Abstract
Despite advances in cancer medicine and research, invasive and potentially risky procedures such as biopsies, venous blood tests, imaging, colonoscopy, and pap smear tests are still primarily used for screening, staging, and assessing response to therapy. The development and interdisciplinary use of biomarkers from urine, feces, saliva, scent, and capillary blood collected with minimally invasive methods represents a potential opportunity for integration with biomarker analysis for cancers, both in clinical practice (e.g., in screening, treatment, and disease monitoring, and improved quality of life for patients) and population-based research (e.g., in epidemiology/public health, studies of social and environmental determinants, and evolutionary medicine). In this article, we review the scientific rationale, benefits, challenges, and potential opportunities for measuring cancer-related biomarkers in samples collected through minimally invasive methods.
1 |. INTRODUCTION
Cancers are a group of diseases in which normal cells become dysregulated and divide uncontrollably. Despite limited data availability in certain regions of the world, it is currently estimated that cancers are the second leading cause of death globally (WHO, 2018). There is considerable variation across nations and regions in the incidence of most cancer types, which has largely been attributed to differences in lifestyle and environment (Larsen et al., 2020; Lortet-Tieulent et al., 2020; Sloan & Gelband, 2007). Low- and middle-income countries (LMICs) tend to have a higher burden of infectious disease than high-income countries (HICs), which is associated with a higher incidence of stomach, liver, esophageal, and cervical cancers; in contrast, in HICs, reproductive patterns and dietary factors are associated with higher incidence of reproductive cancers (breast, prostate, and ovarian) and colorectal cancers (Sloan & Gelband, 2007). Yet, some similarities exist. For example, in both LMICs and HICs, lung cancer is the most common type of cancer in men (mostly due to the global prevalence of smoking), while breast cancer is most common in women (though the reasons for this are less clear). There are still many unknowns when it comes to cancer, and limitations in currently available methods to detect cancers are a major barrier to surveillance and research. Biomarkers collected through minimally invasive methods have the potential to greatly expand multidisciplinary perspectives on cancers, which will improve our understanding of this complex set of diseases.
One-third of countries have no published data on cancer rates and another third lack comprehensive national coverage (WHO, 2018). This is partly from a lack of access to current diagnostic tools, which include imaging and pathology. A lack of incidence reporting is particularly troublesome because projections indicate that cancer cases will increase by 60% worldwide in the next two decades with an 81% increase in LMICs (WHO, 2020). Diagnostic tools based on measurement of biomarkers from minimally invasively collected samples could radically improve accessibility; however, the development of biomarkers with sufficient sensitivity (the rate of detecting true positives) and specificity (the rate of detecting true negatives) for cancer diagnostics and treatment has proven difficult. New technologies and analytical approaches are beginning to expand the possibilities. Considerable research effort is being devoted to developing reliable venous blood biomarkers for use in the detection of cancer and monitoring of treatment outcomes; however, less research has been done to develop biomarkers that use minimally invasive sample types. Venous biomarkers are themselves usually considered minimally invasive in the setting of cancer screening as they are less invasive than common procedures such as biopsies and pap smears, and they do not have the carcinogenic effects of certain imaging technologies. However, for the purpose of this article, “minimally invasively collected biomarkers” refers to those measured in urine, feces, saliva, exhaled breath, and finger prick-obtained capillary blood. These techniques are just beginning to be researched and developed in cancer therapeutics and population-based research, yet they hold enormous potential for revolutionizing these fields.
In this article, we review emerging trends in biomarker research with a focus on minimally invasively collected sample types that can be adopted to detect cancer more quickly and widely with less risk and loss of quality of life. Our goal is to connect the pioneering work on minimally invasive sample collection and analysis in human biology with cancer medicine and epidemiology. Uniting methods and perspectives from these different fields has the potential to improve health globally. Medical research has thoroughly examined many molecules that are suitable for the detection of cancer at a population level, despite presently being of limited use in clinical settings due to more stringent requirements for sensitivity and specificity. Uniting oncology with human biology, which is a leader in the development of minimally invasive techniques for population-based studies (Eick et al., 2016, 2017, 2019, 2020; Gildner, 2021; McDade, 2014; McDade et al., 2007, 2020; Rej et al., 2021; Valeggia, 2007), provides an opportunity to apply well-researched methods to a field that has recognized the need for greater biomarker sampling and less invasive techniques.
2 |. POTENTIAL FOR CANCER BIOMARKERS IN SAMPLES COLLECTED WITH MINIMALLY INVASIVE COLLECTION METHODS
The successful development of biomarkers that can be measured in samples collected through minimally invasive methods for different types of cancer could have widespread benefits, both clinically and in population-based research. Biomarkers of cancer have the potential to be used in screening, cancer classification, risk assessment, staging (i.e., determination of tumor burden), personalization and monitoring of treatment, prognosis, and follow-up (i.e., continued screening for a recurrence). Additionally, they could be used in population-based research to provide key epidemiological data on rates of different cancers globally and to investigate the social, environmental, and evolutionary factors that are associated with cancer rates.
2.1 |. Cancer diagnosis, treatment, and surveillance
Screening entire populations (or, in some cases, at-risk populations such as people with certain germline mutations or a family history of cancer) for specific cancer types have the best probability of successful outcomes because the best predictor of curative success is early detection (e.g., Etzioni et al., 2003; Ginsburg et al., 2020). Once cancer cells spread through the blood to other organs, a process called metastasis, control, and treatment become more difficult. Currently, many cancers are metastatic at presentation. In most cases screening depends on invasive physical exams (e.g., pelvic exams), biopsies of the cancer tissue and subsequent evaluation by a pathologist, and imaging techniques (such as mammograms) that are carcinogenic and increase the risk of cancer by 0.09% to 1.9% for every 10 annual scans (Wen et al., 2013). These suboptimal detection strategies are also not readily available in most rural and other under-resourced areas and are often relegated to expensive private clinics. Analyses of data from 57 countries showed that only 19% of women in LMICs and 63% of women in HICs receive adequate screening for cervical cancer (Gakidou et al., 2008), despite pelvic exams being the most cost-effective strategy for cancer screening (WHO, 2020). Biomarkers measured in minimally invasively collected sample types could be used to screen populations in many settings, including in LMICs, due to the reduced risks associated with collection of these samples compared with venous samples and imaging, and decreased need for the presence of a health clinic for sample collection. Cancer screening using validated biomarker sample types collected through minimally invasive methods could allow more widespread cancer screening and data collection for both population-based research and public health purposes.
Clinical incentives for increased biomarker development in cancer research and therapeutics include improved treatment personalization and increased follow-up surveillance. Treatment personalization involves the detection of patient- and tumor-specific molecular drivers (e.g., somatic mutations in replication pathways) and the use of biologic drugs targeted toward a specific individual’s cancer. These targeted biologic drugs can both complement and, in some cases, replace traditional treatments such as chemotherapy and radiation therapy. For example, when a tumor cell utilizes estrogens (the primary sex hormones in women), using a biologic drug that blocks the estrogen receptor (ER) reduces deaths in patients with ER-sensitive cancers by 31% when detected early, regardless of chemotherapy use (EBCTCG, 2005). Although ER presence is currently detected by histopathology, many blood-based biomarkers are being tested for potential use (Graveel et al., 2015; Mohan et al., 2019; Núñez, 2019). For example, a panel used to detect the ER and 20 other biomarkers is currently being used to determine treatment stratification (McVeigh & Kerin, 2017). Follow-up screening for cancer recurrence usually needs to take place frequently (approximately every 1 to 6 months) for at least the first 5 years after treatment, then must continue at least yearly to detect any possible recurrence of cancer. Making screening for progression more accessible by biomarker screening of samples collected through minimally invasive methods could prevent cancer deaths, especially in areas that lack convenient access to healthcare facilities.
2.2 |. Population-based research
Measurement of cancer biomarkers in minimally invasive sample types could also contribute to a refinement in our understanding of the social determinants of cancer. Social determinants affect cancer incidence and mortality in complex ways. For example, increased incidence of different cancers has been associated with obesity, smoking, alcohol abuse, environmental pollution, occupational carcinogens, dietary factors, socioeconomic status (SES), human papilloma virus (HPV) infection, and structural inequalities based on race (Calle & Kaaks, 2004; Danaei et al., 2005; Driscoll et al., 2005; Lannin et al., 1998; Salgado et al., 2020; Vineis & Xun, 2009; Xia et al., 2013; Yost et al., 2001). To give one example, it was estimated that obesity accounts for ~6% of cancer incidence in the United States (Basen-Engquist & Chang, 2011), and obesity is closely related to SES (Newton et al., 2017). However, the relationship between cancer incidence and social determinants have only been well-studied in a few countries such as the United States, China, and several countries in Europe (e.g., Alcaraz et al., 2020; Fei et al., 2015; Nagel et al., 2007; Vineis & Husgafvel-Pursiainen, 2005; Zhao et al., 2006). Furthermore, the extent and type of social determinants that cause cancer differ based on the cancer type (Tomasetti et al., 2017; Tomasetti & Vogelstein, 2015). Perhaps this complexity is why the social determinants of cancers have not been as well studied as the social determinants of some other diseases, such as cardiovascular disease and infectious diseases (Abrams & Szefler, 2020; Marmot, 2015; Marmot & Wilkinson, 2005). Unlike these other diseases, which tend to have higher incidence and/or prevalence in people with low SES, association with SES is highly variable among cancer types. For instance, in southern Europe there is a negative association between lung, colorectal, and cervical cancer incidence and SES, whereas there is a positive association between prostate and breast cancer incidence and SES (Garcia-Gil et al., 2014). Using biomarkers to detect cancer in conjunction with the collection of sociodemographic and lifestyle data will lead to a better understanding of how specific social and environmental factors are related to the development and progression of certain cancers, as well as the specific physiological pathways through which social conditions are embodied. Furthermore, the identification of specific causal social and environmental factors will allow more effective targeted preventative approaches. This is critical because preventative approaches to cancer control are more effective and cost-efficient than treatment-based approaches.
A key social contributor to cancer risk is through the role of healthcare access in shaping mortality. Less access to preventative measures (such as the HPV vaccine), lower rates of detection/delayed detection, and less aggressive treatment are associated with higher mortality (McDaniel et al., 2019). By improving access to screening, cancer biomarkers measured in minimally invasively collected samples could lessen the association between both lower SES and access to healthcare and higher rates of recurrence and lower rates of survival (Aziz et al., 2010; Barrington et al., 2016; Panagopoulou et al., 2012). Being able to detect cancer in settings with little to no access to health care could help save lives and contribute to cancer management programs.
For these reasons, biomarker development has been a focus of cancer research for at least the past 10 years (Mishra & Verma, 2010). Technical difficulties surrounding biomarker development and a focus on utility and profitability in HICs have hindered the widespread use of biomarkers in epidemiology (Vineis & Perera, 2007). However, to fully understand what factors affect biomarker levels and patterns in cancer, large population-based studies with environmental and non-cancer health components are needed. To illustrate the lack of attention to cancer in the human biology literature, a search for the terms “cancer,” “tumor,” and “malignancy” in the American Journal of Human Biology revealed only one article (Wang et al., 2018) in the past 5 years. Yet, cancers are a major cause of mortality globally and have many social and environmental determinants, which are the main focuses of human biology and closely related disciplines. Furthermore, there are ongoing global, social, and environmental changes (e.g., nutrition transition, market integration/economic development, and changing exposure to pesticides) that are likely driving changing incidence of certain cancer types. There are currently many emerging opportunities for interdisciplinary cancer research, many of which revolve around biomarker applications.
3 |. BIOMARKERS AT THE FRONTIER OF CANCER RESEARCH
3.1 |. A brief history of cancer in humans
The discovery of the structure of DNA in 1953 allowed researchers to show that carcinogens changed the genetic structure and that these changes could cause normal cells to become dysregulated (Sudhakar, 2009). Later, the development of large-scale DNA sequencing methods allowed researchers to begin linking certain genetic pathways to the development of cancer, and the recent increase in accessibility of sequencing technologies is revealing the complexity and extent of variation in molecular drivers for cancer. As this understanding has developed, so too have diagnostic and treatment options. Identification of the molecular drivers of cancer means that measurement of these drivers from minimally invasively collected sample types is possible, which is of increasing interest to biomedical researchers (e.g., Brikun et al., 2019; Cristaldi et al., 2019; Diener et al., 2019; Hirschfeld et al., 2020; Varmus, 2006).
Clinically, the three main areas of ongoing biomarker development are (1) diagnostic biomarkers, which detect cancers; (2) predictive biomarkers, which identify tumor characteristics to target certain molecularly-defined cancers for treatment; and (3) prognostic biomarkers, which inform about the aggressiveness of the cancer and the potential treatment outcomes. The recent increase in accessibility of whole-genome sequencing is allowing researchers to identify the combinations of germline and somatic mutations and molecular drivers of different cancers (Kilpivaara & Aaltonen, 2013; Watson et al., 2013). Recently, a genome-wide comparison of 2658 individual cancers to the corresponding normal tissue for 38 different tumor types showed that on average, tumors have four to five driver mutations in coding and noncoding elements (Campbell et al., 2020). In approximately 5% of cases, no drivers were identified, possibly because the drivers were epigenetic modifications. Many studies of biomarkers have shown you can get better sensitivity and specificity by evaluation methylation markers (Chihara et al., 2013; Costa et al., 2010; Guerrero-Preston et al., 2011; Liyanage et al., 2019; Moreira-Barbosa et al., 2018; Srisuttee et al., 2020; Wang et al., 2016). Some have postulated that these types of discrepancies are because the mutational theory of cancer is incorrect or incomplete (Brücher & Jamall, 2016; Soto & Sonnenschein, 2004, 2011). Genomic studies could potentially lead to new diagnostic panels that simultaneously test for numerous biomarkers from a single sample. Biomarker panels might improve current limitations related to biomarker sensitivity and specificity, which refer to the ability to detect true positives and true negatives, respectively. For example, cancer antigen 125 (CA-125) has a sensitivity and specificity of 89% and 72% in the diagnosis of ovarian cancer (Moss et al., 2005), whereas a panel including CA-125 (CA-125 with CA 19–9, EGFR, G-CSF, Eotaxin, IL-2R, cVCAM, MIF) had a sensitivity and specificity of 98% and 99% respectively (Muinao et al., 2019). Understanding this variation in the molecular drivers and outcomes of cancer could allow us to create diagnostic tools that encompass the entire population of different cancers.
