Significance
The chemical, behavioral, and molecular dissection of sex pheromone communication in parasitoid wasps has not been reported. Here, we have identified two female-derived sex pheromone components, tetradecanal (14:Ald) and 2-heptadecanone (2-Hep) with a ratio of 1:4.6 from Campoletis chlorideae (Ichneumonidae), a parasitoid that can parasitize approximately 30 lepidopteran species. Further, we have discovered that CchlOR18 and CchlOR47 are the two receptors tuned to 14:Ald and 2-Hep, respectively. Additionally, we have demonstrated that the sex pheromones can be applied with a female attractant, (Z)-jasmone to significantly increase the parasitism rate of host larvae. This discovery paves the way for a systematic understanding of sex pheromone communication in parasitoids and points at ways to enhance their parasitic power for pest control.
Keywords: parasitoids, sex pheromone, pheromone receptors, chemical communications
Abstract
Sex pheromones are pivotal for insect reproduction. However, the mechanism of sex pheromone communication remains enigmatic in hymenopteran parasitoids. Here we have identified the sex pheromone and elucidated the olfactory basis of sex pheromone communication in Campoletis chlorideae (Ichneumonidae), a solitary larval endoparasitoid of over 30 lepidopteran pests. Using coupled gas chromatography-electroantennogram detection, we identified two female-derived pheromone components, tetradecanal (14:Ald) and 2-heptadecanone (2-Hep) (1:4.6), eliciting strong antennal responses from males but weak responses from females. We observed that males but not females were attracted to both single components and the blend. The hexane-washed female cadavers failed to arouse males, and replenishing 14:Ald and 2-Hep could partially restore the sexual attraction of males. We further expressed six C. chlorideae male-biased odorant receptors in Drosophila T1 neurons and found that CchlOR18 and CchlOR47 were selectively tuned to 14:Ald and 2-Hep, respectively. To verify the biological significance of this data, we knocked down CchlOR18 and CchlOR47 individually or together in vivo and show that the attraction of C. chlorideae to their respective ligands was abolished. Moreover, the parasitoids defective in either of the receptors were less likely to court and copulate. Finally, we show that the sex pheromone and (Z)-jasmone, a potent female attractant, can synergistically affect behaviors of virgin males and virgin females and ultimately increase the parasitic efficiency of C. chlorideae. Our study provides new insights into the molecular mechanism of sex pheromone communication in C. chlorideae that may permit manipulation of parasitoid behavior for pest control.
Campoletis chlorideae (Ichneumonidae) is an important solitary larval endoparasitoid of many major agricultural noctuid pests, such as Helicoverpa armigera, Spodoptera litura, Spodoptera frugiperda, Agrotis ipsilon, and Mythimna separata (1–4). Field investigation implemented in Hunan Province, China, reveals that the parasitism rate of H. armigera larvae by this parasitoid ranges from 25.1 to 63.1% (2). Because of its high parasitic efficiency, C. chlorideae has been established as a model parasitoid to study the parasite-host interplay (5–7) and tritrophic interactions (8, 9). Mating is essential to obtain normal sex ratios that ensure efficient parasitism and optimize fitness in parasitoids (10). However, the identification of sex pheromones has received less attention in parasitoids. More importantly, the relevant molecular mechanism underlying sex pheromone detection has not been reported before in any parasitoid species.
Sex pheromones are species-selective chemical cues to locate mates (11, 12). The characterization of insect sex pheromones has been initiated and advanced in lepidopteran insects. Lepidopteran sex pheromones are typically a blend of unsaturated C10-18 aldehydes, alcohols, or acetates (13). In Coleoptera, the reported sex pheromone components are highly structurally diversified, ranging from branched and unbranched acyclic compounds, monocyclic lactones, macrolides, and aromatics, to bicyclic oxygen heterocycles (14). Hymenoptera arguably represents the most speciose insect order, largely due to the underestimated species abundance of parasitoids (15). Considering the species richness in parasitoid wasps (>100,000 species) (16), the identification of sex pheromones of hymenopteran parasitoids much lags behind that of lepidopteran and coleopteran insects, in part due to the small body size of parasitoids and a lower amount of sex pheromones released (17). The rare examples of reported sex pheromones in parasitoids include (−)-iridomyrmecin, a major component of female-derived sex pheromones in Leptopilina heterotoma (Figitidae), Leptopilina ryukyuensis (Figitidae), and Leptopilina japonica (Figitidae) (18, 19); (Z,Z)-9,12-octadecadienal, a female-derived volatile sex pheromone of Ascogaster quadridentata (Braconidae), an egg-larval parasitoid of codling moth Cydia pomonella (Tortricidae) (20); nonanal, part of the female-produced attractive sex pheromone in Cotesia glomerata (Braconidae) (21); heptanal, a constituent of the female-produced sex pheromone in Cotesia marginiventris (Braconidae) (22). In stark contrast, some wasps employ an opposite strategy for sex communication. Nasonia vitripennis (Chalcidoidea) males intermittently release (4R,5R)-5-hydroxy-4-decanolide, (4R,5S)-5-hydroxy-4-decanolide, and 4-methylquinazoline to attract virgin females (23–25). Similarly, (E)-bergamotene has been identified as a volatile male-produced sex pheromone in Melittobia digitata (Eulophidae) (26). Besides the airborne sex pheromones, female-derived long-chain CHCs have been suggested as contact sex pheromones in parasitoid wasps (27–31). Many hymenopteran parasitoids are solitary and widely dispersed, which makes mate localization a daunting task, underscoring the importance of olfactory detection of sex pheromones (32).
In insects, sex pheromones are primarily detected by antennae through olfaction. The antennae are decorated with an array of sensilla innervated by the dendritic cilia of the olfactory receptor neurons (ORNs) (33). Residing on the cilia membrane of ORNs, olfactory receptors are deemed as the central molecular player in the olfactory process in the periphery (34–36). Three categories of olfactory receptors including odorant receptors (ORs), inotropic receptors (IRs), and a few gustatory receptors (GRs) have been identified (37, 38). Of these, ORs respond to volatile odors, IRs to biogenic amines and acids, and GRs to carbon dioxide (39–42). Insect ORs are ligand-gated ion channels assembled with two subunits of tuning ORs and two subunits of conserved OR co-receptors (43–46). Pheromone receptors (PRs) are a subtype of ORs and are selectively tuned to sex pheromone-related compounds (47, 48). PRs largely exhibit sex-biased expression patterns, a feature that has been used to predict PRs among ORs. For example, in several noctuid moth species, OR13 is predominantly expressed in ORNs of trichoid sensilla in male antennae detecting (Z)-11-hexadecenal, a major component of female-released sex pheromones (49–51). In Hymenoptera, the ORs involved in sex pheromone detection have only been reported in eusocial bees and ants. In Apis mellifera, the drone-biased OR11 is the receptor specifically detecting the major queen pheromone component 9-oxo-2-decenoic acid (52). Moreover, the nine-exon subfamily of ORs in the ponerine ant Harpegnathos saltator (Formicidae) has been found to be sensitive to CHCs, a group of chemicals involved in sex recognition and mating (27, 53). In parasitoids, the ORs tuned to sex pheromones have not yet been characterized. In C. chlorideae, a total of 210 ORs have been identified from the head and antennae (54). Among them, OR62 is exclusively expressed in female antennae and is selectively tuned to (Z)-jasmone, a component of herbivore-induced plant volatiles (HIPVs), guiding the parasitoid to locate host larvae (54). However, the functions of the other ORs identified in C. chlorideae remain elusive. Six of these ORs exhibit a male-biased expression pattern, suggesting a role in male-specific behaviors such as locating females (54).
Here, we use a combination of chemistry, electrophysiology, molecular biology, and behavior to understand sex pheromone communication in C. chlorideae. We identified tetradecanal (14:Ald) and 2-heptadecanone (2-Hep) as the principal components of its sex pheromone and characterized CchlOR18 and CchlOR47 as the two PRs responsible for detecting 14:Ald and 2-Hep, respectively. In addition, we found that the sex pheromone can work with the potent female attractant, (Z)-jasmone, to greatly increase the parasitic efficiency of C. chlorideae, possibly by facilitating mating by congregating males and females.
Results
Identification of the Sex Pheromone of C. chlorideae.
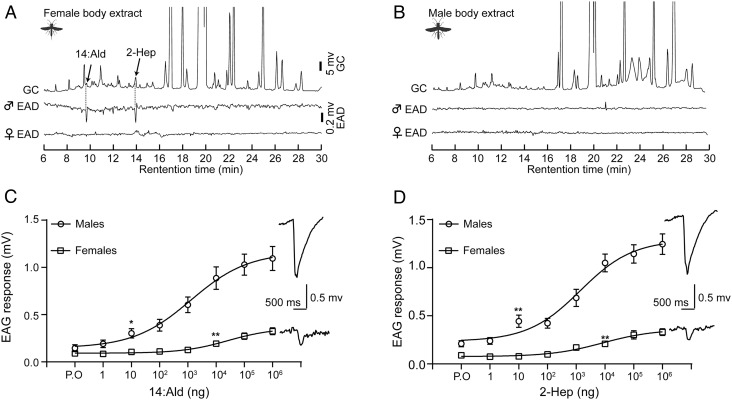
To identify the sex pheromone components of C. chlorideae, we first tested the antennal responses to hexane extracts of female bodies. We found that the female antennae did not respond to, whereas the male antennae robustly and consistently responded to the female body extract (Fig. 1A). In parallel, to check for the existence of a male-derived sex pheromone existed, we challenged the male and female antennae with the hexane extract of male bodies. As shown in Fig. 1B, the male body extract did not elicit appreciable responses from the antennae of either sex, indicating a lack of active compounds from males. Next, we sought to characterize the active components in female hexane extracts. We identified two active compounds by gas chromatography coupled with electroantennogram detection (GC-EAD) and electroantennogram (EAG). Using gas chromatography coupled with mass spectrometry (GC–MS), we determined that these two compounds were tetradecanal (14:Ald) and 2-heptadecanone (2-Hep) based on the MS spectra and retention times (SI Appendix, Fig. S1 A–C). To quantify these two compounds, we constructed an external curve for 2-Hep (X) and integrated area (Y) (Y = 1745117X - 2929561) (SI Appendix, Fig. S1D). Through this analysis, we determined that 14:Ald and 2-Hep were present at 6.3 ± 3.4 ng and 28.8 ± 8.4 ng per female, respectively (SI Appendix, Fig. S1D). We further compared the GC traces of the body extract of males and females and found that 14:Ald and 2-Hep only exist in females (SI Appendix, Fig. S1E). Next we determined the antennal response to different doses of 14:Ald and 2-Hep. Both compounds elicited strong sigmoidal dose responses from male antennae with a threshold dose of 10 ng, while only weak responses from female antennae with a threshold dose of 104 ng (Fig. 1 C and D). Collectively, the results strongly suggest that 14:Ald and 2-Hep are components of the female-derived sex pheromone with a ratio of 1:4.6 in C. chlorideae.
