Abstract
Background
Sleep disturbance is an outcome of multiple factors including environmental and genetic influences. Job stress, a complex environmental factor, likely affects sleep quality, significantly reducing the quality of life of workers. Additionally, FK506 binding protein 51 (FKBP5) may be a pathogenic factor for sleep disturbance as it regulates hypothalamic–pituitary–adrenal (HPA) axis activity, where HPA axis has been found to be involved in the regulation mechanism of sleep and stress response.
Objectives
The main aim of this study was to investigate the association between job stress and FKBP5 gene polymorphism as well as their interaction with sleep disturbance in Chinese workers; to date, these relationships have not been explored.
Methods
This is a cross-sectional study. A total of 675 railway workers (53.8% male) completed a short Effort-Reward Imbalance questionnaire and the Pittsburgh Sleep Quality Index. The SNaPshot single nucleotide polymorphism (SNP) assay was carried out by screening for FKBP5 SNPs in every participant. Generalized multifactor dimensionality reduction (GMDR) was used to identify the strongest G×E interaction combination.
Results
The findings showed that job stress was significantly associated with sleep disturbance; specifically, scores on the PSQI subscales (sleep disturbance, sleep medication, and daytime dysfunction) exhibited significant differences between the two job stress groups (X2 = 18.10, p = 0.01). Additionally, the FKBP5 SNP rs1360780-TT (adjusted odds ratio [AOR] = 4.98, 95% confidence interval [CI] = 2.80–8.84) and rs3800373-CC genotype (AOR = 2.06, CI = 1.10–3.86) were associated with an increased risk of sleep disturbance. Job stress and rs1360780 and rs3800373 variants showed a high-dimensional interaction with sleep disturbance as determined by the GMDR model.
Conclusion
The FKBP5 gene may increase susceptibility to job stress and result in sleep disturbance, especially in the presence of negative work-related events. These findings contribute to the field of sleep disturbance prevention and treatment.
Keywords: Job stress, Sleep disturbance, Gene–environment interaction (G×E), FKBP5
Introduction
Sleep is a critical component of physical and emotional health and well-being, but sleep disturbance is widely prevalent. Studies have revealed that 22.6% of individuals aged 18–25 years experience frequent episodes of insomnia, and 11.3% of people aged 26–40 years and 15.7% of people aged 41–65 years have difficulty maintaining sleep, according to observations in the Netherlands, United Kingdom and United States (Kocevska et al., 2021). Sleep issues are most prevalent in those with paid work (Kocevska et al., 2021). A meta-analysis showed that the prevalence of insomnia in the general population of China reached 15.0% (Cao et al., 2017). Sleep-related health problems not only increase the susceptibility to neuropsychological diseases, but also increase the risks of metabolic diseases, neurodegenerative diseases, and cancer (Daskalakis & Binder, 2015; Duchaine et al., 2020). The negative effects of sleep disturbance lead to lower work capacity and more mistakes by workers; consequently, these workers pose health and safety risks, including accidents and injuries (Parkes, 2017).
It is well- known that environmental and genetic factors contribute to sleep disturbance (Kimura & Winkelmann, 2007). Some of the most influential environmental factors are work-related elements, including the imbalance between effort and reward, job stress, excessive working hours, and high-demand/low-control job training (Li et al., 2021). In the early 1990s, Siegrist introduced the effort-reward imbalance (ERI) model in the field of job stress epidemiology. This theory postulates that an imbalance between high “costs” (exerting great effort at work) and low benefits (salary, the possibility for promotion, and positive feedback) produces job stress that affects both mental and physical health (Theorell, 2017). Workers with higher levels of job stress or ERI are more likely to suffer from difficulty falling asleep or maintaining sleep (Ota et al., 2009; Davies et al., 2017). In addition, individuals who perceive high job stress are vulnerable to sleep disturbances, and those exposed to ERI have a higher risk of absence due to illness (Rugulies et al., 2009; Götz et al., 2018). Cho & Chen (2020) in a 4-year follow-up study, reported that ERI and sleep problems have a reciprocal relationship among older workers. These findings indicate that exploring the relationship between ERI and sleep disturbance may have important implications for sleep health.
Although many studies have identified contributing factors of sleep disturbance, the extent to which the interaction between genetic and environmental factors affects the manifestation of sleep disturbance is still unknown. A large UK twin study reported that the heritability of sleep quality was approximately 43% (Barclay et al., 2010). Several researchers have reported the effect of occupational stress on sleep quality and the role of the PER3 gene in sleep disorders (Peng et al., 2022). In addition, other studies have acknowledged that these factors work in concert to influence sleep via processes such as a gene × job stress interaction (Garfield, 2021; Peng et al., 2022). More interestingly, recent studies have shown interactions between some hypothalamic-pituitary-adrenal (HPA) axis-related gene (e.g., 5-HTTLPR and CRHR1) polymorphisms and stress on sleep (Utge et al., 2010; Huang et al., 2014). The above studies confirm that genetic and environmental factors may interact and influence sleep and that HPA axis genes may play an important role in sleep disturbance.
The effects of these factors on sleep may be modulated by the ability to deal with individual stress, the HPA axis and the neuroendocrine stress response system; these modulators may be promising candidate gene sources to explore with a gene × environment interaction approach. FK506 binding protein 51 (FKBP5), a cochaperone of Hsp90 and a component of the chaperone-receptor heterocomplex, has been shown to promote the homeostatic regulation of the HPA axis through the inhibition of glucocorticoid receptor (GR) activity, which is the principal biological mechanism underlying the stress response (Hartmann et al., 2012). Indeed, a recent study provided evidence that FKBP5 mediates the associations among the HPA axis, GR and the development of sleep disturbance (Buckley, Duggal & Schatzberg, 2008). One of the key regulatory proteins of the GR receptor complex, FKBP5 is located on chromosome 6 p 21.31 and is considered a stress-related gene (Klengel et al., 2013). Moreover, FKBP5 not only plays an important role in regulating the HPA axis and stress response but also has a significant impact on sleep (Albu et al., 2014). FKBP5 single nucleotide polymorphisms (SNPs) (i.e., rs3800373 and rs1360780) may increase suicide risk in individuals with a history of childhood trauma (Roy et al., 2010; Womersley et al., 2022). As many studies have reported, FKBP5 variants have been linked to neuropsychiatric conditions, including depression, anxiety, posttraumatic stress disorder and cognitive impairment, all of which share some symptoms with sleep disturbance (Wang, Shelton & Dwivedi, 2018; Hernández-Díaz et al., 2019; Terrelonge et al., 2022). In addition, a study reported a potential association between the FKBP5 rs9470080 variant and subjective health complaints, in which the FKBP5 rs9470080 CC genotype was associated with poor sleep quality complaints in the female working population (Sannes et al., 2020). Since rs1360780, rs3800373 and rs9470080 are likely functional variants, we decided to focus on these particular SNPs.
