ABSTRACT
Background:
Drug-resistant tuberculosis (DR-TB) is the most exigent and calamitous challenge encountered in treatment of TB. Extra pulmonary (EP) DR-TB poses a complex diagnostic and therapeutic challenge owing to myriad of presentations and paucibacillary nature. Data available on this subset is limited. We studied the prevalence of EPDR-TB cases among the total DR-TB cases visiting our Programmatic management of Drug-Resistant TB (PMDT) site. We also studied the demographic and microbiological profile of these cases and analyzed the prevalence of pre-extensively drug-resistant TB (pre XDR-TB) and extensively drug-resistant TB (XDR-TB) among patients of EPDR-TB in pre Bdq era.
Results:
Of the 1086 DR-TB patients, 64 (5.89%) were cases of EPDR-TB. Seven out of 64 (10.93%) were primary EPDR-TB. The site wise distribution of cases was 34 (53.125%) lymph node DR-TB, 18 (28.125%) pleural DR-TB, 9 (14.0625%) spinal DR-TB/paraspinal abscess/psoas abscess, 1 case (1.5625%) each of abdominal DR-TB, sternal and gluteal abscess. On the basis of the second-line drug susceptibility testing (DST), patients were grouped into: (1) multidrug-resistant TB (MDR-TB), (2) MDR-TB with fluoroquinolone (FQ) resistance {pre XDR XDR-TB (FQ)}, (3) MDR-TB with second-line injectable (SLI) resistance {pre XDR XDR-TB (SLI)}, (4) XDR-TB. Of the 64 patients, 43 (67.185%) had MDR-TB, 19 (29.687%) had preXDR-TB (FQ), none had preXDR-TB (SLI) and 2 (3.125%) had XDR-TB. Gastro esophageal reflux disease (GERD) was the most common comorbidity seen in 26 (40.6%) patients, followed by anemia in 5 (7.8%), psychiatry problems 5 (7.8%), hypertension in 3 (4.6%), renal disorders in 2 (3.1%) while thyroid disorder, HIV and thalassemia in 1 each (1.5%).
Conclusion:
EPDR-TB forms a small but significant proportion of total DR-TB. Lymph node DR-TB is its most common subclass. Our study emphasises the momentousness and essentiality of baseline DST to FQ and SLI in patients of DR-TB. This enables an appropriate modification of therapy at baseline itself to better the treatment outcomes.
We observed a strikingly high proportion of preXDR-TB (FQ) in our study group.
KEY WORDS: BDQ, EPDR-TB, Pre XDR XDR-TB, second-line DST, XDR-TB
INTRODUCTION
The prevalence of the tuberculosis (TB) in India is consequential with its contribution to approximately one fourth of the global burden of the disease.[1] Anti TB therapy (ATT) has served as a potent weapon to combat this contagion. Treatment of drug sensitive TB is executed with short-course chemotherapy with first-line anti TB drugs, such as isoniazid (H), rifampicin (R), pyrazinamide (Z), ethambutol (E), and streptomycin (S) are extremely efficacious with a significant cure rate.[2] However, the soaring intimidation of drug resistance in TB jeopardizes all the measures for its effective control. Drug-resistant TB (DR-TB) is further classified as primary or secondary drug resistance depending on the previous exposure to anti TB drugs.[3] TB is further classified as rifampicin resistant TB which is an infection with TB bacilli resistant to atleast R. Multidrug resistant TB (MDR-TB) which is an infection with TB bacilli resistant to both H and R. Depending on the further drug susceptibility testing (DST) for second-line anti TB drugs, MDR-TB is sub-classified as pre-extensively drug-resistant tuberculosis (Pre XDR-TB) and extensively drug-resistant tuberculosis (XDR-TB). Pre XDR-TB is defined as additional resistance to second-line drugs either fluoroquinolone (FQ), or to at least one of the three injectable second-line injectables {amikacin, kanamycin, and capreomycin (SLI)}. The former is called as pre XDR-TB (FQ) while the latter called as pre XDR-TB (SLI) in pre Bedaquiline era. XDR-TB is defined as MDR-TB with additional resistance to both FQ and SLI.[4] DR-TB is predominantly a solemn man made problem culminating from various factors microbiological, programmatic, doctor, and patient-related factors.[5,6] TB can majorly be classified as pulmonary and extra pulmonary (EP) depending on the presence or absence of lung parenchymal involvement. Extra pulmonary TB (EPTB) is a non-infectious and paucibacillary disease and the affected patients are non-infectious. EPTB accounts for approximately 15% of the total TB burden.[7] However, drug resistance in EPTB is a formidable challenge owing to its heterogenous presentations and barricades in sample procurement for microbiological diagnosis leading to a delay in therapy initiation. The therapy universally available for the management of MDR PTB as well as EPPTB is a DST based therapy under the programmatic management of drug-resistant TB (PMDT) guidelines. Among the second-line drugs, however, the World Health Organization (WHO) endorses baseline testing for FQ and SLI only due reliable and standardized in vitro tests. This standpoint is based on the rationale that a baseline DST enables appropriate modification of the therapy at commencement itself and saliently betters the treatment outcomes.[8] But there is paucity of data pertaining to pattern of baseline drug resistance in EPTB and most of the available studies focus on total MDR or pulmonary MDR-TB patients. Extra pulmonary drug-resistant tuberculosis (EPDR-TB) patients consist of a heterogeneous cohort and accurate cataloging of its attributes is a fundamental step toward a successful management.[9] Hence we conducted this study with an endeavor to study the prevalence, demographic, clinical and microbiological profile of EPDR-TB patients under the programmatic settings with the predominant purpose of studying the prevalence of preXDR-TB and XDR-TB in the study group.
