Abstract
Sex differences in neural and behavioral development are integral to understanding neurodevelopmental, mental health, and neurodegenerative disorders. Much of the literature has focused on late prenatal and early postnatal life as a critical juncture for establishing sex-specific developmental trajectories, and data are now clear that immune signaling plays a central role in establishing sex differences early in life. Adolescence is another developmental period during which sex differences arise. However, we know far less about how immune signaling plays a role in establishing sex differences during adolescence. Herein, we review well-defined examples of sex differences during adolescence, and then survey the literature to speculate how immune signaling might be playing a role in defining sex-specific adolescent outcomes. We discuss open questions in the literature and propose experimental design tenets that may assist in better understanding adolescent neurodevelopment.
Introduction
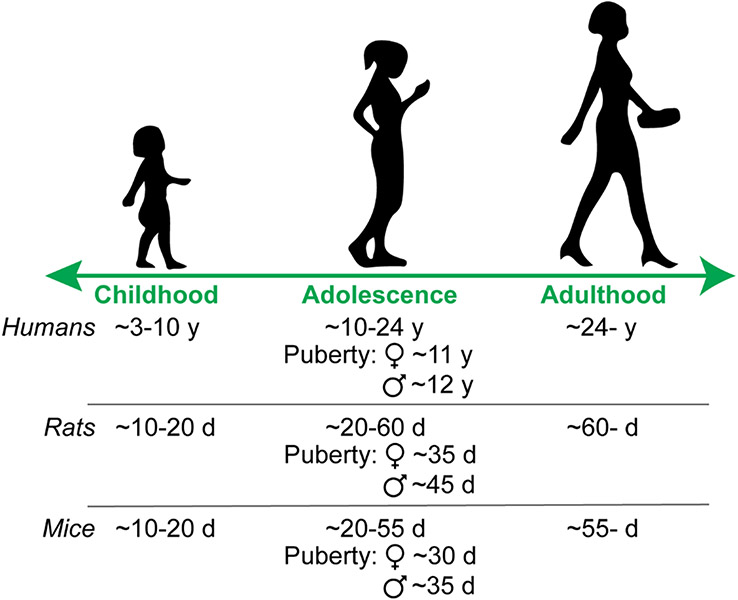
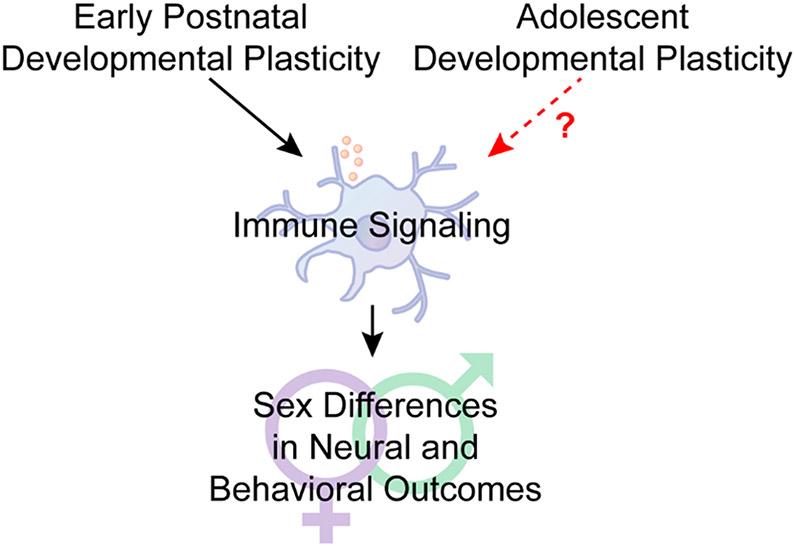
Although once considered immune privileged, there is now copious evidence that the brain is influenced by unique immune signaling profiles. Microglia are the resident immune cells of the brain, but other glial cells, infiltrating peripheral immune cells and even neurons have immune signaling properties. Developmental processes mediated by immune signaling include synaptogenesis and regulating synaptic connectivity, cell and axonal migration, remodeling dendritic complexity, synaptic pruning, and apoptotic clearance [1]. Most of these processes display sex differences. In fact, some of the most fundamental sex differences require immune signaling: the male brain is masculinized by testicular androgen secretion around the time of birth, which is converted to estradiol via aromatase. Estradiol increases prostaglandin E2 immune signaling in the brain to promote male-specific microglial remodeling in multiple brain regions [2, 3]. These early life immune events give rise to sex-specific behaviors later in life. However, sex differences in neural and behavioral outcomes are not solely established in prenatal and early postnatal life. Another crucial developmental period during which numerous sex differences emerge is adolescence. Adolescence is a period of rapid development across the brain, most notably the dopaminergic ‘reward’ circuitry, and is characterized by increased exploration, risk-taking, and peer-centered social behaviors [4-7]. Adolescence begins prior to and extends beyond puberty, which brings forth secondary sexual characteristics [8], in totality encompassing multiple years in humans and multiple weeks in rodents (Fig. 1) [5, 9]. Despite the central role that immune signaling