Abstract

Click chemistry is currently one of the most used tools for the generation of complex organic molecules. The advantages of using click chemistry in organic synthesis are remarkable; in many cases, the reactions occur under mild conditions and are free of solvents, with high yields and short reaction times. This makes it an extraordinarily effective and viable alternative for obtaining complex/conjugated molecules. In this review, the use of click chemistry CuAAC is especially emphasized for polyhydroxylated platforms such as resorcinarenes or calixarenes, focusing mainly on aspects of synthesis, specifically conditions, reagents, and methodologies.
1. Introduction
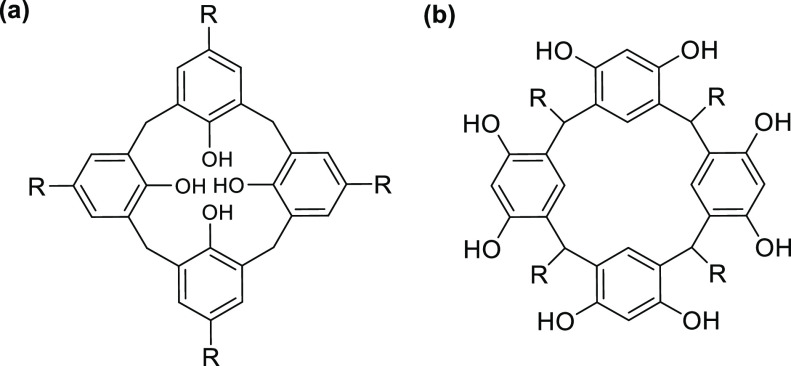
Polyhydroxylated platforms such as calixarenes and resorcinarenes (Figure 1) have become new sources of molecules that are currently being studied for the generation of polyvalent molecules, given their great versatility for synthesis and the reactivity provided by the hydroxyl groups and other reactive positions present in these structures. These polyhydroxylated platforms exhibit great host–host inclusion affinity with a great variety of molecules of organic and inorganic origin. Some examples are in the report by Da Silva et al. in 2003, where they studied their noncovalent inclusion with steroids such as testosterone1 and their interaction with antibacterial drugs such as those reported by Dawn et al. in 2017.2 They also have a high affinity for different metal ions such as Cu2+, Fe2+, Co2+, Ni2+, and Zn2+,3−6 and the interaction of these ligands with proteins have allowed the recognition, detection, modification/modulation, and separation of proteins, as reported by Oshima et al. in 2012.7 Additionally, polyhydroxylated platforms have been found to have several pharmaceutical applications due to their potential for encapsulating drugs, increasing their solubility, bioavailability, oral absorption, and stability under heat, light, and acidic conditions.8
Figure 1.
Representationz of (a) calix[4]arene and (b) calix[4]resorcinarene.
In this way, the exploration of the reactivity of the hydroxyl groups and the versatility in the synthesis of the polyhydroxylated platforms have allowed new synthesis techniques such as click chemistry to be incorporated into the synthesis of derivatives of calixarenes or resorcinarenes (Figure 2). This has allowed new derivatives to emerge, such as carbohydrate binding, reported in 2006 by Dondoni et al.,9 the incorporation of short peptides, the generation of photoactive complexes,10 the affinity and chemical binding to gold surfaces in the generation of biosensors reported by Feng et al.,11 and the inclusion of photoreactive chromophores such as those synthesized by Liu et al.,12 among others. The use of these polyhydroxylated bases also allows obtaining dendrimers with improved pharmaceutical applications, such as for selective delivery in specific drug regions, as reported by Li et al. in 2016, where the affinity of this type of platform to Fe2+ ions was found, which generated a targeting vector and a selective delivery of Dauricin in the treatment of primary intercerebral hemorrhage,5 or the improvement of the solubility of some anti-inflammatory drugs such as ibuprofen and naproxen in an aqueous medium, as reported by Khan et al. in 2017.13
Figure 2.

Use and applications of polyhydroxylated platforms of the calixarene/resorcinarene type.
In this review, the actual and potential applications of click chemistry in the modification of polyhydroxylated platforms via copper-catalyzed alkyne–azide cycloaddition will be discussed, including chemical conditions for the reactions and reagents, among other aspects in the processes described in the specialized literature.
2. Polyhydroxylated Platforms (Calixarenes/Resorcinarenes)
The calix[n]arenes are macrocycles or cyclic oligomers mainly based on the condensation product between para-substituted phenols and formaldehyde. One of the characteristics of this type of compound is the great variety of conformations that can be formed. Specifically, the term calixarene is derived from the term calix, or cup, since geometrically these types of compounds are expressed in this way. Given this characteristic of spatial geometry that they may have, it has been directly correlated with the uptake of ions in the small cavities formed between the different units; these cavities depend on the size of the different units. This is why they have been reported with units of 4, 6, and 8, among the most common ones.8,14
On the other hand, resorcinarenes, also known as calix[4]resorcinarenes, are polyhydroxylated macrocyclic compounds derived from resorcinol, which were synthesized and reported for the first time by Baeyer et al. in the year 1872 from aliphatic or aromatic aldehydes.15,16 They are made up of four resorcinol rings linked together at positions 4 and 6 by a methine bond. These are often functionalized, allowing for a wide spectrum of conformational isomers (stereoisomerism).17−19 Five possible conformers have been reported for resorcinarene: (i) crown, (ii) boat, (iii) saddle, (iv) chair, and (v) diamond.20 Any of these conformations are possible depending on aspects such as the position of the resorcinol units and the substituents on the methine bridges. For most of the cases reported in the synthesis of resorcinarene derivatives, the most stable conformation has been shown to be the crown type, established from the deep cavities formed and a stabilization by hydrogen bonds mediated by the hydroxyl groups present.8,21−23
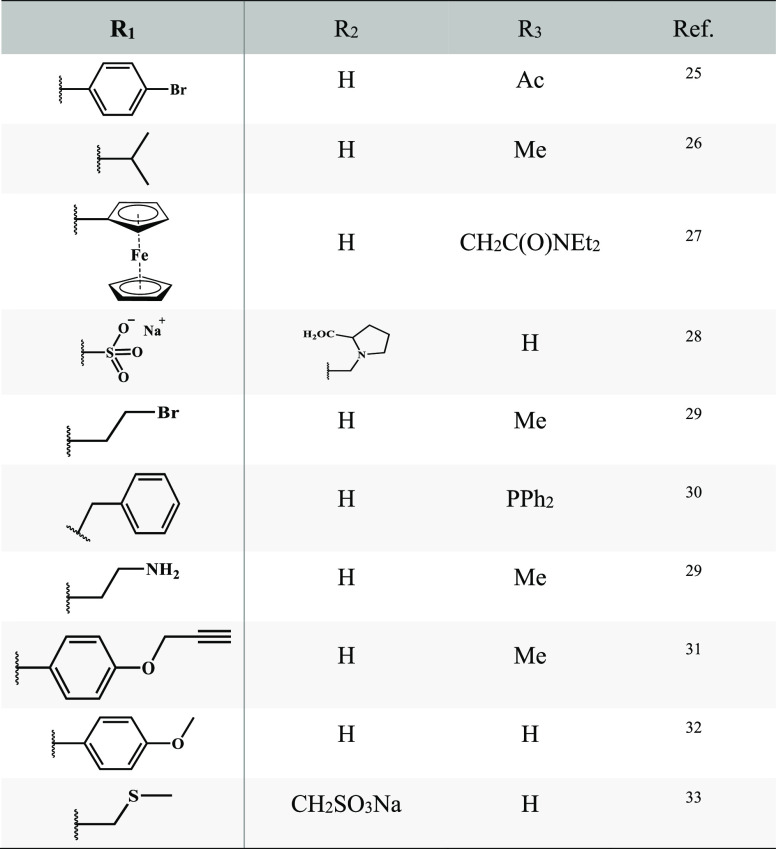
The functionalization at the macrocycle methylene bridge arises from the aldehyde used in the preparation of resorcinarene derivatives, since the group attached to the aldehyde forms the lower edge. A wide variety of aldehydes have been used in the preparation of resorcinarenes, including saturated alkyl aldehydes, unsaturated alkyl aldehydes, and sulfur-containing aldehydes (Table 1). These functionalized aldehydes lead to the construction of resorcinarenes that have various bottom edge functionalities.24
Table 1. Calix[4]resorcinarene Functionalization at R1, R2, and R3 Positions.
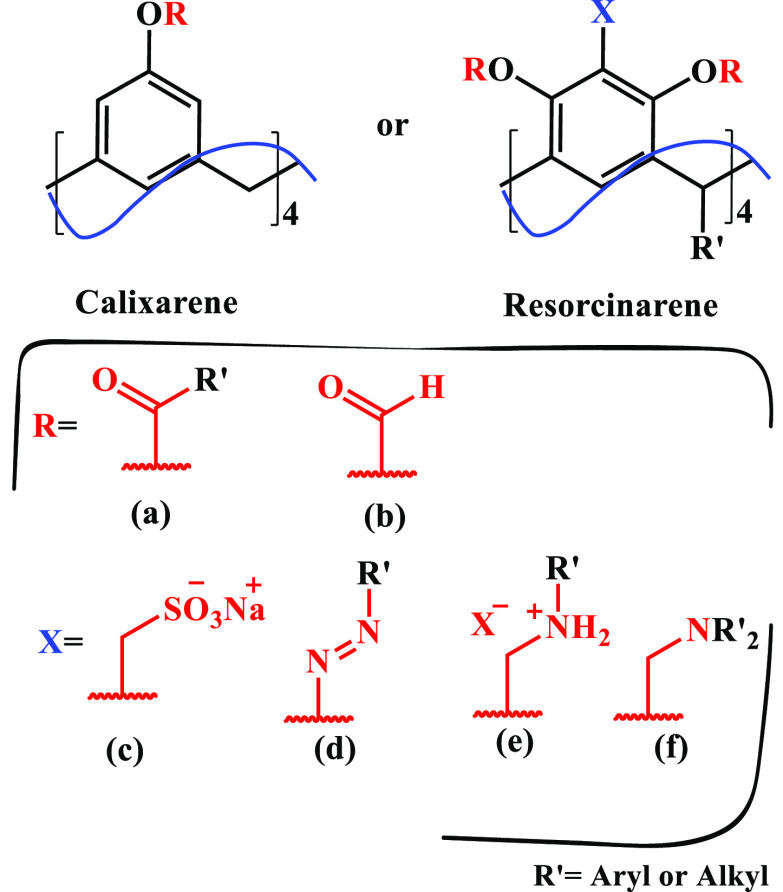
Calixarenes can be functionalized in the aromatic ring from the starting materials by varying the nature of the substituent group on the phenol or in the hydroxyl group,34−36 while the reactivity of the resorcin[4]arenes is mainly located at three points: on the hydroxyl groups, at position 2 of the hydroxyl group, and by modification through the introduction of functionalized aldehyde.23,37−39 Functionalizing substances such as diazonium salts,40−43 methyl sulfonates,44,45 ammonium groups,46,47 acylation,22,48 formylation,49,50 and aminomethylation,51 among others,52,53 can be introduced into the hydroxylated platform by means of easily accessible reactions selectively, with good yields (Figure 3).
