Abstract
The management of R/M HNSCC is rapidly evolving with new available treatment molecules and combination modalities. Anti-EGFR cetuximab (CTX) and immune checkpoint inhibitors (ICI) can be used either alone or in combination with conventional platinum-based doublet chemotherapy (with taxanes or fluorouracil). No data have been reported to date on the association of doublet chemotherapy concomitantly with both CTX and ICI. We present a case series of patients treated with 4 cycles of quadritherapy, every 3 weeks, including paclitaxel 175 mg/m2, carboplatin AUC 5, pembrolizumab 200 mg, and weekly 250 mg/m2 CTX. All patients achieved an objective response (6 complete responses, 2 partial responses). Clinical response was fast, so 1 patient avoided an emergency tracheostomy for laryngeal dyspnea. Four patients furtherly benefited from cisplatin-based chemoradiotherapy on residual tumor sites after the response to quadritherapy. Adverse events were manageable, except for an ICI-related liver toxicity in a patient. Overall, this short series indicates that a quadruple therapy with carboplatin-paclitaxel-CTX and pembrolizumab seems to be safe and active in patients with R/M HNSCC. This observation could be confirmed through further clinical trials.
Keywords: Cetuximab, Pembrolizumab, Paclitaxel, Carboplatin, Head and neck squamous cell carcinoma
Introduction
HNSCC are usually treated with curative intention by surgery and/or concomitant chemoradiotherapy. However, about 50% of patients will relapse with distant metastases and/or locoregional recurrence. About 5% of patients present with upfront metastases [1]. The EXTREME protocol (cisplatin or carboplatin, fluorouracil, and cetuximab (CTX) followed by weekly CTX maintenance) is considered a standard first-line treatment for R/M HNSCC [2]. However the side effects of six cycles of EXTREME limit its administration to patients who are fit. The TPEX protocol (cisplatin-docetaxel-CTX) for 4 cycles and CTX maintenance did not improve the overall survival, but it did lead to fewer toxicities than EXTREME [3]. The non-inferiority and better tolerance of carboplatin-paclitaxel-CTX compared to the EXTREME regimen were also reported [4, 5]. A carboplatin, paclitaxel, and CTX protocol has also been tested in a neoadjuvant setting in a case series of 24 previously untreated patients, who were unfit to receive the TPF protocol [6]. The response rate was 87% after 3 cycles of the PCE protocol, and some patients benefited from a locoregional curative treatment. Carboplatin, paclitaxel, and CTX have been used on a weekly regimen with a good tolerance in frail patients and a 43% overall response rate [7]. After decades of therapeutic stagnation, immunotherapy has emerged as a promising therapy and has transformed the field of cancer therapeutics. The KEYNOTE-048 phase III study established that pembrolizumab, an anti-PD1 antibody, plus standard chemotherapy with platinum and 5-fluorouracil is an appropriate first-line treatment for R/M HNSCC [8]. However, only 15–20% of patients ultimately benefit from anti-PD1 alone or in combination with chemotherapy, highlighting the need to improve the efficacy of immune checkpoint inhibitor for HNSCC treatment. A phase II trial reported that the combination of pembrolizumab and CTX is active and safe [9]. However, combinations of the most active agents in HNSCC, such as platinum-based doublet with taxane chemotherapy combined with CTX and CPI, have not yet been reported. To our knowledge, this combination is not under investigation according to the clinicaltrials.gov database.
In this case series, we discuss the outcomes of 8 patients who received 4 cycles of carboplatin-paclitaxel-CTX-pembrolizumab (further termed quadritherapy) for locally recurrent and/or metastatic HNSCC. Patients were treated in our institution from April 2021 to March 2022. All patients had pathologically confirmed HNSCC and were not immediately suitable for curative therapy (surgery or radiochemotherapy). All patients were smokers and had a negative p16 status. The PD-L1 score was not taken into account to prescribe pembrolizumab. Patients were informed by the investigators about the exploratory aspect of this regimen. Treatment was validated by the specialized medical committee for head and neck carcinomas in our institution.
