Abstract
Rationale
“Forgiveness” charts the ability of a drug or regimen to withstand nonadherence without negative clinical consequences.
Objectives
We aimed to determine the influence of regimen length, regimen drugs, and dosing, and when during treatment nonadherence occurs on the forgiveness of antituberculosis regimens.
Methods
Using data from three randomized controlled trials comparing experimental 4-month regimens for drug-sensitive tuberculosis with the standard 6-month regimen, we used generalized linear models to examine how the risk of a negative composite outcome changed as dose-taking decreased. The percentage of doses taken and the absolute number of doses missed were calculated during the intensive and continuation phases of treatment, and overall. A mediation analysis was undertaken to determine how much the association between intensive phase dose-taking and the negative composite outcome was mediated through continuation phase dose-taking.
Measurements and Main Results
Forgiveness of the 4- and 6-month regimens did not differ for any treatment period. Importantly, 4-month regimens were no less forgiving of small numbers of absolute missed doses than the 6-month regimen (e.g., for 3–7 missed doses vs. no missed doses [baseline], 6-month regimen adjusted risk ratio 1.65 [95% confidence interval, 0.80–3.41] and 4-month regimens 1.80 [1.33–2.45]). No 4-month regimen was conclusively more forgiving than another. We found evidence of mediation by continuation phase dose-taking on the intensive phase dose-taking and negative composite outcome relationship.
Conclusions
With the current appetite for, and progress toward, shorter drug-sensitive tuberculosis regimens worldwide, we offer reassurance that shorter regimens are not necessarily less forgiving of nonadherence. Given the importance of continuation phase adherence, patient support during this period should not be neglected.
Keywords: tuberculosis, forgiveness, adherence, nonadherence, treatment
At a Glance Commentary
Scientific Knowledge on the Subject
Nonadherence to tuberculosis treatment is known to be associated with a greater likelihood of a negative outcome. It is possible that the robustness (“forgiveness”) of shorter treatment regimens for missing even a single dose will be reduced versus longer regimens, as there are fewer doses within the regimen. In addition, regimens may be differentially robust toward missing doses during the intensive phase versus the continuation phase of treatment.
What This Study Adds to the Field
Reassuringly, we did not find a difference in the robustness of the 4- versus 6-month regimens included in this study to missing even small numbers of doses. The intensive phase was not found to be less robust than the continuation phase to nonadherence, despite the higher bacterial load expected in the former. The detrimental impact of missing doses during the intensive phase may be partly explained because these patients go on to miss doses during the continuation phase. Indeed, there will be common causes of missing doses in both periods that we could not adjust for in our modeling. Critically, the continuation phase of treatment should not be neglected when it comes to providing adherence-promoting support to patients.
Progress in reducing the length of treatment for drug-sensitive tuberculosis (TB) during the 20th century culminated in the observation that the use of rifampicin (R) and pyrazinamide (Z) could reduce the duration to 6 months (1). Since the mid-1980s, further reductions have been elusive. Various approaches have been taken, particularly the inclusion of fluoroquinolones in the regimen or increasing the dose of a rifamycin (2). Until the landmark results from Study 31/A5349 (that used both strategies) (3), no 4-month regimen had demonstrated noninferiority.
A frequently-used argument in favor of shortening treatment is that this will decrease the medication burden and thus the likelihood of nonadherence (4). This is for two reasons: 1) shorter regimens mean that the potential for early discontinuation is reduced (i.e., stoppage of medication earlier than initially prescribed); and 2) a shorter duration of treatment means that there is less time during which doses can be skipped (5). Conversely, shortening treatment may increase the relative importance of each dose, and thus missing even a single dose may be problematic (6).
A drug can be “forgiving” of missed doses if its duration of action extends from one dosing interval into the next (7). For example, if a drug is dosed daily and a dose is taken on Day 1 but missed on Day 2, a drug in which the duration of action is longer than 24 hours will be able to withstand this gap in dosing without negative clinical consequences. The drug composition of regimens, as well as dosing, can therefore alter forgiveness for regimens of different lengths (6). Improving regimen forgiveness is a complementary measure to adherence-promoting interventions to combat nonadherence.
Although nonadherence has been found to be strongly associated with negative outcomes from treatment for both 4- and 6-month anti-TB regimens (8), there has been limited research directly comparing the forgiveness of the 6-month and different 4-month regimens.
The phase of treatment in which nonadherence occurs may also be influential. Given the step-down in the number of drugs that participants take between the intensive and continuation phases and the expected reduction in bacterial load, both adherence behaviors and forgiveness may alter as participants progress through treatment.
In our study, we investigated three research gaps: the influence of 1) regimen length; 2) regimen drugs and dosing; and 3) treatment period on forgiveness for nonadherence, as follows: 1) by comparing the risk of a negative composite outcome (treatment failure, death, and recurrence or reinfection) when different percentages of doses are taken or absolute numbers of doses were missed of 4- versus 6-month regimens; 2) by comparing the risk of a negative composite outcome when different percentages of doses are taken in different 4-month regimens; and 3) by comparing the risk of a negative composite outcome when different percentages of doses are taken during the intensive versus continuation phases of treatment.
We used secondary data from three randomized controlled trials (RCTs) of treatment shortening for drug-sensitive TB; these provided high-quality, contemporary data for analysis from both 4- and 6-month treatment regimens. Some of the results of this study have been previously reported in the form of an abstract (9).
Methods
Parent Studies and Population for Analysis
Data for this study were obtained from the OFLOTUB, REMoxTB, and RIFAQUIN RCTs of 4-month fluoroquinolone-containing regimens versus 6-month regimens for drug-sensitive, newly diagnosed, smear-positive pulmonary TB (Table E1 in the online supplement) (10–12). The fluoroquinolones used were either moxifloxacin (M) or gatifloxacin (G). All studies used the standard short-course regimen of 2 months of isoniazid (H), R, Z, and ethambutol (E) followed by 4 months of HR (2HRZE/4HR) as the control regimen against which noninferiority of the 4-month regimens was assessed. We excluded the experimental 6-month regimen from RIFAQUIN and participants with an unknown regimen.
