Abstract
Background
People with cystic fibrosis (CF) experience chronic airway infections as a result of mucus buildup within the lungs. Repeated infections often cause lung damage and disease. Airway clearance therapies aim to improve mucus clearance, increase sputum production, and improve airway function. The active cycle of breathing technique (ACBT) is an airway clearance method that uses a cycle of techniques to loosen airway secretions including breathing control, thoracic expansion exercises, and the forced expiration technique. This is an update of a previously published review.
Objectives
To compare the clinical effectiveness of ACBT with other airway clearance therapies in CF.
Search methods
We searched the Cochrane Cystic Fibrosis Trials Register, compiled from electronic database searches and handsearching of journals and conference abstract books. We also searched clinical trials registries and the reference lists of relevant articles and reviews.
Date of last search: 29 March 2021.
Selection criteria
We included randomised or quasi‐randomised controlled clinical studies, including cross‐over studies, comparing ACBT with other airway clearance therapies in CF.
Data collection and analysis
Two review authors independently screened each article, abstracted data and assessed the risk of bias of each study. We used GRADE to assess our confidence in the evidence assessing quality of life, participant preference, adverse events, forced expiratory volume in one second (FEV1) % predicted, forced vital capacity (FVC) % predicted, sputum weight, and number of pulmonary exacerbations.
Main results
Our search identified 99 studies, of which 22 (559 participants) met the inclusion criteria. Eight randomised controlled studies (259 participants) were included in the analysis; five were of cross‐over design. The 14 remaining studies were cross‐over studies with inadequate reports for complete assessment. The study size ranged from seven to 65 participants. The age of the participants ranged from six to 63 years (mean age 18.7 years). In 13 studies follow up lasted a single day. However, there were two long‐term randomised controlled studies with follow up of one to three years. Most of the studies did not report on key quality items, and therefore, have an unclear risk of bias in terms of random sequence generation, allocation concealment, and outcome assessor blinding. Due to the nature of the intervention, none of the studies blinded participants or the personnel applying the interventions. However, most of the studies reported on all planned outcomes, had adequate follow up, assessed compliance, and used an intention‐to‐treat analysis.
Included studies compared ACBT with autogenic drainage, airway oscillating devices (AOD), high‐frequency chest compression devices, conventional chest physiotherapy (CCPT), positive expiratory pressure (PEP), and exercise. We found no difference in quality of life between ACBT and PEP mask therapy, AOD, other breathing techniques, or exercise (very low‐certainty evidence). There was no difference in individual preference between ACBT and other breathing techniques (very low‐certainty evidence). One study comparing ACBT with ACBT plus postural exercise reported no deaths and no adverse events (very low‐certainty evidence). We found no differences in lung function (forced expiratory volume in one second (FEV1) % predicted and forced vital capacity (FVC) % predicted), oxygen saturation or expectorated sputum between ACBT and any other technique (very low‐certainty evidence). There were no differences in the number of pulmonary exacerbations between people using ACBT and people using CCPT (low‐certainty evidence) or ACBT with exercise (very low‐certainty evidence), the only comparisons to report this outcome.
Authors' conclusions
There is little evidence to support or reject the use of the ACBT over any other airway clearance therapy and ACBT is comparable with other therapies in outcomes such as participant preference, quality of life, exercise tolerance, lung function, sputum weight, oxygen saturation, and number of pulmonary exacerbations. Longer‐term studies are needed to more adequately assess the effects of ACBT on outcomes important for people with cystic fibrosis such as quality of life and preference.
Keywords: Adolescent, Adult, Child, Humans, Middle Aged, Young Adult, Chest Wall Oscillation, Cystic Fibrosis, Cystic Fibrosis/therapy, Mucus, Quality of Life, Respiratory Therapy, Respiratory Therapy/methods
Plain language summary
A comparison of active cycle of breathing technique (ACBT) with other methods of airway clearance therapies in people with cystic fibrosis
Review question
What are the effects of active cycle of breathing technique (ACBT) compared with other methods of airway clearance in people with cystic fibrosis?
Background
Chronic infections are common in cystic fibrosis, and repeated infections can cause lung damage and disease. People with cystic fibrosis use airway clearance therapies to clear mucus and improve lung function. The ACBT uses a combination of three breathing methods to loosen and clear mucus. This is an update of a previously published review.
Search date
The evidence is current to: 29 March 2021.
Study characteristics
While we included 22 studies comparing ACBT with other airway clearance therapies in the review, only eight studies (259 participants) reported data that we could include in the analysis. Each of the eight studies compared different techniques: ACBT was compared with autogenic drainage, airway oscillating devices, high‐frequency chest compression devices, positive expiratory pressure, conventional chest physiotherapy, and ACBT together with exercise. Most studies lasted a single day, but there were two studies that lasted between one and three years. Participants ranged in age from six to 63 years and most (59%) were male.
Key results
We found that ACBT was comparable with other treatments in outcomes such as quality of life, personal preference, exercise tolerance, lung function, sputum weight, oxygen saturation, and the number of pulmonary exacerbations. We were not able to show that any single technique was better than another. Longer studies are needed to better assess the effects of ACBT on outcomes important for people with cystic fibrosis such as quality of life and personal preference.
Certainty of the evidence
We have little or no confidence in the evidence and think that further research is very likely to affect our conclusions of this review for any of the interventions analysed.
Many of the studies did not provide enough details of their methods to determine if there were any biases that might have affected the results. Many studies did not report how they decided who would get which treatment and how they made sure that the people who were putting people into the different treatment groups and those who were assessing the results did not know which group each individual was in. Most of the included studies had a cross‐over design (where people have one treatment and then switch to the second), and many of these did not report the length of time in between different treatments. As it is possible that the first treatment might affect the results of the next treatment, we only included results from the first treatment period. Many of the studies did not report separate results for just the first treatment period, so we did not include their results in our review.
All participants knew which treatment group they were in (it is not possible to disguise different physiotherapy techniques). This could have affected the results for some of the self‐reported outcomes, such as quality of life, personal preference, or exercise tolerance, but is unlikely to have affected the more objective outcomes, such as lung function.
Most of the studies followed those taking part for less than one month and did this for most of the participants for the entire study period. In two out of the three longer studies more than 10% of the people taking part dropped out. The study results could be affected if the people who dropped out of the studies were not evenly spread across the different treatment groups.
Over half of the studies checked that participants were using the airway clearance therapy they were supposed to. Most of the studies reported on all their planned outcomes.
The findings of the review were limited as not many studies made the same comparisons; also, there were not many long‐term studies and the studies we included did not report enough data.
Summary of findings
Summary of findings 1. Summary of findings: ACBT compared with CCPT for people with cystic fibrosis.
| ACBT compared with CCPT for people with cystic fibrosis | ||||||
|
Patient or population: people with cystic fibrosis Settings: outpatients and hospital inpatients Intervention: ACBT Comparison: CCPT | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| CCPT | ACBT | |||||
|
Quality of life Follow‐up: NA |
No studies reported on this outcome. | |||||
|
Personal preference Follow‐up: NA |
No studies reported on this outcome. | |||||
|
Adverse events Follow‐up: NA |
No studies reported on this outcome. | |||||
|
FEV1: % predicted (change from baseline) Follow‐up: at least 6 months |
No studies reported on the absolute change from baseline in % predicted, but 1 study reported the rate of change from baseline in % predicted, 1 study reported FEV1 in L and 1 study reported the outcome narratively (see comments). | One 3‐year study reported mean (SD) rates of decline in FEV1 % predicted (regression slopes) for each group and then a P value for the difference between the groups. The mean (SD) rate of decline from baseline for participants in the ACBT alone group was statistically significant (‐4.7 (7.1 ); P < 0.001); while there was no statistically significant mean (SD) difference from baseline in the CCPT group (‐1.9 (5.8)). The difference in mean rate of decline between groups was also not significant P < 0.08 (Reisman 1988). 1 study reported no difference between groups in FEV1 L at 12 months, MD 0.52 L (95% CI ‐0.25 to 1.29) (Hristara‐Papadopoulou 2005). A further study reported similar results across both groups when FEV1 was measured in % predicted and L (Osman 2010). |
||||
|
FVC: % predicted (change from baseline) Follow‐up: at least 6 months |
No studies reported on change in FVC % predicted but 1 study reported the rate of change from baseline, 1 reported in L and 1 reported narratively (see comments). | The original paper for the 3‐year study reports the mean (SD) rate of decline in FVC % predicted in each group. The mean (SD) rate of decline from baseline for participants in the ACBT alone group as well as the CCPT group was not statistically significant (ACBT ‐1.6 (5.7) and CCPT 0.2 (4.9)). There was no difference between groups in mean rate of decline (Reisman 1988). 1 study reported no difference in FVC L between groups at 12 months, MD 0.70 L (95% CI ‐0.15 to 1.55) (Hristara‐Papadopoulou 2005). A further study reported similar results across both groups when FVC was measured in % predicted and L (Osman 2010). |
||||
|
Sputum weight (change from baseline) Follow‐up: at least 6 months |
No studies reported on this outcome at this time point. See comments. | A cross‐over study reported on sputum weight at 2 days. We are unable to analyse these data because there was only 1 participant in 1 of the arms (Osman 2010). | ||||
|
Number of pulmonary exacerbations Follow‐up: at least 12 months |
167 per 1000 participants experienced an exacerbation | 274 per 1000 (104 to 725) |
RR 1.64 (95% CI 0.62 to 4.34) | 63 (1 study) | ⊕⊕⊝⊝ lowa,b | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ACBT: active cycle of breathing technique; CCPT: conventional chest physiotherapy; CI: confidence interval; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; NA: not applicable; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aEvidence downgraded due to high risk of bias. The study was considered to have a high risk of bias because of the lack of blinding participants or providers and the lack of reporting of other elements. bEvidence downgraded due to imprecision. The sample sizes were small and the confidence intervals around the effect size were wide.
Summary of findings 2. Summary of findings: ACBT compared with PEP mask therapy for people with cystic fibrosis.
| ACBT compared with PEP mask therapy for people with cystic fibrosis | ||||||
|
Patient or population: people with cystic fibrosis Settings: outpatients and hospital inpatients Intervention: ACBT Comparison: PEP mask therapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| PEP mask therapy | ACBT | |||||
|
Quality of life (change from baseline) SF‐36 score and CRQ score Follow‐up: at least 6 months |
There were no significant differences between groups for the physical domain of the SF‐36 (P = 0.99) but both physical and mental domains decreased after 12 months (P = 0.05 and P = 0.002, respectively). There were no significant differences at the end of the study across the treatment groups for each of the 4 domains of the CRQ: dyspnoea (P = 0.7), fatigue (P = 0.85), emotion (P = 0.39), and mastery (P = 0.82). |
30 (1 study) | ⊕⊝⊝⊝ very lowa,b,c | This was a 5‐arm study which included 75 participants (15 in each arm). For this comparison, 30 participants were included (Pryor 2010). | ||
|
Personal preference Follow‐up: NA |
No studies reported on this outcome. | |||||
|
Adverse events Follow‐up: NA |
No studies reported on this outcome. | |||||
|
FEV1: % predicted (change from baseline) Follow‐up: at least 6 months |
No studies reported on this outcome. | None of the included studies that compared ACBT with PEP mask therapy reported on FEV1 % predicted. However, 1 study with 26 participants reported that there was no difference between ACBT and PEP mask therapy in FEV1 L (MD ‐0.08 L; 95% CI ‐0.85 to 0.69 L) (Pryor 2010). |
||||
|
FVC: % predicted (change from baseline) Follow‐up: at least 6 months |
No studies clearly reported on this outcome. See comments. | 1 study reported that across the 5 treatment groups, there were no significant differences in terms of FVC (P = 0.54). It is unclear if the study is reporting FVC % predicted or L (Pryor 2010). | ||||
|
Sputum weight (change from baseline) Follow‐up: NA |
No studies reported analysable data for this outcome. | Osman 2010 reported participants may have produced more sputum with ACBT + CCPT than after PEP therapy, but there was only a single participant in the PEP group precluding formal analysis. | ||||
|
Number of pulmonary exacerbations Follow‐up: NA |
No studies reported on this outcome. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ACBT: active cycle of breathing technique; CI: confidence interval; CRQ: Chronic Respiratory Disease Questionnaire; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; MD: mean difference; NA: not applicable; PEP: positive expiratory pressure; SF‐36: Short Form‐36. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aEvidence downgraded due to high risk of bias. The study was considered to have a high risk of bias because of the lack of blinding participants or providers, incomplete follow‐up, and it did not conduct an intention‐to‐treat analysis. bEvidence downgraded due to imprecision. The sample size was small. cEvidence downgraded due to suspected publication bias.
Summary of findings 3. Summary of findings: ACBT compared with OD for people with cystic fibrosis.
| ACBT compared with OD for people with cystic fibrosis | ||||||
|
Patient or population: people with cystic fibrosis Settings: outpatients and hospital inpatients Intervention: ACBT Comparison: OD | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| ACBT | OD | |||||
|
Quality of life (change from baseline) in SF‐36 score and CRQ score Follow‐up: at least 6 months |
There were no significant differences between groups for the physical domain of the SF‐36 (P = 0.99) but physical and mental domains decreased after 12 months (P = 0.05 and P = 0.002, respectively). There were no significant differences at the end of the study across the treatment groups for each of the 4 domains of the CRQ: dyspnoea (P = 0.7), fatigue (P = 0.85), emotion (P = 0.39), and mastery (P = 0.82). |
30 (1 study) | ⊕⊝⊝⊝ very lowa,b,c | This was a 5‐arm study which included 75 participants (15 in each arm). For this comparison, 30 participants were included (Pryor 2010). | ||
|
Personal preference Follow‐up: at least 6 months |
No studies reported on this outcome at 6 months. See comments. | 2 studies reported on preference after 2 days of using ACBT or OD. There were no consistent findings across these 2 studies regarding personal preference between the 2 treatments (Milne 2004; Phillips 2004). | ||||
|
Adverse events Follow‐up: NA |
No studies reported on this outcome. | |||||
|
FEV1: % predicted (change from baseline) Follow‐up: at least 6 months |
No studies reported on this outcome at 6 months. See comments. | A cross‐over study reported on the FEV1 % predicted after 2 non‐consecutive days of treatment. The change in FEV1 % predicted was similar for ACBT + CPPT and AOD (MD 5.41% predicted; 95% CI ‐15.62 to 26.44% predicted) and for ACBT + CCPT and HFCC (MD 0.30% predicted; 95% CI ‐15.63 to 16.23% predicted) (Osman 2010). | ||||
|
FVC: % predicted (change from baseline) Follow‐up: at least 6 months |
No studies reported on this outcome at 6 months. See comments. | A cross‐over study reported on the FVC % predicted after 2 non‐consecutive days of use. The change in FVC % predicted was similar for ACBT + CPPT and AOD (MD ‐6.49% predicted; 95% CI ‐22.81 to 9.84% predicted) and for ACBT + CCPT and HFCC (MD ‐5.08% predicted; 95% CI ‐20.62 to 10.47% predicted) (Osman 2010). | ||||
|
Sputum weight (change from baseline) Follow‐up: at least 6 months |
No studies reported on this outcome at 6 months. See comments. | 3 studies reported on sputum weight after 1 or 2 days of treatment. The studies differed in terms of their comparisons and study designs and were limited in their reporting of the results. | ||||
|
Number of pulmonary exacerbations Follow‐up: NA |
No studies reported on this outcome. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ACBT: active cycle of breathing technique; AOD: airway oscillating device (Flutter); CCPT: conventional chest physiotherapy; CI: confidence interval; CRQ: Chronic Respiratory Questionnaire; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; HFCC: high‐frequency chest compression devices (Vest); MD: mean difference; NA: not applicable; OD: oscillating devices. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aEvidence downgraded due to high risk of bias. The study was considered to have a high risk of bias because of the lack of blinding participants or providers, incomplete follow‐up, and it did not conduct an intention‐to‐treat analysis. bEvidence downgraded due to imprecision. The sample size was small. cEvidence downgraded due to suspected publication bias.
Summary of findings 4. Summary of findings: ACBT compared with other breathing techniques for people with cystic fibrosis.
| ACBT compared with other breathing techniques for people with cystic fibrosis | ||||||
|
Patient or population: people with cystic fibrosis Settings: outpatients and hospital inpatients Intervention: ACBT Comparison: other breathing techniques | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Other breathing techniques | ACBT | |||||
|
Quality of life (change from baseline) Follow‐up: at least 6 months |
There were no significant differences between groups for the physical domain of the SF‐36 (P = 0.99) but physical and mental domains decreased after 12 months (P = 0.05 and P = 0.002, respectively). There were no significant differences at the end of the study across the treatment groups for each of the 4 domains of the CRQ: dyspnoea (P = 0.7), fatigue (P = 0.85), emotion (P = 0.39), and mastery (P = 0.82). |
30 (1 study) | ⊕⊝⊝⊝ very lowa,b,c | This was a five arm study which included 75 participants (15 in each arm). For this comparison 30 participants were included (Pryor 2010). | ||
|
Personal preference Follow‐up: at least 6 months |
No studies reported on this outcome at 6 months. See comments. | One 2‐day cross‐over study reported similar rates of preference between ACBT and AD (Miller 1995). | ||||
|
Adverse events Follow‐up: NA |
No studies reported on this outcome. | |||||
|
FEV1: % predicted (change from baseline) Follow‐up: at least 6 months |
No studies reported on this outcome at 6 months. See comments. | 1 study reported on FEV1 % predicted after 2 non‐consecutive days of treatment. The change in FEV1 % predicted was similar for ACBT + CCPT and AD (MD ‐8.30% predicted; 95% CI ‐35.22 to 18.62) (Osman 2010). | ||||
|
FVC: % predicted (change from baseline) Follow‐up: at least 6 months |
No studies reported on this outcome at 6 months. See comments. | 1 study reported on FVC % predicted after 2 non‐consecutive days of treatment. The change in FVC % predicted was similar for ACBT + CCPT and AD (MD ‐11.02; 95% CI ‐32.84 to 10.80) (Osman 2010). | ||||
|
Sputum weight (change from baseline) Follow‐up: at least 6 months |
No studies reported on this outcome at 6 months. See comments. | 2 studies compared ACBT with AD after 1 or 2 days of use. There were no statistically significant differences between treatments in terms of sputum weight (Miller 1995; Osman 2010). | ||||
|
Number of pulmonary exacerbations Follow‐up: NA |
No studies reported on this outcome. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ACBT: active cycle of breathing technique; AD: autogenic drainage; CCPT: conventional chest physiotherapy; CI: confidence interval; CRQ: Chronic Respiratory Questionnaire; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; MD: mean difference; NA: not applicable. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
a Evidence downgraded due to high risk of bias. The trial was considered to have a high risk of bias because of the lack of blinding participants or providers, incomplete follow‐up, and did not conduct an intention‐to‐treat analysis. bEvidence downgraded due to imprecision. The sample size was small. cEvidence downgraded due to suspected publication bias.
Summary of findings 5. Summary of findings: ACBT compared with exercise for people with cystic fibrosis.
| ACBT compared with exercise for people with cystic fibrosis | ||||||
|
Patient or population: people with cystic fibrosis Settings: outpatients and hospital inpatients Intervention: ACBT Comparison: ACBT + exercise | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| ACBT + Exercise | ACBT | |||||
|
Quality of life (change from baseline) CFQ‐R score Follow‐up: at least 6 months |
There was no difference between groups in the median CFQ‐R scores for the subdomains of emotional function (P = 0.431) and treatment difficulties (P = 0.579) at 6 months. | N/A | 19 (1 study) | ⊕⊝⊝⊝ very lowa,b,c | ||
|
Personal preference Follow‐up: NA |
No studies reported on this outcome. | |||||
|
Adverse events Follow‐up: at 6 months |
There were no adverse events reported during the study. | NA | 19 (1 study) | ⊕⊝⊝⊝ very lowa,d | ||
|
FEV1: % predicted (change from baseline) Follow‐up: at least 6 months |
There was no difference between groups in the median FEV1 % predicted at 6 months (P = 0.873). | NA | 19 (1 study) | ⊕⊝⊝⊝ very lowa,b |
||
|
FVC: % predicted (change from baseline) Follow‐up: at least 6 months |
There was no difference between groups in the median FVC % predicted at 6 months (P = 0.749). | NA | 19 (1 study) | ⊕⊝⊝⊝ very lowa,b |
||
|
Sputum weight (change from baseline) Follow‐up: NA |
No studies reported on this outcome. | |||||
|
Number of pulmonary exacerbations Follow‐up: NA |
0 out of 11 people. | 1 out of 11 people | NA | 22 (1 study) | ⊕⊝⊝⊝ very lowa,d | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ACBT: active cycle of breathing technique; CI: confidence interval; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; MD: mean difference; NA: not applicable. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aEvidence downgraded due to unclear risk of bias. Information in clinical trials registry entry was insufficient to determine risk of bias. bEvidence downgraded twice due to imprecision. The sample size was small. cEvidence downgraded due to suspected publication bias. Results used for the analysis were published only in the clinical trials registry. The journal article reported on only 2 of the 8 subdomains of the Cystic Fibrosis Questionnaire‐Revised Child Version. dEvidence downgraded twice due to imprecision. Few or no events occurred during the study.
Background
Description of the condition
Cystic fibrosis (CF) is a hereditary multisystem disorder. It is a relatively common autosomal recessive disease. The incidence is estimated to be between 1 in 3000 to 1 in 6000 in Australia, Canada, the USA and Europe, but is less common in other parts of the world (Scotet 2020). It predominately affects the lungs, liver, and exocrine glands of the pancreas and intestines. Individuals with CF have a defect in the gene responsible for the chloride channel that co‐ordinates salt transport across cells (Rowe 2005). Abnormal sodium transport results in the production of viscous mucus and an environment susceptible to chronic airway obstruction. This leads to pulmonary colonisation by pathogenic bacteria. Pulmonary disease is the leading cause of morbidity and mortality in people with CF, accounting for over 60% of deaths (Cystic Fibrosis Foundation Patient Registry 2020; Cystic Fibrosis Trust 2020a).
Currently, there is no cure for CF, but treatment has been developed to increase the life span and quality of life of individuals with CF. In the USA, the predicted mean age of survival increased from 34.1 years in 2004 to 46.2 years in 2019 (Cystic Fibrosis Foundation Patient Registry 2020). The predicted mean age of survival in the UK and Canada in 2019 was 49.1 and 54.3 years, respectively (Cystic Fibrosis Canada 2020; Cystic Fibrosis Trust 2020a), Importance has been placed on early diagnosis as well as effective disease management. Advances in treatment have greatly decreased disease‐related morbidity and mortality over the past 20 years (Giron 2021).
Description of the intervention
There are a number of methods used to remove airway secretions in individuals with CF. These include a variety of medications and inhalation therapies, as well as breathing exercises and devices. The goals of chest physiotherapy (usually initiated soon after diagnosis) are to improve mucus clearance, increase sputum production, and improve airway function. A Cochrane Review concluded that chest physiotherapy was beneficial for mucus transport in people with CF (Warnock 2015). There is a significant increase in the volume of sputum produced when performing chest physiotherapy compared with cough alone (Lorin 1971).
Conventional chest physiotherapy (CCPT) has been the standard treatment used to treat excessive mucus secretions in CF in North America since the 1950s (McIlwaine 1997). Other airway clearance therapies became popular in the 1990s (McIlwaine 2007). These include the active cycle of breathing technique (ACBT), positive expiratory pressure (PEP) mask therapy, high‐pressure PEP (HiPEP) mask therapy, airway oscillating devices (AOD), autogenic drainage (AD), high‐frequency chest compression devices (HFCC), and the resistive inspiratory manoeuvre (RIM). In the early 1990s, concern about oxygen desaturation during chest physiotherapy was addressed with the use of sufficient pauses for relaxation and breathing control during ACBT (Pryor 1990b). In the late 1990s, the use of AOD was shown to enhance mucus expectoration during exacerbations of CF lung disease (Gondor 1999). A number of these therapies can be self‐administered by the individual, while others require the assistance of a trained physiotherapist, parent, or caregiver. The self‐administered techniques can be performed anywhere once the individual is properly trained. The self‐administered techniques included in this review are ACBT, AD, and exercise. The techniques in this review which can be self‐administered but require the use of a device include PEP, HiPEP, AOD, and HFCC. The techniques in this review which require assistance include CCPT and RIM. Descriptions of all interventions can be found in the Types of interventions section.
How the intervention might work