Figure 3.
Modification of calixarenes or resorcinarenes with (a) diazonium salts, (b) methyl sulfonates, (c), ammonium groups, (d), acylation, (e) formylation, and (f) aminomethylation among others.
These modifications allow interaction with other types of more specific reactions, such as click chemistry. The application of these types of compounds is limited, due to their hydrophobic properties, making them poorly soluble in aqueous media; however, by introducing substitutions in their methylene bridges, a great variety of functional groups can be obtained, with which the original molecule can partially modify its hydrophobicity, allowing its applications to be expanded.54
3. Click Chemistry
Click chemistry is an efficient and modern technique applied to the synthesis of complex molecules. The 2022 Nobel Prize in Chemistry recognized its importance and its applications; it sought to make easier some difficult processes. Barry Sharpless and Morten Meldal have laid the groundwork for a functional form of chemistry, click chemistry, in which molecular building blocks come together quickly and efficiently. Carolyn Bertozzi has taken click chemistry to a new dimension and has begun to use it in living organisms.55 The concept of click chemistry was incorporated by Sharpless, where the assembly of small building blocks to form more complex structures is simplified.56,57 Many of the criteria in click chemistry are subjective, and although measurable and objective criteria could be agreed upon, it is unlikely that any one reaction will be perfect for every situation and application. Characteristics have been mentioned for a reaction to be considered within the click concept, for example: (i) stereospecificity, (ii) ease of product purification, (iii) use of low-cost reagents and catalysts, (iv) high efficiency, and (v) mild reaction conditions (it is possible to carry out this type of reaction in polar and environmentally friendly solvents such as water and methanol).58−60
3.1. Copper-Catalyzed Azide–Alkyne Cycloaddition (CuAAC) Reaction
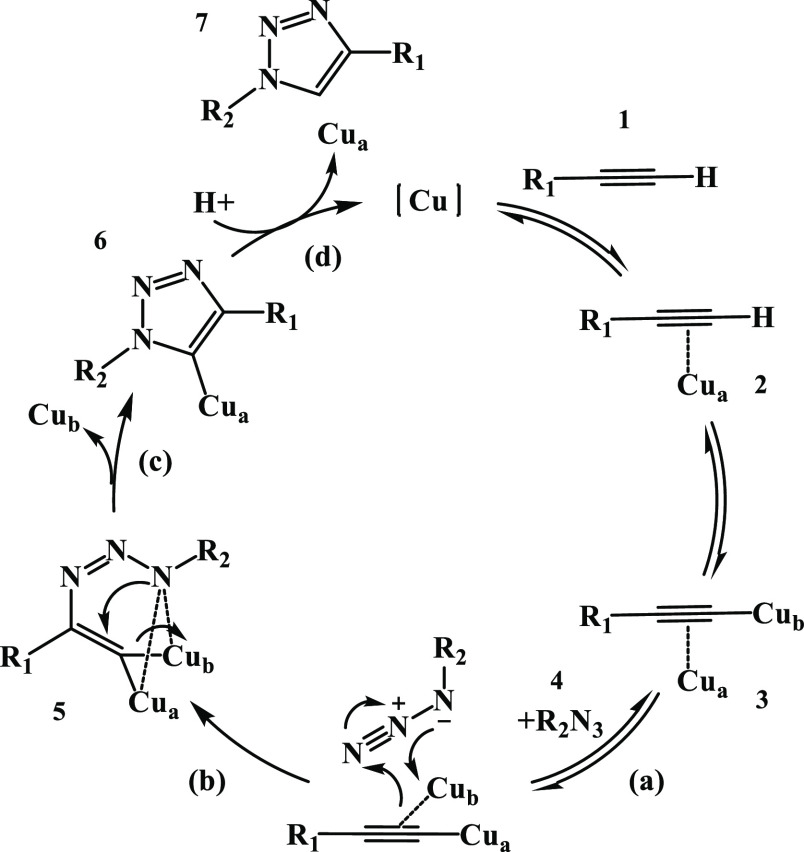
The copper-catalyzed azide–alkyne cycloaddition reaction (CuAAC) was introduced by Meldal et al. in 2001;61 it is based on a 1,3-dipolar Huisgen cycloaddition, which is a nonconcerted reaction where copper(I) acetylides react with azides and nitrile oxides, resulting in 1,4-disubstituted 2,3-triazoles and 3,4-disubstituted isoxazoles.62 Later on, this reaction began to form part of a special group of organic chemistry known as click chemistry reactions.63 The CuAAC reaction mechanism is explained in Scheme 1. First, the copper acetylide complex 2 is formed between the alkyne 1 and the copper(I) species. Then, 2 coordinates a second Cu, generating complex 3. An important feature of this mechanism is that the azide 4 cycloaddition to the resulting dicopper species is a stepwise process: in the first place, (a) azide 4 coordination to the dicopper 3 core yielding the first C–N bond, and this leads to a subsequent formation of the six-membered methacycle 5, followed by (b) intramolecular C–N bond formation yielding the triazolyl-Cu(I) intermediate 6 and dissociating one of the two coppers. This intermediate, which is not very stable, undergoes a final step, a proton transfer from the alkyne to the triazolyl ligand that allows the regeneration of the alkynyl-Cu(I) complex and the release of the 1,4-triazole product 7.57
Scheme 1. CuAAC Mechanism for Triazole Ring Formation57.
The importance of the copper(I) species used as a catalyst has been reported. Variations in reaction conditions have been reported, such as (i) temperature (rt or MW),31 (ii) solvents (DMF, THF, and H2O),64,65 (iii) generation of copper(I) in situ through the use of reducing agents from copper(II),64,66−69 and (iv) use of solvent-free copper nanoparticles (CuNPs).10
A wide variety of copper-derived compounds have been evaluated as effective catalysts in the reaction of CuAAC copper(I) species, such as copper(I) iodide (CuI)66,70 and copper(I) bromide (CuBr),12,71 copper(II), such as copper(II) sulfate pentahydrate (CuSO4·5H2O),72 or the same copper(0) using copper nanoparticles (CuNPs).10 In 2020, for example, Pineda-Castañeda et al. used CuSO4·5H2O together with a reducing agent (ascorbic acid) in EtOH/H2O at 80 °C to produce a chimeric peptide linked by a 1,2,3-triazole ring.73 This same reaction has been used in the synthesis of calixarene derivatives by Tang et al. by controlling the molar ratios of the alkyne and azide precursors. They were able to design and synthesize novel calix[4]resorcinarene-triphenylene with monomeric, dimeric, and tetrameric designs with yields of 50–60% using copper(I) as a catalyst in situ using CuSO4·5H2O and sodium ascorbate reducing agent in DMF. On the other hand, in 2013 Gao et al. used CuBr at reflux in DMF for the generation of Miktoarm A8B4 amphiphilic star copolymers centered on resorcinarene, based on poly(ε-caprolactone) and poly(ethylene glycol) by means of a controlled ring-opening polymerization (CROP) combination.71
The CuAAC reactions have been implemented for obtaining a wide variety of conjugates of calixarenes or resorcinarenes, in which organic and inorganic motifs have been incorporated into them, for example, radiomarkers,74 polymers,71 carbohydrates,70,66,75 gold supports,11 cyclodextrins,76 and peptides,10 among others. All these derivatives will be described later in detail.
3.1.1. Calixarene/Resorcinarene Functionalized via CuAAC
In 2011, derivatives of calixarenes conjugated to carbohydrates through CuAAC were reported by Galante et al. They reported the synthesis of a water-soluble calix[4]arene conjugated glycocluster containing lactose (Scheme 2).77
Scheme 2. Synthesis of Water-Soluble Calix[4]arene Conjugated Glycocluster Containing Lactose77.
