Abstract
Objectives
Determine the association of higher FI-LAB scores, derived from common laboratory values and vital signs, with hospital and post-hospital outcomes in Veterans hospitalized with COVID-19 infection.
Design, Setting, and Participants
A retrospective, multicenter, cohort study of 7 Veterans Health Administration (VHA) medical centers in Florida and Puerto Rico. Patients aged 18 years and older hospitalized with COVID-19 and followed for up to 1 year post discharge or until death.
Clinical Frailty Measure: FI-LAB. Main Outcomes and Measures
Hospital and post-hospital outcomes.
Results
Of the 671 eligible patients, 615 (91.5%) patients were included (mean [SD] age, 66.1 [14.8] years; 577 men [93.8%]; median stay, 8 days [IQR:3-15]. There were sixty-one in-hospital deaths. Veterans in the moderate and high FI-LAB groups had a higher proportion of inpatient mortality (13.3% and 20.6%, respectively) than the low group (4.1%), p <0.001. Moderate and high FI-LAB scores were associated with greater inpatient mortality when compared to the low group, OR:3.22 (95%CI:1.59-6.54), p=.001 and 6.05 (95%CI:2.48-14.74), p<0.001, respectively. Compared with low FI-LAB scores, moderate and high scores were also associated with prolonged length of stay, intensive care unit (ICU) admission, and transfer.
Conclusions and Relevance
In this study of patients admitted to 7 VHA Hospitals during the first surge of the pandemic, higher FI-LAB scores were associated with higher in-hospital mortality and other in-hospital outcomes; FI-LAB can serve as a validated, rapid, feasible, and objective frailty tool in hospitalized adults with COVID-19 that can aid clinical care.
Electronic Supplementary Material
Supplementary material is available for this article at 10.1007/s12603-023-1886-0 and is accessible for authorized users.
Key words: Frailty, FI-LAB, mortality, COVID-19, inpatient, veterans
Introduction
From early in the pandemic, reports from China indicated that older age and the presence of underlying chronic diseases were substantial risk factors for illness and death related to COVID-19 infection (1, 2). COVID-19 posed a disproportionately high threat to older adults worldwide (3–5). Older adults hospitalized with SARS-CoV-2 infection were more likely to suffer serious complications including higher rates of in-hospital and post-discharge morbidity and mortality (6, 7). During the pandemic, hospital-based health care professionals often used chronological age as an objective and quickly obtained characteristic to estimate individuals’ prognosis and make appropriate clinical decisions (8). However, the sole reliance on chronological age ignores the well-known heterogeneity in the aging process (9). Frailty, a common syndrome in older adults, has emerged as a more accurate indicator of biological age (10).
Frailty is an age-related clinical syndrome characterized by decreased reserve and susceptibility to stressors due to multisystem dysregulation (11, 12). Frailty is strongly associated with a wide range of adverse clinical outcomes in older adults (13, 14). Frailty is common in hospitalized older adults (15) and predicts worse clinical outcomes than patients who are not frail (16, 17). Several recent studies in older individuals hospitalized with SARS-CoV-2 infection have demonstrated worse clinical outcomes in, those identified as frail (18). Hence, early recognition of frailty in hospitalized older adults may serve for risk estimation and stratification that may assist hospital clinicians in the management of these patients. There are two accepted conceptual frameworks to characterize frailty, one based on a frailty phenotype (11) and the other on the deficit accumulation model (19). The frailty phenotype defines frailty as displaying at least three out of five indicators: weight loss, self-reported exhaustion, weakness, slow walking speed, and low physical activity. However, the frailty phenotype has limited reliability and feasibility in assessing frailty in hospitalized patients (20, 21). Frailty indexes based on the accumulation of various health deficits may be more suitable for determining frailty in hospitalized patients (19).
Three deficit accumulation-based instruments are currently available: the Hospital Frailty Risk Score (HFRS) (22), Clinical Frailty Scale (CFS) (23), and a frailty index based on laboratory values (FI-LAB) (24). The HFRS is based on International Classification of Diseases (ICD) codes available from electronic health records. In patients with SARS-CoV-2, high HFRS scores were associated with in-hospital death, increased length of hospital stay, ICU admission, and use of mechanical ventilation (25, 26). A major limitation of the HFRS is the requirement for data from an initial hospitalization precluding risk stratification for a first-time presentation (27, 28). The CFS represents a summary estimation of a patient’s multimorbidity, cognition, and functional status (23). Most studies reporting frailty in hospitalized patients with SARS-CoV-2 infection have shown that a CFS>5 is associated with higher in-hospital mortality and lower admission to ICU (18) but no consistent adjusted association with 30-day mortality, discharge to home, or prolonged LOS and increased 6-month mortality (18, 25, 29, 30). CFS requires training, is subjective and susceptible to personal bias (31). FI-LAB scores consisting of laboratory values and vital signs capture the multisystem dysfunction associated with frailty during acute care events and may represent an objective and practical assessment of frailty in hospitalized older adults. Higher FI-LAB scores have shown strong associations with in-hospital mortality in patients with acute exacerbations of chronic obstructive pulmonary disease, and older adults admitted to general medical wards before the pandemic (32–34), as well as long-term mortality of patients after general and geriatric ward hospitalizations (35, 36). The FI-LAB has not been investigated specifically as a prognostic tool during an infectious disease pandemic, such as the COVID-19.