plays in establishing sex differences during prenatal and early life, how immune signaling might contribute to sex differences established during adolescence is less well studied. We propose that just as immune signaling provides a molecular framework for establishing sex differences early in life, immune signaling may also be a crucial contributor to sex differences during adolescent development (Fig. 2) that provides an opportunity for investigation.
Fig. 1: Approximating adolescence across species.
Ages are broad estimates from the literature [5, 9]. y = years, d = postnatal days.
Fig.2: Immune signaling in sex-specific neural and behavioral development: Adolescent opportunity.
Sex differences are established by early life developmental plasticity, and immune signaling is now known to be a critical signaling intermediary for establishing sex-specific neural and behavioral outcomes. Adolescence is also a developmental period in which important sex differences emerge. Far fewer studies have been focused on whether immune signaling is also an intermediary for establishing sex-specific outcomes during adolescence. In this review, we discuss a variety of well characterized sex differences that emerge during adolescence, and then draw attention to literature that predicts ways in which immune signaling may play a role in the sex difference.
To focus this review, we will discuss sex differences that arise from adolescent developmental plasticity in which both a behavioral/physiological outcome and its underlying neural mechanism has been defined. For each example, we will then survey the literature to speculate how immune signaling might be playing a role in defining the sex-specific adolescent outcome. Classification of sex-specific outcomes into groups that distinguish specific features may be helpful for a deeper understanding of sex differences [10], and thus we will further organize this discussion by type of sex difference (Fig. 3). Finally, we will discuss open questions in the literature and propose experimental design tenets that may assist in better understanding adolescent neurodevelopment.
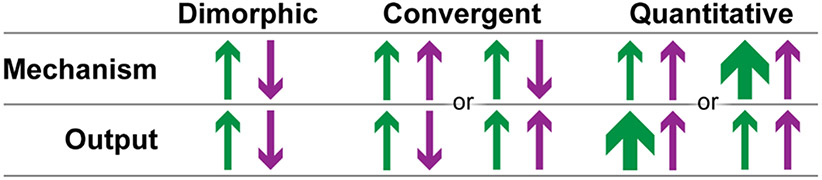
Fig. 3: Different categories of sex differences.
We review examples of sex differences that emerge during adolescence that fall into categories discussed, e.g., in [10]. We define dimorphic sex differences as both the behavioral/physiological output, and its underlying neural mechanism, diverge by sex. Convergent sex differences are when either the same neural mechanism results in divergent outputs (left), or divergent neural mechanisms support a convergent output (right). Quantitative sex differences are when both the neural mechanism and output are the same, but either the mechanism or output is greater in one sex.
