Significance
All positive-sense single-stranded RNA viruses, such as Zika virus (ZIKV), replicate in the cytoplasm of host cells, but they can regulate host gene expression in the nucleus. Here, we report that the ZIKV NS5, the viral RNA-dependent RNA polymerase (RdRp) and essential for the viral replication in the cytoplasm, can directly bind with chromatin DNA in the nuclei. In human neural progenitor cells, NS5 inhibits the transcription of numerous neural genes by binding onto their gene body and blocking PAF1C-mediated transcription elongation. The expression of ZIKV NS5 disrupts neurogenesis in developing mouse brain. Our findings reveal a role of ZIKV RdRp as a DNA binding protein to regulate host gene transcription and provide insights into abnormal neurodevelopment and ZIKV infection.
Keywords: Zika virus, nonstructural protein 5 (NS5), DNA binding, transcriptional elongation, human neural progenitor cells (hNPCs)
Abstract
Zika virus (ZIKV) targets the neural progenitor cells (NPCs) in brain during intrauterine infections and consequently causes severe neurological disorders, such as microcephaly in neonates. Although replicating in the cytoplasm, ZIKV dysregulates the expression of thousands of host genes, yet the detailed mechanism remains elusive. Herein, we report that ZIKV encodes a unique DNA-binding protein to regulate host gene transcription in the nucleus. We found that ZIKV NS5, the viral RNA polymerase, associates tightly with host chromatin DNA through its methyltransferase domain and this interaction could be specifically blocked by GTP. Further study showed that expression of ZIKV NS5 in human NPCs markedly suppressed the transcription of its target genes, especially the genes involved in neurogenesis. Mechanistically, ZIKV NS5 binds onto the gene body of its target genes and then blocks their transcriptional elongation. The utero electroporation in pregnant mice showed that NS5 expression significantly disrupts the neurogenesis by reducing the number of Sox2- and Tbr2-positive cells in the fetal cortex. Together, our findings demonstrate a molecular clue linking to the abnormal neurodevelopment caused by ZIKV infection and also provide intriguing insights into the interaction between the host cell and the pathogenic RNA virus, where the cytoplasmic RNA virus encodes a DNA-binding protein to control the transcription of host cell in the nuclei.
Zika virus (ZIKV) is a member of the Flaviviridae family, which includes many medically important pathogenic viruses, such as Japanese encephalitis virus, yellow fever virus (YFV), dengue virus (DENV), and West Nile virus (1). ZIKV was originally reported in Uganda in 1947 and was declared as a global health emergency by World Health Organization in 2016 because of the widespread epidemic and severe symptoms (2, 3). The intrauterine infection of ZIKV may lead to fetal microcephaly and spontaneous abortion (4, 5). It can also cause the Guillain–Barré syndrome in adults (6). Unfortunately, there is still no available vaccine or approved treatment (7).
As a flavivirus, ZIKV contains a positive-sense, single-stranded RNA genome with about 11k nucleotides in length. The genome encodes a single polyprotein, which is co- or post-translationally cleaved into three structural proteins (capsid, precursor membrane, and envelope) and seven nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5). The nonstructural proteins of flavivirus are responsible for the assembly of the membrane-bound replication complexes in the cytoplasm for viral RNA synthesis, where the NS5 protein plays a central role (8–10). ZIKV NS5 contains a C-terminal RNA-dependent RNA polymerase (RdRp) domain and an N-terminal methyltransferase (MTase) domain (11, 12). The RdRp domain of NS5 mediates the synthesis of viral genomic RNA while the MTase domain is responsible for the viral RNA capping to form a type I RNA cap (m7GpppAmG) at the 5′-end (13, 14). Recently, ZIKV NS5 was reported to bind with the 5′ replication promoter region of the viral RNA to block the polyprotein translation (15). In addition, ZIKV NS5 has been reported to inhibit the host’s innate immune responses by degrading STAT2 (16). It has been well documented that ZIKV NS5 can stimulate the inflammatory responses by either binding to NLRP3 or activating the STAT1-mediated type II interferon and proinflammatory pathways (17–20). In the chicken embryonic neural progenitor cells (NPCs), ZIKV NS5 has been shown to interact with and deplete centrosome proteins at the primary cilia, consequently resulting in ciliopathy and premature neuron delamination (21).
Although the replication of the ZIKV genome occurs in the cytoplasm, ZIKV NS5 predominantly accumulates in the nucleus (22). A typical nuclear localization signal (NLS) has been found in the N-terminal region of the RdRp domain, and the nuclear import of NS5 is relying on the importin α/β-mediated active transport (20, 23). Blocking NS5 nuclear import conferred its rapid degradation and impaired viral RNA replication (23). Thus, the nuclear localization of ZIKV NS5 is crucial for the viral life cycle. ZIKV NS5 was reported to interact with splicing factor SC35, reduce the SC35 protein level, and translocate SC35 from the nuclei to the cytoplasm in NS5-expressing HEK293T cells (24). And a recent proteomic study reported that ZIKV NS5 can interact with splicing factors and the chromatin-associated complex (25). Nevertheless, the role of ZIKV NS5 in the nucleus is not well understood.
In this study, we show that the nuclear ZIKV NS5 associates with chromatin, where ZIKV NS5 is capable to bind with DNA directly through its MTase domain. The interaction of NS5–DNA could be inhibited by a high concentration of Guanosine triphosphate (GTP), a capping substrate of the MTase domain. The ChIP-seq and RNA-seq data provide evidence that ZIKV NS5 binds to the gene body of the key neurofactors and inhibits their transcription, thus disrupting the neurogenesis of NPCs. ZIKV NS5 works as a transcription inhibitor by blocking transcription elongation. We provide evidence that ZIKV NS5 directly binds with chromatin DNA and plays an active role in the regulation of nuclear gene expression.
Results
ZIKV-Encoded NS5 Localizes in Nuclei and Associates with Chromatin.
We first investigated the localization of the ZIKV-encoded proteins in cells (SI Appendix, Fig. S1A). Consistent with previous studies (19, 22), the NS5 protein was mainly localized in the nuclei, while the others were in the cytoplasm. Moreover, disrupting its NLS by point mutation could block the transportation of NS5 into the nuclei (SI Appendix, Fig. S1A). In ZIKV-infected A549 cells, the NS5 protein was detectable in the cytoplasm at 6 h post infection (hpi) and then appeared in the nuclei at 12, 24, 36, and 48 hpi (Fig. 1A and SI Appendix, Fig. S1B). NS5 was also localized in the nuclei of ZIKV-infected U251 cells at 24 hpi (Fig. 1B), indicating that the nuclear localization of NS5 is not cell type-specific. Upon fractionation of virus-infected cells, NS5 protein was present in both cytoplasmic and nucleic fractions but mainly in the nucleic fraction (Fig. 1C).
Fig. 1.
