Abstract
Here, we report a silver-mediated coupling of acetylenes with sulfoximines to synthesize N-α-ketoacyl sulfoximines and N-α,β-unsaturated acyl sulfoximines. The reactions are performed under an open atmosphere using the oxidant K2S2O8 and the ligand 2,2-bipyridyl. However, the fate of the product formation is controlled by the type of substrate used. The coupling between aryl acetylenes and sulfoximines afforded the N-α-ketoacylsulfoximines, while the alkyl acetylenes provided the N-α,β-unsaturated acyl sulfoximines. Controlled experiments reveal the differential reactivity patterns of substrates. The labeling 18O experiments showed that water is the source of the incoming oxygen atom for the keto group of N-α-ketoacyl sulfoximines and N-α,β-unsaturated acyl sulfoximines.
Introduction
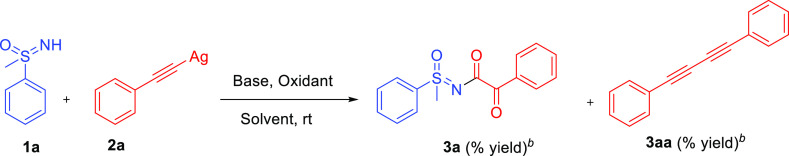
Sulfoximines constitute a significant group in organic chemistry because of their remarkable properties, such as chiral auxiliaries,1−3 ligands,4−6 and building blocks.7−12 Sulfoximines have played a pivotal role in drug discovery13 for improving specificity14 and oral bioavailability.15 They have also been employed for reducing undesired toxicity.16−18 Sulfoximines are also used as bioisosteres for different functional moieties (heterocyclic amidine,15,19 sulfones,20 and secondary hydroxyl groups)21,22 as well as stable transition-state analogue inhibitors.23 Therefore, developing new and efficient methods for their preparation and derivatization is highly needed. Our group has a persistent interest in the functionalization of the sulfoximine moiety and has developed new methods for the N-arylation and N-heteroarylation of sulfoximine.24,25 In the next curiosity, attempts were made for the N-alkynylation of sulfoximine using the combination of silver, oxidants, and additive, but tried conditions provided the N-α-ketoacyl sulfoximines instead of N-alkynyl sulfoximines (Figure 1). On the other hand, one report regarding the synthesis of N-alkynyl sulfoximines employed sensitive bromoacetylenes and copper catalysts.26,27 In the one decade, α-ketoamides have been highlighted due to their remarkable properties as precursors and pharmaceutical agents.28 However, there are many reports related to the synthesis of N-acyl sulfoximines.29,30 However, limited reports are available on the synthesis of N-α-ketoacyl sulfoximines. Zou et al.31 and Cheng and Bolm32 reported the synthesis of N-α-ketoacyl sulfoximines by coupling methyl ketones and NH-sulfoximines in the presence of copper catalysts and oxidants under heating conditions. Later, Cheng and Bolm developed another method for synthesizing N-α-ketoacyl sulfoximines using sulfoximidoyl-containing hypervalent iodine reagents in the presence of light.33 In 2021, Baranwal et al. reported the synthesis of N-α-ketoacyl sulfoximines using selenium dioxide under heating conditions.34 In the recent decade, silver catalysis has also made its impact beyond its use as cocatalysts or bond activators and has been exploited in many organic transformations, and the same has been nicely reviewed also.35a−35c Here, we have exploited the silver catalysis’s use in synthesizing N-α-ketoacyl sulfoximines and N-α,β-unsaturated acyl sulfoximines. The coupling of NH-sulfoximines with aryl acetylenes leads to N-α-ketoacyl sulfoximines. However, aliphatic alkynes provided the N-α,β-unsaturated acyl sulfoximines.
Figure 1.
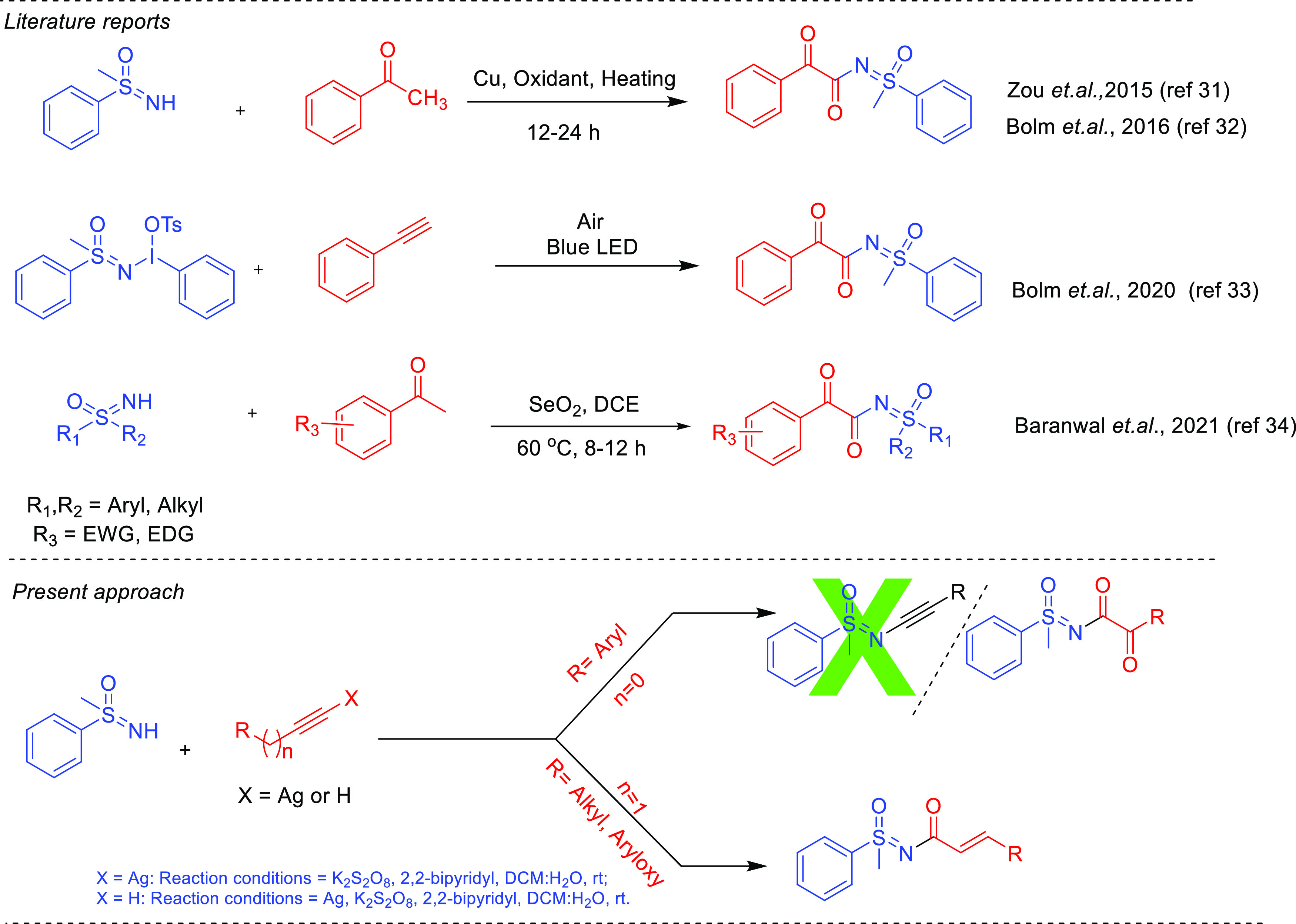
Previous and present approaches for the synthesis of N-α-ketoacyl sulfoximines and N-α,β-unsaturated acyl sulfoximines.