METHODOLOGY
A prospective study was conducted over a period of two years in a tertiary care hospital and its associated PMDT center after ethics committee approval in pre BDQ era. We enrolled the total number of MDR-TB patients who were registered with our PMDT over a two year period from October 01, 2013. Patients of/above 18 years of age and who were able to give a valid and appropriate consent were included. Patients below age of 18 and patients having concomitant pulmonary and extrapulmonary involvement were excluded. Detailed history was taken including past history of TB/history of prior exposure to antituberculosis therapy (ATT) in the past and history of other comorbidities. Blood investigations such as complete blood count with platelets, blood sugar to screen for diabetes mellitus, liver function tests, blood urea, and creatinine to assess the kidney function, thyroid function, screening report for HIV from integrated counseling and testing center and a chest radiograph were performed. The preferred choices of tests for rapid diagnosis of TB and detection drug resistance were as per the PMDT guidelines and in concordance with the logistics of Revised National Tuberculosis Control Program (RNTCP). These were the cartridge-based nucleic acid amplification test (CBNAAT) which detects only R resistance and the first-line line probe assay (LPA) (detects H and R resistance). Baseline second-line liquid culture DST has also been recently integrated in the RNTCP diagnostic algorithm. Second-line rapid DST for SLI and FQ was done by either second-line LPA or liquid culture method (mycobacterial growth indicator tube method) from a RNTCP accredited laboratory in Mumbai. On the basis of the available DST, patients were grouped into: (1) MDR-TB, (2) MDR-TB with FQ resistance {pre XDR-TB (FQ)}, (3) MDR-TB with SLI resistance {pre XDR-TB (SLI)}, and (4) XDR-TB. Data collected were analyzed statistically in the form of frequency and percentage. The final data was reported as prevalence of XDR-TB, pre XDR-TB (FQ) and pre XDR-TB (SLI) in cases of EPTB MDR. The patients were treated according to national PMDT guidelines.