3.2 |. Cancer cell biology as the basis for biomarkers
Unlike many other diseases that have a single physiological cause, cancer is an entire category of diseases with different physiological drivers. This increases the potential utility of biomarkers. Since cancer is the dysregulation of cellular growth, each of the estimated 411 cell types in the human body can form a different type of cancer (Trapnell, 2015; Vickaryous & Hall, 2006). Furthermore, there are multiple physiological mechanisms and mutations that lead to cancers in the various cell types. To take one example, 11 genes have been identified as major molecular drivers of adenocarcinoma of the lung when mutated (EGFR, RAS, ALK, HER2, BRAF, MET, ROS1, NTRK1, MAP2K1, PIK3CA, and RET), and 31% of lung adenocarcinomas have no identified molecular drivers (Hirsch et al., 2016). Squamous cell carcinoma of the lung shares two of these molecular drivers (EGFR and PIK3CA) and has five unique drivers identified thus far (DDR2, FGFR1, PTEN, PDGFRA, and FGFR2). Molecular drivers can also be shared across the same cell types (e.g., glandular cells) from different locations (e.g., mutations in the RAS oncogene can be found in adenocarcinoma of the lung, pancreas, colon, thyroid, and in myeloid leukemia; Bos, 1989). Despite individual molecular drivers sometimes being present across cell types, overall molecular expression patterns of cancers are often different based on cell type (Hoadley et al., 2018). The aggressiveness and treatment outcomes of these different cancers also vary based on these molecular classifications. This makes identification of the molecular drivers of cancer important to improve prognosis and personalize treatment. In addition, technological innovation in instrument platforms is now enabling the simultaneous measurement of multiple molecular markers using ever-smaller volumes of samples.
There are two main kinds of molecular drivers for cancer: mutations in oncogenes that control cell division and differentiation, and mutations in tumor suppressor genes that encode proteins that repair DNA mutations and regulate cell death in dysregulated cells. Driver mutations are often found in the PI3K – AKT – mTOR pathway, which is responsible for cell proliferation, growth, and metabolism (Figure 1; Yuan & Cantley, 2008). Overactivation of this pathway also prevents apoptosis. Normally, the pathway that leads to cell proliferation is initiated by growth factor signaling; however, driver mutations activate it in the absence of signaling molecules. Many additional molecular drivers targeted as cancer biomarkers are a part of this pathway (e.g., PTEN, EGFR, HER, MET, RAS). Additionally, all multicellular organisms have tumor suppressor genes that prevent cell division from becoming dysregulated (Pearson & Alvarado, 2008). One major tumor suppressor, the TP53 gene (which encodes the p53 protein) is mutated or deleted in 50% of all cancers. The p53 protein is responsible for DNA repair, cell cycle arrest, senescence, and apoptosis (Muller & Vousden, 2014; Whibley et al., 2009).
FIGURE 1.

Common cellular pathways detected with cancer biomarkers. Green endpoints are primarily cancer-preventing, and yellow endpoints are primarily cancer-promoting. Expression of many of the biomarkers listed above can be detected at the mRNA or protein levels. Some, such as EGFR are pictured here as genes and proteins, whereas others such as CDKN2A (associated with the protein p16) are only depicted as one or the other. 5-HIAA, 5-hydroxyindoleacetic acid; 5-HT, 5-hydroxytryptamine (serotonin); PSA, prostate specific antigen; PCA3, Prostate cancer antigen 3; BTA, bladder tumor antigen; VMA, vanillylmandelic acid; HMA, homomandelic acid; TNF-α, tumor necrosis factor alpha; VEGF, vascular endothelial growth factor; EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; HER, human epidermal growth factor receptor; RAS and KRAS, rat sarcoma associated proteins; CDKN2a, cyclin dependent kinase inhibitor 2a; BCRP, breast cancer resistance protein; RAF, rapidly accelerated fibrosarcoma protein kinases; MEK, mitogen activated protein kinase; ERK, extracellular signal-regulated kinase; PTEN, phosphatase and tensin homolog; PK-13, protein kinase 13; MTOR, mechanistic target of rapamycin; FOXO, forkhead family of transcription factors. Created with Biorender.com
Molecular drivers of cancer change cellular homeostasis and, therefore, many other pathways in the cell also change to meet the new demands of the malignant cell. These effects have been well-studied in the field of cancer metabolomics. Pathways altered because of cancer change cellular bioenergetics and redox balance, and enhance bio-synthesis in cells without mutations in the underlying genes encoding the components of these pathways (for a more in-depth review see DeBerardinis & Chandel, 2016). Changes to bioenergetics make cancer cells take in glucose and produce lactate, even when no oxygen is available. The cell will use all available oxygen (making the surrounding environment hypoxic) in aerobic glycolysis, which produces precursors needed for anabolic pathways to produce energy in the absence of oxygen. Cancer cells use these and many other molecules that are not normally utilized to create ATP (cellular energy). This increase in metabolism creates potentially damaging levels of reactive oxygen species (viz., O2−, H2O2, and OH−). In response, malignant cells increase production of NADPH and other antioxidants through numerous pathways. Cancer cells also rely on glutamine for increased biosynthesis. By increasing glutamine uptake and stores, malignant cells can create more nonessential amino acids, and uptake more essential amino acids. This increase in amino acid availability inside the cell allows for the creation of macromolecules that support cell division. Even beyond the cell, changes in the demand for nutrients and oxygen lead to an increase in angiogenesis, or the development of new blood vessels to the tumor (Carmeliet & Jain, 2000). Altered intake and production of the molecules reviewed here and others could potentially be detected in minimally invasively collected samples to aid in cancer detection.
4 |. CURRENT ISSUES IN THE DEVELOPMENT OF BIOMARKERS FOR CANCER
The main barrier to biomarker development for applications in cancer is the need for sensitivity and specificity. Biomarkers for cancer can be elevated in other conditions and diseases, making the false-positive rates of these tests generally quite high. For example, the prostate specific antigen (PSA) test has been used routinely to screen for prostate cancer, but because it is also elevated in other prostate conditions, such as benign prostatic hyperplasia and low-grade prostate tumors, this led to unnecessary biopsies and over-treatment while only reducing prostate cancer deaths by a small amount (Andriole et al., 2009; Schröder et al., 2009). Not every person with a certain type of cancer will have an elevated amount of a certain biomarker and not every cancer has unique biomarkers. In addition, distinguishing between aged tissues, precancerous tissues, and cancerous tissues is difficult due to cancer’s gradual evolution (Gerstung et al., 2020). Biomarker samples collected with minimally invasive methods (e.g., finger-prick blood collected as dried blood spots [DBS]) face added technical difficulties with detecting lower levels of the marker of interest compared with venous blood or tissue. However, biomarker panels that can detect patterns of expression of numerous biomarkers are being developed that can overcome limitations related to heterogeneity (Bensalah et al., 2007; Shivapurkar & Gazdar, 2010). For example, the FDA-approved Cologuard (Exact Sciences, Madison, WI) uses 11 different biomarkers for the detection of colorectal cancer, including seven DNA mutation markers, two DNA methylation markers, a hemoglobin marker to detect occult blood, and a beta-actin marker as an internal control to verify sample integrity (Ahlquist, 2015; Imperiale et al., 2014).
Other issues with biomarker development include unforeseen complexity in underlying biological systems, technically inadequate assays, and high normal variation hiding signal (Bensalah et al., 2007; Kern, 2012). Although small studies might make a biomarker seem promising, larger studies do not always produce consistent results (Kern, 2012). This occurs because while a marker might be related to an outcome being measured, there are many other factors that could also affect biomarker levels. For example, the biomarker under investigation might be only transiently expressed (Bensalah et al., 2007). Understanding biological complexity is important for choosing feasible targets. Technically inadequate assays are caused by a lack of testing reliability, a lack of standardization in procedures, a lack of comparison assays, or a lack of appropriate participants. Furthermore, the technologies used are often novel and expensive, which hinders the independent evaluation of the potential biomarker by other laboratories. Finally, false positives can result from normal variation due to aging or the presence of other diseases or environmental factors such as food, drugs, and alternative therapies. In addition, there are methodological errors and inadequacies associated with biomarker development for cancer such as insufficiently accounting for normal variation and hierarchical data structures (Figure 2), deficiencies in study population or study design, and technically inadequate assays (Füzéry et al., 2013; Kern, 2012).
FIGURE 2.
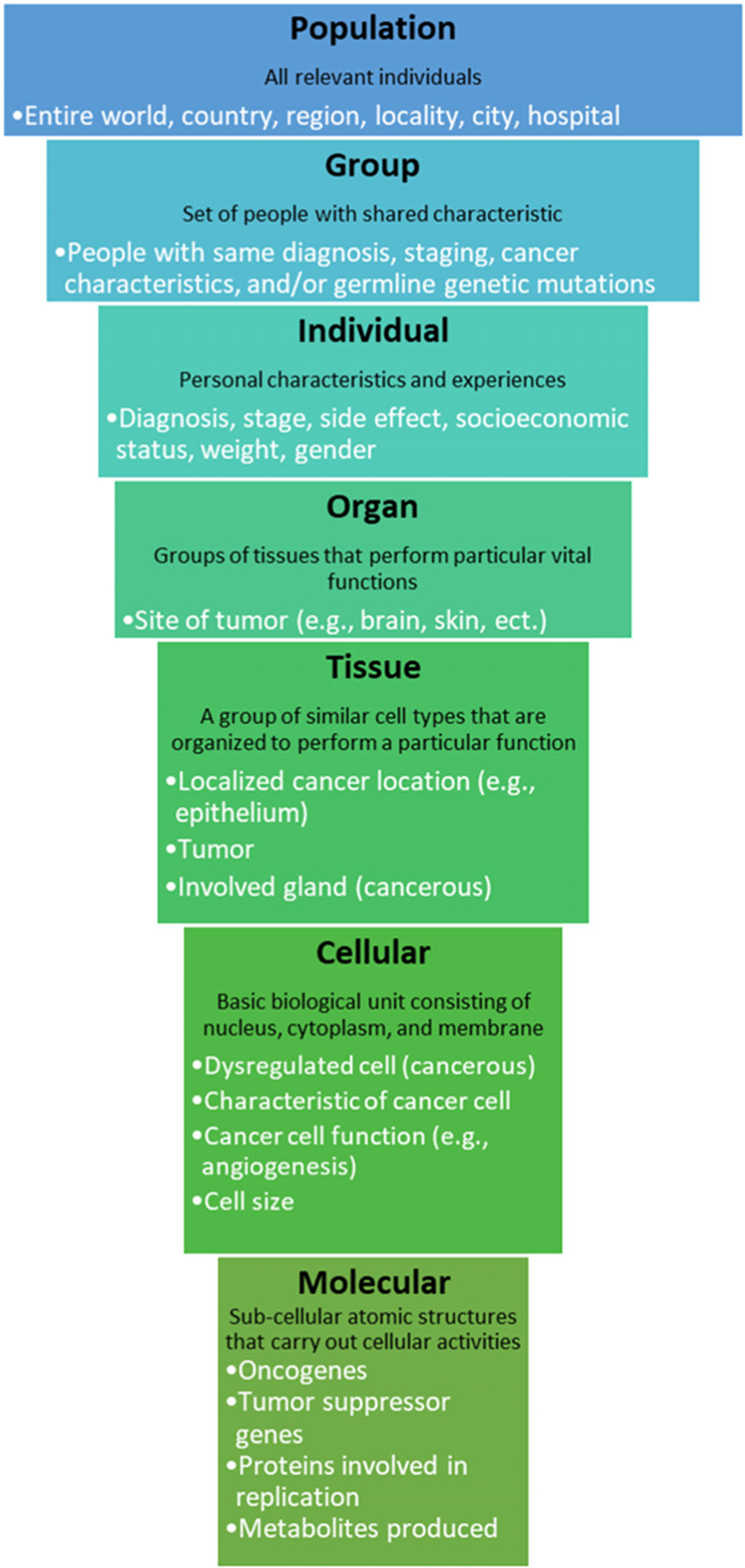
Multilevel structure of human cancer biology. The levels presented were adapted from Novikoff, 1945. This structure should be controlled for statistically using a multilevel model or including group-level control variables
Treatment personalization based on the detection of molecular drivers as cancer biomarkers has also proven difficult. For example, inhibiting the epidermal growth factor receptor (EGFR) was considered a promising therapy for head and neck cancer since its overexpression is a common molecular driver of this cancer type (Byeon et al., 2019). However, the development and use of many EGFR inhibitors improved survival rates very little. Since many other cell functions are altered in malignant cells, the cell will often develop new pathways to continue uncontrolled growth and division when a therapeutic is used to block a molecular driver (de Castro et al., 2013). This has led to treatments that target numerous molecular drivers of certain cancer; while this approach shows more efficacy, it can also lead to more side effects (Karam et al., 2018). For example, upregulation of HER2/HER3 signaling was shown to be a mechanism by which tumors become resistant to EGFR inhibitors (Figure 1), and new combination therapies with EGFR and HER inhibitors show more promise than EGFR inhibitors alone (Byeon et al., 2019). As treatment becomes more personalized, additional cancer biomarkers will need to be detected to adequately assess the characteristics of that particular cancer.
5 |. MINIMALLY INVASIVE SAMPLE TYPES AND BIOMARKERS FOR CANCER DETECTION
Here we review cancer biomarkers that have been detected in samples collected using minimally invasive methods. Many of the biomarkers have been further characterized in studies determining their efficacy and could currently be used in population-based research. Six types of biomarkers are reviewed: DNA, RNA, protein, metabolite, bacteria, and blood cells. However, the same biomarker can often be measured in different sample types (e.g., DNA, RNA, and protein).