Cycles of quadritherapy consisted of paclitaxel given at a dose of 175 mg/m2 and carboplatin (AUC 5 mg/mL/min) intravenous (IV) infusions, every 3 weeks. CTX was administered at 250 mg/m2 weekly. Pembrolizumab 200 mg IV infusion was started on the first day of the second cycle in order to ensure the tolerance of the tritherapy alone, then repeated every 3 weeks. All drugs are approved and available in France for R/M HNSCC. Tumor responses were assessed by clinical examination with nasofibroscopy and positron emission tomography-computed tomography (PET-CT) or cervicothoracic tomodensitometry after the completion of 4 cycles of quadritherapy.
Case Presentation
Patient 1
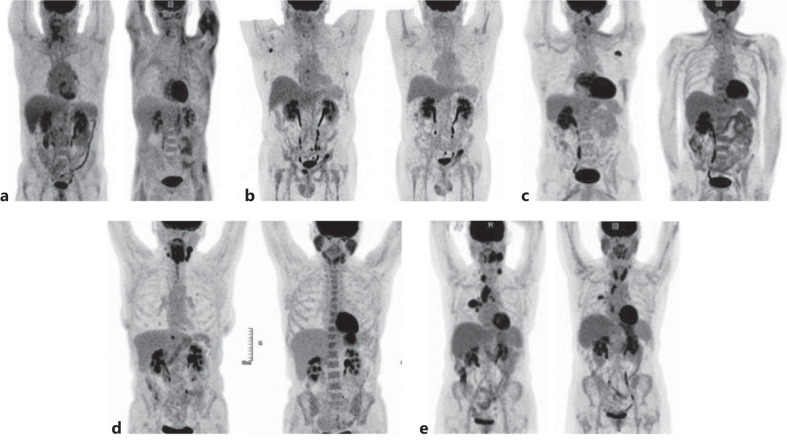
A 56-year-old male current smoker presented with a locally advanced squamous cell carcinoma of the right hypopharynx with homolateral cervical nodes. He received a 70-Gy irradiation with concomitant cisplatin in August–September 2020. In March 2021, clinical examination by nasofibroscopy and PET imaging concluded to a local disease pursuit. Due to a history of myocardial infarction with cerebral stroke, fluorouracil that is part of the EXTREME schedule was contra-indicated. He received 4 cycles of quadritherapy. Partial response (PR) was obtained since the tumor volume was reduced as assessed by a nasofibroscopy examination and SUVmax on PET decreased from 10.3 to 4.7 at the level of the hypopharynx (Fig. 1a). A single cycle of maintenance using CTX (500 mg/m2) and nivolumab (240 mg) was carried out in July 2021. Treatment was stopped because of drug-induced liver injury (AST: x 9 N; ALT: x 15 N; GGT: x 28 N; ALP: x 14 N); however, bilirubin levels remained normal. Liver MRI, liver biopsy, and viral serology were normal. Transaminases returned to a normal level with prednisolone (1 mg/kg). ALP and GGT levels decreased but did not return to normal. In January 2022, clinical progression of the hypopharyngeal tumor led to the resumption of weekly treatment by paclitaxel and carboplatin. Death due to a lung infection occurred in August 2022 (Table 1).
Fig. 1.
a–e PET scan from 5 patients before and after quadritherapy.
Table 1.
Summary of cases
| Gender | Age | ECOG status | Disease status before treatment | Tumor response after 4 cycles of quadritherapy | Duration of response to quadritherapy, months | Major toxicity | Evolution | Living status on September 2022 (survival since treatment, months) | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 56 | 1 | Local relapse after RCT* | PR | 7 | Grade IV liver toxicity | Progression | Dead (16) |
| 2 | Male | 59 | 1 | Bone metastasis after RCT | CR | 5 | Progression | Alive with disease (14) | |
| 3 | Male | 52 | 2 | Local Relapse after RCT | CR | 9 | Progression | Alive with disease (14) | |
| 4 | Male | 64 | 0 | Initially metastatic (lung) | CR | 12 | Secondary RCT on H&N tumor | Alive in CR (14) | |
| 5 | Female | 50 | 4 | Initially metastatic (lung, liver) | CR | 10 | Secondary RCT on H&N tumor | Alive in CR (10) | |
| 6 | Female | 63 | 2 | Initially metastatic (lung) | PR | 10 | Secondary RCT on lung then on H&N tumor | Alive in CR (10) | |
| 7 | Male | 67 | 1 | Lung metastasis after RCT | CR | 10 | Alive in CR (10) | ||
| 8 | Male | 49 | 0 | Locally advanced | CR | 8 | Secondary RCT on H&N tumor | Alive in CR (8) |
PR, partial response; CR, complete response; H&N, head and neck.