Fig. 1.
Identification of the sex pheromone of C. chlorideae. (A) GC-EAD responses of male and female antennae to the hexane extract of female bodies. Male antennae showed responses to two compounds from female body extract, while female antennae were completely anosmic to it. (B) GC-EAD responses of male and female antennae to hexane extract of male bodies. Both male and female antennae were silent to the male body extract. (C) EAG response of antennae to a series of 14:Ald doses (n = 10). The EAG traces represent the response of male and female antennae to 106 ng of 14:Ald. (D) EAG response of antennae to a series of 2-Hep doses (n = 10). The EAG traces represent the response of male and female antennae to 106 ng of 2-Hep. *P < 0.05; **P < 0.01 by two-tailed Student’s t test. P.O represents paraffin oil.
Behavioral Responses of C. chlorideae to 14:Ald and 2-Hep.
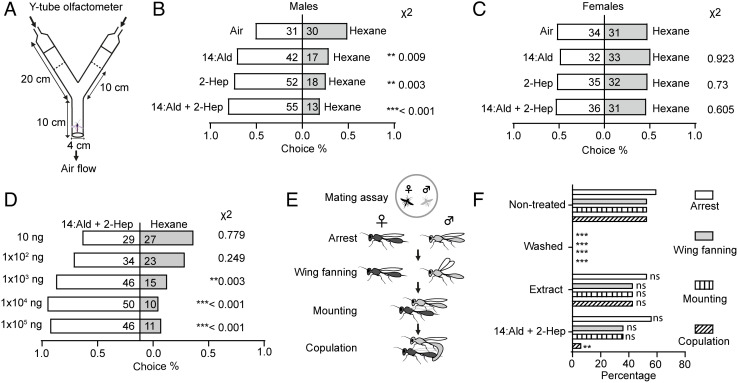
To validate that 14:Ald and 2-Hep are bona fide sex pheromone components of C. chlorideae, we investigated the behavioral responses of both males and females to these two compounds and their blend. We tested the attractiveness of 14:Ald and 2-Hep individually to virgin males and females in a Y-tube olfactometer (Fig. 2A). The males showed a strong preference for 1000 ng of 14:Ald or 2-Hep dissolved in hexane, with 71.2% choosing the 14:Ald-laced arm or 74.3% entering the 2-Hep-laced arm over hexane alone (Fig. 2B). Then, we wondered whether a blend with the natural ratio was more effective than a single component in attraction. We tested the attractiveness of 1000 ng of the blend comprising 14:Ald and 2-Hep (1:4.6) to virgin males (Fig. 2B). We found that around 80.9% of males were attracted by the blend, an effect that was stronger than the attractiveness of a single component (Fig. 2B). In contrast, the virgin females exhibited no attraction to either a single component or the blend (Fig. 2C). Moreover, males displayed a dose response to the blend (Fig. 2 D).
Fig. 2.
Behavioral responses of C. chlorideae to 14:Ald, 2-Hep and their blend (14:Ald/2-Hep = 1/4.6). (A) Schematic representation of the Y-tube olfactometer. (B) Behavioral responses of males to 1000 ng of 14:Ald, 2-Hep, and the blend. (C) Behavioral responses of females to 1000 ng of 14:Ald, 2-Hep, and the blend. (D) Behavioral responses of males to doses of the blend. (E) Schematic representation of the mating assay. (F) Mating behavior of males to untreated female cadavers, hexane-washed female cadavers, and the blend replenished cadavers (n = 30). The numbers in panels B, C, and D represent the replicates. The statistical significance in panels B, C, and D was tested by the binomial test and that of panel F was evaluated by X2 test.
We designed a mating assay to study the effect of these two components on mating efficiency. Virgin males exhibited a stereotypical mating ritual, including locomotor arrest, wing fanning, mounting, and attempted copulation (Fig. 2E). In the arena, the female cadavers could arouse males to court and copulate, while the female cadavers that were washed with hexane completely lost aphrodisiac attraction to males (Fig. 2F). Replenishment of female body extracts restored male arrest, wing fanning, mounting, and copulation (Fig. 2F). Replenishment of 14:Ald and 2-Hep could induce the sequence of mating behaviors except for copulation (Fig. 2F).
Characterization of the ORs Tuned to 14:Ald and 2-Hep.
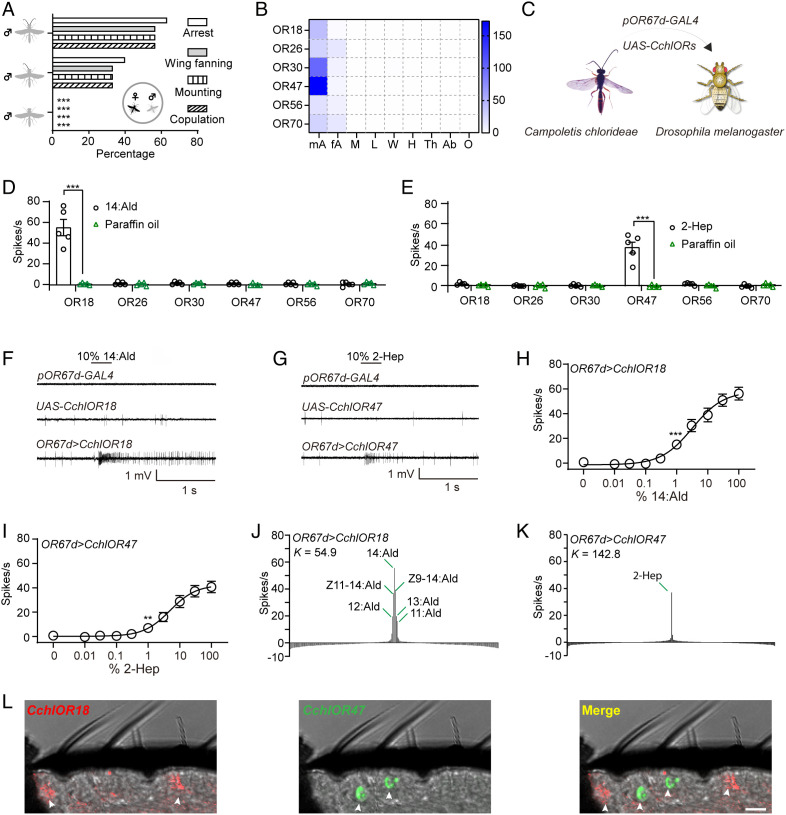
To ascertain the organ that is responsible for detecting the sex pheromone components, we removed the antennae from the head. We observed that the parasitoids with one functional antenna exhibited reduced courtship behavior and copulation attempts, while the fully antennectomized parasitoids ignored the presence of female cadavers, demonstrating that antennae are the organ detecting sex pheromone components (Fig. 3A). Since the electrophysiological and behavioral data indicated that the male antennae strongly responded to 14:Ald and 2-Hep, we reasoned that the ORs that tuned to sex pheromone components should be more abundantly expressed in male than in female antennae. In our previous transcriptome analysis of antennae and heads, we uncovered 98 full-length ORs, and six of them, OR18, OR26, OR30, OR47, OR56, and OR70, show male-biased expression (54). To more precisely detect their expression patterns, we undertook a transcriptome analysis of antennae and other tissues. Fig. 3B shows the FPKM values of the six male-biased ORs (male/female > 1.5) across different tissues. The distribution of these ORs was confined to antennae, suggesting a specific role in olfaction (Fig. 3B). Next, we heterologously expressed the six ORs into the T1 ORNs of D. melanogaster using the OR67d-GAL4 knock-in allele (Fig. 3C). Transgenic expression of each receptor conferred spontaneous spikes to the T1 ORNs, indicating the correct folding and localization of the ectopically expressed CchlORs (SI Appendix, Fig. S2). Then we examined the responses of the CchlOR-expressing T1 neurons to 10% 14:Ald and 10% 2-Hep. Of these, the CchlOR18-expressing neurons exhibited robust responses to 14:Ald, the CchlOR47-expressing neurons exhibited robust responses to 2-Hep, whereas CchlOR26-, CchlOR30-, CchlOR56-, and CchlOR70-expressing neurons did not (Fig. 3 D–G). The responses to 14:Ald and 2-Hep were also evident by the sigmoidal dose-response curves (Fig. 3 H and I). Moreover, to test if CchlOR26, CchlOR30, CchlOR56, and CchlOR70 are tuned to other compounds from female bodies, we investigated the responses of the T1 neurons expressing either of these four receptors to the hexane extracts. We found that these neurons did not respond to the stimuli from the extracts, whereas the neurons expressing CchlOR18 or CchlOR47 did (SI Appendix, Fig. S4).
