal concept in human genetics (random segregation and independent assortment of genes) as proxies to infer whether a modifiable risk factor is causally linked to a health outcome (13). Recently, with an increasing availability of large GWAS summary statistics, MR methods have been used broadly to establish causal relationships between commonly associated traits/diseases and, in so doing, opened new avenues for cost-effective, well-rationalized study designs for clinical trials, including the basis for further hypothesis-driven mechanistic studies. For instance, with respect to asthma, studies have recently established a positive, unidirectional causal effect of obesity and related adiposity traits (i.e., the weighted allele score for body mass index [BMI]) on risk of asthma (20–23). A higher BMI was also identified as a causal risk for AD (47). Of note, Green and colleagues (48) found that central fat distribution (i.e., a higher waist-to-hip ratio), and not BMI, is causally associated with GERD. Because obesity is a potential risk for multiple chronic disorders (16), and new MR approaches to this space are already emerging (21, 49–51), we did not perform MR analysis of obesity and/or obesity-related traits with our study traits (asthma, AD, and GERD) or additional multivariable MR, including genetic associations with potential confounders (i.e., BMI, sex, and smoking status) within the respective cohorts in the nondisclosive summary-level GWASs (26–28).
Recently, Freuer and colleagues (24) reported that the presence of asthma onset in childhood, but not in adulthood, is positively associated with GERD later in life. For this study, they collected genetic IVs for childhood-onset and adult-onset asthma, as well as genetic IVs for GERD, within one study cohort in the UK Biobank (25) and ran one-sample MR in one causal pathway, which may suffer from weak instrument bias and some predicted power. On the one hand, a longitudinal study in the general population of United Kingdom reported that patients with asthma are at increased risk of developing GERD and not vice versa (52). On the other hand, two longitudinal follow-up studies in Korean children (53) and adults (54) detected a reciprocal causality between GERD and asthma. On the basis of these observational studies, here we performed two-sample bidirectional MR analysis to infer the magnitude and direction of causality between asthma and GERD. Interestingly, Zhu and colleagues found that shared genetic loci between asthma and allergic diseases (hay fever/allergic rhinitis or AD) were enriched in immune/inflammatory systems and localized to several epithelial tissues, including the skin, lung, and esophageal tissues (30). Accordingly, we also generated genetic IVs for AD and assessed their exposure commonality with genetically determined asthma on the predisposition to GERD.
In this study, we detected a small but significant effect size for genetically determined asthma on risk of GERD (OR, 1.06; 95% CI, 1.03–1.09; P = 4.94 × 10−4) in European-ancestry populations. For our analysis, asthma SNPs were identified from GWASs in the UK Biobank on a broad asthma definition (26) and may have inflated the nominal causal effect because of an unaccounted natural history (time and course) of patient-reported asthma symptoms. In addition, the genetic variants (asthma, GERD, and AD) were, all or partially, identified from participants in the UK Biobank (26–28) and as such may suffer from sample overlap, one of the limitations of MR studies using publicly available GWAS summary statistics. To this end, we simulated IV bias across the entire range of possible sample overlap (see Table E6) and calculated F statistics of final instruments (see Table E2) but found no evidence of sample overlap in the GWAS summary statistics or weak instrument bias. Further replication studies are needed in non-European populations for the generalizability of our findings, however.