Sex dimorphism during adolescence
We will begin with a discussion of the most stark sex differences, sex dimorphism, which we define as when a behavioral/physiological outcome and its underlying mechanistic processes both diverge by sex. The most classic example of sex dimorphism during adolescence is the effect of puberty-initiated gonadal hormones, which result in several sociosexual behaviors as well as the onset of fertility. Puberty occurs when gonadotropin releasing hormone (GnRH) release activates the hypothalamic-pituitary-gonadal axis, resulting in gonadal hormone production. The neuropeptide kisspeptin is important for puberty initiation [11-14], and may be sex-specific. In humans and other mammals, kisspeptin treatment directly stimulates GnRH neurons [15-17]. Kisspeptin neurons are predominantly found in two hypothalamic areas, the arcuate nucleus (ARC) and the anteroventral periventricular and periventricular nuclei (AVPV/PeN) [18-20]. In females, Kiss1 and kisspeptin expression increases throughout development in both the AVPV/PeN and the ARC nuclei, particularly around puberty and first estrous [21]. In males, Kiss1 and kisspeptin in the AVPV also increases across development, but females express more kisspeptin than do males in this region. Unlike females, males do not show a clear increase in Kiss1 and kisspeptin expression in the ARC nucleus throughout development [17, 21, 22]. Thus, while kisspeptin appears to be important for puberty initiation in females, male puberty may be dependent on absolute changes in kisspeptin levels and involve other signaling factors. A strong relationship between immune and kisspeptin signaling is observed in adult rodents [23-25], but a recent study was the first to examine this relationship during puberty. Pro-inflammatory immune activation via lipopolysaccharide (LPS) treatment in pubertal male and female mice decreased hypothalamic Kiss1 in both sexes, though the magnitude of decrease was larger in females due to their higher baseline expression [26]. These data suggest that puberty initiation, which in turn instructs a plethora of sex differences, can be modulated by immune signaling. Indeed, studies examining patients with irritable bowel syndrome and Crohn’s disease, both associated with chronic inflammation, indicate puberty is delayed, which in the case of Crohn’s disease can be treated by reducing levels of the pro-inflammatory cytokine TNFα [27, 28]. These data suggest that pro-inflammatory immune signaling can modulate kisspeptin expression and puberty onset, but whether natural, non-sickness related immune signaling is an important regulator of kisspeptin and/or puberty has not yet been determined. Either abnormally early or late puberty onset is associated with a number of negative mental health outcomes in both sexes [29]. Female puberty onset occurs about one year earlier than was average in 1977 [30]. Thus, it is important to understand how puberty onset might be regulated, and immune signaling, activated by any number of stressors, may be important lines of research in the coming years.
There are also less obvious sexually dimorphic events occurring during adolescence. A recent report [31] found that prior to puberty, female mice have better spatial memory task performance and lower LTP threshold in the hippocampus than males, which is reversed after puberty. The behavioral and neural change in females was due to female-specific increases in α5 GABAA receptor-containing synapses, whereas the behavioral and neural change in males was not related to α5 GABAA signaling. Pro-inflammatory immune challenge via LPS or ethanol increases GABAergic signaling, at least in part through increased expression of GABAA receptors in mixed sex and male mice, respectively [32, 33]. Pharmacologically manipulating microglia was sufficient to reduce GABAA receptor levels and GABAergic signaling in response to both LPS and ethanol challenge. Whether there is a female-specific increase in microglial immune signaling that supports increased GABAA receptor-containing synapses during adolescence is unknown. Interestingly, there is also a recent report that a subset of microglia which express GABA receptors are selectively tuned to monitor and prune inhibitory synapses during normal cortical development [34]. Whether GABA-receptive microglia could instead increase synaptogenesis of GABAergic synapses is unclear, though microglia are known to induce spine formation during somatosensory development [35].
Convergent sex differences during adolescence
We define convergent sex differences to be when either (a) the behavioral outcome is the same or similar across sex but the underlying mechanisms diverge by sex, or (b) the neural mechanism is the same across sex but the resultant behavior diverges by sex. There are now several studies that show convergent sex differences in the regulation of social play during adolescence. Social play peaks during adolescence and declines into adulthood, and is considered a hallmark of adolescent development in several mammalian species; both sexes express social play, though it is typically more pronounced in males relative to females [36]. A recent study demonstrated that sex-specific immune signaling mediates social play in both sexes [37]. Complement C3, an immune protein, and microglia mediate synaptic pruning during the development of several brain regions. C3-microglia-mediated pruning also occurred in the nucleus accumbens reward region in both male and female rats, but during sex-specific periods: pre-early adolescent females (postnatal day (P)20-30) and early-mid adolescent males (P30-38). This observation is in line with females tending to develop earlier than males during adolescence. In males, microglia-mediated pruning eliminates dopamine D1 receptors, consequently reducing social play behavior. Interfering with complement signaling increased D1 receptors and social play in males. Interfering with complement signaling also increased social play in females by the same magnitude as males, but this was D1 receptor independent. These data suggest that pruning impacts social play in both sexes, but via sex-specific synaptic receptor regulation and during sex-specific adolescent periods. Microglia-mediated pruning also occurs in the prefrontal cortex during adolescence [38, 39], but current studies have not explicitly tested whether there are sex differences in this process.