Fig. 3.
Functional characterization of male-biased odorant receptors in C. chlorideae. (A) The behavioral responses of males with one antenna or without antennae to female cadavers (n = 30). (B) Expression pattern of male-biased receptors. mA: male antennae; fA: female antennae; M: mouthparts; L: legs; W: wings; H: heads; Th: thoraxes; Ab: abdomen; O: ovipositors. (C) Schematic of ectopic expression of CchlORs to the Drosophila T neurons. (D) Reponses of candidate PRs to 10% 14:Ald (n = 5). The electrophysiological recordings revealed that CchlOR18-expressing neurons responded to 14:Ald. (E) Reponses of candidate PRs to 10% 2-Hep (n = 5). The electrophysiological recordings revealed that CchlOR47-expressing neurons responded to 2-Hep. (F) Representative traces of responses of parental lines, pOR67d-GAL4 (genotype: w;+;pOR67d-GAL4) and UAS-CchlOR18 (genotype: w;UAS-CchlOR18;+), as well as OR67d>CchlOR18 (genotype: w;UAS-CchlOR18;pOR67d-GAL4). (G) Representative traces of responses of parental lines, pOR67d-GAL4 (genotype: w;+;pOR67d-GAL4) and UAS-CchlOR47 (genotype: w;UAS-CchlOR47;+), as well as OR67d>CchlOR47 (genotype: w;UAS-CchlOR47;pOR67d-GAL4). (H) Dose response of CchlOR18-expressing neurons to 14:Ald (n = 5). The response of each dose was compared to that of paraffin oil, and the first dose with significant difference was labeled with asterisks. (I) Dose response of CchlOR47-expressing neurons to 2-Hep (n = 5). (J) Tuning breadth of CchlOR18-expressing neurons to a panel of 237 chemical compounds (n = 5). (K) Tuning breadth of CchlOR47-expressing neurons to a panel of 237 chemical compounds (n = 5). (L) Localization of CchlOR18 and CchlOR47 on the antennal sections (Scale bar represents 10 μm.). **P < 0.01, ***P < 0.001 by X2 test for panel A and two-tailed Student’s t test for panels D, E, H, and I.
We defined the tuning spectra of CchlOR18 and CchlOR47 with a panel of 237 chemicals comprising the most commonly found plant odors and derivatives as well as insect sex pheromones and analogs. CchlOR18 was strongly tuned to 14:Ald, moderately to its analogs, (Z)-11-tetradecenal (Z11-14:Ald) and (Z)-9-tetradecenal (Z9-14:Ald), and weakly to undecanal (11:Ald), dodecanal (12:Ald), and tridecanal (13:Ald) (Fig. 3J), while CchOR47 was specifically tuned to 2-Hep (Fig. 3K). Both receptors exhibited high Kurtosis values (K), with 54.9 for CchlOR18 and 142.8 for CchlOR47, indicating that they have high olfactory selectivity (Fig. 3 J and K). To address whether these two receptors are functioning in distinct sensilla, we used mRNA in situ hybridization to localize the two receptors on virgin male antennae and found that the transcripts of CchlOR18 and CchlOR47 were localized to distinct sensilla (Fig. 3L and SI Appendix, Fig. S3). Taken together, these experiments reveal that CchlOR18 and CchlOR47 are the PRs tuned to 14:Ald and 2-Hep in C. chlorideae, respectively.
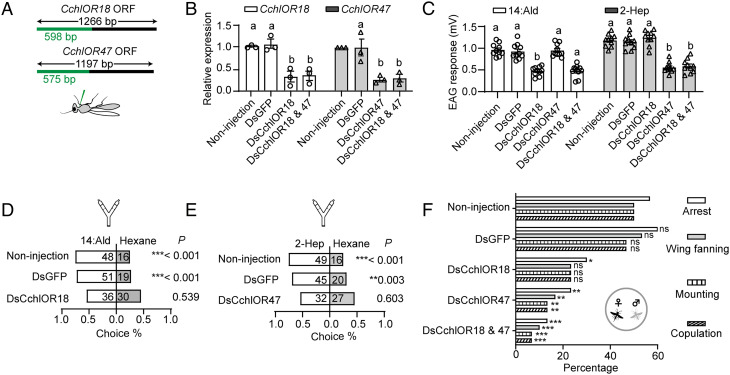
Knockdown of CchlOR18 and CchlOR47 Attenuates the Antennal and Behavioral Responses to 14:Ald and 2-Hep.
To validate the function of CchlOR18 and 47 in vivo, we knocked down their expression by injection of dsRNA (Fig. 4A). qRT-PCR results showed that the dsRNA of each OR effectively reduced the transcript level by around 65% (Fig. 4B). Notably, to study the responses of parasitoids that lack the two receptors together, we knocked down the two receptors simultaneously (Fig. 4B). Electroantennography revealed that the virgin male antennal responses were significantly reduced when single or both receptors were silenced (Fig. 4C). Consistently, the Y-tube olfactometer results indicated the males with CchlOR18 or CchlOR47 knockdown lost preference for 14:Ald and 2-Hep, respectively (Fig. 4 D and E). Similarly, the virgin males with CchlOR18 knockdown exhibited reduced courtship and copulation, although there was a clear trend, the reduction in wing fanning, mounting, and copulation was not significantly different from the non-injected males (Fig. 4F). The males that were defective in CchlOR47 showed significantly attenuated courtship and copulation (Fig. 4F), and the inhibition of behavioral responses of the males with both receptors silenced was more pronounced (Fig. 4F).
Fig. 4.
Knockdown of CchlOR18 and CchlOR47 attenuates antennal and behavioral responses to 14:Ald and 2-Hep. (A) Gene regions for dsRNAs synthesis. A gene fragment of 598 bp and 575 bp at the 5′-end of CchlOR18 and CchlOR47 was cloned as templates for dsRNAs synthesis. (B) qRT-PCR measurements to check the efficiency of CchlOR18 and CchlOR47 knockdown (n = 3). (C) EAG responses of the parasitoids defective in CchlOR18 and CchlOR47 versus the responses of two controls, non-injected and dsGFP injected wasps (n = 10). (D) Behavioral responses of non-injected, dsGFP injected, and dsCchlOR18 injected wasps to 1000 ng of 14:Ald. The parasitoids with CchlOR18 being knocked down lost the preference for 14:Ald. (E) Behavioral responses of non-injected, dsGFP injected, and dsCchlOR47 injected wasps to 1000 ng of 2-Hep. The parasitoids defective in CchlOR47 lost preference for 1000 ng of 2-Hep. (F) The percentage of males attempting to mate. Knocking down of CchlOR18 or CchlOR47 as well as both impaired the mating ability of males (n = 6). The different letters above the graphs indicate statistical differences. Note that each group contains five male wasps; therefore, totally 30 parasitoids were tested. Different letters above columns in panels B and C indicate significant differences tested by one-way ANOVA with post hoc Tukey’s multiple comparisons. *P < 0.05, **P < 0.01, ***P < 0.001 by binomial test for panels D and E and by X2 test for panel F.
Applying 14:Ald and 2-Hep Together with (Z)-jasmone Can Increase the Parasitism Rate of Host Larvae by C. chlorideae.
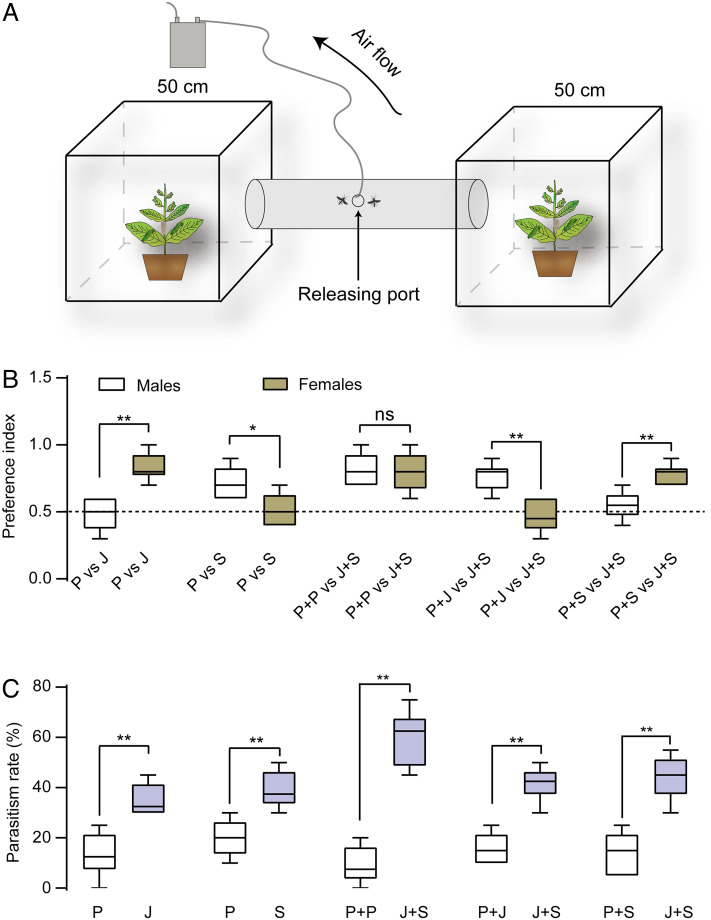
(Z)-jasmone is a herbivore-induced plant volatile that is known to be attractive to C. chlorideae females (54). We hypothesized that the sex pheromone could work with (Z)-jasmone to congregate males and females, thereby facilitating mating and increasing the parasitic power of mated females. To test this, we designed a two-cage preference assay (Fig. 5A). We first tested the attraction of virgin males and females to (Z)-jasmone. The females preferred the cage with (Z)-jasmone-treated plants over the cage with paraffin oil (PO)-treated plants, while the males were equally distributed (Fig. 5B). Then we tested the attraction of virgin wasps to the sex pheromone blend. The males were more attracted to the sex pheromone-treated plants relative to the plants laced with PO, and the females were almost equally found in two cages (Fig. 5B). Moreover, both males and females were attracted to the plants treated with (Z)-jasmone + sex pheromone compared to the PO-treated plant (Fig. 5B). Males, not females were attracted to the plants treated with (Z)-jasmone + sex pheromones compared to the plants treated with (Z)-jasmone + PO (Fig. 5B). Similarly, females, not males were attracted to the plants treated with (Z)-jasmone + sex pheromones compared to the plants treated with sex pheromones + PO (Fig. 5B). These results suggest that the sex pheromone blend and (Z)-jasmone can specifically attract males and females, respectively. Correspondingly, we observed a significant increase in the parasitism rate of host larvae in all treatment groups versus the respective controls (Fig. 5C). Notably, in (Z)-jasmone + sex pheromones versus PO + PO trials, the parasitism rate of H. armigera larvae on the plants treated with (Z)-jasmone + sex pheromones was the highest, presumably because of the highest number of males and females attracted in one cage (Fig. 5C).