5.1 |. Nucleic acids
5.1.1 |. DNA
There are two ways DNA can be used in cancer research: (1) detection of cancer based on detection of tumor cells or DNA released by the tumor into the circulation and (2) detection of familial tendency and/or genetic propensity for certain cancer based on germline DNA. The second is beyond the scope of this article (for review see Bylstra et al., 2021; Chanock & Ostrander, 2020; Chatrath et al., 2020; Gayther et al., 1995; Rotunno et al., 2020). Examples of minimally invasive samples that can be used to obtain DNA for biomarker analysis are fluids containing tumor cells or cell-free tumor DNA (Peng et al., 2017). Cell-free tumor DNA can be shed by tumor cells or released during apoptosis. Tumor DNA can be extracted from minimally invasive sample types such as capillary blood, feces, urine, and saliva (Garcia-Cordero & Maerkl, 2020; Kaczor-Urbanowicz et al., 2019; Nguyen-Dumont et al., 2015; Peng et al., 2017). However, to date tumor DNA has been extracted most successfully from feces, urine, and saliva. Very few studies have been performed to validate the presence of tumor DNA in capillary blood collected via DBS (Nguyen-Dumont et al., 2015; Ruhaak et al., 2012); however, the studies that have been conducted used polymerase chain reaction (PCR) and high-performance liquid chromatography/mass spectrometry (HPLC-MS) to detect tumor DNA. Microfluidic and chip technologies to assess tumor DNA in capillary blood samples also show promise for revolutionizing cancer detection and treatment but are not yet widely accessible (Guo et al., 2021; Iliescu et al., 2019).
Currently, two assays that detect tumor DNA from minimally invasively collected sample types have been FDA approved, with many others showing promise (Table 1). Of those approved by the FDA, the first is a multi-target stool DNA test (Cologuard; Exact Sciences, Madison, WI) that is becoming widespread in the US to detect colorectal cancer (Ahlquist, 2015; Imperiale et al., 2014). Second, UroVysion (Abbott Laboratories Inc, Hoofddorp, The Netherlands) is a fluorescence in situ hybridization (FISH) test used to detect bladder cancer based on analysis of DNA in urine samples; however, it has low sensitivity for low-grade bladder cancers (Oeyen et al., 2019). Other biomarker tests that use tumor DNA in urine to detect urological cancers (Chung et al., 2011; Costa et al., 2010; Larsen et al., 2019) and prostate cancer (Brikun et al., 2019; O’Reilly et al., 2019) are also being developed. Biomarker panels detecting DNA methylation patterns using methylation-specific polymerase chain reaction (MSP) show high sensitivity and specificity for detecting bladder cancer and prostate cancer (Larsen et al., 2019). For example, a preliminary analysis showed that when six or more genes are methylated (including SOX1, TJP2, MYOD, HOXA9_1, HOXA9_2, VAMP8, CASP8, SPP1, IFNG, CAPG, HLADPA1, and/or RIPK3) in DNA from urine you can distinguish people who have bladder cancer from those that do not with nearly perfect accuracy (Chihara et al., 2013). Biomarker panels for DNA extracted from saliva are promising for the detection of oral and oropharangyal cancers (Cristaldi et al., 2019; Liyanage et al., 2019). For example, using PCR to detect a C deletion in exon 4 codon 63 of the p53 gene has shown promising sensitivity and specificity in recent studies (Sukhija et al., 2015; Tekcan et al., 2020). Other studies have shown that pancreatic cancer can be detected from tumor DNA in stool and saliva (Debernardi et al., 2015; Yang et al., 2014). There is also the potential for developing salivary biomarkers for other distant (i.e., nonoral) cancers (Rapado-González et al., 2020).
TABLE 1.
Current biomarkers used for the detection of cancers in minimally invasively collected sample types
| Biomarker name | Sample type | Biomarker type | Method | Cancer(s) detected | Benefits | Challenges | Citation(s) |
|---|---|---|---|---|---|---|---|
| FDA approved | |||||||
| Cologuard | feces | DNA | ELISA, PCR | colorectal cancers | High sensitivity for colorectal cancer | Sensitivity for advanced adenoma (precancerous polyps) is low to moderate, expensive | Onieva-García et al., 2015 |
| UroVysion | urine | DNA | FISH | bladder cancers | More sensitive than urine cytology, can increase sensitivity by repeating test | Not effective if specimen has excessive granulocytes or bacteriuria, sometimes detects nonbladder cancers, does not always detect the earliest stage of cancer, expensive | Hajdinjak, 2008; Oeyen et al., 2019 |
| Progensa PCA3 | Urine | RNA | PCR | Prostate cancers | Higher specificity and predictive value than PSA | Lower sensitivity than PSA, more expensive than PSA | Durand et al., 2011; Eskra et al., 2019 |
| NMP22 | Urine | Protein | ELISA | Bladder cancers | More sensitive than urine cytology | Lower specificity and predictive value than urine cytology | Kumar et al., 2006; Oeyen et al., 2019 |
| BTA TRAK/STAT | Urine | Protein | ELISA | Bladder cancers | High sensitivity except in early tumors, specificity adequate alone and very high if combined with NMP22 | High rate of false positives with gross hematuria | Öge et al., 2000; Öge et al., 2002 |
| Commonly used | |||||||
| PSA | Urine | Protein | ELISA | Prostate cancers | Lots of research, false-positive diagnoses are well known | Low specificity, detects non-clinically significant low-grade malignancies | Eskra et al., 2019; Pezaro et al., 2014 |
| Hematuria | Urine | Cell | Point-of-care dipstick | Bladder cancers | Detects all grades equally, detects earlier than symptom presentation, relatively cost effective | Low specificity, especially for women under 40 | Mariani et al., 1989; Messing et al., 1995 |
| Beta-hCGa | Urine | Protein | Point-of-care dipstick | Choriocarcinoma and germline tumors | High sensitivity, highly specific for malignancya, scales with tumor burden and is indicative of stage, relatively cost-effective | Low specificity for women who are fertile, sometimes detects other tumors | Demirtas et al., 2007; Sisinni & Landriscina, 2015 |
| Serotonin/5-HIAA | Urine | Metabolite | FISH | Carcinoid tumors | Can use filter paper collection, high specificity, relatively cost-effective | Low sensitivity, most accurate in 24 h urine collection | Calanchini et al., 2019; Feldman, 1986; Feldman & O’dorisio, 1986 |
| VMA/HVA | Urine | Metabolite | MS | Neuroendocrine tumors | Very high specificity, can differentiate between early and late-stage disease, relatively cost-effective | Usually done in 24 h urine collection | LaBrosse et al., 1980; Laug et al., 1978 |
Abbreviations: 5-HIAA, 5-hydroxyindoleacetic acid; Beta-hCG, beta-human chorionic gonadotropin; BTA, bladder tumor antigen; DNA, deoxyribonucleic acid; ELISA, enzyme-linked immunosorbent assay; FDA, US Food and Drug Administration; HVA, homovanillic acid; MS, mass spectrometry.; NMP22, nuclear matrix protein 22; PCA3, prostate cancer antigen 3; PCR, polymerase chain reaction; PSA, prostate specific antigen; VMA, vanillylmandelic acid.
In postmenopausal women and men.
5.1.2 |. RNA
Like DNA, RNA signatures associated with tumors have been detected in capillary blood, feces, urine, and saliva (Igaz & Igaz, 2015; Kahraman et al., 2017; Moltzahn et al., 2011). These signatures include detection of messenger RNA associated with proteins that are upregulated in tumors (such as EGFR, transforming growth factor-alpha [TGF-α], and breast cancer resistance protein [BCRP]; Grandis & Tweardy, 1993; Ross et al., 1999) and altered expression of noncoding RNAs that regulate gene expression at transcriptional and posttranscriptional levels (Oliveira et al., 2019). Noncoding RNAs can affect transcription by forming complexes that guide gene activation and repression in association with chromatin-modifying complexes. At a posttranscriptional level, noncoding RNAs are involved in RNA metabolism including splicing, nuclear export, localization, stability/decay, and translation, for instance by acting as molecular scaffolds needed to build proteins. As many protein-based biomarkers to detect cancers already exist and overlap in utility with mRNA methods, the focus has shifted to using noncoding RNAs (which include micro RNAs, short interfering RNAs, piwi-interacting RNAs, long noncoding RNAs, and circular RNAs) as potential biomarkers. Circular RNAs are of particular interest because they sometimes regulate proliferation, are more protected from degeneration than other forms of RNA, and are often overexpressed in malignant cells (Yu & Kuo, 2019; Zhang et al., 2017).
Although many promising tests are in development, only one RNA tests is currently being used to detect cancer. The Progensa PCA3 assay (Hologic Gen-Probe, Marlborough, MA) is used to detect mRNAs in urine samples indicative of prostate cancer (Eskra et al., 2019). Studies have shown that DBS can be used as a substrate for microRNA testing (Diener et al., 2019; Patnaik et al., 2010) based on clustering of genome wide microRNA profiles to discriminate between patients with lung cancer undergoing curative treatment, patients with lung cancer undergoing palliative treatment, and normal controls (Kahraman et al., 2017). Many studies have been performed to detect noncoding RNAs associated with colorectal cancer and colonic adenomas (Yau et al., 2019), but no leading targets have been identified to date. Noncoding RNA signatures in feces have been slightly more successfully used to detect pancreatic cancer (Hasan et al., 2019; Yang et al., 2014); however, detection of these same signatures in urine has been even more successful (Debernardi et al., 2015; Kriz et al., 2020). For example, detection of miR-143, miR-30e, and miR-143 + miR-30e in urine all have a sensitivity of 83% and have specificities of 89%, 81%, and 96% respectively in the detection of pancreatic adenocarcinoma (Debernardi et al., 2015). RNA from urinary and bladder cancers has been detected in urine (Yu et al., 2020), and a recent study reported successful detection of breast cancer in urine samples based on noncoding RNA signatures (Hirschfeld et al., 2020). To summarize, although loco-regional diseases have been the most widely studied using samples collected by minimally invasive methods, these sample types can also potentially be used for detecting more distant cancers.
5.2 |. Proteins and metabolites
Protein and metabolite detection methods for cancer, such as detection of prostate cancer by elevated levels of PSA, have been around longer than nucleic acid-based methods. These tests have been more widely used, and their confounding variables, applications, and shortcomings are better characterized than DNA-based and RNA-based tests. They are therefore currently of more utility than tests using genetic material for targeted research as the data can be corrected for known parameters that affect the tests.
5.2.1 |. Proteins
Many cancer-related proteins can be detected in capillary blood, feces, urine, and saliva using a variety of spectrometry and immunoassay-based methods. The US FDA approved nuclear matrix protein number 22 (NMP22), a biomarker of urothelial cell death that can be measured in urine, for the detection of bladder cancer (Kumar et al., 2006; Oeyen et al., 2019). However, both its specificity and sensitivity are low for an approved test, and false positives are common because NMP22 is released during inflammation and infection. Bladder tumor antigen (BTA) tests based on urine samples have also been approved for use (BTA TRAK; Bard Diagnostics, Redmond, WA), including a point-of-care device (BTA STAT; Bard Diagnostics, Redmond, WA). However, false positives have been noted to occur in people who have hematuria, or blood in the urine, for other reasons. Beta-human chorionic gonadotropin (Beta-hCG) in urine, which is commonly used to detect pregnancy, can also be used to detect choriocarcinoma and germ cell tumors with 100% sensitivity and specificity when pregnancy is controlled for (Demirtas et al., 2007; Sisinni & Landriscina, 2015). Immunoglobulins (most commonly IgG or IgM) in the urine have been used to diagnose multiple myeloma and Waldenström macroglobulinemia (Gertz, 2019; Hoadley et al., 2018; Solomon, 1980). PSA has not been as successfully measured from urine as from whole blood (Eskra et al., 2019); however, another test is in development for detection of urine prostatic exosomal protein, which is elevated in prostate inflammation and associated with false-positive PSA results (Gu et al., 2019).
Salivary proteins, in addition to their potential use in the detection of oral cancers (Sivadasan et al., 2020), have potential uses in the diagnosis of breast cancer and ovarian cancer (Nemeir et al., 2019; Yadav et al., 2019). A recent study evaluated colorectal adenomas from a protein panel using feces but found that this panel was not sensitive enough to detect colorectal adenomas from fecal samples (Komor et al., 2020).
5.2.2 |. Metabolites
Metabolites are small molecules used in energy production and signaling and can be detected in many of the same minimally invasively collected sample types as used in protein applications (capillary blood, feces, urine, and saliva), in addition to exhaled breath. Many spectrometry methods are used to detect metabolites, the most popular being mass spectrometry and nuclear magnetic resonance spectroscopy. Lung cancer has been diagnosed using an electronic aromatic sensor, a condensation device, and trained dogs (Dragonieri et al., 2009; Guerrero-Flores et al., 2017; Saidi et al., 2020; Spivack et al., 2019). Dogs trained to detect organic volatile compounds have also detected prostate cancer from urine samples (Cornu et al., 2011; Taverna et al., 2015). However, these methods have been criticized because of obstacles in using animals (e.g., variability in temperament and behavior), indicating that more reliable methods to detect volatile compounds in breath, urine, and potentially feces are needed (Elliker et al., 2014; Hackner & Pleil, 2017; Pirrone & Albertini, 2017).
Unlike the relatively new approach of detecting volatile organic compounds, urine has been used to test for neurotransmitters and their precursors for half a century. Serotonin and 5-HIAA measured in urine have been used to monitor for carcinoid tumors (Calanchini et al., 2019; Feldman, 1986). Urinary catecholamines such as vanillylmandelic acid (VMA) and homovanillic acid (HVA) have also been used in the detection of neuroblastoma and other neural crest tumors (LaBrosse et al., 1980; Laug et al., 1978). The sensitivity of these tests together is 85%, and there is active research into the reasons why these tests are elevated in individuals without tumors (e.g., Araújo et al., 2020; Smith et al., 2010).
5.3 |. Cellular
5.3.1 |. Bacterial cells
Metabolic products, waste, and signaling outputs of cancerous cells can change the gut microbiome (Vogtmann & Goedert, 2016). In addition, tumor microRNAs can enter bacterial cells and alter signaling pathways. High abundance of F. nucleatum in feces is associated with colorectal cancer and poor survival in colorectal cancer patients (Gethings-Behncke et al., 2020). The abundance of other bacteria in feces also shows promise in the diagnosis of colorectal cancer (Eklöf et al., 2017). Research on detecting pancreatic cancer using the fecal microbiome has shown promise in advanced disease, but not early lesions (Half et al., 2019). The salivary microbiome is also being evaluated for the detection of pancreatic cancer (Lu et al., 2019; Torres et al., 2015) and oropharangeal cancer (Wolf et al., 2017).