Recent studies have shown that FKBP51 mRNA is widely expressed in the hypothalamus and brainstem, which are brain regions important for sleep-wake regulation (Scharf et al., 2011). As FKBP5 is involved in the regulation of HPA activity, which is known to influence sleep, we were interested in determining whether FKBP5 gene variants affect the risk of sleep disturbance. However, research assessing the interplay between FKBP5 gene polymorphisms and job stress as well as their impact on sleep disturbance is scarce. G×E interaction research provides a potential pathway for understanding how genetic differences influence the likelihood that exposure to job stress will result in sleep disturbance.
Therefore, the aims of the present study were to investigate the effects of FKBP5 gene polymorphisms and job stress on sleep disturbance in Chinese workers. Furthermore, we explored the interaction effects through the higher-order GMDR model.
Materials & Methods
Study population
This study was part of the Occupational Health Study of Railway Workers (OHSRW), carried out from October 2019 to May 2020. A detailed questionnaire was used to collect sociodemographic and lifestyle information, accompanied by measurements of sleep disturbance and job stress during the annual occupational health examination. Blood samples were taken from every participant between 7 and 9 AM on the same day as part of the health examination. The inclusion and exclusion criteria and control of confounding factors have been previously described (Wang et al., 2022). Specifically, participants were included who had been working in this particular job (front-line railway) for >1 year and were aged between 20 and 60 years. The present study included 690 participants who consented, of whom 15 participants were excluded due to inadequate information or missing blood samples; thus, 675 participants (363 males and 312 females) from the China Railway Fuzhou Branch were included in the final analysis. This study was approved by the Ethics Committee of Fujian Medical University (No. 2019025). Written informed consent was obtained from each participant.
Job stress
The Effort-Reward Imbalance (ERI) questionnaire is one of the most widely used instruments to estimate job stress, and it is based on Siegrist’s Effort-Reward Imbalance (ERI) model (Siegrist et al., 2009). The job stress test has been previously described (Wang et al., 2022). Specifically, an ERI ratio >1 represents an imbalance between effort and reward, which is considered job stress (Wu et al., 2019). In the present study, Cronbach’s alpha of this questionnaire was 0.882.
Sleep disturbance
The Pittsburgh Sleep Quality Index (PSQI) is a 19-item self-report questionnaire designed to evaluate sleep quality during a one-month interval (Buysse et al., 1989). The PSQI has high internal consistency, reliability and construct validity and consists of seven clinically derived components. Each dimension is scored from 0 to 3; total PSQI scores range from 0 to 21. Participants with a total score higher than 5 were classified as having sleep disturbance (Carpenter & Andrykowski, 1998).
Genotyping
A 5-ml fasting venous blood sample was collected from every participant between 07:00 and 09:00 at the workplace. According to the relevant references (Roy et al., 2010; Fudalej et al., 2015; Sannes et al., 2020; Womersley et al., 2022), the genotype information of SNPs in the Chinese Human Genome (CHB) and the gold standard (i.e., r2 = 0.8, MAF>15% standard), the HapMap database (http://hapmap.ncbi.nlm.nih.gov/) and Haploview software (http://www.broad.mit.edu/mpg) were used to select target tag SNP loci in this study (Wang et al., 2022). Genomic DNA was isolated and purified from the samples using a whole blood genome extraction kit (Beijing Think out Sci-Tech Co., Ltd). Selected FKBP5 SNPs (rs1360780, rs3800373 and rs9470080) were genotyped using SNaPshot analysis (Mehta et al., 2017). Table 1 shows the sequences of the primers.
Table 1. Primer information.
| Primer | Direction | Sequence 5′-3′ |
|---|---|---|
| rs1360780 _F | Forward | GGCATGGGCACTCTGAAAAGAT |
| rs1360780 _R | Reverse | TCTCTTGTGCCAGCAGTAGCAAGT |
| rs3800373 _F | Forward | GGCATGGGAAGCTGTCTTCAAC |
| rs3800373 _R | Reverse | CCAGCATTGCTACTGCTCAGCTTC |
| rs9470080 _F | Forward | TCTTTTCCAGGCTATGAATTGACAAA |
| rs9470080 _R | Reverse | TGTGTCCAGCCATGTGCTTTTT |
Statistical analysis
Statistical analyses were carried out using IBM SPSS version 26.0 (SPSS Inc., Chicago, IL, USA). Chi-square tests were used to compare sociodemographic characteristics. The chi-square goodness-of-fit test was used to determine the Hardy-Weinberg equilibrium (HWE) of the FKBP5 gene rs1360780, rs3800373 and rs9470080 polymorphisms in our sample. The Mann–Whitney U test was used to assess the relationship between job stress and sleep disturbance. Unconditional logistic regression was used to evaluate the relationship of genotypes and job stress with the risk of sleep disturbance, after adjusting for sex, age, ethnicity, marital status, smoking status, and alcohol consumption. Bonferroni correction was applied as a multiplicity correction. Generalized multifactor dimensionality reduction (GMDR, http://sourceforge.net/projects/gmdr/) was used to screen the strongest G×E interaction combination (Lou, 2015). We conducted 10-fold cross-validation (CV) to avoid unstable results and obtained a robust averaged result. All reported P values are two-tailed, and those less than 0.05 were considered statistically significant.