Fig. 5.
Sex pheromone of C. chlorideae works with (Z)-jasmone to increase the parasitism rate of host larvae. (A) Schematic of the two-choice assay. One cage contains a plant decorated with controls, and the other contains a plant spiked with treatments. (B) Preference of males and females in two-choice assays. (C) Parasitism rate of host larvae in two-choice assays. P, J, and S represent paraffin oil, (Z)-jasmone, and sex pheromone blend, respectively. * P < 0.05; **P < 0.01 by the Wilcoxon test.
Discussion
In this work, we identify a blend of 14:Ald and 2-Hep (1:4.6) as the female-derived volatile sex pheromone of the parasitoid, C. chlorideae. The two compounds only existed in the female body extract and elicited much stronger responses from male than from female antennae. Males but not females exhibited strong attraction to 14:Ald, 2-Hep and their blend, and the blend triggered stereotypical male courtship behaviors. We further demonstrate that CchlOR18 and CchlOR47 are the receptors responsible for 14:Ald and 2-Hep detection in C. chlorideae, respectively. Knockdown of the two receptors individually or together resulted in a loss of pheromone attractiveness to male wasps. This sex pheromone combined with (Z)-jasmone, a potent female attractant, can increase the parasitism rate of H. armigera larvae by C. chlorideae.
Insect parasitoid wasps are an important natural control agent of agricultural pests (55). Their host-seeking behaviors through sensing HIPVs have been widely studied. However, relatively few studies are focused on sex pheromone communication. The sex-determination system of parasitoid wasps is haplodiploidy, in which unmated females lay unfertilized eggs that develop into males, and mated females can lay unfertilized eggs that will develop into males or fertilized ones that develop into females. Hence, mating is important for maintaining the balanced sex ratio of the population (56). In Itoplectis conquisitor (Ichneumonidae), geranial and neral (ratio:1.3–1.8) have been found as two sex pheromone components; yet, a ketone component remains unidentified (57). Similarly, nonanal and heptanal were identified from the body extracts of C. glomerata and C. marginiventris females, respectively; however, these two compounds are only effective in attracting males when combined with other body fractions, suggesting other active components of sex pheromones are still missing (21, 22). In Cephalonomia tarsalis (Bethylidae), dodecanal was found in substrates on which females had walked and can arrest males (58). Besides these short-chain aldehydes, some unsaturated long-chain aldehydes have been reported as sex pheromone components in parasitoid wasps. For instance, (Z)-4-tridecenal, (Z)-9-hexadecenal, and (Z,Z)-9,12-octadecadienal have been reported as one sex pheromone component of Macrocentrus grandii (Braconidae) (59), Ascogaster reticulatus (Braconidae) (60), and A. quadridentata (Braconidae) (20), respectively. Moreover, methyl 6-methylsalicylate and (6S,10S)-(2E,4E,8E)-4,6,8,10-tetramethyltrideca-2,4,8-triene were identified as one sex pheromone component of Spalangia endius (Pteromalidae) (61) and Tetrastichus planipennisi (Eulophidae) (62), respectively. In this study, using comprehensive electrophysiological analyses and behavioral tests, we discovered that 14:Ald and 2-Hep comprise the volatile sex pheromone of C. chlorideae. Aldehydes appear to be common in parasitoid wasp pheromones, whereas 2-Hep is the first ketone reported as a sex pheromone component in parasitoid wasps. Although the binary blend was more attractive, the two compounds were also singly attractive to virgin males. This is in stark contrast with the action mode of sex pheromones in lepidopteran moths, which usually requires the presence of full sex pheromone components with accurate ratios (63). An explanation for this difference is that most moths are nocturnal, and olfaction is the principal sensation mediating sex pheromone communication that is critical for mate detection and behavioral isolation. In contrast, parasitoid wasps are diurnal, and besides olfaction, vision and gustation could also guide the location of potential mates. Thus, in parasitoid wasps, evolution may favor the efficiency over the accuracy of sex pheromone communication. In this scenario, parasitoids can find mates by sensing either of the sex pheromone components, which may drastically increase the efficiency of mate localization.
However, the source and synthetic pathway of 14:Ald and 2-Hep in females of C. chlorideae remain elusive. In Aphytis lingnanensis (Aphelinidae), the males could perform full elements of mating behaviors toward the dissected thorax with a particular interest in the region adjoining the wing base and less interest in heads and abdomen, suggesting the sex pheromones are present on the thorax (64). In Apanteles melanoscelus (Braconidae) and Apanteles liparidis (Braconidae), Dufour’s gland in the reproductive system has been found as the source of sex pheromones (65). In contrast, heptanal and the elusive key sex pheromone component(s) are found on the entire body of C. marginiventris and are presumably produced by the air oxidation of double bonds of long-chain unsaturated CHCs (22). Similarly, in Campoletis sonorensis (Ichneumonidae), various female body regions including heads, thorax, abdomen, legs, and wings all indistinguishably elicit the courtship behavior in males, but the internal organs including the acid or poison gland, alkaline or Dufour’s gland, ovary, mandibular glands, digestive tract, hemolymph, and thoracic tissues cannot, suggesting that the sex pheromones may be synthesized through cells or glands associated with the cuticle (66). Considering the phylogenetical similarity between C. chlorideae and C. sonorensis, we speculate that 14:Ald and 2-Hep may be synthesized by cuticle cells and are widely spread on the cuticle. Another question is whether the synthesis of these two sex pheromone components is changed with mating status. In C. glomerata, the mated females become aversive to males, while in C. marginiventris, the mated females just lose their attractiveness to males (67). In C. sonorensis, females can mate multiple times with different males, after which they become less attractive to males (66). In this scenario, females could reduce the production of sex pheromones and/or release an anti-aphrodisiac pheromone after mating. To discern these two possibilities, it is worthwhile to compare the chemical composition of body extracts of virgin and mated females of C. chlorideae in the future.
A blend of 14:Ald and 2-Hep, when added to hexane-washed female cadavers, cannot coax males to copulate, even though it can guide the males to locate the females (Fig. 2F). One explanation is that long-chain CHCs may serve as contact cues to elicit copulation in C. chlorideae. Long-chain CHCs have low volatility yet have been reported as contact sex pheromones of Hymenoptera insects (27–29, 68). Because our GC-EAD experiments showed no other volatile compounds elicited olfactory responses from antennae, long-chain CHCs could be copulation-decisive cues, likely detected by gustation. In the future, screening the response of taste sensilla to CHCs using tip-recording and deorphanization of GRs would nail down this missing copulation factor.
Insects use two olfactory coding mechanisms—combinatory coding and labeled lines—to identify environmental stimuli (69). Combinatory coding posits that one receptor responds to multiple odorants and one odorant activates multiple receptors. Instead, “labeled lines” involve high-specificity ORs to detect odorants with great biological relevance (38). Using expression in Drosophila T1 neurons and screening compounds with SSR, we identified two male-biased ORs, CchlOR18 and CchlOR47 as the receptors tuned to 14:Ald and 2-Hep, respectively (Fig. 3). CchlOR18 predominantly responds to 14:Ald and moderately to the structural analogs, 13:Ald and 12:Ald, a feature that is reminiscent of the tuning of PRs of lepidopteran species. For instance, the OR13 of H. armigera responds not only to the major sex pheromone component, Z11-16:Ald, but also to an analog, Z11-14:Ald (70). This flexibility may provide some freedom for the evolution of sex pheromones and species diversification. Moreover, silencing CchlOR18 or CchlOR47 results in the loss of male chemotaxis behavior to 14:Ald and 2-Hep, respectively (Fig. 4), suggesting a non-redundant olfactory coding mechanism. Collectively, our results indicate that C. chlorideae employs “labeled lines” involving CchlOR18 and CchlOR47 with high olfactory specificity to detect sex pheromones. Since CchlOR18 and CchlOR47 are the first two PRs characterized in parasitoid wasps, whether the “labeled lines” are a common strategy used by parasitoid wasps to encode sex pheromones merits future studies. The OR family has undergone an expansion in parasitoid wasps, resulting in a large repertoire of ORs (71). For instance, N. vitripennis has 225 ORs (72) and C. chlorideae evolved 210 ORs (54). The OR expansion reflects the complexity of the olfactory cues parasitoids might encounter in fields, such as HIPVs, host odors, kairomones, and sex pheromones (73). To date, only five ORs have been deorphanized in different parasitoid wasps. Anastatus japonicus (Eupelmidae) OR35 detects β-caryophyllene and (E)-α-farnesene, two oviposition attractants (74), and Microplitis mediator (Braconidae) OR35 is tuned to (Z)-5-decenyl acetate, a component of the host sex pheromone (75). In C. chlorideae, CchlOR62 responds to (Z)-jasmone, a specific female attractant involved in host-seeking (54), and CchlOR18 and CchlOR47 detect 14:Ald and 2-Hep that only attract males (this study). The “reverse chemical ecology” through functional analysis of ORs is an efficient way to identify behavior-relevant compounds (76). Considering the central role of parasitoids play in interactions of plants—herbivorous insects—parasitoids (55) and the scanty knowledge of OR functions, it is, therefore, imperative to perform a systemic study of OR functions to unravel the olfactory coding mechanisms in parasitoids.