Results and Discussion
The reaction optimization started with the reaction of sulfoximine 1a (0.645 mmol) and silver acetylide 2a (0.967 mmol, 1.5 equiv) as the coupling partners (Table 1). The study was initiated with the reaction of the selected substrates with potassium persulfate (1.935 mmol, 3 equiv) as an oxidant and 2,2-bipyridyl (1.29 mmol, 2 equiv) as an additive under different solvents such as tetrahydrofuran (THF), dimethylformamide (DMF), acetonitrile (CH3CN), toluene, dichloromethane (DCM), and dichloroethane (DCE). All the tried conditions did not provide the required coupled product, but the formation of 1,4-diphenylbuta-1,3-diyne, 3aa, as a sole product is observed (entries 1–6). In the subsequent attempts, a combination of solvents such as toluene/water, DCE/H2O, and DCM/H2O systems was explored; among all the tried conditions, DCE/H2O and DCM/H2O systems provided the corresponding N-α-ketoacyl sulfoximines 3a in a yield of 56 and 63%, respectively, along with 1,4-diphenylbuta-1,3-diyne, 3aa, (entries 7–9). When the reaction was performed in water, no N-α-ketoacyl sulfoximines 3a was observed, but the formation of 1,4-diphenylbuta-1,3-diyne, 3aa, was observed in a yield of 46% (entry 10). When the amount of 2,2′-bipyridyl was decreased to 1.5 and 1 equiv, the yield of N-α-ketoacyl sulfoximine, 3a, was reduced to 32 and 17%, respectively, while no N-α-ketoacyl sulfoximine, 3a, was observed when the concentration of 2,2′-bipyridyl was decreased less than one equivalent (entries 11–13). On the other hand, increasing the concentration of 2,2-bipyridyl to 3 equiv also drastically affect yields (entry 14). When the potassium persulfate was changed with other oxidants such as ammonium persulfate, sodium persulfate, selectfluor, tert-butyl hydroperoxide, hydrogen peroxide, and oxone, 3a was observed with sodium persulfate only (entries 15–20). To suppress the formation of homo-coupled 4-diphenylbuta-1,3-diyne, the reactions were carried out in presence of bases such as cesium carbonate (Cs2CO3), potassium carbonate (K2CO3), and N,N-diisopropyl ethyl amime (DIPEA), but no improvement was observed in the yield of N-α-ketoacyl sulfoximines 3a (entries 21–23). In other attempts, changes in the molar concentration of reactants and additives also did not provide any advantage (results not shown here). When the reaction was performed without additives, no product formation was observed (entry 24). Considering all the attempts, entry no. 9 is considered the best condition (entry 9), and all the next diversity generations were attempted with the same conditions.
Table 1. Optimization studya.
| % yieldb |
|||||
|---|---|---|---|---|---|
| entry | oxidant (equiv) | additive (equiv) | solvent | 3a (%) | 3aa |
| 1 | K2S2O8 (3) | 2,2-bipyridyl (2) | THF | 0 | 67 |
| 2 | K2S2O8 (3) | 2,2-bipyridyl (2) | DMF | 0 | 64 |
| 3 | K2S2O8 (3) | 2,2-bipyridyl (2) | CH3CN | 0 | 62 |
| 4 | K2S2O8 (3) | 2,2-bipyridyl (2) | toluene | 0 | 57 |
| 5 | K2S2O8 (3) | 2,2-bipyridyl (2) | DCM | 0 | 61 |
| 6 | K2S2O8 (3) | 2,2-bipyridyl (2) | DCE | traces | 67 |
| 7 | K2S2O8 (3) | 2,2-bipyridyl (2) | toluene/H2O | 0 | 58 |
| 8 | K2S2O8 (3) | 2,2-bipyridyl (2) | DCE/H2O | 56 | 43 |
| 9 | K2S2O8(3) | 2,2-bipyridyl(2) | DCM/H2O | 63 | 26 |
| 10 | K2S2O8 (3) | 2,2-bipyridyl (2) | H2O | 0 | 46 |
| 11 | K2S2O8 (3) | 2,2-bipyridyl (1.5) | DCM/H2O | 32 | 59 |
| 12 | K2S2O8 (3) | 2,2-bipyridyl (1) | DCM/H2O | 17 | 56 |
| 13 | K2S2O8 (3) | 2,2-bipyridyl (0.8) | DCM/H2O | 0 | 49 |
| 14 | K2S2O8 (3) | 2,2-bipyridyl (3) | DCM/H2O | 0 | 46 |
| 15 | (NH4)2S2O8 (3) | 2,2-bipyridyl (2) | DCM/H2O | traces | 34 |
| 16 | Na2S2O8 (3) | 2,2-bipyridyl (2) | DCM/H2O | 52 | 51 |
| 17 | select fluor | 2,2-bipyridyl (2) | DCM/H2O | 0 | 59 |
| 18 | TBHP (3) | 2,2-bipyridyl (2) | DCM/H2O | 0 | 0 |
| 19 | H2O2 (3) | 2,2-bipyridyl (2) | DCM/H2O | 0 | 0 |
| 20 | Oxone | 2,2-bipyridyl (2) | DCM/H2O | 0 | 0 |
| 21 | K2S2O8 (3) | 2,2-bipyridyl (2) + Cs2CO3 (1.2) | DCM/H2O | 12 | 49 |
| 22 | K2S2O8 (3) | 2,2-bipyridyl (2) + K2CO3 (1.2) | DCM/H2O | 56 | 37 |
| 23 | K2S2O8 (3) | 2,2-bipyridyl (2) + DIPEA (2) | DCM/H2O | 0 | 0 |
| 24 | K2S2O8 (3) | DCM/H2O | 0 | 0 | |
Reaction and conditions: compound 1a (100 mg, 0.645 mmol), compound 2a (0.967 mmol), solvent, rt, 4–10 h.
Isolated yields.
With the best coupling condition, we then explored its diversity using various substituted phenyl acetylides and phenyl sulfoximines, and all the results are presented in Scheme 1. The scope of the approach was found to be very broad and provided the corresponding N-α-ketoacyl sulfoximines with good yields. Phenyl sulfoximine underwent smooth coupling reactions with an electron-donating group containing silver phenylacetylides, such as p-tolylethynylsilver, {(4-propylphenyl)ethynyl}silver, {(4-(tert-butylphenyl)ethynyl)}silver, and {(3-methoxyphenyl)ethynyl}silver, and gave the corresponding coupled products 3b, 3c, 3d, and 3e, respectively, in yields of 53, 54, 56, and 62%, respectively.
Scheme 1. Coupling of Sulfoximines with Phenyl Acetylene Silver.