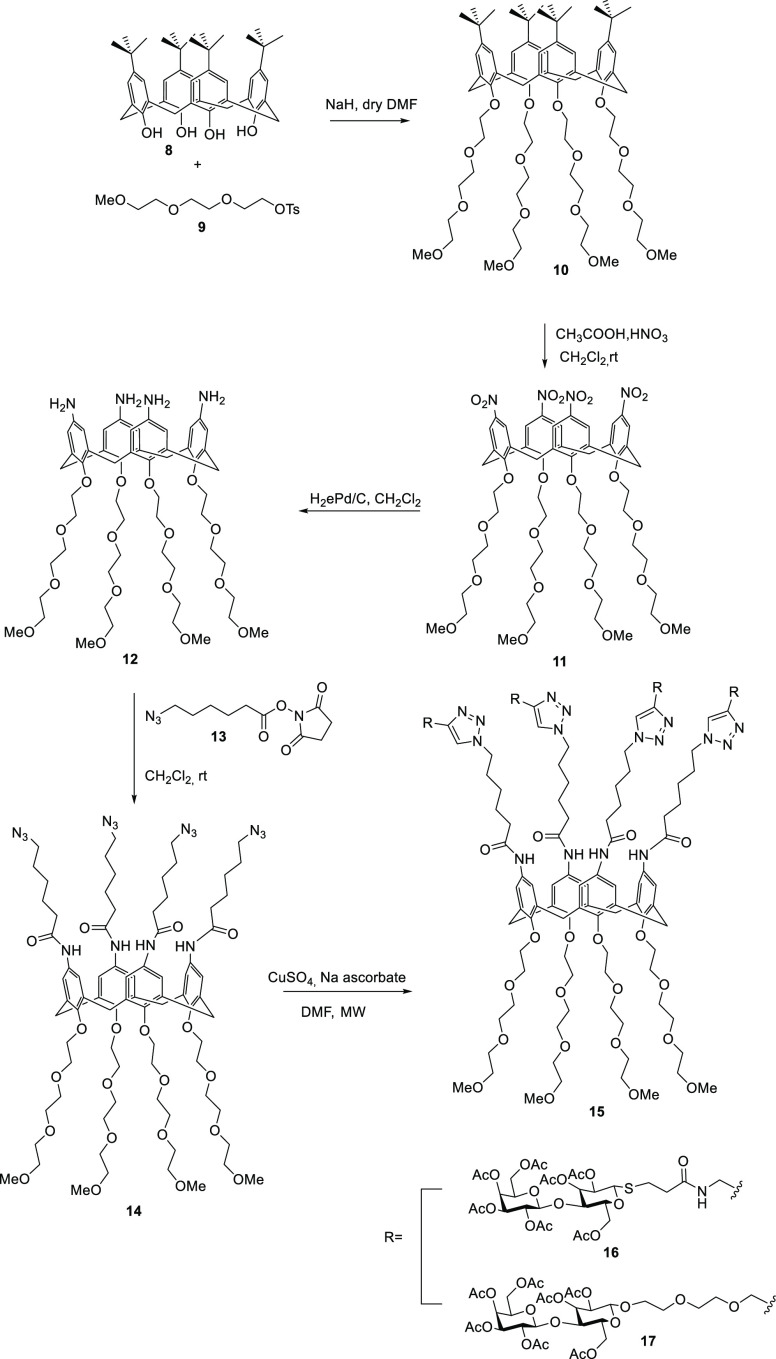
The tetraazide derivative calix[4]arene 14 was generated from p-tert-butylcalix[4]arene 8 in its cone form, which was guaranteed by functionalizing its lower rim with methyl-capped triethylene glycol chains 9. The alkylation of calix[4]arene 8 with tosylate 9 was carried out under standard conditions (NaH in DMF) to provide 10, which was subjected to ipso-nitration, resulting in the substitution of tert-butyl groups with nitro groups in compound 10, generating derivative 11, which was hydrogenated in the presence of a Pd–C catalyst to give tetraamino derivative 12. The reaction of 12 with four molecules of N-succinimidoyl ester 13 led to the derivative of tetraazide calix[4]arene 14. This molecule presented an overall yield of 43%. On the other hand, lactose derivatives functionalized with an alkyne group 16 and 17 reacted give to the calixarene nucleus 14 under conditions suitable for the generation of carbohydrate derivatives; thus, the synthesis was carried out in a microwave reactor in DMF solution at 70 °C in the presence of 0.2 equiv of CuSO4 and 0.4 equiv of sodium ascorbate. The product was purified by chromatography on silica gel, giving the tetralactosyl derivative 15 in a yield of 80%.77
In 2006, Dondoni et al. reported the synthesis of C-glycoside clustering on calix[4]arene. Three functionalized calixarene bases were reported, two of which have the azide group 18 and 19 and another replacement with alkyne groups 20 (Figure 4). In the synthesis of calixarene derivatives 22 and 23 with ethynyl tetra-O-benzyl-β-d-glucopyranoside 21 or tetra-O-acetyl-β-d-glucopyranoside 24 in the presence of CuI and i-Pr2-EtN in toluene at room temperature generated the desired 1,4-disubstituted 1,2,3-triazole rings 22 and 23, each one carrying β-C-glucosyl residues (Figure 5). These reactions occurred with a high degree of efficiency. This was corroborated by the yield between 21a and 18 of 73% and a similar yield between 21a and the tetraazide derivative 19 of 63%. Even higher degrees of efficiency were recorded in the stoichiometric reactions of tetra-O-acetyl-β-d-glucopyranoside 21b with the same polyazide calixarene 18 and 19, which in fact produced the corresponding C-glycosyl-calix[4]arenes 22b and 23b in 96% and 86% yields, respectively.9
Figure 4.
Functionalized calixarene bases, di- and tetrasubstituted and another tetrasubstituted with available alkyne groups.
Figure 5.

Synthesis of calixarene derivatives with ethynyl tetra-O-benzyl-β-d-glucopyranoside or tetra-O-acetyl-β-d-glucopyranoside.
Thus, in both cases each triazole-forming cycloaddition reaction appeared to have occurred in an almost quantitative manner. The authors suggested that the higher reactivity of 21b with respect to 21a is due to steric factors, because the O-acetyl groups are less bulky than the O-benzyl groups and thus result in a less hindered matrix. For the case of the derivative tetraethynylcalix[4]arene 20, the same conditions were used previously; this process exhibited a high degree of efficiency, corroborated by overall yields of 61% and 83% for 25a and 25b, respectively. The spatial arrangement of the four sugar-containing arms in the calix[4]arene derivative 23 and 25 may vary due to some flexibility of the macrocycle ring.9
In the same way, derivatives of tetraethynylcalix[4]resorcinarenes have been reported. In 2019, Husain et al. reported the synthesis of a resorcin[4]arene glycoconjugate consisting of eight β-d-glucopyranoside residues constructed in the phenolic parts of the upper edge of resorcin[4]arene by multiple 1,2,3-triazole bonds, 1,4-disubstituted 30 (Scheme 3).66
Scheme 3. Synthesis of Derivatives of Tetraethynylcalix[4]resorcinarenes66.
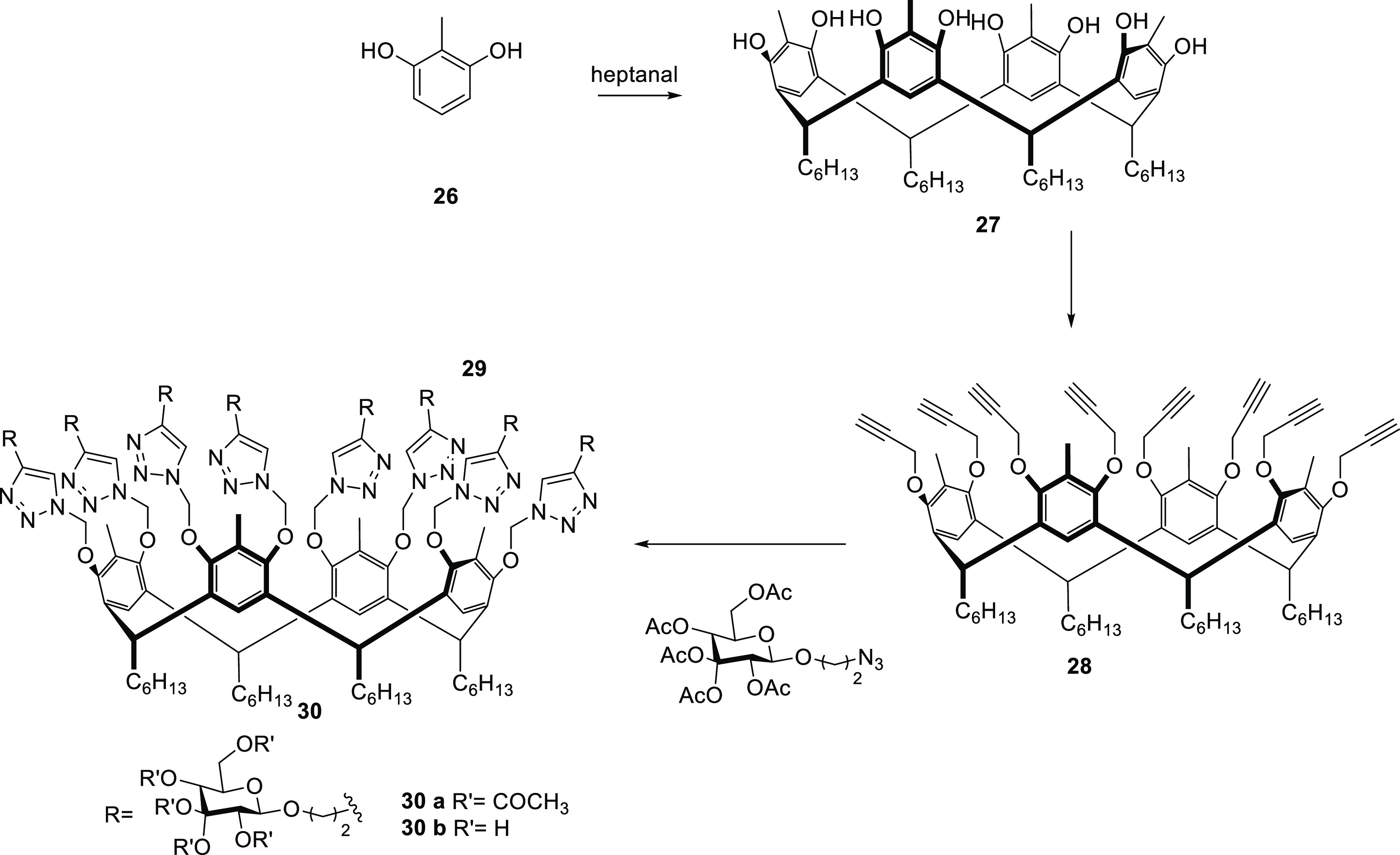
For the synthesis of 30 (Scheme 3), resorcin[4]arene 27 was first synthesized after the acid-catalyzed cyclocondensation reaction of methyl resorcinol 26 with heptanal. Compound 27 was then treated with propargyl bromide in the presence of potassium carbonate in refluxing acetone to achieve the octapropargyl resorcin[4]arene intermediate 28. This was reacted with 2-azidoethyl β-d-glucopyranoside tetraacetate 29a, and the reaction was carried out under chloroform reflux in the presence of CuI and DIPEA to produce the octaacetoxy derivative 30a in 91% yield. Overall deacetylation using a NaOMe solution resulted in 30b with an overall yield of 92%. This new eight-armed resorcin[4]arene glycoconjugate 30b offers an enlarged flexible pseudosaccharide cavity that can act as a molecular container for water-insoluble azide and/or alkyne substrates, presented as an effective catalyst in the reaction of CuAAC species poorly soluble in water with only 1 mol %, with excellent yields and short reaction times.66
The versatility in the synthesis of polyhydroxylated bases makes it possible to select the point reactive group s incorporation. For example, in 2011 Soomro et al. reported the synthesis of a family of tetravalent macrocycles functionalized with galactose and lactose based on a nucleus of resorcin[4]arene. They described the development of diastereoselective synthetic pathways for the formation of lower border propargylated resorcin[4]arenes and their functionalization through copper-catalyzed azide–alkyne click chemistry. Its lower rim was functionalized with specifically prefunctionalized aldehydes 4-(propargyloxy)benzaldehyde 32 (Scheme 4). This direct acid-catalyzed synthesis resulted in products of the form rctt (chair) 33a and rccc (boat) 33b. Glycoconjugate 35 was obtained from 33, which was reacted with galactoside 34a and azidofunctionalized lactoside 34b by means of CuAAC using CuI DIPEA in DMF, assisted by MW with a total reaction time of 15 min to produce acetylated glucoclusters 35Gal and 35Lac. They exhibited yields of between 58% and 85%. Deacetylation was carried out under mild solvolysis conditions in order to obtain the hydroxylated glycoclusters 36Gal and 36Lac. The rccc stereochemistry of propargylated resorcin[4]arene was conserved in the conjugated macromolecules.31
Scheme 4. Synthesis of a Family of Tetravalent Macrocycles Functionalized with Galactose and Lactose Based on a Nucleus of Resorcin[4]arene31.