The primary aim of this study was to assess whether FI-LAB scores are associated with inpatient mortality in older Veterans hospitalized with COVID-19 infection. Secondary aims were to determine the association of the FI-LAB with in-hospital - length of stay, ICU admission, ICU transfers- and post-hospital outcomes-new nursing home placement, 30-day readmissions, and post-discharge mortality at 30-days, six-month, and one-year mortality. We hypothesized that higher FI-LAB scores will be associated with worse hospital and post-hospital outcomes in older Veterans hospitalized with SARS-CoV-2 infection.
Methods
Design, Setting, and Participants
We conducted a retrospective cohort study at seven medical centers part of the Veterans Integrated Service Network 8 (VISN 8), a regional care government-based healthcare system in Florida and Puerto Rico. We included community-dwelling and institutionalized adult US Veterans admitted to a VHA Medical Center with asymptomatic or symptomatic COVID-19 infection as confirmed by reverse transcription-polymerase chain reaction (RT-PCR) or antigen testing between March and August 2020. Staff excluded Veterans from the study if there were less than thirty required results from laboratory tests and vital signs within 72 hours of admission. We followed participants for one year after discharge or until death. Staff obtained all socio-demographics, death dates, ICD-10 codes, relevant laboratory results, and vital signs data from the VA Corporate Data Warehouse (CDW). We complemented this information with in-depth chart audits conducted by trained research associates to ensure that Veterans met inclusion criteria. In addition, we followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline. The Miami VA Healthcare System IRB reviewed and approved this study as a retrospective medical record review, waiving the need for informed consent.
Measurements
Frailty: We categorized frailty status using the FI-LAB, a frailty index that consists of thirty-one blood test results and vital sign values. These values comprised thirteen components of a complete blood count analysis, thirteen elements of a comprehensive metabolic profile, and five vital signs (Appendix 1). Staff scored each one with a value of either 0 (normal) or 1 (abnormal), then calculated the FI-LAB score by dividing the number of abnormal values by 30 or 31. We categorized FI-LAB scores as low (<0.25), moderate (0.25–0.40), and high (>0.40).
Outcomes
In-Hospital and Post-discharge Mortality
Staff identified mortality from data available in the VA CDW. For patients surviving hospital admission, we used the discharge date as Time 0 and documented 30-day, 6-month, and 1-year mortality events.
Readmission
We defined readmission as an unplanned hospital readmission for any cause within 30 days of discharge.
Length of Stay (LOS)
We calculated the LOS by subtracting the discharge date from the admission date. We coded same-day discharges as one and did not leave days from the calculation. We then used the median LOS to dichotomize the length of stay as prolonged (> 8 days) or short (<8 days).
Intensive Care Unit (ICU) Admission and Transfer
We defined ICU admission as Veterans admitted directly from the emergency room to ICU with the care of a critical care physician and ICU as Veterans transferred to the ICU during their hospital stay.
New nursing home placement
We included subjects admitted from the community and discharged to a nursing home defined as a community skilled nursing, extended care unit, or VA community living center (CLC). Existing nursing home residents were excluded from this analysis.
Data Analysis
We calculated descriptive statistics for sociodemographic characteristics and risk factors for severe COVID infection. Categorical variables were reported as frequency (percentage) and continuous variables as means (SDs). FI-LAB score was normally distributed only for age, as assessed by Kolmogorov-Smirnov’s test (p>0.05). Chi-square tests were used for the categorical variables and one-way ANOVA tests for continuous variables to evaluate differences between FI-LAB groups. We replaced values of covariates with missing values with multiple imputations. Odds ratios (OR) and 95% confidence intervals (CI) were calculated using logistic regression, and univariate and multivariate analyses were performed adjusting for gender, age, marital status, race, ethnicity, BMI, substance abuse, smoking status, PTSD, bipolar disorder, and schizophrenia to assess the association between the independent variables, FI-LAB, and outcomes. We also performed a multivariate survival analysis using the Cox proportional hazard regression model with FI-LAB as the independent variable and all-cause inpatient mortality as the dependent one. The proportional hazard ratio assumption was assessed and verified by testing the correlation between the Schoenfeld residuals and survival time. Lower FI-LAB scores served as the reference standard. Lastly, we determined the diagnostic accuracy of the FI-LAB score in predicting inpatient mortality using a Receiver Operator Characteristic (ROC)/area under the curve (AUC) analysis. Only two-tailed statistical tests were used with an assumed significance for a p-value <0.05. We used International Business Machines (IBM) SPSS Statistics, version 28.0.
Results
Patient Characteristics
There were 671 hospitalized patients with COVID-19 infection during our study period, of which 615 (91.65%) met the inclusion criteria. Table 1 shows participant baseline characteristics. The Veterans’ mean age was 66.12 (SD=14.79, range 22–103 years), and were 93.80% (n=577) male, 57.60% (n=354) Caucasian, 81.0% (n=498) non-Hispanic, and with a median BMI of 30.02 (SD=7.55, IQR:25.60–35.99). The baseline socio-demographic characteristics did not differ between the different FI-LAB groups except for age and sex. However, they differed in the frequency of selected comorbidities: chronic kidney disease, congestive heart failure, diabetes mellitus, hypertension, schizophrenia, and bipolar disorder. There were no differences in the number of nursing home residents before admission and independence with self-care between the three groups.
Table 1.
