Abstract

Candida auris is an opportunistic fungal pathogen and an emerging global public health threat, given its high mortality among infected individuals, antifungal resistance, and persistence in healthcare environments. This study explored the applicability of wastewater surveillance for C. auris in a metropolitan area with reported outbreaks across multiple healthcare facilities. Influent or primary effluent samples were collected over 10 weeks from seven sewersheds in Southern Nevada. Pelleted solids were analyzed using an adapted quantitative polymerase chain reaction (qPCR) assay targeting the ITS2 region of the C. auris genome. Positive detection was observed in 72 of 91 samples (79%), with higher detection frequencies in sewersheds serving healthcare facilities involved in the outbreak (94 vs 20% sample positivity). Influent wastewater concentrations ranged from 2.8 to 5.7 log10 gene copies per liter (gc/L), and primary clarification achieved an average log reduction value (LRV) of 1.24 ± 0.34. Presumptive negative surface water and wastewater controls were non-detect. These results demonstrate that wastewater surveillance may assist in tracking the spread of C. auris and serve as an early warning tool for public health action. These findings provide the foundation for future application of wastewater-based epidemiology (WBE) to community- or facility-level surveillance of C. auris and other high consequence, healthcare-associated infectious agents.
Keywords: Candida auris, wastewater-based epidemiology (WBE), healthcare-associated infections (HAIs), antifungal resistance, public health surveillance
Short abstract
This study reports the novel detection of C. auris through community-level wastewater surveillance, suggesting the potential for future application of wastewater-based epidemiology to public health surveillance of this high-priority, healthcare-associated fungal pathogen.
1. Introduction
Candida auris is an opportunistic fungal pathogen capable of causing severe systemic infections and even death, particularly for patients in long-term healthcare settings.1 First identified in 2009, C. auris has now been documented in over 40 countries2,3 and has been designated a priority fungal pathogen of public health importance by the World Health Organization (WHO) as well as an urgent threat by the Centers for Disease Control and Prevention (CDC) in the United States (U.S.), given its increasing incidence and persistence in healthcare settings.4,5 The first case of C. auris infection in the U.S. was reported in New York in 2013,6 and it has since spread to over 25 states, with cases increasing from 63 between 2013 and 2016 to over 1400 in 2021 alone (C. auris became nationally notifiable in 2018). In total, 4782 clinical cases and 11,449 screening cases have been documented in the U.S. to date (as of December 7, 2022).7
Like other Candida species, this fungal pathogen has been detected on multiple sites of the human body, including skin, wounds, blood, and sputum, as well as in urine, stool, vaginal, and rectal swab samples.1,3,8,9 Individuals can be colonized without experiencing symptoms for >24 months following exposure to C. auris,3 and unlike other Candida species, patients with comorbidities or immunocompromising conditions, a history of antifungal exposure, and/or invasive medical devices in long-term healthcare settings (e.g., endotracheal tube, central line, catheter) are at a higher risk of developing serious localized and/or systemic infections.10 Treatment options are limited for C. auris(11) and are becoming increasingly so with the emergence and spread of echinocandin- and pan-resistant strains.12−14 Mortality rates for clinical C. auris cases vary widely, ranging from 20% to nearly 70%,3 and outbreak response and infection control activities (e.g., screening, personal protective equipment, cleaning, staff time) can be costly and resource-intensive.15
Further complicating infection prevention and control efforts, C. auris is notably hardy on surfaces and highly transmissible within healthcare settings.2,4 Sustained growth on dry and moist surfaces has been demonstrated in both controlled laboratory and clinical environments.16 In healthcare facilities with reported C. auris outbreaks, experts have isolated the fungus from several surfaces, including furniture, medical and cleaning equipment, floors, door handles, sinks, sink drains, and even on personal items such as cloth lanyards.17−19C. auris is resistant to many commonly used disinfectants, requiring use of designated antimicrobial products.20
Public health surveillance for colonization cases of C. auris occurs within high-risk healthcare settings like acute-care hospitals, long-term acute-care hospitals (LTACHs), and skilled nursing facilities (SNFs). Typical screening surveillance activities might include point prevalence surveys in contained areas throughout the entire facility,1 testing contacts of cases, and patient screenings prior to facility admission.12 However, conventional approaches to C. auris surveillance underestimate its prevalence,21−23 so the true prevalence of C. auris colonization in the general population is unknown.24
Complementary approaches to traditional surveillance may assist in tracking and better estimating the overall burden of pathogens like C. auris. One such approach is wastewater surveillance, which has gained renewed interest due to the COVID-19 pandemic. Wastewater viral loads of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have been used to estimate COVID-19 clinical cases25 and overall incidence26,27 with the promise of serving as an early detection system.28 Accordingly, there have been calls for the expanded application of wastewater surveillance and wastewater-based epidemiology (WBE) to other pathogens, including C. auris.29 While Candida species (not including C. auris) have been detected previously in hospital wastewater monitored for antifungal susceptibility patterns,13 no peer-reviewed manuscripts have yet documented C. auris in community wastewater or further explored the potential of WBE to inform public health action on this pathogen.
Wastewater surveillance for SARS-CoV-2 has been ongoing since March of 2020 in Southern Nevada, a community that has also experienced a surge in C. auris cases across multiple healthcare facilities beginning in August of 2021.30 Given this ongoing outbreak and growing interest in C. auris as a candidate for WBE, this study set out to determine if C. auris could be detected in wastewater samples collected from wastewater treatment facilities in the Las Vegas metropolitan area. More broadly, the aim of this study was to explore the potential application of WBE to community surveillance of C. auris as a means of generating more comprehensive and accurate estimates of C. auris incidence and/or prevalence.
2. Materials and Methods
2.1. Community Wastewater Sample Collection
Since March 2020, wastewater samples have been routinely collected from local wastewater treatment facilities in Southern Nevada for wastewater surveillance of SARS-CoV-2 RNA. Sample collection, processing, and SARS-CoV-2 analysis have been described previously.26,31 In contrast to this SARS-CoV-2 monitoring approach, which relies on an analysis of the liquid (i.e., supernatant) fraction, C. auris monitoring for this study focused on pelleted solids from the same samples, as this relatively large organism (∼5 μm in length)32 is more likely to settle or adhere to solid particles. Routine wastewater surveillance of C. auris was initiated in late June 2022 and consisted of collecting, processing, and analyzing nine weekly samples from facilities across seven sewersheds (Table 1). Raw influent (Facilities 2–6) or primary effluent (Facility 1) was collected weekly from each treatment facility; raw influent was also collected from Facility 1 starting in mid-July 2022. Sample types (i.e., grab or composite samples) were dependent on logistical considerations but were generally consistent for each facility. It is important to note that Facility 4A receives all solids and some bypass flows from Facility 2, and Facility 4 is the 24 h composite for two distinct sewer trunk lines arriving from the west (Facility 4A) and east (Facility 4B).