Data used in the preparation of this article were obtained from the CPTR (Critical Path to TB Drug Regimens) database. The CPTR initiative is a public–private partnership launched in March 2010 by C-Path (Critical Path Institute), BMGF (Bill and Melinda Gates Foundation), and TB Alliance (Global Alliance for TB Drug Development).
Measuring and Defining Nonadherence to Treatment
Nonadherence to treatment for TB was captured by direct observation/supervision of doses in all three RCTs (Table E1). In the available datasets, the greatest frequency at which dose-taking was reported was weekly (number of doses taken in 7 days), and the lowest frequency was dose-taking in the intensive or continuation phases (number of doses taken in each phase). Data on dose-taking by phase was thus common to all studies.
The percentage of doses taken was calculated across three “periods” (the intensive phase, continuation phase, and overall [the sum of the two phases]). These calculations took into account the frequency of dosing (Table E1) (i.e., percentage of doses taken for a given treatment period = t/p; in which t = the number of doses taken across the given treatment period and p = the number of doses prescribed across the given treatment period, a function of dosing frequency and regimen length). The absolute number of missed doses was also calculated for each of the three periods: absolute number of missed doses for a given treatment period = p – t. Specific data cleaning per trial is documented in Text E1.
Negative Composite Outcome
Broadly (Table E2), our definition of a negative composite outcome arising during or after treatment was taken from the primary efficacy analyses of the original RCTs (i.e., included treatment failed, relapse or recurrence or retreatment of TB, death during or after treatment, adverse events, and lost to follow-up).
In addition, as patients who died during treatment, were lost to follow-up, and had their regimen changed because of adverse events would have taken fewer doses of their treatment because dose-taking was not possible from the date of this event onwards; we also created a restricted negative composite outcome for sensitivity analyses. A negative outcome for this variable consisted of treatment failure (assessed at the end of treatment), relapse or recurrence or retreatment of TB after treatment, and death because of TB after treatment.
Other Variables
See Text E1.
Statistical Methods
Data cleaning and analyses were undertaken in Stata 15.1 and Stata 17. Table E3 documents all the models used.
Forgiveness of the 4- versus 6-month regimens (objective 1)
Objective 1 sought to compare the forgiveness of the 4- versus 6-month regimens for nonadherence measured as either 1) the percentage of doses taken (strictly, a measure of adherence rather than nonadherence); or 2) the absolute number of doses missed.
Generalized linear models with a log link, Gaussian distribution, and robust variance estimator were used to calculate risk ratios (RRs) at different degrees of nonadherence (percentages of doses taken, baseline 100%) for the negative composite outcome for both 4- and 6-month regimens (13). This method was chosen because of convergence issues using a binomial distribution; the robust variance estimator corrects the resulting standard errors. Marginal probabilities were used to calculate risks. Risk differences (RDs; identity link) were also determined. Risks, RRs, and RDs were all calculated from both unadjusted and adjusted models.
In addition to the exposure and outcome, unadjusted models included a three-degree fixed-effect for trial (as this presented a potential source of clustering) and the 4- versus 6-month regimen variable. Causal frameworks determined a priori the additional covariates for adjusted models (age, sex, ethnicity, HIV status and CD4 count, smear status, and cavitation at baseline). The most severe grouping of smear status was used as the default.
Percentage of doses taken
Percentage dose-taking was modeled using fractional polynomials to allow for a nonlinear effect (Text E1).
Within both unadjusted and adjusted multiplicative (RR) and additive (RD) models, the presence of an interaction between percentage dose-taking and the 4-versus 6-month regimen variable was assessed (Wald test).
Models were run separately for the exposures of percentage dose-taking overall, during the intensive phase, and during the continuation phase.
The following sensitivity analyses for the multiplicative models were undertaken for each period: given that the pill burden was greater among those of higher weight, we adjusted for participant weight at screening/baseline. An alternative coding of smear status at baseline (least severe grouping) was used. The impact of an alternative coding of OFLOTUB percentage dose-taking was also assessed (Text E1). Finally, models were rerun using the restricted negative composite outcome.
Absolute number of doses missed
These analyses used the absolute number of pills missed (categorical variable) as the exposure. Unadjusted and adjusted, multiplicative and additive models, were run for the absolute number of doses missed overall, during the intensive phase, and during the continuation phase. The presence of an interaction between the absolute number of doses missed and the 4- versus 6-month regimen variable was assessed.
In sensitivity analyses, these models were rerun using the restricted negative composite outcome.
Forgiveness of different 4-month regimens (objective 2)
Next, we sought to examine the combined impact of drugs and dosing on the forgiveness of the different 4-month regimens. Percentage dose-taking was used as the nonadherence measure. Unadjusted and adjusted, multiplicative and additive, models were run separately for the exposures percentage dose-taking overall, during the intensive phase, and during the continuation phase. The presence of an interaction between percentage dose-taking and the different regimens was assessed.
In sensitivity analyses, these models were rerun using the restricted negative composite outcome.
Forgiveness during each treatment phase (objective 3)
Here, we sought to examine the relative forgiveness of the intensive and continuation phases of treatment. Separately for the 4- and 6-month regimens, RRs for the negative composite outcome were calculated comparing >95%–100% (baseline) versus 0–95% dose-taking.
There is a known association between nonadherence during the intensive and continuation phases of treatment (i.e., individuals who adhere less well during the intensive phase are more likely to adhere less well during the continuation phase) (14), and it seemed likely that nonadherence in both phases would separately influence the likelihood of the negative composite outcome. We hypothesized that the total effect c of percentage dose-taking during the intensive phase of treatment on the risk of the negative composite outcome (as calculated above) is composed of both a direct effect (purely as a result of intensive phase percentage dose-taking, [c’]) and an indirect effect (intensive phase percentage dose-taking influencing continuation phase percentage dose-taking; a product of the pathways a and b, as indicated in Figure 1). This is called mediation.
Figure 1.