RESULTS
Of the 1086 DR-TB patients, 64 were cases of EPDR-TB were included in the study. Thus prevalence of EPDR-TB calculated is 5.89%. Our study population consisted of 42 female and 22 male, i.e. 65.625% patients were female and 34.375% were male. Male to female ratio was 1.90:1, i.e. female preponderance was seen among EPDR-TB patients. Among the 64 patients of EPDR-TB; majority (76.56%, 49 patients) were in the age group 18–30 years, 12 patients (18.75%) were from age group 31–40 years, 2 patients were from group 41–50 years and only 1 patient was >60 years of age. No patients included were from the age group 51–60 years. Mean age among female patients (22.80 years) was less than that of males (31.5 years). Lymph node was the most common site of EPTB followed by pleura. About 34 cases (53.125%) of total 64 were of lymph node DR-TB. Pleural DR-TB contributed to 18 cases (28.125%). Spinal DR-TB/Paraspinal abscess/psoas abscess consisted of 9 cases (14.0625%). Abdominal DR-TB, sternal and gluteal abscess contributed to 1 (1.5625%) case each [Figure 1]. In cases of lymph node DR-TB the most common site of affection was cervical group of lymph nodes. Cervical nodes were involved in 27 of the 34 patients of lymph node, i.e. in 79.40%. Other groups of lymph nodes involved were mediastinal, abdominal, axillary, and inguinal in 3, 2, 1, and 1 patient, respectively, i.e. in 8.8235%, 5.88%, 2.94%, and 2.94%, respectively. Table 1 enlists the site of EPTB. Seven of the 64 patients were cases of primary EPDR-TB, i.e. who did not had any prior history of exposure to ATT. The rest 57 patients had history of previous ATT taken. Out of those 7 patients, 6 were MDR-TB, and 1 was pre XDR-TB (FQ). Hence, the prevalence of primary EPDR-TB in our study is 10.9375%. Among the 64 cases of DR EPTB the DSTs revealed: 43 (67.185%) EP MDR-TB, 19 (29.687%) EP pre XDR-TB (FQ), no pre XDR-TB (SLI), 2 (3.125%) EP XDR-TB [Figure 2]. Table 2 elaborates the DST status as per the EP site. Out of the 64 patients, 21 (32.8%) did not have any comorbidity. Gastro esophageal reflux disease (GERD) was the most common comorbidity seen in 26 (40.6) patients followed by anemia and psychiatry problems in 5 (7.8%) patients each. Other less common comorbidities associated were hypertension in 3 (4.6%), renal disorders in 2 (3.1%), thyroid disorder (hyperthyroid), HIV and thalassemisin 1 (3.1%), each [Figure 3].
Figure 1.
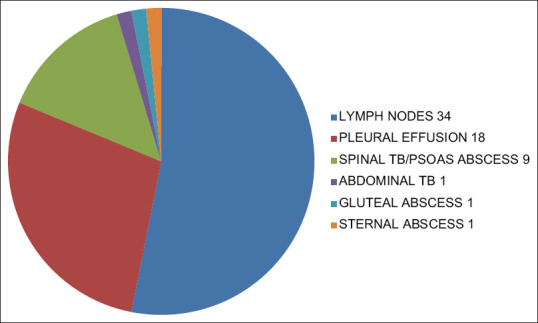
Site wise distribution of EPTB MDR in our study group
Table 1.
Percentage of individual EPTB site of involvement
| Disease | Percentage (%) |
|---|---|
| Lymph node | 53.125 |
| Pleural effusion | 28.125 |
| Spinal tb/paraspinal abscess/psoas abscess | 14.0625 |
| Sternal abscess | 1.5625 |
| Gluteal abscess | 1.5625 |
| Abdominal tb | 1.5625 |
Figure 2.
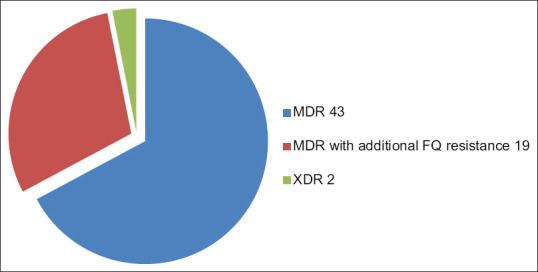
Division of patients among three groups MDR, Pre XDR (FQ), XDR
Table 2.
Percentage prevalence of MDR, MDR with additional FQ resistance, XDR in our study
| Category | Total | Prevalance (%) |
|---|---|---|
| MDR | 43 | 67.185% |
| Pre XDR (FQ) resistance | 19 | 29.687% |
| XDR | 2 | 3.125% |
Figure 3.