Participants Baseline Characteristics According to the FI-LAB
| Low (<0.25) n=266 (43%) | Moderate (0.25–0.40) n=286 (47%) | High (>0.40) n=63 (10%) | All Participants (n=615) Missing Data No. (%) | p-value | |
|---|---|---|---|---|---|
| Age, mean (SD) | 63.7 (15.5) | 68.3 (14.2) | 66.6 (13.0) | 66.1 (14.8) | .001 |
| Males, n (%) | 242a (91) | 273a (95) | 62a (98) | 577 (94) | .03 |
| Married, No. (%) | 123 (46) | 141 (49) | 25 (40) | 289 (47) | .36 |
| Caucasian, n (%) | 162 (61) | 162 (57) | 30 (48) | 354 (58) | .14 |
| Non-Hispanic, n (%) | 211 (79) | 230 (80) | 57 (90) | 498 (81) | .12 |
| BMI, mean (SD) | 30.4 (6.7) | 31.0 (7.3) | 30.8 (8.0) | 30.7 (7.1) 39 (6) | .65 |
| Smoker, n (%) | 71 (27) | 62 (22) | 13 (21) | 146 (24) | .32 |
| Substance Abuse, n (%) | 81 (30) | 70 (24) | 18 (29) | 169 (27) | .28 |
| Diabetes, n (%) | 98a (37) | 136b (48) | 33a,b (52) | 267 (44) 2 (0.3) | .02 |
| CKD, n (%) | 47a (18) | 90b (31) | 25b (40) | 162 (26) 2 (0.3) | <.001 |
| Lung Disease, n (%) | 74 (28) | 67 (23) | 17 (27) | 158 (26) 2 (0.3) | .46 |
| Dementia, n (%) | 66 (25) | 68 (24) | 12 (19) | 146 (24) 2 (0.3) | .61 |
| CAD, n (%) | 64 (24) | 83 (29) | 21 (33) | 168 (27) | .24 |
| HTN, n (%) | 182a (69) | 223b (78) | 49a,b (78) | 454 (74) 2 (0.3) | .04 |
| CHF, n (%) | 25a(9) | 57b(20) | 19b (30) | 101 (16) 2 (0.3) | <.001 |
| Liver Disease, n, (%) | 35 (13) | 26 (9) | 8 (13) | 69 (11) 2 (0.3) | .28 |
| Stroke, n (%) | 37 (14) | 43 (15) | 12 (19) | 92 (15) 2 (0.3) | .60 |
| Cancer, n (%) | 51 (19) | 64 (22) | 7 (11) | 122 (20) 2 (0.3) | .12 |
| Bipolar Disorder, No. (%) | 26a (10) | 22ab (8) | 0b (0) | 48 (8) | .03 |
| PTSD n, (%) | 94a (35) | 65b(23) | 14a,b (22) | 173 (28) | .002 |
| Schizophrenia, No. (%) | 15 (6) | 18 (6) | 3 (5) | 36 (6) | .88 |
| Self-Care, Prior to Admission, n (%) | 193 (73) | 198 (69) | 44 (70) | 435 (71) | .68 |
| Living in a Nursing Home, n (%) | 32 (12) | 31 (11) | 8 (13) | 71 (12) | .87 |
SD= standard deviation; n=number of participants. The assigned superscript letters (a, b, or c) are representative of the low, moderate, and high FI-LAB groups. If a pair of values are not significantly different, the values will have the same superscript letters assigned to them. Data without superscripts is not significantly different between FI-LAB groups. Significant differences are in bold (p<.05)
FI-LAB and Hospital Outcomes
Inpatient Mortality
There were 61 (9.9%) inpatient deaths during the follow-up period. Veterans in the moderate and high FI-LAB groups had a higher proportion of inpatient mortality (13.3%, n=37 and 20.6%, n=13, respectively) than the low group (4.1%, n=11) (p <0.001). Moderate and high FI-LAB scores were associated with higher inpatient mortality when compared to the low group, adjusted OR:3.22 (95%CI:1.59-6.54) and adjusted OR:.6.05 (95%CI:2.48-14.74), respectively (Table 2). Over a median follow-up was eight days (IQR=3-15), patients in the high FI-LAB categories demonstrated greater in-hospital mortality, adjusted HR: 2.59 (95%CI:1.27-5.27), p =.009 and adjusted HR:.4.17 (95%CI: 1.74-9.70), p =.001, respectively, compared to the low FI-LAB group (Figure 1). The predictive value of the FI-LAB for in-hospital mortality was acceptable, AUC of 0.715 (95%CI:.65-.78), p<.001 (Figure 2).
Table 2.
