Abstract
Background:
Trifluridine/tipiracil plus bevacizumab (FTD/TPI + BEV) has shown efficacy and tolerability in refractory metastatic colorectal cancer (mCRC). Because randomized controlled trial (RCT) data comparing FTD/TPI + BEV with FTD/TPI are lacking, this meta-analysis evaluated outcomes with both regimens.
Data Sources and Methods:
Electronic databases, congress proceedings (past 3 years), trial registries, systematic review bibliographies, gray literature, and guidelines through June 2021 were searched for RCTs, non-RCTs, and prospective observational studies involving >20 previously treated patients with mCRC receiving FTD/TPI + BEV or FTD/TPI. Absolute and relative disease control rate (DCR), progression-free survival (PFS), overall survival (OS), adverse event (AE) rates, and discontinuation rates due to AEs were evaluated using fixed-effects and random-effects models. Study quality, heterogeneity, and publication bias were assessed.
Results:
In all, 29 of 875 screened publications were selected (26 studies: 5 RCTs, 11 non-RCTs, and 10 prospective observational studies). One RCT compared FTD/TPI + BEV with FTD/TPI. FTD/TPI + BEV versus FTD/TPI had a higher absolute DCR [64% (6 studies; n = 289) versus 43% (10 studies; n = 2809)], median PFS [4.2 (5 studies; n = 244) versus 2.6 (6 studies; n = 1781) months], 12-month PFS [9% (5 studies; n = 244) versus 3% (6 studies; n = 1781)], median OS [9.8 (5 studies; n = 244) versus 8.1 (6 studies; n = 1814) months], and 12-month OS [38% (5 studies; n = 244) versus 32% (6 studies; n = 1814)]. Grade ⩾3 febrile neutropenia, asthenia/fatigue, diarrhea, nausea, and vomiting rates were similar (1%–7%). Grade ⩾3 neutropenia rate was higher with FTD/TPI + BEV than with FTD/TPI [43% (6 studies; n = 294) versus 29% (12 studies; n = 7139)]. Discontinuation rates due to AEs were similar [8% (5 studies; n = 244) and 7% (10 studies; n = 3724)]. Low study quality, heterogeneity, and/or publication bias were detected in certain instances.
Conclusion:
Despite fewer patients treated with the combination, this meta-analysis consistently suggested that FTD/TPI + BEV provides benefits over FTD/TPI in refractory mCRC and has similar safety, except for more frequent grade ⩾3 neutropenia.
Keywords: bevacizumab, meta-analysis, metastatic colorectal cancer, overall survival, progression-free survival, safety, trifluridine/tipiracil
Highlights
This meta-analysis evaluated efficacy and safety outcomes with trifluridine/tipiracil plus bevacizumab (FTD/TPI + BEV) and FTD/TPI monotherapy in patients with refractory metastatic colorectal cancer (mCRC).
The results suggest that FTD/TPI + BEV provides benefits over FTD/TPI monotherapy in patients with refractory mCRC and has a similar safety profile, except for a higher rate of grade ⩾3 neutropenia.
Given the lack of data from large randomized controlled trials comparing FTD/TPI + BEV with FTD/TPI monotherapy in this population, this meta-analysis may help guide treatment selection.
Introduction
Colorectal cancer (CRC) is the third most common cancer and the second leading cause of cancer-related mortality worldwide, accounting for more than 1.9 million new cases and 935,000 deaths in 2020.1 Approximately 20% of newly diagnosed patients have metastatic CRC (mCRC),2 and 70% of patients with CRC overall will ultimately experience metastatic relapse.3 Patients with mCRC have a poor prognosis, with a median overall survival (OS) of approximately 30 months from initiation of first-line systemic therapy4,5 and a 5-year relative survival of less than 15%.2 Although systemic treatments such as chemotherapy, targeted therapy, immunotherapy, and their combinations have improved OS in these patients over the last decade, more effective therapeutic approaches are needed.4–6
Trifluridine/tipiracil (FTD/TPI), an oral cytotoxic chemotherapy consisting of a thymidine-based nucleoside analog (trifluridine) and a thymidine phosphorylase inhibitor (tipiracil), has demonstrated improved clinical efficacy with a manageable safety profile in patients with heavily pretreated mCRC7,8 and is a standard-of-care treatment for refractory mCRC.4–6 FTD/TPI has a unique mechanism of action that involves incorporation of phosphorylated FTD into DNA (resulting in DNA dysfunction) and inhibition of FTD degradation by thymidine phosphorylase with coadministration of TPI (resulting in increased FTD bioavailability).9 In the phase III RECOURSE trial, FTD/TPI administered beyond second-line treatment was associated with a significant improvement in OS compared with placebo [median OS, 7.1 versus 5.3 months; hazard ratio (HR), 0.68; 95% confidence interval (CI), 0.58–0.81; p < 0.001] in patients with refractory mCRC.8 The most common grade 3–4 adverse events (AEs) with FTD/TPI in that study were neutropenia (38%) and leukopenia (21%). In a subgroup analysis of the RECOURSE trial data, FTD/TPI was shown to be effective regardless of age, geographic region, or KRAS mutation status.10
Findings from preclinical and clinical studies suggest that the combination of FTD/TPI plus bevacizumab (FTD/TPI + BEV; a vascular endothelial growth factor inhibitor) is a feasible treatment option for patients with CRC.11,12 FTD/TPI + BEV has been shown to enhance antitumor activity in human CRC xenografts compared with either treatment alone; phosphorylated FTD levels increased when FTD/TPI was combined with BEV in these tumor models, suggesting that BEV might facilitate FTD accumulation in tumor cell DNA.11 Although FTD/TDI is most commonly used as a single-agent treatment in clinical practice, data from a smaller randomized controlled trial (RCT), the phase II EudraCT trial (n = 93), showed benefits with FTD/TPI + BEV compared with FTD/TPI monotherapy in patients with refractory mCRC.12 In that trial, median progression-free survival (PFS) was 2.6 months with FTD/TPI monotherapy and 4.6 months with FTD/TPI + BEV (HR, 0.45; 95% CI, 0.29–0.72; p = 0.0015), and median OS was 6.7 months with FTD/TPI monotherapy and 9.4 months with FTD/TPI + BEV (HR, 0.55; 95% CI, 0.32–0.94; p = 0.028).12 FTD/TPI + BEV is recommended by the National Comprehensive Cancer Network Clinical Practice Guidelines on Oncology (NCCN Guidelines®) as an option for treating patients with refractory mCRC.13,14 Given the lack of data from larger RCTs comparing FTD/TPI + BEV with FTD/TPI monotherapy in patients with mCRC, we conducted this meta-analysis to evaluate efficacy and safety outcomes with the two regimens.