In ACBT, a cycle of techniques is used to loosen airway secretions. The techniques include breathing control, thoracic expansion exercises, and the forced expiration technique (FET). In breathing control, the individual performs tidal breathing (gentle relaxed breathing) using the lower chest, at his or her own rate and depth (International Physiotherapy Group for CF 2009). Individuals are encouraged to relax their shoulders and upper chest. Breathing control is the resting period between the active parts of ACBT. Thoracic expansion exercises consist of deep breathing with inspiration and passive relaxed expiration. In FET, huffing and breathing control are combined so that one or two forced expirations (huffs) are interspersed with periods of breathing control (International Physiotherapy Group for CF 2009). Huffing is a type of cough which includes inhaling and active exhaling (Cystic Fibrosis Foundation 2023). The length of the huff is altered to optimise clearance. Huffing helps mobilise and clear peripherally‐situated secretions (Pryor 1999). One of the benefits of this technique is that it can be self‐administered by the person with CF.
Why it is important to do this review
People with CF experience chronic airway infections as a result of mucus buildup within the lungs. Repeated infections cause lung damage and disease which are the main causes of death in individuals with CF. For this reason, airway clearance therapies play an important role in the treatment of CF. Scientists have not agreed upon a definitive method of treatment, thus both conventional and alternative treatments are in widespread use. Many treatment centres apply those methods that are most familiar to them and neglect others. Globally, it has been observed that CCPT is widely used in the USA; ACBT is most commonly used in the UK; PEP, flutter (AOD) and AD are commonly used in the rest of Europe; and exercise is the favoured treatment in the Scandinavian countries (Prasad 1998b). Other Cochrane Reviews have explored the effectiveness of airway clearance therapies in CF including CCPT (Main 2005; Warnock 2015), PEP (McIlwaine 2019), AOD (Morrison 2020) and autogenic drainage (Burnham 2021). Three of the reviews included results on comparisons involving ACBT versus other therapies. One review compared ACBT with CCPT (Main 2005), one compared ACBT with AOD (including flutter, acapella, cornet, intrapulmonary percussive ventilation (IPV), and extra‐thoracic oscillations) (Morrison 2020), and one compared ACBT with autogenic drainage (Burnham 2021). A review comparing ACBT with all therapies is needed.
This is an updated version of a previously published review (McKoy 2012; McKoy 2016; Robinson 2010).
Objectives
To compare the clinical effectiveness of ACBT with other airway clearance therapies in CF.
Methods
Criteria for considering studies for this review
Types of studies
Randomised or quasi‐randomised controlled clinical studies, including cross‐over studies, were eligible.
Types of participants
We included individuals with CF diagnosed based on clinical criteria, sweat testing or genotype analysis.
Types of interventions
We compared ACBT with other airway clearance therapies. This includes comparisons as a single technique (e.g. ACBT versus AD or ACBT versus AD and AOD) or in conjunction with other techniques (e.g. ACBT versus ACBT and CCPT).
Airway clearance therapies include the following techniques.
Intervention
ACBT
This self‐administered technique combines breathing control with thoracic expansion and the FET. It may also include postural drainage and chest clapping.
The ACBT methods were initially described as FET. In 1990, the term ACBT was developed to emphasise the importance of breathing control and thoracic expansion, in addition to FET, within the technique (Webber 1998). As a result of this reclassification, we included all studies which described FET interventions that contained all of the components of ACBT outlined above. We used the definitions of the ACBT components as described by the Cystic Fibrosis Foundation and the Cystic Fibrosis Trust in the process (Cystic Fibrosis Foundation 2023; Cystic Fibrosis Trust 2020b).
Comparators
CCPT
CCPT combines a collection of techniques which include postural drainage, percussion, chest shaking, huffing, and coughing. It excludes the use of exercise, PEP, or other mechanical devices. This technique requires assistance.
PEP
PEP therapy
Breathing with a PEP of 10 cm to 25 cm of water; this technique can be self‐administered but requires a device. In adults and adolescents PEP valves or mouthpieces are more commonly used than masks, which are more commonly used by children with CF.
HiPEP therapy
A modification of PEP that includes a full forced expiration against a fixed mechanical resistance, which usually generates pressures ranging from 40 cm to 100 cm of water (McIlwaine 2019). This technique can be self‐administered but requires a device.
Oscillatory devices
AOD
Includes flutter, cornet, acapella, and intrapulmonary percussive ventilation (IPV). This technique can be self‐administered but requires a device.
HFCC
The Vest™ (formerly known as ThAIRapy Vest and manufactured by Hill‐Rom) and the Hayek Oscillator (manufactured by Breasy Medical Equipment Ltd) provide external chest wall compression. This also includes high‐frequency chest wall oscillations (HFCWO) which utilizes The Vest™ with interim periods of huffs or coughs. This technique can be self‐administered but requires a device.
Breathing techniques (excluding ACBT)
AD
A self‐administered breathing technique that uses optimal expiratory flow rates at varying lung volumes to mobilise mucus while avoiding airway closure.
Exercise
A combination of endurance and strength training for the upper and lower body. This technique is self‐administered.
Other therapy
RIM
Includes inspiration against resistance after forced expiration. Repeated inspirations at 80% of the maximum sustained inspiratory pressure are completed in groups of six efforts with rest intervals in between (Chatham 2004). This technique requires assistance and the use of a device.
Types of outcome measures
We assessed the following outcome measures.
Primary outcomes
Quality of life ‐ all instruments that measure the ability of participants to perform activities of daily living (including but not limited to the Cystic Fibrosis Questionnaire (CFQ), Health Assessment Questionnaire (HAQ), Quality of Well Being (QWB) scale, and Nottingham Health Profile (NHP))
Personal preference ‐ the nominated technique of choice by the participant at the conclusion of the study, or by comparison of technique acceptability
Mortality
Secondary outcomes
Adverse events
Exercise tolerance ‐ subjective exercise tolerance or objective measures such as the six‐minute walk test or treadmill test
-
Lung function
forced expiratory volume in one second (FEV1) in L or per cent (%) predicted
forced vital capacity (FVC) in L or % predicted
-
Sputum
dry weight (g)
wet weight (g)
volume (mL)
-
Oxygen saturation
arterial blood gas
pulse oximetry
transcutaneous oximetry
Number of pulmonary exacerbations
Search methods for identification of studies
Studies were eligible for inclusion irrespective of publication status (e.g. abstract or online trial report) or language.
Electronic searches
We identified relevant studies from the Cystic Fibrosis and Genetic Disorders (CFGD) Group's Cystic Fibrosis Trials Register using the terms: active cycle breathing technique (ACBT) OR forced expiration technique (FET or Huff). The Cystic Fibrosis Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of the Cochrane Library), weekly searches of MEDLINE, a search of Embase to 1995 and the prospective handsearching of two journals ‐ Pediatric Pulmonology and the Journal of Cystic Fibrosis. Unpublished work is identified by searching the abstract books of three major cystic fibrosis conferences: the International Cystic Fibrosis Conference; the European Cystic Fibrosis Conference and the North American Cystic Fibrosis Conference. For full details of all searching activities for the register, please see the relevant section of the Cochrane Cystic Fibrosis and Genetic Disorders Group's website.
Date of most recent search: 4 November 2022.
We searched the ClinicalTrials.gov trials registry (clinicaltrials.gov/) and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP); please see the appendices for the search strategies (Appendix 1). The date of the most recent search of the registries was 3 April 2021.
Searching other resources
We searched the reference lists of relevant articles and reviews for additional studies.
Data collection and analysis
Selection of studies
We used a two‐tier screening process to identify relevant articles. Initially, we screened the titles and abstracts of articles identified through searching and obtained the full text versions of those considered potentially relevant. We then screened the full text articles to identify those studies which were eligible for data abstraction and should be included in the review. Two review authors independently screened each article. We resolved any disagreements by consensus or by consulting a third review author.
Data extraction and management
We imported the search results into reference management software (ProCite 1999). We used this software to track the results of the two‐tier screening process. We then abstracted information from eligible review articles and entered data into RevMan 5 (RevMan 2020). The Managing Editor of the CFGD Group translated one paper from German (Hristara‐Papadopoulou 2005).
We grouped studies together based on the time of assessment of outcomes. We considered outcomes as immediate if less than one day in duration; short term if up to one week in duration; medium term if up to one month in duration; and long term if beyond one month in duration.
Assessment of risk of bias in included studies
We assessed the risk of bias in included studies through assessment of sequence generation; allocation concealment; blinding of the study participants, personnel, and outcome assessors; compliance assessment; washout reporting; intention‐to‐treat analysis; adequate follow up; and selective reporting. Two review authors independently applied the methods for evaluating the risk of bias as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). A third author resolved any disagreements.
Measures of treatment effect
We analysed continuous outcomes by mean difference (MD) (or we calculated standardised mean difference (SMD) if study reports used different scales of measurement). We analysed dichotomous outcomes using risk ratios (RR). We have presented all outcomes with the associated 95% confidence intervals (CIs).
Unit of analysis issues
When conducting analyses, we took into consideration the level at which randomisation occurred (Deeks 2011). Randomised controlled studies with parallel group designs are studies where individuals are independently randomised to intervention groups. In randomised cross‐over studies, individuals receive each intervention sequentially in a random order. Cross‐over studies usually contain a washout period, which is a stage after the first treatment but before the second treatment, where time is given for the active effects of the first treatment to wear off before the new treatment begins (i.e. to reduce the carry‐over effect). A concern with the cross‐over design is the risk of a carry‐over effect when the first treatment affects the second. If the carry‐over effect exceeds the washout period, the washout is inadequate. For this review, we considered an adequate washout period for cross‐over studies to be a minimum of one day.
When including both parallel and cross‐over studies with an adequate washout period, we used the inverse variance method, as recommended by Elbourne 2002. In this method, we used the results from paired analyses (including an estimate of treatment effect and its standard error) of the cross‐over studies. In the meta‐analysis, the weight of each study is inversely proportional to the variance (one over the square of the standard error) (Deeks 2011). When including cross‐over studies with an inadequate washout period, we used only the first‐arm data. Even though all information is not considered in this method, this avoids inappropriate consideration of multiple arms.
A total of 19 of the 22 studies were randomised cross‐over studies; for those studies with adequate washout periods, we have included all data (Hristara‐Papadopoulou 2005; Miller 1995; Milne 2004; Phillips 2004). For studies with inadequate washout periods, we planned to include only first‐arm data collected before the first cross‐over. In one study that had no washout period, we contacted the authors and were able to obtain first‐arm data, which we have included (Osman 2010). We have contacted the corresponding authors of the remaining studies with inadequate or not reported washout periods, and are awaiting their responses (Bilton 1992; Chatham 2004; Fauroux 1999; Hofmeyr 1986; Holland 2003; Howard 2000; Hristara‐Papadopoulou 2007; Kofler 1994; Mortensen 1991; Pike 1999; Pryor 1979; Pryor 1994; Steven 1992; Webber 1985). These studies are included in the qualitative synthesis of the review, but excluded from the quantitative synthesis.
Dealing with missing data
We contacted the original investigators of studies when we encountered missing, incomplete, or unclear data. If we could not locate the investigators or they did not send the requested data, we categorised these studies as 'Studies awaiting classification', to be included in future updates of the review, if data are made available.
Assessment of heterogeneity
If we are able to include sufficient data in future updates, we will assess heterogeneity within each outcome between the comparisons using the Chi² test and I² statistic (Deeks 2011).
Under the null hypothesis of homogeneity, we will consider a P value less than 0.10 to indicate the presence of heterogeneity in the Chi² test (Deeks 2011). We will interpret the results with care since the test could have low or high power. Low power is common when studies have a small sample size or there are a small number of studies, which may result in the lack of detection of heterogeneity when it is present. High power is common when there are many studies being analysed, resulting in the detection of heterogeneity that might be insignificant.
The I²statistic measures the proportion of inconsistency in individual studies that cannot be explained by sampling error. In this test the degree of heterogeneity is quantified. The values of I² lie between 0% and 100%. We will consider results for I² which are less than 40% to indicate that heterogeneity might not be important, between 30% and 60% to indicate that heterogeneity may be moderate, between 50% and 90% to indicate that heterogeneity may be substantial, and between 75% and 100% to indicate considerable heterogeneity (Deeks 2011).
Assessment of reporting biases
We assessed outcome reporting bias. Study authors may record more outcome measures than they choose to publish, which can lead to misleading results (Sterne 2011). We compared the 'Methods' section of each included paper to the 'Results' section to ensure all outcomes were reported.
If we are able to include sufficient data in future updates, we will assess reporting bias among the studies using the funnel plot method discussed in the Cochrane Handbook for Systematic Reviews of Interventions (Sterne 2011). If asymmetry is present, we will explore possible causes including publication bias, poor methodological quality, and true heterogeneity.
Data synthesis
We entered data abstracted from included studies into RevMan 5 (RevMan 2020). We analysed each comparison separately.
If we are able to include sufficient data in future updates, we will assess heterogeneity. If we determine that heterogeneity may be moderate, substantial, or considerable, as indicated by an I² result greater than 30%, we will use the random‐effects model to synthesise the results. Otherwise, we will synthesise the results using a fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
If we are able to include sufficient data in future updates, we will investigate heterogeneity by performing the following subgroup analyses:
treatment setting (home versus hospital);
treatment length (one day on and one day off, once daily, twice daily, three times daily, three times per week);
age (paediatric, adolescent, adult);
gender;
disease severity (FEV1 % predicted above 90%, 70% to 89%, 40% to 69%, under 40%).
Sensitivity analysis
If we are able to include sufficient data in future updates, we will perform sensitivity analyses to identify the effects on the results of study size (stratify by sample size), study design (cross‐over versus parallel studies), allocation concealment (high risk of bias versus low risk of bias), assessor blinding (high risk of bias versus low risk of bias), and loss to follow up (high risk of bias versus low risk of bias).
Summary of findings and assessment of the certainty of the evidence
In a post hoc change and in line with current Cochrane guidance, we have prepared a summary of findings table for the following comparisons: ACBT versus CCPT (Table 1), ACBT versus PEP mask therapy (Table 2), ACBT versus OD (Table 3), ACBT versus other breathing technique (Table 4), and ACBT compared with exercise (Table 5). We have listed the population, setting, intervention and comparison and have reported an illustrative risk for the experimental and control intervention (Schünemann 2011). We graded the overall certainty of the body of evidence as high, moderate, low or very low using GRADE (Schünemann 2006). We based our judgements on the risk of bias within the trials, their relevance to our population of interest (indirectness), unexplained heterogeneity or inconsistency, imprecision of the results or high risk of publication bias. We downgraded the evidence once if the risk was serious and twice if the risk was deemed to be very serious and described the rationale for each judgement in footnotes to each table.
For each comparison, where possible, we reported the following outcomes: quality of life (change from baseline to six months), individual preference at six months, adverse events at six months, FEV1 % predicted (change from baseline to six months), FVC % predicted (change from baseline to six months), sputum weight (change from baseline to six months), and number of pulmonary exacerbations at 12 months.
Results
Description of studies
Results of the search
In total, we identified 161 citations representing 99 studies (Figure 1). The electronic searches of the CFGD Trials Register identified 114 citations representing 52 studies. We identified an additional 20 citations, representing 20 studies, by searching the reference lists of relevant articles. Our search of ClinicalTrials.gov yielded 31 citations, one of which had already been identified from the CFGD Trials Register. Our search of the WHO ICTRP yielded 11 citations, three of which were already captured in the ClinicalTrials.gov search. Of the 161 citations representing 99 studies, we excluded 35 at the title and abstract screening stage. We reviewed the full texts for 81 studies (126 citations). We included 22 studies (43 citations) and excluded 59 studies (83 citations) (see below).
1.
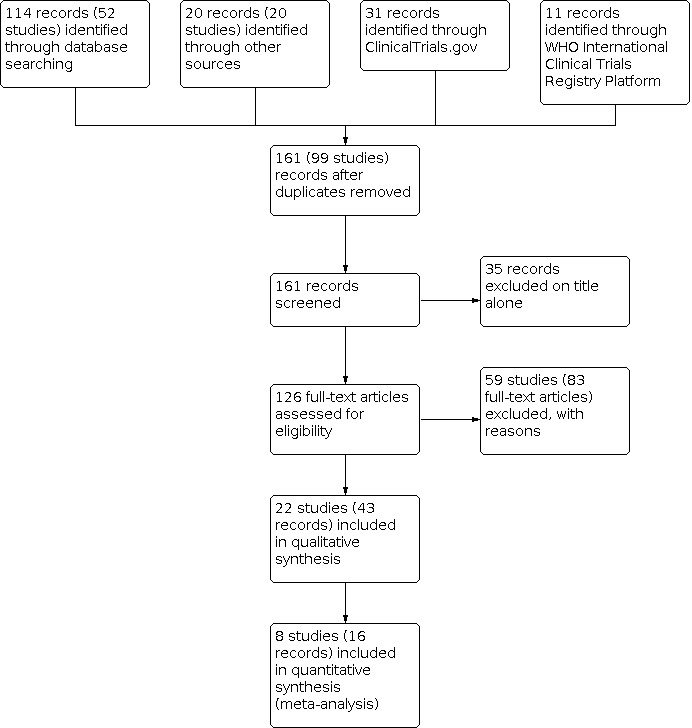
Study flow diagram.
Included studies
We included 43 citations representing 22 studies. Full‐text articles were available for 19 studies (Bilton 1992; Chatham 2004; Fauroux 1999; Gungor 2021; Hofmeyr 1986; Holland 2003; Hristara‐Papadopoulou 2005; Hristara‐Papadopoulou 2007; Miller 1995; Milne 2004; Mortensen 1991; Osman 2010; Phillips 2004; Pryor 1979; Pryor 1994; Pryor 2010; Reisman 1988; Steven 1992; Webber 1985), while only abstracts were available for three studies (Howard 2000; Kofler 1994; Pike 1999). When multiple citations were available for a study, we extracted data from full‐text articles. We reviewed all citations, and when applicable, also extracted date for additional outcomes not included in the full‐text articles.
Of the 22 included studies, we included eight in the analysis: three randomised controlled parallel studies (Gungor 2021; Pryor 2010; Reisman 1988), four randomised cross‐over studies with adequate washout periods (Hristara‐Papadopoulou 2005; Miller 1995; Milne 2004; Phillips 2004), and one randomised cross‐over study with an inadequate washout period for which we have first‐arm data before the first cross‐over period (Osman 2010). The remaining studies were randomised cross‐over studies with inadequate washout periods, and we have attempted to obtain first‐arm data collected before the first cross‐over. We have contacted the study authors and are awaiting their responses.
Seven studies declared funding from either government agencies, patient organisations or charities (Bilton 1992; Fauroux 1999; Hofmeyr 1986; Mortensen 1991; Pryor 2010; Reisman 1988; Webber 1985), and two studies declared funding from these sources plus some industry sponsorship (Chatham 2004; Osman 2010). One study was soley sponsored by industry funding (Miller 1995). One trial specifically reported no funding was received (Gungor 2021), and the remaining 11 studies did not report or describe any funding (Holland 2003; Howard 2000; Hristara‐Papadopoulou 2005; Hristara‐Papadopoulou 2007; Kofler 1994; Milne 2004; Phillips 2004; Pike 1999; Pryor 1979; Pryor 1994; Steven 1992).
We have included information on all 22 included studies in the sections Included studies and Risk of bias in included studies. We have included results on the eight studies included in the analysis in the Effects of interventions section.
Trial design
In 13 studies the intervention duration was less than one day (Bilton 1992; Fauroux 1999; Hofmeyr 1986; Holland 2003; Howard 2000; Miller 1995; Milne 2004; Mortensen 1991; Osman 2010; Phillips 2004; Pike 1999; Pryor 1994; Steven 1992). Three studies had an intervention duration between two days and one week (Chatham 2004; Pryor 1979; Webber 1985). Six studies had an intervention duration of longer than one month (Gungor 2021; Hristara‐Papadopoulou 2005; Hristara‐Papadopoulou 2007; Kofler 1994; Pryor 2010; Reisman 1988). The duration of the intervention in the randomised controlled studies with a parallel design were six weeks, one year, and three years (Gungor 2021; Pryor 2010; Reisman 1988).
We defined adequate washout for cross‐over studies to be a minimum of one day. A total of 19 of the 22 studies were randomised cross‐over studies (Bilton 1992; Chatham 2004; Fauroux 1999; Hofmeyr 1986; Holland 2003; Howard 2000; Hristara‐Papadopoulou 2005; Hristara‐Papadopoulou 2007; Kofler 1994; Miller 1995; Milne 2004; Mortensen 1991; Osman 2010; Phillips 2004; Pike 1999; Pryor 1979; Pryor 1994; Steven 1992; Webber 1985). Of these, only four studies had adequate washout periods (Hristara‐Papadopoulou 2005; Miller 1995; Milne 2004; Phillips 2004).
Participants
There were a total of 559 participants across the 22 studies. The smallest study had seven participants (Milne 2004), while the largest had 75 participants (Pryor 2010). Age ranged from six years to 63 years across all studies, and the mean age was 18.7 years. Of the 506 participants in the 20 studies which reported the sex of participants, 298 (59%) were male. Two studies, only available in abstract form, did not report age or sex of participants (Howard 2000; Kofler 1994).
The participants in a number of the included studies were identified as being infected or colonised with bacteria. In four studies, all analysed participants were infected or colonised with Pseudomonas aeruginosa (Bilton 1992; Chatham 2004; Hofmeyr 1986; Mortensen 1991). In another study, approximately half of the participants were infected or colonised with P aeruginosa and Burkholderia cepacia (Fauroux 1999). In five studies, all participants had an exacerbation of bronchopulmonary infection (Hofmeyr 1986; Phillips 2004; Pryor 1979; Steven 1992; Webber 1985). The authors of one study noted that all of the participants had a history of chronic bronchopulmonary infection (Pryor 1994). One study excluded individuals who had acquired B cepacia within the last three months (Pryor 2010). One study included participants who were stable and did not present with exacerbations (Hristara‐Papadopoulou 2007). One study did not report on participants' infection status (Hristara‐Papadopoulou 2005).
One study stratified randomisation by age, sex, and pulmonary impairment (Reisman 1988) and another study stratified randomisation by pulmonary impairment and sputum expectorated (Pryor 2010). Stratification of results based on the degree of pulmonary impairment was performed in three studies (Fauroux 1999; Miller 1995; Mortensen 1991).
Interventions
Sixteen studies specifically named ACBT as an intervention (Bilton 1992; Chatham 2004; Gungor 2021; Holland 2003; Howard 2000; Hristara‐Papadopoulou 2005; Hristara‐Papadopoulou 2007; Kofler 1994; Miller 1995; Milne 2004; Osman 2010; Phillips 2004; Pike 1999; Pryor 1994; Pryor 2010; Steven 1992). Of these studies, five studies did not describe the components of ACBT (Hristara‐Papadopoulou 2005; Howard 2000; Kofler 1994; Pike 1999; Pryor 2010). Six studies included FET as an intervention, but reported FET to contain all of the components of ACBT as described in the Methods section of this review (Fauroux 1999; Hofmeyr 1986; Mortensen 1991; Pryor 1979; Reisman 1988; Webber 1985). We considered these six studies as including ACBT as an intervention.
Ten studies compared ACBT as a single technique (Gungor 2021; Howard 2000; Hristara‐Papadopoulou 2005; Kofler 1994; Miller 1995; Milne 2004; Phillips 2004; Pike 1999; Pryor 2010; Steven 1992). Twelve studies compared ACBT in conjunction with other techniques (Bilton 1992; Chatham 2004; Fauroux 1999; Hofmeyr 1986; Holland 2003; Hristara‐Papadopoulou 2007; Mortensen 1991; Osman 2010; Pryor 1979; Pryor 1994; Reisman 1988; Webber 1985). Five studies included postural drainage as a component of ACBT (Hristara‐Papadopoulou 2007; Miller 1995; Mortensen 1991; Steven 1992; Webber 1985).
Outcomes
All nine outcomes of this review were reported on by at least one study. Sputum (weight or volume) was the most often reported outcome, discussed in 18 of the 22 studies (Bilton 1992; Chatham 2004; Fauroux 1999; Hofmeyr 1986; Holland 2003; Howard 2000; Hristara‐Papadopoulou 2007; Miller 1995; Milne 2004; Mortensen 1991; Osman 2010; Phillips 2004; Pike 1999; Pryor 1979; Pryor 1994; Pryor 2010; Steven 1992; Webber 1985). The least reported outcomes, which were discussed in only one study each, were mortality (Gungor 2021) and adverse events (Gungor 2021).
Excluded studies
We excluded a total of 59 studies (83 citations).
We excluded 19 studies because they did not address ACBT (ACTRN12619001681145; Asher 1982; Bain 1988; Baldwin 1994; Chatham 1998; Davies 2012; Desmond 1983; Kofler 1998; McDonnell 1986; NCT00164138; NCT00404859; NCT00716664; NCT01943890; NCT02906826; O'Neill 2017; Rossman 1982; Sontag 2010; Sutton 1985; Ward 2018). We excluded 11 studies because they were not randomised (ACTRN12605000471684; Horsley 2007; Klig 1989; Oberwaldner 1986; Orlik 2000; Orlik 2001Pryor 1990; Rogers 1984; Salh 1989; Webber 1986; Wilson 1995). Four studies were review articles with no original data (Prasad 1998a; Prasad 2000; Thomas 1995; Williams 1994). Five studies did not include people with CF (ACTRN12614001233617; ChiCTR1800019989; Hasani 1991; Hasani 1994; van Hengstum 1987). Six studies did not have a comparison of interest (Braggion 1995; Stanford 2020; Verboon 1986; White 1997; Williams 2000; Znotina 2000). We excluded seven studies because the description of the FET intervention did not contain all components of ACBT (ACTRN12619000224123; Andreasson 1987; Falk 1984; Gursli 2017; NCT03078127; RBR‐5g9f6w; Sutton 1983). One additional study was excluded because the intervention of interest was not randomised (Steen 1991).
We excluded six studies (10 citations) previously listed as awaiting classification. Five of the studies were associated with abstracts that provided insufficient information (Castle 1994; Falk 1993; Lannefors 1992; Parker 1984; Petrone 2009), and one study had a mixed population of participants with and without CF (van Hengstum 1988). To date we have not received any response to our requests for additional data to allow us to include these studies and, given the age of the studies, do not expect to receive relevant information now; therefore we have excluded these studies. If we receive any relevant information in future, we will re‐assess these studies.
Risk of bias in included studies
We assessed risk of bias for sequence generation; allocation concealment; blinding of the study participants, personnel, and outcome assessors; incomplete outcome data (intention‐to‐treat analysis, adequate follow up); selective reporting within each study; and other potential sources of bias (compliance or adherence assessment; washout reporting). Please see the figures for a summary of judgements on the risk of bias of all included studies (Figure 2; Figure 3).
2.