The lateral septum is also critical for social play in both sexes, via complex, sex-specific neural mechanisms. Social play increases both GABA and glutamate release in the lateral septum of both sexes. Blocking GABAA receptor signaling in the lateral septum reduces play in both sexes, but blocking glutamatergic signaling (both NMDA and AMPA signaling) reduces play in females only [40]. Additionally, blocking vasopressin receptors (V1aR) in the lateral septum increases social play in the home cage in males and decreases social play in the home cage in females [41]. Surprisingly, this was context-dependent, and blocking V1aRs in the lateral septum did not affect social play in a novel cage in either sex [42]. These data suggest there is an important and largely unexplored interaction between physical context and behavioral endpoint that can mediate sex-specific neural processing. Social play also increased dopamine release within the lateral septum in females, but not males, but blocking dopamine receptors in the lateral septum decreased social play in both sexes. The authors conclude that basal dopamine signaling in lateral septum is important for male social play, but evoked dopamine signaling in lateral septum is important for female social play [43]. Immune signaling is well known to induce vasopressin release in hypothalamus and amygdala [44-46], but this has not been examined in the lateral septum. Prenatal immune activation, which impacts social play behavior in a sex-specific manner, also reduces vasopressin mRNA levels in a sex- and region-specific manner [47] and increases microglia in the lateral septum in females [48]. In general, the role of immune signaling in the lateral septum is entirely unknown.
Just as physical context is important for the relationship between neural signaling and behavior, so too is social context. Both adult male and female rodents are particularly attuned to the opposite sex. In fact, in adult mice, ~80% of medial amygdala (MeA) neurons in both sexes preferentially respond to the opposite sex, a selectivity that only develops after adolescence. Aromotase knockout in male mice abolished this selectivity in adulthood, and early life masculinization with estradiol injections in female mice increased the number of female-responsive neurons [49]. These data suggest that in males, early life masculinization, an immune-dependent event (reviewed above), interacts with adolescent development to produce opposite-sex selectivity in the MeA. The mechanism in females is unclear. Prairie voles are an interesting species in which to study opposite sex behaviors, as they form monogamous pair bonds [50]. Peripheral immune activation with LPS can facilitate partner preference in female, but not male prairie voles [51], though the neural mechanisms underlying this effect are unclear. Taken together these data suggest that both natural and experimentally-activated immune signaling can play a modulatory role in opposite sex-focused behaviors. Whether immune signaling is important for neuronal tuning to any stimulus is an open question, but would be of broad interest across neuroscience disciplines.
Quantitative sex differences during adolescence
We define quantitative sex differences to be when the behavioral outcome and its underlying mechanistic processes are the same, but either the behavior or mechanistic process differs in quantity by sex. Examples of quantitative sex differences in which there is a well-defined relationship between behavioral endpoint and underlying neural mechanism are scarce. For example, while social play is widely recognized to differ in quantity by sex (males>females), there also appears to be clear sex differences in the mechanisms underlying play, as reviewed above. One example of a quantitative sex difference was uncovered in a study examining the effects of vasopressin signaling in the lateral septum on social discrimination and investigation, rather than play. Blocking V1aRs in the lateral septum in adolescent rats did not impact social discrimination, but increased investigation of a novel vs. familiar stimulus in both sexes. However, the increase in novelty-centered investigation was larger in males than in females, which was unique to adolescent animals relative to adults [52]. It is unclear whether adolescent lateral septum vasopressin signaling is a negative regulator of novelty-centered investigation, a positive regulator of familiarity-centered investigation, or some combination. There is, however, evidence that familiarity may be more salient to female rats: female rats will engage in more social play with a familiar social partner vs. a novel partner, while male rats will play equally regardless of familiarity of conspecific [53]. There is interesting evidence in humans suggesting immune signaling is important for motivation to be near familiar vs. unfamiliar individuals. LPS injection in humans too low to induce overt sickness behaviors increased desire to be near family members vs. strangers, which was positively correlated with peripheral IL-6 pro-inflammatory cytokine levels and BOLD fMRI activity in the nucleus accumbens [54]. Moreover, a recent human neuroimaging study showed that activity in the nucleus accumbens also correlated with the shift in responsivity from a familiar (mother’s) voice in childhood to a novel voice in adolescence [55]. How the neural signaling underlying familiarity-induced preference changes during adolescence has not been examined to our knowledge, but immune signaling may be a good starting point.