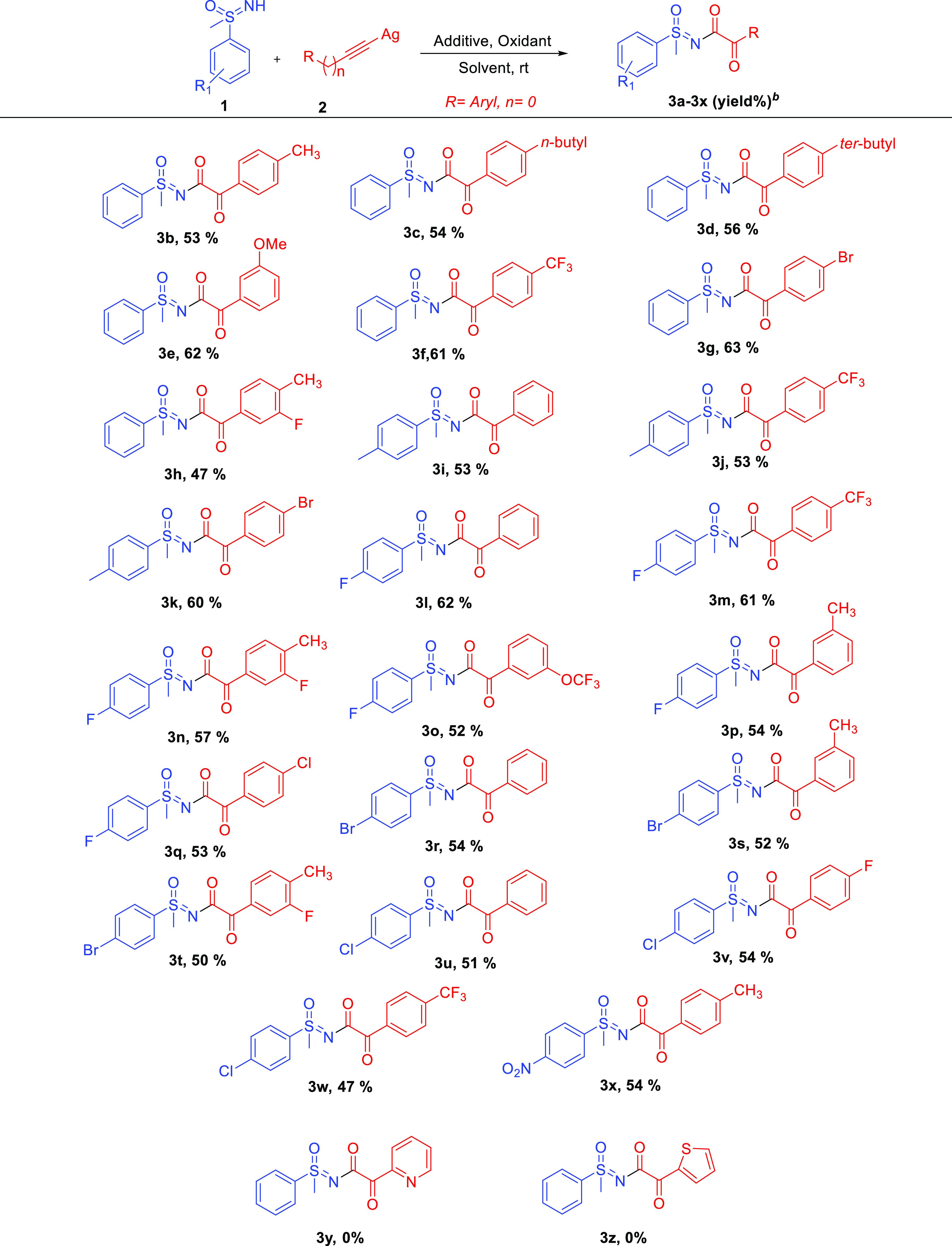
Reaction conditions: compound 1 (100 mg, 0.429–0.645 mmol), compound 2 (1.5 equiv), K2S2O8 (3 equiv), and 2,2-bipyridyl (2 equiv) in DCM/H2O (3:1, 12 mL solvent), rt, 4–8 h. bIsolated yields.
Similarly, electron-withdrawing groups containing silver phenylacetylides such as {(4-trifluoromethylphenyl)ethynyl}silver and {(4-bromophenyl)ethynyl}silver also underwent coupling reactions with sulfoximine 1a and furnished the corresponding coupled products 3f and 3g in yields of 61 and 63%, respectively. Sulfoximine 1a also reacted well with bisubstituted phenylacetylides such as {(3-fluoro-4-methylphenyl)ethynyl}silver to afford the product 3h in a yield of 47%. Substituted phenyl sulfoximines were also used to explore the further possible extension of the optimized method. 4-Tolyl sulfoximine on reaction with (phenylethynyl)silver, {(4-(trifluoromethylphenyl)ethynyl}silver, and {(4-(bromophenyl)ethynyl}silver under optimized conditions provided the corresponding N-α-ketoacyl sulfoximines 3i (53%), 3j (53%), and 3k (60%), respectively. 4-Fluorophenyl containing sulfoximine also reacted well with (phenylethynyl)silver, {(4-(trifluoromethylphenyl)ethynyl}silver, {(3-fluoro-4-methylphenyl)ethynyl}silver, {(3-trifluoromethoxyphenyl)ethynyl} silver, {(3-methylphenyl)ethynyl} silver, {(4-chloro phenyl)ethynyl}silver, and provided the corresponding coupled products 3l (62%), 3m (61%), 3n (57%), 3o (52%), 3p (54%), and 3q (53%), respectively. 4-Bromophenyl-containing sulfoximine reacted with (phenylethynyl)silver, {(3-(methyl)phenyl)ethynyl} silver, and {(3-fluoro-4-methylphenyl)ethynyl}silver and provided coupled products 3r (54%), 3s (52%), and 3t (50%), respectively. Likewise, 4-chlorophenyl containing sulfoximine also reacted well with (phenylethynyl)silver, {(4-fluoromethylphenyl)ethynyl}silver, and {(4-trifluoromethylphenyl)ethynyl}silver and provided sulfoximines 3u (51%), 3v (54%), and 3w (47%), respectively. Interestingly, 4-nitro containing sulfoximine was also compatible under optimized conditions and coupled with {(4-methylphenyl)ethynyl} silver and provided corresponding N-α-ketoacyl sulfoximine 3x in a yield of 54%. Under optimized conditions, when heteroaromatic acetylenes, such as pyridine and thiophene-based silver acetylides, were tried, no coupled products (3y and 3z) were observed. In all the reactions, the formation of corresponding buta-1,3-diynes was observed with yields ranging from 20 to 30%. In further diversification, sulfoximine was replaced with other nucleophiles such as benzamide, sulfonamide, and isatin, but no coupling was observed, suggesting the specificity of the reactions with respect to sulfoximine.