Risk of bias summary: review authors' judgements about risk of bias domains for each included study.
3.

Risk of bias graph: review authors' judgements about risk of bias domains presented as percentages across all included studies.
Allocation
Sequence generation
In one of the 22 studies, which compared HFCWO with usual ACT, participants performed their usual ACT or HFCWO during alternate day treatment, and allocation to either treatment on day one was determined using a computer‐generated randomisation table (Osman 2010). A further study used a computer‐generated randomisation scheme to randomise participants to one of five airway clearance therapies (Pryor 2010). Thus, the risk of bias of sequence generation was low in these two studies.
In 20 of the 22 included studies, the authors did not specify how the random allocation was generated. The studies made statements that participants were randomly allocated to different treatment groups, but did not completely define the approaches. The risk of bias of sequence generation was unclear in these studies (Bilton 1992; Chatham 2004; Fauroux 1999; Gungor 2021; Hofmeyr 1986; Holland 2003; Howard 2000; Hristara‐Papadopoulou 2005; Hristara‐Papadopoulou 2007; Kofler 1994; Miller 1995; Milne 2004; Mortensen 1991; Phillips 2004; Pike 1999; Pryor 1979; Pryor 1994; Reisman 1988; Steven 1992; Webber 1985).
Allocation concealment
Concealment of treatment allocation was reported in only two studies. Both studies used sealed envelopes during randomisation (Gungor 2021; Phillips 2004). We judged there to be a low risk of bias due to allocation concealment associated with these studies. In the remaining 20 studies, there was not sufficient information to make a judgement on allocation concealment; thus, the risk of bias of allocation concealment was unclear.
Blinding
Each intervention within the included studies was associated with a physical activity or mechanical devices (or both) which are necessary to the intervention. For this reason it was not feasible to blind the participants or personnel, as observed in all included studies. Lack of blinding may affect some outcomes more than others. We considered studies that did not blind participants or personnel and reported on subjective outcomes, such as personal preference or quality of life, to have a high risk of bias (Bilton 1992; Fauroux 1999; Gungor 2021; Holland 2003; Kofler 1994; Miller 1995; Milne 2004; Osman 2010; Phillips 2004; Pike 1999; Pryor 1994; Pryor 2010). We considered studies that did not blind participants or personnel and reported only on objective outcomes, such as lung function or sputum weight, to have a low risk of bias (Chatham 2004; Hofmeyr 1986; Howard 2000; Hristara‐Papadopoulou 2005; Hristara‐Papadopoulou 2007; Mortensen 1991; Pryor 1979; Reisman 1988; Steven 1992; Webber 1985).
Five studies reported that the outcome assessors were blinded (Chatham 2004; Gungor 2021; Holland 2003; Pryor 1994; Pryor 2010). In one study it was noted that the laboratory researcher was blinded to the treatment administered to participants (Chatham 2004). In the other four studies, the authors noted that an independent data collector, who was blinded to treatment order or treatment assignment, collected the measurements (Gungor 2021; Holland 2003; Pryor 1994; Pryor 2010). There is a low risk bias of blinding of outcome assessors associated with these four studies. In the remaining 17 studies the blinding of the outcome assessor is not specified, thus the risk of bias is unclear.
Incomplete outcome data
Intention‐to‐treat
The use of intention‐to‐treat analyses was unclear in four studies, therefore we also judged the related risk of bias to be unclear (Howard 2000; Hristara‐Papadopoulou 2005; Hristara‐Papadopoulou 2007; Pike 1999). In eight studies, intention‐to‐treat analyses were not performed, and we judged these studies to have a high risk of bias because they did not include outcome data in the analysis from participants who withdrew (Gungor 2021; Holland 2003; Kofler 1994; Osman 2010; Pryor 1979; Pryor 1994; Pryor 2010; Reisman 1988). The remaining 10 studies are associated with a low risk of bias. In these studies, the participants were analysed in the groups to which they were randomised.
Adequate follow‐up
It is unclear if follow‐up was adequate in three studies (Hristara‐Papadopoulou 2005; Howard 2000; Pike 1999), two of which were only available as conference abstracts (Howard 2000; Pike 1999). The risk of bias due to adequate follow‐up is unclear in these two studies.
Follow‐up was inadequate in six studies (Hristara‐Papadopoulou 2007; Kofler 1994; Gungor 2021; Pryor 1979; Pryor 1994; Pryor 2010). In one study, seven out of 35 participants (20%) were not accounted for in the analysis (Hristara‐Papadopoulou 2007). In another study, which is only available in abstract form, 10 out of 33 (30.30%) children with CF did not complete the program, and there was no description of reasons for loss to follow‐up (Kofler 1994). In a further study, three out of 22 participants (14%) did not complete the study (Gungor 2021). In a fourth study, two out of 18 CF participants (11.11%) withdrew: one person developed a pneumothorax and the other person was unable to produce enough sputum for an accurate assessment (Pryor 1979). In the fifth study, four out of 24 (16.67%) participants withdrew from the study: two participants had their drug regimens changed during the study and two participants withdrew because of technical problems with the oximeter and collection of sputum (Pryor 1994). In the last study, 10 out of 75 participants (13.33%) were lost to follow‐up: one died, one was accepted to the transplantation list, one required a limited pleurodesis for a pneumothorax, three were lost to follow‐up, and three withdrew; the status of one of the participants was not reported (Pryor 2010). Since the loss to follow‐up was greater than 10% in these six studies, they are associated with a high risk of bias.
In the remaining 13 studies the follow‐up was adequate, thus there is a low risk of bias associated with follow‐up in these studies. While all of the participants were accounted for in these 13 studies, authors in three studies reported having participants who withdrew for varying reasons (Holland 2003; Osman 2010, Reisman 1988). In one study, one out of 27 (3.70%) participants withdrew because of pain during respiratory muscle testing (Holland 2003). In another study, one out of 30 (3.33%) participants withdrew due to a hypoglycaemic episode (Osman 2010). In a third study, four out of 67 (5.97%) participants withdrew from the study: two participants from the ACBT + CCPT group relocated and another two participants from the ACBT group withdrew because of family anxiety associated with discontinuation of CCPT used with FET (Reisman 1988). Since the loss to follow‐up was less than 10% in the studies with stated reasons for participant withdrawal, they were all associated with a low risk of bias.
Selective reporting
Since study protocols were unavailable for most studies to allow the comparison of planned and reported outcomes, we assessed selective reporting by comparing the outcomes outlined in the 'Methods' section with those outlined in the 'Results' section of the published papers.
The risk of bias from selective reporting was unclear in three studies that were only available as conference abstracts (Howard 2000; Kofler 1994; Pike 1999). Four studies were thought to potentially involve selective reporting, and we have judged there to be a high risk of bias associated with these studies (Gungor 2021; Pryor 1994; Reisman 1988; Webber 1986). In one study, different outcomes and results were reported in the paper than in the study protocol, which was posted in the trial registry (Gungor 2021). A second study stated that lung function measurements were recorded before and at 5, 10, 15 and 30 minutes after treatment (Pryor 1994); while the third study stated that lung function values were collected before and 30 minutes after the first daily treatment (Webber 1986). In both studies, the authors did not report actual data, only that there were no statistical differences in the lung function measurements collected at the start of treatment each day. The final study mentioned collecting sputum, but did not report on the results of sputum collection (Reisman 1988).
In the remaining 15 studies all outcomes outlined in the 'Methods' section were reported in the 'Results' section, or details were available on the trial registry website; thus, there is a low risk of bias from selective reporting associated with these studies.
Other potential sources of bias
Compliance Assessment
Compliance was assessed in 11 out of the 22 studies (Chatham 2004; Fauroux 1999; Milne 2004; Mortensen 1991; Osman 2010; Phillips 2004; Pryor 1979; Pryor 1994; Pryor 2010; Reisman 1988; Webber 1985). Compliance assessment involved the use of a diary in one study (Reisman 1988), monthly review in another study (Pryor 2010), and supervision in the remaining nine studies; the risk of bias is low in these 11 studies. In the remaining 11 studies it is unclear if compliance was assessed, thus the risk of bias assessment is unclear.
Washout
A total of 19 of the 22 included studies were randomised cross‐over studies (Bilton 1992; Chatham 2004; Fauroux 1999; Hofmeyr 1986; Holland 2003; Howard 2000; Hristara‐Papadopoulou 2005; Hristara‐Papadopoulou 2007; Kofler 1994; Miller 1995; Milne 2004; Mortensen 1991; Osman 2010; Phillips 2004; Pike 1999; Pryor 1979; Pryor 1994; Steven 1992; Webber 1985). Of these, four had adequate washout periods (at least one day): two studies had a one‐day washout period (Milne 2004; Phillips 2004), one study had a seven‐day washout period (Miller 1995), and one study had a two‐month washout period (Hristara‐Papadopoulou 2005); we therefore judged them to have a low risk of bias. One study, for which we were able to obtain first‐arm data before the first cross‐over, had no washout period; thus the risk of bias is high for this study (Osman 2010). The remaining 14 randomised cross‐over studies did not describe a washout period, thus the risk of bias is unclear for these studies.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
In this section, the results are given for the comparisons of ACBT with each comparator. Some comparators (i.e. PEP, oscillatory devices) include multiple techniques or devices, and the results of the comparison of ACBT with each technique or device are discussed separately. We have assessed and graded the certainty of the evidence for predefined outcomes (see above) in the summary of findings tables and definitions of these gradings provided (Table 1; Table 2; Table 3; Table 4; Table 5).
ACBT versus CCPT or combinations
See Table 1.
Six randomised studies (189 participants) compared ACBT with CCPT. Three studies (108 participants) compared ACBT with ACBT + CCPT (Osman 2010; Reisman 1988; Webber 1985), one study (16 participants) compared ACBT + CCPT with CCPT (Pryor 1979), one study (35 participants) compared ACBT + respiratory exercises with CCPT + respiratory exercises (Hristara‐Papadopoulou 2007), and one study (30 participants) compared ACBT with CCPT (Hristara‐Papadopoulou 2005). One study was of parallel design (n = 63) (Reisman 1988), while the remaining studies were of cross‐over design (Hristara‐Papadopoulou 2005; Hristara‐Papadopoulou 2007; Osman 2010; Pryor 1979; Webber 1985). We were only able to include the parallel study and two of the cross‐over studies in the meta‐analysis (Hristara‐Papadopoulou 2005; Osman 2010; Reisman 1988). Four randomised cross‐over studies had insufficient washout periods, but we obtained first‐arm data (before the first cross‐over) comparing ACBT with ACBT + CCPT from the Osman study investigators and included these data in the meta‐analysis where possible (Osman 2010). The study consisted of multiple treatment groups of which ACBT and ACBT + CCPT were just two. Of the 29 participants in the study as a whole, one performed ACBT before the first cross‐over and five performed ACBT + CCPT before the first cross‐over, thus a comparison cannot be made in the analysis (Osman 2010). For the remaining studies with insufficient washout periods, we contacted the study authors to obtain necessary information and are awaiting their response. If new data become available, we will include these in an update of the review.
Primary outcomes
1. Quality of life
None of the included studies assessed this outcome.
2. Personal preference
None of the included studies assessed this outcome.
3. Mortality
None of the included studies assessed this outcome.
Secondary outcomes
1. Adverse events
None of the included studies assessed this outcome.
2. Exercise tolerance
None of the included studies assessed this outcome.
3. Lung function
a. FEV1 in L or % predicted
i. FEV1 (L)
A randomised cross‐over study reported FEV1 in L for ACBT compared to CCPT (Hristara‐Papadopoulou 2005); there was no difference between groups after 12 months, MD 0.52 L (95% CI ‐0.25 to 1.29) (Analysis 1.1). In another cross‐over study, ACBT and ACBT + CCPT had similar effects on FEV1 in L, but the ACBT group had only one person, precluding formal analysis (Osman 2010).
1.1. Analysis.

Comparison 1: ACBT versus CCPT, Outcome 1: FEV 1 (L)
ii. FEV1 (% predicted per year)
The parallel study comparing ACBT with ACBT + CCPT reports the mean rate of decline in FEV1 % predicted in each group. Participants in the ACBT alone group had mean (SD) rates of decline that differed significantly from baseline (‐4.7 (7.1 ); P < 0.001); however, there was no statistically significant difference from baseline in the CCPT group (‐1.9 (5.8)). The difference in mean rate of decline between groups was also reported not to be statistically significant P < 0.08 (Reisman 1988).
In a cross‐over study, ACBT and ACBT + CCPT had similar effects on FEV1 % predicted, but the ACBT group had only one person, precluding formal analysis (Osman 2010).
We have concerns due to the risk of bias and imprecise results, so are uncertain about this evidence.
b. FVC in L or % predicted
i. FVC (L)
One randomised cross‐over study comparing ACBT to CCPT found no difference in FVC (L) between the two interventions after one year (Hristara‐Papadopoulou 2005), MD 0.70 L (95% CI ‐0.15 to 1.55) (Analysis 1.2). In another cross‐over study, FVC in L was higher after a session with ACBT + CCPT than with ACBT alone (Osman 2010). However, there was only one person in the ACBT group, precluding a formal analysis.
1.2. Analysis.

Comparison 1: ACBT versus CCPT, Outcome 2: FVC (L)
ii. FVC (% predicted per year)
The parallel study reported mean rate of decline in FVC % predicted in each group. Participants in both the ACBT alone group and the CCPT group had mean (SD) rates of decline that did not differ significantly from baseline: ACBT ‐1.6 (5.7) and CCPT 0.2 (4.9). Investigators reported no difference between groups in the mean rate of decline (Reisman 1988).
In another cross‐over study, FVC % predicted was higher after a session with ACBT + CCPT than with ACBT alone (Osman 2010). However, there was only one person in the ACBT group, precluding a formal analysis.
We have concerns due to the risk of bias and imprecise results, so are uncertain about this evidence.
4. Sputum
Two studies comparing ACBT with ACBT + CCPT collected sputum; however the parallel study did not report any data for this outcome (Reisman 1988) and while the cross‐over study reported similar weights of sputum with ACBT and with ACBT + CCPT, the ACBT group had only one person, precluding formal analysis (Osman 2010).
5. Oxygen saturation
One cross‐over study comparing ACBT to CCPT reported a non‐significant difference in saturated oxygen levels between the two therapies (Hristara‐Papadopoulou 2005). A further cross‐over study reported similar levels of oxygen saturation after ACBT and ACBT + CCPT, but the ACBT group had only one person, precluding formal analysis (Osman 2010).
6. Number of pulmonary exacerbations
The parallel study comparing ACBT with ACBT + CCPT presented hospitalisation data for pulmonary exacerbations and for non‐pulmonary admissions separately (Reisman 1988). The authors noted that acute exacerbations were treated in the hospital and observed that nine out of 33 participants receiving ACBT and five out of 30 participants receiving ACBT + CCPT experienced pulmonary exacerbations. The nine participants in the ACBT group had 30 hospital admissions and 347 hospital days. One participant in the ACBT group accounted for 15 of the hospital admissions and 150 of the hospital days. The five participants in the ACBT + CCPT group had eight hospital admissions and 73 hospital days. Our analysis showed that group differences in the number of participants, hospital admissions, and hospital days did not reach statistical significance; for pulmonary exacerbations, RR 1.64 (95% CI 0.62 to 4.34) (Analysis 1.3; low‐certainty evidence). The study authors also found no significant difference in pulmonary exacerbation rates between the two therapies. They presented the number of participants, hospital admissions, and hospital days for each therapy. We rated the certainty of evidence as low due to risk of bias concerns and imprecise results.
1.3. Analysis.

Comparison 1: ACBT versus CCPT, Outcome 3: Pulmonary exacerbation
ACBT versus PEP or combinations
See Table 2.
Five studies (110 participants) compared ACBT with PEP: two studies (28 participants) compared ACBT with ACBT + PEP (Hofmeyr 1986; Mortensen 1991), a further two studies (53 participants) compared ACBT with PEP (Kofler 1994; Pryor 2010), and one study (29 participants) compared ACBT + CCPT with PEP (Osman 2010). One study was of parallel design (Pryor 2010) and four studies were cross‐over studies with insufficient washout periods. We obtained first‐arm data (before the first cross‐over) from the Osman study comparing ACBT + CCPT with PEP and included these data in the meta‐analyses where possible. This study consisted of multiple treatment groups of which ACBT + CCPT and PEP were just two. Of the 29 participants, five performed ACBT + CCPT before the first cross‐over and one performed PEP before the first cross‐over. For the remaining three studies with insufficient washout periods, we have contacted the study authors to obtain necessary information and are awaiting their response. If new data become available they will be included in an update of the review.
Primary outcomes
1. Quality of life
The 12‐month, parallel study assessed quality of life using the Short Form‐36 and the Chronic Respiratory Questionnaire (Pryor 2010). This study randomised 75 participants to one of five treatment groups, two of which were ACBT (n = 15) and PEP (n = 15). The authors reported no significant differences across the five treatment groups for either the physical domain (P = 0.99) or the mental domain (P = 0.27) of the Short Form‐36, but both the physical and mental domains decreased after 12 months (P = 0.05 and P = 0.002, respectively). There were no significant differences at the end of the study across the five treatment groups for each of the four domains of the Chronic Respiratory Questionnaire: dyspnoea (P = 0.7), fatigue (P = 0.85), emotion (P = 0.39), and mastery (P = 0.82). The fatigue (P = 0.69), emotion (P = 0.39), and mastery (P = 0.37) domains showed no significant differences over time. However, there were small, clinically‐important improvements in the dyspnoea ratings over the 12‐month study for the ACBT (change of 0.7) and the PEP groups (change of 0.5). This study reported insufficient data for both the Short Form‐36 and the Chronic Respiratory Questionnaire for inclusion in the meta‐analysis. We rated the certainty of the evidence for quality of life when comparing ACBT with PEP mask therapy as very low because of the high risk of bias, the imprecise results, and the suspicion of publication bias.
None of the cross‐over studies reported on quality of life.
2. Personal preference
The included studies did not assess this outcome.
3. Mortality
The included studies did not assess this outcome.
Secondary outcomes
1. Adverse events
The included studies did not assess this outcome.
2. Exercise tolerance
The parallel study assessed exercise tolerance using a modified shuttle test (Pryor 2010). Mean baseline scores for the ACBT and the PEP groups were 1005.4 m and 887.9 m. At the end of the study, there were no statistically significant differences across the five treatment groups (P = 0.52). The authors did not report any additional data on this outcome.
None of the cross‐over studies reported on exercise tolerance.
3. Lung function
a. FEV1 in L or % predicted
i. FEV1 (L)
The parallel study and one cross‐over study reported on this outcome (Osman 2010; Pryor 2010). In the parallel study, there was no significant difference in the final mean (SD) FEV1 L between the ACBT group (1.94 (0.80)) and the PEP group (2.02 (1.17)) (Pryor 2010). We estimated the mean between‐group difference in final values to be MD ‐0.08 L (95% CI ‐0.85 to 0.69) (Analysis 2.1). In the cross‐over study, participants in the ACBT + CCPT group and the PEP group had similar levels of FEV1 in L and % predicted (Osman 2010). However, the PEP group had only one person, precluding formal analysis.
2.1. Analysis.

Comparison 2: ACBT versus PEP, Outcome 1: FEV 1 (L)
b. FVC in L or % predicted
Across the five arms of the parallel study, the investigators reported no significant differences in terms of FVC (P = 0.54) (Pryor 2010). In the cross‐over study, participants in the ACBT + CCPT group and the PEP groups had similar levels of FVC in L and % predicted (Osman 2010). However, the PEP group had only one person, precluding formal analysis.
4. Sputum
In a cross‐over study, participants may have produced more sputum in terms of weight after ACBT + CCPT than after PEP therapy (Osman 2010). However, the PEP group had only one person, precluding formal analysis.
5. Oxygen Saturation
In the cross‐over study, oxygen saturation was similar in the ACBT + CCPT and PEP groups (Osman 2010). However, the PEP group had only one person, precluding formal analysis.
6. Number of pulmonary exacerbations
The included studies did not assess this outcome.
ACBT versus oscillating devices or combinations
See Table 3.
Six studies (152 participants) compared ACBT with oscillating devices: one study (seven participants) compared ACBT with airway oscillating devices (AOD) (Milne 2004), one study (20 participants) compared ACBT with ACBT + AOD (Pryor 1994), one study (21 participants) compared ACBT with AOD + forced expiration (FE) (Pike 1999), one study (10 participants) compared ACBT with HFCC (Phillips 2004), one study (29 participants) compared ACBT + CCPT with AOD (flutter) and ACBT + CCPT to HFCC (HFCWO) (Osman 2010), and one study (65 participants) compared ACBT with two different AOD devices (Cornet and Flutter) (Pryor 2010). One parallel‐designed study (Pryor 2010) and two cross‐over studies with sufficient washout periods were included in the meta‐analysis (Milne 2004; Phillips 2004). Three of the studies were of cross‐over design but had insufficient washout periods (Osman 2010; Pryor 1994; Pike 1999). For one of these cross‐over studies which had multiple treatment groups (of which ACBT + CCPT, AOD, and HFCC were just three), we obtained data from the study authors for results measured before the first cross‐over and included these data in the meta‐analysis (Osman 2010). Of the 29 participants in this study, five performed ACBT + CCPT before the first cross‐over, two performed AOD before the first cross‐over, and 15 performed HFCC before the first cross‐over. For the remaining two cross‐over studies with insufficient washout periods, we have contacted the study authors to obtain necessary information and are awaiting their response. If new data become available, they will be included in an update of the review.
Primary outcomes
1. Quality of life
The 12‐month parallel study assessed quality of life using the Short Form‐36 and the Chronic Respiratory Questionnaire (Pryor 2010). This study randomised 75 participants to one five treatment groups, which included ACBT (n = 15), Cornet (n = 15), and Flutter (n = 15). The authors reported no significant differences across the five treatment groups for either the physical domain (P = 0.99) or the mental domain (P = 0.27) of the Short Form‐36, but both the physical and mental domains decreased after 12 months (P = 0.05 and P = 0.002, respectively). There were no significant differences at the end of the study across the five treatment groups for each of the four domains of the Chronic Respiratory Questionnaire: dyspnoea (P = 0.7), fatigue (P = 0.85), emotion (P = 0.39), and mastery (P = 0.82). The fatigue (P = 0.69), emotion (P = 0.39), and mastery (P = 0.37) domains showed no significant differences over time. However, the dyspnoea ratings improved among the Flutter (change of 1.3, moderate improvement) and ACBT (change of 0.7, small improvement) groups, but not among the Cornet group (change less than 0.5). We rated the certainty of the evidence comparing ACBT with oscillating devices in terms of quality of life as very low because of the high risk of bias, the imprecise results, and the suspicion of publication bias.
2. Personal preference
The two included studies had 17 participants, and each included a different therapy as a comparator. When comparing ACBT with AOD, one study reported that three participants (43%) preferred ACBT, two (29%) preferred AOD, and two (29%) had no preference between the two treatments (Milne 2004). When comparing ACBT with HFCC, one study reported that all 10 participants found ACBT comfortable, while six (60%) found HFCC uncomfortable (Phillips 2004). In addition, eight (80%) participants had difficulty clearing secretions using HFCC (Phillips 2004).
3. Mortality
None of the included studies assessed this outcome.
Secondary outcomes
1. Adverse events
None of the included studies assessed this outcome.
2. Exercise tolerance
One parallel study assessed exercise tolerance using a modified shuttle test (Pryor 2010). Mean baseline scores for the ACBT, Cornet, and Flutter groups were 1005.4 metres, 906.7 metres, and 1044.3 metres respectively. At the end of the study, there were no statistically significant differences across the five treatment groups (P = 0.52). The authors did not report any additional data on this outcome.
None of the cross‐over studies reported on exercise tolerance.
3. Lung function
a. FEV1 in L or % predicted
i. FEV1 (L)
The parallel study reported that there was no significant difference in the final mean (SD) FEV1 (L) between: the ACBT group, 1.94 L (0.80); the Cornet group, 1.90 (0.89) L; or the Flutter group, 2.43 (0.94) L (Pryor 2010). We estimated the mean between‐group difference in final values between the ACBT and Cornet groups to be MD 0.04 L (95% CI ‐0.60 to 0.68) (Analysis 3.1) and between the ACBT and Flutter groups to be MD ‐0.49 L (95% CI ‐1.18 to 0.20) (Analysis 4.1).
3.1. Analysis.

Comparison 3: ACBT versus AOD (Cornet), Outcome 1: FEV 1 (L)
4.1. Analysis.

Comparison 4: ACBT versus AOD (Flutter), Outcome 1: FEV 1 (L)
The two cross‐over studies with adequate washout periods provided no results for FEV1 that were suitable for meta‐analysis. In one study the authors reported a mean FEV1 of 1.34 L after ACBT treatment and 1.38 L after AOD treatment (Milne 2004). In the second study, the authors reported a mean (range) FEV1 of 1.55 L (0.87 to 2.84) after ACBT treatment and 1.48 L (0.73 to 2.76) after HFCC treatment (Phillips 2004). We have contacted both authors for additional information and are awaiting their responses.
In the cross‐over study with first‐arm data, raw data were provided for meta‐analysis (Osman 2010). When comparing ACBT + CCPT with AOD, our analysis showed no significant difference between the two treatments, MD 0.11 L (95% CI ‐0.95 to 1.18) (Analysis 5.1). Similarly, when comparing ACBT + CCPT with HFCC, our analysis showed no significant difference between the two treatments, MD ‐0.06 L (95% CI ‐0.79 to 0.68) (Analysis 6.1).
5.1. Analysis.

Comparison 5: ACBT + CCPT versus AOD (Flutter), Outcome 1: FEV 1 (L)
6.1. Analysis.

Comparison 6: ACBT + CCPT versus HFCC (HFCWO), Outcome 1: FEV 1 (L)
ii. FEV1 (% predicted)
In the cross‐over study with first‐arm data, raw data were provided for meta‐analysis (Osman 2010). Our analyses showed no difference between interventions either when comparing ACBT + CCPT with AOD, MD 5.41% predicted (95% CI ‐15.62 to 26.44) (Analysis 5.2) or when comparing ACBT + CCPT to HFCC, MD 0.30% predicted (95% CI ‐15.63 to 16.23) (Analysis 6.2).
5.2. Analysis.

Comparison 5: ACBT + CCPT versus AOD (Flutter), Outcome 2: FEV 1% predicted
6.2. Analysis.

Comparison 6: ACBT + CCPT versus HFCC (HFCWO), Outcome 2: FEV 1% predicted
b. FVC in L or % predicted
i. FVC (L)
The 12‐month parallel study reported that there were no significant differences in terms of FVC across the five arms (P = 0.54) (Pryor 2010).
The two cross‐over studies with adequate washout periods provided no results on FVC that were suitable for meta‐analysis. When comparing ACBT with AOD in one study, the authors reported a mean FVC of 2.98 L after ACBT treatment and 2.98 L after AOD treatment (Milne 2004). When comparing ACBT with HFCC in the second study, the authors reported a mean (range) FVC of 2.68 L (1.80 to 4.25) after ACBT treatment and 2.64 L (1.59 to 4.14) after HFCC treatment (Phillips 2004). We have contacted both authors for additional information and are awaiting their responses.
In the cross‐over study with first‐arm data, raw data were provided for meta‐analysis (Osman 2010). Our analysis showed no significant difference between the two treatments either when comparing ACBT + CCPT with AOD, MD ‐0.47 L (95% CI ‐1.29 to 0.35) (Analysis 5.3) or when comparing ACBT + CCPT with HFCC, MD ‐0.36 L (95% CI ‐1.29 to 0.56) (Analysis 6.3).
5.3. Analysis.

Comparison 5: ACBT + CCPT versus AOD (Flutter), Outcome 3: FVC (L)
6.3. Analysis.

Comparison 6: ACBT + CCPT versus HFCC (HFCWO), Outcome 3: FVC (L)
ii. FVC (% predicted)
In the cross‐over study with first‐arm data, raw data were provided for meta‐analysis (Osman 2010). Our analysis showed no significant difference between the two treatments either when comparing ACBT + CCPT with AOD, MD ‐6.49% (95% CI ‐22.81 to 9.84) (Analysis 5.4) or when comparing ACBT + CCPT with HFCC, MD ‐5.08% (95% CI ‐20.62 to 10.47) (Analysis 6.4).
5.4. Analysis.

Comparison 5: ACBT + CCPT versus AOD (Flutter), Outcome 4: FVC % predicted
6.4. Analysis.

Comparison 6: ACBT + CCPT versus HFCC (HFCWO), Outcome 4: FVC % predicted
4. Sputum
When comparing ACBT with AOD, our analysis showed no significant difference between the two treatments in terms of sputum weight, MD 1.56 g (95% CI ‐20.53 to 23.65) (Analysis 4.2). The study authors also reported no significant difference between the two treatments with regard to 24‐hour sputum weights (Milne 2004). They presented 24‐hour mean, SD, and the range of sputum weights post‐treatment.
4.2. Analysis.

Comparison 4: ACBT versus AOD (Flutter), Outcome 2: Sputum weight
When comparing ACBT with HFCC, one study had a cross‐over design with an adequate washout, but provided no relevant results on wet sputum weight for meta‐analysis (Phillips 2004). The study authors noted that participants produced a significantly greater amount of sputum during treatment with ACBT than HFCC. Median sputum weight was 4.1 g during ACBT and 0.7 g during HFCC (P < 0.01). We have contacted the authors for additional information and are awaiting their response.
Our analysis showed no significant difference between the two treatments in terms of wet sputum weight when comparing ACBT + CCPT with AOD, MD 36.47 g (95% CI ‐16.71 to 89.65) (Analysis 5.5) or when comparing ACBT + CCPT with HFCC, MD 15.65 g (95% CI ‐39.70 to 71.00) (Analysis 6.5). The study authors noted that significantly more sputum was expectorated during ACBT + CCPT than with HFCC during treatment sessions and over a 24‐hour period of time (including treatment), while the sputum expectorated at all other times (excluding treatment) was not statistically significant (Osman 2010).
5.5. Analysis.

Comparison 5: ACBT + CCPT versus AOD (Flutter), Outcome 5: Sputum weight
6.5. Analysis.

Comparison 6: ACBT + CCPT versus HFCC (HFCWO), Outcome 5: Sputum weight
5. Oxygen saturation
When comparing ACBT with HFCC, one study reported no significant differences in oxygen saturation (no further data reported) (Phillips 2004).
Our analysis of the first‐arm data from the cross‐over study (Osman 2010) showed no significant difference between the two treatments when comparing ACBT + CCPT with AOD, MD ‐0.81% (95% CI ‐2.26 to 0.65) (Analysis 5.6) or when comparing ACBT + CCPT with HFCC, MD ‐1.00% (95% CI ‐2.45 to 0.45) (Analysis 6.6).
5.6. Analysis.

Comparison 5: ACBT + CCPT versus AOD (Flutter), Outcome 6: Oxygen saturation
6.6. Analysis.