Table 1. Summary of Wastewater Treatment Facilities/Sewersheds and Sample Characteristics.
| facility/sewershed | population served | flow rate (mgd) | sample type and source | sample collection volume (mL) | C. auris sampling time frame |
|---|---|---|---|---|---|
| 1 | 872,009 | 100 | grab influent | 150 | 7/11/2022–9/6/2022 |
| grab primary effluent | 10,000a | 6/27/2022–9/6/2022 | |||
| 2 | 86,330 | 5 | composite influent | 10,000a | 6/27/2022–9/6/2022 |
| 3 | 757,418 | 42 | composite/grab influentd | 150 | 7/5/2022–9/6/2022 |
| 4b,c | N/Ab | N/Ab | composite influent | 150 | 7/5/2022–9/6/2022 |
| 4Ab,c | 133,977 | 15 | grab influent | 150 | 7/5/2022–9/6/2022 |
| 4Bb | 114,532 | 6 | grab influent | 150 | 7/5/2022–9/6/2022 |
| 5 | 255,008 | 20 | composite influent | 150 | 7/5/2022–9/6/2022 |
| 6 | 16,399 | 0.8 | grab influent | 150 | 7/5/2022–9/6/2022 |
10,000 mL samples were processed using hollow fiber ultrafiltration (HFUF) prior to pelleting by centrifugation.
Facility 4 is the 24-hour composite of Facility 4A (west trunk line) and Facility 4B (east trunk line).
Facility 4A (and by default Facility 4) receives solids and bypass flows from Facility 2.
Grab influent samples were collected on 8/15, 8/22, and 8/29 (otherwise composite).
2.2. Sample Concentration and Extraction
For grab primary effluent from Facility 1 and composite influent from Facility 2, solids were pelleted (∼3200g for 30 min) from approximately 100 mL of concentrate following hollow fiber ultrafiltration (HFUF) (REXEED-25S dialysis filters, Asahi Kasei Medical Co., Tokyo, Japan) of 10 L samples. HFUF sample processing details are described in Gerrity et al.31 Processing of 10 L samples and collection of primary effluent from Facility 1 were artifacts of the ongoing SARS-CoV-2 wastewater surveillance effort and were not necessarily required for C. auris monitoring. For influent from Facilities 1 and 3–6, 150 mL samples were directly pelleted by centrifugation at ∼3200g for 30 min. Immediately after centrifugation, the supernatant was processed for SARS-CoV-2 wastewater surveillance, during which time the initial pellet typically loosened. To maximize recovery, the initial pellets were resuspended in up to 50 mL of the supernatant (i.e., remaining sample after SARS-CoV-2 processing), transferred to 50 mL conical tubes, and re-centrifuged at ∼3200g for 10 min. The final supernatant was carefully decanted, and a sterile plastic spatula was used to transfer 0.2–0.5 g of each pellet into a DNeasy PowerSoil Pro Kit PowerBead tube (QIAGEN, Germantown, MD). Pellets in PowerBead tubes were stored overnight at 4 °C, and then nucleic acid extraction was performed according to the manufacturer’s instructions. A negative control consisting of Milli-Q nanopure water was included in each extraction. DNA extracts were stored at 4 °C until qPCR analysis was performed the same day.
2.3. Molecular Assay
In the absence of an established quantitative polymerase chain reaction (qPCR) assay for the detection and quantification of C. auris in wastewater, the CDC assay typically used to identify C. auris in clinical specimens was adapted for this study. Originally designed by Leach et al.,33 this highly specific assay targets the C. auris internal transcribed spacer 2 (ITS2) gene (GenBank: ON385998), has no cross-reactivity with closely related pathogens, and shows positive results for all known phylogenetic clades of C. auris.
Reactions were run in triplicate on a CFX384 Touch Real-Time PCR Detection System (BIO-RAD Laboratories, Inc., Hercules, CA). Each reaction contained 2.5 μL of template DNA, 1× iTaq Universal Probes Supermix (BIO-RAD Laboratories, Inc., Hercules, CA), 0.50 μM final concentrations of each primer (Forward: 5′-CAG ACG TGA ATC ATC GAA TCT - 3′ and Reverse: 5′-TTT CGT GCA AGC TGT AAT TT-3′), 0.1 μM of probe (Probe: 5′-FAM-AAT CTT CGC GGT GGC GTT GCA TTC A–BHQ_1-3′), and nanopure water to reach a total reaction volume of 10 μL. The primers, probe, and gBlock Gene Fragment standard were purchased from Integrated DNA Technologies (IDT, Skokie, IL). The standard was resuspended to 10 ng/μL following the manufacturer’s instructions and quantified using the Qubit dsDNA HS Assay kit. Then, a 108 gc/μL stock was made using the online DNA copy number calculator (Thermo Fisher). Each week, a fraction of the stock was serially diluted 10-fold to generate a standard curve, which was included in each qPCR run. A no-template control (NTC) was also included in each standard curve. Thermocycler conditions started with an initial denaturation step at 95 °C for 20 s, which was then followed by 45 cycles of denaturation at 95 °C for 3 s and annealing at 60 °C for 30 s. Additional details about the standard curve preparation and qPCR assay are included in Text S1.34
The standard curves generated for each qPCR run were visually inspected in the Bio-Rad CFX384 Maestro software. Visually identified outlier replicates were excluded from the standard curve, and sample copy numbers (i.e., starting quantities) were recalculated with the adjusted equation. The estimated starting quantities were averaged across any of the three replicates that amplified, and the resulting starting quantities were then adjusted for equivalent sample volume (ESV) to estimate the concentration (in log10 gene copies per liter) in the original wastewater sample. A “quantifiable detection” was defined as a sample with an average Cq from amplification of two or more replicates occurring earlier than the limit of quantification (LoQ: Cq = 33.03). A “nonquantifiable detection” was defined as two or more replicates amplifying but with an average Cq occurring later than the LoQ; a “singular detection” was defined as a single amplification, either above or below the LoQ; and a non-detect was defined as a sample with no qPCR amplification. Additional details related to the assay efficiencies and LoQ, sample-specific ESVs, and quality control are included in Text S1.
2.4. Positive and Presumptive Negative Controls
Additional wastewater samples were analyzed as positive (n = 3) or presumptive negative (n = 1) controls for assessment of the workflow. The Utah Public Health Laboratory provided four influent wastewater samples from a Utah sewershed with no known C. auris cases. Three samples were spiked with different concentrations (10 colony-forming units [CFU]/mL, 100 CFU/mL, and 1000 CFU/mL) of C. auris isolate #0383 from the CDC and U.S. Food and Drug Administration (FDA) Antimicrobial Resistance Isolate Bank. One influent wastewater sample remained unspiked (0 CFU/mL). The C. auris stock suspension of 1 × 106 cells per mL was prepared by measuring cell density on a spectrometer (80% transmittance at a wavelength of 530 nm). Serial dilutions of the stock suspension were used to inoculate 50 mL wastewater samples to yield the appropriate concentrations. Sample preparation, nucleic acid extraction, and qPCR were applied to these 50 mL samples in the same manner as the aforementioned 150 mL wastewater samples (Text S2).