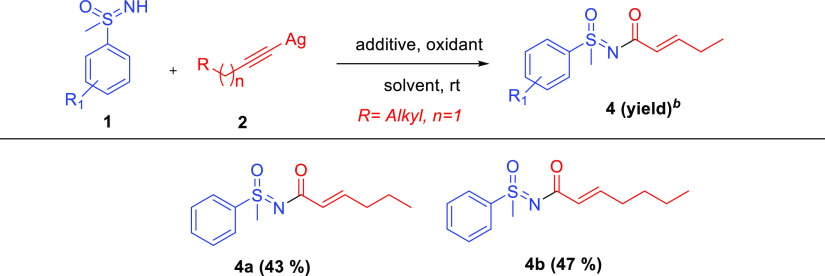
We further explored the reaction conditions for the coupling of sulfoximine with various silver salts of aliphatic acetylenes (Scheme 2); however, the coupled product was identified as N-α,β-unsaturated acyl sulfoximines. The formation of N-α,β-unsaturated acyl sulfoximines has been explained in the latter part of the manuscript. When the sulfoximine 1a was reacted with (hexynyl)silver under the abovementioned optimized conditions, the reactions proceeded smoothly and yielded corresponding N-α,β-unsaturated acyl sulfoximines 4a in a yield of 43%. Similarly, when (heptynyl)silver was used, the corresponding product 4b was formed in a yield of 47%. Interestingly, (3-phenoxyprop-1-yn-1-yl)silver also reacted well with sulfoximine and provided the corresponding coupled product 5 in a yield of 51% (Scheme 3). On the other hand, when aliphatic sulfoximines, such as dibutyl(imino)-λ6-sulfanone, were used, no desired product was obtained.
Scheme 2. Coupling of Sulfoximines with Alkynylsilver.
Reaction conditions: compound 1 (100 mg, 0.429–0.645 mmol), 2 (1.5 equiv), K2S2O8 (3 equiv), and 2,2-bipyridyl (2 equiv) in DCM/H2O (3:1, 12 mL of solvent), rt, 4–8 h. bIsolated yields.
Scheme 3. Coupling of Sulfoximines with Phenoxylpropynyl Silver.
Reaction conditions: compound 1 (100 mg, 0.645 mmol), compound 2 (1.5 equiv 0.967 mmol), K2S2O8 (3 equiv 1.935 mmol), and 2,2-bipyridyl (2 equiv 1.290 mmol), DCM/H2O (3:1, 12 mL), rt, 5 h. bIsolated yields.
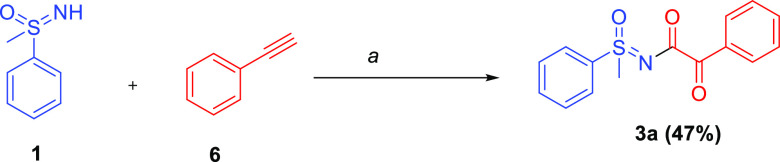
In the next efforts toward extending the scope of the reaction, the coupling was tried with in-situ-generated (phenylethynyl)silver by using the phenyl acetylene and sulfoximine in the presence of silver nitrate, persulfate, and 2,2-bipyridyl, the corresponding product 3a was formed in a yield of 47% (Scheme 4).
Scheme 4. Coupling of Sulfoximines with In-Situ-Generated Phenyl Acetylene Silver.
Reaction condition: compound 1 (100 mg, 0.645 mmol), AgNO3 (0.5 equiv, 0.322 mmol), K2S2O8 (3 equiv, 1.935 mmol), and 2,2-bipyridyl (2 equiv, 1.290 mmol), DCM/H2O (3:1, 12 mL), rt, 7 h.
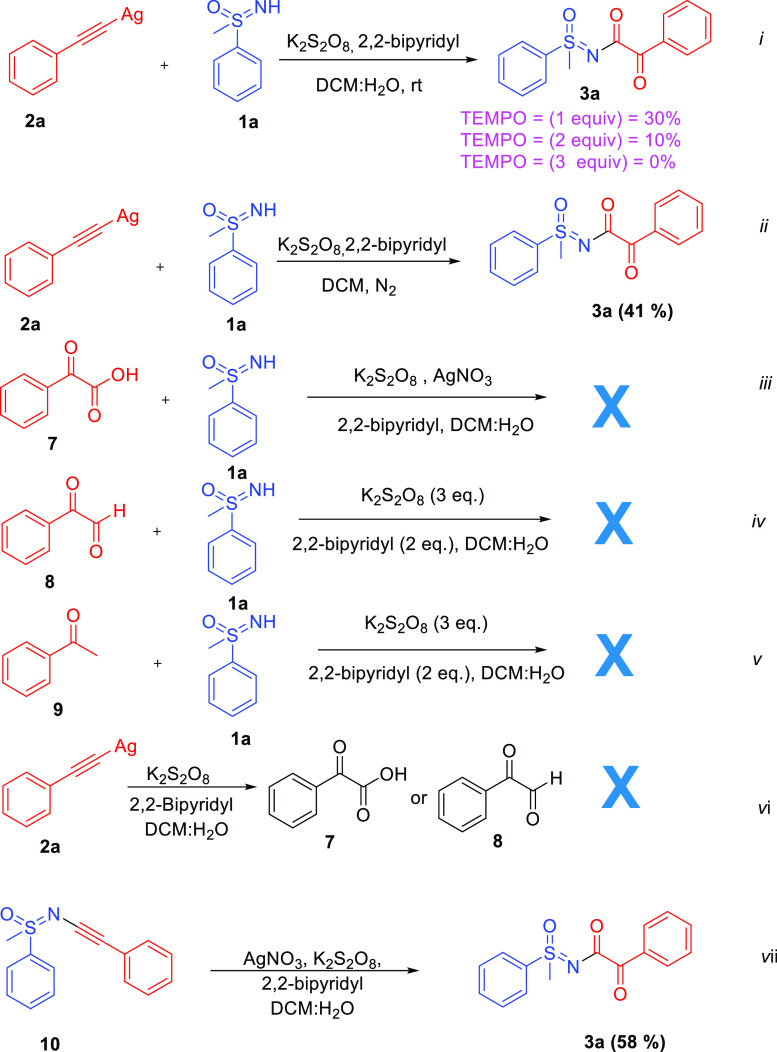
To understand the reaction mechanism, we performed a series of controlled experiments (Scheme 5). When the reactions were performed in the presence of free radical scavengers such as TEMPO the formation of the product 3a was significantly suppressed (eq i), which suggested that there might be involvement of a free radical pathway. When the reaction was conducted under a nitrogen atmosphere after performing freeze–thaw cycles of the solvent system, the reaction worked and provided product 3a, suggesting that the reaction does not have any role with the atmospheric oxygen (eq ii). In the literature, there are reports where phenylacetylenes get converted into glyoxal,36−39 glyoxylic acid,40−43 and acetophenone44−46 under oxidative conditions; therefore, there must be a likely chance of their formation as intermediates and are likely responsible for the formation of coupled product 3a. To rule out the possibility, the reactions were performed with glyoxal/glyoxylic acid/acetophenone (eqs iii–v), but no coupling was observed, suggesting their noninvolvement in the present optimized reaction. In the further reaction, (phenylethynyl)silver was treated with persulfate and additive without sulfoximine and looked for the formation of glyoxal, glyoxylic acid, and acetophenone; however, none of these were observed, which further suggests their noninvolvement in the present optimized conditions (eq vi). When N-alkynyl sulfoximine (10, prepared following known procedure27) was treated under optimized conditions, the corresponding required compound 3a was formed in a yield of 58% (eq vii). The experiment suggested that N-alkynyl sulfoximines could be possible key intermediates during the reaction.
Scheme 5. Controlled Experiments.