5.3.2 |. Blood cells
Hematuria can be detected with a chemical reagent strip in bladder cancer (Messing et al., 1995). Using chemical reagent strips for screening the general population has been shown to be cost effective and save lives (Mariani et al., 1989; Messing et al., 1995). However, this method usually requires follow-up histological tests for diagnosis as hematuria can also be caused by other injuries, infections, and diseases.
6 |. FUTURE DIRECTIONS
The development and application of cancer biomarkers in samples collected using minimally invasive methods have the potential to boost interdisciplinary cancer research. The possibilities for cancer medicine and population-based research are reviewed here.
6.1 |. Cancer diagnosis, treatment, and surveillance
6.1.1 |. Biomarker development
Biomarkers that have research utility but do not meet the stringent demands required for clinical diagnosis could be used to further investigate those factors that decrease test sensitivity for that biomarker, such as environmental factors (e.g., food and drugs) and biological factors (e.g., age, genetics, or other illnesses). Furthermore, the impact of infectious processes on the false-positive rates of many cancer biomarker diagnostics can be investigated by measuring these biomarkers in minimally invasively collected sample types. Validated venous blood biomarkers can also potentially be adapted for use in minimally invasively collected sample types like DBS, which has been extensively used with immune, hormonal, and other biomarkers of physiology and health (Eick et al., 2016, 2017, 2019, 2020; McDade et al., 2007, 2012; McDade & Shell-Duncan, 2002; Rej et al., 2021). However, more basic research is needed to develop cancer biomarkers that can be measured in minimally invasively collected sample types and to ascertain which technological platform can be used for optimal quantitation and cost-effectiveness.
6.1.2 |. Cancer detection
There are large disparities in cancer detection and treatment globally, leading to late detection and poor outcomes in most countries. For example, mortality is significantly reduced when breast cancer is detected in Stages 1 or 2, and while 79% of breast cancers are detected in these early stages in Canada, only 21% are in Nigeria (Smith et al., 2006; Unger-Saldaña, 2014). Cancer biomarkers in samples collected using minimally invasive methods that are validated for clinical use could help public health officials understand the landscape of cancer in different regions so that adequate prevention and treatment systems can be developed and deployed. Cancer biomarker evaluation in minimally invasively collected sample types could also be used to research barriers to treatment at the national, regional, and personal levels and to develop prevention strategies in regions with high incidences of cancers linked to behavioral and lifestyle factors. By increasing detection ability, cancer biomarkers measured in minimally invasively collected sample types could prevent cancer deaths.
6.1.3 |. Personalized medicine
Clinically, research into cancer biomarkers could lead to the development of more personalized medicine (i.e., treatment tailored to the characteristics of a small group or a single person’s disease; de Castro et al., 2013). Although the incidence of most cancers has been reduced by current treatments, it has been reduced very little compared with their overall rates (Arnold et al., 2019); treatment personalization using targeted biologic drugs may change this. Detecting certain molecular drivers, then targeting those drivers using biologic drugs does not require the same level of medical access and equipment as traditional chemotherapy and radiation. Therefore, an expansion of these treatments might be a promising option for treating people who do not have ready access to healthcare. Expanding treatment options using biomarkers measured in minimally invasively collected sample types could decrease the social inequities in cancer treatment globally.
6.2 |. Population-based research
Newly developed cancer biomarkers have the potential for several nonclinical uses, but this potential has not yet been realized. Although clinical application of biomarkers in minimally invasively collected sample types has stringent requirements regarding reliability and validity, the same is not true for population-based research. There are cancer biomarkers available for several different minimally invasively collected sample types that are suitable for basic research purposes (Table 1). For example, beta-hCG can detect choriocarcinoma with nearly perfect sensitivity and specificity in men and postmenopausal women (Sisinni & Landriscina, 2015). Using these methods in population-based research would broaden understanding of cancer rates and causes, and the development of public health systems. In order to expand knowledge about cancers, work must be done at the intersection of the natural and social/behavioral sciences. A greater focus on evolutionary perspectives of cancer also has potential in furthering our theoretical understanding of cancers. Cancer biomarkers measured from minimally invasively collected samples could be used to expand basic research on cancers globally.
6.2.1 |. Epidemiology
Cancer biomarkers that use samples collected through minimally invasive methods could potentially also contribute to epidemiology and public health. Making biomarkers for cancer more accessible would improve the current problem of lack of data and/or data coverage in most nations (WHO, 2018). However, population-based research raises some ethical challenges that need to be carefully considered, including whether participants need to be informed of their results (especially when tests have low diagnostic standards) and if diagnosis is appropriate when treatments are not available. The World Health Organization (WHO, 2020) is advocating for increased diagnostic coverage even when treatments are not readily available because: (1) early detection lessens treatment burden if healthcare is available but hard to access; (2) palliative treatments, such as pain medications, are more easily accessed than cancer treatments; (3) prevention programs are more easily enacted than treatment programs and are more effective when the rates of different types of cancers are known; and (4) data on diagnosis rates in a region can help develop treatment programs for cancers with curative treatments, which will be needed as cancer rates continue to rise in LMICs.
6.2.2 |. Social and environmental determinants of cancers
At a population level, measurement of cancer biomarkers in minimally invasive collected sample types could be employed in research on carcinogens and social risk factors to understand cancer rates and patterns globally. Prevention of cancer-causing factors has been shown to have the most widespread public health effects, including limiting tobacco consumption to reduce lung cancer incidence, and preventing hepatitis-B virus (Hep-B) and HPV through vaccination to reduce liver and cervical cancers, respectively (WHO, 2020). Metabolic syndrome, a clustering of high central adiposity, blood pressure, plasma glucose, inflammation, and triglycerides, is another risk factor for cancer that can be evaluated through biomarker studies (Braun et al., 2011; Cowey & Hardy, 2006). Environmental factors, such as occupational and residential exposure to chemicals and pollutants have also been shown to contribute to cancer and are likely underestimated in LMICs because of data availability issues (Boffetta, 2006; Patel & Gomes, 2010; Vineis & Xun, 2009). In addition, PSA level and prostate cancer risk are higher for many populations of African Americans than European Americans in the US (Vijayakumar et al., 1998). However, the cause of this discrepancy has still not been well characterized. Differences in inflammation and obesity in these populations due to structural inequalities do not account for this increased risk in African Americans (Fowke et al., 2006; Zhang et al., 2000). Studies that include data on these social and environmental causes in addition to biomarker data will be useful as countries move to implement cancer programs.
6.2.3 |. Evolutionary medicine
Evolutionary science could also benefit from integrating cancer biomarkers measured in minimally invasive collected sample types. The lack of selective pressure after reproduction ceases leads to reduced selection against cancers in older adults (Williams, 1957), which account for ~90% of cancers globally (NIH, 2015). Measurement of cancer biomarkers from minimally invasively collected sample types could allow for an assessment of the proportion of cancers in older adult populations that are associated with germline mutations to better understand the evolutionary characteristics of some cancers.
Another evolutionary hypothesis for the development of cancer is mismatch between our evolved biology and the environment due to recent rapid environmental and social changes (Stearns & Medzhitov, 2015). For example, research has shown that Dogon women in Mali, a traditional West African population that does not use contraceptives, have an average of approximately nine children, whereas women in the US, which has widespread contraceptive access, have an average of around two children (Strassmann, 1999). Societies with access to contraceptives have three times the amount of menstrual cycling across the lifespan, and higher overall levels of estrogens and progesterone throughout the lifespan, leading to 12 times the risk of breast cancer compared with societies that do not use contraceptives and have more births and longer lactation periods. In response, contraceptives that suppress menstrual cycling have been developed; while these reduce ovarian cancer rates, they do not affect breast cancer since they are still based on the administration of estrogen and progesterone. In addition, populations in HICs have concentrations of sex hormones that are notably higher than those of LMICs due to excess energy availability (high-calorie diets and decreases in physical activity; Jasieńska & Thune, 2001). Integrating cancer biomarkers with other biomarkers (such as sex hormones) measured in minimally invasively collected sample types will aid in our understanding of cancer from an evolutionary perspective, which may lead to more appropriate interventions.
Our immune systems evolved with repeated exposure to diverse parasites and pathogens that are now relatively rare in HICs due to evolutionarily novel environments, hygienic measures, and the development of medical treatments (Rook, 2019; Rook et al., 2017). This novel microbial environment almost certainly has major consequences for immune molecules that eliminate cancers. A study with Tsimané of Bolivia, a population with high rates of hook-worm, Giardia, and roundworm (Ascaris lumbricoides) infection, showed that many of their biomarkers of immune response were elevated when compared with populations with low rates of these infections (Blackwell et al., 2016). Furthermore, cancer-killing natural killer cells generally decline with age in the US but increase with age in Tsimané, which could have implications for variability in cancer rates. Tsimané have a very low burden of reproductive cancers, including endometrial, ovarian, breast, and prostate cancers, but a greater occurrence of cancers associated with infectious disease such as cervical cancer (Gurven et al., 2017). This is highly suggestive that cancer risk in contemporary populations, especially in HICs, is at least in part an evolutionary mismatch (Blackwell et al., 2016; Stearns, 2012; Wu & Lanier, 2003). Different components of the immune system have been linked with both risk for certain cancers and survival from certain cancers (de Visser et al., 2006), and expanding research into different environments could help explain this paradox. Cancer biomarkers in samples collected through minimally invasive methods could expand the knowledge of cancer rates in these different environments, which would aid in our evolutionary understanding of cancer. This is critical given the rapidly changing social and environmental factors occurring with economic development and globalization that are affecting the risk factors and, therefore, incidence and mortality risk of certain cancers.
7 |. CONCLUSION
Once technical limitations related to biomarker development and measurement in minimally invasively collected sample types are overcome, these biomarkers could usher in a new era of cancer research and treatment characterized by improved accessibility, lower costs, improved patient quality of life, and better outcomes. There are currently only five FDA-approved cancer tests based on the measurement of biomarkers in minimally invasively collected sample types, but there are numerous biomarkers that are now in development. Clinically, using biomarkers in samples collected through minimally invasive methods is a promising way to improve global outcomes. Detecting cancer earlier in the general population will lead to increased treatment, which has the potential to save and improve lives. Furthermore, the quality of life of cancer patients can be improved by utilizing minimally invasive collection methods instead of venous biomarkers, imaging, and invasive medical exams. These benefits also make biomarkers in samples collected through minimally invasive methods more acceptable in communities that are resource-limited and/or opposed to more invasive techniques (McDade et al., 2007; Su et al., 2018). These communities also often have higher risk of disease due to social determinants of health, including being medically underserved. Widespread adoption of these minimally invasive methods for screening and tracking different cancers could expand cancer research and treatment globally.
Cancer research benefits from an interdisciplinary perspective. While some disciplines have invested heavily in cancer research, others such as human biology have yet to apply their expertise. Cancer rates are expected to increase significantly in the near future (WHO, 2020), and more global health researchers are needed to improve data coverage (Gupta et al., 2014). Currently, there are available biomarkers that can be measured in samples collected using minimally invasive methods to detect cancer that has not been adequately utilized in scientific investigations. Specifically, global data on the rates of different cancers is lacking (WHO, 2018), and methods and studies that fill this gap are needed. With more scientists turning toward biomarkers in samples collected using minimally invasive methods, more attention should be given to cancers.
ACKNOWLEDGMENTS
The authors thank participants in the “Minimally Invasive Biomarkers in Human Population Biology Research: State of the Science and Future Directions” poster session at the annual meeting of the American Association of Physical Anthropology in 2019 for their feedback on an earlier version of this work.