Methods
Data sources
A meta-analysis was conducted using studies identified in a systematic literature review. The systematic literature review involved searching electronic databases (MEDLINE®, Embase®, and Cochrane Library databases); congress proceedings (the American Society of Clinical Oncology, European Society of Medical Oncology, and American Association for Cancer Research) for the past 3 years; clinical trial registries (Clinicaltrials.gov and UMIN registry); systematic review bibliographies; gray literature; and clinical guidelines through June 2021 to identify studies involving patients with mCRC treated with FTD/TPI + BEV or FTD/TPI monotherapy. Specific inclusion and exclusion criteria were applied as a two-stage screening process to identify relevant publications (Supplemental Table 1); the electronic search strategy used for one of the databases, MEDLINE®, is shown in Supplemental Table 2. In the first stage, abstracts returned by the search strategy were examined independently by two researchers and screened based on the inclusion and exclusion criteria. In the event of any conflict, a third independent reviewer was consulted whose decision would be considered final. In the second stage, full texts of all the studies included in the first stage were obtained. Two reviewers examined these independently for inclusion or exclusion, and disagreements were resolved by a third independent reviewer, whose decision was considered final.
Quantitative synthesis
A feasibility analysis was carried out to evaluate the possibility of data synthesis for relevant outcomes based on study design, patient setting, and consistency in reporting outcomes across studies. Based on the composition of the identified studies, the quantitative synthesis was limited to previously treated patients with mCRC in RCTs, non-RCTs, and prospective observational studies. Only studies with a sample size of more than 20 patients were analyzed in the quantitative synthesis. In addition, only patients who were treated with FTD/TPI + BEV or FTD/TPI monotherapy were included in the meta-analysis; patients who received placebo were excluded. Data from the included publications were extracted by one reviewer. To identify and rectify any errors in data extraction, a second reviewer checked and validated all the data by conducting an independent internal data check.
Outcomes
Outcomes evaluated in this meta-analysis included objective response rate (ORR), disease control rate (DCR), PFS, OS, AE rates, and discontinuation rates due to AEs. Grade ⩾3 neutropenia, febrile neutropenia, asthenia/fatigue, diarrhea, nausea, and vomiting were reported in most studies; therefore, these AEs were used in the safety analysis. Because neutropenia is frequently observed during the 2-week rest period after initiation of FTD/TPI treatment15 and granulocyte colony-stimulating factor (G-CSF) is often used to treat neutropenia, data were collected for AE monitoring schedule and G-CSF use to determine if cases of neutropenia were accurately captured and appropriately treated.
Statistical analysis
The meta-analysis assessed absolute (pooled) and relative outcomes using fixed- and random-effects models, which were premised on an inverse-variance weighting approach.16 Dichotomous outcomes (ORR, DCR, AE rates, and discontinuation rates due to AEs) were analyzed as proportions. Time-to-event outcomes (OS and PFS) were analyzed as rates with corresponding 95% CIs at specific landmark time points (e.g. 12 and 24 months) and were determined by pooling the Kaplan–Meier curves using Guyot’s algorithm.17 For sparse dichotomous outcomes, relative treatment effects were estimated as risk ratios (RRs) or risk differences (RDs). For time-to-event outcomes, relative treatment effects were analyzed as HRs with corresponding 95% CIs.
Quality assessment of the included studies was carried out using the Cochrane risk-of-bias tool for RCTs,18 the Downs and Black checklist for non-RCTs,19 and the Newcastle–Ottawa scale for observational studies.20 Heterogeneity (i.e. differences in patient characteristics) between the studies was assessed using I2 statistics21,22 and τ2 statistics.23 Publication bias (i.e. the tendency to publish studies with beneficial outcomes or statistically significant findings) was evaluated using funnel plots (i.e. plots of effect estimates against sample sizes)24 and Egger’s weighted regression p values.23 An asymmetrical funnel plot and an Egger’s weighted regression p < 0.1 suggested significant publication bias.23,25 Due to low testing power, funnel plots and Egger’s weighted regression p value were not considered when the meta-analysis included fewer than 10 studies.
The statistical analysis was carried out using R software. All comparative assessments were performed by way of a pairwise meta-analysis; indirect treatment comparisons were not carried out. Missing parameters (e.g. standard deviations and standard errors) required for the meta-analysis were estimated based on Cochrane guidelines.18
Results
Study selection
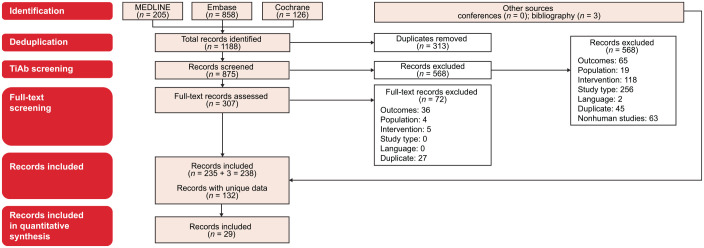
Among 875 screened publications, 29 publications were selected, which reported on 26 studies: 5 RCTs,7,8,12,26–30 11 non-RCTs,31–41 and 10 prospective observational studies15,42–50 (Figure 1; Supplemental Table 3). Six studies involved FTD/TPI + BEV as an intervention. Only one RCT was identified comparing FTD/TPI + BEV with FTD/TPI monotherapy in pretreated patients with mCRC, the phase II EudraCT trial (n = 93).12 Pooled across all 26 studies, FTD/TPI monotherapy and FTD/TPI + BEV were used in 9383 and 289 patients, respectively.
Figure 1.
PRISMA diagram. Only records for RCTs, non-RCTs, and prospective observational studies with a sample size of more than 20 patients were included in the quantitative synthesis.
RCTs, randomized controlled trials; TiAb, title and abstract.