Results
Sociodemographic characteristics of participants
The demographic characteristics and sleep disturbances of participants are summarized in Table 2. In this study, 363 males (53.8%) and 312 females (46.2%) were included. There were significant differences in the distribution of sleep disturbances between different job stress groups (x2 = 18.10, p = 0.01). We found that there were no significant differences in demographic characteristics between the people that did not report sleep disturbance and sleep disturbance groups (p > 0.05).
Table 2. Demographic characteristics of job stress and sleep disturbance (n = 675).
| Variables | N | Non-sleep disturbance (%) | Sleep disturbance (%) | χ 2 | p-value |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 363 | 221 (60.9) | 142 (39.1) | 0.57 | 0.45 |
| Female | 312 | 181 (58.0) | 131 (42.0) | ||
| Age (years) | |||||
| ≤ 30 | 160 | 95 (59.4) | 65 (40.6) | 3.68 | 0.30 |
| 31–40 | 236 | 147 (62.3) | 89 (37.7) | ||
| 41–50 | 200 | 109 (54.5) | 91 (45.5) | ||
| >51 | 79 | 51 (64.6) | 28 (35.4) | ||
| Ethnicity | |||||
| Han | 530 | 318 (60.0) | 212 (40.0) | 0.20 | 0.65 |
| Minority | 145 | 84 (57.9) | 61 (42.1) | ||
| Marital status | |||||
| Unmarried | 119 | 68 (57.1) | 51 (42.9) | 1.97 | 0.37 |
| Married | 520 | 316 (60.8) | 204 (39.2) | ||
| Divorced or widowed | 36 | 18 (50.0) | 18 (50.0) | ||
| Smoking status | |||||
| Non-smoker | 263 | 156 (59.3) | 107 (40.7) | 0.01 | 0.92 |
| Smoker | 412 | 246 (59.7) | 166 (40.3) | ||
| Alcohol consumption | |||||
| Non-drinker | 362 | 219 (60.5) | 143 (39.5) | 0.29 | 0.60 |
| Drinker | 313 | 183 (58.5) | 130 (41.5) | ||
| Job stress | |||||
| Non-job stress | 366 | 245 (60.9) | 121 (44.3) | 18.10 | 0.01 |
| Job stress | 309 | 157 (39.1) | 152 (55.7) |
Association between job stress and sleep disturbance
The PSQI total and subscale scores were compared between the two job stress groups using the Mann–Whitney U test. Significant differences in the PSQI (sleep disturbance, sleep medication, and daytime dysfunction subscales and total) scores were found between the two job stress groups (p = 0.019, p = 0.001, p = 0.005, and p = 0.003, respectively), as shown in Table 3. Moreover, after adjusting for confounding factors, the logistic regression investigating job stress and sleep disturbance showed that participants with job stress (ERI ratio >1) had a higher risk of sleep disturbance (OR = 1.98, 95% CI = 1.45−2.71).
Table 3. Association between the job stress and sleep disturbance and its subscale scores (n = 675).
| Job stress, mean (SD) | |||
|---|---|---|---|
| Sleep disturbance | ERI ≤1 | ERI>1 | P-values |
| Subjective sleep quality | 0.44 (0.75) | 0.49 (0.80) | 0.521 |
| Sleep latency | 0.86 (0.88) | 0.81 (0.65) | 0.919 |
| Sleep duration | 0.16 (0.42) | 0.19 (0.51) | 0.915 |
| Sleep efficiency | 0.68 (0.94) | 0.59 (0.92) | 0.117 |
| Sleep disturbance | 0.66 (0.66) | 0.64 (0.84) | 0.019 |
| Sleep medication | 0.43 (0.68) | 0.77 (0.66) | 0.001 |
| Daytime dysfunction | 0.63 (0.65) | 0.80 (0.74) | 0.005 |
| PSQI total scores | 3.86 (2.56) | 4.29 (2.49) | 0.003 |
Notes.
P-values for the two-groups comparison were determined by the Mann–Whitney U test.
Relationships of three SNPs of the FKBP5 gene with sleep disturbance
All genotypes in the control group were distributed according to HWE (p > 0.05). For information about linkage disequilibrium between these SNPs, see Fig. S1. The rs1360780-TT genotype frequency was 18.3% in the sleep disturbance group and 4.7% in the non-sleep disturbance group. The rs3800373-CC genotype frequency was 9.5% in the sleep disturbance group and 4.7% in the non-sleep disturbance group. As shown in Table 4, The rs1360780-TT genotypes and job stress were associated with increased sleep disturbance risk (OR = 4.98, 95% CI = 2.80−8.84, p < 0.001, Bonferroni corrected p < 0.01); this relationship remained significant after controlling for covariates (AOR = 2.06, 95% CI = 1.10−3.86, p < 0.001, Bonferroni corrected p < 0.01). We also found no significant correlation between rs3800373 (after Bonferroni correction) or rs9470080 and susceptibility to sleep disturbance.
Table 4. Association analysis for 3 target SNPs of FKBP5 gene with sleep disturbance.
| SNPs | Genotypes and alleles | Frequencies N (%) | OR (95%CI)a | P-values | |
|---|---|---|---|---|---|
| Non-sleep disturbance (n = 402) | Sleep disturbance (n = 273) | ||||
| rs1360780 | |||||
| CC | 231 (57.5) | 123 (45.1) | Ref | ||
| CT | 152 (37.8) | 100 (36.6) | 1.24 (0.86–1.73) | ||
| TT | 19 (4.7) | 50 (18.3) | 4.98 (2.80-8.84)** | ||
| C allele | 614 (76.4) | 346 (63.4) | Ref | ||
| T allele | 190 (23.6) | 200 (36.6) | 1.88 (1.48-2.39)** | ||
| HWE test for controls | 0.811 | ||||
| rs3800373 | |||||
| AA | 234 (58.2) | 156 (57.1) | Ref | ||
| CA | 149 (37.1) | 91 (33.3) | 0.92 (0.66–1.24) | ||
| CC | 19 (4.7) | 26 (9.5) | 2.06 (1.10-3.86)* | ||
| A allele | 617 (76.7) | 403 (73.8) | Ref | ||
| C allele | 187 (23.3) | 143 (26.2) | 1.18 (0.92–1.52) | ||
| HWE test for controls | 0.855 | ||||
| rs9470080 | |||||
| CC | 187 (46.5) | 140 (51.3) | Ref | ||
| CT | 170 (42.3) | 112 (41.0) | 0.88 (0.64–1.22) | ||
| TT | 45 (11.2) | 21 (7.7) | 0.63 (0.36–1.12) | ||
| C allele | 544 (67.7) | 392 (71.8) | Ref | ||
| T allele | 260 (32.3) | 154 (28.2) | 0.83 (0.66–1.06) | ||
| HWE test for controls | 0.891 | ||||
Notes.