Comparison 6: ACBT + CCPT versus HFCC (HFCWO), Outcome 6: Oxygen saturation
6. Number of pulmonary exacerbations
None of the included studies assessed this outcome.
ACBT versus other breathing techniques
See Table 4.
One parallel study and two cross‐over studies compared ACBT with other breathing techniques (Miller 1995; Osman 2010; Pryor 2010). The parallel study included 75 participants across five arms, two of which were ACBT and AD (Pryor 2010). We included one cross‐over study (n = 18) with a sufficient washout period in the meta‐analysis comparing ACBT with AD (Miller 1995). The second cross‐over study, with 29 participants, compared ACBT + CCPT with AD (Osman 2010). Although the study had an insufficient washout period, we obtained data from before the first cross‐over from the study authors and included these data in the meta‐analysis. The study consisted of multiple treatment groups of which ACBT + CCPT and AD were just two; of the 29 participants, five performed ACBT + CCPT before the first cross‐over and five performed AD before the first cross‐over.
Primary outcomes
1. Quality of life
The 12‐month parallel study assessed quality of life using the Short Form‐36 and the Chronic Respiratory Questionnaire (Pryor 2010). This study randomised 75 participants to one of five treatment groups, which included ACBT (n = 15) and AD (n = 15). The authors reported no significant differences across the five treatment groups for either the physical domain (P = 0.99) or the mental domain (P = 0.27) of the Short Form‐36, but both the physical and mental domains decreased after 12 months (P = 0.05 and (P = 0.002, respectively). There were no significant differences at the end of the study across the five treatment groups for each of the four domains of the Chronic Respiratory Questionnaire: dyspnoea (P = 0.7), fatigue (P = 0.85), emotion (P = 0.39), and mastery (P = 0.82). The fatigue (P = 0.69), emotion (P = 0.39), and mastery (P = 0.37) domains showed no significant differences over time. However, there were small, clinically‐important improvements in the dyspnoea ratings over the 12‐month study period for the ACBT (change of 0.7) and the AD groups (change of 0.5). We rated the certainty of the evidence for quality of life when comparing ACBT with other breathing techniques as very low because of the high risk of bias, the imprecise results, and the suspicion of publication bias.
2. Personal preference
When comparing ACBT with AD, one cross‐over study reported that nine participants (50%) preferred AD, while eight preferred (44%) ACBT (Miller 1995). One study participant (6%) had no preference between the two treatments.
3. Mortality
The included studies did not assess this outcome.
Secondary outcomes
1. Adverse events
The included studies did not assess this outcome.
2. Exercise tolerance
The parallel study assessed exercise tolerance using a modified shuttle test (Pryor 2010). Mean baseline scores for the ACBT and the AD groups were 1005.4 metres and 985.0 metres, respectively. At the end of the 12‐month study period, there were no statistically significant differences across the five treatment groups (P = 0.52). The authors did not report any additional data on this outcome.
None of the cross‐over studies reported on exercise tolerance.
3. Lung function
a. FEV1 in L or % predicted
i. FEV1 (L)
In the parallel study, there was no significant difference in the final mean (SD) FEV1 between the ACBT group, 1.94 (0.80) L, and the AD group, 2.64 (1.22) L (Pryor 2010). We estimated the mean between‐group difference in final values between the ACBT and AD groups to be ‐0.70 L (95% CI ‐1.49 to 0.09) (Analysis 7.1).
7.1. Analysis.

Comparison 7: ACBT versus AD, Outcome 1: FEV 1 (L)
When comparing ACBT with AD, one study had a cross‐over design with an adequate washout, but no results on FEV1 suitable for meta‐analysis were provided (Miller 1995). The study authors noted that there was no difference in pulmonary function between the two therapies. We have contacted the authors for additional information and are awaiting their response.
A further study compared ACBT + CCPT with AD (Osman 2010); our analysis showed no significant difference between the treatments, MD ‐0.51 L (95% CI ‐1.72 to 0.70) (Analysis 8.1).
8.1. Analysis.

Comparison 8: ACBT + CCPT versus AD, Outcome 1: FEV 1 (L)
ii. FEV1 (%)
One study compared ACBT + CCPT with AD (Osman 2010); our analysis showed no significant difference between the treatments, MD ‐8.30% predicted (95% CI ‐35.22 to 18.62) (Analysis 8.2).
8.2. Analysis.

Comparison 8: ACBT + CCPT versus AD, Outcome 2: FEV 1% predicted
b. FVC in L or % predicted
i. FVC (L)
The 12‐month parallel study reported that there were no significant differences in terms of FVC across the five arms (P = 0.54) (Pryor 2010).
When comparing ACBT with AD, one included study had a cross‐over design with an adequate washout, but no results on FVC suitable for meta‐analysis were provided (Miller 1995). The study authors noted that there was no difference in pulmonary function between the two therapies, yet more participants had improved FVC with ACBT than AD. Results for FVC were presented as the number of tests which showed an improvement. We have contacted the authors for additional information and are awaiting their response.
A further study compared ACBT + CCPT with AD (Osman 2010); our analysis showed no significant difference between the treatments, MD ‐0.85 L (95% CI ‐2.13 to 0.44) (Analysis 8.3).
8.3. Analysis.

Comparison 8: ACBT + CCPT versus AD, Outcome 3: FVC (L)
i. FVC (%)
One study compared ACBT + CCPT with AD (Osman 2010); our analysis showed no significant difference between the treatments, MD ‐11.02% predicted (95% CI ‐32.84 to 10.80) (Analysis 8.4).
8.4. Analysis.

Comparison 8: ACBT + CCPT versus AD, Outcome 4: FVC % predicted
4. Sputum
Two studies reported on this outcome (Miller 1995; Osman 2010). When comparing ACBT with AD, our analysis of data from the Miller study showed no significant difference in sputum weights between the two therapies, MD ‐0.40 g (95% CI ‐3.93 to 3.13) (Analysis 7.2). The study authors presented the MD and standard error (SE) between the two treatments and also noted that there was no significant difference in sputum weights between the two therapies (Miller 1995).
7.2. Analysis.

Comparison 7: ACBT versus AD, Outcome 2: Sputum weight
In the second study, Osman compared ACBT + CCPT with AD (Osman 2010). Our analysis showed no significant difference in sputum weights between the two therapies, MD ‐3.52 g (95% CI ‐68.49 to 61.46) (Analysis 8.6).
8.6. Analysis.

Comparison 8: ACBT + CCPT versus AD, Outcome 6: Sputum weight
There were no statistically significant differences between treatments in terms of sputum weights.
5. Oxygen saturation
When comparing ACBT with AD, one cross‐over study with an adequate washout noted that there was no difference in mean oxygen saturation between the two therapies, but provided no results which we could enter into a meta‐analysis (Miller 1995). We have contacted the authors for additional information, and are awaiting their response.
One study compared ACBT + CCPT with AD (Osman 2010). Our analysis showed no significant difference in oxygen saturation between the two therapies, MD ‐1.08 % (‐3.17 to 1.01) (Analysis 8.5).
8.5. Analysis.