In 2013, Gao et al. reported the synthesis of a derivative of a resorcinarene coupled to polyethylene glycol (PEG) and polycaprolactone (PCL). This amphiphilic star copolymer was synthesized by a click chemistry reaction and controlled ring-opening polymerization (CROP). The azide group was incorporated at its upper edge by means of an nucleophilic substitution reaction of a halide derivative with sodium azide to give the formation of functionalized resorcin[4]arene 37 (Scheme 5). In the first instance, PEG-functionalized alkyne 38 was incorporated via CuAAC at 37 using PMDETA in DMF as a CuBr catalyst, with a reaction time of 48 h at 40 °C. Product 39 was recovered with column chromatography and a subsequent lyophilization with a yield of 84%. The synthesis of copolymer 40 was carried out by means of the controlled ring-opening polymerization of ε-CL as a tetrafunctional macromolecular initiator and Sn(Oct)2 as a catalyst in dry ethanol at 100 °C for 24 h. The crude product was dissolved in THF and poured into cold diethyl ether to precipitate the final product, which was dried under vacuum to constant weight. Finally, 40 was obtained with a yield of 95%. The importance of these new derivatives of resorcinarene polymers is because both PCL and PEG are biocompatible and nontoxic polymers, so that the resorcinarene–PEG–PCL 40 copolymer synthesized via CuAAC in this article would have potential application in biomedical areas, in addition to the importance of the reactivity provided by the hydroxyl at the end of the PCL chains, which provides the possibility of conjugating them with drugs, making them a viable strategy for drug administration.71
Scheme 5. Synthesis of a Derivative of a Resorcinarene Coupled to Polyethylene Glycol (PEG) and Polycaprolactone (PCL)71.
Peptide sequences and nitrogenous bases have been incorporated into polyhydroxylated structures to generate multivalent systems that can provide selective selectivity for cells or ions.10,78 Ruthenium-based photoactive complexes have been actively studied for their biological applications as potential diagnostic agents against cancer. To provide selective cell selection, Kajouj et al. developed a multivalent system composed of a ruthenium(II) photoreactive complex bound to a calix[4]arene platform with multiple cyclopentapeptides containing the RGD sequence (Scheme 6).10
Scheme 6. Synthesis of a Ruthenium(II) Photoreactive Complex Bound to a Calix[4]arene Platform with Multiple Cyclopentapeptides Containing the RGD Sequence10.
They started from a derivative of p-tert-butylcalix[4]arene 8, which was functionalized on its upper border with azide groups 42. This was reacted with C-[RGDfK]-alkyne 43, a peptide sequence functionalized with an alkyne group. The copper(I) species used as a catalyst was generated in situ from a mixture of CuSO4·5H2O and sodium ascorbate. Unfortunately, this methodology led to low yields and a lack of reproducibility in the case of derivative 44, even when MW heating was used. They thereupon decided to switch to the use of copper nanoparticles (CuNPs), since it is known that nanomaterials efficiently catalyze a wide range of organic reactions and especially azide–alkyne cycloaddition. They brought the mixture to 50 °C with the assistance of MW (100 W) for 1 h, where they obtained product 44 with a yield of 31% after a purification step in semipreparative RP-HLPC.10
On the other hand, nitrogenous bases (cytosine (C), thymine (T), adenine (A), and guanine (G)) were incorporated into structures of the calixarene type by means of click chemistry CuAAC (Scheme 7a). The nitrogenous bases were functionalized with an alkyne group, 45–48, which reacted under click chemistry conditions, generating the copper(I) species in situ by means of the reduction of Cu2+; 45–48 react with functionalized calixarene with an azide group on its upper edge 5-methylazidocalix[4]arene 49, thus giving the formation of the nucleobase calix[4]arenes corresponding to 50–53, with yields that ranged from 66% to 85%. The four derivatives of calix[4]arene efficiently complexed with alkali-metal ions. The presence of nitrogenous bases on the upper border did not alter the selectivity to this type of ion but allowed understanding the recognition between nucleic bases in the assembly of derivatives in CDCl3 in the construction of high-order molecular structures 54, as shown in Scheme 7b.78
Scheme 7. Formation of the Nucleobase Calix[4]arenes Using the Nitrogenous Bases Cytosine (C), Thymine (T), Adenine (A), and Guanine (G)78.
The incorporation of radiomarkers into calixarene-type platforms has been reported as possible additional evaluation agents in antiangiogenic cancer therapy. In 2015, Läppchen et al. reported the design of two close calixarene mimetics derivates of calixarene 0118 55 (Figure 6), containing a terminal alkynyl functional group, developed through a semiautomated procedure optimized for radiolabeling with 2-[18F]-fluoroethylazide using click chemistry. For this, they generated the copper(I) species in situ using CuSO4·5H2O and sodium ascorbate as a reducing agent. This was carried out in a DMF/H2O solution at rt overnight. After purification and formulation by semipreparative HPLC, the [18F] lower rim tagged [18F] analogue 56 and the [18F] equatorially tagged 57 were obtained with a radiochemical purity of >97%.74
Figure 6.
Synthesis of two close calixarene mimetics derivates of calixarene.
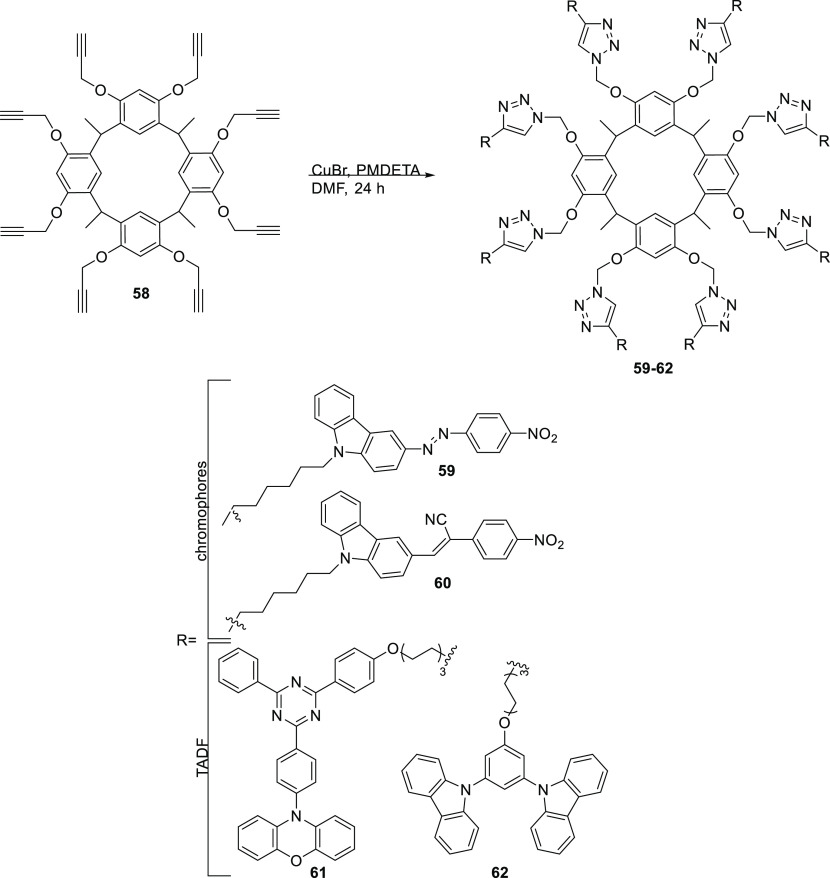
In platforms of the resorcinarene type, chromophores and compounds with thermally activated delayed fluorescence (TADF) were incorporated (Scheme 8). Liu et al.12 in 2016 and Wang et al. in 202079 reported resorcinarene conjugates obtained with click chemistry (CuAAC) using as a functionalized platform octaalkylcalix[4]resorcinarene 58. In both syntheses, the copper(I) species was used directly, using CuBr and PMDETA stabilizer in DMF for 24 h. For the case of compound 59, it was obtained with a yield of 40% compared to compound 60, which exhibited a yield of 75%. This type of compound can have applications in the generation of new photosensitive materials.
Scheme 8. Derived Resorcinarene with Chromophores and Compounds with Thermally Activated Delayed Fluorescence (TADF)79.