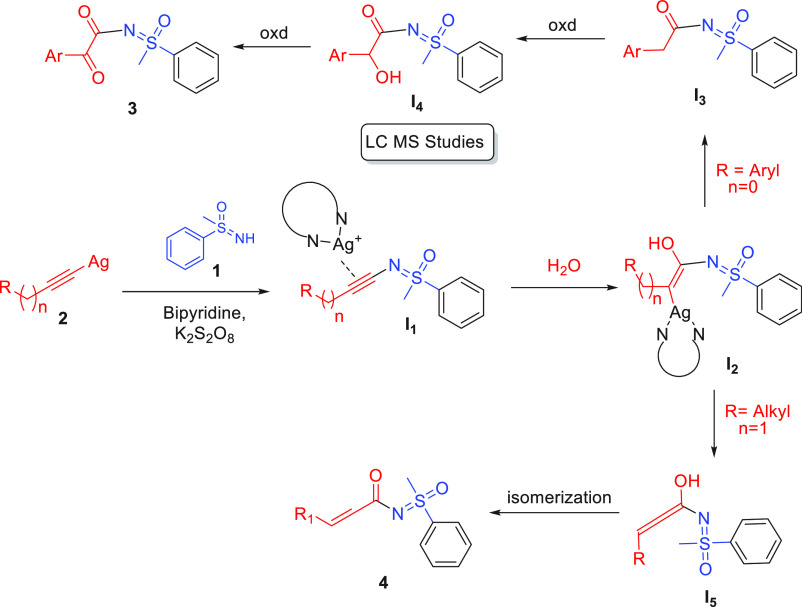
Next, we performed the reaction in the presence of 18O-labeled water to check the source of oxygen in the product. The LC–MS experiment confirmed that 18O is present in the product in 30% (Figure 2), which indicated oxygen atom is coming from water in the acylated product.
Figure 2.
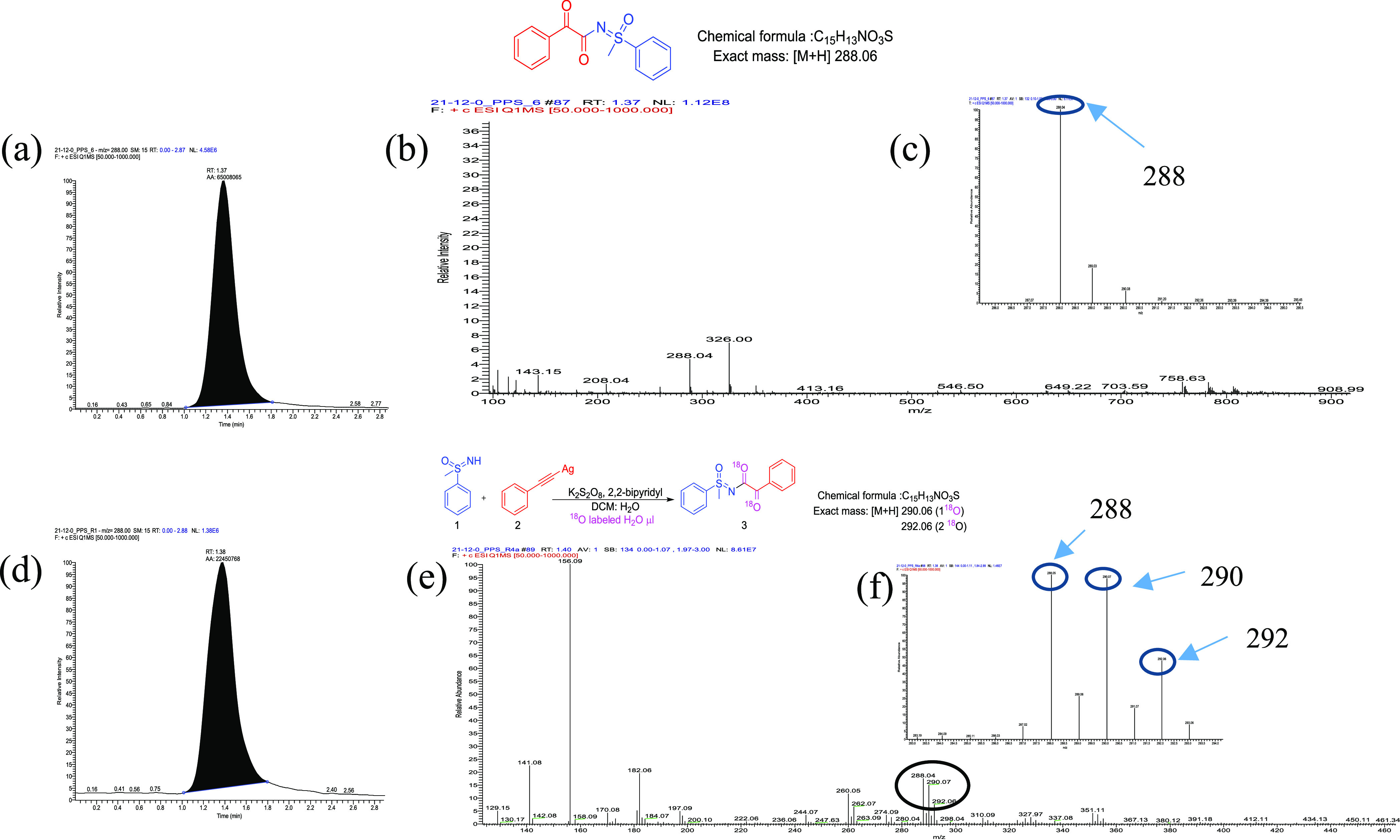
Labeling experiment (a) LC–MS chromatogram of the reaction mixture with DCM and normal water; (b) MS of the reaction mixture for 3a; (c) expanded MS of 3a; (d) LC–MS chromatogram of the reaction mixture with DCM and 18O labeled H2O; (e) MS of 18O labeled 3a; (f) expanded MS of 18O labeled 3a.
To get insight into the involvement of reaction intermediates, the reaction was also performed and examined through the LC–MS study. The aliquots were taken at different time intervals and analyzed under LC–MS. The LC–MS revealed one intermediate I4 (m/z = 290.25) (experimental details are given on Page nos. 16 and 17, Supporting Information).
Based on the controlled and labeling experiments as well as LC–MS study and literature reports,47−49 we proposed the plausible pathways for the synthesis of N-α-ketoacyl sulfoximines and N-α,β-unsaturated acyl sulfoximines, as shown in Figure 3. Initially, (phenylethynyl)silver reacted with sulfoximines, leading to the formation of intermediate I1 (confirmed by a controlled experiment, eq vii). The intermediate I1 gets attacked by water molecules and generates the intermediate I2. In the case of aryl acetylenes, the intermediate I2 undergoes rearrangement and provides the intermediate I3. The intermediate I3 underwent oxidation in the presence of silver, and the oxidant generates the N-α-hydroxyacyl sulfoximines I4, and subsequent oxidation affords N-α-ketoacyl sulfoximines 3. However, in the case of alkyl acetylenes, the intermediate I2 underwent deprotonation at the β-position and generated the allenyl intermediate I5 followed by the rearrangement afford the N-α,β-unsaturated acyl sulfoximines 4.
Figure 3.
Plausible reaction mechanism for the formation of N-α-ketoacyl sulfoximines and N-α,β-unsaturated acyl sulfoximines.