Quality assessment
Applying the Cochrane risk-of-bias tool18 to the five RCTs, three studies were judged low risk in all seven categories; one study was judged low risk in four categories and high risk in three categories; and one study was judged low risk in three categories, high risk in two categories, and unclear risk in two categories (Supplemental Table 4). Applying the Downs and Black checklist19 to the 11 non-RCTs, one study was good quality, five studies were fair quality, and five studies were poor quality (Supplemental Table 5). Applying the Newcastle–Ottawa scale20 to the 10 observational studies, one study was high quality, six studies were medium quality, and three studies were low quality (Supplemental Table 6).
Absolute (pooled) efficacy
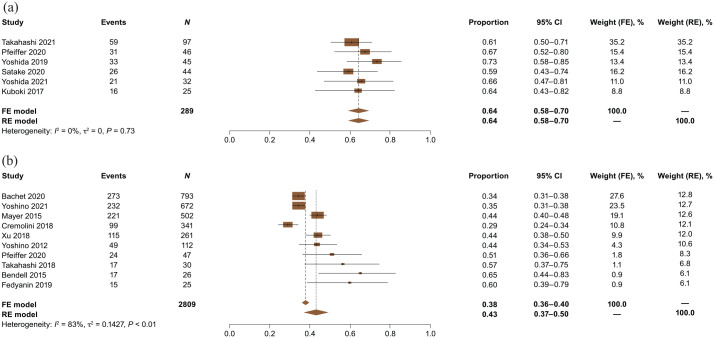
Absolute ORR was 4% with FTD/TPI + BEV (6 studies; n = 289) and 2% with FTD/TPI monotherapy (9 studies; n = 2784) (Supplemental Figure 1). Absolute DCR was 64% with FTD/TPI + BEV (6 studies; n = 289) and 43% with FTD/TPI monotherapy (10 studies; n = 2809) (Figure 2).
Figure 2.
Absolute (pooled) DCRs for (a) FTD/TPI + BEV and (b) FTD/TPI monotherapy.
CI, confidence interval; DCRs, disease control rates; FE, fixed effects; FTD/TPI, trifluridine/tipiracil; FTD/TPI + BEV, trifluridine/tipiracil + bevacizumab; RE, random effects.
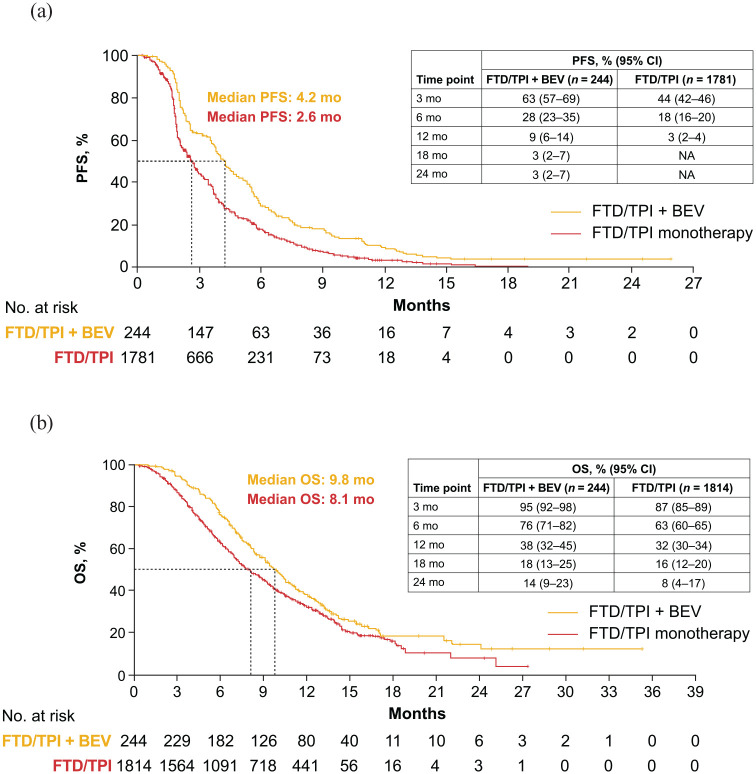
Absolute median PFS was 4.2 months with FTD/TPI + BEV (5 studies; n = 244) and 2.6 months with FTD/TPI monotherapy (6 studies; n = 1781); absolute 12-month PFS was 9% (95% CI, 6%–14%) and 3% (95% CI, 2%–4%), respectively (Figure 3; Supplemental Table 7). Absolute median OS was 9.8 months with FTD/TPI + BEV (5 studies; n = 244) and 8.1 months with FTD/TPI monotherapy (6 studies; n = 1814); absolute 12-month OS was 38% (95% CI, 32%–45%) and 32% (95% CI, 30%–34%), respectively (Figure 3; Supplemental Table 8).
Figure 3.
Pooled absolute (a) PFS and (b) OS for FTD/TPI + BEV and FTD/TPI monotherapy.
FTD/TPI, trifluridine/tipiracil; FTD/TPI + BEV, trifluridine/tipiracil + bevacizumab; NA, not applicable; OS, overall survival; PFS, progression-free survival.
For the absolute DCR analysis for FTD/TPI monotherapy, which included 10 studies, the funnel plot and Egger’s weighted regression p value suggested the presence of publication bias. Funnel plots and Egger’s weighted regression p values were not considered for the other absolute efficacy analyses because fewer than 10 studies were included.
Relative efficacy
The relative efficacy analysis only included the EudraCT trial.12 In that trial, FTD/TPI + BEV, compared with FTD/TPI monotherapy, was associated with numerically (although not statistically significantly) higher ORR (RD, 0.02; 95% CI, −0.02 to 0.07; p = 0.35) and DCR (RR, 1.32; 95% CI, 0.94–1.86; p = 0.11).12 FTD/TPI + BEV, compared with FTD/TPI monotherapy, was associated with a 55% significant reduction in the risk for progression (HR, 0.45; 95% CI, 0.29–0.72; p = 0.0015) and a 45% significant reduction in the risk for death (HR, 0.55; 95% CI, 0.32–0.94; p = 0.03).12 Funnel plots and Egger’s weighted regression p values were not considered for the relative efficacy analyses because only one study was included.