NS5 protein localizes in nuclei and associates with chromatin. (A) The localization of NS5 in A549 cells infected by ZIKV–SMGC-1 at the indicated time points. NS5 (red) and DAPI (blue). hpi, hours post infection. (Scale bar, 5 μm.) (B) The localization of NS5 in ZIKV-infected A549 and U251 cells at 24 hpi. NS5 (red), actin (green), and DAPI (blue). (Scale bar, 5 μm.) (C) The distribution of NS5 in cytoplasmic and nuclear fractions of ZIKV-infected A549 cells at 24 hpi. GAPDH and lamin B serve as markers of cytoplasmic and nuclear fractions, respectively. (D) Preextraction of cells with 0.25% Triton X-100 prior to fixation revealed proteins associated with chromatin. NS5–GFP (green) and DAPI (blue). (Scale bar, 5 μm.) (E) NS5 associates with chromatin. HEK293T cells transfected with NS5–GFP plasmid were separated into cytoplasmic, nucleoplasmic, and chromatin fractions, and NS5–GFP was detected by immunoblotting. GAPDH and histone H3 serve as markers of cytoplasm and chromatin, respectively. (F and G) The association of NS5 with chromatin is DNA dependent. After cellular fractionation, the chromatin fractions were treated with (G) or without (F) Turbo DNase and then were sequentially extracted with increasing concentrations of NaCl (80–800 mM). The distribution of NS5–GFP was detected by immunoblotting. GAPDH and H3 serve as markers of cytoplasmic and chromatin fractions, respectively. (H) The distribution of virus-encoded NS5 in the sequential salt extraction (80–800 mM NaCl) experiments with ZIKV-infected A549 cells at 24 hpi were detected by immunoblotting. (I and J) Colocalization of NS5 and dsDNA in the hNPCs and U251 cells expressing NS5–GFP (I) or hNPCs infected by Zika virus at 24 hpi (J). Cells were fixed and stained with the related antibodies, and the colocalization signal was analyzed by ZEN microscope software and Imaris. NS5–GFP/NS5 (green), dsDNA (red), and DAPI (blue). (Scale bar, 5 μm.) (K) Schematic diagram of the proximity ligation assay (PLA) to detect protein–DNA interaction. (L) Visualization of the association between NS5 and chromatin DNA by PLA in hNPCs expressing NS5–GFP or infected by Zika virus. Cells were fixed, and PLA was performed as described in Materials and Methods section. The NS5–dsDNA interaction signals (red), NS5–GFP (green), and DAPI (blue). (Scale bar, 5 μm.)
Nuclear proteins can attach to chromatin or exist in a soluble form within the nucleoplasm. The soluble proteins in the nucleoplasm could be extracted and washed away by the nonionic detergent, Triton X-100, while proteins associated with chromatin remain in the nucleus (26, 27). To investigate whether the NS5 protein is in the nucleoplasm or associates with chromatin, the cells expressing NS5–GFP were treated with 0.25% (vol/vol) Triton X-100 prior to fixation. As shown in Fig. 1D, the NS5–GFP still existed in the nuclei after detergent treatment, implying that NS5 may associate with chromatin in the nuclei. To further confirm this observation, cells were fractionated into cytoplasmic, nucleoplasmic, and chromatin fractions. Western blotting showed that NS5 protein was predominantly enriched in the chromatin fraction (Fig. 1E). The isolated chromatin was then sequentially extracted with increasing concentrations of NaCl to investigate the relationship between NS5 and chromatin. As shown in Fig. 1 F and G, NS5 was detected in the high salt extractions and it could be released into the low salt buffer by DNase digestion, implying that NS5 is tightly associated with chromatin in a DNA-dependent manner. Furthermore, NS5 is also stably associated with chromatin in ZIKV-infected cells (Fig. 1H).
An anti-dsDNA-specific antibody was then applied to visualize the genomic DNA, and ZIKV NS5 was found to be apparently colocalized with the dsDNA signals in the nuclei of NS5-expressing or ZIKV-infected cells (Fig. 1 I and J). The proximity ligation assay (PLA) is an antibody-based method to detect protein–protein and protein–DNA interactions in situ (28, 29). To better visualize the interaction between NS5 and chromatin DNA, we performed PLA with anti-dsDNA and anti-NS5 antibodies (Fig. 1K). As shown in Fig. 1L, plenty of PLA signals were detected in the nuclei of NS5–expressing or ZIKV-infected cells, while minimal signals were seen in control cells. Taken together, all these results demonstrated that the NS5 of ZIKV is associated with chromatin DNA in the nuclei of host cells.
NS5 Protein Directly Binds to Chromatin DNA through Its MTase Domain.
Given the association between NS5 and chromatin DNA, we wonder whether the ZIKV NS5 could directly bind to dsDNA. The recombinant GST–NS5 was expressed in HEK293T cells, and cell lysates were treated with benzonase nuclease to deplete host DNA before the protein purification (SI Appendix, Fig. S2A). Glutathione agarose beads prebound with GST or GST-NS5 were incubated with the sonicated and purified chromatin DNA (SI Appendix, Fig. S2B). After thorough washing, the beads were stained with PicoGreen, a dsDNA-specific fluorescent sensor. Bright fluorescence signals were visualized on the surface of GST-NS5-bound beads, with minimal signals on the control beads (Fig. 2 A and B and SI Appendix, Fig. S2 C and D), implying that the chromatin DNA was concentrated on the surface of beads by NS5. Electrophoretic mobility shift assays (EMSA) were further performed to investigate the interaction between NS5 and dsDNA. The results showed that NS5 apparently retarded the mobility of either the chromatin DNA mixture (SI Appendix, Fig. S2E) or a defined DNA duplex (Fig. 2C), indicating the formation of a DNA–protein complex. Together, these data suggested that ZIKV NS5 could directly bind to chromatin DNA.
Fig. 2.
NS5 protein directly binds to chromatin DNA through the MTase domain. (A and B) Glutathione agarose beads prebound with GST or GST–NS5 were incubated with purified chromatin DNA. The dsDNA on the beads was visualized by staining with PicoGreen (A). (Scale bar, 20 μm.) The fluorescence intensities of these beads were measured by using Image J software (B). Data are mean ± SEM (n = 20). Two-tailed unpaired Student’s t test was used for statistical analysis. ****P < 0.0001 and n.s. = not significant. (C) Electrophoretic mobility shift assay (EMSA) for detecting DNA binding activity of NS5. Recombinant GST (10 μM) or GST–NS5 (2–5 μM) were incubated with biotin-labeled DNA (5 μM) in binding buffer at 4°C for 1 h. DNA migration was performed in 4% polyacrylamide gel electrophoresis without sodium dodecylsulfate (SDS) and detected by Streptavidin-HRP Conjugate. (D) Schematic diagram showing the constructing strategy for MTase, RdRp, and MTase–NLS truncations of NS5. (E and F) Glutathione agarose beads prebound with GST, GST–NS5, GST–MTase, or GST–RdRp were incubated with purified chromatin DNA and then were stained by PicoGreen (E). The fluorescence intensities of these beads were measured by using Image J software (F). Data are mean ± SEM (n = 15). One-way ANOVA followed by Dunnett’s multiple comparisons test was used for statistical analysis. ****P < 0.0001 and n.s. = not significant. (G) EMSA for detecting DNA binding activity of MTase domain of NS5. The recombinant GST–MTase (2–10 μM) was incubated with biotin-labeled DNA (5 μM) and DNA migration was detected as above. (H and I) The distribution of RdRp–Flag (H) and MTase–NLS–Flag (I) in the sequential salt extraction (80–800 mM NaCl) experiments with HEK293T cells were detected by immunoblotting. (J–L) Quantitative analysis of MTase–DNA binding with isothermal titration calorimetry (ITC) assay. ITC thermograms show an exothermic reaction when MTase binds with DNA (J and L), and the binding affinity (KA = 1/KD), reaction stoichiometry (n), enthalpy (∆H), and entropy (ΔS) were provided (L). The control ITC thermogram in which the MTase protein was injected into the buffer alone was provided (K).
As mentioned above, NS5 contains an N-terminal MTase domain and a C-terminal RdRp domain (11, 12). To investigate which domain of NS5 is responsible for its binding to DNA, we constructed and purified the MTase and RdRp domains of NS5 tagged with GST (Fig. 2D and SI Appendix, Fig. S2A). The PicoGreen staining experiments showed that apparent fluorescence signals were visualized on the surface of both the MTase-bound and full-length NS5-bound beads, while the RdRp-bound beads failed to concentrate DNA, just like the GST control beads (Fig. 2 E and F). Moreover, the EMSA results further supported that only the MTase domain, but not the RdRp domain, formed a stable complex with dsDNA and retarded its migration (Fig. 2G and SI Appendix, Fig. S2 F and G). These data indicated that the NS5–DNA binding was mediated by the MTase domain. We further verified the binding of MTase and RdRp domains with chromatin in cells. The MTase–NLS recombinant protein was constructed to help it localize into the nuclei, like the RdRp domain (Fig. 2D and SI Appendix, Fig. S2H). The cellular fractionation and sequential salt extraction experiments showed that the RdRp domain was mainly distributed in the nucleoplasmic fraction, but the MTase–NLS protein remained in the high-salt fraction (Fig. 2 H and I), thus confirming the stable association of MTase domain with chromatin DNA.