Applications such as the generation of diagnostic electrodes have also been the focus of research in recent years using these type of polyhydroxylated platforms conjugated by click chemistry. In 2013, Feng et al. reported the design and development of an electrode using a functionalized gold surface (Scheme 9); the amino alkylene groups were first assembled on a gold surface by S–Au bonds after CS2 was added, and then the end azide of 63 reacted with alkynyl groups of 64 with a Cu+ catalyst.11
Scheme 9. Design and Development of an Electrode Using a Functionalized Gold Surface11.
Simultaneously, the two steps in the gold surface modification process were successfully observed via X-ray photoelectron spectroscopy (XPS). This type of electrode exhibited selectivity in the detection of o-phenylenediamine compared to its m- and p-isomers.11 On the other hand, in 2016 Buttress et al. reported the synthesis of calixarene derivatives 66 and 68 suitable for attachment to a vitreous carbon electrode (GCE) surface on the upper or lower rim (Scheme 10). Once attached to the GCE surface, the exposed face of calixarene was designed for easy modification, using the copper-catalyzed alkyne azide cycloaddition reaction (CuAAC), where they obtained functionalized electrodes 67 and 69.80
Scheme 10. Synthesis of Calixarene Derivatives Suitable for Their Attachment to a Vitreous Carbon Electrode (GCE) Surface on the Upper or Lower Edge80.
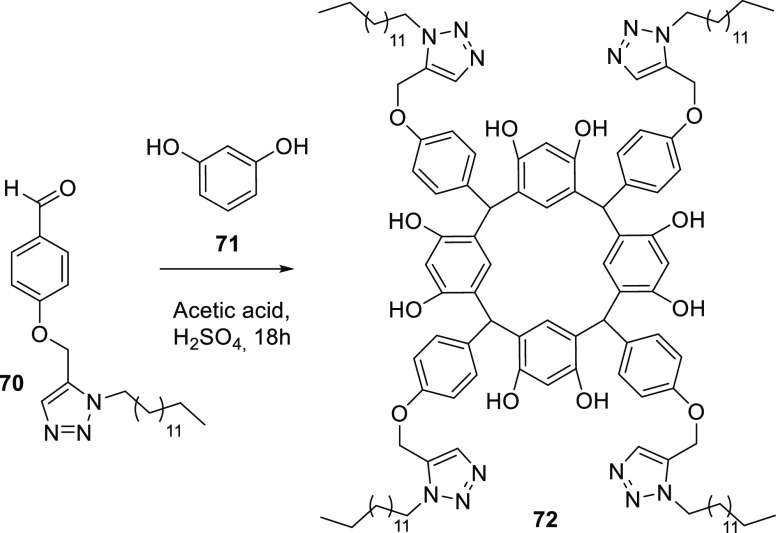
In 2020, Qadri et al. reported the synthesis of a new macrocycle of calix[4]resorcinarene functionalized with tetratriazole 72 used for the detection of copper ions in an aqueous medium (Scheme 11). The photophysical potential of compound 72 can be determined by a variety of cations (Ba2+, Ca2+, Co2+, Hg2+, K+, Mg2+, Mn2+, Na+, NH4+, and Pd2+). The macrocycle of calix[4]resorcinarene based on triazole 68 interacts with the Cu2+ ion in preference to other cations. This new tetratriazole compound 72 was synthesized from an aldehyde functionalized with an aliphatic chain 70 via click chemistry for a subsequent reaction with resorcinol 71 in an acid medium.81
Scheme 11. Synthesis of a New Macrocycle of Calix[4]resorcinarene Functionalized with Tetratriazole81.
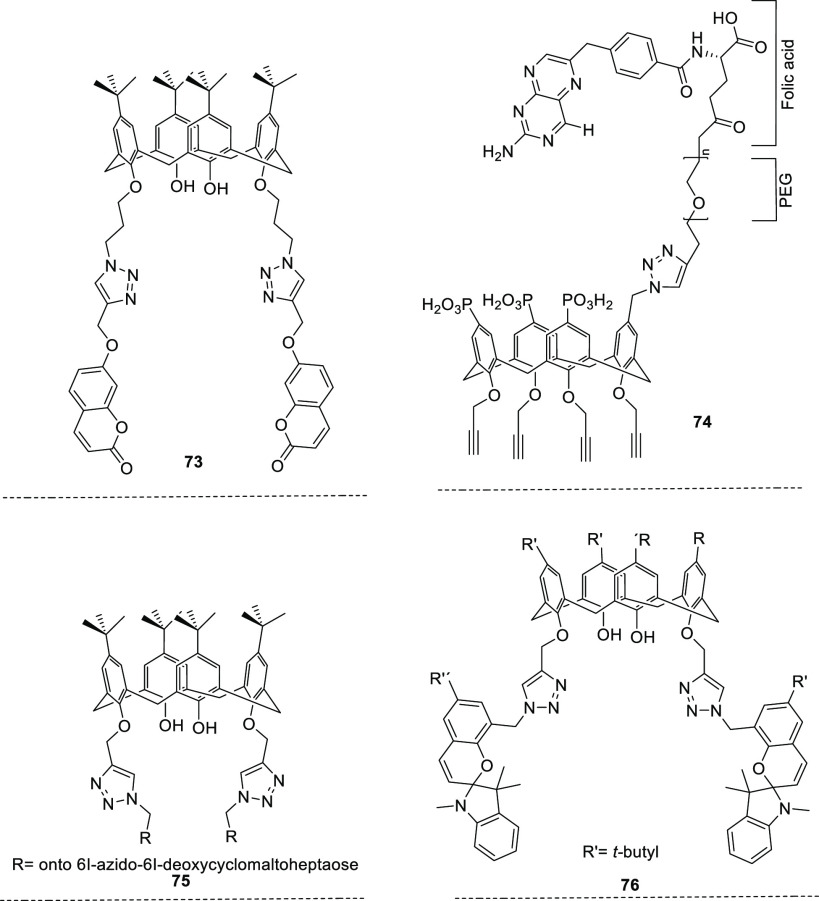
Biologically active molecules such as coumarin,72 folic acid,82 cyclodextrins,76 and spiroindoline3 have also been incorporated by means of click chemistry to these functionalized calixarene/resorcinarene-type platforms (Figure 7). These four derivatives were obtained under the same synthetic route of click chemistry, using Cu(I) generated in situ as a catalyst through the reaction of CuSO4·5H2O and sodium ascorbate, with rt temperature variation for 73, 74, and 76 assisted by MW for 75, all in a mixture of polar solvents.
Figure 7.
Biologically active molecules such as coumarin 73, folic acid 74, cyclodextrins 75, and spiroindoline 76 have also been incorporated in functionalized calixarene/resorcinarene platforms.
The generation of macrocycles of calixarene derivatives linked together through CuAAC click chemistry has already been reported. In 2020/2021, Burilov et al. reported that a new imidazolium amphiphilic calix[4]arene with terminal acetylene fragments in the polar region was synthesized according to a two-step scheme including regioselective chloromethylation of distal di-O-butyl calix[4]arene and subsequent interaction with 1-(hex-5-yn-1-yl)-1H-imidazole. The aggregation properties (CAC and the size and ζ potential of aggregates) of alkynyl calix[4]arene 78, as well as of previously synthesized azidopropyl calix[4]arene 77 and their 1/1 mixture, were announced. Macrocycles with azide and alkyne fragments in the polar region were covalently cross-linked under CuAAC conditions in water (Scheme 12A). For the synthesis of target macrocycles containing azidoalkylimidazolium/alkynylimidazolium groups, it was carried out through the alkylation of the corresponding N-substituted imidazoles with dichloromethyl calix[4]arene derivative 82 (Scheme 12B). The foregoing synthetic strategy allows introducing any alkyl/imidazolium fragments into the lower/upper rims of a calix[4]arene macrocyclic platform in just three steps with high yields. p-H-calix[4]arene 80, presynthesized from p- -butyl-calix[4]arene using de-tert-butylation with AlCl3 in the presence of phenol, was treated by butyl bromide to give bis-O-butyl derivative 81, which was then incorporated into a Blanc chloromethylation to give calix[4]arene 82 with a nearly qualitative yield (Scheme 12B). The final step was the reaction of calix[4]arene 83 with 1-(3-azidopropyl)-1H-imidazole or 1-(hex-5-yn-1-yl)-1H-imidazole.83,84
Scheme 12. (A) Representation of the Formation of the Polymeric Nanoparticles Using a Supramolecular Approach and (B) Synthetic Pathway for Macrocycles 84 and 85 Containing Azidoalkylimidazolium/Alkynylimidazolium Groups on the Upper Rim83,84.
Finally, Table 2(85−93) shows examples of the incorporation of other organic molecules, such as cationic, ionic, nonionic, allyl, benzyl, triphenylene, thiazole acetate, dimethyl acetylenedicarboxylate, and polyammonium, along with their required conditions. This comparative table allows us to show the great versatility that the copper-catalyzed cycloaddition reaction can have between an alkyne group and an azide group (CuAAC) in the generation of new calixarene/resorcinarene derivatives.
Table 2. Examples of the Incorporation of Other Organic Molecules.
4. Conclusions
In summary, this review has shown the most significant advances in copper-catalyzed azide–alkyne cycloaddition (CuAAC) used in the generation of new calixarenes/resorcinarenes. The reaction between alkynes and azide derivatives using copper(I) as a catalyst for the functionalization of polyhydroxylated platforms is a powerful tool in molecular design. This review opens up a new opportunity for researchers from all areas who want to synthesize macromolecules with calixarene/resorcinarene-type nuclei using click chemistry for its functionalization. Thus, we consolidated synthetic conditions and routes that will allow the advancement of complex molecule synthesis in a more efficient and faster way. These new molecules generated from triazole rings will allow extending the applications of polyhydroxylated compounds by incorporating organic and/or inorganic molecules with potential pharmaceutical applications.