CONFLICT OF INTEREST
Dr. Sana D. Karam receives clinical trial funding from AstraZeneca and preclinical research funding from Roche for work unrelated to this research.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- Abrams EM, & Szefler SJ (2020). COVID-19 and the impact of social determinants of health. The Lancet Respiratory Medicine, 8(7), 659–661. 10.1016/S2213-2600(20)30234-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlquist DA (2015). Multi-target stool DNA test: A new high bar for noninvasive screening. Digestive Diseases and Sciences, 60(3), 623–633. 10.1007/s10620-014-3451-5 [DOI] [PubMed] [Google Scholar]
- Alcaraz KI, Wiedt TL, Daniels EC, Yabroff KR, Guerra CE, & Wender RC (2020). Understanding and addressing social determinants to advance cancer health equity in the United States: A blueprint for practice, research, and policy. CA: a Cancer Journal for Clinicians, 70(1), 31–46. 10.3322/caac.21586 [DOI] [PubMed] [Google Scholar]
- Andriole GL, Crawford ED, Grubb RL, Buys SS, Chia D,Church TR, Fouad MN, Gelmann EP, Kvale PA, Reding DJ, Weissfeld JL, Yokochi LA, O’Brien B, Clapp JD, Rathmell JM, Riley TL, Hayes RB, Kramer BS, Izmirlian G, … Berg CD (2009). Mortality results from a randomized prostate-cancer screening trial. The New England Journal of Medicine, 360(13), 1310–1319. 10.1056/NEJMoa0810696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo BR, Furtado DZS, de Moura Leite FBV, deAssunção NA, & Carrilho E (2020). Metabolic profiling of organic acids in urine samples of cri Du chat syndrome individuals by gas chromatography-mass spectrometry. Journal of Chromatography B, 1153, 122267. 10.1016/j.jchromb.2020.122267 [DOI] [PubMed] [Google Scholar]
- Arnold M, Rutherford MJ, Bardot A, Ferlay J,Andersson TM-L, Myklebust TÅ, Tervonen H, Thursfield V, Ransom D, Shack L, Woods RR, Turner D, Leonfellner S, Ryan S, Saint-Jacques N, de P, McClure C, Ramanakumar AV, Stuart-Panko H, … Bray F (2019). Progress in cancer survival, mortality, and incidence in seven high-income countries 1995–2014 (ICBP SURVMARK-2): A population-based study. The Lancet Oncology, 20(11), 1493–1505. 10.1016/S1470-2045(19)30456-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz Z, Iqbal J, Akram M, & Anderson BO (2010). Worsened oncologic outcomes for women of lower socio-economic status (SES) treated for locally advanced breast cancer (LABC) in Pakistan. The Breast, 19(1), 38–43. 10.1016/j.breast.2009.10.005 [DOI] [PubMed] [Google Scholar]
- Barrington DA, Dilley SE, Landers EE, Thomas ED,Boone JD, Straughn JM, McGwin G, & Leath CA (2016). Distance from a Comprehensive Cancer Center: A proxy for poor cervical cancer outcomes? Gynecologic Oncology, 143(3), 617–621. 10.1016/j.ygyno.2016.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basen-Engquist K, & Chang M (2011). Obesity and cancer risk: Recent review and evidence. Current Oncology Reports, 13(1), 71–76. 10.1007/s11912-010-0139-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensalah K, Montorsi F, & Shariat SF (2007). Challenges of cancer biomarker profiling. European Urology, 52(6), 1601–1609. 10.1016/j.eururo.2007.09.036 [DOI] [PubMed] [Google Scholar]
- Blackwell AD, Trumble BC, Maldonado Suarez I, Stieglitz J, Beheim B, Snodgrass JJ, Kaplan H, & Gurven M (2016). Immune function in Amazonian horticulturalists. Annals of Human Biology, 43(4), 382–396. 10.1080/03014460.2016.1189963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boffetta P (2006). Human cancer from environmental pollutants: The epidemiological evidence. Mutation Research, Genetic Toxicology and Environmental Mutagenesis, 608(2), 157–162. 10.1016/j.mrgentox.2006.02.015 [DOI] [PubMed] [Google Scholar]
- Bos JL (1989). Ras oncogenes in human cancer: A review. CancerResearch, 49(17), 4682–4689. [PubMed] [Google Scholar]
- Braun S, Bitton-Worms K, & LeRoith D (2011). The link between the metabolic syndrome and cancer. International Journal of Biological Sciences, 7(7), 1003–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brikun I, Nusskern D, & Freije D (2019). An expanded biomarker panel for the detection of prostate cancer from urine DNA. Experimental Hematology & Oncology, 8(1), 13. 10.1186/s40164-019-0137-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brücher BLDM, & Jamall IS (2016). Somatic mutation theory—Why it’s wrong for most cancers. Cellular Physiology and Biochemistry, 38(5), 1663–1680. 10.1159/000443106 [DOI] [PubMed] [Google Scholar]
- Byeon HK, Ku M, & Yang J (2019). Beyond EGFR inhibition: Multilateral combat strategies to stop the progression of head and neck cancer. Experimental & Molecular Medicine, 51(1), 1–14. 10.1038/s12276-018-0202-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylstra Y, Lim WK, Kam S, Tham KW, Wu RR, Teo JX,Davila S, Kuan JL, Chan SH, Bertin N, Yang CX, Rozen S, Teh BT, Yeo KK, Cook SA, Jamuar SS, Ginsburg GS, Orlando LA, & Tan P (2021). Family history assessment significantly enhances delivery of precision medicine in the genomics era. Genome Medicine, 13(1), 3. 10.1186/s13073-020-00819-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calanchini M, Tadman M, Krogh J, Fabbri A, Grossman A, & Shine B (2019). Measurement of urinary 5-HIAA: Correlation between spot versus 24-h urine collection. Endocrine Connections, 8(8), 1082–1088. 10.1530/EC-19-0269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calle EE, & Kaaks R (2004). Overweight, obesity and cancer: Epidemiological evidence and proposed mechanisms. Nature Reviews Cancer, 4(8), 579–591. 10.1038/nrc1408 [DOI] [PubMed] [Google Scholar]
- Carmeliet P, & Jain RK (2000). Angiogenesis in cancer and other diseases. Nature, 407(6801), 249–257. 10.1038/35025220 [DOI] [PubMed] [Google Scholar]
- Chanock SJ, & Ostrander EA (2020). 21—Discovery and characterization of Cancer genetic susceptibility alleles. In Niederhuber JE, Armitage JO, Kastan MB, Doroshow JH, & Tepper JE (Eds.), Abeloff’s clinical oncology (sixth edition) (pp. 323–336.e3). Elsevier. 10.1016/B978-0-323-47674-4.00021-9 [DOI] [Google Scholar]
- Chatrath A, Przanowska R, Kiran S, Su Z, Saha S, Wilson B,Tsunematsu T, Ahn JH, Lee KY, Paulsen T, Sobierajska E, Kiran M, Ta T, Kumar P, Ratan A, & Dutta A (2020). The pan-cancer landscape of prognostic germline variants in 10,582 patients. Genome Medicine, 12(1), 15. 10.1186/s13073-020-0718-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chihara Y, Kanai Y, Fujimoto H, Sugano K, Kawashima K,Liang G, Jones PA, Fujimoto K, Kuniyasu H, & Hirao Y (2013). Diagnostic markers of urothelial cancer based on DNA methylation analysis. BMC Cancer, 13, 275. 10.1186/1471-2407-13-275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung W, Bondaruk J, Jelinek J, Lotan Y, Liang S,Czerniak B, & Issa J-PJ (2011). Detection of bladder cancer using novel DNA methylation biomarkers in urine sediments. Cancer Epidemiology and Prevention Biomarkers, 20(7), 1483–1491. 10.1158/1055-9965.EPI-11-0067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornu J-N, Cancel-Tassin G, Ondet V, Girardet C, & Cussenot O (2011). Olfactory detection of prostate cancer by dogs sniffing urine: A step forward in early diagnosis. European Urology, 59(2), 197–201. 10.1016/j.eururo.2010.10.006 [DOI] [PubMed] [Google Scholar]
- Costa VL, Henrique R, Danielsen SA, Duarte-Pereira S,Eknaes M, Skotheim RI, Rodrigues Â, Magalhães JS, Oliveira J, Lothe RA, Teixeira MR, Jerónimo C, & Lind GE (2010). Three epigenetic biomarkers, GDF15, TMEFF2, and VIM, accurately predict bladder cancer from DNA-based analyses of urine samples. Clinical Cancer Research, 16(23), 5842–5851. 10.1158/1078-0432.CCR-10-1312 [DOI] [PubMed] [Google Scholar]
- Cowey S, & Hardy RW (2006). The metabolic syndrome: A high-risk state for cancer? The American Journal of Pathology, 169(5), 1505–1522. 10.2353/ajpath.2006.051090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristaldi M, Mauceri R, Di Fede O, Giuliana G, Campisi G, & Panzarella V (2019). Salivary biomarkers for oral squamous cell carcinoma diagnosis and follow-up: Current status and perspectives. Frontiers in Physiology, 10(1476). 10.3389/fphys.2019.01476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danaei G, Hoorn SV, Lopez AD, Murray CJ, & Ezzati M (2005). Causes of cancer in the world: Comparative risk assessment of nine behavioural and environmental risk factors. The Lancet, 366(9499), 1784–1793. 10.1016/S0140-6736(05)67725-2 [DOI] [PubMed] [Google Scholar]
- de Castro DG, Clarke PA, Al-Lazikani B, & Workman P (2013). Personalized cancer medicine: Molecular diagnostics, predictive biomarkers, and drug resistance. Clinical Pharmacology & Therapeutics, 93(3), 252–259. 10.1038/clpt.2012.237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira JC, Oliveira LC, Mathias C, Pedroso GA, Lemos DS, Salviano-Silva A, Jucoski TS, Lobo-Alves SC, Zambalde EP, Cipolla GA, & Gradia DF (2018). Long non-coding RNAs in cancer: Another layer of complexity. The Journal of Gene Medicine, 21(1), e3065. 10.1002/jgm.3065 [DOI] [PubMed] [Google Scholar]
- de Visser KE, Eichten A, & Coussens LM (2006). Paradoxical roles of the immune system during cancer development. Nature Reviews Cancer, 6(1), 24–37. 10.1038/nrc1782 [DOI] [PubMed] [Google Scholar]
- DeBerardinis RJ, & Chandel NS (2016). Fundamentals of cancer metabolism. Science Advances, 2(5), e1600200. 10.1126/sciadv.1600200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debernardi S, Massat NJ, Radon TP, Sangaralingam A, Banissi A, Ennis DP, Dowe T, Chelala C, Pereira SP, Kocher HM, Young BD, Bond-Smith G, Hutchins R, & Crnogorac-Jurcevic T (2015). Noninvasive urinary miRNA biomarkers for early detection of pancreatic adenocarcinoma. American Journal of Cancer Research, 5(11), 3455–3466. [PMC free article] [PubMed] [Google Scholar]
- Demirtas E, Krishnamurthy S, & Tulandi T (2007). Elevated serum β-human chorionic gonadotropin in nonpregnant conditions. Obstetrical & Gynecological Survey, 62(10), 675–679. 10.1097/01.ogx.0000281557.04956.61 [DOI] [PubMed] [Google Scholar]
- Diener C, Galata V, Keller A, & Meese E (2019). MicroRNA profiling from dried blood samples. Critical Reviews in Clinical Laboratory Sciences, 56(2), 111–117. 10.1080/10408363.2018.1561641 [DOI] [PubMed] [Google Scholar]
- Dragonieri S, Annema JT, Schot R, van der Schee MPC, Spanevello A, Carratú P, Resta O, Rabe KF, & Sterk PJ (2009). An electronic nose in the discrimination of patients with non-small cell lung cancer and COPD. Lung Cancer, 64(2), 166–170. 10.1016/j.lungcan.2008.08.008 [DOI] [PubMed] [Google Scholar]
- Driscoll T, Nelson DI, Steenland K, Leigh J, Concha-Barrientos M, Fingerhut M, & Prüss-Ustün A (2005). The global burden of disease due to occupational carcinogens. American Journal of Industrial Medicine, 48(6), 419–431. 10.1002/ajim.20209 [DOI] [PubMed] [Google Scholar]
- Durand X, Moutereau S, Xylinas E, & de la Taille A (2011). Progensa™ PCA3 test for prostate cancer. Expert Review of Molecular Diagnostics, 11(2), 137–144. 10.1586/erm.10.122 [DOI] [PubMed] [Google Scholar]
- EBCTCG, Early Breast Cancer Trialists’ Collaborative Group.(2005). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. The Lancet, 365(9472), 1687–1717. [DOI] [PubMed] [Google Scholar]
- Eick G, Urlacher SS, McDade TW, Kowal P, & Snodgrass JJ (2016). Validation of an optimized ELISA for quantitative assessment of epstein-barr virus antibodies from dried blood spots. Biodemography and Social Biology, 62(2), 222–233. 10.1080/19485565.2016.1169396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eick GN, Cepon-Robins TJ, Devlin MJ, Kowal P,Sugiyama LS, & Snodgrass JJ (2020). Development and validation of an ELISA for a biomarker of thyroid dysfunction, thyroid peroxidase autoantibodies (TPO-ab), in dried blood spots. Journal of Physiological Anthropology, 39(1), 16. 10.1186/s40101-020-00228-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eick GN, Devlin MJ, Cepon-Robins TJ, Kowal P, Sugiyama LS, & Snodgrass JJ (2019). A dried blood spot-based method to measure levels of tartrate-resistant acid phosphatase 5b (TRACP-5b), a marker of bone resorption. American Journal of Human Biology, 31(3), e23240. 10.1002/ajhb.23240 [DOI] [PubMed] [Google Scholar]
- Eick GN, Kowal P, Barrett T, Thiele EA, & Snodgrass JJ (2017). Enzyme-linked immunoassay-based quantitative measurement of apolipoprotein B (ApoB) in dried blood spots, a biomarker of cardiovascular disease risk. Biodemography and Social Biology, 63(2), 116–130. 10.1080/19485565.2017.1283582 [DOI] [PubMed] [Google Scholar]
- Eklöf V, Löfgren-Burström A, Zingmark C, Edin S, Larsson P,Karling P, Alexeyev O, Rutegård J, Wikberg ML, & Palmqvist R (2017). Cancer-associated fecal microbial markers in colorectal cancer detection. International Journal of Cancer, 141(12), 2528–2536. 10.1002/ijc.31011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliker KR, Sommerville BA, Broom DM, Neal DE, Armstrong S, & Williams HC (2014). Key considerations for the experimental training and evaluation of cancer odour detection dogs: Lessons learnt from a double-blind, controlled trial of prostate cancer detection. BMC Urology, 14, 22. 10.1186/1471-2490-14-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskra JN, Rabizadeh D, Pavlovich CP, Catalona WJ, & Luo J (2019). Approaches to urinary detection of prostate cancer. Prostate Cancer and Prostatic Diseases, 22(3), 362–381. 10.1038/s41391-019-0127-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etzioni R, Urban N, Ramsey S, McIntosh M, Schwartz S, Reid B, Radich J, Anderson G, & Hartwell L (2003). The case for early detection. Nature Reviews Cancer, 3(4), 243–252. 10.1038/nrc1041 [DOI] [PubMed] [Google Scholar]