Assessment of Hospital and Post-Discharge Outcomes Among FI-LAB groups
| Moderate FI-LAB Univariate OR (95%CI), p-value | Moderate FI-LAB Multivariate OR (95%CI), p-value | High FI-LAB Univariate OR (95%CI), p-value | High FI-LAB Multivariate OR (95%CI), p-value | |
|---|---|---|---|---|
| Hospital Outcomes | ||||
| In-hospital Mortality | 3.45 (1.72–6.90) p<0.001 | 3.22 (1.59–6.54) p=.001 | 6.03 (2.56-14.21) p<.001 | 6.05 (2.48-14.74) p<.001 |
| Length of stay | 1.88 (1.34–2.64) p<.001 | 1.86 (1.28–2.70) p=.001 | 2.93 (1.64-5.22) p<.001 | 3.15 (1.69-5.86) p<.001 |
| ICU Admission | 1.96 (1.14–3.38) p=.02 | 2.16 (1.23–3.80) p=.008 | 5.55 (2.81-10.97) p<.001 | 7.48 (3.60-15.56) p<.001 |
| ICU Transfer | 1.71 (1.01–2.88) p<.05 | 1.58 (0.92–2.73) p=.10 | 3.01 (1.48-6.13) p=.002 | 2.75 (1.32-5.76) p<.01 |
| Nursing Home Placement | .88 (.46–1.68) p=.69 | .81(.41–1.61) p=.55 | 1.06 (.38-2.94) p=.91 | 0.95(0.32-2.79) p=0.93 |
| Post-Discharge Outcomes | ||||
| 30-day Readmission | 0.95 (.59–1.52) p=.82 | 0.97 (.58–1.95) p=.90 | 1.10 (0.52-2.34) p=.81 | 1.12 (.50-2.48) p=.78 |
| 30-day Mortality | 1.56 (.39–6.59) p=.55 | 1.40 (0.32–6.14) p=.66 | 5.94 (1.30-27.27) p=.02 | 4.28 (0.87-21.07) p=.07 |
| 6-month Mortality | 1.93 (0.88–4.19) p=.10 | 1.88 (.84–4.22) p=0.13 | 2.70 (0.94-7.72) p=0.07 | 2.46 (0.81-7.50) p=0.11 |
| 1-Year Mortality | 2.03 (1.05–3.93) p=.04 | 1.82(0.90–3.66) p=.10 | 2.62 (1.05-8.30) p=.04 | 2.34 (0.88-6.25) p=.09 |
a. Low FI-LAB Group=Reference Standard. Multivariate logistic regression adjusted for gender, age, marital status, race, ethnicity, BMI, substance abuse, smoking status, PTSD, bipolar disorder, schizophrenia due to potentially significant differences between FI-LAB groups
Figure 1.
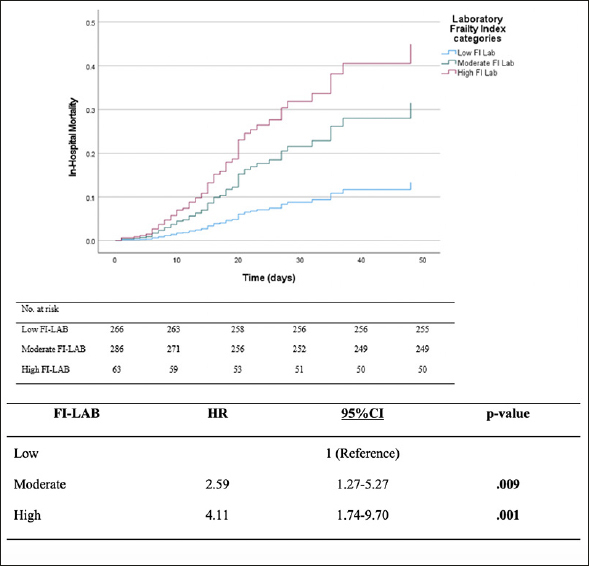
Mortality Over Time During Hospital Admission Among FI-LAB Groups (Survival Analysis)
Figure 2.
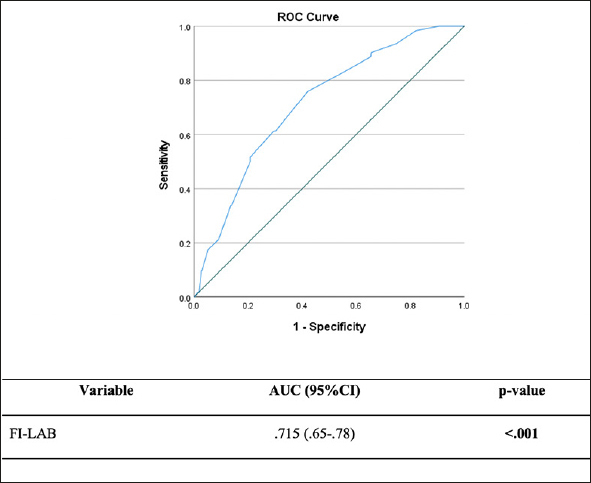
FI-LAB Receiver Operating Characteristics Curve for All-Cause In-Hospital Mortality
HR= hazard ratio; CI= confidence interval; FI-LAB= laboratory frailty index. Significant differences are in bold (p<.05). Adjusted for age, gender, marital status, race, ethnicity, BMI, PTSD, bipolar disorder, schizophrenia, substance abuse, and tobacco use.
ICU Admissions and Transfers
86 Veterans (14.0%) were directly admitted to the ICU. Moderate and high FI-LAB scores were associated with direct ICU admissions, with adjusted ORs of 2.16 (95%CI:1.23-3.80), p=.008 and 7.48 (95%CI:3.60-15.56), p<.001, respectively (Table 2). Seventy-eight patients were transferred to the ICU after an initial admission to a general medical or surgical ward. High FI-LAB scores were associated with ICU transfers compared to the low group, adjusted OR: 2.75(95%CI:1.32-5.76), p<.01 (Table 2).