Hypothesized mediation model. The total effect c of the exposure E (intensive phase percentage dose-taking) on the outcome O (negative composite outcome) is composed of direct and indirect effects. The direct effect c’ measures the extent to which the risk of the negative composite outcome changes when intensive phase percentage dose-taking alters by one unit but the mediator variable M (continuation phase percentage dose-taking) is fixed. The indirect effect, a combination of a and b, measures the extent to which the risk of the negative composite outcome changes when intensive phase percentage dose-taking is fixed, and continuation phase percentage dose-taking changes by the amount it would have changed had intensive phase percentage dose-taking altered by one unit.
To examine the hypothesis that continuation phase percentage dose-taking is a mediator of the intensive phase percentage dose-taking ->negative composite outcome relationship, we used two approaches, 1) a ‘traditional’ approach comparing regression models with and without conditioning on the mediator and 2) the medeff package in Stata (Text E1) (15–17). For both methods, we grouped dose-taking as a binary variable.
Within the traditional approach, we approximated the direct effect c’ of intensive phase percentage dose-taking on the composite outcome by adjusting for continuation phase percentage dose-taking. We examined the association between intensive phase percentage dose-taking and continuation phase percentage dose-taking (path a) using a multiplicative model with continuation phase percentage dose-taking as the outcome and intensive phase dose-taking as the exposure. Path b was approximated by the RR for continuation phase percentage dose-taking on the composite outcome, including adjusting for intensive phase percentage dose-taking and/or culture status at 2 months. Models were run separately for the 6- and 4-month regimens. In sensitivity analyses, these models were rerun using the restricted negative composite outcome.
The use of medeff extended this analysis by including an interaction term between the two phases of percentage dose-taking and calculated the proportion of the total effect of intensive phase percentage dose-taking mediated through continuation phase percentage dose-taking (Text E1). In sensitivity analyses, these models were rerun using the restricted negative composite outcome.
Results
Characteristics of the Study Population
A total of 3,686 participants were available from the three RCTs and met the inclusion criteria for this study (Figure E1). A total of 1,565 received 6 months of treatment with 2HRZE/4HR, and 2,121 received 4 months of treatment with one of several regimens; 1,491 (95.3%) participants who received 6 months of treatment had nonadherence and outcome data, and 2,045 (96.4%) who received 4 months of treatment.
The characteristics of the study cohort are given in Table 1. A total of 2,473/3,536 included study participants (69.9%) were male. The median age was 29 years (interquartile range, 24–38), 3,026/3,536 (85.6%) were HIV-negative, participants overwhelmingly had smear-positive disease, and 2,153/3,418 (60.9%) had cavitation. Percentage dose-taking was very high for both 4- and 6-month regimens (median 100%, lowest decile 95%; median 100%, lowest decile 92%; respectively) and across all treatment periods (Table 1 and Figure E2). Within the cohort, 678/3,536 (19.2%) participants had a negative composite outcome (Table 1).
Table 1.
Characteristics of the Study Population, Excluding Participants Missing Outcome or Nonadherence Data
| Exposure Variables | Overall Cohort |
Negative Outcome |
||
|---|---|---|---|---|
| N | Col. % | n | Row % | |
| Overall | 3,536 | 100.0 | 678 | 19.2 |
| Overall percentage of doses taken, % | ||||
| 100 | 2,642 | 74.7 | 339 | 12.8 |
| >95–<100 | 479 | 13.5 | 78 | 16.3 |
| >90–95 | 139 | 3.9 | 40 | 28.8 |
| >85–90 | 31 | 0.9 | 7 | 22.6 |
| >80–85 | 24 | 0.7 | 10 | 41.7 |
| >60–80 | 59 | 1.7 | 43 | 72.9 |
| 0–60 | 162 | 4.6 | 161 | 99.4 |
| Intensive phase percentage of doses taken, % | ||||
| 100 | 3,015 | 85.3 | 464 | 15.4 |
| >95–<100 | 267 | 7.6 | 59 | 22.1 |
| >90–95 | 76 | 2.1 | 20 | 26.3 |
| >85–90 | 48 | 1.4 | 18 | 37.5 |
| >80–85 | 11 | 0.3 | 4 | 36.4 |
| >60–80 | 32 | 0.9 | 28 | 87.5 |
| 0–60 | 87 | 2.5 | 85 | 97.7 |
| Continuation phase percentage of doses taken, % | ||||
| 100 | 2,903 | 82.1 | 392 | 13.5 |
| >95–<100 | 222 | 6.3 | 32 | 14.4 |
| >90–95 | 64 | 1.8 | 13 | 20.3 |
| >85–90 | 85 | 2.4 | 20 | 23.5 |
| >80–85 | 14 | 0.4 | 5 | 35.7 |
| >60–80 | 45 | 1.3 | 22 | 48.9 |
| 0–60 | 203 | 5.7 | 194 | 95.6 |
| Length of treatment, mo | ||||
| 6 | 1,491 | 42.2 | 216 | 14.5 |
| 4 | 2,045 | 57.8 | 462 | 22.6 |
| Sex | ||||
| Male | 2,473 | 69.9 | 514 | 20.8 |
| Female | 1,063 | 30.1 | 164 | 15.4 |
| Age, yr | ||||
| Median (IQR) | 29 (24–38) | 32 (25–41) | ||
| 16–<26 | 1,204 | 34.0 | 187 | 15.5 |
| 26–<36 | 1,241 | 35.1 | 223 | 18.0 |
| 36–<46 | 644 | 18.2 | 157 | 24.4 |
| 46–<56 | 332 | 9.4 | 83 | 25.0 |
| 56–<66 | 86 | 2.4 | 20 | 23.3 |
| 66+ | 25 | 0.7 | 6 | 24.0 |
| Missing | 4 | 0.1 | 2 | 50.0 |
| Ethnicity* | ||||
| Black | 2,534 | 71.7 | 461 | 18.2 |
| Asian | 527 | 14.9 | 123 | 23.3 |
| Other | 475 | 13.4 | 94 | 19.8 |
| HIV status/CD4 count, cells/mm3 | ||||
| HIV-negative | 3,026 | 85.6 | 538 | 17.8 |