- Fei X, Wu J, Kong Z, & Christakos G (2015). Urban-rural disparity of breast cancer and socioeconomic risk factors in China. PLoS One, 10(2), e0117572. 10.1371/journal.pone.0117572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JM (1986). Urinary serotonin in the diagnosis of carcinoid tumors. Clinical Chemistry, 32(5), 840–844. 10.1093/clinchem/32.5.840 [DOI] [PubMed] [Google Scholar]
- Feldman JM, & O’dorisio TM (1986). Role of neuropeptides and serotonin in the diagnosis of carcinoid tumors. The American Journal of Medicine, 81(6, Supplement 2), 41–48. 10.1016/0002-9343(86)90583-8 [DOI] [PubMed] [Google Scholar]
- Fowke JH, Signorello LB, Chang SS, Matthews CE, Buchowski MS, Cookson MS, Ukoli FM, & Blot WJ (2006). Effects of obesity and height on prostate-specific antigen (PSA) and percentage of free PSA levels among African-American and Caucasian men. Cancer, 107(10), 2361–2367. 10.1002/cncr.22249 [DOI] [PubMed] [Google Scholar]
- Füzéry AK, Levin J, Chan MM, & Chan DW (2013). Translation of proteomic biomarkers into FDA approved cancer diagnostics: Issues and challenges. Clinical Proteomics, 10(1), 13. 10.1186/1559-0275-10-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gakidou E, Nordhagen S, & Obermeyer Z (2008). Coverage of cervical cancer screening in 57 countries: Low average levels and large inequalities. PLoS Medicine, 5(6), e132. 10.1371/journal.pmed.0050132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Cordero JL, & Maerkl SJ (2020). Microfluidic systems for cancer diagnostics. Current Opinion in Biotechnology, 65, 37–44. 10.1016/j.copbio.2019.11.022 [DOI] [PubMed] [Google Scholar]
- Garcia-Gil M, Elorza J-M, Banque M, Comas-Cufí M, Blanch J, Ramos R, Méndez-Boo L, Hermosilla E, Bolibar B, & Prieto-Alhambra D (2014). Linking of primary care records to census data to study the association between socioeconomic status and cancer incidence in southern Europe: A nation-wide ecological study. PLoS One, 9(10), e109706. 10.1371/journal.pone.0109706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayther SA, Warren W, Mazoyer S, Russell PA, Harrington PA, Chiano M, Seal S, Hamoudi R, van Rensburg EJ, Dunning AM, Love R, Evans G, Easton D, Clayton D, Stratton MR, & Ponder BAJ (1995). Germline mutations of the BRCA1 gene in breast and ovarian cancer families provide evidence for a genotype–phenotype correlation. Nature Genetics, 11(4), 428–433. 10.1038/ng1295-428 [DOI] [PubMed] [Google Scholar]
- Gertz MA (2019). Waldenström macroglobulinemia: 2019 update on diagnosis, risk stratification, and management. American Journal of Hematology, 94(2), 266–276. 10.1002/ajh.25292 [DOI] [PubMed] [Google Scholar]
- Gethings-Behncke C, Coleman HG, Jordao HW, Longley DB, Crawford N, Murray LJ, & Kunzmann AT (2020). Fusobacterium nucleatum in the colorectum, and its association with cancer risk and survival: A systematic review and meta-analysis. Cancer Epidemiology and Prevention Biomarkers, 29(3), 539–548. 10.1158/1055-9965.EPI-18-1295 [DOI] [PubMed] [Google Scholar]
- Gildner TE (2021). Reproductive hormone measurement from minimally invasive sample types: Methodological considerations and anthropological importance. American Journal of Human Biology, 33(1), e23535. 10.1002/ajhb.23535 [DOI] [PubMed] [Google Scholar]
- Ginsburg O, Yip C-H, Brooks A, Cabanes A, Caleffi M, Dunstan Yataco JA, Anderson BO, McCormack V, McLaughlin de Anderson M, Mehrotra R, Mohar A, Murillo R, Pace LE, Paskett ED, Romanoff A, Rositch AF, Scheel JR, Schneidman M, Unger-Saldaña K, … Anderson BO (2020). Breast cancer early detection: A phased approach to implementation. Cancer, 126 (S10), 2379–2393. 10.1002/cncr.32887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandis JR, & Tweardy DJ (1993). Elevated levels of transforming growth factor α and epidermal growth factor receptor messenger RNA are early markers of carcinogenesis in head and neck cancer. Cancer Research, 53(15), 3579–3584. [PubMed] [Google Scholar]
- Graveel CR, Calderone HM, Westerhuis JJ, Winn ME, & Sempere LF (2015). Critical analysis of the potential for microRNA biomarkers in breast cancer management. Breast Cancer: Targets and Therapy, 7, 59–79. 10.2147/BCTT.S43799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C-Y, Huang Y-Q, Han C-T, Zhu Y, Bo D, Meng J, Qin X-J, & Ye D-W (2019). Clinical significance of urine prostatic exosomal protein in the diagnosis of prostate cancer. American Journal of Cancer Research, 9(5), 1074–1078. [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Flores H, Apresa-García T, Garay-Villar Ó, Sánchez-Pérez A, Flores-Villegas D, Bandera-Calderón A, García-Palacios R, Rojas-Sánchez T, Romero-Morelos P, Sánchez-Albor V, Mata O, Arana-Conejo V, Badillo-Romero J, Taniguchi K, Marrero-Rodríguez D, Mendoza-Rodríguez M, Rodríguez-Esquivel M, Huerta-Padilla V, Martínez-Castillo A, … Salcedo M (2017). A non-invasive tool for detecting cervical cancer odor by trained scent dogs. BMC Cancer, 17(1), 79. 10.1186/s12885-016-2996-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Preston R, Soudry E, Acero J, Orera M, Moreno-López L, Macía-Colón G, Jaffe A, Berdasco M, Ili-Gangas C, Brebi-Mieville P, Fu Y, Engstrom C, Irizarry RA, Esteller M, Westra W, Koch W, Califano J, & Sidransky D (2011). NID2 and HOXA9 promoter hypermethylation as biomarkers for prevention and early detection in oral cavity squamous cell carcinoma tissues and saliva. Cancer Prevention Research, 4(7), 1061–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Zhang L, Liu J, Li Z, Li J, Zhou W, Wang H, Li JQ, Liu DY, Yu XY, & Zhang J (2021). Multifunctional microfluidic chip for cancer diagnosis and treatment. Nano, 5(1), 73–89. 10.7150/ntno.49614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Venkatesh A, Ray S, & Srivastava S (2014). Challenges and prospects for biomarker research: A current perspective from the developing world. Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics, 1844(5), 899–908. 10.1016/j.bbapap.2013.12.020 [DOI] [PubMed] [Google Scholar]
- Gurven M, Stieglitz J, Trumble B, Blackwell AD, Beheim B, Davis H, Hooper P, & Kaplan H (2017). The Tsimane health and life history project: Integrating anthropology and biomedicine. Evolutionary Anthropology: Issues, News, and Reviews, 26(2), 54–73. 10.1002/evan.21515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackner K, & Pleil J (2017). Canine olfaction as an alternative to analytical instruments for disease diagnosis: Understanding “dog personality” to achieve reproducible results. Journal of Breath Research, 11(1), 012001. 10.1088/1752-7163/aa5524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajdinjak T (2008). UroVysion FISH test for detecting urothelial cancers: Meta-analysis of diagnostic accuracy and comparison with urinary cytology testing. Urologic Oncology: Seminars and Original Investigations, 26(6), 646–651. 10.1016/j.urolonc.2007.06.002 [DOI] [PubMed] [Google Scholar]
- Half E, Keren N, Reshef L, Dorfman T, Lachter I, Kluger Y,Reshef N, Knobler H, Maor Y, Stein A, Konikoff FM, & Gophna U (2019). Fecal microbiome signatures of pancreatic cancer patients. Scientific Reports, 9(1), 1–12. 10.1038/s41598-019-53041-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan S, Jacob R, Manne U, & Paluri R (2019). Advances in pancreatic cancer biomarkers. Oncology Reviews, 13(1), 69–76. 10.4081/oncol.2019.410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch FR, Suda K, Wiens J, & Bunn PA (2016). New and emerging targeted treatments in advanced non-small-cell lung cancer. The Lancet, 388(10048), 1012–1024. 10.1016/S0140-6736(16)31473-8 [DOI] [PubMed] [Google Scholar]
- Hirschfeld M, Rücker G, Weiß D, Berner K, Ritter A,Jäger M, & Erbes T (2020). Urinary exosomal microRNAs as potential non-invasive biomarkers in breast cancer detection. Molecular Diagnosis & Therapy, 24(2), 215–232. [DOI] [PubMed] [Google Scholar]
- Hoadley KA, Yau C, Hinoue T, Wolf DM, Lazar AJ, Drill E, Shen R, Taylor AM, Cherniack AD, Thorsson V, Akbani R, Bowlby R, Wong CK, Wiznerowicz M, Sanchez-Vega F, Robertson AG, Schneider BG, Lawrence MS, Noushmehr H, … Mariamidze A (2018). Cell-of-origin patterns dominate the molecular classification of 10,000 tumors from 33 types of cancer. Cell, 173(2), 291–304.e6. 10.1016/j.cell.2018.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igaz I, & Igaz P (2015). Diagnostic relevance of microRNAs in other body fluids including urine, feces, and saliva. In Igaz P (Ed.), Circulating microRNAs in disease diagnostics and their potential biological relevance (pp. 245–252). Springer. 10.1007/978-3-0348-0955-9_11 [DOI] [PubMed] [Google Scholar]
- Iliescu FS, Poenar DP, Yu F, Ni M, Chan KH, Cima I, Taylor HK, Cima I, & Iliescu C (2019). Recent advances in microfluidic methods in cancer liquid biopsy. Biomicrofluidics, 13(4), 041503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperiale TF, Ransohoff DF, Itzkowitz SH, Levin TR, Lavin P, Lidgard GP, Ahlquist DA, & Berger BM (2014). Multitarget stool DNA testing for colorectal-cancer screening. New England Journal of Medicine, 370(14), 1287–1297. 10.1056/NEJMoa1311194 [DOI] [PubMed] [Google Scholar]
- Jasieńska G, & Thune I (2001). Lifestyle, hormones, and risk of breast cancer. British Medical Journal (Clinical Research Ed.), 322(7286), 586–587. 10.1136/bmj.322.7286.586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczor-Urbanowicz KE, Wei F, Rao SL, Kim J, Shin H, Cheng J, Tu M, Wong DTW, & Kim Y (2019). Clinical validity of saliva and novel technology for cancer detection. Biochimica et Biophysica Acta. Reviews on Cancer, 1872(1), 49–59. 10.1016/j.bbcan.2019.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahraman M, Laufer T, Backes C, Schrörs H, Fehlmann T, Ludwig N, Kohlhaas J, Meese E, Wehler T, Bals R, & Keller A (2017). Technical stability and biological variability in microRNAs from dried blood spots: A lung cancer therapy-monitoring showcase. Clinical Chemistry, 63(9), 1476–1488. 10.1373/clinchem.2017.271619 [DOI] [PubMed] [Google Scholar]
- Karam SD, Reddy K, Blatchford PJ, Waxweiler T, DeLouize AM, Oweida A, Somerset H, Marshall C, Young C, Davies KD, Kane M, Tan AC, Wang XJ, Jimeno A, Aisner DL, Bowles DW, & Raben D (2018). Final report of a phase I trial of Olaparib with Cetuximab and radiation for heavy smoker patients with locally advanced head and neck cancer. Clinical Cancer Research, 24(20), 4949–4959. 10.1158/1078-0432.CCR-18-0467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern SE (2012). Why your new cancer biomarker may never work: Recurrent patterns and remarkable diversity in biomarker failures. Cancer Research, 72(23), 6097–6101. 10.1158/0008-5472.CAN-12-3232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpivaara O, & Aaltonen LA (2013). Diagnostic cancer genome sequencing and the contribution of germline variants. Science, 339(6127), 1559–1562. 10.1126/science.1233899 [DOI] [PubMed] [Google Scholar]
- Komor MA, Bosch LJ, Coupé VM, Rausch C, Pham TV, Piersma SR, Mongera S, Mulder CJJ, Dekker E, Kuipers EJ, Wiel MA, Carvalho B, Fijneman RJA, Jimenez CR, Meijer GA, & Wit M (2020). Proteins in stool as biomarkers for non-invasive detection of colorectal adenomas with high risk of progression. The Journal of Pathology, 250(3), 288–298. 10.1002/path.5369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriz D, Ansari D, & Andersson R (2020). Potential biomarkers for early detection of pancreatic ductal adenocarcinoma. Clinical & Translational Oncology: Official Publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico, 22(12), 2170–2174. 10.1007/s12094-020-02372-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Kumar R, & Gupta NP (2006). Comparison of NMP22 BladderChek test and urine cytology for the detection of recurrent bladder cancer. Japanese Journal of Clinical Oncology, 36(3), 172–175. 10.1093/jjco/hyi244 [DOI] [PubMed] [Google Scholar]
- LaBrosse EH, Com-Nougué C, Zucker JM, Comoy E, Bohuon C, Lemerle J, & Schweisguth O (1980). Urinary excretion of 3-methoxy-4-hydroxymandelic acid and 3-methoxy-4-hydroxyphenylacetic acid by 288 patients with neuroblastoma and related neural crest tumors. Cancer Research, 40(6), 1995–2001. [PubMed] [Google Scholar]
- Lannin DR, Mathews HF, Mitchell J, Swanson MS, Swanson FH, & Edwards MS (1998). Influence of socioeconomic and cultural factors on racial differences in late-stage presentation of breast cancer. JAMA, 279(22), 1801–1807. 10.1001/jama.279.22.1801 [DOI] [PubMed] [Google Scholar]
- Larsen IK, Myklebust TÅ, Babigumira R, Vinberg E, Møller B, & Ursin G (2020). Education, income and risk of cancer: Results from a Norwegian registry-based study. Acta Oncologica, 59(11), 1300–1307. 10.1080/0284186X.2020.1817548 [DOI] [PubMed] [Google Scholar]
- Larsen LK, Lind GE, Guldberg P, & Dahl C (2019). DNA-methylation-based detection of urological cancer in urine: Overview of biomarkers and considerations on biomarker design, source of DNA, and detection technologies. International Journal of Molecular Sciences, 20(11), 2657. 10.3390/ijms20112657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laug WE, Siegel SE, Shaw KN, Landing B, Baptista J, & Gutenstein M (1978). Initial urinary catecholamine metabolite concentrations and prognosis in neuroblastoma. Pediatrics, 62, 77–83. [PubMed] [Google Scholar]