For further quantitative analysis, the isothermal titration calorimetry (ITC) assay was performed to access the binding strength of MTase–DNA interaction. As shown in Fig. 2 J–L, the MTase–DNA binding showed an exothermic reaction, and the dissociation constant (Kd) was determined to be 0.427 μM by ITC, and the binding reaction is enthalpically driven (ΔH = −9.27 kJ/mol) with a favorable total entropy change (−TΔS = −32.8 kJ/mol). Taken together, our results revealed that ZIKV NS5 could directly bind to dsDNA through the MTase domain.
GTP Inhibits the Interaction between NS5 and DNA.
Flavivirus NS5 is a multifunctional domain protein with GTP and S-adenosyl-methionine (SAM)-binding pocket in the MTase domain, which can transfer the guanosine monophosphate from GTP to form the cap of the genomic RNA and transfer the methyl group from SAM to the RNA cap (14, 30–32). To define whether these factors would affect the DNA-NS5 interaction, we treated the isolated chromatin of HEK293T cells expressing NS5 with GTP or SAM during the sequential salt extraction experiments. Intriguingly, the results showed that the distribution of NS5 shifted from high salt buffers to the lower salt buffers when GTP was added, whereas SAM had minimal effects on the NS5 distribution as compared with the mock (Fig. 3 A–C). Furthermore, GTP also inhibited the NS5–chromatin association in ZIKV-infected cells (Fig. 3 D and E). To investigate whether this inhibitory effect is specific to GTP, other NTPs, such as ATP, CTP, and dGTP, were also tested in the salt extraction buffers. As shown in Fig. 3F, the inhibitory effect of GTP was most predominant than others (SI Appendix, Fig. S3 A and E). These results suggested that the association between NS5 and chromatin could be specifically inhibited by GTP. Furthermore, both the results of PicoGreen staining experiments and EMSA revealed that the GTP could abolish NS5–DNA interaction in a dose-dependent manner (Fig. 3 G–I and SI Appendix, Fig. S3F). Taken together, these data suggested that the GTP binding to the MTase domain could specifically inhibit and regulate the interaction between NS5 and chromatin DNA.
Fig. 3.
GTP inhibits the interaction between NS5 and chromatin DNA. (A–C) Effects of GTP and SAM on NS5–chromatin association. In the sequential salt extraction experiments, mock (A), 2 mM GTP (B), or 2 mM SAM (C) were added in the extraction buffer. The distribution of NS5–GFP was detected by immunoblotting. (A–E) The protein level was quantified by using Image J software. (D and E) Effects of GTP on NS5–chromatin association in virus infected cells. A549 cells were infected with ZIKV for 48 h. Chromatin fractions were isolated and extracted in gradient concentration of NaCl buffer added with mock (D) or 2 mM GTP (E). (F) Effects of NTP on NS5–chromatin association. Chromatin of HEK293T cells expressing NS5–GFP was isolated and eluted in gradient concentration of NaCl buffer added with NTP (2 mM), including GTP, CTP, ATP, and dGTP. (G and H) Effects of GTP on NS5–DNA interaction. Glutathione agarose beads prebound with GST–NS5 were incubated with purified chromatin DNA and increasing concentrations of GTP. The dsDNA on the beads was visualized by staining with PicoGreen (G). (Scale bar, 20 μm.) The fluorescence intensities of these beads were measured by using Image J software (H). Data are mean ± SEM (n = 15). One-way ANOVA followed by Dunnett’s multiple comparisons test was used for statistical analysis. ****P < 0.0001. (I) EMSA was used to evaluate the effects of GTP on NS5–DNA interaction. GST–NS5 (5 μM) and GTP (0–20 mM) were incubated with biotin-labeled DNA (5 μM) at 4°C for 1 h, and DNA migration was detected as above.
NS5 Protein Represses Gene Transcription in Human Neural Progenitor Cells (hNPCs).
In order to investigate the biological function of NS5, we transiently expressed NS5 in hNPCs and analyzed the transcription profiles by RNA sequencing (RNA-seq). A total of 1,760 differentially expressed genes (DEGs) (|log2 FC| >0.5 and P-value <0.01) were found in NS5-expressing hNPCs compared to the control cells (Fig. 4A), and their expression patterns were further analyzed by hierarchical clustering (Fig. 4B). Among these DEGs, 701 (39.8%) genes were upregulated, whereas 1059 (60.2%) genes were downregulated (Fig. 4C). We further performed the genes ontology (GO) enrichment analysis for the down- or up-regulated genes, respectively. The results showed that the downregulated genes were significantly enriched in GO terms involved in nucleosome organization, neural development, cellular response to type I interferon, DNA repair, and cell cycle process (Fig. 4D). In contrast, the upregulated genes were enriched in translation initiation, protein targeting to ER, metabolic process, cell cycle arrest, and RNA splicing (SI Appendix, Fig. S4A). To validate the reliability of the RNA-seq experiment, a group of the up- and down-regulated genes were selected for RT-qPCR analysis, and the results were highly correlated with the RNA-seq data (Fig. 4 E–G and SI Appendix, Fig. S4 B and C).
Fig. 4.
Effects of NS5 on gene expression in hNPCs. (A) Volcano plot of RNA-seq data showing the gene expression changes in hNPCs expressing NS5–GFP versus GFP. The red dots and blue dots represented the up- and down-regulated genes, respectively. (B) Heat map view of differentially expressed genes. (C) The pie chart showing the number and percentage of up- and down-regulated genes. (D) Gene ontology (GO) analysis showing the enrichment of downregulated genes in biological process, cellular component, and molecular function. (E–G) RT-qPCR validation of the downregulated genes involved in interferon response (IFITM1, IFI44L and IFI6) (E), nucleosome organization (H3C7, H2AC18, and H2AW) (F), and neural development (Sox2, Tubb3, Nestin, Sox8, MAP2, and Pax6) (G). n = 3 biological replicates. Data are mean ± SEM. Two-tailed unpaired Student’s t test was used for statistical analysis. *P < 0.05, **P < 0.01, and ***P < 0.001.
A group of genes involved in cellular response to type I interferon and defense response to virus, including IFITM1, IFI44L, and IFI6, were downregulated in NS5-expressing hNPCs (Fig. 4 D and E), which is consistent with the previously reported function of NS5 to block IFN pathway (16, 25). Moreover, NS5 also inhibited the transcription of genes that are associated with chromatin assembly and nucleosome organization, including H2AC18, H2AW, and H3C7, in hNPCs (Fig. 4 D and F). This indicated that the chromatin status of host cells might be changed by NS5. Coincidentally, these genes were also inhibited in ZIKV-infected human neural crest cells (33). Notably, the downregulated genes in NS5-expressing hNPCs were enriched in multiple neural-related terms, such as neural tube development, spinal cord development, neural crest cell migration, and regulation of neuron projection development (Fig. 4D). And the downregulation of these neural-related genes, including Sox2, Tubb3, Nestin, Sox8, MAP2, Pax6, and Neurod1, was confirm by qPCR (Fig. 4G and SI Appendix, Fig. S4C). These data indicated that NS5 may disturb the normal homeostasis of hNPCs.
NS5 Protein Binds to Host Genes Involved in Neurogenesis.