Comparison 8: ACBT + CCPT versus AD, Outcome 5: Oxygen saturation
6. Number of pulmonary exacerbations
The included studies did not assessed this outcome.
ACBT versus exercise or versus ACBT plus exercise
See Table 5.
One cross‐over RCT compared ACBT with exercise (Bilton 1992), and one parallel RCT compared ACBT to ACBT plus exercise (Gungor 2021). Bilton 1992 included 18 participants and compared ACBT with exercise. This study had a cross‐over design with an insufficient washout period; therefore, we did not include it in the meta‐analysis. We have contacted study authors to obtain necessary information and are awaiting their response. If new data become available, we will include these in a future update of the review. In the parallel study, the participants received the intervention for six weeks, but were followed for six months. The study randomised 22 participants to either ACBT plus postural exercise or ACBT alone (Gungor 2021). Investigators have reported their results as a journal article and posted results on their clinical trial registry entry, but the reporting of these results is inconsistent between the two resources. We have primarily presented the results reported in the journal article, and noted when we report results that were extracted from the clinical trial registry entry.
Primary outcomes
1. Quality of life
One study (22 participants) assessed quality of life using the CFQ‐Revised and scores did not change significantly from baseline over the course of the study (Gungor 2021). Median scores for the emotional function and treatment difficulties subdomains were not statistically significant between groups at six weeks (P = 0.093 on the emotional function subdomain and P = 0.062 on the treatment difficulties subdomain ) or six months (P = 0.431 on the emotional function subdomain and P = 0.579 on the treatment difficulties subdomain). The study did not report on the between‐group differences for the other subdomains. These measurements differ from the results on ClinicalTrials.gov which reports the mean (SE) score of all domains for each group. We rated our certainty of the evidence as very low because of our concerns with risk of bias, imprecision, and publication bias.
2. Personal preference
The included parallel study did not address this outcome (Gungor 2021).
3. Mortality
The included parallel study reported no deaths in either treatment group during the six months of follow‐up (Gungor 2021). Mortality data were only reported in the clinical trials registry entry.
Secondary outcomes
1. Adverse events
The included parallel study reported no adverse events in either treatment group during the six months of follow‐up (Gungor 2021). We rated our certainty of the evidence as very low because of our concerns with risk of bias and our very serious concerns with the imprecise results.
2. Exercise tolerance
The included parallel study used the modified shuttle test to assess exercise tolerance (Gungor 2021). The median distance covered by participants in both groups increased from baseline over the course of the study, but the differences in median distances between groups were not statistically significant at six weeks (990 m in the ACBT + exercise group versus 760 m in the ACBT alone group) or at six months (1235 m in the ACBT + exercise group versus 960 m in the ACBT alone group). These measurements differ from the results on ClinicalTrials.gov which reports the mean (SE) distance walked.
3. Lung function
a. FEV1 in L or % predicted
i. FEV1 % predicted
The median FEV1 % predicted did not change significantly in either group during follow‐up and there were no differences seen between groups at either six weeks (90.5% predicted in the ACBT + exercise group versus 86% predicted in the ACBT alone group) or six months (88.5% predicted in the ACBT + exercise group versus 95.5% predicted in the ACBT alone group; P = 0.873) (Gungor 2021). These measurements differ from the results on ClinicalTrials.gov which reports the mean (SD) values at each time point. We rated our certainty of the evidence as very low because of our concerns with the risk of bias and our very serious concerns with the imprecise results.
b. FVC in L or % predicted
The mean FVC % predicted did not change significantly in either group during follow‐up and there were no differences seen between groups at either six weeks or six months (P = 0.749) (Gungor 2021). These measurements differ from the results on ClinicalTrials.gov which reports the mean (SD) values at each time point. We rated our certainty of the evidence as very low because of our concerns with the risk of bias and our serious concerns with the imprecise results.
4. Sputum
The included study did not address this outcome.
5. Oxygen saturation
The included study did not address this outcome.
6. Number of pulmonary exacerbations
One participant in the ACBT only group (1 out of 11 people; 9.1%) was hospitalised due to an exacerbation and was discontinued from the trial (Gungor 2021). There were no pulmonary exacerbations in the ACBT plus postural exercise group. We rated our certainty of the evidence as very low because of our concerns with risk of bias and our very serious concerns with the imprecise results.
ACBT versus other therapy
Five studies (106 participants) compared ACBT with other therapies. One study (26 participants) compared ACBT with ACBT + non‐invasive ventilation (NIV) (Holland 2003), one study (16 participants) compared ACBT with ACBT + pressure support ventilation (PSV) (Fauroux 1999), one study (20 participants) compared ACBT with the test of incremental respiratory endurance (TIRE) (Howard 2000), one study (24 participants) compared ACBT with coughing (Steven 1992), and one study (20 participants) compared ACBT + CCPT with RIM (Chatham 2004). All five studies had a cross‐over design with insufficient washout periods and hence none of these were included in the meta‐analyses. We have contacted study authors to obtain necessary information and are awaiting their response. If new data become available they will be included in an update of the review.
Discussion
Summary of main results
This review compared ACBT with other airway clearance therapies. Following searching and screening, we included 22 studies in the review. Of these, we were only able to include eight in the analyses; the remaining 14 studies are cross‐over studies with inadequate washout periods. Due to the risk of a carry‐over effect in cross‐over studies, we only used data from the period before the first cross‐over. When possible we have contacted the study authors and in some cases are still awaiting their responses. Of the eight studies that are included in the analysis, ACBT was compared with eight different therapies: AD, AOD, HFCC, PEP, ACBT + CCPT, CCPT + respiratory exercises, CCPT, and ACBT + postural exercise.
For each of the primary outcomes of this review (quality of life, personal preference, and mortality), we considered the certainty of the evidence to be low or very low. Each comparison for the primary outcomes was addressed by only one or two studies with either a high or an unclear risk of bias, imprecise results, and limited reporting of the study results. One of these studies was available only as a clinical trials registry entry. We were unable to assess the certainty of the evidence for many of the outcomes for many of the comparisons because of a lack of data.
ACBT versus CCPT
Six randomised studies (189 participants) were eligible for this comparison (Hristara‐Papadopoulou 2005; Hristara‐Papadopoulou 2007; Osman 2010; Pryor 1979; Reisman 1988; Webber 1985). None of the studies evaluated any of the primary outcomes. We found no difference in the effects on FEV1 and FVC % predicted between ACBT and CCPT (Hristara‐Papadopoulou 2005; Osman 2010; Reisman 1988). One study reported similar sputum weight in both groups (Osman 2010), and two studies reported similar levels of oxygen saturation in both groups (Hristara‐Papadopoulou 2005; Osman 2010). There were no differences in the number of pulmonary exacerbations between people using ACBT and people using CCPT (low‐certainty evidence) (Reisman 1988). None of the studies reported on adverse events or exercise tolerance.
ACBT versus PEP
Five studies (110 participants) are included in this comparison (Hofmeyr 1986; Kofler 1994; Mortensen 1991; Osman 2010; Pryor 2010).
We found no evidence of an effect on quality of life when comparing ACBT to PEP mask therapy (very low‐certainty evidence); no study reported on the other two primary outcomes. One study found no difference between therapies in exercise tolerance using a modified shuttle test (Pryor 2010). We found no difference in the effects on FEV1 and FVC % predicted (Osman 2010; Pryor 2010). In one study participants may have produced more sputum ACBT + CCPT than after PEP therapy, but there was only a single participant in the PEP group precluding formal analysis (Osman 2010). The same study reported similar levels of oxygen (Osman 2010). No study reported on adverse events or pulmonary exacerbations.
ACBT versus oscillating devices
Six studies (152 participants) compared ACBT with oscillating devices (Milne 2004; Osman 2010; Phillips 2004; Pike 1999; Pryor 1994; Pryor 2010).
In one study, there were no differences between the five treatment groups in any of the four domains of the Chronic Respiratory Questionnaire (very low‐certainty evidence) (Pryor 2010). Two studies reported on personal preference after two days of treatment, but found no clear preference for any individual therapy (Milne 2004; Phillips 2004). One study found no difference between therapies in exercise tolerance using a modified shuttle test (Pryor 2010). Four studies assessed lung function and found no difference between treatments (Milne 2004; Osman 2010; Phillips 2004; Pryor 2010). Three studies did not identify any differences between groups in expectorated sputum (Milne 2004; Osman 2010; Phillips 2004); two of these studies additionally reported no differences in oxygen saturation (Osman 2010; Phillips 2004). No study reported mortality, adverse events or pulmonary exacerbations.
ACBT versus other breathing techniques
Three studies compared ACBT with other breathing techniques (Miller 1995; Osman 2010; Pryor 2010).
One study reported no differences between the five treatment groups in any of the four domains of the Chronic Respiratory Questionnaire (very low‐certainty evidence) (Pryor 2010). A second study found no clear preference for a particular technique in a comparison of ACBT and AD (Miller 1995). Only one study reported on exercise tolerance and found no difference between treatments (Pryor 2010). None of the three studies in this comparison found any difference between groups in any measure of lung function (Miller 1995; Osman 2010; Pryor 2010). Two studies reported sputum weight and oxygen saturation; neither found any difference between treatment groups for either outcome (Miller 1995; Osman 2010). No study assessed mortality, adverse events or pulmonary exacerbations.
ACBT versus exercise
Two studies compared ACBT with exercise (Bilton 1992; Gungor 2021). One study had a cross‐over design with an insufficient washout period, so we have not included the results in our analysis (Bilton 1992). We have concerns surrounding the results of the second study, which seem to differ between those published online at ClinicalTrials.gov and the published paper (Gungor 2021).
The study found no difference in quality of life but did not report personal preference (Gungor 2021). There were no deaths or adverse events in either treatment arm. The study also did not find any differences in any measure of lung function, although data were reported as median (IQR) in the published paper and mean (SD) on the trials registry. The study did not report on sputum or oxygen levels. One participant out of 11 in the ACBT‐only group was hospitalised due to an exacerbation, but there were no pulmonary exacerbations in the ACBT plus postural exercise group (Gungor 2021).
ACBT versus other therapy
Five studies (106 participants) compared ACBT with other therapies: with ACBT + non‐invasive ventilation (NIV) (Holland 2003), with ACBT + pressure support ventilation (PSV) (Fauroux 1999), with the test of incremental respiratory endurance (TIRE) (Howard 2000), with coughing (Steven 1992), and with RIM (Chatham 2004). All five studies had a cross‐over design with insufficient washout periods and hence none of these were included in our analysis.
Overall completeness and applicability of evidence
This review includes analyses and a summary of 22 studies (eight studies in the quantitative analysis). The studies had different intervention groups, thus they could not be compared with each other. Two of the therapies of interest (hPEP and RIM) were not included as interventions in any of the studies. We included studies that assessed children and adults as well as those who have been hospitalised for exacerbations and those with stable conditions. Of the eight studies that were included in our analyses, three followed participants for at least one year (Hristara‐Papadopoulou 2005; Pryor 2010; Reisman 1988); and four followed participants for less than one week (Miller 1995; Milne 2004; Osman 2010; Phillips 2004). All the studies included fewer than 100 participants.
Quality of the evidence
We were unable to assess the certainty of the evidence for many of the outcomes for many of the comparisons because of a lack of data. Overall, we rated the certainty of the evidence as low or very low. We downgraded the certainty because of concerns regarding study limitations, imprecise results, and suspected publication bias. Many of the studies were of short duration (less than one week), limiting our ability to draw conclusions about the effectiveness of the long‐term use of ACBT (Table 1; Table 2; Table 3; Table 4; Table 5).
With regards to sequence generation, allocation concealment, blinding (of outcome assessors), and washout periods, the majority of the studies are associated with an unclear risk of bias (Figure 3). A total of 20 out of the 22 studies reported that the participants were randomly allocated, but the methods of randomisation were not defined, thus the risk of bias for sequence generation is unclear in these studies. In the remaining two studies, there was a low risk of bias for sequence generation. Allocation concealment was reported in two of the 22 studies, and the remaining studies are associated with an unclear risk of bias for allocation concealment. Blinding of the outcome assessors was observed in five studies and was unclear in the remaining 17 studies (Figure 2). A washout period was not reported in 14 of the 19 randomised cross‐over studies, thus they were associated with a unclear risk of bias. One of the randomised cross‐over studies clearly did not have a washout period, thus it had a high risk of bias. Four of the 19 randomised cross‐over studies had washout periods of at least a day, which we regarded as adequate and thus they were associated with low risk of bias (Figure 2).
In regards to intention‐to‐treat analysis, adequate follow up, selective reporting, and compliance assessment, the majority of the studies are associated with a low risk of bias (Figure 3). An intention‐to‐treat analysis was used in 10 out of the 22 studies. Follow‐up was adequate with a of low risk of bias in 13 of the 22 studies. With regards to selective reporting, 16 out of the 22 studies had a low risk of bias, and we assessed the risk of bias due to compliance as low in 11 of the 22 studies (Figure 2).
Potential biases in the review process
It is unlikely that we have missed any studies that address ACBT. We have hand‐searched all reference lists in the studies identified from the electronic searches, as well as the included studies presented both as full text articles and conference abstracts. In addition, because of the renaming of FET as ACBT in 1990 (Webber 1998), the electronic search included both terms (FET and ACBT) to capture all relevant studies. Also, all study authors that were contacted for additional information were sent a list of the studies included in the review and asked if they could provide the references of additional studies they thought may be relevant.
Bias may have been introduced because we only included studies of FET if FET was reported as containing all of the components of ACBT. As stated in the Types of interventions section, we used the definitions of the ACBT components as described by the Cystic Fibrosis Foundation and the Cystic Fibrosis Trust as standards in the process (Cystic Fibrosis Foundation 2023; Cystic Fibrosis Trust 2020b). Of the 53 excluded studies, seven were excluded solely because FET was not reported as including all of the components of ACBT (ACTRN12619000224123; Andreasson 1987; Falk 1984; Gursli 2017; NCT03078127; RBR‐5g9f6w; Sutton 1983).
Agreements and disagreements with other studies or reviews
Consistent with previous Cochrane Reviews on airway clearance therapies for people with cystic fibrosis (Burnham 2021; Main 2005; McIlwaine 2019; Morrison 2020), we did not identify any advantage of ACBT compared to other airway clearance therapies.
Authors' conclusions
Implications for practice.
There is little evidence to support or reject the use of active cycle of breathing technique (ACBT) over any other airway clearance therapy in people with cystic fibrosis (CF). It is our opinion that ACBT is comparable with other therapies in outcomes such as personal preference, exercise tolerance, lung function, sputum weight, oxygen saturation, and number of pulmonary exacerbations.
Implications for research.
The majority of studies in this review were cross‐over studies with insufficient washout periods, increasing the risk of carry‐over effects. More randomised controlled studies comparing ACBT with other airway clearance therapies are needed. Because of the concern of carry‐over effects, cross‐over study authors should allow adequate washout periods between treatments.
The majority of included studies had immediate outcomes, as defined by intervention durations of less than one day. Long‐term studies with interventions greater than one month are needed to more adequately assess the effects of the interventions. Such studies may provide more data for outcomes that are important to people with CF, including quality of life and personal preference.
What's new
| Date | Event | Description |
|---|---|---|
| 30 January 2023 | New citation required but conclusions have not changed | With the inclusion of a new study, we were able to provide limited additional evidence on the effects of ACBT versus exercise. Some of the conclusions have been reworded based on current Cochrane guidance. Two authors are no longer able to contribute to the review and have been added to the acknowledgements (NAM, OAO). |
| 30 January 2023 | New search has been performed | A search of the Cochrane Cystic Fibrosis and Genetic Disorders (CFGD) Review Group's Cystic Fibrosis Trials Register identified 24 references potentially eligible for inclusion in the review. Searches of clinical trials registers yielded 42 references. Of these, three were duplicates and four references were captured in the search of the Cochrane Cystic Fibrosis and Genetic Disorders Review Group's Cystic Fibrosis Trials Register. Three studies were included, each with a single reference; two from the CFGD Group's register search (Hristara‐Papadopoulou 2005; Hristara‐Papadopoulou 2007) and one from the clinical trials registers (Gungor 2021). Five were additional references to already included studies (Fauroux 1999; Holland 2003; Osman 2010; Phillips 2004; Pryor 2010). Seven were additional references to six already excluded studies (Asher 1982; Braggion 1995; Chatham 1998; Gursli 2017; Rossman 1982; Steen 1991). Four new studies (10 references) from the CFGD Group's register search were excluded (Davies 2012; Horsley 2007; O'Neill 2017; Stanford 2020), along with 13 studies identified from the clinical trials registers. We have excluded the six studies that were awaiting assessment pending further information which had been requested from the investigators; to date we have not received any replies and so have excluded these studies now (Castle 1994; Falk 1993; Lannefors 1992; Parker 1984; Petrone 2009; van Hengstum 1988). We have added the summary of findings tables, per current guidance from Cochrane. Some of the conclusions have been reworded based on the new guidance. |
History
Protocol first published: Issue 3, 2009 Review first published: Issue 11, 2010
| Date | Event | Description |
|---|---|---|
| 30 June 2016 | New citation required but conclusions have not changed | A new author (Lisa Wilson) has joined the review team. Despite the inclusion of new data from the Pryor study, our conclusions have not changed. |
| 30 June 2016 | New search has been performed | A search of the Cystic Fibrosis and Genetic Disorders Group's Cystic Fibrosis Trials Register identified four new references potentially eligible for inclusion in this update. One was the full paper to an abstract previously listed as awaiting classification, but which has now been included (Pryor 2010). The second reference was to a study that has been excluded (Gursli 2017). Two references to one study have been listed as 'Awaiting classification' (Petrone 2009). Two studies previously listed as 'Awaiting classification' have now been excluded as we do not believe them to have been randomised (Orlik 2000; Orlik 2001). |
| 22 October 2012 | New citation required but conclusions have not changed | Despite the inclusion of new data, there is still insufficient evidence to support or reject the use of active cycle of breathing technique (ACBT) over any other airway clearance therapy and hence our conclusions have not changed. |
| 22 October 2012 | New search has been performed | The Cysitic Fibrosis Trial Register was search and no new references were identified. One study, which was initially listed under 'Studies awaiting classification', has now been included (Osman 2010). The study authors, who we had previously contacted for additional information, provided us with raw data for each participant clarifying which treatment they received and first‐arm data before the cross‐over. There was no washout period. |
Acknowledgements
We would like to thank Naomi A. McKoy and Olaide A. Odelola for their work on the original review.
We thank Laura Barnes, BS, for her clerical assistance, including her help with article retrieval.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search strategies for online trials registries
| Database | Search terms | Date last searched |
| ClinicalTrials.gov | "cystic fibrosis" AND "active cycle of breathing technique" OR "forced expiration technique" OR "huff" | 3 April 2021 |
| World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) | "cystic fibrosis" AND"active cycle of breathing technique" OR"forced expiration technique" OR "huff" | 3 April 2021 |
Data and analyses
Comparison 1. ACBT versus CCPT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 FEV 1 (L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1.1 At 1 year | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.2 FVC (L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.2.1 At 1 year | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.3 Pulmonary exacerbation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.3.1 At 3 years | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 2. ACBT versus PEP.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 FEV 1 (L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1.1 Up to 1 week | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 3. ACBT versus AOD (Cornet).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 FEV 1 (L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1.1 Up to 1 week | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 4. ACBT versus AOD (Flutter).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 FEV 1 (L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1.1 Up to 1 week | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.2 Sputum weight | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.2.1 Up to 1 week | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 5. ACBT + CCPT versus AOD (Flutter).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 FEV 1 (L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1.1 Day 1 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.95, 1.18] | |
| 5.2 FEV 1% predicted | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.2.1 Day 1 | 1 | Mean Difference (IV, Fixed, 95% CI) | 5.41 [‐15.62, 26.44] | |
| 5.3 FVC (L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.3.1 Day 1 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.4 FVC % predicted | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.4.1 Day 1 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.5 Sputum weight | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.5.1 Day 1 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.6 Oxygen saturation | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.6.1 Day 1 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 6. ACBT + CCPT versus HFCC (HFCWO).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 FEV 1 (L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1.1 Day 1 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.2 FEV 1% predicted | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.2.1 Day 1 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.3 FVC (L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.3.1 Day 1 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.4 FVC % predicted | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.4.1 Day 1 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.5 Sputum weight | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.5.1 Day 1 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.6 Oxygen saturation | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.6.1 Day 1 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 7. ACBT versus AD.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 FEV 1 (L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1.1 Up to 1 week | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.2 Sputum weight | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.2.1 Up to 1 week | 1 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐3.93, 3.13] |
Comparison 8. ACBT + CCPT versus AD.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 FEV 1 (L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1.1 Day 1 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.2 FEV 1% predicted | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.2.1 Day 1 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.3 FVC (L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.3.1 Day 1 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.4 FVC % predicted | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.4.1 Day 1 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.5 Oxygen saturation | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.5.1 Day 1 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.6 Sputum weight | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.6.1 Day 1 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bilton 1992.
| Study characteristics | ||
| Methods | Study type: RCT (cross‐over). Each participant used 4 treatment regimens in randomised order over 4 consecutive days. The treatments were ACBT, exercise, exercise followed by ACBT, and ACBT followed by exercise. Each day consist of 2 identical treatment sessions with each session lasting 20 minutes. Usual medications were unchanged. |
|
| Participants | 18 enrolled; 18 evaluated; 13 male (72.7% male). Age: mean (21 years); median (NR); SD (NR); range (16 to 34 years). Inclusion criteria: NR. Exclusion criteria: NR. Characteristics: all participants were infected with Pseudomonas aeruginosa. |
|
| Interventions | ACBT: thoracic expansion exercise, breathing control and the FET. The intervention was conducted in a gravity‐assisted position for 20 minutes. Exercise: cycling at 60% VO2 max for 20 minutes. Exercise followed by ACBT: exercise for 10 minutes followed by 10 minutes of ACBT. ACBT followed by exercise: ACBT for 10 minutes followed by 10 minutes of exercise. |
|
| Outcomes | Outcome measures: participant preference, lung function, sputum weight. Additional outcomes: perceived effectiveness. |
|
| Notes | Funding: D. Bilton and J. Abbott were supported by the Cystic Fibrosis Trust. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The subjects were studied on four consecutive days. The study period contained four treatment days. The order of these was randomly allocated to each patient." |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) Participants | High risk | Blinding was not possible. The lack of blinding may have a high risk of bias for subjective outcomes, such as patient preference. The lack of blinding may have less of an effect on objective outcomes, such as lung function and sputum weight. |
| Blinding (performance bias and detection bias) Personnel | High risk | Not possible. |
| Blinding (performance bias and detection bias) Outcome Assessors | Unclear risk | Not discussed. |
| Selective reporting (reporting bias) | Low risk | All outcomes outlined in the methods section were reported in the results. |
| Adequate follow up? | Low risk | "All 18 patients completed the study." |
| Compliance/ adherence assessed? | Unclear risk | Not reported. |
| Intention‐to‐treat? | Low risk | Participants were analysed in the groups to which they were randomised. |
| Washout? | Unclear risk | No description of a washout period. |
Chatham 2004.
| Study characteristics | ||
| Methods | Study Type: RCT (cross‐over). Each participant was randomly allocated to alternate day treatment for 4 days. Treatments were applied once a day. The 2 treatment regimens used were CCPT + ACBT and RIM. Inhaled or nebulised treatments, or both, were administered before all study interventions and usual medications were unchanged. All participants also received intravenous antibiotics for worsening respiratory symptoms. |
|
| Participants | 20 enrolled; 20 evaluated; 10 male (50% male). Age: mean (NR); median (NR); SD (NR); range (NR). Inclusion criteria: adult participants. Exclusion criteria: NR. Characteristics: all 20 participants were infected with Pseudomonas aeruginosa. |
|
| Interventions | CCPT + ACBT: postural drainage (with percussion administered by a physiotherapist) and FET. There were periods of relaxed breathing and thoracic expansion exercises as described in ACBT. The session durations were 30 minutes. This intervention was identified as the standardised physiotherapy. RIM: maximum of 36 manoeuvres. Every 6 inspiratory efforts was accompanied by a short rest interval (maximum of 1 minute). The session duration varied among the participants. The RIM protocol required the use of the RT2 hand‐held manometer. |
|
| Outcomes | Outcome measures: sputum weight. Additional outcomes: concentration of protein, concentration of interleukin‐8 (IL‐8), concentration of HNE, FFM. |
|
| Notes | FEV1 % predicted results were provided for the group whose alternate day treatment began with CCPT + ACBT and for the group whose alternate day treatment began with RIM. Results were not provided for each intervention group separately. Funding: A.A. Ionescu and L.S. Nixon were supported by CF Trust UK project grants. Other support was from the Astra Foundation UK and GlaxoSmithKline UK. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Participants were randomly allocated to alternate day treatment ....." |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) Participants | Low risk | Blinding was not possible. However, the risk of bias from a lack of blinding may be low for objective outcomes, such as sputum weight. |
| Blinding (performance bias and detection bias) Personnel | Low risk | Blinding was not possible. However, the risk of bias from a lack of blinding may be low for subjective outcomes, such as sputum weight. |
| Blinding (performance bias and detection bias) Outcome Assessors | Low risk | "The laboratory researcher was blind to the treatment administered to patients." |
| Selective reporting (reporting bias) | Low risk | All outcomes outlined in the methods section were reported in the results. |
| Adequate follow up? | Low risk | All participants were accounted for. |
| Compliance/ adherence assessed? | Low risk | "All treatment sessions were performed under supervision..." |
| Intention‐to‐treat? | Low risk | Participants were analysed in the groups to which they were randomised. |
| Washout? | Unclear risk | No description of a washout period. |
Fauroux 1999.
| Study characteristics | ||
| Methods | Study Type: RCT (cross‐over). Each participant used 2 treatment regimens in randomised order over 2 days. The treatments were FET and FET + PSV. Each day consisted of 2 different treatment sessions with each session lasting 20 minutes. Usual medications were unchanged. |
|
| Participants | 16 enrolled; 16 evaluated; 7 male (43.8% male). Age: mean (13 years); median (NR); SD (4 years); range (6 to 18 years). Inclusion criteria: age greater than 6 years; clinically stable. Exclusion criteria: NR. Characteristics: 9 participants were colonized with Pseudomonas aeruginosa, 7 were colonised with Burkholderia cepacia, 6 were on inhaled bronchodilators, 7 were on corticosteroids, 11 were on rhDNase, 1 participant was on a lung transplant list and had been receiving PSV for the previous 6 years. |
|
| Interventions | FET: As described in Pryor 1979. FET + PSV: FET manoeuvres with PSV applied during inspiration and resting periods. PSV required the use of a nasal mask and the pressure support generator ARM25. | |
| Outcomes | Outcome measures: participant preference, lung function, sputum weight, oxygen saturation. Additional outcomes: heart rate; respiratory rate; maximal expiratory pressure; maximal inspiratory pressure; PEF; FEF at 50%, 25% and 25‐75%; airway resistance % predicted value. |
|
| Notes | Results were stratified by pulmonary disease severity. Funding: Association Francaise de Lutte contre la Mucoviscidose (AFLM). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "During the study, each patient received two chest physiotherapy sessions in random order on two different days..." |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) Participants | High risk | Blinding was not possible. The lack of blinding may have a high risk of bias for subjective outcomes, such as patient preference. The lack of blinding may have less of an effect on objective outcomes, such as lung function, sputum weight, and oxygen saturation. |
| Blinding (performance bias and detection bias) Personnel | High risk | Blinding was not possible. The lack of blinding may have a high risk of bias for subjective outcomes, such as patient preference. The lack of blinding may have less of an effect on objective outcomes, such as lung function, sputum weight, and oxygen saturation. |
| Blinding (performance bias and detection bias) Outcome Assessors | Unclear risk | Not discussed. |
| Selective reporting (reporting bias) | Low risk | All outcomes outlined in the methods section were reported in the results. |
| Adequate follow up? | Low risk | "All the patients completed the protocol." |
| Compliance/ adherence assessed? | Low risk | A physiotherapist supervised the sessions. |
| Intention‐to‐treat? | Low risk | Participants were analysed in the groups to which they were randomised. |
| Washout? | Unclear risk | No description of a washout period. |