Acknowledgments
This research was conducted with the financial support of COLCIENCIAS, Project “Diseño y obtención de nuevos agentes antibacterianos basados en dendrímeros péptido-resorcinareno: Una alternativa para combatir la resistancia bacteriana. RC-846 2019. HERMES Code 45746”.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
References
- Da Silva E.; Valmalle C.; Becchi M.; Cuilleron C. Y.; Coleman A. W. The Use of Electrospray Mass Spectrometry (ES/MS) for the Differential Detection of Some Steroids as Calix-[n]-arene Sulphonate Complexes. J. Incl. Phenom. 2003, 46, 65–69. 10.1023/A:1025685628493. [DOI] [Google Scholar]
- Dawn A.; Chandra H.; Ade-Browne C.; Yadav J.; Kumari H. Multifaceted Supramolecular Interactions from C-Methylresorcin[4]arene Lead to an Enhancement in In Vitro Antibacterial Activity of Gatifloxacin. Chem. - A Eur. J. 2017, 23, 18171–18179. 10.1002/chem.201704291. [DOI] [PubMed] [Google Scholar]
- Nag R.; Polepalli S.; Althaf Hussain M.; Rao C. P. Ratiometric Cu2+ Binding, Cell Imaging, Mitochondrial Targeting, and Anticancer Activity with Nanomolar IC50 by Spiro-Indoline-Conjugated Calix[4]arene. ACS Omega 2019, 4, 13231–13240. 10.1021/acsomega.9b01402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeerupan J.; Konishi G. I.; Nemoto T.; Shin D. M.; Nakamoto Y. Synthesis of multifunctional poly(calix[4]resorcinarene). Polym. J. 2007, 39, 762–763. 10.1295/polymj.PJ2006274. [DOI] [Google Scholar]
- Li M.; Liu G.; Wang K.; Wang L.; Fu X.; Lim L. Y.; Chen W.; Mo J. Metal ion-responsive nanocarrier derived from phosphonated calix[4]arenes for delivering dauricine specifically to sites of brain injury in a mouse model of intracerebral hemorrhage. J. Nanobiotechnology 2020, 18, 1–19. 10.1186/s12951-020-00616-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahojb Noruzi E.; Kheirkhahi M.; Shaabani B.; Geremia S.; Hickey N.; Asaro F.; Nitti P.; Kafil H. S. Design of a Thiosemicarbazide-Functionalized Calix[4]arene Ligand and Related Transition Metal Complexes: Synthesis, Characterization, and Biological Studies. Front. Chem. 2019, 10.3389/fchem.2019.00663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima T.; Baba Y. Recognition of exterior protein surfaces using artificial ligands based on calixarenes, crown ethers, and tetraphenylporphyrins. J. Incl. Phenom. Macrocycl. Chem. 2012, 73, 17–32. 10.1007/s10847-011-0088-2. [DOI] [Google Scholar]
- Español E. S.; Villamil M. M. Calixarenes: Generalities and their role in improving the solubility, biocompatibility, stability, bioavailability, detection, and transport of biomolecules. Biomolecules 2019, 9, 90. 10.3390/biom9030090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dondoni A.; Marra A. C-glycoside clustering on calix[4]arene, adamantane, and benzene scaffolds through 1,2,3-triazole linkers. J. Org. Chem. 2006, 71, 7546–7557. 10.1021/jo0607156. [DOI] [PubMed] [Google Scholar]
- Kajouj S.; Marcelis L.; Mattiuzzi A.; Grassin A.; Dufour D.; Van Antwerpen P.; Boturyn D.; Defrancq E.; Surin M.; De Winter J.; et al. Synthesis and photophysical studies of a multivalent photoreactive Ru II -calix[4]arene complex bearing RGD-containing cyclopentapeptides. Beilstein J. Org. Chem. 2018, 14, 1758–1768. 10.3762/bjoc.14.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng N.; Luo L.; Zhang G.; Miao F.; Wang C.; Tian D.; Li H. Wettability recognition for isomeric phenylenediamine by nitro-calix[4]arene click chemistry. RSC Adv. 2013, 3, 19278–19281. 10.1039/c3ra42325e. [DOI] [Google Scholar]
- Liu W.; Yang H.; Wu W.; Gao H.; Xu S.; Guo Q.; Liu Y.; Xu S.; Cao S. Calix[4]resorcinarene-based branched macromolecules for all-optical photorefractive applications. J. Mater. Chem. C 2016, 4, 10684–10690. 10.1039/C6TC04062D. [DOI] [Google Scholar]
- Khan K.; Lal Badshah S.; Ahmad N.; Rashid H. U.; Mabkhot Y. Inclusion complexes of a new family of non-ionic amphiphilic dendrocalix[4]arene and poorly water-soluble drugs naproxen and ibuprofen. Molecules 2017, 22, 783. 10.3390/molecules22050783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R.; Sharma A.; Singh H.; Suating P.; Kim H. S.; Sunwoo K.; Shim I.; Gibb B. C.; Kim J. S. Revisiting Fluorescent Calixarenes: From Molecular Sensors to Smart Materials. Chem. Rev. 2019, 119, 9657–9721. 10.1021/acs.chemrev.8b00605. [DOI] [PubMed] [Google Scholar]
- Agrawal Y. K.; Patadia R. N. Studies on Resorcinarenes and their Analytical Applications. Rev. Anal. Chem. 2006, 25, 155–239. 10.1515/REVAC.2006.25.3.155. [DOI] [Google Scholar]
- Shah M. D.; Agrawal Y. Calixarene: A new architecture in the analytical and pharmaceutical technology. J. Sci. Ind. Res. (India). 2012, 71, 21–26. [Google Scholar]
- Jain V. K.; Kanaiya P. H. Chemistry of calix[4]resorcinarenes. Russ. Chem. Rev. 2011, 80, 75–102. 10.1070/RC2011v080n01ABEH004127. [DOI] [Google Scholar]
- Tian H. W.; Liu Y. C.; Guo D. S. Assembling features of calixarene-based amphiphiles and supra-amphiphiles. Mater. Chem. Front. 2020, 4, 46–98. 10.1039/C9QM00489K. [DOI] [Google Scholar]
- Georghiou P. E. Calixarenes and fullerenes. Calixarenes and Beyond 2016, 879–919. 10.1007/978-3-319-31867-7_33. [DOI] [Google Scholar]
- Moore D.; Watson G. W.; Gunnlaugsson T.; Matthews S. E. Selective formation of the rctt chair stereoisomers of octa-O-alkyl resorcin[4]arenes using Brønsted acid catalysis. New J. Chem. 2008, 32, 994–1002. 10.1039/b714735j. [DOI] [Google Scholar]
- Ziaja P.; Krogul A.; Pawłowski T. S.; Litwinienko G. Structure and stoichiometry of resorcinarene solvates as host-guest complexes - NMR, X-ray and thermoanalytical studies. Thermochim. Acta 2016, 623, 112–119. 10.1016/j.tca.2015.10.018. [DOI] [Google Scholar]
- Velásquez-Silva A.; Cortés B.; Rivera-Monroy Z. J.; Pérez-Redondo A.; Maldonado M. Crystal structure and dynamic NMR studies of octaacetyl-tetra(propyl)calix[4]resorcinarene. J. Mol. Struct. 2017, 1137, 380–386. 10.1016/j.molstruc.2017.02.059. [DOI] [Google Scholar]
- Castillo-Aguirre A. A.; Pérez-Redondo A.; Maldonado M. Influence of the hydrogen bond on the iteroselective O-alkylation of calix[4]resorcinarenes. J. Mol. Struct. 2020, 1202, 127402. 10.1016/j.molstruc.2019.127402. [DOI] [Google Scholar]
- Das S.; Das M. K.. Surface Modification of Nanoparticles for Targeted Drug Delivery; Pathak Y. V, Ed.; Springer International Publishing: 2019. [Google Scholar]
- Palmer K. J.; Wong R. Y.; Jurd L.; Stevens K. The Structures of the Oetaaeetate Esters of Two Condensation Tetramers of Resoreinol with. Acta Crystallogr. B 1976, B32, 847–852. 10.1107/S0567740876004056. [DOI] [Google Scholar]
- Iwanek W. The synthesis of octamethoxyresorc[4]arenes catalysed by Lewis acids. Tetrahedron 1998, 54, 14089–14094. 10.1016/S0040-4020(98)00859-X. [DOI] [Google Scholar]
- Han J.; Yan C. G. Synthesis, crystal structure and configuration of resorcinarene amides. J. Incl. Phenom. Macrocycl. Chem. 2008, 61, 119–126. 10.1007/s10847-007-9403-3. [DOI] [Google Scholar]
- O’Farrell C. M.; Chudomel J. M.; Collins J. M.; Dignam C. F.; Wenzel T. J. Water-soluble calix[4]resorcinarenes with hydroxyproline groups as chiral NMR solvating agents. J. Org. Chem. 2008, 73, 2843–2851. 10.1021/jo702751z. [DOI] [PubMed] [Google Scholar]
- Botta B.; Pierini M.; Monache G. D.; Subissati D.; Subrizi F.; Zappia G. Synthesis of amino and ammonium resorcin[4]arenes as potential receptors. Synthesis (Stuttg) 2008, 2008, 2110–2116. 10.1055/s-2008-1067111. [DOI] [Google Scholar]
- Xu W.; Rourke J. P.; Vittal J. J.; Puddephatt R. J. Transition Metal Rimmed-Calixresorcinarene Complexes. Inorg. Chem. 1995, 34, 323–329. 10.1021/ic00105a050. [DOI] [Google Scholar]
- Soomro Z. H.; Cecioni S.; Blanchard H.; Praly J. P.; Imberty A.; Vidal S.; Matthews S. E. CuAAC synthesis of resorcin[4]arene-based glycoclusters as multivalent ligands of lectins. Org. Biomol. Chem. 2011, 9, 6587–6597. 10.1039/c1ob05676j. [DOI] [PubMed] [Google Scholar]
- Sardjono R. E.; Kadarohman A.; Mardhiyah A. Green Synthesis of Some Calix[4]Resorcinarene Under Microwave Irradiation. Procedia Chem. 2012, 4, 224–231. 10.1016/j.proche.2012.06.031. [DOI] [Google Scholar]