Given that NS5 regulated expression of a large number of host genes and it could associate with chromatin, we performed the chromatin immunoprecipitation coupled with sequencing (ChIP-seq) in NS5-expressing hNPCs to analyze the direct target gene profile of NS5. Immunoblotting confirmed the efficient immunoprecipitation of NS5–GFP (SI Appendix, Fig. S5A). We detected 149841 peaks normalized to the input (P-value <0.01 and fold enrichment >2), in which 18724 genes were annotated, suggesting a wide genomic distribution of NS5 in hNPCs. The peak density of NS5 showed that a large proportion of them located in the gene body (12.41% first intron, 39.55% other introns, 0.63% first exon, and 9.30% other exons) and 30.53% of them localized in the intergenic region (Fig. 5A). Only a small subset of NS5 peaks (7.57%) is localized in the transcription start site (TSS) of gene promoters (Fig. 5 A and B). Then, the de novo motif analysis of NS5 binding sites was calculated with Homer de novo motif research tool, and the motifs for MET28, TRB2, and Isl1 were among the most enriched motifs (SI Appendix, Fig. S5B).
Fig. 5.
NS5 targets genes involved in neurogenesis and represses gene transcription in hNPCs. (A) The pie chart showing the genomic distribution of NS5 ChIP-seq peaks in hNPCs. (B) Heat maps of the distribution of NS5 binding or input peaks within 2.0 kb of the gene transcription start sites (TSS). Scales indicated ChIP-seq reads intensities. (C) Venn diagram showing overlaps between NS5-binding genes and the differentially expressed genes (Upper panel). Among the 856 overlapped genes, 182 genes (21.3%) were upregulated and 674 genes (78.7%) were downregulated (Lower panel). (D) Average binding profile of NS5 along the overlapped genes. TSS: transcription start site. TES: transcript end sites. (E) Gene ontology (GO) analysis showing the enrichment of downregulated target genes in biological process. Pathways related to neural development were marked in red. (F) Integrative genomics viewer (IGV) shots showing the read density profiles of representative NS5 binding genes (Sox2, Tubb3, Nestin, Pax6, and Sox8). (G) ChIP–PCR validation of a set of the NS5 binding targets in NS5–GFP expressing hNPCs. DNA enrichment was calculated as percentage of input, with normal IgG as control. n = 3 biological replicates. Data are mean ± SEM. Two-tailed unpaired Student’s t test was used for statistical analysis. *P < 0.05, **P < 0.01, and ***P < 0.001.
Next, we investigated the direct target genes of NS5 through combinational analysis of the ChIP-seq data with the transcriptome data. A total of 856 genes were directly bound and transcriptionally regulated by NS5. Among them, 182 genes (21.3%) were upregulated and 674 (78.7%) genes were downregulated (Fig. 5C), implying that NS5 mainly inhibits the transcription of NS5-bound DEGs in hNPCs. NS5 is mainly distributed along the gene body instead of the promoter-TSS region of these downregulated target genes (Fig. 5D). Furthermore, GO enrichment analysis showed that the downregulated target genes were associated with Semaphorin-plexin signaling pathway, neural crest cell migration, negative regulation of glial cell proliferation, and neurogenesis, as well as type I interferon signaling pathway and DNA repair (Fig. 5E), indicating that NS5 might inhibit gene transcription to disturb neurogenesis in hNPCs. The binding of NS5 on the representative neurogenesis-related target genes is shown in Fig. 5F and SI Appendix, Fig. S5C, and they were further confirmed by ChIP–PCR analysis (Fig. 5G). Altogether, these data suggested that NS5 could bind to chromatin DNA and localize at the gene body to regulate gene transcription of neurogenesis-related genes.
NS5 Represses Gene Transcription through Blocking the Transcriptional Elongation Mediated by the PAF1 Complex.
The next question is how NS5 inhibits the expression of its target genes, after binding onto their gene body regions. To answer this question, we measured the interaction complex of NS5 in the nuclei by immunoprecipitation followed by mass spectrometry (MS) assay (Dataset S1). All the components of the PAF1 complex (PAF1C), including PAF1, CTR9, CDC73, LEO1, RTF1, and WDR61, were detected by the MS assay, and the interaction between NS5 and PAF1C were further confirmed by Co-IP experiment (Fig. 6 A and B, SI Appendix, Table S3, and Dataset S1). PAF1C is a critical elongation factor in eukaryotic cells which can directly interact with Pol II and promote the transcriptional elongation of active genes (34, 35). Although previous proteomic study showed the association between PAF1C and NS5 (25), the detailed mechanism of NS5 in regulating gene expression remains unknown.
Fig. 6.
NS5 represses gene transcription through blocking the elongation mediated by the PAF1 complex in a gene body region. (A and B) NS5 interacts with the PAF1 complex in the nucleus. The nuclei of HEK293T cells expressing NS5–Flag were isolated and then the immunoprecipitates were visualized by silver staining (A). Components of the PAF1 complex were detected by immunoblotting (B). (C) The distribution of the PAF1 complex in the sequential salt extraction experiments with A549 cells in the presence or absence of ZIKV NS5. (D) NS5 blocked the PAF1 complex in the gene body region. ChIP was performed with normal IgG or anti-PAF1 antibody in the control or NS5-expressing hNPCs. The enrichment of PAF1 on the gene body of NS5 targeting genes, including Sox2, Nestin, Pax6, and Sox8, were detected by qPCR. (E) GTP promoted PAF1 occupancy in the gene body region in NS5-expressing hNPCs. NS5-expressing hNPCs were treated with 2 mM GTP for 24 h, and ChIP was performed with normal IgG or anti-PAF1 antibody to detect NS5 targeting genes by qPCR. (F) GTP rescued the transcription of NS5-targeting genes. NS5-expressing hNPCs were treated with 2 mM GTP for 24 h, and the transcription levels of NS5 targeting genes were measured by RT-qPCR. (D–F) n = 3 biological replicates. Data are mean ± SEM. Two-tailed unpaired Student’s t test was used for statistical analysis. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, and n.s. = not significant.
The sequential salt extraction of chromatin experiments showed that, same as NS5, the components of PAF1C were predominantly distributed in the chromatin fraction (Fig. 6C), indicating that the interaction between NS5 and PAF1C might happen on chromatin. PAF1C components are mainly distributed in the low salt fractions in the control cells, implying their relatively low affinity in association with the chromatin. This is consistent with their biological function in mediating the transcriptional elongation (34, 35). It is noteworthy that a portion of PAF1C components, such as LEO1, CTR9, and WDR61, moved to the high salt fraction in NS5-expressing cells (Fig. 6C). These results suggested that the affinities of NS5–chromatin interaction and NS5–PAF1C binding were stronger than the affinity of PAF1C–chromatin association. Thus, we hypothesized that the transcriptional elongation mediated by PAF1C may be blocked when PAF1C encounters and binds to NS5 on the gene body.
To validate this hypothesis, we performed ChIP–PCR with an anti-PAF1-specific antibody to detect the occupancy of PAF1 on the gene body of the target genes in the control or NS5-expressing hNPCs. The results showed that NS5 could decrease the abundance of PAF1 on these gene body regions distal to the TSS (Fig. 6D). Furthermore, inhibiting the NS5–chromatin interaction by GTP treatment increased the occupancy of PAF1 on the gene body of these NS5-targeting genes (Fig. 6E). Consistently, the transcription levels of these genes were also restored by GTP treatment in NS5-expressing hNPCs (Fig. 6F). All together, these results indicated that the interaction between NS5 and chromatin DNA could inhibit the gene transcription through blocking the transcriptional elongation mediated by PAF1C.
NS5 Protein Disrupts Neurogenesis.