Absolute (pooled) safety
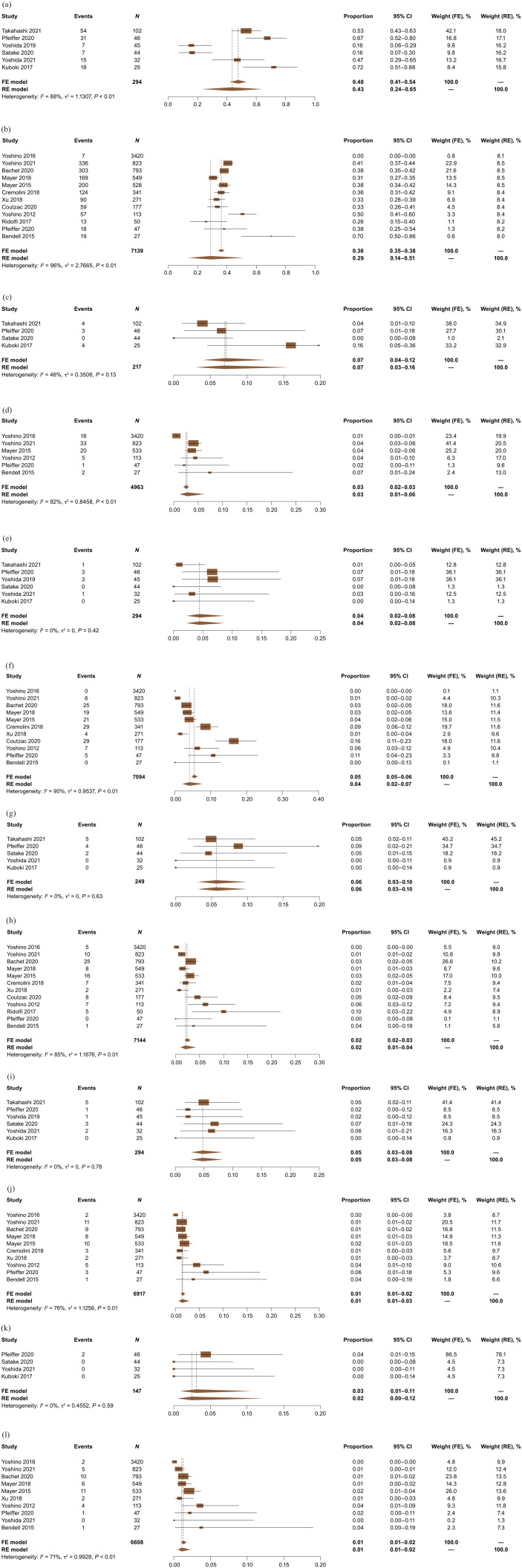
AEs were not consistently reported across the studies, and AE grading followed the National Cancer Institute Common Terminology Criteria for Adverse Events v3.0, v4.0, or v4.03, depending on when the respective studies were conducted. Data for the following six most commonly reported AEs were pooled across the studies: neutropenia, febrile neutropenia, asthenia/fatigue, diarrhea, nausea, and vomiting. Absolute rates for grade ⩾3 neutropenia were 43% with FTD/TPI + BEV (6 studies; n = 294) and 29% with FTD/TPI monotherapy (12 studies; n = 7139) (Figure 4(a) and (b)). Absolute rates for grade ⩾3 febrile neutropenia were 7% with FTD/TPI + BEV (4 studies; n = 217) and 3% with FTD/TPI monotherapy (6 studies; n = 4963) (Figure 4(c) and (d)). Absolute rates for grade ⩾3 asthenia/fatigue were 4% with FTD/TPI + BEV (6 studies; n = 294) and 4% with FTD/TPI monotherapy (11 studies; n = 7094) (Figure 4(e) and (f)). Absolute rates for grade ⩾3 diarrhea were 6% with FTD/TPI + BEV (5 studies; n = 249) and 2% with FTD/TPI monotherapy (12 studies; n = 7144) (Figure 4(g) and (h)). Absolute rates for grade ⩾3 nausea were 5% with FTD/TPI + BEV (6 studies; n = 294) and 1% with FTD/TPI monotherapy (10 studies; n = 6917) (Figure 4(i) and (j)). Absolute rates for grade ⩾3 vomiting were 3% with FTD/TPI + BEV (4 studies; n = 147) and 1% with FTD/TPI monotherapy (10 studies; n = 6608) (Figure 4(k) and (l)). Absolute discontinuation rates due to AEs were 8% with FTD/TPI + BEV (5 studies; n = 244) and 7% with FTD/TPI monotherapy (10 studies; n = 3724) (Supplemental Figure 2).
Figure 4.
Absolute (pooled) rates for grade ⩾3 AEs for FTD/TPI + BEV and FTD/TPI monotherapy. (a) FTD/TPI + BEV: Grade ⩾3 neutropenia. (b) FTD/TPI monotherapy: Grade ⩾3 neutropenia. (c) FTD/TPI + BEV: Grade ⩾3 febrile neutropenia. (d) FTD/TPl monotherapy: Grade ⩾3 febrile neutropenia. (e) FTD/TPI + BEV: Grade ⩾3 asthenia/fatigue. (f). FTD/TPI monotherapy: Grade ⩾3 asthenia/fatigue. (g) FTD/TPI + BEV: Grade ⩾3 diarrhea. (h) FTD/TPI monotherapy: Grade ⩾3 diarrhea. (i) FTD/TPI + BEV: Grade ⩾3 nausea. (j) FTD/TPI monotherapy: Grade ⩾3 nausea. (k) FTD/TPI + BEV: Grade ⩾3 vomiting. (l) FTD/TPI monotherapy: Grade ⩾3 vomiting.
AEs, adverse events; CI, confidence interval; FE, fixed effects; FTD/TPI, trifluridine/tipiracil; FTD/TPI + BEV, trifluridine/tipiracil + bevacizumab; RE, random effects.
For absolute safety analyses for FTD/TPI monotherapy that included 10 or more studies, funnel plots and Egger’s weighted regression p values suggested no publication bias for neutropenia, asthenia/fatigue, diarrhea, nausea, and vomiting, but publication bias was suggested for discontinuation rates due to AEs. Funnel plots and Egger’s weighted regression p values were not considered for the other absolute safety analyses because fewer than 10 studies were included.