Three additional samples from Southern Nevada were also included as presumptive negative environmental controls: one effluent-impacted surface water (Las Vegas Wash) and two untreated drinking waters (Lake Mead/Colorado River). The effluent-impacted surface water consists primarily of disinfected wastewater effluent from the treatment facilities included in this study; the untreated drinking waters are also influenced by these effluent discharges. Sample preparation, nucleic acid extraction, and qPCR were applied to these 20 L samples in the same manner as the aforementioned 10 L wastewater samples (Text S2).
2.5. Characteristics of Southern Nevada’s C. auris Outbreak
In mid-April 2022, Nevada’s Healthcare-Associated Infection Program, which is managed by the Division of Public and Behavioral Health (DBPH), announced an investigation of C. auris cases in acute-care hospitals, LTACHs, and SNFs. The earliest case of C. auris infection in Nevada was traced back to August 2021, and by June 2022, 300 infection and colonization cases had been reported for 22 healthcare facilities in the Las Vegas metropolitan area,30 a semi-urban area with a population of ∼2.3 million people.35
2.6. Overlaying Wastewater and Clinical Data
A map of qPCR results by sewershed was created using R Statistical Computing Software version 4.2.0 in RStudio36 using the ggplot2 package.37 Healthcare facility locations were layered on top of the sewershed catchment areas, which were defined using sewershed shapefiles provided by the Southern Nevada Water Authority and the City of Henderson (Nevada), along with the city limits for Facility 6. A list of facilities reporting C. auris cases during the ongoing outbreak was obtained from the Nevada Office of Public Health Investigations and Epidemiology (personal communication, August 19, 2022).
3. Results and Discussion
3.1. Wastewater Surveillance Data
C. auris was detected in at least one sample from all Southern Nevada wastewater treatment facility locations (and all sample types), as well as in the spiked influent wastewater from Utah. Overall, C. auris was detected in 79% of the Southern Nevada wastewater samples analyzed (72 of 91 total samples). Concentrations, in gene copies per liter (gc/L) of wastewater, varied in magnitude between facilities, but facility-specific results were generally consistent between weeks (Figure 1). Influent wastewater concentrations ranged from 2.8 to 5.7 log10 gc/L across all samples that were above the LoQ, indicating a relatively high concentration of individual yeast organisms, considering each C. auris cell carries ∼37 copies of the target gene of interest.38C. auris concentrations in the grab influent from Facility 1 were significantly higher than the corresponding grab primary effluent (p < 0.0001; t-test on log10-transformed concentrations), yielding an average log reduction value (LRV) for primary clarification of 1.24 ± 0.34. Facility 2 concentrations ranged from 2.8 to 4.7 log10 gc/L. Facilities 3 and 4 had a 100% detection rate with concentrations ranging from 4.1 to 5.1 and 4.1 to 5.6 log10 gc/L, respectively. Finally, Facilities 4B, 5, and 6 had very few quantifiable detections (1, 1, and 0 of 10 samples, respectively), although Facility 5 also had numerous nonquantifiable or singular detections.
Figure 1.
C. auris concentrations (log10 gene copies per liter) in Southern Nevada wastewater samples. Data are shown by wastewater treatment facility and sample type (for Facility 1), sampling date, and qPCR outcome. Solid circles represent quantifiable detections (≥2 qPCR reactions; >LoQ), open circles represent nonquantifiable detections (≥2 qPCR reactions; <LoQ), asterisks represent singular detections (1 qPCR reaction; <LoQ or >LoQ), and red crosses represent non-detects (0 qPCR reactions).
Given the different characteristics of the sewersheds, C. auris concentrations were also normalized by average daily flow rate and sewershed population, yielding a similar pattern of results (Text S3 and Figure S1). Population size was recognized as a potential determinant of the locations of hospitals and SNFs within the sewersheds (Text S3).
3.2. Controls
The spiked and unspiked wastewater controls as well as the presumptive negative environmental controls supported the validity of the qPCR assay. The spiked influent wastewater from Utah amplified consistently, whereas C. auris was non-detect in the unspiked wastewater from Utah and the environmental controls from Southern Nevada. Since C. auris isolate #0383 contains ∼37 copies of the target ITS2 gene per cell,38 the expected concentration by qPCR for wastewater spiked with 10, 100, and 1000 CFU/mL (4, 5, and 6 log10 CFU/L) would be 5.6, 6.6, and 7.6 log10 gc/L. The observed concentrations by qPCR were consistent with expectations at 6.0, 6.7, and 7.6 log10 gc/L, respectively. The slight differences between the expected and observed concentrations could be due to several competing factors, including experimental error, detection of DNA from nonculturable cells, clustering of C. auris in the pure culture and spiked wastewater, and DNA loss during concentration and extraction.
3.3. Wastewater-Based Epidemiology of C. auris in Southern Nevada
The Office of Public Health Investigations and Epidemiology provided a list of facilities with reported C. auris clinical and colonization cases, allowing for allocation of these facilities to specific sewersheds (Table 2).
Table 2. State-Licensed Healthcare Facilities and C. auris Outbreak Involvement in the Las Vegas Metropolitan Area by Wastewater Treatment Facility/Sewershed, August 2021–August 2022.
| number
of state-licensed healthcare facilities,
Las Vegas metropolitan areaa |
|||
|---|---|---|---|
| facility/sewershed | hospitalsb | skilled nursing facilities | number of hospitals or skilled nursing facilities with reported C. auris clinical or colonization cases |
| 1 | 17 | 12 | 7 |
| 2 | 4 | 2 | 2 |
| 3 | 13 | 17 | 11 |
| 4A | 2 | 3 | 1 |
| 4B | 0 | 1 | 0 |
| 5 | 2 | 2 | 1 |
| 6 | 1c | 2 | 0 |
| total | 39 | 39 | 22 |
Source: Nevada Department of Health and Human Services Division of Public and Behavioral Health Licensee Search (accessed October 5, 2022).
Includes acute-care and specialty hospitals.
Licensed as a rural hospital.
Figure 2 shows the locations of state-licensed healthcare facilities reporting C. auris clinical or colonization cases between August 2021 and August 2022, along with average C. auris wastewater concentrations by sewershed.
Figure 2.