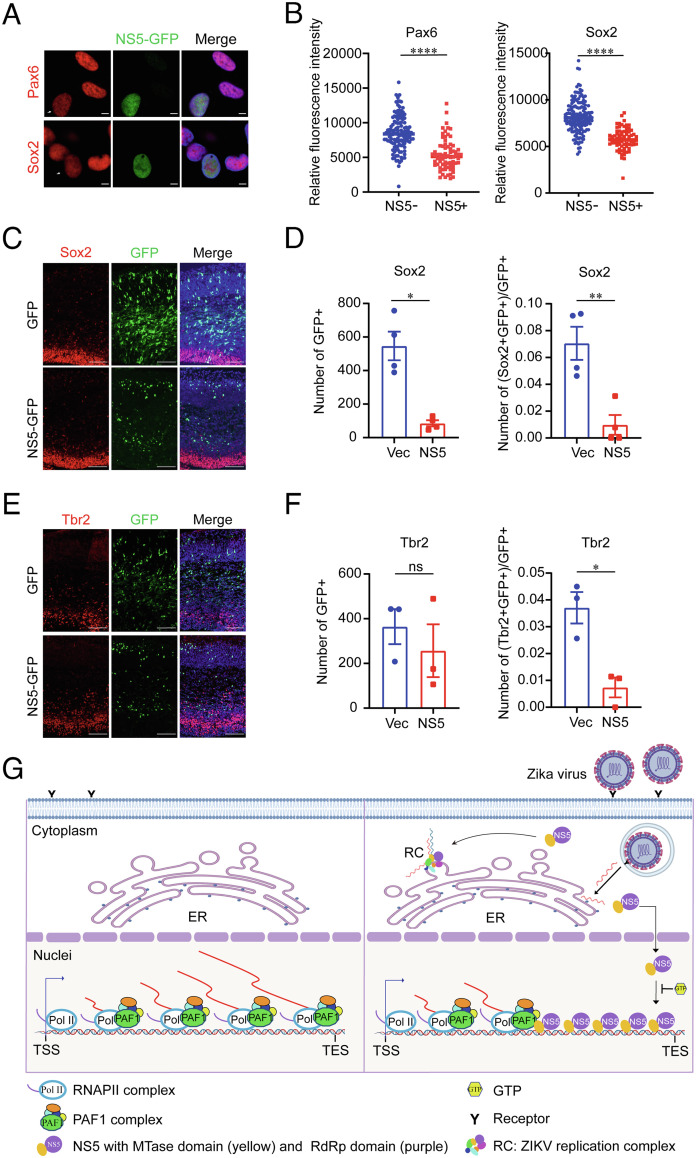
To analyze the potential role of NS5 in neurogenesis, we expressed the NS5 in hNPCs and detected the expression of neural markers, including Pax6 and Sox2. The immunostaining results showed that the expression levels of the two markers were significantly reduced in NS5-expressing hNPCs (Fig. 7 A and B). To further investigate the biological function of NS5 in fetal brain development, we performed utero electroporation in C57BL/6 pregnant mice to express NS5 in the fetal brain at E13.5, where the GFP group was set as control. The pregnant mice were sacrificed at E16.5, and the fetal brains were isolated. The immunostaining for neural makers were performed, and the ratio of cells expressing Sox2 or Tbr2 in GFP-positive cells were calculated. As shown in Fig. 7 C–F, the ratios of Sox2 or Tbr2-positive cells were dramatically decreased in the NS5-expressing group compared with the GFP control, indicating that ZIKV NS5 could cause abnormal neurogenesis in mouse. Thus, consistent with ZIKV infection, expression of NS5 alone could disrupt brain development through affecting neurogenesis.
Fig. 7.
NS5 disrupts the neurogenesis. (A and B) hNPCs infected with NS5–GFP lentivirus were performed immunostaining to analysis the expression of neuronal progenitor markers, Pax6 and Sox2 (A). (Scale bar, 5 μm.) The fluorescence intensities of PAX6 and Sox2 in NS5 negative or positive cells were analyzed by image J software (B). Data are mean ± SEM (n = 125) (n = 72). Two-tailed unpaired Student’s t test was used for statistical analysis. ****P < 0.0001. (C–F) In utero electroporation was performed at E13.5 to express NS5–GFP or GFP in embryonic mouse cortex. The pregnant mice and fetus were sacrificed at E16.5 and fetal brains were isolated for immunostaining of Sox2 (C) and Tbr2 (E). And staining for DAPI and GFP are shown. (Scale bar, 100 μm.) The number of GFP positive cells (Left panel) and the percentage of Sox2- or Tbr2-expressing GFP cells in total GFP positive cells (Right panel) were counted and shown in (D) and (F). Sections were from three or four animals. Data are mean ± SEM. Two-tailed unpaired t test with Welch’s was used for statistical analysis. *P < 0.05, **P < 0.01, and n.s. = not significant. (G) Schematic model of the inhibitory role of ZIKV NS5 in regulating host gene transcription. After virus infection, most of ZIKV NS5 is transported into the nuclei and binds to chromatin DNA through its MTase domain. A portion of the NS5 protein localizes on the gene body of its target genes. PAF1 complex associates with pol II and mediates gene transcriptional elongation. When the elongation complex meets NS5 on the gene body, PAF1C interact with NS5 and then the transcriptional elongation is blocked. Furthermore, GTP can inhibit the NS5–chromatin interaction and restore the transcription of NS5-targeting genes.
Discussion
As a member of flavivirus, ZIKV accomplishes its whole life cycle including the entering, replication, translation, packaging, and releasing in the host cytoplasm and cell membrane (8, 36, 37). However, the ZIKV NS5 is mainly localized in the nucleus of the host cell both in the exogenous expression and virus infection conditions (Fig. 1 A–C) (22). Here, we reported a function of NS5 in the nucleus where it directly regulates the expression of host genes by binding to chromatin with its MTase domain. A subset of NS5 target genes was enriched in neurogenesis, thus implying a molecular clue linking to the abnormal neurodevelopment caused by ZIKV infection.
In the present study, we found that the ZIKV NS5 associates with the chromatin even in high concentration of NaCl conditions, which could be released by DNase treatment. Moreover, the PLA assay further confirmed the association between NS5 and chromatin DNA in situ (Fig. 1). The in vitro results from Picogreen staining and EMSA demonstrated that the recombinant NS5 is capable to bind with dsDNA directly. The MTase domain but not RdRp domain is responsible for its DNA binding capacity and the MTase domain in association with the NLS sequence could tightly associate with the host cell chromatin. The ITC assay showed that the MTase binds to the DNA duplex strongly, with the Kd of 0.427 μM. In comparison, the Kd of ZIKV MTase and the viral RNA promoter stem-loop A (SLA) binding is 0.59 μM and the Kd of ZIKV NS5 and STAT2 binding is about 3.2 μM (38, 39). In further comparison, cGAS binds dsDNA with a Kd of ∼20 μM in an ITC assay (40). In the future, the structural biology study can further help to clarify the details of the interaction between NS5 and dsDNA.
The MTase domain of NS5, which is multifunctional with N7-MTase, 2′-O-MTase, and GTase activities (13, 30, 41), contains three different binding sites to recognize SAM, GTP, and RNA, respectively (14, 31, 42). Our further results implied that GTP could inhibit the DNA binding capacity of NS5 and release NS5 from chromatin in a relatively low NaCl concentration (Fig. 3). GTP is the guanylyl supplier to form the RNA cap structure during viral RNA replication (32). A previous study also mentioned that GTP could disrupt the dimerization/oligomerization of NS5 (43). Our study further expanded the role of GTP in regulating the function of NS5.
To elucidate the function of NS5 in association with chromatin, the gene expression profile was analyzed by RNA-seq and the distribution of NS5 on the host genome was mapped by ChIP-seq. We found that 1,059 genes were downregulated and 701 genes were upregulated by ZIKV NS5 expression. Among these DEGs, 856 genes were identified as transcriptional targets of ZIKV NS5. About 78.7% of these related genes were downregulated, while only 21.3% were upregulated, implying that ZIKV NS5 functions mainly to suppress the expression of its target genes. The cluster analysis of these downregulated genes showed the enrichment of GO terms including neural development, nucleosome organization, cellular response to type I interferon, DNA repair, and cell cycle process (Fig. 5). Instead of ingene promoters, ZIKV NS5 mainly enriched in the gene body regions of its target genes. Interestingly, a previous proteome study reported that both DENV NS5 and ZIKV NS5 interacted with the PAF1C (25), a chromatin-associated complex that is required for transcriptional elongation (44, 45). In the present study, we also captured and confirmed the interaction between NS5 and PAF1C, and they both were found to associate with chromatin (Fig. 6). The sequential salt extraction of chromatin experiments demonstrated that the affinities of NS5–chromatin interaction and NS5–PAF1C binding were stronger than the affinity of PAF1C–chromatin association. And the ChIP results indicated that NS5 bound to the gene body of its target genes and then decreased the abundance of PAF1 on these gene body regions distal to the TSS (Figs. 5 F and G and 6D). Thus, in our hNPCs system, ZIKV NS5 seems to inhibit gene transcription directly through binding to its target genes and then blocking the PAF1C-mediated transcriptional elongation when they meet each other on the gene body of these target genes (Fig. 7G).