Sex divergence with unclear mechanism or behavior: Opportunities
Many adolescent data show a sex difference in either behavior or mechanism, but the behavior-mechanism relationship is not fully defined and thus does not fit clearly into one of the former three categories. These data represent an experimental opportunity. For example, prepubertal gonadectomy resulted in increased neuron numbers in females [56], and increased spine density in males [57]. Microglia were recently shown to be important for synaptic homeostasis during adolescence in the prefrontal cortex of male rats, which had several cognitive implications [39]. Whether immune signaling is important for sex-specific prefrontal cortex neuron numbers, to what behavioral consequence this contributes, and whether microglia also mediate synaptic homeostasis in females, are all undetermined. There are also data indicating that dopamine receptor overexpression during adolescence, a frequent developmental program prior to pruning, is more robust in males than in females in several brain regions [58, 59], resulting in long-term sex differences in dopamine receptor expression (males>females). How this occurs and its behavioral consequences are unclear. However, dopaminergic axon outgrowth during adolescence is an important process mediated by netrin and its receptor, DCC (Deleted in Colorectal Cancer) in male mice [60]. Earlier in life, microglia are important for dopaminergic axon outgrowth [61], but whether immune signaling is important for netrin/DCC-mediated outgrowth during adolescence, and whether the process occurs in females, is unknown.
Conclusions
Several psychiatric disorders emerge during adolescence in sex-specific ways [62], and basal sex differences in neuro-immune signaling has been hypothesized to underlie sex-specific vulnerability to psychiatric disorders [63]. Studies exploring sex specific adolescent neurodevelopment are vital to our understanding and interpretation of clinical observations. Post hoc classifications of sex differences is one way to formalize collective knowledge and quickly identify opportunities to fill gaps in the literature. Existing data emphasize some key design points for future studies, including (1) prospective use of sex as a biological variable, (2) selecting behavioral tasks matched to the brain region under investigation (or vice versa) to assess mechanism-behavior concordance, (3) consideration of a developmental time course given that females develop earlier than males across species, and (4) examining physical- and social context-dependency in endpoints. We propose that immune signaling is often a good candidate mechanism to explore, as it is quickly becoming relevant to every healthy and pathological biological state, both neural or non-neural in nature.
Highlights.
Immune signaling is important for establishing sex differences early in life.
Sex differences are influenced by, and emerge during, adolescence.
The role of immune signaling in adolescent sex differences is under studied.
Immune signaling presents an opportunity to explore adolescent sex differences.
Acknowledgments
This work was supported by the National Institutes of Health [R03AG07011 and R01DA052889 to AMK, and R00HD092894 to SBZS] and Albany Medical College start-up funds to AMK and SBZS.
Footnotes
Disclosure Statement
DNK and AMK conceived of the manuscript. All authors wrote and edited the manuscript. The authors declare no conflicts of interest.