Gungor 2021.
| Study characteristics | ||
| Methods | Study type: RCT. Parallel design. Duration: 6 weeks. Location: single centre in Turkey. |
|
| Participants | 22 participants enrolled; 19 analysed; 11 male (57.8% male). Age, mean (SD): 9.36 (2) years). Inclusion criteria: aged 6 to 14 years old, FEV1 > 30% Exclusion criteria: presence of cor pulmonale, spinal fracture history, currently under intravenous medication, severe gastroesophageal reflux. |
|
| Interventions | Each intervention was applied by a therapist once per week for 6 weeks. ACBT: 3 phases ‐ breathing control, chest expansion exercise, and huff coughing. ACBT + postural exercise: ACBT as above plus postural exercise, which included thoracic vertebra mobilization, pectoral stretching, scapula and thoracic extension strengthening, and core stability exercises. |
|
| Outcomes | Outcome measures: exercise tolerance (modified shuttle test), quality of life (CFQ‐R), FEV1, FVC, mortality, adverse events, pulmonary exacerbations. Additional measures: postural stability, spinal deformity, FEV1/FVC, PEF. |
|
| Notes | Funding: Authors received no financial support and declared no conflicts of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not currently available. |
| Allocation concealment (selection bias) | Low risk | "The patients ... were equally randomized into two groups according to the sealed opaque envelope system with blocking." |
| Blinding (performance bias and detection bias) Participants | High risk | Blinding was not possible. The lack of blinding may have a high risk of bias for subjective outcomes, such as quality of life. The lack of blinding may have less of an effect on objective outcomes, such as lung function and mortality. |
| Blinding (performance bias and detection bias) Personnel | High risk | Blinding was not possible. The lack of blinding may have a high risk of bias for subjective outcomes, such as quality of life. The lack of blinding may have less of an effect on objective outcomes, such as lung function and mortality. |
| Blinding (performance bias and detection bias) Outcome Assessors | Low risk | All evaluations were performed by a blinded independent rehabilitation specialist. |
| Selective reporting (reporting bias) | High risk | There are major discrepancies between the outcomes reported in the study protocol, the NCT registry entry, and the paper. For instance, FVC is mentioned in the paper, but not in the study protocol or NCT registry entry. |
| Adequate follow up? | High risk | In ACBT + postural exercise group, 1/11 participants (9%) was lost to follow‐up. In the ACBT only group, 1/11 participants (9%) was lost to follow‐up and 1/11 (9%) was hospitalised. |
| Compliance/ adherence assessed? | Unclear risk | Not currently available. |
| Intention‐to‐treat? | High risk | Study used a per‐protocol analysis. |
Hofmeyr 1986.
| Study characteristics | ||
| Methods | Study type: RCT (cross‐over). Each participant used 3 treatment regimens in randomised order over 3 consecutive days. The treatments were FET (gravity‐assisted position), PEP + FET (gravity‐assisted position) and PEP + FET (sitting position). Each regimen was used for a 24‐hour period which included 4 treatment sessions. The mean time for each session was 21 minutes (range:10 to 31). The mean time for each intervention on a treatment day was 83 minutes/day (range: 59 to 105). Bronchodilators were continued before physiotherapy if this was a part of the participants' normal regimen. 15 participants were receiving intravenous antibiotic treatment and 3 were receiving oral antibiotic treatment. |
|
| Participants | 18 enrolled; 18 evaluated; 12 male (66.7% male). Age: mean (22.5 years); median (NR); range (13 to 37 years). Inclusion criteria: producing at least 20g of sputum in 24 hours; fit enough to carry out own chest physiotherapy. Exclusion criteria: participants with pneumothorax or a history of pneumothorax. Characteristics: all 18 participants had an exacerbation of their bronchopulmonary infection. |
|
| Interventions | FET (gravity‐assisted position): 4 deep inspirations with relaxed expiration, breathing control, FET, and coughing as needed. PEP + FET (gravity‐assisted position): 6 breaths with PEP, breathing control, FET, and coughing as needed. PEP required the use of a PEP mask, one‐way valve, and manometer. PEP + FET (sitting position): 6 breaths with PEP, breathing control, FET, and coughing as needed. PEP required the use of a PEP mask, one‐way valve, and manometer. |
|
| Outcomes | Outcome measures: lung function, sputum weight, oxygen saturation. | |
| Notes | The lowest and highest points of SaO2 were provided. Funding: Cystic Fibrosis Research Trust |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Each patient used the three treatment regimens in randomised order over three consecutive days..." |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) Participants | Low risk | Blinding was not possible. However, the risk of bias from a lack of blinding may be low for objective outcomes, such as lung function, sputum weight, and oxygen saturation. |
| Blinding (performance bias and detection bias) Personnel | Low risk | Blinding was not possible. However, the risk of bias from a lack of blinding may be low for objective outcomes, such as lung function, sputum weight, and oxygen saturation. |
| Blinding (performance bias and detection bias) Outcome Assessors | Unclear risk | Not discussed. |
| Selective reporting (reporting bias) | Low risk | All outcomes outlined in the methods section were reported in the results. |
| Adequate follow up? | Low risk | All participants were accounted for. |
| Compliance/ adherence assessed? | Unclear risk | Not discussed. |
| Intention‐to‐treat? | Low risk | Participants were analysed in the groups to which they were randomised. |
| Washout? | Unclear risk | No description of a washout period. |
Holland 2003.
| Study characteristics | ||
| Methods | Study type: RCT (cross‐over). Each participant used 2 treatment regimens in randomised order over 2 consecutive days. The treatments were ACBT and ACBT + NIV. Each day consisted of 2 treatment sessions with each session lasting 30 minutes. Bronchodilator and rhDNase were used on study days. The study had a run‐in period of 2 days. |
|
| Participants | 27 enrolled; 26 evaluated; 21 male (80.8% male). Age: mean (27 years); median (NR); SD (6.4 years); range (NR). BMI mean: 20.6 kg/m². Inclusion criteria: aged 18 years or over; admitted to a university hospital with an acute exacerbation of CF; producing more than 20 g sputum in 24 hours. Exclusion criteria: required continuous NIV, decreased level of consciousness, pneumothorax, symptomatic gastro‐oesophageal reflux requiring modification of treatment, major haemoptysis (200 mL or more over 24 hours), oxygen saturation less than 90% on room air at study entry, started home antibiotic treatment before day 5 of admission. Characteristics: 26 participants were infected with Pseudomonas aeruginosa, none were colonized with Burkholderia cepacia. |
|
| Interventions | ACBT: a sequence of 6 thoracic expansion exercises, breathing control, 6 thoracic expansion exercises, breathing control, FET, and coughing as needed. ACBT + NIV: ACBT with NIV administered during the entire duration of the treatment. NIV required the use of a nasal mask and bilevel device. |
|
| Outcomes | Outcome measures: participant preference; lung function; sputum weight; oxygen saturation. Additional outcomes: inspiratory and expiratory muscle strength; breathlessness. |
|
| Notes | Funding: Not described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "A within‐subject cross‐over design was used with subjects randomly allocated to treatment order." |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) Participants | High risk | Blinding was not possible. The lack of blinding may have a high risk of bias for subjective outcomes, such as patient preference. The lack of blinding may have less of an effect on objective outcomes, such as lung function, sputum weight, and oxygen saturation. |
| Blinding (performance bias and detection bias) Personnel | High risk | Blinding was not possible. The lack of blinding may have a high risk of bias for subjective outcomes, such as patient preference. The lack of blinding may have less of an effect on objective outcomes, such as lung function, sputum weight, and oxygen saturation. |
| Blinding (performance bias and detection bias) Outcome Assessors | Low risk | "All measurements were obtained by an independent data collector who was blinded to treatment order." |
| Selective reporting (reporting bias) | Low risk | All outcomes outlined in the methods section were reported in the results. |
| Adequate follow up? | Low risk | One of 27 participants withdrew at the start of the study because of pain experienced during respiratory muscle testing. The loss to follow up was 3.70% (< 10%). |
| Compliance/ adherence assessed? | Unclear risk | Not reported. |
| Intention‐to‐treat? | High risk | Participants lost to follow up were not included in the analysis. |
| Washout? | Unclear risk | No description of a washout period. |
Howard 2000.
| Study characteristics | ||
| Methods | Study type: RCT (cross‐over). Each participant was randomly allocated to alternate day treatment for 2 days. The 2 treatment regimens used were ACBT and TIRE. There was a run‐in period of 10 days. |
|
| Participants | 20 enrolled; number evaluated (NR); gender split (NR). Age: Details NR. Inclusion criteria: NR. Exclusion criteria: NR. Characteristics: 5 participants had FEV1 less than 30% predicted, 8 participants had FEV1 30% to 70% predicted, 7 participants had FEV1 > 70% predicted. |
|
| Interventions | ACBT: physiotherapy using ACBT. TIRE: TIRE at 80% of sustained maximum inspiratory pressure until failure was indicated by a computer. |
|
| Outcomes | Outcome measures: sputum weight. | |
| Notes | Funding: Not described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...patients were randomly allocated..." |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) Participants | Low risk | Blinding was not possible. However, the risk of bias from a lack of blinding may be low for objective outcomes, such as sputum weight. |
| Blinding (performance bias and detection bias) Personnel | Low risk | Blinding was not possible. However, the risk of bias from a lack of blinding may be low for objective outcomes, such as sputum weight. |
| Blinding (performance bias and detection bias) Outcome Assessors | Unclear risk | Not discussed. |
| Selective reporting (reporting bias) | Unclear risk | Not reported. |
| Adequate follow up? | Unclear risk | Not reported. |
| Compliance/ adherence assessed? | Unclear risk | Not reported. |
| Intention‐to‐treat? | Unclear risk | Unclear. Only available as an abstract. |
| Washout? | Unclear risk | No description of a washout period. |
Hristara‐Papadopoulou 2005.
| Study characteristics | ||
| Methods | Study type: RCT. Cross‐over design with 2‐month washout period. Duration: 1 year. Location: Greece. Each participant used 2 treatment regimens in a randomised order for 1 year, with a 2‐month washout period. |
|
| Participants | 30 children and young people with CF enrolled; 30 evaluated; 16 male (53% male). Age, mean (SD): 13.13 (4.01) years. Characteristics: all 20 participants were infected with Pseudomonas aeruginosa. |
|
| Interventions | ACBT: 1 breath control cycle, breast expansion, and forced exhale; includes a 3‐month learning period; performed under the supervision of physiotherapists or parents for 25 minutes/day. CCPT: positioning, tremors, pressings or vibrations, and cough applied by physiotherapists or parents for 15 minutes/day. |
|
| Outcomes | Outcome measures: FEV1, FVC, SaO2 Additional outcomes: Maximum expiratory flow rate, FEF50 |
|
| Notes | We believe that the author of this paper is actually Alexandra Hristara‐Papadopoulou and there was an error in the citation. It is highly unlikely that there are two people working in the same department in the same institution who have the same name except for a single letter. Funding: Not described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported in study. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) Participants | Low risk | Blinding was not possible. However, the risk of bias from a lack of blinding may be low for objective outcomes, such as lung function and oxygen saturation. |
| Blinding (performance bias and detection bias) Personnel | Low risk | Blinding was not possible. However, the risk of bias from a lack of blinding may be low for objective outcomes, such as lung function and oxygen saturation. |
| Blinding (performance bias and detection bias) Outcome Assessors | Unclear risk | Not reported in study. |
| Selective reporting (reporting bias) | Low risk | All outcomes outlined in the methods section were reported in the results. |
| Adequate follow up? | Unclear risk | Not reported in study. |
| Compliance/ adherence assessed? | Unclear risk | Not reported in study. |
| Intention‐to‐treat? | Unclear risk | Not reported in study. |
| Washout? | Low risk | 2 months. |
Hristara‐Papadopoulou 2007.
| Study characteristics | ||
| Methods | Study type: RCT. Cross‐over design. Each participant used 2 treatment regimens in randomised order for 3 months. Duration: 3 months. Location: single centre in Greece. |
|
| Participants | 35 participants enrolled; 28 evaluated; 14 male (40% male). Age, mean (SD): 12.4 (3.9) years; range 8 to 20 years. Inclusion and exclusion criteria: NR. Characteristics: all participants were stable and did not present with exacerbations of symptoms. |
|
| Interventions | ACBT: diaphragmatic breathing (5 to 10 times), thoracic breathing (4 times deep breathing + 5 seconds of holding breath), percussion, pressure vibration, FET, or huffing, cough, and active respiratory exercises (unilateral and bilateral, 10 minutes). The intervention was performed in a drainage position. Each session lasted 55 minutes. CCPT with respiratory exercises: PD, percussion, pressure‐vibration, cough and active respiratory exercises (unilateral and bilateral, 10 minutes). Each session lasted 60 minutes. |
|
| Outcomes | Outcome measures: sputum volume. Additional outcomes: sputum colour |
|
| Notes | The same author team undertook the earlier year‐long study in the same institution using the same comparison in children with CF. We do not know whether some children who participated in the earlier study also participated in this later study, potentially being counted twice in the results. Funding: Not described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "All children received the same 2 methods of respiratory physiotherapy ... in random order of respiratory physiotherapy for approximately 3 months" States random order but does not state how sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed.. |
| Blinding (performance bias and detection bias) Participants | Low risk | Blinding was not possible. However, the risk of bias from a lack of blinding may be low for objective outcomes, such as sputum volume. |
| Blinding (performance bias and detection bias) Personnel | Low risk | Blinding was not possible. However, the risk of bias from a lack of blinding may be low for objective outcomes, such as sputum volume. |
| Blinding (performance bias and detection bias) Outcome Assessors | Unclear risk | Not discussed |
| Selective reporting (reporting bias) | Low risk | All outcomes outlined in methods are reported in results. |
| Adequate follow up? | High risk | Study does not account for the missing 7 participants. |
| Compliance/ adherence assessed? | Unclear risk | Not discussed. |
| Intention‐to‐treat? | Unclear risk | Not discussed.. |
| Washout? | Unclear risk | No description of a washout period. |
Kofler 1994.
| Study characteristics | ||
| Methods | Study type: RCT (cross‐over). Each participant was randomly allocated to alternate 4‐month treatments for 8 months. The 2 treatment regimens used were ACBT and PEP. Each group followed conventional therapy for CF (including antibiotics and enzymes). |
|
| Participants | 33 enrolled; 23 evaluated; gender split (NR). Age: NR. Inclusion criteria: NR. Exclusion criteria: NR. Characteristics: NR. |
|
| Interventions | ACBT: no description. PEP: no description. |
|
| Outcomes | Outcome measures: participant preference, lung function. | |
| Notes | Funding: Not described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Paper states "children with cystic fibrosis were randomly assigned". |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) Participants | High risk | Blinding was not possible. The lack of blinding may have a high risk of bias for subjective outcomes, such as patient preference. The lack of blinding may have less of an effect on objective outcomes, such as lung function. |
| Blinding (performance bias and detection bias) Personnel | High risk | Blinding was not possible. The lack of blinding may have a high risk of bias for subjective outcomes, such as patient preference. The lack of blinding may have less of an effect on objective outcomes, such as lung function. |
| Blinding (performance bias and detection bias) Outcome Assessors | Unclear risk | Not discussed. |
| Selective reporting (reporting bias) | Unclear risk | Not discussed. |
| Adequate follow up? | High risk | 10 of 33 CF children (> 10%) did not complete the program, and there was no description of those participants lost to follow up. The loss to follow up was 30.30% (> 10%). Only available as an abstract. |
| Compliance/ adherence assessed? | Unclear risk | Not reported. |
| Intention‐to‐treat? | High risk | Participants lost to follow up were not included in the analysis. Only available as an abstract. |
| Washout? | Unclear risk | No description of a washout period. |
Miller 1995.
| Study characteristics | ||
| Methods | Study type: RCT (cross‐over). Each participant used 2 treatment regimens in randomised order over 2 days. The treatments were ACBT and AD. Each day consisted of 2 identical treatment sessions with each session lasting 30 minutes. There was a 1‐week washout period between the 2 treatment days. Pre‐treatment included nebulised bronchodilators for some participants, and saline for others. |
|
| Participants | 18 enrolled; 18 evaluated; 10 male (55.6% male) Age: mean (NR); median (NR); SD (NR); range (11 to 32 years). Inclusion criteria: age greater than or equal to 11 years; clinically stable participants not receiving intravenous antibiotics. Exclusion criteria: NR. Characteristics: all 18 participants were clinically stable at the time of the study and not receiving intravenous antibiotics. |
|
| Interventions | ACBT: a postural drainage regimen was performed with ACBT (including breathing control, deep breathing, and forced expirations). AD: breathing control in conjunction with cough suppression to mobilise mucus. After multiple cycles, sputum was expectorated. The position was either sitting or supine. |
|
| Outcomes | Outcome measures: participant preference; lung function; sputum secretion; oxygen saturation. Additional outcomes: heart rate; xenon‐133 gas ventilation study. |
|
| Notes | Funding: MedicAid provided the Optimist nebulisers. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Eighteen patients with cystic fibrosis took part in a randomised two‐day cross‐over trial." |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) Participants | High risk | Blinding was not possible. The lack of blinding may have a high risk of bias for subjective outcomes, such as patient preference. The lack of blinding may have less of an effect on objective outcomes, such as lung function, sputum weight, and oxygen saturation. |
| Blinding (performance bias and detection bias) Personnel | High risk | Blinding was not possible. The lack of blinding may have a high risk of bias for subjective outcomes, such as patient preference. The lack of blinding may have less of an effect on objective outcomes, such as lung function, sputum weight, and oxygen saturation. |
| Blinding (performance bias and detection bias) Outcome Assessors | Unclear risk | Not discussed. |
| Selective reporting (reporting bias) | Low risk | All outcomes outlined in the methods section were reported in the results. |
| Adequate follow up? | Low risk | All participants were accounted for. |
| Compliance/ adherence assessed? | Unclear risk | Not reported. |
| Intention‐to‐treat? | Low risk | Participants were analysed in the groups to which they were randomised. |
| Washout? | Low risk | 1‐week washout period. |
Milne 2004.
| Study characteristics | ||
| Methods | Study type: RCT (cross‐over). Each participant was randomised to alternate‐day treatment on 2 days. Each day consist of 2 identical treatment sessions with each session lasting 15 minutes. There was a 1‐day washout period between the 2 treatment days. The 2 treatment regimens used were ACBT and AOD (flutter). Nebulisation therapy was administered before all study interventions. |
|
| Participants | 7 enrolled; 7 evaluated; 4 male (57.1% male). Age: mean (28 years); median (NR); SD (NR); range (16 to 42 years). Inclusion criteria: participants admitted for a course of intravenous antibiotics, participants old enough to perform lung function test. Exclusion criteria: pneumothorax or frank haemoptysis; participant admitted for terminal care. Characteristics: NR. |
|
| Interventions | ACBT: thoracic expansions; controlled breathing; FET; and coughing in a sitting position. AOD (flutter): the device was place in the participant's mouth. Participants exhaled 10 to 15 times through the flutter and then performed FET. AOD required the use of a flutter device. |
|
| Outcomes | Outcome measures: participant preference; lung function; sputum weight. | |
| Notes | Funding: Not described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Participants were randomised to two groups..." |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) Participants | High risk | Blinding was not possible. The lack of blinding may have a high risk of bias for subjective outcomes, such as patient preference. The lack of blinding may have less of an effect on objective outcomes, such as lung function and sputum weight. |
| Blinding (performance bias and detection bias) Personnel | High risk | Blinding was not possible. The lack of blinding may have a high risk of bias for subjective outcomes, such as patient preference. The lack of blinding may have less of an effect on objective outcomes, such as lung function and sputum weight. |
| Blinding (performance bias and detection bias) Outcome Assessors | Unclear risk | Not discussed. |
| Selective reporting (reporting bias) | Low risk | All outcomes outlined in the methods section were reported in the results. |
| Adequate follow up? | Low risk | All participants were accounted for. |
| Compliance/ adherence assessed? | Low risk | "The researcher supervised the physiotherapy sessions.". |
| Intention‐to‐treat? | Low risk | Participants were analysed in the groups to which they were randomised. |
| Washout? | Low risk | 1‐day washout period. |
Mortensen 1991.
| Study characteristics | ||
| Methods | Study type: RCT (cross‐over). Each participant was randomised to 3 groups over 3 days. The groups included a control (spontaneous coughing) group, an FET group, and a PEP + FET group. Each treatment day consisted of one session lasting 20 minutes. Usual medications were unchanged, including bronchodilators, mucolytic drugs and antibiotics taken before treatments. |
|
| Participants | 10 enrolled; 10 evaluated; 6 male (60% male). Age: mean (NR); median (20 years); SD (NR); range (15 to 26 years). Height: mean (NR); median (165.5 cm); range (154 to 185 cm) Inclusion criteria: NR. Exclusion criteria: NR. Characteristics: all 10 participants were infected with Pseudomonas aeruginosa and were non‐smokers. |
|
| Interventions | Control (spontaneous coughing): spontaneous coughing. FET: FET; breathing control; and postural drainage (including thoracic expansion exercises, relaxation, breathing control). PEP + FET: tidal volume breathing with PEP followed by FET and coughing. The intervention was conducted in a sitting position. PEP required the use of a PEP mask, 1‐way valve, and manometer. |
|
| Outcomes | Outcome measures: lung function, sputum weight. Additional measures: number of huffs; urine content; tracheobronchial clearance. |
|
| Notes | Funding: Danish Medical Research Council and the National Union for the Fight Against Lung Diseases | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The study was a randomised, single‐blinded cross‐over trial." |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) Participants | Low risk | Blinding was not possible. However, the risk of bias from a lack of blinding may be low for objective outcomes, such as lung function and sputum weight. |
| Blinding (performance bias and detection bias) Personnel | Low risk | Blinding was not possible. However, the risk of bias from a lack of blinding may be low for objective outcomes, such as lung function and sputum weight. |
| Blinding (performance bias and detection bias) Outcome Assessors | Unclear risk | Unclear. Not enough information was presented to make an assessment. |
| Selective reporting (reporting bias) | Low risk | All outcomes outlined in the methods section were reported in the results. |
| Adequate follow up? | Low risk | All participants were accounted for. |
| Compliance/ adherence assessed? | Low risk | "Physiotherapy treatments were supervised by a physiotherapist". |
| Intention‐to‐treat? | Low risk | Participants were analysed in the groups to which they were randomised. |
| Washout? | Unclear risk | No description of a washout period. |
Osman 2010.
| Study characteristics | ||
| Methods | Study type: RCT (cross‐over). Each participant was randomised to alternate day treatment over 4 consecutive days. The treatments regimens were HFCWO and usual therapy. Participants received either HFCWO on days 1 and 3 and their usual ACT on days 2 and 4 or vice versa. Treatment session were 2 times daily for 30 min. All nebulised and inhaled medications were taken before treatment sessions. |
|
| Participants | 30 enrolled; 29 evaluated; 21 male (72% male). Age: mean (29.4 years); median (NR); SD (8.4 years); range (NR). Height: mean (171 cm); median (NR); SD (9 cm); range (NR) Inclusion criteria: FEV1 ≥ 20% predicted, age ≥ 16 years, infective pulmonary exacerbations. Exclusion criteria: current severe haemoptysis, rib fractures, pregnancy, inability to give consent, persons whose usual ACT was HFCWO. Characteristics: NR. |
|
| Interventions | HFCWO (The Vest): This is the same as HFCC. Each participant was fitted with the appropriate sized vest. Participants remained in a upright sitting position throughout the 30 minute treatment session. HFCWO was applied for 8 minutes at each of 3 frequencies in sequence (10, 13, and 15 Hz) with each frequency followed by a 2‐minute resting period. Participants were instructed to huff or cough as they felt necessary to expectorate secretions. ACBT with modified PD&P and with modified PD alone: In accordance with the guidelines of the International Physiotherapy Group for Cystic Fibrosis (International Physiotherapy Group for CF 2009). AD in sitting and with modified PD: In accordance with the guidelines of the International Physiotherapy Group for Cystic Fibrosis (International Physiotherapy Group for CF 2009). PEP: In accordance with the guidelines of the International Physiotherapy Group for Cystic Fibrosis (International Physiotherapy Group for CF 2009). Not stated if using mask or mouthpiece. Flutter: In accordance with the guidelines of the International Physiotherapy Group for Cystic Fibrosis (International Physiotherapy Group for CF 2009). |
|
| Outcomes | Outcome measures: participant preference; lung function; sputum weight; oxygen saturation. Additional measures: perceived efficacy; comfort; incidence of urinary leakage. |
|
| Notes | In the published article, all usual therapies are group together, including ACBT, and compared to HFCWO. HFCWO was described by the study authors as The Vest, which is an HFCC device. The study authors were contacted and provided us with raw data for each participant including what their usual therapies were and all first‐arm data before the first cross‐over. Funding: Robert Luff Foundation and Hill‐Rom Ltd. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation to HFCWO or usual ACT on day 1 was determined using a computer‐generated randomisation table. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) Participants | High risk | Blinding was not possible. The lack of blinding may have a high risk of bias for subjective outcomes, such as patient preference. The lack of blinding may have less of an effect on objective outcomes, such as lung function, sputum weight, and oxygen saturation. |
| Blinding (performance bias and detection bias) Personnel | High risk | Blinding was not possible. The lack of blinding may have a high risk of bias for subjective outcomes, such as patient preference. The lack of blinding may have less of an effect on objective outcomes, such as lung function, sputum weight, and oxygen saturation. |
| Blinding (performance bias and detection bias) Outcome Assessors | Unclear risk | It is noted that an independent observer, blinded to the method of airway clearance used, performed spirometry, weighed the sputum samples, and collected visual analogue scales regarding perceived efficacy, comfort, and urinary leakage. It is not noted whether an independent observer assessed oxygen saturation. |
| Selective reporting (reporting bias) | Low risk | All outcomes outlined in the methods section were report in the results. |
| Adequate follow up? | Low risk | One of the participants withdrew due to a hypoglycaemic episode. The loss to follow up was 3.33% (< 10%). |
| Compliance/ adherence assessed? | Low risk | Each airway clearance treatment sessions was supervised by a physiotherapist to ensure optimisation and standardization. |
| Intention‐to‐treat? | High risk | Participants lost to follow up were not included in the analysis. |
| Washout? | High risk | There was no washout period. Raw data were obtained from the study authors before first crossover. |
Phillips 2004.
| Study characteristics | ||
| Methods | Study type: RCT (cross‐over). Each participant used 2 treatment regimens in randomised order over 2 days. Each day consisted of 2 identical treatment sessions each lasting 20 minutes. There was a 1‐day washout period between the 2 treatment days. The treatment regimens used were ACBT and HFCC. |
|
| Participants | 10 enrolled; 10 evaluated; 7 male (70% male). Age: mean (13.7 years); median (14 years); SD (NR); range (9 to 16 years). Inclusion criteria: acute respiratory exacerbation. Exclusion criteria: pneumothorax or haemoptysis; any vision, hearing or balance disturbance; chest trauma. Characteristics: NR. |
|
| Interventions | ACBT: relaxed breathing control; 3‐4 thoracic expansion exercises; and FET. HFCC: 4x 2‐phase cycles of 600 oscillations per minute for 3 minutes followed by 60 oscillations per minute for 2 minutes. HFCC required the use of the Hayek Oscillator 1000. |
|
| Outcomes | Outcome measures: participant preference; lung function; sputum weight. | |
| Notes | Funding: Not described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used. |
| Blinding (performance bias and detection bias) Participants | High risk | Blinding was not possible. The lack of blinding may have a high risk of bias for subjective outcomes, such as patient preference. The lack of blinding may have less of an effect on objective outcomes, such as lung function and sputum weight. |
| Blinding (performance bias and detection bias) Personnel | High risk | Blinding was not possible. The lack of blinding may have a high risk of bias for subjective outcomes, such as patient preference. The lack of blinding may have less of an effect on objective outcomes, such as lung function and sputum weight. |
| Blinding (performance bias and detection bias) Outcome Assessors | Unclear risk | Study reported, "An independent, blinded observer measured the weight of wet sputum." The study did not report if those assessing other outcomes were blinded. |
| Selective reporting (reporting bias) | Low risk | All outcomes outlined in the methods section were reported in the results. |
| Adequate follow up? | Low risk | All participants were accounted for. |
| Compliance/ adherence assessed? | Low risk | Both treatments were supervised. |
| Intention‐to‐treat? | Low risk | Participants were analysed in the groups to which they were randomised. |
| Washout? | Low risk | 1‐day washout period. |
Pike 1999.
| Study characteristics | ||
| Methods | Study type: RCT (cross‐over). Each participant used 2 treatment regimens in randomised order over 2 days. Each day consisted of 2 identical treatment sessions. The 2 treatment regimens used were ACBT and AOD (Flutter) + FE. |
|
| Participants | 21 enrolled; 21 evaluated; 12 male (57.1% male). Age: mean (NR); median (26 years); SD (NR); range (NR). Inclusion criteria: NR. Exclusion criteria: NR. Characteristics: NR. |
|
| Interventions | ACBT: no description. AOD (Flutter) + FE: no description. |
|
| Outcomes | Outcome measures: participant preference; lung function; sputum weight; oxygen saturation. | |
| Notes | Funding: Not described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The participants were randomised, but method not described. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) Participants | High risk | Blinding was not possible. The lack of blinding may have a high risk of bias for subjective outcomes, such as patient preference. The lack of blinding may have less of an effect on objective outcomes, such as lung function, sputum weight, and oxygen saturation. |
| Blinding (performance bias and detection bias) Personnel | High risk | Blinding was not possible. The lack of blinding may have a high risk of bias for subjective outcomes, such as patient preference. The lack of blinding may have less of an effect on objective outcomes, such as lung function, sputum weight, and oxygen saturation. |
| Blinding (performance bias and detection bias) Outcome Assessors | Unclear risk | Study reported that "sputum weight, pulmonary function, and oxygen saturation were measured by an independent observer." |
| Selective reporting (reporting bias) | Unclear risk | Not discussed. |
| Adequate follow up? | Unclear risk | Not discussed. |
| Compliance/ adherence assessed? | Unclear risk | Not reported. |
| Intention‐to‐treat? | Unclear risk | Unclear. Only available as an abstract. |
| Washout? | Unclear risk | No description of a washout period. |
Pryor 1979.
| Study characteristics | ||
| Methods | Study type: RCT (cross‐over). Each participant used 2 treatment regimens in randomised order over 4 days. The treatments were CCPT and FET. The frequency of the treatment sessions varied. 12 participants needed treatment 4x daily, 3 needed treatment 3x daily, 1 needed treatment 2x daily. Usual medications were unchanged. |
|
| Participants | 18 enrolled; 16 evaluated; 8 male (50% male). Age: mean (20.5 years); median (NR); SD (NR); range (14 to 28 years). Inclusion criteria: NR. Exclusion criteria: admitted for terminal care. Characteristics: all 18 participants had an acute exacerbation of their bronchopulmonary infection. |
|
| Interventions | CCPT: postural drainage (breathing expansion exercises, coughing, and percussion); chest percussion; and shaking. FET + CCPT: Postural drainage and FET with expansion breathing exercise; coughing; and percussion and chest compression. |