Conclusions
In conclusion, a novel synthetic methodology has been developed for C–N bond formation and for the synthesis of N-α-ketoacyl sulfoximines and N-α,β-unsaturated acyl sulfoximines. The reaction worked well at room temperature with the use of mild reaction conditions and inexpensive silver catalysts. Further, the labeling experiment suggested that water plays a major role as a source of oxygen in the acylated product.
Experimental Section
General Information
The reactions were carried out at room temperature under an open atmosphere and detected with the help of thin-layer chromatography (60 F254, 20 × 20 cm). Rotavapor was used for the concentration of the solvents as well as for drying the compounds. The column chromatography was done using 230–400 mesh silica gel to purify the compounds. The 400 and 101 MHz spectrometers were used to record the 1H NMR and 13C NMR spectra of all the compounds. The units for chemical data for protons are given in parts per million (ppm, scale) relative to TMS. The coupling constant (J) is in Hz. Also, the LC–MS study and mass spectra were obtained by using a Q-TOF-LC/MS spectrometer using electron spray ionization.
General Procedure for the Synthesis of N-α-Ketoacyl Sulfoximines (Table 1 and Scheme 1)
The reactants phenylethynyl silver 2 (1.5 equiv) and 2,2-bipyridyl (2 equiv) were dissolved in 12 mL of DCM/H2O in a 50 mL round bottom flask and stirred for about 5 min at room temperature. Then potassium persulfate and sulfoximine 1 were added to the above-resulting mixture. This solution was then kept at room temperature for 4–8 h with thorough stirring. The development of the reaction was examined with the help of thin-layer chromatography. As soon as the reaction was completed, the workup was done by transferring the whole reaction mixture to a separating funnel and then extracting it with a dilute solution of hydrochloric acid and dichloromethane. Sodium sulfate was added to the organic layer so as to absorb water molecules if present. The organic layer was then dried using a rotavapor. The crude material obtained was then purified with the help of column chromatography to give the purified compounds, N-α-ketoacyl sulfoximines 3a–3x in a yield of 47–63%. The elution system was 30% ethyl acetate/hexane. All the synthesized compounds were then characterized with the help of 1H NMR, 13C NMR, and HR MS spectra.
General Procedure for the Coupling of Sulfoximines with Alkynylsilver (Schemes 2 and 3)
Here, alkynyl silver 2 (1.5 equiv) and 2,2-bipyridyl (2 equiv) were mixed in a 100 mL round bottom flask, and both were dissolved in DCM/H2O in a 3:1 ratio. The resulting solution was allowed to stir continuously for about 5–10 min. After 10 min, potassium persulfate (3 equiv) and sulfoximine 1 (100 mg) were added to the abovementioned reaction mixture. This solution was then kept at room temperature for 4–8 h with thorough stirring. The development of the reaction was examined with the help of thin-layer chromatography. As soon as the reaction was completed, the workup was done in the separating funnel. To the reaction mixture was added a dilute solution of hydrochloric acid and extracted with dichloromethane. Sodium sulfate was added to the organic layer so as to absorb water molecules if present. The organic layer was then dried using a rotavapor. The crude material obtained was then purified with the help of column chromatography using ethyl acetate/hexane as eluent to give the purified compounds, 4a–4b and 5 in good yields (43–51%). The synthesized compounds were then characterized with the help of 1H NMR, 13C NMR, and HR MS spectra.
General Procedure for the Coupling of Sulfoximines with In-Situ-Generated Phenyl Acetylene Silver (Scheme 4)
In this case, phenylacetylene 6 (1.5 equiv), silver nitrate (0.8 equiv), and 2,2-bipyridyl (2 equiv) were taken in a 50 mL round bottom flask and dissolved in DCM/H2O in a 3:1 ratio. The resulting solution was kept at room temperature and stirred continuously for 4 h. After this, potassium persulfate (3 equiv) was added to the abovementioned mixture, and the reaction mixture was allowed to stir thoroughly at room temperature for about 1 h. Finally, sulfoximine 1 (100 mg) was added to the abovementioned mixture. This final solution was then kept at room temperature for 4–8 h with thorough stirring. The development of the reaction was examined with the help of thin-layer chromatography. As soon as the reaction was completed, the workup was done in the separating funnel. To the reaction mixture was added a dilute solution of hydrochloric acid and extracted with dichloromethane. Sodium sulfate was added to the organic layer so as to absorb water molecules if present. The organic layer was then dried using a rotavapor. The crude material obtained was then purified with the help of column chromatography by using ethyl acetate/hexane as eluent to give the purified compound N-α-ketoacylsulfoximine 3a in a yield of 47%. The synthesized compound was then characterized with the help of 1H NMR, 13C NMR, and HR MS spectra.
Acknowledgments
R.G. is thankful to DST-INSPIRE for the fellowship. R.A. is thankful to UGC for the grant of funds. Z.A. is thankful to the CSIR for the fellowship. M.K. is thankful to the CSIR IIIM for the fellowship. Also, we are thankful to the CSIR-IIIM Jammu (MLP21003) and SERB-DST (GAP2181) for the financial support. This article bears the Institutional Publication No. CSIR-IIIM/IPR/00440.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c05894.
General procedures, details of controlled experiments, isotope labeling experiments, LC–MS experiments, and spectral data (NMR and mass spectra) of all compounds (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Craig D.; Grellepois F.; White A. J. Asymmetric decarboxylative Claisen rearrangement reactions of sulfoximine-substituted allylic tosylacetic esters. J. Org. Chem. 2005, 70, 6827–6832. 10.1021/jo050747d. [DOI] [PubMed] [Google Scholar]