Most strikingly, as opposed to the findings of Freuer and colleagues (24), we detected the presence of a reciprocal causal effect of GERD on asthma (genetically determined GERD increased the risk for asthma by 21%). Although MR analysis does not provide or suggest the underlying biology, here we speculated different mechanisms, including those involving acid reflux effects on the lung. Barbas and colleagues (55) showed that chronic aspiration of gastric fluid evokes a shift of immune responses (from T helper type 1 [Th1] to Th2) in a mouse model of asthma. Using isolated human airway smooth muscle (ASM) cells in culture as a physiological model, we have reported that small reductions in extracellular pH evoke contraction through OGR1 (ovarian cancer G protein–coupled receptor 1 or GPR68 [G protein–coupled receptor 68]) expressed on ASM (56), suggesting that alterations in extracellular pH (i.e., airway acidification caused by exogenous and/or endogenous acids or as a consequence of airway inflammation) has direct effects on the contractility of an end-effector cell of acute airway narrowing in asthma (57). In addition, reflex-mediated increases in ASM contraction and airway resistance (58), mediated by autonomic nerves innervating the airways (59), or by mechanical perturbation to the mucosa of the upper airways and esophagus (5, 60), can contribute to asthma pathogenesis. Together these studies highlight an importance of characterizing reflex and/or reflux mechanisms regulating ASM tone and contractility in patients with asthma and GERD (59). Our results also warrant further investigation into the diagnosis and treatment of GERD comorbidity in patients with asthma.
Among the three traits studied, we detected the largest effect size for asthma predisposition to AD (increasing AD risk by 46%). This is a marked contrast to the widely held notion that AD is an early causal factor in the ultimate development of atopic asthma (31, 32). Although the causal mechanisms for this atopic march remain subject to debate (32), one plausible mechanism is the loss-of-function mutations in the FLG (filaggrin) gene in patients with AD that lead to dysregulation of epidermal skin barrier function and consequent induction of allergic sensitization and airway hyperresponsiveness through an antigen-specific Th2 responses (61). In addition to variants in FLG that define the shared genetic pathways in AD and asthma (61–63), Sliz and colleagues (28) have also identified other novel missense variants, including DSC1 (desmocollin 1) and SERPINB7 (serpin family B member 7), that may contribute to altered mechanical stability and epidermal barrier properties in individuals of European ancestry. Accordingly, our results not only confirmed a plausible causal effect of AD on asthma but also identified a previously unrecognized association suggesting that asthma may be a causal risk factor for AD.
Interestingly, GERD equally increased the risks for asthma (OR, 1.21; 95% CI, 1.09–1.35; P = 5.63 × 10−4) and AD (OR, 1.21; 95% CI, 1.07–1.37; P = 3.32 × 10−3); AD did not increase risk of GERD. These results suggested that the predisposition to AD, including increased risk of asthma, can arise from specific pathogenic mechanisms manifested by GERD, a disease of the gastrointestinal tract. To the best of our knowledge, a causal effect of GERD on AD has not been established. Although further studies are warranted to establish the specific mechanisms, we speculate that the intestinal neuroimmune axis (64), including interkingdom microbial cross-talk (65), is a possible homeostatic mechanism regulating inflammatory responses in the lung and skin and shaping the ultimate development of atopic disorders: asthma and AD. We believe further insights into the causal pathway to these complex traits may come from advances in MR that incorporate tissue-specific microbiome, metabolome, transcriptome, and phenome-wide analyses (17, 66–69). Such findings would not only provide greater granularity to disease etiology, but also reveal new biomarker(s) and advance the development of new targeted intervention strategies.
Conclusions
Taken together, this study established a complex genetic interplay among AD, asthma, and GERD in European-ancestry populations. Importantly, our findings not only substantiated the association between asthma and GERD but also uncovered previously unrecognized associations that have clinical implications: 1) asthma is a causal risk factor for AD, and 2) the predisposition to AD, including increased risk of asthma, can arise from specific pathogenic mechanisms manifested by GERD. Further studies are needed for the identification and characterization of the gut–lung–skin axis.