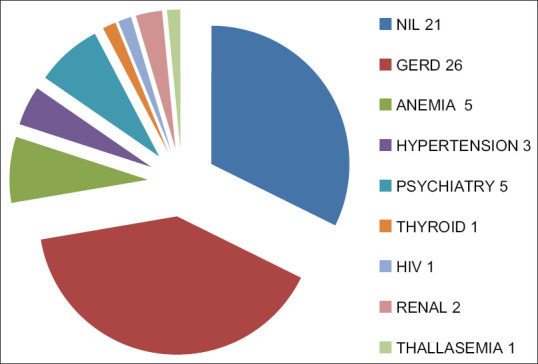
Various comorbidities seen among our EPTB MDR patients. EPTB: Extrapulmonary Tuberculosis. MDR EPTB: Multidrug Resistant Extrapulmonary Tuberculosis. Pre XDR (FQ) EPTB: Pre Extensively Drug-Resistant Extrapulmonary Tuberculosis cases with additional Fluoroquinolone resistance. XDR EPTB: Extensively Drug-Resistant Extrapulmonary Tuberculosis
DISCUSSION
Drug resistance in TB is the most critical challenge in the effective control of TB in the current era. There is abundance of literature pertaining to pulmonary DR-TB however on the other hand there is dejecting scarcity of data on EPDR-TB. Our study is unique in this fact that it focuses on EPDR-TB and uncovers various facets of its DST pattern based sub-classification and demographic aspects. The prevalence of EPDR-TB among DR-TB patients observed in our study was 5.89%. This prevalence is comparable and only slightly higher than the results in the Maharashtra data under PMDT (2011–2012cohort) which had 3% of EP DR-TB cases.[10] Comparing with the international data, one of the US-based studies from 1993–2006 had a total of 2664 MDR patients out of which 299 were EPTB MDR, i.e. 11%.[11] This US-based study had estimated slightly more prevalence than our study which could be because of several reasons like longer study period and the larger number of cases included. The prevalence data from our study gives us an insight into the distribution of EPDR-TB in Mumbai. There was a female preponderance observed in our study. Female to male ratio was 1.9:1. This female predominance among EPDR-TB patients has been documented by several studies earlier in literature.[12] While many other studies have shown a male predominance among DR-TB patients, but these studies have focused on overall DR-TB patients without the subdivision of DR-TB patients into pulmonary and extra pulmonary.[13-15] In a study by Mukherjee et al.,[16] 61% affected patients were men. A study by Dholakia and Shah,[15] showed male to female patient ratio of 1:1, however only 34 patients were a part of this study. Our study had majority (76%) of EPDR-TB cases in the younger age group (19–30 years) and the mean age was 26.5 years. This is in agreement with other studies done in various parts of India like Kolkota and Mumbai on DR-TB patients where majority of patients (upto 70%) were in the mean age between 25–35 years. Mean age among female patients was 22.80 years while that of male patients was 31.50 years, which is in agreement with the study done by Mukherjee et al.[16] at Kolkata on sociodemographic and clinical profile of DR-TB patients. The most common site of involvement among EPDR-TB patients in our study was lymph node. About 53% of patients had lymph node involvement. The next most common site involved was pleura (28%). This is consistent with available literature from Maharashtra which states lymph node as the most common site of involvement among EPTB patients, followed by pleural effusion.[10] The US-based study done by Peto et al.[11] also found lymph nodes to be the most common site among EP TB patients (40%) followed by pleural effusion in 20%. Indian studies from our states also reveal that lymph node predominance is seen among EP TB patients. The study by Sharma et al.[14] cites lymph node as the most common site among EP TB patients in 35% patients, the next common being pleural effusion in 20%. Cervical group of lymph nodes were most commonly involved in our group of patients. Cervical lymph nodes involvement was seen in 79% and mediastinal nodes were involved in approximately 9% patients. Above-mentioned various studies also suggest that cervical nodes are most commonly involved followed by mediastinal nodes. We observed that prevalence of primary EPDR-TB was 10.93%. The higher prevalence may be due to the study design as our patient cohort consisted of referrals to a tertiary care center and needs larger population-based studies for confirmation. In India and across the globe prevalence of primary DR-TB has been variably reported, ranging from 1%–5% in most of them. However, there are reports which have shown a higher prevalence of In a study done at Uttar Pradesh the prevalence of primary DR-TB was 13%.[17] Geographical differences in prevalence among various studies done in different parts across India and globe could be a contributing factor. About 89% of EPDR-TB patients in our study had prior exposure to ATT, reiterating the fact that WHO recommended rapid TB diagnostics and management with judicious and guideline-based use of ATT is the need of the hour. Adherence to treatment is the key to prevention of secondary DR-TB. The data on the DST in EP DR-TB is available majorly from a microbiologists perspective from various laboratory-based studies. However, our study provides the distribution of these cases as per DST at a PMDT center of a tertiary care hospital in the field settings. The prevalence of Pre XDR-TB (FQ) was found to be 29% in our study. Data on this subgroup are scarce in literature. Udwadia et al.[13] found prevalence as high as 50% of pre XDR-TB (FQ) and 7% for XDR patients among 78 MDR patients. The prevalence of preXDR-TB (FQ) cases in our study is consistent with studies from Mumbai.[18] However, this percentage is lower than that reported in another study done from our center which was done on our pulmonary DR-TB cases.[1] This difference can be attributed to the difference in prevalence and in the sensivity of DST of Pulmonary DR-TB versus EPDR-TB. The high level of Pre XDR-TB (FQ) can be imputed to the indiscriminate and rampant use of FQ as antibiotics for run-of-the-mill infections. This propagates the selective growth of FQ resistant MTB mutants. This circumstance is compounded by the addition phenomenon in which often FQ are standalone added to a failing regime. We did not find any patient with pre XDR-TB (SLI) in our cohort. Studies by Porwal et al.[19] have reported SLI resistance of 5% in India. The percentage prevalence of EP XDR-TB was 3% in our study. This is in accordance with the prevalence of XDR-TB mentioned in the literature. In a study done at AIIMS, New Delhi, prevalence of XDR-TB was also found to be 2.4%, i.e. in a similar range as our study.[20] Also, this prevaence was slightly lower than the prevalence of XDR-TB among our pulmonary DR-TB patients (4.85%) an earlier in a study done from our department.