- Moessner C.; Bolm C. Cu(OAc)2-Catalyzed N-Arylations of Sulfoximines with Aryl Boronic Acids. Org. Lett. 2005, 7, 2667–2669. 10.1021/ol050816a. [DOI] [PubMed] [Google Scholar]
- Gais H.-J.; Babu G. S.; Günter M.; Das P. Asymmetric Synthesis of Cycloalkenyl and Alkenyloxiranes From Allylic Sulfoximines and Aldehydes and Application to Solid-Phase Synthesis. Eur. J. Org. Chem. 2004, 1464–1473. 10.1002/ejoc.200300726. [DOI] [Google Scholar]
- Harmata M.; Hong X. The Intramolecular, Stereoselective Addition of Sulfoximine Carbanions to α,β-Unsaturated Esters. J. Am. Chem. Soc. 2003, 125, 5754–5756. 10.1021/ja034744z. [DOI] [PubMed] [Google Scholar]
- Koep S.; Gais H.-J.; Raabe G. Asymmetric synthesis of unsaturated, fused bicyclic proline analogues through amino alkylation of cyclic bis(allylsulfoximine)titanium complexes and migratory cyclization of delta-amino alkenyl aminosulfoxonium salts. J. Am. Chem. Soc. 2003, 125, 13243–13251. 10.1021/ja030324y. [DOI] [PubMed] [Google Scholar]
- Reddy L. R.; Gais H.-J.; Woo C.-W.; Raabe G. Asymmetric synthesis of anti-homopropargylic alcohols from aldehydes and chiral sulfonimidoyl substituted bis(allyl)titanium complexes through generation and elimination of novel chiral alkylidenecarbene (dimethylamino)sulfoxonium ylides. J. Am. Chem. Soc. 2002, 124, 10427–10434. 10.1021/ja020570u. [DOI] [PubMed] [Google Scholar]
- Senthil Kumar P.; Bharatam P. V. Theoretical studies on the S–N interactions in sulfoximine. Tetrahedron 2005, 61, 5633–5639. 10.1016/j.tet.2005.03.070. [DOI] [Google Scholar]
- Hackenberger C. P. R.; Raabe G.; Bolm C. Synthetic and Spectroscopic Investigation of N-Acylated Sulfoximines. Chem.—Eur. J. 2004, 10, 2942–2952. 10.1002/chem.200306016. [DOI] [PubMed] [Google Scholar]
- Bolm C.; Müller D.; Dalhoff C.; Hackenberger C. P. R.; Weinhold E. The stability of pseudopeptides bearing sulfoximines as chiral backbone modifying element towards proteinase K. Bioorg. Med. Chem. Lett. 2003, 13, 3207–3211. 10.1016/S0960-894X(03)00697-8. [DOI] [PubMed] [Google Scholar]
- Bolm C.; Müller D.; Hackenberger C. P. R. Turn formation initiated by a bissulfoximine motif: synthesis and structural investigation. Org. Lett. 2002, 4, 893–896. 10.1021/ol017188r. [DOI] [PubMed] [Google Scholar]
- Bolm C.; Moll G.; Kahmann J. D. Synthesis of Pseudopeptides with Sulfoximines as Chiral Backbone Modifying Elements. Chem.—Eur. J. 2001, 7, 1118–1128. . [DOI] [PubMed] [Google Scholar]
- Mock W. L.; Tsay J. T. Sulfoximine and sulfodiimine transition state analog inhibitors for carboxypeptidase A. J. Am. Chem. Soc. 1989, 111, 4467–4472. 10.1021/ja00194a049. [DOI] [Google Scholar]
- Lücking U. A Neglected Opportunity in Medicinal Chemistry. Angew. Chem., Int. Ed. 2013, 52, 9399–9408. 10.1002/anie.201302209. [DOI] [PubMed] [Google Scholar]
- Griffith O. W.; Meister A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J. Biol. Chem. 1979, 254, 7558–7560. 10.1016/S0021-9258(18)35980-5. [DOI] [PubMed] [Google Scholar]
- Pandya V.; Jain M.; Chakrabarti G.; Soni H.; Parmar B.; Chaugule B.; Patel J.; Jarag T.; Joshi J.; Joshi N.; et al. Synthesis and structure–activity relationship of potent, selective and orally active anthranilamide-based factor Xa inhibitors: Application of weakly basic sulfoximine group as novel S4 binding element. Eur. J. Med. Chem. 2012, 58, 136–152. 10.1016/j.ejmech.2012.10.005. [DOI] [PubMed] [Google Scholar]
- Walker D. P.; Zawistoski M. P.; McGlynn M. A.; Li J.-C.; Kung D. W.; Bonnette P. C.; Baumann A.; Buckbinder L.; Houser J. A.; Boer J.; et al. Sulfoximine-substituted trifluoromethylpyrimidine analogs as inhibitors of proline-rich tyrosine kinase 2 (PYK2) show reduced hERG activity. Bioorg. Med. Chem. Lett. 2009, 19, 3253–3258. 10.1016/j.bmcl.2009.04.093. [DOI] [PubMed] [Google Scholar]
- Park S. J.; Buschmann H.; Bolm C. Bioactive sulfoximines: Syntheses and properties of Vioxx® analogs. Bioorg. Med. Chem. Lett. 2011, 21, 4888–4890. 10.1016/j.bmcl.2011.06.029. [DOI] [PubMed] [Google Scholar]
- Park S. J.; Baars H.; Mersmann S.; Buschmann H.; Baron J. M.; Amann P. M.; Czaja K.; Hollert H.; Bluhm K.; Redelstein R.; et al. N-Cyano Sulfoximines: COX Inhibition, Anticancer Activity, Cellular Toxicity, and Mutagenicity. ChemMedChem 2013, 8, 217–220. 10.1002/cmdc.201200403. [DOI] [PubMed] [Google Scholar]
- Dillard R. D.; Yen T. T.; Stark P.; Pavey D. E. Synthesis and blood pressure lowering activity of 3-(substituted-amino)-1,2,4-benzothiadiazine 1-oxide derivatives. J. Med. Chem. 1980, 23, 717–722. 10.1021/jm00181a003. [DOI] [PubMed] [Google Scholar]
- Kahraman M.; Sinishtaj S.; Dolan P. M.; Kensler T. W.; Peleg S.; Saha U.; Chuang S. S.; Bernstein G.; Korczak B.; Posner G. H. Potent, Selective and Low-Calcemic Inhibitors of CYP24 Hydroxylase: 24-Sulfoximine Analogues of the Hormone 1α,25-Dihydroxyvitamin D3. J. Med. Chem. 2004, 47, 6854–6863. 10.1021/jm040129+. [DOI] [PubMed] [Google Scholar]
- Lu D.; Sham Y. Y.; Vince R. Design, asymmetric synthesis, and evaluation of pseudosymmetric sulfoximine inhibitors against HIV-1 protease. Bioorg. Med. Chem. 2010, 18, 2037–2048. 10.1016/j.bmc.2010.01.020. [DOI] [PubMed] [Google Scholar]
- Lu D.; Vince R. Discovery of potent HIV-1 protease inhibitors incorporating sulfoximine functionality. Bioorg. Med. Chem. Lett. 2007, 17, 5614–5619. 10.1016/j.bmcl.2007.07.095. [DOI] [PubMed] [Google Scholar]
- Gutierrez J. A.; Pan Y.-X.; Koroniak L.; Hiratake J.; Kilberg M. S.; Richards N. G. J. An Inhibitor of Human Asparagine Synthetase Suppresses Proliferation of an L-Asparaginase-Resistant Leukemia Cell Line. Chem. Biol. 2006, 13, 1339–1347. 10.1016/j.chembiol.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aithagani S. K.; Dara S.; Munagala G.; Aruri H.; Yadav M.; Sharma S.; Vishwakarma R. A.; Singh P. P. Metal-Free Approach for the Synthesis of N-Aryl Sulfoximines via Aryne Intermediate. Org. Lett. 2015, 17, 5547–5549. 10.1021/acs.orglett.5b02804. [DOI] [PubMed] [Google Scholar]