Footnotes
Supported by the National Center for Advancing Translational Sciences, a component of the NIH, under award UL1TR003017, and NHLBI grant P01HL114471, NIH grant R01HL058506, and National Institute of Diabetes and Digestive and Kidney Diseases grant R01DK035385. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author Contributions: K.A. and S.S.A. conceived and designed the research; K.A. and S.S.A. performed the analysis; K.A., R.B.P., S.R., R.A.P., B.F.V., and S.S.A. analyzed the data, interpreted the results, and wrote the paper.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202205-0951OC on October 10, 2022
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R, Global Consensus Group The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol . 2006;101:1900–1920. doi: 10.1111/j.1572-0241.2006.00630.x. [DOI] [PubMed] [Google Scholar]
- 2. Leggett JJ, Johnston BT, Mills M, Gamble J, Heaney LG. Prevalence of gastroesophageal reflux in difficult asthma: relationship to asthma outcome. Chest . 2005;127:1227–1231. doi: 10.1378/chest.127.4.1227. [DOI] [PubMed] [Google Scholar]
- 3. Harding SM, Guzzo MR, Richter JE. The prevalence of gastroesophageal reflux in asthma patients without reflux symptoms. Am J Respir Crit Care Med . 2000;162:34–39. doi: 10.1164/ajrccm.162.1.9907072. [DOI] [PubMed] [Google Scholar]
- 4. Reddel HK, Bacharier LB, Bateman ED, Brightling CE, Brusselle GG, Buhl R, et al. Global Initiative for Asthma Strategy 2021: executive summary and rationale for key changes. Am J Respir Crit Care Med . 2022;205:17–35. doi: 10.1164/rccm.202109-2205PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mansfield LE, Stein MR. Gastroesophageal reflux and asthma: a possible reflex mechanism. Ann Allergy . 1978;41:224–226. [PubMed] [Google Scholar]
- 6. Kennedy JH. Silent gastroesophageal reflux—an important but little known cause of pulmonary complications. Dis Chest . 1962;42:42–45. [Google Scholar]
- 7. Antus B, Barta I, Kullmann T, Lazar Z, Valyon M, Horvath I, et al. Assessment of exhaled breath condensate pH in exacerbations of asthma and chronic obstructive pulmonary disease: a longitudinal study. Am J Respir Crit Care Med . 2010;182:1492–1497. doi: 10.1164/rccm.201003-0451OC. [DOI] [PubMed] [Google Scholar]
- 8. Hunt JF, Fang K, Malik R, Snyder A, Malhotra N, Platts-Mills TA, et al. Endogenous airway acidification: implications for asthma pathophysiology. Am J Respir Crit Care Med . 2000;161:694–699. doi: 10.1164/ajrccm.161.3.9911005. [DOI] [PubMed] [Google Scholar]
- 9. Ricciardolo FL, Rado V, Fabbri LM, Sterk PJ, Di Maria GU, Geppetti P. Bronchoconstriction induced by citric acid inhalation in guinea pigs: role of tachykinins, bradykinin, and nitric oxide. Am J Respir Crit Care Med . 1999;159:557–562. doi: 10.1164/ajrccm.159.2.9804022. [DOI] [PubMed] [Google Scholar]
- 10. Denlinger LC, Phillips BR, Ramratnam S, Ross K, Bhakta NR, Cardet JC, et al. National Heart, Lung, and Blood Institute’s Severe Asthma Research Program-3 Investigators Inflammatory and comorbid features of patients with severe asthma and frequent exacerbations. Am J Respir Crit Care Med . 2017;195:302–313. doi: 10.1164/rccm.201602-0419OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. DiMango E, Holbrook JT, Simpson E, Reibman J, Richter J, Narula S, et al. American Lung Association Asthma Clinical Research Centers Effects of asymptomatic proximal and distal gastroesophageal reflux on asthma severity. Am J Respir Crit Care Med . 2009;180:809–816. doi: 10.1164/rccm.200904-0625OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harding SM, Guzzo MR, Richter JE. 24-h esophageal pH testing in asthmatics: respiratory symptom correlation with esophageal acid events. Chest . 1999;115:654–659. doi: 10.1378/chest.115.3.654. [DOI] [PubMed] [Google Scholar]