Mated females are more efficient than virgin females to parasitize, which is reflected by more time spent on host searching, more eggs laid, and higher parasitism rates (77–79). The identification of parasitoid wasp sex pheromones could help increase the efficiency of parasitoid wasps by facilitating mating. Our results suggest that the plants spiked with the sex pheromone and (Z)-jasmone act as a rendezvous site for mating, which culminates in an increased parasitism rate of host larvae. This extrapolation is reasonable as plants have been reported being rendezvous sites for mate-seeking insects, and plant volatiles can stimulate the production of sex pheromones and render the mates more receptive (80). Thus, the enhancement of the parasitism by the sex pheromone and (Z)-jasmone paves the way for developing a pragmatic paradigm to leverage the parasitic capacity of C. chlorideae to control pest populations in crop fields. However, it is worth pointing out that if not used properly, synthetic volatiles may confuse the searching parasitoids in the treated area to find mates or hosts. In the field, odor plumes from plants, parasitoids, and their hosts are often intermingled and fluctuate, and parasitoids employ sophisticated olfactory coding mechanisms to detect the chemical space. The successful use of synthetic volatiles to manipulate parasitoid behaviors will require a much better fundamental understanding of how parasitoids actually exploit odors in complex and highly variable environments. On the other hand, the bottleneck of indoor breeding of this parasitoid wasp is the skewed sex ratios which ultimately results in colony collapse. Identification of its sex pheromone may help to design a new approach to maintain the normal sex ratio, thereby allowing for the breeding of this parasitoid wasp on a large scale and releasing them in the field to control pest damage. Furthermore, synthetic sex pheromones have been used to monitor the spatial and temporal occurrence of parasitoids and their hosts in the field (81). In this case, we could purposely release the wasps according to the population ratio of wasps and hosts to maximize the parasitic power of parasitoids.
In sum, we have characterized that 14:Ald and 2-Hep (1:4.6) are two principal components of the sex pheromone of C. chlorideae, an important parasitoid wasp of many agricultural noctuid pests. We found that CchlOR18 and CchlOR47 are the PRs for detecting 14:Ald and 2-Hep, respectively. Our work delineates the molecular mechanisms of sex pheromone communication in an important parasitoid, providing a new perspective to design more efficient and reliable programs for pest control.
Materials and Methods
Insects.
The early instar larvae of H. armigera potentially parasitized by C. chlorideae were collected from tobacco fields located in the suburb of Luoyang City, Henan Province, China. Emerged wasps were reared with 20% honey at 25 ± 1°C with 60 ± 5% RH and a 16-h L/8-h D cycle. The GAL4 knock-in allele of OR67d (OR67d-GAL42) was originally obtained from Barry Dickson’s lab, the Research Institute of Molecular Pathology, Vienna (60). The generation of transgenic flies was previously described (91).
Chemicals.
In total, 237 chemical compounds were used for the functional analysis of ORs. Each chemical was of the highest purity available commercially. The detailed information of each compound was compiled in SI Appendix, Table S1.
Extraction of Body Chemicals.
A total of 25 5-d-old wasp adults were immersed in 500 μL of hexane (Sigma-Aldrich, St Louis, MO, USA) for 1 h at room temperature. Then, the hexane extract was dehydrated by running through a Na2SO4-filled Pasteur pipette (ANPEL, Shanghai, China) and was finally concentrated to 25 μL using a high-purity nitrogen gas flow. The extract was stored at −20°C for further chemical, electrophysiological, and behavioral analysis.
Gas Chromatography-Electroantennograhic Detection (GC-EAD).
The equipment consisted of an Agilent 7820 GC with a flame ionization detector (FID) equipped with an HP-5 capillary column (30 m × 0.25 mm × 0.25 μm, J&W Scientific, Folsom, CA, USA) and electroantennographic detector (Syntech, Buchenbach, Germany). The injector temperature was set at 250°C. The GC oven temperature program was held at 80°C for 2 min, increased to 180°C at a speed of 10°C per min, held at 180°C for 2 min, increased to 260°C at a speed of 30°C per min, and finally held at 260°C for 10 min. The detector temperature was 300°C. An amount of 2 μL of hexane extract was injected into the GC. A Y-shaped glass tube was used to split the flow with a ratio of 1:1 between FID and the antenna. The signal was amplified by PRG-3 (Syntech, Buchenbach, Germany), digitized by a serial data acquisition interface (IDAC4, Syntech), and sorted by GcEad2014 v1.2.5 (Syntech). To mount the antenna, the antennal tip was cut off from an excised antenna using a Vannas scissor (TW150-4, World Precision Instruments, Sarasota, FL, USA). Then one borosilicate glass capillary (approximate 0.5 mm openness at the tip) (TW105-4, World Precision Instruments) was filled with 0.9% NaCl, and the base of the antenna was inserted into the glass capillary. After, the capillary with the antenna was mounted onto the reference electrode of PRG-3. Next, the tip of the antenna was inserted into another capillary that was already mounted on the recording electrode of PRG-3 under a stereomicroscope (DGM-1, Dinggan Biotech, Tangshan, China). Air filtered by active charcoal and controlled by CS-55 (Syntech, Buchenbach, Germany) was used as the carrier gas.
GC–MS.
The body extract was analyzed by Agilent 5973 MS coupled with an Agilent 6890N GC equipped with an HP-5 capillary column (30 m × 0.25 mm ID, 0.25 m film, J&W Scientific, Folsom, CA, USA). The GC oven temperature program was held at 80°C for 2 min, linearly ramped to 180°C at 10°C/min, held at 180°C for 2 min, increased to 260°C at 30°C/min, and finally held at 260°C for 10 min. The injection was at split less mode, helium was used as carrier gas, and the electron impact (EI) ionization at 70 eV was used to ionize chemicals in MS. The temperatures of the ion source and the interface were 230°C and 300°C, respectively. To examine the antennal responses, five virgin males or females were tested. To quantify the sex pheromone candidate, a standard curve of synthetic 2-Hep was established based on the correspondence between doses of synthetic compounds and integrated areas in GC profiles. Then, an introgression equation was established, and the amount of sex pheromone candidates from a single parasitoid was determined.
EAG.
The protocol of EAG was previously reported (54). For EAG measurement, one antenna was cut off from the head at the base. Then, the proximal tip of the antenna was finely excised. Subsequently, the antenna was attached to the two forks of the electrode with conductive gel (Spectra 360, Parker Lab, NJ, USA). The stimulation puff was 200 ms, and the responses were recorded for 3 s. To avoid sensory adaptation, two puffs were separated by 1 min intervals. A constant airflow (30 mL/s) from an air stimulus controller (Model CS-55, Syntech, Buchenbach, Germany) was filtered by activated carbon-filtered air passed over the antenna. The EAG signals were amplified by IDAC4 (Syntech, Buchenbach, Germany) and were recorded by EAG pro-2000 software (Syntech, Buchenbach, Germany). To check the response to 14:Ald and 2-Hep, ten antennae from ten wasps were tested.
Y-tube Olfactometer.
The protocol of the Y-tube olfactometer was previously described (54). The Y-tube olfactometer consisted of two air samplers (Model QC-1S, Institute of Urban Safety and Environmental Science, Beijing, China), two 250 mL glass flasks, two active charcoal air filters, and a glass Y-tube (a 10-cm-long main stem and two 20-cm-long arms with an angle of 45° with an inner diameter of 3.5 cm). A series of doses (10 ng, 102 ng, 103 ng, 104 ng, and 105 ng) of compounds were tested. For each treatment, at least 50 virgin wasps were tested.
Mating Assay.
For a mating assay, females were treated as follows: (1) The females were killed by refrigeration at −20°C for 30 min, and the cadavers were recovered to room temperature before the experiment (non-treated); (2) five females were washed in 5 mL of hexane for 1 min for 3 times with intermittent vortex (washed); (3) the abdomen of hexane-washed cadavers was replenished with 5 μL of female extract that is quantitatively equivalent to the amount of compound from one female body (extract); (4) the abdomen of washed female cadavers was added with 50 ng of 14:Ald/2-Hep with a ratio of 1:4.6 (14:Ald + 2-Hep). One virgin male was introduced to a 9 cm Petri dish and rested for 5 min followed by the placement of a female cadaver around 5 cm away from the male’s location. The male’s behavior was observed for 5 min. Four typical mating behaviors were observed and recorded: (1) arrest as defined by a sudden stop of the male’s movement; (2) wing finning as defined by back and forth wing flip; (3) mounting as reflected by the quick crawl onto the back of female’s cadavers; (4) copulation as characterized by the bend of the abdomen. For each treatment, the mating behavior of 30 virgin males was observed.