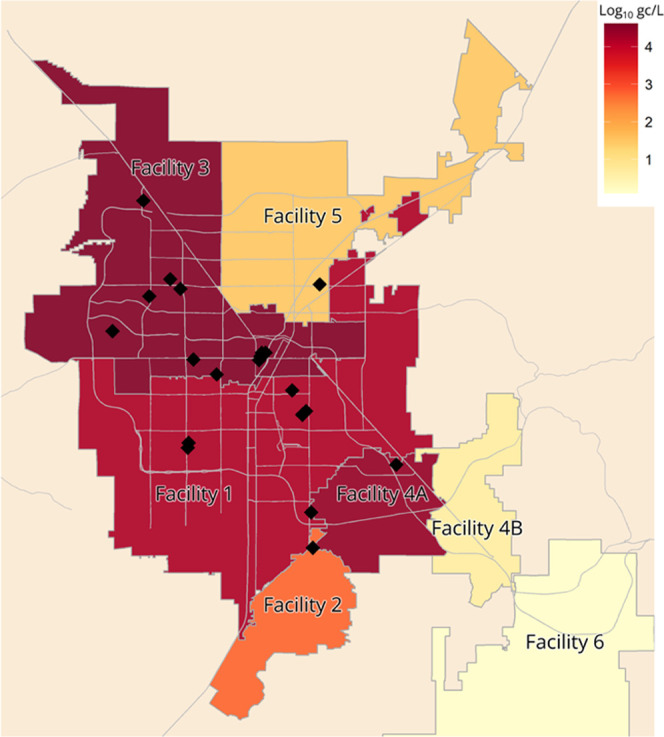
Map of Southern Nevada sewersheds in the context of C. auris wastewater surveillance. Sewershed coloring represents average influent wastewater concentration of C. auris (in log10 gc/L) from July through September of 2022. Average concentrations exclude the Facility 1 primary effluent and the Facility 4 composite influent. Recall that Facility 4A receives all solids and bypass flows from Facility 2. Black diamonds denote locations of state-licensed healthcare facilities (n = 22) reporting C. auris clinical or colonization cases between August 2021 and August 2022. For visualization purposes (i.e., relative comparisons), samples below the LoQ were set to 1 log10 gc/L and non-detects were set to 0 log10 gc/L. Note that some healthcare facility locations overlap, making it difficult to distinguish them on the map.
This 10-week analysis of influent wastewater from seven sewersheds in Southern Nevada demonstrates the ability to detect C. auris DNA in wastewater from a large, metropolitan area experiencing an outbreak. The fact that C. auris DNA was non-detect in the unspiked influent wastewater from Utah, an area with no known C. auris cases, also highlights the potential value of this monitoring approach in detecting the introduction of this antifungal-resistant pathogen into a community. If C. auris infections are suspected (e.g., after a novel wastewater detection) but clinically unconfirmed in the healthcare facilities contributing to the sewershed, this approach could be used in conjunction with increased sampling resolution (e.g., strategic manholes or building-level wastewater surveillance) to identify high-priority locations for individual screening or increased infection control education to prevent potential spread.
In this study, concentrations varied by wastewater treatment facility; however, since C. auris shedding in human waste (i.e., feces, urine, sputum, etc.) has not been characterized in human subjects, we cannot confidently correlate wastewater concentrations with exact C. auris case counts. Also, longer-term sampling would be needed to better understand and characterize potential trends in concentration. But importantly, for sewersheds serving facilities with confirmed C. auris cases (n = 5 sewersheds), 94% of the corresponding influent wastewater samples [47 of 50; excludes the redundant Facility 1 primary effluent samples (n = 11) and Facility 4 composite influent samples (n = 10)] showed amplification for the C. auris target in ≥1 qPCR reaction, and 82% of samples (41 of 50) showed amplification in ≥2 qPCR reactions (Table S3). The percentage of samples with ≥2 amplifications was also correlated with the proportion of healthcare facilities with confirmed cases (Pearson correlation coefficient r = 0.87; p-value = 0.01).
Furthermore, sample positivity (i.e., the percentage of samples with ≥1 amplification) differed significantly between sewersheds with and without C. auris-positive healthcare facilities (Kruskal–Wallis p-value = 0.04). Although Facility 4B and Facility 6 had no known C. auris cases in their respective sewersheds, their wastewater detections [20% of samples (4 of 20) with ≥1 amplification] do not necessarily represent false positives. Instead, unconfirmed infections and/or asymptomatically colonized individuals may have been shedding into the wastewater. While the extent of colonized patients’ shedding in urine and feces has not been characterized to date, potentially high shedding rates from colonized patients’ skin into the surrounding healthcare environment39 may be a contributing source.
3.4. Study Strengths
Renewed interest in WBE has fueled the exploration of new research questions around pathogens of public health importance. This study, performed within the context of the Southern Nevada C. auris outbreak, represents the first instance of C. auris detection by qPCR through community-scale wastewater surveillance. This 10-week study included multiple environmental water matrices (wastewater influent, primary effluent, effluent-impacted surface water, and untreated drinking water) through both grab and composite sampling. Concentrations varied by sewershed, but sewershed-specific detection frequencies displayed notable consistency over the 10 weeks of sampling and were correlated with the proportion of healthcare-associated facilities with confirmed C. auris cases. The study design allowed for C. auris surveillance of nearly the entire population of the Las Vegas metropolitan area, except for the small number of residents who use septic tanks40 and in outlying areas, during one of the largest recent outbreaks of healthcare-associated C. auris in the U.S.
This study also offers an effective procedure for sample processing, preparation, and qPCR analysis of pelleted wastewater solids for C. auris, adapted from the work of Leach et al.33 and the CDC assay41 for clinical samples. Solids appear to be preferred for this application, as a supplementary analysis of the post-HFUF supernatant from Facility 2 was non-detect for C. auris but positive for SARS-CoV-2, while the corresponding pelleted solids from Facility 2 were positive for C. auris (Cq = 30.65). Though the supernatants for non-HFUF samples were not screened for C. auris in this study, this supplementary finding demonstrates how wastewater samples can be partitioned and tested for various targets, including supernatant analysis for SARS-CoV-231 and poliovirus RNA42 and solids analysis for C. auris and monkeypox DNA.43,44 This approach yielded robust results over time and by wastewater treatment facility, and the qPCR assay reliably identified C. auris in positive controls and indicated its absence in negative controls. Our research suggests that primary settled sludge is also a viable option for monitoring of C. auris and could potentially be more convenient than our current method, although additional research is needed for confirmation.
To date, only a single study exists that has used wastewater surveillance to detect an outbreak of a healthcare-associated infection in a hospital setting. Acosta et al.45 used qPCR on wastewater samples collected from hospital drainage outflows to detect a hospital-acquired outbreak of SARS-CoV-2 and successfully distinguished the signal from community-acquired infections. However, the current study and its culture-based analogue46 are the first to use community-scale wastewater surveillance in the context of an ongoing outbreak of healthcare-associated C. auris infections. Additionally, unlike previous detections in environmental samples, the method described here is the first to quantify C. auris rather than just detecting its presence or absence via culture-based methods. Thus, in developing and disseminating this methodology, the field of WBE now expands to include monitoring healthcare-associated fungal pathogens through wastewater surveillance.
3.5. Study Limitations
There are several important considerations for interpreting our findings. Due to privacy issues, facility-level C. auris case data and reporting dates were not available for further analysis. A list of facilities experiencing outbreaks was the finest resolution of data we were able to obtain through public health partners, and as such, time-dependent correlations between concentrations and case counts could not be assessed. Also, C. auris shedding rates in human waste (i.e., feces, urine, sputum, skin, etc.) have not been established for either clinical or colonization cases, though one study has provided shedding estimates for C. auris in both feces (104 to 105 CFU/μL) and urine (102 CFU/μL) of intravenously infected neutropenic mice.47 Thus, the extent to which C. auris concentrations in wastewater relate to reported clinical and/or colonization cases in a given sewershed is not yet known. Clarifying human shedding rates to wastewater and the relationship between shedding and severity of infection is a critical next step in this area.