Various comorbidities were observed in our study. Out of the 64 patients, 21 (32.8%) did not have any comorbidity. GERD was the most common comorbidity seen in 26 (40.6) patients followed by anemia and psychiatry problems in 5 (7.8%) patients each. Other less common comorbidities associated were hypertension in 3 (4.6%), renal disorders in 2 (3.1%), thyroid disorder (hyperthyroid), HIV and thalassemia in 1 (3.1%), each. It is extremely pertinent to manage comorbidities holistically considering the prolonged nature of therapy and the therapy related adverse drug reactions. This significantly betters the treatment adherence and improves the outcomes. In our study only 1 patient had HIV coinfection. There have been studies finding a positive correlation between HIV and TB/DR TB. Simultaneously there have been eminent studies supporting our finding that HIV does not increase the risk of an individual developing EPDR-TB. A study from AIIMS included 211 patients of DR-TB over a time span of 6 years, all the patients were sero-negative for HIV infection.[20] In a study conducted at Ahmedabad, of the 81 MDR-TB patients, 1 patient was sero-positive for HIV.[21] The US-based study found equal probability of EPTB and PTB among patients infected with HIV which was contrary to the other studies which mention increased risk of EPTB among HIV-infected individuals. We conclude contrary to the prior belief that HIV increases the risk of DR-TB.
Our study is noteworthy from various aspects. The study group selected was only EPDR-TB cases. Our findings provide an insight to the prevalence, demography, drug susceptibility patterns and comorbidities in EPDR-TB patients prompting the need for more population-based studies for the same. We highlight the importance of baseline DST to FQ and SLI in all patients of diagnosed or suspected DR-TB to enable an appropriate therapy modification at baseline itself and thus improve the outcome. However, our study had certain limitations too. It was performed at the single PMDT site attached to a tertiary care hospital in Mumbai. So the results and data cannot be generalized to entire population. Also, a referral bias was unavoidable. We conclude that EPTDR-TB comprises a relatively small but definitely salvageable proportion of the total DR-TB cohort. The microbiological diagnosis although challenging can be achieved by pursuance for a sample for microbiology and rapid DST. Meticulous patient and care giver counseling, timely therapy initiation, direct observation of therapy (DOT), management of comorbidities and prompt addressing of ADRs are the cornerstones of successful management.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Advani S, Desai UD, Joshi JM. Prevalence of pre-extensively drug-resistant tuberculosis (Pre XDR-TB) and extensively drug-resistant tuberculosis (XDR-TB) among pulmonary multidrug resistant tuberculosis (MDR-TB) at a tertiary care center in Mumbai. J Krishna Inst Med Sci Univ. 2016;5:13–9. [Google Scholar]
- 2.Joshi JM. Tuberculosis chemotherapy in the 21st century: Back to Basics. Lung India. 2011;28:193–200. doi: 10.4103/0970-2113.83977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gothi D. Clinical and laboratory observations of tuberculosis at a Mumbai (India) clinic. Postgrad Med J. 2004;80:97–100. doi: 10.1136/pmj.2003.008185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balaji V, Daley P, Anand A, Sudarsanam T, Michael J, Sahni R, et al. Risk factors for MDR and XDR-TB in a tertiary referral hospital in India. PLoS One. 2010;5:e9527. doi: 10.1371/journal.pone.0009527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kant S, Maurya AK, Kushwaha RAS, Nag VL, Prasad R. Multi-drug resistant tuberculosis: An iatrogenic problem. Biosci Trends. 2010;4:48–55. [PubMed] [Google Scholar]