RNA Extraction and cDNA Synthesis.
Around 50 mg of dissected tissues was homogenized in 1 mL of Trizol reagent (Invitrogen, Carlsbad, California, USA) followed by the separation and purification by the RNeasy Plus Universal Mini Kit (QIAGEN GmbH, Hilden, Germany). The integration of RNA was examined by agarose gel electrophoresis, and the RNA was quantified with a NanoDrop Spectrometer (Thermo Fischer Scientific, MA, USA). Then, the first-strand cDNA was synthesized from 2 μg of the total RNA using M-MLV Reverse Transcriptase (Promega, Madison, USA) at 42°C for 1 h.
Transcriptome Sequencing.
For preparing high-quality RNAs for transcriptome analysis, around 50 pairs of male antennae, 50 pairs of female antennae, 20 heads, 50 mouth parts, 20 thoraxes, 150 pairs of legs, including forelegs, middle legs, and hind legs, 20 abdomens, 50 pairs of wings, and 50 ovipositors were collected for each replicate. Each tissues has three replicates. Except for the antennae, the tissues from both sexes were collected and mixed. The method for assembling transcriptomic data was previously reported (82). Sequencing from one end was performed on an Illumina HiSeq4000 platform (Illumina, Inc., San Diego, CA, USA). Adapter sequences, poly-N, and low-quality reads were removed by Trimmomatic, and the clean reads were assembled by Trinity v2. 114.0. Then, BLASTn and BLASTx searches (E-value < 1e−5) against the non-redundant protein database were implemented to annotate the unigenes. RSEM v1.2.15 was used to calculate the FPKM (fragments per kilobase of exon model per million mapped fragments).
Single Sensillum Recording (SSR).
The protocol of single sensillum recording was described in detail previously (70). Briefly, a 4–7-d-old male fly was placed into a yellow pipette (Axygen, USA) and air-pushed to the tip. Next, the tip was cut off by a sharp blade and the fly head with antennae protruded. Reference and recording electrodes (World Precision Instruments, Sarasota, Florida USA) were inserted in the compound eye and Drosophila T1 sensilla, respectively. The airflow was 30 mL/s, and the puff duration was 300 ms. Extracellular action potentials were amplified by 1000 folds by IDAC4 (Syntech, Buchenbach, Germany) and sorted and visualized by Autospike 3.4 (Syntech, Buchenbach, Germany). At least five flies were recorded for each experiment. To test the response to chemicals, 30 μL of different concentrations of compounds dissolved in paraffin oil was added to a piece of filter paper (2 cm × 2 cm). To test the response to the hexane extracts, 5 μL of concentrated extracts (equivalent to the extract from five wasps) was loaded onto the same-sized filter paper.
In Situ Hybridization.
The fluorescent in situ hybridization was performed according to the protocol previously reported (83). Briefly, the probes of CchlOR18 and OR47 were synthesized by RNA labeling kit version 12 (SP6/T7) (Roche, Mannheim, Germany). Sections of 12 µm were cut with a Leica CM 1950 microtome at −22°C. Then the sections were fixed with 4% paraformaldehyde for 30 min and treated with phosphate buffer saline for 1 min, 0.2 M HCl for 10 min, and 50% deionized formamide (MP Biomedicals, Solon, OH, USA)/5× SSC (10× SSC: 1.5 M NaCl, 0.15 M Na-citrate, pH 7.0) for 15 min. Each slide was treated with 100 µL of hybridization buffer (Boster, Wuhan, China) containing probes (dilution 1:100) and was incubated at 55°C for 14 h followed by two washes with 0.1× SSC for 30 min at 60°C. After a brief rinse in TBS (100 mM Tris, pH 7.5, 150 mM NaCl), the slides were treated in 1% blocking reagent (Roche, Mannheim, Germany) in TBS plus 0.03% Triton-X100 (Merck, Darmstadt, Germany) for 30 min at room temperature. Then, 100 µL of antidioxigenin alkaline phosphatase-conjugated antibody (Roche, Mannheim, Germany) diluted 1:500 in 1% blocking reagent was applied to each slide and incubated at 37°C for 1 h. Following three washes for 5 min with TBS plus 0.05% Tween-20 and a short rinse in DAP buffer (100 mM Tris, pH 9.5, 100 mM NaCl, 50 mM MgCl2), DIG and Biotin signals were developed by HNPP/Fast Red (Roche) and Biotin-TSA kit (Perkin Elmer, MA, USA), respectively.
RNA Interference.
First, the gene fragments of CchlOR18 and CchlOR47 were amplified from the cDNA of male antennae by LA Taq DNA polymerase (Takara, Beijing, China) and sub-cloned into pGEM-T vectors (Promega, Madison, USA). T sequences were then amplified using specific forward primers containing T7 promoter and respective reverse primers. Using 3 µg of purified PCR products as templates, double-strand RNAs were synthesized by T7 RiboMAX Express RNAi System (Promega, Madison, WI, USA) as per the manufacturer’s protocol. In parallel, the dsRNA of GFP (green fluorescent protein, GenBank #AAX31732.1) was synthesized as a control. For injection, the newly emerged wasps (less than 1 day) were anesthetized with carbon dioxide on a fly pad (Genesee Scientific, CA, USA) and manually flipped with forceps to expose a soft area around the pharynx. Then, 0.1 μL of each dsRNA (2 μg/μL) was injected using a PLI-100A microinjector (Harvard Apparatus, Holliston, MA, USA) under a stereomicroscope (Olympus, Japan). Notably, for double-gene silencing, both types of dsRNAs were mixed well by pipetting on ice and a total of 0.2 μL of dsRNA mixture was injected. After 3 d, qRT-PCR was performed to check the efficiency of RNAi. The primers for cloning and qRT-PCR are provided in SI Appendix, Table S2.
Two-Cage Olfactometer Assay.
This assay was composed of two cages (50 cm × 50 cm × 50 cm) and a connecting cylinder (50 cm long and 10 cm in diameter) with a releasing port (2 cm in diameter) in the middle. Each cage contained one tobacco plant (NC89 with 5–6 leaves) on which 20 second-instar larvae of H. armigera were released. Stock compound solutions of 10 μg/μL were prepared using paraffin oil as solvents. Before each experiment, 10 μL of stock solution was loaded to a rubber septum. Five pairs of comparisons were designed: (1) paraffin oil (100 μg) versus (Z)-jasmone (100 μg); (2) paraffin oil (100 μg) versus sex pheromone (100 μg of 14:Ald and 2-Hep with a ratio of 1:4.6); (3) paraffin oil (200 μg) versus (Z)-jasmone (100 μg) + sex pheromone (100 μg); (4) (Z)-jasmone (100 μg) + paraffin oil (100 μg) versus (Z)-jasmone (100 μg) + sex pheromone (100 μg); (5) sex pheromone (100 μg) + paraffin oil (100 μg) versus (Z)-jasmone (100 μg) + sex pheromone (100 μg). A rubber septum loaded with either solvent (paraffin oil) or the compounds was hung on the main stem of the plant. An air sampler (Model QC-1S, Institute of Urban Safety and Environmental Science, Beijing, China) was used to create air filaments (30 mL/s) flowing from the plants to the releasing sites. First, ten virgin females of 3 d old were released through the port. After 1 h, ten virgin males of 3 d old were released. After 12 h, the number of males and females in each cage was counted, and the larvae were collected. Each preference test was repeated six times. In total, 60 virgin males and 60 virgin females were used for each test. After the test, the host larvae were collected and reared individually in a single vial with an artificial diet, and the parasitism rate was finally calculated and presented in percentage. The preference index was calculated by the following formula: PI = the number choosing treatment/the total number.
Data Analysis.
Two-tailed Student’s t test was used to analyze the EAG response of antennae to sex pheromone components and paraffin oil as well as to analyze the response of CchlOR-expressing neurons to compounds and hexane body extracts. One-way ANOVA with post hoc Tukey’s multiple comparisons was employed to compare the knockdown efficiency of dsRNAs and the EAG responses of antennae of individuals of non-injection, dsGFP injection, and Ds-CchlOR injection to the sex pheromone components. Two-tailed binomial test with a 50:50 distribution was performed to compare the preference of wasps to odorant stimuli in the Y-tube olfactometer. X2 test was used to test the significance of the differences in the mating behaviors of parasitoid wasps with different treatments. The t test, X2, and one-way ANOVA analyses were performed with Graphpad Prism 9 software (GraphPad Software, San Diego, CA, USA), and the two-tailed binomial test was done with SPSS Statistics 20 (IBM, Armonk, NY, USA). Data were presented as mean ± SEM. The level of significance was set as P < 0.05. *P < 0.05; **P < 0.01; ***P < 0.001. Kurtosis values indicated the tuning breadth of receptors, with high values representing high receptor specificity.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We are grateful to Mr. Rui Wang, Institute of Zoology, Chinese Academy of Sciences for assistance in GC–MS analyses. We are indebted to Mrs. Cui-Wei Liu at Tangshan Dinggan Technology Co., LTD for assistance in GC-EAD. We thank our lab mate, Sohaib Shahid for his insightful suggestions for drafting this manuscript. This work was supported by the National Natural Science Foundation of China (Grant Nos. 31830088, 32130090) and Strategic Priority Research Program of Chinese Academy of Sciences (Grant No. XDPB16).