C. auris clinical cases are relatively rare in comparison to other pathogens studied in wastewater (e.g., SARS-CoV-2); only approximately 4800 clinical cases of C. auris have been reported in the U.S. between 2013 and 2022.7 However, traditional public health surveillance methods likely underestimate C. auris prevalence, given variable screening requirements and the potential for laboratory misidentification.21−23 Further, there may be colonized individuals in the community not detected through traditional surveillance, although transmission outside of healthcare settings is thought to be rare.48 This clearly highlights the potential value of wastewater surveillance in developing a more accurate estimate of C. auris prevalence throughout the U.S. and the world.
Given the ability of C. auris to form biofilms,49 which has already been documented in healthcare settings,50 environmental reservoirs such as premise plumbing or sewer collection systems could be a confounding factor when applying WBE principles. It is not clear whether this issue had any impact on the current study, but the environmental control samples suggest that C. auris is not ubiquitous in treated wastewater effluent, effluent-impacted surface water (e.g., Las Vegas Wash), or other source waters (e.g., Lake Mead and the Colorado River). While C. auris is known to be heat- and salt-resistant in cell culture,3 the persistence and genomic stability of C. auris in wastewater is still unknown. Further research is needed to understand the fate and transport of C. auris through the sewer system, as this information is critical for the potential use of C. auris as an early warning signal, a means of estimating incidence/prevalence, or a means of confirming clearance from a facility or community.
A key advantage of WBE is its ability to consider entire communities connected to a shared sewer collection system, but that feature also limits the resolution of the data and related conclusions. Specifically, community-scale wastewater surveillance may not be useful in identifying specific source(s) of a C. auris signal, particularly in sewersheds with large populations and numerous healthcare facilities. However, high-frequency building-level wastewater surveillance at healthcare facilities may not always be feasible due to resource limitations. Community-scale wastewater surveillance may help identify areas for higher resolution sampling (e.g., strategic manholes), building-level wastewater surveillance, and, ultimately, individual screening. At these fine spatial scales, the potential for generating personally or demographically identifiable data and a lack of informed consent raises ethical considerations. Kwiatkowska et al.51 and Coffman et al.52 have both explored these scenarios and suggest that for wastewater surveillance of small groups, careful consultation with stakeholders, public health professionals, and data subjects themselves should be undertaken. This consultation might involve convening a working group composed of decision-makers from healthcare, academia, and other relevant fields. This group could develop clear guidelines and protocols for data sharing that could help address liability and privacy concerns as well as elucidate the benefits and services provided by WBE.
3.6. Implications for Public Health Surveillance and Response
The results of this study highlight the potential to detect markers of emerging fungal pathogens in pooled wastewater samples at the community scale. In particular, developing a better understanding of C. auris is a priority for public health agencies in the U.S. and globally, given its recent emergence, resistance to antifungals, persistence in acute-care settings, and high risk of morbidity and mortality for affected patients.12 Routine monitoring of large sewersheds for C. auris may not yield actionable data at the building scale, but it does offer the ability to detect new introductions of the organism into a sewershed. In a community with no reported C. auris cases nor wastewater-based detections (e.g., Utah), a novel wastewater detection could prompt public health investigations into sources of C. auris shedding, health alert notifications, increased infection control reinforcements, and public health screening efforts. Where C. auris is already established (e.g., Nevada), routine wastewater monitoring can be implemented to track trends in concentration and ultimately assess changes in prevalence/incidence, as has been demonstrated for SARS-CoV-2. Estimating C. auris infections through WBE is not currently possible, given the lack of published waste-shedding data, but due to the high-priority nature of this pathogen, the scientific community may quickly fill this knowledge gap. Finally, if it is possible to implement high-resolution wastewater surveillance, the opportunity to conserve or more efficiently mobilize limited public health resources may justify the additional costs and logistical challenges.
Wastewater surveillance can also aid in characterizing the genomic diversity of target pathogens and identifying new variants or functionally important mutations. Whole genome sequencing (WGS) of clinical C. auris isolates suggests the nearly concurrent emergence of multiple geographically diverse clades since 2009,10 with clade V being identified more recently in 2018.53 In 2021, the Antibiotic Resistance Laboratory Network identified simultaneous, independent clusters of pan- and echinocandin-resistant C. auris exhibiting healthcare-associated transmission in the U.S.14 Genomic sequencing of wastewater isolates or development of amplicon-based WGS panels represent alternative means of characterizing circulating strains or detecting the emergence of new genetic variation, including genes that might confer antifungal resistance.
Interpretation of these findings to inform public health action will depend on the further elucidation of C. auris shedding rates into wastewater from both clinical and colonization cases. Currently, it is not clear how C. auris concentrations in wastewater reflect incidence or prevalence nor whether increased concentrations are sufficient to trigger public health responses. Persistent environmental sources of C. auris could also be explored, as it and other Candida species have been detected in sand and beach water environmental samples elsewhere.54−57 Given the recent and rapid emergence of C. auris, expanding antifungal resistance,10,14 and its hypothesized link to climate change,58 ongoing monitoring of this fungal pathogen will remain a public health priority for the foreseeable future.
Acknowledgments
The authors would like to acknowledge Lauryn Massic at the Nevada State Public Health Laboratory for contributing to the initiation of this study, Kimisha Causey and Chidinma Njoku in the Nevada Department of Health and Human Services for discussions related to the C. auris outbreak investigation, the collaborating wastewater agencies for their assistance with sampling logistics, and Mitchell Stoker and Kai Chung for providing the environmental surface water samples. The graphical abstract was created with BioRender.com.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.2c07763.
Additional qPCR assay, data interpretation, and quality control details; positive and negative control sample details; qPCR results normalized for flow and population (PDF)
Author Contributions
⊥ C.B. and K.C. contributed equally to this work. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
This work was partially supported by funding from the U.S. Centers for Disease Control and Prevention (CDC) and the Association of Public Health Laboratories (APHL) under Cooperative Agreement Numbers NH75OT000057-01-00 and NU60OE000104. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of CDC or the APHL. The authors declare no competing financial interest.
The authors declare no competing financial interest.
Supplementary Material
References
- Pacilli M.; Kerins J. L.; Clegg W. J.; Walblay K. A.; Adil H.; Kemble S. K.; Xydis S.; McPherson T. D.; Lin M. Y.; Hayden M. K.; Froilan M. C.; Soda E.; Tang A. S.; Valley A.; Forsberg K.; Gable P.; Moulton-Meissner H.; Sexton D. J.; Jacobs Slifka K. M.; Vallabhaneni S.; Walters M. S.; Black S. R. Regional Emergence of Candida auris in Chicago and Lessons Learned From Intensive Follow-up at 1 Ventilator-Capable Skilled Nursing Facility. Clin. Infect. Dis. 2020, 71, e718–e725. 10.1093/cid/ciaa435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vila T.; Sultan A. S.; Montelongo-Jauregui D.; Jabra-Rizk M. A. Candida auris: a fungus with identity crisis. Pathog. Dis. 2020, 78, ftaa034 10.1093/femspd/ftaa034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad S.; Alfouzan W. Candida auris: Epidemiology, Diagnosis, Pathogenesis, Antifungal Susceptibility, and Infection Control Measures to Combat the Spread of Infections in Healthcare Facilities. Microorganisms 2021, 9, 807 10.3390/microorganisms9040807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2019. https://www.cdc.gov/drugresistance/biggest-threats.html.