| HIV-positive, CD4 count <200 | 55 | 1.6 | 11 | 20.0 |
| HIV-positive, CD4 count 200–<500 | 274 | 7.7 | 75 | 27.4 |
| HIV-positive, CD4 count ⩾500 | 86 | 2.4 | 24 | 27.9 |
| Missing | 95 | 2.7 | 30 | 31.6 |
| Smear status, most severe† | ||||
| Negative | 47 | 1.3 | 18 | 38.3 |
| 1+ | 490 | 13.9 | 82 | 16.7 |
| 2+ | 798 | 22.6 | 113 | 14.2 |
| 3+ or more | 2,157 | 61.0 | 453 | 21.0 |
| Missing | 44 | 1.2 | 12 | 27.3 |
| Smear status, least severe† | ||||
| Negative | 234 | 6.6 | 59 | 25.2 |
| 1+ | 786 | 22.2 | 122 | 15.5 |
| 2+ | 785 | 22.2 | 106 | 13.5 |
| 3+ or more | 1,687 | 47.7 | 379 | 22.5 |
| Missing | 44 | 1.2 | 12 | 27.3 |
| Cavitation | ||||
| Yes | 2,153 | 60.9 | 422 | 19.6 |
| No | 1,161 | 32.8 | 202 | 17.4 |
| Missing | 222 | 6.3 | 54 | 24.3 |
| Weight, kg | ||||
| ⩽45 | 711 | 20.1 | 161 | 22.6 |
| >45–⩽50 | 728 | 20.6 | 141 | 19.4 |
| >50–⩽55 | 808 | 22.9 | 155 | 19.2 |
| >55–⩽70 | 1,168 | 33.0 | 197 | 16.9 |
| >70 | 121 | 3.4 | 24 | 19.8 |
| Culture status at 2 mo | ||||
| Negative | 2,618 | 74.0 | 376 | 14.4 |
| Positive | 625 | 17.7 | 159 | 25.4 |
| Missing | 293 | 8.3 | 143 | 48.8 |
Definition of abbreviations: CI = confidence interval; Col = column; IQR = interquartile range; N/A = not applicable.
Demographic and clinical characteristics of the study population at baseline (unless otherwise indicated) and nonadherence data, excluding individuals missing outcome or nonadherence data. Both smear and cavitation status were recorded at baseline.
Ethnicity imputed for OFLOTUB.
Two alternative groupings of smear status, one taking the most severe result recorded and one the least.
Forgiveness of the 4- versus 6-month Regimens (Objective 1)
Percentage of doses taken
For all three periods of treatment (overall, intensive, and continuation) and in both unadjusted and adjusted models, RRs (baseline 100% of doses taken) showed that the risk of a negative composite outcome increased steeply with reducing percentage dose-taking for both 4- and 6-month regimens (Table E4 and Figures 2B, 2E, and 2H). Comparing the RRs, 4-month regimens seemed more robust to missed doses than the 6-month regimen (Wald P values for test for interaction between regimens grouped by length and percentage of doses taken, all P < 0.0001). Examination of the marginal risks, however, demonstrated that even at 100% dose-taking, the 4-month regimens had a greater risk of a negative composite outcome than the 6-month regimen (Figures 2A, 2D, and 2G). As dose-taking reduced, the risk curves for the 4- and 6-month regimens started to converge; thus, in fact, the 4-month regimens were not more robust. RDs were similar for the 4- and 6-month regimens (Figure 2C, 2F, and 2I). Wald P values for test for interaction were: overall, 0.06; intensive phase, 0.06; and continuation phase, 0.07.
Figure 2.

Forgiveness of the 4- versus 6-month regimens. Adjusted marginal risks (A, D, and G), risk ratios (B, E, and H), and risk differences (C, F, and I) for the negative composite outcome by the percentage of doses taken (modeled as fractional polynomials of the functional form x3) across the entire treatment period (overall, [A–C]), intensive phase (D–F), and continuation phase (G–I) presented stratified by regimens grouped by length. One model per period of treatment, 4- and 6-month regimens in the same model. The baseline for the multiplicative and additive models is 100% of doses taken. For the multiplicative models, Wald P values for interaction between regimens grouped by length and percentage of doses taken, all P < 0.0001; horizontal dotted line charts a risk ratio of 1. For the additive models, Wald P values for interaction between regimens grouped by length and percentage of doses taken were 0.06 (overall), 0.06 (intensive phase), and 0.07 (continuation phase); horizontal dotted line charts a risk difference of 0. All models adjusted for sex, age (fitted using a fractional polynomial), ethnicity, HIV and CD4 status, smear status at baseline (most severe), cavitation at baseline, and a three-degree fixed-effect for trial. All models contain data for 3,180 participants. Data presented for 80–100% of doses taken because of data sparsity at lower degrees, but the full range of values was included in the statistical models. Panels (A and B) from model 3; (C) from model 4; (D and E) from model 7; (F) model 8; (G and H) from model 11; and (I) model 12. aRD = adjusted risk difference; aRisk = adjusted risk; aRR = adjusted risk ratio; CI = confidence interval.
Sensitivity analyses (weight, smear status, and alternative coding of percentage dose-taking) gave similar results (Tables E5–E7). The use of the restricted negative composite outcome reduced the number of negative outcomes to 399; there were too few to fit fractional polynomials. Instead, percentage dose-taking was grouped in 5% categories and used as the (linear) exposure. As expected, the effect estimates were reduced in these models. These results also suggested that the 4-month regimens were no more or less robust to lower degrees of dose-taking than the 6-month regimen (Table E8).
Absolute number of doses missed
The 4-month regimens appeared no less robust to small absolute numbers of missed doses than the 6-month regimen across any period of treatment (Table E9). This also held true in the sensitivity analysis using the restricted negative composite outcome (Table E10).