- 13. Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet . 2014;23:R89–R98. doi: 10.1093/hmg/ddu328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harbaum L, Rhodes CJ, Wharton J, Lawrie A, Karnes JH, Desai AA, et al. U.K. National Institute for Health Research BioResource Rare Diseases Consortium, U.K. Pulmonary Arterial Hypertension Cohort Study Consortium, and U.S. Pulmonary Arterial Hypertension Biobank Consortium Mining the plasma proteome for insights into the molecular pathology of pulmonary arterial hypertension. Am J Respir Crit Care Med . 2022;205:1449–1460. doi: 10.1164/rccm.202109-2106OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jones TK, Feng R, Kerchberger VE, Reilly JP, Anderson BJ, Shashaty MGS, et al. Plasma sRAGE acts as a genetically regulated causal intermediate in sepsis-associated acute respiratory distress syndrome. Am J Respir Crit Care Med . 2020;201:47–56. doi: 10.1164/rccm.201810-2033OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Larsson SC, Burgess S. Causal role of high body mass index in multiple chronic diseases: a systematic review and meta-analysis of Mendelian randomization studies. BMC Med . 2021;19:320. doi: 10.1186/s12916-021-02188-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lord J, Jermy B, Green R, Wong A, Xu J, Legido-Quigley C, et al. Mendelian randomization identifies blood metabolites previously linked to midlife cognition as causal candidates in Alzheimer’s disease. Proc Natl Acad Sci U S A . 2021;118:e2009808118. doi: 10.1073/pnas.2009808118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rhodes CJ, Otero-Núñez P, Wharton J, Swietlik EM, Kariotis S, Harbaum L, et al. Whole-blood RNA profiles associated with pulmonary arterial hypertension and clinical outcome. Am J Respir Crit Care Med . 2020;202:586–594. doi: 10.1164/rccm.202003-0510OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Trinder M, Genga KR, Kong HJ, Blauw LL, Lo C, Li X, et al. Cholesteryl ester transfer protein influences high-density lipoprotein levels and survival in sepsis. Am J Respir Crit Care Med . 2019;199:854–862. doi: 10.1164/rccm.201806-1157OC. [DOI] [PubMed] [Google Scholar]
- 20. Granell R, Henderson AJ, Evans DM, Smith GD, Ness AR, Lewis S, et al. Effects of BMI, fat mass, and lean mass on asthma in childhood: a Mendelian randomization study. PLoS Med . 2014;11:e1001669. doi: 10.1371/journal.pmed.1001669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Richardson TG, Crouch DJM, Power GM, Morales-Berstein F, Hazelwood E, Fang S, et al. Childhood body size directly increases type 1 diabetes risk based on a lifecourse Mendelian randomization approach. Nat Commun . 2022;13:2337. doi: 10.1038/s41467-022-29932-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xu S, Gilliland FD, Conti DV. Elucidation of causal direction between asthma and obesity: a bi-directional Mendelian randomization study. Int J Epidemiol . 2019;48:899–907. doi: 10.1093/ije/dyz070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhu Z, Guo Y, Shi H, Liu CL, Panganiban RA, Chung W, et al. Shared genetic and experimental links between obesity-related traits and asthma subtypes in UK Biobank. J Allergy Clin Immunol . 2020;145:537–549. doi: 10.1016/j.jaci.2019.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Freuer D, Linseisen J, Meisinger C. Asthma and the risk of gastrointestinal disorders: a Mendelian randomization study. BMC Med . 2022;20:82. doi: 10.1186/s12916-022-02283-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ferreira MAR, Mathur R, Vonk JM, Szwajda A, Brumpton B, Granell R, et al. 23andMe Research Team eQTLGen Consortium; BIOS Consortium. Genetic architectures of childhood- and adult-onset asthma are partly distinct. Am J Hum Genet . 