- Han X.; Park J.; Wu W.; Malagon A.; Wang L.; Vargas E.; Wikramanayake A.; Houk K. N.; Leblanc R. M. A resorcinarene for inhibition of Aβ fibrillation. Chem. Sci. 2017, 8, 2003–2009. 10.1039/C6SC04854D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales A.; Santana A.; Althoff G.; Melendez E. Host-guest interactions between calixarenes and Cp2NbCl 2. J. Organomet. Chem. 2011, 696, 2519–2527. 10.1016/j.jorganchem.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liška A.; Ludvík J. Stereoelectrochemistry of calixarenes – Molecules with multiple redox centers. Curr. Opin. Electrochem. 2018, 8, 45–51. 10.1016/j.coelec.2017.12.006. [DOI] [Google Scholar]
- Bakas I.; Hayat A.; Piletsky S.; Piletska E.; Chehimi M. M.; Noguer T.; Rouillon R. Electrochemical impedimetric sensor based on molecularly imprinted polymers/sol-gel chemistry for methidathion organophosphorous insecticide recognition. Talanta 2014, 130, 294–298. 10.1016/j.talanta.2014.07.012. [DOI] [PubMed] [Google Scholar]
- Castillo-Aguirre; Maldonado Preparation of Methacrylate-based Polymers Modified with Chiral Resorcinarenes and Their Evaluation as Sorbents in Norepinephrine Microextraction. Polymers (Basel) 2019, 11, 1428. 10.3390/polym11091428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velásquez-Silva B. A.; Castillo-Aguirre A.; Rivera-Monroy Z. J.; Maldonado M. Aminomethylated calix[4]resorcinarenes as modifying agents for glycidyl methacrylate (GMA) rigid copolymers surface. Polymers (Basel) 2019, 11, 1147. 10.3390/polym11071147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-Aguirre A.; Rivera-Monroy Z.; Maldonado M. Selective o-alkylation of the crown conformer of tetra(4-hydroxyphenyl)calix[4]resorcinarene to the corresponding tetraalkyl ether. Molecules 2017, 22, 1660. 10.3390/molecules22101660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado M.; Sanabria E.; Batanero B.; Esteso M.Á. Apparent molal volume and viscosity values for a new synthesized diazoted resorcin[4]arene in DMSO at several temperatures. J. Mol. Liq. 2017, 231, 142–148. 10.1016/j.molliq.2017.01.093. [DOI] [Google Scholar]
- Jain V. K.; Kanaiya P. H.; Bhojak N. Synthesis, spectral characterization of azo dyes derived from calix[4]resorcinarene and their application in dyeing of fibers. Fibers Polym. 2008, 9, 720–726. 10.1007/s12221-008-0113-2. [DOI] [Google Scholar]
- Elçin S.; Ilhan M. M.; Deligöz H. Synthesis and spectral characterization of azo dyes derived from calix[4]arene and their application in dyeing of fibers. J. Incl. Phenom. Macrocycl. Chem. 2013, 77, 259–267. 10.1007/s10847-012-0240-7. [DOI] [Google Scholar]
- Maldonado M.; Sanabria E.; Velasquez-Silva A.; Casas-Hinestroza J. L.; Esteso M. A. Comparative study of the volumetric properties of three regioisomers of diazoted C-tetra(propyl)resorcin[4]arene in DMSO at various temperatures. J. Mol. Liq. 2021, 325, 115252. 10.1016/j.molliq.2020.115252. [DOI] [Google Scholar]
- Sanabria E.; Esteso M.; Pérez-Redondo A.; Vargas E.; Maldonado M. Synthesis and Characterization of Two Sulfonated Resorcinarenes: A New Example of a Linear Array of Sodium Centers and Macrocycles. Molecules 2015, 20, 9915–9928. 10.3390/molecules20069915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartenstein J. E.; Lucas N. T. Reduced symmetry triflate-resorcin[4]arenes. Supramol. Chem. 2012, 24, 618–626. 10.1080/10610278.2012.703324. [DOI] [Google Scholar]
- Utomo S. B.; Saputro A. N. C.; Rinanto Y.. Functionalization of C-4-methoxyphenylcalix[4]resorcinarene with several ammonium compounds. In Proceedings of the IOP Conference Series: Materials Science and Engineering; Institute of Physics Publishing: 2016; Vol. 107, p 012042.
- Hong M.; Zhang Y. M.; Liu Y. Selective binding affinity between quaternary ammonium cations and water-soluble calix[4]resorcinarene. J. Org. Chem. 2015, 80, 1849–1855. 10.1021/jo502825z. [DOI] [PubMed] [Google Scholar]
- Casas-Hinestroza J.; Maldonado M. Conformational Aspects of the O-acetylation of C-tetra(phenyl)calixpyrogallol[4]arene. Molecules 2018, 23, 1225. 10.3390/molecules23051225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballistreri F. P.; Pappalardo A.; Tomaselli G. A.; Sfrazzetto G. T.; Vittorino E.; Sortino S. Synthesis and photophysics of a fullerene-triquinoxaline ensemble. New J. Chem. 2010, 34, 2828–2834. 10.1039/c0nj00481b. [DOI] [Google Scholar]
- Pappalardo A.; Amato M. E.; Ballistreri F. P.; Notti A.; Tomaselli G. A.; Toscano R. M.; Sfrazzetto G. T. Synthesis and topology of [2 + 2] calix[4]resorcarene-based chiral cavitand-salen macrocycles. Tetrahedron Lett. 2012, 53, 7150–7153. 10.1016/j.tetlet.2012.10.101. [DOI] [Google Scholar]
- Castillo-Aguirre A.; Esteso M. A.; Maldonado M. Resorcin[4]arenes: Generalities and Their Role in the Modification and Detection of Amino Acids. Curr. Org. Chem. 2020, 24, 2412–2425. 10.2174/1385272824999200510232141. [DOI] [Google Scholar]
- Han J.; Cai Y. H.; Liu L.; Yan C. G.; Li Q. Syntheses, crystal structures, and electrochemical properties of multi-ferrocenyl resorcinarenes. Tetrahedron 2007, 63, 2275–2282. 10.1016/j.tet.2006.12.073. [DOI] [Google Scholar]
- Yang Q.; Yan C.; Zhu X. A fluorescent chemosensor for paeonol based on tetramethoxy resorcinarene tetraoxyacetic acid. Sensors Actuators, B Chem. 2014, 191, 53–59. 10.1016/j.snb.2013.09.044. [DOI] [Google Scholar]
- Charnley M.; Fairfull-Smith K.; Williams N. H.; Haycock J. W. Evaluation of anti-inflammatory calixarene-peptides for biomaterial modification. Eur. Cells Mater. 2006, 11, 26. [Google Scholar]
- Press release: The Nobel Prize in Chemistry 2022 - NobelPrize.org Available online: https://www.nobelprize.org/prizes/chemistry/2022/press-release/ (accessed on Oct 28, 2022).
- Kolb H. C.; Sharpless K. B. The growing impact of click chemistry on drug discovery. Drug Discovery Today 2003, 8, 1128–37. 10.1016/S1359-6446(03)02933-7. [DOI] [PubMed] [Google Scholar]
- Bock V. D.; Hiemstra H.; Van Maarseveen J. H. Cu I-catalyzed alkyne-azide “click” cycloadditions from a mechanistic and synthetic perspective. Eur. J. Org. Chem. 2006, 2006, 51–68. 10.1002/ejoc.200500483. [DOI] [Google Scholar]
- Kaur J.; Saxena M.; Rishi N. An Overview of Recent Advances in Biomedical Applications of Click Chemistry. Bioconjugate Chem. 2021, 32 (8), 1455–1471. 10.1021/acs.bioconjchem.1c00247. [DOI] [PubMed] [Google Scholar]
- Shin J. A.; Lim Y. G.; Lee K. H. Copper-catalyzed azide-alkyne cycloaddition reaction in water using cyclodextrin as a phase transfer catalyst. J. Org. Chem. 2012, 77, 4117–4122. 10.1021/jo3000095. [DOI] [PubMed] [Google Scholar]
- Buckley B. R.; Figueres M. M. P.; Khan A. N.; Heaney H. A New Simplified Protocol for Copper(I) Alkyne-Azide Cycloaddition Reactions Using Low Substoichiometric Amounts of Copper(II) Precatalysts in Methanol. Synlett 2015, 27, 51–56. 10.1055/s-0035-1560526. [DOI] [Google Scholar]
- Meldal M.; Diness F. Recent Fascinating Aspects of the CuAAC Click Reaction. Trends Chem. 2020, 2, 569–584. 10.1016/j.trechm.2020.03.007. [DOI] [Google Scholar]
- Himo F.; Lovell T.; Hilgraf R.; Rostovtsev V. V.; Noodleman L.; Sharpless K. B.; Fokin V. V. Copper(I)-catalyzed synthesis of azoles. DFT study predicts unprecedented reactivity and intermediates. J. Am. Chem. Soc. 2005, 127, 210–216. 10.1021/ja0471525. [DOI] [PubMed] [Google Scholar]
- Huisgen R. Proc. Chem. Soc. 1961, 357–396. 10.1039/PS9610000357. [DOI] [Google Scholar]
- Knyazeva I. R.; Abdrafikova D. K.; Mukhamedyanova K. M.; Syakaev V. V.; Gabidullin B. M.; Gubaidullin A. T.; Habicher W. D.; Burilov A. R.; Pudovik M. A. Synthesis of novel highly functionalized triazole-linked calix[4]resorcinols via click reaction. Mendeleev Commun. 2017, 27, 556–558. 10.1016/j.mencom.2017.11.005. [DOI] [Google Scholar]
- Tang H.; Guo H.; Yang F.; Zhu S. Synthesis and mesomorphic properties of calix[4]resorcinarene–triphenylene oligomers. Liq. Cryst. 2017, 44, 1566–1574. 10.1080/02678292.2017.1305459. [DOI] [Google Scholar]