Length of Stay
Median hospital length of stay was eight days (IQR = 12). More than half of the patients had a prolonged stay (n=311, 50.6%). Veterans in the high and moderate FI-LAB groups had a higher proportion of prolonged hospitalizations (67%, n=42 and 56%, n=161 respectively) than the low FI-LAB group (41%, n=108), p<0.001. Moderate and high FI-LAB scores were associated with a prolonged length of stay, adjusted OR:1.86 (95%CI:1.28-2.70), p=.001 and adjusted OR: 3.15 (95%CI:1.69-5.86), p=<.001, respectively (Table 2).
Nursing home placement
There were 44 (7.2%) patients admitted to a nursing home. Veterans in the high or moderate FI-LAB groups did not have a higher proportion of new nursing home placements (9%, n=5 and 7%, n=19 respectively) compared with the low FI-LAB group (9%, n=20), p=0.87. There were no differences in the risk of new nursing home placements between the FI-LAB groups: adjusted OR:0.81 (95%CI:0.41-1.61), p=.55, adjusted OR:0.95(95%CI:0.32-2.79), p=.93, for the moderate and high FILAB score groups respectively when compared to the low FI-LAB group (Table 2).
FI-LAB and Post-Discharge Outcomes
Readmissions
There were a total of eighty-nine patients (14.5%) readmitted within 30 days, thirty-nine were in the low (14.7%), forty in the moderate (14.0%), and 10 (15.9%) in the high score groups. However, there were no differences between the three FI-LAB groups (p=0.71). There were no differences in the risk of readmissions (30-day or overall) between the moderate and high FI-LAB scores, adjusted OR:0.97 (95%CI:0.58-1.95), p=.90, and 1.12 (95%CI:0.50-2.48), p=.78, respectively when compared to the low score group (Table 2).
Post-Discharge Mortality
There were 12 (2.0%) additional deaths within 30 days from hospital discharge. A larger proportion of patients in the high FI-LAB group died within 30 days of discharge (8%, n=4), than the moderate (2%, n=5), and low FI-LAB groups (1%, n=3), p=.01. Moderate and high FI-LAB scores were not associated with a greater risk of death within 30 days of discharge, when compared to the low FI-LAB group: adjusted OR:1.40 (95%CI:0.32-6.14), p=.66, and 4.28 (0.87–21.07), p=.07 respectively (Table 2).
Mortality within 6 Months: At 6 months from discharge, there were 36 (5.9%) deaths. Veterans with moderate and high FI-LAB scores did not have a greater risk of death within 6 months of discharge adjusted OR:1.88 (95%CI:0.84-4.22), p=.13, and 2.46 (95%CI:0.81-7.50), p=.11, respectively compared to the low FI-LAB group (Table 2).
Mortality within one year: At one-year post-discharge, there were fifty-one deaths (8.3%). A higher proportion of patients in the high and moderate FI-LAB groups died within one year of discharge (16%, n=8 and 12%, n=29 respectively) than in the low FI-LAB group (5%, n=14), p=0.01 (Table 3). Moderate and high FI-LAB scores did not show an association with one-year mortality, adjusted OR:1.82 (95%CI:0.90- 3.66), p=.10, and 2.34 (95%CI:0.88-6.25), p=.09, respectively (Table 2).
Discussion
In this retrospective cohort study, we investigated the association between the FI-LAB and several in-hospital and post-hospitalization outcomes in Veterans admitted with COVID-19 infection. We also assessed and compared the diagnostic accuracy of the FI-LAB in predicting in-hospital mortality. In our study, higher FI-LAB scores were associated with a higher risk for all-cause in-hospital mortality. The FI-LAB demonstrated a moderate accuracy for all-cause in-hospital mortality as assessed through AUC/ROC analyses. Higher FI-LAB scores were also associated with prolonged length of stay, more ICU direct admissions, and ICU transfers. High FI-LAB scores were not associated with new nursing home placement, 30-day post-discharge readmissions, 30-day, 6-month, and 1-year post-discharge mortality.
Our findings align with previous studies of frailty indexes based on laboratory values upon hospital admission demonstrating associations with in-hospital mortality before the COVID-19 pandemic (32–36). Unlike the CFS, higher FI-LAB scores show an association with ICU admissions and transfers, suggesting that the FI-LAB may be a better indicator of rapid changes in medical condition and risk stratification for ICU placement. We did not show a difference seen in six-month mortality as seen in one South American study looking at the impact of CFS of >5 (29). As with the CFS and HFRS, higher FI-LAB scores did not show an association with nursing home placement (18, 37). Additionally, comparing higher FI-LAB scores to higher HFRS scores, the FI-LAB scores seem to show a higher risk for in-hospital death (18, 37–40), prolonged length of stay, and ICU admission (18, 39, 41). We did not see a difference in outcomes in 30-day readmission rate, new nursing home placement, and post-discharge mortality (34–36) previously seen in a study of hospitalized patients before the COVID-19 pandemic. However, this may be due to the dynamic of the early pandemic where patients and caregivers wanted to discharge home, and in the case of the frail, as early as possible, regardless of physical status, due to fear of skilled nursing facilities being centers of COVID-19 transmission and outbreaks leading to an increased number of readmissions and decreased nursing home placement (42). We also likely did not have the statistical power to detect differences in post-discharge outcomes due to the relative scarcity of events.