Adjusted for sex, age, ethnicity, marital status, smoking status, and alcohol consumption.
P < 0.05.
P < 0.001.
Interaction between job stress and FKBP5 gene SNPs on sleep disturbance
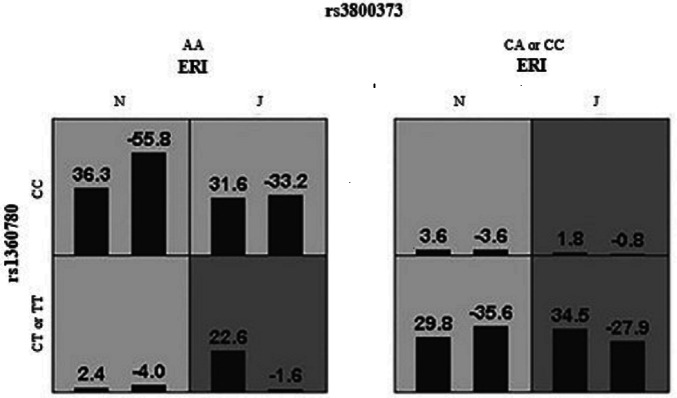
Gene × environment interaction models were determined by GMDR analysis. As shown in Table 5, the strongest model of the three factors was the interaction between rs1360780 and rs3800373 and job stress, with a CV consistency of 10/10 and testing accuracy of 0.589 (P value = 0.011). Figure 1 shows the details of the results. We found that participants with job stress, the CA or CC genotype of rs3800373 and the CT or TT genotype of rs1360780 had the highest risk of sleep disturbances.
Table 5. Strongest gene-environment interaction models, as identified by GMDR.
| Model | Testing accuracy (%) | Training accuracy (%) | P-value | Cross-validation consistency |
|---|---|---|---|---|
| ERI | 0.583 | 0.585 | p = 0.011 | 10/10 |
| ERI ×rs1360780 | 0.585 | 0.575 | p = 0.055 | 10/10 |
| ERI ×rs1360780×rs3800373 | 0.589 | 0.585 | p = 0.011 | 10/10 |
Notes.
Adjusted for gender, age, ethnicity, marital status, smoking status and alcohol consumption.
Figure 1. The GMDR results of the interaction between FKBP5 gene and job stress.
High- and low- risk factors are inherently determined by the model, in which the dark gray box represents the high-risk factors, and the light gray represents the low-risk factors. Bars represent the maximum likelihood estimation of case weights. In the same box. the left column is positive score and the right column is negative score. N and J denote normal and job stress (ERI>1), respectively. Among them, individuals with job stress, CA or CC genotype of rs3800373 and CT or TT genotype of rs1360780 had the highest sum score.
Discussion
The present study examined the interaction between FKBP5 gene polymorphisms and job stress on sleep disturbances in a sample of Chinese workers. Job stress was significantly associated with sleep disturbance, as indicated by significant differences between the PSQI total score and its subscale (sleep disturbance, sleep medication and daytime dysfunction) scores between the two job stress groups. Moreover, the FKBP5 SNP rs1360780-TT genotypes was related to an increased risk of sleep disturbances. In the GMDR model, job stress, rs1360780 and rs3800373 showed a high-level interaction that influenced sleep disturbance.
Most epidemiological data have shown that job stress puts individuals at a high risk for sleep disturbance (Linton et al., 2015). In the present study, we observed that 40.4% of participants reported sleep disturbance, indicating that sleep disturbance among Chinese workers may be a serious public health problem. We found that job stress was a significantly associated with sleep disturbance and that job stress was an independent risk factor for sleep disturbance. These effects of job stress on sleep disturbance might be associated with physiological arousal due to activation of the HPA axis in stressful environments (Buckley & Schatzberg, 2005). When excessive job stress exceeds the body’s ability to cope, the imbalance affects the sleep of worker sleep, resulting in insomnia or sleep disturbance (Halonen et al., 2017). Moreover, long-term job stress increases adrenal sensitivity to adrenocorticotropic hormones, cortisol levels, and changes in glucocorticoid and growth hormone levels on the HPA axis, which may lead to inhibition of sleep and sleep disturbance (Matenchuk, Mandhane & Kozyrskyj, 2020; Asarnow, 2020).
Dysregulation of FKBP5 gene may disrupt the feedback loop of the HPA axis, ultimately leading to disruption of HPA axis homeostasis (Albu et al., 2014). Moreover, most stress-related hormones are known to promote wakefulness, elevated HPA activity appears to contribute to stress-induced sleep disorder (Buckley & Schatzberg, 2005). We observed an association between the FKBP5 genotype and sleep disturbance. In our study, participants with the TT genotype of rs1360780 had an increased risk of sleep disturbance after adjusting for confounding factors. Indeed, FKBP5 SNPs were previously found to be associated with affective disorders such as those arising from maladaptation to stress (De Kloet, Joëls & Holsboer, 2005), with diagnosed patients displaying altered sleep patterns (Steiger & Kimura, 2010). Mice lacking the gene encoding FKBP5 (51KO mice) demonstrated more active stress-coping behavior and improved sleep profiles (Hartmann et al., 2012). In contrast, individuals carrying the T allele of rs1360780 who were exposed to early-life stress had a higher risk of posttraumatic stress disorder; thus, the interaction between these genes may increase the likelihood of developing stress-related disorders (Wang, Shelton & Dwivedi, 2018).