References
* Of special interest
** Of outstanding interest
- 1.Wu Y, et al. , Microglia: Dynamic Mediators of Synapse Development and Plasticity. Trends Immunol, 2015. 36(10): p. 605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lenz KM, et al. , Microglia are essential to masculinization of brain and behavior. J Neurosci, 2013. 33(7): p. 2761–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.VanRyzin JW, et al. , Microglial Phagocytosis of Newborn Cells Is Induced by Endocannabinoids and Sculpts Sex Differences in Juvenile Rat Social Play. Neuron, 2019. 102(2): p. 435–449 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spear LP, The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev, 2000. 24(4): p. 417–63. [DOI] [PubMed] [Google Scholar]
- 5.Schneider M, Adolescence as a vulnerable period to alter rodent behavior. Cell Tissue Res, 2013. 354(1): p. 99–106. [DOI] [PubMed] [Google Scholar]
- 6.Kopec AM, Smith CJ, and Bilbo SD, Neuro-Immune Mechanisms Regulating Social Behavior: Dopamine as Mediator? Trends Neurosci, 2019. 42(5): p. 337–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuhrmann D, Knoll LJ, and Blakemore SJ, Adolescence as a Sensitive Period of Brain Development. Trends Cogn Sci, 2015. 19(10): p. 558–566. [DOI] [PubMed] [Google Scholar]
- 8.Sisk CL and Foster DL, The neural basis of puberty and adolescence. Nat Neurosci, 2004. 7(10): p. 1040–7. [DOI] [PubMed] [Google Scholar]
- 9.Sawyer SM, et al. , The age of adolescence. Lancet Child Adolesc Health, 2018. 2(3): p. 223–228. [DOI] [PubMed] [Google Scholar]
- 10. Arambula SE and McCarthy MM, Neuroendocrine-Immune Crosstalk Shapes Sex-Specific Brain Development. Endocrinology, 2020. 161(6). *This paper elaborates on defining sex differences in neural development and provides an overview of the role of immune signaling establishing sex differences early in life.
- 11.Lapatto R, et al. , Kiss1−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology, 2007. 148(10): p. 4927–36. [DOI] [PubMed] [Google Scholar]
- 12.de Roux N, et al. , Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A, 2003. 100(19): p. 10972–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seminara SB, et al. , The GPR54 gene as a regulator of puberty. N Engl J Med, 2003. 349(17): p. 1614–27. [DOI] [PubMed] [Google Scholar]
- 14.d'Anglemont de Tassigny X, et al. , Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci U S A, 2007. 104(25): p. 10714–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.d'Anglemont de Tassigny X, et al. , Kisspeptin can stimulate gonadotropin-releasing hormone (GnRH) release by a direct action at GnRH nerve terminals. Endocrinology, 2008. 149(8): p. 3926–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irwig MS, et al. , Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology, 2004. 80(4): p. 264–72. [DOI] [PubMed] [Google Scholar]
- 17.Han SK, et al. , Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci, 2005. 25(49): p. 11349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gottsch ML, et al. , A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology, 2004. 145(9): p. 4073–7. [DOI] [PubMed] [Google Scholar]
- 19.Smith JT, et al. , Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology, 2005. 146(9): p. 3686–92. [DOI] [PubMed] [Google Scholar]
- 20.Smith JT, et al. , Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology, 2005. 146(7): p. 2976–84. [DOI] [PubMed] [Google Scholar]
- 21.Takase K, et al. , Possible role of oestrogen in pubertal increase of Kiss1/kisspeptin expression in discrete hypothalamic areas of female rats. J Neuroendocrinol, 2009. 21(6): p. 527–37. [DOI] [PubMed] [Google Scholar]
- 22.Clarkson J and Herbison AE, Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology, 2006. 147(12): p. 5817–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Makowski KN, et al. , Peripheral interleukin-1beta inhibits arcuate kiss1 cells and LH pulses in female mice. J Endocrinol, 2020. 246(2): p. 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long KLP, et al. , Endotoxin rapidly desensitizes the gonads to kisspeptin-induced luteinizing hormone release in male Siberian hamsters (Phodopus sungorus). J Exp Biol, 2018. 221(Pt 23). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwasa T, et al. , Hypothalamic Kiss1 and RFRP gene expressions are changed by a high dose of lipopolysaccharide in female rats. Horm Behav, 2014. 66(2): p. 309–16. [DOI] [PubMed] [Google Scholar]
- 26. Smith KB, et al. , Pubertal immune challenge suppresses the hypothalamic-pituitary-gonadal axis in male and female mice. Brain Res Bull, 2021. 170: p. 90–97. *This paper suggests that puberty is uniquely vulnerable to immune dysregulation, as a single pro-inflammatory event has long-term consequences for endocrine and immune outcomes.