Forgiveness of Different 4-month Regimens (Objective 2)
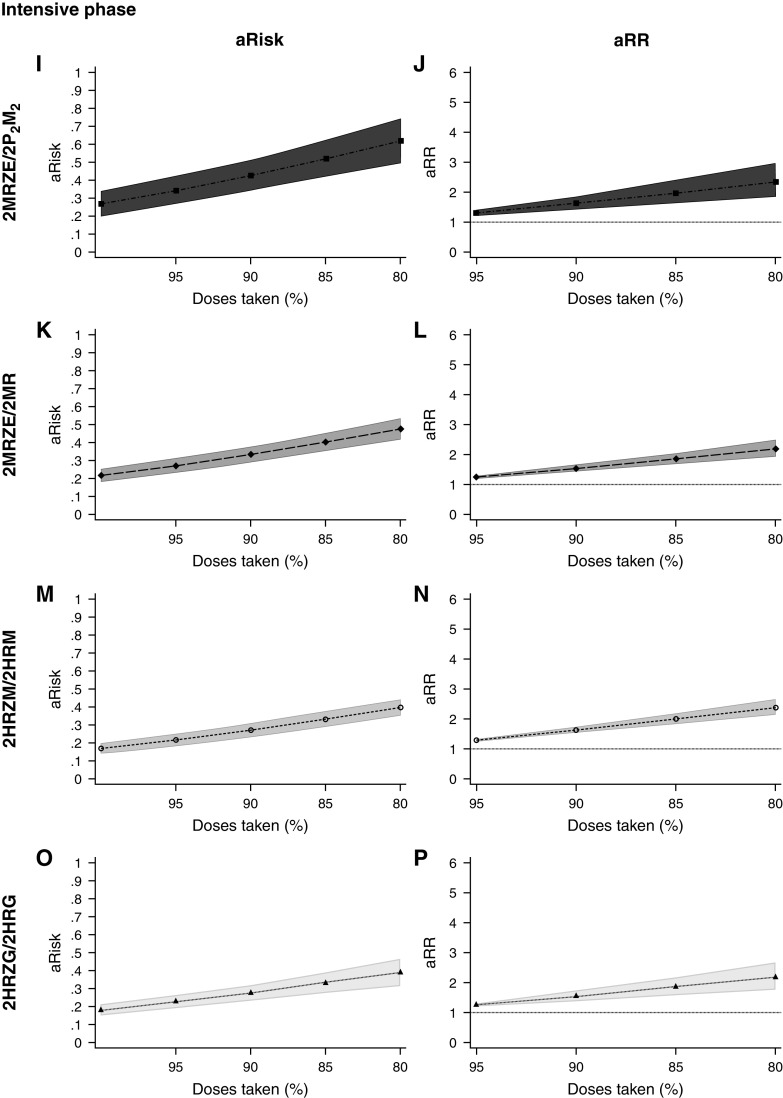
The M and rifapentine (P) regimen dosed twice-weekly during the second half of treatment (2MRZE/2P2M2) appeared potentially more forgiving than other 4-month regimens on the multiplicative scale in the continuation phase (Wald P value for interaction, 0.004) but had a greater marginal risk of a negative composite outcome even at 100% dose-taking than the other regimens (Figure 3, Table E11, and Figure E3). The larger marginal risk for 2MRZE/2P2M2 was also seen for the overall and intensive phase models, but there was no evidence of differing effects of dose-taking by different regimens on the additive or multiplicative scale for these periods.
Figure 3.
Forgiveness of different 4-month regimens. Adjusted risks (A, C, E, G, I, K, M, O, Q, S, U, and W) and risk ratios (B, D, F, H, J, L, N, P, R, T, V, and X) for the negative composite outcome by the percentage of doses taken (modeled as fractional polynomials of the functional form x3) across the entire treatment period (A–H), intensive phase (I–P), and continuation phase (Q–X), stratified by 4-month regimen. The baseline for multiplicative and additive models is 100% dose-taking. One model per period of treatment, all 4-month regimens in the same model. For the multiplicative models, Wald P values for interaction between regimens grouped by length and percentage of doses taken were 0.10 (overall), 0.76 (intensive phase), and 0.004 (continuation phase); horizontal dotted line charts a risk ratio of 1. For the additive models, Wald P values for interaction between regimens grouped by length and percentage of doses taken were 0.84 (overall), 0.50 (intensive phase), and 0.004 (continuation phase); horizontal dotted line charts a risk difference of 0. Models adjusted for sex, age (fitted using a fractional polynomial), ethnicity, HIV and CD4 status, smear status at baseline (most severe), and cavitation at baseline. No adjustment for study because of collinearity with the regimen. Models contain data for 1,837 participants. Data presented for 80–100% of doses taken because of data sparsity at lower degrees, but the full range of values was included in the statistical models. Overall treatment from model 34; intensive phase from model 37; continuation phase from model 40. 2 = twice weekly dosing; aRisk = adjusted risk; aRR = adjusted risk ratio; CI = confidence interval; E = ethambutol; G = gatifloxacin; H = isoniazid; M = moxifloxacin; P = rifapentine; R = rifampicin; Z = pyrazinamide.
In the sensitivity analysis using the restricted negative composite outcome, differences between regimens were not detected; data were sparse (Table E12).
Forgiveness During Each Treatment Phase (Objective 3)
In models unadjusted for percentage dose-taking in the other phase of treatment, the association between percentage dose-taking (grouped as 0–95% vs. >95%–100%, latter baseline) and the risk of a negative composite outcome for the 6-month regimen was: intensive phase adjusted risk ratio (aRR), 5.75 (95% confidence interval [CI], 4.13–8.00) and continuation phase aRR, 10.23 (95% CI, 7.70–13.59) (Figure 4A). The marginal risks at >95%–100% dose-taking were 0.10 (95% CI, 0.08–0.12) and 0.06 (95% CI, 0.05–0.08), respectively. For the 4-month regimens, estimates were intensive phase aRR, 3.06 (95% CI, 2.57–3.63) and continuation phase aRR, 3.59 (95% CI, 3.07–4.19) (Figure 4B). The marginal risks at >95%–100% dose-taking were 0.19 (95% CI, 0.17–0.21) and 0.18 (95% CI, 0.16–0.20), respectively.
Figure 4.