Interestingly, we found significant G×E interactions by performing GMDR. GMDR is an emerging method of G×E analysis that reduces the false positive rate and improve accuracy through cross validation and permutation tests (Lou et al., 2008). Using GMDR, we found that individuals carrying the CA/CC genotype (rs3800373) and CT/TT genotype (rs1360780) were at greatest risk of sleep disturbance, when experiencing job stress. Thus, FKBP5 polymorphisms may affect sleep disturbance through interactions with job stress. Our results suggest that environmental and genetic factors interact to influence sleep disturbance, as reported in some studies (Li et al., 2021; Garfield, 2021). Gene × environment interaction research has been primarily guided by the diathesis-stress model (Belsky & Pluess, 2009; Belsky & Hartman, 2014) which establishes that individuals carrying “vulnerable genes” are more susceptible to the effect of environmental adversity and thus more prone to developing psychological or behavioral problems (Monroe & Simons, 1991; Belsky & Pluess, 2013; He et al., 2019). In this study, the CT/TT genotype in rs1360780 of FKBP5 are probably the genetic variants or polymorphisms that make individuals vulnerable to stressful environments. Furthermore, the FKBP5 rs3800373 variant is located in the 3′ prime untranslated region and likely alters the stability and half-life of the mRNA and modulates glucocorticoid signaling and HPA axis function (Fudalej et al., 2015); this variant has been linked to symptoms directly associated with sleep disturbances such as anxiety, depression and pain (Knisely et al., 2019). On the other hand, previous studies have reported that the rs9470080 CC genotype was linked to an increased risk of low diurnal cortisol levels and likely leads to inattention, irritability and sleep problems through dysregulation of the HPA axis and FKBP5 (Isaksson et al., 2015). However, our study found no significant correlation of rs9470080 or rs3800373 (after Bonferroni correction) with susceptibility to sleep disturbance. The role of these two SNPs in job stress and sleep disturbance needs to be investigated in future studies.
To the best of our knowledge, this is the first study to analyze the high-dimensional interaction among job stress and FKBP5 genetic variants or polymorphisms on sleep disturbance. However, this study has several limitations. First, all of the subjects were railway workers, and occupational peculiarities may result in a lack of external validity due to selection bias. Second, we could not draw any definitive causal conclusions about relationships among job stress, the FKBP5 gene, and sleep disturbance or their interaction since this study used a cross-sectional design. Third, the limited sample size (n = 675) may lead to false-positive or false-negative results due to the lack of statistical power. Therefore, our current findings need to be validated in studies with larger sample sizes before any firm conclusions can be drawn. Finally, the PSQI scores were used to evaluate sleep disturbance; thus, the study still lacks objectivity compared with common diagnostic methods. Therefore, future studies should include objective measurement methods (such as actigraphy and objective PSG indices), use an extended sample size, take people in other professions as subjects and explore how these particular polymorphisms affect HPA axis homeostasis to provide better sleep quality profiles.
Conclusions
In summary, our results showed that chronic exposure to job stress tends to be associated with sleep disturbance and that FKBP5 rs1360780 gene polymorphisms were associated with sleep disturbance. More importantly, these two polymorphisms and job stress interacted to affect sleep disturbance, indicating that the impact of job stress on sleep disturbance may be regulated by genotype. Our results suggest that the CA or CC genotype of rs3800373 and the CT or TT genotype of rs1360780 may be stress-responsive risk genotypes, supporting the diathesis-stress model.
Supplemental Information
The linkage disequilibrium heatmap between the SNPs was measured using r2 and the absolute value of D’. The relative positions of FKBP5 SNPs and the numbers in the squares refer to pair-wise linkage disequilibrium.
Acknowledgments
The authors want to express their sincere gratitude to all participants for participating in the study.
Funding Statement
This work was supported by the Natural Science Foundation of Fujian Province, China (grant number 2020J01642), and the Fujian Medical University’s Research Foundation for Talented Scholars (Grant number XRCZX2018011). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Peixin Li performed the experiments, analyzed the data, authored or reviewed drafts of the article, and approved the final draft.
Yuxi Wang performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Baoying Liu performed the experiments, prepared figures and/or tables, and approved the final draft.
Chuancheng Wu performed the experiments, prepared figures and/or tables, and approved the final draft.
Chenzhou He performed the experiments, prepared figures and/or tables, and approved the final draft.
Xuejie Lv performed the experiments, prepared figures and/or tables, and approved the final draft.
Yu Jiang conceived and designed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Human Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
This study was approved by the institutional ethical committees of Fujian Medical University (No. 2019025).
Data Availability
The following information was supplied regarding data availability:
The raw measurements are available in the Supplemental Files.