- 27.Ballinger AB, Savage MO, and Sanderson IR, Delayed puberty associated with inflammatory bowel disease. Pediatr Res, 2003. 53(2): p. 205–10. [DOI] [PubMed] [Google Scholar]
- 28.DeBoer MD, et al. , Increases in Sex Hormones during Anti-Tumor Necrosis Factor alpha Therapy in Adolescents with Crohn's Disease. J Pediatr, 2016. 171: p. 146–52 e1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graber JA, Pubertal timing and the development of psychopathology in adolescence and beyond. Horm Behav, 2013. 64(2): p. 262–9. [DOI] [PubMed] [Google Scholar]
- 30.Eckert-Lind C, et al. , Worldwide Secular Trends in Age at Pubertal Onset Assessed by Breast Development Among Girls: A Systematic Review and Meta-analysis. JAMA Pediatr, 2020. 174(4): p. e195881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Le AA, et al. , Prepubescent female rodents have enhanced hippocampal LTP and learning relative to males, reversing in adulthood as inhibition increases. Nat Neurosci, 2022. 25(2): p. 180–190. **This study uncovers a sexually dimorphic developmental event underlying sex differences in learning and memory that emerges during adolescence.
- 32.Warden AS, et al. , Microglia Control Escalation of Drinking in Alcohol-Dependent Mice: Genomic and Synaptic Drivers. Biol Psychiatry, 2020. 88(12): p. 910–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang J, et al. , Systemic LPS-induced microglial activation results in increased GABAergic tone: A mechanism of protection against neuroinflammation in the medial prefrontal cortex in mice. Brain Behav Immun, 2022. 99: p. 53–69. [DOI] [PubMed] [Google Scholar]
- 34. Favuzzi E, et al. , GABA-receptive microglia selectively sculpt developing inhibitory circuits. Cell, 2021. 184(22): p. 5686. *This study showed that transcriptionally unique subsets of microglia attend to specific synapses during development. Sex differences were not explored.
- 35.Miyamoto A, et al. , Microglia contact induces synapse formation in developing somatosensory cortex. Nat Commun, 2016. 7: p. 12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.VanRyzin JW, Marquardt AE, and McCarthy MM, Developmental origins of sex differences in the neural circuitry of play. Int J Play, 2020. 9(1): p. 58–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kopec AM, et al. , Microglial dopamine receptor elimination defines sex-specific nucleus accumbens development and social behavior in adolescent rats. Nat Commun, 2018. 9(1): p. 3769. **This study examines a convergent sex difference mediated by immune signaling and emphasizes the importance of examining sex-specific time courses during development.
- 38.Mallya AP, et al. , Microglial Pruning of Synapses in the Prefrontal Cortex During Adolescence. Cereb Cortex, 2019. 29(4): p. 1634–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schalbetter SM, et al. , Adolescence is a sensitive period for prefrontal microglia to act on cognitive development. Sci Adv, 2022. 8(9): p. eabi6672. **This study uses temporally and spatially precise microglial ablation to demonstrate their importance for prefrontal cortical and cognitive development during adolescence. Sex differences were not explored.
- 40.Bredewold R, et al. , Dynamic changes in extracellular release of GABA and glutamate in the lateral septum during social play behavior in juvenile rats: Implications for sex-specific regulation of social play behavior. Neuroscience, 2015. 307: p. 117–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Veenema AH, Bredewold R, and De Vries GJ, Sex-specific modulation of juvenile social play by vasopressin. Psychoneuroendocrinology, 2013. 38(11): p. 2554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bredewold R, et al. , Sex-specific modulation of juvenile social play behavior by vasopressin and oxytocin depends on social context. Front Behav Neurosci, 2014. 8: p. 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bredewold R, et al. , Involvement of dopamine, but not norepinephrine, in the sex-specific regulation of juvenile socially rewarding behavior by vasopressin. Neuropsychopharmacology, 2018. 43(10): p. 2109–2117. **This study examines a convergent sex difference in dopaminergic signaling utilization underlying social play.