|
| Outcomes | Outcome measures: lung function; sputum weight. Additional outcomes: rate of sputum production. |
|
| Notes | Analysis included part I of the study only. Part II compares ACBT (self‐administered) to ACBT (physiotherapist administered). This is not a comparison of interest in this review. Funding: Not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The treatments were given in random order. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) Participants | Low risk | Blinding was not possible. However, the risk of bias from a lack of blinding may be low for objective outcomes, such as lung function and sputum weight. |
| Blinding (performance bias and detection bias) Personnel | Low risk | Blinding was not possible. However, the risk of bias from a lack of blinding may be low for objective outcomes, such as lung function and sputum weight. |
| Blinding (performance bias and detection bias) Outcome Assessors | Unclear risk | Not discussed. |
| Selective reporting (reporting bias) | Low risk | All outcomes outlined in the methods section were reported in the results. |
| Adequate follow up? | High risk | Two of 18 participants withdrew from the study: 1 developed pneumothorax and the other was unable to produce enough sputum for an accurate assessment. The loss to follow‐up was 11.11% (> 10%). |
| Compliance/ adherence assessed? | Low risk | "Three physiotherapists took part in the treatment sessions throughout the study." |
| Intention‐to‐treat? | High risk | Participants lost to follow up were not included in the analysis. |
| Washout? | Unclear risk | No description of a washout period. |
Pryor 1994.
| Study characteristics | ||
| Methods | Study type: RCT (cross‐over). The treatments were randomised and remained the same for a 24‐hour period. There were 3 sessions in a day (2 monitored and 1 unmonitored). The treatments were ACBT and ACBT + AOD (flutter). Usual medications were unchanged. |
|
| Participants | 24 enrolled; 20 evaluated; 14 male (70% male). Age: mean (24.4 years); median (NR); SD (NR); range (16 to 36 years). Inclusion criteria: participants admitted to the hospital as clinically stable (clinical stability was measured by the absence of any clinical changes such as fever, FEV1, FVC, FEF50, FEF75, ability to do their own chest physiotherapy); available for 2 consecutive days as close to discharge as possible. Exclusion criteria: participants admitted to the hospital for terminal care or with a pneumothorax or frank haemoptysis. Characteristics: all 24 participants had a history of bronchopulmonary infection. |
|
| Interventions | ACBT: breathing control; thoracic expansion; FET. ACBT + AOD (flutter): flutter for the first 10 minutes of the session; ACBT for the remainder. |
|
| Outcomes | Outcome measures: participant preference; sputum weight; oxygen saturation. | |
| Notes | Sputum weight is reported for both the morning and afternoon sessions. Only baseline information is extractable for lung function. Funding: Not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Treatment regimens were randomised..." |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) Participants | High risk | Blinding was not possible. The lack of blinding may have a high risk of bias for subjective outcomes, such as patient preference. The lack of blinding may have less of an effect on objective outcomes, such as sputum weight and oxygen saturation. |
| Blinding (performance bias and detection bias) Personnel | High risk | Blinding was not possible. The lack of blinding may have a high risk of bias for subjective outcomes, such as patient preference. The lack of blinding may have less of an effect on objective outcomes, such as sputum weight and oxygen saturation. |
| Blinding (performance bias and detection bias) Outcome Assessors | Low risk | "The measurement of the monitored session were taken by independent observers." |
| Selective reporting (reporting bias) | High risk | It is stated that lung function measurements were recorded before and at 5, 10, 15 and 30 minutes after treatment, but results of lung function measurement are not provided. |
| Adequate follow up? | High risk | 4 of 24 participants withdrew from the study; 2 participants had their drug regimens changed during the study and another 2 participants withdrew because of technical problems with the oximeter and collection of sputum. The loss to follow up was 16.67% (> 10%). |
| Compliance/ adherence assessed? | Low risk | The treatment sessions were monitored. |
| Intention‐to‐treat? | High risk | Participants lost to follow up were not included in the analysis. |
| Washout? | Unclear risk | No description of a washout period. |
Pryor 2010.
| Study characteristics | ||
| Methods | `Study type: RCT (parallel design). | |
| Participants | 75 participants with CF Age: median (range) 27 (17 to 63) years. Gender split: 47 males and 28 females. Inclusion criteria: 16 years of age or older, FEV1 ≥ 25% predicted. Exclusion criteria: evidence of current respiratory exacerbation, past history of pneumothorax, current severe haemoptysis, awaiting lung or heart transplantation, pregnancy, and recent (within 3 months) acquisition of Burkholderia cepacia. |
|
| Interventions | ACBT versus AD versus cornet versus flutter versus PEP (exact device not clear i.e. mask or mouthpiece). | |
| Outcomes | Measured monthly for 1 year: FEV1; FVC; MEF25; RV/TLC%; BMI; exercise capacity (modified shuttle test); QoL. | |
| Notes | Lung function data available for 65 participants. The author has been contacted about obtaining additional data, and we are awaiting their response. Funding: An award from the Clinical Research Committee, Royal Brompton & Harefield Charitable Trust Foundation, provided the equipment and the Debbie Shearer Fund financed travel for the proponents of AD (Belgium) and PEP (Denmark) to visit Royal Brompton Hospital; and the travel expenses of the physiotherapists training subjects on the AD arm of the study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was computerized and used a random number sequence stratified by FEV1% predicted..." |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) Participants | High risk | Blinding was not possible. The lack of blinding may have a high risk of bias for subjective outcomes, such as quality of life. The lack of blinding may have less of an effect on objective outcomes, such as lung function. |
| Blinding (performance bias and detection bias) Personnel | High risk | Blinding was not possible. The lack of blinding may have a high risk of bias for subjective outcomes, such as quality of life. The lack of blinding may have less of an effect on objective outcomes, such as lung function. |
| Blinding (performance bias and detection bias) Outcome Assessors | Low risk | "The measurements of lung function and body mass index at 0, 6, and 12 months and the statistical analyses were undertaken by observers (physiologists and statistician) blind to the regimen to which the subjects had been randomised." |
| Selective reporting (reporting bias) | Low risk | All outcomes outlined in the methods section were reported in the results. |
| Adequate follow up? | High risk | 10 of 75 (13%) of participants were lost to follow up. Additionally, 13 participants changed their airway clearance technique, but the details on this are not provided. |
| Compliance/ adherence assessed? | Low risk | "Subjects were requested to attend monthly, for a review of their ACT..." |
| Intention‐to‐treat? | High risk | Participants who were lost to follow up were not included in the analysis. |
Reisman 1988.
| Study characteristics | ||
| Methods | Study type: RCT (parallel design). Participants were stratified according to sex, age, and pulmonary function. Within each stratum, participants were then randomised to 1 of the treatment groups. The treatments were FET and CCPT + FET. In both groups participants took pancreatic enzyme supplements, antistaphylococcal antibiotics, and inhaled β2‐bronchodilators. 3 year study. |
|
| Participants | 67 enrolled; 63 evaluated; 38 male (60.3 % male). Age: no mean; SD; median or range reported. Inclusion criteria: 7 < years to < 21 years; FEV1 > 40% of the predicted value for height and sex; participants with mild to moderate pulmonary disease only. Exclusion criteria: participants involved in any other studies. |
|
| Interventions | FET: maximal and normal inspirations, forced expiration, and breathing control. CCPT + FET: postural drainage; percussion; and FET. |
|
| Outcomes | Outcome measures: lung function; number of pulmonary exacerbations. | |
| Notes | Participants were stratified by age, sex, and pulmonary impairment. Funding: Supported in part by grant aid from the Canadian Federal Department of Health and Welfare. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Subjects were randomly assigned within each stratum to one of two groups." |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) Participants | Low risk | Blinding was not possible. However, the risk of bias from a lack of blinding may be low for objective outcomes, such as lung function and the number of pulmonary exacerbations. |
| Blinding (performance bias and detection bias) Personnel | Low risk | Blinding was not possible. However, the risk of bias from a lack of blinding may be low for objective outcomes, such as lung function and the number of pulmonary exacerbations. |
| Blinding (performance bias and detection bias) Outcome Assessors | Unclear risk | Not discussed. |
| Selective reporting (reporting bias) | High risk | Not all of the outcomes outlined in the methods section were reported in the results. For instance, sputum was collected but results were not reported. |
| Adequate follow up? | Low risk | 4 of 67 participants withdrew from the study: 2 participants from the CCPT + FET group relocated and another 2 participants from the FET group withdrew because of family anxiety associated with discontinuation of conventional chest physiotherapy (used with FET). The loss to follow up was 5.97% (<10%). |
| Compliance/ adherence assessed? | Low risk | "... they were asked to keep a diary reporting adherence to their own physiotherapy regimens.". |
| Intention‐to‐treat? | High risk | Lost to follow‐up participants were not included in the analysis. |
Steven 1992.
| Study characteristics | ||
| Methods | Study type: RCT (cross‐over). Each participant used 3 treatment regimens for 24‐hour periods in randomised order over 3 consecutive days.The treatments were coughing, ACBT (gravity‐assisted position), and ACBT (sitting). The frequency of the treatment sessions varied between 2 to 4 times per day. The mean duration of the treatment sessions was 22 minutes. |
|
| Participants | 24 enrolled; 24 evaluated; 16 male (66.7% male). Age: mean (25 years); median (NR); SD (NR); range (17 to 33 years). Inclusion criteria: participants who were clinically stable; producing more than 20 g of sputum in 24 hours; and fit enough to carry out their own chest physiotherapy. Exclusion criteria: participants with a pneumothorax; frank haemoptysis; or an FEV1 which increased more than 15% after bronchodilators. Characteristics: all 24 participants had an exacerbation of their bronchopulmonary infection. |
|
| Interventions | Coughing (sitting): coughing and breathing control. ACBT (gravity‐assisted position): postural drainage and ACBT; including breathing control, thoracic expansion, and FET. ACBT (sitting): ACBT including breathing control; thoracic expansion; and FET. |
|
| Outcomes | Outcome measures: lung function; sputum weight; oxygen saturation. | |
| Notes | Funding: Not described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Each patient used each of the three treatment regimens for a 24 hour period, in randomised order, over three consecutive days." |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) Participants | Low risk | Blinding was not possible. However, the risk of bias from a lack of blinding may be low for objective outcomes, such as lung function, sputum weight, and oxygen saturation. |
| Blinding (performance bias and detection bias) Personnel | Low risk | Blinding was not possible. However, the risk of bias from a lack of blinding may be low for objective outcomes, such as lung function, sputum weight, and oxygen saturation. |
| Blinding (performance bias and detection bias) Outcome Assessors | Unclear risk | Not discussed. |
| Selective reporting (reporting bias) | Low risk | All outcomes outlined in the methods section were reported in the results. |
| Adequate follow up? | Low risk | All participants were accounted for. |
| Compliance/ adherence assessed? | Unclear risk | Not reported. |
| Intention‐to‐treat? | Low risk | Participants were analysed in the groups to which they were randomised. |
| Washout? | Unclear risk | No description of a washout period. |
Webber 1985.
| Study characteristics | ||
| Methods | Study type: RCT (cross‐over). Each participant used 2 treatment regimens in randomised order over 4 consecutive days. The treatments were FET and FET + self‐percussion. The treatment regimen remained unchanged for a 24‐hour period. 6 participants needed treatment 4x daily, 8 needed treatment 3 x daily, 2 needed treatment 2 x daily.The individual treatment time ranged from 10‐38 minutes, while the daily treatment times ranged from 51‐107 minutes. 12 participants received intravenous antibiotic treatment and four received oral antibiotic treatment. 4 participants did not use chest compression. |
|
| Participants | 16 enrolled; 16 evaluated; 10 male (62.5% male). Age: mean (21.1 years); median (NR); SD (NR); range (13 to 35 years). Inclusion criteria: NR. Exclusion criteria: NR. Characteristics: all 18 participants were admitted with an acute exacerbation of their bronchopulmonary infection. |
|
| Interventions | FET: postural drainage including thoracic expansion and FET including breathing control. FET + self‐percussion: postural drainage including thoracic expansion; self‐percussion; and FET including breathing control. |
|
| Outcomes | Outcome measures: sputum weight. | |
| Notes | Funding: Cystic Fibrosis Research Trust | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Treatment days were randomised." |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) Participants | Low risk | Blinding was not possible. However, the risk of bias from a lack of blinding may be low for objective outcomes, such as sputum weight. |
| Blinding (performance bias and detection bias) Personnel | Low risk | Blinding was not possible. However, the risk of bias from a lack of blinding may be low for objective outcomes, such as sputum weight. |
| Blinding (performance bias and detection bias) Outcome Assessors | Unclear risk | Not discussed. |
| Selective reporting (reporting bias) | High risk | Lung function results were not reported. |
| Adequate follow up? | Low risk | All participants were accounted for. |
| Compliance/ adherence assessed? | Low risk | Treatment regimens were performed under the supervision of 3 physiotherapists. |
| Intention‐to‐treat? | Low risk | Participants were analysed in the groups to which they were randomised. |
| Washout? | Unclear risk | No description of a washout period. |
ACBT: active cycle of breathing technique AD: autogenic drainage AOD: airway oscillating devices BMI: body mass index CCPT: conventional chest physiotherapy CF: cystic fibrosis CFQ‐R: Cystic Fibrosis Questionnaire ‐ Revised FE: forced expiration FEF: forced expiratory flow FET: forced expiratory technique FEV1: forced expiratory volume at one second FFM: fat‐free mass FVC: forced vital capacity HFCC: high‐frequency chest compression HFCWO: high‐frequency chest wall oscillation HNE: human neutrophil elastase Hz: herz NIV: non‐invasive ventilation NR: not reported PD: postural drainage PD&P: postural drainage and percussion PEF: peak expiratory flow PEP: positive expiratory pressure PSV: pressure support ventilation QoL: quality of life RCT: randomised controlled trial rhDNase: dornase alfa RIM: resistance inspiratory manoeuvre SaO2: oxygen saturation SD: standard deviation TIRE: test of incremental respiratory endurance VO2: oxygen consumption
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTRN12605000471684 | Not an RCT. |
| ACTRN12614001233617 | Study does not include people with CF. |
| ACTRN12619000224123 | Study used FET which did not include all components of ACBT. |
| ACTRN12619001681145 | Study does not evaluate ACBT. |
| Andreasson 1987 | Study used FET which did not include all components of ACBT. |
| Asher 1982 | Study did not address ACBT. |
| Bain 1988 | Study did not address ACBT. |
| Baldwin 1994 | Study did not address ACBT. |
| Braggion 1995 | A cross‐over RCT, but no comparison of interest as all participants received FET after treatment periods. |
| Castle 1994 | To date we have not received any response to our requests for additional data to allow us to include this study. Given its age, we do not expect to receive relevant information now. Therefore we have excluded the study, but if we receive any relevant information in future, we will re‐assess this decision. |
| Chatham 1998 | Study did not address ACBT. |
| ChiCTR1800019989 | Study does not include people with CF. |
| Davies 2012 | This study was excluded because it did not address ACBT. |
| Desmond 1983 | Study did not address ACBT. |
| Falk 1984 | A cross‐over RCT, but FET did not include all components of ACBT. |
| Falk 1993 | To date we have not received any response to our requests for additional data to allow us to include this study. Given its age, we do not expect to receive relevant information now. Therefore we have excluded the study, but if we receive any relevant information in future, we will re‐assess this decision. |
| Gursli 2017 | A cross‐over RCT, which did not describe the components of FET. |
| Hasani 1991 | This article did not address CF. |
| Hasani 1994 | This article did not address CF. |
| Horsley 2007 | A non‐randomised study with no outcome of interest. |
| Klig 1989 | A non‐randomised cross‐over study. |
| Kofler 1998 | Study did not address ACBT. |
| Lannefors 1992 | To date we have not received any response to our requests for additional data to allow us to include this study. Given its age, we do not expect to receive relevant information now. Therefore we have excluded the study, but if we receive any relevant information in future, we will re‐assess this decision. |
| McDonnell 1986 | Study did not address ACBT. |
| NCT00164138 | This study was excluded because it did not address ACBT. |
| NCT00404859 | Study did not address ACBT. |
| NCT00716664 | Study did not address ACBT. |
| NCT01943890 | Study did not address ACBT. |
| NCT02906826 | Study did not address ACBT. |
| NCT03078127 | A cross‐over study using FET, which did not include all components of ACBT. |
| O'Neill 2017 | Study did not address ACBT. |
| Oberwaldner 1986 | Non‐randomised controlled study. |
| Orlik 2000 | Non‐randomised controlled study. |
| Orlik 2001 | Non‐randomised controlled study. |
| Parker 1984 | To date we have not received any response to our requests for additional data to allow us to include this study. Given its age, we do not expect to receive relevant information now. Therefore we have excluded the study, but if we receive any relevant information in future, we will re‐assess this decision. |
| Petrone 2009 | To date we have not received any response to our requests for additional data to allow us to include this study. Given its age, we do not expect to receive relevant information now. Therefore we have excluded the study, but if we receive any relevant information in future, we will re‐assess this decision. |
| Prasad 1998a | A review on physiotherapy treatments in CF, thus there were no original data. |
| Prasad 2000 | A review on physiotherapy treatments in CF, thus there were no original data. |
| Pryor 1990 | A non‐randomised study. |
| RBR‐5g9f6w | Study used FET, which did not include all components of ACBT. |
| Rogers 1984 | Not an RCT. |
| Rossman 1982 | Study did not address ACBT. |
| Salh 1989 | A non‐randomised cross‐over study. |
| Sontag 2010 | Study did not address ACBT. |
| Stanford 2020 | No comparison of interest. |
| Steen 1991 | While the majority of the arms were randomised, the intervention of interest was not randomised. |
| Sutton 1983 | Cross‐over RCT using FET, which did not include all components of ACBT. |
| Sutton 1985 | Did not address ACBT. |
| Thomas 1995 | A review on physiotherapy treatments in CF, thus there were no original data. |
| van Hengstum 1987 | Did not address CF. |
| van Hengstum 1988 | Results were presented for the 8 participants involved in the study (6 with CF and 2 with agammaglobulinaemia). We contacted the study authors to obtain data for CF participants separately, but have not received any reply so are not able to include this study. |
| Verboon 1986 | Cross‐over RCT did not include a comparison of interest. The 2 treatments only differed by PD, which is considered a component of ACBT in our definition. |
| Ward 2018 | Study does not evaluate ACBT. |
| Webber 1986 | A non‐randomised study. |
| White 1997 | Cross‐over RCT which did not include a comparison of interest. Participants were randomised to receive ACBT or ACBT without thoracic expansion. |
| Williams 1994 | A review on physiotherapy treatments in CF, thus there were no original data. |
| Williams 2000 | Cross‐over RCT with no comparison of interest. CF participants were randomised to therapy‐assisted ACBT or independent ACBT. |
| Wilson 1995 | A non‐randomised cross‐over study. |
| Znotina 2000 | No comparison of interest. RCT comparing PEP/ oscillating PEP + FET with a physiotherapist (Group A) or without a physiotherapist (Group B). |
ACBT: active cycle of breathing technique CF: cystic fibrosis FET: forced expiration technique PD: postural drainage PEP: positive expiratory pressure RCT: randomised controlled trial
Differences between protocol and review
We reorganised the comparator interventions. They were previously listed separately, but we have grouped them for ease of analysis and in accordance with other physiotherapy reviews of the Cochrane CFGD Group into the following:
Conventional chest physiotherapy (CCPT) (postural drainage, percussion, chest shaking, huffing, and coughing; excludes the use of exercise, FET, PEP, or other mechanical devices);
PEP (PEP mask therapy, high pressure PEP mask therapy);
Oscillatory devices (airway oscillating devices, high frequency chest compression devices);
Breathing techniques (excluding ACBT, but including autogenic drainage);
Exercise;
Other therapy (resistive inspiratory manoeuvre).
In line with current Cochrane guidance, we have generated summary of findings tables for each comparison listed above and have graded the evidence using the GRADE criteria.
We have added sputum volume as an outcome.
In addition to our electronic searching and hand searching, we have searched the ClinicalTrials.gov and WHO ICTRP clinical trial registries.
Contributions of authors
Karen Robinson provided the link with the Cochrane Cystic Fibrosis and Genetic Disorders Editorial Base.
Protocol
All authors were responsible for drafting the protocol.
Original review
The Editorial Base and all authors developed the search strategy and searched for studies. Naomi Mckoy obtained copies of studies, entered data into RevMan and carried out the analysis. All authors were responsible for selecting which studies to include, extracting data, interpreting the analysis and drafting the final review.
Updated review 2022
Karen Robinson, Ian Saldanha, and Lisa Wilson were involved in updating the review.
Sources of support
Internal sources
No sources of support provided
External sources
-
CF Foundation, USA
Partially funded by the Cystic Fibrosis Foundation.
-
National Institute for Health Research, UK
This systematic review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group.
Declarations of interest
Lisa Wilson declares no known potential conflict of interest.
Ian Saldahna declares no known potential conflict of interest.
Karen Robinson declares no known potential conflict of interest.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bilton 1992 {published data only}
- Bilton D, Dodd M, Webb AK. The benefits of exercise combined with physiotherapy in cystic fibrosis. Pediatric Pulmonology 1990;9(Suppl 5):253-4. [CFGD REGISTER: PE41a] [Google Scholar]
- Bilton D, Dodd ME, Abbot JV, Webb AK. The benefits of exercise combined with physiotherapy in the treatment of adults with cystic fibrosis. Respiratory Medicine 1992;86(6):507-11. [CFGD REGISTER: PE41b] [DOI] [PubMed] [Google Scholar]
Chatham 2004 {published data only}
- Chatham K, Ionescu AA, Nixon LS, Shale DJ. A short-term comparison of two methods of sputum expectoration in cystic fibrosis. European Respiratory Journal 2004;23(3):435-9. [CFGD REGISTER: PE149] [DOI] [PubMed] [Google Scholar]
Fauroux 1999 {published data only}
- Fauroux B, Boulé M, Lofaso F, Zérah F, Clément A, Harf A, et al. Chest physiotherapy in cystic fibrosis: improved tolerance with nasal pressure support ventilation. Pediatrics 1999;103(3):E32. [CFGD REGISTER: PE103a] [DOI] [PubMed] [Google Scholar]
- Fauroux B, Boule M, Escurat G, Pouzet C, Harf A, Isabey D. Chest physiotherapy in cystic fibrosis: improvement of tolerance with nasal pressure support ventilation. European Respiratory Journal 1997;10 Suppl 25:79S. [CFGD REGISTER: PE103b] [Google Scholar]
Gungor 2021 {published data only}
- Gungor S, Gencer-Atalay K, Bahar-Ozdemir Y, Kenis-Coskun O, Karadag-Saygi E. The clinical effects of combining postural exercises with chest physiotherapy in cystic fibrosis: a single-blind, randomized-controlled trial. Turkish Journal of Physical Medicine and Rehabilitation 2021;67(1):91-8. [CFGD REGISTER: PE319b] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03295201. Clinical effects of exercise program added to pulmonary rehabilitation in patients with cystic fibrosis. clinicaltrials.gov/ct2/show/NCT03295201 (first received 27 September 2017). [CFGD REGISTER: PE319a]
Hofmeyr 1986 {published data only}
- Hofmeyr JL, Webber BA, Hodson ME. Evaluation of positive expiratory pressure as an adjunct to chest physiotherapy in the treatment of cystic fibrosis. Thorax 1986;41(12):951-4. [CFGD REGISTER: PE9b] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber BA, Hofmeyr JL, Hodson ME, Batten JC. Evaluation of positive expiratory pressure as an adjunct to postural drainage. In: 13th Annual Meeting of the European Working Group for Cystic Fibrosis. 1985:95. [CFGD REGISTER: PE9a]
Holland 2003 {published data only}
- Holland A, Denehy L, Ntoumenopoulos G, Naughton M, et al. Non-invasive ventilation preserves inspiratory muscle strength and prevents oxygen desaturation during airway clearance in adults with exacerbations of cystic fibrosis. Australian Journal of Physiotherapy 2004;50(3):A6. [CFGD REGISTER: PE143e] [Google Scholar]
- Holland AE, Denehy L, Ntoumenopoulos G, McMeeken J, Wilson JW. Non-invasive ventilation prevents inspiratory muscle fatigue and oxygen desaturation during airway clearance in adults with acute exacerbations of cystic fibrosis. In: Thoracic Society of Austrailia & New Zealand Annual Scientific Meeting. 2003 April 4-9. [ABSTRACT NO.: P140] [CFGD REGISTER: PE143d]
- Holland AE, Denehy L, Ntoumenopoulos G, Naughton M, Wilson J. Non-invasive ventilation prevents inspiratory muscle fatigue and oxygen desaturation during airway clearance in adults with acute exacerbations of cystic fibrosis. Journal of Cystic Fibrosis 2003;2(Suppl1):S62. [CFGD REGISTER: PE143a] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland AE, Denehy L, Ntoumenopoulos G, Naughton MT, Wilson JW. Non-invasive ventilation assists chest physiotherapy in adults with acute exacerbations of cystic fibrosis. Thorax 2003;58(10):880-4. [CFGD REGISTER: PE143c] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland AE, Denehy L, Ntoumenopoulos G, Wilson JW. Non-invasive ventilation prevents inspiratory muscle fatigue and oxygen desaturation during airway clearance in adults with exacerbations of cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 2003;167:D041. [CFGD REGISTER: PE143b] [Google Scholar]
Howard 2000 {published data only}
- Howard J, Bradley J, Hewitt O, Elborn S. The active cycle of breathing (ACBT) is a more effective method of airway clearance in cystic fibrosis (CF) patients than the test of incremental respiratory endurance (TIRE). Pediatric Pulmonology 2000;30(Suppl 20):304. [CFGD REGISTER: PE116] [Google Scholar]
Hristara‐Papadopoulou 2005 {published data only}
- Christara-Papadopoulou A, Varsamis P, Diomou G. Physiotherapy in cystic fibrosis: an empirical comparison of two methods [Physiotherapie bei Zystisher Fibrose: Ein empirisher Vergleich zweier Methoden]. Zeitschrift fur Physiotherapeuten 2005;57(3):536-42. [CFGD REGISTER: PE240b] [Google Scholar]
Hristara‐Papadopoulou 2007 {published data only}
- Hristara-Papadopoulou A, Tsanakas J. Results of active cycle of breathing techniques and conventional physiotherapy in mucociliary clearance in children with cystic fibrosis. Hippokratia 2007;11:202-4. [PMC free article] [PubMed] [Google Scholar]
Kofler 1994 {published data only}
- Kofler AM, Belluscio M, Bressan T, Carlesi A, Leone P, Lucidi V, et al. PEP-mask and active cycle of breathing techniques. What is better in children with cystic fibrosis. In: 19th European Cystic Fibrosis Conference; 1994 May 29-June 3; Paris, France. 1994:O66. [CFGD REGISTER: PE50]
Miller 1995 {published data only}
- Hall DO, Miller S, Clayton CB, Nelson R. Chest physiotherapy in cystic fibrosis: A comparative study of autogenic drainage and the active cycle of breathing technique. In: 19th European Cystic Fibrosis Conference; 1994 May 29-June 3; Paris, France. 1994:O64. [CFGD REGISTER: PE28a] [DOI] [PMC free article] [PubMed]
- Miller S, Hall DO, Clayton CB, Nelson R. Chest physiotherapy in cystic fibrosis: a comparative study of autogenic drainage and the active cycle of breathing techniques with postural drainage. Thorax 1995;50(2):165-9. [CFGD REGISTER: PE28c] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S, Hall DO, Clayton CB, Nelson R. Chest physiotherapy in cystic fibrosis (CF) a comparative study of autogenic drainage (AD) and active cycle of breathing technique (ACBT) (formerly FET). Pediatric Pulmonology 1993;16(Suppl 9):267. [CFGD REGISTER: PE28b] [DOI] [PMC free article] [PubMed] [Google Scholar]
Milne 2004 {published data only}
- Milne SM, Eales CJ. A pilot study comparing two physiotherapy techniques in patients with cystic fibrosis. South African Journal of Physiotherapy 2004;60(2):3-6. [CFGD REGISTER: PE161] [Google Scholar]
Mortensen 1991 {published data only}
- Falk M, Mortensen J, Jensen C, Groth S, Jensen T. Postural drainage or PEP. effects on tracheobronchial clearance in cystic fibrosis. Pediatric Pulmonology 1990;9(Suppl 5):250. [CFGD REGISTER: PE15a] [Google Scholar]
- Mortensen J, Falk M, Groth S, Jensen C. The effects of postural drainage and positive expiratory pressure physiotherapy on tracheobronchial clearance in cystic fibrosis. Chest 1991;100(5):1350-7. [CFGD REGISTER: PE15b] [DOI] [PubMed] [Google Scholar]
- Mortensen J, Groth S, Falk M, Jensen C, Jensen T. Assessment of tracheobronchial clearance by sputum expectorated during chest physiotherapy in cystic fibrosis. European Respiratory Journal 1990;3(Suppl 10):260s-261s. [CFGD REGISTER: PE15c] [Google Scholar]
Osman 2010 {published and unpublished data}
- NCT00817947. Airway clearance using high frequency chest wall oscillation [An assessment of the short-term effects of the airway clearance technique of high frequency chest wall oscillation in people with cystic fibrosis]. clinicaltrials.gov/show/NCT00817947 (first received 07 January 2009). [CENTRAL: CN-01523471] [CFGD REGISTER: PE171c]
- Osman LP, Roughton M, Hodson ME, Pryor JA. High frequency chest wall oscillation in cystic fibrosis. Journal of Cystic Fibrosis 2008;7(Suppl 2):S73. [ABSTRACT NO.: 295] [CFGD REGISTER: PE171a] [Google Scholar]
- Osman LP, Roughton M, Hodson ME, Pryor JA. Short-term comparative study of high frequency chest wall oscillation and European airway clearance techniques in patients with cystic fibrosis. Thorax 2010;65(3):196-200. [CFGD REGISTER: PE171b] [DOI] [PMC free article] [PubMed] [Google Scholar]
Phillips 2004 {published data only}
- Phillips GE, Pike S, Jaffe A, Bush A. Comparison of the active cycle of breathing techniques and external high frequency oscillation jacket for clearance of secretions in children with cystic fibrosis. Thorax 1998;53(Suppl 4):A61. [CFGD REGISTER: PE114a] [Google Scholar]
- Phillips GE, Pike SE, Jaffe A, Bush A. Comparison of active cycle of breathing and high-frequency oscillation jacket in children with cystic fibrosis. Pediatric Pulmonology 2004;37(1):71-5. [CFGD REGISTER: PE114b] [DOI] [PubMed] [Google Scholar]
Pike 1999 {published data only}
- Pike SE, Machin AC, Dix KJ, Pryor JA, Hodson ME. Comparison of flutter VRPI and forced expirations (FE) with active cycle of breathing techniques (ACBT) in subjects with cystic fibrosis (CF). Netherlands Journal of Medicine 1999;54:S55-S56. [CFGD REGISTER: PE102] [Google Scholar]
Pryor 1979 {published data only}
- Hodson ME, Batten JC, Pryor JA, Webber BA. Evaluation of the forced expiration technique as an adjunct to postural drainage in the treatment of cystic fibrosis. In: 9th Meeting European Working Group for Cystic Fibrosis; 1979 June 12-13; Noordwijkerhout, The Netherlands. 1979:57. [CFGD REGISTER: PE78b]
- Pryor JA, Webber BA, Hodson ME, Batten JC. Evaluation of the forced expiration technique as an adjunct to postural drainage in treatment of cystic fibrosis. BMJ 1979;2(6187):417-8. [CFGD REGISTER: PE78a] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor JA, Webber BA. An evaluation of the forced expiration technique as an adjunct to postural drainage. Physiotherapy 1979;65(10):304-7. [CFGD REGISTER: PE78c] [PubMed] [Google Scholar]
Pryor 1994 {published data only}
- Pryor JA, Webber BA, Hodson ME, Warner JO. The Flutter VRP1 as an adjunct to chest physiotherapy in cystic fibrosis. In: 11th International Cystic Fibrosis Congress; 1992; Dublin, Ireland. 1992:WP 102. [CFGD REGISTER: PE25a] [DOI] [PubMed]
- Pryor JA, Webber BA, Hodson ME, Warner JO. The Flutter VRP1 as an adjunct to chest physiotherapy in cystic fibrosis. Respiratory Medicine 1994;88(9):677-81. [CFGD REGISTER: PE25b] [DOI] [PubMed] [Google Scholar]
Pryor 2010 {published data only}
- NCT00890370. Should any one airway clearance technique BE recommended for people with cystic fibrosis? [A comparison of five airway clearance techniques in the treatment of adults with cystic fibrosis]. clinicaltrials.gov/show/NCT00890370 (first received 29 April 2009). [CENTRAL: CN-01500662] [CFGD REGISTER: PE164c]
- Pryor JA, Tannenbaum E, Cramer D, Scott SF, Burgess J, Gyi K, et al. A comparison of five airway clearance techniques in the treatment of people with Cystic Fibrosis. Journal of Cystic Fibrosis 2006;5(Suppl):S76. [CFGD REGISTER: PE164a] [DOI] [PubMed] [Google Scholar]
- Pryor JA, Tannenbaum E, Scott SF, Burgess J, Cramer D, Gyi K, et al. Beyond postural drainage and percussion: Airway clearance in people with cystic fibrosis. Journal of Cystic Fibrosis 2010;9(3):187-92. [CENTRAL: 759356] [CFGD REGISTER: PE164b] [PMID: ] [DOI] [PubMed] [Google Scholar]
Reisman 1988 {published data only}
- Reisman JJ, Rivington-Law B, Corey M, Marcotte J, Wannamaker E, Harcourt D, Levison H. Role of conventional physiotherapy in cystic fibrosis. Journal of Pediatrics 1988;113(4):632-6. [CFGD REGISTER: PE12] [DOI] [PubMed] [Google Scholar]
Steven 1992 {published data only}