- World Health Organization. First meeting of the WHO antifungal expert group on identifying priority fungal pathogens: meeting report; Geneva, Switzerland, 2020; pp 1–13.
- Vallabhaneni S.; Kallen A.; Tsay S.; Chow N.; Welsh R.; Kerins J.; Kemble S. K.; Pacilli M.; Black S. R.; Landon E.; Ridgway J.; Palmore T. N.; Zelzany A.; Adams E. H.; Quinn M.; Chaturvedi S.; Greenko J.; Fernandez R.; Southwick K.; Furuya E. Y.; Calfee D. P.; Hamula C.; Patel G.; Barrett P.; Lafaro P.; Berkow E. L.; Moulton-Meissner H.; Noble-Wang J.; Fagan R. P.; Jackson B. R.; Lockhart S. R.; Litvintseva A. P.; Chiller T. M. Investigation of the First Seven Reported Cases of Candida auris, a Globally Emerging Invasive, Multidrug-Resistant Fungus-United States, May 2013-August 2016. MMWR. Morb. Mortal. Wkly. Rep. 2016, 65, 1234–1237. 10.15585/mmwr.mm6544e1. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Tracking Candida auris. https://www.cdc.gov/fungal/candida-auris/tracking-c-auris.html.
- Welsh R. M.; Bentz M. L.; Shams A.; Houston H.; Lyons A.; Rose L. J.; Litvintseva A. P. Survival, Persistence, and Isolation of the Emerging Multidrug-Resistant Pathogenic Yeast Candida auris on a Plastic Health Care Surface. J. Clin. Microbiol. 2017, 55, 2996–3005. 10.1128/JCM.00921-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg K.; Woodworth K.; Walters M.; Berkow E. L.; Jackson B.; Chiller T.; Vallabhaneni S. Candida auris: The recent emergence of a multidrug-resistant fungal pathogen. Med. Mycol. 2019, 57, 1–12. 10.1093/mmy/myy054. [DOI] [PubMed] [Google Scholar]
- Lockhart S. R.; Etienne K. A.; Vallabhaneni S.; Farooqi J.; Chowdhary A.; Govender N. P.; Colombo A. L.; Calvo B.; Cuomo C. A.; Desjardins C. A.; Berkow E. L.; Castanheira M.; Magobo R. E.; Jabeen K.; Asghar R. J.; Meis J. F.; Jackson B.; Chiller T.; Litvintseva A. P. Simultaneous Emergence of Multidrug-Resistant Candida auris on 3 Continents Confirmed by Whole-Genome Sequencing and Epidemiological Analyses. Clin. Infect. Dis. 2017, 64, 134–140. 10.1093/cid/ciw691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frías-De-León M. G.; Hernández-Castro R.; Vite-Garín T.; Arenas R.; Bonifaz A.; Castañón-Olivares L.; Acosta-Altamirano G.; Martínez-Herrera E. Antifungal Resistance in Candida auris: Molecular Determinants. Antibiotics 2020, 9, 568 10.3390/antibiotics9090568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. General Information about Candida auris. https://www.cdc.gov/fungal/candida-auris/candida-auris-qanda.html.
- Mataraci-Kara E.; Ataman M.; Yilmaz G.; Ozbek-Celik B. Evaluation of antifungal and disinfectant-resistant Candida species isolated from hospital wastewater. Arch. Microbiol. 2020, 202, 2543–2550. 10.1007/s00203-020-01975-z. [DOI] [PubMed] [Google Scholar]
- Lyman M.; Forsberg K.; Reuben J.; Dang T.; Free R.; Seagle E.; Sexton D. J.; Soda E.; Jones H.; Hawkins D.; Anderson A.; Bassett J.; Lockhart S. R.; Merengwa E.; Iyengar P.; Jackson B. R.; Chiller T. Notes from the Field: Transmission of Pan-Resistant and Echinocandin-Resistant Candida auris in Health Care Facilities ― Texas and the District of Columbia, January–April 2021. MMWR. Morb. Mortal. Wkly. Rep. 2021, 70, 1022–1023. 10.15585/mmwr.mm7029a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taori S. K.; Khonyongwa K.; Hayden I.; Athukorala G. I. D. D. A. D.; Letters A.; Fife A.; Desai N.; Borman A. M. Candida auris outbreak: Mortality, interventions and cost of sustaining control. J. Infect. 2019, 79, 601–611. 10.1016/j.jinf.2019.09.007. [DOI] [PubMed] [Google Scholar]
- Piedrahita C. T.; Cadnum J. L.; Jencson A. L.; Shaikh A. A.; Ghannoum M. A.; Donskey C. J. Environmental Surfaces in Healthcare Facilities are a Potential Source for Transmission of Candida auris and Other Candida Species. Infect. Control Hosp. Epidemiol. 2017, 38, 1107–1109. 10.1017/ice.2017.127. [DOI] [PubMed] [Google Scholar]
- Escandón P.; Chow N. A.; Caceres D. H.; Gade L.; Berkow E. L.; Armstrong P.; Rivera S.; Misas E.; Duarte C.; Moulton-Meissner H.; Welsh R. M.; Parra C.; Pescador L. A.; Villalobos N.; Salcedo S.; Berrio I.; Varón C.; Espinosa-Bode A.; Lockhart S. R.; Jackson B. R.; Litvintseva A. P.; Beltran M.; Chiller T. M. Molecular Epidemiology of Candida auris in Colombia Reveals a Highly Related, Countrywide Colonization With Regional Patterns in Amphotericin B Resistance. Clin. Infect. Dis. 2018, 68, 15–21. 10.1093/cid/ciy411. [DOI] [PubMed] [Google Scholar]
- Kumar J. A.; Eilertson B.; Cadnum J. L.; Whitlow C. S.; Jencson A. L.; Safdar N.; Krein S. L.; Tanner W. D.; Mayer J.; Samore M. H.; Donskey C. J. Environmental Contamination with Candida Species in Multiple Hospitals Including a Tertiary Care Hospital with a Candida auris Outbreak. Pathog. Immun. 2019, 4, 260–270. 10.20411/pai.v4i2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson C. A.; Wyncoll D.; Patel A.; Ceesay Y.; Newsholme W.; Chand M.; Mitchell H.; Tan M.; Edgeworth J. D. Cloth Lanyards as a Source of Intermittent Transmission of Candida auris on an ICU. Crit. Care Med. 2021, 49, 697–701. 10.1097/CCM.0000000000004843. [DOI] [PubMed] [Google Scholar]
- United States Environmental Protection Agency. List P: Antimicrobial Products Registered with EPA for Claims Against Candida auris. https://www.epa.gov/pesticide-registration/list-p-antimicrobial-products-registered-epa-claims-against-candida-auris.