- 44.Wilkinson MF, et al. , Central interleukin-1 beta stimulation of vasopressin release into the rat brain: activation of an antipyretic pathway. J Physiol, 1994. 481 ( Pt 3): p. 641–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raber J and Bloom FE, Arginine vasopressin release by acetylcholine or norepinephrine: region-specific and cytokine-specific regulation. Neuroscience, 1996. 71(3): p. 747–59. [DOI] [PubMed] [Google Scholar]
- 46.Kostoglou-Athanassiou I, et al. , Endotoxin stimulates an endogenous pathway regulating corticotropin-releasing hormone and vasopressin release involving the generation of nitric oxide and carbon monoxide. J Neuroimmunol, 1998. 86(1): p. 104–9. [DOI] [PubMed] [Google Scholar]
- 47.Taylor PV, et al. , Sexually dimorphic effects of a prenatal immune challenge on social play and vasopressin expression in juvenile rats. Biol Sex Differ, 2012. 3(1): p. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Leao E, et al. , Lateral septum microglial changes and behavioral abnormalities of mice exposed to valproic acid during the prenatal period. J Chem Neuroanat, 2021. 111: p. 101875. [DOI] [PubMed] [Google Scholar]
- 49.Bergan JF, Ben-Shaul Y, and Dulac C, Sex-specific processing of social cues in the medial amygdala. Elife, 2014. 3: p. e02743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Loth MK and Donaldson ZR, Oxytocin, Dopamine, and Opioid Interactions Underlying Pair Bonding: Highlighting a Potential Role for Microglia. Endocrinology, 2021. 162(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bilbo SD, et al. , Lipopolysaccharide facilitates partner preference behaviors in female prairie voles. Physiol Behav, 1999. 68(1-2): p. 151–6. [DOI] [PubMed] [Google Scholar]
- 52.Veenema AH, Bredewold R, and De Vries GJ, Vasopressin regulates social recognition in juvenile and adult rats of both sexes, but in sex- and age-specific ways. Horm Behav, 2012. 61(1): p. 50–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Argue KJ and McCarthy MM, Characterization of juvenile play in rats: importance of sex of self and sex of partner. Biol Sex Differ, 2015. 6: p. 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Inagaki TK, et al. , The role of the ventral striatum in inflammatory-induced approach toward support figures. Brain Behav Immun, 2015. 44: p. 247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abrams DA, et al. , A Neurodevelopmental Shift in Reward Circuitry from Mother's to Nonfamilial Voices in Adolescence. J Neurosci, 2022. 42(20): p. 4164–4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koss WA, et al. , Gonadectomy before puberty increases the number of neurons and glia in the medial prefrontal cortex of female, but not male, rats. Dev Psychobiol, 2015. 57(3): p. 305–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Delevich K, et al. , Sex and Pubertal Status Influence Dendritic Spine Density on Frontal Corticostriatal Projection Neurons in Mice. Cereb Cortex, 2020. 30(6): p. 3543–3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Andersen SL, et al. , Sex differences in dopamine receptor overproduction and elimination. Neuroreport, 1997. 8(6): p. 1495–8. [DOI] [PubMed] [Google Scholar]
- 59.Andersen SL, et al. , Pubertal changes in gonadal hormones do not underlie adolescent dopamine receptor overproduction. Psychoneuroendocrinology, 2002. 27(6): p. 683–91. [DOI] [PubMed] [Google Scholar]
- 60.Reynolds LM, et al. , DCC Receptors Drive Prefrontal Cortex Maturation by Determining Dopamine Axon Targeting in Adolescence. Biol Psychiatry, 2018. 83(2): p. 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Squarzoni P, et al. , Microglia modulate wiring of the embryonic forebrain. Cell Rep, 2014. 8(5): p. 1271–9. [DOI] [PubMed] [Google Scholar]
- 62.Paus T, Keshavan M, and Giedd JN, Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci, 2008. 9(12): p. 947–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McCarthy MM, Sex differences in neuroimmunity as an inherent risk factor. Neuropsychopharmacology, 2019. 44(1): p. 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]