References
- Albu et al. (2014).Albu S, Romanowski CPN, Letizia Curzi M, Jakubcakova V, Flachskamm C, Gassen NC, Hartmann J, Schmidt MV, Schmidt U, Rein T, Holsboer F, Hausch F, Paez-Pereda M, Kimura M. Deficiency of FK506-binding protein (FKBP) 51 alters sleep architecture and recovery sleep responses to stress in mice. Journal of Sleep Research. 2014;23(2):176–185. doi: 10.1111/jsr.12112. [DOI] [PubMed] [Google Scholar]
- Asarnow (2020).Asarnow LD. Depression and sleep: what has the treatment research revealed and could the HPA axis be a potential mechanism? Current Opinion in Psychology. 2020;34:112–116. doi: 10.1016/j.copsyc.2019.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay et al. (2010).Barclay NL, Eley TC, Buysse DJ, Archer SN, Gregory AM. Diurnal preference and sleep quality: same genes? A study of young adult twins. Chronobiology International. 2010;27(2):278–296. doi: 10.3109/07420521003663801. [DOI] [PubMed] [Google Scholar]
- Belsky & Hartman (2014).Belsky J, Hartman S. Gene-environment interaction in evolutionary perspective: differential susceptibility to environmental influences. World Psychiatry. 2014;13(1):87–89. doi: 10.1002/wps.20092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky & Pluess (2009).Belsky J, Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychological Bulletin. 2009;135(6):885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Belsky & Pluess (2013).Belsky J, Pluess M. Beyond risk, resilience, and dysregulation: phenotypic plasticity and human development. Development and Psychopathology. 2013;25(4 Pt 2):1243–1261. doi: 10.1017/S095457941300059X. [DOI] [PubMed] [Google Scholar]
- Buckley, Duggal & Schatzberg (2008).Buckley T, Duggal V, Schatzberg AF. The acute and post-discontinuation effects of a glucocorticoid receptor (GR) antagonist probe on sleep and the HPA axis in chronic insomnia: a pilot study. Journal of Clinical Sleep Medicine. 2008;4(3):235–241. doi: 10.5664/jcsm.27186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley & Schatzberg (2005).Buckley TM, Schatzberg AF. On the interactions of the hypothalamic-pituitary-adrenal (HPA) axis and sleep: normal HPA axis activity and circadian rhythm, exemplary sleep disorders. Journal of Clinical Endocrinology & Metabolism. 2005;90(5):3106–3114. doi: 10.1210/jc.2004-1056. [DOI] [PubMed] [Google Scholar]
- Buysse et al. (1989).Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cao et al. (2017).Cao XL, Wang SB, Zhong BL, Zhang L, Ungvari GS, Ng CH, Li L, Chiu HFK, Lok GKI, Lu JP, Jia FJ, Xiang YT. The prevalence of insomnia in the general population in China: a meta-analysis. PLOS ONE. 2017;12(2):e0170772. doi: 10.1371/journal.pone.0170772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter & Andrykowski (1998).Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh Sleep Quality Index. Journal of Psychosomatic Research. 1998;45(1):5–13. doi: 10.1016/s0022-3999(97)00298-5. [DOI] [PubMed] [Google Scholar]
- Cho & Chen (2020).Cho E, Chen TY. The bidirectional relationships between effort-reward imbalance and sleep problems among older workers. Sleep Health. 2020;6(3):299–305. doi: 10.1016/j.sleh.2020.01.008. [DOI] [PubMed] [Google Scholar]
- Daskalakis & Binder (2015).Daskalakis NP, Binder EB. Schizophrenia in the spectrum of gene-stress interactions: the FKBP5 example. Schizophrenia Bulletin. 2015;41(2):323–329. doi: 10.1093/schbul/sbu189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies et al. (2017).Davies G, Haddock G, Yung AR, Mulligan LD, Kyle SD. A systematic review of the nature and correlates of sleep disturbance in early psychosis. Sleep Medicine Reviews. 2017;31:25–38. doi: 10.1016/j.smrv.2016.01.001. [DOI] [PubMed] [Google Scholar]
- De Kloet, Joëls & Holsboer (2005).De Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nature Reviews Neuroscience. 2005;6(6):463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Duchaine et al. (2020).Duchaine CS, Aubé K, Gilbert-Ouimet M, Vézina M, Ndjaboué R, Massamba V, Talbot D, Lavigne-Robichaud M, Trudel X, Pena-Gralle A-PB, Lesage A, Moore L, Milot A, Laurin D, Brisson C. Psychosocial stressors at work and the risk of sickness absence due to a diagnosed mental disorder: a systematic review and meta-analysis. JAMA Psychiatry. 2020;77(8):842–851. doi: 10.1001/jamapsychiatry.2020.0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudalej et al. (2015).Fudalej S, Kopera M, Wołyńczyk-Gmaj D, Fudalej M, Krajewski P, Wasilewska K, Szymański K, Chojnicka I, Podgórska A, Wojnar M, Płoski R. Association between FKBP5 functional polymorphisms and completed suicide. Neuropsychobiology. 2015;72(2):126–131. doi: 10.1159/000441659. [DOI] [PubMed] [Google Scholar]
- Garfield (2021).Garfield V. Sleep duration: a review of genome-wide association studies (GWAS) in adults from 2007 to 2020. Sleep Medicine Reviews. 2021;56:101413. doi: 10.1016/j.smrv.2020.101413. [DOI] [PubMed] [Google Scholar]
- Götz et al. (2018).Götz S, Hoven H, Müller A, Dragano N, Wahrendorf M. Age differences in the association between stressful work and sickness absence among full-time employed workers: evidence from the German socio-economic panel. International Archives of Occupational and Environmental Health. 2018;91(4):479–496. doi: 10.1007/s00420-018-1298-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halonen et al. (2017).Halonen JI, Lallukka T, Pentti J, Stenholm S, Rod NH, Virtanen M, Salo P, Kivimäki M, Vahtera J. Change in job strain as a predictor of change in insomnia symptoms: analyzing observational data as a non-randomized pseudo-trial. Sleep. 2017;40(1):zsw007. doi: 10.1093/sleep/zsw007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann et al. (2012).Hartmann J, Wagner KV, Liebl C, Scharf SH, Wang XD, Wolf M, Hausch F, Rein T, Schmidt U, Touma C, Cheung-Flynn J, Cox MB, Smith DF, Holsboer F, Müller MB, Schmidt MV. The involvement of FK506-binding protein 51 (FKBP5) in the behavioral and neuroendocrine effects of chronic social defeat stress. Neuropharmacology. 2012;62(1):332–339. doi: 10.1016/j.neuropharm.2011.07.041. [DOI] [PubMed] [Google Scholar]