Forgiveness during each treatment phase. To compare forgiveness during the two treatment phases, intensive phase and continuation phase percentage dose-taking were categorized into 0–95% versus >95%–100% (baseline) and adjusted risk ratios calculated for (A) the 6-month regimen and (B) the 4-month regimens, as follows: 1) intensive phase dose-taking was the exposure and continuation phase dose-taking the outcome (models 51 and 52); 2) continuation phase dose-taking was the exposure and the negative composite outcome the outcome (models 53, 55, 57, 59, 61, 63, 65, and 67); and 3) intensive phase dose-taking was the exposure and negative composite outcome the outcome (models 43, 45, 47, and 49). Results from models 1) and 3) are presented without (*) and with (**) adjustment for dose-taking during the other treatment phase, assuming no interaction. For model 2), results are also presented with (^) adjustment for culture status at 2 months. Models adjusted for sex, age (fitted using a fractional polynomial), ethnicity, HIV and CD4 status, smear status at baseline (most severe), cavitation at baseline, and a three-degree fixed-effect for trial. aRR = adjusted risk ratio; CI = confidence interval.
Adjustment of the intensive phase models for percentage dose-taking during the continuation phase (an estimate of the direct effect, c’) resulted in all aRRs being attenuated toward one, a 74% reduction for the 6-month regimen (5.75–1.52) and a 44% reduction for the 4-month regimen (3.06–1.71). Adjustment of the continuation phase models for percentage dose-taking during the intensive phase and/or culture status at 2 months (indicated by ** and ^ in Figure 4) made a relatively minimal difference to the effect estimates.
Furthermore, a strong association was detected between dose-taking in the intensive phase and continuation phase for both regimen lengths. These data suggested that continuation phase percentage dose-taking was a mediator of the intensive phase percentage dose-taking negative composite outcome association.
In the sensitivity analysis using the restricted negative composite outcome, for the 6-month regimen the estimates for the association between dose-taking and the negative composite outcome were more similar between the intensive and continuation phases than previously (Figure E4). For the 4-month regimen, the estimates were very similar.
Allowing for a potential interaction between percentage dose-taking in the two phases using medeff, the direct effects (c’) indicated a small remaining increase in the odds of a negative composite outcome if intensive phase dose-taking changed from >95%–100% to 0–95% but continuation phase dose-taking was fixed (Table 2), which was in line with the traditional analysis results (Figure 4). Also, in line with the analyses above, for the 6-month regimen, 64% (95% CI, 49–90%) of the total effect of intensive phase dose-taking was because of the impact dose-taking during this phase had on dose-taking during the continuation phase. These figures were 51% (95% CI, 42–66%) for the 4-month regimen.
Table 2.
Forgiveness During Each Treatment Phase: Mediation Analysis
| Regimens Grouped by Length | Direct Effect 0 | Indirect Effect 1 | Direct Effect 1 | Indirect Effect 0 | Proportion of Total Effect Mediated |
|---|---|---|---|---|---|
| 6-mo | 1.12 (1.01–1.32) | 1.34 (1.19–1.50) | 1.20 (1.07–1.34) | 1.25 (1.17–1.34) | 0.64 (0.49–0.90) |
| 4-mo | 1.15 (1.04–1.27) | 1.30 (1.21–1.40) | 1.29 (1.18–1.41) | 1.16 (1.10–1.23) | 0.51 (0.42–0.66) |
Direct effects and indirect effects are expressed as odds ratios and (95% confidence intervals); 0–95% versus >95%–100% (baseline) dose-taking compared. Direct effect 0: how much the risk of the negative composite outcome would change if intensive phase dose-taking changed from >95%–100% to 0–95% but, for each individual, continuation phase dose-taking was fixed at the degree it would have taken, for that individual, when intensive phase dose-taking was >95%–100%. Direct effect 1: as per direct effect 0, but when continuation phase dose-taking is fixed at the degree it would have taken, for that individual, when intensive phase dose-taking (exposure) was ⩽95%. Indirect effect 0: how much the outcome would change, on average, if intensive phase dose-taking was fixed at >95%–100%, but continuation phase dose-taking changed from the degree it would take if intensive phase dose-taking was >95%–100% to if intensive phase dose-taking was ⩽95%. Indirect effect 1: as per indirect effect 0, but when intensive phase dose-taking fixed at ⩽95%. Models adjusted for sex, age (fitted using a fractional polynomial), ethnicity, HIV and CD4 status, smear status at baseline (most severe), cavitation at baseline, and a three-degree fixed-effect for trial.
In the sensitivity analysis for the medeff analyses using the restricted composite negative outcome, the percentage of the total effect of intensive phase dose-taking because of the impact dose-taking during this phase had on dose-taking during the continuation phase was reduced to 11% (5–73%) for the 6-month regimen and to 1% (1–8%) for the 4-month regimen (Table E13).
Discussion
In this study of nonadherence data from three RCTs, we did not find a difference in the forgiveness of (i.e., the robustness of) the included 4-month regimens versus the 6-month regimen 2HRZE/4HR to different degrees of percentage dose-taking across any period of treatment, or to lower numbers of absolute missed doses (objective 1). Even at 100% dose-taking, the 4-month regimens had a higher risk of a negative composite outcome. Among the 4-month regimens, none convincingly appeared differentially forgiving of lower degrees of percentage dose-taking (objective 2). The intensive phase of treatment may be more robust to different degrees of percentage dose-taking than the continuation phase for the 6-month regimen (although we note the limitations of comparing nonnested models), and more than 50% of the intensive phase dose-taking effect on the risk of a negative composite outcome was found to be mediated through continuation phase dose-taking (objective 3). In sensitivity analyses restricting the definition of a negative composite outcome to avoid overemphasizing the dose-taking and negative composite outcomes relationship, we observed greater similarity between the two phases and less mediation than before. We note that this restricted definition, although useful, is not the complete picture of the dose-taking and outcomes relationship as, for example, it does not account for the impact of dose-taking on the likelihood of death during treatment.