2019;104:665–684. doi: 10.1016/j.ajhg.2019.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Valette K, Li Z, Bon-Baret V, Chignon A, Bérubé JC, Eslami A, et al. Prioritization of candidate causal genes for asthma in susceptibility loci derived from UK Biobank. Commun Biol . 2021;4:700. doi: 10.1038/s42003-021-02227-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. An J, Gharahkhani P, Law MH, Ong JS, Han X, Olsen CM, et al. BEACON 23andMe Research Team. Gastroesophageal reflux GWAS identifies risk loci that also associate with subsequent severe esophageal diseases. Nat Commun . 2019;10:4219. doi: 10.1038/s41467-019-11968-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sliz E, Huilaja L, Pasanen A, Laisk T, Reimann E, Magi R, et al. FinnGen Estonian Biobank Research Team. Uniting biobank resources reveals novel genetic pathways modulating susceptibility for atopic dermatitis. J Allergy Clin Immunol . 2022;149:1105–1112.e9. doi: 10.1016/j.jaci.2021.07.043. [DOI] [PubMed] [Google Scholar]
- 29. Wolfe R, Carlin JB, Oswald H, Olinsky A, Phelan PD, Robertson CF. Association between allergy and asthma from childhood to middle adulthood in an Australian cohort study. Am J Respir Crit Care Med . 2000;162:2177–2181. doi: 10.1164/ajrccm.162.6.9812019. [DOI] [PubMed] [Google Scholar]
- 30. Zhu Z, Lee PH, Chaffin MD, Chung W, Loh PR, Lu Q, et al. A genome-wide cross-trait analysis from UK Biobank highlights the shared genetic architecture of asthma and allergic diseases. Nat Genet . 2018;50:857–864. doi: 10.1038/s41588-018-0121-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Davidson WF, Leung DYM, Beck LA, Berin CM, Boguniewicz M, Busse WW, et al. Report from the National Institute of Allergy and Infectious Diseases workshop on “atopic dermatitis and the atopic march: mechanisms and interventions”. J Allergy Clin Immunol . 2019;143:894–913. doi: 10.1016/j.jaci.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dharmage SC, Lowe AJ, Tang ML.Revisiting the atopic march: current evidence Am J Respir Crit Care Med 2022;206:925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bulik-Sullivan BK, Loh PR, Finucane HK, Ripke S, Yang J, Patterson N, et al. Schizophrenia Working Group of the Psychiatric Genomics Consortium LD score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet . 2015;47:291–295. doi: 10.1038/ng.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pierce BL, Burgess S. Efficient design for Mendelian randomization studies: subsample and 2-sample instrumental variable estimators. Am J Epidemiol . 2013;178:1177–1184. doi: 10.1093/aje/kwt084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol . 2015;44:512–525. doi: 10.1093/ije/dyv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bowden J, Del Greco M F, Minelli C, Davey Smith G, Sheehan N, Thompson J. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat Med . 2017;36:1783–1802. doi: 10.1002/sim.7221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet . 2018;50:693–698. doi: 10.1038/s41588-018-0099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife . 2018;7:e34408. doi: 10.7554/eLife.34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Burgess S, Davies NM, Thompson SG. Bias due to participant overlap in two-sample Mendelian randomization. Genet Epidemiol . 2016;40:597–608. doi: 10.1002/gepi.21998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Faure S, de Santa Barbara P. Molecular embryology of the foregut. J Pediatr Gastroenterol Nutr . 2011;52:S2–S3. doi: 10.1097/MPG.0b013e3182105a1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Morrisey EE, Hogan BL. Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev Cell . 2010;18:8–23. doi: 10.1016/j.devcel.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Morrisey EE, Rustgi AK. The lung and esophagus: developmental and regenerative overlap. Trends Cell Biol . 2018;28:738–748. doi: 10.1016/j.tcb.2018.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shaker R, Dodds WJ, Ren J, Hogan WJ, Arndorfer RC. Esophagoglottal closure reflex: a mechanism of airway protection. Gastroenterology . 1992;102:857–861. doi: 10.1016/0016-5085(92)90169-y. [DOI] [PubMed] [Google Scholar]