Relative safety
As in the relative efficacy analysis, the relative safety analysis only included the EudraCT trial.12 In that trial, the risk for grade ⩾3 neutropenia was significantly higher with FTD/TPI + BEV than with FTD/TPI monotherapy (RD, 0.29; 95% CI, 0.10–0.49; p = 0.0033). Risks were similar between the two regimens for grade ⩾3 febrile neutropenia (RD, 0.04; 95% CI, −0.04 to 0.13; p = 0.2961), asthenia/fatigue (RD, −0.04; 95% CI, −0.15 to 0.07; p = 0.48), diarrhea (RD, 0.09; 95% CI, 0.00–0.17; p = 0.04), nausea (RD, −0.04; 95% CI, −0.12 to 0.04; p = 0.31), and vomiting (RD, 0.02; 95% CI, −0.05 to 0.09; p = 0.55). The risk of discontinuation due to AEs in the EudraCT trial was similar with FTD/TPI + BEV and FTD/TPI monotherapy (RD, −0.02; 95% CI, −0.09 to 0.05; p = 0.57) despite a longer treatment duration with combination therapy than with monotherapy (median, 4.9 versus 2.4 months). Funnel plots and Egger’s weighted regression p values were not considered for the relative safety analyses because only one study was included.
AE monitoring schedule and G-CSF use
To determine if cases of neutropenia were accurately captured and appropriately treated, data were collected for the AE monitoring schedule and G-CSF use. The AE monitoring schedule, which was reported in 10 of the 26 studies (38%), was approximately every week or every 2 weeks (Supplemental Table 9). G-CSF was used in five studies (19%), not allowed in two studies (8%), and not reported in 19 studies (73%) (Supplemental Table 9).
Discussion
The results of this meta-analysis, which pooled data across 26 studies, suggest that the addition of BEV to FTD/TPI is a feasible treatment for patients with refractory mCRC. Given the lack of data from large RCTs comparing FTD/TPI + BEV with FTD/TPI monotherapy, this meta-analysis provides further important insights into the use of FTD/TPI + BEV that may help guide treatment decisions in this patient population.
Efficacy results were consistently superior with FTD/TPI + BEV than with FTD/TPI monotherapy in this meta-analysis. Absolute median PFS was 4.2 and 2.6 months with FTD/TPI + BEV and FTD/TPI monotherapy, respectively, and absolute median OS was 9.8 and 8.1 months, respectively. Efficacy findings of our meta-analysis were consistent with those of a recent meta-analysis with mCRC (pooled across 25 studies) in which median PFS was 4.35 and 2.53 months with FTD/TPI + BEV and FTD/TPI monotherapy, respectively, and median OS was 10.41 and 6.95 months, respectively.51 Furthermore, efficacy results with FTD/TPI + BEV in our meta-analysis appeared to be superior to those with any later-line regimen reported in a systematic literature review of phase II and phase III trials (67 studies; 7556 patients) in refractory mCRC (median PFS, 3.2 months; median OS, 8.8 months),52 although it is difficult to make comparisons between different analyses.
FTD/TPI + BEV and FTD/TPI monotherapy were shown to be safe and well tolerated in this meta-analysis. Absolute rates for grade ⩾3 febrile neutropenia, asthenia/fatigue, diarrhea, nausea, and vomiting were low with FTD/TPI + BEV and FTD/TPI monotherapy (1–7%). However, grade ⩾3 neutropenia was reported more frequently with FTD/TPI + BEV than with FTD/TPI monotherapy (43% and 29%, respectively). These safety results are in line with those of the previous meta-analysis with mCRC patients in which FTD/TPI + BEV, compared with FTD/TPI monotherapy, was associated with a significantly higher incidence of grade ⩾3 AEs (odds ratio, 2.19; 95% CI, 1.40–3.44).51 In another meta-analysis, the addition of BEV to other cancer therapies increased the risk of serious neutropenic events in patients with various tumor types.53 Because neutropenia is an overlapping AE with FTD/TPI and BEV, the benefits and risks of adding BEV to FTD/TPI should be considered carefully in patients with mCRC. However, the increased incidence of grade ⩾3 neutropenia with FTD/TPI + BEV may not be clinically significant as absolute discontinuation rates due to AEs were low with both the combination therapy and monotherapy (8% and 7%, respectively), suggesting that both regimens were well tolerated and that toxicity was generally manageable.
Data were collected for AE monitoring schedule and G-CSF use to determine if cases of neutropenia with FTD/TPI, which are frequently observed during the 2-week rest period after treatment initiation,15 were accurately captured and appropriately treated. In the studies that reported the AE monitoring schedule, AEs were monitored approximately every week or every 2 weeks; thus, the monitoring schedules were sufficient to capture cases of neutropenia. G-CSF use was not consistently reported, so a relationship between the occurrence of neutropenia and use of G-CSF could not be determined.
RCTs are needed to provide definitive evidence on the use of FTD/TPI + BEV in patients with refractory mCRC. The only RCT comparing FTD/TPI + BEV and FTD/TPI monotherapy in this population was the phase II EudraCT trial, which enrolled 93 patients.12 The international, open-label, phase III SUNLIGHT trial (NCT04737187) compared the efficacy and safety of FTD/TPI + BEV and FTD/TPI monotherapy in patients with unresectable mCRC in the third-line setting.54,55 The SUNLIGHT trial had an estimated enrollment of 490 patients and a predicted primary completion date of December 2022.55 The study sponsor announced positive results in a press release (September 12, 2022).56 Upcoming SUNLIGHT trial results are expected to be consistent with the findings of this meta-analysis.