- He et al. (2019).He SC, Wu S, Du XD, Jia Q, Wang C, Wu F, Ning Y, Wang D, Wang L, Zhang XY. Interactive effects of corticotropin-releasing hormone receptor 1 gene and work stress on burnout in medical professionals in a Chinese Han population. Journal of Affective Disorders. 2019;252:1–8. doi: 10.1016/j.jad.2019.03.084. [DOI] [PubMed] [Google Scholar]
- Hernández-Díaz et al. (2019).Hernández-Díaz Y, González-Castro TB, Tovilla-Zárate CA, Juárez-Rojop IE, López-Narváez ML, Pérez-Hernández N, Rodríguez-Pérez JM, Genis-Mendoza AD. Association between FKBP5 polymorphisms and depressive disorders or suicidal behavior: a systematic review and meta-analysis study. Psychiatry Research. 2019;271:658–668. doi: 10.1016/j.psychres.2018.12.066. [DOI] [PubMed] [Google Scholar]
- Huang et al. (2014).Huang C, Li J, Lu L, Ren X, Li Y, Huang Q, Lan Y, Wang Y. Interaction between serotonin transporter gene-linked polymorphic region (5-HTTLPR) and job-related stress in insomnia: a cross-sectional study in Sichuan, China. Sleep Medicine. 2014;15(10):1269–1275. doi: 10.1016/j.sleep.2014.01.023. [DOI] [PubMed] [Google Scholar]
- Isaksson et al. (2015).Isaksson J, Allen M, Nilsson KW, Lindblad F. Polymorphisms in the FK506 binding protein 5 gene are associated with attention deficit hyperactivity disorder and diurnal cortisol levels. Acta Paediatrica. 2015;104(9):910–915. doi: 10.1111/apa.13056. [DOI] [PubMed] [Google Scholar]
- Kimura & Winkelmann (2007).Kimura M, Winkelmann J. Genetics of sleep and sleep disorders. Cellular and Molecular Life Sciences. 2007;64(10):1216–1226. doi: 10.1007/s00018-007-6532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klengel et al. (2013).Klengel T, Mehta D, Anacker C, Rex-Haffner M, Pruessner JC, Pariante CM, Pace TWW, Mercer KB, Mayberg HS, Bradley B, Nemeroff CB, Holsboer F, Heim CM, Ressler KJ, Rein T, Binder EB. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nature Neuroscience. 2013;16(1):33–41. doi: 10.1038/nn.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knisely et al. (2019).Knisely MR, Maserati M, Heinsberg LW, Shah LL, Li H, Zhu Y, Ma Y, Graves LY, Merriman JD, Conley YP. Symptom science: advocating for inclusion of functional genetic polymorphisms. Biological Research for Nursing. 2019;21(4):349–354. doi: 10.1177/1099800419846407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocevska et al. (2021).Kocevska D, Lysen TS, Dotinga A, Koopman-Verhoeff ME, Luijk MPCM, Antypa N, Biermasz NR, Blokstra A, Brug J, Burk WJ, Comijs HC, Corpeleijn E, Dashti HS, De Bruin EJ, De Graaf R, Derks IPM, Dewald-Kaufmann JF, Elders PJM, Gemke RJBJ, Grievink L, Hale L, Hartman CA, Heijnen CJ, Huisman M, Huss A, Ikram MA, Jones SE, Velderman MK, Koning M, Meijer AM, Meijer K, Noordam R, Oldehinkel AJ, Groeniger JO, Penninx BWJH, Picavet HSJ, Pieters S, Reijneveld SA, Reitz E, Renders CM, Rodenburg G, Rutters F, Smith MC, Singh AS, Snijder MB, Stronks K, Ten Have M, Twisk JWR, Van de Mheen D, Van der Ende J, Van der Heijden KB, Van der Velden PG, Van Lenthe FJ, Van Litsenburg RRL, Van Oostrom SH, Van Schalkwijk FJ, Sheehan CM, Verheij RA, Verhulst FC, Vermeulen MCM, Vermeulen RCH, Verschuren WMM, Vrijkotte TGM, Wijga AH, Willemen AM, Ter Wolbeek M, Wood AR, Xerxa Y, Bramer WM, Franco OH, Luik AI, Van Someren EJW, Tiemeier H. Sleep characteristics across the lifespan in 1.1 million people from the Netherlands, United Kingdom and United States: a systematic review and meta-analysis. Nature Human Behaviour. 2021;5(1):113–122. doi: 10.1038/s41562-020-00965-x. [DOI] [PubMed] [Google Scholar]
- Li et al. (2021).Li Y, Cao Z, Wu S, Wang C, He S, Dong Y, Zhang X. Association of job stress, CLOCK gene polymorphism and their interaction with poor sleep quality. Journal of Sleep Research. 2021;30(1):e13133. doi: 10.1111/jsr.13133. [DOI] [PubMed] [Google Scholar]
- Linton et al. (2015).Linton SJ, Kecklund G, Franklin KA, Leissner LC, Sivertsen B, Lindberg E, Svensson AC, Hansson SO, Sundin Ö, Hetta J, Björkelund C, Hall C. The effect of the work environment on future sleep disturbances: a systematic review. Sleep Medicine Reviews. 2015;23:10–19. doi: 10.1016/j.smrv.2014.10.010. [DOI] [PubMed] [Google Scholar]
- Lou (2015).Lou XY. UGMDR: a unified conceptual framework for detection of multifactor interactions underlying complex traits. Heredity. 2015;114(3):255–261. doi: 10.1038/hdy.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou et al. (2008).Lou XY, Chen GB, Yan L, Ma JZ, Mangold JE, Zhu J, Elston RC, Li MD. A combinatorial approach to detecting gene-gene and gene-environment interactions in family studies. American Journal of Human Genetics. 2008;83(4):457–467. doi: 10.1016/j.ajhg.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matenchuk, Mandhane & Kozyrskyj (2020).Matenchuk BA, Mandhane PJ, Kozyrskyj AL. Sleep, circadian rhythm, and gut microbiota. Sleep Medicine Reviews. 2020;53:101340. doi: 10.1016/j.smrv.2020.101340. [DOI] [PubMed] [Google Scholar]
- Mehta et al. (2017).Mehta B, Daniel R, Phillips C, McNevin D. Forensically relevant SNaPshot® assays for human DNA SNP analysis: a review. International Journal of Legal Medicine. 2017;131(1):21–37. doi: 10.1007/s00414-016-1490-5. [DOI] [PubMed] [Google Scholar]