- Liyanage C, Wathupola A, Muraleetharan S, Perera K, Punyadeera C, & Udagama P (2019). Promoter hypermethylation of tumor-suppressor genes p16INK4a, RASSF1A, TIMP3, and PCQAP/MED15 in salivary DNA as a quadruple biomarker panel for early detection of oral and oropharyngeal cancers. Biomolecules, 9(4), 148. 10.3390/biom9040148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lortet-Tieulent J, Georges D, Bray F, & Vaccarella S (2020). Profiling global cancer incidence and mortality by socioeconomic development. International Journal of Cancer, 147(11), 3029–3036. 10.1002/ijc.33114 [DOI] [PubMed] [Google Scholar]
- Lu H, Ren Z, Li A, Li J, Xu S, Zhang H, Jiang J, Yang J, Luo Q, Zhou K, Zheng S, & Li L (2019). Tongue coating microbiome data distinguish patients with pancreatic head cancer from healthy controls. Journal of Oral Microbiology, 11(1), 1563409. 10.1080/20002297.2018.1563409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani AJ, Mariani MC, Macchioni C, Stams UK,Hariharan A, & Moriera A (1989). The significance of adult hematuria: 1,000 hematuria evaluations including a risk-benefit and cost-effectiveness analysis. Journal of Urology, 141(2), 350–355. 10.1016/S0022-5347(17)40763-4 [DOI] [PubMed] [Google Scholar]
- Marmot M (2015). The health gap: The challenge of an unequal world. Bloomsbury Press. [DOI] [PubMed] [Google Scholar]
- Marmot M, & Wilkinson R (Eds.). (2005). Social determinants of health. Oxford: Oxford University Press. [Google Scholar]
- McDade TW (2014). Development and validation of assay protocols for use with dried blood spot samples. American Journal of Human Biology, 26(1), 1–9. 10.1002/ajhb.22463 [DOI] [PubMed] [Google Scholar]
- McDade TW, Miller A, Tran TT, Borders AEB, & Miller G (2020). A highly sensitive multiplex immunoassay for inflammatory cytokines in dried blood spots. American Journal of Human Biology, e23558. 10.1002/ajhb.23558 [DOI] [PubMed] [Google Scholar]
- McDade TW, & Shell-Duncan B (2002). Whole blood collected on filter paper provides a minimally invasive method for assessing human transferrin receptor level. The Journal of Nutrition, 132(12), 3760–3763. 10.1093/jn/132.12.3760 [DOI] [PubMed] [Google Scholar]
- McDade TW, Williams S, & Snodgrass JJ (2007). What a drop can do: Dried blood spots as a minimally invasive method for integrating biomarkers into population-based research. Demography, 44(4), 899–925. 10.1353/dem.2007.0038 [DOI] [PubMed] [Google Scholar]
- McDade TW, Woodruff TK, Huang Y, Funk WE, Prewitt M, Kondapalli L, & Gracia CR (2012). Quantification of anti-Müllerian hormone (AMH) in dried blood spots: Validation of a minimally invasive method for assessing ovarian reserve. Human Reproduction, 27(8), 2503–2508. 10.1093/humrep/des194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel JT, Nuhu K, Ruiz J, & Alorbi G (2019). Social determinants of cancer incidence and mortality around the world: An ecological study. Global Health Promotion, 26(1), 41–49. 10.1177/1757975916686913 [DOI] [PubMed] [Google Scholar]
- McVeigh TP, & Kerin MJ (2017). Clinical use of the Oncotype DX genomic test to guide treatment decisions for patients with invasive breast cancer. Breast Cancer: Targets and Therapy, 9, 393–400. 10.2147/BCTT.S109847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing EM, Young TB, Hunt VB, Gilchrist KW, Newton MA, Bram LL, Hisgen WJ, Barry Greenberg E, Kuglitsch ME, & Wegenke JD (1995). Comparison of bladder cancer outcome in men undergoing hematuria home screening versus those with standard clinical presentations. Urology, 45(3), 387–397. 10.1016/S0090-4295(99)80006-5 [DOI] [PubMed] [Google Scholar]
- Mishra A, & Verma M (2010). Cancer biomarkers: Are we ready for the prime time? Cancers, 2(1), 190–208. 10.3390/cancers2010190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan S, Lawton R, Palmer C, & Rojas AC (2019). Competitive ELISA method for novel estrogen-negative breast cancer biomarker quantitation. Journal of Immunological Methods, 474, 112671. 10.1016/j.jim.2019.112671 [DOI] [PubMed] [Google Scholar]
- Moltzahn F, Olshen AB, Baehner L, Peek A, Fong L,Stöppler H, Simko J, Hilton JF, Carroll P, & Blelloch R (2011). Microfluidic-based multiplex qRT-PCR identifies diagnostic and prognostic microRNA signatures in the sera of prostate cancer patients. Cancer Research, 71(2), 550–560. 10.1158/0008-5472.CAN-10-1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira-Barbosa C, Barros-Silva D, Costa-Pinheiro P, Torres-Ferreira J, Constâncio V, Freitas R, Oliveira J, Antunes L, Henrique R, & Jerónimo C (2018). Comparing diagnostic and prognostic performance of two-gene promoter methylation panels in tissue biopsies and urines of prostate cancer patients. Clinical Epigenetics, 10, 132. 10.1186/s13148-018-0564-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss EL, Hollingworth J, & Reynolds TM (2005). The role of CA125 in clinical practice. Journal of Clinical Pathology, 58(3), 308–312. 10.1136/jcp.2004.018077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muinao T, Deka Boruah HP, & Pal M (2019). Multi-biomarker panel signature as the key to diagnosis of ovarian cancer. Heliyon, 5(12), e02826. 10.1016/j.heliyon.2019.e02826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller PAJ, & Vousden KH (2014). Mutant p53 in cancer: New functions and therapeutic opportunities. Cancer Cell, 25(3), 304–317. 10.1016/j.ccr.2014.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel G, Linseisen J, Boshuizen HC, Pera G, del Giudice G, Westert GP, Gonzalez CA, Allen NE, Key TJ, Numans ME, Peeters PH, Sieri S, Siman H, Berglund G, Hallmans G, Stenling R, Martinez C, Arriola L, Barricarte A, … Gonzalez CA (2007). Socioeconomic position and the risk of gastric and oesophageal cancer in the European prospective investigation into cancer and nutrition (EPIC-EURGAST). International Journal of Epidemiology, 36(1), 66–76. 10.1093/ije/dyl275 [DOI] [PubMed] [Google Scholar]
- Nemeir IA, Saab J, Hleihel W, Errachid A, & Zine N (2019). Salivary protein antigens for breast cancer biomarkers. Journal of Cancer Immunology, 1(1), 24–30. 10.33696/cancerimmunol.1.004 [DOI] [Google Scholar]
- Newton S, Braithwaite D, & Akinyemiju TF (2017). Socioeconomic status over the life course and obesity: Systematic review and meta-analysis. PLoS One, 12(5), e0177151. 10.1371/journal.pone.0177151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Dumont T, Mahmoodi M, Hammet F, Tran T, Tsimiklis H, Kathleen Cuningham Foundation Consortium for Research into Familial Breast Cancer (kConFab), Giles GG, Hopper JL, Australian Breast Cancer Family Registry, Southey MC, & Park DJ (2015). Hi-Plex targeted sequencing is effective using DNA derived from archival dried blood spots. Analytical Biochemistry, 470, 48–51. 10.1016/j.ab.2014.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH, National Institute of Health. (2015). Age and cancer risk. Bethesda. https://www.cancer.gov/about-cancer/causes-prevention/risk/age [Google Scholar]
- Novikoff AB (1945). The concept of integrative levels and biology. Science, 101(2618), 209–215. 10.1126/science.101.2618.209 [DOI] [PubMed] [Google Scholar]
- Núñez C (2019). Blood-based protein biomarkers in breast cancer. Clinica Chimica Acta, 490, 113–127. 10.1016/j.cca.2018.12.028 [DOI] [PubMed] [Google Scholar]
- O’Reilly E, Tuzova AV, Walsh AL, Russell NM, O’Brien O, Kelly S, Dhomhnallain ON, DeBarra L, Dale CM, Brugman R, Clarke G, Schmidt O, O’Meachair S, Patil D, Pellegrini KL, Fleshner N, Garcia J, Zhao F, Finn S, …Perry AS (2019). epiCaPture: A urine DNA methylation test for early detection of aggressive prostate cancer. JCO Precision Oncology, 3, 1–18. 10.1200/PO.18.00134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeyen E, Hoekx L, De Wachter S, Baldewijns M, Ameye F, & Mertens I (2019). Bladder cancer diagnosis and follow-up: The current status and possible role of extracellular vesicles. International Journal of Molecular Sciences, 20(821), 1–18. 10.3390/ijms20040821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öge O, Atsü N, Sahin A, & Ozen H (2000). Comparison of BTA stat and NMP22 tests in the detection of bladder cancer. Scandinavian Journal of Urology and Nephrology, 34(6), 349–351. 10.1080/003655900455404 [DOI] [PubMed] [Google Scholar]
- Öge Ö, Kozaci D, & Gemalmaz H (2002). The BTA stat test is nonspecific for hematuria: An experimental hematuria model. Journal of Urology, 167(3), 1318–1320. 10.1016/S0022-5347(05)65290-1 [DOI] [PubMed] [Google Scholar]
- Onieva-García MÁ, Llanos-Méndez A, Baños-Álvarez E, & Isabel-Gómez R (2015). A systematic review of the clinical validity of the Cologuard™ genetic test for screening colorectal cancer. Revista Clínica Española (English Edition), 215(9), 527–536. 10.1016/j.rceng.2015.08.006 [DOI] [PubMed] [Google Scholar]
- Panagopoulou P, Gogas H, Dessypris N, Maniadakis N, Fountzilas G, & Petridou ET (2012). Survival from breast cancer in relation to access to tertiary healthcare, body mass index, tumor characteristics and treatment: A Hellenic cooperative oncology group (HeCOG) study. European Journal of Epidemiology, 27(11), 857–866. 10.1007/s10654-012-9737-z [DOI] [PubMed] [Google Scholar]
- Patel P, & Gomes J (2010). The environmental causes of cancer: A literature review. Revue Interdisciplinaire Des Sciences de La Santé - Interdisciplinary Journal of Health Sciences, 1(1), 60–65. 10.18192/riss-ijhs.v1i1.1536 [DOI] [Google Scholar]
- Patnaik S, Mallick R, & Yendamuri S (2010). Detection of microRNAs in dried serum blots. Analytical Biochemistry, 407(1), 147–149. 10.1038/npre.2010.4785.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- PCAWG Evolution & Heterogeneity Working Group, PCAWG Consortium, Gerstung M, Jolly C, Leshchiner I, Dentro SC, Gonzalez S, Rosebrock D, Mitchell TJ, Rubanova Y, Anur P, Yu K, Tarabichi M, Deshwar A, Wintersinger J, Kleinheinz K, V azquez-García I, Haase K, Jerman L, …van Loo P (2020). The evolutionary history of 2,658 cancers. Nature, 578(7793), 122–128. 10.1038/s41586-019-1907-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson BJ, & Alvarado AS (2008). Regeneration, stem cells, and the evolution of tumor suppression. Cold Spring Harbor Symposia on Quantitative Biology, 73, 565–572. 10.1101/sqb.2008.73.045 [DOI] [PubMed] [Google Scholar]
- Peng M, Chen C, Hulbert A, Brock MV, & Yu F (2017). Non-blood circulating tumor DNA detection in cancer. Oncotarget, 8(40), 69162–69173. 10.18632/oncotarget.19942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezaro C, Woo HH, & Davis ID (2014). Prostate cancer: Measuring PSA. Internal Medicine Journal, 44(5), 433–440. 10.1111/imj.12407 [DOI] [PubMed] [Google Scholar]
- Pirrone F, & Albertini M (2017). Olfactory detection of cancer by trained sniffer dogs: A systematic review of the literature. Journal of Veterinary Behavior, 19, 105–117. 10.1016/j.jveb.2017.03.004 [DOI] [Google Scholar]
- Rapado-González Ó, Martínez-Reglero C, Salgado-Barreira Á,Takkouche B, López-López R, Su arez-Cunqueiro MM, & Muinelo-Romay L (2020). Salivary biomarkers for cancer diagnosis: A meta-analysis. Annals of Medicine, 52, 1–14. 10.1080/07853890.2020.1730431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rej PH, Bondy MH, Lin J, Prather AA, Kohrt BA,Worthman CM, & Eisenberg DTA (2021). Telomere length analysis from minimally-invasively collected samples: Methods development and meta-analysis of the validity of different sampling techniques. American Journal of Human Biology, 33(1), e23410. 10.1002/ajhb.23410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook G (2019). Immune System. In Brüne M & Schiefenhövel W (Eds.), The Oxford handbook of evolutionary medicine. Oxford University Press. [Google Scholar]
- Rook G, Bäckhed F, Levin BR, McFall-Ngai MJ, & McLean AR (2017). Evolution, human-microbe interactions, and life history plasticity. The Lancet, 390(10093), 521–530. 10.1016/S0140-6736(17)30566-4 [DOI] [PubMed] [Google Scholar]
- Ross DD, Yang W, Abruzzo LV, Dalton WS, Schneider E,Lage H, Dietel M, Greenberger L, Cole SPC, & Doyle LA (1999). Atypical multidrug resistance: Breast cancer resistance protein messenger RNA expression in mitoxantrone-selected cell lines. Journal of the National Cancer Institute, 91(5), 429–433. 10.1093/jnci/91.5.429 [DOI] [PubMed] [Google Scholar]
- Rotunno M, Barajas R, Clyne M, Hoover E, Simonds NI,Lam TK, Mechanic LE, Goldstein AM, & Gillanders EM (2020). A systematic literature review of whole exome and genome sequencing population studies of genetic susceptibility to cancer. Cancer Epidemiology and Prevention Biomarkers, 29(8), 1519–1534. 10.1158/1055-9965.EPI-19-1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhaak LR, Miyamoto S, Kelly K, & Lebrilla CB (2012). N-glycan profiling of dried blood spots. Analytical Chemistry, 84 (1), 396–402. 10.1021/ac202775t [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saidi T, Moufid M, de Jesus Beleño-Saenz K, Welearegay TG, el Bari N, Lisset Jaimes-Mogollon A, Ionescu R, Bourkadi JE, Benamor J, el Ftouh M, & Bouchikhi B (2020). Non-invasive prediction of lung cancer histological types through exhaled breath analysis by UV-irradiated electronic nose and GC/QTOF/MS. Sensors and Actuators B: Chemical, 311, 127932. 10.1016/j.snb.2020.127932 [DOI] [Google Scholar]