*RCT, radiochemotherapy with concomitant cisplatin.
Patient 2
A 59-year-old male current smoker presented with a left cervical node in October 2019. Biopsy of the left tonsil confirmed the presence of a squamous cell carcinoma. Cervical left node surgery in January–February 2020 was followed by 70-Gy radiotherapy of the oropharyngeal tumor and cervical lymph nodes with concomitant carboplatin and fluorouracil. Recurrent cervical lymph nodes and diffuse bone metastasis were diagnosed by PET scan in April 2021. After 4 cycles of quadritherapy from June to August 2021, PET imaging showed a complete metabolic response of the lymph nodes and bone metastases (Fig. 1b). A maintenance treatment with CTX 500 mg/m2 and nivolumab 240 mg, every 2 weeks was started. However, PET imaging showed a progression of bone metastasis in December 2021. Weekly paclitaxed was resumed in January 2022, then carboplatin and gemcitabine since July 2022.
Patient 3
A 52-year-old male current smoker was treated in June 2017 by 70-Gy radiotherapy and concomitant cisplatin in August–September 2020 for a squamous cell carcinoma of the hypopharynx. He complained of dysphagia, cervical pain, and headache in June 2021. Tumor recurrence was evidenced by PET imaging at the level of the pharynx with left cervical and left axillary lymph nodes. An isolated right cerebellar metastasis was treated by a single dose of 18-Gy irradiation under stereotaxic conditions. The patient received 4 cycles of quadritherapy from July to September 2021. Clinical examination was normalized. PET examination showed a complete metabolic response of all lesions in October 2021 (Fig. 1c). A maintenance treatment was started with CTX 500 mg/m2 and nivolumab 240 mg, every 2 weeks until February 2022. Gemcitabine and carboplatin were started due to a reprogression of the pharyngeal tumor and lymph nodes in March 2022. The patient is alive with stable tumor on treatment in September 2022.
Patient 4
A 64-year-old male current smoker presented in July 2021 with a locally advanced squamous cell carcinoma of the hypopharynx that extended to the esophageal sphincter, with bilateral cervical nodes and 2 lung metastases on PET scan. PR of the hypopharyngeal lesion was observed at clinical examination after 4 cycles of quadritherapy (August–September 2021). A complete regression of cervical lymph nodes and lung metastasis was recorded by CT scanning in October 2021. A maintenance treatment with CTX and nivolumab was done from October to December 2021. As lack of recurrence of lung metastasis on the CT-scan was confirmed, a 70-Gy irradiation with concomitant cisplatin was carried out on the hypopharynx and the cervical nodes in January–February 2022. Complete response (CR) is ongoing on September 2022.
Patient 5
A 50-year-old female current smoker presented in October 2021 with dyspnea at rest, complete dysphagia, and cervical nodes. Endoscopic examination showed an advanced squamous cell carcinoma of the hypopharynx that extended to the larynx and the esophageal orifice. Lung and liver metastases were detected on the PET scan. A quick improvement in dyspnea was obtained after starting quadritherapy, avoiding tracheostomy. After 4 cycles of quadritherapy from November 2021 to January 2022, CR was registered at clinical examination and on the PET scan (Fig. 1d). A 70-Gy irradiation with concomitant cisplatin was given on the initial hypopharyngeal tumor and cervical nodes in April–June 2022. CR is ongoing on September 2022.
Patient 6
A 63-year-old female current smoker presented in October 2021 with a 25-mm left cervical lymph node. A squamous cell carcinoma of the left base of the tongue was seen at endoscopy. Initial PET imaging showed hyperfixation of the base of the tongue, cervical lymph nodes, multiple mediastinal lymph nodes, and a single metastasis in the upper lobe of the right lung. Normalization of tongue and cervical fixation on PET was observed after 4 cycles of quadritherapy from December 2021 to February 2022 (Fig. 1e). However, only a partial attenuation of SUV was observed at the level of the right lung and mediastinal nodes. Pharyngoscopy examination did not detect any tumor. Radiotherapy with concomitant cisplatin and vinorelbine was performed on the upper right lung and mediastinum. In July 2022, PET showed an increased fixation of the pharyngeal area but no fixation at the lung and mediastinum level. Radiotherapy of the pharynx and cervical nodes was started with concomitant carboplatin (August 2022).