- Kordalewska M.; Zhao Y.; Lockhart S. R.; Chowdhary A.; Berrio I.; Perlin D. S. Rapid and Accurate Molecular Identification of the Emerging Multidrug-Resistant Pathogen Candida auris. J. Clin. Microbiol. 2017, 55, 2445–2452. 10.1128/JCM.00630-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirhendi H.; Charsizadeh A.; Aboutalebian S.; Mohammadpour M.; Nikmanesh B.; de Groot T.; Meis J. F.; Badali H. South Asian (Clade I) Candida auris meningitis in a paediatric patient in Iran with a review of the literature. Mycoses 2022, 65, 134–139. 10.1111/myc.13396. [DOI] [PubMed] [Google Scholar]
- Sengupta S.; Marimuthu K.; Stewardson A.; Harbarth S.; Durante A.; Singh S. Challenges in Identification of Candida auris in Hospital Laboratories: Comparison Between HIC and LMIC. Infect. Control Hosp. Epidemiol. 2020, 41, s158. 10.1017/ice.2020.681. [DOI] [Google Scholar]
- Sikora A. Z. F.Candida auris; StatPearls Publishing: Treasure Island, FL, 2022. [Google Scholar]
- Shah S.; Gwee S. X. W.; Ng J. Q. X.; Lau N.; Koh J.; Pang J. Wastewater surveillance to infer COVID-19 transmission: A systematic review. Sci. Total Environ. 2022, 804, 150060 10.1016/j.scitotenv.2021.150060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vo V.; Tillett R. L.; Papp K.; Shen S.; Gu R.; Gorzalski A.; Siao D.; Markland R.; Chang C.-L.; Baker H.; Chen J.; Schiller M.; Betancourt W. Q.; Buttery E.; Pandori M.; Picker M. A.; Gerrity D.; Oh E. C. Use of wastewater surveillance for early detection of Alpha and Epsilon SARS-CoV-2 variants of concern and estimation of overall COVID-19 infection burden. Sci. Total Environ. 2022, 835, 155410 10.1016/j.scitotenv.2022.155410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz B. W.; Innes G. K.; Prasek S. M.; Betancourt W. Q.; Stark E. R.; Foster A. R.; Abraham A. G.; Gerba C. P.; Pepper I. L. Enumerating asymptomatic COVID-19 cases and estimating SARS-CoV-2 fecal shedding rates via wastewater-based epidemiology. Sci. Total Environ. 2021, 801, 149794 10.1016/j.scitotenv.2021.149794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClary-Gutierrez J. S.; Mattioli M. C.; Marcenac P.; Silverman A. I.; Boehm A. B.; Bibby K.; Balliet M.; de Los Reyes F. L. 3rd; Gerrity D.; Griffith J. F.; Holden P. A.; Katehis D.; Kester G.; LaCross N.; Lipp E. K.; Meiman J.; Noble R. T.; Brossard D.; McLellan S. L. SARS-CoV-2 Wastewater Surveillance for Public Health Action. Emerging Infect. Dis. 2021, 27, 1–8. 10.3201/eid2709.210753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims N.; Kasprzyk-Hordern B. Future perspectives of wastewater-based epidemiology: Monitoring infectious disease spread and resistance to the community level. Environ. Int. 2020, 139, 105689 10.1016/j.envint.2020.105689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevada Department of Health and Human Services Division of Public and Behavioral Health (NV DHHS DPBH) Candida auris training from Nevada Division of Public and Behavioral Health, 2022.
- Gerrity D.; Papp K.; Stoker M.; Sims A.; Frehner W. Early-pandemic wastewater surveillance of SARS-CoV-2 in Southern Nevada: Methodology, occurrence, and incidence/prevalence considerations. Water Res.: X 2021, 10, 100086 10.1016/j.wroa.2020.100086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh K.; Makimura K.; Hasumi Y.; Nishiyama Y.; Uchida K.; Yamaguchi H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol. Immunol. 2009, 53, 41–44. 10.1111/j.1348-0421.2008.00083.x. [DOI] [PubMed] [Google Scholar]
- Leach L.; Zhu Y.; Chaturvedi S. Development and Validation of a Real-Time PCR Assay for Rapid Detection of Candida auris from Surveillance Samples. J. Clin. Microbiol. 2018, 56, e01223-17 10.1128/JCM.01223-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin S. A.; Benes V.; Garson J. A.; Hellemans J.; Huggett J.; Kubista M.; Mueller R.; Nolan T.; Pfaffl M. W.; Shipley G. L.; Vandesompele J.; Wittwer C. T. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- Las Vegas Convention and Visitors Authority. LVCVA Research Reports: Clark County Population (Las Vegas Metro Area); August 2021.
- R Core Team., R: A Language and Environment for Statistical Computing. R: A language and environment for statistical computing.: Vienna, Austria, 2022.
- Wickham H., ggplot2: Elegant Graphics for Data Analysis; Springer-Verlage: New York, 2016.
- National Center for Biotechnology Information Candida auris (txid498019). https://www.ncbi.nlm.nih.gov/genome/?term=txid498019[Organism:noexp].
- Proctor D. M.; Dangana T.; Sexton D. J.; Fukuda C.; Yelin R. D.; Stanley M.; Bell P. B.; Baskaran S.; Deming C.; Chen Q.; Conlan S.; Park M.; Mullikin J.; Thomas J.; Young A.; Bouffard G.; Barnabas B.; Brooks S.; Han J.; Ho S.-l.; Kim J.; Legaspi R.; Maduro Q.; Marfani H.; Montemayor C.; Riebow N.; Schandler K.; Schmidt B.; Sison C.; Stantripop M.; Black S.; Dekhtyar M.; Masiello C.; McDowell J.; Thomas P.; Vemulapalli M.; Welsh R. M.; Vallabhaneni S.; Chiller T.; Forsberg K.; Black S. R.; Pacilli M.; Kong H. H.; Lin M. Y.; Schoeny M. E.; Litvintseva A. P.; Segre J. A.; Hayden M. K. Integrated genomic, epidemiologic investigation of Candida auris skin colonization in a skilled nursing facility. Nat. Med. 2021, 27, 1401–1409. 10.1038/s41591-021-01383-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochhead C.Water authority looks to curb another group of water wasters — septic systems Las Vegas Rev.-J. 2022.
- Centers for Disease Control and Prevention. Real-Time PCR Based Identification of Candida auris Using Applied biosystems 7500 Fast Real-Time PCR Platform; August 2019, 2019.