Our objective 3 findings have interesting implications for adherence support during the treatment course for TB participants. Importantly, stepping down nonadherence monitoring and promotion efforts during the continuation phase would likely be detrimental, even if the patient has done well to date. Indeed, close healthcare worker engagement across the full treatment period is important given how (often fluctuating) life events can derail treatment (18). As degrees of nonadherence can be linked between the two phases of treatment, it will be important to establish good and lasting habits and relationships between participants and healthcare workers early on (19). Previous pharmacokinetics-pharmacodynamics simulations have highlighted the importance of good adherence during the intensive phase of treatment (20); moving forward, understanding how such models translate to population degree effects and the common causes of nonadherence and negative treatment outcomes will be critical.
Within objectives 1 and 2, we found that the different regimens were no more or less forgiving than each other; this was particularly encouraging for the 4-month regimens when the absolute number of doses missed was analyzed. Within the recently published Study 31/A5349, the exclusion of participants with at least 5% or 25% nonadherence suggested a potential shift in the effect estimates in favor of the 6-month regimen 2HRZE/4HR (3); future work to examine the relative forgiveness of 2HPZM/2PHM versus 2HRZE/4HR would be pertinent.
This is the first study of its kind to examine in depth the relationship between nonadherence and outcomes in TB. A major strength was the availability of large datasets of nonadherence and outcomes data from three RCTs that tested different treatment regimens. Our analyses could have been improved by the availability of daily nonadherence data to allow assessment of the implications of different nonadherence patterns (6, 21). Dose-taking within these trials was very high, so relatively few data points were available to fit the fractional polynomials at low dose-taking concentrations, resulting in lower statistical certainty. As the 4-month regimens were specific to each trial (although REMoxTB had two), these regimens may be acting as a proxy for trial in the analyses. Outcomes were measured from the time of randomization, which may have disadvantaged the 4-month regimens, as they had greater time after treatment during which relapse could occur.
We were unable to adjust for postrandomization risk factors for nonadherence (22). Incomplete adjustment for the propensity of participants to adhere to treatment (known to be influenced by a complex dynamic of economic, structural, patient-related, regimen, health provider, and healthcare delivery factors) (23) could be influencing both the regression and mediation analyses. For the latter, this would overestimate the association between nonadherence in the intensive and continuation phases, leading to the degree of mediation being overemphasized. We note that the large number of factors influencing the propensity to adhere means confounding has rarely been fully adjusted for in observational studies in this area (24).
There is substantial interest globally in shortening treatment for drug-sensitive TB from 6 months to 4 as this may decrease degrees of nonadherence. Critically, our study suggests that even 4-month regimens previously found to be inferior to 2HRZE/4HR (at least in specific population groups) (8) are no more susceptible to absolute small numbers of missed doses than the standard 6-month regimen. Work to better understand 1) the most important nonadherence patterns for the risk of negative outcomes; 2) how common these patterns are and where/in whom they occur; and 3) if some regimens are more forgiving of important and common nonadherence patterns, may aid decisions about how to deploy different regimens globally. (Indeed, the importance of documenting and analyzing different nonadherence patterns is part of the World Health Organization’s position statement on innovative trial design (25).) Such studies can also inform discussions about relative investment in interventions to prevent nonadherence versus regimens that are forgiving of nonadherence.
Conclusions
With the current appetite for, and progress toward, shorter drug-sensitive tuberculosis regimens worldwide, we offer reassurance that shorter regimens do not necessarily equate to higher vulnerability to nonadherence. The importance of continuation phase adherence should not be underestimated, of which clinical and public health programs should be mindful. As new regimens for drug-sensitive TB (and indeed, drug-resistant TB) are formulated and trialed, detailed consideration of forgiveness and its interplay with pharmacokinetics will be important to maximize operational efficacy.
Acknowledgments
Acknowledgment
The authors wish to acknowledge Elizabeth F. Walker for additional code verification as part of the analysis for this work, Anna Schauer for the background research that inspired this paper, and Charlotte Jackson for her thoughtful and helpful comments on a draft of the manuscript. Data used in the preparation of this article were obtained from the Critical Path to TB Drug Regimens (CPTR) Database. The CPTR initiative is a public–private partnership launched in March 2010 by Critical Path Institute (C-Path), the Bill & Melinda Gates Foundation (BMGF), and the Global Alliance for TB Drug Development (TB Alliance). The authors thank the participants in the included trials, the staff at the trial sites, all of the trial investigators, and the data management team at the Critical Path Institute.
Footnotes
Supported by United Kingdom Research and Innovation (the United Kingdom Medical Research Council [MRC]) (MR/R008345/1) (H.R.S. and M.F.); the National Institute for Health Research (NIHR) Health Technology Assessment Programme, United Kingdom (16/88/06) (H.R.S. and M.C.I.L.); J.A.T. is jointly funded by the United Kingdom MRC and the United Kingdom Foreign, Commonwealth, and Development Office (FCDO) under the MRC/FCDO Concordat agreement and is also part of the European & Developing Countries Clinical Trials Partnership (EDCTP2) program supported by the European Union (grant ref: MR/R010161/1); K.L.F. is partly funded by the United Kingdom MRC and the United Kingdom FCDO under the MRC/FCDO Concordat agreement and is also part of the EDCTP2 program supported by the European Union (grant ref: MR/R010161/1). The views expressed are those of the authors and not necessarily those of the National Health Service, United Kingdom, the NIHR, or the Department of Health and Social Care.
Author Contributions: H.R.S., K.L.F., and J.A.T. conceptualized and designed the work. H.R.S. analyzed the data with methodological guidance from K.L.F. and J.A.T. and verification by M.F. All authors interpreted the data for the work. H.R.S. drafted the work, and all other authors revised it critically for important intellectual content. All authors give final approval of the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors had full access to all the data in the study and accept responsibility to submit for publication.
Data used in the preparation of this article were obtained from the Critical Path to TB Drug Regimens (CPTR) Database. The CPTR initiative is a public–private partnership launched in March 2010 by Critical Path Institute (C-Path), the Bill and Melinda Gates Foundation (BMGF), and the Global Alliance for TB Drug Development (TB Alliance).