Author contributions
H.G. and C.-Z.W. designed research; H.G., B.-T.M., G.-C.L., Z.-L.L., L.-Q.H., Y.-L.S., J.-F.D., and C.-Z.W. performed research; H.G., L.-Q.H., and C.-Z.W. contributed new reagents/analytic tools; H.G., B.-T.M., G.-C.L., and C.-Z.W. analyzed data; and H.G., D.P.S., and C.-Z.W. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Dhillon M. K., Sharma H. C., Survival and development of Campoletis chlorideae on various insect and crop hosts: Implications for Bt-transgenic crops. J. Appl. Entomol. 131, 179–185 (2007). [Google Scholar]
- 2.You L.-S., Lei L.R.-H., Jiang J.-X., Lian-Yang B. O., Xiao Z.-S., Bionomic of Campoletis chlorideae (Hym: Ichneumonidae) as a parasitoid of the cotton bollworm Helicoverpa armigera (Lep: Noctuidae). Insect Sci. 9, 29–37 (2002). [Google Scholar]
- 3.Shylesha A. N., Studies on new invasive pest Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) and its natural enemies. J. Biol. Control. 32, 145–151 (2018). [Google Scholar]
- 4.Yan Z.-G., Wang C.-Z., Identification of Mythmna separata-induced maize volatile synomones that attract the parasitoid Campoletis chlorideae. J. Appl. Entomol. 130, 213–219 (2006). [Google Scholar]
- 5.Han L.-B., Yin L.-H., Huang L.-Q., Wang C.-Z., Differential immunosuppression by Campoletis chlorideae eggs and ichnovirus in larvae of Helicoverpa armigera and Spodoptera exigua. J. Invertebr. Pathol. 130, 88–96 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Han L.-B., Huang L.-Q., Wang C.-Z., Host preference and suitability in the endoparasitoid Campoletis chlorideae is associated with its ability to suppress host immune responses. Ecol. Entomol. 38, 173–182 (2013). [Google Scholar]
- 7.Tian S.-P., Zhang J.-H., Wang C.-Z., Cloning and characterization of two Campoletis chlorideae ichnovirus vankyrin genes expressed in parasitized host Helicoverpa armigera. J. Insect Physiol. 53, 699–707 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Guo H., Wang C.-Z., The ethological significance and olfactory detection of herbivore-induced plant volatiles in interactions of plants, herbivorous insects, and parasitoids. Arthropod Plant Interact. 13, 161–179 (2019). [Google Scholar]
- 9.Yan Z.-G., Wang C.-Z., Similar attractiveness of maize volatiles induced by Helicoverpa armigera and Pseudaletia separata to the generalist parasitoid Campoletis chlorideae. Entomol. Exp. Appl. 118, 87–96 (2006). [Google Scholar]
- 10.Ode P. J., Hardy I. C. W., "Parasitoid sex ratios and biological control" in Behavioral Ecology of Insect Parasitoids, Wajnberg É., Bernstein C., van Alphen J., Eds. (John Wiley & Sons Ltd, 2008), pp. 253–291. [Google Scholar]
- 11.Karlson P., Lüscher M., ‘Pheromones’: A new term for a class of biologically active substances. Nature 183, 55–56 (1959). [DOI] [PubMed] [Google Scholar]
- 12.Yew J. Y., Chung H., Insect pheromones: An overview of function, form, and discovery. Prog. Lipid Res. 59, 88–105 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Ando T., Yamakawa R., Analyses of lepidopteran sex pheromones by mass spectrometry. TrAC Trends Anal. Chem. 30, 990–1002 (2011). [Google Scholar]
- 14.Vanderwel D., Oehlschlager A. C., "6 - Biosynthesis of pheromones and endocrine regulation of pheromone production in Coleoptera" in Pheromone Biochemistry, Prestwich G. D., Blomquist G. J., Eds. (Academic Press, 1987), pp. 175–215. [Google Scholar]
- 15.Forbes A. A., Bagley R. K., Beer M. A., Hippee A. C., Widmayer H. A., Quantifying the unquantifiable: Why Hymenoptera, not Coleoptera, is the most speciose animal order. BMC Ecol. 18, 21 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Askew R. R., Parasitic Insects (Heinemann, London, 1971). [Google Scholar]
- 17.Ayasse M., Paxton R. J., Tengö J., Mating behavior and chemical communication in the order Hymenoptera. Annu. Rev. Entomol. 46, 31–78 (2001). [DOI] [PubMed] [Google Scholar]
- 18.Böttinger L. C., Hofferberth J., Ruther J., Stökl J., Semiochemicals mediating defense, intraspecific competition, and mate finding in Leptopilina ryukyuensis and L. japonica (Hymenoptera: Figitidae), parasitoids of Drosophila. J. Chem. Ecol. 45, 241–252 (2019). [DOI] [PubMed] [Google Scholar]
- 19.Weiss I., et al. , A nonspecific defensive compound evolves into a competition avoidance cue and a female sex pheromone. Nat. Commun. 4, 2767 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delury N. C., Gries G., Gries R., Judd G. J. R., Brown J. J., Sex pheromone of Ascogaster quadridentata, a parasitoid of Cydia pomonella. J. Chem. Ecol. 25, 2229–2245 (1999). [Google Scholar]
- 21.Xu H., et al. , The combined use of an attractive and a repellent sex pheromonal component by a gregarious parasitoid. J. Chem. Ecol. 45, 559–569 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Xu H., et al. , Distinct roles of cuticular aldehydes as pheromonal cues in two Cotesia parasitoids. J. Chem. Ecol. 46, 128–137 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Ruther J., Stahl L. M., Steiner S., Garbe L. A., Tolasch T., A male sex pheromone in a parasitic wasp and control of the behavioral response by the female’s mating status. J. Exp. Biol. 210, 2163–2169 (2007). [DOI] [PubMed] [Google Scholar]
- 24.Ruther J., Steiner S., Garbe L.-A., 4-Methylquinazoline is a minor component of the male sex pheromone in Nasonia vitripennis. J. Chem. Ecol. 34, 99–102 (2008). [DOI] [PubMed] [Google Scholar]
- 25.Niehuis O., et al. , Behavioural and genetic analyses of Nasonia shed light on the evolution of sex pheromones. Nature 494, 345–348 (2013). [DOI] [PubMed] [Google Scholar]
- 26.Cônsoli F. L., Williams H. J., Vinson S. B., Matthews R. W., Cooperband M. F., Trans-bergamotenes—male pheromone of the ectoparasitoid Melittobia digitata. J. Chem. Ecol. 28, 1675–1689 (2002). [DOI] [PubMed] [Google Scholar]
- 27.Steiner S., Hermann N., Ruther J., Characterization of a female-produced courtship pheromone in the parasitoid Nasonia vitripennis. J. Chem. Ecol. 32, 1687–1702 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Ruther J., Döring M., Steiner S., Cuticular hydrocarbons as contact sex pheromone in the parasitoid Dibrachys cavus. Entomol. Exp. Appl. 140, 59–68 (2011). [Google Scholar]
- 29.Würf J., Pokorny T., Wittbrodt J., Millar J. G., Ruther J., Cuticular hydrocarbons as contact sex pheromone in the parasitoid wasp Urolepis rufipes. Front. Ecol. Evol. 8, 180 (2020). [Google Scholar]
- 30.Fauvergue X., Hopper K. R., Antolin M. F., Mate finding via a trail sex pheromone by a parasitoid wasp. Proc. Natl. Acad. Sci. U.S.A. 92, 900–904 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kühbandner S., Bello J. E., Mori K., Millar J. G., Ruther J., Elucidating structure-bioactivity relationships of methyl-branched alkanes in the contact sex pheromone of the parasitic wasp Lariophagus distinguendus. Insects 4, 743–760 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Böttinger L. C., Stökl J., Dispersal from natal patch correlates with the volatility of female sex pheromones in parasitoid wasps. Front. Ecol. Evol. 8, (2020). [Google Scholar]
- 33.Hansson B. S., Stensmyr M. C., Evolution of insect olfaction. Neuron 72, 698–711 (2011). [DOI] [PubMed] [Google Scholar]
- 34.Hallem E. A., Carlson J. R., Coding of odors by a receptor repertoire. Cell 125, 143–160 (2006). [DOI] [PubMed] [Google Scholar]
- 35.Su C.-Y., Menuz K., Carlson J. R., Olfactory perception: Receptors, cells, and circuits. Cell 139, 45–59 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Touhara K., Vosshall L. B., Sensing odorants and pheromones with chemosensory receptors. Annu. Rev. Physiol. 71, 307–332 (2009). [DOI] [PubMed] [Google Scholar]
- 37.Joseph R. M., Carlson J. R., Drosophila chemoreceptors: A molecular interface between the chemical world and the brain. Trends Genet. 31, 683–695 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leal W. S., Odorant reception in insects: Roles of receptors, binding proteins, and degrading enzymes. Annu. Rev. Entomol. 58, 373–391 (2013). [DOI] [PubMed] [Google Scholar]
- 39.Ai M., et al. , Acid sensing by the Drosophila olfactory system. Nature 468, 691–695 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benton R., Vannice K. S., Gomez-Diaz C., Vosshall L. B., Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell 136, 149–162 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hallem E. A., Ho M. G., Carlson J. R., The molecular basis of odor coding in the Drosophila antenna. Cell 117, 965–979 (2004). [DOI] [PubMed] [Google Scholar]
- 42.Jones W. D., Cayirlioglu P., Grunwald Kadow I., Vosshall L. B., Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature 445, 86–90 (2007). [DOI] [PubMed] [Google Scholar]
- 43.del Mármol J., Yedlin M. A., Ruta V., The structural basis of odorant recognition in insect olfactory receptors. Nature 597, 126–131 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Larsson M. C., et al. , Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron 43, 703–714 (2004). [DOI] [PubMed] [Google Scholar]