Patient 7
A 67-year-old male current smoker presented in August 2021 with voice modification. A large squamous cell carcinoma of the upper larynx was evidenced at endoscopy with bilateral lymph nodes on a CT scan. Radiotherapy with concomitant cisplatin was performed in August–September 2021. On February 2022, there were no residual tumor and cervical nodes at endoscopy and a CT scan, but multiple lung metastases appeared. Normalization of the lung scan was obtained after 4 cycles of quadritherapy from February 2022 to April 2022. A maintenance treatment with pembrolizumab is ongoing (September 2022).
Patient 8
A 49-year-old male current smoker presented in March 2022 with a voluminous right lymph node (50 mm diameter). A squamous cell of the right tonsil and tongue base was diagnosed after endoscopy. Due to the volume of the cervical lymphadenopathy, immediate radiochemotherapy was not deemed possible. The patient benefited from 4 courses of quadritherapy from June to July 2022 which resulted in CR. Radiotherapy with concomitant cisplatin was performed on the tumor and the cervical lymph nodes in July–August 2022.
The CARE Checklist has been completed by the authors for this case report, attached as online supplementary material (for all online suppl. material, see www.karger.com/doi/10.1159/000528326).
Discussion
Eight patients received quadritherapy with carboplatin-paclitaxel-CTX and pembrolizumab between March 2021 and April 2022. Three had locally recurrent head and neck disease or metastatic disease after previous radiotherapy and concomitant cisplatin. Four had distant metastases at the time of diagnosis and had not received previous treatment. One had a locally advanced disease not deeming initial radiochemotherapy. All patients but one were alive at the time of this analysis (September 15, 2022). Grade IV liver toxicity was related to the immune checkpoint inhibitors therapy in patient 1 (biological cholestasis without a bilirubin increase). Hepatic biology slowly normalized after corticosteroid therapy. Immunotherapy was definitively stopped for this patient. Other toxicities were mild in the series, mainly grade 1 or 2 hematological toxicities with some cycle delay. Also, there were some cutaneous effects, as can be expected with CTX therapy. We did not observe any drug-related interstitial pneumonitis. All patients responded to 4 cycles of quadritherapy (4 CR, 2 PR). Clinical response was quickly observed, most often after cycle 1, as judged by the cervical node volume and pain. Tracheostomy was avoided in 1 female patient. Interestingly, 4 patients with distant metastases at the time of diagnosis who had not received prior treatments obtained a very good PR and were irradiated on the residual tumor site, leading to CR. By comparison, response rates were 36% and 59% in the EXTREME and TPEx trials, respectively. In the keynote-048 study, response rate to pembrolizumab monotherapy was 17%, which increased to 36% when pembrolizumab was combined with standard cisplatin-fluorouracil chemotherapy regimen [7]. A sustained innate immunity activation by CTX through NK activation and antibody-dependent complement cytotoxicity (ADCC) could explain its synergy with the PD1 inhibitors as in NSCLC patients [10].
Conclusion
Our observation indicates that quadritherapy seems highly active in the first-line treatment of recurrent, initially metastatic, or locally advanced HNSCC. The additive role of combining an anti-PD1 antibody to a paclitaxel/carboplatin/CTX backbone could be investigated through a prospective randomized trial.
Acknowledgments
We thank Zuzana SAIDAK for the help in revising the manuscript. We thank Dr. Abdelkrim BOULANOUAR, Dr. Ahmed ABDAOUI, Dr. Alexandre COUTTE, and Dr. Reda GARIDI, for their helpful discussion.
Statement of Ethics
Ethics approval was not required in accordance with local guidelines for this retrospective unplanned study. Most patients were treated in emergency by four drugs that are commercially authorized in France for advanced head and neck cancers. Written informed consent was obtained from each patient for the treatment and for the publication of these case reports and any accompanying images. Written informed consent was obtained from the deceased patient’s next of kin for publication of the details. Authors avoided providing identifying information on patients.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
No funding received.