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202201-0144OC on August 11, 2022
Author disclosures are available with the text of this article at www.atsjournals.org
References
- 1. Iseman MD. Tuberculosis therapy: past, present and future. Eur Respir J Suppl . 2002;36:87s–94s. doi: 10.1183/09031936.02.00309102. [DOI] [PubMed] [Google Scholar]
- 2. Lee A, Xie YL, Barry CE, Chen RY. Current and future treatments for tuberculosis. BMJ . 2020;368:m216. doi: 10.1136/bmj.m216. [DOI] [PubMed] [Google Scholar]
- 3. Dorman SE, Nahid P, Kurbatova EV, Phillips PPJ, Bryant K, Dooley KE, et al. AIDS Clinical Trials Group; Tuberculosis Trials Consortium Four-month rifapentine regimens with or without moxifloxacin for tuberculosis. N Engl J Med . 2021;384:1705–1718. doi: 10.1056/NEJMoa2033400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zumla AI, Gillespie SH, Hoelscher M, Philips PP, Cole ST, Abubakar I, et al. New antituberculosis drugs, regimens, and adjunct therapies: needs, advances, and future prospects. Lancet Infect Dis . 2014;14:327–340. doi: 10.1016/S1473-3099(13)70328-1. [DOI] [PubMed] [Google Scholar]
- 5. Munro SA, Lewin SA, Smith HJ, Engel ME, Fretheim A, Volmink J. Patient adherence to tuberculosis treatment: a systematic review of qualitative research. PLoS Med . 2007;4:e238. doi: 10.1371/journal.pmed.0040238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stagg HR, Flook M, Martinecz A, Kielmann K, Abel Zur Wiesch P, Karat AS, et al. All nonadherence is equal but is some more equal than others? Tuberculosis in the digital era. ERJ Open Res . 2020;6:00315–2020. doi: 10.1183/23120541.00315-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Urquhart J. The electronic medication event monitor. Lessons for pharmacotherapy. Clin Pharmacokinet . 1997;32:345–356. doi: 10.2165/00003088-199732050-00001. [DOI] [PubMed] [Google Scholar]
- 8. Imperial MZ, Nahid P, Phillips PPJ, Davies GR, Fielding K, Hanna D, et al. A patient-level pooled analysis of treatment-shortening regimens for drug-susceptible pulmonary tuberculosis. Nat Med . 2018;24:1708–1715. doi: 10.1038/s41591-018-0224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stagg HR, Flook M, Fielding K. LB-2046-24 temporal non-adherence and TB treatment outcomes? ‘O art of subtlety and secrecy!’. Int J Tuberc Lung Dis . 2020;24:S408. [Google Scholar]
- 10. Gillespie SH, Crook AM, McHugh TD, Mendel CM, Meredith SK, Murray SR, et al. REMoxTB Consortium Four-month moxifloxacin-based regimens for drug-sensitive tuberculosis. N Engl J Med . 2014;371:1577–1587. doi: 10.1056/NEJMoa1407426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jindani A, Harrison TS, Nunn AJ, Phillips PP, Churchyard GJ, Charalambous S, et al. RIFAQUIN Trial Team High-dose rifapentine with moxifloxacin for pulmonary tuberculosis. N Engl J Med . 2014;371:1599–1608. doi: 10.1056/NEJMoa1314210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Merle CS, Fielding K, Sow OB, Gninafon M, Lo MB, Mthiyane T, et al. OFLOTUB/Gatifloxacin for Tuberculosis Project A four-month gatifloxacin-containing regimen for treating tuberculosis. N Engl J Med . 2014;371:1588–1598. doi: 10.1056/NEJMoa1315817. [DOI] [PubMed] [Google Scholar]
- 13. Cummings P. Methods for estimating adjusted risk ratios. Stata J . 2009;9:175–196. [Google Scholar]
- 14. Stagg HR, Lewis JJ, Liu X, Huan S, Jiang S, Chin DP, et al. Temporal factors and missed doses of tuberculosis treatment. A causal associations approach to analyses of digital adherence data. Ann Am Thorac Soc . 2020;17:438–449. doi: 10.1513/AnnalsATS.201905-394OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hicks R, Tingley D. Causal mediation analysis. Stata J . 2011;11:605–619. [Google Scholar]
- 16. Imai K, Keele L, Tingley D. A general approach to causal mediation analysis. Psychol Methods . 2010;15:309–334. doi: 10.1037/a0020761. [DOI] [PubMed] [Google Scholar]
- 17. Imai K, Keele L, Yamamoto T. Identification, inference, and sensitivity analysis for causal mediation effects. Stat Sci . 2010;25:51–71. [Google Scholar]
- 18. Kielmann K, Vidal N, Riekstina V, Krutikov M, van der Werf MJ, Biraua E, et al. “Treatment is of primary importance, and social assistance is secondary”: a qualitative study on the organisation of tuberculosis (TB) care and patients’ experience of starting and staying on TB treatment in Riga, Latvia. PLoS One . 2018;13:e0203937. doi: 10.1371/journal.pone.0203937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Karumbi J, Garner P. Directly observed therapy for treating tuberculosis. Cochrane Database Syst Rev . 2015:CD003343. doi: 10.1002/14651858.CD003343.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fors J, Strydom N, Fox WS, Keizer RJ, Savic RM. Mathematical model and tool to explore shorter multi-drug therapy options for active pulmonary tuberculosis. PLOS Comput Biol . 2020;16:e1008107. doi: 10.1371/journal.pcbi.1008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vernon A, Fielding K, Savic R, Dodd L, Nahid P. The importance of adherence in tuberculosis treatment clinical trials and its relevance in explanatory and pragmatic trials. PLoS Med . 2019;16:e1002884. doi: 10.1371/journal.pmed.1002884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Murray EJ, Hernán MA. Improved adherence adjustment in the coronary drug project. Trials . 2018;19:158. doi: 10.1186/s13063-018-2519-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization. 2003. https://apps.who.int/iris/handle/10665/42682
- 24. Jones ASK, Bidad N, Horne R, Stagg HR, Wurie FB, Kielmann K, et al. Determinants of non-adherence to anti-TB treatment in high income, low TB incidence settings: a scoping review. Int J Tuberc Lung Dis . 2021;25:483–490. doi: 10.5588/ijtld.21.0024. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization. 2021. https://www.who.int/publications/i/item/9789240030800