Strengths of this study were comprehensive socio-demographic and clinical information from electronic health records and an in-depth chart review that complemented EHR information. Our study also adhered to recommendations for constructing the FI-LAB -over thirty items in contrast to 17–27 items in other studies (24, 32–35). Another strength of the study was an evaluation of the wide range of more clinical and utilization outcomes previously seen in studies of frailty tools and COVID-19 outcomes. There are, however, some limitations. Shortcomings of the retrospective cohort study design include the possibility of bias, such as missing data that may limit control of confounding, misclassification of comorbid conditions or diagnostic measures, and selection bias. Our study included a predominantly male group of hospitalized Veterans at VHA medical centers in Florida and Puerto Rico, where socio-demographics may differ from other facilities. Our study did not include Veterans admitted to non-VHA hospitals where they may have received hospital care (43). The acuity and severity of Veterans admitted for COVID-19 to non-VHA hospitals may have been different than those admitted to a VHA facility. Evidence shows that the quality of inpatient care at VHA hospitals may be better than at non-VHA hospitals, thereby potentially influencing study outcomes (44, 45). The timing of the study, as subsequent waves after the introduction of COVID-19 vaccines and therapeutics may have altered the associations of the FI-LAB with the outcomes. We need more extensive prospective studies in health care systems inside and outside VHA, with more diverse populations who received COVID-19 vaccines and therapeutics to confirm the association of FI-LAB scores with these clinical outcomes, as well as studies in other infectious disease epidemics.
In terms of clinical implications, the FI-LAB may represent a valuable tool that enables hospital-based clinicians to estimate and stratify risk for these patients. Armed with this information, hospital-based clinicians would implement further diagnostic tests and therapies to decrease the impact of COVID 19 in hospitalized older adults with frailty. By providing more accurate insights into patients’ risk and prognosis, the information can stimulate discussions between clinicians and patients, and loved ones regarding the person’s care goals and prognosis (28). For example, clinicians can use the FI-LAB to determine the most appropriate level of care for these patients and incorporate this information during goals of care discussions (46). Hospitalized patients with COVID-19 and moderate to high FI-LAB scores may benefit from the early involvement of geriatric consultation teams and the implementation of interprofessional care transition interventions. Research shows that comprehensive in-hospital geriatric assessment may lead to improved adherence to recommendations and better clinical and cost outcomes for hospitalized adults with frailty (47). The application of interprofessional geriatrics models of care during hospitalization may also help adults with frailty. One example is the Hospital Elder Life Program (HELP), which reduced the incidence of delirium and falls, the number of days in the hospital, and delayed institutionalization (48).
In conclusion, the FI-LAB higher scores were associated with higher in-hospital mortality and other in-hospital and posthospital outcomes in older adults with COVID-19 infection. Using the FI-LAB to identify hospitalized older adults with frailty and COVID 19 infection may assist clinicians in risk estimation and stratification that may help with management decisions.
Key Points
- Question: In patients hospitalized with COVID-19 infection at seven VHA medical centers, was there a positive association between higher scores in a frailty index from common laboratory values and vital signs (FI-LAB) and in-hospital and post-hospital outcomes?
- Findings: In this retrospective cohort study of 615 Veterans, those with high and moderate FI-LAB scores indicating frailty, had worse hospital and post-hospital outcomes than those with lower scores.
- Meaning: Using the FI-LAB may assist clinicians in the identification of hospitalized patients with COVID 19 and frailty at highest risk for poor clinical outcomes.
Electronic supplementary material
Supplementary material, approximately 18.5 KB.
Acknowledgments
We want to acknowledge Dr. Fei Tang PhD for her assistance with frameworks for the statistical analysis.
Author contributions: Natasha Melo Resendes: Study concept and design, data collection, acquisition of subjects and data, analysis and interpretation of data, and preparation of manuscript. Marlena Fernandez: Study concept and design, acquisition of subjects and data, analysis and interpretation of data, and preparation of manuscript. Angelica Torres-Morales, MD: Study concept and design, analysis and interpretation of data, and preparation of manuscript. Lorena Burton, Andria Chada, Alma Diaz-Quiñones, Christian Gomez, Shivaan Oomrigar: Data collection, analysis and interpretation of data, and preparation of manuscript. Jorge G. Ruiz: Study concept and design, acquisition of subjects and data, analysis and interpretation of data, and preparation of manuscript.
Funding: This material is the result of work supported with resources and the use of facilities at the Miami VA Healthcare System GRECC.
Conflict of interest disclosure form: The authors declare that they have no known competing fiscal interests or personal relationships, perceived to have influenced the work reported in this article.
References
- 1.World Health Organization. Coronavirus disease 2019 (COVID-19): situation report-1. January 20, 2020. 2020 [cited 2022 April 5, 2022]; Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200121-sitrep-1-2019-ncov.pdf?sfvrsn=20a99c10_4.
- 2.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Coronavirus disease 2019 (COVID-19): situation report-51. March 11, 2020 2020 [cited 2022 April 5, 2022]; Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200311-sitrep-51-covid-19.pdf?sfvren=1ba62e57_10.
- 4.Centers for Disease Control Severe Outcomes Among Patients with Coronavirus Disease 2019 (COVID-19) - United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343–346. doi: 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control. COVID-19: Hospitalization and Death by Age. Last Updated June 27, 2022. 2022 [cited 2022 July 5, 2022]; Available from: https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-age.html.