There are limitations in the review process and evidence that should be considered when interpreting the results of this analysis. As with any meta-analysis, these findings may have been confounded by study heterogeneity and publication bias. Specifically, the results of this meta-analysis may have been influenced by differences in prognostic characteristics (e.g. age, histology, number of metastases, mutational status, and performance status) between patients treated with FTD/TPI + BEV and patients treated with FTD/TPI monotherapy who were pooled across the various studies. In particular, study heterogeneity may have had a significant impact on pooled Kaplan–Meier curves, impeding interpretation of those results. In addition, a disparity existed in the number of patients treated with FTD/TPI monotherapy (n = 9383) and FTD/TPI + BEV (n = 289) in this analysis. However, the evaluation of efficacy included absolute outcomes, which were independently determined. Thus, the difference in the number of pooled patients for the two regimens was not expected to materially affect the analysis. The relative outcomes analysis was limited by the inclusion of only one RCT (EudraCT trial12) that compared FTD/TPI + BEV with FTD/TPI monotherapy in patients with mCRC who were pretreated, in which sample sizes for the two regimens were similar. Another limitation was that publication bias was detected in certain instances, and some studies were deemed to be of low quality. Furthermore, safety could not be fully assessed because the overall incidences of grade ⩾3 AEs were not consistently reported in the studies. Consequently, six commonly reported AEs (neutropenia, febrile neutropenia, asthenia/fatigue, diarrhea, nausea, and vomiting) were examined. Despite these limitations, the precision of the calculated estimates may have increased by the inclusion of large sample sizes and the use of two models (fixed and random effects).
Conclusion
In summary, the results of this meta-analysis suggest that FTD/TPI + BEV provides benefits over FTD/TPI monotherapy in patients with refractory mCRC, with a higher DCR and longer PFS and OS, and has a similar safety profile. However, the combination therapy is also associated with a higher rate of grade ⩾3 neutropenia, although this finding is of uncertain clinical significance as absolute discontinuation rates due to AEs were low with both regimens. Given the lack of data from large RCTs comparing FTD/TPI + BEV with FTD/TPI monotherapy in this population, these findings may help guide treatment decisions. Results of the phase III SUNLIGHT trial54–56 will more directly delineate the role of FTD/TPI + BEV in mCRC.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359221146137 for Trifluridine/tipiracil with or without bevacizumab in metastatic colorectal cancer: results of a systematic review and meta-analysis by Takayuki Yoshino, Julien Taieb, Yasutoshi Kuboki, Per Pfeiffer, Amit Kumar and Howard S. Hochster in Therapeutic Advances in Medical Oncology
Acknowledgments
These results were presented, in part, at the 2022 American Society of Clinical Oncology (ASCO) Annual Meeting; June 3–7, 2022; Chicago, IL, USA. Medical writing and editorial support for the development of this manuscript, under the direction of the authors, were provided by Mark Palangio and Jennifer Robertson of Ashfield MedComms, an Inizio company, and funded by Taiho Oncology, Inc.
Footnotes
ORCID iDs: Per Pfeiffer  https://orcid.org/0000-0002-2925-0586
https://orcid.org/0000-0002-2925-0586
Amit Kumar  https://orcid.org/0000-0001-8777-9456
https://orcid.org/0000-0001-8777-9456
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Takayuki Yoshino, Department of Gastroenterology and Gastrointestinal Oncology, National Cancer Center Hospital East, 6-5-1, Kashiwanoha, Kashiwa, Chiba 277-8577, Japan.
Julien Taieb, Hôpital Européen Georges-Pompidou, Université Paris-Cité, SIRIC CARPEM, Paris, France.
Yasutoshi Kuboki, National Cancer Center Hospital East, Kashiwa, Japan.
Per Pfeiffer, Odense University Hospital, Odense, Denmark.
Amit Kumar, SmartAnalyst India Pvt Ltd, Gurugram, Haryana, India.
Howard S. Hochster, Rutgers Cancer Institute of New Jersey, New Brunswick, NJ, USA
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contribution(s): Takayuki Yoshino: Conceptualization; Data curation; Investigation; Writing – review & editing.
Julien Taieb: Conceptualization; Data curation; Investigation; Writing – review & editing.
Yasutoshi Kuboki: Conceptualization; Data curation; Investigation; Writing – review & editing.
Per Pfeiffer: Conceptualization; Data curation; Investigation; Writing – review & editing.
Amit Kumar: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing – review & editing.
Howard S. Hochster: Conceptualization; Data curation; Investigation; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was sponsored by Taiho Oncology, Inc.
Takayuki Yoshino reports medical writing support for the submitted work from Taiho Oncology; lecture fees and/or honoraria from Bayer Yakuhin, MSD K.K., Chugai Pharmaceutical, Eli Lilly Japan K.K, Merck Biopharma, Ono Pharmaceutical, and Taiho Pharmaceutical; research funds to his institution from Amgen K.K., Pfizer Japan, Chugai Pharmaceutical, Genomedia, MSD K.K., Nippon Boehringer Ingelheim, Ono Pharmaceutical, Parexel International, Sanofi K.K, Daiichi Sankyo, Sysmex Corporation, and Taiho Pharmaceutical.
Julien Taieb reports medical writing support for the submitted work from Taiho Oncology; consulting fees, payment or honoraria, and support for attending meetings and/or travel from Amgen, Astellas, AstraZeneca, Bristol Myers Squibb, Merck, Merck Sharp & Dohme, Novartis, Pierre Fabre, Roche, and Servier.
Yasutoshi Kuboki reports medical writing support for the submitted work from Taiho Oncology; grants or contracts Abbie, Astelas, AstraZeneca, Boehringer Ingelheim, Chugai, Daiichi-Sankyo, Genmab, GlaxoSmithKline, Incyte, Lilly, Taiho Oncology, and Takeda; consulting fees from Amgen, Boehringer Ingelheim, Taiho, and Takeda; payment or honoraria from Bristol Myers Squibb, Lilly, Ono, and Taiho Oncology.
Per Pfeiffer reports medical writing support for the submitted work from Taiho Oncology and grants to his institution from Servier.
Amit Kumar reports medical writing support for the submitted work from Taiho Oncology and is an employee of SmartAnalyst, the consulting firm contracted by Taiho Oncology to conduct the analysis for the submitted work and by Bristol Myers Squibb, Janssen, Pfizer, and Merck for unrelated work.
Howard S. Hochster reports medical writing support for the submitted work and payment for expert testimony from Taiho Oncology, Inc.
Availability of data and materials: All relevant data are included in the published article and supplemental material.
References
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71: 209–249. [DOI] [PubMed] [Google Scholar]
- 2. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer stat facts: colorectal cancer, https://seer.cancer.gov/statfacts/html/colorect.html (2022, accessed 18 February 2022).