- Husain A. A.; Bisht K. S. Synthesis of a novel resorcin[4]arene-glucose conjugate and its catalysis of the CuAAC reaction for the synthesis of 1,4-disubstituted 1,2,3-triazoles in water. RSC Adv. 2019, 9, 10109–10116. 10.1039/C9RA00972H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamata K.; Nakagawa Y.; Yamaguchi K.; Mizuno N. 1,3-Dipolar cycloaddition of organic azides to alkynes by a dicopper-substituted silicotungstate. J. Am. Chem. Soc. 2008, 130, 15304–15310. 10.1021/ja806249n. [DOI] [PubMed] [Google Scholar]
- Brotherton W. S.; Michaels H. A.; Tyler Simmons J.; Clark R. J.; Dalai N. S.; Zhu L. Apparent copper(II)-accelerated azide - alkyne cycloaddition. Org. Lett. 2009, 11, 4954–4957. 10.1021/ol9021113. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Silva K.; Rendon-Nava D.; Alvarez-Hernández A.; Mendoza-Espinosa D. Copper(II) accelerated azide–alkyne cycloaddition reaction using mercaptopyridine-based triazole ligands. New J. Chem. 2019, 43, 16538–16545. 10.1039/C9NJ03974K. [DOI] [Google Scholar]
- Burilov V. A.; Fatikhova G. A.; Dokuchaeva M. N.; Nugmanov R. I.; Mironova D. A.; Dorovatovskii P. V.; Khrustalev V. N.; Solovieva S. E.; Antipin I. S. Synthesis of new p-tert-butylcalix[4]arene-based polyammonium triazolyl amphiphiles and their binding with nucleoside phosphates. Beilstein J. Org. Chem. 2018, 14, 1980–1993. 10.3762/bjoc.14.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C.; Wang Y.; Gou P.; Cai X.; Li X.; Zhu W.; Shen Z. Synthesis and characterization of resorcinarene-centered amphiphilic A 8B4 miktoarm star copolymers based on poly(ε- caprolactone) and poly(ethylene glycol) by combination of CROP and “click” chemistry. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 2824–2833. 10.1002/pola.26670. [DOI] [Google Scholar]
- Hosseinzadeh R.; Domehri E.; Tajbakhsh M.; Bekhradnia A. New fluorescent sensor based on a calix[4]arene bearing two triazole–coumarin units for copper ions: application for Cu 2+ detection in human blood serum. J. Incl. Phenom. Macrocycl. Chem. 2019, 93, 245–252. 10.1007/s10847-018-0872-3. [DOI] [Google Scholar]
- Pineda-Castañeda H. M.; Bonilla-Velásquez L. D.; Leal-Castro A. L.; Fierro-Medina R.; García-Castañeda J. E.; Rivera-Monroy Z. J. Use of Click Chemistry for Obtaining an Antimicrobial Chimeric Peptide Containing the LfcinB and Buforin II Minimal Antimicrobial Motifs. ChemistrySelect 2020, 5, 1655–1657. 10.1002/slct.201903834. [DOI] [Google Scholar]
- Läppchen T.; Dings R. P. M.; Rossin R.; Simon J. F.; Visser T. J.; Bakker M.; Walhe P.; Van Mourik T.; Donato K.; Van Beijnum J. R.; et al. Novel analogs of antitumor agent calixarene 0118: Synthesis, cytotoxicity, click labeling with 2-[18F]fluoroethylazide, and in vivo evaluation. Eur. J. Med. Chem. 2015, 89, 279–295. 10.1016/j.ejmech.2014.10.048. [DOI] [PubMed] [Google Scholar]
- Gajjar J. A.; Vekariya R. H.; Parekh H. M. Recent advances in upper rim functionalization of resorcin[4]arene derivatives: Synthesis and applications. Synth. Commun. 2020, 50, 2545–2571. 10.1080/00397911.2020.1766080. [DOI] [Google Scholar]
- Garska B.; Tabatabai M.; Ritter H. Calix[4]arene-click-cyclodextrin and supramolecular structures with watersoluble NIPAAM-copolymers bearing adamantyl units: “Rings on ring on chain.. Beilstein J. Org. Chem. 2010, 6, 784–788. 10.3762/bjoc.6.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galante E.; Geraci C.; Sciuto S.; Campo V. L.; Carvalho I.; Sesti-Costa R.; Guedes P. M. M.; Silva J. S.; Hill L.; Nepogodiev S. A.; et al. Glycoclusters presenting lactose on calix[4]arene cores display trypanocidal activity. Tetrahedron 2011, 67, 5902–5912. 10.1016/j.tet.2011.06.065. [DOI] [Google Scholar]
- Liu W.; Minier M. A.; Franz A. H.; Curtis M.; Xue L. Synthesis of nucleobase-calix[4]arenes via click chemistry and evaluation of their complexation with alkali metal ions and molecular assembly. Supramol. Chem. 2011, 23, 806–818. 10.1080/10610278.2011.632824. [DOI] [Google Scholar]
- Wang F.; Qiu W.; Zeng J.; Yuan P.; Zong W.; Wu W.; Liu Y.; Xu S.; Su S. J.; Cao S. Calix[4]resorcinarene-based hyper-structured molecular thermally activated delayed fluorescence yellow-green emitters for non-doped OLEDs. J. Mater. Chem. C 2020, 8, 4469–4476. 10.1039/C9TC05817F. [DOI] [Google Scholar]
- Buttress J. P.; Day D. P.; Courtney J. M.; Lawrence E. J.; Hughes D. L.; Blagg R. J.; Crossley A.; Matthews S. E.; Redshaw C.; Bulman Page P. C.; et al. Janus” Calixarenes: Double-Sided Molecular Linkers for Facile, Multianchor Point, Multifunctional, Surface Modification. Langmuir 2016, 32, 7806–7813. 10.1021/acs.langmuir.6b02222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qadri T.; Ali I.; Hussain M.; Ahmed F.; Shah M. R.; Hussain Z. Synthesis of New Tetra Triazole Functionalized Calix[4]resorcinarene and Chemosensing of Copper Ions in Aqueous Medium. Curr. Org. Chem. 2020, 24, 332–337. 10.2174/1385272824666200211114211. [DOI] [Google Scholar]
- Mo J.; Eggers P. K.; Yuan Z. X.; Raston C. L.; Lim L. Y. Paclitaxel-loaded phosphonated calixarene nanovesicles as a modular drug delivery platform. Sci. Rep. 2016, 6, 1–12. 10.1038/srep23489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burilov V.; Garipova R.; Mironova D.; Sultanova E.; Bogdanov I.; Ocherednyuk E.; Evtugyn V.; Osin Y.; Rizvanov I.; Solovieva S.; et al. New poly-imidazolium-triazole particles by CuAAC cross-linking of calix[4]arene bis-azide/alkyne amphiphiles - a prospective support for Pd in the Mizoroki-Heck reaction. RSC Adv. 2021, 11, 584–591. 10.1039/D0RA09740C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burilov V.; Mironova D.; Sultanova E.; Garipova R.; Evtugyn V.; Solovieva S.; Antipin I. Nhc polymeric particles obtained by self-assembly and click approach of calix[4]arene amphiphiles as support for catalytically active pd nanoclusters. Molecules 2021, 26, 6864. 10.3390/molecules26226864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu E. H.; Zhao Y. Efficient synthesis of water-soluble calixarenes using click chemistry. Org. Lett. 2005, 7, 1035–1037. 10.1021/ol047468h. [DOI] [PubMed] [Google Scholar]
- Sliwa W.; Deska M. Syntheses and reactivity of calixarenes functionalized at meso positions. Arkivoc 2012, 2012, 173–210. 10.3998/ark.5550190.0013.106. [DOI] [Google Scholar]
- Gorbunov A.; Kuznetsova J.; Deltsov I.; Molokanova A.; Cheshkov D.; Bezzubov S.; Kovalev V.; Vatsouro I. Selective azide-alkyne cycloaddition reactions of azidoalkylated calixarenes. Org. Chem. Front. 2020, 7, 2432–2441. 10.1039/D0QO00650E. [DOI] [Google Scholar]
- Burilov V. A.; Artemenko A. A.; Garipova R. I.; Amirova R. R.; Fatykhova A. M.; Borisova J. A.; Mironova D. A.; Sultanova E. D.; Evtugyn V. G.; Solovieva S. E.; et al. New Calix[4]arene-Fluoresceine Conjugate by Click Approach-Synthesis and Preparation of Photocatalytically Active Solid Lipid Nanoparticles. Molecules 2022, 27, 2436–2436. 10.3390/molecules27082436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews S. E.; Cecioni S.; O’Brien J. E.; MacDonald C. J.; Hughes D. L.; Jones G. A.; Ashworth S. H.; Vidal S. Fixing the Conformation of Calix[4]arenes: When Are Three Carbons Not Enough?. Chem. - A Eur. J. 2018, 24, 4436–4444. 10.1002/chem.201705955. [DOI] [PubMed] [Google Scholar]
- Ahmed F.; Perveen S.; Shah K.; Shah M. R.; Ahmed S. Synthesis and characterization of triazole based supramolecule for interaction with cefuroxime in tap water and blood plasma. Ecotoxicol. Environ. Saf. 2018, 147, 49–54. 10.1016/j.ecoenv.2017.08.023. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Ippel H.; Miller M. C.; Wong T. J.; Griffioen A. W.; Mayo K. H.; Pieters R. J. Hybrid ligands with calixarene and thiodigalactoside groups: galectin binding and cytotoxicity. Org. Chem. Front. an Int. J. Org. Chem. 2019, 6, 2981–2990. 10.1039/C9QO00810A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlüter D.; Korsching K. R.; Azov V. A. Lower-Rim-Modified Calix[4]arene-Pyrrolotetrathiafulvalene Molecular Tweezers. Eur. J. Org. Chem. 2021, 2021, 4469–4476. 10.1002/ejoc.202100676. [DOI] [Google Scholar]
- Malakhova M.; Gorbunov A.; Ozerov N.; Korniltsev I.; Ermolov K.; Bezzubov S.; Kovalev V.; Vatsouro I. Triazolated calix[4]semitubes: assembling strategies towards long multicalixarene architectures. Org. Chem. Front. 2022, 9, 3084–3092. 10.1039/D2QO00432A. [DOI] [Google Scholar]