- 6.Shaw PA, Yang JB, Mowery DL, et al. Determinants of hospital outcomes for patients with COVID-19 in the University of Pennsylvania Health System. PLoS One. 2022;17(5):e0268528. doi: 10.1371/journal.pone.0268528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang CW, Park JS, Song H, et al. Disease-Specific Factors Associated with Readmissions or Mortality After Hospital Discharge in COVID-19 Patients: a Retrospective Cohort Study. J Gen Intern Med, 2022: p. 1–6. doi: 10.1007/s11606-022-07610-5 [DOI] [PMC free article] [PubMed]
- 8.Henri KH. Statement - Older people are at highest risk from COVID-19, but all must act to prevent community spread. https://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/statements/statement-older-people-are-at-highest-risk-from-covid-19,-but-all-must-act-to-prevent-community-spread. Published 2020. Accessed May 5, 2022.
- 9.Lowsky DJ, Olshansky SJ, Bhattacharya J, Goldman DP. Heterogeneity in healthy aging. J Gerontol A Biol Sci Med Sci. 2014;69(6):640–649. doi: 10.1093/gerona/glt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kravvariti E, Ntouros PA, Vlachogiannis NI, Pappa M, Souliotis VL, Sfikakis PP. Geriatric frailty is associated with oxidative stress, accumulation and defective repair of DNA double-strand breaks independent of age and comorbidities. J Gerontol A Biol Sci Med Sci. 2022. doi: 10.1093/gerona/glac214 [DOI] [PubMed]
- 11.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–156. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- 12.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14(6):392–397. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc. 2010;58(4):681–687. doi: 10.1111/j.1532-5415.2010.02764.x. [DOI] [PubMed] [Google Scholar]
- 15.Ligthart-Melis GC, Luiking YC, Kakourou A, Cederholm T, Maier AB, de van der Schueren MAE. Frailty, Sarcopenia, and Malnutrition Frequently (Co-)occur in Hospitalized Older Adults: A Systematic Review and Meta-analysis. J Am Med Dir Assoc. 2020;21(9):1216–1228. doi: 10.1016/j.jamda.2020.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Muscedere J, Waters B, Varambally A, et al. The impact of frailty on intensive care unit outcomes: a systematic review and meta-analysis. Intensive Care Med. 2017;43(8):1105–1122. doi: 10.1007/s00134-017-4867-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panayi AC, Orkaby AR, Sakthivel D, et al. Impact of frailty on outcomes in surgical patients: A systematic review and meta-analysis. Am J Surg. 2019;218(2):393–400. doi: 10.1016/j.amjsurg.2018.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dumitrascu F, Branje KE, Hladkowicz ES, Lalu M, McIsaac DI. Association of frailty with outcomes in individuals with COVID-19: A living review and meta-analysis. J Am Geriatr Soc. 2021;69(9):2419–2429. doi: 10.1111/jgs.17299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal. 2001;1:323–336. doi: 10.1100/tsw.2001.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chong E, Ho E, Baldevarona-Llego J, Chan M, Wu L, Tay L. Frailty and Risk of Adverse Outcomes in Hospitalized Older Adults: A Comparison of Different Frailty Measures. J Am Med Dir Assoc. 2017;18(7):638. doi: 10.1016/j.jamda.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 21.Chong E, Ho E, Baldevarona-Llego J, et al. Frailty in Hospitalized Older Adults: Comparing Different Frailty Measures in Predicting Short- and Long-term Patient Outcomes. J Am Med Dir Assoc. 2018;19(5):450–457. doi: 10.1016/j.jamda.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Gilbert T, Neuburger J, Kraindler J, et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet. 2018;391(10132):1775–1782. doi: 10.1016/S0140-6736(18)30668-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. Cmaj. 2005;173(5):489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rockwood K, McMillan M, Mitnitski A, Howlett SE. A Frailty Index Based on Common Laboratory Tests in Comparison With a Clinical Frailty Index for Older Adults in Long-Term Care Facilities. J Am Med Dir Assoc. 2015;16(10):842–847. doi: 10.1016/j.jamda.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 25.Ramos-Rincon JM, Moreno-Perez O, Pinargote-Celorio H, et al. Clinical Frailty Score vs Hospital Frailty Risk Score for predicting mortality and other adverse outcome in hospitalised patients with COVID-19: Spanish case series. Int J Clin Pract. 2021;75(10):e14599. doi: 10.1111/ijcp.14599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Caoimh R, Cooney MT, Cooke J, O’Shea D. The challenges of using the Hospital Frailty Risk Score. Lancet. 2019;392(10165):2693. doi: 10.1016/S0140-6736(18)32424-3. [DOI] [PubMed] [Google Scholar]
- 27.Conroy S, Gilbert T, Street A, Roberts HC, Parker S. The challenges of using the Hospital Frailty Risk Score - Author’s reply. Lancet. 2018;392(10165):2693–2694. doi: 10.1016/S0140-6736(18)33194-5. [DOI] [PubMed] [Google Scholar]