This inhibition mechanism of ZIKV NS5 on gene transcription in hNPCs is reminiscent of the recently published report that the ZIKV NS5 directly blocks translation of the viral polyprotein through directly binding to the SLA at the 5′ end of the viral genome and then decreasing ribosome occupancy (15). As with a limited volume of viral genome, RNA viruses frequently encode proteins with multiple functions. Thus, it is tempting to speculate that ZIKV may use a similar mechanism to block the viral protein translation and inhibit host gene transcription by exploiting the nucleic acid-binding capabilities of the viral NS5 (Fig. 7G).
Multiple ZIKV-coded proteins have been reported to participate in the abnormal neural development. ZIKV with S139E mutation in prM increased the infectivity in human and mouse NPCs and caused more severe microcephaly and higher mortality rates in neonatal mice (46). ZIKV capsid can interact with MDM2 to activate the p53-mediated apoptosis pathway and cause neural cell death (47), and it also interacts with Dicer to dampen global miRNA production in NSCs, thus driving neurodevelopment defects (48). The NS2A is able to interact with the adheres junction complex to disrupt the neuroepithelium integrity and impair NPC proliferation (49). The NS2B-NS3 heterodimer inhibits NPC cytokinesis by cleaving the host protein Septin-2 (50). The NS4A suppresses key functions of Ankle2 to inhibit the brain development in Drosophila (25). The NS4A and NS4B cooperatively increase autophagy and impede the neurogenesis in fetal neural stem cells by deregulating the Akt-mTOR pathway (51). During the preparation of this manuscript, Saade and colleagues reported that ZIKV NS5 led to abnormal neurogenesis in the chicken embryo (21). They showed that despite the majority of NS5 localized in the nucleus, a small subset of NS5 interacted with multiple centrosome proteins at the cilium base and consequently caused ciliopathy and premature neuron delamination. Together with our results that the NS5 in the nucleus remains tightly associated with chromatin in human NPCs and inhibits the expression of important genes of neurogenesis, ZIKV NS5 appears to be one of the major viral factors responsible for the microcephaly caused by ZIKV infection.
In this study, we showed that ZIKV NS5 is mainly localized in the nucleus and directly bound to the chromatin DNA through its MTase domain, consequently inhibiting the transcription of the key neurogenesis-associated factors and disrupting the neurogenesis of NPCs. In addition to hNPCs, ZIKV also targets some other tissues such as testis, eyes, placenta, etc (52–54). It will be interesting to explore whether ZIKV NS5 could also inhibit the highly expressed functional genes in these tissues in the future research. Notably, not only ZIKV NS5, the NS5 encoded by other flaviviruses such as Dengue virus serotype 2 and 3 and YFV also localized predominantly in the host cell nucleus (55, 56). Further studies are needed to investigate whether these NS5 proteins also associate with chromatin and directly regulate gene expression. Controlling host cells at the transcriptional level may appear to be the common strategy for these flaviviruses to block the normal cell functions and hijack the cell resources for more efficient virus replication. In summary, our study provides insights into the virus–host interactions and provides potential therapeutic targets for the treatment of the diseases caused by virus infection.
Materials and Methods
Cell Lines.
ReNcell CX cells, a cell line of hNPC, were cultured in an ReNcell NSC maintenance medium (SCM005, Millipore) with 20 ng/mL FGF-2 (100-18B-100, PeproTech) and 20 ng/mL EGF (315-09-100, PeproTech) in 20 µg/mL laminin-coated dishes at 37°C with 5% CO2. HEK293T, A549, U251, and Vero cells were cultured in a Dulbecco's Modified Eagle Medium (DMEM, Corning) and supplemented with 10% fetal bovine serum (FBS, Gibco) at 37°C with 5% CO2. Cell lines were tested for mycoplasma. Cell culture plates and dishes were purchased from Jet Biofil.
Antibodies.
Primary antibodies used in this study are listed: mouse monoclonal antibody anti-Flag (F1804, Sigma-Aldrich), rabbit polyclonal antibody anti-GFP (G1544, Sigma-Aldrich), rabbit polyclonal antibody anti-Zika NS5 protein (GTX133329, GeneTex), rabbit polyclonal antibody anti-H3 (17168-1-AP, Proteintech), rabbit polyclonal antibody anti-GAPDH (10494-1-AP, Proteinteach), rabbit polyclonal antibody anti-lamin B1 (12987-1-AP, Proteinteach), rabbit polyclonal antibody anti-Actin (23660-1-AP, Proteinteach), rabbit polyclonal antibody anti-PAF1/PD2 (ab20662, Abcam), mouse monoclonal antibody anti-dsDNA (sc-58749, Santa Cruz), and rabbit anti-IgG (2,729, CST). Secondary antibodies for immunoblotting and immunofluorescence were obtained from Invitrogen.
Viruses and Infection.
ZIKV strain ZIKA-SMGC-1 (GenBank accession number KX266255) was kindly provided by Prof. Y. Wu (57). Cells were infected by ZIKA-SMGC-1 in a DMEM for 2 h and then replaced with a DMEM supplemented with 2% FBS at 37°C with 5% CO2. The recombinant lentiviral vector expressing GFP and ZIKV NS5–GFP were packaged with PSPAX2 and PMD2.G and transfected in HEK293T cells. Cells were infected by lentivirus for 8 h and then replaced with fresh culture.
Plasmid Construction.
Plasmids including pLV_Zika_Cv_Flag (# 79628), pLV_Zika_PrM_Flag (# 79631), pLV_Zika_NS3_Flag (# 79635), pLV_Zika_Flag_NS4A (# 79636), pLV_Zika_NS2B_Flag (# 79637), pLV_Zika_NS4B_Flag (# 79640), pLV_Zika_NS1_Flag (Variant: W98G) (# 79641), and pLV_Zika_NS5_Flag (# 79639) were gifts from Vaithi Arumugaswami Lab provided by Addgene. And plasmids pLV_Zika_E_Flag and pLV_Zika_NS2A_Flag were constructed in the same vector using restriction enzyme digestion and ligation method with ZIKA-SMGC-1 genome as the template. The ZIKV NS5 was also constructed with a GFP tag into a lentiviral vector with an EF1α promoter, which promoted protein expression in stem cells. The full length of ZIKV NS5 and NS5 truncations, including MTase and RdRp domain proteins, with the GST tag were constructed into plasmid pGEX 4T-1 for expression and purification. And all plasmids and mutations were confirmed by sequencing.
Expression and Purification of Recombinant Proteins.
The extended protocol for expression and purification of recombinant proteins is provided in SI Appendix, Materials and Methods.
Immunofluorescence.
Immunofluorescence was performed in A549, U251, hNPCs, and HEK293T cells. Cells were seeded in chamber slides either for transfection or infection with ZIKV. Cells for immunofluorescence were preextracted as described previously (58). And these extended protocols are provided in SI Appendix, Materials and Methods.
Coimmunoprecipitation and Immunoblotting.
The coimmunoprecipitation and immunoblotting are performed using standard procedures, and the detailed protocols are provided in SI Appendix, Materials and Methods.
Separation of Cytoplasm and Nuclei, and Chromatin Fractionation.
Salt fractionation of nuclei was adapted from established protocols (59). And the extended protocol is provided in SI Appendix, Materials and Methods.
GST Pull-Down and PicoGreen Staining Assay.