- 45.Sato K., et al. , Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature 452, 1002–1006 (2008). [DOI] [PubMed] [Google Scholar]
- 46.Wicher D., et al. , Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature 452, 1007–1011 (2008). [DOI] [PubMed] [Google Scholar]
- 47.Fleischer J., Krieger J., Insect pheromone receptors – key elements in sensing intraspecific chemical signals. Front. Cell. Neurosci. 12, 425 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ha T., Smith D., Odorant and pheromone receptors in insects. Front. Cell. Neurosci. 3, 10 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang X.-J., et al. , Sequence similarity and functional comparisons of pheromone receptor orthologs in two closely related Helicoverpa species. Insect Biochem. Mol. Biol. 48, 63–74 (2014). [DOI] [PubMed] [Google Scholar]
- 50.Liu Y., Liu C., Lin K., Wang G., Functional specificity of sex pheromone receptors in the cotton bollworm Helicoverpa armigera. PLoS One 8, e62094 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang K., Wang C.-Z., Review of pheromone receptors in heliothine species: Expression, function, and evolution. Entomol. Exp. Appl. 169, 156–171 (2021). [Google Scholar]
- 52.Wanner K. W., et al. , A honey bee odorant receptor for the queen substance 9-oxo-2-decenoic acid. Proc. Natl. Acad. Sci. U.S.A. 104, 14383–14388 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pask G. M., et al. , Specialized odorant receptors in social insects that detect cuticular hydrocarbon cues and candidate pheromones. Nat. Commun. 8, 297 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun Y.-L., et al. , An odorant receptor mediates the attractiveness of cis-jasmone to Campoletis chlorideae, the endoparasitoid of Helicoverpa armigera. Insect Mol. Biol. 28, 23–34 (2019). [DOI] [PubMed] [Google Scholar]
- 55.Turlings T. C. J., Erb M., Tritrophic interactions mediated by herbivore-induced plant volatiles: Mechanisms, ecological relevance, and application potential. Annu. Rev. Entomol. 63, 433–452 (2018). [DOI] [PubMed] [Google Scholar]
- 56.Cowan D. P., Stahlhut J. K., Functionally reproductive diploid and haploid males in an inbreeding hymenopteran with complementary sex determination. Proc. Natl. Acad. Sci. U.S.A. 101, 10374–10379 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Robacker D. C., Hendry L. B., Neral and geranial: Components of the sex pheromone of the parasitic wasp, Itoplectis conquisitor. J. Chem. Ecol. 3, 563–577 (1977). [Google Scholar]
- 58.Collatz J., Tolasch T., Steidle J. L. M., Mate finding in the parasitic wasp Cephalonomia tarsalis (Ashmead): More than one way to a female’s heart. J. Chem. Ecol. 35, 761–768 (2009). [DOI] [PubMed] [Google Scholar]
- 59.Swedenborg P. D., Jones R. L., (Z)-4-Tridecenal, a pheromonally active air oxidation product from a series of (Z,Z)-9,13 dienes in Macrocentrus grandii Goidanich (Hymenoptera: Braconidae). J. Chem. Ecol. 18, 1913–1931 (1992). [DOI] [PubMed] [Google Scholar]
- 60.Kainoh Y., Mating behavior of Ascogaster reticulatus Watanabe (Hymenoptera: Braconidae), an egg-larval parasitoid of the smaller tea tortrix, Adoxophyes sp. (Lepidoptera:Tortricidae): III. identification of a sex pheromone. Appl. Entomol. Zool. 26, 543–549 (1991). [Google Scholar]
- 61.Nichols W. J., Cossé A. A., Bartelt R. J., King B. H., Methyl 6-methylsalicylate: A female-produced pheromone component of the parasitoid wasp Spalangia endius. J. Chem. Ecol. 36, 1140–1147 (2010). [DOI] [PubMed] [Google Scholar]
- 62.Cossé A. A., et al. , Female-produced sex pheromone of Tetrastichus planipennisi, a parasitoid introduced for biological control of the invasive emerald ash borer, Agrilus planipennis. J. Chem. Ecol. 46, 508–519 (2020). [DOI] [PubMed] [Google Scholar]
- 63.Ando T., Inomata S., Yamamoto M., "Lepidopteran sex pheromones" in The Chemistry of Pheromones and Other Semiochemicals I, Topics in Current Chemistry, Schulz S., Ed. (Springer, 2004), pp. 51–96. [DOI] [PubMed] [Google Scholar]
- 64.Rao S., DeBach P., Experimental studies on hybridization and sexual isolation between some Aphytis species (Hymenoptera: Aphelinidae): I. Experimental hybridization and an interpretation of evolutionary relationships among the species. Hilgardia 39, 515–553 (1969). [DOI] [PubMed] [Google Scholar]
- 65.Weseloh R. M., Dufour’s gland: Source of sex pheromone in a hymenopterous parasitoid. Science 193, 695–697 (1976). [DOI] [PubMed] [Google Scholar]
- 66.Vinson S. B., Courtship behavior and evidence for a sex pheromone in the parasitoid Campoletis sonorensis (Hymenoptera: Ichneumonidae). Environ. Entomol. 1, 409–414 (1972). [Google Scholar]
- 67.Xu H., Veyrat N., Degen T., Turlings T. C. J., Exceptional use of sex pheromones by parasitoids of the genus Cotesia: Males are strongly attracted to virgin females, but are no longer attracted to or even repelled by mated females. Insects 5, 499–512 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sprenger P. P., Menzel F., Cuticular hydrocarbons in ants (Hymenoptera: Formicidae) and other insects: How and why they differ among individuals, colonies, and species. Myrmecol. News 30, 1–26 (2020). [Google Scholar]
- 69.Haverkamp A., Hansson B. S., Knaden M., Combinatorial codes and labeled lines: How insects use olfactory cues to find and judge food, mates, and oviposition sites in complex environments. Front. physiol. 9, 49 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guo H., Huang L.-Q., Gong X.-L., Wang C.-Z., Comparison of functions of pheromone receptor repertoires in Helicoverpa armigera and Helicoverpa assulta using a Drosophila expression system. Insect Biochem. Mol. Biol. 141, 103702 (2022). [DOI] [PubMed] [Google Scholar]
- 71.Zhou X., et al. , Chemoreceptor evolution in Hymenoptera and its implications for the evolution of eusociality. Genome. Biol. Evol. 7, 2407–2416 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Robertson H. M., Gadau J., Wanner K. W., The insect chemoreceptor superfamily of the parasitoid jewel wasp Nasonia vitripennis. Insect Mol. Biol. 19, 121–136 (2010). [DOI] [PubMed] [Google Scholar]
- 73.Wäschke N., Meiners T., Rostás M., "Foraging strategies of parasitoids in complex chemical environments" in Chemical Ecology of Insect Parasitoids, Wajnberg E., Colazza S., Eds. (John Wiley & Sons Ltd, 2013), pp. 37–63. [Google Scholar]
- 74.Wang Y., et al. , Molecular basis of peripheral olfactory sensing during oviposition in the behavior of the parasitic wasp Anastatus japonicus. Insect Biochem. Mol. Biol. 89, 58–70 (2017). [DOI] [PubMed] [Google Scholar]
- 75.Shan S., A female-biased odorant receptor tuned to the lepidopteran sex pheromone in parasitoid Microplitis mediator guiding habitat of host insects. J. Adv. Res., 10.1016/j.jare.2022.03.006 (2022) (September 4, 2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Choo Y.-M., et al. , Reverse chemical ecology approach for the identification of an oviposition attractant for Culex quinquefasciatus. Proc. Natl. Acad. Sci. U.S.A. 115, 714–719 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kugimiya S., Shimoda T., Wajnberg E., Uefune M., Takabayashi J., Host-searching responses to herbivory-associated chemical information and patch use depend on mating status of female solitary parasitoid wasps. Ecol. Entomol. 35, 279–286 (2010). [Google Scholar]
- 78.Michaud J. P., Mackauer M., Oviposition behavior of Monoctonus paulensis (Hymenoptera: Aphidiidae): Factors influencing reproductive allocation to hosts and host patches. Ann. Entomol. Soc. Am. 88, 220–226 (1995). [Google Scholar]
- 79.Tagawa J., Yoshida C., Hashimoto T., Sudare A., Effects of mating on the oviposition behaviour of the parasitic wasp, Apanteles glomeratus L. (Hymenoptera: Braconidae). J. Ethol. 5, 37–41 (1987). [Google Scholar]
- 80.Xu H., Turlings T. C. J., Plant volatiles as mate-finding cues for insects. Trends Plant Sci. 23, 100–111 (2018). [DOI] [PubMed] [Google Scholar]
- 81.Suckling D. M., Gibb A. R., Burnip G. M., Delury N. C., Can parasitoid sex pheromones help in insect biocontrol? A case study of codling moth (Lepidoptera: Tortricidae) and its parasitoid Ascogaster quadridentata (Hymenoptera: Braconidae). Environ. Entomol. 31, 947–952 (2002). [Google Scholar]
- 82.Guo H., et al. , Functional analysis of pheromone receptor repertoire in the fall armyworm, Spodoptera frugiperda. Pest Manag. Sci. 78, 2052–2064 (2022). [DOI] [PubMed] [Google Scholar]
- 83.Guo H., Guo P.-P., Sun Y.-L., Huang L.-Q., Wang C.-Z., Contribution of odorant binding proteins to olfactory detection of (Z)-11-hexadecenal in Helicoverpa armigera. Insect Biochem. Mol. Biol. 131, 103554 (2021). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
All study data are included in the article and/or SI Appendix.