- 28.Rockwood K, Theou O. Using the Clinical Frailty Scale in Allocating Scarce Health Care Resources. Can Geriatr J. 2020;23(3):210–215. doi: 10.5770/cgj.23.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aliberti MJR, Szlejf C, Avelino-Silva VI, et al. COVID-19 is not over and age is not enough: Using frailty for prognostication in hospitalized patients. J Am Geriatr Soc. 2021;69(5):1116–1127. doi: 10.1111/jgs.17146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Subramaniam A, Shekar K, Afroz A, et al. Frailty and mortality associations in patients with COVID-19: a systematic review and meta-analysis. Intern Med J. 2022;52(5):724–739. doi: 10.1111/imj.15698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Theou O, Pérez-Zepeda MU, van der Valk AM, Searle SD, Howlett SE, Rockwood K. A classification tree to assist with routine scoring of the Clinical Frailty Scale. Age Ageing. 2021;50(4):1406–1411. doi: 10.1093/ageing/afab006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gu JJ, Liu Q, Zheng LJ. A Frailty Assessment Tool to Predict In-Hospital Mortality in Patients with Acute Exacerbations of Chronic Obstructive Pulmonary Disease. Int J Chron Obstruct Pulmon Dis. 2021;16:1093–1100. doi: 10.2147/COPD.S300980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ellis HL, Wan B, Yeung M, et al. Complementing chronic frailty assessment at hospital admission with an electronic frailty index (FI-Laboratory) comprising routine blood test results. Cmaj. 2020;192(1):E3–e8. doi: 10.1503/cmaj.190952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ritt M, Jäger J, Ritt JI, Sieber CC, Gaßmann K-G. Operationalizing a frailty index using routine blood and urine tests. Clinical interventions in aging. 2017;12:1029–1040. doi: 10.2147/CIA.S131987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klausen HH, et al. Association between routine laboratory tests and long-term mortality among acutely admitted older medical patients: a cohort study. BMC Geriatr. 2017;17(1):62. doi: 10.1186/s12877-017-0434-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ysea-Hill O, Gomez CJ, Mansour N, et al. The association of a frailty index from laboratory tests and vital signs with clinical outcomes in hospitalized older adults [published online ahead of print, 2022 Aug 6]. J Am Geriatr Soc. 2022; doi:10.1111/jgs.17977 [DOI] [PubMed]
- 37.Rottler M, Ocskay K, Sipos Z, et al. Clinical Frailty Scale (CFS) indicated frailty is associated with increased in-hospital and 30-day mortality in COVID-19 patients: a systematic review and meta-analysis. Ann Intensive Care. 2022;12(1):17. doi: 10.1186/s13613-021-00977-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hägg S, Jylhävä J, Wang Y, et al. Age, Frailty, and Comorbidity as Prognostic Factors for Short-Term Outcomes in Patients With Coronavirus Disease 2019 in Geriatric Care. J Am Med Dir Assoc. 2020;21(11):1555–1559. doi: 10.1016/j.jamda.2020.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Navaratnam AV, Gray WK, Day J, Wendon J, Briggs TWR. Patient factors and temporal trends associated with COVID-19 in-hospital mortality in England: an observational study using administrative data. Lancet Respir Med. 2021;9(4):397–406. doi: 10.1016/S2213-2600(20)30579-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kundi H, Çetin EH, Canpolat U, et al. The role of Frailty on Adverse Outcomes Among Older Patients with COVID-19. J Infect. 2020;81(6):944–951. doi: 10.1016/j.jinf.2020.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Apea VJ, Wan YI, Dhairyawan R, et al. Ethnicity and outcomes in patients hospitalised with COVID-19 infection in East London: an observational cohort study. BMJ Open. 2021;11(1):e042140. doi: 10.1136/bmjopen-2020-042140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gustavson AM, Toonstra A, Johnson JK, Ensrud KE. Reframing Hospital to Home Discharge from “Should We?” to “How Can We?”: COVID-19 and Beyond. J Am Geriatr Soc. 2021;69(3):608–609. doi: 10.1111/jgs.17036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sherman RL, Judon KM, Koufacos NS, et al. Utilizing a health information exchange to facilitate COVID-19 VA primary care follow-up for Veterans diagnosed in the community. JAMIA Open. 2021;4(1):ooab020. doi: 10.1093/jamiaopen/ooab020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nuti SV, Qin L, Rumsfeld JS, et al. Association of Admission to Veterans Affairs Hospitals vs Non-Veterans Affairs Hospitals With Mortality and Readmission Rates Among Older Men Hospitalized With Acute Myocardial Infarction, Heart Failure, or Pneumonia. JAMA. 2016;315(6):582–592. doi: 10.1001/jama.2016.0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anhang Price R, Sloss EM, Cefalu M, Farmer CM, Hussey PS. Comparing Quality of Care in Veterans Affairs and Non-Veterans Affairs Settings. J Gen Intern Med. 2018;33(10):1631–1638. doi: 10.1007/s11606-018-4433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Austin CA, Mohottige D, Sudore RL, Smith AK, Hanson LC. Tools to Promote Shared Decision Making in Serious Illness: A Systematic Review. JAMA Intern Med. 2015;175(7):1213–1221. doi: 10.1001/jamainternmed.2015.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ellis G, Langhorne P. Comprehensive geriatric assessment for older hospital patients. Br Med Bull. 2005;71:45–59. doi: 10.1093/bmb/ldh033. [DOI] [PubMed] [Google Scholar]
- 48.Hshieh TT, Yang T, Gartaganis SL, Yue J, Inouye SK. Hospital Elder Life Program: Systematic Review and Meta-analysis of Effectiveness. Am J Geriatr Psychiatry. 2018;26(10):1015–1033. doi: 10.1016/j.jagp.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material, approximately 18.5 KB.