The detailed protocols for GST pull-down and PicoGreen staining assay are carried out in SI Appendix, Materials and Methods.
Electrophoretic mobility shift assay (EMSA).
The EMSA was performed according to the instruction of the EMSA kit (Beyotime). The extended protocol is provided in SI Appendix, Materials and Methods.
Isothermal titration calorimetry (ITC) Assay.
The ITC assay was described in the extended protocol, which was provided in SI Appendix, Materials and Methods.
Proximity ligation assay (PLA).
The PLA was performed according to the instructions of Duolink In Situ Orange Starter Kit Mouse/Rabbit (Sigma-Aldrich). And the extended protocol is provided in SI Appendix, Materials and Methods.
Chromatin Immunoprecipitation (ChIP).
The ChIP was performed according to the instructions of EZ-Magna ChIP A/G kit (Millipore). And the extended protocol is provided in SI Appendix, Materials and Methods.
RNA-seq.
hNPC cells expressing NS5–GFP or GFP were lysed in a Trizol reagent (Invitrogen) 48 h post infection by lentivirus. RNA was extracted by chloroform-Isoamyl alcohol methods. Libraries were prepared by deleting the ribosomal RNA depletion, and then the remaining RNA was performed the UID (Unique Identifier Digital) sequencing with HiSeq2500 (Illumina). RNA-seq reads were mapped to the human genome (hg38) with TopHat2, and differential expression analysis was performed using DESeq2.
Real-Time RT-PCR.
Total RNA was extracted using a Trizol reagent and PrimeScript™ RT Master Mix reverse transcription kit (Takara), and 500 ng of RNA was utilized for reverse transcription to synthesize cDNA. Quantitative PCR assays were performed with Power Up SYBR Green PCR Master Mix (Invitrogen) in an ABI Q5 Detection 682 System (Applied Biosystems) using specific primers listed in SI Appendix, Tables S1 and S2.
Mouse Experiments and In Utero Electroporation (IUE).
C57BL/6 pregnant mice were bred in pathogen-free animal facilities of Sun Yat-Sen University (SYSU). All animal studies were approved and supervised by the SYSU Institutional Animal Care and Use Committee (SYSU IACUC) under protocol no. SYSU-IACUC-MED-2019-B0002, and all animal experiments were performed in accordance with the guidelines by SYSU IACUC. In utero electroporation was performed as described previously (60). The extended protocol for IUE is provided in SI Appendix, Materials and Methods.
Statistical Analyses.
The details of statistical analysis are described in each figure legend. Statistical analysis is performed on at least three biological replicates, unless otherwise stated in the figure legend. The data are shown as the mean ± SEM. The statistical significance between different groups was calculated by two-tailed unpaired Student t test, one-way ANOVA test, or two-tailed unpaired t test with Welch’s connection. A P value <0.05 was significant. Data were analyzed with GraphPad Prism 8.3 software.
Supplementary Material
Appendix 01 (PDF)
Dataset S01 (XLSX)
Acknowledgments
The work is supported by the National Natural Science Foundation of China (NSFC Grant #82230075 and # 81620108020 to D.G. and Grant # 32270159 and # 31800151 to J.W.), National Key Research and Development Program of China (Grant #2021YFC2300100 to C.L.), Zhujiang Leading Talents Program of Guangdong province (Grant #2016LJ06Y540 to D.G. and Grant #2019ZT08Y464 to C.L.), Shenzhen Science and Technology Program (Grant # KQTD20180411143323605 and # JCYJ20200109142201695 to D.G.; Grant #GXWD20201231165807008 and 20200825183117001 to J.W.; and Gran #RCBS20200714114923232 to F.X.). National Ten-thousand Talents Program to D.G. We are grateful to Prof. Y. Wu of State Key Laboratory of Virology, School of Basic Medical Sciences, Wuhan University for sharing the ZIKV SMGC-1(GenBank accession number: KX266255) and to Prof. M. Li of School of Public Health, Sun Yat-Sen University for sharing the human neural progenitor cells.
Author contributions
D. G supervised the study and P. L., J. W. and D. G. contributed to the conception of the study. P. L., J. W., S.L., R. L., H. J., N. W. and Z. Z. performed the experiments and analyzed the data. P.L., J. W. and D.G. prepared the figures and wrote the manuscript. M. L., L. G., J. X., L. B., L. L. and S. L. assisted with the experiments. F. X., P. H., C. L., L. M., B. Z., and Z. Z. oversaw the study design and data analysis of a part of the experiments.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission. J.W.-L. is a guest editor invited by the Editorial Board.
Contributor Information
Junyu Wu, Email: wujy68@mail.sysu.edu.cn.
Deyin Guo, Email: guodeyin@mail.sysu.edu.cn.
Data, Materials, and Software Availability
All the sequencing data have been deposited in the NCBI Gene Expression Omnibus (GEO). RNA sequencing and ChIP sequencing data are accessible through GEO Series accession numbers GSE165510 and GSE165893. They are publicly available. All the other study data are included in manuscript and Supplementary information.
Supporting Information
References
- 1.Simmonds P., et al. , ICTV virus taxonomy profile: Flaviviridae. J. Gen. Virol. 98, 2–3 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dick G. W., Kitchen S. F., Haddow A. J., Zika virus. I., Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 46, 509–520 (1952). [DOI] [PubMed] [Google Scholar]
- 3.Weaver S. C., et al. , Zika virus: History, emergence, biology, and prospects for control. Antiviral Res. 130, 69–80 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schuler-Faccini L., et al. , Possible association between Zika virus infection and microcephaly - Brazil, 2015. Mmwr Morb. Mortal. Wkly. Rep. 65, 59–62 (2016). [DOI] [PubMed] [Google Scholar]
- 5.de Araujo T. V. B., et al. , Association between Zika virus infection and microcephaly in Brazil, January to May, 2016: Preliminary report of a case-control study. Lancet Infect. Dis. 16, 1356–1363 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oehler E., et al. , Zika virus infection complicated by Guillain-Barre syndrome - case report, French Polynesia, December 2013. Eurosurveillance 19, 4–6 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Modhiran S. H., et al. , A broadly protective antibody that targets the flavivirus NS1 protein. Science 371, 190–194 (2021). [DOI] [PubMed] [Google Scholar]
- 8.Abrams R. P. M., Solis J., Nath A., Therapeutic approaches for Zika virus infection of the nervous system. Neurotherapeutics 14, 1027–1048 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cortese M., et al. , Ultrastructural characterization of Zika virus replication factories. Cell Rep. 18, 2113–2123 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin D. L., et al. , The ER membrane protein complex promotes biogenesis of dengue and Zika virus non-structural multi-pass transmembrane proteins to support infection. Cell Rep. 27, 1666–1674.e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao B. Y., et al. , Structure and function of the Zika virus full-length NS5 protein. Nat. Commun. 8, 14762 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang B. X., et al. , The structure of Zika virus NS5 reveals a conserved domain conformation. Nat. Commun. 8, 14763 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egloff M. P., Benarroch D., Selisko B., Romette J. L., Canard B., An RNA cap (nucleoside-2’-O-)-methyltransferase in the flavivirus RNA polymerase NS5: Crystal structure and functional characterization. EMBO J. 21, 2757–2768 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geiss B. J., et al. , Analysis of flavivirus NS5 methyltransferase cap binding. J. Mol. Biol. 385, 1643–1654 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fajardo T., et al. , The flavivirus polymerase NS5 regulates translation of viral genomic RNA. Nucleic Acids Res. 48, 5081–5093 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grant A., et al. , Zika virus targets human STAT2 to inhibit type I interferon signaling. Cell Host Microbe 19, 882–890 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He Z., et al. , NLRP3 Inflammasome activation mediates Zika virus-associated inflammation. J. Infect. Dis. 217, 1942–1951 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Wang W. B., et al. , Zika virus infection induces host inflammatory responses by facilitating NLRP3 inflammasome assembly and interleukin-1 beta secretion. Nat. Commun. 9, 106 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ng I. H. W., et al. , Zika virus NS5 forms supramolecular nuclear bodies that sequester importin-alpha and modulate the host immune and pro-inflammatory response in neuronal cells. Acs Infect. Dis. 5, 932–948 (2019). [DOI] [PubMed] [Google Scholar]