- 3. Wang J, Li S, Liu Y, et al. Metastatic patterns and survival outcomes in patients with stage IV colon cancer: a population-based analysis. Cancer Med 2020; 9: 361–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 2016; 27: 1386–1422. [DOI] [PubMed] [Google Scholar]
- 5. Biller LH, Schrag D. Diagnosis and treatment of metastatic colorectal cancer: a review. JAMA 2021; 325: 669–685. [DOI] [PubMed] [Google Scholar]
- 6. Yoshino T, Arnold D, Taniguchi H, et al. Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: a JSMO-ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann Oncol 2018; 29: 44–70. [DOI] [PubMed] [Google Scholar]
- 7. Yoshino T, Mizunuma N, Yamazaki K, et al. TAS-102 monotherapy for pretreated metastatic colorectal cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol 2012; 13: 993–1001. [DOI] [PubMed] [Google Scholar]
- 8. Mayer RJ, Van Cutsem E, Falcone A, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med 2015; 372: 1909–1919. [DOI] [PubMed] [Google Scholar]
- 9. Lenz HJ, Stintzing S, Loupakis F. TAS-102, a novel antitumor agent: a review of the mechanism of action. Cancer Treat Rev 2015; 41: 777–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Van Cutsem E, Mayer RJ, Laurent S, et al. The subgroups of the phase III RECOURSE trial of trifluridine/tipiracil (TAS-102) versus placebo with best supportive care in patients with metastatic colorectal cancer. Eur J Cancer 2018; 90: 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tsukihara H, Nakagawa F, Sakamoto K, et al. Efficacy of combination chemotherapy using a novel oral chemotherapeutic agent, TAS-102, together with bevacizumab, cetuximab, or panitumumab on human colorectal cancer xenografts. Oncol Rep 2015; 33: 2135–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pfeiffer P, Yilmaz M, Möller S, et al. TAS-102 with or without bevacizumab in patients with chemorefractory metastatic colorectal cancer: an investigator-initiated, open-label, randomised, phase 2 trial. Lancet Oncol 2020; 21: 412–420. [DOI] [PubMed] [Google Scholar]
- 13. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Colon Cancer: V.1.2022. ©National Comprehensive Cancer Network, Inc. All rights reserved. (2022, accessed June 7, 2022). [Google Scholar]
- 14. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Rectal Cancer: V.1.2022. ©National Comprehensive Cancer Network, Inc. All rights reserved (2022, accessed June 7, 2022). [Google Scholar]
- 15. Yoshino T, Uetake H, Fujita N, et al. TAS-102 safety in metastatic colorectal cancer: results from the first postmarketing surveillance study. Clin Colorectal Cancer 2016; 15: e205–e211. [DOI] [PubMed] [Google Scholar]
- 16. Borenstein M, Hedges LV, Higgins JP, et al. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods 2010; 1: 97–111. [DOI] [PubMed] [Google Scholar]
- 17. Guyot P, Ades AE, Ouwens MJ, et al. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol 2012; 12: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Higgins JPT, Green S. (eds). Cochrane handbook for systematic reviews of interventions version 5.1.0. www.handbook.cochrane.org (2011, accessed 28 February 2022).
- 19. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998; 52: 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses, www.ohri.ca/programs/clinical_epidemiology/oxford.asp (2013, accessed 28 February 2022).
- 21. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 22. Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Borenstein M, Hedges LV, Higgins JPT, et al. Introduction to meta-analysis. Chichester: Wiley, 2009. [Google Scholar]
- 24. Stuck AE, Rubenstein LZ, Wieland D. Bias in meta-analysis detected by a simple, graphical test. Asymmetry detected in funnel plot was probably due to true heterogeneity. BMJ 1998; 316: 469–471. [PMC free article] [PubMed] [Google Scholar]
- 25. Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011; 343: d4002. [DOI] [PubMed] [Google Scholar]
- 26. Karthaus M, Van Cutsem E, Mayer RJ, et al. TAS-102 versus placebo plus best supportive care in patients with metastatic colorectal cancer refractory to standard therapies: final survival results of the phase III RECOURSE trial. Oncol Res Treat 2016; 39: 1–294. [Google Scholar]
- 27. Van Cutsem E, Falcone A, Garcia-Carbonero R, et al. Proxies of quality of life in metastatic colorectal cancer: analyses in the RECOURSE trial. ESMO Open 2017; 2: e000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xu J, Kim TW, Shen L, et al. Results of a randomized, double-blind, placebo-controlled, phase III trial of trifluridine/tipiracil (TAS-102) monotherapy in Asian patients with previously treated metastatic colorectal cancer: the TERRA study. J Clin Oncol 2018; 36: 350–358. [DOI] [PubMed] [Google Scholar]
- 29. Yoshino T, Shinozaki E, Yamazaki K, et al. Final survival results and onset of neutropenia as an indicator of therapeutic effect in phase 2 of TAS-102 vs placebo with metastatic colorectal cancer (J003-10040030). Ann Oncol 2016; 27: ii102–ii117. [Google Scholar]
- 30. Kasper S, Hofheinz RD, Stintzing S, et al. Interim safety analysis of the phase IIb study of ramucirumab in combination with TAS102 vs. TAS102 monotherapy in metastatic colorectal cancer: The RAMTAS trial of the German AIO. Ann Oncol 2020; 31: S409–S461. [Google Scholar]
- 31. Bachet JB, Wyrwicz L, Price T, et al. Safety, efficacy and patient-reported outcomes with trifluridine/tipiracil in pretreated metastatic colorectal cancer: results of the PRECONNECT study. ESMO Open 2020; 5: e000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fedyanin M, Chekini D, Chubenko V, et al. Trifluridine/tipiracil safety and efficacy in Russian patients with metastatic colorectal cancer and refractory or intolerant to standard chemotherapies: results of the primary analysis. Ann Oncol 2019; 30(Suppl. 4): iv16. [Google Scholar]