- Aithagani S. K.; Kumar M.; Yadav M.; Vishwakarma R. A.; Singh P. P. Metal-Free, Phosphonium Salt-Mediated Sulfoximination of Azine N-Oxides: Approach for the Synthesis of N-Azine Sulfoximines. J. Org. Chem. 2016, 81, 5886–5894. 10.1021/acs.joc.6b00593. [DOI] [PubMed] [Google Scholar]
- Pirwerdjan R.; Becker P.; Bolm C. Exploring the Reactivity of N-Alkynylated Sulfoximines: Regioselective Hydroacyloxylations and Hydroaminations. Org. Lett. 2015, 17, 5008–5011. 10.1021/acs.orglett.5b02477. [DOI] [PubMed] [Google Scholar]
- Chen X. Y.; Wang L.; Frings M.; Bolm C. Copper-Catalyzed N-Alkynylations of Sulfoximines with Bromoacetylenes. Org. Lett. 2014, 16, 3796–3799. 10.1021/ol5016898. [DOI] [PubMed] [Google Scholar]
- Sai K. K. S.; Esteves P. M.; da Penha E. T.; Klumpp D. A. Superacid-Promoted Reactions of α-Ketoamides and Related Systems. J. Org. Chem. 2008, 73, 6506–6512. 10.1021/jo801208m. [DOI] [PubMed] [Google Scholar]
- Wang L.; Priebbenow D. L.; Zou L.-H.; Bolm C. The Copper-Catalyzed OxidativeN-Acylation of Sulfoximines. Adv. Synth. Catal. 2013, 355, 1490–1494. 10.1002/adsc.201300273. [DOI] [Google Scholar]
- Zou Y.; Xiao J.; Peng Z.; Dong W.; An D. Transition metal-free aroylation of NH-sulfoximines with methyl arenes. Chem. Commun. 2015, 51, 14889–14892. 10.1039/C5CC05483D. [DOI] [PubMed] [Google Scholar]
- Zou Y.; Peng Z.; Dong W.; An D. CuI-Mediated α-Ketoacylation of Sulfoximines under Solvent-Free Conditions. Eur. J. Org. Chem. 2015, 4913. 10.1002/ejoc.201500410. [DOI] [Google Scholar]
- Cheng H.; Bolm C. Copper-Catalyzed Oxidative#±-Ketoacylations of Sulfoximines with Aryl Methyl Ketones and Dioxygen as Terminal Oxidant. Synlett 2016, 27, 769–772. 10.1055/s-0035-1560989. [DOI] [Google Scholar]
- Wang C.; Ma D.; Tu Y.; Bolm C. Use of Hypervalent Iodine Reagents in Visible Light-Promoted α-Ketoacylations of Sulfoximines with Aryl Alkynes. Org. Lett. 2020, 22, 8937–8940. 10.1021/acs.orglett.0c03338. [DOI] [PubMed] [Google Scholar]
- Baranwal S.; Gupta S.; Kandasamy J. Selenium Dioxide Promoted α-Keto N-Acylation of Sulfoximines Under Mild Reaction Conditions. Asian J. Org. Chem. 2021, 10, 1835–1845. 10.1002/ajoc.202100298. [DOI] [Google Scholar]
- a Yoo K.; Jwa D. G.; Lee H.-E.; Kim H. J.; Kim C.; Kim M. Recent Organic Transformations with Silver Carbonate as a Key External Base and Oxidant. Catalysts 2019, 9, 1032. 10.3390/catal9121032. [DOI] [Google Scholar]; b Li C.-J.; Bi X.. Silver Catalysis in Organic Synthesis; Wiley, 2019. [Google Scholar]; c Abbiati G.; Rossi E. Silver and gold-catalyzed multicomponent reactions. Beilstein J. Org. Chem. 2014, 10, 481–513. 10.3762/bjoc.10.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manikandan T. S.; Ramesh R.; Semeril D. Synthesis and characterisation of cycloruthenated benzhydrazone complexes: catalytic application to selective oxidative cleavage of olefins to aldehydes. RSC Adv. 2016, 6, 97107–97115. 10.1039/C6RA19044H. [DOI] [Google Scholar]
- Mi C.; Li L.; Meng X.-G.; Yang R.-Q.; Liao X.-H. Highly selective oxidation of unsaturated hydrocarbons to carbonyl compounds by two-phase catalysis. Tetrahedron 2016, 72, 6705–6710. 10.1016/j.tet.2016.09.003. [DOI] [Google Scholar]
- Muthumari S.; Ramesh R. Synthesis and Structure of Ru(II) Complexes of Thiosemicarbazone: Highly Selective Catalysts for Oxidative Scission of Olefins to Aldehydes. ChemistrySelect 2018, 3, 3036–3041. 10.1002/slct.201800163. [DOI] [Google Scholar]
- Gómez-Herrera A.; Hashim I. I.; Porré M.; Nahra F.; Cazin C. S. J. Au(I)-Catalyzed Hydration of 1-Iodoalkynes Leading to α-Iodoketones. Eur. J. Org. Chem. 2020, 6790–6794. 10.1002/ejoc.202001238. [DOI] [Google Scholar]
- Mckillop A.; Mills L. S. Phase-Transfer Catalysed Permanganate Oxidations Using Tris[2-(2-Methoxyethoxy)Ethyl]Amine (Tda-1). Synth. Commun. 1987, 17, 647–655. 10.1080/00397918708075738. [DOI] [Google Scholar]
- Zhu Z.; Espenson J. H. Oxidation of Alkynes by Hydrogen Peroxide Catalyzed by Methylrhenium Trioxide. J. Org. Chem. 1995, 60, 7728–7732. 10.1021/jo00129a010. [DOI] [Google Scholar]
- Sultan S.; Rizvi M. A.; Kumar J.; Shah B. A. Acyl Radicals from Terminal Alkynes: Photoredox-Catalyzed Acylation of Heteroarenes. Chem.—Eur. J. 2018, 24, 10617–10620. 10.1002/chem.201801628. [DOI] [PubMed] [Google Scholar]
- Zeller K.-P.; Kowallik M.; Haiss P. The dimethyldioxirane-mediated oxidation of phenylethyne. Org. Biomol. Chem. 2005, 3, 2310–2318. 10.1039/B504296H. [DOI] [PubMed] [Google Scholar]
- Xie Y.; Wang J.; Wang Y.; Han S.; Yu H. Hydration of Alkynes to Ketones with an Efficient and Practical Polyoxomolybdate-based Cobalt Catalyst. ChemCatChem 2021, 13, 4985–4989. 10.1002/cctc.202101180. [DOI] [Google Scholar]
- Lai J.-W.; Liu Z.-Y.; Chen X.-Y.; Zhang H.; Liu H.-Y. Hydration of terminal alkynes catalyzed by cobalt corrole complex. Tetrahedron Lett. 2020, 61, 152426. 10.1016/j.tetlet.2020.152426. [DOI] [Google Scholar]
- Zheng B.; Jin X.; Liu J.; Cheng H. Accelerated Metal-Free Hydration of Alkynes within Milliseconds in Microdroplets. ACS Sustainable Chem. Eng. 2021, 9, 4383–4390. 10.1021/acssuschemeng.1c00887. [DOI] [Google Scholar]
- Wang S.; Kaltashov I. A. An 18O-labeling assisted LC/MS method for assignment of aspartyl/isoaspartyl products from Asn deamidation and Asp isomerization in proteins. Anal. Chem. 2013, 85, 6446–6452. 10.1021/ac400984r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M.; Ahmed R.; Singh M.; Sharma S.; Thatikonda T.; Singh P. P. Functionalization of Alkynes and Alkenes Using a Cascade Reaction Approach: Synthesis of β-Keto Sulfones under Metal-free Conditions. J. Org. Chem. 2020, 85, 716–725. 10.1021/acs.joc.9b02779. [DOI] [PubMed] [Google Scholar]
- Sharma S.; Kumar M.; Vishwakarma R. A.; Verma M. K.; Singh P. P. Room Temperature Metal-Catalyzed Oxidative Acylation of Electron-Deficient Heteroarenes with Alkynes, Its Mechanism, and Application Studies. J. Org. Chem. 2018, 83, 12420–12431. 10.1021/acs.joc.8b01475. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.