- 6.Sharma SK, Mohan A. Multidrug-resistant tuberculosis: A menace that threatens to destabilize tuberculosis control. Chest. 2006;130:261–72. doi: 10.1378/chest.130.1.261. [DOI] [PubMed] [Google Scholar]
- 7.Sharma SK, Mohan A. Extrapulmonary tuberculosis. Indian J Med Res. 2004;120:316–23. [PubMed] [Google Scholar]
- 8.Waghmare MA, Utpat K, Joshi JM. Treatment outcomes of drug-resistant pulmonary tuberculosis under programmatic management of multidrug-resistant tuberculosis, at tertiary care center in Mumbai. Med J DY Patil Univ. 2017;10:41–5. [Google Scholar]
- 9.Maurya A, Kant S, Nag V, Kushwaha R, Dhole T. Trends of anti-tuberculosis drug resistance pattern in new cases and previously treated cases of extrapulmonary tuberculosis cases in referral hospitals in northern India. J Postgrad Med. 2012;58:185–9. doi: 10.4103/0022-3859.101379. [DOI] [PubMed] [Google Scholar]
- 10.Suryawanshi SL, Shewade HD, Nagaraja SB, Nair SA, Parmar M. Unfavorable outcomes among patients with MDR-TB on standard 24 month regimen in Maharashtra, India. Public Health Action. 2017;7:116–22. doi: 10.5588/pha.17.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peto HM, Pratt RH, Harrington TA, LoBue PA, Armstrong LR. Epidemiology of extrapulmonary tuberculosis in the United States, 1993-2006. Clin Infect Dis. 2009;49:1350–7. doi: 10.1086/605559. [DOI] [PubMed] [Google Scholar]
- 12.Forssbohm M, Zwahlen M, Loddenkemper R, Rieder HL. Demographic characteristics of patients with extrapulmonary tuberculosis in Germany. Eur Respir J. 2008;31:99–105. doi: 10.1183/09031936.00020607. [DOI] [PubMed] [Google Scholar]
- 13.Udwadia ZF, Moharil G. Multidrug-resistant-tuberculosis treatment in the Indian private sector: Results from a tertiary referral private hospital in Mumbai. Lung India. 2014;31:336–41. doi: 10.4103/0970-2113.142101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma SK, Kumar S, Saha PK, George N, Arora SK, Gupta D, et al. Prevalence of multidrug-resistant tuberculosis among Category II pulmonary tuberculosis patients. Indian J Med Res. 2011;133:312–5. [PMC free article] [PubMed] [Google Scholar]
- 15.Dholakia YN, Shah DP. Clinical profile and treatment outcomes of drug-resistant tuberculosis before directly observed treatment strategy plus: Lessons for the program. Lung India. 2013;30:316–20. doi: 10.4103/0970-2113.120608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mukherjee P, Karmakar PR, Basu R, Lahiri SK. Sociodemographic and clinical profile of multi drug resistant tuberculosis patients: A study at drug resistant tuberculosis centers of Kolkata. IOSR J Dent Med Sci. 2015;14:52–8. [Google Scholar]
- 17.Jain A, Mondal R, Prasad R, Singh K, Ahuja RC. Prevalence of multidrug resistant Mycobacterium tuberculosis in Lucknow, Uttar Pradesh. Indian J Med Res. 2008;128:300–6. [PubMed] [Google Scholar]
- 18.Dalal A, Pawaskar A, Das M, Desai R, Prabhudesai P, Chhajed P, et al. Resistance patterns among multi drug resistant tuberculosis patients in greater metropolitan Mumbai: Trends over time. PLoS One. 2015;10:e0116798. doi: 10.1371/journal.pone.0116798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porwal C, Kaushik A, Makkar N, Banavaliker JN, Hanif M, Singla R, et al. Incidence and risk factors for extensively drug-resistant tuberculosis in Delhi region. PLoS One. 2013;8:e55299. doi: 10.1371/journal.pone.0055299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma SK, George N, Kadhiravan T, Saha PK, Mishra HK, Hanif M. Prevalence of extensively drug-resistant tuberculosis among patients with multidrug-resistant tuberculosis: A retrospective hospital-based study. Indian J Med Res. 2009;130:392–5. [PubMed] [Google Scholar]
- 21.Bhatt G, Vyas S, Trivedil K. An epidemiological study of multi drug resistant tuberculosis cases registered under Revised National Tuberculosis Control Program of Ahmedabad City. Indian J Tuberc. 2012;59:18–27. [PubMed] [Google Scholar]