- 44. Cantarutti A, Barbiellini Amidei C, Valsecchi C, Scamarcia A, Corrao G, Gregori D, et al. Association of treated and untreated gastroesophageal reflux disease in the first year of life with the subsequent development of asthma. Int J Environ Res Public Health . 2021;18:9633. doi: 10.3390/ijerph18189633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Harding SM, Richter JE, Guzzo MR, Schan CA, Alexander RW, Bradley LA. Asthma and gastroesophageal reflux: acid suppressive therapy improves asthma outcome. Am J Med . 1996;100:395–405. doi: 10.1016/S0002-9343(97)89514-9. [DOI] [PubMed] [Google Scholar]
- 46. Zheng Z, Luo Y, Li J, Gao J. Randomised trials of proton pump inhibitors for gastro-oesophageal reflux disease in patients with asthma: an updated systematic review and meta-analysis. BMJ Open . 2021;11:e043860. doi: 10.1136/bmjopen-2020-043860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yew YW, Loh M, Thng STG, Chambers JC. Investigating causal relationships between body mass index and risk of atopic dermatitis: a Mendelian randomization analysis. Sci Rep . 2020;10:15279. doi: 10.1038/s41598-020-72301-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Green HD, Beaumont RN, Wood AR, Hamilton B, Jones SE, Goodhand JR, et al. Genetic evidence that higher central adiposity causes gastro-oesophageal reflux disease: a Mendelian randomization study. Int J Epidemiol . 2020;49:1270–1281. doi: 10.1093/ije/dyaa082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Budu-Aggrey A, Brumpton B, Tyrrell J, Watkins S, Modalsli EH, Celis-Morales C, et al. Evidence of a causal relationship between body mass index and psoriasis: a Mendelian randomization study. PLoS Med . 2019;16:e1002739. doi: 10.1371/journal.pmed.1002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Leyden GM, Shapland CY, Davey Smith G, Sanderson E, Greenwood MP, Murphy D, et al. Harnessing tissue-specific genetic variation to dissect putative causal pathways between body mass index and cardiometabolic phenotypes. Am J Hum Genet . 2022;109:240–252. doi: 10.1016/j.ajhg.2021.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Xue H, Pan W. Robust inference of bi-directional causal relationships in presence of correlated pleiotropy with GWAS summary data. PLoS Genet . 2022;18:e1010205. doi: 10.1371/journal.pgen.1010205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ruigómez A, Rodríguez LA, Wallander MA, Johansson S, Thomas M, Price D. Gastroesophageal reflux disease and asthma: a longitudinal study in UK general practice. Chest . 2005;128:85–93. doi: 10.1378/chest.128.1.85. [DOI] [PubMed] [Google Scholar]
- 53. Kim SY, Kim HR, Min C, Oh DJ, Park B, Choi HG. Bidirectional association between GERD and asthma in children: two longitudinal follow-up studies using a national sample cohort. Pediatr Res . 2020;88:320–324. doi: 10.1038/s41390-020-0749-1. [DOI] [PubMed] [Google Scholar]
- 54. Kim SY, Min C, Oh DJ, Choi HG. Bidirectional association between GERD and asthma: two longitudinal follow-up studies using a national sample cohort. J Allergy Clin Immunol Pract . 2020;8:1005–1013.e9. doi: 10.1016/j.jaip.2019.10.043. [DOI] [PubMed] [Google Scholar]
- 55. Barbas AS, Downing TE, Balsara KR, Tan HE, Rubinstein GJ, Holzknecht ZE, et al. Chronic aspiration shifts the immune response from Th1 to Th2 in a murine model of asthma. Eur J Clin Invest . 2008;38:596–602. doi: 10.1111/j.1365-2362.2008.01976.x. [DOI] [PubMed] [Google Scholar]