- 33. Takahashi T, Yamazaki K, Oki E, et al. Phase II study of trifluridine/tipiracil plus bevacizumab by RAS mutation status in patients with metastatic colorectal cancer refractory to standard therapies: JFMC51-1702-C7. ESMO Open 2021; 6: 100093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yoshida Y, Yamada T, Kamiyama H, et al. Combination of TAS-102 and bevacizumab as third-line treatment for metastatic colorectal cancer: TAS-CC3 study. Int J Clin Oncol 2021; 26: 111–117. [DOI] [PubMed] [Google Scholar]
- 35. Takahashi M, Gamoh M, Ohori H, et al. Possible predictive value of G8 score and the drug concentrations for efficacy and toxicity of trifluridine/tipiracil for elderly patients with advanced colorectal cancer: a multicenter, phase II study (T-CORE1401). J Clin Oncol 2018; 36: e15516. [Google Scholar]
- 36. Yoshida Y, Yamada T, Matsuoka H, et al. Biweekly TAS-102 and bevacizumab as a third-line chemotherapy for metastatic colorectal cancer: a phase II multicenter clinical trial (TASCC4 study). Ann Oncol 2019; 30: V235. [Google Scholar]
- 37. Satake H, Kato T, Oba K, et al. Phase Ib/II study of biweekly TAS-102 in combination with bevacizumab for patients with metastatic colorectal cancer refractory to standard therapies (BiTS Study). Oncologist 2020; 25: e1855–e1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kuboki Y, Nishina T, Shinozaki E, et al. TAS-102 plus bevacizumab for patients with metastatic colorectal cancer refractory to standard therapies (C-TASK FORCE): an investigator-initiated, open-label, single-arm, multicentre, phase 1/2 study. Lancet Oncol 2017; 18: 1172–1181. [DOI] [PubMed] [Google Scholar]
- 39. Bendell JC, Rosen LS, Mayer RJ, et al. Phase 1 study of oral TAS-102 in patients with refractory metastatic colorectal cancer. Cancer Chemother Pharmacol 2015; 76: 925–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ridolfi C, Tamburini E, Rudnas B, et al. Trifluridine tipiracil (TAS 102) in patients with stage IV pretreated colorectal neoplasm: an experience from Rimini City Hospital. Ann Oncol 2017; 28: III117–III118. [Google Scholar]
- 41. Mayer RJ, Hochster HS, Cohen SJ, et al. Safety of trifluridine/tipiracil in an open-label expanded-access program in elderly and younger patients with metastatic colorectal cancer. Cancer Chemother Pharmacol 2018; 82: 961–969. [DOI] [PubMed] [Google Scholar]
- 42. Yoshino T, Uetake H, Funato Y, et al. Post-marketing surveillance study of trifluridine/tipiracil in patients with metastatic colorectal cancer. Jpn J Clin Oncol 2021; 51: 700–706. [DOI] [PubMed] [Google Scholar]
- 43. Cheung WY, Kavan P, Dolley A. Quality of life in a real-world study of patients with metastatic colorectal cancer treated with trifluridine/tipiracil. Curr Oncol 2020; 27: e451–e458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jalali A, Wong H.-L, Wong R, et al. Initial experience of TAS-102 chemotherapy in Australian patients with chemorefractory metastatic colorectal cancer. J Clin Oncol 2021; 39: 71. [DOI] [PubMed] [Google Scholar]
- 45. Coutzac C, Trouilloud I, Artru P, et al. Trifluridine/tipiracil or regorafenib in refractory metastatic colorectal cancer patients: an AGEO prospective “real life” study. J Clin Oncol 2020; 38: 4036–4036. [DOI] [PubMed] [Google Scholar]
- 46. Cremolini C, Rossini D, Martinelli E, et al. Trifluridine/tipiracil (TAS-102) in refractory metastatic colorectal cancer: a multicenter register in the frame of the Italian compassionate use program. Oncologist 2018; 23: 1178–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kasper S, Kisro J, Fuchs M, et al. Safety profile of trifluridine/tipiracil monotherapy in clinical practice: results of the German compassionate-use program for patients with metastatic colorectal cancer. BMC Cancer 2018; 18: 1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Garcia-Alfonso P, Ruiz A, Carrato A, et al. Compassionate use program with FDT-TPI (trifluridine-tipiracil) in pre-treated metastatic colorectal cancer patients: Spanish real world data. J Clin Oncol 2017; 35: e15019. [Google Scholar]
- 49. Salvatore L, Niger M, Bellu L, et al. Compassionate use program for trifluridine/tipiracil (TAS-102) in metastatic colorectal cancer: a real-life overview. Ann Oncol 2016; 27: vi149–vi206. [Google Scholar]
- 50. Hamers P, Vink G, Elferink M, et al. QUAlity of LIfe and survival of meTAStatic colorectal cancer patients treated with trifluridine-tipiracil (QUALITAS). Ann Oncol 2020; 31: S434–S435. [DOI] [PubMed] [Google Scholar]
- 51. Liu CJ, Hu T, Shao P, et al. TAS-102 monotherapy and combination therapy with bevacizumab for metastatic colorectal cancer. Gastroenterol Res Pract 2021; 2021: 4014601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Petrelli F, Perego G, Ghidini A, et al. A systematic review of salvage therapies in refractory metastatic colorectal cancer. Int J Colorectal Dis 2020; 35: 783–794. [DOI] [PubMed] [Google Scholar]
- 53. Zhou F, Shao JH, Wu LQ, et al. Risk of serious neutropenic events in cancer patients treated with bevacizumab: a meta-analysis. Asian Pac J Cancer Prev 2013; 14: 2453–2459. [DOI] [PubMed] [Google Scholar]
- 54. Tabernero J, Taieb J, Prager GW, et al. Trifluridine/tipiracil plus bevacizumab for third-line management of metastatic colorectal cancer: SUNLIGHT study design. Future Oncol 2021; 17: 1977–1985. [DOI] [PubMed] [Google Scholar]
- 55. ClinicalTrials.gov. Phase III study of trifluridine/tipiracil in combination with bevacizumab vs trifluridine/tipiracil single agent in patients with refractory metastatic colorectal cancer (SUNLIGHT), https://clinicaltrials.gov/ct2/show/NCT04737187 (2022, accessed 3 June 2022).
- 56. Drug combination meets survival endpoint in phase III pivotal trial involving participants with refractory metastatic colorectal cancer [press release]. Paris, France: Servier, Taiho Oncology, Inc., and Taiho Pharmaceutical Co., Ltd., 2022. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359221146137 for Trifluridine/tipiracil with or without bevacizumab in metastatic colorectal cancer: results of a systematic review and meta-analysis by Takayuki Yoshino, Julien Taieb, Yasutoshi Kuboki, Per Pfeiffer, Amit Kumar and Howard S. Hochster in Therapeutic Advances in Medical Oncology