- 20.Chaudhary V., et al. , Selective activation of type II interferon signaling by Zika virus NS5 protein. J. Virol. 91, e00163–17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saade M., et al. , Multimerization of Zika virus-NS5 causes ciliopathy and forces premature neurogenesis. Cell Stem Cell 27, 920–936.e8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hou W., et al. , Molecular cloning and characterization of the genes encoding the proteins of Zika virus. Gene 628, 117–128 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ji W., Luo G. X., Zika virus NS5 nuclear accumulation is protective of protein degradation and is required for viral RNA replication. Virology 541, 124–135 (2020). [DOI] [PubMed] [Google Scholar]
- 24.Hou W. H., et al. , Molecular cloning and characterization of the genes encoding the proteins of Zika virus. Gene 628, 117–128 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah P. S., et al. , Comparative flavivirus-host protein interaction mapping reveals mechanisms of Dengue and Zika Virus pathogenesis. Cell 175, 1931–1945.e18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murzina N., Verreault A., Laue E., Stillman B., Heterochromatin dynamics in mouse cells: Interaction between chromatin assembly factor 1 and HP1 proteins. Mol. Cell 4, 529–540 (1999). [DOI] [PubMed] [Google Scholar]
- 27.Nguyen H. D., et al. , Functions of replication protein A as a sensor of R loops and a regulator of RNaseH1. Mol. Cell 65, 832–847.e4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soderberg O., et al. , Characterizing proteins and their interactions in cells and tissues using the in situ proximity ligation assay. Methods 45, 227–232 (2008). [DOI] [PubMed] [Google Scholar]
- 29.Dai S., et al. , Suppression of the HSF1-mediated proteotoxic stress response by the metabolic stress sensor AMPK. EMBO J. 34, 275–293 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dong H., et al. , Flavivirus RNA methylation. J. Gen. Virol. 95, 763–778 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Zhou Y., et al. , Structure and function of flavivirus NS5 methyltransferase. J. Virol. 81, 3891–3903 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bollati M., et al. , Recognition of RNA cap in the Wesselsbron virus NS5 methyltransferase domain: Implications for RNA-capping mechanisms in Flavivirus. J. Mol. Biol. 385, 140–152 (2009). [DOI] [PubMed] [Google Scholar]
- 33.Oh Y., et al. , Zika virus directly infects peripheral neurons and induces cell death. Nat. Neurosci. 20, 1209–1212 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu Y., et al. , Architecture of the RNA polymerase II-Paf1C-TFIIS transcription elongation complex. Nat. Commun. 8, 15741 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hou L., et al. , Paf1C regulates RNA polymerase II progression by modulating elongation rate. Proc. Natl. Acad. Sci. U.S.A. 116, 14583–14592 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murray C. L., Jones C. T., Rice C. M., Architects of assembly: Roles of Flaviviridae non-structural proteins in virion morphogenesis. Nat. Rev. Microbiol. 6, 699–708 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gillespie L. K., Hoenen A., Morgan G., Mackenzie J. M., The endoplasmic reticulum provides the membrane platform for biogenesis of the flavivirus replication complex. J. Virol. 84, 10438–10447 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang B., et al. , Structural basis for STAT2 suppression by flavivirus NS5. Nat. Struct. Mol. Biol. 27, 875–885 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bujalowski P. J., Bujalowski W., Choi K. H., Identification of the viral RNA promoter stem loop A (SLA)-binding site on Zika virus polymerase NS5. Sci. Rep. 10, 13306 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li X., et al. , Cyclic GMP-AMP synthase is activated by double-stranded DNA-induced oligomerization. Immunity 39, 1019–1031 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coutard B., et al. , Zika Virus methyltransferase: Structure and functions for drug design perspectives. J. Virol. 91, e02202–16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao Y., et al. , Molecular basis for specific viral RNA recognition and 2’-O-ribose methylation by the dengue virus nonstructural protein 5 (NS5). Proc. Natl. Acad. Sci. U.S.A. 112, 14834–14839 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferrero D. S., et al. , Supramolecular arrangement of the full-length Zika virus NS5. PLoS Pathog. 15, e1007656 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu M., et al. , RNA polymerase II-associated factor 1 regulates the release and phosphorylation of paused RNA polymerase II. Science 350, 1383–1386 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim J., Guermah M., Roeder R. G., The human PAF1 complex acts in chromatin transcription elongation both independently and cooperatively with SII/TFIIS. Cell 140, 491–503 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuan L., et al. , A single mutation in the prM protein of Zika virus contributes to fetal microcephaly. Science 358, 933–936 (2017). [DOI] [PubMed] [Google Scholar]
- 47.Teng Y., et al. , An integrative analysis reveals a central role of P53 activation via MDM2 in Zika virus infection induced cell death. Front. Cell. Infect. Microbiol. 7, 327 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeng J., et al. , The Zika virus capsid disrupts corticogenesis by suppressing dicer activity and miRNA biogenesis. Cell Stem Cell 27, 618–632.e9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoon K. J., et al. , Zika-virus-encoded NS2A disrupts mammalian cortical neurogenesis by degrading adherens junction proteins. Cell Stem Cell 21, 349–358.e6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li H., et al. , Zika virus protease cleavage of host protein septin-2 mediates mitotic defects in neural progenitors. Neuron 101, 1089–1098.e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liang Q. M., et al. , Zika virus NS4A and NS4B proteins deregulate Akt-mTOR signaling in human fetal neural stem cells to inhibit neurogenesis and induce autophagy. Cell Stem Cell 19, 663–671 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Furtado J. M., Esposito D. L., Klein T. M., Teixeira-Pinto T., da Fonseca B. A., Uveitis associated with Zika virus infection. N. Engl. J. Med. 375, 394–396 (2016). [DOI] [PubMed] [Google Scholar]
- 53.Ma W., et al. , Zika virus causes testis damage and leads to male infertility in mice. Cell 168, 542 (2017). [DOI] [PubMed] [Google Scholar]
- 54.Miner J. J., et al. , Zika virus infection during pregnancy in mice causes placental damage and fetal demise. Cell 165, 1081–1091 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tay M. Y., et al. , Nuclear localization of dengue virus (DENV) 1–4 non-structural protein 5; protection against all 4 DENV serotypes by the inhibitor Ivermectin. Antiviral Res. 99, 301–306 (2013). [DOI] [PubMed] [Google Scholar]
- 56.Laurent-Rolle M., et al. , The Interferon signaling antagonist function of yellow fever virus NS5 protein is activated by type I interferon. Cell Host Microbe 16, 314–327 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hui L., et al. , Matrix metalloproteinase 9 facilitates Zika virus invasion of the testis by modulating the integrity of the blood-testis barrier. PLoS Pathog. 16, e1008509 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nguyen H. D., et al. , Functions of replication protein A as a sensor of R loops and a regulator of RNaseH1. Mol. Cell 65, 832–847 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mendez J., Stillman B., Chromatin association of human origin recognition complex, cdc6, and minichromosome maintenance proteins during the cell cycle: Assembly of prereplication complexes in late mitosis. Mol. Cell Biol. 20, 8602–8612 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gruber R., et al. , MCPH1 regulates the neuroprogenitor division mode by coupling the centrosomal cycle with mitotic entry through the Chk1-Cdc25 pathway. Nat. Cell Biol. 13, 1325–1334 (2011). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Dataset S01 (XLSX)
Data Availability Statement
All the sequencing data have been deposited in the NCBI Gene Expression Omnibus (GEO). RNA sequencing and ChIP sequencing data are accessible through GEO Series accession numbers GSE165510 and GSE165893. They are publicly available. All the other study data are included in manuscript and Supplementary information.