- Salgado M, Madureira J, Mendes AS, Torres A,Teixeira JP, & Oliveira MD (2020). Environmental determinants of population health in urban settings. A systematic review. BMC Public Health, 20(1), 853. 10.1186/s12889-020-08905-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder FH, Hugosson J, Roobol MJ, Tammela TLJ, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H, Zappa M, Denis LJ, Recker F, Berenguer A, Määttänen L, Bangma CH, Aus G, Villers A, Rebillard X, van der Kwast T, … Auvinen A (2009). Screening and prostate-cancer mortality in a randomized European study. The New England Journal of Medicine, 360(13), 1320–1328. 10.1056/NEJMoa0810084 [DOI] [PubMed] [Google Scholar]
- Shivapurkar N, & Gazdar AF (2010). DNA methylation based biomarkers in non-invasive cancer screening. Current Molecular Medicine, 10(2), 123–132. 10.2174/156652410790963303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisinni L, & Landriscina M (2015). The role of human chorionic gonadotropin as tumor marker: Biochemical and clinical aspects. In Advances in Cancer biomarkers (pp. 159–176). Springer. [DOI] [PubMed] [Google Scholar]
- Sivadasan P, Gupta MK, Sathe G, Sudheendra HV, Sunny SP, Renu D, Hari PS, Gowda H, Suresh A, Kuriakose MA, & Sirdeshmukh R (2020). Salivary proteins from dysplastic leukoplakia and oral squamous cell carcinoma and their potential for early detection. Journal of Proteomics, 212, 103574. 10.1016/j.jprot.2019.103574 [DOI] [PubMed] [Google Scholar]
- Sloan FA, & Gelband H (2007). The cancer burden in low-and middle-income countries and how it is measured. In Cancer control opportunities in low-and middle-income countries. National Academies Press; (US: ). [PubMed] [Google Scholar]
- Smith RA, Caleffi M, Albert U-S, Chen THH, Duffy SW, Franceschi D, & Nyström L (2006). Breast cancer in limited-resource countries: Early detection and access to care. The Breast Journal, 12(s1), S16–S26. 10.1111/j.1075-122X.2006.00200.x [DOI] [PubMed] [Google Scholar]
- Smith SJ, Diehl NN, Smith BD, & Mohney BG (2010). Urine catecholamine levels as diagnostic markers for neuroblastoma in a defined population: Implications for ophthalmic practice. Eye, 24(12), 1792–1796. 10.1038/eye.2010.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon A (1980). Monoclonal immunoglobulins as biomarkers of cancer. In Sell S (Ed.), Cancer markers: Diagnostic and developmental significance (pp. 57–87). Humana Press. 10.1007/978-1-4612-6117-9_3 [DOI] [Google Scholar]
- Soto AM, & Sonnenschein C (2004). The somatic mutation theory of cancer: Growing problems with the paradigm? BioEssays, 26(10), 1097–1107. 10.1002/bies.20087 [DOI] [PubMed] [Google Scholar]
- Soto AM, & Sonnenschein C (2011). The tissue organization field theory of cancer: A testable replacement for the somatic mutation theory. BioEssays, 33(5), 332–340. 10.1002/bies.201100025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivack S, Shi MK, Patel D, Desai A, Dobkin J, Shah C,Hosgood D, Ye K, Qiu Y, & Kurland I (2019). Initial discovery of exhaled small polar energetics-related metabolites by GC-MS for lung cancer risk assessment. Journal of Thoracic Oncology, 14(10), S519. 10.1016/j.jtho.2019.08.1084 [DOI] [Google Scholar]
- Srisuttee R, Arayataweegool A, Mahattanasakul P,Tangjaturonrasme N, Kerekhanjanarong V, Keelawat S, Mutirangura A, & Kitkumthorn N (2020). Evaluation of NID2 promoter methylation for screening of oral squamous cell carcinoma. BMC Cancer, 20, 218. 10.1186/s12885-020-6692-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns SC (2012). Evolutionary medicine: Its scope, interest and potential. Proceedings of the Royal Society B: Biological Sciences, 279(1746), 4305–4321. 10.1098/rspb.2012.1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns SC, & Medzhitov R (2015). Evolutionary medicine. Oxford University Press. [Google Scholar]
- Strassmann BI (1999). Menstrual cycling and breast cancer: An evolutionary perspective. Journal of Women’s Health, 8(2), 193–202. 10.1089/jwh.1999.8.193 [DOI] [PubMed] [Google Scholar]
- Su X, Carlson BF, Wang X, Li X, Zhang Y, Montgomery JP, Ding Y, Wagner AL, Gillespie B, & Boulton ML (2018). Dried blood spots: An evaluation of utility in the field. Journal of Infection and Public Health, 11(3), 373–376. 10.1016/j.jiph.2017.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhakar A (2009). History of cancer, ancient and modern treatment methods. Journal of Cancer Science & Therapy, 1(2), 1–4. 10.4172/1948-5956.100000e2 [DOI] [PubMed] [Google Scholar]
- Sukhija H, Krishnan R, Balachander N, Raghavendhar K,Ramadoss R, & Sen S (2015). C-deletion in exon 4 codon 63 of p53 gene as a molecular marker for oral squamous cell carcinoma: A preliminary study. Contemporary Clinical Dentistry, 6(Suppl 1), S227–S234. 10.4103/0976-237X.166840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverna G, Tidu L, Grizzi F, Torri V, Mandressi A, Sardella P, la Torre G, Cocciolone G, Seveso M, Giusti G, Hurle R, Santoro A, & Graziotti P (2015). Olfactory system of highly trained dogs detects prostate cancer in urine samples. The Journal of Urology, 193(4), 1382–1387. 10.1016/j.juro.2014.09.099 [DOI] [PubMed] [Google Scholar]
- Tekcan A, Gümüşay Ö, Nursal AF, Yiğit S, Yildiz S, & Tümer MK (2020). C deletion in exon 4 codon 63 of p53 gene in Turkish patients with oral squamous cell carcinoma. Turkish Journal of Oncology, 35(2), 163–166. 10.5505/tjo.2019.2092 [DOI] [Google Scholar]
- The ICGC/TCGA Pan-Cancer Analysis of Whole Genomes Consortium. (2020). Pan-cancer analysis of whole genomes. Nature, 578, 82–93. 10.1038/s41586-020-1969-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasetti C, Li L, & Vogelstein B (2017). Stem cell divisions, somatic mutations, cancer etiology, and cancer prevention. Science, 355(6331), 1330–1334. 10.1126/science.aaf9011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasetti C, & Vogelstein B (2015). Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science, 347(6217), 78–81. 10.1126/science.1260825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres PJ, Fletcher EM, Gibbons SM, Bouvet M,Doran KS, & Kelley ST (2015). Characterization of the salivary microbiome in patients with pancreatic cancer. PeerJ, 3, e1373. 10.7717/peerj.1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C (2015). Defining cell types and states with single-cell genomics. Genome Research, 25(10), 1491–1498. 10.1101/gr.190595.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger-Saldaña K (2014). Challenges to the early diagnosis and treatment of breast cancer in developing countries. World Journal of Clinical Oncology, 5(3), 465–477. 10.5306/wjco.v5.i3.465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valeggia CR (2007). Taking the lab to the field: Monitoring reproductive hormones in population research. Population and Development Review, 33(3), 525–542. 10.1111/j.1728-4457.2007.00183.x [DOI] [Google Scholar]
- Varmus H (2006). The new era in cancer research. Science, 312 (5777), 1162–1165. 10.1126/science.1126758 [DOI] [PubMed] [Google Scholar]
- Vickaryous MK, & Hall BK (2006). Human cell type diversity, evolution, development, and classification with special reference to cells derived from the neural crest. Biological Reviews, 81(3), 425–455. 10.1017/S1464793106007068 [DOI] [PubMed] [Google Scholar]
- Vijayakumar S, Winter K, Sause W, Gallagher MJ, Michalski J, Roach M, Porter A, & Bondy M (1998). Prostate-specific antigen levels are higher in african-american than in white patients in a multicenter registration study: Results of RTOG 94–12. International Journal of Radiation Oncology, 40 (1), 17–25. 10.1016/S0360-3016(97)00834-1 [DOI] [PubMed] [Google Scholar]
- Vineis P, & Husgafvel-Pursiainen K (2005). Air pollution and cancer: Biomarker studies in human populations. Carcinogenesis, 26(11), 1846–1855. 10.1093/carcin/bgi216 [DOI] [PubMed] [Google Scholar]
- Vineis P, & Perera F (2007). Molecular epidemiology and biomarkers in etiologic cancer research: The new in light of the old. Cancer Epidemiology and Prevention Biomarkers, 16(10), 1954–1965. 10.1158/1055-9965.EPI-07-0457 [DOI] [PubMed] [Google Scholar]
- Vineis P, & Xun W (2009). The emerging epidemic of environmental cancers in developing countries. Annals of Oncology, 20 (2), 205–212. 10.1093/annonc/mdn596 [DOI] [PubMed] [Google Scholar]
- Vogtmann E, & Goedert JJ (2016). Epidemiologic studies of the human microbiome and cancer. British Journal of Cancer, 114 (3), 237–242. 10.1038/bjc.2015.465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Lu H, Li K-K, Bai C-Y, & Ma Z-B (2018). Digit ratio (2D:4D) in Chinese women with gastric cancer. American Journal of Human Biology, 30(3), e23109. 10.1002/ajhb.23109 [DOI] [PubMed] [Google Scholar]
- Wang Y, Yu Y, Ye R, Zhang D, Li Q, An D, Fang L, Lin Y,Hou Y, Xu A, Fu Y, Lu W, Chen X, Chen M, Zhang M, Jiang H, Zhang C, Dong P, Li C, … Wu S (2016). An epigenetic biomarker combination of PCDH17 and POU4F2 detects bladder cancer accurately by methylation analyses of urine sediment DNA in Han Chinese. Oncotarget, 7(3), 2754–2764. 10.18632/oncotarget.6666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson IR, Takahashi K, Futreal PA, & Chin L (2013). Emerging patterns of somatic mutations in cancer. Nature Reviews Genetics, 14(10), 703–718. 10.1038/nrg3539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen JC, Sai V, Straatsma BR, & McCannel TA (2013). Radiation-related cancer risk associated with surveillance imaging for metastasis from choroidal melanoma. JAMA Ophthalmology, 131(1), 56. 10.1001/jamaophthalmol.2013.564 [DOI] [PubMed] [Google Scholar]
- Whibley C, Pharoah PDP, & Hollstein M (2009). p53 polymorphisms: Cancer implications. Nature Reviews Cancer, 9(2), 95–108. [DOI] [PubMed] [Google Scholar]
- WHO (2018). Cancer incidence and mortality data: Sources and methods by country. Geneva, Switzerland. https://gco.iarc.fr/today/data/methods/GLOBOCAN2018_Annex_A.XLSX. [Google Scholar]
- WHO (2020). WHO report on cancer: Setting priorities, investing wisely and providing care for all. Geneva, Switzerland. https://apps.who.int/iris/bitstream/handle/10665/330745/9789240001299-eng.pdf. [Google Scholar]
- Williams GC (1957). Pleiotropy, natural selection, and the evolution of senescence. Evolution, 11(4), 398–411. 10.1111/j.1558-5646.1957.tb02911.x [DOI] [Google Scholar]
- Wolf A, Moissl-Eichinger C, Perras A, Koskinen K, Tomazic PV, & Thurnher D (2017). The salivary microbiome as an indicator of carcinogenesis in patients with oropharyngeal squamous cell carcinoma: A pilot study. Scientific Reports, 7(1), 1–10. 10.1038/s41598-017-06361-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, & Lanier LL (2003). Natural killer cells and cancer. Advances in Cancer Research, 90, 127–156. 10.1016/s0065-230x(03)90004-2 [DOI] [PubMed] [Google Scholar]
- Xia Z, Duan X, Tao S, Qiu W, Liu D, Wang Y, Wei S, Wang B, Jiang Q, Lu B, Song Y, & Hu X (2013). Pollution level, inhalation exposure and lung cancer risk of ambient atmospheric polycyclic aromatic hydrocarbons (PAHs) in Taiyuan, China. Environmental Pollution, 173, 150–156. 10.1016/j.envpol.2012.10.009 [DOI] [PubMed] [Google Scholar]
- Yadav S, Tajmul M, Chandra KB, Saini A, Mathur SR,Sharma JB, & Kumar S (2019). P156 saliva: Hub of plausible ovarian cancer proteomic markers. International Journal of Gynecological Cancer, 29(4). 10.1136/ijgc-2019-ESGO.217 [DOI] [Google Scholar]
- Yang J-Y, Sun Y-W, Liu D-J, Zhang J-F, Li J, & Hua R(2014). MicroRNAs in stool samples as potential screening biomarkers for pancreatic ductal adenocarcinoma cancer. American Journal of Cancer Research, 4(6), 663–673. [PMC free article] [PubMed] [Google Scholar]
- Yau TO, Tang C-M, Harriss EK, Dickins B, & Polytarchou C (2019). Faecal microRNAs as a non-invasive tool in the diagnosis of colonic adenomas and colorectal cancer: A meta-analysis. Scientific Reports, 9(1), 1–13. 10.1038/s41598-019-45570-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost K, Perkins C, Cohen R, Morris C, & Wright W (2001). Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes & Control, 12 (8), 703–711. 10.1023/A:1011240019516 [DOI] [PubMed] [Google Scholar]
- Yu C-Y, & Kuo H-C (2019). The emerging roles and functions of circular RNAs and their generation. Journal of Biomedical Science, 26(1), 29. 10.1186/s12929-019-0523-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Wang R, Han C, Wang Z, & Jin X (2020). A panel of urinary long non-coding RNAs differentiate bladder cancer from urocystitis. Journal of Cancer, 11(4), 781–787. 10.7150/jca.37006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan TL, & Cantley LC (2008). PI3K pathway alterations in cancer: Variations on a theme. Oncogene, 27(41), 5497–5510. 10.1038/onc.2008.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Sesterhenn IA, Connelly RR, Mostofi FK, & Moul JW (2000). Inflammatory infiltrate (prostatitis) in whole mounted radical prostatectomy specimens from black and white patients is not an etiology for racial difference in prostate specific antigen. The Journal of Urology, 163(1), 131–136. 10.1016/S0022-5347(05)67988-8 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Liang W, Zhang P, Chen J, Qian H, Zhang X, & Xu W (2017). Circular RNAs: Emerging cancer biomarkers and targets. Journal of Experimental & Clinical Cancer Research, 36(1), 152. 10.1186/s13046-017-0624-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Wang S, Aunan K, Martin Seip H, & Hao J (2006). Air pollution and lung cancer risks in China—A meta-analysis. Science of the Total Environment, 366(2), 500–513. 10.1016/j.scitotenv.2005.10.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