- Link-Gelles R.; Lutterloh E.; Ruppert P. S.; Backenson P. B.; George K. S.; Rosenberg E. S.; Anderson B. J.; Fuschino M.; Popowich M.; Punjabi C.; Souto M.; McKay K.; Rulli S.; Insaf T.; Hill D.; Kumar J.; Gelman I.; Jorba J.; Ng T. F. F.; Gerloff N.; Masters N. B.; Lopez A.; Dooling K.; Stokley S.; Kidd S.; Oberste M. S.; Routh J.; et al. Public Health Response to a Case of Paralytic Poliomyelitis in an Unvaccinated Person and Detection of Poliovirus in Wastewater--New York, June-August 2022. MMWR. Morb. Mortal. Wkly. Rep. 2022, 71, 1065–1068. 10.15585/mmwr.mm7133e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe M. K.; Yu A. T.; Duong D.; Rane M. S.; Hughes B.; Chan-Herur V.; Donnelly M.; Chai S.; White B. J.; Vugia D. J.; Boehm A. B.. Wastewater Surveillance for Monkeypox Virus in Nine California Communities medRxiv 2022.
- Wolfe M. K.; Duong D.; Hughes B.; Chan-Herur V.; White B. J.; Boehm A. B., Detection of monkeypox viral DNA in a routine wastewater monitoring program. medRxiv 2022, 2022.07.25.22278043. [Google Scholar]
- Acosta N.; Bautista M. A.; Waddell B. J.; McCalder J.; Beaudet A. B.; Man L.; Pradhan P.; Sedaghat N.; Papparis C.; Bacanu A.; Hollman J.; Krusina A.; Southern D. A.; Williamson T.; Li C.; Bhatnagar S.; Murphy S.; Chen J.; Kuzma D.; Clark R.; Meddings J.; Hu J.; Cabaj J. L.; Conly J. M.; Dai X.; Lu X.; Chekouo T.; Ruecker N. J.; Achari G.; Ryan M. C.; Frankowski K.; Hubert C. R. J.; Parkins M. D. Longitudinal SARS-CoV-2 RNA wastewater monitoring across a range of scales correlates with total and regional COVID-19 burden in a well-defined urban population. Water Res. 2022, 220, 118611 10.1016/j.watres.2022.118611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi A.; Chavez J.; Iverson T.; Hergert J.; Oakeson K.; LaCrosse N.; Njoku C.; Gorzalski A.; D G.. Culture-based isolation and genomic characterization of Candida auris through community-level wastewater surveillance and its association with an ongoing healthcare outbreak-Nevada Emerging Infect. Dis. Dispatches 2022. [DOI] [PMC free article] [PubMed]
- Torres S. R.; Pichowicz A.; Torres-Velez F.; Song R.; Singh N.; Lasek-Nesselquist E.; Jesus M. D. Impact of Candida auris Infection in a Neutropenic Murine Model. Antimicrob. Agents Chemother. 2020, 64, e01625-19 10.1128/AAC.01625-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabino R.; Veríssimo C.; Pereira Á. A.; Antunes F. Candida auris, An Agent of Hospital-Associated Outbreaks: Which Challenging Issues Do We Need to Have in Mind? In. Microorganisms 2020, 8, 181 10.3390/microorganisms8020181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry L.; Ramage G.; Kean R.; Borman A.; Johnson E. M.; Richardson M. D.; Rautemaa-Richardson R. Biofilm-Forming Capability of Highly Virulent, Multidrug-Resistant Candida auris. Emerging Infect. Dis. 2017, 23, 328–331. 10.3201/eid2302.161320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton M. V.; Nett J. E. Candida auris infection and biofilm formation: going beyond the surface. Curr. Clin. Microbiol. Rep. 2020, 7, 51–56. 10.1007/s40588-020-00143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowska R.; Ruhaak A.; Kasprzyk-Hordern B.; Hassard F.; Lundy L.; Di Cesare M.; Hickman M.; Singer A.. Wastewater-Based Epidemiology and Group Privacy: the Elephant in the Sewer? 2021.
- Coffman M. M.; Guest J. S.; Wolfe M. K.; Naughton C. C.; Boehm A. B.; Vela J. D.; Carrera J. S. Preventing Scientific and Ethical Misuse of Wastewater Surveillance Data. Environ. Sci. Technol. 2021, 55, 11473–11475. 10.1021/acs.est.1c04325. [DOI] [PubMed] [Google Scholar]
- Chow N. A.; de Groot T.; Badali H.; Abastabar M.; Chiller T.; Meis J. Potential Fifth Clade of Candida auris, Iran, 2018. Emerging Infect. Dis. 2019, 25, 1780–1781. 10.3201/eid2509.190686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora P.; Singh P.; Wang Y.; Yadav A.; Pawar K.; Singh A.; Padmavati G.; Xu J.; Chowdhary A. Environmental Isolation of Candida auris from the Coastal Wetlands of Andaman Islands, India. mBio 2021, 12, e03181-20 10.1128/mBio.03181-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandão J.; Gangneux J. P.; Arikan-Akdagli S.; Barac A.; Bostanaru A. C.; Brito S.; Bull M.; Çerikçioğlu N.; Chapman B.; Efstratiou M. A.; Ergin Ç.; Frenkel M.; Gitto A.; Gonçalves C. I.; Guégan H.; Gunde-Cimerman N.; Güran M.; Irinyi L.; Jonikaitė E.; Kataržytė M.; Klingspor L.; Mares M.; Meijer W. G.; Melchers W. J. G.; Meletiadis J.; Meyer W.; Nastasa V.; Babič M. N.; Ogunc D.; Ozhak B.; Prigitano A.; Ranque S.; Rusu R. O.; Sabino R.; Sampaio A.; Silva S.; Stephens J. H.; Tehupeiory-Kooreman M.; Tortorano A. M.; Velegraki A.; Veríssimo C.; Wunderlich G. C.; Segal E. Mycosands: Fungal diversity and abundance in beach sand and recreational waters — Relevance to human health. Sci. Total Environ. 2021, 781, 146598 10.1016/j.scitotenv.2021.146598. [DOI] [PubMed] [Google Scholar]
- Sabino R.; Veríssimo C.; Cunha M. A.; Wergikoski B.; Ferreira F. C.; Rodrigues R.; Parada H.; Falcão L.; Rosado L.; Pinheiro C.; Paixão E.; Brandão J. Pathogenic fungi: An unacknowledged risk at coastal resorts? New insights on microbiological sand quality in Portugal. Mar. Pollut. Bull. 2011, 62, 1506–1511. 10.1016/j.marpolbul.2011.04.008. [DOI] [PubMed] [Google Scholar]
- Shah A. H.; Abdelzaher A. M.; Phillips M.; Hernandez R.; Solo-Gabriele H. M.; Kish J.; Scorzetti G.; Fell J. W.; Diaz M. R.; Scott T. M.; Lukasik J.; Harwood V. J.; McQuaig S.; Sinigalliano C. D.; Gidley M. L.; Wanless D.; Ager A.; Lui J.; Stewart J. R.; Plano L. R. W.; Fleming L. E. Indicator microbes correlate with pathogenic bacteria, yeasts and helminthes in sand at a subtropical recreational beach site. J. Appl. Microbiol. 2011, 110, 1571–1583. 10.1111/j.1365-2672.2011.05013.x. [DOI] [PubMed] [Google Scholar]
- Casadevall A.; Kontoyiannis D. P.; Robert V. On the Emergence of Candida auris: Climate Change, Azoles, Swamps, and Birds. mBio 2019, 10, e01397-19 10.1128/mBio.01397-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