Author Contributions
Yuedan Zhou, Liza Medjkoune, Ali Youssef, Bruno Chauffert, and Mohamad Chehimi contributed to the patient’s treatment and elaborated the manuscript. Abdenour Ouikene, Agnes Galez, Farid Belkhir, and Pierre Saint Germain contributed to the treatment choice and to the patient’s treatment.
Funding Statement
No funding received.
Data Availability Statement
All data that support the findings of this study are included in this article. Data are taken from the individual medical files of Centre Hospitalier de Saint-Quentin. They are not publicly available on legal or ethical grounds. Any inquiries can be sent to the corresponding author, Bruno CHAUFFERT.
Supplementary Material
References
- 1. Machiels JP, René Leemans C, Golusinski W, Grau C, Licitra L, Gregoire V, et al. Squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(11):1462–75. 10.1016/j.annonc.2020.07.011. [DOI] [PubMed] [Google Scholar]
- 2. Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359(11):1116–27. 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 3. Guigay J, Aupérin A, Fayette J, Saada-Bouzid E, Lafond C, Taberna M, et al. Cetuximab, docetaxel, and cisplatin versus platinum, fluorouracil, and cetuximab as first-line treatment in patients with recurrent or metastatic head and neck squamous-cell carcinoma (GORTEC 2014-01 TPExtreme): a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2021;22(4):463–75. 10.1016/S1470-2045(20)30755-5. [DOI] [PubMed] [Google Scholar]
- 4. Tsakonas G, Specht L, Kristensen CA, Moreno MHC, Cange HH, Soderstrom K, et al. Randomized phase II study with cetuximab in combination with 5-FU and cisplatin or carboplatin versus. cetuximab in combination with paclitaxel and carboplatin for treatment of patients with relapsed or metastatic squamous cell carcinoma of the head and neck (CETMET trial). Cancers. 2020;12(11):3110–21. 10.3390/cancers12113110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tahara M, Kiyota N, Yokota T, Hasegawa Y, Muro K, Takahashi S, et al. Phase II trial of combination treatment with paclitaxel, carboplatin and cetuximab (PCE) as first-line treatment in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck (CSPOR-HN02). Ann Oncol. 2018;29(4):1004–9. 10.1093/annonc/mdy040. [DOI] [PubMed] [Google Scholar]
- 6. Shirasu H, Yokota T, Kawakami T, Hamauchi S, Onozawa Y, Ogawa H, et al. Efficacy and feasibility of induction chemotherapy with paclitaxel, carboplatin and cetuximab for locally advanced unresectable head and neck cancer patients ineligible for combination treatment with docetaxel, cisplatin, and 5-fluorouracil. Int J Clin Oncol. 2020;25(11):1914–20. 10.1007/s10147-020-01742-6. [DOI] [PubMed] [Google Scholar]
- 7. Carinato H, Burgy M, Ferry R, Fischbach C, Kalish M, Guihard S, et al. Weekly paclitaxel, carboplatin, and cetuximab as first-line treatment of recurrent and/or metastatic head and neck squamous cell carcinoma for patients ineligible to cisplatin-based chemotherapy: a retrospective monocentric study in 60 patients. Front Oncol. 2021;11:714551. 10.3389/fonc.2021.714551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G Jr, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394(10212):1915–28. 10.1016/S0140-6736(19)32591-7. [DOI] [PubMed] [Google Scholar]
- 9. Sacco AG, Chen R, Worden FP, Wong DJL, Adkins D, Swiecicki P, et al. Pembrolizumab plus cetuximab in patients with recurrent or metastatic head and neck squamous cell carcinoma: an open-label, multi-arm, non-randomised, multicentre, phase 2 trial. Lancet Oncol. 2021;22(6):883–92. 10.1016/S1470-2045(21)00136-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Della Corte CM, Fasano M, Ciaramella V, Cimmino F, Cardnell R, Gay CM, et al. Anti-tumor activity of cetuximab plus avelumab in non-small cell lung cancer patients involves innate immunity activation: findings from the CAVE-Lung trial. J Exp Clin Cancer Res. 2022;41(1):109–19. 10.1186/s13046-022-02332-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data that support the findings of this study are included in this article. Data are taken from the individual medical files of Centre Hospitalier de Saint-Quentin. They are not publicly available on legal or ethical grounds. Any inquiries can be sent to the corresponding author, Bruno CHAUFFERT.