- Steven MH, Pryor JA, Webber BA, Hodson MR. Physiotherapy versus cough alone in the treatment of cystic fibrosis. New Zealand Journal of Physiotherapy 1992;20(August):31-7. [CFGD REGISTER: PE65] [Google Scholar]
Webber 1985 {published data only}
- Webber B, Parker R, Hofmeyr J, Hodson M. Evaluation of self-percussion during postural drainage using the forced expiration technique. Physiotherapy Theory and Practice 1985;1(1):42-5. [CFGD REGISTER: PE33a] [Google Scholar]
- Webber B, Parker RA, Hofmeyr JL, Hodson ME. Evaluation of self percussion during postural drainage using the forced expiration technique (FET). In: 9th International Cystic Fibrosis Congress. 1984:2-12. [CFGD REGISTER: PE33b]
References to studies excluded from this review
ACTRN12605000471684 {published data only}
- ACTRN12605000471684. Chest physiotherapy use and gastro-oesophageal reflux symptoms in adults with cystic fibrosis. anzctr.org.au/ACTRN12605000471684.aspx (first received 23 September 2005).
ACTRN12614001233617 {published data only}
- ACTRN12614001233617. Does the bubble-positive expiratory pressure (PEP) device improve secretion clearance compared to the active cycle of breathing technique (ACBT) or no intervention (control) in people with non-cystic fibrosis bronchiectasis? anzctr.org.au/ACTRN12614001233617.aspx (first received 25 November 2014).
ACTRN12619000224123 {published data only}
- ACTRN12619000224123. Modified forced expiration technique using expiratory resistance in adults with cystic fibrosis: A pilot study. anzctr.org.au/ACTRN12619000224123.aspx (first received 15 February 2019). [CFGD REGISTER: PE333]
ACTRN12619001681145 {published data only}
- ACTRN12619001681145. Does the MetaNeb®, a new airway clearance device, change lung function in adults. anzctr.org.au/ACTRN12619001681145.aspx (first received 29 November 2019).
Andreasson 1987 {published data only}
- Andreasson B, Jonson B, Kornfalt R, Nordmark E, Sandstrom S. Long-term effects of physical exercise on working capacity and pulmonary function in cystic fibrosis. Acta Paediatrica Scandinavica 1987;76(1):70-5. [DOI] [PubMed] [Google Scholar]
Asher 1982 {published data only}
- Asher M, Pardy R, Coates A, Thomas E, Macklem PT. The effects of inspiratory muscle training in patients with cystic fibrosis. Australian and New Zealand Journal of Medicine 1983;13:204. [CFGD REGISTER: PE127a] [DOI] [PubMed] [Google Scholar]
- Asher MI, Pardy RL, Coates AL, Thomas E, Macklem PT. The effects of inspiratory muscle training in patients with cystic fibrosis. American Review of Respiratory Disease 1982;126(5):855-9. [CENTRAL: 997710] [CFGD REGISTER: PE127b] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bain 1988 {published data only}
- Bain J, Bishop J, Olinsky A. Evaluation of directed coughing in cystic fibrosis. British Journal of Diseases of the Chest 1988;82(8):134-48. [CFGD REGISTER: PE11] [DOI] [PubMed] [Google Scholar]
Baldwin 1994 {published data only}
- Baldwin DR, Hill AL, Peckham DG, Knox AJ. Effect of addition of exercise to chest physiotherapy on sputum expectoration and lung function in adults with cystic fibrosis. Respiratory Medicine 1994;88(1):49-53. [CFGD REGISTER: PE19] [DOI] [PubMed] [Google Scholar]
Braggion 1995 {published data only}
- Braggion C, Cappelletti LM, Cornacchia M, Zanolla L, Mastella G. Short-term effects of three chest physiotherapy regimens in patients hospitalized for pulmonary exacerbations of cystic fibrosis: a cross-over randomized study. Pediatric Pulmonology 1995;19(1):16-22. [CFGD REGISTER: PE26b] [DOI] [PubMed] [Google Scholar]
- Cappelletti LM, Cornacchia M, Braggion C, Zanolla L, Mastella G. Short-term effects of 3 physiotherapy (CPT) regimens in cystic fibrosis (CF) patients hospitalized for a pulmonary exacerbation: a cross-over randomized trial. In: 18th European Cystic Fibrosis Conference; 1993 May 21-26; Madrid, Spain. 1993:W9.3. [CFGD REGISTER: PE26a] [DOI] [PubMed]
Castle 1994 {published data only}
- Castle T, Metcalfe C, Knox A. A comparison between the active cycle of breathing technique (A.C.B.T.) and positive expiratory pressure (PEP) mask plus A.C.B.T. on sputum production and lung volumes in adults with cystic fibrosis. In: 19th European Cystic Fibrosis Conference; 1994 May 29-June 3; Paris, France. 1994:O17. [CFGD REGISTER: PE49]
Chatham 1998 {published data only}
- Chatham K, Nixon LS, Ionescu AA, Garwood R, Premier G, Shale DJ. Increased sputum expectoration in cystic fibrosis patients after repeated resisted mueller manoeuvres. Pediatric Pulmonology 1998;26(Suppl 17):348. [CFGD REGISTER: PE98a] [Google Scholar]
- Chatham K, Nixon LS, Ionescu AA, Shale DJ. Repeated inspiratory manoeuvres against a fixed resistance with biofeed back is more effective than standard chest physiotherapy in aiding sputum expectoration in cystic fibrosis. Pediatric Pulmonology 1999;Suppl 19:289. [CFGD REGISTER: PE98b] [Google Scholar]
ChiCTR1800019989 {published data only}
- ChiCTR1800019989. Study for rehabilitation effect of the active cycle of breathing technique combined with 5A model in patients undergoing lung cancer surgery. www.chictr.org.cn/showproj.aspx?proj=32782 (first registered 11 December 2018).
Davies 2012 {published data only}
- Banks A, Davies G, Agent P, Osman L, Bilton D, Hodson M. The use of high frequency chest wall oscillation during an acute infective pulmonary exacerbation of cystic fibrosis. European Respiratory Journal 2012;40(Suppl 56):Abstract no: 3483. [CFGD REGISTER: PE197b] [Google Scholar]
- Davies GA, Banks AE, Agent P, Osman LP, Bilton D, Hodson ME. The use of high frequency chest wall oscillation during an acute infective pulmonary exacerbation of cystic fibrosis. Pediatric Pulmonology 2012;47(S35):366, Abstract no: 396. [CFGD REGISTER: PE197a] [Google Scholar]
- NCT01057524. High frequency chest wall oscillation and cystic fibrosis [The use of high frequency chest wall oscillation during an acute infective pulmonary exacerbation of cystic fibrosis]. clinicaltrials.gov/show/NCT01057524 (first received 27 January 2010). [CENTRAL: CN-01527868] [CFGD REGISTER: PE197c]
Desmond 1983 {published data only}
- Desmond KJ, Schwenk WF, Thomas E, Beaudry PH, Coates AL. Immediate and long-term effects of chest physiotherapy in patients with cystic fibrosis. Journal of Pediatrics 1983;103(4):538-42. [CFGD REGISTER: PE77] [DOI] [PubMed] [Google Scholar]
Falk 1984 {published data only}
- Falk M, Kelstrup M, Andersen JB, Kinoshita T, Falk P, Stovring S, et al. Improving the ketchup bottle method with positive expiratory pressure, PEP, in cystic fibrosis. European Journal of Respiratory Diseases 1984;65(6):423-32. [CFGD REGISTER: PE7] [PubMed] [Google Scholar]
Falk 1993 {published data only}
- Falk M, Mortensen J, Kelstrup M, Lanng S, Larsen L, Ulrik CS. Short-term effects of positive expiratory pressure and the forced expiration technique on mucus clearance and lung function in CF. Pediatric Pulmonology 1993;16(Suppl 9):241. [CFGD REGISTER: PE48a] [Google Scholar]
- Larsen L, Mortensen J, Falk M, Kelstrup M, Lanng S, Ulrik CS. Radiolabelled mucus clearance in patients with cystic fibrosis is improved by physiotherapy with positive expiratory pressure and the forced expiration technique. Clinical Physiology 1994;14:365. [CFGD REGISTER: PE48b] [Google Scholar]
- Mortensen J, Falk M, Kelstrump M, Lanng S, Ulrik CS. Effect of positive expiratory pressure and the forced expiration technique on mucus clearance in patients with cystic fibrosis. European Respiratory Journal 1993;6(Suppl 17):4409S. [CFGD REGISTER: PE48c] [Google Scholar]
Gursli 2017 {published data only}
- Gursli S, Quittner A, Jahnsen RB, Skrede B, Stuge B, Bakkeheim E. Airway clearance physiotherapy and health-related quality of life in cystic fibrosis - a substudy of a series of n-of-1 randomised controlled trials. Journal of Cystic Fibrosis 2022;21(Suppl):S126. [CFGD REGISTER: PE206d] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gursli S, Sandvik L, Bakkeheim E, Skrede B, Stuge B. Airway clearance therapy and quality of life in cystic fibrosis: a pilot study of a series of n-of-1 randomised controlled trials. Pediatric Pulmonology 2017;52 Suppl 47:395-6. [CFGD REGISTER: PE206c] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gursli S, Sandvik L, Bakkeheim E, Skrede B, Stuge B. Evaluation of a novel technique in airway clearance therapy - Specific Cough Technique (SCT) in cystic fibrosis: a pilot study of a series of N-of-1 randomised controlled trials. SAGE open medicine 2017;5:no pagination. [CFGD REGISTER: PE206b] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gursli S, Sandvik L, Skrede B, Stuge B. Individual efficacy of cough technique versus forced expiration technique in cystic fibrosis. Journal of Cystic Fibrosis 2013;12 Suppl 1:S103. [ABSTRACT NO.: 214] [CENTRAL: 875195] [CFGD REGISTER: PE206a] [Google Scholar]
- NCT01266473. Study of individualized physiotherapy for airway clearance in cystic fibrosis [Efficacy study of physiotherapy for airway clearance in cystic fibrosis. Randomized controlled trials in single subjects (N of 1 RCT`s)]. www.clinicaltrials.gov/show/NCT01266473 (first registered 24 December 2010). [CFGD REGISTER: PE206e]
Hasani 1991 {published data only}
- Hasani A, Pavia D, Agnew JE, Clarke SW. The effect of unproductive coughing/FET on regional mucus movement in the human lungs. Respiratory Medicine 1991;85(Suppl A):23-6. [DOI] [PubMed] [Google Scholar]
Hasani 1994 {published data only}
- Hasani A, Pavia D, Agnew JE, Clarke SW. Regional lung clearance during cough and forced expiration technique (FET): effects of flow and viscoelasticity. Thorax 1994;49(6):557-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasani A, Pavia D, Agnew JE, Clarke SW. Regional mucus transport following unproductive cough and forced expiration technique in patients with airways obstruction. Chest 1994;105(5):1420-5. [DOI] [PubMed] [Google Scholar]
Horsley 2007 {published data only}
- Horsley A, Ridley S, Beattie C, Macleod K, Greening A, Innes JA. Changes in lung gas mixing after physiotherapy in adults with cystic fibrosis. European Respiratory Journal 2007;30(Suppl 51):223s. [CFGD REGISTER: PE252a] [Google Scholar]
- Macleod K, Dhouieb E, Riding K, Horsley A, Bell N, Alistair Innes J, et al. Acute changes in ventilation heterogeneity following routine physiotherapy in children with cystic fibrosis. In: European Respiratory Society Annual Congress; 2008 Oct 4-8; Berlin, Germany. 2008:P3903. [CFGD REGISTER: PE252b]
Klig 1989 {published data only}
- Klig S, Denning C, Jacoby J, Xia F, Gaerlan P, Bisberg D, et al. A biopsychosocial examination of two methods of pulmonary therapy. Pediatric Pulmonology 1989;7(Suppl 4):145. [ABSTRACT NO.: 128] [CFGD REGISTER: PE39] [Google Scholar]
Kofler 1998 {published data only}
- Kofler AM, Carlesi A, Cutrera R, Leone P, Lucidi V, Rosati S, et al. BiPAP versus PEP as chest physiotherapy in patients with cystic fibrosis. Pediatric Pulmonology 1998;26(Suppl 17):344. [CFGD REGISTER: PE95] [Google Scholar]
Lannefors 1992 {published data only}
- Lannefors L, Wollmer P. Mucus clearance in cystic fibrosis (CF) - a comparison between postural drainage, PEP-mask and physical exercise. In: 11th International Cystic Fibrosis Congress; 1992; Dublin, Ireland. 1992:AHP31. [CFGD REGISTER: PE46a] [PubMed]
- Lannefors L, Wollmer P. Mucus clearance with three chest physiotherapy regimes in cystic fibrosis: a comparison between postural drainage, PEP and physical exercise. European Respiratory Journal 1992;5(6):748-73. [CFGD REGISTER: PE46b] [PubMed] [Google Scholar]
McDonnell 1986 {published data only}
- McDonnell T, McNicholas WT, FitzGerald MX. Hypoxaemia during chest physiotherapy in patients with cystic fibrosis. Irish Journal of Medical Science 1986;155(10):345-8. [CFGD REGISTER: OV9] [DOI] [PubMed] [Google Scholar]
NCT00164138 {published data only}
- NCT00164138. Prevalence and treatment of urinary incontinence in women with cystic fibrosis and chronic obstructive pulmonary disease [Development of an intervention and education program for adult women with urinary incontinence and chronic lung disease including cystic fibrosis (CF) and chronic obstructive pulmonary disease (COPD)]. clinicaltrials.gov/show/NCT00164138 (first received 14 September 2005).
NCT00404859 {published data only}
- NCT00404859. FLAME: airway inflammation monitoring in asthma and cystic fibrosis [Monitoring chronic airway inflammation in children with asthma and cystic fibrosis]. clinicaltrials.gov/show/NCT00404859 (first received 29 November 2006).
NCT00716664 {published data only}
- NCT00716664. Do musculoskeletal techniques improve forced expiratory volume in one second in adults with cystic fibrosis? [Pilot study: Do musculoskeletal techniques improve forced expiratory volume in one second in adults with cystic fibrosis?]. clinicaltrials.gov/show/NCT00716664 (first received 16 July 2008). [CFGD REGISTER: PE311]
NCT01943890 {published data only}
- NCT01943890. High volumes of hypertonic saline and chest physiotherapy in CF patients [Establishment and implementation of nebulised high volumes of hypertonic saline in cystic fibrosis patients]. clinicaltrials.gov/show/NCT01943890 (first received 17 September 2013).
NCT02906826 {published data only}
- NCT02906826. Adherence to airway clearance. Novel approaches to improving adherence. clinicaltrials.gov/show/NCT02906826 (first received 20 September 2016).
NCT03078127 {published data only}
- NCT03078127. Researching the effects of airway clearance therapies in cystic fibrosis [Researching the effects of airway clearance therapies in cystic fibrosis (REACT-CF)]. clinicaltrials.gov/show/NCT03078127 (first received 13 March 2017).
O'Neill 2017 {published data only}
- NCT01753869. Timing of hypertonic saline inhalation relative to airways clearance in cystic fibrosis. clinicaltrials.gov/show/nct01753869 (first received 20 December 2012). [CFGD REGISTER: BD231c]
- O'Neill K, Moran F, Bradbury I, Downey DG, Rendall J, Tunney MM, et al. Exploring the timing of hypertonic saline (HTS) and airways clearance techniques (ACT) in cystic fibrosis (CF): a crossover study. Thorax 2016;71 Suppl 3:A134, Abstract no: P95. [CFGD REGISTER: BD231a] [Google Scholar]
- O'Neill K, Moran F, Tunney MM, Elborn JS, Bradbury I, Downey DG, et al. Timing of hypertonic saline and airway clearance techniques in adults with cystic fibrosis during pulmonary exacerbation: pilot data from a randomised crossover study. BMJ Open Respiratory Research 2017;4(1):e000168. [CFGD REGISTER: BD231b] [DOI] [PMC free article] [PubMed] [Google Scholar]
Oberwaldner 1986 {published data only}
- Oberwaldner B, Evans JC, Zach MS. Forced expirations against a variable resistance: a new chest physiotherapy method in cystic fibrosis. Pediatric Pulmonology 1986;2(6):358-67. [DOI] [PubMed] [Google Scholar]
Orlik 2000 {published data only}
- Orlik T. Evaluation of autodrainage methods in a selected group of cystic fibrosis patients with home environment factors taken into consideration. Medycyna Wieku Rozwojowego 2000;4(3):247-59. [CFGD REGISTER: PE141] [PubMed] [Google Scholar]
- Orlik T. Evaluation of the efficiency of selected thoracic physiotherapy methods used in the treatment of patients with cystic fibrosis. Medycyna Wieku Rozwojowego 2000;4(3):233-46. [PubMed] [Google Scholar]
Orlik 2001 {published data only}
- Orlik T, Sands D. Long-term evaluation of effectiveness for selected chest physiotherapy methods used in the treatment of cystic fibrosis. Medycyna Wieku Rozwojowego 2001;5(3):245-57. [PubMed] [Google Scholar]
- Orlik T, Sands D. Long-term study of efficiencies of select physiotherapy methods used in the treatment of cystic fibrosis. In: Proceedings of 24th European Cystic Fibrosis Conference; 2001 June 6-9; Vienna, Austria. 2001:P113.
Parker 1984 {published data only}
- Parker RA, Webber BA, Sutton PP, Newman SP, Garland N, Lopez-Vidriero MT, et al. Evaluation of three individual components of a postural drainage treatment. In: 9th International Cystic Fibrosis Congress; 1984 June 9-15; Brighton, England. 1984:2.13. [CFGD REGISTER: PE32]
Petrone 2009 {published data only}
- Petrone A, Quartieri M, Tirone F, Tirone C, Mauro GF. Management of patients with cystic fibrosis: Role of non invasive ventilation (NIV) and chest physiotherapy. In: European Respiratory Society Annual Congress; 2010 Sep 18-22; Barcelona, Spain. 2010:[E3907]. [CENTRAL: 777469] [CFGD REGISTER: PE220b]
- Petrone A, Quartieri M, Tolisano A, Tirone C, Tirone F. Non invasive ventilation (NIV) assists chest physiotherapy for treatment of patients with cystic fibrosis. In: European Respiratory Society Annual Congress; 2009 Sep 12-16; Vienna, Austria. 2009:[P1262]. [CENTRAL: 758852] [CFGD REGISTER: PE220a]
Prasad 1998a {published data only}
- Prasad SA, Main E. Finding evidence to support airway clearance techniques in cystic fibrosis. Disability and Rehabilitation 1998;20(6/7):235-46. [DOI] [PubMed] [Google Scholar]
Prasad 2000 {published data only}
- Prasad SA, Tannenbaum EL, Mikelsons C. Physiotherapy in cystic fibrosis. Journal of the Royal Society of Medicine 2000;93(Suppl 38):27-36. [PMC free article] [PubMed] [Google Scholar]
Pryor 1990 {published data only}
- Pryor JA, Webber BA, Hodson ME. Effect of chest physiotherapy on oxygen saturation in patients with cystic fibrosis. Thorax 1990;45(1):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
RBR‐5g9f6w {published data only}
- RBR-5g9f6w. Rehabilitation of children and adolescents with cystic fibrosis. ensaiosclinicos.gov.br/rg/RBR-5g9f6w (first received 17 June 2020).
Rogers 1984 {published data only}
- Rogers D, Tottle J, Pickering DM, Plews E, Davies V, Newcombe RG, et al. Comparison of physiotherapy techniques employed in cystic fibrosis. In: Lawson D, editors(s). Cystic Fibrosis Horizons. Chichester: John Wiley, 1984:238. [Google Scholar]
Rossman 1982 {published data only}
- Rossman C, Waldes R, Sampson D, Newhouse M. Does chest physiotherapy improve mucus removal in patients with cystic fibrosis? In: 8th International Cystic Fibrosis Congress; 1980; Toronto, Canada. 1980:32a. [CFGD REGISTER: PE6a]
- Rossman CM, Waldes R, Sampson D, Newhouse MT. Effect of chest physiotherapy on the removal of mucus in patients with cystic fibrosis. American Review of Respiratory Disease 1982;126(1):131-5. [CFGD REGISTER: PE6b] [DOI] [PubMed] [Google Scholar]
Salh 1989 {published data only}
- Salh W, Bilton D, Dodd M, Webb AK. Effect of exercise and physiotherapy in aiding sputum expectoration in adults with cystic fibrosis. Thorax 1989;44(12):1006-8. [CFGD REGISTER: PE63] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sontag 2010 {published data only}
- Accurso FJ, Sontag MK, Koenig JM, Quittner AL. Multi-center airway secretion clearance study in cystic fibrosis. Pediatric Pulmonology 2004;38(Suppl 27):314, Abstract no. 363. [CFGD REGISTER: PE152a] [Google Scholar]
- Modi AC, Cassedy AE, Quittner AL, Accurso F, Sontag M, Koenig JM, et al. Trajectories of adherence to airway clearance therapy for patients with cystic fibrosis. Journal of Pediatric Psychology 2010;35(9):1028-37. [CFGD REGISTER: PE152e] [DOI] [PubMed] [Google Scholar]
- Modi AC, Sontag MK, Koenig JM, Accurso FJ, Quittner AL, Investigators and Coordinators of the Airway Secretion Clearance Study. Adherence to airway clearance therapies in patients with cystic fibrosis. Journal of Cystic Fibrosis 2006;5 Suppl 1:S97, Abstract no. 436. [CFGD REGISTER: PE152c] [Google Scholar]
- NCT00839644. Airway secretion clearance in cystic fibrosis. clinicaltrials.gov/show/NCT00839644 (first received 9 February 2009). [CFGD REGISTER: PE152g]
- Quittner AL, Modi AC, Accurso FJ, Koenig JM, Sontag MK, Oermann C, et al. Treatment satisfaction, health-related quality of life and airway clearance therapies in patients with cystic fibrosis. Pediatric Pulmonology 2004;38(Suppl 27):314, Abstract no. 364. [CFGD REGISTER: PE152b] [Google Scholar]
- Sontag MK, Quittner AL, Modi AC, Koenig JM, Giles D, Oermann CM, et al. Lessons learned from a randomized trial of airway secretion clearance techniques in cystic fibrosis. Pediatric Pulmonology 2010;45(3):291-300. [CFGD REGISTER: PE152d] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontag MK, Quittner AL, Modi AC, Koenig JM, Giles D, Oermann CM, et al. Lessons learned from a randomized trial of airway secretion clearance techniques in cystic fibrosis. Pediatric Pulmonology 2010;45(3):291-300. Online supplement. [CFGD REGISTER: PE152f] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stanford 2020 {published data only}
- ISRCTN11220163. Investigating outcome measures for trials of airway clearance techniques in adults with cystic fibrosis. isrctn.com/ISRCTN11220163 (first received 19 September 2016). [CFGD REGISTER: PE281d]
- ISRCTN11220163. Investigating outcome measures for trials of airway clearance techniques in adults with cystic fibrosis. isrctn.com/ISRCTN11220163 (first received 19 September 2016).
- NCT02721498. Improving outcome measures for adult CF ACT Trials. clinicaltrials.gov/show/NCT02721498 (first received 29 March 2016). [CFGD REGISTER: PE281b]
- Stanford G, Cathcart F, Beverley Z, Short C, Jones M, Bilton D, et al. Investigating outcome measures for physiotherapy trials of airway clearance in adult patients with cystic fibrosis. Journal of Cystic Fibrosis 2019;18 Suppl 1:S3. [CFGD REGISTER: PE281a] [Google Scholar]
- Stanford G, Davies JC, Usmani O, Banya W, Charman S, Jones M, et al. Investigating outcome measures for assessing airway clearance techniques in adults with cystic fibrosis: protocol of a single-centre randomised controlled crossover trial. BMJ Open Respiratory Research 2020;7(1):e000694. [CFGD REGISTER: PE281c] [DOI: 10.1136/bmjresp-2020-000694] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford GE, Cathcart F, Beverley Z, Short C, Jones M, Bilton D, et al. Outcome measures for airway clearance in adults with cystic fibrosis (CF): a randomised controlled crossover trial. Thorax 2019;74:A222. [CFGD REGISTER: PE281e] [Google Scholar]
Steen 1991 {published data only}
- Steen HJ, Redmond AOB, O'Neil D, Beattie F. Evaluation of the PEP mask in cystic fibrosis. Acta Paediatrica Scandinavica 1991;80(1):51-6. [CFGD REGISTER: PE14b] [DOI] [PubMed] [Google Scholar]
- Steen HJ, Redmond AOB, O' Neill D, Beattie F. Has the PEP mask a role in the management of teenage patients? In: Proceedings of 13th Annual Meeting of the European Working Group for Cystic Fibrosis; 1985 Nov 3-8; Jerusalem, Israel. 1985:94. [CFGD REGISTER: PE14a]
Sutton 1983 {published data only}
- Sutton PP, Parker RA, Webber BA, Newman SP, Garland N, Lopez-Vidriero MT, et al. Assessment of the forced expiration technique, postural drainage and directed coughing in chest physiotherapy. European Journal of Respiratory Diseases 1983;64(1):62-8. [PubMed] [Google Scholar]
Sutton 1985 {published data only}
- Sutton PP, Lopez-Vidriero MT, Pavia D, Newman SP, Clay MM, Webber B, et al. Assessment of percussion, vibratory-shaking and breathing exercises in chest physiotherapy. European Journal of Respiratory Diseases 1985;66(2):147-52. [CFGD REGISTER: PE81] [PubMed] [Google Scholar]
Thomas 1995 {published data only}
- Thomas J, Cook DJ, Brooks D. Chest physical therapy management of patients with cystic fibrosis. A meta-analysis. American Journal of Respiratory and Critical Care Medicine 1995;151(3 (Pt 1)):846-50. [DOI] [PubMed] [Google Scholar]
van Hengstum 1987 {published data only}
- Hengstum M, Festen J, Beurskens C, Hankel M, den Broek W, Buijs W, et al. The effect of positive expiratory pressure (PEP) versus forced expiration technique (FET) on tracheobronchial clearance in chronic bronchitis. In: 15th Annual Meeting of the European Working Group for Cystic Fibrosis; 1987; Oslo, Norway. 1987. [CFGD REGISTER: PE35]
van Hengstum 1988 {published data only}
- Van Hengstum M, Festen J, Beurskens C, Hankel M, Beekman F, Corstens F. Conventional physiotherapy and forced expiration manoeuvres have similar effects on tracheobronchial clearance. European Respiratory Journal 1988;1(8):758-61. [CFGD REGISTER: PE64] [PubMed] [Google Scholar]
Verboon 1986 {published data only}
- Verboon JM, Bakker W, Sterk PJ. The value of the forced expiration technique with and without postural drainage in adults with cystic fibrosis. European Journal of Respiratory Diseases 1986;69(3):169-74. [CFGD REGISTER: PE8b] [PubMed] [Google Scholar]
- Verboon JML, Bakker W, Dijkman JH. Effect of the forced expiration technique and postural drainage in adults with cystic fibrosis. In: 9th International Cystic Fibrosis Congress. June 9-15; Brighton, England. 1984:2.17. [CFGD REGISTER: PE8a]
- Verboon JML, Bakker W, Sterk PJ. The value of the "forced expiration technique" (fet) [De waarde van de 'forced expiration technique' (FET)]. Nederlandische Tijdschrift Fysiotherapie 1987;97:62-4. [CFGD REGISTER: PE8c] [Google Scholar]
Ward 2018 {published data only}
- ACTRN12615001361594. Airway clearance by exercising in mild cystic fibrosis (ACE-CF): a feasibility study. anzctr.org.au/ACTRN12615001361594.aspx (first reeived 15 December 2015).
- Ward N, Stiller K, Rowe H, Morrow S, Morton J, Greville H, et al. Airway clearance by exercising in mild cystic fibrosis: clinical outcomes. Respirology 2018;23(Suppl 1):140. [CFGD REGISTER: PE257a] [DOI] [PubMed] [Google Scholar]
- Ward N, Stiller K, Rowe H, Morrow S, Morton J, Greville H, et al. Airway clearance by exercising in mild cystic fibrosis (ACE-CF): a feasibility study. Respiratory Medicine 2018;142:23-8. [CFGD REGISTER: PE257b] [DOI] [PubMed] [Google Scholar]
Webber 1986 {published data only}
- Webber BA, Hofmeyr JL, Morgan MD, Hodson ME. Effects of postural drainage, incorporating the forced expiration technique, on pulmonary function in cystic fibrosis. British Journal of Diseases of the Chest 1986;80(4):353-9. [DOI] [PubMed] [Google Scholar]
White 1997 {published data only}
- Stiller K. Are thoracic expansion exercises necessary during the active cycle of breathing techniques for adult cystic fibrosis patients? Israel Journal of Medical Sciences 1996;32(Suppl):S275. [CFGD REGISTER: PE61a] [Google Scholar]
- White D, Stiller K, Willson K. The role of thoracic expansion exercises during the active cycle of breathing techniques. Physiotherapy Theory Practice 1997;13(2):155-62. [CFGD REGISTER: PE61b] [Google Scholar]
Williams 1994 {published data only}
- Williams MT. Chest physiotherapy and cystic fibrosis. Why is the most effective form of treatment still unclear? Chest 1994;106(6):1872-82. [DOI] [PubMed] [Google Scholar]
Williams 2000 {published data only}
- Parsons DW, Williams MT, Frick RA, Ellis ER, Martin AJ, Giles SE, et al. Chest physiotherapy: Improvements in lung function and ventilation are associated with physiotherapy-assisted treatment. Pediatric Pulmonology 1995;Suppl 12:271. [CFGD REGISTER: PE57a] [Google Scholar]
- Williams M, Parsons D, Martin A, Ellis E, Giles S, Staugas REM, et al. Chest physiotherapy (CPT) and cystic fibrosis: physiotherapist-assisted treatment is more energy efficient. Australian and New Zealand Journal of Medicine 1995;25:441. [CFGD REGISTER: PE57c] [Google Scholar]
- Williams M, Parsons D, Martin A, Giles S, Staugas REM. Energy expenditure during chest physiotherapy in normal and cystic fibrosis (CF) subjects. Australian and New Zealand Journal of Medicine 1994;25:445. [CFGD REGISTER: PE57b] [Google Scholar]
- Williams MT, Parsons DW, Frick RA, Ellis ER, Martin AJ, Giles SE, et al. Acute respiratory infection in patients with cystic fibrosis with mild pulmonary impairment: comparison of two physiotherapy regimens. Australian Journal of Physiotherapy 2001;47(4):227-36. [CFGD REGISTER: PE57d] [DOI] [PubMed] [Google Scholar]
- Williams MT, Parsons DW, Frick RA, Ellis ER, Martin AJ, Giles SE, et al. Energy expenditure during physiotherapist-assisted and self-treatment in cystic fibrosis. Physiotherapy Theory & Practice 2000;16(2):57-67. [CFGD REGISTER: PE57e] [Google Scholar]
Wilson 1995 {published data only}
- Wilson GE, Baldwin AL, Walshaw MJ. Chest physiotherapy in patients with cystic fibrosis (CF) - a comparison of traditional methods with the active cycle of breathing. In: 20th European Cystic Fibrosis Conference; 1995 June 18-21; Brussels, Belgium. 1995:P58. [CFGD REGISTER: PE52]
Znotina 2000 {published data only}
- Znotina I, Svabe V. The effectiveness of physiotherapy for children with cystic fibrosis. In: XIIIth International Cystic Fibrosis Congress, 4-8 June 2000, Stockholm, Sweden. 2000:152. [CFGD REGISTER: PE113]
Additional references
Burnham 2021
- Burnham P, Stanford G, Stewart R. Autogenic drainage for airway clearance in cystic fibrosis. Cochrane Database of Systematic Reviews 2021, Issue 12. Art. No: CD009595. [DOI: 10.1002/14651858.CD009595.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cystic Fibrosis Canada 2020
- Cystic Fibrosis Canada. The Canadian Cystic Fibrosis Registry 2019 Annual Data Report. Available at www.cysticfibrosis.ca/registry/2019AnnualDataReport.pdf 2020.
Cystic Fibrosis Foundation 2023
- Cystic Fibrosis Foundation. Airway Clearance Techniques. www.cff.org/managing-cf/airway-clearance (accessed 24 January 2023).
Cystic Fibrosis Foundation Patient Registry 2020
- Cystic Fibrosis Foundation Patient Registry. 2019 Annual Data Report. Available at www.cff.org/sites/default/files/2021-10/2019-Patient-Registry-Annual-Data-Report.pdf 2020.
Cystic Fibrosis Trust 2020a
- Cystic Fibrosis Trust. Cystic Fibrosis Strength in Numbers: UK Cystic Fibrosis Registry 2019 Annual Data Report; 2020. Available at www.cysticfibrosis.org.uk/sites/default/files/2020-12/2019%20Registry%20Annual%20Data%20report_Sep%202020.pdf.
Cystic Fibrosis Trust 2020b
- Association of Chartered Physiotherapist in Cystic Fibrosis (ACPCF). Clinical Guidelines For the Physiotherapy Management of Cystic Fibrosis. www.cysticfibrosis.org.uk/sites/default/files/2020-12/Standards of Care and Good Clinical Practice for the Physiotherapy Management of Cystic Fibrosis Fourth edition December 2020.pdf 2020.
Deeks 2011
- Deeks J, Higgins J, Altman D. Chapter 9 Analysing data and undertaking meta-analysis. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140-9. [DOI] [PubMed] [Google Scholar]
Giron 2021
- Giron Moreno RM, Garcia-Clemente M, Diab-Caceres L, Martinez-Vergara A, Martinez-Garcia MA, Gomez-Punter RM. Treatment of pulmonary disease of cystic fibrosis: a comprehensive review. Antibiotics 2021;10(5):486. [DOI: 10.3390/antibiotics10050486] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gondor 1999
- Gondor M, Nixon PA, Mutich R, Rebovich P, Orenstein DM. Comparison of flutter device and chest physical therapy in the treatment of cystic fibrosis pulmonary exacerbation. Pediatric Pulmonology 1999;28(4):255-60. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
International Physiotherapy Group for CF 2009
- International Physiotherapy Group for CF. Physiotherapy for people with Cystic Fibrosis: from infant to adult. www.cfww.org/docs/ipg-cf/bluebook/bluebooklet2009websiteversion.pdf (accessed 10 August 2010).
Lorin 1971
- Lorin MI, Denning CR. Evaluation of postural drainage measurement of sputum volume and consistency. American Journal of Physical Medicine 1971;50(5):215-9. [PubMed] [Google Scholar]
Main 2005
- Main E, Prasad A, Schans CP. Conventional chest physiotherapy compared to other airway clearance techniques for cystic fibrosis. Cochrane Database of Systematic Reviews 2005, Issue 1. Art. No: CD002011. [DOI: 10.1002/14651858.CD002011.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
McIlwaine 1997
- McIlwaine PM, Wong L, Peacock D, Davidson AG. Long-term comparative trial of conventional postural drainage and percussion versus positive expiratory pressure physiotherapy in the treatment of cystic fibrosis. Journal of Pediatrics 1997;131(4):570-4. [DOI] [PubMed] [Google Scholar]
McIlwaine 2007
- McIlwaine M. Chest physical therapy, breathing techniques and exercise in children with CF. Paediatric Respiratory Reviews 2007;8(1):8-16. [DOI] [PubMed] [Google Scholar]
McIlwaine 2019
- McIlwaine M, Button B, Nevitt SJ. Positive expiratory pressure physiotherapy for airway clearance in people with cystic fibrosis. Cochrane Database of Systematic Reviews 2019, Issue 11. Art. No: CD003147. [DOI: 10.1002/14651858.CD003147.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morrison 2020
- Morrison L, Milroy S. Oscillating devices for airway clearance in people with cystic fibrosis. Cochrane Database of Systematic Reviews 2020, Issue 4. Art. No: CD006842. [DOI: 10.1002/14651858.CD006842.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Prasad 1998b
- Prasad SA, Main E. Finding evidence to support airway clearance techniques in cystic fibrosis. Disability and Rehibilitation 1998;20(6/7):235-46. [DOI] [PubMed] [Google Scholar]
ProCite 1999 [Computer program]
- ProCite. Version 5. Stamford (CT): Thomson Reuters, 1999. Available at www.adeptscience.co.uk/sysreq/procite.
Pryor 1990b
- Pryor JA, Webber BA, Hodson ME. Effect of chest physiotherapy on oxygen saturation in patients with cystic fibrosis. Thorax 1990;45(1):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pryor 1999
- Pryor JA. Physiotherapy for airway clearance in adults. European Respiratory Journal 1999;14:1418-24. [DOI] [PubMed] [Google Scholar]
RevMan 2020 [Computer program]
- Review Manager (RevMan). Version 5.4. The Cochrane Collaboration, 2020.
Rowe 2005
- Rowe SM, Miller S, Sorscher EJ. Mechanisms of Disease: Cystic Fibrosis. New England Journal of Medicine 2005;352(19):1992-2001. [DOI] [PubMed] [Google Scholar]
Schünemann 2006
- Schünemann HJ, Fretheim A, Oxman AD. Improving the use of research evidence in guideline development: 9. Grading evidence and recommendations. Health Research and Policy Systems 2006;4(21). [DOI: 10.1186/1478-4505-4-21] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ , Oxman AD , Higgins JP, Vist GE, Glasziou P, Guyatt GH on behalf of the Cochrane Applicability and Recommendations Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Presenting results and ‘Summary of findings’ tables. n: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Scotet 2020
- Scotet V, L'Hostis C, Ferec C. The changing epidemiology of cystic fibrosis: incidence, survival and impact of the CFTR gene discovery. Genes 2020;11(6):589. [DOI: 10.3390/genes11060589] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2011
- Sterne J, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011].The CochraneCollaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Warnock 2015
- Warnock L, Gates A. Chest physiotherapy compared to no chest physiotherapy for cystic fibrosis. Cochrane Database of Systematic Reviews 2015, Issue 12. Art. No: CD001401. [DOI: 10.1002/14651858.CD001401.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Webber 1998
- Webber BA, Pryor JA, Bethune DD. Physiotherapy techniques (Chapter 8). In: Pryor JA, Webber BA, editors(s). Physiotherapy for Respiratory and Cardiac Problems. 2nd edition. Edinburgh: Churchill Livingstone, 1998:143-208. [Google Scholar]
References to other published versions of this review
McKoy 2012
- Mckoy NA, Saldanha IJ, Odelola OA, Robinson KA. Active cycle of breathing technique for cystic fibrosis. Cochrane Database of Systematic Reviews 2012, Issue 12. Art. No: CD007862. [DOI: 10.1002/14651858.CD007862.pub3] [DOI] [PubMed] [Google Scholar]
McKoy 2016
- Mckoy NA, Saldanha IJ, Odelola OA, Robinson KA. Active cycle of breathing technique for cystic fibrosis. Cochrane Database of Systematic Reviews 2016, Issue 7. Art. No: CD007862. [DOI: 10.1002/14651858.CD007862.pub4] [DOI] [Google Scholar]
Robinson 2010
- Robinson KA, Mckoy NA, Saldanha IJ, Odelola OA. Active cycle of breathing technique for cystic fibrosis. Cochrane Database of Systematic Reviews 2010, Issue 11. Art. No: CD007862. [DOI: 10.1002/14651858.CD007862.pub2] [DOI] [PubMed] [Google Scholar]