- 56. Saxena H, Deshpande DA, Tiegs BC, Yan H, Battafarano RJ, Burrows WM, et al. The GPCR OGR1 (GPR68) mediates diverse signalling and contraction of airway smooth muscle in response to small reductions in extracellular pH. Br J Pharmacol . 2012;166:981–990. doi: 10.1111/j.1476-5381.2011.01807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. An SS, Mitzner W, Tang WY, Ahn K, Yoon AR, Huang J, et al. An inflammation-independent contraction mechanophenotype of airway smooth muscle in asthma. J Allergy Clin Immunol . 2016;138:294–297.e4. doi: 10.1016/j.jaci.2015.12.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Han L, Limjunyawong N, Ru F, Li Z, Hall OJ, Steele H, et al. Mrgprs on vagal sensory neurons contribute to bronchoconstriction and airway hyper-responsiveness. Nat Neurosci . 2018;21:324–328. doi: 10.1038/s41593-018-0074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Canning BJ. Reflex regulation of airway smooth muscle tone. J Appl Physiol (1985) . 2006;101:971–985. doi: 10.1152/japplphysiol.00313.2006. [DOI] [PubMed] [Google Scholar]
- 60. Stein MR. Advances in the approach to gastroesophageal reflux (GER) and asthma. J Asthma . 1999;36:309–314. doi: 10.3109/02770909909068223. [DOI] [PubMed] [Google Scholar]
- 61. Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet . 2006;38:441–446. doi: 10.1038/ng1767. [DOI] [PubMed] [Google Scholar]
- 62. Palmer CN, Ismail T, Lee SP, Terron-Kwiatkowski A, Zhao Y, Liao H, et al. Filaggrin null mutations are associated with increased asthma severity in children and young adults. J Allergy Clin Immunol . 2007;120:64–68. doi: 10.1016/j.jaci.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 63. Rice NE, Patel BD, Lang IA, Kumari M, Frayling TM, Murray A, et al. Filaggrin gene mutations are associated with asthma and eczema in later life. J Allergy Clin Immunol . 2008;122:834–836. doi: 10.1016/j.jaci.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jacobson A, Yang D, Vella M, Chiu IM. The intestinal neuro-immune axis: crosstalk between neurons, immune cells, and microbes. Mucosal Immunol . 2021;14:555–565. doi: 10.1038/s41385-020-00368-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Enaud R, Prevel R, Ciarlo E, Beaufils F, Wieërs G, Guery B, et al. The gut-lung axis in health and respiratory diseases: a place for inter-organ and inter-kingdom crosstalks. Front Cell Infect Microbiol . 2020;10:9. doi: 10.3389/fcimb.2020.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Richardson TG, Hemani G, Gaunt TR, Relton CL, Davey Smith G. A transcriptome-wide Mendelian randomization study to uncover tissue-dependent regulatory mechanisms across the human phenome. Nat Commun . 2020;11:185. doi: 10.1038/s41467-019-13921-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zheng J, Haberland V, Baird D, Walker V, Haycock PC, Hurle MR, et al. Phenome-wide Mendelian randomization mapping the influence of the plasma proteome on complex diseases. Nat Genet . 2020;52:1122–1131. doi: 10.1038/s41588-020-0682-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Taylor K, Davey Smith G, Relton CL, Gaunt TR, Richardson TG. Prioritizing putative influential genes in cardiovascular disease susceptibility by applying tissue-specific Mendelian randomization. Genome Med . 2019;11:6. doi: 10.1186/s13073-019-0613-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hughes DA, Bacigalupe R, Wang J, Rühlemann MC, Tito RY, Falony G, et al. Genome-wide associations of human gut microbiome variation and implications for causal inference analyses. Nat Microbiol . 2020;5:1079–1087. doi: 10.1038/s41564-020-0743-8. [DOI] [PMC free article] [PubMed] [Google Scholar]