- Monroe & Simons (1991).Monroe SM, Simons AD. Diathesis-stress theories in the context of life stress research: implications for the depressive disorders. Psychological Bulletin. 1991;110(3):406–425. doi: 10.1037/0033-2909.110.3.406. [DOI] [PubMed] [Google Scholar]
- Ota et al. (2009).Ota A, Masue T, Yasuda N, Tsutsumi A, Mino Y, Ohara H, Ono Y. Psychosocial job characteristics and insomnia: a prospective cohort study using the Demand-Control-Support (DCS) and Effort-Reward Imbalance (ERI) job stress models. Sleep Medicine. 2009;10(10):1112–1117. doi: 10.1016/j.sleep.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Parkes (2017).Parkes KR. Work environment, overtime and sleep among offshore personnel. Accident Analysis and Prevention. 2017;99(Pt B):383–388. doi: 10.1016/j.aap.2015.11.022. [DOI] [PubMed] [Google Scholar]
- Peng et al. (2022).Peng X, Li J, Han B, Zhu Y, Cheng D, Li Q, Du J. Association of occupational stress, period circadian regulator 3 (PER3) gene polymorphism and their interaction with poor sleep quality. Journal of Sleep Research. 2022;31(1):e13390. doi: 10.1111/jsr.13390. [DOI] [PubMed] [Google Scholar]
- Roy et al. (2010).Roy A, Gorodetsky E, Yuan Q, Goldman D, Enoch M-A. Interaction of FKBP5, a stress-related gene, with childhood trauma increases the risk for attempting suicide. Neuropsychopharmacology. 2010;35(8):1674–1683. doi: 10.1038/npp.2009.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugulies et al. (2009).Rugulies R, Norborg M, Sørensen TS, Knudsen LE, Burr H. Effort-reward imbalance at work and risk of sleep disturbances. Cross-sectional and prospective results from the Danish Work Environment Cohort Study. Journal of Psychosomatic Research. 2009;66(1):75–83. doi: 10.1016/j.jpsychores.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Sannes et al. (2020).Sannes AC, Christensen JO, Nielsen MB, Gjerstad J. The influence of age, gender and the FKBP5 genotype on subjective health complaints in the Norwegian working population. Journal of Psychosomatic Research. 2020;139:110264. doi: 10.1016/j.jpsychores.2020.110264. [DOI] [PubMed] [Google Scholar]
- Scharf et al. (2011).Scharf SH, Liebl C, Binder EB, Schmidt MV, Müller MB. Expression and regulation of the Fkbp5 gene in the adult mouse brain. PLOS ONE. 2011;6(2):e16883. doi: 10.1371/journal.pone.0016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegrist et al. (2009).Siegrist J, Wege N, Pühlhofer F, Wahrendorf M. A short generic measure of work stress in the era of globalization: effort-reward imbalance. International Archives of Occupational and Environmental Health. 2009;82(8):1005–1013. doi: 10.1007/s00420-008-0384-3. [DOI] [PubMed] [Google Scholar]
- Steiger & Kimura (2010).Steiger A, Kimura M. Wake and sleep EEG provide biomarkers in depression. Journal of Psychiatric Research. 2010;44(4):242–252. doi: 10.1016/j.jpsychires.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Terrelonge et al. (2022).Terrelonge M, LaHue SC, Tang C, Movsesyan I, Pullinger CR, Dubal DB, Leung J, Douglas VC. KIBRA, MTNR1B, and FKBP5 genotypes are associated with decreased odds of incident delirium in elderly post-surgical patients. Scientific Reports. 2022;12(1):556. doi: 10.1038/s41598-021-04416-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theorell (2017).Theorell T. On effort-reward imbalance and depression. Scandinavian Journal of Work Environment & Health. 2017;43(4):291–293. doi: 10.5271/sjweh.3642. [DOI] [PubMed] [Google Scholar]
- Utge et al. (2010).Utge S, Soronen P, Partonen T, Loukola A, Kronholm E, Pirkola S, Nyman E, Porkka-Heiskanen T, Paunio T. A population-based association study of candidate genes for depression and sleep disturbance. American Journal of Medical Genetics Part B-Neuropsychiatric Genetics. 2010;153B(2):468–476. doi: 10.1002/ajmg.b.31002. [DOI] [PubMed] [Google Scholar]
- Wang, Shelton & Dwivedi (2018).Wang Q, Shelton RC, Dwivedi Y. Interaction between early-life stress and FKBP5 gene variants in major depressive disorder and post-traumatic stress disorder: a systematic review and meta-analysis. Journal of Affective Disorders. 2018;225:422–428. doi: 10.1016/j.jad.2017.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2022).Wang Y, Zhao M, Li P, Wu C, Lv Y, Jiang Y. Gene-environment interaction between circadian clock gene polymorphisms and job stress on the risk of sleep disturbances. Psychopharmacology. 2022;239(10):3337–3344. doi: 10.1007/s00213-022-06219-0. [DOI] [PubMed] [Google Scholar]
- Womersley et al. (2022).Womersley JS, Nothling J, Toikumo S, Malan-Müller S, Van den Heuvel LL, McGregor NW, Seedat S, Hemmings SMJ. Childhood trauma, the stress response and metabolic syndrome: a focus on DNA methylation. European Journal of Neuroscience. 2022;55(9-10):2253–2296. doi: 10.1111/ejn.15370. [DOI] [PubMed] [Google Scholar]
- Wu et al. (2019).Wu WT, Ss T, Cc W, Yj L, Tn W, Ts S, Sh L. Professional Driver’s Job Stress and 8-year Risk of Cardiovascular Disease: The Taiwan Bus Driver Cohort Study. Epidemiology. 2019;30(Supp 1):S39–S47. doi: 10.1097/EDE.0000000000001003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The linkage disequilibrium heatmap between the SNPs was measured using r2 and the absolute value of D’. The relative positions of FKBP5 SNPs and the numbers in the squares refer to pair-wise linkage disequilibrium.
Data Availability Statement
The following information was supplied regarding data availability:
The raw measurements are available in the Supplemental Files.